IncobotulinumtoxinA for the treatment of lower-limb spasticity in children and adolescents with cerebral palsy: A phase 3 study1
Abstract
PURPOSE:
Investigate the efficacy and safety of multipattern incobotulinumtoxinA injections in children/adolescents with lower-limb cerebral palsy (CP)-related spasticity.
METHODS:
Phase 3 double-blind study in children/adolescents (Gross Motor Function Classification System – Expanded and Revised I–V) with unilateral or bilateral spastic CP and Ashworth Scale (AS) plantar flexor (PF) scores
RESULTS:
Among 311 patients, AS-PF and AS scores in all treated clinical patterns improved from baseline to 4-weeks post-injection and cumulatively across injection cycles. GICS-PF and GICS scores confirmed global spasticity improvements. GMFM-66 scores indicated better motor function. No significant differences between doses were evident. Treatment was well-tolerated, with no unexpected treatment-related adverse events or neutralising antibody development.
CONCLUSION:
Children/adolescents with lower-limb spasticity experienced multipattern benefits from incobotulinumtoxinA, which was safe and well-tolerated in doses up to 16 U/kg, maximum 400 U.
1.Introduction
Cerebral palsy (CP), the most common cause of chronic disability in children [1], is defined as a group of permanent disorders of movement and/or posture and of motor function, which are due to a nonprogressive interference, lesion, or abnormality of the developing/ immature brain. The core motor dysfunction symptoms are often accompanied by impairments in sensation, perception, cognition, communication, and behaviour as well as epilepsy and secondary musculoskeletal problems [2].
Spasticity affects approximately 70–90% of children with CP [3, 4, 5]. The increased muscle tone due to spasticity results in a limited range of passive and active motion in joints and contributes to development of joint contractures, poor muscular control, and hyperactive reflexes [2]. In the lower limb (LL), it often manifests as pes equinus, a deformity associated with insufficient dorsiflexion of the ankle that prevents the heel from contacting the ground, which may mean that walking is done on the toes [6]. Pes equinus is most common in children with CP and LL spasticity [7], but a number of other spastic patterns including knee flexion and hip flexion/adduction may also present [8]. Pediatric spasticity has been associated with reduced health-related quality of life [9, 10], which may be attributed in part to factors such as reduced mobility [11], inability to self-care [12], and pain [13, 14, 15].
A well-rounded treatment plan for a child with CP generally includes systematic rehabilitation, pharmacotherapy, physiotherapy, and perhaps surgical interventions [2, 16] to reduce muscle spasms, facilitate mobility and dexterity, improve ease of care, improve posture, minimize contractures and deformity, reduce pain, and improve quality of life [17]. Factors to consider when determining a treatment plan to optimize function for a child with CP include age, stage of development, and distribution of muscle impairment, including the level (i.e., upper limb [UL] and/or LL), and pattern (i.e., unilateral vs. bilateral; pes equinus only or in combination with other muscle groups).
An individualized, multilevel, multipattern focal treatment approach to target specific muscle groups, especially those underlying particular functional deficits, can help address the diverse clinical presentation of spasticity in children with CP [18, 19]. Botulinum neurotoxin type A (BoNT-A) is a recommended therapy for pediatric spasticity [20, 21, 22, 23, 24] that has been demonstrated to be effective and well-tolerated and can be well integrated in such a multimodal, multiprofessional, interdisciplinary treatment approach [25].
Three BoNT-A formulations, onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA, are currently available in the European Union (EU) [26, 27, 28] and North America [29, 30, 31] for the treatment of spasticity. Specific indications for these BoNT-As vary by region and product in adults and in children/adolescents.
The phase 3 Treatment with IncobotulinumtoxinA in Movement (TIM) study investigated the efficacy and safety of three dose levels of incobotulinumtoxinA administered to a heterogeneous group of children and adolescents with LL spastic CP. TIM included patients who manifested all levels of CP disease severity and ambulatory ability. Each patient was treated in a multipattern approach with two clinical LL patterns. Depending on the investigator’s clinical judgement, treatment for pes equinus could be bilateral or unilateral and, if unilateral, include additional ipsilateral muscle groups. The study utilized several types of assessments to measure efficacy and considered the patients’, parents’/caregivers’, and clinicians’ perspectives. The unique study design allowed for individualization of treatment within standardized trial guidelines, reflective of real-world clinical patient needs.
Figure 1.
Treatment according to (A) clinical patterns and (B) study design.
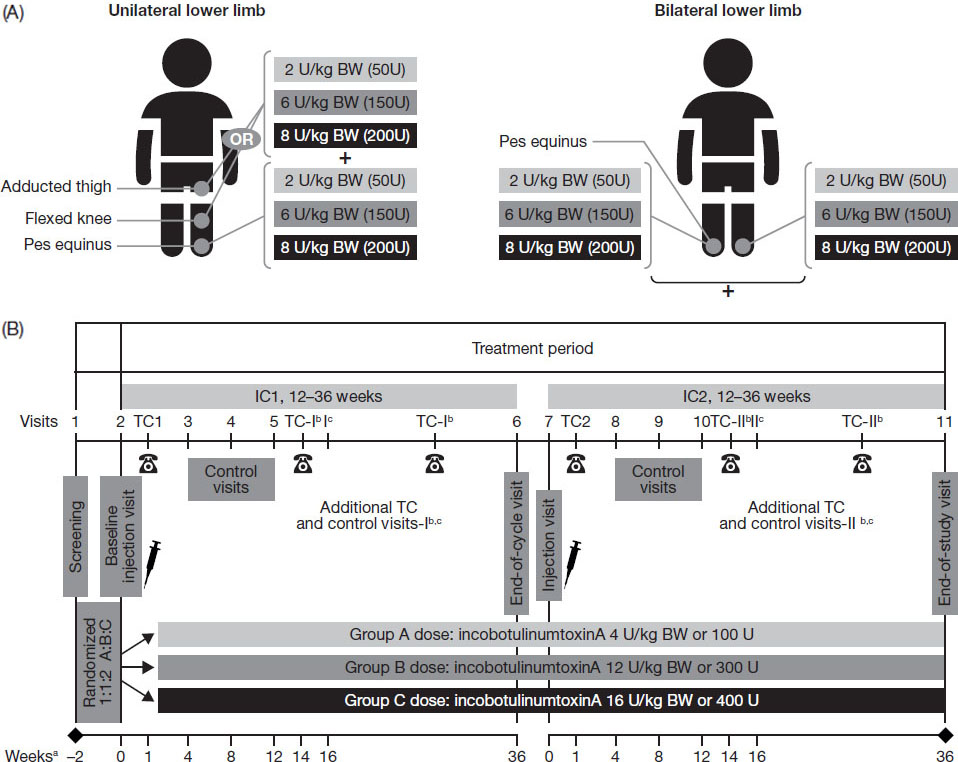
2.Methods
2.1Participants
The TIM study recruited ambulant and non-ambulant children and adolescents, aged 2–17 years, with unilateral or bilateral LL spasticity due to CP. To be enrolled, patients were required to have a clinical need determined by clinicians for treatment of either unilateral pes equinus with treatment of ipsilateral flexed knee or adducted thigh, or bilateral pes equinus. In addition, patients had to have a clinical need for incobotulinumtoxinA 16 U/kg treatment of LL spasticity and an Ashworth Scale [32] of the plantar flexor (AS-PF) score of
Key exclusion criteria included fixed contracture or predominant forms of muscle hypertonia other than spasticity (e.g., dystonia) in the target limbs, surgery for pes equinus in the target limbs within 12 months prior to screening or planned within the study period, hip flexion requiring orthopedic management and/or BoNT-A injection or limitation of hip abduction to
2.2Study design and treatment
The TIM study was a prospective, double-blind, randomized, multicenter, parallel-group, phase 3 study conducted in 45 sites across 14 countries worldwide. Eligible patients were randomized 1:1:2 to three parallel incobotulinumtoxinA dose groups, respectively: low dose: 4 units/kilogram (U/kg) body weight (BW), maximum total dose 100 U; mid dose: 12 U/kg BW, maximum total dose 300 U; high dose: 16 U/kg BW, maximum total dose 400 U.
