Cecostomy tubes improve bowel continence for pediatric patients with spina bifida: A retrospective analysis of outcomes from a single clinic
Abstract
PURPOSE:
Pediatric patients with spina bifida often experience neurogenic bowel dysfunction. Although cecostomy tubes could improve bowel continence, their effectiveness is not well established in this population. The aims of this study were to better understand the effectiveness of cecostomy tubes relative to other management strategies (between-subject) and to explore their effectiveness among patients who received these placements (within-subject).
METHODS:
Retrospective analysis of data from pediatric patients enrolled in a national spina bifida patient registry (n = 297) at a single multidisciplinary clinic was performed, covering visits between January 2014 –December 2021. Linear and ordinal mixed effect models (fixed and random effects) tested the influence of cecostomy status (no placement vs placement) and time (visits) on bowel continence while controlling for demographic and condition-specific covariates.
RESULTS:
Patients with cecostomy tubes had higher bowel continence compared to patients without placements (B = 0.695, 95% CI [0.333, 1.050]; AOR = 2.043, p = .007). Patients with cecostomy tubes had higher bowel continence after their placements compared to before (B = 0.834, 95% CI [0.142, 1.540]; AOR = 3.259, p = 0.011).
CONCLUSION:
Results indicate cecostomy tubes are effective for improving bowel continence in this pediatric population. Future research is needed to conduct risk analyses and determine the clinical significance of these effects.
1Introduction
Spina bifida is the most common neural tube birth defect in the United States [1] with a prevalence of ∼1, 400 newborns per year [2]. This birth defect is often associated with many physical complications, including neurogenic bowel dysfunction. In the most severe form (i.e., myelomeningocele), spinal nerves innervating lower extremities (e.g., bowel sphincters) lose their function and reflexes [3] leading to bowel incontinence [4, 5]. Importantly, bowel incontinence has been associated with reduced quality of life and depressive symptoms during adolescence [6, 7]. Addressing bowel incontinence earlier in life has been shown to mitigate these negative outcomes [8, 9] while allowing these patients to engage in life in ways previously limited by incontinence. Leaders in the spina bifida community have designated addressing bowel incontinence as a primary research agenda [1].
The Spina Bifida Association outlined a stepwise approach to bowel management starting with dietary and lifestyle changes (e.g., fiber, fluids, exercise) and moving on to pharmacological adjuncts (e.g., sennosides, polyethylene glycol) and rectal stimulants (e.g., glycerin, docusate sodium, bisacodyl suppositories) [4]. Subsequently, physical rectal interventions are recommended (e.g., transanal irrigation, cone enemas). Although this stepwise approach has been associated with improved bowel continence among these patients, surgical interventions including cecostomy tubes (e.g., Malone appendicostomies, Chait tubes) could be more effective [10].
Bevill and colleagues assessed the effectiveness of cecostomy placements (i.e., Chait tubes) against this stepwise approach to bowel management at a large hospital center in the U.S. Midwest [11]. Bowel incontinence was cross-sectionally evaluated among 86 pediatric patients with spina bifida (and other spinal dysraphisms) who either had cecostomy placements (n = 53) or used this stepwise approach (n = 33). Their results demonstrated that patients with cecostomy placements reported significantly higher bowel continence compared to patients who used the stepwise approach [11]. Cecostomy tubes were also found to be associated with improved hygiene, independence, and social confidence [11]. Using a large national sample, researchers have also shown the likelihood of cecostomy tube placement varies by sociodemographic and condition-specific variables, and may vary based on the clinic in which a patient is served [12, 13].
The International Children’s Continence Society (ICCS) reported a lack of research on the various bowel management strategies, with many providers relying on their clinical experience as a substitute for formal research [14]. Although the ICCS report was issued in 2012, this notion is still relevant today [1]. There is an even greater dearth of research on the effectiveness of surgical interventions and particularly cecostomy tubes [15]. This lack of research hinders the ability of providers to make evidence-informed decisions while addressing bowel incontinence among these patients. There are no randomized trials or longitudinal cohort studies on the effectiveness of cecostomy tubes. Although randomized trials are considered the highest tier of evidence, they present both ethical and practical concerns in these circumstances [15]. Therefore, longitudinal cohort studies are an ideal middle-ground to provide high level evidence while avoiding the ethical and practical concerns associated with randomized trials.
The objective of the current study was to address this gap in the literature by evaluating the effectiveness of cecostomy tubes on bowel continence in a longitudinal cohort of pediatric patients seen in a single multi-disciplinary spina bifida clinic. The primary aim was to better understand the effectiveness of cecostomy placements relative to other management strategies (between-subject effects) by comparing bowel continence over time between patients with and without these placements. The secondary (exploratory) aim was to explore the effectiveness of cecostomy placements for patients who received these placements (within-subject effects) by comparing bowel continence before versus after their cecostomy placement. Importantly, the longitudinal paradigm may improve aspects of causal inference compared to cross-sectional designs [11] while allowing for the exploration of within-subject effects that have never been described in the literature.
