Spasticity-related pain in children/adolescents with cerebral palsy. Part 2: IncobotulinumtoxinA efficacy results from a pooled analysis
Abstract
PURPOSE:
This pooled analysis of data from three Phase 3 studies investigated the effects of incobotulinumtoxinA on spasticity-related pain (SRP) in children/adolescents with uni-/bilateral cerebral palsy (CP).
METHODS:
Children/adolescents (ambulant and non-ambulant) were evaluated for SRP on increasingly difficult activities/tasks 4 weeks after each of four incobotulinumtoxinA injection cycles (ICs) using the Questionnaire on Pain caused by Spasticity (QPS; six modules specific to lower limb [LL] or upper limb [UL] spasticity and respondent type [child/adolescent, interviewer, or parent/caregiver]). IncobotulinumtoxinA doses were personalized, with all doses pooled for analysis.
RESULTS:
QPS key item responses were available from 331 and 155 children/adolescents with LL- and UL-spasticity, respectively, and 841/444 (LL/UL) of their parents/caregivers. IncobotulinumtoxinA efficacy was evident with the first IC. Efficacy was sustained and became more robust with further subsequent ICs. By Week 4 of the last (i.e. fourth) IC, 33.8–53.3% of children/adolescents reported complete SRP relief from their baseline pain for respective QPS items. Children/adolescents reported reductions in mean LL SRP intensity at levels that surpassed clinically meaningful thresholds. Similarly, parents/caregivers observed complete SRP relief and less frequent SRP with incobotulinumtoxinA. Similar results were found for UL SRP.
CONCLUSION:
These findings indicate that incobotulinumtoxinA could bring considerable benefit to children/adolescents with spasticity by reducing SRP, even during strenuous activities.
1Introduction
Pain, including spasticity-related pain (SRP), is common in children/adolescents with cerebral palsy (CP) [1–4], negatively affecting health-related quality of life [5] and interfering with physical function, attendance and performance in school, daily care activities, participation in physical leisure activities, sleep, and mental health [4–10].
Botulinum neurotoxinA (BoNT-A) is recommended and its use established to relieve CP-related spasticity in children [11–14], and it has demonstrated efficacy for improving muscle tone and helping patients achieve individualized goals of motor function [11, 15, 16].
There is less evidence available regarding the use of BoNT-A formulations for the control of SRP, especially in children/adolescents. A recent review of the analgesic effect of BoNT-A formulations found one high-quality study (level II evidence) and two observational studies that support botulinum neurotoxin therapy for muscle hypertonia-related pain in non-ambulant children and contradictory evidence for the analgesic effects of this treatment for ambulant children with CP [17]. Michelsen and colleagues [5] have suggested that BoNT-A may have direct pain-relieving activity, while Roscigno [18] discussed the question of whether medication-induced pain relief stems indirectly from reduced spasticity or directly via an intrinsic analgesic mechanism of action.
IncobotulinumtoxinA is a highly purified formulation of BoNT-A (150 kD) that does not contain complexing proteins [19]. A large, international Phase 3 study program was initiated in 2013 to investigate the efficacy and safety of incobotulinumtoxinA for the treatment of pediatric CP-related lower limb (LL) and/or upper-limb (UL) spasticity. The study program included TIM (Treatment with IncobotulinumtoxinA in Movement, NCT01893411) [20], TIMO (Treatment with IncobotulinumtoxinA in Movement Open-label, NCT01905683) [21], and XARA (incobotulinumtoXinA in aRm treatment in cerebral pAlsy, NCT02002884) [22]. Results showed incobotulinumtoxinA to be effective at reducing CP-related spasticity over multiple injection cycles spanning 24–98 weeks [20, 22–24]. The data indicated that repeated injections of incobotulinumtoxinA are safe in children/adolescents with CP [21, 22, 25] and reduce spasticity when administered according to multiple clinical patterns and levels as needed.
To assess SRP and evaluate the effects of incobotulinumtoxinA on SRP, the Questionnaire on Pain caused by Spasticity (QPS) [26, 27]— a comprehensive and validated tool designed for children 2 years of age and older with CP— was incorporated into all patient visits before and during treatment in TIM, TIMO, and XARA. The QPS allows the collection of data directly reported by children themselves or through trained interviewers, as well as observed behavior from parents and caregivers. The QPS consists of separate modules (three each for LL and UL, respectively, for a total of six modules) that ask about SRP during different activities: the self-report module for children/adolescents (12 items), the interviewer-administered module for children/adolescents (12 items), and the observer-report module for parents/caregivers on SRP behaviors (13 items). Information gained from parents’/caregivers’ observations was designed to complement the SRP intensity information gathered from the child/adolescent or to provide SRP information when the patient was too young or unable to report.
The TIM, TIMO, and XARA studies shared many common features in study design and participants, which made the collected data suitable for a combined analysis. In this two-part report, pooled QPS data from the TIM, TIMO, and XARA studies were analyzed to learn more about the prevalence of SRP and its treatment with incobotulinumtoxinA in children and adolescents with CP. In the first publication, it was demonstrated that at baseline— prior to treatment with incobotulinumtoxinA— more than 80% of children/adolescents with LL spasticity and almost 70% with UL spasticity reported SRP. The parents and caregivers of these children observed even higher SRP prevalence [28]. SRP was reported in multiple body sites and had widespread and detrimental effects on activities and behavior, highlighting the need for better SRP control for most children with CP.
In this second publication, the pooled QPS data is now presented from the TIM, TIMO, and XARA studies describing and analyzing the effects of incobotulinumtoxinA on SRP over multiple treatment cycles in children/adolescents with CP.
2Methods
Full details of all three studies included in this analysis have been published [20–22]. The following provides a brief overview only.
2.1Study designs
The objectives of TIM, TIMO, and XARA were to investigate the efficacy and safety of incobotulinumtoxinA in children/adolescents with spasticity due to CP. The designs for the three studies are illustrated in Fig. 1A, and the clinical patterns treated are presented in Fig. 1B. The study design allowed physicians to treat each child/adolescent for spasticity according to clinical patterns and dosage requirements that could be adjusted to meet each individual’s needs (LL, UL, or combined).
Fig. 1
A: Study designs of TIM, TIMO, and XARAa. B: Summary of clinical treatment patterns for 1) LL (TIM, TIMO, and XARA) and 2) UL (XARA and TIMO). aFor full details of the TIM, TIMO, and XARA studies, see the primary publications of each study [20–22]. bPatients previously enrolled in TIM who then entered TIMO only provided data for the current pain analyses during TIM. cInjections were administered bilaterally for pes equinus or unilaterally for pes equinus with the addition of another LL site (either flexed knee or adducted thigh) in TIM [20], bilaterally for pes equinus with potential to add an UL site or unilaterally for pes equinus and another ipsilateral LL site with or without UL sites (with doses for some combinations of clinical pattern dependent on GMFCS-E&R level) in TIMO [21], or unilaterally/bilaterally to a main UL clinical target pattern (flexed elbow or wrist) and additional UL clinical patterns, with further optional bilateral and uni/bilateral LL injections in one of five topographical distributions (with doses for some combinations of clinical pattern dependent on GMFCS-E&R level) in XARA [22]. BW = body weight; GMFCS-E&R = Gross Motor Function Classification System Expanded and Revised; LL = lower limb; QPS = Questionnaire on Pain caused by Spasticity; TIM = Treatment with IncobotulinumtoxinA in Movement; TIMO = Treatment with IncobotulinumtoxinA in Movement Open-label; UL = upper limb; XARA = incobotulinumtoXinA in aRm treatment in cerebral pAlsy.
![A: Study designs of TIM, TIMO, and XARAa. B: Summary of clinical treatment patterns for 1) LL (TIM, TIMO, and XARA) and 2) UL (XARA and TIMO). aFor full details of the TIM, TIMO, and XARA studies, see the primary publications of each study [20–22]. bPatients previously enrolled in TIM who then entered TIMO only provided data for the current pain analyses during TIM. cInjections were administered bilaterally for pes equinus or unilaterally for pes equinus with the addition of another LL site (either flexed knee or adducted thigh) in TIM [20], bilaterally for pes equinus with potential to add an UL site or unilaterally for pes equinus and another ipsilateral LL site with or without UL sites (with doses for some combinations of clinical pattern dependent on GMFCS-E&R level) in TIMO [21], or unilaterally/bilaterally to a main UL clinical target pattern (flexed elbow or wrist) and additional UL clinical patterns, with further optional bilateral and uni/bilateral LL injections in one of five topographical distributions (with doses for some combinations of clinical pattern dependent on GMFCS-E&R level) in XARA [22]. BW = body weight; GMFCS-E&R = Gross Motor Function Classification System Expanded and Revised; LL = lower limb; QPS = Questionnaire on Pain caused by Spasticity; TIM = Treatment with IncobotulinumtoxinA in Movement; TIMO = Treatment with IncobotulinumtoxinA in Movement Open-label; UL = upper limb; XARA = incobotulinumtoXinA in aRm treatment in cerebral pAlsy.](https://content.iospress.com:443/media/prm/2023/16-1/prm-16-1-prm220020/prm-16-prm220020-g001.jpg)
Briefly, the TIM study was a randomized, double-blind, parallel-group, multicenter study of children/adolescents with LL spasticity, all of whom had pes equinus, conducted across 45 sites in 14 countries (two injection cycles [ICs], N = 311) [20]. Injections were administered bilaterally for pes equinus or unilaterally for pes equinus with the addition of another LL site (either flexed knee or adducted thigh) [20]. Patients were randomized to three different incobotulinumtoxinA dose groups: 2 U/kg body weight (BW), maximum 50 U; 6 U/kg BW, maximum 150 U; or 8 U/kg BW, maximum 200 U per LL clinical pattern (Fig. 1).
