Advice to People with Parkinson’s in My Clinic: Probiotics and Prebiotics
Abstract
There is increasing evidence that microbial-based therapies can be useful in people with Parkinson’s disease (PD). In this viewpoint, we provide a state-of-the-art review of the clinical and pre-clinical evidence for probiotics and prebiotics in PD. Currently, short-term clinical studies, including double-blind placebo-controlled randomized clinical trials, have demonstrated safety, and efficacy primarily in improving constipation-related symptoms. Pre-clinical studies consistently reported improvements in a range of biological markers and outcomes, including evidence for attenuation of gut dysfunction and neuroprotection. Bacteria from the genus Lactobacillus and Bifidobacterium have been the most frequently studied both in clinical and pre-clinical probiotics studies, while research into prebiotics is still limited and primarily involved resistant starch and fructooligosaccharides. We provide practical suggestions for clinicians on how to advise patients in the clinic regarding these popular treatments, and important caveats to be aware of. Finally, areas for further advancements are highlighted. It is envisaged that in the future, microbial-based therapies may benefit from personalization based on an enhanced understanding of a whole range of host factors and host-microbiome interactions.
INTRODUCTION
The role of the microbiome-gut-brain axis has garnered significant attention in Parkinson’s disease (PD), in which gastrointestinal dysfunction is a prominent feature.1–5 A growing number of clinical studies (>50) in the field have found an alteration of gut microbiome and metabolome in patients with PD, although specific microbial changes are quite heterogenous across different research populations.1,6–9 Meanwhile, several pre-clinical studies have demonstrated that gut microbes and their microbial products (e.g., lipopolysaccharide or amyloid curli protein) can promote gut hyperpermeability and inflammation, leading to systemic inflammation and neuroinflammation, as well as increased α-synuclein pathology in the gut and/or the brain.4,10,11 Notably, gut microbes in the stomach (i.e., Helicobacter pylori) and small bowel (e.g., tyrosine-decarboxylase-producing bacteria) have been implicated in the metabolism or absorption of levodopa which is the mainstay pharmacological treatment for PD.12–14
Excitingly, various microbial-directed therapies such as dietary modification, supplementation with probiotics and/or prebiotics, as well as fecal microbiota transplantation, are rapidly emerging as promising treatment strategies for PD.1,2,15,16 Among them, probiotics and prebiotics which have been widely used as health supplements, are propitious and popular candidates as they are deemed to be affordable, accessible, safe, and effective.17 Indeed, beliefs regarding the health-promoting attributes of microbial-based products date far back into early civilization, when humans started consuming fermented foods.18 It is not surprising that their potential benefits in PD have been one of the commonly asked questions by patients in the clinic. This is also due in part to the high frequency of gastrointestinal symptoms in this patient population, particularly constipation with a prevalence of up to 70%.19 In this Viewpoint, we summarize the clinical and pre-clinical evidence for probiotics and prebiotics (and their combination) as potential symptomatic or disease-modifying treatments in PD. Additionally, we discuss current recommendations and safety considerations for their usage in PD patients and offer perspectives on future development of these non-invasive gut-modulating therapies.
WHAT ARE PROBIOTICS AND PREBIOTICS?
Probiotics have been defined by expert consensus as “live microorganisms, which when administered in adequate amounts, confer a health benefit on the host”.20 Since Metchnikoff’s observation on the potential link between consumption of fermented milk and extreme longevity in the early 1900s to the recent advances in microbiome science, there has been an exponential growth in the multibillion dollar probiotics industry, with an estimated value ∼USD70 billion in 2023.18 Unfortunately, the term “probiotics” has been frequently misused and exploited, with products sold as such without manufacturers providing evidence for their health claims. While various national food and safety regulatory bodies have established guidelines, the International Scientific Association for Probiotics and Prebiotics (ISAPP) recommends that the term probiotic be used only on products that contain well-defined probiotic strain(s) with proof of delivery of viable