Psychological predictors of performance-based physical functioning among pediatric pain program participants
Abstract
PURPOSE:
The purpose of the present study was to evaluate changes in performance-based physical functioning and investigate psychological predictors of physical functioning over time in pediatric patients with chronic pain who completed an interdisciplinary rehabilitation intensive outpatient program (IOP).
METHODS:
Participants (N = 55; mean age = 14.92 years; 12.7% male, 87.3% female; 83.6% White, 5.6% African-American/Black; 9.1% Latinx) completed baseline measures assessing pain intensity and modifiable psychological factors (i.e., pain catastrophizing, kinesiophobia, anxiety and depressive symptoms). Participants were administered performance-based assessments of physical functioning (i.e., physical endurance, high-level motor abilities) before and after IOP completion.
RESULTS:
Pain intensity was not significantly associated with physical functioning at either timepoint. There was significant improvement on measures of physical functioning after completion of the IOP when controlling for the effects of sex, race, and ethnicity. Depressive symptoms were associated with baseline physical endurance, β = − .28, p = .047, while pain catastrophizing was associated with baseline gross motor abilities, β = − .28, p = .032.
CONCLUSION:
Participation in an IOP led to significant improvement in physical endurance and high-level motor ability. Depressive symptoms and pain catastrophizing were associated with physical functioning at baseline but not post-program completion. Integration of pain psychology and physical therapy in an IOP can help address the interrelated psychological and physical factors impacting physical functioning to improve outcomes for children with chronic pain.
1Introduction
Pediatric chronic pain is noted by the World Health Organization to be a significant health problem and a leading cause of morbidity in children [1]. The three most common forms of chronic pain in youth are headaches, abdominal pain, and musculoskeletal pain syndromes [2]. Nationally, pediatric chronic pain costs $19.5 billion to treat [3]. Pediatric chronic pain affects between 11–38% of youth [2] and is commonly associated with decreases in children’s and adolescents’ academic, social, and physical functioning. Youth with chronic pain are at increased risk for a number of negative functional outcomes, including physical functioning decline, fatigue, and diminished endurance to participate in hobbies and social activities [4, 5]. As such, cornerstones of evidence-based treatment for pediatric chronic pain are physical therapy (PT) and cognitive behavioral therapy (CBT). Specifically, PT aimed at graded aerobic exercise of a moderate intensity is useful to enhance exercise tolerance, physical function, and pain modulation [6–8]. CBT for pediatric chronic pain is another evidence-based approach designed to help children and adolescents with chronic pain manage their symptoms and improve their functioning. Grounded in the biopsychosocial understanding of chronic pain, protocols for CBT are typically multicomponent in nature and focus on skills development. Though tailored more individually in clinical practice, common components generally include education about pain, behavioral activation, exposure, problem solving, relaxation training, and cognitive reframing. The focus will often include the individual and the family system as well [9–11].
Comprehensive chronic pain rehabilitation programs that incorporate PT and CBT are globally associated with reduced pain and functional impairment [12, 13]. However, few studies have examined changes in performance-based physical functioning outcomes, focusing instead on parent- or self-report. Although one study did find that scores across multiple PT measures improved significantly in youth participating in a multidisciplinary treatment program, it focused specifically on children with chronic pain in one lower extremity only [14]. Youth and their caregivers seek treatment for wide arrays of pain presentations. One of the most common types of musculoskeletal pain, amplified musculoskeletal pain syndrome (AMPS) [15] is a form of nociplastic pain and can present as localized, e.g., complex regional pain syndrome, or diffuse (widespread pain such as juvenile fibromyalgia), with or without accompanying autonomic symptoms like energy regulation difficulties (fatigue and poor sleep), cognitive difficulties like brain fog, or affective distress. Emerging evidence indicates that some commonly used PT measures may not be appropriate for pediatric chronic pain populations given they may not adequately capture variability in functional impairment [16]. Given the widespread, complex, and diverse nature of chronic pain locations, such as in the case of AMPS, examining whether youth with chronic pain experience an improvement in PT outcomes after participation in a pain rehabilitation program is a crucial next step in understanding mechanisms of change in pediatric pain treatment. Importantly, there have been calls to include performance-based (versus just parent- or self-report) assessment of physical functioning [17] for a more comprehensive understanding of the effects of these programs on functioning.
