Effectiveness of simple and basic home-based exercise programs including pediatric massage executed by caregivers at their homes in the management of children with spastic cerebral palsy: A randomized controlled trial
Abstract
PURPOSE:
This study aimed to assess the effectiveness of simple and basic home-based exercise programs (HEPs), including pediatric massage (PM), executed by caregivers at their homes in the management of children with spastic cerebral palsy (CP).
METHODS:
Sixty-eight children with spastic CP (diplegia) aged 4–12 years were randomly assigned to PM and HEP groups for a randomized controlled trial continuing from November 01, 2021 to June 2022. Parents provided home-based exercises to both groups, five times a week for 12 weeks. However, the PM group was additionally provided with PM. Modified Ashworth Scale (MAS), Gross Motor Function Measure (GMFM-88) and Gross Motor Function Classification System (GMFCS) were used for evaluation of spasticity and gross motor activity at baseline as well as after six and 12 weeks of intervention. Comparative analysis of data was carried out with SPSS-20.
RESULTS:
Mean age in HEP and PM groups was 6.65±2.12 and 7.09±2.22 years respectively. Data revealed homogeneity of both groups at the beginning of study. The PM group showed a statistically significant decrease in MAS scores after six and 12 weeks of intervention (p < 0.05) when compared with the HEP group, but similar changes did not happen in GMFM scores and GMFCS levels. However, comparative analysis revealed statistically significant change in GMFM scores and GMFCS levels (p < 0.05) when compared from baseline to 12 weeks of intervention in both groups.
CONCLUSION:
PM along with HEPs can be used effectively to reduce spasticity and to improve gross motor ability if performed for a period of at least six and 12 weeks respectively. In conjunction with HEPs, PM has better outcomes in the management of tone and movement disorders of spastic CP than HEPs alone
1Introduction
Cerebral palsy (CP), initially termed as “insult to the immature brain,” is now an umbrella term comprising neurological conditions that result in disorders of the development of patterns of movement and posture. These disorders lead to activity limitation that is ascribed to mostly non-progressive lesions of the fetal or infant brain. Motor disorders of CP are frequently accompanied by disturbances of perception, sensation, cognition, behavior, and communication, as well as convulsive disorders and musculoskeletal problems [1, 2].
No credible, reliable, and central data regarding prevalence/incidence of CP exists in Pakistan. However, in nearby countries like China and India, its incidence is quite similar to Western countries at 2–2.5/1000 live births. Moreover, it is a well-known fact that obtaining rates of neurological disorders in resource-poor countries is quite difficult [3]. Spasticity and the subtype spastic diplegia, accounting for 60–70% of all tone disorders, are the most common clinical manifestations of CP and result in muscular stiffness, soft tissue contractures, and joint deformities. This eventually limits the affected person’s activities and performance, leading to compromised quality of life [4].
Healthcare professionals use many strategies and interventions to manage the disabling lifelong consequences of CP. In one systematic review, 64 such interventions were identified in current use for the management of CP [5]. However, most of these remain beyond the reach of many due to financial and resource constraints. Conditions in low-income countries are much worse because resources needed for this purpose are mostly not made available by the state. So the disability, which is common in these countries, multiplies its burden manyfold and results in emotional distress and social isolation of those disabilities and their families. In resource-poor countries, developmental disabilities are serious public health concerns, as these have social and economic impacts on families. A study conducted in Pakistan suggested that cost-effective, feasible, and community-level interventions are needed to facilitate healthcare availability and reduce burden on caregivers of children with developmental delay [6]. The World Health Organization has also argued for research into and use of interventions that are accessible, affordable, and achievable by communities in low-income countries [7]. In Pakistan, home-based exercise programs (HEPs) and pediatric massage (PM) fall into this category and should be considered in management strategies as they have no financial implications on caregivers.
In Pakistan, due to the mismatch between patient load and provider availability, daily “hands on” physical therapy services by qualified professionals are not always feasible, available, or affordable for all CP patients presenting in outpatient settings. Consequently, physical therapists have to teach simple and basic exercises to caregivers to be practiced at their homes as an alternative therapeutic approach. On one hand, no standard clinical guidelines are available for healthcare professionals regarding the use of these HEPs. On the other hand, PM is being practiced by caregivers in a way quite different to Swedish massage, which is considered a standard amongst the professional community. Caregivers of children with spastic CP are not trained in the application of PM by healthcare professionals. They practice PM in a manner that they learn traditionally from their community members. No study has been carried out to find the effectiveness of this type of PM and HEP in the management of spastic CP in Pakistan. In a double blind study, Novak et al. observed that home-based exercises are very effective and produce good results and hence should be recommended by health care professionals [8]. However, studies carried out by Hernandez et al. and Alizad about the effectiveness of Swedish massage in the management of spasticity have produced contradictory results and created a need for further research in this field [9, 10]. Many knowledgeable and credible health professionals in Pakistan also differ in opinion about the use of PM in the management of spastic CP, which puts caregivers in a dilemma. Therefore, a randomized controlled trial (RCT) was planned with the aim of investigating the effects of these simple and basic treatment modalities (HEP and PM) in the management of spastic CP.
2Materials and methods
The current study (an RCT) was completed from November 2021 to June 2022. Non-probability, purposive sampling was used for recruitment of the participants, who were randomly allocated through a sealed envelope method to HEP and PM groups. The children included in the study were aged 4–12 years with diagnosis of spastic diplegic CP. After referral from medical health professionals, children were received in the physical therapy department for rehabilitation. All children with soft tissue contractures, deformities, severe mental retardation, attention deficiency syndromes, behavioral ailments, uncontrolled seizures, and degenerative brain disease were excluded.