Two LL clinical patterns were selected for treatment for each patient, one of which was required to be pes equinus on one side of the body. The patterns chosen by the investigator reflected the patient’s clinical need for therapy, with consideration given to the severity of the involved spastic muscles of the clinical pattern, subject age/weight and muscle size, activity, and experience from previous BoNT treatments. In the bilateral group, patients were treated for pes equinus on both sides of the body (Fig. 1A). In the unilateral group, patients were treated for pes equinus and ipsilateral flexed knee or adducted thigh. In this group, patients with an AS score
At the initial screening visit, each patient was evaluated medically for inclusion in the study, including Gross Motor Function Classification System (GMFCS) classification, AS score, and presence of pain; participants were also questioned about past and concomitant medications within the last 4 weeks, and prior BoNT-A medications. After a 14-day screening period which allowed investigators to check each subject’s eligibility for study participation, treatments were administered during two consecutive double-blind injection cycles, each followed by 12–36 weeks of observation (Fig. 1B), giving an overall study duration of 26–74 weeks. The injections were administered according to the study’s standardized treatment plans with predefined dose ranges and injection-site numbers for each muscle. Equal injection volumes were administered in all dose groups (total volume up to 8 mL; 4 mL/clinical pattern), with dose ranges and injection volumes adjusted for patients with
Eligibility for reinjection was assessed regularly from 12–36 weeks post-injection. The treatment plan defined for the first injection cycle was continued in the second injection. Patients were eligible for re-treatment if they had an investigator- and patient-agreed clinical need for reinjection in the LL(s) and clinical patterns chosen at the injection visit of injection cycle 1, and an AS score
Participants were allowed to maintain prior usual and concomitant therapies. These included nonpharmacological therapies such as physical therapy, orthotic management other than casting and rehabilitation, and pharmacological treatments, such as muscle relaxants and antidepressants. Patients who completed the TIM study had the option of enrolling in the open-label Treatment with IncobotulinumtoxinA in Movement Open Label (TIMO) study with 4 further injection cycles.
2.3Standard protocol approvals, registrations, and patient consent
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and registered on clinicaltrials.gov (NCT01893411). The study protocol, informed consent forms, and other appropriate study-related documents were reviewed and approved by the local independent ethics committees and institutional review boards. Parents/guardians of all patients provided written informed consent, and patients provided assent if applicable.
2.4Efficacy endpoints
The primary efficacy endpoint was the change from baseline in the AS-PF on the primary body side chosen for treatment at week 4 of injection cycle 1. The 5-point AS-PF scale was used, ranging from 0 (no increase in muscle tone) to 4 (limb rigid in flexion or extension). The coprimary efficacy endpoint was the investigator’s Global Impression of Change of Plantar Flexor Spasticity Scale (GICS-PF) score on the primary body side chosen for treatment at week 4 compared with the condition before the last injection. The GICS-PF is a 7-point Likert scale from
Secondary endpoints included AS scores for the knee flexors and thigh adductor muscles and the investigator’s Global Impression of Change Scales (GICS) to assess global changes after treatment. For the latter, overall LL spasticity change was assessed on a 7-point Likert scale from
Change in a patient’s gross motor function over time was assessed using the Gross Motor Function Measure (GMFM)-66 (scores range from 0 to 100, with higher scores reflecting better function). The GMFM-66, a shorter version of the GMFM-88, is a standardized observational instrument designed and validated to measure change in gross motor function over time in people with CP [34, 35, 36]. It has been shown to be a reliable and valid tool in children with different disabilities and is useful to assess children aged
2.5Safety
Safety endpoints assessed throughout the study included the occurrence of treatment-emergent adverse events (TEAEs), TEAEs of special interest (TEAESIs) potentially indicating distant toxin spread, and treatment-emergent serious adverse events (TESAEs). BoNT antibody testing was conducted in patients
2.6Statistical analysis
Efficacy data were analyzed descriptively in the full analysis set (a subset of patients in the safety evaluation set [SES] who had at least a baseline AS-PF score or the investigator’s GICS-PF at week 4 of injection cycle 1 available). Safety variables were analyzed descriptively in the SES, which included all patients who received at least one study treatment.
Comparison of dose groups was the primary statistical analysis. The primary and coprimary efficacy endpoints, the change from study baseline in AS-PF and investigator’s GICS-PF for the primary side at week 4, were analyzed using a mixed-model for repeated measures (MMRM; two-sided, significance level
Secondary and other analyses included the change from baseline in AS-PF and investigator’s GICS-PF for the primary side at week 4 of injection cycle 2 and at additional times during injection cycle 1 and injection cycle 2 and changes from baseline in secondary endpoints at various times during both injection cycles. Statistical analyses were performed using the statistical analysis system (SAS
Safety data were analyzed descriptively.
2.7Sample size calculations and study power
It was estimated that the sample size of
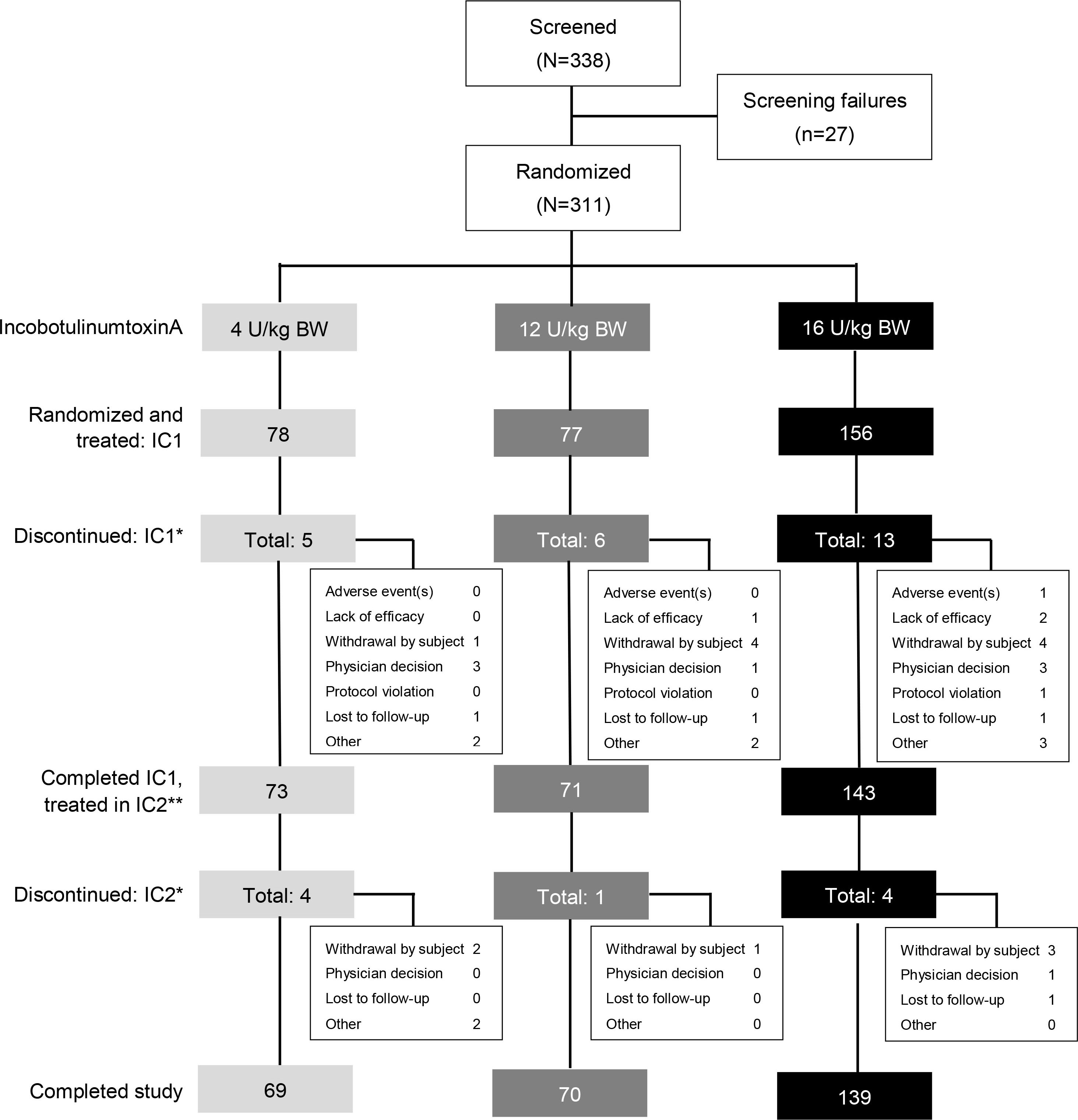
Figure 2.