strain(s) at efficacious doses, and convincing evidence of health effects in human studies.20 Meanwhile, fermented foods with undefined microbial content should be described as “containing live and active cultures”, but should not be called probiotics.20 More recently, to regulate and distinguish the use of live microorganisms as medicinal drugs vs. nutritional supplements, the United States Food and Drug Administration (FDA) and the European Pharmacopeia have released guidelines for the development and testing of “live biotherapeutic products (LBPs)” in clinical trials, which require stringent standards of safety, reliability, robustness, and consistency of each produced batch of probiotic strain(s).21
The concept of prebiotics is relatively newer, where in the 1990s, microbial-targeted substrates such as non-digestible dietary oligosaccharides were recognized for their abilities to promote beneficial change in host microbial composition and/or activities.22 The definition of prebiotics was updated by the ISAPP in 2017, as “a substrate that is selectively utilized by host microorganisms conferring a health benefit”.22 Selectivity to microbial fermentation is considered central to the prebiotics concept, and distinguishes prebiotic compounds such as fructooligosaccharides (FOS) and galactooligosaccharides (GOS) known to enrich Lactobacillus spp. and Bifidobacterium spp., from other dietary fibers that are broadly metabolized.22 These microbial substrates can also be co-administered with live microorganisms for complementary or synergistic effects on host health, and are defined as synbiotics.23
Probiotics and prebiotics act through diverse mechanisms, in part, mediated through modification of the microbiota and/or its function.15,17,24 Their potential beneficial effects on system-wide metabolic and physiological functions include modulation of immune function, defense against pathogens, production of organic acids, and improvement of gut barrier function.15,17,24 Notably, their benefits on human health may be tied to specific strains and specific diseases, e.g., a particular probiotic strain or mixture of strains may be effective for one disease but ineffective for other diseases.17,25 Given the complex inter-individual variability in the gut microbiome, these microbial-based therapies may also require personalization based on host microbiome profiles.26
EVIDENCE FOR PROBIOTICS IN PARKINSON’S DISEASE
Probiotics have been most extensively studied to assess their efficacy in managing gut-related symptoms. Several meta-analyses have demonstrated that certain probiotics (e.g., Bifidobacterium lactis) improve chronic constipation in adults (including older adults), but the evidence is mixed with large heterogeneity across studies.27
In PD, five double-blind placebo-controlled randomized clinical trials (RCTs), with sample sizes ranging from 27 to 128, showed that single-strain or multi-strain probiotics significantly improved bowel movement, stool consistency, constipation severity scores, constipation-related quality of life, and/or laxative usage, for up to 12 weeks (Table 1).28–32 Two other double-blind placebo-controlled RCTs (n = 55–120) combined multi-strain probiotics with prebiotics (i.e., prebiotic fiber and FOS) and found similar improvements in constipation symptoms,33,34 with one of the studies additionally documenting a reduction in colonic transit time.34
Table 1
Probiotics, Prebiotics, and Probiotics with Prebiotics Clinical Studies in Parkinson’s Disease
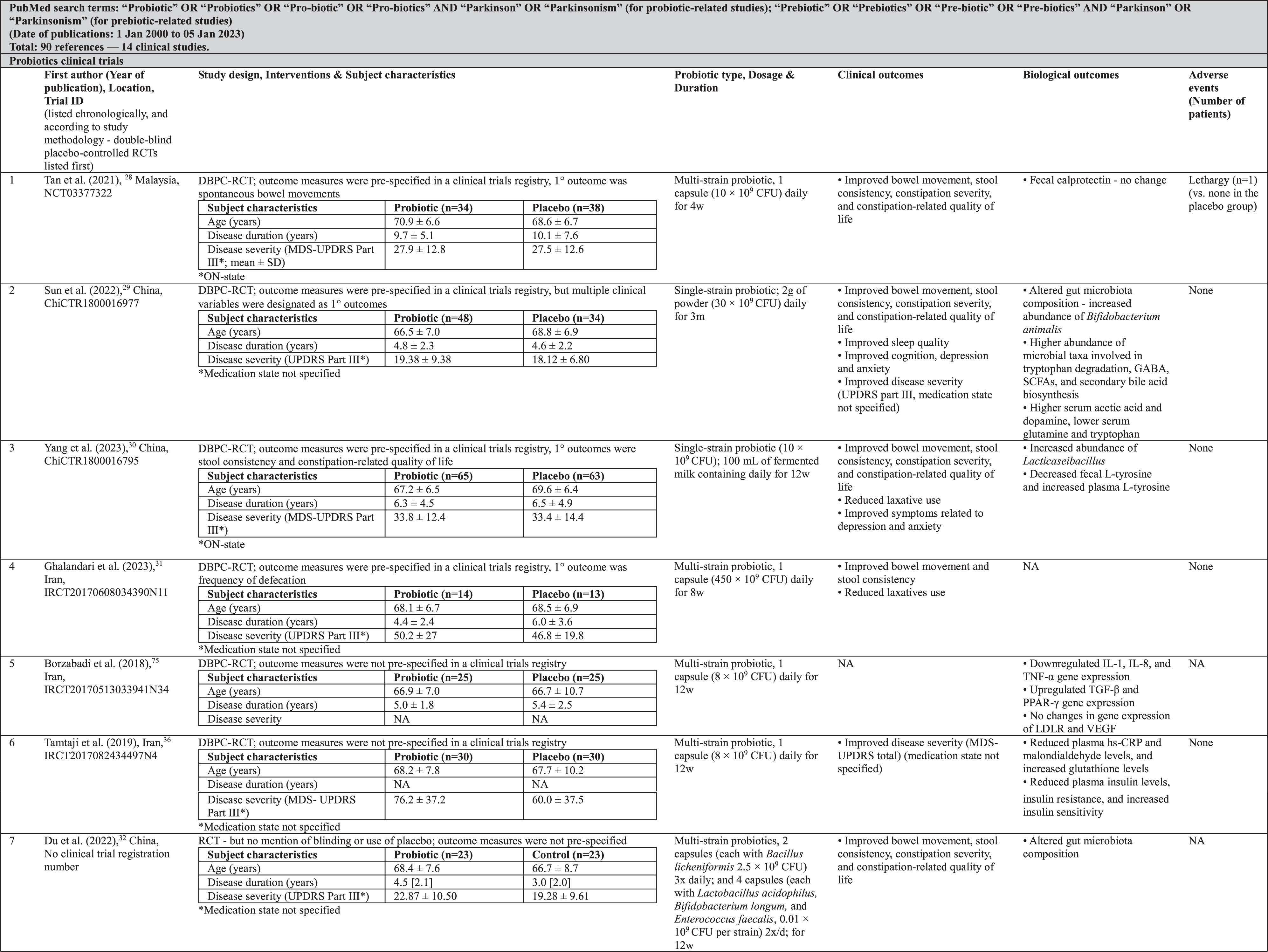 |
Probiotics clinical studies also noted improvements in PD severity,29,35,36 as well as in the duration of the ON and OFF periods.35 However, major limitations of these studies were the lack of specification whether motor evaluations were done in the medication-ON vs. medication-OFF state,29,36 reporting of only total scores of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) without separate reporting of subscores (parts I–IV) as has been recommended (raising concerns about selective reporting36),37 and open-label methodology with a high risk of bias.35 Investigators also reported improved sleep quality,29 cognition,29 depression,29,30 anxiety,29,30 and PD-related quality of life.35 Adverse events in these clinical trials appear to be very infrequent (Table 1).
The commonly used probiotics bacteria in these studies were from the genus Lactobacillus (L. acidophilus , L. casei [3], L. plantarum [3], L. reuteri [3]) and Bifidobacterium (B. longum [4], B. bifidum [3], and B. infantis [3]) (Table 2). These were most frequently presented as capsules containing multiple strains and consumed 1–2× daily for 4–12 weeks.