The TIMO study was an open label, non-controlled, long-term, multi-center study in LL or combined LL and UL spasticity conducted across 30 sites in 12 countries (four ICs) [21]. The study population (N = 370) included selected TIM completers and newly recruited children/adolescents. All children/adolescents received treatment for bilateral pes equinus with potential to add an UL site or unilateral pes equinus and another ipsilateral LL site with or without UL sites (8 U/kg per LL pattern, total dose per cycle of 16–20 U/kg BW, maximum 400–500 U incobotulinumtoxinA, depending on Gross Motor Function Classification System Expanded and Revised [GMFCS-E&R] level) (Fig. 1).
XARA was a randomized, double-blind, parallel-group, multicenter study (one IC) with open-label long-term follow-up (three ICs in UL or combined UL/LL spasticity [N = 351]) [22]. In the double-blind main period, UL injections of incobotulinumtoxinA were administered at least unilaterally to one main clinical target pattern (flexed elbow or wrist) and additional clinical patterns, with further optional bilateral and uni-/bilateral LL injections in one of five topographical distributions. Patients were randomized to three different incobotulinumtoxinA dose groups for UL treatment: 2 U/kg BW, maximum 50 U; 6 U/kg BW, maximum 150 U; or 8 U/kg BW, maximum 200 U per UL clinical pattern (Fig. 1). Bilateral UL treatment followed the same dosing approach, and, for additional LL treatment, the same dose group relationship was maintained. Based on selected uni-/bilateral limbs and clinical patterns in the XARA main period, patients continued in the open-label extension period to receive further injections with the highest dose regimen of incobotulinumtoxinA 8–20 U/kg BW, maximum 200–500 U (depending on GMFCS-E&R level) (Fig. 1).
Common design features of the three studies were: the option to provide multilevel treatment for children/adolescents with LL and/or UL spasticity; treatment of multiple clinical patterns simultaneously; and personalized flexibility in muscular dosing to meet the clinical needs of each child/adolescent within the standardized trial guidelines. Eligible children/adolescents in all three studies were representative of those seen in clinical practice, that is, aged 2 to 17 years and ambulant or non-ambulant (GMFCS-E&R levels I to V), with uni- or bilateral spasticity associated with CP, an Ashworth Scale score≥2 in prespecified clinical patterns for treatment, and a clinical need for treatment. All three studies enrolled children/adolescents with LL CP-related spasticity but only TIMO and XARA also included children/adolescents with UL CP-related spasticity. Since SRP was not an eligibility criterion, each trial included children/adolescents whether they experienced SRP or not. For 2 weeks prior to the screening visit, at the baseline injection visit, and during the entire study duration, intrathecal baclofen, oral anticoagulants, drugs acting as peripheral muscle relaxants, casting, serial casting, and functional electrical stimulation of the target joints for injection were not allowed. No BoNT treatment was allowed within 2 weeks prior to the screening visit or at the baseline injection visit, and the only BoNT allowed during the study period was incobotulinumtoxinA in the target patterns. Treatments such as physiotherapy, occupational therapy, or any other rehabilitation measures to treat spasticity were prohibited prior to study assessments on the day of any visit.
2.2Assessments
SRP was assessed using the QPS [26, 27] in children/adolescents with LL (TIM, TIMO, and XARA) and UL spasticity (TIMO and XARA). The QPS comprises six modules specific to the level of spasticity (LL and UL) and respondent (self-reports by children/adolescents, interviewer-reported children/adolescents, and parents/caregivers). In each module, SRP was assessed for different levels of task difficulty, ranging from rest to self-described hard tasks (see Table 1 of Part 1 of the accompanying publication [Heinen et al. 2021] [28] for further details of QPS items). For children/adolescents, SRP intensity was assessed with the following QPS key items: General SRP (Item 2); SRP at rest (Item 4); SRP with usual activities (Item 6); SRP with exercises (Item 8); and SRP with a self-defined hard task (Item 11). Intensity was rated by children/adolescents from 0 (no hurt) to 10 (worst hurt) on the Wong-Baker FACES scale. Answers were limited to a 7-day recall period.
Table 1
Demographics and baseline characteristics for children/adolescents enrolled in and providing any QPS data from the pooled TIM, TIMO, and XARA studiesa
| Characteristic | Enrolled patientsa | QPS respondentsb | ||
| LL treated N = 849 | UL treated N = 454 | LL QPS N = 340 | UL QPS N = 160 | |
| Male sex, n (%) | 507 (59.7) | 287 (63.2) | 208 (61.2) | 99 (61.9) |
| Age, years; mean (SD) | 6.5 (4.2) | 7.1 (4.4) | 9.3 (3.8) | 10.3 (3.7) |
| Weight, kg; mean (SD) | 22.9 (13.4) | 24.4 (14.7) | 32.6 (14.8) | 36.8 (16.5) |
| GMFCS-E&R IV-V, n (%) | 226 (26.6) | 122 (26.9) | 33 (9.7) | 16 (10.0) |
| Body side affected by cerebral palsy, n (%) | ||||
| LL unilateral | 304 (35.8) | 193 (42.5) | 157 (46.2) | 97 (70.3) |
| LL bilateral | 545 (64.2) | 203 (44.7) | 183 (53.8) | 41 (29.7) |
| UL unilateral | 365 (43.0) | 402 (88.5) | 128 (92.8) | 144 (90) |
| UL bilateral | 31 (3.7) | 52 (11.5) | 10 (7.2) | 16 (10.0) |
| Duration since first diagnosis of spasticity, months; mean (SD) | (N = 848) | (N = 453) | (N = 292) | (N = 147) |
| 69.1 (49.9) | 76.9 (52.3) | 95.4 (47.8) | 109.7 (46.3) | |
aChildren/adolescents enrolled in one of the three studies (new recruits only for TIMO) and then treated for at least LL or UL spasticity. bChildren/adolescents treated for at least LL or UL spasticity in the first injection cycle and who provided QPS data at any time during the study. Includes children/adolescents who completed the QPS via an interviewer as well as by self-report. MFCS-E&R = Gross Motor Function Classification System–expanded & revised; LL = lower limb; QPS = Questionnaire on Pain caused by Spasticity; SD = standard deviation; TIM = Treatment with IncobotulinumtoxinA in Movement; TIMO = Treatment with IncobotulinumtoxinA in Movement Open-label; XARA = incobotulinumtoXinA in aRm treatment in cerebral pAlsy; UL = upper limb.
The child/adolescent self-completed module was completed by those with sufficient cognitive, communicative, and motor abilities (usually those who were older or had fewer disabilities); otherwise, the interviewer-completed module was used. Thus, no QPS information was collected from children who were too young or were unable to report. While the QPS was investigated at every visit, only the Week 4 data of each IC were analyzed to assess treatment effects.
Parents/caregivers reported on the observed frequency of behaviors associated with SRP in their child during different activities, beginning with whether such behaviors were present with the activity (Yes/No) and then how often they were observed based on a 5-point scale from 0 (never) to 4 (always). The QPS key items for the parent/caregiver analysis were: General SRP (Item 8); SRP at rest (Item 9b); SRP with usual activities (Item 10b); SRP with exercises (Item 11b); and SRP with a self-defined hard task (Item 13b) (adapted from [27]). Further details regarding the QPS can be found in Table 1 of Part 1 [28].
2.3Analysis populations
QPS data in TIM was collected for LL SRP only, whereas in TIMO and XARA the respective LL and/or UL QPS module data was collected when the patient presented with, and received treatment for, LL or UL spasticity. Patients with both LL and UL spasticity could therefore be included in the LL and UL analyses. QPS self-reported and interviewer-reported data from children/adolescents were pooled accordingly. Generally, all of the collected TIM, TIMO, and XARA study data from children/adolescents and parent/caregiver QPS LL and UL modules were utilized for analysis. Data were pooled across these studies according to QPS LL and UL children/adolescent and parent/caregiver modules and analyzed by visit order and ICs. To gain greater insight into SRP in the overall study population, the three incobotulinumtoxinA dose groups of the TIM and XARA studies were also combined.
A child/adolescent was considered to have SRP if any QPS key item score was reported > 0 at baseline in the child/adolescent modules or the parent/caregiver modules. These different baseline pain (i.e., SRP) populations were then utilized to analyze SRP relief and changes in SRP after incobotulinumtoxinA treatment.
2.4Statistical analyses
The QPS LL and UL modules of the children/adolescent and parent/caregiver were analyzed separately using frequency tables and descriptive summary statistics at all visits and for changes from baseline to all post-baseline visits.
QPS child/adolescent scores and the QPS parent/caregiver scores were evaluated by mixed model repeated measures (MMRMs). The dependent variable was the change from baseline in the QPS key item score. The independent variables were treatment group (high dose and, in the second step, mid-dose versus low dose), pooled sites, BoNT-A pre-treatment status (pre-treated, treatment-naïve) as fixed factors and visit as repeated factor, and covariates were QPS score at baseline (Day 1 of the main period [XARA]) and GMFCS-E&R level at the screening visit. Observed case (OC) analyses were performed.
