Self-Management Systems for Patients and Clinicians in Parkinson’s Disease Care: A Scoping Review
Abstract
Background:
Digital self-management tools including mobile apps and wearables can enhance personalized care in Parkinson’s disease, and incorporating patient and clinician feedback into their evaluation can empower users and nurture patient-clinician relationships, necessitating a review to assess the state of the art and refine their use.
Objective:
This review aimed to summarize the state of the art of self-management systems used in Parkinson’s disease management, detailing the application of self-management techniques and the integration of clinicians. It also aimed to provide a concise synthesis on the acceptance and usability of these systems from the clinicians’ standpoint, reflecting both patient engagement and clinician experience.
Methods:
The review was organized following the PRISMA extension for Scoping Reviews and PICOS frameworks. Studies were retrieved from PubMed, CINAHL, Scopus, ACM Digital Library, and IEEE Xplore. Data was collected using a predefined form and then analyzed descriptively.
Results:
Of the 15,231 studies retrieved, 33 were included. Five technology types were identified, with systems combining technologies being the most evaluated. Common self-management strategies included educational material and symptom journals. Only 11 studies gathered data from clinicians or reported evidence of clinician integration; out of those, six studies point out the importance of raw data availability, data visualization, and integrated data summaries.
Conclusions:
While self-management systems for Parkinson’s disease are well-received by patients, the studies underscore the urgency for more research into their usability for clinicians and integration into daily medical workflows to enhance overall care quality.
Plain Language Summary
Digital tools, such as smartphone applications and wearable devices, could help people with Parkinson’s disease manage their symptoms by using data and technology to provide support that is personalized to them and by supporting communication between patients and healthcare providers. This review studies current literature on these digital self-management systems for people with Parkinson’s disease. Of the 33 studies included in our review, we found that many of these systems combine different types of digital technologies (for example, a mobile app and a wearable sensor). The most common strategies to help support patients with self-management included in these digital tools were providing educational health content and symptom diaries. Only a few studies have considered healthcare providers’ perspectives on these systems. Those that did highlighted a need for better access to patient data, improved data presentation, and summaries of key health insights. While patients find digital self-management tools favorable, further research is needed to ensure they meet healthcare providers’ professional needs and can fit easily into daily clinical routines, ultimately improving care for individuals with Parkinson’s disease.
INTRODUCTION
Parkinson’s disease (PD) is the most rapidly expanding neurological disorder globally, affecting 1 in 37 people in the UK during their lifetime and currently impacting approximately 153,000 UK residents.1This figure is projected to rise to 172,000 by 2030 with diagnoses occurring at a rate of two every hour.1PD progression and response to treatments vary widely among individuals, with some experiencing minimal progression while others face rapid cognitive and motor declines.2The complexity of PD symptoms demands for personalized treatment solutions, which benefits from digital self-management interventions.2,3To enhance patient support via these systems, understanding the dynamics of the patient-clinician relationship is essential. Although digital health tools are continually developed and improved, many lack the ability to consolidate clinical data at the point of care, limiting their utility in enhancing patient-clinician communication.4By enabling continuous data collection and optimizing usability for healthcare professionals, digital health technology can make the treatment process more efficient and patient-centric and enhance the patient-clinician relationship.5,6Despite the important role of clinicians in supporting digital self-management, there remains a notable lack of insight into how clinicians perceive and use these digital self-management systems.6
Strong communication is essential for fostering good relationships between patients and healthcare providers, which can significantly impact health outcomes, and the nature of care provided. Integrating clinicians’ insights into the systems for treatment can enhance cross-disciplinary collaboration, streamline data collection, assess treatment suitability, and ultimately, aid patient outcomes.6,7For example, the interdisciplinary network Parkinson’s Network Münsterland+ (PNM+) found that PNM+ increased collaboration between professionals and improved both diagnosis and therapy for PD patients through knowledge transfer, highlighting the importance of effective communication and collaboration in PD care.8The effectiveness of telehealth has shown also potential to improve communication skills within dyads of PD patients and their CPs9, demonstrating the positive effects of collaboration and improved communication between patients and their clinician.9
Past studies have explored the impact of digital tools on PD care, focusing on self-management strategies to alleviate physical and cognitive symptoms in patients.10–23There are numerous studies focusing on the effectiveness and acceptability of self-management systems for patients, including the examination of the patient’s usability,24–47but few studies have systematically examined the standardization of acceptability and usability assessments for clinicians.27–33Examples of recent literature that have started to examine how digital health can bridge the gap between patients and clinicians included Tenison et al.48and Eggers et al.49who explored innovative integrated care models for PD, emphasizing proactive management, multidisciplinary care, and patient empowerment. The PRIME Parkinson model focuses on clinician integration through personalized care management and technology support48whereas the Cologne Parkinson’s network integrates neurologists, specialists, and PD nurses to improve the patient’s quality of life.49Despite their contributions, these studies revealed a gap in effectively reaching the clinician and their personal needs in treating people with Parkinson’s disease (PwP), underlining a need for further research on strategies that ensure comprehensive care and support throughout the care pathway.
There is a significant gap in literature aggregating findings from qualitative research on the acceptability and usability of self-management systems in PD and how clinician experiences and their integration into the systems is embodied to enable interconnectedness of clinician involvement and patient engagement. Previous reviews which include the clinician’s experience were published over four years ago.27–33Due to the rapid advancement in technology, an updated overview of more recent research on digital PD solutions is needed. This review aimed to explore “how self-management systems for Parkinson’s disease are being assessed and implemented and to what extent do they incorporate active clinician participation?”. To address this aim, the objectives included assessing the different types of technologies in use and the application of self-management techniques to the system with a particular emphasis on the engagement between patients and clinicians.
METHODS
Study design
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR Checklist; Supplementary Table 1) and Population, Intervention, Comparator, Outcome, and Studies (PICOS) framework (Table 1) were used to build the search strategy and provide a framework for the review.
