Exposure to Glycolysis-Enhancing Drugs and Risk of Parkinson’s Disease: A Meta-Analysis
Abstract
Background:
Impaired glucose and energy metabolism has been suggested as a pathogenic mechanism underlying Parkinson’s disease (PD). In recent cohorts, phosphoglycerate kinase 1 activators (PGK1a) have been associated with a lower incidence of PD when compared with other antiprostatic agents that do not activate PGK1.
Objective:
We aimed to perform a systematic review and meta-analysis comparing the incidence of PD in patients taking PGK1a versus tamsulosin.
Methods:
We searched PubMed, Embase, and Cochrane Library for studies comparing PGK1a vs. tamsulosin in adults and elderly. The primary outcome was the incidence of PD. We computed hazard ratios (HR) for binary endpoints, with 95% confidence intervals (CIs). Statistical analysis was performed using Review Manager 5.4 and R (version 4.3.1).
Results:
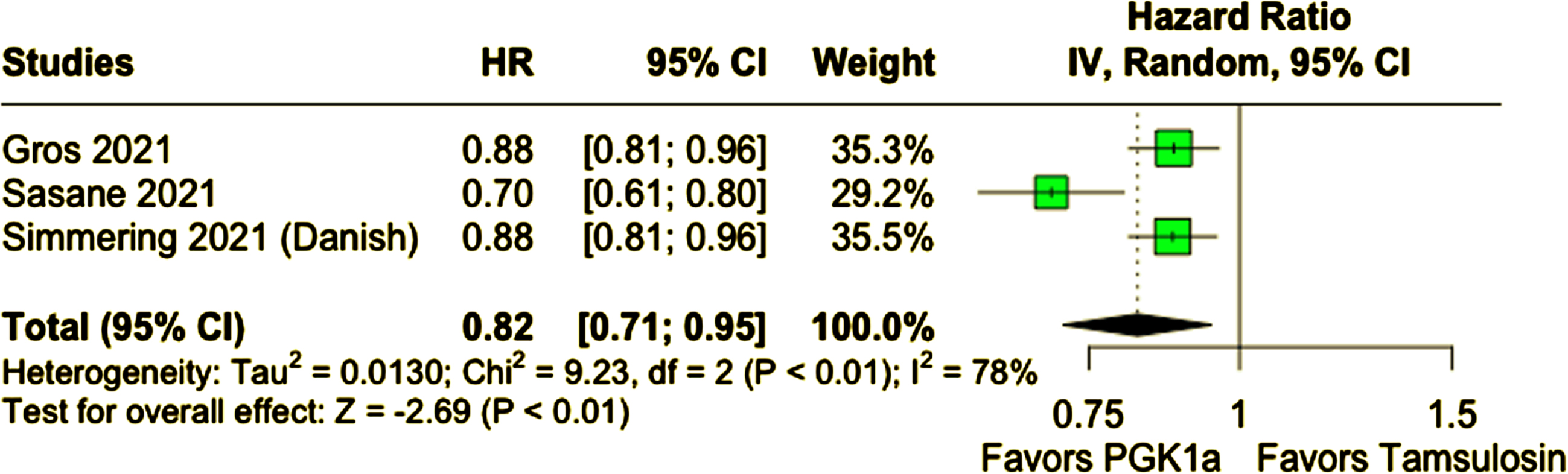
A total of 678,433 participants from four cohort studies were included, of whom 287,080 (42.3%) received PGK1a. Mean age ranged from 62 to 74.7 years and nearly all patients were male. Patients taking PGK1a had a lower incidence of PD (PGK1a 1.04% vs. tamsulosin 1.31%; HR 0.80; 95% CI 0.71–0.90; p < 0.01). This result remained consistent in a sensitivity analysis excluding patients of age 60 years old or younger (PGK1a 1.21% vs. tamsulosin 1.42%; HR 0.82; 95% CI 0.71–0.95; p < 0.01).
Conclusions:
Glycolysis-enhancing drugs are associated with a lower incidence of PD when compared with tamsulosin in adults and elderly individuals with prostatic disease in use of alpha-blockers. Our findings support the notion of glycolysis as a potential neuroprotective mechanism in PD. Future investigations with randomized controlled trials are needed.