Patient disposition. *Multiple entries possible. **Subjects who completed IC1 and continued to IC2. BW
2.8Data availability statement
Key elements of the study protocol, design, and statistical analysis plan were deposited in the U.S. National Library of Medicine database (www.clinicaltrials.gov, NCT01893411) and EU Clinical Trials Register (https: //eudract.ema.europa.eu/, 2012-005054-30). All relevant information is contained within this manuscript and the supplementary material.
Table 1
Patient demographics and baseline characteristics, SES/FAS
| Characteristic | Low dose 4 U/kg, maximum 100 U | |||
| Mid dose 12 U/kg, maximum 300 U | ||||
| High dose 16 U/kg, maximum 400 U | ||||
| Total | ||||
| Sex, | ||||
| Male | 42 (53.8) | 44 (57.1) | 83 (53.2) | 169 (54.3) |
| Female | 36 (46.2) | 33 (42.9) | 73 (46.8) | 142 (45.7) |
| Age, years; mean (SD) | 7.1 (4.6) | 6.6 (3.8) | 6.4 (3.9) | 6.6 (4.1) |
| Weight, kg; mean (SD) | 24.6 (16.0) | 22.7 (11.9) | 22.3 (11.8) | 22.9 (13.0) |
| GMFCS-E&R | ||||
| Level I | 14 (17.9) | 17 (22.1) | 34 (21.8) | 65 (20.9) |
| Level II | 24 (30.8) | 25 (32.5) | 50 (32.1) | 99 (31.8) |
| Level III | 19 (24.4) | 16 (20.8) | 33 (21.2) | 68 (21.9) |
| Level IV | 17 (21.8) | 12 (15.6) | 24 (15.4) | 53 (17.0) |
| Level V | 4 (5.1) | 7 (9.1) | 15 (9.6) | 26 (8.4) |
| Affected body side, | ||||
| Unilateral right | 11 (14.1) | 11 (14.3) | 15 (9.6) | 37 (11.9) |
| Unilateral left | 9 (11.5) | 5 (6.5) | 15 (9.6) | 29 (9.3) |
| Bilateral | 58 (74.4) | 61 (79.2) | 126 (80.8) | 245 (78.8) |
| Baseline AS-PF score | ||||
| Mean (SD) | 2.7 (0.6) | 2.7 (0.5) | 2.8 (0.5) | 2.7 (0.5) |
| Median (interquartile range) | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) | 3.0 (2.0, 3.0) |
| BoNT pretreatment, | ||||
| Yes | 40 (51.3) | 54 (70.1) | 97 (62.2) | 191 (61.4) |
| No | 38 (48.7) | 23 (29.9) | 59 (37.8) | 120 (38.6) |
| Concomitant diseases, | ||||
| Patients with at least one | 55 (70.5) | 57 (74.0) | 105 (67.3) | 217 (69.8) |
| Most common | ||||
| Intellectual disability | 19 (24.4) | 9 (11.7) | 18 (11.5) | 46 (14.8) |
| Epilepsy | 13 (16.7) | 14 (18.2) | 23 (14.7) | 50 (16.1) |
| Strabismus | 10 (12.8) | 13 (16.9) | 35 (22.4) | 58 (18.6) |
| Foot deformity | 8 (10.3) | 10 (13.0) | 16 (10.3) | 34 (10.9) |
3.Results
A total of 338 patients were screened, of whom 311 were randomized and treated with incobotulinumtoxinA. Of these, 78 patients were treated with low-dose incobotulinumtoxinA (4 U/kg, maximum 100 U), 77 were treated with mid-dose incobotulinumtoxinA (12 U/kg, maximum 300 U), and 156 were treated with high-dose incobotulinumtoxinA (16 U/kg, maximum 400 U). A total of 278 (89.4%) patients completed both injection cycles. The discontinuation rate was low and similarly distributed across all incobotulinumtoxinA dose groups (Fig. 2).
Demographics were similar across dose groups (Table 1). Patients were generally young (mean age 6.6 years). Three-quarters of patients had low- to mid-level ambulatory gross motor impairment (GMFCS - Expanded and Revised [GMFCS-E&R] Level I–III) and one-quarter was more severely impaired and non-ambulant (GMFCS-E&R Level IV–V). Most patients presented with bilateral LL spasticity (78.8%), with 11.9% presenting with unilateral right-sided symptoms and 9.3% with unilateral left-sided symptoms. Almost 70% of participants had at least one concomitant disease. The most frequently reported were epilepsy (42.8%), strabismus (18.6%), intellectual disability (16.1%), hypokinesia (14.8%), and foot deformity (10.9%). More than one-half (61.4%) of patients received a BoNT treatment before study enrolment, with a mean (standard deviation [SD]) of 3 (2.9) pretreatments.
During the study, the majority of patients were treated for bilateral pes equinus (72.7% injection cycle 1, 73.5% injection cycle 2). Of those receiving unilateral treatment, more patients were treated for unilateral pes equinus and flexed knee in injection cycle 1 and injection cycle 2 (19.3% and 18.5%, respectively) than unilateral pes equinus and adducted thigh (8.0% in both injections). IncobotulinumtoxinA doses administered overall and by pattern and muscle group during each injection cycle are summarized in the Supplementary Table.
The median time to reinjection or discontinuation was 14.3 (range 4–37) weeks overall in injection cycle 1 and 14.6 (range 11–38) weeks overall in injection cycle 2. The median (range) time to reinjection was similar in all three dose groups (incobotulinumtoxinA 4, 8, and 16 U/kg [maximum 100, 300, and 400 U]) in injection cycle 1, being 14.1 (4–37), 14.4 (8–36), and 14.3 (8–37) weeks, respectively, with a mean (SD) time to reinjection of 15.7 (5.9), 15.9 (5.7), and 15.3 (4.6) weeks, respectively. Injection intervals were 12 to
Figure 3.
The effect of incobotulinumtoxinA on mean change from baseline at week 4 on the AS-PF on the primary body side, FAS, OC. AS score: 5-point scale from 0 (no increase in muscle tone) to 4 (limb rigid in flexion or extension). The change in the AS-PF from baseline to week 4 was the primary efficacy variable. ***
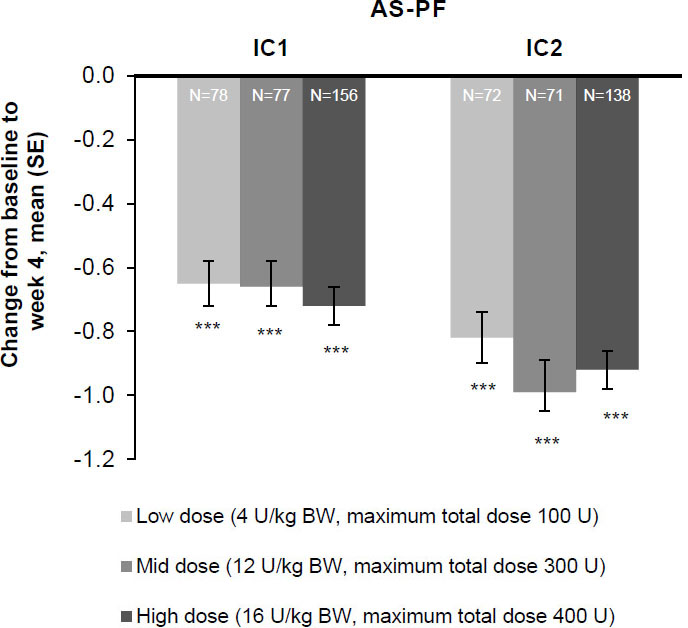
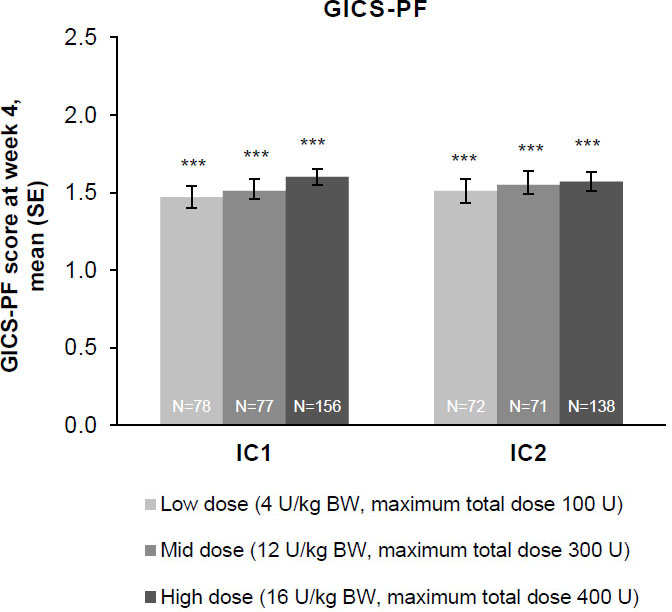
Figure 4.