2Methods
2.1Ethical considerations
The Institutional Review Board at the academic health center approved procedures for this study, a retrospective secondary analysis of single clinic data gathered from a longitudinal cohort of pediatric patients receiving care at a multidisciplinary spina bifida clinic. All data were de-identified and stored on a secure server to maintain patient confidentiality.
2.2Participants
Participants included 297 pediatric patients (age: 11.53±5.6; 48% female) at a single multi-disciplinary spina bifida clinic. Eligibility criteria consisted of participation in the clinic, enrollment in the National Spina Bifida Patient Registry (NSBPR; requiring qualifying diagnoses of myelomeningocele, lipomeningocele, meningocele, fatty filum, terminal myelocystocele, or split cord malformation [16]), visiting the clinic between January 2014 –December 2021, and being age 3 –21 years at the time of the visit. All NSBPR clinic-specific data remain available locally. This clinic enrolls over 98% of eligible patients into the NSBPR; thus, registry data (and this analysis) represent the entire population at this single clinic.
2.3Measures
2.3.1Bowel continence
Bowel continence was the outcome variable in all analyses and was defined as the frequency of bowel incontinence during the month preceding each annual visit. Patients or their parent/legal guardian reported bowel continence using a five-point Likert-type scale (1 = daily, 2 = weekly, 3 = monthly, 4 = less than monthly, 5 = never).
2.3.2Cecostomy status
Cecostomy tube placement was the primary explanatory variable and was assessed as a binary composite during each annual clinic visit (0 = no placement, 1 = placement), including: (a) Malone appendicostomies, (b) Chait tubes, and (c) other types of cecostomy placements. For patients who did not receive any placements during the study period (no cecostomy group), all visits were labeled as no placement. For patients who received placements anytime during the study period (cecostomy group), visits prior to their placement were labeled as no placement, while visits after their placement were labeled as placement.
2.3.3Demographic and condition-specific variables
Demographic variables were selected based on their theoretical relevance including age, biological sex, ethnicity, and race [12, 13, 16–18]. Condition-specific variables included diagnosis type, shunt status, level of lesion, ambulation status, and other bowel and bladder management strategies.
2.4Data analysis
The primary aim was addressed by comparing bowel continence scores over time between patients with and without cecostomy placements. This was accomplished with linear mixed effect models (fixed and random effects) using restricted maximum likelihood estimation. Within these models cecostomy status and time (annual clinic visits) were included as main effects and tested in an initial model without an interaction term and subsequently in a model with an interaction term (cecostomy status x time). References included “no placement” (cecostomy status) and “visit one” (time); unstandardized coefficients were reported for the fixed effects (B). All demographic and condition-specific variables were included as covariates; models were restricted to the first five visits given the reduced sample in subsequent visits. Sensitivity analyses tested: (a) the inclusion of all visits, (b) the exclusion of patients younger than five years of age, and (c) the exclusion of patients who were continent (4 = less than monthly, 5 = never) with no bowel management (cecostomy tubes or other management strategies). Comparative analyses tested each type of placement against one another: (a) Malone vs Chait, (b) Malone vs other, and (c) Chait vs other.
Model fit for the random effects were assessed comparatively using likelihood-ratio tests (with appropriate p-value corrections [19]) between models with only random intercepts (participants) and models with both random intercepts (participants) and slopes (time). The random effect structure with better model fit was always reported. When neither random effect structure demonstrated better model fit, the simpler structure was always reported (only random intercepts). The amount of outcome variation attributed to the random effect structure was assessed using the conditional intraclass correlation coefficient. Assumptions for the mixed effect models were evaluated using diagnostic plots (normality of the residuals [histograms] and homogeneity of variances [scatter plots]).
Importantly, it must be acknowledged that linear mixed effect models assume the outcome is represented on a continuous scale. While bowel continence represents an ordered sequence of frequencies (continuous concept), there is a high likelihood this outcome would distort the standard errors and not provide appropriate coverage of the confidence intervals. Therefore, even though the point estimates would be valid, determining the statistical significance of the estimates is problematic within these models. To address this concern, statistical significance was determined using non-parametric bootstraps (1,000 bootstrapped samples) for the standard errors and confidence intervals (without distributional assumptions). Further, this outcome was tested within ordinal mixed effect models (cumulative mixed link models) using logit links, maximum likelihood estimation (Laplace approximation), and the same random effect structure as the corresponding linear mixed effect model.
The exploratory aim was addressed by restricting the sample only to patients who received a cecostomy placement during the study period (cecostomy group) and comparing bowel continence before vs after their placement. All modeling aspects were retained from the primary aim with the exceptions of including all visits (given the restricted sample size within these models) and not interpreting the main effect of time, nor including the interaction between cecostomy status and time (as cecostomy status accounted for the relevant effects of time within these models). For each patient, all visits prior to their placement contributed to the no placement (total) estimate while all visits after their placement contributed to the placement (total) estimate.
Data were analyzed in R (3.6.3) using the lmerTest package for the mixed effect models, the lmeresampler package for the bootstrapped samples, the ordinal package for the ordinal mixed effect models, the performance package for the intraclass correlation coefficients, and the ggplot2 package for model diagnostics and graphical visualizations.
