Naturalistic Eye Movement Tasks in Parkinson’s Disease: A Systematic Review
Abstract
Background:
Eye tracking assessments in the laboratory have previously highlighted clear differences in eye movements between Parkinson’s disease (PD) and healthy aging. However, laboratory-based eye movement tasks are artificial and limit the ecological validity of observed results. Eye movement tasks utilizing more naturalistic scenarios may provide more accurate insight into cognitive function but research in this area is limited.
Objective:
This systematic review aims to ascertain what naturalistic tasks have revealed about oculomotor deficits in PD and what this information may help us understand about the underlying sensorimotor and cognitive processes.
Methods:
Adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, a literature search of PsycInfo, Medline, Scopus, and Web of Science was conducted using predetermined search terms. Articles including both individuals with PD and healthy older adults completing eye tracking tasks involving naturalistic eye movements (e.g., reading, video-watching, unrestricted visual search) or naturalistic stimuli were included.
Results:
After screening, 30 studies were identified as matching the inclusion criteria. Results revealed consistent findings across tasks, including longer fixation durations and smaller saccadic amplitudes in PD compared to healthy aging. However, inconsistencies in the literature and a lack of standardization in tasks limit interpretation of these results.
Conclusions:
Naturalistic eye movement tasks highlight some consistent differences in eye movements between people with PD and healthy aging. However, future research should expand the current literature in this area and strive towards standardization of naturalistic tasks that can preferably be conducted remotely.
Plain Language Summary
Previous studies using eye tracking in a laboratory environment have shown clear differences in eye movements between people with Parkinson’s disease and healthy older adults. However, eye movements produced during lab-based tasks do not fully imitate eye movements produced during real-life situations, making findings from these studies less applicable to everyday life. Collecting eye movement data during tasks that mimic everyday activities might therefore provide more accurate information regarding cognitive ability in Parkinson’s disease. We reviewed studies that explored eye movements during everyday tasks in Parkinson’s disease to better understand what these studies might be able to tell us about the underlying cognitive processes involved in these tasks. 30 studies were summarized, including studies exploring eye movements during reading, real-life simulations, free viewing, and goal-oriented tasks with natural stimuli. A common finding from the reviewed studies was that Parkinson’s patients fixated their gaze for longer and produced smaller eye movements when viewing the presented visual stimuli (e.g., pictures, texts). Nevertheless, it is difficult to draw firm conclusions about differences in eye movements between people with Parkinson’s disease and healthy controls, as there were many inconsistent findings across the studies. These inconsistencies could be attributed to differences in how the tasks were designed and the types of data collected. We therefore encourage researchers in this field to work towards improving the designs of these tasks to allow for easier comparisons, ideally producing tasks that can be conducted remotely to allow for large quantities of data collection.
INTRODUCTION
Parkinson’s disease (PD) is the second most common neurodegenerative disorder after Alzheimer’s disease, affecting approximately 8.5 million people globally.1 According to the 2016 Global Burden of Disease study, the worldwide prevalence of PD increased by 74% between 1990 and 2016.2 PD is characterized by progressive dopaminergic cell death in the basal ganglia caused by the aggregation of misfolded alpha-synuclein proteins within the brainstem and cortex (Lewy bodies) and also within neuronal cell processes (Lewy neurites).3,4 People with PD may experience a range of both motor (e.g., bradykinesia, tremor, rigidity) and non-motor symptoms (e.g., cognitive abnormalities, psychiatric symptoms) with neuronal loss significantly preceding the appearance of motor features.5 As the disease progresses, and Lewy bodies accumulate in frontal brain areas, cognitive deficits may also begin to occur.5