It was assumed that not all children/adolescents would be able to complete the self-administered or interviewer-administered QPS at all visits, which could lead to a high number of missing values for QPS child/adolescent scores and the child/adolescent general SRP intensity item (Yes/No). For this reason, OC analysis of QPS key items was used, and the child/adolescent general SRP intensity item (Yes/No) was of main interest.
To evaluate changes in child/adolescent self-reported and interviewer-obtained child/adolescent QPS key item data, the change in each QPS key item score from baseline to Week 4 of each IC was analyzed using MMRM analyses in children/adolescents who reported SRP with an item score of > 0 at baseline. To evaluate changes in SRP frequency as observed by parents/caregivers, the mean change in each QPS key item score from baseline to Week 4 of each IC was analyzed using MMRM analyses in children/adolescents for whom SRP was reported with an item score of > 0 at baseline. The proportion of children/adolescents achieving complete SRP relief was determined in children/adolescents with a QPS key item score > 0 at baseline and was defined as the proportion of children/adolescents achieving complete relief (score of “0 = no hurt”) of the item at Week 4 of each IC, as analyzed using an exact one-sample binomial test (one-sided) on the hypothesis that the response rate would lie above 10%, with a significance level of α= 0.025 in the Full Analysis Set (FAS).
3Results
The total population with LL spasticity at baseline (alone or with UL spasticity) consisted of 849 children/adolescents who were treated in the first IC of TIM, TIMO or XARA, of whom 177 were in the incobotulinumtoxinA 2 U/kg group, 275 were in the 6 U/kg group, and 397 were in the 8 U/kg group. Of 454 children in the full population with UL spasticity (alone or with LL spasticity) who were treated in the double-blind injection periods of TIMO or XARA, 72 were in the incobotulinumtoxinA 2 U/kg group, 190 were in the 6 U/kg group, and 192 were in the 8 U/kg group.
At least one item of the QPS was completed at any time of the study by 340 children/adolescents with LL spasticity and 160 with UL spasticity; of these 331 and 155, respectively, completed at least one QPS key item at baseline. For those treated for LL spasticity, 75 were in the incobotulinumtoxinA 2 U/kg group, 107 were in the 6 U/kg group and 149 were in the 8 U/kg group; for those with UL spasticity, 39, 75, and 41 were treated with incobotulinumtoxinA 2 U/kg, 6 U/kg, and 8 U/kg, respectively.
Overall, 841 and 444 parents/caregivers of children/adolescents with LL and UL spasticity, respectively, completed at least one QPS key item at baseline. Of the 841 children/adolescents with LL spasticity, 175 were in the incobotulinumtoxinA 2 U/kg group, 269 were in the 6 U/kg group, and 397 were in the 8 U/kg group. Of the 444 children/adolescents with UL spasticity, 112, 214, and 118 were treated with incobotulinumtoxinA 2 U/kg, 6 U/kg, and 8 U/kg, respectively.
3.1Demographics
Demographics of the pooled LL and pooled UL full populations were generally similar and in line with the distribution in the pooled QPS completers populations, although QPS completers tended to be older and less likely to have a higher level of disability (GMFCS-E&R IV–V) than the full populations (Table 1). Approximately 60% of each group was male. About 10% of the QPS completers were severely disabled with poor ambulation (GMFCS-E&R levels IV and V) versus almost 27% of the full populations.
3.2SRP changes in LL spasticity
3.2.1QPS child/adolescent self-reported and interviewer-reported modules
The overall proportion of children/adolescents reporting LL SRP on any QPS key item decreased from 81.9% at baseline to 68.3% after the first incobotulinumtoxinA IC (all measurements were conducted 4 weeks after each IC). The proportion then gradually decreased with each further IC to 54.9% at IC4 (Fig. 2).
Fig. 2
The overall proportion of children with lower limb and upper limb cerebral palsy-related spasticity reporting any SRP on any QPS key item at baseline and at Week 4 of each of four incobotulinumtoxinA injections cycles according to children/adolescents and parents/caregiversa. aThe decrease in the number of patients with LL spasticity after IC2 reflects the design and contribution to the pooled data of the TIM study, which included only two injection cycles. BL = baseline; IC = injection cycle; n/N = number of subjects reporting any SRP (on one or more of items 8, 9b, 10b, 11b, and 13b) per assessment visit/number of parent/caregiver QPS subjects evaluated for SRP per assessment visit; QPS = Questionnaire on Pain caused by Spasticity; SRP = spasticity-related pain; TIM = Treatment with IncobotulinumtoxinA in Movement.
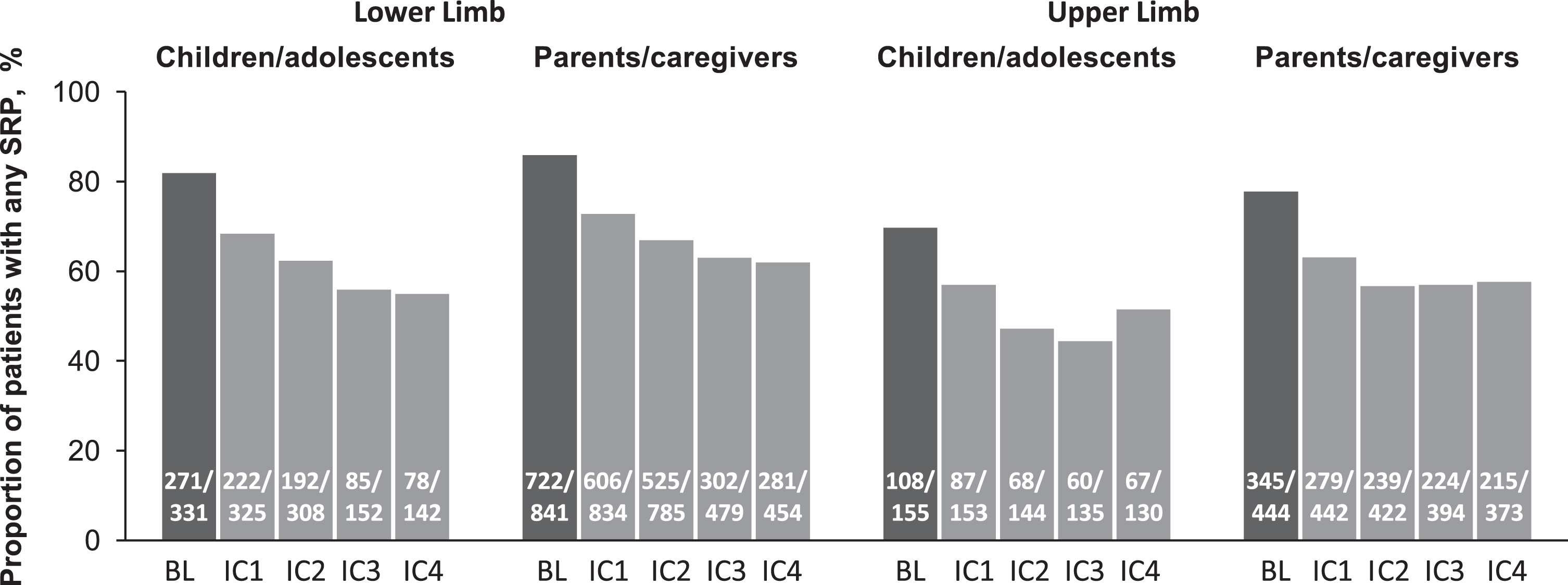
Table 2A shows the percentages of children/adolescents with LL SRP at baseline who reported complete SRP relief on QPS key items. Between 28.4% and 45.3% of children/adolescents with LL spasticity reported complete SRP relief for each key item, depending on the strenuousness of the activities, with the first IC. A pattern suggesting an increase in percentages of children/adolescents experiencing complete SRP relief was seen with each subsequent IC, so that by IC4, 33.8% to 53.3% of children/adolescents experienced complete SRP relief in individual QPS key items. For all ICs and QPS key items, significant proportions of children/adolescents reported complete SRP relief (p < 0.001).