Table 1
PICOS framework50
| PICOS | Planned | Implemented* |
| Population | Healthcare Professionals (HCPs) | Healthcare Professionals (HCPs); PwP, HCP and carer |
| Intervention | Digital interventions for self-management for PwP, e.g., telehealth, exergaming, websites, smart homes, mobile application, web-based systems, wearable devices, which offer a clinician portal or similar which may enable, e.g., e-record integration, messaging feature, remote monitoring, or treatment decision support (later expanded to all systems which don’t include features mentioned) | All digital interventions for self-management for PwP also those which don’t include previously mentioned features |
| Comparator | None | None |
| Outcomes | Primary outcome: Acceptability and usability of digital interventions for self-management in PD care by healthcare professionals. | Primary outcome: Types of technologies and self-management techniques applied to systems and the level of clinician integration in digital interventions in PD care. |
| Secondary outcomes: Acceptability and usability of clinicians evaluated in captured systems | Secondary outcomes: the evaluation of acceptability and usability of clinicians in captured systems | |
| Study types | Qualitative and quantitative studies, e.g., case-control studies, case series, longitudinal studies, cohort studies, or Randomized controlled trials (RCTs) | Qualitative and quantitative studies, e.g., case-control studies, case series, longitudinal studies, cohort studies, or Randomized controlled trials (RCTs) |
Search strategy
The database search was performed on January 12th, 2024, using PubMed, CINAHL, Scopus, ACM digital library, and IEEE Xplore databases. PubMed was chosen due to its biomedical literature relevant to PD and the other databases were selected because they capture multidisciplinary resources blending the area of healthcare and technology. The review included studies that utilized qualitative, quantitative, and mixed methods to examine general views on the effectiveness and user-friendliness of digital health interventions from all parties. There was no limit on the publication date. A preliminary exploration of relevant terms based on the PICOS framework was established to identify digital self-management interventions for PwP and healthcare professionals from peer-reviewed sources. The search strategy included a set of keywords relating to digital self-management tools and their user-friendliness and user acceptance (Table 2) and keywords were derived from Medical Subject Headings related to the subject and used as search terms.50
Table 2
MeSH terms and keywords used50
| Category | MeSH | Keyword (in Title/Abstract) |
| Parkinson’s Disease | Parkinson disease OR parkinsonian disorders | “Parkinson’s disease” OR parkinson OR parkinson’s OR “parkinson disease” |
| Self-management intervention | Electronic OR technology OR data collection OR internet-based intervention OR digital health OR telemedicine OR computing methodologies OR software OR wearable electronic devices OR self-help devices OR rehabilitation OR computer-user training | “Digital intervention” OR technology OR system OR portal OR remote OR home-based OR database management system OR internet-mediated therapy OR remote consultation OR personal health services |
| Evaluation | Quality of healthcare OR healthcare evaluation mechanism OR program evaluation OR attitude OR behaviour OR acceptance process OR acceptance processes OR treatment adherence and compliance OR communication methods, total OR security measures OR educational measurement OR time management OR efficiency, organizational | Evaluation OR attitude OR user experience OR acceptability OR usability OR impact OR acceptance OR compliance OR conformity OR efficiency |
Rationale for deviations from protocol
The protocol manuscript for this paper has been accepted for publication in the peer-reviewed journal JMIR Research Protocols. Originally, the protocol for our review aimed to concentrate on the acceptability and usability of digital PD interventions as reported by clinicians. This focus was predicated on the assumption that healthcare professionals’ perspectives bring rich insights for informing future system development and recommendations for incorporating preferences of healthcare professionals and their daily workflow. The initial search yielded only eleven studies with a focus on clinicians’ assessments of usability across various self-management systems. To ensure that the review was providing a good overview of the state of the literature around PD self-management interventions, the scope of the review was broadened through the search string and used keywords, not only including clinicians, but all affected individuals living with PD, alongside their carers (both professional and familial). This expansion aimed to capture a broader spectrum of experiences and perceptions regarding digital PD intervention tools. By adopting this more inclusive research strategy, the review now offers a more holistic view of the digital PD intervention landscape. This comprehensive approach not only augments the study’s initial aims but also enhances its potential to inform more nuanced system development and recommendations, thereby significantly widening its impact. To address our original research question focusing on clinicians’ experiences, the subset of studies evaluating clinicians were specifically analyzed. The data extraction items were amended accordingly, whereas we initially planned to focus on the system features exclusively for clinicians after being informed by the experiences and perceptions of clinicians, we then focused on the data revealing what kind of self-management techniques the systems incorporated, how these were measured and what overall recommendation their expressed for the system benefitting patient, carer and or clinician.
Inclusion criteria
Studies selected for inclusion were those investigating self-management systems for PwP and their healthcare practitioners (HCPs), applying accredited evaluation measures or presenting clinical evidence of acceptability and/or usability. A comprehensive definition of self-management interventions was employed to capture a wide variety of intervention types. This included any digital intervention, like websites or mobile applications, aimed at enhancing any aspect of patient self-management in PD. Studies published at any point in time and evaluating the intervention in question, be it through randomized controlled trials (RCTs), cohort studies, or case-control studies were considered. There was no restriction on the publication date, as the review sought to offer an exhaustive overview of evaluations of self-management systems for PD patients.
Table 3
Full data charting list50
| Study related information | |
| Author | |
| Title | |
| Link | |
| Year | |
| Study type | |
| System related information | |
| Name | |
| Year of launch | |
| Technology Domain (e.g., IoT, mobile app or website) | |
| Elements of combined technology | |
| Type of self-management intervention | |
| Description of self-management intervention | |
| Outcomes examined | |
| Evaluation method (how outcomes were measured) | |
| Costs | |
| Inclusion of clinician/clinical management? | |
| Were Clinicians asked on any usability/acceptability aspect? | |
| Key takeaways for future research |
Exclusion criteria
Studies not examining digital self-management interventions for PD, along with editorials, perspective pieces, conference papers, and protocols, were excluded. Literature not in English was also omitted due to the research team’s limitations in assessing such materials.
Screening and selection
References were organized and duplicates removed using EndNote X21 citation management software. An initial screening employing search strategy keywords was conducted using EndNote X21 (Supplementary Tables 2 and 3). Subsequently, the remaining titles and abstracts were assessed for final eligibility. The studies that passed the screening were hand-searched to ensure no studies meeting the inclusion criteria were overlooked in the initial search, then added to the list for a full-text review. Screening of titles and abstracts was divided among three authors (SB, MMI, and AA) and a full-text review to determine final eligibility was performed by the lead author (SB).
Data extraction
Data extraction items were modified to realign the study’s emphasis as previously mentioned in the deviations from Protocol section. Furthermore, this revised focus entailed an in-depth analysis of whether the studies in question solicited feedback from clinicians regarding the usability of the systems and their integration into clinical practice. This strategic shift aims to provide a nuanced understanding of how digital self-management tools are perceived and utilized within the healthcare ecosystem, enhancing the relevance and applicability of the research findings. Data extraction was performed by the first author (SB) by collecting pertinent information from the study manuscripts into a pre-developed form (see Table 3).50
Data analysis
A descriptive analysis was employed to synthesize the data collected from the research studies, offering a comprehensive summary of the current literature on self-management interventions for PD. The significance and potential impact of these results are further explored in the discussion section.
RESULTS
Included studies
The initial literature search yielded 15,231 results from the five databases. After automated duplicate removal and screening in EndNote X21, 1,583 references were included for title and abstract screening and 92 references for full text review. After assessing and deploying the eligibility criteria in detail, 33 references were included in the analyses for this review (Fig. 1).
Fig. 1
PRISMA flow chart.