Table 2
Commonly used probiotic species in probiotic clinical studies in Parkinson’s disease
| Study | Tan | Sun | Yang | Ghalandari | Borzabadi | Tamtaji | Du | Georgescu | Lu | Cassani | Barichella | Ibrahim | Number of reports in Clinical Studies | Number of reports in Pre-Clinical Studies |
| (2021) | (2022) | (2023) | (2023) | (2018) | (2019) | (2022) | (2016) | (2021) | (2011) | (2016) | (2020) | |||
| Duration | 4 weeks | 3 months | 12 weeks | 8 weeks | 12 weeks | 12 weeks | 12 weeks | 3 months | 12 weeks | 5 weeks | 4 weeks | 8 weeks | ||
| Total Species | 8 | 1 | 1 | 8 | 4 | 4 | 4 | 2 | 1 | 1 | 9 | 5 | ||
| Bacillus licheniformis | X | 1 | 0 | |||||||||||
| Bifidobacterium animalis | X | X | 2 | 5 | ||||||||||
| Bifidobacterium bifidum | X | X | X | 3 | 5 | |||||||||
| Bifidobacterium breve | X | X | 2 | 7 | ||||||||||
| Bifidobacterium infantis | X | X | X | 3 | 4 | |||||||||
| Bifidobacterium longum | X | X | X | X | 4 | 6 | ||||||||
| Enterococcus faecalis | X | X | 2 | 0 | ||||||||||
| Enterococcus faecium | X | X | 2 | 3 | ||||||||||
| Lactobacillus acidophilus | X | X | X | X | X | X | X | X | 8 | 11 | ||||
| Lactobacillus bulgaricus | X | 1 | 0 | |||||||||||
| Lactobacillus casei | X | X | X | 3 | 5 | |||||||||
| Lactobacillus delbrueckii | X | 1 | 4 | |||||||||||
| Lactobacillus fermentum | X | X | 2 | 4 | ||||||||||
| Lactobacillus gasseri | X | 1 | 1 | |||||||||||
| Lactobacillus lactis | X | 1 | 4 | |||||||||||
| Lactobacillus paracasei | X | X | 2 | 4 | ||||||||||
| Lactobacillus plantarum | X | X | X | 3 | 16 | |||||||||
| Lactobacillus reuteri | X | X | X | 3 | 3 | |||||||||
| Lactobacillus rhamnosus | X | X | 2 | 12 | ||||||||||
| Streptococcus salivarius | X | 1 | 1 | |||||||||||
| Streptococcus thermophilus | X | 1 | 3 |
Biological processes investigated in these trials included probiotics’ ability to alter host gut microbiota composition and metabolic capacity, modulate host metabolism, and reduce host inflammatory pathology and gut barrier dysfunction. Among the various biological markers assessed were short-chain fatty acids (SCFAs), dopamine, tryptophan, glutamine, insulin, calprotectin, interleukins, hs-CRP, and zonulin, measured in faeces or in biological fluids such as blood or urine (Table 1). While alterations in biological markers were often (although not always) observed with probiotics treatment, their biological and clinical significance, and the mechanisms underlying these effects, are currently uncertain.
In this regard, pre-clinical animal and cell models provide valuable opportunities to further dissect the mechanistic effects of probiotics, particularly those mechanisms that cannot be easily investigated in human subjects. We have previously discussed these in detail and provide an updated compilation of these studies in Supplementary Table 1.15 This is evidently an active field of research with a relatively large number (∼40) of studies published. The vast majority were in murine models, and in line with human studies mostly commonly involved the administration of bacteria from the genus Lactobacillus (L. plantarum, L. rhamnosus, L. acidophilus) and Bifidobacterium (B. breve, B. animalis, and B. bifidum) (Table 1 and Supplementary Table 4). A variety of positive effects were reported in pre-clinical models of probiotics in PD, besides recapitulating those observed in human studies. These included evidence for neuroprotection, e.g., the rescue of dopaminergic cells;38–42 reduction of α-synuclein aggregation,43–45 neuroinflammation and oxidative stress;39,40,44–50 and restoration of neurotrophic pathways;38 with “clinical” benefits on motor,38,40–44,47,49–52 and non-motor (such as cognitive) functions.40,50,53 While the consistency of beneficial probiotics effects across different experimental systems would appear to give confidence regarding their health benefits, publication bias (with positive findings much more likely to be published than negative ones) suggests caution in interpreting the literature.1,54 Emphasis should increasingly be given to replication studies, both positive and negative, in the basic and clinical sciences.55
EVIDENCE FOR PREBIOTICS IN PARKINSON’S DISEASE
In general, the evidence on prebiotics in PD has lagged behind that for probiotics. As discussed above, prebiotics administered together with probiotics have demonstrated efficacy in managing constipation-related symptoms in PD (Table 1).33,34
There are thus far only two clinical trials specifically studying prebiotics in PD (Table 1). Although this research has provided valuable insights into the possible mechanistic effects of prebiotics interventions in PD patients (discussed further below), the studies were non-randomized and open-label and thus have a high risk of bias. The study by Hall et al., using a prebiotics bar (containing a mixture of resistant starch, rice bran, resistant maltodextrin, and inulin) for ten days, reported improved gastrointestinal symptoms in the subgroup of patients on PD medications, but not in the de novo (medication-untreated) patients, and not in constipation symptoms.56 Similarly, constipation symptoms were not improved in another study utilizing resistant starch, although overall non-motor symptom burden and depressive symptoms were reported to be reduced post-intervention.57 Exploratory analyses in the former study also suggested a small improvement in total UPDRS score after prebiotics administration.56
In these studies, prebiotics significantly altered the gut microbiome by reducing the abundance of pro-inflammatory bacteria and increasing the levels of SCFA-producing bacteria.56,57 This finding was further corroborated by the restoration of fecal butyrate levels,57 and increased plasma SCFA levels post-intervention.56 Additionally, reduced levels of plasma zonulin,56 and fecal calprotectin seen after prebiotics administration suggested improvement in intestinal barrier integrity and gut inflammation,56,57 respectively.