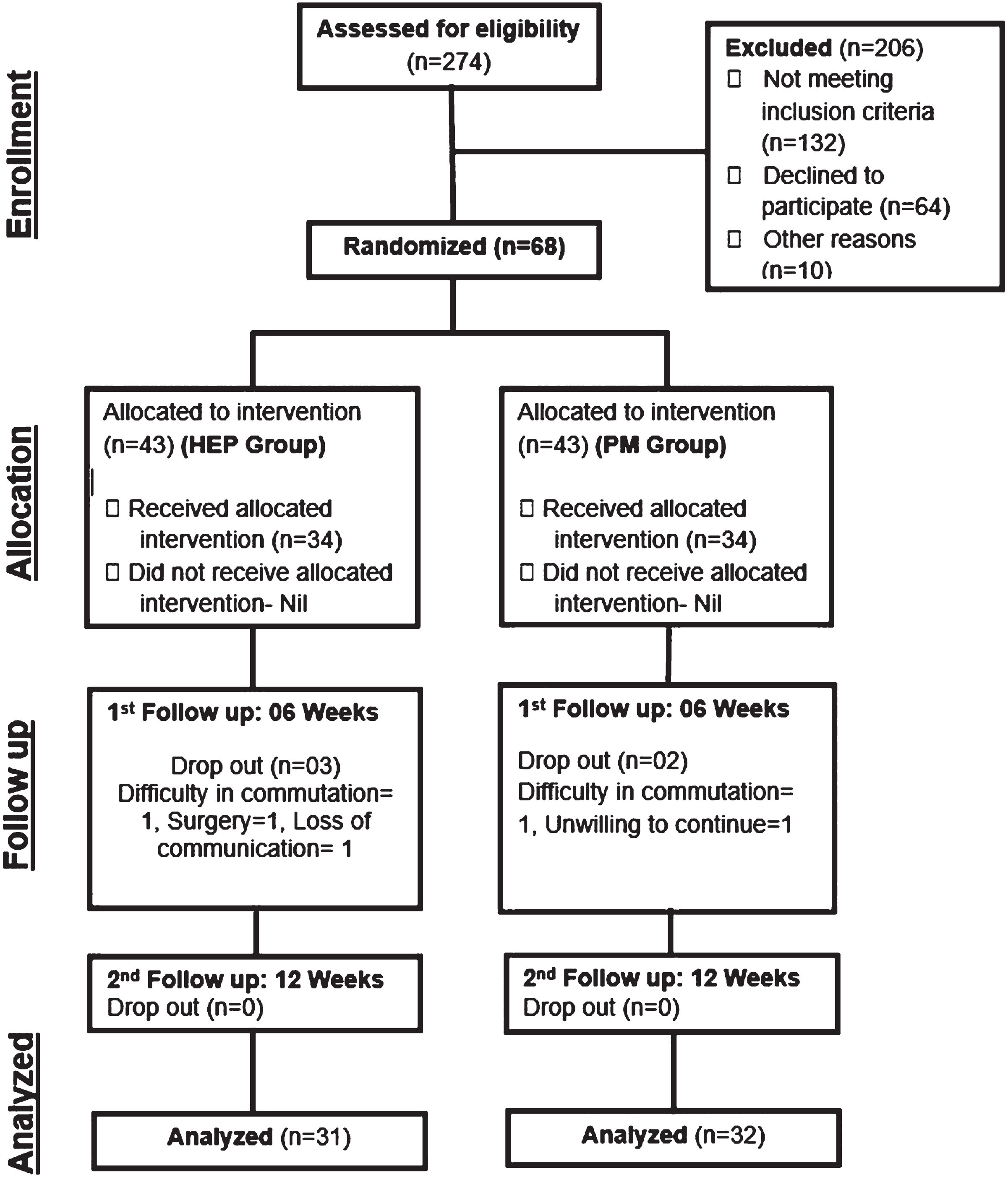
OpenEpi software, version 3, was used for calculation of sample size with a 95% confidence interval and power of 80%; this provided a sample size of 34 participants in each group. The metric of interest in the current study was improvement in motor function, which was assessed using the Gross Motor Function Measure (GMFM). It was planned to use mixed methods ANOVA to compare the two groups with respect to within-subjects changes, between-subjects changes, and interaction effects of these changes with time. Based on previous studies and clinical expertise, an effect size of at least 0.5 standard deviations was anticipated, which is considered a moderate effect size in this context [11]. A study conducted by Elgawish et al. was used as a reference [12]. Thus, 68 children diagnosed with spastic diplegia were recruited to ensure sufficient statistical power to detect the anticipated effect size(Fig. 1).
Fig. 1
Participant flow chart.
Simple and basic HEPs were provided to both groups. These were comprised of a session of 30 minutes duration daily, five times a week for 12 weeks. PM was additionally provided to the PM group; it was comprised of a session of 30 minutes duration before the exercise program. The HEP included a strengthening protocol, stretching protocol, handling, positioning, and gait training. Children were provided with stretching of spastic muscles up to a level of gentle discomfort with hold time of 30 seconds with repetition of five times in a session. Each weak muscle was given resistive exercise, with 10 repetitions in a session. Parents were guided regarding sitting posture of their children with open legs on bench/block so that both heels reached the ground. They were also advised to keep the back of the child straight and to avoid scissoring and W-sitting postures of legs on the floor. Standing against a wall with separated legs (in moderate abduction and lateral rotation) for 15 minutes was also advised.
The massage technique employed in this study, referred to as PM, involved the gentle and soothing application of oil to all four limbs. This process followed a precise pattern of gentle rubbing in a proximal to distal direction for the upper limbs (from shoulder to wrist) and a similar approach for the lower limbs (from hip to knee joint). Additionally, the thoracic area received PM on both the front and back, with a delicate touch extending from the center to the peripheral regions. Notably, the application of this technique was characterized by gentle, superficial, and longitudinal movements, deliberately avoiding the incorporation of deep intermittent pressures, circular driven movements, stroking, and kneading –elements typically associated with Swedish massage. Each limb, along with the front and back of the thoracic area, received five minutes of this specialized pediatric massage.
To ensure the efficacy of the HEPs and PM at home, caregivers underwent comprehensive training. This included hands-on practice under supervision, detailed handouts, visual aids such as still pictures, and video recordings. The objective was to empower caregivers to seamlessly replicate these standardized protocols, ensuring both efficiency and confidence in their application –a crucial aspect of the therapeutic process that aligns with the expectations of physical therapists.