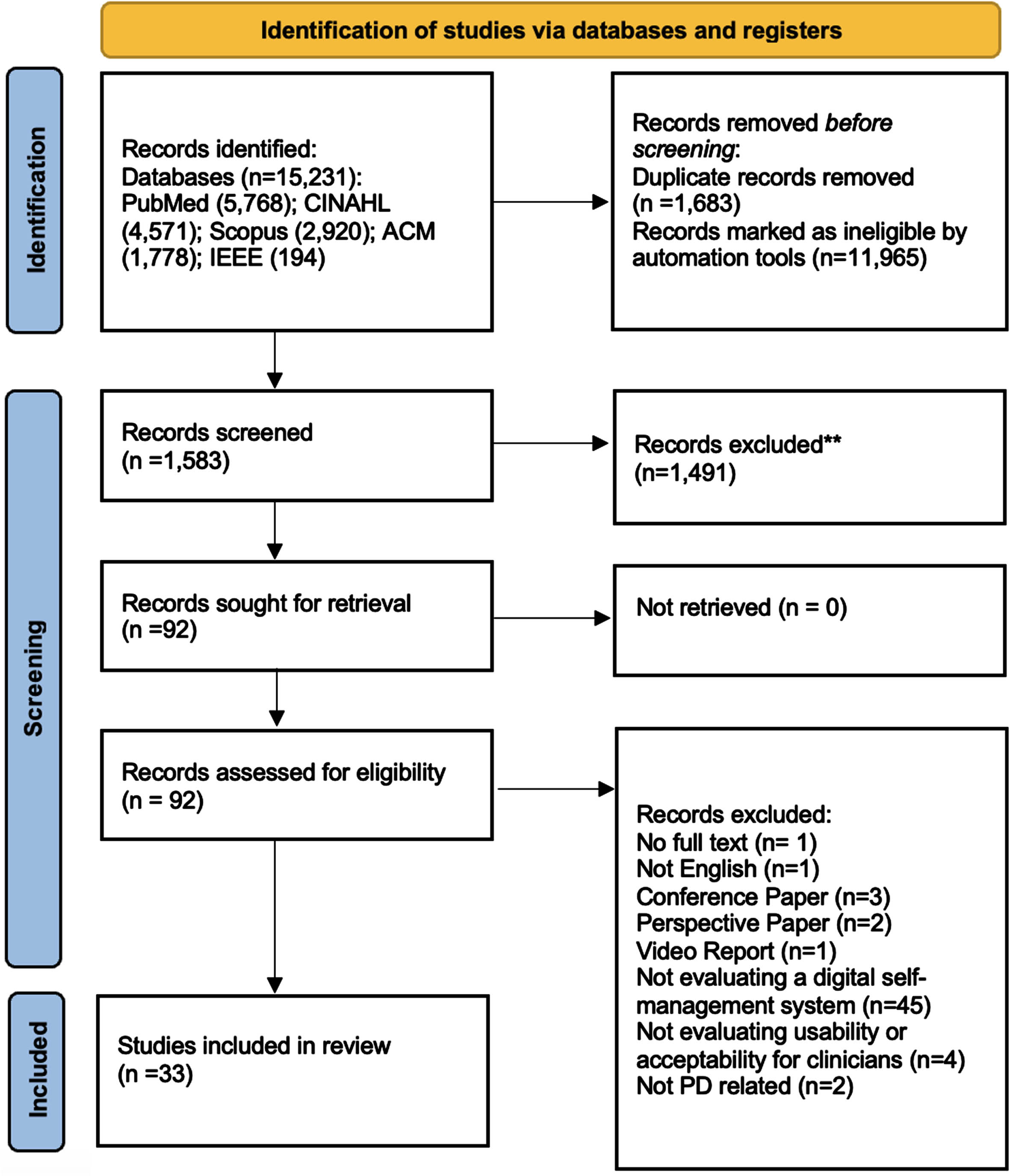
The results are organized based on the following sections: (1) outcomes measured, and evaluation methods used, (2) types of technology, (3) integration of self-management techniques into digital tools, and (4) clinician involvement. The earliest studies included in the review were published in 201051,52and the latest in 202353–55but two thirds of the studies (22/33) were published in 2020 or later. A variety of study designs were used to evaluate self-management systems, but mixed method pilot studies were the most common (28/33). Three studies focused on qualitative methods examining the acceptability and deeper insight into various perceptions of self-management systems through group discussions and semi-structured interviews with patients and clinicians.23,56,57Two studies used quantitative methods to evaluate the performance of data collection by the system through wearable sensors measuring motor and non-motor symptoms in patients.58,59A summary of system characteristics for included articles can be seen in Table 4 (see Supplementary Table 4 for a more detailed overview).
Table 4
Summary of system characteristics for included articles
| Author | Name of System | Type of Technology | Component(s) of technology |
| Albani et al.66 | Not reported | Combination | Vision system for the upper limb analysis and a wearable sensors-based system for the analysis of lower limbs. |
| Beijer et al.51 | EST (e-learning based speech therapy) | IoT | Web application |
| Bendig et al.43 | 3D-camera system (Motognosis Amsa; Motognosis GmbH), wearable system (PD Neurotechnology Ltd) and tablet app (TelePark tablet app; Intecsoft group) | Combination | Camera + wearable + tablet app |
| Brown et al.26 | PD-BRIDGE | IoT | Web platform/ dashboard |
| Chang et al.53 | Not reported | IoT | Smartphone |
| Chaudhuri et al.55 | Parkinson’s KinetiGraph (PKG) | Wearable | Wrist-worn device |
| Connor et al.27 | Siebens Domain Management ModelTM + Siebens Health Care Notebook | IoT | Practical clinical tool + self-reported notebook tool |
| Debelle et al.58 | DHTS | Combination | Smartwatch, smartphone and IMU can be utilized to monitor mobility and assess medication adherence |
| Dorsey et al.52 | Not reported | IoT | Telemedicine visits usingVSee Video Conferencing freeware run on Dell Notebook Computers |
| Erb et al.28 | Not reported | Combination | Mobile app + wearables |
| Ferreira et al.67 | SENSE-PARK System | Combination | Set of wearable sensors (3 to be used during the day and one at night), a Wii Balance Board, software and a smartphone app |
| Fleisher et al.68 | IN-HOME-PD | Combination | Telemedicine connection using a wireless hotspot, tablet, and a Health Insurance Portability and Accountability Act (HIPAA)-secure video conferencing platform. |
| Flynn et al.69 | Physiotherapyexercises.com | IoT | Website |
| Gassner et al.29 | PatientConcept | Mobile app | Smartphone |
| Gao et al.70 | Care-PD program | Combination | IoT + smartphone |
| Karni et al.30 | EMPARK | Combination | Wearables + digital platform |
| Landers et al.61 | 9zest | Mobile app | Smartphone |
| LoBuono et al.31 | Not reported | Combination | Telehealth (interactive videoconferencing) and wearable devices (technology that collects continuous data overtime |
| Maggio et al.62 | NeuroNation Brain Training | Mobile app | Smartphone |
| Morgan et al.25 | Not reported | Combination | Wearables, home sensors, IoT |
| Omberg et al.71 | Not reported | Combination | Wearables + smartphone |
| Ozanne et al.56 | Not reported | Wearable | Wearable sensors, such as accelerometers, gyroscopes, and magnetometers integrated in garments or accessories |
| Ozinga et al.59 | Not reported | Mobile app | Smartphone |
| Pastana et al.54 | Not reported | IoT | Remote individual rehabilitation program |
| Piro et al.72 | Not reported | Combination | IoT + Wearables mobile app |
| Rodrigues et al.33 | Vivendo com Parkinson | Mobile app | Smartphone |
| Santos et al.57 | Not reported | IoT | Telemedicine |
| Schmidt et al.63 | Not reported | IoT | Exergame |
| Tzallas et al.65 | PERFORM | Combination | Wearable Multi-Sensor Monitor Unit, the Local Base Unit and the Centralized Hospital Unit |
| Virmani et al.64 | Not reported | Combination | Telemedicine enhanced research visits (digital survey instruments for self-assessments, digital voice recordings and digitized spiral drawings) |
| Xu et al.60 | Not reported | IoT | Telehealth |
| Zhang et al.19 | PD Move | Combination | IoT + wearables + mobile app |
| Zhao et al.73 | Google Glass | Wearable | Smart glasses |
(1) Measures and evaluation of outcomes
Most of the studies focused on the feasibility, acceptability, and usability of the systems (24/33). Half of the studies measured and evaluated cognitive and physical improvement (17/33) and almost a third of the studies covered gait performance measures in patients (10/33). A quarter of studies assessed overall patient satisfaction (8/33) and quality of life of patients (8/33). Only one study focused on assessing cost-utility.55This review specifically examined the outcome measures of acceptability and usability of the self-management systems, which were well-represented by the results. In addition to the quantifiable improvements in physical capabilities, the systems focusing on physical improvement centered primarily on the impact on the patient, particularly in terms of their usability and satisfaction. We found that the most used evaluation techniques for usability in the literature were adapted questionnaires designed for a specific study (16/33), validated questionnaires (14/33) and interviews (11/33). Despite the breadth of research on patient data and their perceptions on self-management systems, the inclusion of clinicians as participants in evaluations of system acceptability and usability remains limited with only a third (11/33) of studies addressing this aspect.