Eye movements such as saccades are widely accepted to differ between PD and healthy aging6–10 (for latest review, see Antoniades & Spering11). Prosaccadic and antisaccadic eye movement abnormalities during the early stages of PD have been associated with cognitive dysfunction.12 Prosaccade tasks require participants to direct their gaze towards a suddenly presented target. By contrast, antisaccades involve a more complex top-down control task requiring participants to inhibit shifting their gaze towards a target and instead direct their gaze towards the opposite (target-absent) side.13,14 In PD patients, visually guided saccadic abnormalities such as those elicited by prosaccade tasks include frequent interruptions during the prosaccade, increased prosaccadic latencies resulting from abnormal saccadic trajectories, and slower saccade velocity.7 PD patients also produce a greater number of antisaccadic directional errors resulting from a failure to inhibit reflexive prosaccades towards a target.12
In memory-guided saccade tasks, participants are required to direct their gaze towards a remembered peripheral target at the onset of a cue. PD patients’ memory-guided saccades are often characterized by a multiple-step eye movement pattern involving multiple saccades made in the same direction during one gaze shift.15,16 Smooth pursuit eye movement tasks require participants to fixate and follow a moving target with their gaze as smoothly as possible. PD patients demonstrate poorer gain (hypometria) relative to healthy controls (HC) during smooth pursuit, due to increased saccadic intrusions (inappropriate saccades away from a target).15 Finally, square wave jerks, comprising rapid microsaccades away from and back to the target during gaze holding, are necessary for visual stability in the healthy population. Disrupted visual clarity in PD is associated with square wave jerks that are too numerous and too large during gaze-holding tasks.17 Overall, the range of eye movement tasks available allows for a diverse assessment and indication of cognitive function in healthy adults and people with neurodegenerative disease.
Cortico-basal loops comprising separate parallel frontal cortex loops converging on the basal ganglia are implicated in these eye movements. The prefrontal loop is responsible for higher functions such as response inhibition, which feed into motor and oculomotor loops via the striatum to influence action selection.18 Simple and reflexive prosaccades likely rely purely on the oculomotor loop, whereas the inhibition of the prosaccadic reflex during more complicated volitional antisaccadic eye movements likely involves the dorsolateral prefrontal cortex, part of the prefrontal loop.19 Low firing rates of striatal medium spiny neurons are integral to successful interloop information transfer.20 In PD, medium spiny neuron firing rates are abnormally high.21,22 Increased antisaccade error rates in PD plausibly reflect a breakdown in interloop information transfer and a resulting lack of prefrontal loop influence over the oculomotor loop.19 Furthermore, increased prosaccadic latencies in PD suggests dysfunctional oculomotor intraloop information transfer.19 Antisaccade impairments are observed during the early stages of the disease, suggesting that interloop information transfer is more affected than intraloop transfer in PD.19
Whilst the commonly utilized eye movement tasks described above have robustly demonstrated important differences in eye movement measures between PD and HC, these tasks have certain limitations.23 They are artificial, not natural, and often counterintuitive, which may decrease the ecological validity of the observed results. In contrast, naturalistic eye movement tasks, such as reading and watching television, are familiar to participants and can often be undertaken in a more comfortable environment (see Fig. 1). As a result, naturalistic eye movement tasks are less stressful and more ecologically valid and may thus provide greater insight into a patient’s oculomotor and cognitive capabilities.23 Our previous review exploring naturalistic eye movements in Alzheimer’s disease revealed that eye movement deficits were present across a range of naturalistic eye movement tasks with some evidence to suggest that such naturalistic eye movement deficits may also occur in mild cognitive impairment (see Readman et al.23; also see Seligman and Giovannetti24).
Fig. 1
Figure to show example stimuli and analysis for both standard laboratory and naturalistic eye movement tasks. Currently available recording equipment can be easily utilized for both types of eye movement tasks.