Pre-clinical studies of prebiotics in PD are also relatively fewer (7 published), compared to probiotics studies (Supplementary Table 3). Various types of fibers, including wheat bran, resistant maltodextrin, inulin, and nutriose, and potential prebiotics like flavanol and dioscin, have been tested on different animal and cell models to explore the effects and mechanisms of prebiotics in PD. Consistent with the findings from clinical trials, prebiotics supplementation was found to remodel the altered gut microbiome towards an increased abundance of taxa associated with potentially protective effects.58–60 A range of other benefits similar to those observed with probiotics was also reported, including evidence for neuroprotection and “clinical” motor and non-motor improvements.58,60–66 Interestingly, one study reported much better neuroprotective effects when prebiotic was combined with probiotic (in this case, polymannuronic acid and L. rhamnosus GG, respectively),66 suggesting that further study of probiotics-prebiotics combinations could be fruitful (Supplementary Table 2).
WHAT WE TELL PEOPLE WITH PARKINSON’S IN OUR CLINIC?
While pre-clinical and clinical evidence suggest promising benefits of probiotics and prebiotics in PD, careful consideration of indications and safety issues, adherence to regulatory guidelines, and individualized risk assessment are essential to ensure their safe and effective use. Here, we summarize recommendations for patients based on the limited but growing studies in the PD field, as well as general guidelines and knowledge on these supplements. The discussion with patients should obviously also take into account their level of understanding, and desire for knowledge, regarding PD and its management.67–70
• Indications: There is reasonably good evidence that usage of probiotics, with or without prebiotic fiber,28,33 can improve bowel movements in patients with PD who are experiencing constipation. Potential benefits of probiotics or prebiotics to treat motor symptoms or other non-motor symptoms (e.g., sleep, anxiety, depression, and cognition) in PD are still being explored (NCT04871464, NCT04722211, NCT03968133, NCT05568498, NCT06019117, NCT06118294, NCT04140760, and NCT05576818).
• Formulation and storage: Probiotics are not all the same. Data remain limited regarding the ideal formulations of probiotics (e.g., single- vs. multi-strain, type of strain(s), etc.) for patients with PD. Strains from the genus Lactobacillus (e.g., L. acidophilus, L. casei, L. plantarum, L. reuteri) and Bifidobacterium (e.g., B. longum, B. bifidum, B. infantis) are better studied for their potential benefits in PD patients, compared to others. Meanwhile, there is growing evidence on the role of gut microbes in levodopa metabolism;13,14,71,72 for example, Enterococcus faecalis strains were shown to reduce levodopa bioavailability through tyrosine decarboxylase-dependent metabolism in two pre-clinical studies,13,71 raising caution for the use of probiotics containing these strains. Although none of the RCTs in PD reported worsening motor symptoms or motor response complications after probiotics/prebiotics intervention, health practitioners should keep their mind open to this possibility.
Multi-strain probiotics are hypothesized to confer greater benefits through additive or synergistic actions, but some strains may compete for nutrient sources and inhibit each other’s growth. It is therefore important that multi-strain formulations have in vitro evidence demonstrating symbiosis between selected strains.73
Data are even more scarce for prebiotics, where the exact ingredients of prebiotic fiber are infrequently reported. Resistant starch and FOS are among the prebiotics used in PD studies.
Probiotics/prebiotics are frequently packaged in capsule, powder, or liquid forms. Freeze-dried probiotics, delivered in capsules or powder, generally contain higher doses of probiotics, are more convenient for storage (usually in cool, dry, and dark places), and have a longer shelf-life.74 Meanwhile, fermented milk containing probiotics requires cold-chain preservation.
• Dosing and duration of treatment: While it is well recognized that appropriate dosing is important to achieve the intended clinical effect of probiotics treatment, there is no standard recommendation for probiotics dosage. Most probiotics supplements contain 1–10 billion colony forming units (CFUs) per dose. Dosages used in PD RCTs ranged widely from 2.5–450 billion CFUs per dose; most studies administered between 8 to 30 billion CFUs per dose (Table 1).28–30,35,36,75 Higher CFU counts do not necessarily equate to greater health benefits. Notably, the number of live microorganisms can drop during storage; patients should look for products labelled with the number of CFUs at the end of the product’s shelf-life, not just at the time of manufacture. As the duration of RCTs was between 4 to 12 weeks, the longer-term efficacy and side effect profiles of probiotics and prebiotics in PD patients remain open questions.
• Safety: Many probiotics strains are derived from species with a long history of safe use in foods or from gut commensal bacteria and are therefore unlikely to cause harm in healthy individuals. Side effects of probiotics/prebiotics are usually minor and consist mainly of self-limiting gastrointestinal symptoms. Notably, none of the RCTs in PD reported serious adverse events. However, probiotics should be used with caution in individuals who are severely ill or immunocompromised (e.g., organ transplant recipients, those undergoing chemotherapy or radiation therapy),76 as use of probiotics has been linked to systemic infections, excessive immune stimulation, and antimicrobial resistance.76
Regulatory bodies have established safety criteria for the assessment of probiotics for human use (encompassing records of isolation history, taxonomic identification, and absence of virulence, infectivity, toxicity, and transferable antibiotic resistance genes). In the USA and Europe, strains that have passed safety criteria are designated as “Generally Recognized as Safe (GRAS)” or have “qualified presumption of safety (QPS)” status, respectively.77
• Product label and quality: The ISAPP has developed an informative consumer guide to decipher a probiotics product label (https://isappscience.org/wp-content/uploads/2019/04/Probiotic_labeling-_rev1029-1.pdf). The probiotics product must clearly identify all microorganisms contained in the product (species and/or strain), which should be deposited in an internationally recognized culture collection.
Marketing regulatory guidelines for prebiotics are less clear and vary between countries. Most prebiotics products are currently marketed as dietary fiber, or as complementary ingredients in probiotics and/or nutritional milk supplements. Nevertheless, prebiotics product labels should contain standard information for dietary supplements including serving size and suggested dosage, quantity, and percentage composition of active ingredients per unit dose, other ingredients, and expiration date.