Plain Language Summary
It has been suggested that impairment in glucose and energy metabolism is one of the mechanisms underlying the development of Parkinson’s disease. In recent studies, medications traditionally prescribed for prostate diseases, called phosphoglycerate kinase 1 activators (PGK1a), have been associated with a lower incidence of Parkinson’s disease when compared to other medications for the same purpose that do not activate the same energetic pathway. Therefore, we thoroughly reviewed the literature and combined the results of studies that compared both medications (PGK1a versus another medication that does not activate this energetic pathway, called tamsulosin), evaluating the incidence of Parkinson’s disease in both groups. We included a total of 678,433 individuals, of whom 42.3% received PGK1a and 57.7% received tamsulosin. In our analysis, patients taking PGK1a had a lower incidence of Parkinson’s disease when compared to the other group, even when we excluded patients younger than 60 years of age. As a result, our findings support the notion that the increase of energy metabolism is a potential neuroprotective mechanism in Parkinson’s disease and future investigations are needed.
INTRODUCTION
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders in the world, with an estimated prevalence of 8.5 million individuals worldwide and 572 per 100,000 individuals ≥45 years in North America.1,2 Although the exact disease mechanism remains uncertain, recent evidence from animal models has suggested that impaired glucose and energy metabolism might be one of the pathogenic mechanisms involved in PD, with attenuated disease progression and cognitive symptoms in models who received glycolysis-enhancing drugs, such as terazosin (TZ).3,4 A possible explanation includes the restoration of tyrosine hydroxylase enzyme activity, with increased levels of dopamine in the substantia nigra and striatum.3
TZ is an alpha-1 adrenergic receptor antagonist (alpha-blocker) commonly prescribed for the treatment of benign prostatic hyperplasia (BPH) and, less frequently, hypertension. Recent studies have shown that TZ also acts on phosphoglycerate kinase 1 (PGK1) activity, increasing the product of glycolysis and, therefore, adenosine triphosphate (ATP) concentration.5 Alfuzosin (AZ) and doxazosin (DZ) are also PGK1 activators (PGK1a) with a similar mechanism of action. On the other hand, tamsulosin is an alpha-1 blocker consisting of a structure that lacks a quinazoline group that binds to PGK1. Therefore, this drug has been used as a control group in cohort studies investigating the incidence of PD in patients with BPH.3,6–9
Some retrospective studies have shown a lower incidence of PD in patients using PGK1a medications when compared with tamsulosin.3,7 Moreover, a recent double-blind randomized pilot study demonstrated increased ATP levels in the brain and serum of patients receiving TZ.10 In contrast, a recent cohort study from 2021 found a higher incidence of PD in tamsulosin users when compared with patients taking PGK1a and matched controls not taking any antiprostatic medication.8 This last study raises the concern that tamsulosin could induce neurodegeneration, instead of a potential neuroprotective effect of PGK1a. Contrary to all these studies, a large cohort also published in 2021 found a reduction in the incidence of PD in patients with cumulative exposure to either PGK1a and tamsulosin compared with matched controls.9 To validate tamsulosin as a comparison group, a 2022 cohort compared patients taking PGK1a to both tamsulosin and 5-alpha-reductase inhibitors. This study found a lower incidence of PD in patients taking PGK1a when compared to both groups.7
Therefore, given the controversy in the literature regarding the neuroprotection of alpha-1 adrenergic receptor antagonists and reduction of PD incidence, we aimed to perform a meta-analysis to compare the incidence of PD in patients using PGK1a versus tamsulosin.
MATERIALS AND METHODS
This systematic review and meta-analysis was performed and reported in accordance with the Cochrane Collaboration Handbook for Systematic Review of Interventions and the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) Statement guidelines.11,12 The prospective meta-analysis protocol was registered on PROSPERO on June 12, 2023, under protocol CRD42023431769.
Study eligibility
Inclusion in the meta-analysis was restricted to studies that met all the following eligibility criteria: 1) randomized clinical trials (RCTs) or nonrandomized cohorts; 2) enrolling patients without PD; 3) comparing PGK1a (TZ/AZ/DZ) with tamsulosin; and 4) reporting the incidence of PD in both groups. Exclusion criteria included: 1) participants with a prior diagnosis or symptoms of PD; 2) studies with no description of PD incidence after the use of PGK1a; or 3) studies with overlapping populations. In the specific case of overlapping populations, only the report with the highest number of patients was included.
Search strategy and data extraction
PubMed, Embase, and Cochrane Library were systematically searched from inception to August 1, 2023. Additional details regarding the search strategy are detailed in Supplementary Table 1A. References from all included studies and previous systematic reviews were also searched manually for any additional studies. Two authors (G.R. and F.R.) independently extracted data following predefined search criteria.