(2) Types of technology
The identified literature categorizes self-management systems and their evaluation into different domains of technology (Table 5). We found that existing research places a significant emphasis on integrated technological solutions for PD care. Among the 33 papers examined, nearly 45% focused on exploring how combined digital technologies, such as mobile apps and wearable devices like wristbands or sensors, can be synergistically used to improve self-management strategies using different or combined technology. In contrast, the investigation into singular technology-based interventions reveals a more varied distribution of research interest, with ten papers focusing on Internet of Things (IoT)24,25,51–54,56–59and five studies on mobile applications.27,31,60–63Wearable devices as standalone interventions present the least coverage in chosen literature, with only three papers examining their feasibility for patients and their cost effectiveness.55,64,65Results in literature which discuss wearables indicate a need for real-time monitoring and the comfortable sensor wear, emphasizing comfort and the potential for remote assessment feasibility across diverse populations.54We found a predominant interest in leveraging a combination of digital technologies for PD self-management, reflecting an acknowledgment of the complexities inherent in treating and managing the condition.
Table 5
Summary of self-management characteristics for mentioned systems
| Author | Name of System | Type of self-management intervention | Description of self-management intervention | Inclusion of clinician/clinical management? (Y/N) | Were Clinicians asked about any usability/acceptability aspect? (Y/N) |
| Albani et al.66 | Not reported | Remote Automated Assessment | Analyzing upper and lower limb movements during specific tasks, using machine learning for automated UPDRS score prediction. | Y | N |
| Beijer et al.51 | EST (e-learning based speech therapy) | Speech Therapy Platform | Enables speech training remotely, improving access for neurologically impaired patients. | N | N |
| Bendig et al.43 | 3D-camera system (Motognosis Amsa; Motognosis GmbH), wearable system (PD Neurotechnology Ltd) and tablet app (TelePark tablet app; Intecsoft group) | Integrated Digital Health Dashboard | Collates EHR and PROs for easy review by physicians and patients. | Y | Y |
| Brown et al.26 | PD-BRIDGE | Telerehabilitation for Speech Therapy | Provides online speech therapy via video calls. | N | N |
| Chang et al.53 | Not reported | Parkinson’s KinetiGraph (PKG) | Wrist-worn system for continuous movement tracking and medication adherence monitoring. | Y | N |
| Chaudhuri et al.55 | Parkinson’s KinetiGraph (PKG) | Siebens Domain Management ModelTM | Organizes health-related data into four domains for ongoing tracking and communication. | Y | Y |
| Connor et al.27 | Siebens Domain Management ModelTM + Siebens Health Care Notebook | IMU and Smartwatch Reminder System | Monitors movement and provides medication reminders. | N | N |
| Debelle et al.58 | DHTS | Telemedicine Specialty Care Access | Enhances access to specialty care for remote patients. | N | N |
| Dorsey et al.52 | Not reported | Electronic VA Patient Motor Diary | Continuous monitoring using wearable technology for PD symptoms. | Y | Y |
| Erb et al.28 | Not reported | Sensor-Based Activity Monitoring | Collects data on PD symptoms and activities using sensors. | N | N |
| Ferreira et al.67 | SENSE-PARK System | Telehealth Home Visits | Interdisciplinary visits for homebound individuals. | N | N |
| Fleisher et al.68 | IN-HOME-PD | Physiotherapy Exercise Program | Tailored exercise routines for PD patients. | N | N |
| Flynn et al.69 | https://www.physiotherapyexercises.com/ | Personalized Training App | Provides customized training videos for home exercise. | Y | Y |
| Gassner et al.29 | PatientConcept | Care-PD Medication Management Platform | Supports medication adherence and rehabilitation training. | Y (as supervision/ monitoring element by clinician) | N |
| Gao et al.70 | Care-PD program | Longitudinal Home Monitoring | Collects data over time for self-assessment and clinical decision-making. | Y | Y |
| Karni et al.30 | EMPARK | NeuroNation VR Tele-cognitive App | Offers personalized brain training exercises. | N | N |
| Landers et al.61 | 9zest | Multimodal Sensor Platform | Uses ambient and wearable sensors for symptom quantification. | N | Y |
| LoBuono et al.31 | Not reported | Mobile Application for Activity Assessment | Records various activities using phone sensors. | Y | N |
| Maggio et al.62 | NeuroNation Brain Training | Wearable Sensor Integration | Integrates sensors for outpatient evaluation. | N | N |
| Morgan et al.25 | Not reported | Centre of Mass Movement Characterization | Measures postural stability using mobile device sensors. | N | N |
| Omberg et al.71 | Not reported | Telerehabilitation with Real-time Feedback | Conducts remote physiotherapy with visual cues. | N | N |
| Ozanne et al.56 | Not reported | Telemonitoring System for Motion Recording | Records patient motions using inertia sensors for remote monitoring. | Y | Y |
| Ozinga et al.59 | Not reported | Educational App for PD Care | Informs caregivers and patients about PD and its care. | Y | N |
| Pastana et al.54 | Not reported | Teleconsultations for Developing Countries | Enables remote consultations for patients in underserved areas. | N | N |
| Piro et al.72 | Not reported | Custom Exergame System for Arm Reaching | Engages patients in arm-reaching exercises through custom games. | Y | Y |
| Rodrigues et al.33 | Vivendo com Parkinson | Real-time Adjustment of Treatment Plan | Collects daily data to inform physicians for treatment adjustment. | Y | Y |
| Santos et al.57 | Not reported | Telephone and video consultation | Teleconsultations for patients with parkinsonian syndromes in developing countries | N | N |
| Schmidt et al.63 | Not reported | Self-reported Logbooks | self-reported logbooks and electronic records of adherence from a 12-week minimally-supervised, home-based exercise | Y | N |
| Tzallas et al.65 | PERFORM | Patient-Specific Profile Building | Constructs personalized profiles based on sensor data and other inputs. | Y | Y |
| Virmani et al.64 | Not reported | Telemedicine Research Visits | Evaluates motor and non-motor symptoms remotely for research purposes. | N | Y |
| Xu et al.60 | Not reported | Video Visits for PD Assessments | Allows remote assessments of motor symptoms and medication management. | N | N |
| Zhang et al.19 | PD Move | Gait Collector with Smartphone Sensors | Collects gait data using smartphone sensors for medication response assessment. | N | N |
| Zhao et al.73 | Google Glass | Smart Glasses for Gait Management | Aids gait management using wearable displays with advanced cueing features. | N | N |
(3) Integration of self-management
Included reviews show the implementation of self-management methodologies integrated into the digital health interventions. The literature search revealed that educational components are central to digital interventions for PD management, aiming to empower patients through home-based exercises available via videos, audios, and digital information sheets.26–31,65,67,68These elements represent a pedagogical strategy embraced by many systems to disseminate PD knowledge, promoting self-learning and condition management. Included literature also highlights systems integrating self-reporting of symptoms with biomechanical data collection, offering a comprehensive self-management approach. Patients are prompted to document daily symptoms in digital journals, while wearables track physiological metrics like heart rate variability, facilitating a deeper understanding of symptom patterns and their physiological underpinnings.26,28,29,31,65,68The findings from the literature emphasize that the choice of self-management techniques is closely tied to the technology used: mobile apps require active user input for symptom logging, while wearables passively gather data, such as gait metrics. 27,65,67,68This distinction between active and passive data collection methods reflects the diverse technological approaches in PD management and the importance of customizing interventions to meet the varied needs of PwP.