Additionally, stamps of approval from local regulatory bodies, good manufacturing practice (GMP) certification and/or other accreditations from recognized third party testing laboratories, signify that the product manufacturer has conformed to local safety standards and best practices to ensure quality-controlled production and delivery of these supplements. Importantly, health claims on the product label should be interpreted carefully; probiotics/prebiotics products in the USA carry a disclaimer statement from the FDA that the health claim statements have not beenevaluated.
CONCLUSION AND FUTURE PERSPECTIVES
Notably, existing probiotics and prebiotics products in PD trials have yet to meet regulatory requirements to be marketed as a drug (i.e., intended for use in the diagnosis, cure, treatment, or prevention of disease). In this regard, two novel probiotics strains, Parabacteroides distasonis (MRX0005) and Megasphaera massiliensis (MRX0029) were shown to have potent anti-inflammatory and antioxidant effects,78 and have recently received FDA approval as LBPs with an ongoing phase 1 trial. There are emerging microbial therapeutic innovations to optimize the clinical efficacy of live microorganisms, including the development of symbiotic microbial consortia, which are assemblies of well-characterized microbial strains that work synergistically towards specific microbial functions.79 For example, the microbial consortium GUT-108 consists of 11 microbial strains that have the ability to reverse gut inflammation by synthesis of SCFAs, indole derivatives, and deconjugation of bile salts into therapeutic bile acids.80 Besides pre-clinical evidence in treating colitis, GUT-108 holds promise to treat a range of conditions that are affected by dysbiosis-mediated intestinal inflammation and hyperpermeability, such as PD.80
Genetically-engineered probiotics bacteria with specific desired phenotypic traits that can be transferred to the host, such as the expression of glucagon-like peptide-1 (GLP-1, which plays key functions in neurogenesis, neuronal metabolism, and synaptic plasticity) have shown promise in two pre-clinical studies in PD (Supplementary Table 1).15,81,82 Meanwhile, in a mouse model study, administration of a human probiotic strain Escherichia coli Nissle 1917 that was genetically engineered to produce levodopa continuously led to good pharmacodynamic responses with no side effects.83 Additionally, genetic engineering strategies can be used to improve the survival and delivery of LBPs, which encounter various physiological challenges during passage from the stomach to the large bowel.84 To our knowledge, these “next-generation” biotherapeutics have yet to be tested in human PD clinical trials.81,82,85–89
Importantly, as with management of PD overall,68,70,90 a key principle with microbial-directed therapeutics in PD is that “one size does not fit all”. Host factors (e.g., baseline gut microbial profiles, genetics, diet, lifestyle, comorbidities, and/or medications) and host-microbiome interactions are likely key factors influencing disease manifestations and the response to treatments,91–94 including probiotics and prebiotics therapy.1,24 With continued research efforts into these therapies, it is envisioned that more personalized recommendations for probiotics and prebiotics in PD can be made in the future.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the funding support from the Ministry of Higher Education Malaysia (FRGS/1/2018/SKK02/UM/02/1) and the Malaysia Science Toray Foundation, for their previous and ongoing research work on probiotics in Parkinson’s disease.
FUNDING
The authors did not receive funding support for the preparation of this article.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available within the article and/or its supplementary material.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-240172.
REFERENCES
1. | Tan AH , Lim SY and Lang AE . The microbiome-gut-brain axis in Parkinson disease – from basic research to the clinic. Nat Rev Neurol (2022) ; 18: : 476–495. |
2. | Tan AH , Chuah KH , Beh YY , et al. Gastrointestinal dysfunction in Parkinson’s disease: neuro-gastroenterology perspectives on a multifaceted problem. J Mov Disord (2023) ; 16: : 138–151. |
3. | Hor JW , Lim SY , Khor ES , et al. Fecal calprotectin in Parkinson’s disease and multiple system atrophy. J Mov Disord (2022) ; 15: : 106–114. |
4. | Perez-Pardo P , Dodiya HB , Engen PA , et al. Role of TLR4 in the gut-brain axis in Parkinson’s disease: A translational study from men to mice. Gut (2019) ; 68: : 829–843. |
5. | Chiang HL and Lin CH . Altered gut microbiome and intestinal pathology in Parkinson’s disease. J Mov Disord (2019) ; 12: : 67–83. |
6. | Tan AH , Chong CW , Lim SY , et al. Gut microbial ecosystem in Parkinson disease: New clinicobiological insights from multi-omics. Ann Neurol (2021) ; 89: : 546–559. |
7. | Toh TS , Chong CW , Lim SY , et al. Gut microbiome in Parkinson’s disease: New insights from meta-analysis. Parkinsonism Relat Disord (2022) ; 94: : 1–9. |
8. | Boktor JC , Sharon G , Verhagen Metman LA , et al. Integrated multi-cohort analysis of the Parkinson’s disease gut metagenome. Mov Disord (2023) ; 38: : 399–409. |
9. | Wallen ZD , Demirkan A , Twa G , et al. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat Commun (2022) ; 13: : 6958. |