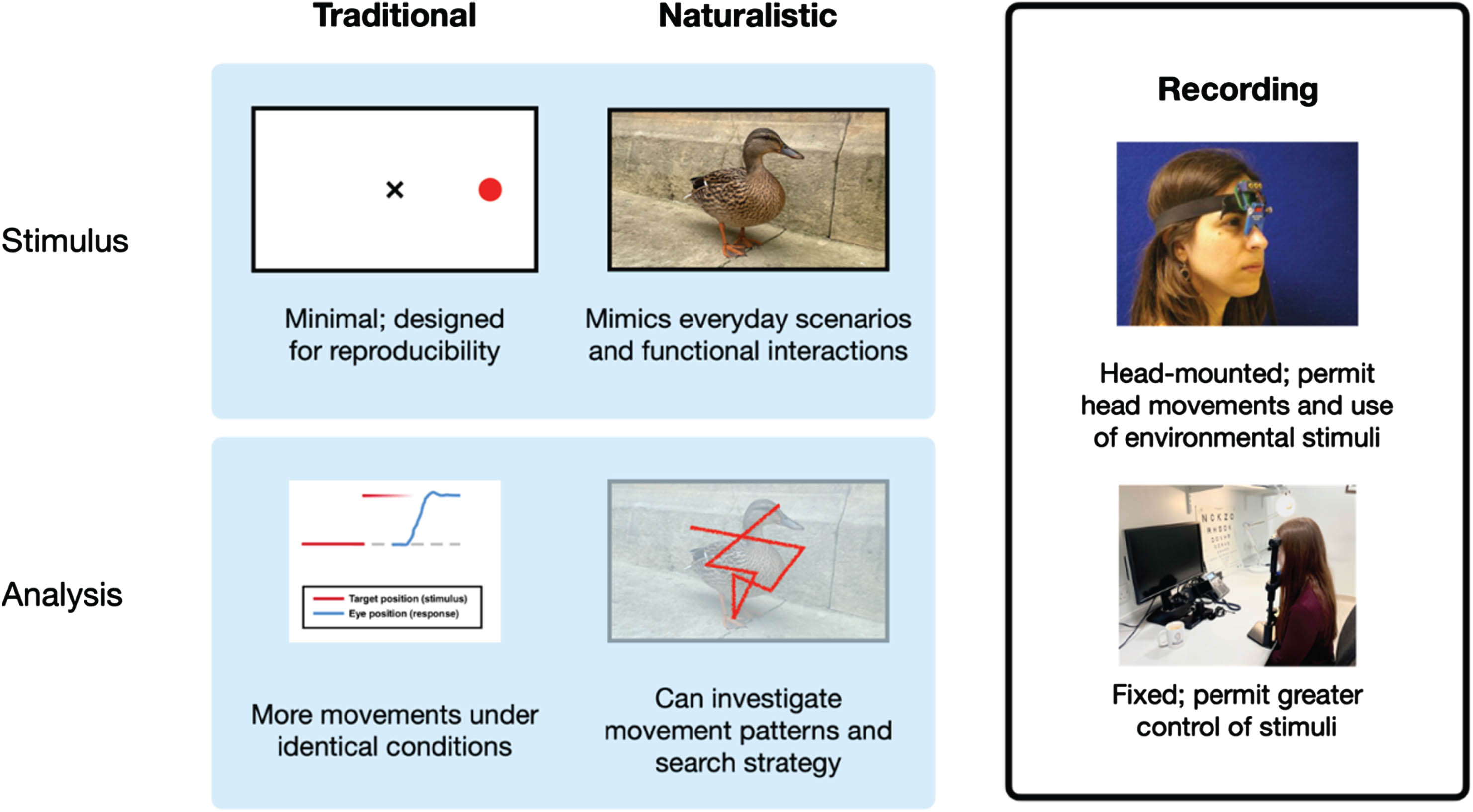
The analysis of naturalistic eye movements is more complex than data from standard laboratory eye movement tasks such as the previously mentioned prosaccades and antisaccades. However, naturalistic tasks can be readily implemented using largely the same equipment as standard tasks. As such, the aim of this review is to explore the range of naturalistic eye movement tasks utilized in the present literature and ascertain what these tasks have revealed about oculomotor as well as cognitive deficits in PD. As volitional eye movements are more impaired than automatic eye movements in PD,25 we hypothesized that a greater impairment in volitional compared to automatic naturalistic eye movements would be reported.
MATERIALS AND METHODS
The systematic literature review protocol was pre-registered on Prospero (ID: CRD42022347606). We adhered to the Non-Interventional, Reproducible, and Open Systematic Review guidelines (V1)26 when conducting and writing the review (see the Supplementary Material for a more detailed extended methods). Papers that explored eye movements during naturalistic locomotion and balance tasks were collected as part of the pre-registered search strategy and these papers will be summarized in a separate review. There were no further deviations from the pre-registration.
