Spasticity-related pain in children/adolescents with cerebral palsy. Part 1: Prevalence and clinical characteristics from a pooled analysis
Abstract
PURPOSE:
A large prospective database from three Phase 3 studies allowed the study of spasticity-related pain (SRP) in pediatric cerebral palsy (CP).
METHODS:
Baseline (pretreatment) SRP data occurring during different activities in children/adolescents (aged 2–17 years, ambulant/nonambulant) with uni-/bilateral spastic CP was obtained using the Questionnaire on Pain caused by Spasticity (QPS; six modules specific to spasticity level [lower limb (LL) or upper limb (UL)] and type of respondent [child/adolescent, interviewer, or parent/caregiver]).
RESULTS:
At baseline, 331 children/adolescents with LL- and 155 with UL-spasticity completed at least one key item of their modules; LL/UL QPS modules of parent/caregivers were at least partially completed (key items) by 841/444 parents/caregivers. SRP with at least one activity at baseline was self-reported in 81.9% /69.7% (LLs/ULs) of children/adolescents with spasticity. Parents/caregivers observed LL/UL SRP behaviors in 85.9% /77.7% of their children, with multiple body regions affected. SRP negatively affected the great majority of the children in various ways. Child/adolescent-reported mean SRP intensity and parent/caregiver-observed mean SRP behavior frequencies were higher for LLs than ULs, and the level of SRP increased with more physically demanding activities.
CONCLUSION:
These data suggest SRP is more common and intense in pediatric CP than generally thought, emphasizing the need for effective, long-term pain management.
1Introduction
Chronic pain is one of the most common physical complaints of patients with cerebral palsy (CP) but is often underrecognized and undertreated [1, 2]. In children with CP, pain has been attributed to spasticity (hypertonic muscles) [3] as well as medical and surgical interventions, including rehabilitative procedures [1]. In addition to negatively affecting health-related quality of life (QoL) [4], pain often interferes with the already limited physical function, performance, and presentism in school, daily care activities, participation, sleep, and mental health [1, 5, 6] in those with CP. Spasticity-related pain (SRP) can be continuous or recurrent [7] and some patients report that SRP can be more disabling than the spasticity itself [8].
A systematic review of 57 studies (106 publications) of pain in children and adolescents with CP demonstrated prevalence rates of 14% to 76% [9]. Generally, pain prevalence studies in children/adolescents with CP have not specified the source of pain, for example if the pain is spasticity-related [10–13], although Ostojic and colleagues [14] and McKinnon and colleagues [15, 16] have described pain related to muscle tone in children with mixed spasticity/dyskinesic CP. Consequently, little is known about the epidemiology of pain specific to spasticity since most studies focus on general pain or interventional treatment outcomes on spasticity. In the few studies that break down their populations according to the predominant CP motor type, pain prevalence rates for those with CP-associated spasticity range from 33% to 58.2% [6, 14, 17]. These studies differed with respect to patient characteristics, assessment tools, and data collection methods, which likely contributed to the variation in results. Therefore, SRP in children with CP remains poorly understood, frequently unrecognized, and poorly controlled [1, 7, 13, 18–20].
Botulinum neurotoxinA (BoNT-A) is used to relieve pediatric CP-associated spasticity [21–26], which affects approximately 70% to 90% of children with CP [27–29]. BoNT-A has demonstrated efficacy for improving muscle tone and helping patients achieve individualized patient goals [21–23].
Starting in 2013, a large, international Phase 3 study program was initiated to investigate the efficacy and safety of incobotulinumtoxinA for the treatment of pediatric lower limb (LL) and/or upper limb (UL) spasticity associated with CP. IncobotulinumtoxinA is a highly purified formulation of BoNT-A (150 kD) that does not contain BoNT complexing proteins [30]. The study program included TIM (Treatment with IncobotulinumtoxinA in Movement, NCT01893411) [31], TIMO (Treatment with IncobotulinumtoxinA in Movement Open-label, NCT01905683) [32], and XARA (incobotulinumtoXinA in aRm treatment in cerebral pAlsy, NCT02002884) [33]. All three studies enrolled children/adolescents with CP and LL spasticity; in TIMO and XARA, patients could also present with UL spasticity. Results from these studies showed that incobotulinumtoxinA is effective and safe for reducing pediatric LL and UL spasticity over multiple injection cycles spanning a total of 24 to 98 weeks [31–36].
The comprehensive and validated Questionnaire on Pain caused by Spasticity (QPS) [7, 37] was incorporated into all patient visits to allow evaluation of the effects of incobotulinumtoxinA on SRP. The studies used all the established QPS modules for LL and UL (three each for LL and UL, respectively, for a total of six modules), which ask about SRP during different activities: the self-report module for children/adolescents (12 items), the interviewer-administered module for children/adolescents (12 items), and the observer-report module for parents/caregivers on SRP behaviors (13 items). Information gained from parent’s/caregiver’s observations is designed to complement the SRP intensity information gained from the child/adolescent or to provide SRP information when the patient is too young or not capable of self-reporting.
TIM, TIMO, and XARA shared common features in study design and participants, which made the data suitable for pooled analyses. Results are presented within two separate publications. In Part 1, baseline QPS data shed light on the prevalence, intensity, and clinical characteristics of SRP in children/adolescents with CP, as well as the effects of SRP on behavior and modifying factors, from the perspectives of patients and parents/caregivers. In Part 2, the effects of incobotulinumtoxinA on SRP over multiple treatment cycles in children and adolescents with CP are presented.
2Methods
Full details of all three incobotulinumtoxinA studies included in this analysis have already been described [31–33]. The following provides a brief overview only.
2.1Study designs
The aims of TIM, TIMO, and XARA were to investigate the efficacy and safety of incobotulinumtoxinA in children/adolescents with spasticity due to CP. The TIM study was a prospective, multicenter, randomized, double-blind, parallel-group study of children/adolescents with LL spasticity—all of whom had pes equinus—conducted across 45 sites in 14 countries (two injection cycles; N = 311) [31]. The TIMO study was an open-label, non-controlled, multicenter, long-term study in LL or combined LL and UL spasticity conducted across 30 sites in 12 countries (four injection cycles) [32]. The TIMO study population (N = 370) included patients who completed TIM and newly recruited children/adolescents. XARA (N = 351) was a multinational, multicenter, randomized, double-blind, parallel-group study (one injection cycle) in children/adolescents with UL or combined UL and LL spasticity with an open-label long-term follow-up (three injection cycles) [33].
Eligible children/adolescents in all three studies were aged 2 to 17 years and were ambulant or non-ambulant (Gross Motor Function Classification System Expanded and Revised [GMFCS-E&R] level I–V), with uni- or bilateral spasticity associated with CP. To ensure a proper level of spasticity for incobotulinumtoxinA treatment, an Ashworth Scale (AS) score≥2 in prespecified clinical patterns was required. The three studies included patients with a range of LL and/or UL presentations [31–33], as would be expected in clinical practice. Within 14 weeks prior to the screening visit and/or within the screening period, no BoNT-A treatment was allowed for any body region. Within 2 weeks prior to the screening visit, and within the screening period, participants were prohibited from treatment with intrathecal baclofen, oral anticoagulants, drugs acting as peripheral muscle relaxants, casting, serial casting, or functional e-stim of the target joints. Treatments such as physiotherapy, occupational therapy, or any other rehabilitation methods to treat spasticity were allowed throughout the study but not permitted prior to any study assessment. Central muscle relaxants (including benzodiazepines) and antidepressants were allowed if administered at a stable dose for at least 2 weeks prior to the screening visit. Concomitantly administered pain medication in studies was not considered likely to have influenced QPS outcomes; only a small fraction (e.g., 7.1% of subjects in XARA) of patients received analgesics as concomitant medications (including anesthesia/analogsedation offered before injection treatments). The QPS was assessed at each study visit starting with the baseline visit. Since SRP was not an eligibility criterion, each trial included children/adolescents with spasticity due to CP, whether they experienced SRP or not.
2.2Questionnaire on pain caused by spasticity
SRP was assessed using the QPS [7, 37] in children/adolescents with LL (TIM, TIMO, and XARA) and UL spasticity (TIMO and XARA). The six modules of the QPS (Table 1) are specific to the level of spasticity (LL/UL) and respondent (self-reports by children/adolescents/interviewer-reported children/adolescents/parents/caregivers). The QPS includes SRP assessments associated with performing tasks of different levels of difficulty, from rest to self-defined hard task (Table 1). All answers are provided with a 7-day recall period. The QPS was to be completed independently at the beginning of the site visit by patients and their parents/caregivers to reduce bias from any other activities.