Poorer outcomes from integrated, comprehensive pain treatment programs are associated with maladaptive but modifiable psychological factors [18]. However, despite clinical integration of psychology and PT in these programs, no known study has examined modifiable psychological predictors of PT outcomes in the pediatric chronic pain population, even though psychological factors are linked to PT outcomes in other pain populations (e.g., adults with low back pain) [19]. Commonly studied modifiable psychological factors in pediatric pain research include pain catastrophizing, kinesiophobia, and internalizing symptoms [5, 20]. Examining whether and which modifiable psychological factors are linked to performance-based PT measures may provide pain psychologists with further guidance for creating targeted treatment goals to facilitate physical functioning.
As such, the purpose of the present study was to examine changes in performance-based assessment of physical functioning among pediatric chronic pain patients in an intensive outpatient program (IOP), as well as modifiable psychological factors associated with performance-based physical ability. It was hypothesized that there would be a significant improvement in performance-based physical functioning after completion of the IOP. Also, greater levels of baseline modifiable psychological factors were expected to be associated with poorer physical functioning both pre- and post-IOP participation.
2Method
Institutional Review Board (HUM00155312) approval was obtained for review of patients’ medical records.
2.1Participants
Fifty-five pediatric patients who enrolled in an IOP treating chronic pain conditions at an academic medical center in a mid-sized Midwestern city participated in the present study. See Table 1 for demographic information. Participants had a variety of chronic pain diagnoses, with the majority having a diagnosis within the AMPS category (n = 45); 14 had multiple pain diagnoses.
Table 1
Sample Characteristics
| n (%) | M (range) | |
| Race | ||
| White | 46 (83.6) | |
| African-American/Black | 3 (5.6) | |
| Other | 6 (10.1) | |
| Hispanic/Latinx | 5 (9.1) | |
| Sex | ||
| Female | 48 (87.3) | |
| Male | 7 (12.7) | |
| Age | 14.9 (9.3–18.2) y |
Note. M = mean; y = years. Total N = 55.
2.2Measures
2.2.1Physical functioning measures
Participants were administered the Fitkids Treadmill Test (FTT), which assesses aerobic exercise capacity in children and adolescents. The FTT follows a specific protocol in which participants are placed on a treadmill; speed and incline are adjusted in 90-second increments until the volitional time to exhaustion (TTE) is reached, which is defined as when the participants choose to stop the test despite considerable encouragement from the therapist. Participants are not permitted to hold onto railings during testing. Participants ambulate for a 90-second warm-up at 2.2 miles per hour (mph) and 0% incline, then the test is initiated with an increase in incline to 1% . The speed increases by 0.3 mph and the incline increases by 2% every 90 seconds, with a maximum incline of 15% [21]. The FTT has been found to be a valid, reproducible measure with normative values established based on age and sex in children ages 6–18 [22]. At this time, no minimal detectable change has been published.
High-level gross motor skills are an important aspect of physical capacity which, in turn, is an important aspect of participation in various functional mobility, social, sport, and leisure activities for children and adolescents [23]. Participants were administered the revised High-Level Mobility Assessment Tool (HiMAT) to assess high-level gross motor skills [24]. The HiMAT involves performance of eight items that gradually increase in difficulty: walking, walking backwards, walking on toes, walking over an obstacle, running, skipping, hopping forwards on the affected leg, and bouncing onto the less affected leg. Time to complete each task over the course of 10 meters or distance in centimeters is recorded and scored from 0–4. The scores for all eight items are added together for a maximum of 32 points. Higher scores indicate better capability for high-level gross motor skills.
The original and revised HiMAT have excellent interrater and retest reliability as well as internal validity in typical adults and adults with neurological conditions [24]. There are established normative values for people aged 18–25 for the original HiMAT [24]. In a recent study published in Australia, normative values for the revised HiMAT were established for typically developing children aged 5–12 [23]. Total mean scores on the revised HiMAT in the normative population ranged from 17.5 for age 5–6 years to 26.8 for age 11–12 years. Currently, no normative data exist for adolescents.
2.2.2Modifiable psychological factors
The Tampa Scale for Kinesiophobia [25] is a 17-item patient-reported measure assessing fear of physical movement due to fear of injury/reinjury. Higher scores indicate greater levels of kinesiophobia. It demonstrates adequate internal consistency and reliability [26].
