Pain and health status in adults with myelomeningocele living in Sweden
Abstract
PURPOSE:
To increase knowledge about pain and general health in adults with myelomeningocele, a health condition with several risk factors for pain such as musculoskeletal deformities, shunt dysfunctions, bowel problems, and urinary tract infections/stones.
METHODS:
Descriptive correlational pilot study (
RESULTS:
Seventy-three percent reported pain in the past four weeks. No significant sex or age differences were associated with the presence of pain. Women were significantly more likely to report that pain interfered with work,
CONCLUSIONS:
Pain was frequent, and pain sites differed widely. Women were more likely to report that pain interfered with work, and scored lower on health, as did older persons.
1.Introduction
Myelomeningocele (MMC) is the most severe type among several different spinal neural tube defects (NTDs) [1]. The term “spina bifida” (SB) is often used synonymously with MMC; however, SB may sometimes include also other types of spinal NTDs. The live birth incidence of MMC differs substantially across the world and was 0.7, 0.8, and 1.3 per 10,000 live births during the years 2008–2015 in Thailand, Denmark, and Sweden respectively [2, 3]. In Sweden, the live birth incidence of NTDs (of which more than half were spinal NTDs) has decreased more than six-fold during the last 40 years. This decrease is mainly due to the termination of pregnancies after early prenatal diagnosis. However, over the past 20 years, there has also been a decrease in the number of pregnancies complicated by NTDs [4]. The prevalence rate of MMC in 15–18 year-olds was 3.8 per 10,000 among those born in 1986-89 and residing in Sweden in 2004 [5]. However, the corresponding prevalence rate for adults with MMC is unknown. The condition is complex, which is compounded by the fact that many body systems are affected simultaneously, resulting in a variety of urological, neurological, and musculoskeletal complications [6]. Comorbidities and secondary conditions are frequent and wide-ranging [6], and can substantially affect quality of life (QoL) [7], and social involvement [8]. The number and severity of the comorbidities tend to correlate to the type and the level of the SB/MMC. Approximately 30 percent of individuals with SB have typical cognitive development (IQ
It is only in the recent 40–50 years that children born with MMC could expect to survive into adulthood, which can be attributed to improvements in medical and public health [16, 17]. Although multidisciplinary care to manage MMC is often available in childhood (depending on the geographical location of the child), finding appropriate care as an adult is more challenging. Coordinated multidisciplinary services are still rare, and many adult providers do not receive training on or are not experienced with the condition. Moreover, in adulthood, individuals are expected to assume responsibility of their own healthcare, a difficult task given the involvement of numerous body systems coupled with the difficulties related to executive function and the lack of experience of managing one’s own care. Hence, it is possible that healthcare lapses in adults with MMC might translate into poorer health and the development or undertreatment of pain.
1.1MMC and pain
Pain has been defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage [18]. Pain is subjective, complex and challenging to measure. Nociception (activity in the neuronal pain system) results in sensations that may or may not be experienced as pain. Nociceptive pain is the general sensation of pain due to the activation of nociceptors in the skin, musculoskeletal system, or visceral organs. Nociceptive pain is a warning system designed to protect us from injury. On the other hand, neuropathic pain is caused by a lesion or disease in the neuronal pain system and has no such protective function. Although non-functional neuronal tissue (neuropathy) may lead to lost or impaired sensation, the same tissue may be the origin of neuropathic pain. Neuropathic pain may occur without any associated stimulation. At times, neuropathy may result in the distorted perception of a stimulus, so that, for example, a light touch is experienced as severe pain. Nociceptive pain and neuropathic pain must be treated differently in terms of removal of painful stimuli as well as pharmacological or neurophysiological interventions. Removal of neuropathic pain may be possible if the symptoms are caused by conditions such as tethering of the spinal cord, syringomyelia, or compression of the spinal cord and nerves. Neuropathic pain may be either intermittent or continuous for extended periods, and may co-occur with nociceptive pain. Acute nociceptive pain signals that something is wrong (e.g., physiological harm), and, if caused by an injury, the pain usually disappears before the injury has completely healed. Nociceptive pain can become chronic and be persistent or recurrent [18, 19].
Surprisingly little attention has been paid to pain in SB/MMC, although individuals with these conditions frequently have one or more well-established risk factors for both types of pain [20], such as musculoskeletal deformities, dysfunctional or infected shunts, urinary tract infections, kidney stones, bowel problems, tethered cord syndrome, syringomyelia, spinal root or nerve compressions, suboptimal positioning, and pressure injuries [21, 2, 23]. The lesion level may be classified as either the vertebral level or the muscle function level (as in the current study), but these descriptions do not correlate perfectly to one another. These lesions may cause neuropathic pain, which is also possible in insensate areas, meaning that individuals with MMC with high lesions can still experience pain in the lower parts of the body. These are additional risk factors beyond the regular causes of pain that may affect the general population without SB/MMC.