Table 1
The QPS modules and items [37]
| The six QPS modules | |||||
| Child/adolescent self-administered LL assessmenta | Child/adolescent self-administered UL assessmenta | ||||
| Child/adolescent interviewer-administered LL assessmenta | Child/adolescent interviewer-administered UL assessmenta | ||||
| Parent/caregiver observational report LL assessment | Parent/caregiver observational report UL assessment | ||||
| Concepts in SRP included in the QPS | |||||
| Child/adolescent modules (UL and LL)a | Parent/caregiver modules (UL and LL)c | ||||
| Targeted symptom concepts | Item number | Response scale | Targeted symptom concepts | Item number | Response scale |
| Spasticity | 1 | Yes/no | Spasticity (observed) | 5 | Yes/no |
| General SRP | 2 | Yes/no | General SRP (verbalization) | 6 | Yes/no |
| General SRP severity | 3 | WBFb | General SRP (observed signs) | 7 | Yes/no |
| SRP at rest | 4 | Yes/no | General SRP observed frequency | 8 | Frequencyd |
| SRP at rest severity | 5 | WBFb | SRP while at rest (observed signs) | 9 | Yes/no |
| SRP during usual activities | 6 | Yes/no | SRP while at rest (observed frequency) | 9b | Frequencyd |
| SRP during usual activities severity | 7 | WBFb | SRP during usual activities (observed signs) | 10 | Yes/no |
| SRP during exercises / active mobilization | 8 | Yes/no | SRP during usual activities (observed frequency) | 10b | Frequencyd |
| SRP during exercises / active mobilization severity | 9 | WBFb | SRP during exercises / active mobilization (observed signs) | 11 | Yes/no |
| SRP during difficult activity | 11 | Yes/no | SRP during exercises / active mobilization (observed frequency) | 11b | Frequencyd |
| SRP during difficult activity severity | 12 | WBFb | SRP during difficult activity (observed signs) | 13 | Yes/no |
| SRP during difficult activity (observed frequency) | 13b | Frequencyd | |||
aChild/adolescent self-administered and child/adolescent interviewer-administered modules are often combined if the child or adolescent cannot provide independent responses. bWong-Baker FACES scale, includes six faces ranging from smiling to crying with scores of no hurt (0), hurts little bit (2), hurts little more (4), hurts even more (6), hurts whole lot (8), and hurts worst (10). cFor parents/caregivers, items 1–4 described the observed SRP status of their children/adolescents and the role of the parent/caregiver. Item 1 = presence of SRP in specific body locations. Item 2 = number of hours parents/caregivers were in direct contact with child. Item 3 = relationship of QPS respondent to child/adolescent. Item 4 = observed changes in behaviors. dThe frequency scale includes five points with scores of never (0), rarely (1), sometimes (2), often (3), and always (4). QPS = Questionnaire on Pain caused by Spasicity; SRP = spasticity-related pain; WBF = Wong-Baker FACES scale.
2.2.1QPS child/adolescent self-reported and child/adolescent interviewer-reported modules
Self-reported and interviewer-reported modules for LL and UL share the same content and structure for the respective body region, but the latter module includes additional specific interviewer instructions. The child/adolescent self-completed module was completed by those patients with sufficient cognitive, communicative and motor abilities (usually older children with no/slight impairments); otherwise, the interviewer-completed module was used for younger children. If children were too young or were unable—as a result of CP-associated impairment—to respond, no QPS information was collected from them.
Children/adolescents reported the presence and intensity of SRP in general and with respect to different everyday activities through specific questions for both LL and UL SRP. For all activities, the respondents were first asked in the QPS if SRP is present (yes/no) and then intensity was rated by children/adolescents from 0 (no hurt) to 10 (worst hurt) on the Wong-Baker FACES scale. The key items for QPS analysis are: General SRP (Item 2), SRP at rest (Item 4), SRP with usual activities (Item 6), SRP with exercises (Item 8), and SRP with a self-defined hard task (Item 11) (Table 1) [7]. For an example of child/adolescent items, please refer to Fig. 1A.
Fig. 1
Item examples of the Questionnaire on Pain caused by Spasticity (QPS). (A) Items 6 and 7 of the upper limb children/adolescents module and (B) Item 10 of the upper limb parent/caregiver module. Reproduced with permission of Merz Therapeutics GmbH.

2.2.2Parent/caregiver observational module
The LL and UL modules share the same content and structure but always refer to the respective body region. First, the parent/caregiver was asked to report whether SRP was observed to be present in specific locations of their child’s LL or UL (Item 1). The next items queried how many hours of direct contact per day they spend with the child (Item 2) and who completed the QPS (e.g., parent or caregiver, Item 3). Observed changes in behavior of the child were then documented (e.g., changes in activity level, mood, sleep, or sound/verbal expressions; Item 4). These first items (1–4) thus describe observed SRP status of the children/adolescents and the role of the parent/caregiver. The parent/caregiver then reported on the observed frequency of the previously identified behaviors associated with SRP in their child during different activities, beginning with whether such behaviors were present with the activity (yes/no) and then how often they were observed based on a 5-point scale from 0 (never) to 4 (always). The key items for QPS analysis are: General SRP (Item 8), SRP at rest (Item 9b), SRP with usual activities (Item 10b), SRP with exercises (Item 11b), and SRP with a self-defined hard task (Item 13b) (Table 1) [7]. For an example of parent/caregiver items, please refer to Fig. 1B.
2.3Analysis populations
QPS data were collected for LL SRP only in TIM, whereas in TIMO and XARA, the LL and/or UL module data were collected when the patient presented with, and received treatment for, LL or UL spasticity, respectively. Patients with both LL and UL spasticity could therefore be included in each of the LL analyses and the UL analyses. QPS self-report and interviewer-report data from children/adolescents were pooled. Generally, all TIM, TIMO, and XARA study data that were received from the child/adolescent and parent/caregiver QPS LL and UL modules were utilized for analysis. Baseline data were pooled across these studies according to QPS LL and UL children/adolescent and parent/caregiver modules. For this publication on SRP prevalence, the QPS baseline data (prior to incobotulinumtoxinA treatment) of the first injection cycle were utilized.
A child/adolescent was considered to have SRP if any QPS key item score was reported > 0 at baseline. Similarly, a child/adolescent was defined as presenting with SRP if parents/caregivers observed any key item score > 0 at baseline.
The enrolled population included all children/adolescents with LL/UL CP-related spasticity who were enrolled in the TIM, TIMO (new recruits), and XARA studies and were later treated with incobotulinumtoxinA. QPS completers were those children/adolescents with CP-related spasticity who provided baseline data for at least one key item of the QPS.
2.4Statistical analyses
The QPS modules were analyzed separately (i.e., the LL and UL modules of the child/adolescent and parent/caregiver questionnaires). The QPS items of interest (see above) were analyzed using frequency tables and descriptive summary statistics.
Logistic regression analyses were performed to determine baseline child/adolescent demographics and characteristics that influenced baseline SRP in children with LL spasticity who were treated in the first injection cycle of TIM, TIMO, or XARA, or children with UL spasticity who were treated in the first injection cycle of TIMO or XARA.
Factors considered in the analyses for children/adolescents with SRP in at least one item in the QPS at baseline (LL and UL) were: age, GMFCS-E&R level, physiotherapy or rehabilitation therapy at baseline (yes/no), sex, AS score at baseline, parent/caregiver QPS Item 4 (SRP behavior; yes/no) and parent/caregiver QPS Item 8 (general SRP observed frequency; yes/no). Factors considered in the parent/caregiver assessment for their child with SRP in at least one item in the QPS at baseline (LL) were: age, GMFCS-E&R level, physiotherapy or rehabilitation therapy at baseline (yes/no), sex, AS score at baseline, parent/caregiver QPS Item 2 (hours of direct contact per day) and parent/caregiver QPS Item 3 (questionnaire respondent) at baseline. Factors considered for parent/caregiver QPS (UL) were the same as those listed for LL with the addition of the parent/caregiver QPS Item 3 (questionnaire respondent).