Endpoint and subgroup analyses
The primary outcome of interest was the incidence of PD. Definitions of PD varied between studies, including International Classification of Diseases (ICD) and dispensing events for anti-PD medications. We performed a prespecified subgroup analysis restricted to patients aged 61 or older.
Risk of bias and quality assessment
Studies were assessed with the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool.13 Certainty of evidence was assessed by the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework.14 Two independent authors completed the tasks (G.R. and F.R.). Disagreements were resolved through a consensus after discussing reasons for discrepancy.
Statistical analysis
Hazard-ratios (HR) with 95% confidence intervals (CI) were computed to compare intervention effects for categorical endpoints. To minimize the risk of confounding, we used multivariable adjusted data, when available. Heterogeneity was evaluated with I2 statistics and Cochran Q test; p-values < 0.10 and I2 > 40% were considered significant for heterogeneity. Restricted Maximum Likelihood (REML) methodology was used. Review Manager 5.4 (Cochrane Center, The Cochrane Collaboration, Denmark) and R version 4.3.1 (R Foundation for Statistical Computing) were used for statistical analysis.
RESULTS
Study selection and characteristics
The initial search yielded 9,275 results (Fig. 1). After removal of duplicate records and ineligible studies, five remained and were fully reviewed based on inclusion criteria (Supplementary Table 1B).3,6–9 One study was excluded due to overlapping population.3 The Truven cohort from Simmering 2021 was also excluded due to overlapping population.7 Four prospective cohort studies were included, comprising 678,433 patients.6–9
Fig. 1
PRISMA flow diagram of study screening and selection.
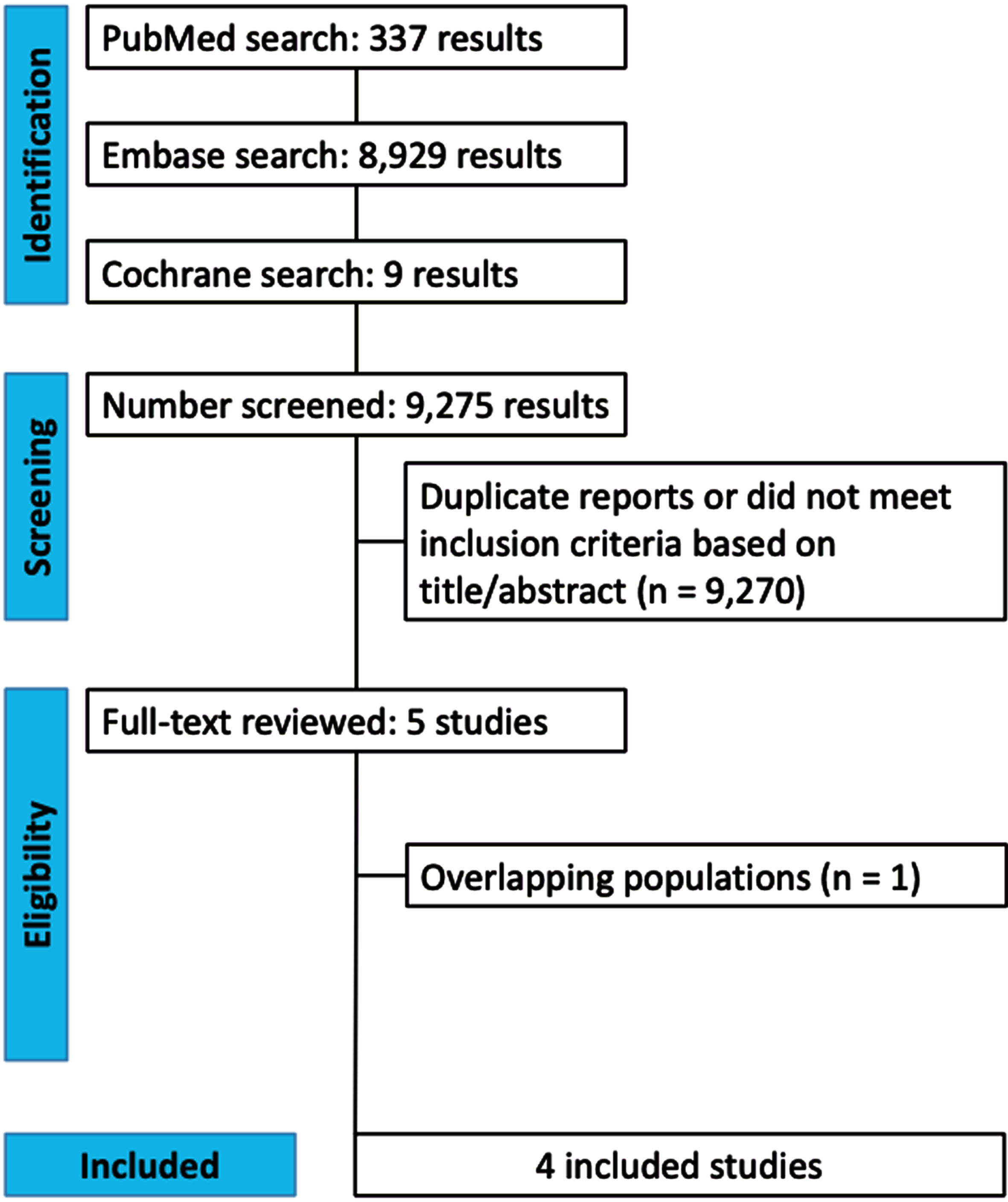
A total of 287,080 (42.3%) patients received PGK1a (TZ/DZ/AZ), and 391,353 (57.6%) received tamsulosin. The mean age ranged from 62–74.74 years and the minimum follow-up time was a median of 45 months. Patients were mostly male (99.9%). Study characteristics are reported in Table 1.
