COVID-19 vaccination in individuals with spina bifida: A national survey
Abstract
PURPOSE:
This study aimed to conduct a national survey of individuals with spina bifida (SB) and their care partners to assess COVID-19 vaccination behaviors and vaccine uptake.
METHODS:
A survey instrument was designed to assess current vaccination status, general perceptions towards vaccinations, and barriers to vaccination within the SB community. Surveys were administered to individuals with SB or their representing care partner. Chi-squared and independent-samples t-tests were used to analyze the relationship between vaccine uptake and demographics. Multivariable logistic regression modeling was used to test which predictors impacted the odds that a participant received a COVID vaccine.
RESULTS:
A total of 1,412 participants completed the questionnaire, and 1,145 participants reported their COVID-19 vaccine status. The most common reason for not getting vaccinated was a concern about vaccine safety and efficacy. Overall, healthcare professional recommendations played a significant (OR 2.77 p < 0.001) role in whether to get vaccinated.
CONCLUSION:
About one in five individuals with SB have not received any COVID-19 vaccine. Actionable and modifiable factors were identified which may help increase vaccine uptake. Importantly, health providers play a critical role in COVID-19 vaccination messaging and should emphasize vaccine safety and efficacy.
1Introduction
More than one million deaths in the United States (US) have been attributed to COVID-19 [1]. The US Food and Drug Administration (FDA) has given full approval to the Pfizer-BioNTech and Moderna vaccines for use in persons six months or older [2–4] and given emergency use authorization for the Janssen vaccine [5]. While the COVID-19 vaccines have been proven safe and effective in reducing COVID-19 related severe illness and death, vaccine hesitancy remains a significant obstacle in curtailing the pandemic. Vaccine hesitancy is the refusal of or delay in the acceptance of a vaccine. According to the latest data from the Centers for Disease Control and Prevention (CDC), 20.8% of eligible Americans have not received their first vaccine dose, and 32.5% are not fully vaccinated [1]. In the general population, the most common reason for vaccine hesitancy is a concern about vaccine safety and efficacy; hesitancy has been disproportionately higher in those who self-identify as female gender, Black race, and/or Latinx ethnicity [6–9].
Most studies exploring US COVID-19 vaccination preferences and experiences have been conducted on the general population without focusing on disability status. The pandemic has had a significantly negative impact on individuals with disabilities through increasing sedentary behaviors [10], exacerbating health inequities [11], and worsening mental health [12]. Individuals with disabilities are more likely to have chronic health conditions, face social inequality, and meet barriers to care, all factors which increase the risk of COVID-19 related illness and death [13]. The COVID-19 vaccine hesitancy rate among individuals with disabilities is about 25% [14]. Within this population, vaccine hesitancy has been associated with COVID-19 vaccine safety concerns, inability to receive reliable information, and prior negative experiences with vaccines [15, 16]. Decreased hesitancy has been associated with worries about getting COVID-19, trust in experts, and a higher education level [14, 17].
No studies to date have specifically examined COVID-19 vaccine hesitancy in individuals with spina bifida (SB). SB is the most common permanently disabling congenital defect in the US, with over 166,000 individuals living with this condition [18, 19]. Understanding the underlying factors driving COVID-19 vaccination beliefs and behavior in this community is essential for guiding messaging, targeting barriers, and implementing campaigns to promote vaccination. Thus, the objective of this study was to conduct a national survey of individuals with SB to assess current vaccination status and general perceptions towards vaccinations and identify barriers to receiving the COVID-19 vaccine.
2Methods
2.1Survey development
After a critical review of national surveys utilized by the CDC for COVID-19 vaccination status and hesitancy, a panel of stakeholders from the Spina Bifida Association (SBA), clinicians, and researchers convened to create the study survey. The survey was pretested for language and clarity with eight individuals who were either adults with SB or the parent of a child with SB. The final survey had a maximum of 22 questions. The exact number of questions presented to each individual depended on branching logic from their responses. The survey was written in English and translated into Spanish. The survey was programmed into the Qualtrics (Provo, UT) survey platform. This research study was exempt from the university’s institutional review board (Pro00109800; approved on 11/30/2021).
2.2Survey distribution
The survey was sent via email to the 22,922 addresses registered with the SBA. Tailored reminder emails were sent to non-respondents. Two general reminders were sent to the entire list of registered email addresses. The SBA also advertised the survey, which was open from 1/11/22-1/25/22, on social media platforms. The survey was voluntary and respondents were not compensated for their time. Respondents were asked to complete the survey only once to represent each individual with SB. Respondents could have been those with a diagnosis of SB and over 18 years of age or care partners (representing children or adults with a diagnosis of SB).