3Results
A total of 297 pediatric patients with spina bifida were included in this study with an average of three visits (±two visits) per participant, resulting in a total of 916 visits. Participant characteristics for the overall sample and subgroups (cecostomy group [n = 54] or no cecostomy group [n = 243]) during the last visit are presented in Table 1 (to ensure that all patients in the cecostomy group had received their placement).
Table 1
Participant characteristics by cecostomy status
| Mean±SD or N (%) | ||||
| Total (n = 297) | Cecostomy (n = 54) | No Cecostomy (n = 243) | p | |
| Key Demographics | ||||
| Age (Years) | 11.5 (±5.6) | 15.5 (±4.2) | 10.7 (±5.5) | .001† |
| Female | 141 (47.5%) | 29 (53.7%) | 112 (46.1%) | .31 |
| Shunt Placement | 149 (50.2%) | 41 (75.9%) | 108 (44.4%) | .001 |
| Hispanic or Latino | 74 (24.9%) | 10 (18.5%) | 64 (26.3%) | .23 |
| White | 259 (87.2%) | 49 (90.7%) | 210 (86.4%) | .39 |
| Diagnosis | ||||
| Myelomeningocele | 194 (65.3%) | 49 (90.7%) | 145 (59.7%) | .001 |
| Level of Lesion | ||||
| Sacral | 139 (46.8%) | 12 (22.2%) | 127 (52.3%) | .001 |
| Low Lumbar | 43 (14.5%) | 9 (16.7%) | 34 (14.0%) | .61 |
| Mid Lumbar | 61 (20.5%) | 14 (25.9%) | 47 (19.3%) | .27 |
| High Lumbar | 18 (6.1%) | 6 (11.1%) | 12 (4.9%) | .11‡ |
| Thoracic | 36 (12.1%) | 13 (24.1%) | 23 (9.5%) | .003 |
| Ambulation | ||||
| Community | 186 (62.6%) | 23 (42.6%) | 163 (67.1%) | .001 |
| Household | 21 (7.1%) | 5 (9.3%) | 16 (6.6%) | .55‡ |
| Therapeutic | 17 (5.7%) | 2 (3.7%) | 15 (6.2%) | .75‡ |
| Non-Ambulator | 73 (24.6%) | 24 (44.4%) | 49 (20.2%) | .001 |
| Other Bowel Management | ||||
| None | 98 (33.0%) | 0 (0.0%) | 98 (40.3%) | .001 |
| Annual Clinic Visits | ||||
| Visits | 3.0 (±2.0) | 4.0 (±2.0) | 3.0 (±2.0) | .001 |
Note. All data were obtained from the last annual clinic visit (to ensure that all patients in the cecostomy group had received their placement). P-values reference between-group differences using independent t-tests (M±SD) or chi-square tests (N) for the corresponding characteristic with appropriate corrections (degree of freedom corrections† or exact tests‡, respectively) whenever assumptions were violated.
3.1Participant characteristics
Relative to patients without cecostomy placements, patients with placements typically were older (p< .001), had shunt placements (p< .001), attended more visits (p< .001), used more (additional) bowel management strategies (p< .001), and experienced more severe condition states (see Table 1). For instance, patients with placements more commonly reported diagnoses of meningomyelocele (p< .001), thoracic lesions (p= .003), and non-ambulator status (p< .001). No significant differences were noted for biological sex (p= .31), race (p= .39), or ethnicity (p= .23) among patients with and without cecostomy placements.
3.2Mixed effect models
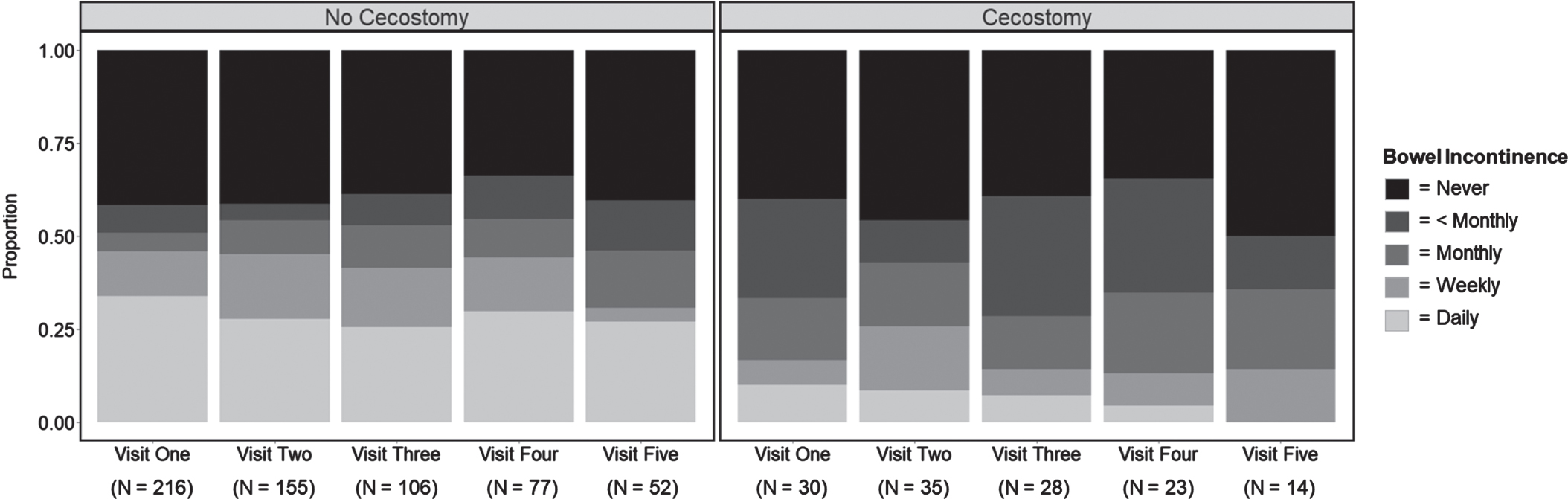
For the mixed effect models, seven patients in the cecostomy group were excluded for either having an unknown placement date (n = 6) or not providing any outcome data (n = 1), while six patients in the no cecostomy group were excluded for not providing any outcome data (n = 6). Among the remaining 284 patients, 97 visits were excluded for either missing outcome data (94 visits) or missing covariate data (3 visits). This resulted in a total of 783 visits included in the mixed effect models with 82% labeled as no placement (643 visits) and 18% labeled as placement (140 visits). Stacked bar charts were used to visualize bowel continence scores across the first five visits (783 → 736 visits) delineated by no placement vs placement (Fig. 1).