Table 1
Baseline characteristics of included studies
| Study | Design | Country of database | Number of patients | Age* | ||
| TZ/AZ/DZ | Tamsulosin | TZ/AZ/DZ | Tamsulosin | |||
| Sasane 20218 | Retrospective cohort | USA | 22,690 | 45,380 | 70.3 (9.9) | 70.3 (9.9) |
| Gros 20219 | Retrospective cohort | Canada | 92,081 | 173,664 | 74.2 (6.5) | 74.7 (6.9) |
| Simmering 20217 | Retrospective cohort | Denmark | 52,365 | 52,365 | 67.9 (10.4) | 67.9 (10.4) |
| Simmering 20226 | Retrospective cohort | USA | 119,944 | 119,944 | 62 (55–70) | 62 (55–70) |
| Study | Males, % | Follow-up, months | ||||
| TZ/AZ/DZ | Tamsulosin | TZ/AZ/DZ | Tamsulosin | |||
| Sasane 20218 | 98.8 | 98.8 | ≥60 | ≥60 | ||
| Gros 20219 | 100 | 100 | 45 (15–89) | 50 (28–86) | ||
| Simmering 20217 | 100 | 100 | 59.9 (27.5–105.8) | 64.2 (29.9–112.1) | ||
| Simmering 20226 | 100 | 100 | ≥120 | ≥120 |
AZ, alfuzosin; DZ, doxazosin; TZ, terazosin; USA, United States of America. *Values are: mean (SD) or median and interquartile range.
The diagnosis of PD was made by using the International Classification of Diseases (ICD) codes on all cohorts. Two studies added dispensing events for anti-PD medications as an additional method for diagnosis.6,7 Three of four studies reported multivariable adjusted data, whereas one study only provided unadjusted results.
Pooled analysis of all studies
Compared with tamsulosin, patients who received PGK1a (TZ/DZ/AZ) had a lower incidence of PD during follow-up (PGK1a 1.04% vs. tamsulosin 1.31%; HR 0.80; 95% CI 0.71–0.90; p < 0.01; I2 = 84%; Fig. 2A). In a subanalysis excluding patients 60 years of age or younger, patients who received PGK1a (TZ/DZ/AZ) also had a lower incidence of PD (PGK1a 1.21% vs. tamsulosin 1.42%; HR 0.82; 95% CI 0.71–0.95; p < 0.01; I2 = 78%; Fig. 2B). The incidence of PD was slightly higher in this older subgroup, as expected.
Fig. 2A
PD incidence was significantly lower in patients who received PGK1a.