Table 1
Demographic and baseline characteristics of study population
| Demographics | Study | HEP Group | PM Group | P-value |
| Population | (n = 31) | (n = 32) | ||
| (n = 63) | ||||
| Age, mean (SD) | 6.87 (2.16) | 6.65 (2.12) | 7.09 (2.22) | 0.416a |
| Gender, n (%) | 0.687b | |||
| Male | 47 (59) | 19 (61) | 18 (56) | |
| Female | 26 (41) | 12 (39) | 14 (44) | |
| Birth Place, n (%) | 0.424b | |||
| Govt. Setting | 27 (43) | 13 (42) | 14 (44) | |
| Pvt. Setting | 23 (36) | 9 (29) | 14 (44) | |
| Home | 13 (21) | 9 (29) | 4 (12) | |
| Nature of Pregnancy, n (%) | 0.716b | |||
| Full term | 48 (76) | 23 (74) | 25 (78) | |
| Premature | 15 (24) | 8 (26) | 7 (22) | |
| Post-Natal Complications, n (%) | 0.845b | |||
| Fever | 11 (17) | 4 (13) | 7 (18) | |
| Delayed Cry | 33 (52) | 18 (58) | 18 (48) | |
| Ventilation | 3 (5) | 2 (6) | 2 (5) | |
| Others | 16 (26) | 7 (23) | 11 (29) | |
| GMFCS Levels, n (%) | 0.798b | |||
| Level I | 0 (0) | 0 (0) | 0 (0) | |
| Level II | 26 (38) | 14 (41) | 12 (35) | |
| Level III | 24 (35) | 11 (32) | 13 (38) | |
| Level IV | 17 (25) | 9 (27) | 8 (24) | |
| Level V | 1 (2) | 0 (0) | 1 (3) |
a- p value calculation with independent samples t-test. b- p value calculation with chi-squared test. GMFCS- Gross Motor Function Classification System. Percentages have been rounded to whole numbers. HEP- Home exercise program. PM- Pediatric massage.
The Institutional Review Board and Ethics Committee conferred ethical approval and allowed for data collection. Caregivers were informed about the study process, and written consent was secured. They were briefed about the safety of the study involving negligible risk and least chance of any harm to their children. The following questionnaires/scales were used for data collection at baseline, as well as after six and twelve weeks of intervention:
• A self-structured questionnaire recording gender, age, birth/labor history, medical history, family history, parent’s qualification, associated post-natal problems and history of seizures.
• Modified Ashworth Scale (MAS): This scale is mostly used for assessment of spasticity. It entails six grades (0, 1, 1+, 2, 3, 4), where zero is used for normal tone and four for severe spasticity. This is measured in resting supine position by moving the limb through full range of motion; any resistance felt is graded. This scale has well established inter-rater and intra-rater reliability [13]. In this study, the MAS was applied to four muscle groups of upper limbs (finger flexors, wrist flexors, elbow flexors, and shoulder adductors) and four muscle groups of lower limbs (ankle plantar flexors, knee flexors, hip flexors, and hip adductors). Then, mean MAS grades were calculated for each limb by adding the grades of the four muscles of each limb and dividing the total by four to get a mean for that limb (for example, MAS right upper limb). Similarly, MAS grades for all four limbs were computed by adding the scores of all four limbs, then dividing this by four to get a mean grade, called MAS all four limbs.
• GMFM-88: This is typically used for the assessment of gross motor function and consists of 88 items to be assessed in five subdomains: lying and rolling (total items: 17), sitting (total items: 20), crawling/kneeling (total items: 14), standing (total items: 13), and walking/running and Jumping (total items: 24). The relative reliability of this method has been reported as excellent, with an intraclass correlation coefficient (ICC) ranging from 0.952 to 1.000 [14].
• Gross Motor Function Classification System (GMFCS): This consists of five levels which represent the mobility of the participant. Levels I to III are used for ambulatory conditions with or without aids, while levels IV and V are for non-ambulatory status. Its inter-rater and intra-rater reliability is excellent (ICCs = 0.994 and 0.972 respectively) [15].
A single blinded procedure was adopted to ensure that outcome assessors remained unaware of the group allocation of children during all follow-up assessments. SPSS-20 software was used to compare and analyze the data.
3Results
Three (4%) and two (3%) participants dropped out of the HEP and PM groups, leaving 31 (46%) and 32 (47%) respectively. Reasons for dropping out included difficulties in transportation from distant areas, loss of interest in the study, telecommunication problems, and surgery in two participants. One-way ANOVA in MAS grades, GMFCS levels, and GMFM scores at baseline showed no statistically significant differences between the groups, indicating homogeneity with respect to outcome variables. Similarly, demographic and baseline characteristics of both groups such as gender, birth place, gestational duration (full-term or preterm), and post-natal complications were assessed through a chi-squared test, and no significant differences were found between the groups. This showed that the groups were homogenous with respect to these characteristics at the start of the study (Table 1).
Several statistical methods were employed in this study. To compare means between groups, one-way ANOVA was used. For the repeated measures analysis of spasticity and motor function over time, a mixed-effects ANOVA was utilized, taking into account the within-subject correlation. Prior to conducting the analysis, the assumptions of sphericity and normality of residuals were evaluated. Sphericity was assessed using Mauchly’s test, and when violations were detected, the Greenhouse-Geisser correction was applied. Additionally, the distribution of residuals was examined graphically and a Shapiro-Wilk test for normality was conducted. The residuals approximated a normal distribution, validating the assumption for repeated measures analysis. These steps were taken to ensure the validity and reliability of statistical analyses in the study [11].