The Catastrophizing subscale of the Coping Strategies Questionnaire [27] is a six-item patient-reported subscale; higher scores indicate higher levels of pain catastrophizing. The questionnaire demonstrates adequate internal consistency [27].
The Hospital Anxiety and Depression Scale (HADS) is a patient-reported 14-item measure consisting of two subscales: anxiety and depressive symptoms [28]. Scores of nine or greater on the anxiety subscale and seven or greater on the depression subscale indicate clinically elevated levels of symptoms in youth [29]. The HADS has been validated in youth and demonstrates adequate internal consistency [29].
2.2.3Pain intensity
A single item from the Pain Severity subscale of the Brief Pain Inventory was used to assess pain intensity [30]. Specifically, patients were asked to rate their average pain in the past week on an 11-point scale ranging from 0–10, with higher scores indicating greater pain intensity.
2.2.4Covariates
Participants reported on their sex, race, and ethnicity. Given small sample sizes of those who self-identified as people of color, race was dichotomized as White/person of color in analyses.
2.3Procedure
Pediatric patients with chronic pain enrolled in a four-week (three days per week) multidisciplinary IOP consisting of PT (three times per week, individual and group format), occupational therapy (if indicated, once per week, individual or paired format), pain psychology (four times per week, individual, family, and parent and participant groups format), art therapy (once per week, parent and participant groups format), and therapeutic recreation (once per week, paired format). The focus of the program was on restoring function and decreasing pain-related disability and pain behaviors.
Participants completed questionnaires about pain and associated domains at their initial pain clinic evaluation (T1) as part of standard clinical intake; no follow-up regarding pain and associated domains was collected as this was not part of clinic protocol at the time. Licensed physical therapists with expertise in pediatric chronic pain conducted the FTT and HiMAT assessments at participants’ first (T1) and last PT sessions (T2).
2.4Statistical analyses
All analyses were conducted in IBM SPSS (v.28) [31].
First, bivariate correlations were examined between baseline pain intensity and physical and psychological factors at T1 in order to determine whether to include pain intensity as a covariate. If pain intensity was significantly correlated with a study variable, it was included as a covariate in that particular model.
Repeated measures analyses of covariance (ANCOVAs) were conducted to determine whether there was a significant change in performance on the FTT or HiMAT from T1 to T2, when controlling for effects of covariates (i.e., sex, race, and ethnicity, as well as pain intensity if correlated with outcome variable).
Hierarchical linear regression analyses were conducted to determine whether baseline psychological factors were associated with the FTT or HiMAT at T1 or T2. Covariates (i.e., sex, race, and ethnicity, as well as pain intensity if correlated with outcome variable) were entered as Step 1 in each model. Pain-specific cognitive (i.e., kinesiophobia, pain catastrophizing) and general affective (i.e., depressive symptoms, anxiety symptoms) variables were entered as Step 2 in separate models. Outcome variables included the FTT and HiMAT at T1 and T2. In models that included physical functioning variables at T2, the corresponding physical functioning variable at T1 was also added as a covariate in Step 1.
3Results
Demographic and diagnostic characteristics are reported in Table 1. Descriptives for outcome measures are reported in Table 2.
Table 2
Descriptive information of clinical outcome measures
| Measure | Time 1 | Time 2 |
| M (SD) | M (SD) | |
| Average Pain Intensity | 5.66 (1.64) | |
| Anxiety Symptoms | 11.60 (3.79) | |
| Depressive Symptoms | 8.48 (4.49) | |
| Pain Catastrophizing | 18.26 (8.62) | |
| Kinesiophobia | 42.43 (8.14) | |
| Fitkids Time to Exhaustion | 408.37 (117.90) | 482.53 (102.27) |
| HiMAT Total | 17.94 (5.85) | 23.11 (5.20) |
Note. HiMAT = Revised High-Level Mobility Assessment Tool; M = mean; SD = standard deviation.
Average pain intensity was in the moderate range (mean [M] = 5.66, standard deviation [SD] = 1.64). See Table 1 for descriptive information on study variables. Bivariate correlations did not reveal significant associations between average pain at baseline and the FTT and HiMAT at T1 and T2. As such, pain intensity was not included in subsequent models.