10. | Sampson TR , Debelius JW , Thron T , et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell (2016) ; 167: : 1469–1480.e1412. |
11. | Sampson TR , Challis C , Jain N , et al. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. Elife (2020) ; 9: : e53111. |
12. | Tan AH , Lim SY , Mahadeva S , et al. Helicobacter pylori eradication in Parkinson’s disease: A randomized placebo-controlled trial. Mov Disord (2020) ; 35: : 2250–2260. |
13. | Maini Rekdal V , Bess EN , Bisanz JE , et al. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science (2019) ; 364: : eaau6323. |
14. | van Kessel SP , de Jong HR , Winkel SL , et al. Gut bacterial deamination of residual levodopa medication for Parkinson’s disease. BMC Biol (2020) ; 18: : 137. |
15. | Tan AH , Hor JW , Chong CW , et al. Probiotics for Parkinson’s disease: Current evidence and future directions. JGH Open (2021) ; 5: : 414–419. |
16. | Bruggeman A , Vandendriessche C , Hamerlinck H , et al. Safety and efficacy of faecal microbiota transplantation in patients with mild to moderate Parkinson’s disease (GUT-PARFECT): A double-blind, placebo-controlled, randomised, phase 2 trial. EClinicalMedicine (2024) ; 71: : 102563. |
17. | Sanders ME , Merenstein DJ , Reid G , et al. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat Rev Gastroenterol Hepatol (2019) ; 16: : 605–616. |
18. | Britton RA , Hoffmann DE and Khoruts A . Probiotics and the microbiome-how can we help patients make sense of probiotics? Gastroenterology (2021) ; 160: : 614–623. |
19. | Knudsen K , Krogh K , Østergaard K , et al. Constipation in Parkinson’s disease: Subjective symptoms, objective markers, and new perspectives. Mov Disord (2017) ; 32: : 94–105. |
20. | Hill C , Guarner F , Reid G , et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol (2014) ; 11: : 506–514. |
21. | Charbonneau MR , Isabella VM , Li N , et al. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat Commun (2020) ; 11: : 1738. |
22. | Gibson GR , Hutkins R , Sanders ME , et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol (2017) ; 14: : 491–502. |
23. | Swanson KS , Gibson GR , Hutkins R , et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol (2020) ; 17: : 687–701. |
24. | Suez J , Zmora N , Segal E , et al. The pros, cons, and many unknowns of probiotics. Nat Med (2019) ; 25: : 716–729. |
25. | McFarland LV , Evans CT and Goldstein EJC . Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis. Front Med (Lausanne) (2018) ; 5: : 124. |
26. | Zmora N , Zilberman-Schapira G , Suez J , et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell (2018) ; 174: : 1388–1405.e1321. |
27. | van der Schoot A , Helander C , Whelan K , et al. Probiotics and synbiotics in chronic constipation in adults: A systematic review and meta-analysis of randomized controlled trials. Clin Nutr (2022) ; 41: : 2759–2777. |
28. | Tan AH , Lim SY , Chong KK , et al. Probiotics for constipation in Parkinson disease: A randomized placebo-controlled study. Neurology (2021) ; 96: : e772–e782. |
29. | Sun H , Zhao F , Liu Y , et al. Probiotics synergized with conventional regimen in managing Parkinson’s disease. NPJ Parkinsons Dis (2022) ; 8: : 62. |
30. | Yang X , He X , Xu S , et al. Effect of Lacticaseibacillus paracasei strain Shirota supplementation on clinical responses and gut microbiome in Parkinson’s disease. Food Funct (2023) ; 14: : 6828–6839. |
31. | Ghalandari N , Assarzadegan F , Habibi SAH , et al. Efficacy of probiotics in improving motor function and alleviating constipation in Parkinson’s disease: A randomized controlled trial. Iran J Pharm Res (2023) ; 22: : e137840. |
32. | Du Y , Li Y , Xu X , et al. Probiotics for constipation and gut microbiota in Parkinson’s disease. Parkinsonism Relat Disord (2022) ; 103: : 92–97. |
33. | Barichella M , Pacchetti C , Bolliri C , et al. Probiotics and prebiotic fiber for constipation associated with Parkinson disease: An RCT. Neurology (2016) ; 87: : 1274–1280. |
34. | Ibrahim A , Ali RAR , Manaf MRA , et al. Multi-strain probiotics (Hexbio) containing MCP BCMC strains improved constipation and gut motility in Parkinson’s disease: A randomised controlled trial. PLoS One (2020) ; 15: : e0244680. |
35. | Lu CS , Chang HC , Weng YH , et al. The add-on effect of Lactobacillus plantarum PS128 in patients with Parkinson’s disease: A pilot study. Front Nutr (2021) ; 8: : 650053. |
36. | Tamtaji OR , Taghizadeh M , Daneshvar Kakhaki R , et al. Clinical and metabolic response to probiotic administration in people with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr (2019) ; 38: : 1031–1035. |
37. | Goetz CG , Tilley BC , Shaftman SR , et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord (2008) ; 23: : 2129–2170. |
38. | Srivastav S , Neupane S , Bhurtel S , et al. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J Nutr Biochem (2019) ; 69: : 73–86. |
39. | Castelli V , d’Angelo M , Lombardi F , et al. Effects of the probiotic formulation SLAB51 in in vitro and in vivo Parkinson’s disease models. Aging (Albany N Y) (2020) ; 12: : 4641–4659. |
40. | Alipour Nosrani E , Tamtaji OR , Alibolandi Z , et al. Neuroprotective effects of probiotics bacteria on animal model of Parkinson’s disease induced by 6-hydroxydopamine: A behavioral, biochemical, and histological study. J Immunoassay Immunochem (2021) ; 42: : 106–120. |
41. | Sun J , Li H , Jin Y , et al. Probiotic Clostridium butyricum ameliorated motor deficits in a mouse model of Parkinson’s disease via gut microbiota-GLP-1 pathway. Brain Behav Immun (2021) ; 91: : 703–715. |