3Results
3.1Demographics
A total of 849 children/adolescents with LL spasticity and 454 with UL spasticity were newly enrolled in the TIM, TIMO, and XARA studies and treated with incobotulinumtoxinA in these studies. Of those, 340 and 160 children/adolescents with LL and UL spasticity, respectively, completed any part of the QPS at any time of the study and these four populations were used for reporting baseline demographic and characteristic data. The child/adolescent QPS completer populations (i.e., those who provided data for at least one key QPS item at baseline) consisted of 324 to 331 children/adolescents with LL spasticity and 148 to 155 children/adolescents with UL spasticity, depending on the number of responses per item. For parent/caregivers, up to 841 and 444 provided key QPS item data for the LL and UL modules, respectively.
Generally, children/adolescents participating in the studies were young (but covered the complete age spectrum: 2 to 17 years), represented the full range of possible disabilities, and were affected by CP unilaterally as well as bilaterally in ULs and LLs (Table 2, but see also [31–33] for further details). Approximately 60% of the patients were male. Children/adolescents who were able to complete the QPS by themselves or through interview were about 3 years older and, thus, had greater body weight when compared to the full populations (Table 2). About 10% of the QPS completers had poor ambulation status (GMFCS-E&R Levels IV and V) versus almost 27% of the full populations, for both those with LL and UL spasticity.
Table 2
Demographics and baseline characteristics for children/adolescents enrolled in and providing any QPS data from the pooled TIM, TIMO, and XARA studies
| Characteristic | Enrolled patientsa | QPS respondentsb | ||
| LL treated | UL treated | LL QPS | UL QPS | |
| N = 849 | N = 454 | N = 340 | N = 160 | |
| Male sex, n (%) | 507 (59.7) | 287 (63.2) | 208 (61.2) | 99 (61.9) |
| Age, years; mean (SD) | 6.5 (4.2) | 7.1 (4.4) | 9.3 (3.8) | 10.3 (3.7) |
| Weight, kg; mean (SD) | 22.9 (13.4) | 24.4 (14.7) | 32.6 (14.8) | 36.8 (16.5) |
| GMFCS-E&R IV–V, n (%) | 226 (26.6) | 122 (26.9) | 33 (9.7) | 16 (10.0) |
| Body side affected by cerebral palsy, n (%) | ||||
| LL unilateral | 304 (35.8) | 193 (42.5) | 157 (46.2) | 97 (70.3) |
| LL bilateral | 545 (64.2) | 203 (44.7) | 183 (53.8) | 41 (29.7) |
| UL unilateral | 365 (43.0) | 402 (88.5) | 128 (92.8) | 144 (90) |
| UL bilateral | 31 (3.7) | 52 (11.5) | 10 (7.2) | 16 (10.0) |
| Duration since first diagnosis of | (N = 848) | (N = 453) | (N = 292) | (N = 147) |
| spasticity, months; mean (SD) | 69.1 (49.9) | 76.9 (52.3) | 95.4 (47.8) | 109.7 (46.3) |
aChildren/adolescents enrolled in one of the three studies (new recruits only for TIMO) and then treated for at least LL or UL spasticity. bChildren/adolescents treated for at least LL or UL spasticity in the first injection cycle and who provided QPS data at any time during the study. Includes children/adolescents who completed the QPS via an interviewer as well as by self-reports. GMFCS-E&R = Gross Motor Function Classification System–expanded & revised; LL = lower limb; QPS = Questionnaire on Pain caused by Spasticity; SD = standard deviation; TIM = Treatment with IncobotulinumtoxinA in Movement; TIMO = Treatment with IncobotulinumtoxinA in Movement Open-label; UL = upper limb; XARA = incobotulinumtoXinA in aRm treatment in cerebral palsy.
Usually, the child’s mother completed the QPS parent/caregiver module (89.5% of those with LL spasticity and 90.6% of those with UL spasticity) while the child’s father was responsible for just a small proportion of reports (7.5% and 7.9% of those with LL and UL spasticity, respectively). Parents/caregivers who completed the QPS spent a mean of about 15 hours each day with their children, regardless of the level of spasticity (LL: 15.4 [standard deviation 7.5] hours/day; N = 839 vs. UL: 15.6 [7.3] hours/day; N = 387).
3.2SRP prevalence
At baseline, parents reported that they had observed SRP in many body regions of their children’s lower and upper extremities in the last week (Fig. 2). For children with LL spasticity, the most common locations of parent/caregiver-observed SRP were foot, lower leg, and knee. For children with UL spasticity, parents most commonly observed SRP in their child’s elbow, hand, and forearm. Parents/caregivers confirmed that they recognized SRP in LLs and ULs based on many altered behaviors of their child, such as activity level, posture, mood, facial expression, eating, sleeping, and interaction with others (Table 3). Of note, for half of the behaviors questioned, a change due to SRP was confirmed by 40% of parents/caregivers.
Fig. 2
Location of SRP as observed by parents/caregivers (QPS Item 1 pain areas). An additional 1.0% and 1.4% of parents/caregivers observed pain in another LL or UL site, respectively, and 29.4% and 31.1% observed no LL or UL pain, respectively.
LL = lower limb; QPS = Questionnaire on Pain caused by Spasticity; UL = upper limb.
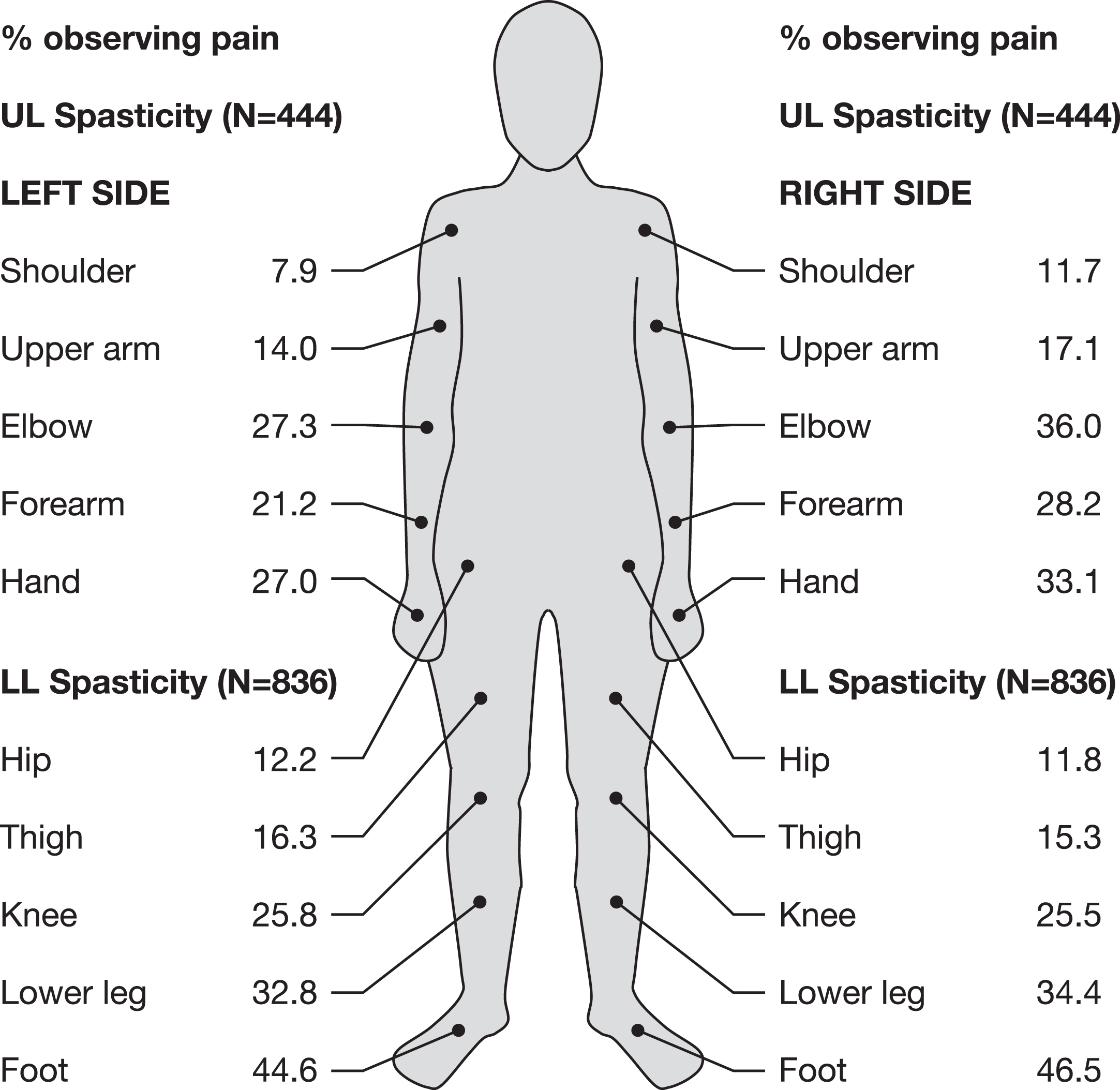
Table 3
Frequency of observed SRP behaviors as rated by parents/caregivers at baseline (QPS item 4)
| Observed SRP behavior | LL | UL |
| (N = 744) | (N = 379) | |
| I have seen that my child’s: n (%) | ||
| Activity level changes | 334 (44.9) | 161 (42.5) |
| Interaction with others changes | 162 (21.8) | 78 (20.6) |
| Body changes | 215 (28.9) | 105 (27.7) |
| Posture changes | 341 (45.8) | 171 (45.1) |
| Eating pattern changes | 99 (13.3) | 50 (13.2) |
| Facial expression changes | 330 (44.4) | 185 (48.8) |
| Mood changes | 403 (54.2) | 232 (61.2) |
| Sleep pattern changes | 219 (29.4) | 120 (31.7) |
| Makes sounds | 324 (43.5) | 159 (42.0) |
| Touches/points to areas of pain | 331 (44.5) | 147 (38.8) |
LL = lower limb; QPS = Questionnaire on Pain caused by Spasticity; SRP = spasticity-related pain; UL = upper limb.