Although surgeries and rehabilitation efforts can be used to treat pain, they may inadvertently result in acute or chronic pain. The literature on pain in SB/MMC is largely inconclusive in terms of prevalence, etiologies, and prevention/treatment strategies, partly due to different inclusion criteria (e.g., diagnoses or ages included), and methodologies (e.g., measures or definitions) used. Compared to typically developing young adults, young adults with SB and hydrocephalus in the Netherlands reported significantly worse scores on bodily pain (head, neck, and back) [17]. In a recent US study where secondary conditions were assessed, 90 percent of the participants with SB reported pain, and the majority stated that the pain affected their lives “somewhat” to “greatly” [24]. In yet another small US study on individuals with MMC, increased pain was associated with reduced physical and psychological health, and pain was most commonly reported in the shoulder, back, and head areas [25]. High rates of chronic idiopathic headache (not due to intracranial pressure) in adults with SB and hydrocephalus have also been reported [26]. In a Swedish study that included adults with MMC specifically, 29 percent reported pain in general, with 19 percent reporting nociceptive pain and ten percent neuropathic pain [27].
1.2Purpose of study
As part of a larger inventory of medical needs in adults with MMC, the purpose of this specific study was to investigate pain and general health in adults with MMC. The specific aims were to (1) assess the prevalence of self-reported pain, (2) explore the relationships of pain impact, severity, and body site with gender, age, and muscle function level, (3) assess self-reported general health (today), (4) explore the relationship of general health (today) with gender, age, and muscle function level, and (5) determine if general health had declined, improved, or remained stable in the past three years, and if participants needed referrals following the study.
It was hypothesized that pain prevalence and general health (today) would differ based on gender, age, and muscle function level.
2.Methods
2.1Participants and procedure
A combined national quality registry and follow-up program (MMCUP) was recently launched in Sweden for individuals with spinal dysraphisms, including MMC. This study was part of a broader MMCUP pilot project on health, medical status, physical function, and social situation to investigate the healthcare need of adults with MMC. The study included individuals with open spinal NTDs, skin covered myelomeningoceles or lipo-MMC (also described as lipomatous spinal NTDs with a subcutaneous mass [1]) and all included participants had neurological deficits from birth. Individuals with intellectual disabilities (ID) were included; all but three participants had mild ID and were able to self-report general health (today) on the visual analogue scale (VAS) that was included. No instrument to differentiate between nociceptive and neuropathic pain was included in the study.
Individuals older than 18 years of age who met the additional inclusion criteria (see above) were identified in four Swedish healthcare regions through a variety of sources that included medical record reviews, clinic attendee lists, and collaboration with the professionals working with individuals with MMC in the catchment area. In total, 190 individuals with MMC, aged 19–75 years (born 1941 to 1997), who were currently or had been residing in the catchment area were identified. Of those, ten were deceased, 19 had moved out of the region, and two could not be located, resulting in 159 individuals eligible to participate in the study. Invitations to participate were done by letters, and thereafter verbally by study personnel who were familiar with the eligible participants at the pediatric or adult habilitation services. The goal of the pilot study was to recruit approximately 50 participants during the project period. Following the consent process, trained professionals working at the respective clinics performed the study assessments which lasted approximately three hours each. The EQ-5D-5L was filled out by the participants, and if needed they received assistance from someone not involved with the healthcare of the specific participant. The professionals who performed the assessments varied from a single pediatric physical therapist in one region, to a habilitation team for adults including physical and occupational therapists, urotherapist/nurse, physician, and a social worker in another region. An experienced pediatric neurologist (principal investigator (PI) and last author LW) reviewed all assessments, validated the diagnoses of MMC, and confirmed the presence of comorbid and secondary conditions through medical record reviews. The data collected at the study sites were recorded using paper and pencil onto forms that were later delivered to the PI, who subsequently entered the data into a database. A convenience sample of 51 adults from the known total population of adults with MMC living in the region (
The project was part of MMCUP and was approved by the local Institutional Review Board (Regionala etikprövningsnämnden, Lund, dnr 2009/241).
2.2Measures
The medical inventory relied on material originally developed for MMCUP by the MMCUP-network, and consisted of (1) a general questionnaire that included items on medical diagnoses, social situation, healthcare use, health information including kidney function, cognitive function, and surgeries undergone, (2) a urology questionnaire that included items on urinary bladder and bowel functions, emptying procedures, and leakage, and (3) a motor function protocol that included assessments of motor functional level of lesion, joint range of motion, functional mobility, use of mobility devices and orthoses, presence of pressure injuries, and information on pain. The EQ-5D-5L, a brief patient reported questionnaire that measures health in five domains, was also included [28], however only one of the five domains (pain) and the VAS (health) from this questionnaire were included in the current study. Studies have shown that the VAS scale of the EQ-5D can be used reliably even with children as young as 9 years of age [28].
2.2.1Pain
The pain items used were as follows: “Have you experienced pain in the past four weeks?” (yes or no). If the participants answered yes, they were asked to indicate the pain site/s (head, neck, back, chest, abdomen, shoulders, arms/hands, hips, thighs/knees, lower leg/feet, and “other”). In addition, participants were asked “During the past four weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?” and “During the past four weeks how much did pain interfere with sleep”? The response items were on a Likert scale (“not at all”, “a little bit”, “moderately”, “quite a bit”, and “extremely”). These two items were recoded to “did not interfere at all”, or “did interfere” respectively (i.e., “a little bit”, “moderately”, “quite a bit”, and “extremely” were combined into one group). The severity of pain (today) was assessed with the pain/discomfort dimension from the EQ-5D-5L (“Which statement best describes your health today – Pain/Discomfort”). The corresponding response items were: “I have no pain or discomfort”, “I have slight pain or discomfort”, “I have moderate pain or discomfort”, “I have severe pain or discomfort”, or “I have extreme pain or discomfort”. This variable was dichotomized into “no pain” versus “pain”.