The effect of incobotulinumtoxinA on investigator’s GICS-PF score at week 4; FAS, OC. Investigators were asked to rate their overall impression of change in spasticity of the PFs compared with the condition before the last injection; positive values indicate better results. Investigator’s GICS-PF score at week 4 was the coprimary efficacy variable. ***
3.1Efficacy
3.1.1Primary and coprimary endpoint results
Patients in all three incobotulinumtoxinA dose groups (total body doses of 4, 8, and 16 U/kg [maximum 100, 300, and 400 U]) experienced significant improvements in AS-PF scores on the primary body side chosen for treatment 4 weeks post-injection in injection cycle 1 versus the study baseline (
Greater improvement was associated with higher baseline AS scores. Of note, treatments produced comparable improvements in AS-PF scores across all GMFCS-E&R levels.
3.1.2Further results
AS
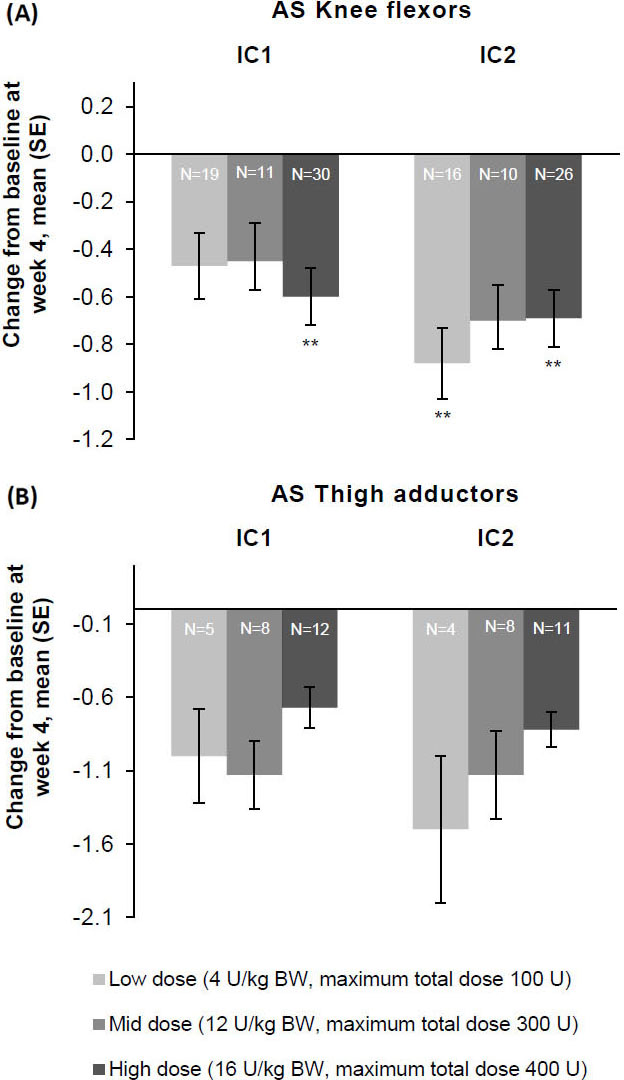
Consistent improvements for all patterns with each injection cycle were seen at weeks 4 and 8. AS-PF scores of the primary body side further improved from baseline to week 4 of the second injection cycle in all three dose groups (Fig. 3). The treatment effects were greatest 4 and 8 weeks following each injection, after which changes decreased by week 12. Following unilateral LL treatment, all doses of incobotulinumtoxinA resulted in improvements from baseline in AS scores of the knee flexors (Fig. 5A) and the thigh adductors (Fig. 5B) 4 weeks following each injection. Wherever estimable, all doses of incobotulinumtoxinA produced comparable improvements.
Figure 5.
The effect of incobotulinumtoxinA on mean change from baseline on week 4 on AS as measured on the (A) knee flexors and (B) thigh adductor muscles, FAS, OC. **
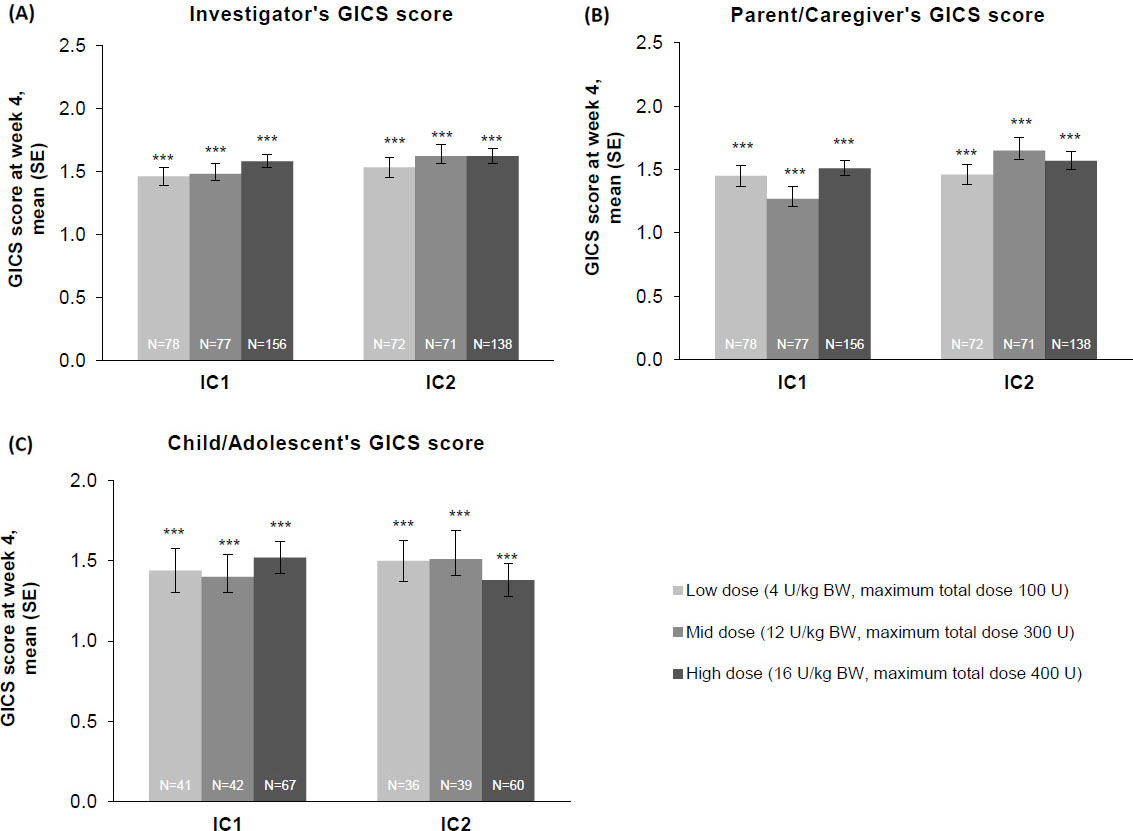
Investigator’s, child/adolescent’s, and parent/caregiver’s GICS scores
Whether from the perspective of the investigator, the parent/caregiver, or the patient, GICS scores confirmed a consistent and global improvement in LL spasticity at 4 weeks post-injection for all three incobotulinumtoxinA dose groups and across both injection cycles (Fig. 6A–C). Across both injection cycles, investigators indicated improvement rates of
Motor functioning
GMFM-66 scores indicated that motor function improved in all three treatment groups from the end of injection cycle 1 to the end-of-study visit 8 weeks later. Mean (SD) GMFM-66 scores increased from baseline by 1.8 (2.8), 1.2 (3.5), and 1.4 (3.1) at the end of injection cycle 1 and by 3.1 (3.4), 3.3 (4.5), and 2.8 (4.1) at the end-of-study visit in patients who received incobotulinumtoxinA 4, 8, and 16 U/kg (maximum 100, 300, and 400 U), respectively.