42. | Nápoles-Medina AY , Aguilar-Uscanga BR , Solís-Pacheco JR , et al. Oral administration of Lactobacillus inhibits the permeability of blood-brain and gut barriers in a parkinsonism model. Behav Neurol (2023) ; 2023: : 6686037. |
43. | Goya ME , Xue F , Sampedro-Torres-Quevedo C , et al. Probiotic Bacillus subtilis protects against α-synuclein aggregation in C. elegans. Cell Rep (2020) ; 30: : 367–380.e367. |
44. | Pan S , Wei H , Yuan S , et al. Probiotic Pediococcus pentosaceus ameliorates MPTP-induced oxidative stress via regulating the gut microbiota-gut-brain axis. Front Cell Infect Microbiol (2022) ; 12: : 1022879. |
45. | Wang L , Zhao Z , Zhao L , et al. Lactobacillus plantarum DP189 reduces α-SYN aggravation in MPTP-Induced Parkinson’s disease mice via regulating oxidative damage, inflammation, and gut microbiota disorder. J Agric Food Chem (2022) ; 70: : 1163–1173. |
46. | Magistrelli L , Amoruso A , Mogna L , et al. Probiotics may have beneficial effects in Parkinson’s disease: in vitro evidence. Front Immunol (2019) ; 10: : 969. |
47. | Perez Visñuk D , Savoy de Giori G , LeBlanc JG , et al. Neuroprotective effects associated with immune modulation by selected lactic acid bacteria in a Parkinson’s disease model. Nutrition (2020) ; 79–80: : 110995. |
48. | Ghyselinck J , Verstrepen L , Moens F , et al. Influence of probiotic bacteria on gut microbiota composition and gut wall function in an in-vitro model in patients with Parkinson’s disease. Int J Pharm X (2021) ; 3: : 100087. |
49. | Li T , Chu C , Yu L , et al. Neuroprotective effects of Bifidobacterium breve CCFMin MPTP-induced mouse models of Parkinson’s disease. Nutrients (2022) ; 14: : 4678. |
50. | Chu C , Yu L , Li Y , et al. Lactobacillus plantarum CCFM405 against rotenone-induced Parkinson’s disease mice via regulating gut microbiota and branched-chain amino acids biosynthesis. Nutrients (2023) ; 15: : 1737. |
51. | Hsieh TH , Kuo CW , Hsieh KH , et al. Probiotics alleviate the progressive deterioration of motor functions in a mouse model of Parkinson’s disease. Brain Sci (2020) ; 10: : 206. |
52. | Cuevas-Carbonell SG , Vásquez-Celaya L , García-López D , et al. Chronic treatment with the probiotics Lacticaseibacillus rhamnosus GG and Bifidobacterium lactis BB12 attenuates motor impairment, striatal microglial activation, and dopaminergic loss in rats with 6-hydroxydopamine-induced hemiparkinsonism. Neuroscience (2022) ; 507: : 79–98. |
53. | Xie C and Prasad AA . probiotics treatment improves hippocampal dependent cognition in a rodent model of Parkinson’s disease. Microorganisms (2020) ; 8: : 1661. |
54. | Walter J , Armet AM , Finlay BB , et al. Establishing or exaggerating causality for the gut microbiome: lessons from human microbiota-associated rodents. Cell (2020) ; 180: : 221–232. |
55. | Albanese F , Bloem BR and Kalia LV . Addressing the “replication crisis” in the field of Parkinson’s disease. J Parkinsons Dis (2023) ; 13: : 849–850. |
56. | Hall DA , Voigt RM , Cantu-Jungles TM , et al. An open label, non-randomized study assessing a prebiotic fiber intervention in a small cohort of Parkinson’s disease participants. Nat Commun (2023) ; 14: : 926. |
57. | Becker A , Schmartz GP , Gröger L , et al. Effects of resistant starch on symptoms, fecal markers, and gut microbiota in Parkinson’s disease — The RESISTA-PD Trial. Genomics Proteomics Bioinformatics (2022) ; 20: : 274–287. |
58. | Tsao SP , Nurrahma BA , Kumar R , et al. Probiotic enhancement of antioxidant capacity and alterations of gut microbiota composition in 6-hydroxydopamin-induced Parkinson’s disease rats. Antioxidants (Basel) (2021) ; 10: : 1823. |
59. | Abdel-Haq R , Schlachetzki JCM , Boktor JC , et al. A prebiotic diet modulates microglial states and motor deficits in α-synuclein overexpressing mice. Elife (2022) ; 11: : e81453. |
60. | Mao Z , Hui H , Zhao X , et al. Protective effects of dioscin against Parkinson’s disease via regulating bile acid metabolism through remodeling gut microbiome/GLP-1 signaling. J Pharm Anal (2023) ; 13: : 1153–1167. |
61. | Ho L , Zhao D , Ono K , et al. Heterogeneity in gut microbiota drive polyphenol metabolism that influences α-synuclein misfolding and toxicity. J Nutr Biochem (2019) ; 64: : 170–181. |
62. | Perez-Pardo P , de Jong EM , Broersen LM , et al. Promising effects of neurorestorative diets on motor, cognitive, and gastrointestinal dysfunction after symptom development in a mouse model of Parkinson’s disease. Front Aging Neurosci (2017) ; 9: : 57. |
63. | Krishna G and Muralidhara . Oral supplements of inulin during gestation offsets rotenone-induced oxidative impairments and neurotoxicity in maternal and prenatal rat brain. Biomed Pharmacother (2018) ; 104: : 751–762. |
64. | Yamasaki TR , Ono K , Ho L , et al. Gut microbiome-modified polyphenolic compounds inhibit α-synuclein seeding and spreading in α-synucleinopathies. Front Neurosci (2020) ; 14: : 398. |
65. | Nurrahma BA , Tsao SP , Wu CH , et al. Probiotic supplementation facilitates recovery of 6-OHDA-induced motor deficit via improving mitochondrial function and energy metabolism. Front Aging Neurosci (2021) ; 13: : 668775. |
66. | Liu X , Du ZR , Wang X , et al. Polymannuronic acid prebiotic plus Lacticaseibacillus rhamnosus GG probiotic as a novel synbiotic promoted their separate neuroprotection against Parkinson’s disease. Food Res Int (2022) ; 155: : 111067. |