Surgeries might have bearing on pain and general health (today), and therefore the types of surgeries and the surgery dates were investigated. Based on medical record reviews and participant self-reports, a total of 110 surgeries were reported. Shunt revisions were the most common type of surgery performed, followed by surgeries of the feet. Three shunt revisions, one tethered cord release, one ileostomy, one surgery for pressure injuries, and one surgery for kidney stones were performed less than one year before the study’s start, but none of them were performed less than six months before the study assessments. It is unlikely, albeit possible, that any of these surgeries would have resulted in post-operative pain lasting long enough to be reflected in the current study.
2.2.2General health (today)
General health today (“How good or bad is your health today?”) was measured using the EQ-5D-5L VAS with endpoints labeled “the best health you can imagine” (100) and “the worst health you can imagine” (0). An item on the stability of health (medical) during the past three years was included (response options: “stable”, “improved”, or “deteriorated”). In addition, after reviewing the study data, the PI made a decision whether a subsequent referral for medical follow-up was warranted based on the data collected, and this was coded as “referral yes” or “referral no”.
Table 1
Level of muscle function
| Muscle function level* | |||
|---|---|---|---|
| Level | Description of function | ||
| I | Sacral | Low-lesion | Weakness of intrinsic foot muscles. Good-to-normal foot plantarflexion. |
| II | Low lumbar | Fair or less foot plantarflexion, fair or better knee flexion, poor to fair or better hip extension and/or hip abduction | |
| III | Middle lumbar | Good-to-normal hip flexion and knee extension, fair or less knee flexion, trace of hip extension, hip abduction and below-knee muscles. | |
| IV | High lumbar | High-lesion | No knee extension activity, poor or less hip flexion, fair or good pelvic elevation |
| V | Thoracic | No muscle activity in the lower limbs, no pelvic elevation | |
2.2.3Additional variables
Based on the manual testing of muscle strength of the lower limbs, the muscle function levels (MFLs) were used to classify function into one of five levels (Table 1). The MFL classification system was developed by Bartonek and colleagues [29, 30] after comparing six previous common classifications of neurological levels of lesion and is based on muscle strength and the theoretical relation to ambulatory level. In the event of asymmetric weakness, the most affected side is used to determine the MFL. The age variable was dichotomized (19–30 years of age versus older than 30 years of age) in Aim 1 but continuous in Aim 2.
2.3Statistical analysis
Frequencies (
Table 2
Medical and functional characteristics of sample (
| % |
| |
|---|---|---|
| Shunted hydrocephalus* | 80 | 41 |
| Severe vision limitation** or blind | 12 | 6 |
| Confirmed intellectual disability (ICD codes F70-79) | 22 | 11 |
| Kidney function | ||
| Normal and near normal (eGFR | 75 | 38 |
| At least one urinary tract infection in the past year | 53 | 27 |
| Bowel leakage | 55 | 28 |
| Urinary leakage | 65 | 33 |
| Urinary voiding | ||
| Normal | 4 | 2 |
| Clean and intermittent catheterization | 79 | 40 |
| Urinary diversion | 16 | 8 |
| Muscle function level | ||
| I Sacral | 22 | 11 |
| I Female | 27 | 3 |
| II Low lumbar | 16 | 8 |
| II Female | 38 | 3 |
| III Middle lumbar | 18 | 9 |
| III Female | 44 | 4 |
| IV High lumbar | 16 | 8 |
| IV Female | 63 | 5 |
| V High lumbar/thoracic | 22 | 11 |
| V Female | 55 | 6 |
| Unknown | 8 | 4 |
| Female | 75 | 3 |
In Aim 2, a General Linear Model univariate ANOVA was used to analyze the association among sex, age, and general health (today). General health (today) was entered as the dependent variable, and sex and age (continuous) were entered as independent variables. In Aim 3, percentages of how many participants’ health in the past three years had remained stable, improved, or declined as well as how many had needed referrals following the study assessment were calculated. Pairwise deletion was used and the level of significance was set at
3.Results
3.1Sample characteristics
Forty-nine participants (96%) had a diagnosis of MMC confirmed by a physician. One female participant included in the study had a lipomatous mass in addition to MMC (“lipo MMC”). Another female, born abroad with a clinical diagnosis of MMC, had a bifid dorsal spine from L1 down to include the whole sacrum and an atrophic spinal cord from C6 and downwards, and imaging showed probable neuroenteric cysts rather than a previously operated MMC. This was discovered after the study and the participant was kept in the analyses. The age range spanned from 19 to 56 years (mean
Figure 1.
Pain interference with work or sleep during the past 4 weeks.