3.2Safety
IncobotulinumtoxinA treatment was generally well-tolerated over both injection cycles, with TEAEs reported in 42.8% of patients overall (Table 2). The incidence of TEAEs was slightly higher in patients who received the highest dose of incobotulinumtoxinA, followed by the lowest dose, and then the mid dose. Most TEAEs were mild or moderate in intensity. TEAEs assessed by investigators as treatment-related also occurred at a low frequency (4.8% of patients overall). These events were localized muscular weakness (
Table 2
Summary of TEAEs by treatment over two injection cycles, SES
| Adverse events | Low dose 4 U/kg, maximum 100 U | |||
| Mid dose 12 U/kg, maximum 300 U | ||||
| High dose 16 U/kg, maximum 400 U | ||||
| Total | ||||
| Any TEAE, | 30 (38.5) | 26 (33.8) | 77 (49.4) | 133 (42.8) |
| Mild | 19 (24.4) | 14 (18.2) | 41 (26.3) | 74 (23.8) |
| Moderate | 10 (12.8) | 11 (14.3) | 33 (21.2) | 54 (17.4) |
| Severe | 1 (1.3) | 1 (1.3) | 3 (1.9) | 5 (1.6) |
| Treatment-related | 2 (2.6) | 2 (2.6) | 11 (7.1) | 15 (4.8) |
| Any TEAESI, | 1 (1.3) | 1 (1.3) | 5 (3.2) | 7 (2.3) |
| Treatment-related | 0 (0.0) | 1 (1.3) | 4 (2.6) | 5 (1.6) |
| Any TESAE, | 6 (7.7) | 1 (1.3) | 7 (4.5) | 14 (4.5) |
| Treatment-related | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any TEAE leading to discontinuation | 0 (0.0) | 0 (0.0) | 1 (0.6) | 1 (0.3) |
| Treatment-related | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Any fatal TEAE, | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Figure 6.
The effect of incobotulinumtoxinA on (A) investigator, (B) parent/caregiver, and (C) child/adolescent GICS scores
TEAESIs were reported in 2.3% of patients overall. The most frequent TEAESI, localized muscular weakness, was reported in five patients and, in all cases, affected the treated LL and was considered by the investigator to be related to treatment. Given the low incidence of TEAESIs, no meaningful conclusions could be drawn concerning any relationship between CP severity and TEAESIs.
TESAEs occurred at low frequencies in all dose groups for each injection cycle (high dose: 2.6% and 2.1%; mid dose: 0.0% and 1.4%; low dose: 3.8% and 4.1% in the first and second cycles, respectively). No treatment-related TESAE or fatal TEAE was reported. One patient in the high-dose group discontinued the study because of a non-treatment-related nonserious TEAE (asthma).
At the screening visit, 19 of 127 (15.0%) subjects eligible for testing were tested positive on the FIA-AB. Three patients subsequently tested positive for NABs according to HDA test results; all three patients had been pretreated with other BoNT-As. Of these, one had a negative HDA test at the end-of-study visit, and the other two patients did not undergo further HDA testing. Based on a protocol deviation, ten patients at screening and two patients at the end-of-study visit were FIA-AB positive but were not HDA tested (HDA missing). All patients with positive HDA or HDA missing based on protocol deviation responded to treatment, based on the investigator’s GICS-PF assessment.
4.Discussion
The TIM study is one of the most extensive BoNT-A studies of its kind investigating incobotulinumtoxinA in pediatric and adolescent patients with CP-related LL spasticity across all GMFCS levels (ambulatory and non-ambulatory), reflecting real-world CP distribution. Consequently, this study fills an important gap in the literature and existing clinical evidence. IncobotulinumtoxinA treatment with doses of 4, 12, and 16 U/kg (maximum 100, 300, and 400 U, respectively) per patient divided over two clinical patterns showed consistent improvement in all LL clinical patterns treated, as measured by the AS. These results were supported by better global and functional outcomes from the perspective of patients, parents/caregivers, and physicians, all of whom reported notable improvements in GICS scores in 80% or more of patients. Furthermore, sustained and cumulative improvement in spasticity and functional outcomes was observed with repeated incobotulinumtoxinA treatment to the same clinical patterns.
No significant differences between doses were observed for either the primary or the secondary outcomes, and so the primary efficacy analyses of the study were not met. In planning the TIM study, it was assumed that the higher incobotulinumtoxinA doses could be differentiated from the lower, but ultimately the results demonstrated a measurable response for patients receiving all doses tested. For example, in our study, the AS-PF LS-mean change was -0.70 for the high-dose group and -0.66 for the low-dose incobotulinumtoxinA group (
The original AS and the MAS [40] are routinely used to assess the treatment effects of BoNT on spasticity in adults [41, 42, 43] and children with CP [25, 44]. The MAS, a 6-point scale, would be expected to yield a greater effect size than the original 5-point AS scale. However, the changes in muscle tone from baseline in our study using the original AS were comparable to the LL spasticity effect sizes in studies that used the MAS and included placebo controls [38, 39].
As already reported, incobotulinumtoxinA (total doses of 2–16 U) was also equally efficacious at producing partial paralysis of the extensor digitorum brevis muscle of the foot in a double-blind study in healthy volunteers [45]. Furthermore, demonstration of significant differences between dose groups can be more difficult in LL spasticity than in UL spasticity. Indeed, the recently published IncobotulinumtoXinA in aRm treatment in cerebral pAlsy (XARA) trial (NCT02002884) demonstrated that incobotulinumtoxinA 8 U/kg BW produced AS scores that were significantly superior to those of 2 U/kg BW for UL CP-related spasticity [46].
The results also indicate that incobotulinumtoxinA had a favorable safety and tolerability profile at doses of up to 16 U/kg (maximum 400 U) for patients in all GMFCS severity groups. No new or unexpected safety concerns were identified over two injection cycles (up to 72 weeks).
Overall, the incobotulinumtoxinA treatments were well-tolerated, as evidenced by the high patient retention rate (89.4% completed the study). Our results also align with a previous retrospective report that found incobotulinumtoxinA to be safe and effective for CP-related spasticity in children [47].
Despite using an incobotulinumtoxinA dose of up to 400 U and repeated treatments, no secondary nonresponse to treatment due to NABs occurred. This is of importance for the anticipated long-term treatment of spastic CP as a chronic condition occurring in young patients [18]. In a study of BoNT-A use in patients with a variety of neurological impairments, 13.9% of 596 patients developed measurable NABs [48]. The rate of NABs varied between BoNT-A formulations: 6% for abobotulinumtoxinA and 7% for onabotulinumtoxinA compared with 0% for incobotulinumtoxinA. It should be noted that not all patients who did not respond to BoNT-A therapy had high NAB levels, and some patients with high NAB levels still responded to BoNT-As [48]. The current results add to the growing body of evidence that incobotulinumtoxinA may carry less risk of inducing an immunogenic response relative to other BoNT-As – and hence less risk of nonresponse to therapy – because it is a highly purified formulation of BoNT-A [48] that does not contain BoNT-A accessory proteins [18]. For this reason, it may be the preferred choice for a chronic condition in a pediatric population [50].