Of the children/adolescents who completed the QPS, a large proportion reported SRP to be an issue. For patients with LL spasticity, 81.9% reported the presence of SRP with at least one activity at baseline, as did 69.7% of those with UL spasticity (Table 4). The percentages of children/adolescents with SRP as reported by parents/caregivers (including observations of younger children or those unable to self-report) were slightly higher, with parents/caregivers observing SRP in at least one QPS activity in 85.9% of children with LL spasticity and 77.7% with UL spasticity (Table 4).
Table 4
Prevalence of SRP in children and adolescents with CP by individual QPS item at baseline, pooled TIM, TIMO, and XARA populations
| Reported by: | Children/Adolescents: | Parents/Caregivers | ||
| LL SRP | UL SRP | LL SRP | UL SRP | |
| SRP reported by children/adolescents at baseline, by QPS itema,b, n (%) [N] | SRP behavior observed by parents/caregivers at baseline, by QPS itemb,c, n (%) [N] | |||
| SRP in at least one activity | 271 (81.9) [331] | 108 (69.7%) [155] | 722 (85.9) [841] | 345 (77.7) [444] |
| General SRP | 178 (53.9) [330] | 69 (45.4) [152] | 568 (67.7) [839] | 294 (66.5) [442] |
| SRP at rest | 86 (26.5) [324] | 34 (23.0) [148] | 321 (39.2) [818] | 160 (37.7) [424] |
| SRP with usual activities | 178 (54.3) [328] | 52 (34.7) [150] | 485 (59.4) [816] | 248 (58.4) [425] |
| SRP with exercises | 234 (70.9) [330] | 99 (64.7) [153] | 674 (81.3) [829] | 317 (72.9) [435] |
| SRP with hard task | 202 (61.2) [330] | 83 (53.5) [155] | 594 (71.5) [831] | 294 (67.4) [436] |
| Meana,d (SD) SRP intensity reported by children/adolescents, by QPS item | Meanc,d (SD) SRP behavior frequency observed by parents/caregivers, by QPS item | |||
| General SRP | 4.3 (2.15) [178] | 3.6 (1.59) [69] | 2.3 (0.84) [568] | 2.3 (0.85) [294] |
| SRP at rest | 3.7 (1.90) [86] | 3.5 (1.66) [34] | 1.9 (0.84) [321] | 1.9 (0.86) [160] |
| SRP with usual activities | 4.0 (1.95) [178] | 3.6 (1.65) [52] | 2.2 (0.84) [485] | 2.2 (0.86) [248] |
| SRP with exercises | 4.5 (2.34) [234] | 4.0 (1.96) [99] | 2.6 (0.98) [674] | 2.6 (0.96) [317] |
| SRP with hard task | 4.6 (2.43) [202] | 3.9 (2.06) [83] | 2.7 (0.99) [594] | 2.5 (1.02) [294] |
aChildren/adolescents reported the intensity of SRP using the 10-point graphic Wong-Baker FACES scale, includes six faces ranging from smiling to crying with scores of no hurt (0), hurts little bit (2), hurts little more (4), hurts even more (6), hurts whole lot (8), and hurts worst (10). bA baseline score > 0 for the item defined the presence of SRP. cThe frequency of SRP signs and symptoms observed by parents/caregivers was rated on a 5-point scale from never (0), sometimes (2), often (3), to always (4). dMean scores were calculated using SRP data with a value > 0. GMFCS = Gross Motor Function Classification System; LL = lower limb; N = total children/adolescents assessed for a given characteristic; QPS = Questionnaire on Pain caused by Spasticity; SD = standard deviation; SRP = spasticity-related pain; TIM = Treatment with IncobotulinumtoxinA in Movement; TIMO = Treatment with IncobotulinumtoxinA in Movement Open-label; UL = upper limb; XARA = incobotulinumtoXinA in aRm treatment in cerebral palsy.
Considering each of the activities in the QPS separately, SRP was reported less frequently for activities such as “at rest” but more often with higher demanding activities (Table 4). For example, general SRP was reported to be present by 53.9% of children/adolescents with LL spasticity and 45.4% of children/adolescents with UL spasticity. Notably, similar results were found for usual activities as for general SRP reports. However, for activities at rest, only 26.5% of children/adolescents with LL and 23.0% of children/adolescents with UL spasticity, respectively, reported SRP. These percentages are much lower than those seen for SRP with exercises, during which 70.9% and 64.7% of children with LL and UL spasticity, respectively, reported SRP. The parent/caregiver results are similar to this described pattern with somewhat higher percentages regarding each observed activity compared to the self-reports of the children/adolescents (Table 4).
Mean SRP intensity scores reported by children/adolescents indicate that higher scores are associated in a stepwise fashion with each more demanding task, from at-rest activities up to self-defined hard tasks (Table 4). The same pattern is seen with the parent/caregiver reports. For both child/adolescent and parent/caregiver QPS information, the mean scores usually lay in the middle of the response options. Thus, these scores generally represented “hurt a little more” to “hurt even more” for child-/adolescent-experienced SRP intensity and “sometimes” to “often” for parent-/caregiver-observed SRP behaviors. Nevertheless, it should be noted that 19.3% of the total LL spasticity population reported a score of “8 –Hurt a whole lot” or “10 –Hurt worst” in at least one of the five QPS items. For those with UL spasticity, 7.1% reported such high SRP intensity in at least one item.
3.2.1Factors affecting the presence of SRP
Logistic regression analyses were performed to determine whether demographic or other factors were associated with baseline SRP. Using data from the child/adolescent QPS, results indicate significant associations between baseline SRP and level of disability (GMFCS-E&R score) for children/adolescents with LL spasticity who had SRP in at least one QPS item (p = 0.0199) with an odds ratio (OR) of 1.6 and a confidence interval for the OR (CIOR) of [1.1; 2.4], meaning that children/adolescents with higher GMFCS-E&R scores had a 1.6-fold higher chance of having baseline SRP than those with lower scores. For age or sex, or for physiotherapy/rehabilitation therapy or degree of spasticity (AS at baseline) the respective CIOR contained the value of “1,” meaning that no association was found for these factors. For children/adolescents with UL spasticity, none of the above factors had an association with SRP that was statistically significant. The CIOR of these factors all contained the value of “1.”