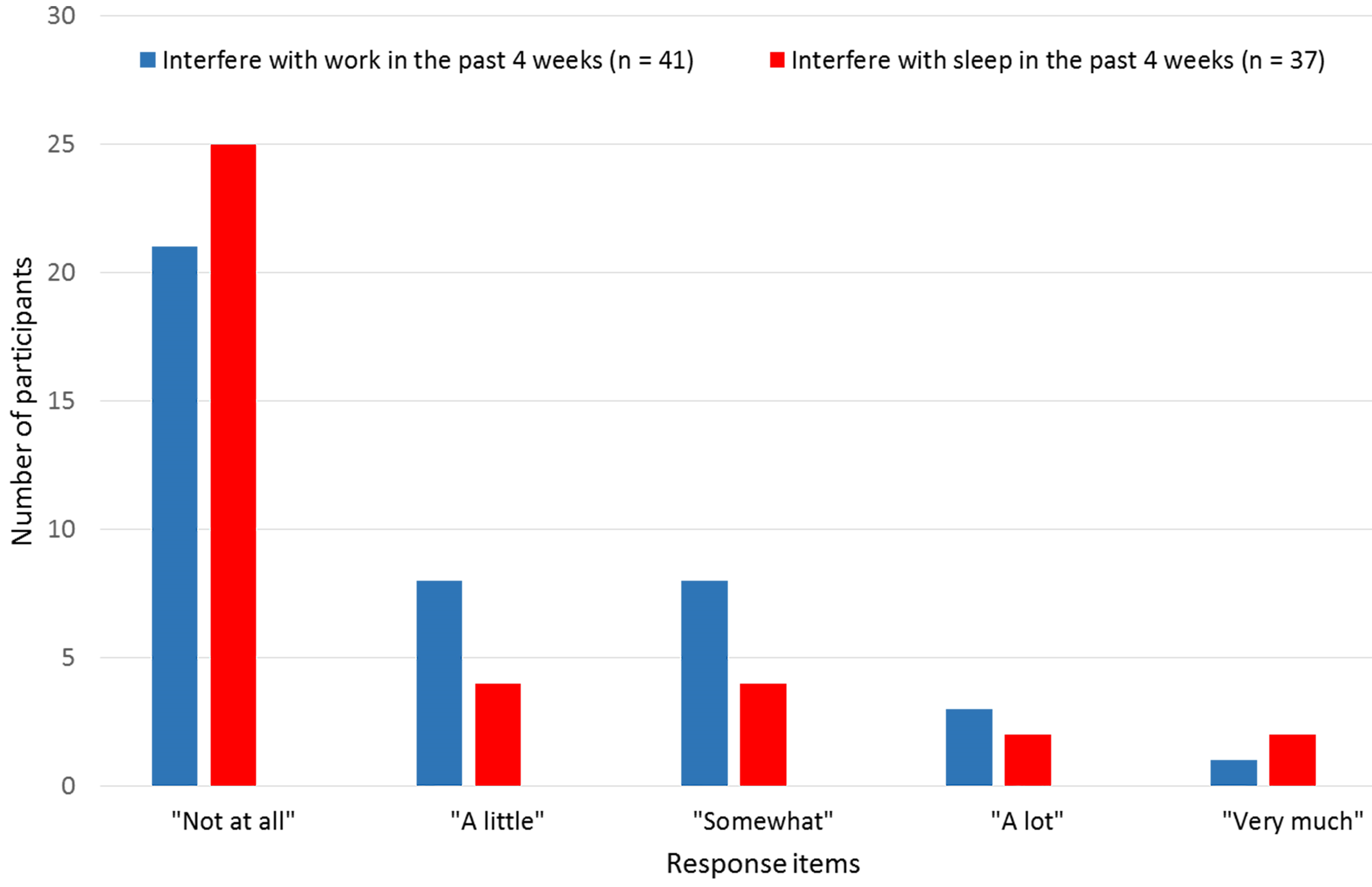
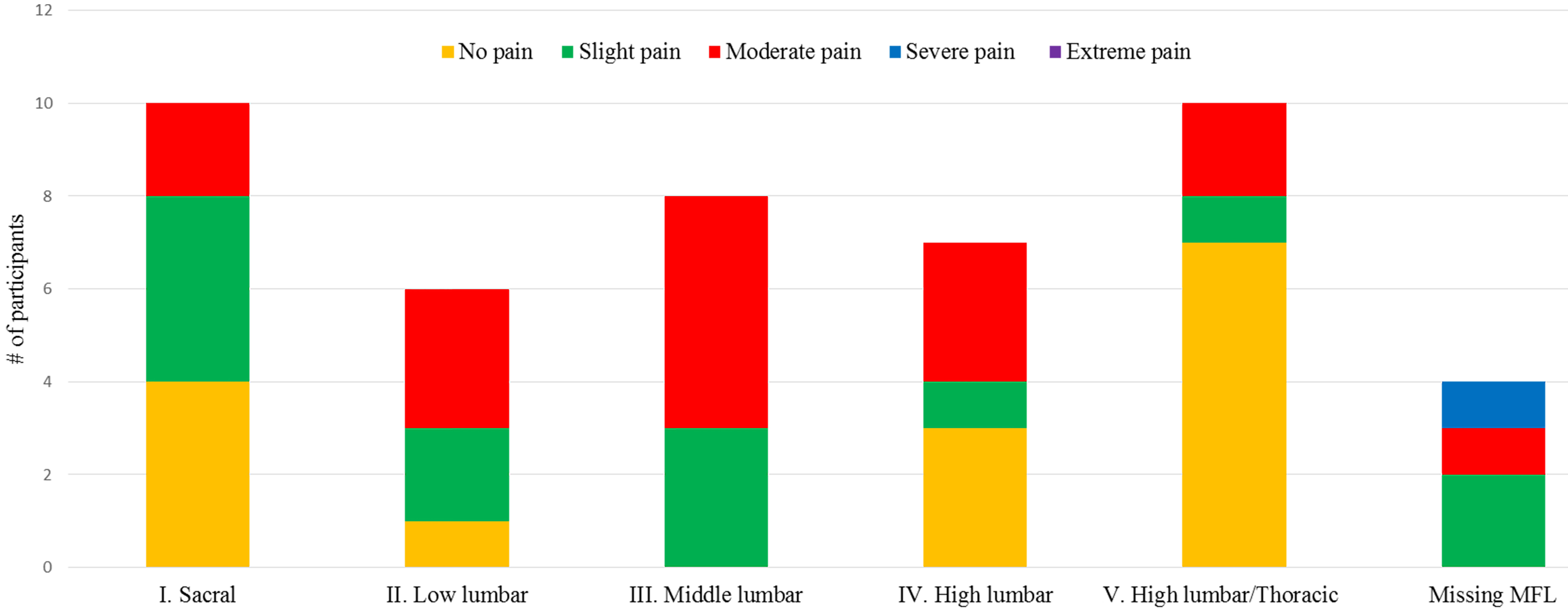
Figure 2.