Fig. 1
Bowel continence scores by cecostomy status. Note. Stacked bar charts visualizing bowel continence scores across the first five visits delineated by cecostomy status “no cecostomy” (no placement) vs “cecostomy” (placement). “N” refers to the number of visits (participants) during each timepoint. For patients who received placements during the study period, visits prior to their placement were labeled as “no cecostomy” (no placement) while visits after their placement were labeled as “cecostomy” (placement).
3.2.1Primary aim
The main effects model (without interaction terms) included random effects for both the intercepts (participants) and slopes (time) which explained 36.2% of the total variance for bowel continence. Diagnostic plots provided no evidence of violations regarding assumptions for the normality of the residuals or homogeneity of variances. This model provided evidence of a significant main effect for cecostomy status (B = 0.695, 95% CI [0.333, 1.050]) while the main effect for time was not significant (B = 0.021, 95% CI [–0.048, 0.094]). The interaction model was then tested by including an interaction term (cecostomy status x time) that was also not significant (B = –0.049, 95% CI [–0.239, 0.129]). Therefore, the results were interpreted from the main effects model without interaction terms. Independent of time, pediatric spina bifida patients with cecostomy placements had bowel continence scores 0.70 points higher on average (95% CI [0.333, 1.050]) and twice the odds of reporting higher bowel continence (AOR = 2.043, 95% CI [1.220, 3.421], p = .007) compared to patients without placements (Table 2).
Table 2
Mixed effect models (Primary Aim)
| Estimate | 95% CI (Boot) | Model Fit | ICC | AOR | 95% CI (AOR) | Visits | |
| Primary Analyses | |||||||
| Main Effects Model | 0.695 | 0.333, 1.050 | X2(2) = 5.57* | 0.326 | 2.043** | 1.220, 3.421 | 736 |
| Interaction Model | 0.820 | 0.246, 1.420 | X2(2) = 5.22* | 0.326 | 2.838** | 1.323, 6.085 | 736 |
| Sensitivity Analyses | |||||||
| Sensitivity Analysis (1) | 0.668 | 0.295, 1.050 | X2(2) = 10.70** | 0.344 | 2.042** | 1.215, 3.432 | 783 |
| Sensitivity Analysis (2) | 0.709 | 0.343, 1.050 | X2(2) = 8.68** | 0.333 | 2.107** | 1.298, 3.419 | 605 |
| Sensitivity Analysis (3) | 0.909 | 0.562, 1.280 | X2(2) = 3.23 | 0.262 | 2.812*** | 1.724, 4.586 | 582 |
| Comparative Analyses | |||||||
| Chait vs Malone | 0.232 | –0.411, 0.865 | X2(2) = 5.06 | 0.132 | 1.611 | 0.638, 4.065 | 161 |
| Other vs Malone | 0.771 | –0.080, 1.620 | – | – | 2.723 | 0.810, 9.155 | 161 |
| Other vs Chait | 0.540 | -0.180, 1.270 | – | – | 1.691 | 0.576, 4.966 | 161 |
Note. This table integrates the results from the mixed effect models used to test the primary aim. Estimates (unstandardized), bootstrapped confidence intervals (CI; 95%), model fit (random effects), and conditional intraclass correlation coefficients (ICCs) were derived from the linear mixed effect models. Significant model fit (likelihood-ratio tests) indicates the model with both random intercepts and slopes demonstrates better fit than the model with only random intercepts (random intercept and slope model reported); non-significant model fit indicates no difference between the random effect structures (random intercept only model reported). The conditional ICC describes the proportion of total variance within bowel continence that is attributed to the random effects. The odds ratio (adjusted for the demographic and condition-specific covariates; AOR) and the confidence interval for the AOR (95%) were derived from the ordinal mixed effect models. AORs indicate the odds of patients in the cecostomy group reporting higher bowel continence vs patients in the no cecostomy group. Visits refer to the total number of datapoints included in each analysis. Sensitivity analyses tested (1) the inclusion of all visits, (2) the exclusion of patients younger than five years of age, and (3) the exclusion of patients who were continent (4 = less than monthly, 5 = never) with no bowel management (cecostomy tubes or other management strategies). Comparative analyses were all within the same model (using different reference groups) and thus model fit and the conditional ICC are redundant. *p < 0.05; **p < 0.01; ***p < 0.001.