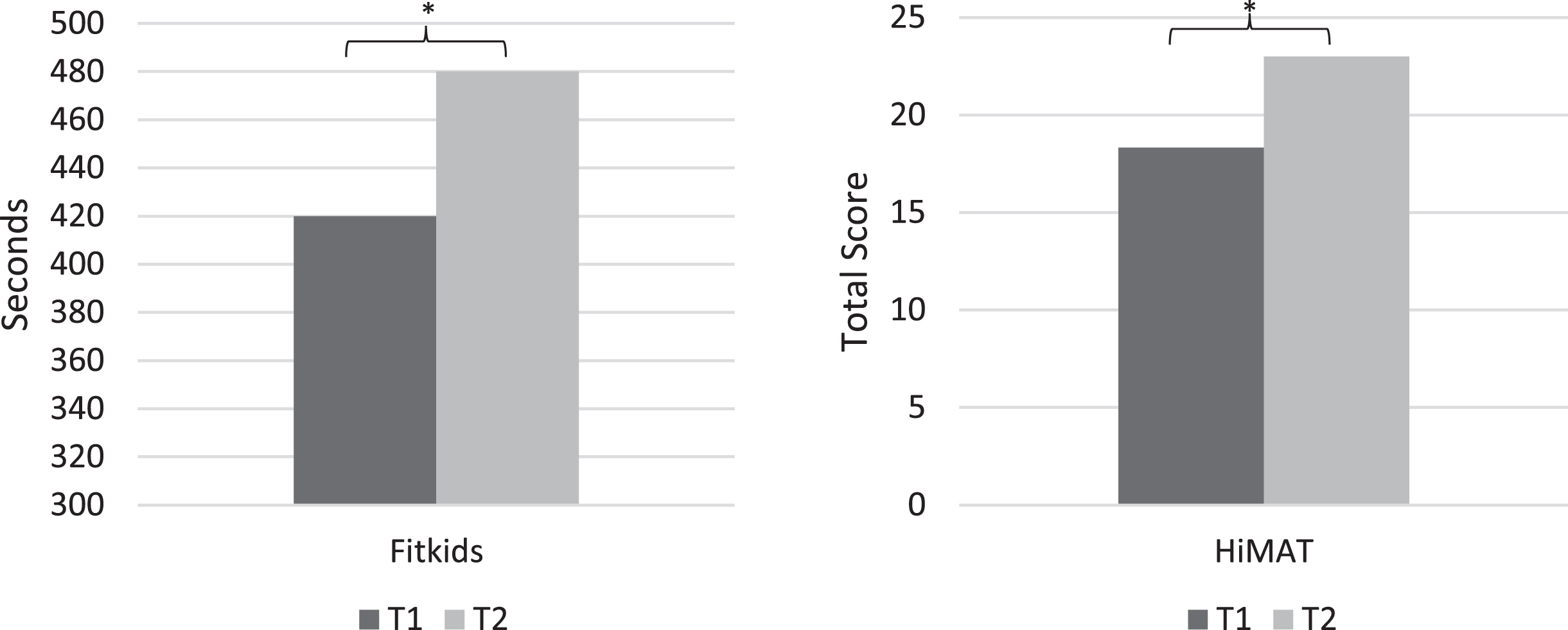
A repeated measures ANCOVA (Fig. 1) indicated a significant change in scores on the FTT from T1 (M = 420.36 seconds [sec]) to T2 (M = 479.98 sec) when controlling for effects of sex, race, and ethnicity, F (1, 41)=15.17, p < .001. Another repeated measures ANCOVA (Fig. 1) also revealed a significant change in score on the HiMAT from T1 (M = 18.33) to T2 (M = 23.00) when controlling for effects of sex, race, and ethnicity, F (1, 39)=34.77, p < .001.
Fig. 1
Pre- and Post-Treatment Measures of Physical Functioning. Note. * p<.001. HiMAT = Revised High-Level Mobility Assessment Tool; T1 = Pre-Treatment; T2 = Post-Treatment. Analyses included sex, race, and ethnicity as covariates.
When controlling for the effects of race, ethnicity, and sex, depressive symptoms at T1 were associated with the FTT at T1, β = − .28, R 2 = .15, ΔR 2 = .07, ΔF (1, 48) = 4.16, p = .047. This meant that the more depressive symptoms participants endorsed, the shorter their FTT time was, indicating worse endurance; the effect size of this association was in the small-to-medium range. Depressive symptoms were not associated with the FTT at T2 or with HiMAT scores at T1 or T2. Pain catastrophizing was associated with the HiMAT at T1, β = − .28, R 2 = .20, ΔR 2 = .08, ΔF (1, 49) =4.86, p = .032, but not at T2. This meant that the more pain catastrophizing participants endorsed, the lower their HiMAT score was, indicating poorer high-level gross motor abilities; the effect size of this association was in the small-to-medium range. Pain catastrophizing was not associated with the FTT at T1 or T2. Kinesiophobia and anxiety symptoms were not associated with the FTT or HiMAT at T1 or T2.