Self-reported severity of current pain in relation to muscle function level.
3.2Pain
Pain in the past four weeks was reported by 37 participants (73%;
Overall, 15 (29%) reported “no pain today”, 13 (26%) reported “slight pain”, 16 (31%) reported “moderate pain”, and one reported (2%) “severe pain” (
The median number of pain sites was one, and the number of pain sites ranged from zero to nine sites. Number of pain site did not significantly differ based on sex and age. The pain sites in relation to MFLs and wheelchair status are presented in Table 3. Pain in the upper body (back, shoulder, and neck) was most commonly reported.
Table 3
| Pain site | ||||||||||||
| Muscle function level | Head | Neck | Back | Chest | Stomach | Shoulders | Arms/ | Hips | Thighs/ | Lower leg/ | Other | Total |
| hands | knee | feet | ||||||||||
| I. Sacral ( | 1 | 2 | 5 | 1 | 2 | 1 | 1 | 13 | ||||
| II. Low lumbar ( | 2 | 2 | 3 | 1 | 4 | 1 | 3 | 16 | ||||
| III. Middle lumbar ( | 3 | 1 | 2 | 1 | 2 | 9 | ||||||
| IV. High lumbar ( | 1 | 2 | 3 | 1 | 1 | 4 | 2 | 2 | 1 | 1 | 18 | |
| V. High lumbar/thoracic ( | 2 | 4 | 1 | 4 | 2 | 1 | 14 | |||||
| Unknown ( | 1 | 1 | 1 | 1 | 1 | 5 | ||||||
| Has wheelchair* ( | 5 | 8 | 11 | 0 | 2 | 13 | 3 | 4 | 4 | 1 | 1 | 52 |
| Does not have wheelchair ( | 1 | 2 | 4 | 0 | 1 | 0 | 0 | 2 | 2 | 2 | 0 | 14 |
| Total ( | 7 | 11 | 16 | 1 | 3 | 14 | 4 | 7 | 7 | 4 | 1 | |
*The majority had both manual and electric wheelchair. No information on which wheelchair was primarily used, or how many hrs/day. One participant did not have information on wheelchair use.
3.3General health (today)
Forty-eight participants answered the item on general health (today); three persons were unable because of ID. The scores for general health (today) ranged from 10 to 100. The mean score of general health (today) for the entire group was 70.90 (SD
3.4Stability of health and need for referral
In terms of self-reported (sometimes together with a proxy) stability of health (medical parameters) during the past three years, 32 (63%) reported that their health had remained stable, 25 percent reported that their health had declined, three participants (6%) reported that their health had improved, one participant (2%) did not know, and the rest (4%) were missing. All study assessments and self-reports for each individual were reviewed by the PI, and related to a comprehensive review of present and past medical records for that individual; only 25% were considered to have received satisfactory medical follow-ups and 51% needed referrals to specialized care for medical, urological or neurosurgical evaluations/interventions. Participants were also in need of basic regular follow-up care for monitoring blood pressure, kidney function, weight, and pain. Regular care for bladder and bowel function was especially necessary.
4.Discussion
In this study, pain and general health (today) in adults with MMC were assessed. While almost two thirds reported that they had experienced pain during the past four weeks, most reported that the pain had not interfered with work or sleep. Women were more likely than men to report that pain had interfered with work. These findings might imply that the pain experienced was not severe. However, individuals living with chronic pain, which may or may not have been the case in this study, might gradually start to modify and adapt their behaviors as a coping mechanism in response to the pain. For instance, engaging in fewer activities and cutting back on work or school hours in order to preserve energy and avoid mental fatigue might occur insidiously over time, eventually becoming the “new normal”. Consequently, the individual may not be aware that the pain had indeed affected their work life. The participants, for the most part, were young adults (median age 29 years), and it is possible that the pain reported did not yet affect them as such. In a study by Wagner et al., 90 percent perceived pain, and the vast majority (52–94 percent) reported that this pain affected their lives. These adults were on average older, with 67 percent being 25 years of age or older. The inclusion of relatively young adults in our sample likely also helps explain why an association between pain and age was not found. A positive correlation between age and pain has been noted in other complex chronic disabilities, and in the general population overall [32, 33].