2.3Inclusion criteria
Respondents self-verified that they were older than 18 years of age. Eligible survey participants included individuals with SB or the care partner representing an individual of any age with SB. Care partners were asked about the vaccine status of the individual with SB for whom they care. Participants self-verified their residence within the United States or territories to be eligible to complete the survey.
2.4Statistical analysis
Only those whose vaccine status was reported on the survey were included in the analysis. For this study, vaccine uptake was defined as receiving at least one dose of a COVID-19 vaccine. This study analyzed (1) the relationship between vaccine uptake and demographics, (2) where respondents obtained health information, and (3) beliefs about vaccines in general and specifically the COVID-19 vaccine.
An epidemiological analysis was completed to examine vaccine uptake. For care partners who responded on behalf of an individual with SB, the analysis was carried out based on the individual with SB and will be referenced as individuals with SB throughout. The sample was then stratified into those who reported receiving a COVID-19 vaccine and those who did not receive a COVID-19 vaccine, and differences in their demographic variables were tested.
Chi-squared analysis was used to test for differences among categorical variables. Independent-sample t-tests were used to test for differences among continuous variables. Multivariable logistic regression modeling was used to test for independent predictors of the odds that a participant received a COVID-19 vaccine.
For those who had not received a COVID-19 vaccine, a descriptive analysis was performed to determine the perceived likelihood of receiving the COVID-19 vaccine in the future and the reasons for failure to obtain the COVID-19 vaccine. Similarly, for those who received a vaccine, a descriptive analysis was performed for their reasons for accepting the vaccine.
3Results
3.1Response rate
Of the 22,922 emails sent, 78 were returned due to invalid addresses; 26% (n = 5,860) opened the email, with 38% of them using the link to access the survey. Of those who accessed the survey, 89% completed it (n = 1,412). Thirteen surveys were completed in Spanish; the rest were completed in English.