4Discussion
The present study was the first to examine change in performance-based physical functioning and the effects of modifiable psychological (i.e., kinesiophobia, pain catastrophizing, depressive and anxiety symptoms) factors on performance-based physical functioning before and immediately after participation in an IOP among pediatric patients with chronic pain. Depressive symptoms were significantly negatively associated with endurance at baseline but not upon program completion above and beyond any effects of race, sex, and ethnicity as well as baseline levels of endurance. Similarly, pain catastrophizing was significantly negatively associated with high-level gross motor skills at baseline but not post-program. Interestingly, pain intensity at baseline was not associated with any measures of physical functioning.
These findings indicated that poorer performance on physical functioning assessments was linked to low mood and pain catastrophizing but not pain intensity. This lends support to the biopsychosocial model of pain and functioning [32, 33], specifically the contribution of psychological factors to pain-related functional impairment. The present study suggests this model is nuanced; whereas some psychological factors are associated with physical ability, others are not, revealing the need for further examination of a tailored treatment approach that focuses on mood (e.g., via behavioral activation) and pain catastrophizing (e.g., via cognitive restructuring). Additionally, the findings add to the well-developed evidence base showing the negative effects of pain catastrophizing and mood on youth and parent-reported functioning by confirming an association with performance-based indices of physical performance [34, 35]. The lack of association between these factors and physical functioning post-treatment is reassuring, as it suggests that participation in an IOP leads to overall improvement in physical outcomes regardless of baseline mood or catastrophizing. This may have been due in part to the integration of pain psychology and PT services in the IOP, which together addressed these interrelated factors.
Kinesiophobia and anxiety were not associated with physical functioning in the present study, diverging from prior findings of associations between these factors and self-reported functioning [20]. However, the present study included adolescents enrolled in IOP services, the majority of whom were diagnosed with AMPS, which differed from samples included in other studies of pediatric chronic pain [20]. In contrast, the current results may suggest that there is a disconnect between fear of movement as well as anxiety and PT-assessed performance ability for pediatric chronic pain patients participating in the IOP. This further underscores the importance of incorporating performance-based assessments of physical ability to gain a more thorough understanding of treatment targets in pain treatment, rather than relying solely on patient or parent report of functioning.
Although the present study had notable strengths, including longitudinal design, covariates, and, importantly, use of both subjective and objective measures, some limitations existed and should be addressed in follow-up studies. First, psychological factors were measured only pre-treatment; collecting these data over several months post-treatment would lead to better understanding of whether changes in these factors are associated with changes in physical functioning outcomes. Prior studies have shown that comparable treatment results in moderate decreases in kinesiophobia and internalizing symptoms but not pain catastrophizing [12, 36]. This suggests that changes observed in physical functioning in the present study may have been attributed in part to unmeasured changes in depressive symptoms but not catastrophizing. Second, the present study assessed physical outcomes pre- and post-treatment. Collecting longer-term follow-up (e.g., three and six months post-treatment) as well as real-time data (e.g., via actigraphy) could provide information on the sustainability of improvements as well as nuanced understanding of the mechanisms of these changes. Additionally, the lack of a control or treatment-as-usual sample precluded the ability to definitively ascertain that physical functioning improvements were due to the IOP. However, given very high test-retest reliability of the physical functioning measures used in this study [21, 24], it is likely that the changes in physical functioning found in the present study were beyond those that would occur with no treatment. Third, the study had a relatively small sample size; future work should replicate the study in a larger sample. Lastly, the study was comprised largely of White non-Latinx youth, as frequently seen across pediatric pain clinics. As such, it is not known whether the findings may generalize to youth from other racial/ethnic groups; future studies should examine whether findings would differ across youth from diverse demographic backgrounds. There are several factors likely contributing to the over-representation of White participants in this study. First, according to the 2020 US census data for the State of Michigan (where the majority of the clinic’s patients reside), the state averages 15 percentage points higher than the US general population identifying as White, non-Hispanic/Latinx. Second, this sample was majority White and female, which parallels previous literature examining racial and ethnic characteristics of participants within pediatric chronic pain clinics [37–39]. Third, since the current study was a retrospective review of patients seeking care from a medical center and did not prospectively recruit, the sample represents those who sought healthcare, which has also been shown to be influenced by self-selection bias and systematic barriers to treatment [40, 41]. Additionally, this sample appeared to have higher rates of AMPS diagnosis (76.4%) than samples from other tertiary pain centers (7–54%), although diagnostic categories or definitions are not always consistent across the broader literature and rates of pain conditions represented in the literature vary widely [42].