Data from the parent/caregiver QPS modules also showed that that GMFCS-E&R levels were associated with baseline SRP for patients with both LL (p = 0.0053) and UL spasticity (p = 0.0497). The ORs for GMFCS-E&R were 1.5 (CIOR = [1.1; 1.9]) and 1.8 (CIOR = [1.0; 3.1]) for LL and UL, respectively; that is, children/adolescents with higher GMFCS-E&R scores had a 1.5- and 1.8-fold higher chance of having baseline SRP than those with lower scores.
Data from these modules additionally indicate that the number of hours per day parents/caregivers were in direct contact with the child/adolescent with LL spasticity was strongly associated with baseline SRP (QPS Item 2, p = 0.0006, OR = 1.1, CIOR = [1.0; 1.1]), whereas physiotherapy/rehabilitation therapy was associated with baseline SRP in children with UL spasticity (p = 0.0207, OR = 3.5, CIOR = [1.2; 10.2]); that is, children/adolescents with physiotherapy/rehabilitation therapy at baseline had a 3.6-fold higher chance of baseline SRP than those without such therapies.
Several analyses confirm consistency between self-reports of patients and observations made by parents/caregivers in the reporting of SRP. These include significant associations in the child/adolescent LL QPS module between baseline SRP and observed SRP behavior (parent/caregiver LL QPS Item 4; p = 0.0480, OR = 0.4, CIOR = [0.2; 1.0]) and in both the child/adolescent LL and UL QPS modules between baseline SRP and observed general SRP (parent/caregiver QPS Item 8; p < 0.0001 for both; OR = 0.2 with CIOR = [0.1; 0.4] and OR = 0.1 with CIOR = [0.0; 0.2] for children/adolescents LL and UL QPS modules, respectively). For these factors the chances of having baseline SRP were lower with the presence of these QPS items compared to not being present. In the parent/caregiver UL QPS module, the effect of general SRP severity (child/adolescent UL QPS Item 3) was also significant (p = 0.0001, OR = 8.1, CIOR = [2.8; 23.6]); that is, subjects with higher general SRP have an 8.1-fold higher chance of baseline SRP than those with lower general SRP.
4Discussion
This pooled analysis of three Phase 3 studies provides the largest prospective database of SRP in children/adolescents available to date and, as such, allows valuable insights into this challenging but common health issue for those with CP-associated spasticity. The database covers a broad representation of age groups (2 to 17 years), all levels of CP disease severity (GMFCS-E&R levels I–V), and topographic SRP distributions (see also [31–33]). In addition, the proportions of children/adolescents at each GMFCS-E&R level were generally representative of those described in epidemiological real-world studies [38–40]. The findings are also strengthened by the use of a validated instrument designed specifically to assess SRP in children and adolescents with CP-associated spasticity, including those who, because of age or disability, have communication difficulties. As expected, children/adolescents were generally only able to complete the QPS by themselves or by interview if they were older (as shown by their higher mean age) and had fewer disabilities (based on the lower proportion in GMFCS level IV–V), than the total pooled population. Therefore, the parent/caregiver QPS results are more complete as they additionally include observer information on the youngest and more disabled patients. Our data show that, based on the amount of time spent with their children, parents/caregivers could be expected to be very well informed about their children’s daily SRP behavior.
Analysis of the QPS data shows that, at baseline, more than 80% of children/adolescents with LL spasticity and nearly 70% of those with UL spasticity reported SRP in at least one QPS activity. Our findings also show a good correlation between self-reported SRP and observed frequencies of these SRP behaviors, although the percentages reported by parents/caregivers were higher, with almost 86% observing SRP in at least one QPS activity at baseline in children/adolescents with LL spasticity and almost 78% observing SRP in children/adolescents with UL spasticity. Specifically, the reports by parents/caregivers representing the complete patient population indicate that SRP in children and adolescents with CP-associated spasticity is nearly universal.
The data also reveal the everyday circumstances where SRP becomes most relevant. Generally, the level of SRP was in the mid-range of the response scales including that of patients experiencing the most severe SRP. When considering the general location of pain, SRP was reported more often in those with LL versus UL spasticity, which probably relates to the importance of mobility in daily lives. Children/adolescents also reported that SRP intensity was generally higher in LLs than ULs and increased with more physically demanding activities. These findings highlight the need for sufficient pain-relieving measures that can allow children/adolescents with SRP to participate in exercise and other activities that otherwise would cause pain. Most previously published studies reported a lower prevalence of baseline pain in children and adolescents with CP [6, 12, 14, 17] than that reported in the current study. In a systematic review by McKinnon and colleagues [9], eight studies reported on pain prevalence, with rates ranging from 14% to 76%; the studies used carer, clinician, or a combination of self-reports and carer reports. Although the systematic review concluded that CP motor type was not a predictor of pain prevalence, the conclusion was based on only two relevant studies. The variability in reported pain prevalence among studies has been attributed to factors such as sampling bias, inconsistent outcome measurement, varying recall periods (0 to 4 weeks), different age ranges of participants [9], or the use of non-comprehensive or non-standardized questions adapted from health-related tools not designed to detect pain prevalence. When only studies specific to children/adolescents with CP-associated spasticity are considered, SRP prevalence rates still vary considerably, with reported rates ranging from 33% to 79% [6, 14, 15, 17]. The highest rates are reported in children/adolescents with bilateral versus unilateral spasticity [6, 17] and spasticity versus mixed spasticity/dyskinesia [14].
The findings of the current report suggest CP-associated pediatric SRP may be more prevalent than generally thought. The higher prevalence of SRP found by the current study compared to reports from other investigators may reflect the QPS’s format of multi-question modules specific to the child’s spasticity level and the type of respondent. Gathering data from multiple perspectives allows access to information from children/adolescents with CP who, because of young age or disability, might have been excluded from other investigations that only rely on self-reports. We believe the higher pain prevalence demonstrated in the current study may also reflect the close association between spasticity and pain [41], since spasticity was an inclusion criterion for the clinical trials. This would not be surprising since limited range of motion and misloading of joints, together with muscle tightness/stiffness, can be painful.
The current results also add insight into factors that influence SRP, including disease severity (GMFCS-E&R level) in most modules and, for the parent/caregiver UL (but not LL) module, physiotherapy or rehabilitation therapy. The association of SRP with the number of hours parents/caregivers spent with their child might be linked to having more time available for parents/caregivers to detect and observe SRP behaviors. Factors found not to have a significant impact here included age, gender, and baseline AS score. The AS/modified AS score, which is derived from the short passive movement of the clinical pattern, may not be a good predictor for patients’ weekly experienced tightness and associated pain, as other studies have also found that AS and pain levels do not necessarily closely correlate [7, 42]. Thus, while being well suited to assess general muscular spasticity and respective treatment interventions, it may be less informative for more complex topics such as function and SRP. Therefore, further research is warranted to better understand these relationships and dependencies.
When compared with the findings of other investigators, our results agree with reports that children/adolescents with CP and higher GMFCS-E&R levels experience pain more frequently [9–11, 14, 43] and more intensely [11] than those at lower GMFCS-E&R levels; that SRP is most commonly located in the lower extremities [9–11, 43]; and that pain often occurs in multiple body locations [15]. However, the current findings do not indicate that pain in children/adolescents with CP is more prevalent in girls than boys [9–11, 43] or that pain worsens with age [9–11, 13, 43–45], as reported by others.
In common with other reports [43, 46], the current results showed good concordance between SRP frequency reported by children/adolescents and observed by parents/caregivers, although parents/caregivers tended to more frequently report SRP in their children. Although reasons for the slightly higher reported rate are unclear, it should be remembered that the parent/caregiver reports include data from younger children and those with greater disability, and that the parents/caregivers could either overestimate pain [9] or more accurately report SRP because bias and pain tolerance are not issues [43]. Other studies have found that parents tended to underestimate [2] or be in concordance with [43, 46] patient self-reports. When establishing the QPS, a certain level of SRP denial from children was observed [37], probably because they wanted to please their parents or doctors. Thus, several factors may play a role in the variation between the pain reported by children/adolescents and that observed by parents/caregivers. While self-reports are considered the gold standard, we agree with others [44, 47] that proxy reports can be especially helpful for children with poor communication skills or disabilities and can provide important information and insights that would otherwise not be accessible. The overall consistency and patterns of our data underscore that observer reports are an important and valid addition for this topic.