Table 2
MAS grades (all four limbs)
| Within subjects, between subjects & interaction results (mixed method anova) | ||||||
| Source | SS | DF | MS | F | p-value | Partial Eta-squared |
| Between-subjects | 1.275 | 1 | 1.275 | 2.972 | 0.184 | 0.039 |
| Within-subjects | 6.790 | 2 | 3.395 | 65.539 | 0.000 | 0.473 |
| Interaction Time*group | 0.368 | 2 | 0.184 | 3.552 | 0.031 | 0.046 |
| Comparison between groups (One-way ANOVA, in rows horizontally) and within groups (Repeated measure ANOVA, in columns vertically, top to bottom) | |||
| MAS grades all four limbs | HEP group (Mean±SD) (F = 19.9; DF = 1.8) | PM group (Mean±SD) (F = 49.47; DF = 1.3) | P-value |
| Baseline | 0.90±0.41 | 0.89±0.45 | 0.722 |
| After 6 weeks | 0.81±0.45 | 0.57±0.43 | 0.049 |
| 0.098a | 0.001a | ||
| After 12 weeks | 0.61±0.44 | 0.39±0.35 | 0.019 |
| 0.001b | 0.000b | ||
| 0.000c | 0.000c | ||
a- p value baseline to six weeks (within group). b- p value six to 12 weeks (within group). c- p value baseline to 12 weeks (within group). MAS- Modified Ashworth Scale. SS = sum of squares, DF = degrees of freedom, MS = mean squares, F = F-value. HEP- Home exercise program. PM- Pediatric massage.
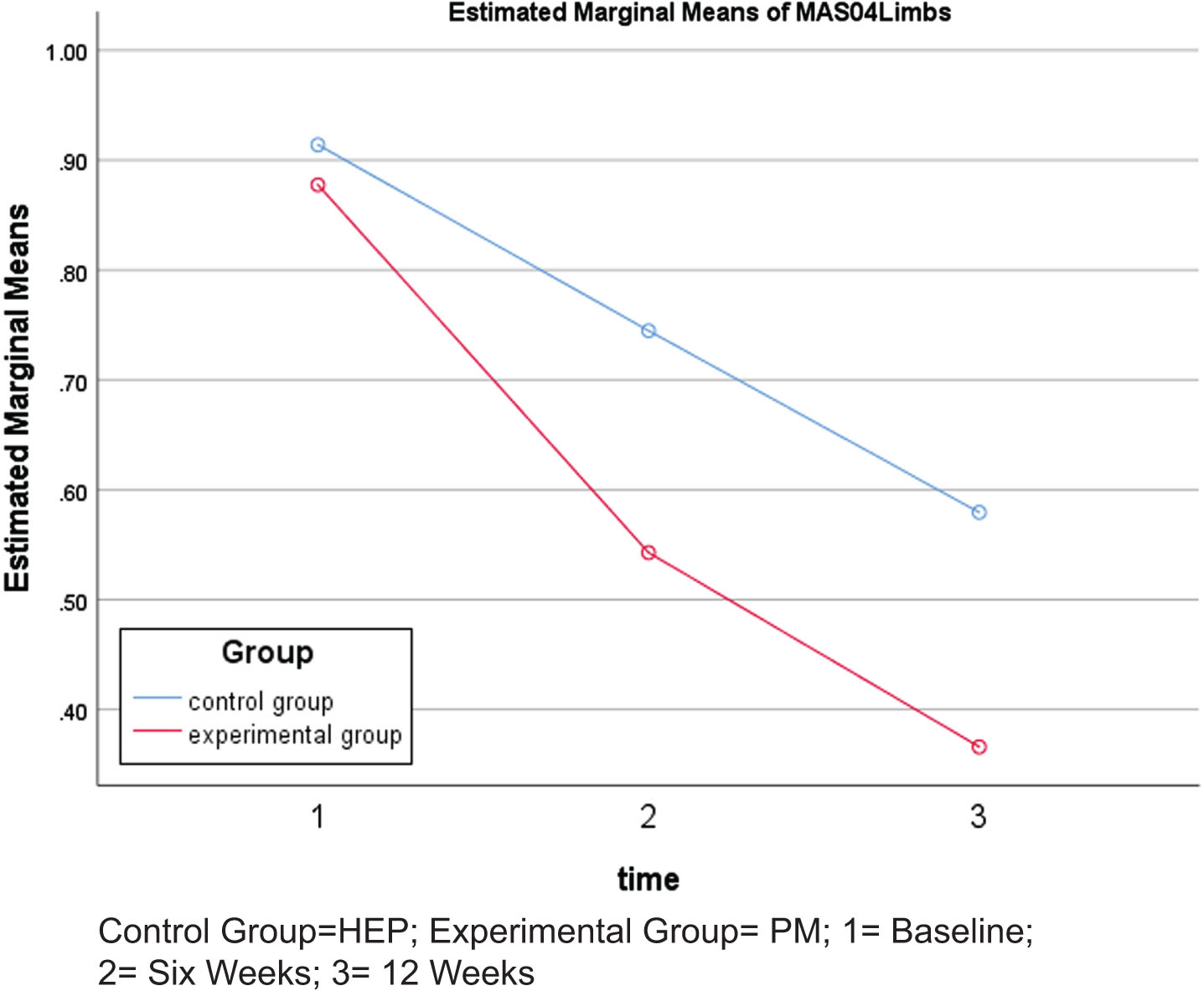
MAS grades of all four limbs revealed statistically significant decreases in the PM group after six and 12 weeks of intervention (p < 0.05) when compared to the HEP group. Similarly, intra-group comparison showed a statistically significant reduction in MAS grades from baseline to six weeks, six weeks to 12 weeks, and baseline to 12 weeks (p < 0.05) in the PM group. However, in the HEP group, reduction in MAS grades was not significant from baseline to six weeks of intervention, but became significant from six to 12 weeks and baseline to 12 weeks of intervention. In the case of MAS grades, lower scores indicate reduction in spasticity (Table 2, Fig. 2).
Fig. 2
Changes in Modified Ashworth Scale (MAS) grades.