Findings from the present study highlight the ways PT and psychology can work together in the assessment and treatment of pediatric chronic pain. First, depressive symptoms and pain catastrophizing should be assessed before the start of pain treatment among youth with chronic pain and may need to be targeted specifically among youth with poorer physical functioning. Second, the study highlights how optimal nonpharmacological pediatric pain management relies on integration between PT and psychology as this may reduce or even eliminate any association between depressive symptoms and pain catastrophizing with physical ability outcomes. Lastly, given that these findings support the biopsychosocial model of pain perception and functional impairment [33], patients and families should be educated on this model to promote engagement with both psychology and PT. In summary, the present study was the first to examine and support the association between modifiable psychological factors and performance-based measures of physical functioning in youth with a variety of pain concerns completing an IOP, bolstering the evidence for incorporation of psychological treatment in pediatric chronic pain care.
Acknowledgments
The authors have no acknowledgements.
Conflict of interest
The authors have no conflict of interest to report.
Funding
The authors have no sources of funding pertinent to this research to disclose.
References
[1] | Guidelines on the management of chronic pain in children. Geneva:World Health Organization; 2020. Available from: https://www.who.int/publications/i/item/9789240017870 |
[2] | King S , Chambers CT , Huguet A et al. The epidemiology of chronic pain in children and adolescents revisited: A systematic review, Pain (2011) ;152: (12):2729–38. Doi: 10.1016/j.pain.2011.07.016. |
[3] | Groenewald CB , Essner BS , Wright D , Fesinmeyer MD , Palermo TM . The economic costs of chronic pain among a cohort of treatment-seeking adolescents in the United States, J Pain. (2014) ;15: (9):925–33. Doi: 10.1016/j.jpain.2014.06.002. |
[4] | Roth-Isigkeit A , Thyen U , Stöven H , Schwarzenberger J , Schmucker P . Pain among children and adolescents: Restrictions in daily living and triggering factors, Pediatrics (2005) ;115: (2):e152–62. Doi: 10.1542/peds.2004-0682. |
[5] | Zernikow B , Wager J , Hechler T et al. Characteristics of highly impaired children with severe chronic pain: A 5-year retrospective study on 2249 pediatric pain patients. BMC Pediatr (2012) ;12: :54. doi: 10.1186/1471-2431-12-54 |
[6] | Öte Karaca Ş , Demirsoy N , Günendi Z . Effects of aerobic exercise on pain sensitivity, heart rate recovery, and health-related quality of life in patients with chronic musculoskeletal pain, Int J Rehabil Res (2017) ;40: (2):164–70. Doi: 10.1097/MRR.0000000000000212. |
[7] | Häuser W , Klose P , Langhorst J et al. Efficacy different types of aerobic exercise in fibromyalgia syndrome: A systematic review and meta-analysis of randomised controlled trials, Arthritis Res Ther (2010) ;12: (3):R79. Doi: 10.1186/ar3002. |
[8] | Kichline T , Cushing CC . A systematic review and quantitative analysis on the impact of aerobic exercise on pain intensity in children with chronic pain, Child Health Care. (2019) ;48: (2):244–61. Doi: 10.1080/02739615.2018.1531756. |
[9] | Coakley R , Wihak T . Evidence-based psychological interventions for the management of pediatric chronic pain: New directions in research and clinical practice. Children (Basel).9 (2017) ;4: (2):9. doi: 10.3390/children4020009 |
[10] | Fisher E , Law EF , Dudeney J , Palermo TM , Stewart G , Eccleston C Psychological therapies for the management of chronic and recurrent pain in children and adolescents, Cochrane Database Syst Rev (2018) ;9: (9):CD003968. Doi: 10.1002/14651858.CD003968.pub5. |