Sensitivity analysis indicated that the main effect of cecostomy status remained significant while including all visits (B = 0.668, 95% CI [0.295, 1.050]; AOR = 2.042, p = .007), excluding patients younger than five years old (B = 0.709, 95% CI [0.343, 1.050]; AOR = 2.107, p = .003), and excluding patients who were continent with no bowel management (B = 0.909, 95% CI [0.562, 1.280]; AOR = 2.812, p < .001). Comparative analyses indicated no significant differences among the different types of cecostomy placements: (a) Chait vs Malone (B = 0.232, 95% CI [–0.411, 0.865]), (b) other vs Malone (B = 0.771, 95% CI [–0.080, 1.620]), and (c) other vs Chait (B = 0.540, 95% CI [–0.180, 1.270]).
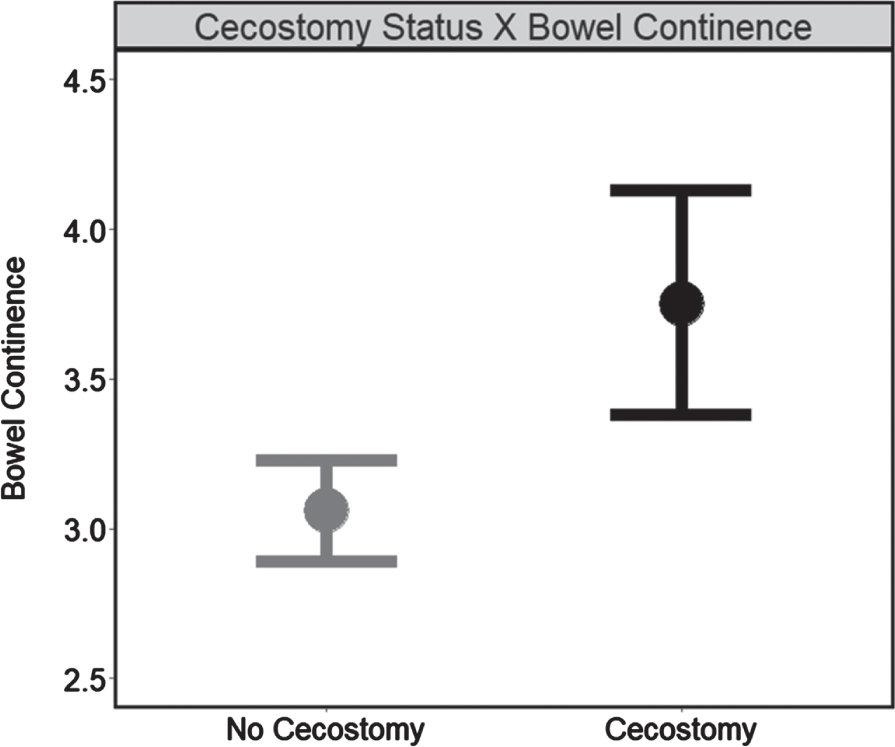
Together, these findings indicate that, while the slope of the relationship between time and bowel continence did not differ by cecostomy status, patients with cecostomy placements had higher bowel continence, in general (Fig. 2), and remained higher over time, on average, relative to patients without placements.
Fig. 2
Cecostomy status on bowel continence independent of time (N = 284). Note. Estimated marginal means for bowel continence delineated by cecostomy status “no cecostomy” (no placement; 606 visits) vs “cecostomy” (placement; 130 visits) obtained from the main effects model (without interaction terms). Higher scores indicate greater continence while error bars represent confidence intervals (95%).