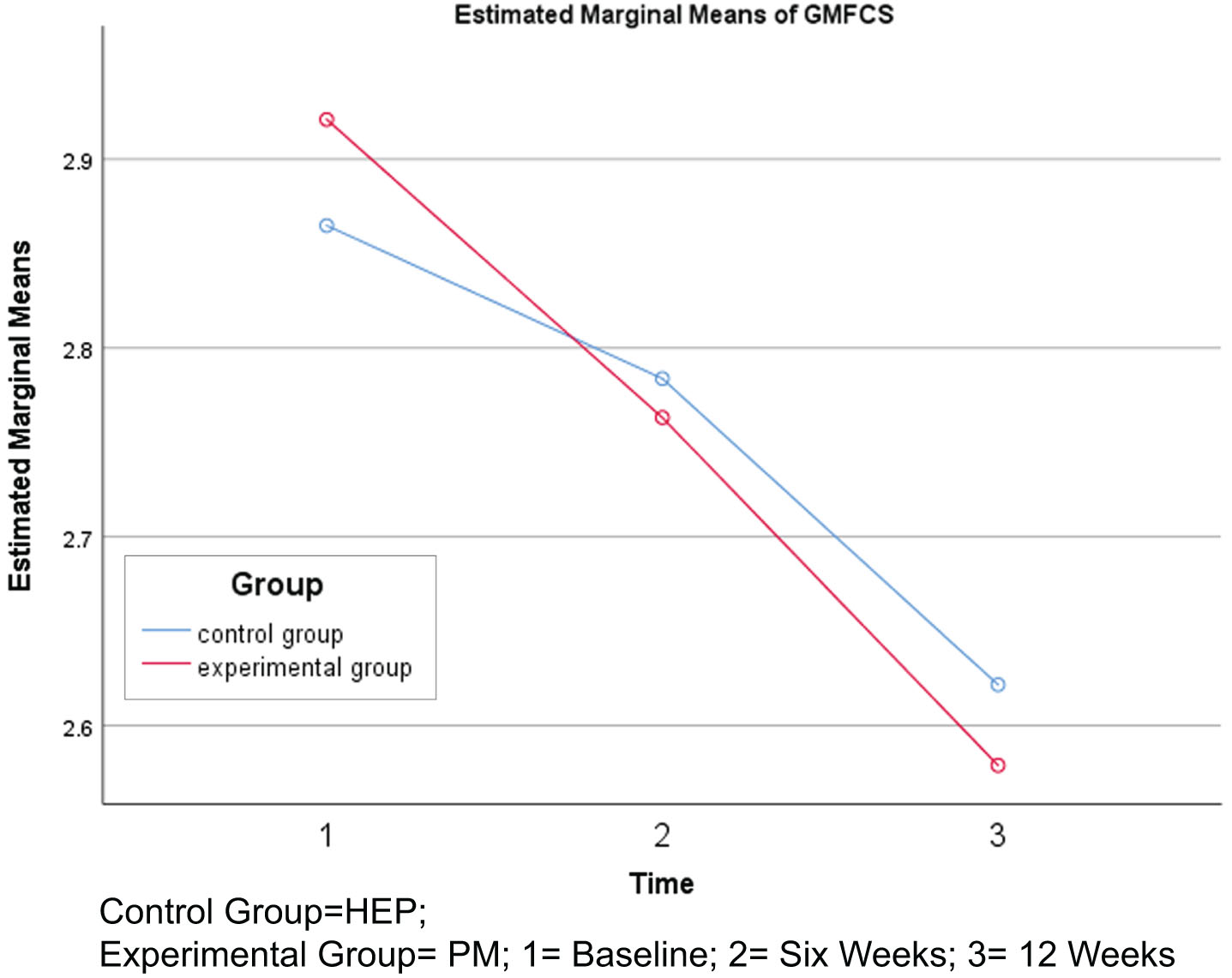
GMFCS levels showed no statistically significant reduction after six and 12 weeks of intervention in the PM group (p > 0.05) when compared to the HEP group. However, intra-group comparison showed a statistically significant reduction in GMFCS levels from baseline to six weeks, six weeks to 12 weeks, and baseline to 12 weeks (p < 0.05) in the PM group. However, in the HEP group, reduction in GMFCS levels was not significant from baseline to six weeks of intervention, but became significant from six to 12 weeks and baseline to 12 weeks of intervention. In the case of GMFCS levels, lower scores represent improved mobility (Table 3, Fig. 3).
Table 3
GMFCS levels
| Within subjects, between subjects &interaction results (mixed method ANOVA) | ||||||
| Source | SS | DF | MS | F | p-value | Partial Eta-squared |
| Between-subjects | 0.000 | 1 | 0.000 | 0.000 | 0.991 | 0.000 |
| Within-subjects | 3.248 | 2 | 1.624 | 16.970 | 0.000 | 0.189 |
| Interaction Time*group | 0.101 | 2 | 0.050 | 0.528 | 0.591 | 0.007 |
| Comparison between groups (One-way ANOVA, in rows horizontally) and within groups (Repeated measure ANOVA, in columns vertically, top to bottom) | |||
| GMFCS levels | HEP group (Mean±SD) (F = 8.4; DF=1.62) | PM group (Mean±SD) (F = 9.05; DF=1.68) | P-value |
| Baseline | 2.87±0.85 | 2.94±0.88 | 0.966 |
| After 6 Weeks | 2.84±0.86 | 2.72±0.88 | 0.914 |
| 0.325a | 0.017a | ||
| After 12 Weeks | 2.65±1.08 | 2.59±0.95 | 0.992 |
| 0.037b | 0.019b | ||
| 0.006c | 0.003c | ||
a- p value baseline to six weeks (within group). b- p value six to 12 weeks (within group). c- p value baseline to 12 weeks (within group) GMFCS- Gross Motor Function Classification System. SS = sum of squares, DF = degrees of freedom, MS = mean squares, F = F-value. HEP- Home exercise program. PM- Pediatric massage.
Fig. 3
Changes in Gross Motor Function Classification System (GMFCS) levels.