[11] | Palermo TM Cognitive-behavioral therapy for chronic pain in children and adolescents. New York: Oxford University Press; 2012. |
[12] | Claus BB , Stahlschmidt L , Dunford E et al. Intensive interdisciplinary pain treatment for children and adolescents with chronic noncancer pain: A preregistered systematic review and individual patient data meta-analysis. Pain (2022) ;163: (12):2281–301. doi: 10.1097/j.pain.0000000000002636 |
[13] | Simons LE , Sieberg CB , Conroy C et al. Children with chronic pain: Response trajectories after intensive pain rehabilitation treatment. J Pain (2018) ;19: (2):207–18. 10.1016/j.jpain.2017.10.005. |
[14] | Mirek E , Logan D , Boullard K , Hall AM , Staffa SJ , Sethna N . Physical therapy outcome measures for assessment of lower extremity chronic pain-related function in pediatrics, Pediatr Phys Ther. (2019) ;31: (2):200–7. Doi: 10.1097/PEP.0000000000000587. |
[15] | Landry BW , Fischer PR , Driscoll SW et al. Managing chronic pain in children and adolescents: A clinical review, PM R. (2015) ;7: (11 Suppl):S295–315. Doi: 10.1016/j.pmrj.2015.09.006. |
[16] | Homan K , Collins A , Crowley S et al. A psychometric investigation of objective and therapist-rated performance measures in a pediatric chronic pain population: How well do these measure work? J Pain (2022) ;23: (5 Suppl):50. Doi: 10.1016/j.jpain.2022.03.190. |
[17] | Taylor A , Phillips K , Patel K et al. Assessment of physical function and participation in chronic pain clinical trials: IMMPACT/OMERACT recommendations, Pain (2016) ;157: (9):1836–50. Doi: 10.1097/j.pain.0000000000000577. |
[18] | Simons LE , Kaczynski KJ , Conroy C , Logan DE . Fear of pain in the context of intensive pain rehabilitation among children and adolescents with neuropathic pain: Associations with treatment response. J Pain (2012) ;13: (12):1151–61. doi: 10.1016/j.jpain.2012.08.007 |
[19] | Beneciuk JM , Bishop MD , Fritz JM et al. The STarT Back screening tool and individual psychological measures: Evaluation of prognostic capabilities for low back pain clinical outcomes in outpatient physical therapy settings, Phys Ther (2013) ;93: (3):321–33. Doi: 10.2522/ptj.20120207. |
[20] | McGarrigle L , Wesson C , DeAmicis L , Connoly S , Ferreira N . Psychological mediators in the relationship between paediatric chronic pain and adjustment: An investigation of acceptance, catastrophising and kinesiophobia, J Context Behav Sci. (2020) ;18: :294–305. doi: 10.1016/j.jcbs.2020.10.009. |
[21] | Kotte EMW , De Groot JF , Bongers BC , Winkler AMF , Takken T . Validity and Reproducibility of a new treadmill protocol: The Fitkids Treadmill Test, Med Sci Sports Exerc (2015) ;47: (10):2241–7. Doi: 10.1249/MSS.0000000000000657. |
[22] | Kotte EMW , de Groot JF , Bongers BC , Winkler AMF , Takken T . Fitkids Treadmill Test: Age- and sex-related normative values in dutch children and adolescents, Phys Ther (2016) ;96: (11):1764–72. Doi: 10.2522/ptj.20150399. |
[23] | Eldridge BJ , Galea MP , Kissane AL et al. High-Level Mobility Assessment Tool normative values for children, Phys Ther. (2020) ;100: (2):324–31. Doi: 10.1093/ptj/pzz168. |
[24] | Williams G , Hill B , Pallant JF , Greenwood K . Internal validity of the revised HiMAT for people with neurological conditions, Clin Rehabil (2012) ;26: (8):741–7. Doi: 10.1177/0269215511429163. |
[25] | Miller RP , Kori SH , Todd DD . The Tampa Scale: A measure of kinisophobia, Clin J Pain. (1991) ;7: (1):51. Doi: 10.1097/00002508-199103000-00053. |