Table 2A
Complete relief of LL SRP and change in QPS key item scores at Week 4 of each incobotulinumtoxinA injection cycle as reported by (A) children/adolescents and (B) parents/caregivers in the pooled analysis population A. Child/adolescents LL QPS (self- and interviewer-reported)
| Child/adolescent LL SRP intensity [0–10]a | Overall population at baseline | Baseline SRP population b | Complete SRP relief after incobotulinumtoxinA treatmentc (% of children/adolescents)d,e | LS mean change in item score in the baseline SRP populationf | |||||||
| FAS | N | N (%) | Mean score | Injection cycle 1 | Injection cycle 2 | Injection cycle 3 | Injection cycle 4 | Injection cycle 1 | Injection cycle 2 | Injection cycle 3 | Injection cycle 4 |
| General SRP Hurt when tight (Item 3) | 330 | 178 (53.9) | 4.3 | 35.3 | 48.1 | 45.7 | 49.4 | –2.1 | –2.5 | –2.7 | –2.8† |
| SRP at rest Sitting, watching TV, or trying to sleep (Item 5) | 324 | 86 (26.5) | 3.7 | 43.4 | 46.2 | 39.5 | 51.4 | –1.8 | –1.9 | –2.2 | –2.6 |
| SRP with usual activities Moving, walking, or playing (Item 7) | 328 | 178 (54.3) | 4.0 | 45.3 | 45.2 | 44.2 | 53.3 | –2.1 | –2.3 | –2.4 | –2.7 |
| SRP with exercises Physical therapy or stretching exercises (Item 9) | 330 | 234 (70.9) | 4.5 | 32.3 | 33.0 | 39.4 | 41.6 | –1.9 | –2.2 | –2.4 | –2.6 |
| SRP with hard task Self-defined hard thing (Item 12) | 330 | 202 (61.2) | 4.6 | 28.4 | 33.3 | 30.7 | 33.8 | –1.9 | –2.4 | –2.5 | –2.7† |
aSRP was rated in the QPS by the child/adolescent themself or via an interviewer using the graphic Wong-Baker FACES scale: 0 = No hurt, 2 = Hurt a little bit, 4 = Hurt a little more, 6 = Hurt even more, 8 = Hurt a whole lot, 10 = Hurt worst.
bChildren/adolescents from the total population irrespective of dose group who reported SRP with an item score > 0 at baseline.
cChildren/adolescents from the baseline SRP population who reported no SRP at the Week 4 visit with an item score of “0 = No hurt.”
dFor children/adolescents with LL spasticity, size of population per QPS item per cycle (injection cycle 1– 4): Item 3: 173, 162, 81, 77; Item 5: 83, 78, 38, 37; Item 7: 172, 157, 77, 75; Item 9: 229, 215, 109, 101; Item 12: 194, 183, 88, 80. The decrease in the number of patients with LL spasticity after injection cycle 2 reflects the design and contribution to the pooled data of the TIM study, which included only two injection cycles.
eValues in bold indicate p < 0.001 for the Week 4 responder rate being significantly above 10% (exact one-sample binomial test, one-sided, significance level alpha = 0.025).
fValues in bold indicate p < 0.001 for the Week 4 change being differentiated significantly against the mean item baseline (MMRM).
†p < 0.01 for the Week 4 change being differentiated significantly against the mean item baseline (MMRM).
FAS = full analysis set; LL = lower limb; MMRM = mixed model repeated measures; N = number of non-missing observations; QPS = Questionnaire on Pain caused by Spasticity; SRP = spasticity-related pain; TIM = Treatment with IncobotulinumtoxinA in Movement.
IncobotulinumtoxinA treatment provided complete SRP relief for some children/adolescents for even the most strenuous tasks. For instance, after IC4, 41.6% of children/adolescents with LL spasticity reported complete SRP relief with exercise and 33.8% reported being free of SRP when performing a hard task.
Children/adolescents also reported significant reductions from baseline in least squares (LS) mean LL SRP intensity scores for all QPS key items (p < 0.001) (Table 2A) over each of the four ICs. At the first IC, LS mean changes ranged from –1.8 to –2.1 across QPS key items; these improvements were equivalent to one grade on the 6-point Wong-Baker FACES response scale and represent relative reductions in scores from baseline of 41.3% to 52.0%. At IC4, LS mean score reductions ranged from –2.6 to –2.8, representing relative reductions from baseline across QPS key items of 56.9% to 69.7%. Scores improved with each subsequent IC, and the magnitude of improvement generally increased with the difficulty of the task.
The proportion of children/adolescents experiencing the most severe LL SRP (i.e., a score of 8: “Hurt a whole lot” to 10: “Hurt worst” in at least one of the five items) decreased progressively from 19.3% at baseline to 8.3% (IC1), 5.5% (IC2), 1.3% (IC3), and 2.1% (IC4).
3.2.2QPS parent/caregiver modules
The proportion of parents/caregivers observing any signs of LL SRP in their children was highly consistent with the children’s/adolescents’ assessments. Parents/caregivers reported observing any LL SRP in 85.9% of children/adolescents at baseline in QPS key items, which decreased to 72.7% by IC1 and then to 61.9% by IC4 (Fig. 2).
Parent/caregivers observed complete SRP relief for increasing percentages of children/adolescents with each subsequent IC for all QPS key items (Table 2B). At IC1, parents/caregivers observed that 20.5% to 36.8% of children/adolescents with LL SRP had complete SRP relief on one QPS key item, which increased to 32.5% to 44.8% at IC4. For the most difficult activities, such as exercise, 32.9% of the parent/caregivers reported complete LL SRP relief by IC4 and 32.5% reported children/adolescents experienced complete LL SRP relief with a hard task. For all ICs and QPS key items, significant proportions of parents/caregivers observed complete SRP relief in their children (p < 0.001).
Table 2B
Parent/caregiver LL QPS
| Parent/caregiver observed LL SRP behavior frequency [0–5]a | Overall population at baseline | Baseline SRP populationb | Complete SRP relief after incobotulinumtoxinA treatmentc (% of children/adolescents)d,e | LS Mean change in item score in the baseline SRP populationf | |||||||
| FAS | N | N (%) | Mean score | Injection cycle 1 | Injection cycle 2 | Injection cycle 3 | Injection cycle 4 | Injection cycle 1 | Injection cycle 2 | Injection cycle 3 | Injection cycle 4 |
| General SRP Hurt when tight (Item 8) | 839 | 568 (67.7) | 2.3 | 25.2 | 31.9 | 32.5 | 34.1 | –0.9 | –1.1 | –1.2 | –1.2 |
| SRP at rest Sitting, watching TV, or trying to sleep (Item 9b) | 818 | 321 (39.2) | 1.9 | 36.8 | 44.8 | 37.4 | 44.8 | –0.9 | –1.0 | –1.0 | –1.1 |
| SRP with usual activities Moving, walking, or playing (Item 10b) | 816 | 485 (59.4) | 2.2 | 27.6 | 33.9 | 33.3 | 37.5 | –0.9 | –1.1 | –1.1 | –1.2 |
| SRP with exercises Physical therapy or stretching exercises (Item 11b) | 829 | 674 (81.3) | 2.6 | 22.2 | 27.9 | 29.1 | 32.9 | –0.9 | –1.1 | –1.2 | –1.4 |
| SRP with hard task Self-defined hard thing (Item 13b) | 831 | 594 (71.5) | 2.7 | 20.5 | 27.5 | 30.2 | 32.5 | –0.9 | –1.1 | –1.3 | –1.3 |
aObserved SRP frequency was rated in the QPS by the parent/caregiver using the 5-point response scale: 0 = Never, 1 = Rarely, 2 = Sometimes, 3 = Often, 4 = Always.
bParents/caregivers from the total population irrespective of dose group who reported SRP behavior with an item score at baseline >0.
cParents/caregivers from the baseline SRP population who reported no SRP behavior at the Week 4 visit with an item score of “0 = Never.”
dValues in bold indicate p < 0.001 for the week 4 responder rate being significantly above 10% (exact one-sample binomial test, one-sided, significance level alpha = 0.025).
eFor parents/caregivers observations of children/adolescents with LL spasticity, size of population per QPS item per cycle (injection cycle 1– 4): Item 8: 563, 526, 326, 317; Item 9b: 310, 290, 179, 172; Item 10b: 478, 446, 276, 267; Item 11b: 658, 620, 378, 362; Item 13b: 577, 535, 318, 295. The decrease in the number of patients with LL spasticity after injection cycle 2 reflects the design and contribution to the pooled data of the TIM study, which included only two injection cycles.
fValues in bold indicate p<0.001 for the Week 4 change being differentiated significantly against the mean item baseline (MMRM).
FAS = full analysis set; LL = lower limb; MMRM=mixed model repeated measures; N = number of non-missing observations; QPS = Questionnaire on Pain caused by Spasticity; SRP = spasticity-related pain; TIM = Treatment with IncobotulinumtoxinA in Movement.
Parent/caregiver-observed QPS LS mean frequency scores also significantly decreased from baseline in all observed LL SRP behaviors in a similar pattern to those reported by children/adolescents (p < 0.001) (Table 2B). Scores generally showed increasing improvement with each subsequent IC, and the magnitude of improvement generally increased with the difficulty of the task. At baseline, 54.3% of parents/caregivers observed that their children/adolescents experienced SRP often or always. This percentage dropped to 31.2% at IC1, 23.8% at IC2, 20.5% at IC3, and 19.8% at IC4.
3.3SRP changes in UL spasticity
3.3.1QPS child/adolescent self-reported and interviewer-reported modules
Children/adolescents reported similar changes in UL SRP as reported for LL SRP, but UL SRP was slightly less frequently reported. The overall proportion of children/adolescents reporting UL SRP on any of the QPS key item decreased from 69.7% at baseline to 56.9% at the first incobotulinumtoxinA IC (Fig. 2). This proportion then gradually decreased with each further IC down to 51.5% by IC4.
Table 3A shows the percentages of children/adolescents with UL SRP at baseline who reported complete SRP relief on individual QPS key items over the four ICs. Depending on the level of activity, between 26.6% and 39.7% of children/adolescents reported complete SRP relief at IC1. With additional injections, the percentage of children/adolescents experiencing complete UL SRP relief increased for all QPS key items at IC4, reaching 54.8% for SRP with usual activities, 44.3% for SRP with exercise, and 48.4% for SRP with hard task. For all ICs and QPS key items, significant proportions of children/adolescents reported complete SRP relief (p < 0.025).