Search protocol
The systematic literature search was conducted on 25 August 2023 using PsycInfo, Medline, Scopus, and Web of Science. The search strings encompassed search terms relating to the populations (PD, healthy older adults) and tasks (naturalistic eye movement tasks) of interest (see Supplementary Material for the full search strategy). The.ris files acquired from each database were exported into CADIMA, an online open-access tool designed to facilitate conducting a systematic review.27,28 The records were deduplicated using CADIMA’s automatic duplicate removal tool. In addition, we completed forward and backward citation tracking using Google Scholar and searched for relevant pre-prints using PsyArXiv, MedArXiv, and bioArXiv.
Inclusion and exclusion criteria
Screening was conducted in two phases: (1) title and abstract screening, and (2) full-text screening (see Supplementary Material for the screening criteria applied during both phases of screening).
Naturalistic eye movement tasks were defined as tasks that either (a) incorporate naturalistic stimuli, (b) allow free unrestricted visual exploration of stimuli that are present for a minimum duration of 5 s, or (c) tasks identical to or closely mirroring tasks undertaken in normal daily life. We regard prosaccade tasks incorporating naturalistic stimuli as naturalistic paradigms, as they replicate eye movement regularly executed in daily life. In contrast, as antisaccade tasks do not require fixation on an object of interest, and instead require inhibition of eye movements away from an object of interest, we argue that these eye movements are counterintuitive to those performed in daily life and are not included under this definition (see the Supplementary Material for full inclusion and exclusion criteria).
Studies were included if they utilized a naturalistic eye movement task (as above) and featured both idiopathic PD and HC groups. As research has observed significant differences in eye movements between idiopathic PD and other etiologies of parkinsonism,29–31 we excluded studies with atypical parkinsonian syndromes. Therefore, while studies that recruited both idiopathic PD patients and atypical parkinsonian syndromes were included, only the idiopathic group with be discussed in this review.
Psychiatric comorbidities are common in PD,32,33 along with additional neurological conditions such as rapid eye movement sleep disorder34 and can lead to disruptions in eye movements. Therefore, we excluded patients with reported psychiatric and neurological comorbidities.35 Finally, we excluded studies that only included PD patients with reported comorbid mild cognitive impairment or PD dementia.
Screening
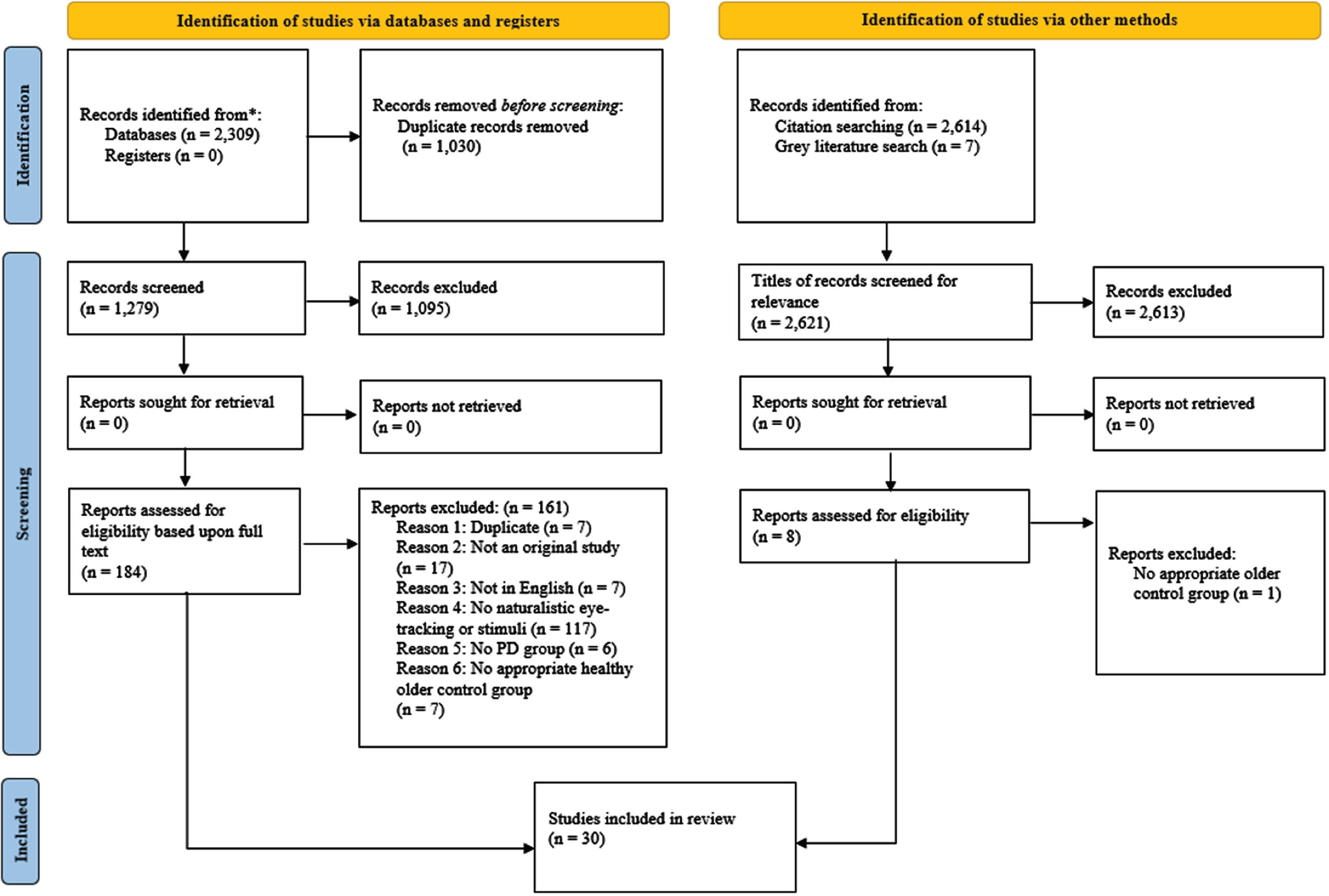