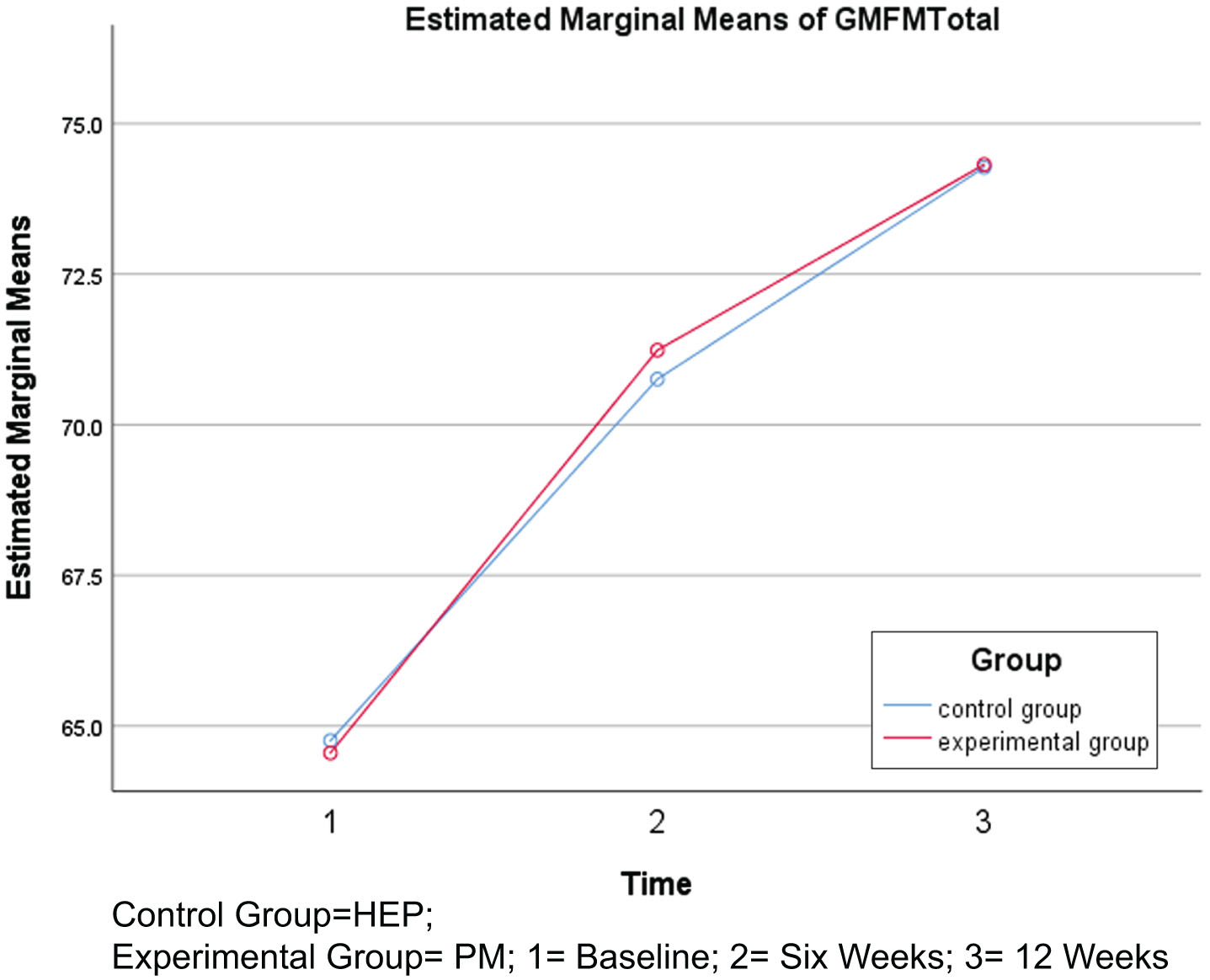
GMFM scores showed no statistically significant increase after six and 12 weeks of intervention in the PM group (p > 0.05) when compared to the HEP group. However, intra-group comparison revealed a significant increase in GMFM scores from baseline to six weeks, six weeks to 12 weeks, and baseline to 12 weeks of intervention in both groups (p < 0.05). In the case of GMFM, higher scores indicate improvement in gross motor function (Table 4, Fig. 4).
Table 4
GMFM Total Scores
| Within subjects, Between subjects & Interaction results (Mixed Method ANOVA) | ||||||
| Source | SS | DF | MS | F | p-value | Partial Eta-squared |
| Between-Subjects | 0.646 | 1 | 0.646 | 0.001 | 0.981 | 0.000 |
| Within-Subjects | 3598.984 | 2 | 1799.492 | 134.082 | 0.000 | 0.647 |
| Interaction Time*group | 4.495 | 2 | 2.247 | 0.167 | 0.846 | 0.002 |
| Comparison between groups (One-way ANOVA, in rows horizontally) and within groups (Repeated Measure ANOVA, in columns vertically, top to bottom) | |||
| GMFM Total Scores | HEP Group (Mean±SD) (F = 71.84, DF=1.5) | PM Group (Mean±SD) (F = 63.61, DF=1.4) | P-value |
| Baseline | 63.81±22.89 | 63.84±19.32 | 0.966 |
| After Six Weeks | 69.65±21.81 | 70.75±17.46 | 0.914 |
| 0.000a | 0.000a | ||
| After 12 Weeks | 73.16±21.99 | 74.25±17.42 | 0.992 |
| 0.000b | 0.000b | ||
| 0.000c | 0.000c | ||
a- p value baseline to six weeks (within group). b- p value six to 12 weeks (within group). c- p value baseline to 12 weeks (within group). GMFM- Gross Motor Function Measure. SS = sum of squares, DF = degrees of freedom, MS = mean squares, F = F-value. HEP-Home exercise program. PM- Pediatric massage.
Fig. 4
Changes in Gross Motor Function Measure (GMFM) total scores.