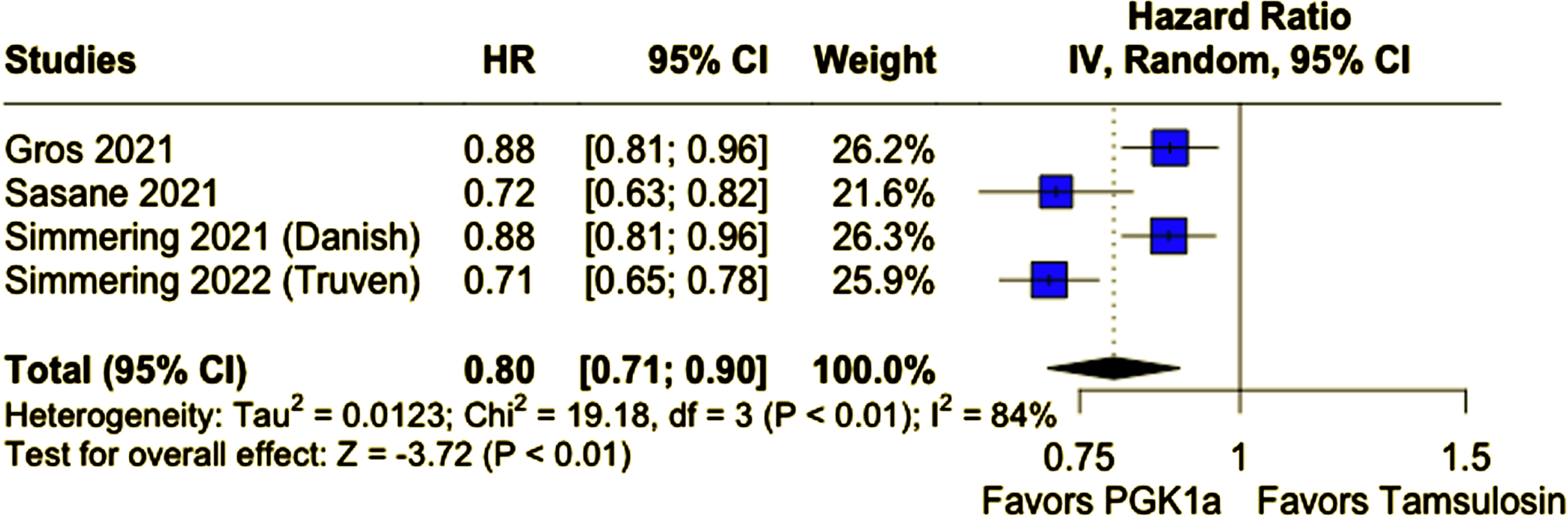
Fig. 2B
PD incidence was lower in patients who received PGK1a in a subanalysis excluding patients 60 years old or younger.
Quality assessment
Risk of bias was assessed by the ROBINS-I tool. No study was considered at high risk of bias (Supplementary Table 1C). Certainty of evidence was deemed moderate overall, as per GRADE (Supplementary Table 1D).14 Analysis of funnel plots showed no evidence of publication bias, although this analysis has limited sensitivity in the setting of a low number of included studies (Supplementary Table 1E).
DISCUSSION
In this systematic review and meta-analysis of four studies and 678,433 patients, we compared the incidence of PD in individuals who received PGK1a versus tamsulosin. We found that patients who received PGK1a (TZ/DZ/AZ) had a 20% relative reduction in the incidence of PD during a median follow-up of at least 45 months, with a consistent result among patients age 61 or older. To the best of our knowledge, this is the first meta-analysis evaluating the risk of PD in participants using PGK1a agents.
There is no consensus in the literature regarding the potential neuroprotection related to alpha-blockers. Hypothesized mechanisms include PGK1 activation and/or alpha-1 adrenergic receptor antagonism. Only the latter is an effect of tamsulosin.15 TZ and analogs, including AZ and DZ, increase intracellular ATP content and decrease reactive oxygen species (ROS) through PGK1 activation.16 Neuroprotection in PD remains a topic of interest with several new potential drugs in the pipeline.
The potential neuroprotective effect of alpha blockers may also be a function of time and cumulative exposure to PGK1 or tamsulosin, as recently described by Gros et al.9 To preserve the effect of time in our analyses, we utilized the hazard as a measure of association and used the results for the longest time of follow-up available in each of the individual studies.
Our findings are in agreement with a previous study published by Cai et al.,3 who also observed slower disease progression in individuals with PD taking TZ/AZ/DZ. A subsequent cohort published by Sasane et al. described a higher incidence of PD in tamsulosin users when compared with PGK1a; however, a higher incidence of PD was also noticed when compared with matched controls not taking prostatic medications.7 A subsequent cohort published by Simmering et al. also observed a lower incidence of PD in patients taking PGK1a when compared with 5-alpha-reductase inhibitors.6 Our findings corroborate this data and are highly suggestive of a potential neuroprotection by glycolysis-enhancing drugs.