Two raters (MG, JH) independently screened all records (see the Supplementary Material for a decision log regarding the resolution of inconsistencies). Overall, 30 papers are included in the review (see Fig. 2 for a pictorial depiction of the number of records excluded at each stage of the screening process).36 All records meeting the full text screening criteria were checked for retraction using the Retraction Watch Database on 23 October 2023 (http://retractiondatabase.org/) with none of the included records being found within this database.
Fig. 2
PRISMA flowchart outlining number of papers excluded at each stage of screening, detailing the number of records at each stage of the search and screening process.36 Only the first reason for exclusion is reported for each paper (see the Supplementary Material for each reason for exclusion for each paper). The PRISMA flowchart allows for transparent reporting, so that our searches can be reproduced.
Data extraction
Data extraction for each record was conducted by a single reviewer (MR) and checked by another reviewer (MP) using Excel (see the Supplementary Material for the data extracted from each included paper).
Quality assessment
As no appropriate tool currently exists for assessing the quality of non-interventional research, the Downs and Black risk of bias checklist37 was adapted to be more appropriate (see Supplementary Material for the modified risk of bias tool). This tool has been commonly modified for assessing the quality of non-intervention research in previous systematic reviews (e.g., Readman et al.23 and Luo et al.38). Each record was independently assessed by two reviewers (MG, JH) with any inconsistencies in ratings being resolved by a third reviewer (MP) (see the Supplementary Material for a log of decision making). The maximum score for our modified risk of bias checklist was 34 (mean = 25.0, range = 16–31).
RESULTS
This systematic review has shown that previous studies (N = 30) examining naturalistic eye movements in people with PD and HC can be broadly classified into five domains: goal-directed paradigms with naturalistic stimuli (e.g., visual search within a naturalistic scene), reading tasks, real-life simulations and everyday tasks (e.g., video-watching), free viewing of static images, and facial processing tasks (see Table 1 for the quality assessment scores of each included study). Due to the distinct eye movement patterns elicited by the five domains identified and the heterogenous nature of the tasks utilized between these five domains, drawing comparisons between the results of these domains is impractical. Consequently, the results of each domain of literature are presented separately.