4Discussion
Spasticity contributes to disability by restricting movements, developing musculo-skeletal stiffness leading to contractures, impairing mobility, creating difficulties in performing activities of daily living, restricting participation, and compromising quality of life [16]. For better management of complications of CP, a decline in spasticity and enhancement in motor control are the desired objectives of any intervention. The current study was conducted to evaluate the effectiveness of simple and basic HEPs including PM in decreasing spasticity and improving gross motor activity of children with spastic CP. Analytic comparison within the groups revealed statistically significant change indicating that spasticity decreased and motor activity improved in both groups from baseline to 12 weeks of intervention. However, the rate of this change from baseline to six weeks of intervention was more evident in the PM group compared to the HEP group. This shows that HEPs and PM in conjunction are simple, basic, effective, easily available, and doable strategies that should be incorporated in the rehabilitation of spastic CP. In a comparison of no HEPs to four weeks and eight weeks of HEPs with a double blind protocol, Novak et al. reported that HEPs are effective and yield good results; therefore, these should be prescribed/endorsed by health professionals [8]. Multiple studies in health-related literature have supported the effectiveness of a HEP model in the management of CP and recommended that it should be used as an alternative to clinical interventions [17, 18].
When a pediatrician advises regular physical therapy, many caregivers find it hard to arrange this for their children due to the acute shortage of certified professionals, lack of financial resources, lengthy waiting lists in public sector hospitals, and scarcity of time available for this purpose. Physical therapists and pediatricians should inform and guide parents that an effective therapy protocol in the form of a HEP and PM is available as an alternative to costly clinical care. Moreover, therapy provided in the home environment has the advantage of being more useful because it is more conducive, friendly, meaningful, and comfortable for children compared to intimidating hospitals or clinical settings. Involvement of parents as effective and collaborative members of the rehabilitation team will result in useful, long-lasting, and functional gains, which in turn will improve the quality of life of their children with CP [19].
Previous studies regarding the effects of PM in decreasing spasticity have yielded contradictory results. On one hand, Hernandez et al. observed a decrease in spasticity after 12 weeks of Swedish massage in an intervention group as compared to a control group that received reading activities [9]; on the other hand, Alizad et al. did not find such effectiveness after using Swedish massage for 12 weeks in conjunction with occupational therapy techniques in an intervention group [10]. Similarly, two studies did not find any significant reduction in spasticity: one by applying localized transverse deep friction massage on the muscles of the lower leg along with conventional physiotherapy, and the other using Swedish massage for 12 weeks in five children with spastic CP [20, 21]. However, no existing study has reported adverse effects of massage on spasticity. The current study showed significant reduction in spasticity after six and 12 weeks of intervention in the PM group and after 12 weeks of intervention in the HEP group, and no adverse effects were observed in the PM group. This is contrary to the misconception that massage increases spasticity. Healthcare professionals involved in the management of spastic CP should consider the benefits of HEPs and PM when advising and educating patients, caregivers, their communities, and other professionals. These effective, low-cost, easily available, and easy to implement rehabilitation tools can be utilized to improve the management of spastic CP, therefore lowering the workload on healthcare professionals in hospitals [22].
In the current study, results showed that GMFM scores did not increase and GMFCS levels did not decrease significantly in the PM group when compared to the HEP group after six and 12 weeks of intervention. Rasool reached the same conclusion in a study assessing motor function via an RCT using of a self-structured nine-point questionnaire that was not tested for validity and reliability [21]. Within-group comparative analysis of the current study revealed significant increases in GMFM scores and significant decreases of GMFCS levels in both groups. Healthcare publications support the results of the current study and mirror the usefulness of HEPs in decreasing spasticity, building strength, regaining range of motion, and enhancing fine and gross motor activities of children with CP [5, 23, 24].
The current study indicated that PM has the potential to reduce spasticity after intervention of six weeks, and it can also produce significant improvement in gross motor function when assessed through the GMFM scale. But improvement in gross motor function was not significant after six weeks of intervention when assessed using GMFCS levels. However, after intervention of 12 weeks, significant improvement was observed in gross motor function when assessed using the GMFM and GMFCS in both groups. Therefore, healthcare professionals should consider recommending these measures (i.e., HEPs and PM) for a period of 12 weeks to get maximum benefits of the interventions. As continuity of HEPs and PM depends on active participation and sustained motivation of caregivers/parents, it is therefore very important to remain in frequent contact with them with the use of telecommunication. Use of HEPs and PM as therapeutic tools can help to cope with the ever increasing burden of disability, provided that caregivers are well-trained and receive regular, effective, and meaningful follow ups. It is important to mention that, at present, clinical guidelines regarding intensity and frequency of HEPs do not exist. Therefore, healthcare professionals should work in close collaboration with public sector officials to develop these guidelines.
5Conclusion
PM along with HEPs can be used to effectively reduce spasticity and to improve gross motor ability in children with spastic CP if performed for a period of at least six and 12 weeks respectively. No harmful effects of PM and HEPs have been documented in this or past studies, so these techniques are safe to be administered by parents at home. These techniques should be prescribed/advised by health care professionals in the life-long management of children with spastic CP, ensuring regular, effective, and meaningful follow ups. PM in conjunction with HEPs has better outcomes in the management of tone and movement disorders of spastic CP than HEPs alone.
5.1Limitations
As the parents were responsible for the provision of intervention at home, its quantity, frequency, intensity, quality, and continuity was therefore not under control of the researcher. However, every effort was made to train the parents to administer the exercise plan and pediatric massage in a standardized manner.
Because the age group of the current study was 4–12 years, its results therefore cannot be generalized to other age groups.
5.2Recommendations
Further research is needed to develop guidelines for HEPs and PM, exploring the answers of the following questions in detail.
1. What is the most useful and effective quantity, frequency, and intensity of HEPs and PM, which should be practiced by parents at home for their children with spastic CP?
2. How and when is decreased spasticity due to HEPs and PM actually transformed into improved motor function? This RCT with ID: NCT05111236 is registered with the National Institutes of Health in the USA and is available on ClinicalTrials.gov.
Acknowledgments
The authors have no acknowledgements regarding grant support or financial implications of any nature. However, all children and their parents are well acknowledged for their participation in this study. I am also really thankful to Dr. Mian Imran Amjad for his sustained, committed, and valuable support.