67. | Tan AH , Tan CT , Marras C , et al. Knowledge of Parkinson’s disease in a multiethnic urban asian setting. J Parkinsons Dis (2015) ; 5: : 865–879. |
68. | Lim SY , Tan AH , Fox SH , et al. Integrating patient concerns into Parkinson’s disease management. Curr Neurol Neurosci Rep (2017) ; 17: : 3. |
69. | Choo XY , Lim SY , Chinna K , et al. Understanding patients’ and caregivers’ perspectives and educational needs in Parkinson’s disease: A multi-ethnic Asian study. Neurol Sci (2020) ; 41: : 2831–2842. |
70. | Bhidayasiri R , Panyakaew P , Trenkwalder C , et al. Delivering patient-centered care in Parkinson’s disease: Challenges and consensus from an international panel. Parkinsonism Relat Disord (2020) ; 72: : 82–87. |
71. | van Kessel SP , Frye AK , El-Gendy AO , et al. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat Commun (2019) ; 10: : 310. |
72. | Cirstea MS , Creus-Cuadros A , Lo C , et al. A novel pathway of levodopa metabolism by commensal Bifidobacteria. Sci Rep (2023) ; 13: : 19155. |
73. | Toscano M , De Grandi R , Pastorelli L , et al. A consumer’s guide for probiotics: 10 golden rules for a correct use. Dig Liver Dis (2017) ; 49: : 1177–1184. |
74. | Kiepś J and Dembczyński R . Current trends in the production of probiotic formulations. Foods (2022) ; 11: : 2330. |
75. | Borzabadi S , Oryan S , Eidi A , et al. The effects of probiotic supplementation on gene expression related to inflammation, insulin and lipid in patients with Parkinson’s disease: A randomized, double-blind, placeb ocontrolled trial. Arch Iran Med (2018) ; 21: : 289–295. |
76. | Doron S and Snydman DR . Risk and safety of probiotics. Clin Infect Dis (2015) ; 60: Suppl 2: S129–S134. |
77. | de Melo Pereira GV , de Oliveira Coelho B , Magalhães Júnior AI , et al. How to select a probiotic? A review and update of methods and criteria. Biotechnol Adv (2018) ; 36: : 2060–2076. |
78. | Ahmed S , Busetti A , Fotiadou P , et al. In vitro characterization of gut microbiota-derived bacterial strains with neuroprotective properties. Front Cell Neurosci (2019) ; 13: : 402. |
79. | Sorbara MT and Pamer EG . Microbiome-based therapeutics. Nat Rev Microbiol (2022) ; 20: : 365–380. |
80. | van der Lelie D , Oka A , Taghavi S , et al. Rationally designed bacterial consortia to treat chronic immune-mediated colitis and restore intestinal homeostasis. Nat Commun (2021) ; 12: : 3105. |
81. | Fang X , Zhou X , Miao Y , et al. Therapeutic effect of GLP-1 engineered strain on mice model of Alzheimer’s disease and Parkinson’s disease. AMB Express (2020) ; 10: : 80. |
82. | Wu H , Wei J , Zhao X , et al. Neuroprotective effects of an engineered Escherichia coli Nissle on Parkinson’s disease in mice by delivering GLP-1 and modulating gut microbiota. Bioeng Transl Med (2023) ; 8: : e10351. |
83. | Padhi P , Abdalla A , Backes N , et al. Emerging microbiome genetic engineering technology for stable levodopa delivery in Parkinson’s disease. FASEB J (2022) ; 36: (s1): doi: 10.1096/fasebj.2022.36.S1.R6272. |
84. | Heavey MK , Durmusoglu D , Crook N , et al. Discovery and delivery strategies for engineered live biotherapeutic products. Trends Biotechnol (2022) ; 40: : 354–369. |
85. | Fang X , Tian P , Zhao X , et al. Neuroprotective effects of an engineered commensal bacterium in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine Parkinson disease mouse model via producing glucagon-like peptide-1. J Neurochem (2019) ; 150: : 441–452. |
86. | Pan H , Sun T , Cui M , et al. Light-sensitive Lactococcus lactis for microbe– gut– brain axis regulating via upconversion optogenetic micro-nano system. ACS Nano (2022) ; 16: : 6049–6063. |
87. | Yue M , Wei J , Chen W , et al. Neurotrophic role of the next-generation probiotic strain L. lactis MG-pMG36e-GLP-1 on Parkinson’s disease via inhibiting ferroptosis. Nutrients (2022) ; 14: : 4886. |
88. | Zhang X , Pang G , Sun T , et al. A red light-controlled probiotic bio-system for in-situ gut-brain axis regulation. Biomaterials (2023) ; 294: : 122005. |
89. | Wang Y , Chen Wj , Han Yy , et al. Neuroprotective effect of engineered Clostridium butyricum-pMTL007-GLP-1 on Parkinson’s disease mice models via promoting mitophagy. Bioeng Transl Med (2023) ; 8: : e10505. |
90. | Bloem BR , Okun MS and Klein C . Parkinson’s disease. Lancet (2021) ; 397: : 2284–2303. |
91. | Paul KC , Chuang YH , Shih IF , et al. The association between lifestyle factors and Parkinson’s disease progression and mortality. Mov Disord (2019) ; 34: : 58–66. |
92. | Müller-Nedebock AC , Dekker MCJ , Farrer MJ , et al. Different pieces of the same puzzle: A multifaceted perspective on the complex biological basis of Parkinson’s disease. NPJ Parkinsons Dis (2023) ; 9: : 110. |
93. | Lim SY and Klein C . Parkinson’s disease is predominantly a genetic disease. J Parkinsons Dis (2024) ; 14: : 467–482. |
94. | Yong VW , Tan YJ , Ng YD , et al. Progressive and accelerated weight and body fat loss in Parkinson’s disease: A three-year prospective longitudinal study. Parkinsonism Relat Disord (2020) ; 77: : 28–35. |
95. | Georgescu D , Ancusa OE , Georgescu LA , et al. Nonmotor gastrointestinal disorders in older patients with Parkinson’s disease: Is there hope? Clin Interv Aging (2016) ; 11: : 1601–1608. |
96. | Cassani E , Privitera G , Pezzoli G , et al. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol Dietol (2011) ; 57: : 117–121. |