Most of the participants when asked if they had pain today reported that they did. Furthermore, one third reported that the pain was moderate, indicating that the pain was indeed problematic, even in this rather young population. The fact that no statistical differences were found in terms of sex (or age) was potentially due to low statistical power. The pain was mainly concentrated in the upper body, with back, shoulder, and neck being the most commonly reported sites. Pain in the upper body may reflect overuse of muscles, possibly by using manual wheelchairs or perhaps caused by poor positioning. That fewer participants reported pain in the lower limbs might reflect the reduced sensation in the areas below the lesions. The pain sites reported in this study were consistent with those observed in previous research [24, 25]. In terms of self-reported general health (today), women reported lower average health scores than did men. Moreover, an inverse relationship between age and health was noted. That women tend to self-report worse health than men has been found previously [34]. Overall, the variance explained by the variables included in this study was small (
Sweden, like most parts of the world, does not prioritize adult clinics for persons with MMC, and it is difficult to find experienced adult specialists and general practitioners with knowledge about MMC. It is noteworthy that the participants in the study performed by Werhagen et al. [27], with an overall pain prevalence of 28 percent, all attended an adult SB clinic. It is possible that the participants in the Werhagen et al. study experienced less pain overall due to more comprehensive ongoing multidisciplinary care. If adults with MMC are less likely to seek and receive care, and if they are treated by providers not familiar with the condition, it is possible that health declines and that pain goes unnoticed and undertreated by healthcare providers. The co-occurrence of ID in this population is high. Including individuals with IDs in research studies can be complicated, given that varying cognitive levels makes accurate and reliable self-report more difficult. To introduce proxy-reports complicate matters further because caregivers may either over- or under-report. Nevertheless, individuals with MMC and ID might be at an even higher risk for poor health and how to address this issue in research and clinical practice needs to be considered.
Self-reported outcomes in terms of health and pain have to be considered the gold standard. Whereas subjective measures typically tap into how one’s health is perceived, objective measures request factual information about health [35]. Objectively and subjectively measured outcomes may indeed differ. People with life-long disabilities or chronic conditions may adapt to their current situations and under-report certain outcomes. We must also consider the ‘disability-paradox’, i.e., those who do not have disabilities tend to expect that individuals with disabilities have reduced QoL and poorer health by default [36, 37]. Nevertheless, the fact that 26 of the 51 participants in the current study were considered to be in need of medical follow-up and probable intervention by a physician (and that 14 of these participants were referred for urgent care and treatment) highlights the importance of both subjective and objective measures of health.
4.1Limitations
There were some clear limitations to the study, most notably the sample size. It is likely that the small sample size resulted in reduced statistical power and the possibility of Type II error, and it also prevented us from performing certain statistical analyses. However, this was a pilot study and the data collection will be expanded in the future, as the nation-wide MMCUP started including adults in 2017. Given the sample size, the pain item was dichotomized which means that relevant nuances related to pain might have been lost. For instance, intense pain likely affects work and sleep more than mild pain. Additionally, we used a convenience sample, and caution is warranted when considering the generalizability of the results. As in many studies that rely on active recruitment of participants, selection bias might be a concern. In a study on pain, for instance, those who experience pain might be more likely to participate. However, this study was based on secondary data from a larger protocol, where data on numerous variables were collected. Eligible participants were not recruited to a study on pain per se, and the recruiters did not specifically “seek out” those they knew were in pain. Selection bias might still have been present; those who participated might have had poorer health or had more comorbidities, or had more leisure time because they were less likely to be employed than those who declined participation. Furthermore, those with comorbid ID might have been less likely to participate which means that the current findings would not be generalizable to them.
No distinction between nociceptive and neuropathic pain was made, and it was not known if the pain was actively being treated. This is explained by the fact that the study was part of MMCUP; as a combined follow-up program and registry it is necessary to be strict on what items to include, or else the program becomes too time-consuming and cumbersome for both patients and professionals. The pain items used herein primarily serve as a screening for pain. In clinic, the practitioners are to follow-up clinically when warranted, but that data are not entered into the registry.
Unfortunately, it was not possible to analyze differences between those using a manual wheelchair versus those using an electric wheelchair. Virtually all who had wheelchairs had both a manual and an electric wheelchair, and the study questionnaire did not differentiate how much the participants used one versus the other. Where the pain was manifested (back, shoulders, and neck) would have been useful information. Furthermore, mental fatigue was not assessed. Mental fatigue is often associated with chronic pain and may at times affect activities and participation as much as or more than the actual pain, either directly or through a mediating pathway.