3.2.2Exploratory aim
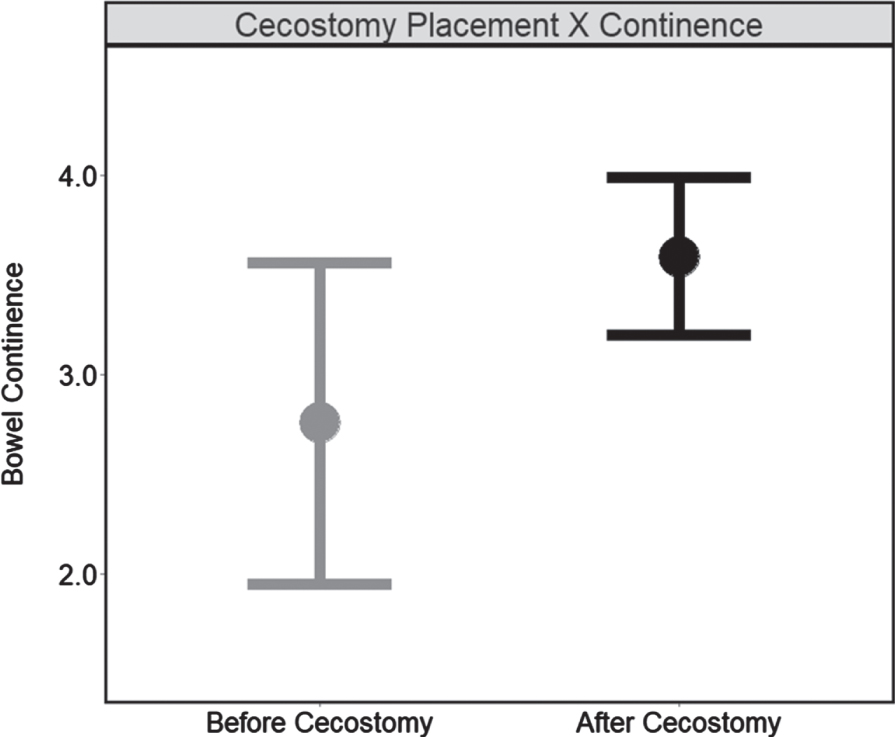
The mixed effects model included random effects only for the intercepts (participants), which explained 19.8% of the total variance for bowel continence. Diagnostic plots provided no evidence of violations regarding assumptions for the normality of the residuals or homogeneity of variances. This model provided evidence of a significant main effect for cecostomy status (B = 0.834, 95% CI [0.142, 1.540]) among pediatric patients with cecostomy placements. The main effect indicated that patients with placements had bowel continence scores 0.83 points higher on average (95% CI [0.142, 1.540]) after their placement (relative to before their placement). Further, after receiving their placements, pediatric patients had triple the odds of reporting higher bowel continence (AOR = 3.259, 95% CI [1.311, 8.098], p = 0.011). These findings indicate that cecostomy placements could be an effective strategy for improving bowel continence among pediatric patients without placements (Fig. 3).
Fig. 3
Cecostomy placement on bowel continence (N = 47). Note. Estimated marginal means for bowel continence delineated by cecostomy status “before cecostomy” (no placement; 21 visits) vs “cecostomy” (placement; 140 visits) obtained from the mixed effect model testing the secondary aim (within-subject). Higher scores indicate greater continence while error bars represent confidence intervals (95%).
4Discussion
In this longitudinal cohort of 297 pediatric patients with spina bifida, there was evidence of cecostomy tubes improving bowel continence. Patients with cecostomy tubes had higher bowel continence after their tube placement and higher continence overall when compared to patients without cecostomy tubes. Importantly, patients with cecostomy tubes typically presented with a more severe form of spina bifida (i.e., myelomeningocele) and condition state, which are associated with lower bowel continence [17]. Regardless, patients with cecostomy tubes still experienced higher bowel continence relative to patients without cecostomy tubes. This evidence further exemplifies the effectiveness of cecostomy tubes among these pediatric patients.
The results of this study aligned with past research [11–13, 20] and expand this limited body of evidence [15]. While Bevill and colleagues found that cecostomy tubes (Chait tubes) were effective in improving bowel continence in a cross-sectional sample of 86 pediatric patients [11], the current findings are based on a longitudinal cohort of 297 pediatric patients across a seven-year period while also evaluating various types of cecostomy tubes (i.e., Malone, Chait, and other types). Similar to other studies [20], no differences in effectiveness among the various types of cecostomy tubes were noted.
Importantly, bowel continence was evaluated by categorical degrees within this study (i.e., never, less than monthly, monthly, weekly, daily), warranting a slightly different interpretation of the results compared to a binary indicator (i.e., continence or incontinence). Specifically, it was found that patients with cecostomy tubes had bowel continence scores almost one point (categorical level) higher on average relative to patients without cecostomy placements. This one level increase is substantial, as it would be the difference between being incontinent, for example, daily versus weekly, or weekly versus monthly, which could be quite meaningful for patients.
The approach used in this study had several strengths including the longitudinal cohort, analytical techniques, and the evaluation of various types of cecostomy tubes. The longitudinal cohort provided a large sample size (n = 297) with data collection spanning seven years (i.e., 2014 –2021) and across a wide age range (i.e., 3 –21 years). The sample size was considerably larger than prior studies on this topic [11], and the longitudinal paradigm may improve causal inference relative to cross-sectional designs. The analytical techniques also evaluated both between-subject (i.e., primary aim) and within-subject (i.e., exploratory aim) effects using mixed effect models. The use of these modeling techniques is important as they offer significant advantages over typical analyses of variance including the management of missing data (i.e., restricted maximum likelihood estimation), the focus on individual level change and variance (i.e., random effects), and the ability to use unstructured data with time-varying covariates [21].