Although the majority of participants in TIM required bilateral injections for pes equinus, almost 20% were treated for unilateral pes equinus and ipsilateral flexed knee and 8% for unilateral pes equinus and ipsilateral adducted thigh. IncobotulinumtoxinA therapy demonstrated efficacy in all these patterns, adding to evidence supporting the use of incobotulinumtoxinA in multipattern spasticity.
The TIM study is part of a large, international phase 3 pediatric study program investigating the efficacy and safety of incobotulinumtoxinA for the treatment of CP-related spasticity. All of these studies, including the TIM study, were designed to incorporate important aspects of individualized treatment within the perspective of a class I phase 3 study based on distribution of spasticity, i.e., multiple affected clinical patterns. The physician was able to tailor treatment using standardized dose ranges for the complete set of muscles of a pattern, defining injection sites based on anatomy, and adjust injection intervals. Overall, the reports from this and two other trials (TIMO: NCT01905683; and XARA: NCT02002884) confirm that incobotulinumtoxinA is safe and effective for multipattern spasticity in children and adolescents with CP [46, 51, 52], including those with severe symptoms [53].
The mean time to reinjection was 15.6 (5.2) weeks, although 29.3% of patients did not need a second dose until 16 weeks or later. Currently, minimal intervals of 12–16 weeks are recommended for the treatment of spasticity in children [29, 30, 31], although other reports have suggested longer injection intervals may be appropriate at times [54, 55]. These results from the TIM study suggest that some flexibility in time to reinjection based on a patient’s clinical needs may be warranted in clinical practice.
The changes in motor function, as assessed by GMFM-66 scores over time, were consistent with those of previous studies of BoNT-A for LL spasticity [56, 57, 58]. Although GMFM-66 improvements should be considered in the context of the patient’s global rehabilitation plan and cannot be solely attributed to incobotulinumtoxinA treatment, improvements after a single injection in the current study were within changes being reported as minimum clinically important differences by Oeffinger et al. [59], who defined medium and large effects as changes of 0.7–1.7 and 1.2–2.7, respectively, in ambulatory children with CP.
Spasticity is just one factor among many others that could interfere with gross motor development [60], even in children with severe forms of spastic CP. For instance, strength (impaired by the underlying paresis) [61] and selective motor control [62] have also been found to be contributory. A meaningful improvement, as shown here, is thus a promising result, especially when considering that the GMFM was assessed only at the end-of-cycle visits (when the effect was waning) and not at week 4 visits (the time point of expected maximum effect).
4.1Strengths and limitations
The strengths of the TIM study include its patient-centric approach to treatment. Individualized treatment options within standardized, multipattern treatment protocols for LL spasticity were available to address the real-world needs of a large, heterogeneous population of children and adolescents with CP. Participants represented a broad age range (2–17 years) and all levels of disease severity (GMFCS-E&R levels I–V). The efficacy and safety of incobotulinumtoxinA were assessed across a wide dose range. The study design allowed for the treatment of several muscle groups at once, giving physicians the freedom to adjust dose, injection site, number of injections per site, and time to reinjection as required, based on individual patient need. Additional strengths were the generation of a comprehensive dataset, which included efficacy and safety data for other LL muscle groups in addition to the PFs and the uni/bilateral combination treatment. The outcomes provided data on the effect of incobotulinumtoxinA on muscle resistance, global improvement, and gross motor function, from the point of view of the patient, parent/caregiver, and physician.
The lack of a placebo control in the TIM study may be viewed as a limitation, but use of placebo in this vulnerable young patient population raises concerns in terms of ethical aspects and feasibility. Furthermore, the effect sizes, as discussed earlier, were within established ranges, and the current patient-centric approach allowed for a thorough investigation of long-term efficacy and safety across a broad dose range of incobotulinumtoxinA in children and adolescents with spastic CP.
5.Conclusion
This large phase 3 study demonstrated that incobotulinumtoxinA used according to an individualized treatment plan within standardized guidelines is beneficial in improving muscle tone and motor function for children with spasticity-related CP. IncobotulinumtoxinA total doses up to 16 U/kg BW (maximum 400 U) were effective and well-tolerated for the multipattern treatment of LL spasticity due to CP for ambulant and non-ambulant children and adolescents presenting with all levels of disease severity. IncobotulinumtoxinA had a good safety and tolerability profile and did not induce NAB formation.
Ethical considerations
The study protocol, informed consent forms and other appropriate study-related documents were reviewed and approved by the local independent ethics committees and institutional review boards. Parents/ guardians of all patients provided written informed consent, and patients provided assent if applicable.
Supplementary data
The supplementary files are available to download from http://dx.doi.org/10.3233/PRM-210040.
Acknowledgments
The authors wish to thank all study subjects and investigators. They would like to acknowledge Hanna Dersch and Annette Lehn for statistical support and Amy Rothman Schonfeld and Caroline Spencer (Rx Communications, Mold, UK) for medical writing assistance with the preparation of this manuscript, funded by Merz Pharmaceuticals GmbH. This study was supported by Merz Pharmaceuticals GmbH, Frankfurt am Main, Germany.
Conflict of interest
Florian Heinen has received speaker’s honoraria from Allergan plc, Desitin, Ipsen Biopharmaceuticals, Merz Pharmaceuticals, and Novartis and unrestricted educational grants from Allergan and Merz Pharmaceuticals. Petr Kanovský has received speaker’s honoraria from Desitin, Ipsen Biopharmaceuticals, Merz Pharmaceuticals, and Medtronic. A. Sebastian Schroeder has received speaker’s honoraria from and participated in advisory boards for Allergan plc, Ipsen Biopharmaceuticals, and Merz Pharmaceuticals. Henry G. Chambers serves as a consultant for Orthopediatrics Corp and Allergan Corporation. Edward Dabrowski has participated in an advisory board and speaker bureau for Ipsen Biopharmaceuticals. Thorin L. Geister is an employee of Merz Pharmaceuticals GmbH. Angelika Hanschmann is an employee of Merz Pharmaceuticals GmbH. Francisco J. Martinez-Torres is a former employee of Merz North America LLC. Irena Pulte is an employee of Merz Pharmaceuticals GmbH. Marta Banach has served as a consultant and speaker and participated in an advisory board for Merz Pharmaceuticals and has served as a speaker for Allergan, Ipsen, and Kedrion. Deborah Gaebler-Spira has served as a consultant for Teva and Kashiva.