Table 1
Quality assessment ratings using the modified Downs and Black (1998) checklist 37 for each paper that passed through our inclusion criteria
| Reference | Quality Assessment Rating (out of 34) |
| Beylergil et al.39 | 23 |
| Bek et al.40 | 24 |
| Bek et al.41 | 25 |
| Nagai et al.42 | 27 |
| Archibald et al.43 | 31 |
| Aveni et al 44 | 29 |
| Stock et al.45 | 27 |
| Park46 | 29 |
| Tsitsi et al.47 | 30 |
| Waldthaler et al.48 | 20 |
| Kannan49 | 25 |
| Yu et al.50 | 20 |
| Terao et al.51 | 28 |
| Watanabe et al.52 | 22 |
| Sacrey et al.53 | 20 |
| Sacrey et al.54 | 18 |
| Tseng et al.55 | 16 |
| Habibi et al.56 | 21 |
| Fischer et al.57 | 28 |
| Zhang et al.58 | 23 |
| Matsumoto et al.59 | 27 |
| Dietz et al.60 | 26 |
| Revankar et al.61 | 31 |
| Buhmann et al.62 | 23 |
| Crutcher et al.63 | 30 |
| Clark et al.64 | 25 |
| Polet et al.65 | 26 |
| Bek et al.66 | 23 |
| Waldthaler et al.67 | 26 |
| Ciccarelli et al.68 | 27 |
Studies employing goal-directed paradigms with naturalistic stimuli
Out of the included studies, six incorporated goal-directed paradigms with naturalistic stimuli.39–44 Goal-directed paradigms include those studies where participants were asked to perform a specific task while looking at naturalistic stimuli, such as photographs or drawings (see Table 2 for a summary of the main results). The following studies include a complex visual search task,39 clock-matching tasks,42,43 action observation and imitation tasks,40,41 and sentence-picture matching task.44
Table 2
Summary of main results of papers employing goal-directed paradigms with naturalistic stimuli. All comparisons are between people with PD and HC. PD, Parkinson’s disease; ROI, region of interest; HC, healthy control; CN, cognitively normal
| Study | Groups (n) | Task | Main results |
| Beylergil et al.39 | PD on medication (13) Age-matched HC (7) | Complex visual search | PD patients were slower to look at ROI in the unexpected condition. Higher fixational saccade frequencies in PD. Lower non-fixational saccade frequencies in PD. |
| Bek et al.40 | PD (21) Age-matched HC (23) Medication state unreported | Observation and imitation | No significant differences. |
| Bek et al.41 | PD, on medication (18) Age-matched HC (21) | Observation and imitation | Unlike HC, PD patients did not modulate eye movements based on predictability. |
| Nagai et al.42 | PD, on medication (13) Age-matched HC (17) Young HC (36) | Clock matching Inverted clock matching | PD exhibited more saccades to ROI and array ROIs and larger deficiency score on inverted clock-matching. |
| Archibald et al.43 | PD-CN, on medication (35) HC (29) | Clock matching Inverted clock matching Angle matching Shape position Overlapping figures Data from all tasks analyzed together. | PD-CN made longer fixations during viewing. |
| Aveni et al.44 | PD (24), on medication (23) Age-matched HC (24) | Sentence-picture matching | No significant differences. |
Beylergil et al.’s39 complex visual search task involved searching for everyday objects inside cluttered photographic scenes. In a number of trials, the target object was in a location semantically associated with the scene (e.g., a kettle on a kitchen counter), hereafter referred to as the expected condition. Other trials had the target object presented in a location that it would not commonly be found (e.g., a kettle on a chair), hereafter referred to as the unexpected condition. PD patients were significantly slower than HC to look at the target object in unexpected conditions. Non-fixational saccade frequency was significantly lower in PD compared to HC, whereas fixational saccade frequency was significantly higher. Further analyses revealed that disease duration and PD patient age at the time of testing were positively correlated with time taken to look at the target object in both conditions. Additionally, total motor severity scores from part III of the Unified Parkinson’s Disease Rating Scale (UPDRS-III), as well as gait and axial sub-scores, were positively correlated with time taken to look at the target object in both conditions.