4.2Future directions
Based on these results and the small but emerging body of research on pain in adults with MMC, it is clear that more evidence is needed. Research is also needed in terms of evidence-based pain management strategies in this population. Investigating the occurrence and distinction between neuropathic and nociceptive pain is of particular interest in this population in order to tailor appropriate treatments. More information is needed regarding the nature of the pain, how it develops over time, how it affects people at different muscle function levels, and different imaging findings in the nervous system, in order to intervene appropriately or proactively prevent pain occurrence in the first place.
Not all pain is preventable. However, pain needs to be actively surveyed by the professionals working with patients with spina bifida. It should not be assumed that patients will always bring up issues of pain, because they may believe or have been taught that ‘pain comes with the territory’. Pain should actively be screened for, and when present, a pain management plan for the individual should be developed to ensure that all possible pain reduction strategies are exhausted.
Acknowledgments
We would like to thank all who participated in the Swedish professional and patient network who helped to create the study forms, and the re/habilitation professionals who performed the assessments. We would also like to express our gratitude towards the Centrum for Sallsynta Diagnoser and the Kockska Foundation who helped support the study financially.
Conflict of interest
The authors have no conflicts of interest to report.
References
[1] | McComb JG. A practical clinical classification of spinal neural tube defects. Childs Nerv Syst (2015) ; 31: (10): 1641-57. doi: 10.1007/s00381-015-2845-9. |
[2] | Jaruratanasirikul S, Kor-anantakul O, Limpitikul W, Dissaneevate P, Khunnarakpong N, Sattapanyo A. Prevalence of neural tube defect in southern Thailand: A population-based survey during 2009–2012. Childs Nerv Syst (2014) ; 30: (7): 1269-75. doi: 10.1007/s00381-014-2410-y. |
[3] | Bodin CR, Rasmussen MM, Tabor A, Westbom L, Tiblad E, Ekelund CK, et al. Ultrasound in prenatal diagnostics and its impact on the epidemiology of spina bifida in a national cohort from Denmark with a comparison to Sweden. Biomed Res Int (2018) ; 9203985: . doi: 10.1155/2018/9203985. eCollection 2018. |
[4] | Retrieved at the Birth defects 2016, the National Board of Health and Welfare 2018, http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/20888/2018-3-13.pdf. |
[5] | Olsson I, Dahl M, Mattsson S, Wendelius M, Aström E, Westbom L. Medical problems in adolescents with myelomeningocele (MMC): An inventory of the Swedish MMC population born during 1986–1989. Acta Paediatr (2007) ; 96: (3): 446-9. |
[6] | Alriksson-Schmidt AI, Thibadeau JK, Swanson ME, Marcus D, Carris KL, Ward E. The natural history of spina bifida in children pilot project: Research protocol. JMIR Res Protoc (2013) ; 2: (1): e2. doi: 10.2196/resprot.2209. |
[7] | Simeonsson R, Sturtz McMillen J, Huntington GS. Secondary conditions in children with disabilities: Spina bifida as a case example. Ment Retard Dev Disabil Res Rev (2002) ; 8: (3): 198-205. |
[8] | Oddson BE, Clancy CA, McGrath PJ. The role of pain in reduced quality of life and depressive symptomology in children with spina bifida. Clin J Pain (2006) ; 22: (9): 784-9. |
[9] | Lindquist B, Carlsson G, Persson EK, Uvebrant P. Learning disabilities in a population-based group of children with hydrocephalus. Acta Paediatr (2005) ; 94: (7): 878-83. |
[10] | Barf HA, Verhoef M, Jennekens-Schinkel A, Post MW, Gooskens RH, Prevo AJ. Cognitive status of young adult with spina bifida. Dev Med Child Neurol (2003) ; 45: (12): 813-20. |
[11] | Essner BS, Murray CB, Holmbeck GN. The influence of condition parameters and internalizing symptoms on social outcomes in youth with spina bifida. J Pediatr Psychol (2014) ; 39: (7): 718-34. doi: 10.1093/jpepsy/jsu036. |
[12] | Yeates KO, Loss N, Colvin AN, Enrile BG. Do children with myelomeningocele and hydrocephalus display nonverbal learning disabilities? An empirical approach to classification. J Int Neuropsychol Soc (2003) ; 9: (4): 653-62. |
[13] | Burmeister R, Hannay HJ, Copeland K, Fletcher JM, Boudousquie A, Dennis M. Attention problems and executive functions in children with spina bifida and hydrocephalus. Child Neuropsychol (2005) ; 11: (3): 265-83. |
[14] | Vinck A, Maassen B, Mullaart R, Rotteveel J. Arnold-Chiari-II malformation and cognitive functioning in spina bifida. J Neurol Neurosurg Psychiatry (2006) ; 77: (9): 1083-6. |
[15] | Burmeister R, Hannay HJ, Copeland K, Fletcher JM, Boudousquie A, Dennis M. Attention problems and executive functions in children with spina bifida and hydrocephalus. Child Neuropsychol (2005) ; 11: (3): 265-83. |