3.2Vaccine uptake
A total of 1,412 respondents with SB or on behalf of people with SB completed the questionnaire. Of those, 1,145 (81%) reported whether they had received a COVID-19 vaccine (Fig. 1). Most SB individuals reported being vaccinated (n = 896, 78.3%) (Table 1). Vaccine uptake was not uniform across groups. Children under 18 years of age with SB had significantly lower vaccine uptake (n = 151, 50.3%) compared to adults with SB (Table 2).
Fig. 1
Response flow diagram representing the vaccination status of the sample.
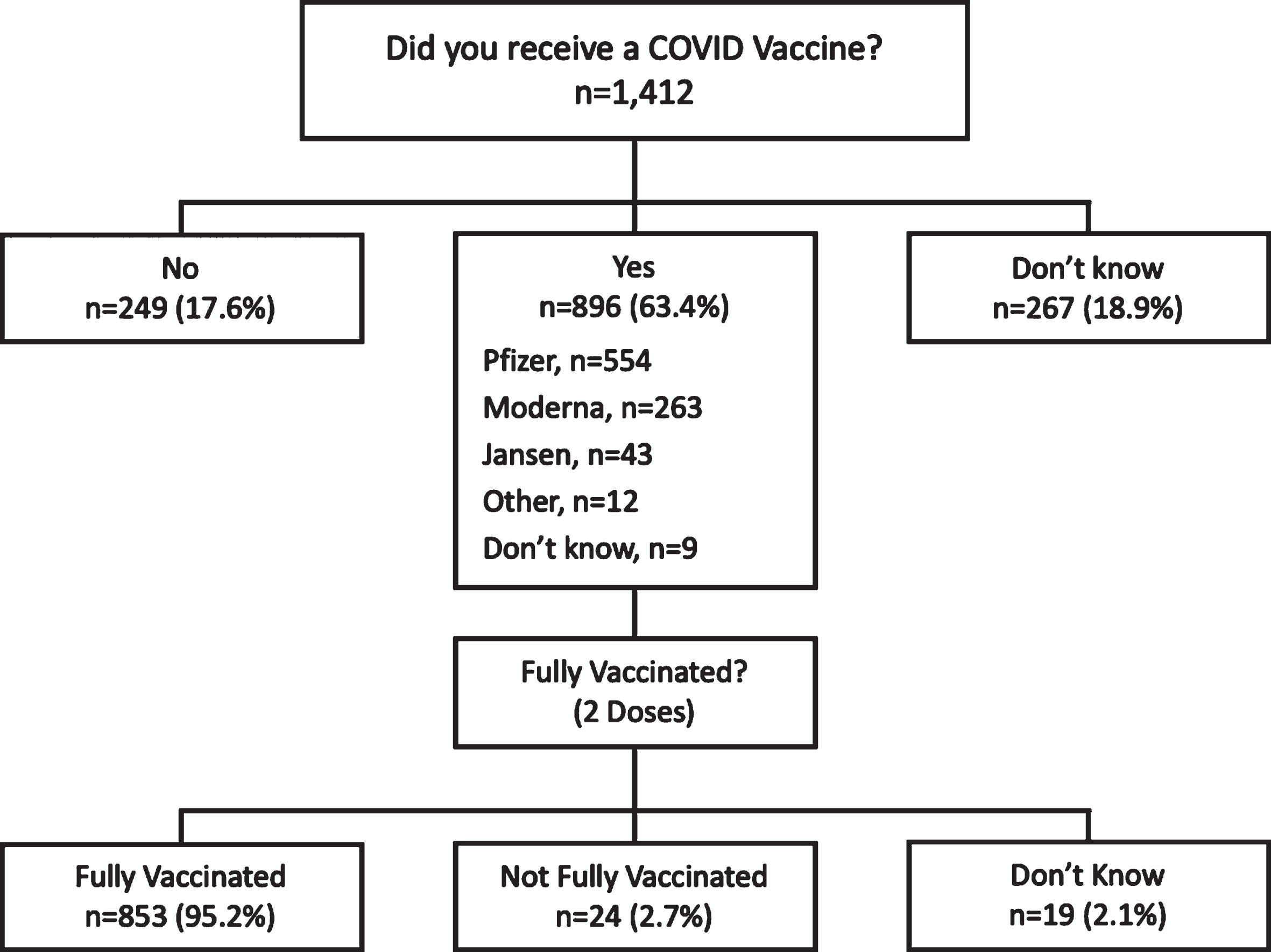
Table 1
Individuals with spina bifida demographics stratified by whether they reported they had received a vaccine or not
| Vaccinated N=896 (78.3%) | Not Vaccinated N=249 (21.7%) | p-value | |
| Adult (≥18 years old) with SB | 745 (83.1%) | 100 (40.2%) | <0.001* |
| Child (<18 years old) with SB | 151 (16.9%) | 149 (59.8%) | |
| Age of individual with SB (years) | 0.001* | ||
| <5 | 41 (4.9%) | 12 (5.1%) | |
| 5–11 | 93 (11.2%) | 26 (11.0%) | |
| 12–17 | 79 (9.5%) | 43 (18.2%) | |
| 18–24 | 57 (6.9%) | 21 (8.9%) | |
| 25–34 | 142 (17.1%) | 45 (19.1%) | |
| 35–44 | 204 (24.6%) | 41 (17.4%) | |
| 45–54 | 113 (13.6%) | 32 (13.6%) | |
| 55–64 | 66 (8.0%) | 15 (6.4%) | |
| 65+ | 34 (4.1%) | 1 (0.4%) | |
| Female gender | 555 (66.7%) | 138 (60.5%) | 0.156 |
| Race | 0.007* | ||
| White | 725 (80.9%) | 193 (77.5%) | |
| Black | 30 (3.3%) | 16 (6.4%) | |
| Other | 141 (15.7%) | 58 (23.3%) | |
| Latinx ethnicity | 98 (11.8%) | 24 (10.6%) | 0.338 |
| Has health insurance | 868 (96.9%) | 233 (93.6%) | 0.011* |
| Reports typically getting vaccines | 855 (95.4%) | 216 (86.7%) | <0.001* |
| Has a high-risk condition | 435 (48.5%) | 72 (28.9%) | <0.001* |
| Clinician recommended COVID-19 vaccine | 699 (78.0%) | 98 (39.4%) | <0.001* |
| Lives in . . . . | <0.001* | ||
| Large city (>250,000) | 232 (27.7%) | 50 (21.9%) | |
| Suburban (>2,500) | 510 (60.9%) | 129 (56.6%) | |
| Rural (<2,500) | 96 (11.5%) | 49 (21.5%) |
*=significant at the 0.05 level.