[26] | Swinkels-Meewisse EJCM , Swinkels RAHM , Verbeek ALM , Vlaeyen JWS , Oostendorp RAB . Psychometric properties of the Tampa Scale for kinesiophobia and the fear-avoidance beliefs questionnaire in acute low back pain, Man Ther. (2003) ;8: (1):29–36. Doi: 10.1054/math.2002.0484. |
[27] | Robinson ME , Riley JLI , Myers CD et al. The Coping Strategies Questionnaire: A large sample, item level factor analysis, Clin J Pain (1997) ;13: (1):43–9. Doi: 10.1097/00002508-199703000-00007. |
[28] | Zigmond AS , Snaith RP . The Hospital Anxiety and Depression Scale, Acta Psychiatr Scand (1983) ;67: (6):361–70. Doi: 10.1111/j.1600-0447.1983.tb09716.x. |
[29] | White D , Leach C , Sims R , Atkinson M , Cottrell D . Validation of the Hospital Anxiety and Depression Scale for use with adolescents, Br J Psychiatry (1999) ;175: :452–4. doi: 10.1192/bj175.5.452. |
[30] | Keller S , Bann CM , Dodd SL , Schein J , Mendoza TR , Cleeland CS . Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain, Clin J Pain (2004) ;20: (5):309–18. Doi: 10.1097/00002508-200409000-00005. |
[31] | IBM SPSS Statistics for Windows. IBM; 2021. |
[32] | Collins AB . Chronic pediatric pain management: A review of multidisciplinary care and emerging topics, Curr Phys Med Rehabil Rep (2019) ;7: :30–9. doi: 10.1007/s40141-019-0211-7. |
[33] | Gatchel RJ , Peng YB , Peters ML , Fuchs PN , Turk DC . The interplay of parent and adolescent catastrophizing and its impact on adolescents’ pain, functioning, and pain behavior. Psychol Bull. (2007) ;133: (4):581–624. doi: 10.1037/0033-2909.133.4.581 |
[34] | Lynch-Jordan AM , Kashikar-Zuck S , Szabova A , Goldschneider KR . The interplay of parent and adolescent catastrophizing and its impact on adolescents’ pain, functioning, and pain behavior, Clin J Pain. (2013) ;29: (8):681–8. Doi: 10.1097/AJP.0b013e3182757720. |
[35] | Vervoort T , Goubert L , Eccleston C , Bijttebier P , Crombez G . Catastrophic thinking about pain is independently associated with pain severity, disability, and somatic complaints in school children and children with chronic pain, J Pediatr Psychol. (2006) ;31: (7):674–83. Doi: 10.1093/jpepsy/jsj059. |
[36] | Wicksell RK , Melin L , Lekander M , Olsson GL . Evaluating the effectiveness of exposure and acceptance strategies to improve functioning and quality of life in longstanding pediatric pain - A randomized controlled trial, Pain (2009) ;141: (3):248–57. Doi: 10.1016/j.pain.2008.11.006. |
[37] | Evans S , Taub R , Tsao JC , Meldrum M , Zeltzer LK Sociodemographic factors in a pediatric chronic pain clinic: The roles of age, sex and minority status in pain and health characteristics, J Pain Manag (2010) ;3: (3):273–81. |
[38] | Ibeziako P , Randall E , Vassilopoulos A , Choi C , Thomson K , Ribeiro M et al. Prevalence, patterns, and correlates of pain in medically hospitalized pediatric patients with somatic symptom and related disorders, J Acad Consult-Liaison Psychiatry (2021) ;62: (1):46–55. Doi: 10.1016/j.psym.2020.05.008. |
[39] | Wilson AC , Stone AL , Poppert Cordts KM et al. Baseline characteristics of a dyadic cohort of mothers with chronic pain and their children, Clin J Pain (2020) ;36: (10):782. Doi: 10.1097/AJP.0000000000000864. |
[40] | Janevic MR , Mathur VA , Booker SQ et al. Making pain research more inclusive: Why and how, J Pain (2022) ;23: (5):707–28. Doi: 10.1016/j.jpain.2021.10.004. |
[41] | Palermo TM , Davis KD , Bouhassira D et al. Promoting inclusion, diversity, and equity in pain science, Pain Med (2023) ;24: (2):105–9. Doi: 10.1093/pm/pnac204. |
[42] | Hechler T , Kanstrup M , Lewandowski Holley A et al. Systematic review on intensive interdisciplinary pain treatment of children with chronic pain, Pediatrics (2015) ;136: (1):115–27. Doi: 10.1542/peds.2014-3319. |