(4) Clinician involvement
Our research initially focused narrowly on the acceptability and usability of self-management systems by clinicians in PD care, aiming to make digital tools intuitive and integral to their practice for better patient management. Due to limited findings, we broadened our scope to include the general acceptability and usability of such systems among all stakeholders, including patients and healthcare providers, to gain a more comprehensive understanding of their impact. Out of these search results we included 33 studies, whereas 15 papers mention that their addressed system includes the clinician or incorporates clinical elements in some form while 11 studies actively involved the interrogation of clinician’s acceptability and usability of self-management systems as part of the system’s evaluation.26–33,63,65,66In those studies, assessments primarily utilized surveys and semi-structured or dyadic interviews to gather insights from healthcare professionals. Common themes in these interviews were personalized management for patients and clinicians through, e.g., notification centers, data visualization or voice note features.26In the evaluation of the system “EMPARK”, physicians and nurses’ feedback included the request to access and visualize raw data in addition to integrated summaries, an overview graph representing the daily summary with scores from different variables, such as medication, mealtime, exercise, self-assessment, or sleep score. Other requests were a flexible and interchangeable time axis for the score results, allowing discoveries of associations between different disease activities and treatment.30Additionally, results suggest that the training of the service should be provided prior to implementing an intervention, specifically for PwP, developers should consider both PwP and caregiver views, needs and preferences for enhanced personalized management.31The discussed studies imply that the ability for clinicians to access real-time data on patient symptoms, medication adherence, and response to treatment allows for timely adjustments to treatment plans and can influence patient outcomes.31Further, integrating clinician feedback into digital care platforms enhances the system’s relevance and efficacy.30The studies suggest that clinicians contributing their expertise to refine data collection tools and interfaces ensures that care systems are user-friendly and meet clinical needs, which ultimately improves the patient-clinician relationship.25,31
DISCUSSION
Overview of findings
The review identified 33 studies that evaluated the acceptability and usability of self-management systems for patients with PD, but less than half of them (15/33) considered the clinician or clinical elements within the system. The studies examined a variety of different outcome measures, with more than half (17/33) testing for the effectiveness and performance of the systems, and almost three quarters (24/33) assessing the acceptability and usability of the system. Multiple types of technologies were used including IoT, mobile apps, websites, wearable devices, and a combination of multiple technological systems. The use of different data collection technologies identified as the most used practice within the implementation of self-management systems, highlights the complexity of PD and the variety of possible symptoms.
Studies integrating multiple technologies, such as vision systems with wearable sensors, found that combining data sources offers comprehensive evaluations of motor and non-motor symptoms. Systems like these use gesture-based Human-Machine Interfaces (HMIs) to enhance usability and improve patient interaction. For instance, integrated optical and wearable sensor systems were noted for their objective patient performance characterization, mitigating subjectivity in PD assessments, and ensuring consistent automated evaluations. In the mobile app domain, usability and accessibility are critical. The Siebens Domain Management ModelTM emphasizes centralized care management and adaptability to disease severity, receiving high clinician acceptability due to its flexible data organization.27Similarly, PD Move demonstrated high usability by optimizing medication prescriptions based on real-time gait variability data, emphasizing personalized care. Simplified interfaces, such as voice input and gesture-based controls, were consistently found to improve user satisfaction and adherence. Wearable devices like the Parkinson’s KinetiGraph (PKG) support cost-related evaluations and highlight the importance of design for ease of use. Participants preferred wearables with attractive designs, breathable materials, and easy don and doff features.55This feedback highlights the necessity of combining functionality with comfort to enhance the acceptability of wearable technologies among users with PD. IoT and telemedicine systems, such as PD-BRIDGE and other telehealth platforms, stress the need for accurate data entry and patient education.26Systems in the telemedicine domain showed general high user acceptance, with participants expressing self-reliance and a preference for in-home visits. Continued remote assessments are advocated to track disease progression and potentially reduce healthcare costs. The importance of overcoming technological and cultural barriers to improve telemedicine’s accessibility was also emphasised.57
Study outcomes consistently highlighted the significance of user-centered design, simplified interfaces, and real-time data integration. Improving usability and accessibility is crucial across all domains. By addressing these factors, digital tools can better meet the specific needs of PD patients and bridge gaps in care, particularly for populations experiencing health disparities.
Although the acceptability and usability of self-management systems is widely researched, the inclusion of clinician perspectives in the evaluation and implementation of these systems was notably limited, suggesting a gap in understanding clinician’s preferences and daily work routines. The 11 papers which asked clinicians for their perception and insights are qualitative in nature, indicating that clinician perceptions are best captured through qualitative means. The publication dates of studies which emphasize the inclusion of clinicians vary, but the majority (8/11) were published after 2020. This may indicate a development towards technological solutions serving both patients and clinicians.27–31,33,65Educational components stand out as a central component of self-management interventions, providing patients with accessible home-based exercises and information. This approach, combined with symptom reporting and physiological data collection, forms a comprehensive self-management strategy. The study by Rodrigues Pereira et al. highlighted a significant information and training gap for clinicians in using digital systems in their treatment service, emphasizing the need for professional guidance in PD self-care.33This differentiation underscores the necessity for customized interventions to meet the PD population’s varied needs.
The observation of the least represented focus of wearable as a standalone intervention suggests a benefit of using wearables in combination with another type of technology.12Despite their limited representation in the literature, wearable technologies possess the capability to provide real-time monitoring and feedback, which can be instrumental in managing the day-to-day variability of symptoms experienced by individuals with PD and data can also be collected and presented in files, linked applications or even websites to review data, which strengthens the argument of blended use of different technologies.30,65The use of combined devices highlights the growing recognition of the potential benefits that can be derived from leveraging a combination of digital tools to monitor and manage the complex symptoms associated with PD. Challenges with traditional motor fluctuation assessments, including poor adherence to motor diaries and unreliable self-reports of dyskinesia, point to the necessity of integrating mobile and wearable technologies for high-resolution monitoring. These technologies, coupled with machine learning algorithms, can offer a nuanced understanding of PD symptom severity, and underline the importance of continuous improvement and adaptability in monitoring strategies.
Strengths and limitations
This study provides a comprehensive analysis of digital self-management systems for PD, covering a wide range of technologies from both patient and clinician perspectives. It summarizes the evaluations of mobile apps, wearables, telehealth, exergaming, and more, distinguishing between patient-centric and clinician-centric solutions and highlighting aspects relating to acceptability and usability. By evaluating the strengths and limitations of each, the research offers recommendations and implications for future work as summarized below, highlighting its breadth in examining varied study types and digital tools for effective self-management.