Table 2
Reported vaccination rates stratified by group
| Percent who had received a vaccine | |
| Adult with SB | 88.2% (745/845) |
| Child with SB | 50.3% (151/300) |
3.3Demographic differences
As depicted in Table 2, those who were vaccinated were more likely to be an adult with SB (n = 745, 88.2%) than a child under 18 years of age with SB (n = 151, 50.3%). This was consistent when tested for age effects and who actually completed the survey (adult –self-report, adult –care partner-report, child –care partner-report, and adult –spouse-report). Those who were vaccinated tended to be older. When the mid-point for the age group was imputed and means were compared, the vaccinated group was significantly older (43.9 years vs. 38.7 years, p < 0.001). There was a racial disparity (p = 0.007). Those vaccinated were more likely to be insured (n = 868, 96.9% vs. n = 233, 93.6%; p = 0.011), typically get other vaccines (n = 855, 95.4% vs. n = 216, 86.7%; p < 0.001), have a high-risk condition (n = 435, 48.5% vs. n = 72, 28.9%; p < 0.001), have had a clinician recommend the COVID-19 vaccine (n = 699, 78.0% vs. n = 98, 39.4%; p < 0.001), and live in either a large city (n = 232, 27.7% vs. n = 50, 21.9%; p < 0.001) or a suburban area (n = 510, 60.9% vs. n = 129, 56.6%; p < 0.001).
Whether vaccine uptake was self-reported or reported by a care partner did not impact the odds of receiving the COVID-19 vaccine.
3.4Vaccine beliefs
Sources of health information differed between those who had been vaccinated and those who had not (Table 3). Those who had had a COVID-19 vaccine were more likely to sometimes (n = 290, 32.4% vs. n = 65, 26.1%) or often (n = 264, 29.5% vs. n = 58, 23.3%) seek information about COVID-19 vaccines. Those who had not been vaccinated were most likely to believe the COVID-19 vaccines were not safe (n = 88, 36.1%), that it was not at all important to get (n = 96, 39.3%), and that they were definitely not going to be getting the vaccine (n = 116, 47.5%).
Table 3
Individuals’ sources of health information, beliefs about COVID-19 vaccine safety, and likelihood of receiving the COVID-19 vaccine, stratified by COVID-19 vaccination status
| Received a vaccine | Have not received a vaccine | |
| n = 896 (%) | n = 249 (%) | |
| Where health information is obtained* | ||
| Family/friends/work colleagues | 197 (22.0%) | 44 (17.7%) |
| Online searches | 333 (37.2%) | 89 (35.7%) |
| Primary care provider | 563 (62.8%) | 129 (51.8%) |
| Spina bifida health provider | 206 (23.0%) | 70 (28.1%) |
| Social media | 174 (19.4%) | 56 (22.5%) |
| Online medical sources | 397 (44.3%) | 95 (38.2%) |
| News sources | 339 (37.8%) | 57 (22.9%) |
| Centers for Disease Control and Prevention | 271 (30.2%) | 50 (20.1%) |
| Other | 40 (4.5%) | 11 (4.4%) |
| How often in the past month information about COVID-19 was sought | ||
| Never | 176 (19.6%) | 77 (30.9%) |
| Rarely | 150 (16.7%) | 49 (19.7%) |
| Sometimes | 290 (32.4%) | 65 (26.1%) |
| Often | 264 (29.5%) | 58 (23.3%) |
| Opinion of COVID-19 vaccine safety | ||
| Not safe | – | 88 (36.1%) |
| Somewhat safe | – | 41 (16.8%) |
| Very safe | – | 21 (8.6%) |
| Completely safe | – | 27 (11.1%) |
| Opinion of how important it is to get COVID-19 vaccine | ||
| Not at all important | – | 96 (39.3%) |
| A little important | – | 27 (11.1%) |
| Somewhat important | – | 18 (7.4%) |
| Very important | – | 50 (20.5%) |
| Belief of likelihood that individual with spina bifida will get | ||
| COVID-19 vaccine in the next 6 months | ||
| Definitely will get the vaccine | – | 22 (9.0%) |
| Probably will get the vaccine | – | 11 (4.5%) |
| Probably will not get the vaccine | – | 43 (17.6%) |
| Definitely will not get the vaccine | – | 116 (47.5%) |
| Don’t know | – | 52 (21.3%) |
* = participants were allowed to pick multiple responses causing the total percentage to add to more than 100%.
3.5Odds of vaccine uptake
The odds of COVID-19 vaccine uptake were not significantly different between children and adults (OR 0.61, CI 0.10–3.60). The odds of vaccine uptake were significantly higher in people who were insured (OR = 3.77, 95% CI 2.50–3.13, p < 0.001), typically received other vaccines (OR = 2.64, 95% CI 1.46–4.14, p < 0.001), had a high-risk condition (OR = 1.85, 95% CI 1.45–2.33, p = 0.002), and had a clinician recommend a COVID-19 vaccine (OR = 2.72, 95% CI 2.09–3.53), p < 0.001) (Table 4).