References
[1] | Pavone V, Testa G, Restivo DA, Cannavò L, Condorelli G, Portinaro NM, et al. Botulinum toxin treatment for limb spasticity in childhood cerebral palsy. Front Pharmacol. (2016) ; 7: : 29. doi: 10.3389/fphar.2016.00029. |
[2] | Sadowska M, Sarecka-Hujar, Kopyta I. Cerebral palsy: Current opinions on definition, epidemiology, risk factors, classification and treatment options. Neuropsychiatr Dis Treat. (2020) ; 16: : 1505-1518. doi: 10.2147/NDT.S235165. |
[3] | Centers for Disease Control and Prevention. Cerebral palsy. Accessed March 14, 2021. Available from: https://www.cdc.gov/ncbddd/cp/facts.html. |
[4] | Surveillance of Cerebral Palsy in Europe. Surveillance of cerebral palsy in Europe: A collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev Med Child Neurol. (2000) ; 42: (12): 816-824. doi: 10.1017/s0012162200001511. |
[5] | Hagglund G, Wagner OP. Development of spasticity with age in a total population of children with cerebral palsy. BMC Musculoskelet Disord. (2008) ; 9: : 150-159. doi: 10.1186/1471-2474-9-150. |
[6] | Lampe R, Mitternacht J. Research on the performance of the spastic calf muscle of young adults with cerebral palsy. J Clin Med Res. (2011) ; 3: : 8-16. doi: 10.4021/jocmr483w. |
[7] | Horsch A, Götze M, Geisbüsch A, Beckmann N, Tsitlakidis S, Berrsche G, et al. Prevalence and classification of equinus foot in bilateral spastic cerebral palsy. World J Pediatr. (2019) ; 15: : 276-280. doi: 10.1007/s12519-019-00238-2. |
[8] | Rethlefsen SA, Blumstein G, Kay RM, Dorey F, Wren TAL. Prevalence of specific gait abnormalities in children with cerebral palsy revisited: Influence of age, prior surgery, and gross motor function classification system level. Dev Med Child Neurol. (2017) ; 59: : 79-88. doi: 10.1111/dmcn.13205. |
[9] | Colver A, Rapp M, Eisemann N, Ehlinger V, Thyen U, Dickinson HO, et al. Self-reported quality of life of adolescents with cerebral palsy: A cross-sectional and longitudinal analysis. Lancet. (2015) ; 385: : 705-716. doi: 10.1016/S0140-6736(14)61229-0. |
[10] | Park EY. Path analysis of strength, spasticity, gross motor function, and health-related quality of life in children with spastic cerebral palsy. Health Qual Life Outcomes. (2018) ; 16: : 70-76. doi: 10.1186/s12955-018-0891-1. |
[11] | Akodu AK, Oluwale OAT, Adegoke ZO, Ahmed UA, Akinola TO. Relationship between spasticity and health related quality of life in individuals with cerebral palsy. Nig Q J Hosp Med. (2012) ; 22: : 99-102. |
[12] | Öhrvall AM, Eliasson AC, Löwing K, Ödman P, Krumlinde-Sundholm L. Self-care and mobility skills in children with cerebral palsy, related to their manual ability and gross motor function classifications. Dev Med Child Neurol. (2010) ; 52: : 1048-1055. doi: 10.1111/j.1469-8749.2010.03764.x. |
[13] | Geister TL, Quintanar-Solares M, Martin M, Aufhammer S, Asmus F. Qualitative development of the ’Questionnaire on Pain caused by Spasticity (QPS),’ a pediatric patient-reported outcome for spasticity-related pain in cerebral palsy. Qual Life Res. (2014) ; 23: : 887-896. doi: 10.1007/s11136-013-0526-2. |
[14] | Poirot I, Laudy V, Rabilloud M, Roche S, Ginhoux T, Kassaï B, et al. Prevalence of pain in 240 non-ambulatory children with severe cerebral palsy. Ann Phys Rehabil Med. (2017) ; 60: : 371-375. doi: 10.1016/j.rehab.2017.03.011. |
[15] | Penner M, Xie WY, Binepal N, Switzer L, Fehlings D. Characteristics of pain in children and youth with cerebral palsy. Pediatrics. (2013) ; 132: : E407-E413. doi: 10.1542/peds.2013-0224. |
[16] | Hutchinson R, Graham HK. Management of spasticity in children. In: Barnes MP, Johnson GR, editors. Upper motor neurone syndrome and spasticity. Clinical management and neurophysiology. 2nd ed. Cambridge, MA: Cambridge University Press. (2008) ; 214-234. |
[17] | Koman LA, Smith BP, Balkrishnan R. Spasticity associated with cerebral palsy in children: Guidelines for the use of botulinum A toxin. Pediatr Drugs. (2003) ; 5: : 11-23. doi: 10.2165/00128072-200305010-00002. |
[18] | Li S, Francisco GE. The use of botulinum toxin for treatment of spasticity. Handb Exp Pharmacol. (2021) ; 263: : 127-146. doi: 10.1007/164_2019_315. |
[19] | Satila H. Over 25 years of pediatric Botulinum Toxin treatments: What have we learned from injection techniques, doses, dilutions, and recovery of repeated injections? Toxins. (2020) ; 12: : 440-460. doi: 10.3390/toxins12070440. |
[20] | Fehlings D, Novak I, Berweck S, Hoare B, Stott NS, Russo RN, et al. Botulinum toxin assessment, intervention and follow-up for paediatric upper limb hypertonicity: International consensus statement. Eur J Neurol. (2010) ; 17: : 38-56. doi: 10.1111/j.1468-1331.2010.03127.x. |
[21] | Love SC, Novak I, Kentish M, Desloovere K, Heinen F, Molenaers G, et al. Botulinum toxin assessment, intervention and after-care for lower limb spasticity in children with cerebral palsy: International consensus statement. Eur J Neurol. (2010) ; 17: : 9-37. doi: 10.1111/j.1468-1331.2010.03126.x. |
[22] | Delgado MR, Hirtz D, Aisen M, Ashwal S, Fehlings DL, McLaughlin J, et al. Practice parameter: Pharmacologic treatment of spasticity in children and adolescents with cerebral palsy (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. (2010) ; 74: : 336-343. doi: 10.1212/WNL.0b013e3181cbcd2f. |
[23] | Heinen F, Desloovere K, Schroeder AS, Berweck S, Borggraefe I, van Campenhout A, et al. The updated European Consensus 2009 on the use of Botulinum toxin for children with cerebral palsy. Eur J Paediatr Neurol. (2010) ; 14: : 45-66. doi: 10.1016/j.ejpn.2009.09.005. |
[24] | National Institute for Health and Care Excellence. Spasticity in under 19s: Management. Clinical guideline [CG145]. Published 25 July 2012 [cited 2021 03 11]. Available from: www.nice.org.uk/guidance/cg145. |
[25] | Multani I, Manji J, Hastings-Ison T, Khot A, Graham K. Botulinum toxin in the management of children with cerebral palsy. Pediatr Drugs. (2019) ; 21: : 261-281. doi: 10.1007/s40272-019-00344-8. |
[26] | Botox |
[27] | Dysport 500U. Summary of product characteristics. Cambridge, MA: Ipsen Biopharm Ltd, 2017 [cited 2021 Jan 19]. Available from: https://www.medicines.org.uk/emc/medicine/32114. |
[28] | Merz Pharma UK Ltd. Xeomin 200 units powder for solution for injection. Herts: Merz Pharma UK Ltd, 2020 [cited 2021 Jan 25]. Available from: https://www.medicines.org.uk/emc/product/2162/smpc. |
[29] | BOTOX (onabotulinumtoxinA) for injection, for intramuscular, intradetrusor, or intradermal use. Highlights of prescribing information – Botox®. Dublin: Allergan Inc., 2019 [cited 2021 Feb 23]. Available from: https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/20190620-BOTOX-100-and-200-Units-v3-0USPI1145-v2-0MG1145.pdf. |
[30] | DYSPORT (abobotulinumtoxinA) for injection, for intramuscular use. Highlights of prescribing information. Cambridge, MA: Ipsen Biopharm Ltd, 2020 [cited 2021 Feb 4]. Available from: https://www.ipsen.com/websites/Ipsen_Online/wp-content/uploads/2020/07/10002305/DYS-US-004998_Dysport-PI-July-2020.pdf. |
[31] | XEOMIN (incobotulinumtoxinA) for injection, for intramuscular or intraglandular use: US prescribing information. Raleigh, NC: Merz Pharmaceuticals LLC, 2020 [cited 2021 Feb 5]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125360s078lbl.pdf. |
[32] | Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. (1964) ; 192: : 540-542. |
[33] | Elovic EP, Munin MC, Kanovský P, Hanschmann A, Hiersemenzel R, Marciniak C. Randomized, placebo-controlled trial of incobotulinumtoxina for upper-limb post-stroke spasticity. Muscle Nerve. (2016) ; 53: : 415-421. doi: 10.1002/mus.24776. |