Conflict of interest
The authors have no conflict of interest to report.
References
[1] | Rosenbaum P , Paneth N , Leviton A , et al. A report: The definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. (2007) ;109: (Suppl 109)8–14. doi:10.1017/s001216220500112x |
[2] | Chiu HC , Ada L . Constraint-induced movement therapy improves upper limb activity and participation in hemiplegic cerebral palsy: A systematic review. J Physiother. (2016) ;62: (3):130–7. doi:10.1016/j.jphys.2016.05.013 |
[3] | Gladstone M . A review of the incidence and prevalence, types and aetiology of childhood cerebral palsy in resource-poor settings. Ann Trop Paediatr. (2010) ;30: (3):181–96. doi:10.1179/146532810x12786388978481 |
[4] | Verschuren O , Smorenburg AR , Luiking Y , Bell K , Barber L , Peterson MD . Determinants of muscle preservation in individuals with cerebral palsy across the lifespan: A narrative review of the literature. J Cachexia Sarcopenia Muscle. (2018) ;9: (3):453–64. doi:10.1002/jcsm.12287 |
[5] | Novak I , Mcintyre S , Morgan C , et al A systematic review of interventions for children with cerebral palsy: State of the evidence. Dev Med Child Neurol. (2013) ;55: (10):885–910. doi:10.1111/dmcn.122466 |
[6] | Mirza I , Tareen A , Davidson L , Rahman A . Community management of intellectual disabilities in Pakistan: A mixed methods study. J Intellect Disabil Res. (2009) ;53: (6):559–70. doi:10.1111/j.1365-2788.2009.01176.x |
[7] | Developmental difficulties in early childhood: Prevention, early identification, assessment and intervention in low-and middle-income countries: A review. World Health Organizaiton; 2012. |
[8] | Novak I , Cusick A , Lannin N . Occupational therapy home programs for cerebral palsy: Double-blind, randomized, controlled trial. Pediatrics. (2009) ;124: (4):e606–14. doi:10.1542/peds.2009-0288 |
[9] | Hernandez-Reif M , Field T , Largie S , et al Cerebral palsy symptoms in children decreased following massage therapy. Early Child Dev Care. (2005) ;175: (5):445–56. doi:10.1080/0300443042000230546 |
[10] | Alizad V , Sajedi F , Vameghi R . Muscle tonicity of children with spastic cerebral palsy: How effective is Swedish massage? Iran J Child Neurol. (2009) ;3: (2):25–9. doi:10.22037/ijcn.v3i2.1268 |
[11] | Response from ChatGPT, an AI language model developed by OpenAI. OpenAI [cited 2023 July, 13]; Available from:https://www.openai.com/chatgpt. |
[12] | Elgawish MH , Zakaria MA . The effectiveness of intensive versus standard physical therapy for motor progress in children with spastic cerebral palsy. Egyptian Rheumatol Rehabil. (2015) ;42: (1):1.doi:10.4103/1110-161x.155622 |
[13] | Bohannon RW , Smith MB . Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. (1987) ;67: (2):206–7. doi:10.1093/ptj/67.2.206 |
[14] | Ko J , Kim M . Reliability and responsiveness of the gross motor function measure-88 in children with cerebral palsy. Phys Ther. (2013) ;93: (3):393–400. doi:10.2522/ptj.20110374 |
[15] | Ko J , Woo JH , Her J-G . The reliability and concurrent validity of the GMFCS for children with cerebral palsy. J Phys Ther Sci. (2011) ;23: (2):255–8. doi:10.1589/jpts.23.255 |
[16] | Barnes MP An overview of the clinical management of spasticity. Upper motor neurone syndrome and spasticity:Clinical management and neurophysiology. In: Barnes MP,Johhnson GR, editors. Upper Motor Neurone Syndrome and Spasticity. 1st edition. Cambridge University Press; 2001. |
[17] | Eliasson A-C , Krumlinde-Sundholm L , Shaw K , Wang C . Effects of constraint-induced movement therapy in young children with hemiplegic cerebral palsy: An adapted model. Dev Med Child Neurol. (2005) ;47: (4):266–75. doi:10.1017/s0012162205000502 |
[18] | Lin K-c , Wang T-n , Wu C-y , et al Effects of home-based constraint-induced therapy versus dose-matched control intervention on functional outcomes and caregiver well-being in children with cerebral palsy. Res Dev Disabil. (2011) ;32: (5):1483–91.10.1016/j.ridd.2011.01.023 |
[19] | Bazyk S . Changes in attitudes and beliefs regarding parent participation and home programs: An update. Am J Occup Ther. (1989) ;43: (11):723–8. doi:10.5014/ajot.43.11.723 |
[20] | Macgregor R , Campbell R , Gladden MH , Tennant N , Young D . Effects of massage on the mechanical behaviour of muscles in adolescents with spastic diplegia: A pilot study. Dev Med Child Neurol. (2007) ;49: (3):187–91. doi:10.1111/j.1469-8749.2007.00187.x |
[21] | Rasool F , Memon AR , Kiyani MM , Sajjad AG . The effect of deep cross friction massage on spasticity of children with cerebral palsy: A double-blind randomised controlled trial. J Pakistan Med Assoc ((2017) ;67: (1), 87–91. |
[22] | Schreiber JM , Effgen SK , Palisano RJ . Effectiveness of parental collaboration on compliance with a home program. Pediatric Physical Therapy. (1995) ;7: (2):59–64.10.1097/00001577-199500720-00003 |
[23] | Damiano DL . Activity, activity, activity: Rethinking our physical therapy approach to cerebral palsy. Phys Ther ((2006) ;86: (11):1534–40. doi:10.2522/ptj.20050397 |
[24] | Harris SR , Roxborough L . Efficacy and effectiveness of physical therapy in enhancing postural control in children with cerebral palsy. Neural Plast. (2005) ;12: (2–3):229–43. doi:10.1155/n2005.229 |