[16] | Bowman RM, Boshnjaku V, McLone DG. The changing incidence of myelomeningocele and its impact on pediatric neurosurgery: A review from the Children’s Memorial Hospital. Childs Nerv Syst (2009) ; 25: (7): 801-6. doi: 10.1007/s00381-009-0865-z. |
[17] | Verhoef M, Post MWM, Barf HA, van Asbeck FWA, Gooskens RHJM, Prevo AJH. Perceived health in young adults with spina bifida. Dev Med Child Neurol (2007) ; 49: (3): 192-7. |
[18] | International Association for the Study of Pain. IASP Taxonomy [Internet]. Washington: International Association for the Study of Pain; (2012) (cited 2017 February 15). Available from: http//www.iasp-pain.org/Taxonomy. |
[19] | Merskey H, Bogduk N. International Association for the Study of Pain. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. Seattle: IASP Press; (1994) . |
[20] | Clancy CA, McGrath CJ, Oddson BE. Pain in children and adolescents with spina bifida. Dev Med Child Neurol (2005) ; 47: (1): 27-34. Erratum in: Dev Med Child Neurol (2006) ; 48: (2): 159. |
[21] | Thomson JD, Segal LS. Orthopedic management of spina bifida. Dev Disabil Res Rev (2010) ; 16: (1): 96-103. doi: 10.1002/ddrr.97. |
[22] | Wang HS, Wiener JS, Ross SS, Routh JC. Emergent care pattern in patients with spina bifida. A case-control study. J Urol (2015) ; 193: (1): 268-73. doi: 10.1016/j.juro.2014.06.085. |
[23] | Weenboer PW, Procee AI, Verheijden JMA, Ruud Bosch JLH, van Asbeck FWA, de Kort LMO. Medical and psychosocial problems in middle-aged spina bifida patients: Survey among members of the Dutch patients’ association. Disabil Rehabil (2014) ; 36: (7): 539-45. doi: 10.3109/09638288.2013.801522. |
[24] | Wagner R, Linroth R, Gangl C, Mitchell N, Hall M, Cady R, et al. Perception of secondary conditions in adults with spina bifida and impact on daily life. Disabil Health J (2015) ; 8: (4): 492-8. doi: 10.1016/j.dhjo.2015.03.012. |
[25] | Bellin MH, Dicianno BE, Osteen P, Dosa N, Aparicio E, Braun P, et al. Family satisfaction, pain, and quality-of-life in emerging adults with spina bifida: A longitudinal analysis. Am J Phys Med Rehabil (2013) ; 92: (8): 641-55. doi: 10.1097/PHM.0b013e31829b4bc1. |
[26] | Edwards RJ, Witchell C, Pople IK. Chronic headaches in adults with spina bifida and associated hydrocephalus. Eur J Pediatr Surg (2003) ; 13: (Suppl 1): S13-7. |
[27] | Werhagen L, Hulting C, Borg K. Pain, especially neuropathic pain, in adults with spina bifida, and its relation to age, neurological level, completeness, gender and hydrocephalus. J Rehabil Med (2010) ; 42: (4): 374-6. doi: 10.2340/16501977-0529. |
[28] | Burström K, Egmar A, Sun S, Eriksson M, Svartengren M. Utveckling av EQ-5D-Y – en barnvänlig version av det hälsorelaterade livskvalitetsinstrumentet EQ-5D. A report from the Karolinska Institute’s Public Health Academy (2010) ; 1-30. Swedish only. |
[29] | Bartonek A, Saraste H, Knutson LM. Comparison of different systems to classify the neurological level of lesion in patients with myelomeningocele. Dev Med Child Neurol (1999) ; 41: (12): 796-805. |
[30] | Bartonek A, Saraste H. Factors influencing ambulation in myelomeningocele: A cross-sectional study. Dev Med Child Neurol (2001) ; 43: (4): 253-60. |
[31] | Alriksson-Schmidt A, Hägglund G. Pain in children and adolescents with cerebral palsy: A population-based registry study. Acta Paediatr (2016) ; 105: (6): 665-70. doi: 10.1111/apa.13368. |
[32] | Cheung CW, Choi SW, Wong SS, Lee Y, Irwin MG. Changes in prevalence, outcomes, and help-seeking behavior of chronic pain in an aging population over the last decade. Pain Pract (2017) ; 5: : 643-654. doi: 10.1111/papr.12496. |
[33] | Ahacic K, Kåreholt I. Prevalence of musculoskeletal pain in the general Swedish population from 1968 to 2002: Age, period, and cohort patterns. Pain (2010) ; 151: (1): 206-14. doi: 10.1016/j.pain.2010.07.011. |
[34] | Balaj M, Huijts T, McNamara CL, Stornes P, Bambra C, Eikemo TA. Non-communicable diseases and the social determinants of health in the Nordic countries: Findings from the European Social Survey (2014) special module on the social determinants of health. Scand J Public Health (2017) ; 45: (2): 90-102. doi: 10.1177/1403494816686026. |
[35] | Alriksson-Schmidt AI, Wallander J, Biasini F. Quality of life and resilience in adolescents with a mobility disability. J Pediatr Psychol (2007) ; 32: (3): 370-9. |
[36] | Fellinghauer B, Reinhardt JD, Stucki G, Bickenbach J. Explaining the disability paradox: A cross-sectional analysis of the Swiss general population. BMC Public Health (2012) ; 12: : 655. doi: 10.1186/1471-2458-12-655. |
[37] | Albrecht GL, Devlieger PJ. The disability paradox: High quality of life against all odds. Soc Sci Med (1999) ; 48: (8): 977-988. |