Results of the current analyses also illustrate some of the many adverse consequences of SRP in children/adolescents with CP. Parents reported SRP-affected behavior, with regard to mood, posture, activity levels, sleep, and social interactions, in many patients. Other studies have also described negative effects of pain in children and adolescents with CP, including disrupted sleep [11, 14, 43, 48], disturbed daily activities [11, 43], missed school days, less participation [12, 49, 50], reduced ambulation [12, 49, 50], poor QoL (as reported by caregivers but not by patients) [16], and reduced enjoyment [14]. Consequently, Michelsen and colleagues [49] suggested that experiencing pain is the greatest contributor to reduced QoL in children with CP, as some of the earlier studies have suggested [4].
The adverse effects and the high prevalence of SRP in children/adolescents with CP emphasize the need for early, effective, and safe long-term pain management strategies for daily life [51], and for support during demanding therapy regimens, even for the very young. Since pain in children with CP has multiple and complex etiologies (including hypertonia and muscle spasms, hip subluxation, surgical and other management procedures, constipation, and gastroesophageal reflux [3]), optimal pain management should encompass treatments that are begun early in the disease process and that target specific causes [2]. In a systematic review, Ostojic and colleagues [3] highlighted the paucity of high-quality research investigating interventions to manage pain in children and adolescents with CP and concluded that for children with SRP, few good treatment options are available. These authors suggested that current available evidence is limited by a lack of standardization in methods of pain assessment and by weak study designs. Similarly, McKinnon and colleagues [15] highlighted the lack of coordinated, evidence-based, and multidisciplinary pain management for young people with CP. Although clinical guidelines on CP from the National Institute for Health and Care Excellence (NICE) in the United Kingdom encourage physicians to ask about pain at each clinical interaction [52] it is not clear how widespread this practice is. In fact, a significant disconnect was recognized between pain identified by screening versus pain recognition and management by treating clinicians in a Swedish registry study of 185 children with CP reporting pain [17, 51]. A few management strategies have been described for CP-associated pain [3, 16, 20, 47, 53, 54], and a growing body of evidence indicates that BoNT-A can offer targeted pain relief for children [55–61] with CP-associated SRP. In addition, limited data suggest some pain-relief benefit from BoNT-A in adults with CP-associated SRP, albeit exploratory [61, 62]. In a companion publication, we present pooled data from TIM, TIMO, and XARA regarding the therapeutic effects of incobotulinumtoxinA on SRP in children and adolescents with CP [58].
4.1Strengths and limitations
The strength of this study, which combined data from three Phase 3 clinical trials, is the inclusion of a large, heterogeneous population of children and adolescents with CP-associated SRP. While larger registry studies exist of children with CP, they tend to focus on QoL rather than pain (e.g., in SPARCLE, pain was broadly assessed with two questions from the Child Health Questionnaire [4]). Our study is unique in that SRP was investigated in detail in a sizable number of children/adolescents and more than 800 parents/caregivers of affected children using an instrument specifically designed to assess SRP in this population. Participants presented with a range of clinical patterns requiring treatment for spasticity and represented a broad age range and all levels of CP-associated disability. The three studies have generated a comprehensive dataset that includes data on the presence of SRP in children/adolescents with CP, together with an assessment of spasticity and other functional outcomes; because the studies did not focus on SRP and we found consistent patterns in our findings, we believe the results obtained were unlikely to be affected by bias.
An additional strength of this analysis was the use of the disease- and condition-specific QPS, which was designed to include input from both children/adolescents (even those with limited communicative abilities) and parents/caregivers [7, 37]. The QPS provides a comprehensive, reliable, and valid measure of SRP, which is relevant to a child’s spasticity-associated experience and is easy to understand. It has been tested in a population sample that was regarded as being representative and suitable for SRP assessment in children with LL and UL SRP. The incorporation of responses from children/adolescents (through self-reports and interviews) and parents/caregivers is important for providing a better understanding of pain and its everyday effects on children with CP [5].
All three studies required participants to fulfill the inclusion and exclusion criteria for enrollment, and therefore the current analysis population may differ in some respects from the general real-world CP population. This might have limited some of the analyses; for example, including an even broader population may have revealed more details on predictors shaping SRP presence and intensity. Another limitation is that the SRP data were pooled from three studies with similar but not identical study designs: TIM enrolled only children/adolescents with LL spasticity, while TIMO and XARA included children/adolescents with both LL and UL spasticity.
5Conclusion
This pooled analysis of QPS data obtained from both children/adolescents and parents/caregivers indicates that most children/adolescents with CP and LL or UL spasticity have SRP during usual activities, and both the intensity and frequency of SRP increase with more demanding levels of activity. The data also suggest that SRP is more intense and ubiquitous in pediatric CP than is generally thought across all ages and disability levels, which negatively impacts mood, sleep, social interactions, and other activities of daily living. This high rate of SRP, along with its negative consequences, suggests that pain assessment should begin early in life for those with CP and emphasizes the need for effective and long-term pain monitoring, prevention, and treatment for children and adolescents with CP. In addition to improving motor activity, it may be time to consider pain relief as a distinct treatment goal for those living with CP.
Acknowledgments
The authors wish to thank all study subjects and investigators. They would like to acknowledge Hanna Dersch and Dieter Matthias for statistical support and Amy Rothman Schonfeld and Caroline Spencer (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Merz Therapeutics GmbH. This study was supported by Merz Therapeutics GmbH, Frankfurt am Main, Germany.
Conflict of interest
Florian Heinen has received speaker’s honoraria from Allergan plc, Desitin, Ipsen Biopharmaceuticals, Merz Therapeutics, and Novartis, and unrestricted educational grants from Allergan and Merz Therapeutics. Michaela Bonfert has received research grants from the HABA Foundation, the Deutsche Rentenversicherung, the Deutsche Migräne und Kopfschmerzgesellschaft, the European Research Council, ERA-NET Neuron, CSL Behring, ZNS - Hannelore Kohl Foundation, and a research scholarship of the Bavarian Gender Equality Grant of the Free State of Bavaria, Germany. Petr Kaňovský has received speaker’s honoraria from Desitin, Ipsen Biopharmaceuticals, Merz Therapeutics, and Medtronic. A. Sebastian Schroeder has received speaker’s honoraria from and participated in advisory boards for Allergan plc, Ipsen Biopharmaceuticals, and Merz Therapeutics. Henry G. Chambers serves as a consultant for Orthopediatrics Corp and Allergan Corporation. Edward Dabrowski has participated in an advisory board and speaker bureau for Ipsen Biopharmaceuticals. Thorin L. Geister is an employee of Merz Therapeutics GmbH. Angelika Hanschmann is an employee of Merz Therapeutics GmbH. Michael Althaus is an employee of Merz Therapeutics GmbH. Marta Banach has served as a consultant and speaker and participated in an advisory board for Merz Therapeutics and has served as a speaker for Allergan, Ipsen, and Kedrion. Deborah Gaebler-Spira has served as a consultant for Teva and Kashiva.
Ethical considerations
The study protocol, informed consent forms, and other appropriate study-related documents were reviewed and approved by the local independent ethics committees and institutional review boards. Parents/guardians of all patients provided written informed consent, and patients provided assent if applicable.