However, the approach also had several limitations that should be considered when interpreting the results. First, although data were from a longitudinal cohort, they represented an accelerated longitudinal design. Specifically, the patients differed by age (i.e., 3–21 years) at enrollment (i.e., visit one) which may have occurred anywhere from 2014–2021. Thus, planned missingness was a byproduct of the design, with only 15 participants providing annual data (clinic visits) over the full seven-year period. In this context, planned missingness was considered to be missing completely at random (MCAR) and facilitated time unstructured data, warranting the use of maximum likelihood estimation and mixed effect models in analytical procedures [21]. Second, the outcome variable was an unvalidated, retrospective, and self-reported (single-item) measure of bowel continence that could not differentiate between patients who were incontinent once vs multiple times per day. However, there are currently no validated measures of bowel continence, and the risk of recall bias was likely negligible as patients recalled their bowel continence during the month preceding each annual visit. Third, the sample included patients from a single clinic who were primarily white (87%) or non-Hispanic (75%) with a low prevalence of cecostomy placements (18%). This limits the generalizability of the results, especially considering the high prevalence of spina bifida among Hispanic patients [22]. Fourth, data were obtained from a single clinic (local data) rather than using the entire NSBPR (aggregated data). However, the intention was to assess local outcomes first to build a foundation to spur further investigation using the aggregated data. Fifth, data were not collected on compliance with clinical recommendations for cecostomy tubes, complications, other bowel managements, or specific symptoms related to bowel incontinence experienced by the patients.
Further, only a small number of patients with cecostomy tubes were included in the primary and exploratory analyses (n = 47), which may have influenced effect sizes and significance (even though these patients did have more visits on average). This limitation was particularly relevant for the comparative analyses given the small number of patients with Malone (n = 10) and other types of placements (n = 8) vs Chait tubes (n = 29), although these findings did align with past research [20]. Importantly, this limitation was even more significant for the exploratory aim especially given that 72% of the cecostomy patients (n = 34) already had their cecostomy placement during visit one. Therefore, 28% of these patients (n = 13; 21 visits) contributed data to the no placement (total) estimate and 100% of these patients (n = 47; 140 visits) contributed data to the placement (total) estimate (the patients differed by their number of visits and the date of their placement). Although not ideal, this limitation was partially addressed using maximum likelihood estimation, which maximized data from all patients observed at least once. Specifically, parameter values were estimated based on existing outcome data across all waves of measurement to obtain unbiased estimates of change for each patient, adjusted for missingness [21]. Predicted trajectories were then weighted via the available data for each patient, with the estimates being closer to the mean for patients with less data. Also, no differences were observed between patients who had their placement during vs after visit one with the exception of age; during visit one, patients who already had their placement were older (13.06±4.09 years) than patients who had not received their placement yet (7.15±4.49 years).
Despite these limitations, there was evidence of cecostomy tubes improving bowel continence (relative to other management strategies) among pediatric patients with spina bifida. In light of this, it is recommended that providers consider the potential and unique challenges associated with each type of cecostomy placement when working with individual cases to select the type that best aligns with the patient and their family. In order to support these individualized decisions, providers may need to educate patients and their families about unique challenges (e.g., complications [10, 15, 20]).
Researchers are encouraged to expand this limited body of evidence while addressing the limitations of this study to mitigate bias and improve generalizability. It is important to offer providers the most accurate and generalizable estimates to reduce ambiguity around clinical decisions for these patients. Further research is needed to conduct risk analyses and determine the clinical significance of these effects, which were beyond the scope of the current study. Furthermore, researchers are encouraged to investigate the effects of cecostomy tubes on quality of life and mental health outcomes, especially for children as they transition into school and other social settings. Importantly, bowel continence could mediate these associations, and longitudinal designs are needed to support causal inferences. Alas, this limited body of evidence is not strong enough to inform clinical decisions among these patients, although this study represents a stride in the right direction. Addressing these avenues of further research could lead to reaching this climacteric, even in the near future.
Cecostomy tubes may not serve as an initial approach in bowel management strategies for pediatric patients with spina bifida. However, surgical and biotechnological advancements have resulted in these placements becoming more feasible for younger patients by reducing their invasiveness [10]. Despite these advances, the potential risks and benefits of early placements remain unclear; future research is needed to better understand both the short- and long-term effects of early placements. Should risk analyses reveal that the potential benefits outweigh the potential risks, early placements may become more practical within bowel management strategies. This approach could be particularly beneficial for patients experiencing more severe complications. Ideally, these placements would occur prior to transitioning into school and other social settings, enabling these individuals to engage in life in ways otherwise limited by bowel incontinence.
Acknowledgments
This project was by the Cooperative Agreements numbers 1U01DDD0007722, 1U01DD001071, and 1U01DD001275 from the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention (K. Freeman). We also acknowledge Ms. Karin Ide, MS, CPNP, Director of the Spina Bifida Clinic at Oregon Health & Science University, for her ongoing support of quality improvement initiatives as a clinic partner.
Conflicts of interest
None.
Ethical considerations
This study was reviewed by the university’s Institutional Review Board and determined to be exempt (IRB # 00023847).