Table 4
Logistic regression modeling predicting the odds of an individual with spina bifida (SB) receiving a vaccination for COVID-19
| OR | 95% CI of OR | p-value | |
| Adult (≥18 years old) with SB | Ref | 0.10–3.60 | 0.587 |
| Child (<18 years old) with SB | 0.61 | ||
| Age of individual with SB (years) | |||
| <5 | Ref | ||
| 5–11 | 1.09 | 0.50–2.42 | 0.825 |
| 12–17 | 2.18 | 1.02–4.69 | 0.046* |
| 18–24 | 0.85 | 0.12–6.05 | 0.874 |
| 25–34 | 0.74 | 0.11–5.04 | 0.758 |
| 35–44 | 0.50 | 0.07–3.40 | 0.475 |
| 45–54 | 0.67 | 0.10–4.68 | 0.688 |
| 55–64 | 0.56 | 0.09–4.05 | 0.567 |
| 65+ | 0.08 | 0.01–1.21 | 0.068 |
| Female gender | 1.18 | 0.86–1.61 | 0.320 |
| Race | |||
| White | Ref | ||
| Black | 1.65 | 0.71–3.75 | 0.274 |
| Other | 0.92 | 0.50–1.70 | 0.792 |
| Latinx ethnicity | 1.44 | 0.94–2.21 | 0.094 |
| Has health insurance | 3.77 | 2.50–3.13 | <0.001* |
| Reports typically getting vaccines | 2.64 | 1.46–4.14 | <0.001* |
| Has a high risk condition | 1.85 | 1.45–2.33 | 0.002* |
| Clinician recommended COVID-19 vaccine | 2.72 | 2.09–3.53 | <0.001* |
| Lives in | |||
| Large city (>250,000) | Ref | ||
| Suburban (>2,500) | 1.42 | 0.71–2.77 | 0.317 |
| Rural (<2,500) | 1.97 | 0.93–3.09 | 0.202 |
* = significant at the 0.05 level.
3.6Reasons for not getting a COVID-19 vaccine
The most common reasons for not getting a COVID-19 vaccine were concerns about COVID-19 vaccine side effects (n = 109, 43.8%), concerns about the safety of the COVID-19 vaccine (n = 107, 43.0%), concerns about curtailing COVID-19 infections (n = 85, 34.1%), distrust in the vaccine (n = 78, 31.3%) and knowing people who had a negative COVID-19 vaccine experience (n = 60, 24.1%) (Table 5). Thirty-nine parents (15.7%) of unvaccinated children with SB reported that their child was not old enough to receive a vaccine (Table 5).
Table 5
Reasons reported for having received or not received a COVID-19 vaccine
| Reason for not receiving the COVID-19 vaccine | n = 249 (%) |
| Concerned of the effects of COVID-19 vaccine | 109 (43.8%) |
| Concerned about safety of COVID-19 vaccine | 107 (43.0%) |
| Doesn’t believe COVID-19 vaccine stops the spread of COVID-19 | 85 (34.1%) |
| Doesn’t trust COVID-19 vaccine | 78 (31.3%) |
| Doesn’t believe COVID-19 vaccines work | 72 (28.9%) |
| Negative previous experience with other vaccines | 24 (9.6%) |
| Friends have had negative COVID-19 vaccine experience | 60 (24.1%) |
| Doesn’t believe they need COVID-19 vaccine | 43 (17.3%) |
| Not old enough to receive the COVID-19 vaccine | 39 (15.7%) |
| Had COVID-19 and feels protected | 37 (14.9%) |
| Feel they have an adequate immune system without the COVID-19 vaccine | 30 (12.0%) |
| Want to wait until fully approved (not emergency use) for 5–11 year olds | 14 (5.6%) |
| Doesn’t believe in any vaccines | 9 (3.6%) |
| Transportation issues | 4 (1.6%) |
| Reasons for receiving the COVID-19 vaccine | n = 896 (%) |
| Concerned about getting COVID-19 | 629 (70.2%) |
| Didn’t want to transmit COIVD-19 to someone else | 478 (53.3%) |
| Wanted to prevent the spread of COVID-19 | 466 (52.0%) |
| Clinician recommended the COVID-19 vaccine | 382 (42.6%) |
| In high-risk health group | 358 (40.0%) |
| To reduce the chance of other COIVD-19 variants from developing | 311 (34.7%) |
| Lives with or has close contact with an individual in a high-risk health group | 191 (21.3%) |
| COVID-19 vaccine received FDA approval | 182 (20.3%) |
| Recommended by family/friends | 171 (19.1%) |
| Friend/family was hospitalized/died from COVID-19 | 110 (12.3%) |
| Required for work/school | 98 (10.9%) |
| Recommended by faith community | 23 (2.6%) |
3.7Reasons for getting a COVID-19 vaccine
The most common reasons for getting a COVID-19 vaccine were concerns about getting COVID-19 (n = 629, 70.2%), a desire not to transmit COVID-19 to other people (n = 478, 53.3%), a desire to prevent the general spread of COVID-19 (n = 466, 52.0%), due to clinician recommendation (n = 382, 42.6%) and being in a high-risk group (n = 358, 40.0%) (Table 5).