References
[1] | Blackman JA , Svensson CI , Marchand S . Pathophysiology of chronic pain in cerebral palsy: implications for pharmacological treatment and research. Dev Med Child Neurol. (2018) ;60: (9):861–5. doi: 10.1111/dmcn.13930 |
[2] | Penner M , Xie WY , Binepal N , Switzer L , Fehlings D . Characteristics of pain in children and youth with cerebral palsy. Pediatrics. (2013) ;132: (2):e407–13. doi: 10.1542/peds.2013-0224 |
[3] | Ostojic K , Paget SP , Morrow AM . Management of pain in children and adolescents with cerebral palsy: a systematic review. Dev Med Child Neurol. (2019) ;61: (3):315–21. doi: 10.1111/dmcn.14088 |
[4] | Dickinson HO , Parkinson KN , Ravens-Sieberer U , Schirripa G , Thyen U , Arnaud C , et al. Self-reported quality of life of 8- to 12-year-old children with cerebral palsy: a cross-sectional European study. Lancet. (2007) ;369: (9580):2171–8. doi: 10.1016/S0140-6736(07)61013-7 |
[5] | Badia M , Riquelme I , Orgaz B , Acevedo R , Longo E , Montoya P . Pain, motor function and health-related quality of life in children with cerebral palsy as reported by their physiotherapists. BMC Pediatr. (2014) ;14: :192. doi: 10.1186/1471-2431-14-192 |
[6] | Parkinson KN , Dickinson HO , Arnaud C , Lyons A , Colver A ; SPARCLE group. Pain in young people aged 13 to 17 years with cerebral palsy: cross-sectional, multicentre European study. Arch Dis Child. (2013) ;98: (6):434–40. doi: 10.1136/archdischild-2012-303482 |
[7] | Geister TL , Bushnell DM , Yang J , Zhang Y , Martin ML , Heilbronn A , et al. Initial psychometric validation of the questionnaire on pain caused by spasticity (QPS). Health Qual Life Outcomes. (2017) ;15: (1):229. doi: 10.1186/s12955-017-0804-8 |
[8] | Jabbari B . Botulinum neurotoxins for relief of pain associated with spasticity. In: Botulinum toxin treatment of pain disorders. New York, NY: Springer; (2015) . pp. 153–66. ISBN: 978-1-4939-2501-8 |
[9] | McKinnon CT , Meehan EM , Harvey AR , Antolovich GC , Morgan PE . Prevalence and characteristics of pain in children and young adults with cerebral palsy: a systematic review. Dev Med Child Neurol. (2019) ;61: (3):305–14. doi: 10.1111/dmcn.14111 |
[10] | Alriksson-Schmidt A , Hägglund G . Pain in children and adolescents with cerebral palsy: a population-based registry study. Acta Paediatr. (2016) ;105: (6):665–70. doi: 10.1111/apa.13368 |
[11] | Eriksson E , Hägglund G , Alriksson-Schmidt AI . Pain in children and adolescents with cerebral palsy - a cross-sectional register study of 3545 individuals. BMC Neurol. (2020) ;20: (1):15. doi: 10.1186/s12883-019-1597-7 |
[12] | Østergaard CS , Pedersen NSA , Thomasen A , Mechlenburg I , Nordbye-Nielsen K . Pain is frequent in children with cerebral palsy and negatively affects physical activity and participation. Acta Paediatr. (2021) ;110: (1):301–6. doi: 10.1111/apa.15341 |
[13] | Parkinson KN , Gibson L , Dickinson HO , Colver AF . Pain in children with cerebral palsy: a cross-sectional multicentre European study. Acta Paediatr. (2010) ;99: (3):446–51. doi: 10.1111/j.1651-2227.2009.01626.x |
[14] | Ostojic K , Paget S , Kyriagis M , Morrow A . Acute and chronic pain in children and adolescents with cerebral palsy: prevalence, interference, and management. Arch Phys Med Rehabil. (2020) ;101: (2):213–19. doi: 10.1016/j.apmr.2019.08.475 |
[15] | McKinnon CT , Morgan PE , Antolovich GC , Clancy CH , Fahey MC , Harvey AR . Pain in children with dyskinetic and mixed dyskinetic/spastic cerebral palsy. Dev Med Child Neurol. (2020) a;62: (11):1294–301. doi: 10.1111/dmcn.14615 |
[16] | McKinnon CT , White JH , Morgan PE , Antolovich GC , Clancy CH , Fahey MC , et al. The lived experience of chronic pain and dyskinesia in children and adolescents with cerebral palsy. BMC Pediatr. (2020) b;20: (1):125. doi: 10.1186/s12887-020-2011-8 |
[17] | Westbom L , Rimstedt A , Nordmark E . Assessments of pain in children and adolescents with cerebral palsy: a retrospective population-based registry study. Dev Med Child Neurol. (2017) ;59: (8):858–63. doi: 10.1111/dmcn.13459 |
[18] | Roscigno CI . Addressing spasticity-related pain in children with spastic cerebral palsy. J Neurosci Nurs. (2002) ;34: (3):123–33. doi: 10.1097/01376517-200206000-00005 |
[19] | Van Sant AF . The challenge of pain in children and adolescents with cerebral palsy. Pediatr Phys Ther. (2010) ;22: (1):1. doi: 10.1097/PEP.0b013e3181ce11e1 |
[20] | Peck J , Urits I , Kassem H , Lee C , Robinson W , Cornett EM , et al. Interventional approaches to pain and spasticity related to cerebral palsy. Psychopharmacol Bull. (2020) ;50: (4 Suppl 1):108–20. |
[21] | Fehlings D , Novak I , Berweck S , Hoare B , Stott NS , Russo RN , et al. Botulinum toxin assessment, intervention and follow-up for paediatric upper limb hypertonicity: international consensus statement. Eur J Neurol. (2010) ;17: (Suppl 2):38–56. doi: 10.1111/j.1468-1331.2010.03127.x |
[22] | Love SC , Novak I , Kentish M , Desloovere K , Heinen F , Molenaers G , et al. Botulinum toxin assessment, intervention and after-care for lower limb spasticity in children with cerebral palsy: international consensus statement. Eur J Neurol. (2010) ;17: (Suppl 2):9–37. doi: 10.1111/j.1468-1331.2010.03126.x |
[23] | Delgado MR , Hirtz D , Aisen M , Ashwal S , Fehlings DL , McLaughlin J , et al. Practice parameter: pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. (2010) ;74: :336–43. doi: 10.1212/WNL.0b013e3181cbcd2f |
[24] | Delgado MR , Tilton A , Carranza-Del Río J , Dursun N , Bonikowski M , Aydin R , et al. Efficacy and safety of abobotulinumtoxinA for upper limb spasticity in children with cerebral palsy: a randomized repeat-treatment study. Dev Med Child Neurol. (2021) ;63: :592–600. doi: 10.1111/dmcn.14733 |
[25] | Heinen F , Desloovere K , Schroeder AS , Berweck S , Borggraefe I , van Campenhout A , et al. The updated European Consensus on the use of Botulinum toxin for children with cerebral palsy. Eur J Paediatr Neurol. (2010) ;14: :45–66. doi: 10.1016/j.ejpn.2009.09.005 |
[26] | NICE. National Institute for Health and Care Excellence. Spasticity in under 19s: management. Clinical guideline [CG145]. 25 July 2012 [cited 2021 March 11]. Available from: www.nice.org.uk/guidance/cg |
[27] | SCPE. Surveillance of Cerebral Palsy in Europe. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Dev Med Child Neurol. (2000) ;42: :816–24. doi: 10.1017/s0012162200001511 |
[28] | Centers for Disease Control and Prevention. Cerebral palsy. 2020 [cited 2021 March 15]. Available from: https://www.cdc.gov/ncbddd/cp/facts.html |
[29] | Hagglund G , Wagner OP . Development of spasticity with age in a total population of children with cerebral palsy. BMC Musculoskelet Disord. (2008) ;9: :150–9. doi: 10.1186/1471-2474-9-150 |
[30] | Li S , Francisco GE . The use of botulinum toxin for treatment of spasticity. Handb Exp Pharmacol. (2021) ;263: :127–46. doi: 10.1007/164_2019_315 |
[31] | Heinen F , Kaňovský P , Schroeder AS , Chambers HG , Dabrowski E , Geister TL , et al. IncobotulinumtoxinA for the treatment of lower-limb spasticity in children and adolescents with cerebral palsy: A Phase 3 study. J Pediatr Rehabil Med. (2021) ;14: (2):183–97. doi: 10.3233/PRM-210040 |
[32] | Kaňovský P , Heinen F , Schroeder AS , Chambers HG , Dabrowski E , Geister TL , et al. Safety and efficacy of repeat long-term incobotulinumtoxinA treatment for lower limb or combined upper/lower limb spasticity in children with cerebral palsy. J Pediatr Rehabil Med. 2021. doi: 10.3233/PRM-210041 |