Table 3A
Complete relief of UL SRP and change in QPS key item scores at Week 4 of each incobotulinumtoxinA injection cycle as reported by (A) children/adolescents and (B) parents/caregivers in the pooled analysis population. A. Child/adolescent UL QPS (self- and interviewer-reported)
| Child/adolescent UL SRP intensity [0–10]a | Overall population at baseline | Baseline SRP populationb | Complete SRP relief after incobotulinumtoxinA treatmentc (% of children/adolescents)d,e | LS Mean change in item score in the baseline SRP populationf | |||||||
| FAS | N | N (%) | Mean score | Injection cycle 1 | Injection cycle 2 | Injection cycle 3 | Injection cycle 4 | Injection cycle 1 | Injection cycle 2 | Injection cycle 3 | Injection cycle 4 |
| General SRP Hurt when tight (Item 3) | 152 | 69 (45.4) | 3.6 | 39.7 | 40.0 | 43.1 | 41.8 | –1.7 | –2.0 | –2.1 | –2.2 |
| SRP at rest Sitting, watching TV, or trying to sleep (Item 5) | 148 | 34 (23.0) | 3.5 | 35.3 | 25.0* | 32.3 | 36.7 | –1.1† | –1.6† | –1.8 | –1.9 |
| SRP with usual activities Moving, walking, or playing (Item 7) | 150 | 52 (34.7) | 3.6 | 34.0 | 33.3 | 45.2 | 54.8 | –1.2† | –1.9 | –2.2 | –2.6 |
| SRP with exercises Physical therapy or stretching exercises (Item 9) | 153 | 99 (64.7) | 4.0 | 35.7 | 41.4 | 42.4 | 44.3 | –1.7 | –2.1 | –2.2 | –2.5 |
| SRP with hard task Self-defined hard thing (Item 12) | 155 | 83 (53.5) | 3.9 | 26.6 | 43.7 | 43.1 | 48.4 | –1.3 | –2.2 | –2.2 | –2.7 |
aSRP was rated in the QPS by the child/adolescent themself or via an interviewer using the graphic Wong-Baker FACES scale: 0 = No hurt, 2 = Hurt a little bit, 4 = Hurt a little more, 6 = Hurt even more, 8 = Hurt a whole lot, 10 = Hurt worst.
bChildren/adolescents from the total population irrespective of dose group who reported SRP with an item score > 0 at baseline.
cChildren/adolescents from the baseline SRP population who reported no SRP at the Week 4 visit with an item score of “0 = No hurt.”
dFor children/adolescents with UL spasticity, size of population per QPS item per cycle (injection cycle 1– 4): Item 3: 68, 60, 58, 55; Item 5: 34, 32, 31, 30; Item 7: 50, 45, 42, 42; Item 9: 98, 87, 85, 79; Item 12: 79, 71, 65, 64.
eValues in bold indicate p < 0.001 for the Week 4 responder rate being significantly above 10% (exact one-sample binomial test, one-sided, significance level alpha = 0.025).
fValues in bold indicate p < 0.001 for the Week 4 change being differentiated significantly against the mean item baseline (MMRM).
*p < 0.025 for the Week 4 responder rate being significantly above 10% (exact one-sample binomial test, one-sided, significance level alpha = 0.025).
†p < 0.01 for the Week 4 change being differentiated significantly against the mean item baseline (MMRM).
FAS = full analysis set; MMRM = mixed model repeated measures; N = number of non-missing observations; QPS = Questionnaire on Pain caused by Spasticity; SRP = spasticity-related pain; UL = upper limb.
Children/adolescents reported significant reductions in LS mean UL SRP intensity scores for all individual QPS key items from baseline of 3.5 (at rest) to 4.0 (with exercise) over each of the four ICs (p < 0.01) (Table 3A). At the first IC, the LS mean improvement was –1.1 to –1.7 across QPS key items (Table 3A), representing relative mean reductions from baseline scores of 32.0% to 46.7%. At IC4, the LS mean changes ranged from –1.9 to –2.7, representing relative mean reductions from baseline scores across QPS key items of 54.6% to 71.4%. Scores showed increasing improvement with each subsequent IC, and the magnitude of improvement generally increased with the difficulty of the task.
The proportion of children/adolescents experiencing the most severe UL SRP (i.e., a score of 8: “Hurt a whole lot” to 10: “Hurt worst” in at least one of the five items) decreased progressively from 7.1% at baseline to 5.2% (IC1), 0.7% (IC2), 2.2% (IC3), and 1.5% (IC4) after each respective treatment cycle.
3.3.2QPS parent/caregiver modules
The proportion of parents/caregivers observing any SRP in their children was highly consistent with the children’s/adolescents’ assessments. Parents/caregivers observed any UL SRP in 77.7% of children/adolescents at baseline, which decreased to 57.6% by IC4 (Fig. 2).
Parent/caregiver observations showed patterns suggesting an increase in percentages of children/adolescents with complete SRP relief on each QPS key item with each subsequent IC (Table 3B). At the first incobotulinumtoxinA IC, 24.5% to 30.3% of parents/caregivers observed complete SRP relief in their children, which rose to 32.8% to 44.1% by IC4. For the most difficult activities, such as exercise, 32.8% of the parent/caregivers reported children/adolescents experienced complete UL SRP relief by IC4, and 37.6% experienced complete UL SRP relief when performing a hard task. For all injection cycles and QPS key items, significant proportions of parents/caregivers observed complete SRP relief in their children (p < 0.001).
Table 3B
Parent/caregiver UL QPS
| Parent/Caregiver Observed UL SRP behavior frequency [0–5]a | Overall population at baseline | Baseline SRP Populationb | Complete SRP relief after incobotulinumtoxinA treatmentc (% of children/adolescents)d,e | LS Mean change in item score in the baseline SRP populationf | |||||||
| FAS | N | N (%) | Mean score | Injection cycle 1 | Injection cycle 2 | Injection cycle 3 | Injection cycle 4 | Injection cycle 1 | Injection cycle 2 | Injection cycle 3 | Injection cycle 4 |
| General Pain Hurt when tight (Item 8) | 442 | 294 (66.5) | 2.3 | 28.3 | 34.9 | 34.0 | 38.2 | –1.0 | –1.1 | –1.2 | –1.4 |
| Pain at rest Sitting, watching TV, or trying to sleep (Item 9b) | 424 | 160 (37.7) | 1.9 | 29.1 | 40.7 | 32.6 | 44.1 | –0.7 | –1.0 | –0.9 | –1.1 |
| Pain with usual activities Moving, walking, or playing (Item10b) | 425 | 248 (58.4) | 2.2 | 30.3 | 36.5 | 35.8 | 40.5 | –0.9 | –1.1 | –1.1 | –1.2 |
| Pain with exercises Physical therapy or stretching exercises (Item 11b) | 435 | 317 (72.9) | 2.6 | 24.5 | 31.4 | 29.6 | 32.8 | –0.9 | –1.2 | –1.1 | –1.3 |
| Pain with hard task Self-defined hard thing (Item 13b) | 436 | 294 (67.4) | 2.5 | 27.4 | 35.3 | 32.9 | 37.6 | –0.9 | –1.2 | –1.2 | –1.4 |
aObserved SRP frequency was rated in the QPS by the parent/caregiver using the 5-point response scale: 0 = Never, 1 = Rarely, 2 = Sometimes, 3 = Often, 4=Always.
bParent/caregivers from the total population irrespective of dose group who reported SRP behavior with an item score at baseline >0.
cParent/caregivers from the baseline SRP population who reported no SRP behavior at the Week 4 visit with an item score of “0 = Never.”
dValues in bold indicate p < 0.001 for the Week 4 responder rate being significantly above 10% (exact one-sample binomial test, one-sided, significance level alpha = 0.025).
eFor parents/caregiver observations of children/adolescents with UL spasticity, size of population per QPS item per cycle (injection cycle 1– 4): Item 8: 290, 278, 262, 251; Item 9b: 151, 140, 132, 127; Item 10b: 238, 230, 215, 210; Item 11b: 306, 293, 277, 268; Item 13b: 277, 258, 240, 226.
fValues in bold indicate p < 0.001 for the Week 4 change being differentiated significantly against the mean item baseline (MMRM).
FAS = full analysis set; MMRM = mixed model repeated measures; N = number of non-missing observations; QPS = Questionnaire on Pain caused by Spasticity; SRP = spasticity-related pain; UL = upper limb.
Parent/caregiver-observed QPS frequency scores also showed significant reductions from baseline in all observed activities in a similar pattern to those self-reported by children/adolescents (p < 0.001). With the fourth injection, parents/caregivers observed LS mean reductions in UL QPS SRP frequency scores ranged from –1.1 for SRP with rest to –1.4 for SRP with a hard task, representing relative mean reductions from baseline scores across QPS key items of 50.0% to 59.1% (Table 3B). Scores generally showed increasing improvement with each subsequent IC, while the magnitude of improvement increased with the difficulty of the task.
At baseline, 46.6% of parents/caregivers observed that their children/adolescents experienced SRP often or always. This percentage dropped to 25.6% at IC1, 14.9% at IC2, 17.0 % at IC3, and 12.3% at IC4.