[34] | Russell DJ, Avery LM, Rosenbaum PL, Raina PS, Walter SD, Palisano RJ. Improved scaling of the gross motor function measure for children with cerebral palsy: Evidence of reliability and validity. Phys Ther. (2000) ; 80: : 873-885. |
[35] | Harvey A, Robin J, Morris ME, Graham HK, Baker R. A systematic review of measures of activity limitation for children with cerebral palsy. Dev Med Child Neurol. (2008) ; 50: : 190-198. doi: 10.1111/j.1469-8749.2008.02027.x. |
[36] | Alotaibi M, Long T, Kennedy E, Bavishi S. The efficacy of GMFM-88 and GMFM-66 to detect changes in gross motor function in children with cerebral palsy (CP): A literature review. Disabil Rehabil. (2014) ; 36: : 617-627. doi: 10.3109/09638288.2013.805820. |
[37] | Rosenbaum PL, Walter SD, Hanna SE, Palisano RJ, Russell DJ, Raina P, et al. Prognosis for gross motor function in cerebral palsy: Creation of motor development curves. JAMA. (2002) ; 288: : 1357-1363. doi: 10.1001/jama.288.11.1357. |
[38] | Delgado MR, Tilton A, Russman B, Benavides O, Bonikowski M, Carranza J, et al. AbobotulinumtoxinA for equinus foot deformity in cerebral palsy: A randomized controlled trial. Pediatrics. (2016) ; 137: : e20152830. doi: 10.1542/peds.2015-2830. |
[39] | Kim H, Meilahn J, Liu C, Chambers HG, McCusker E, Dimitrova R. Efficacy and safety of onabotulinumtoxinA for the treatment of pediatric lower limb spasticity: Primary results. Neurology. (2018) ; 90: (15 Suppl): S29.007. |
[40] | Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. (1987) ; 67: : 206-207. doi: 10.1093/ptj/67.2.206. |
[41] | Moccia M, Frau J, Carotenuto A, Butera C, Coghe G, Barbero P, et al. Botulinum toxin for the management of spasticity in multiple sclerosis: The Italian botulinum toxin network study. Neurol Sci. (2020) ; 41: : 2781-2792. doi: 10.1007/s10072-020-04392-8. |
[42] | Palazón-García R, Alcobendas-Maestro M, Esclarin-de Ruz A, Benavente-Valdepeñas AM. Treatment of spasticity in spinal cord injury with botulinum toxin. J Spinal Cord Med. (2019) ; 42: : 281-287. doi: 10.1080/10790268.2018.1479053. |
[43] | Wein T, Esquenazi A, Jost WH, Ward AB, Pan G, Dimitrova R. OnabotulinumtoxinA for the treatment of poststroke distal lower limb spasticity: A randomized trial. PM R. (2018) ; 10: : 693-703. doi: 10.1016/j.pmrj.2017.12.006. |
[44] | Choi JY, Kim SK, Park ES. The effect of botulinum toxin injections on gross motor function for lower limb spasticity in children with cerebral palsy. Toxins (Basel). (2019) ; 11: : 651-664. doi: 10.3390/toxins11110651. |
[45] | Wohlfarth K, Müller C, Sassin I, Comes G, Grafe S. Neurophysiological double-blind trial of a botulinum neurotoxin type A free of complexing proteins. Clin Neuropharmacol. (2007) ; 30: : 86-94. doi: 10.1097/01.WNF.0000240951.18821.50. |
[46] | Dabrowski E, Chambers HG, Gaebler-Spira D, Banach M, Kanovsky P, Dersch H, et al. Efficacy and safety of incobotulinumtoxinA for upper- or combined upper- and lower-limb spasticity in children and adolescents with cerebral palsy: Results of the phase 3 XARA study. Toxicon. (2021) ; 190: : S14-S15. doi: 10.1016/j.toxicon.2020.11.369. |
[47] | Leon-Valenzuela A, Palacios JS, Del Pino Algarrada R. IncobotulinumtoxinA for the treatment of spasticity in children with cerebral palsy – a retrospective case series focusing on dosing and tolerability. BMC Neurol. (2020) ; 20: : 126. doi: 10.1186/s12883-020-01702-7. |
[48] | Albrecht P, Jansen A, Lee JI, Moll M, Ringelstein M, Rosenthal D, et al. High prevalence of neutralizing antibodies after long-term botulinum neurotoxin therapy. Neurology. (2019) ; 92: : e48-e54. doi: 10.1212/WNL.0000000000006688. |
[49] | Frevert J. Pharmaceutical, biological, and clinical properties of botulinum neurotoxin type A products. Drugs R D. (2015) ; 15: : 1-9. doi: 10.1007/s40268-014-0077-1. |
[50] | Hefter H, Brauns R, Ürer B, Rosenthal D, Albrecht P. Effective long-term treatment with incobotulinumtoxin (Xeomin®) without neutralizing antibody induction: A monocentric, cross-sectional study. J Neurol. (2020) ; 267: : 1340-1347. doi: 10.1007/s00415-019-09681-7. |
[51] | Heinen F, Kanovsky P, Schraeder S, Chambers HG, Dabrowski E, Geister TL, et al. Pooled efficacy analysis of incobotulinumtoxinA in the multipattern treatment of upper- and lower-limb spasticity in children and adolescents with cerebral palsy. Toxicon. (2021) ; 190: : S32-S33. doi: 10.1016/j.toxicon.2020.11.405. |
[52] | Banach M, Kanovsky P, Schroeder AS, Chambers HG, Dabrowski E, Geister TL, et al. Safety of incobotulinumtoxinA in multipattern treatment of upper- and lower-limb spasticity in children/adolescents with cerebral palsy: A pooled analysis of 3 large phase 3 studies. Toxicon. (2021) ; 190: : S7. doi: 10.1016/j.toxicon.2020.11.353. |
[53] | Kanovsky P, Gaebler-Spira D, Schroeder AS, Chambers HG, Dabrowski E, Geister TL, et al. Pooled efficacy and safety analysis of incobotulinumtoxinA in the treatment of upper- and lower-limb spasticity in children with severe cerebral palsy (GMFCS level IV and V). Toxicon. (2021) ; 190: : S36. doi: 10.1016/j.toxicon.2020.11.414. |
[54] | Hastings-Ison T, Blackburn C, Rawicki B, Fahey M, Simpson P, Baker R, et al. Injection frequency of botulinum toxin A for spastic equinus: A randomized clinical trial. Dev Med Child Neurol. (2016) ; 58: : 750-757. doi: 10.1111/dmcn.12962. |
[55] | Kanovský P, Bares M, Severa S, Richardson A; Dysport Paediatric Limb Spasticity Study Group. Long-term efficacy and tolerability of 4-monthly versus yearly botulinum toxin type A treatment for lower-limb spasticity in children with cerebral palsy. Dev Med Child Neurol. (2009) ; 51: : 436-445. doi: 10.1111/j.1469-8749.2008.03264.x. |
[56] | Baker R, Jasinski M, Maciag-Tymecka I, Michalowska-Mrozek J, Bonikowski, Carr L, et al. Botulinum toxin treatment of spasticity in diplegic cerebral palsy: A randomized, double-blind, placebo-controlled, dose-ranging study. Dev Med Child Neurol. (2002) ; 44: : 666-675. doi: 10.1017/s0012162201002730. |
[57] | Chang HJ, Hong BY, Lee SJ, Lee S, Park JH, Kwon JY. Efficacy and safety of letibotulinum toxin A for the treatment of dynamic equinus foot deformity in children with cerebral palsy: A randomized controlled trial. Toxins (Basel). (2017) ; 9: : 252. doi: 10.3390/toxins9080252. |
[58] | Kim K, Shin HI, Kwon BS, Kim SJ, Jung IY, Bang MS. Neuronox versus BOTOX for spastic equinus gait in children with cerebral palsy: A randomized, double-blinded, controlled multicentre clinical trial. Dev Med Child Neurol. (2011) ; 53: : 239-244. doi: 10.1111/j.1469-8749.2010.03830.x. |
[59] | Oeffinger D, Bagley A, Rogers S, Gorton G, Kryscio R, Abel M, et al. Outcome tools used for ambulatory children with cerebral palsy: Responsiveness and minimum clinically important differences. Dev Med Child Neurol. (2008) ; 50: : 918-925. doi: 10.1111/j.1469-8749.2008.03150.x. |
[60] | Katusic A, Alimovic S. The relationship between spasticity and gross motor capability in nonambulatory children with spastic cerebral palsy. Int J Rehabil Res. (2013) ; 36: : 205-210. doi: 10.1097/MRR.0b013e32835d0b11. |
[61] | Ross SA, Engsberg JR. Relationships between spasticity, strength, gait, and the GMFM-66 in persons with spastic diplegia cerebral palsy. Arch Phys Med Rehabil. (2007) ; 88: : 1114-1120. doi: 10.1016/j.apmr.2007.06.011. |
[62] | Noble JJ, Gough M, Shortland AP. Selective motor control and gross motor function in bilateral spastic cerebral palsy. Dev Med Child Neurol. (2019) ; 61: : 57-61. doi: 10.1111/dmcn.14024. |