4Discussion
About one in five (21.7%) individuals with SB surveyed had not received a single COVID-19 vaccine, which is slightly more than the general US population (20.8%) [1] and lower than the COVID-19 vaccine hesitancy rate (25%) in the general community of individuals with disabilities [14]. Overall, this study showed that being insured, having a high-risk condition, typically getting vaccines, and having a clinician recommend the COVID-19 vaccine were associated with higher odds of having had a COVID-19 vaccine. The most common reasons for not getting a vaccine were concerns about the safety and efficacy of the vaccine. The results support prior research on vaccine hesitancy and add important insights into vaccination behavior and perceptions within the SB community.
Similar to previous data from the general population and those in the disabled community [14, 17], this study found that the most common reason for not getting vaccinated was due to a concern about the COVID-19 vaccine’s safety and efficacy. In a study by Myers et al., concerns about the COVID-19 vaccine and lack of trust in experts regarding COVID-19 were significant factors in predicting vaccine hesitancy [14]. This study demonstrated that the vaccination rate of children with SB was significantly less than adults with SB. This should be cautiously interpreted as the survey was sent out only a few months after the first COVID-19 vaccine was given emergency FDA approval for children 5–11 years old [20]. This could reflect the cautious approach to vaccination that many parents of children under 12 years old in the general population had at the time: four in 10 parents reported that they “will wait and see how it is working” before vaccinating their child [21]. The significance did not change when this group age group was excluded from the analysis. The adjustment model did not find that education, gender, ethnicity, or geographic area influenced vaccination status. This is in contrast to other studies, which have suggested that those without a bachelor’s degree [14], of the female gender [6, 7, 9], of the Black race, of Latinx ethnicity [6, 7], and living in rural areas [7] are more COVID-19 vaccine-hesitant. While the predispositions for vaccine hesitancy may slightly differ between studies and populations, the important question of why individuals are reluctant to get the vaccine remains constant –they do not think it is safe or effective. This is true in studies on the general population [6, 7] and those with disabilities [14, 17].
To improve COVID-19 vaccine uptake in the COVID-19 vaccine-hesitant, the factors that drive COVID-19 vaccination must be considered. In this study sample, insured patients, those who typically get vaccines, those with high-risk conditions, and those who had their clinician recommend vaccination were more likely to have received a COVID-19 vaccine. Importantly, insurance status and clinician recommendation were significant predictors of vaccination in all subgroups except children under 18 years old.
Insurance status was found to be a predictor of COVID-19 vaccinations; this is interesting given that the vaccines are covered by the federal government for the uninsured [22]. This could be due to uninsured individuals’ lack of awareness of this fact, or insurance status may be more reflective of improved healthcare access. One straightforward way to target this would be through informational messaging directed at the uninsured.
Importantly, this data suggests that healthcare professional recommendation plays a significant role in whether to get vaccinated or not. The vaccinated and unvaccinated report that their primary care provider is their most common source of health information. However, only four in 10 individuals in the non-vaccinated group (compared to eight in 10 in the vaccinated group) said that a healthcare professional recommended getting the COVID-19 vaccine. While the low level of vaccine recommendation is alarming, this represents a clear opportunity to improve COVID-19 vaccine messaging.
Given this study and the context discussed above, there are significant implications for improving the current approach to COVID-19 vaccine communication within the SB community. The data supports the critical role of healthcare providers in personal vaccine decision-making. This role should not fall only on primary care providers of SB patients but also the specialty SB providers such as urologists, neurosurgeons, and orthopedic surgeons. This is especially pertinent since not all healthcare providers are perfect messengers; about three in 10 primary care physicians in the US lack very high confidence in the COVID-19 vaccine [23]. Given this, the more frequently a COVID-19 vaccine-positive message can be delivered, the better. This responsibility should fall on all providers caring for individuals with SB.
State and national organizations such as the SBA should aim to structure COVID-19 vaccination messages to healthcare providers, individuals with SB, and their care partners. Individuals who are undecided about the importance of getting vaccinated or unsure about getting vaccinated may be the best targets to reach with messaging. The safety and efficacy of the vaccines should be essential themes in the messaging. Other targets for messaging can be taken from the reasons why vaccinated people chose to get vaccinated, including concerns about getting COVID-19, a desire to prevent a loved one from getting COVID-19, and a desire to help prevent COVID-19 spread.