A limitation of this review is the exclusion of systems that are not available in English, introducing a potential bias towards English-language resources. Another limitation is that it does not thoroughly evaluate the factors influencing acceptability and usability from clinicians’ perspectives, but rather assesses whether such evaluations have been conducted and their breadth. The reason for this was the limited or restricted accessibility of self-management systems from a clinical perspective which is governed by regulatory measures, ensuring that only certified healthcare professionals or sanctioned clinicians are granted comprehensive access to the system’s capabilities, both software and hardware. These regulations ensure that full features of these systems remain exclusive to the clinical environment, aligning with patient privacy and data security protocols. Consequently, the general public may find that these systems are not widely available on app stores or online platforms.
Further, the limited number of studies that consider clinician perspectives restricted a comprehensive comparison of systems’ effectiveness. Additionally, the review’s insights are limited by a single reviewer’s potential bias in data selection and interpretation. This sole perspective may affect the objectivity of the conclusions. The potential bias introduced by socio-economic factors, technological literacy, and access disparities among different patient populations, can affect both the conduct and interpretation of studies involving digital tools, potentially skewing the results and their applicability to a broader population. Recognizing and addressing these biases through inclusive study designs and targeted interventions can help ensure the findings are more representative and applicable to diverse patient groups. Another limitation arises from the reliance on published literature, which may not capture recent technological advances, excluding emergent tools post-literature search.
Implications and future work
There is a growing number of studies examining the acceptability and usability of PD self-management systems. This review demonstrates the variety of different types of remote systems available, which mostly and directly address the patient. Several studies examined the potential in including the feedback, experiences, and preferences of clinicians as an essential part of the system’s evaluation and implementation, which is likely to grow in demand due to rising emphasis on the patient-clinician relationship and inclusive healthcare.
The results of this review reflect the complexity of digital PD care and research with varying study types, technologies, and application of self-management techniques. The synthesis of findings across the diverse studies emphasizes the importance of integrating clinician and healthcare professional perspectives alongside patient care in the development and implementation of self-management systems for PD. Implementing features which enable interactions between patient and clinician can enhance usability and user satisfaction, demonstrating the significance of easy-to-use interfaces and fostering a valuable synergy between patients and clinicians.69,70The findings suggest the necessity for care models to be multidisciplinary, integrating insights from both patients and healthcare providers to tailor care approaches that effectively address individual requirements.6,30,31
Key recommendations include further research into usability for clinicians, exploring how they can work most efficiently with these systems, the optimal management and presentation of their data, and the integration of such digital solutions into both non-digital and fully digital clinical workflows. This additional research is crucial for ensuring that digital health technologies not only cater to patient needs but also enhance clinician efficiency and effectiveness in practice. Optimizing digital health technologies for clinician use and integrating them smoothly into clinical workflows is crucial for enhancing patient care.67,68,73Such improvements not only aid clinician efficiency but also lead to better patient outcomes through timely, informed, and personalized healthcare interventions.68,73Results suggest that future research should also focus on improving data visualization and interaction for clinicians.6This includes providing access to raw data, integrated summaries, and flexible visualization tools to enhance patient monitoring and treatment personalization. The collaborative approach promises to refine self-management practices in PD care, ensuring interventions are both clinically relevant and aligned with patient needs.30Digital self-management tools have the potential to significantly impact populations experiencing health disparities by providing accessible and cost-effective means of managing PD. However, the studies reviewed did not consistently evaluate how these tools perform across different socio-economic and demographic groups. This finding demonstrates that the benefits of such technologies may not be equally distributed, potentially exacerbating existing health disparities. Future research should focus on assessing the effectiveness and accessibility of these tools among diverse populations to ensure equitable healthcare outcomes. Achieving this balance requires ongoing innovation in technology development, usability studies, and care models, underpinned by a commitment to person-centered and scalable solutions.
Conclusions
There has been a growing number of studies examining self-management systems in PD care, particularly in recent years. This review highlighted the variety of different types of technologies in use, with the biggest category being a combined approach of remote monitoring and sensor measurement via wearables. Several studies examined the efficacy of those systems and their integration of self-management techniques. As the focus on personalized and preventive medicine increases, this aspect is expected to become a more significant part of ongoing research. The review found a few studies beginning to study the interplay between level of clinician involvement and improved patient outcomes, which is an essential element of care communication and thereby improved patient care. The findings from this review underscore a significant gap in the literature concerning self-management systems in PD care, particularly regarding clinician involvement. This oversight highlights a critical need for developing systems that are not only intuitive for clinicians to use but also effectively integrated into the care pathway. Such advancements are essential for enhancing care delivery and improving treatment outcomes, underscoring the importance of clinician-focused research in the realm of digital PD care.
ACKNOWLEDGMENTS
The review was written by SB and supervised by MMI and EM. Clinical feedback was provided by CC. SB conducted the searches and initial screening and the title and abstract screening. SB, MMI and AA conducted the full text screening. SB performed the data extraction and analysis and drafted the first version of the review. AA, MMI, and EM contributed revisions.
FUNDING
This review was supported by external funding by Parkinson’s UK Non-Drug Awards Programme (Grant Number: NDA-21). The views expressed in this review are those of the authors and are not funding agencies or their affiliated institutions. The review is supported by the National Institute for Health Research (NIHR) Newcastle Biomedical Research Centre (BRC) based at The Newcastle upon Tyne Hospital NHS Foundation Trust, Newcastle University and the Cumbria, Northumberland and Tyne and Wear (CNTW) NHS Foundation Trust.
CONFLICT OF INTEREST
Camille Carroll is an Editorial Board member of this journal but was not involved in the peer-review process of this article nor had access to any information regarding its peer review. The other authors have no conflict of interest to report.
DATA AVAILABILITY
The datasets generated and/or analyzed in this review are available from the corresponding author on reasonable request. The full review, including all findings and conclusions, has been submitted for consideration in a peer-reviewed journal to ensure rigorous evaluation and wide dissemination among the scientific community. Upon acceptance, the review will be accessible in the published journal.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-240137.