4Discussion
The current results provide evidence of substantial, clinically meaningful reductions in the frequency and intensity of SRP after incobotulinumtoxinA treatment in a large pediatric population with CP-related spasticity. As reported and discussed in Part 1 of this two-part report, pain, i.e., SRP as measured by the QPS, was highly prevalent in this CP population [28]. A significant reduction in the intensity of SRP for children/adolescents reporting SRP at baseline was demonstrated after one cycle of incobotulinumtoxinA treatment. The proportion of children/adolescents reporting complete SRP relief on at least one QPS key item increased with successive incobotulinumtoxinA injections. The trend for complete SRP relief, reported by children/adolescents and parents/caregivers, was evident for all reported activities of the QPS. Indeed, the most striking finding was that complete relief from SRP during a hard task and exercise was reported by 34% and 42% of children/adolescents with LL spasticity, respectively, and 48% and 44% of those with UL spasticity, respectively, after four ICs of incobotulinumtoxinA. Parent/caregiver observations indicate that even patients most severely affected by SRP and those not able to actively report SRP benefitted from incobotulinumtoxinA treatment.
The SRP-relieving effects of incobotulinumtoxinA injections reported in this study exceed the recommended thresholds for clinical meaningfulness. For chronic pain such as SRP in adults, improvements of 2 points on a 0- to 10-point pain intensity scale or reductions in pain intensity in the range of 10% (minimally important) up to 50% (substantially important) are commonly used as thresholds for clinically meaningful changes [29, 30]. Recommended thresholds for children are lower (1-point improvements and ∼10% reductions in pain intensity) [31]. At the first IC, children/adolescents reported maximum relative LS mean reductions in SRP intensity of 52.0% /46.7% for those with LL/UL spasticity, which reached 69.7% /71.4% by IC4.
With regard to the management of pain and SRP, a systematic review by Ostojic and colleagues [32] highlighted the paucity of high-quality research investigating the efficacy of pain-relieving interventions for children/adolescents with CP, concluding that such children have few good treatment options. In a follow-up report, Ostojic and colleagues [4] found that both acute and chronic pain in children/adolescents with CP were still most often managed with acetaminophen and ibuprofen as well as massage, rest, thermotherapy, and hydrotherapy. Other investigators have called for a strategy for implementing effective chronic pain management for children/adolescents with CP [33].
The use of BoNT-A to relieve SRP in children with CP may represent one option for these patients. A few, small observational studies reported that BoNT-A treatment reduces CP-related pain intensity and/or frequency (n = 3–63) [34–37]. Misra and colleagues [37] treated 63 children with CP-related spasticity with onabotulinumtoxinA and followed them for 6 months to 2 years. Sixty percent had > 50% reduction in pain as measured on a 10-point Visual Analog Scale by patients and proxies. In a prospective, observational study of 282 children with CP, BoNT-A (formulation not specified) significantly reduced pain at rest and during mobilization, lasting up to 12 months post-injection, in a subset of 46 children (16%) with pain at baseline [38]. Jabbari [39] provided anecdotal evidence from clinical experience with more than 200 children with CP (some of whom were followed for up to 8 years), noting that pain was reduced in children with CP after BoNT-A injections (further details not provided). In a small, prospective, double-blind study of 41 non-ambulatory children with SRP, onabotulinumtoxinA reduced pain compared to baseline at 4 and 16 weeks in 18 children who reported pain on the Pediatric Pain Profile at baseline. Pain was significantly reduced from baseline with BoNT-A but not for those undergoing a sham procedure, suggesting that BoNT-A had an effect on pain; however, no differences in pain control were found between those injected with onabotulinumtoxinA compared to those undergoing a sham procedure. There are several explanations for this result, such as using a pain scale not designed to assess SRP or the small sample size [40]. In a single-blind, randomized, controlled trial of 43 children with CP, 21 received onabotulinumtoxinA plus occupational therapy and 22 received occupational therapy alone; on a Visual Analog Scale, pain improved over time for both groups, although no statistical analysis was reported, with the authors suggesting that further study was required [41]. Larger registry studies of pain in young people with CP tend to focus on quality of life and participation rather than the results of any specific treatment [3, 9, 42]. It is in this context that a large pool of data that details the prevalence of SRP in children with CP and the promising results of incobotulinumtoxinA treatment is presented.
The effects of SRP on children with CP should be considered as a cornerstone for participation, which is often underestimated. SRP can prevent a child with CP from performing usual daily activities and, importantly, from the physiotherapy and strenuous exercise that are vital parts of CP therapy [43–45]. The overall consequences can feed a vicious cycle of less movement, changes in muscle structure, tightness/stiffness, contractures, and subsequently more pain. Increased sedentary behavior and reduced physical activity in children with CP have their own negative health consequences, including weight gain and elevated blood pressure [46]. In addition to improving quality of life [42, 47–49], the data suggest that incobotulinumtoxinA treatment has the potential to add broader therapeutic options through attenuation of SRP combined with reduced spasticity.
It has been proposed that the pain-relieving effects of BoNT-A in SRP may be related to muscle relaxation (via inhibition of acetylcholine release), inhibition of the release of pain mediators from nerves, and modulation of the excitability of spinal cord nociceptive pathways [5, 7, 39, 50]. Further investigation is needed to clarify whether the SRP relief provided by incobotulinumtoxinA results from lessening spasticity or direct antinociceptive action, or both.
As a general rule, BoNT-A injections have the advantage of a long duration of action with a low risk of adverse effects even after repeated injections [51]. A pooled analysis of TIM, TIMO, and XARA (N = 907) showed that incobotulinumtoxinA was safe and well tolerated for LL, UL, or combined multipattern treatment over (up to) six ICs in a population of ambulant and non-ambulant pediatric patients with spasticity (GMFCS-E&R levels I–V) within dose ranges of up to 20 U/kg BW (maximum 500 U). The most common adverse events were nasopharyngitis/pharyngitis, bronchitis, and upper respiratory tract infection. Treatment-related adverse events (N = 10) and serious adverse events (N = 2) were low. Discontinuation rates due to adverse events were very low (N = 3, 0.3%) [25]. IncobotulinumtoxinA was found to have no risk of neutralizing antibody formation in BoNT-A-naïve children [52].
4.1Strengths and limitations
When compared with other published studies evaluating the effects of BoNT-A formulations on SRP in children with CP, the current analysis generally included larger numbers of self-reporting children/adolescents (340 with LL spasticity and 160 with UL spasticity), evaluated a longer treatment period (64–72 weeks), followed a more standardized treatment protocol, and utilized a validated SRP outcome assessment specific for the population (the QPS). Notably, the modular design approach of the QPS provided insights into SRP behaviors as observed by more than 800 parent/caregivers, allowing the SRP improvements to be measured in the overall population including the very young and children with higher levels of impairment, as well in children able to provide information directly. The study designs allowed multipattern injections across multiple ICs, which could be tailored to meet individual patient needs. This fits very well with the QPS evaluative approach to SRP, considering that the QPS asks more globally about LL or UL SRP and not, for example, about SRP in a single clinical pattern. Analyses were performed on a background of stable antispastic medications and physical/occupational therapy, and a high number of participants were retained until completion. Therefore, the resulting large data population represents the most detailed view into the topic of SRP treatment with BoNT-A to date.
A limitation to be discussed is that this report consolidates SRP data from three studies with similar but not identical study designs and pools data from different incobotulinumtoxinA doses. TIM enrolled only children/adolescents with LL spasticity while TIMO and XARA included children/adolescents with both LL and UL spasticity. The studies also differed in further details such as duration, number of allowed injections, and incobotulinumtoxinA dose requirements, although all studies utilized the QPS [20–22]. However, this heterogeneity of the data presents a more comprehensive evaluation of the topic as is similarly achieved in registry studies, which usually have more flexible inclusion criteria than clinical trials and aim more strongly towards the general, real-world patient population.
The decision to pool dose groups and data from three studies was made for two reasons. First, the sample size for each IC was able to be maximized by pooling the data, which was possible because the studies shared many enrollment criteria. Second, although improvements in spasticity can differ for the investigated dose groups [22], useful levels of improvement in spasticity can be achieved with the investigated lower dose regimens [22, 23]. By pooling data from each dose group, more cohesive sets of QPS data have been obtained for patients with LL or UL CP-related spasticity. Post-hoc QPS analyses (data not shown) revealed no dose differences between QPS responses, supporting the study assumptions and that the data are suitable for pooling.
Furthermore, the lack of a control group, such as a placebo group, is a limitation. The decision not to include a placebo group in the studies was based on fundamental ethical concerns for this pediatric patient population. Nevertheless, the placebo response (setting response) in pain studies is an issue that must be taken into account to fully understand any pain model and should be considered carefully when interpreting the clinical meaningfulness of treatment. Another limitation of the present study is that, although spasticity is frequently associated with pain in people with CP, there can be other sources of pain in this population [45]. To minimize this, pain was assessed using the QPS, which recorded information on pain caused by spasticity only [26, 27].
Based on the consistent treatment effects and substantial improvements found across QPS modules and activities, this study suggests that the data provided are comprehensive and provide evidence to support incobotulinumtoxinA as a promising treatment for SRP; however, further research is warranted.