One final notable point about this study is that 18.9% of the respondents did not know if they or their child had received the COVID-19 vaccine. While this is a concerning finding, as it suggests a lack of health awareness and low health literacy, it cannot be concluded because it was not the aim of this study. Prior studies have suggested that levels of health literacy in care partners of individuals with SB are similar to the general population and there is inadequate health literacy among adolescents with SB compared to controls in the general population [24].
The results of this study need to be considered within the bounds of several limitations. The study was a self-reported survey and thus may be limited by recall bias and socially desirable responses. Furthermore, other characteristics could drive vaccination preferences that were not captured by the study. One factor in particular, political affiliation, likely does drive behavior [7, 14]. Political affiliation was not included since the association has been well-established previously and the designers of the survey did not want to give the participants the impression that it was politicized. Given the study’s cross-sectional nature, a snapshot of vaccine preferences and intentions at a single moment in time was captured. This study may not have perfectly captured the demographics of SB; for instance, the Latinx population was 10% of the survey respondents while 22% of patients registered in the National Spina Bifida Patient Registry are of Latinx ethnicity [25]. While the response rate for the email survey was 6% and thus may not have captured the whole SB population, the margin of error is±2.6% (assuming a confidence interval of 95% and that the US SB population is 166,000 individuals [18]) and thus provides some confidence in the reliability of the findings. Finally, future studies should focus on how vaccine preferences change over time and what factors drive that change. This survey was administered over email, which limited the external validity to those who had access to email, computer/mobile devices, and the ability to navigate a survey.
5Conclusions
About one in five individuals with SB have not received a single COVID-19 vaccine. Concerns over vaccine safety and efficacy are the most common reasons for vaccine hesitancy. Uninsured individuals are more likely not to be vaccinated. In those vaccinated, healthcare provider recommendations play a significant role in their decisions. Vaccination efforts should be directed toward SB healthcare providers and uninsured individuals. These efforts should emphasize the safety and efficacy of the COVID-19 vaccines.
Acknowledgments
This work was supported by the Association of University Centers on Disabilities (ACUD) 1 NU51DD000001-01-00 and CDC-RFA-DD21-2105.
Conflict of interest
The authors have no conflict of interest to report.
References
[1] | Centers for Disease Control and Prevention. COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services; 2022 [cited 2022 September 06]. Available from: https://covid.cdc.gov/covid-data-tracker |
[2] | United States Food and Drug Administration. FDA Approves First COVID-19 Vaccine. Silver Spring, MD: US Food and Drug Administration; 2021 [cited 2022 August 20]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine |
[3] | United States Food and Drug Administration. Coronavirus (COVID-19) Update: FDA takes key action by approving second COVID-19 vaccine. Silver Spring, MD: US Food and Drug Administration; 2021 [cited 2022 August 20]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-takes-key-action-approving-second-covid-19-vaccine |
[4] | United States Food and Drug Administration. Coronavirus (COVID-19) Update: FDA authorizes moderna 433 and Pfizer-BioNTech COVID-19 vaccines for children down to 6 months of age: FDA. Silver Spring, MD: US Food and Drug Administration; 2021 [cited 2022 September 06]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-and-pfizer-biontech-covid-19-vaccines-children |
[5] | United States Food and Drug Administration. FDA issues emergency use authorization for third COVID-19 vaccine. Silver Spring, MD: US Food and Drug Administration; 2021 [cited 2022 September 06]. Available from: https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine |
[6] | Callaghan T , Moghtaderi A , Lueck JA , et al. Correlates anddisparities of intention to vaccinate against COVID-19. Soc Sci Med. (2021) ;272: :113638. doi: 10.1016/j.socscimed.2020.113638 |