REFERENCES
[1] | Reporting on Parkinson’s: information for journalists. Parkinson’s UK, https://www.parkinsons.org.uk/about-us/reporting-parkinsons-information-journalists (accessed July 30, (2024) ). |
[2] | Mulroy E , Erro R and Bhatia KP. Refining the clinical diagnosis of Parkinson’s disease. Parkinsonism Relat Disord (2024) ; 122: : 106041. |
[3] | Kouli A , Torsney KM and Kuan W-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In: Stoker TB and Greenland JC (eds)Parkinson’s Disease: Pathogenesis and Clinical Aspects. Brisbane (AU): Codon Publications, (2018) , pp. 3–26. |
[4] | Kuosmanen E , Huusko E , van Berkel N , et al. Exploring crowdsourced self-care techniques: A study on Parkinson’s disease. Int J Hum Comput Stud (2023) ; 177: : 103062. |
[5] | Bounsall K , Milne-Ives M , Hall A , et al. Artificial intelligence applications for assessment, monitoring, and management of Parkinson disease symptoms: Protocol for a systematic review. JMIR Res Protoc (2023) ; 12: : e46581. |
[6] | Brown EG and Tanner CM. Impaired cognition and the risk of Parkinson disease. JAMA Neurol (2017) ; 74: : 1398. |
[7] | Hall AM , Aroori S , Carroll CB , et al. Impact of digital technologies on self-efficacy in people with Parkinson’s: a scoping review protocol. BMJ Open (2023) ; 13: : e069929. |
[8] | Kerkemeyer L , Achtert K , Claus I , et al. Quickcard-based approach to guiding specific nonpharmacological treatments in a German Parkinson’s network. J Clin Med (2020) ; 9: : 2272. |
[9] | Clay P , Beeke S , Volkmer A , et al. A communication partner training program delivered via telehealth for people living with Parkinson’s (Better Conversations with Parkinson’s): Protocol for a feasibility study. JMIR Res Protoc (2023) ; 12: : e41416. |
[10] | Dixit S , Bohre K , Singh Y , et al. A comprehensive review on AI-enabled models for Parkinson’s disease diagnosis. Electronics (Basel) (2023) ; 12: : 783. |
[11] | Freytag J , Chu J , Hysong SJ , et al. Acceptability and feasibility of video-based coaching to enhance clinicians’ communication skills with patients. BMC Med Educ (2022) ; 22: : 85. |
[12] | Perski O and Short CE. Acceptability of digital health interventions: embracing the complexity. Transl Behav Med (2021) ; 11: : 1473–1480. |
[13] | Meinert E , Milne-Ives M , Chaudhuri KR , et al. The impact of a digital artificial intelligence system on the monitoring and self-management of nonmotor symptoms in people with Parkinson disease: Proposal for a phase 1 implementation study. JMIR Res Protoc (2022) ; 11: : e40317. |
[14] | Mattison G , Canfell O , Forrester D , et al. The influence of wearables on health care outcomes in chronic disease: Systematic review. J Med Internet Res (2022) ; 24: : e36690. |
[15] | Lee J , Yeom I , Chung ML , et al. Use of mobile apps for self-care in people with Parkinson disease: Systematic review. JMIR MHealth UHealth (2022) ; 10: : e33944. |
[16] | Triantafyllidis A , Segkouli S , Zygouris S , et al. Mobile app interventions for Parkinson’s disease, multiple sclerosis and stroke: A systematic literature review. Sensors (Basel) (2023) ; 23: : 3396. |
[17] | Özden F . The effect of mobile application-based rehabilitation in patients with Parkinson’s disease: A systematic review and meta-analysis. Clin Neurol Neurosurg (2023) ; 225: : 107579. |
[18] | Marotta N , Calafiore D , Curci C , et al. Integrating virtual reality and exergaming in cognitive rehabilitation of patients with Parkinson disease: a systematic review of randomized controlled trials. Eur J Phys Rehabil Med (2022) ; 58: : 818–826. |
[19] | Zhang J , Luximon Y , Pang MYC , et al. Effectiveness of exergaming-based interventions for mobility and balance performance in older adults with Parkinson’s disease: systematic review and meta-analysis of randomised controlled trials. Age Ageing (2022) ; 51: : afac175. |
[20] | Giannakopoulou K-M , Roussaki I and Demestichas K. Internet of Things technologies and machine learning methods for Parkinson’s disease diagnosis, monitoring and management: A systematic review. Sensors (Basel) (2022) ; 22: : 1799. |
[21] | Tripathi S , Malhotra A , Qazi M , et al. Clinical review of smartphone applications in Parkinson’s disease. Neurologist (2022) ; 27: : 183–193. |
[22] | Gallou-Guyot M , Nuic D , Mandigout S , et al. Effectiveness of home-based rehabilitation using active video games on quality of life, cognitive and motor functions in people with Parkinson’s disease: a systematic review. Disabil Rehabil (2022) ; 44: : 8222–8233. |
[23] | Abou L , Peters J , Wong E , et al. Gait and balance assessments using smartphone applications in Parkinson’s disease: A systematic review. J Med Syst (2021) ; 45: : 87. |
[24] | Ellis TD and Earhart GM. Digital therapeutics in Parkinson’s disease: Practical applications and future potential. J Parkinsons Dis (2021) ; 11: : S95–S101. |
[25] | Morgan C , Rolinski M , McNaney R , et al. Systematic review looking at the use of technology to measure free-living symptom and activity outcomes in Parkinson’s disease in the home or a home-like environment. J Parkinsons Dis (2020) ; 10: : 429–454. |
[26] | Brown EG , Schleimer E , Bledsoe IO , et al. Enhancing clinical information display to improve patient encounters: human-centered design and evaluation of the Parkinson Disease-BRIDGE Platform. JMIR Hum Factors (2022) ; 9: : e33967. |
[27] | Connor KI , Siebens HC , Mittman BS , et al. Stakeholder perceptions of components of a Parkinson disease care management intervention, care coordination for health promotion and activities in Parkinson’s disease (CHAPS). BMC Neurol (2020) ; 20: : 437. |
[28] | Erb MK , Karlin DR , Ho BK , et al. mHealth and wearable technology should replace motor diaries to track motor fluctuations in Parkinson’s disease. NPJ Digit Med (2020) ; 3: : 6. |
[29] | Gaßner H , Friedrich J , Masuch A , et al. The effects of an individualized smartphone-based exercise program on self-defined motor tasks in Parkinson disease: Pilot interventional study. JMIR Rehabil Assist Technol (2022) ; 9: : e38994. |
[30] | Karni L , Jusufi I , Nyholm D , et al. Toward improved treatment and empowerment of individuals with Parkinson disease: Design and evaluation of an internet of things system. JMIR Form Res (2022) ; 6: : e31485. |
[31] | LoBuono DL , Shea KS , Tovar A , et al. Acceptance and perception of digital health for managing nutrition in people with Parkinson’s disease and their caregivers and their digital competence in the United States: A mixed-methods study. Health Sci Rep (2021) ; 4: : e412. |
[32] | Baumann L , Tengler M , Piro L , et al. Telemonitoring of patients with Parkinson’s disease using inertia sensors. Appl Clin Inform (2014) ; 05: : 503–511. |
[33] | Rodrigues Pereira NR , Celeste LC , Barros de Sales A , et al. Usability study of a smartphone app entitled: Living with Parkinson’s disease. Heliyon (2023) ; 9: : e17572. |
[34] | Milne-Ives M , Carroll C and Meinert E. Self-management interventions for people with Parkinson disease: Scoping review. J Med Internet Res (2022) ; 24: : e40181. |
[35] | Park Y , Kim SR , So HY , et al. Effect of mobile health intervention for self-management on self-efficacy, motor and non-motor symptoms, self-management, and quality of life in people with Parkinson’s disease: Randomized controlled trial. Geriatr Nurs (2022) ; 46: : 90–97. |
[36] | Nousia A , Martzoukou M , Tsouris Z , et al. The beneficial effects of computer-based cognitive training in Parkinson’s disease: A systematic review. Arch Clin Neuropsychol (2020) ; 35: : 434–447. |
[37] | Shahhar AZM , Qasheesh M and Shaphe MA. Effectiveness of Nintendo Wii on balance in people with Parkinson’s disease: A systematic review. J Lifestyle Med (2022) ; 12: : 105–112. |