5Conclusion
In the first publication of this two-part report, it was demonstrated that children/adolescents with CP have a high prevalence of SRP and that they experience SRP in multiple body locations and during typical daily activities that may have broad, long-lasting negative consequences. In this second publication, evidence has been presented that incobotulinumtoxinA provides clinically meaningful relief of SRP for young people with CP while they are engaged in typical daily tasks as well as strenuous activities such as exercise and physiotherapy. The SRP-relieving effects of incobotulinumtoxinA are evident to both children/adolescents and their parents/caregivers after the first injection, and the clinical benefit is sustained with further improvements after subsequent treatments and as the activities become more challenging. Promoting activity should be the undisputed mantra for treating children with CP and, in this context, it is suggested that BoNT-A treatment should be considered as an innovative yet established procedure for effective pain management to promote physical activity. In this regard, incobotulinumtoxinA could bring considerable clinical benefit to the lives of children/adolescents with LL and/or UL spasticity through the reduction of SRP, in addition to the well-known muscle tone regulation.
Acknowledgments
The authors wish to thank all study subjects and investigators. They would like to acknowledge Hanna Dersch and Dieter Matthias for statistical support and Amy Rothman Schonfeld and Caroline Spencer (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Merz Therapeutics GmbH. This study was supported by Merz Therapeutics GmbH, Frankfurt am Main, Germany.
Conflict of interest
Florian Heinen has received speaker’s honoraria from Allergan plc, Desitin, Ipsen Biopharmaceuticals, Merz Therapeutics, and Novartis and unrestricted educational grants from Allergan and Merz Therapeutics. Michaela Bonfert has received research grants from the HABA Foundation, the Deutsche Rentenversicherung, the Deutsche Migräne und Kopfschmerzgesellschaft, the European Research Council, ERA-NET Neuron, CSL Behring, and ZNS - Hannelore Kohl Foundation, and a research scholarship of the Bavarian Gender Equality Grant of the Free State of Bavaria, Germany. Petr Kaňovský has received speaker’s honoraria from Desitin, Ipsen Biopharmaceuticals, Merz Therapeutics, and Medtronic. A. Sebastian Schroeder has received speaker’s honoraria from and participated in advisory boards for Allergan plc, Ipsen Biopharmaceuticals, and Merz Therapeutics. Henry G. Chambers serves as a consultant for Orthopediatrics Corp and Allergan Corporation. Edward Dabrowski has participated in an advisory board and speaker bureau for Ipsen Biopharmaceuticals. Thorin L. Geister, Angelika Hanschmann, and Michael Althaus are employees of Merz Therapeutics GmbH. Marta Banach has served as a consultant and speaker and participated in an advisory board for Merz Therapeutics and has served as a speaker for Allergan, Ipsen, and Kedrion. Deborah Gaebler-Spira has served as a consultant for Teva and Kashiva.
Ethical considerations
The study protocol, informed consent forms, and other appropriate study-related documents were reviewed and approved by the local independent ethics committees and institutional review boards. Parents/guardians of all patients provided written informed consent, and patients provided assent if applicable.
References
[1] | Westbom L , Rimstedt A , Nordmark E , Assessments of pain in children and adolescents with cerebral palsy: a retrospective population-based registry study, Dev Med Child Neurol (2017) ;59: (8):858–63. doi: 10.1111/dmcn.13459. |
[2] | Parkinson KN , Dickinson HO , Arnaud C , Lyons A , Colver A , Pain in young people aged 13 to 17 years with cerebral palsy: cross-sectional, multicentre European study, Arch Dis Child (2013) ;98: (6):434–40. doi: 10.1136/archdischild-2012-303482. |
[3] | Parkinson KN , Gibson L , Dickinson HO , Colver AF , Pain in children with cerebral palsy: a cross-sectional multicentre European study, Acta Paediatr (2010) ;99: (3):446–51. doi: 10.1111/j.1651-2227.2009.01626.x. |
[4] | Ostojic K , Paget S , Kyriagis M , Morrow A , Acute and chronic pain in children and adolescents with cerebral palsy: prevalence, interference, and management, Arch Phys Med Rehabil (2020) ;101: (2):213–19. doi: 10.1016/j.apmr.2019.08.475. |
[5] | Michelsen JS , Normann G , Wong C , Analgesic effects of botulinum toxin in children with CP, Toxins (Basel) (2018) ;10: (4):162. doi: 10.3390/toxins10040162. |
[6] | Eriksson E , Hägglund G , Alriksson-Schmidt AI . Pain in children and adolescents with cerebral palsy - a cross-sectional register study of individuals, BMC Neurol (2020) ;20: (1):15. doi: 10.1186/s12883-019-1597-7. |
[7] | Hägglund G , Burman-Rimstedt A , Czuba T , Alriksson-Schmidt AI . Self-versus proxy-reported pain in children with cerebral palsy: a population-based registry study of children, J Prim Care Community Health (2020) ;11: :1523. doi: 10.1177/2150132720911523. |
[8] | Horwood L , Li P , Mok E , Shevell M , Constantin E . A systematic review and meta-analysis of the prevalence of sleep problems in children with cerebral palsy: how do children with cerebral palsy differ from each other and from typically developing children? Sleep Health (2019) ;5: (6):555–71. doi: 10.1016/j.sleh.2019.08.006. |
[9] | Østergaard CS , Pedersen NSA , Thomasen A , Mechlenburg I , Nordbye-Nielsen K , Pain is frequent in children with cerebral palsy and negatively affects physical activity and participation, Acta Paediatr (2021) ;110: (1):301–6. doi: 10.1111/apa.15341. |
[10] | Riquelme I , do Rosário RS , Vehmaskoski K , Natunen P , Montoya P . Influence of chronic pain in physical activity of children with cerebral palsy, NeuroRehabilitation (2018) ;43: (2):113–23. doi: 10.3233/NRE-172409. |
[11] | Delgado MR , Hirtz D , Aisen M , Ashwal S , Fehlings DL , McLaughlin J , et al., Practice parameter: pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society, Neurology (2010) ;74: (4):336–43. doi: 10.1212/WNL.0b013e3181cbcd2f. |
[12] | Spasticity in under 19s: management. Clinical guideline [CG145]. National Institute for Health and Care Excellence (NICE); 25 Jul 2012 [updated 29 Nov 2016; cited 11 Mar 2021]. Available from: http://www.nice.org.uk/guidance/cg145. |
[13] | Hareb F , Bertoncelli CM , Rosello O , Rampal V , Solla F , Botulinum toxin in children with cerebral palsy: An update, Neuropediatrics (2020) ;51: (1):1–5. doi: 10.1055/s-0039-1694988. |
[14] | Sadowska M , Sarecka-Hujar B , Kopyta I , Cerebral palsy: current opinions on definition, epidemiology, risk factors, classification and treatment options, Neuropsychiatr Dis Treat (2020) ;16: :1505–18. doi: 10.2147/NDT.S235165. |
[15] | Fehlings D , Novak I , Berweck S , Hoare B , Stott NS , Russo RN , et al., Botulinum toxin assessment, intervention and follow-up for paediatric upper limb hypertonicity: international consensus statement, Eur J Neurol (2010) ;17: (Suppl 2):38–56. doi: 10.1111/j.1468-1331.2010.03127.x. |
[16] | Love SC , Novak I , Kentish M , Desloovere K , Heinen F , Molenaers G , et al., Botulinum toxin assessment, intervention and after-care for lower limb spasticity in children with cerebral palsy: international consensus statement, Eur J Neurol (2010) ;17: (Suppl 2):9–37. doi: 10.1111/j.1468-1331.2010.03126.x. |
[17] | Almina S , Karile Y , Audrone P , Indre B , Analgesic effect of botulinum toxin in children with cerebral palsy: A systematic review, Toxicon (2021) ;199: :60–7. doi: 10.1016/j.toxicon.2021.05.012. |
[18] | Roscigno CI , Addressing spasticity-related pain in children with spastic cerebral palsy, J Neurosci Nurs (2002) ;34: (3):123–33. doi: 10.1097/01376517-200206000-00005. |
[19] | Li S , Francisco GE , The use of botulinum toxin for treatment of spasticity, Handb Exp Pharmacol (2021) ;263: :127–46. doi: 10.1007/164_2019_315. |
[20] | Heinen F , Kanovský P , Schroeder AS , Chambers HG , Dabrowski E , Geister TL , et al., IncobotulinumtoxinA for the treatment of lower-limb spasticity in children and adolescents with cerebral palsy: a phase 3 study, J Pediatr Rehabil Med (2021) ;14: (2):183–97. doi: 10.3233/PRM-210040. |