[7] | Khubchandani J , Sharma S , Price JH , Wiblishauser MJ , Sharma M , Webb FJ . COVID-19 vaccination hesitancy in the United States: A rapid national assessment. J Community Health. (2021) ;46: (2):270–7. doi: 10.1007/s10900-020-00958-x |
[8] | Malik AA , McFadden SM , Elharake J , Omer SB . Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine. (2020) ;26: :100495. doi: 10.1016/j.eclinm.2020.100495 |
[9] | Zintel S , Flock C , Arbogast AL , Forster A , von Wagner C , Sieverding M . Gender differences in the intention to get vaccinated against COVID- A systematic review and meta-analysis. Z Gesundh Wiss. (2022) ;19: :1–25. doi: 10.1007/s10389-021-01677-w |
[10] | Bates LC , Conners R , Zieff G , et al. Physical activity and sedentary behavior in people with spinal cord injury: Mitigation strategies during COVID-19 on behalf of ACSM-EIM and HL-PIVOT. Disabil Health J. (2022) ;15: (1):101177. doi: 10.1016/j.dhjo.2021.101177 |
[11] | Kavanagh A , Hatton C , Stancliffe RJ , et al. Health and healthcare for people with disabilities in the UK during the COVID-19 pandemic. Disabil Health J. (2022) ;15: (1):101171. doi: 10.1016/j.dhjo.2021.101171 |
[12] | Na L , Yang L . Psychological and behavioral responses during the COVID-19 pandemic among individuals with mobility and/or self-care disabilities. Disabil Health J. (2022) ;15: (1):101216. doi: 10.1016/j.dhjo.2021.101216 |
[13] | Ryerson AB , Rice CE , Hung M-C , et al. Disparities in COVID-19 vaccination status, intent, and perceived access for noninstitutionalized adults, by disability status —national immunization survey adult COVID module, United States, 2021. MMWR Morb Mortal Wkly Rep. (2021) ;70: (39):1365–71. doi: 10.15585/mmwr.mm7039a2 |
[14] | Myers A , Ipsen C , Lissau A . COVID-19 vaccination hesitancy among Americans with disabilities aged 18- An exploratory analysis. Disabil Health J. (2022) ;15: (1):101223. doi: 10.1016/j.dhjo.2021.101223 |
[15] | Umucu E , Lee B , Bezyak J . Measuring COVID-19 vaccine hesitancy among college students with disabilities: Sociodemographic and psychological correlates of COVID-19 vaccine hesitancy. J Am Coll Health. 2022: :1–7. doi: 10.1080/07448481.2022.2071619 |
[16] | Forber-Pratt AJ , Burdick CE , Narasimham G . Perspectives aboutCOVID-19 vaccination among the paralysis community in the UnitedStates. Rehabil Psychol. (2022) ;67: (1):9–19. doi: 10.1037/rep0000426 |
[17] | Iadarola S , Siegel JF , Gao Q , McGrath K , Bonuck KA . COVID-19 vaccine perceptions in New York State’s intellectual and developmental disabilities community. Disabil Health J. (2022) ;15: (1):101178. doi: 10.1016/j.dhjo.2021.101178 |
[18] | Lloyd JC , Wiener JS , Gargollo PC , Inman BA , Ross SS , Routh JC . Contemporary epidemiological trends in complex congenital genitourinary anomalies. J Urol. (2013) ;190: (4 Suppl):1590–5. doi: 10.1016/j.juro.2013.04.034 |
[19] | Spina Bifida Association. What is Spina Bifida? Arlington, VA: Spina Bifida Association; 2022 [cited 2022 Aug 20]. Available from: https://www.spinabifidaassociation.org/what-is-spina-bifida-2/ |
[20] | United States Food and Drug Administration. FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 through 11 Years of Age. Silver Spring, MD: US Food and Drug Administration; 2021 [cited 2022 Sept 06]. Available from: https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age |
[21] | Hamel L , Lopes L , Kearney A , et al. KFF COVID-19 Vaccine Monitor: Parents and the pandemic. San Francisco, CA: Kaiser Family Foundation; 2021 [cited 2022 April 01]. Available from: https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-parents-and-the-pandemic/ |
[22] | Heath Resources & Services Administration. COVID-19 claims reimbursement to health care providers and facilities for testing, treatment, and vaccine administration for the uninsured: Rockville,MD:Health Resources & Services Administration; 2022 [cited 2022 April 01].Available from: https://www.hrsa.gov/CovidUninsuredClaim |
[23] | Callaghan T , Washburn D , Goidel K , et al. Imperfect messengers? An analysis of vaccine confidence among primary care physicians. Vaccine. (2022) ;40: (18):2588–603. doi: 10.1016/j.vaccine.2022.03.025 |
[24] | Lightfoot MA , Cheng JW , Hu X , et al. Assessment of health literacyin adolescents with spina bifida and their caregivers: Amulti-institutional study. J Pediatr Urol. (2020) ;16: (2):167.e1–167.e6. doi: 10.1016/j.jpurol.2019.11.016 |
[25] | Centers for Disease Control and Prevention. Findings from the National Spina Bifida Patient Registry. Atlanta, GA: US Department of Health and Human Services; 2022. Available from: www.cdc.gov/ncbddd/spinabifida/nsbprregistryfindings.html |