[38] | Garcia-Agundez A , Folkerts A-K , Konrad R , et al. Recent advances in rehabilitation for Parkinson’s disease with exergames: a systematic review. J Neuroeng Rehabil (2019) ; 16: : 17. |
[39] | Linares-del Rey M , Vela-Desojo L and Cano-de la Cuerda R. Aplicaciones móviles en la enfermedad de Parkinson: una revisión sistemática. Neurologia (2019) ; 34: : 38–54. |
[40] | Spreadbury JH , Young A and Kipps CM. A comprehensive literature search of digital health technology use in neurological conditions: Review of digital tools to promote self-management and support. J Med Internet Res (2022) ; 24: : e31929. |
[41] | Pigott JS , Kane EJ , Ambler G , et al. Systematic review and meta-analysis of clinical effectiveness of self-management interventions in Parkinson’s disease. BMC Geriatr (2022) ; 22: : 45. |
[42] | Hellqvist C , Berterö C , Dizdar N , et al. Self-management education for persons with Parkinson’s disease and their care partners: A quasi-experimental case-control study in clinical practice. Parkinsons Dis (2020) ; 2020: : 6920943. |
[43] | Bendig J , Spanz A , Leidig J , et al. Measuring the usability of eHealth solutions for patients with Parkinson disease: Observational study. JMIR Form Res (2022) ; 6: : e39954. |
[44] | Owen CL , Ibrahim K , Dennison L , et al. Falls self-management interventions for people with Parkinson’s disease: A systematic review. J Parkinsons Dis (2019) ; 9: : 283–299. |
[45] | Lökk J . Lack of information and access to advanced treatment for Parkinson’s disease patients. J Multidiscip Healthc (2011) ; 4: : 433–439. |
[46] | So HY , Kim SR , Kim S , et al. Effect of home-based self-management intervention for community-dwelling patients with early Parkinson’s disease: A feasibility study. J Community Health Nurs (2023) ; 40: : 133–146. |
[47] | Guo CC , Chiesa PA , de Moor C , et al. Digital devices for assessing motor functions in mobility-impaired and healthy populations: Systematic literature review. J Med Internet Res (2022) ; 24: : e37683. |
[48] | Tenison E , Smink A , Redwood S , et al. Proactive and integrated management and empowerment in Parkinson’s disease: designing a new model of care. Parkinsons Dis (2020) ; 2020: : 8673087. |
[49] | Eggers C , Dano R , Schill J , et al. Access to end-of life Parkinson’s disease patients through patient-centered integrated healthcare. Front Neurol (2018) ; 9: : 627. |
[50] | Boege S , Milne-Ives M and Carroll C. Self-management systems for patients and clinicians in Parkinson’s care: protocol for an integrated systematic review, product evaluation and thematic synthesis. JMIR Res Protoc (2024) . doi: 10.2196/58845. |
[51] | Beijer LJ , Rietveld TCM , Hoskam V , et al. Evaluating the feasibility and the potential efficacy of e-learning-based speech therapy (EST) as a web application for speech training in dysarthric patients with Parkinson’s disease: A case study. Telemed J E Health (2010) ; 16: : 732–738. |
[52] | Dorsey ER , Deuel LM , Voss TS , et al. Increasing access to specialty care: A pilot, randomized controlled trial of telemedicine for Parkinson’s disease. Mov Disord (2010) ; 25: : 1652–1659. |
[53] | Chang HJ , Kim J , Joo JY , et al. Feasibility and efficacy of video-call speech therapy in patients with Parkinson’s disease: A preliminary study. Parkinsonism Relat Disord (2023) ; 114: : 105772. |
[54] | Pastana Ramos LF , Vilacorta-Pereira T de CS , Duarte J dos S , et al. Feasibility and effectiveness of a remote individual rehabilitation program for people with Parkinson’s disease living in the Brazilian Amazon: a randomized clinical trial. Front Neurol (2023) ; 14: : 1244661. |
[55] | Chaudhuri KR , Hand A , Obam F , et al. Cost-effectiveness analysis of the Parkinson’s KinetiGraph and clinical assessment in the management of Parkinson’s disease. J Med Econ (2022) ; 25: : 774–782. |
[56] | Ozanne A , Johansson D , Hällgren Graneheim U , et al. Wearables in epilepsy and Parkinson’s disease—A focus group study. Acta Neurol Scand (2018) ; 137: : 188–194. |
[57] | Santos DT , Camelo DMF , Strelow MZ , et al. Feasibility of telemedicine for patients with parkinsonism in the Brazilian public health system. Arq Neuropsiquiatr (2022) ; 80: : 914–921. |
[58] | Debelle H , Packer E , Beales E , et al. Feasibility and usability of a digital health technology system to monitor mobility and assess medication adherence in mild-to-moderate Parkinson’s disease. Front Neurol (2023) ; 14: : 1111260. |
[59] | Ozinga SJ , Koop MM , Linder SM , et al. Three-dimensional evaluation of postural stability in Parkinson’s disease with mobile technology. Neurorehabilitation (2017) ; 41: : 211–218. |
[60] | Xu Y , Feeney MP , Surface M , et al. Attitudes toward telehealth services among people living with Parkinson’s disease: A survey study. Mov Disord (2022) ; 37: : 1289–1294. |
[61] | Landers MR , Ellis TD . A mobile app specifically designed to facilitate exercise in Parkinson disease: Single-cohort pilot study on feasibility, safety, and signal of efficacy. JMIR MHealth UHealth (2020) ; 8: : e18985. |
[62] | Maggio MG , Luca A , D’Agate C , et al. Feasibility and usability of a non-immersive virtual reality tele-cognitive app in cognitive rehabilitation of patients affected by Parkinson’s disease. Psychogeriatrics (2022) ; 22: : 775–779. |
[63] | Schmidt M , Paul SS , Canning CG , et al. The accuracy of self-report logbooks of adherence to prescribed home-based exercise in Parkinson’s disease. Disabil Rehabil (2022) ; 44: : 1260–1267. |
[64] | Virmani T , Lotia M , Glover A , et al. Feasibility of telemedicine research visits in people with Parkinson’s disease residing in medically underserved areas. J Clin Transl Sci (2022) ; 6: : e133. |
[65] | Tzallas A , Tsipouras M , Rigas G , et al. PERFORM: A system for monitoring, assessment and management of patients with Parkinson’s disease. Sensors (Basel) (2014) ; 14: : 21329–21357. |
[66] | Albani G , Ferraris C , Nerino R , et al. An integrated multi-sensor approach for the remote monitoring of Parkinson’s disease. Sensors (Basel) (2019) ; 19: : 4764. |
[67] | Ferreira JJ , Godinho C , Santos AT , et al. Quantitative home-based assessment of Parkinson’s symptoms: The SENSE-PARK feasibility and usability study. BMC Neurol (2015) ; 15: : 89. |
[68] | Fleisher JE , Hess SP , Klostermann EC , et al. IN-HOME-PD: The effects of longitudinal telehealth-enhanced interdisciplinary home visits on care and quality of life for homebound individuals with Parkinson’s disease. Parkinsonism Relat Disord (2022) ; 102: : 68–76. |
[69] | Flynn A , Preston E , Dennis S , et al. Home-based exercise monitored with telehealth is feasible and acceptable compared to centre-based exercise in Parkinson’s disease: A randomised pilot study. Clin Rehabil (2021) ; 35: : 728–739. |
[70] | Gao S , Hou Y , Ma R , et al. A novel management platform based on personalized home care pathways for medicine management and rehabilitation of persons with Parkinson’s disease—requirements and implementation plan of the care-PD program. Front Neurol (2021) ; 12: : 672208. |
[71] | Omberg L , Chaibub Neto E , Perumal TM , et al. Remote smartphone monitoring of Parkinson’s disease and individual response to therapy. Nat Biotechnol (2022) ; 40: : 480–487. |
[72] | Piro NE , Baumann L , Tengler M , et al. Telemonitoring of patients with Parkinson’s disease using inertia sensors. Appl Clin Inform (2014) ; 5: : 503–511. |
[73] | Zhao Y , Nonnekes J , Storcken EJM , et al. Feasibility of external rhythmic cueing with the Google Glass for improving gait in people with Parkinson’s disease. J Neurol (2016) ; 263: : 1156–1165. |