In contrast to a regular review, the rigorous methodology of the systematic search provided a thorough assessment of current literature and the pooled analysis yielded quantitative data with statistical significance. However, our study has several limitations. First, the observational nature of the data is subject to confounding factors. To minimize the effect of confounding, we used multivariable adjusted data for studies wherein the data was available. Nevertheless, we cannot exclude the possibility of residual confounding. For instance, patients with prodromal PD and orthostatic hypotension may be over-represented in the tamsulosin group. Second, due to the case definition for PD, we cannot exclude the possibility of incorrect adjudication of atypical parkinsonism as PD. Third, due to the limited number of studies, we could not perform advanced analyses, such as meta-regression to explore potential reasons for heterogeneity. And, finally, generalizability to populations beyond male sex (99.9%) and North America (84.5%) is limited due to the predominant inclusion of these patient groups.
In this meta-analysis evaluating more than 670,000 adults and elderly individuals, the use of glycolysis-enhancing drugs, such as TZ, AZ, and DZ was associated with a lower incidence of PD when compared with tamsulosin. The underlying mechanism may relate to the neuroprotective effects of glycolysis, although this remains to be determined. Future randomized controlled trials in large and diverse populations are warranted to support these findings, and further explore the role of neuroprotective agents in PD.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available within the article and/or its supplementary material.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD240104.
REFERENCES
1. | GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet (2020) ; 396: : 1204–1222. Erratum in: Lancet 2020; 396: 1562. |
2. | Marras C , Beck JC , Bower JH , et al. Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis (2018) ; 4: : 21. |
3. | Cai R , Zhang Y , Simmering JE , et al. Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases. J Clin Invest (2019) ; 129: : 4539–4549. |
4. | Weber MA , Sivakumar K , Tabakovic EE , et al. Glycolysis-enhancing α1-adrenergic antagonists modify cognitive symptoms related to Parkinson’s disease. NPJ Parkinsons Dis (2023) ; 9: : 32. |
5. | Chen X , Zhao C , Li X , et al. Terazosin activates Pgk1 and Hsp90 to promote stress resistance. Nat Chem Biol (2015) ; 11: : 19–25. |
6. | Simmering JE , Welsh MJ , Schultz J , et al. Use of glycolysis-enhancing drugs and risk of Parkinson’s disease. Mov Disord (2022) ; 37: : 2210–2216. |
7. | Simmering JE , Welsh MJ , Liu L , et al. Association of glycolysis-enhancing α-1 blockers with risk of developing Parkinson disease. JAMA Neurol (2021) ; 78: : 407–413. |
8. | Sasane R , Bartels A , Field M , et al. Parkinson disease among patients treated for benign prostatic hyperplasia with α1 adrenergic receptor antagonists. J Clin Invest (2021) ; 131: : e145112. |
9. | Gros P , Wang X , Guan J , et al. Exposure to phosphoglycerate kinase 1 activators and incidence of Parkinson’s disease. Mov Disord (2021) ; 36: : 2419–2425. |
10. | Schultz JL , Brinker AN , Xu J , et al. A pilot to assess target engagement of terazosin in Parkinson’s disease. Parkinsonism Relat Disord (2022) ; 94: : 79–83. |
11. | Higgins JPT , Thomas J , Chandler J , et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook. |
12. | Page MJ , McKenzie JE , Bossuyt PM , et al. The PRISMA statement: An updated guideline for reporting systematic reviews. BMJ (2021) ; 372: : n71. |
13. | Sterne JA , Hernán MA , Reeves BC , et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (2016) ; 355: : i4919. |
14. | Schünemann H , Brożek J , Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. Available from guidelinedevelopment.org/handbook. |
15. | Hertz L , Lovatt D , Goldman SA , et al. Adrenoceptors in brain: Cellular gene expression and effects on astrocytic metabolism and [Ca2+]i. Neurochem Int (2010) ; 57: : 411–420. |
16. | Wang Y , Qian S , Zhao F , et al. Terazosin analogs targeting Pgk1 as neuroprotective agents: Design, synthesis, and evaluation. Front Chem (2022) ; 10: : 906974. |