[21] | Kanovský P , Heinen F , Schroeder AS , Chambers HG , Dabrowski E , Geister TL , et al. Safety and efficacy of repeat long-term incobotulinumtoxinA treatment for lower limb or combined upper/lower limb spasticity in children with cerebral palsy, J Pediatr Rehabil Med (2022) ;15: (1):113–27. doi: 10.3233/PRM-210041. |
[22] | Dabrowski E , Chambers HG , Gaebler-Spira D , Banach M , Kanovský P , Dersch H , et al., IncobotulinumtoxinA efficacy/safety in upper-limb spasticity in pediatric cerebral palsy: Randomized controlled trial, Pediatr Neurol (2021) ;123: :10–20. doi: 10.1016/j.pediatrneurol.2021.05.014. |
[23] | Heinen F , Kanovsky P , Schroeder AS , Chambers HG , Dabrowski E , Geister T , et al., Pooled efficacy analysis of incobotulinumtoxinA in the multipattern treatment of upper- and lower-limb spasticity in children and adolescents with cerebral palsy. Poster presented at TOXINS 2021 Virtual Conference, January 16-17, 2021. |
[24] | Schroeder AS , Kanovsky P , Chambers HG , Dabrowski E , Geister TL , Dersch H , et al., Sustained efficacy of incobotulinumtoxinA over six injection cycles for the treatment of lower-limb spasticity in children and adolescents with cerebral palsy. Poster presented at TOXINS 2021 Virtual Conference, January 16-17, 2021. |
[25] | Banach M , Kanovsky P , Schraeder AS , Chambers HG , Dabrowski E , Geister T , et al., Safety of incobotulinumtoxinA in multipattern treatment of upper- and lower-limb spasticity in children/adolescents with cerebral palsy: a pooled analysis of three large phase 3 studies. Poster pre- sented at TOXINS 2021 Virtual Conference, January 16-17, 2021. |
[26] | Geister TL , Quintanar-Solares M , Martin M , Aufhammer S , Asmus F , Qualitative development of the ‘Questionnaire on Pain caused by Spasticity (QPS),’ a pediatric patient-reported outcome for spasticity-related pain in cerebral palsy, Qual Life Res (2014) ;23: (3):887–96. doi: 10.1007/s11136-013-0526-2. |
[27] | Geister TL , Bushnell DM , Yang J , Zhang Y , Martin ML , Heilbronn A , et al. Initial psychometric validation of the questionnaire on pain caused by spasticity (QPS), Health Qual Life Outcomes. (2017) ;15: (1):229. doi: 10.1186/s12955-017-0804-8. |
[28] | Heinen F , Bonfert M , Kanovský P , Schroeder AS , Chambers HG , Dabrowski E , et al., Spasticity-related pain in children/adolescents with cerebral palsy, Part prevalence and clinical characteristics from a pooled analysis. J Pediatr Rehabil Med. (2022) ;15: (1):129–43. doi: 10.3233/PRM-220011. |
[29] | Bhardwaj P , Yadav RK Measuring pain in clinical trials: Pain scales, endpoints, and challenges, Int J Clin Exp Physiol (2015) ;2: (3):43151–6. |
[30] | Dworkin RH , Turk DC , Wyrwich KW , Beaton D , Cleeland CS , Farrar JT , et al., Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations, J Pain (2008) ;9: (2):105–21. doi: 10.1016/j.jpain.2007.09.005. |
[31] | Hirschfeld G , Wager J , Schmidt P , Zernikow B , Minimally clinically significant differences for adolescents with chronic pain –Variability of ROC-based cut points, J Pain (2014) ;15: (1):32–9. doi: 10.1016/j.jpain.2013.09.006. |
[32] | Ostojic K , Paget SP , Morrow AM , Management of pain in children and adolescents with cerebral palsy: a systematic review, Dev Med Child Neurol (2019) ;61: (3):315–21. doi: 10.1111/dmcn.14088. |
[33] | McKinnon C , White J , Morgan P , Harvey A , Clancy C , Fahey M , et al., Clinician perspectives of chronic pain management in children and adolescents with cerebral palsy and dyskinesia, Phys Occup Ther Pediatr (2021) ;41: (3):244–58. doi: 10.1080/01942638.2020.1847236. |
[34] | Gooch JL , Sandell TV . Botulinum toxin for spasticity and athetosis in children with cerebral palsy, Arch Phys Med Rehabil (1996) ;77: (5):508–11. doi: 10.1016/s0003-9993(96)90042-8. |
[35] | Rivard PF , Nugent AC , Symons FJ . Parent-proxy ratings of pain before and after botulinum toxin type A treatment for children with spasticity and cerebral palsy, Clin J Pain (2009) ;25: (5):413–7. doi: 10.1097/AJP.0b013e31819a6d07. |
[36] | Lundy CT , Doherty GM , Fairhurst CB . Botulinum toxin type A injections can be an effective treatment for pain in children with hip spasms and cerebral palsy, Dev Med Child Neurol (2009) ;51: (9):705–10. doi: 10.1111/j.1469-8749.2009.03315.x. |
[37] | Misra AK , Kumar S , Biswas A , Das SK , Botulinum toxin type A in subjects with spastic cerebral palsy from Eastern India, J Pediatr Neurol (2010) ;8: (4):349–57. doi: 10.3233/PN-2010-0410. |
[38] | Chaléat-Valayer E , Parratte B , Colin C , Denis A , Oudin S , Bérard C , et al., A French observational study of botulinum toxin use in the management of children with cerebral palsy: BOTULOSCOPE, Eur J Paediatr Neurol (2011) ;15: (5):439–48. doi: 10.1016/j.ejpn.2010.04.006. |
[39] | Jabbari B Chapter 11. Botulinum neurotoxins for relief of pain associated with spasticity. In: Jabbari B. Botulinum toxin treatment of pain disorders. New York: Springer Science++ Business Media; 2015. pp. 153-66. |
[40] | Copeland L , Edwards P , Thorley M , Donaghey S , Gascoigne-Pees L , Kentish M , et al., Botulinum toxin A for nonambulatory children with cerebral palsy: a double blind randomized controlled trial, J Pediatr (2014) ;165: (1):140–146.e4. doi: 10.1016/j.jpeds.2014.01.050. |
[41] | Russo RN , Crotty M , Miller MD , Murchland S , Flett P , Haan E . Upper-limb botulinum toxin A injection and occupational therapy in children with hemiplegic cerebral palsy identified from a population register: a single-blind, randomized, controlled trial, Pediatrics (2007) ;119: (5):e1149–58. doi: 10.1542/peds.2006-2425. |
[42] | Dickinson HO , Parkinson KN , Ravens-Sieberer U , Schirripa G , Thyen U , Arnaud C , et al.. Self-reported quality of life of 8-12-year-old children with cerebral palsy: a cross-sectional European study, Lancet. (2007) ;369: (9580):2171–8. doi: 10.1016/S0140-6736(07)61013-7. |
[43] | Swiggum M , Hamilton ML , Gleeson P , Roddey T . Pain in children with cerebral palsy: implications for pediatric physical therapy, Pediatr Phys Ther (2010) ;22: (1):86–92. doi: 10.1097/PEP.0b013e3181cd18a7. |
[44] | Houx L , Pons C , Saudreau H , Dubois A , Creusat M , Le Moine P , et al. No pain, no gain? Children with cerebral palsy and their experience with physiotherapy, Ann Phys Rehabil Med (2021) ;64: (3):101448.. doi: 10.1016/j.rehab.2020.10.002. |
[45] | Penner M , Xie WY , Binepal N , Switzer L , Fehlings D . Characteristics of pain in children and youth with cerebral palsy, Pediatrics (2013) ;132: (2):e407–13. doi: 10.1542/peds.2013-0224. |
[46] | Ryan JM , Hensey O , McLoughlin B , Lyons A , Gormley J . Associations of sedentary behaviour, physical activity, blood pressure and anthropometric measures with cardiorespiratory fitness in children with cerebral palsy, PLoS One. (2015) ;10: (4):e0123267. doi: 10.1371/journal.pone.0123267. |
[47] | Badia M , Riquelme I , Orgaz B , Acevedo R , Longo E , Montoya P . Pain, motor function and health-related quality of life in children with cerebral palsy as reported by their physiotherapists, BMC Pediatr. (2014) ;14: :192. doi: 10.1186/1471-2431-14-192. |
[48] | Colver A , Rapp M , Eisemann N , Ehlinger V , Thyen U , Dickinson HO , et al.. Self-reported quality of life of adolescents with cerebral palsy: a cross-sectional and longitudinal analysis, Lancet (2015) ;385: (9969):705–16. doi: 10.1016/S0140-6736(14)61229-0. |
[49] | Michelsen SI , Flachs EM , Damsgaard MT , Parkes J , Parkinson K , Rapp M , et al., European study of frequency of participation of adolescents with and without cerebral palsy, Eur J Paediatr Neurol (2014) ;18: (3):282–94. doi: 10.1016/j.ejpn.2013.12.003. |
[50] | De Icco R , Perrotta A , Berra E , Allena M , Alfonsi E , Tamburin S , et al., OnabotulinumtoxinA Reduces Temporal Pain Processing at Spinal Level in Patients with Lower Limb Spasticity, Toxins (Basel) (2019) ;11: (6):359. doi: 10.3390/toxins11060359. |
[51] | Matak I , Bölcskei K , Bach-Rojecky L , Helyes Z , Mechanisms of Botulinum toxin type A action on pain, Toxins (Basel) (2019) ;11: (8):459. doi: 10.3390/toxins11080459. |
[52] | Chambers HG , Kanovsky P , Schroeder AS , Dabrowski E , Geister TL , Dersch H , et al. Absence of neutralizing antibody formation during incobotulinumtoxinA treatment of spasticity in botulinum toxin-naïve children with cerebral palsy: pooled analysis of three phase 3 studies. TOXINS 2021 Virtual Conference, January 16-17, 2021. |