References
[1] | Struwe S , Thibadeau J , Kelly MS , Widener-Burrows D . Establishing the first community-centered Spina Bifida research agenda. J Pediatr Urol. (2022) ;18: (6):800.e1–800.e7. doi: 10.1016/j.jpurol.2022.06.014. |
[2] | National Institute of Neurological Disorders & Stroke. Spina Bifida. Bethesda, MD: National Institutes of Health; [updated 20 September 2023; cited 14 November 2022]. Available from: https://www.ninds.nih.gov/spinabifida-fact-sheet |
[3] | Rocque BG , Hopson BD , Blount JP . Caring for the Child with Spina Bifida. Pediatr Clin North Am. (2021) ;68: (4):915–27. doi: 10.1016/j.pcl.2021.04.013. |
[4] | Guidelines for the care of people with spina bifida. Spina Bifida Association; 2018 [cited 22 November 2022]. Available from: https://www.spinabifidaassociation.org/wp-content/uploads/Guidelines-for-the-Care-of-People-with-Spina-Bifida-2018.pdf |
[5] | Chaney JY , Taha AA , Pinter JD . Demystifying Spina Bifida Guidelines Using a Periodicity Schedule. Top Spinal Cord Inj Rehabil. (2022) ;28: (3):1–8. doi: 10.46292/sci21-00097. |
[6] | Mosiello G , Safder S , Marshall D , Rolle U , Benninga MA . Neurogenic Bowel Dysfunction in Children and Adolescents. J Clin Med. (2021) ;10: (8):1669. doi: 10.3390/jcm10081669. |
[7] | Taha AA , Eisen AM , Abdul-Rahman HQ , Zouros A , Norman S . The moderating role of spirituality on quality of life and depression among adolescents with spina bifida. J Adv Nurs. (2020) ;76: (7):1627–37. doi: 10.1111/jan.14374. |
[8] | Freeman JJ , Simha S , Jarboe MD , Ehrlich PF , Teitelbaum DH Antegrade continent enema procedures performed prior to starting school may improve functional stooling and quality of life. Pediatr Surg Int. (2014) ;30: (7):715–22. doi: 10.1007/s00383-014-3520-z. |
[9] | Sawin KJ , Brei TJ , Houtrow AJ Quality of life: Guidelines for the care of people with spina bifida. J Pediatr Rehabil Med. (2020) ;13: (4):565–82. doi: 10.3233/PRM-200732. |
[10] | Gor RA , Katorski JR , Elliott SP . Medical and surgical management of neurogenic bowel. Curr Opin Urol. (2016) ;26: (4):369–75. doi: 10.1097/MOU.0000000000000299. |
[11] | Bevill MD , Bonnett K , Arlen A , Cooper C , Baxter C , Storm DW . Outcomes and satisfaction in pediatric patients with Chait cecostomy tubes. J Pediatr Urol. (2017) ;13: (4):365–70. doi: 10.1016/j.jpurol.2017.04.008. |
[12] | Routh JC , Joseph DB , Liu T , et al. Variation in surgical management of neurogenic bowel among centers participating in National Spina Bifida Patient Registry. J Pediatr Rehabil Med. (2017) ;10: (3-4):303–12. doi: 10.3233/PRM-170460. |
[13] | Wiener JS , Suson KD , Castillo J , et al. Bowel management and continence in adults with spina bifida: Results from the National Spina Bifida Patient Registry –15. J Pediatr Rehabil Med.. (2017) ;10: (3-4):335–43. doi: 10.3233/PRM-170466. |
[14] | Rawashdeh YF , Austin P , Siggaard C , et al. International children’s continence society’s recommendations for therapeutic intervention in congenital neuropathic bladder and bowel dysfunction in children. Neurourol Urodyn. (2012) ;31: (5):615–20. doi: 10.1002/nau.22248. |
[15] | Kelly MS . Malone antegrade continence enemas vs cecostomy vs. transanal irrigation— What is new and how do we counsel our patients? Curr Urol Rep. (2019) ;20: (8):41. doi: 10.1007/s11934-019-0909-1. |
[16] | Sawin KJ , Liu T , Ward E , et al. The National Spina Bifida Patient Registry: Profile of a large cohort of participants from the first 10 clinics. J Pediatr. (2015) ;166: (2):444–50.e1. doi: 10.1016/j.jpeds.2014.09.039. |
[17] | Freeman KA , Castillo H , Castillo J , et al. Variation in bowel and bladder continence across US spina bifida programs: A descriptive study. J Pediatr Rehabil Med. (2017) ;10: (3-4):231–41. doi: 10.3233/PRM-170450. |
[18] | Schechter MS , Liu T , Soe M , Swanson M , Ward E , Thibadeau J . Sociodemographic attributes and spina bifida outcomes. Pediatrics. (2015) ;135: (4):e957–64. doi: 10.1542/peds.2014-2576. |
[19] | West BT , Welch KB , Galecki AT Linear mixed models: A Practical guide using statistical software. 3rd ed. New York: Chapman & Hall; 2022. |
[20] | Li C , Shanahan S , Livingston MH , Walton JM . Malone appendicostomy versus cecostomy tube insertion for children with intractable constipation: A systematic review and meta-analysis. J Pediatr Surg. (2018) ;53: (5):885–91. doi: 10.1016/j.jpedsurg.2018.02.010. |
[21] | Singer JD , Willett JB Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. |
[22] | Mai CT , Isenburg JL , Canfield MA et al. National Birth Defects Prevention Network. National population-based estimates for major birth defects, 2010–2014. Birth Defects Res. (2019) ;111: (18):1420–35. doi: 10.1002/bdr2.1589. |