[33] | Dabrowski E , Chambers HG , Gaebler-Spira D , Banach M , Kaňovský P , Dersch H , et al. IncobotulinumtoxinA efficacy/safety in upper-limb spasticity in pediatric cerebral palsy: Randomized controlled trial. Pediatric Neurology. (2021) ;123: :10–20. doi: 10.1016/j.pediatrneurol.2021.05.014 |
[34] | Heinen F , Kanovsky P , Schroeder AS , Chambers HG , Dabrowski E , Geister TL , et al. Pooled efficacy analysis of incobotulinumtoxinA in the multipattern treatment of upper- and lower-limb spasticity in children and adolescents with cerebral palsy. 2021. Poster presented at TOXINS 2021 Virtual Conference, January 16-17, 2021. |
[35] | Schroeder AS , Kanovsky P , Chambers HG , Dabrowski E , Geister TL , Dersch H , et al. Sustained efficacy of incobotulinumtoxinA over six injection cycles for the treatment of lower-limb spasticity in children and adolescents with cerebral palsy. Poster presented at TOXINS 2021 Virtual Conference, January 16-17, 2021. |
[36] | Banach M , Kanovsky P , Schroeder AS , Chambers HG , Dabrowski E , Geister TL , et al. Safety of incobotulinumtoxinA in multipattern treatment of upper- and lower-limb spasticity in children/adolescents with cerebral palsy: a pooled analysis of three large phase 3 studies. Poster presented at TOXINS 2021 Virtual Conference, January 16-17, 2021. |
[37] | Geister TL , Quintanar-Solares M , Martin M , Aufhammer S , Asmus F . Qualitative development of the “Questionnaire on Pain caused by Spasticity (QPS),” a pediatric patient-reported outcome for spasticity-related pain in cerebral palsy. Qual Life Res. (2014) ;23: (3):887–96. doi: 10.1007/s11136-013-0526-2 |
[38] | Reid SM , Carlin JB , Reddihough DS . Using the gross motor function classification system to describe patterns of motor severity in cerebral palsy. Dev Med Child Neurol. (2011) ;53: (11):1007–12. doi: 10.1111/j.1469-8749.2011.04044.x |
[39] | Bugler KE , Gaston MS , Robb JE . Distribution and motor ability of children with cerebral palsy in Scotland: a registry analysis. Scott Med J. (2019) ;64: (1):16–21. doi: 10.1177/0036933018805897 |
[40] | Himmelmann K , Beckung E , Hagberg G , Uvebrant P . Gross and fine motor function and accompanying impairments in cerebral palsy. Dev Med Child Neurol. (2006) ;48: (6):417–23. doi: 10.1017/S0012162206000922 |
[41] | Shaikh A , Phadke CP , Ismail F , Boulias C . Relationship between botulinum toxin, spasticity, and pain: a survey of patient perception. Can J Neurol Sci. (2016) ;43: :311–15. doi: 10.1017/cjn.2015.321 |
[42] | Flanigan M , Gaebler-Spira D , Kocherginsky M , Garrett A , Marciniak C . Spasticity and pain in adults with cerebral palsy. Dev Med Child Neurol. (2020) ;62: (3):379–85. doi: 10.1111/dmcn.14368 |
[43] | Hägglund G , Burman-Rimstedt A , Czuba T , Alriksson-Schmidt AI . Self-versus proxy-reported pain in children with cerebral palsy: a population-based registry study of 3783 children. J Prim Care Community Health. (2020) ;11: :2150132720911523. doi: 10.1177/2150132720911523 |
[44] | Jayanath S , Ong LC , Marret MJ , Fauzi AA . Parent-reported pain in non-verbal children and adolescents with cerebral palsy. Dev Med Child Neurol. (2016) ;58: (4):395–401. doi: 10.1111/dmcn.12943 |
[45] | Larsen SM , Terjesen T , Jahnsen RB , Ramstad K . Recurrent pain in adolescents with cerebral palsy: a longitudinal population-based study. Dev Med Child Neurol. (2022) ;64: (3):357–63. doi: 10.1111/dmcn.15040 |
[46] | Fairhurst C , Shortland A , Chandler S , Will E , Scrutton D , Simonoff E , et al. Factors associated with pain in adolescents with bilateral cerebral palsy. Dev Med Child Neurol. (2019) ;61: (8):929–36. doi: 10.1111/dmcn.14113 |
[47] | Raiter AM , Burkitt CC , Merbler A , Lykken L , Symons FJ . Caregiver-reported pain management practices for individuals with cerebral palsy. Arch Rehabil Res Clin Transl. (2021) ;3: (1):100105. doi: 10.1016/j.arrct.2021.100105 |
[48] | Horwood L , Li P , Mok E , Shevell M , Constantin E . A systematic review and meta-analysis of the prevalence of sleep problems in children with cerebral palsy: how do children with cerebral palsy differ from each other and from typically developing children? Sleep Health. (2019) ;5: (6):555–71. doi: 10.1016/j.sleh.2019.08.006 |
[49] | Michelsen JS , Normann G , Wong C . Analgesic effects of botulinum toxin in children with CP. Toxins (Basel). (2018) ;10: (4):162. doi: 10.3390/toxins10040162 |
[50] | Riquelme I , do Rosário RS , Vehmaskoski K , Natunen P , Montoya P . Influence of chronic pain in physical activity of children with cerebral palsy. NeuroRehabilitation. (2018) ;43: (2):113–23. doi: 10.3233/NRE-172409 |
[51] | Fehlings D . Pain in cerebral palsy: a neglected comorbidity. Dev Med Child Neurol. (2017) ;59: (8):782–3. doi: 10.1111/dmcn.13477 |
[52] | NICE. National Institute for Health and Care Excellence Guideline. Cerebral palsy in adults. 15 January 2019 [cited 2022 Jan 10]. Available from: https://www.nice.org.uk/guidance/ng119 |
[53] | McKinnon C , White J , Morgan P , Harvey A , Clancy C , Fahey M , et al. Clinician perspectives of chronic pain management in children and adolescents with cerebral palsy and dyskinesia. Phys Occup Ther Pediatr. (2021) ;41: (3):244–58. doi: 10.1080/01942638.2020.1847236 |
[54] | NICE. National Institute for Health 1095 and Care Excellence Guideline. Cerebral palsy in under 25s: assessment and management. 2017 [cited 2021 April 12]. Available from: https://www.nice.org.uk/guidance/ng62 |
[55] | Almina S , Karile Y , Audrone P , Indre B . Analgesic effect of botulinum toxin in children with cerebral palsy: A systematic review. Toxicon. (2021) ;199: :60–7. doi: 10.1016/j.toxicon.2021.05.012 |
[56] | Chaléat-Valayer E , Parratte B , Colin C , Denis A , Oudin S , Bérard C , et al. A French observational study of botulinum toxin use in the management of children with cerebral palsy: BOTULOSCOPE. Eur J Paediatr Neurol. (2011) ;15: (5):439–48. doi: 10.1016/j.ejpn.2010.04.006 |
[57] | Copeland L , Edwards P , Thorley M , Donaghey S , Gascoigne-Pees L , Kentish M , et al. Botulinum toxin A for nonambulatory children with cerebral palsy: a double blind randomized controlled trial. J Pediatr. (2014) ;165: (1):140–6.e4. doi: 10.1016/j.jpeds.2014.01.050 |
[58] | Heinen F , Kaňovský P , Schroeder AS , Chambers HG , Dabrowski E , Geister TL , et al. Improvement of spasticityrelated pain with incobotulinumtoxinA treatment in children/adolescents with cerebral palsy: a pooled analysis of three phase 3 studies. 2021. Poster presented at TOXINS 2021 Virtual Conference, January 16-17, 2021. |
[59] | Lundy CT , Doherty GM , Fairhurst CB . Botulinum toxin type A injections can be an effective treatment for pain in children with hip spasms and cerebral palsy. Dev Med Child Neurol. (2009) ;51: (9):705–10. doi: 10.1111/j.1469-8749.2009.03315.x |
[60] | Rivard PF , Nugent AC , Symons FJ . Parent-proxy ratings of pain before and after botulinum toxin type A treatment for children with spasticity and cerebral palsy. Clin J Pain. (2009) ;25: (5):413–7. doi: 10.1097/AJP.0b013e31819a6d07 |
[61] | Wissel J , Müller J , Dressnandt J , Heinen F , Naumann M , Topka H , et al. Management of spasticity associated pain with botulinum toxin A. J Pain Symptom Manage. (2000) ;20: (1):44–9. doi: 10.1016/s0885-3924(00)00146-9 |
[62] | Jacobson D , Löwing K , Kullander K , Rydh BM , Tedroff K . A first clinical trial on Botulinum toxin-A for chronic muscle-related pain in cerebral palsy. Front Neurol. (2021) ;12: :696218. doi: 10.3389/fneur.2021.696218 |