Two studies by the same research group explored eye movements in PD patients with mild to moderate disease during observation and imitation of movement sequences demonstrated by either a human hand (biological stimulus) or a blue square (non-biological stimulus).40,41 In their first study, no differences in eye movements were found between PD and HC.40 In their second study, HC exhibited significantly smaller saccadic amplitudes when presented with predictable compared to unpredictable movements following an elevated, but not a direct, trajectory.41 This difference was not observed in PD.
Both Nagai et al.42 and Archibald et al.43 recorded eye movements while participants completed an inverted clock-matching task. Participants were presented an upright clock face displaying a time and instructed to select the matching clock face from an array of clocks orientated at 180° to the original. Nagai et al.42 observed that PD patients made significantly more saccades within, and repeated saccades to, regions of interest of inverted clock faces than HC. Clinical characteristics of PD did not correlate with eye movement measures. Archibald et al.43 further found significantly increased fixation durations in PD compared to HC. Fixation durations were negatively correlated with cognitive scores and positively correlated with UPDRS-III scores.
Finally, Aveni et al.44 recorded eye movements as participants heard sentences and matched them to one of four presented images. Anticipatory fixations are fixations on the target image made before the point at which the correct target image was identifiable. Anticipatory fixations did not differ between PD and HC and were not correlated with motor severity.
Overall, the tasks utilized to investigate eye movements during goal-directed paradigms are highly heterogeneous. Nevertheless, increased fixational saccades were observed by both Nagai et al.42 and Beylergil et al.39 It was further found that older age at time of testing in PD patients, higher UPDRS-III scores, and worse cognitive scores were associated with greater eye movement abnormalities (Beylergil et al.39; Archibald et al.43; but see Nagai et al.42). Overall, these tasks demonstrate that eye movement deficits in PD are present during a range of goal-directed tasks and may affect performance (Beylergil et al.39; Bek et al.40,41; Nagai et al.42; Archibald et al.43; but see Aveni et al.44).
Reading tasks
Of the 30 studies included, eight used eye tracking while participants read silently45–49 or aloud50–52 short sentences, passages, or single word lists in their native language (see Table 3 for summary of main results).
Table 3
Summary of main results of papers employing Reading tasks. All comparisons are between people with PD and HC. PD, Parkinson’s disease; HC, Healthy control, MCI, mild cognitive impairment; CN, cognitively normal
| Study | Groups (n) | Task | Main results |
| Stock et al.45 | PD-CN, on medication (38) – 25 tested ON and OFF medication. Age-matched HC (29) | Silent reading | PD-CN exhibited longer fixation durations OFF medication compared to ON medication. |
| Park46 | PD, on medication (13) Age-matched HC (17) | Silent reading and reading aloud | Silent reading and reading aloud: PD patients had longer first and average fixation durations. |
| Tsitsi et al.47 | PD-CN, on medication (32) Age-matched HC (42) | Silent reading | PD-CN exhibited lower rates of fixation compared to HC. Pooled PD-CN and PD-MCI exhibited longer fixation durations compared to HC. Pooled PD-CN and PD-MCI exhibited higher fixation duration variability compared to HC. |
| Waldthaler et al.48 | PD, tested ON and OFF medication (19) Age-matched HC (13) | Silent reading | PD patients read significantly fewer words per minute. PD exhibited an increased number of regressive saccades. PD exhibited longer fixation durations. Disease duration showed correlation with reading speed. |
| Kannan49 | PD (64) HC (43) Medication state unreported. | Silent reading | The duration of reading forward saccades was longer in PD. |
| Yu et al.50 | PD, on medication (1) Age-matched HC (1) | Reading aloud | PD patient made more saccades per line with smaller amplitudes. Slower word reading in PD was related to longer fixation duration and more regressive saccades. Fixation duration in PD was also longer during number reading. |
| Terao et al.51 | PD, on medication (18) Age-matched HC (30) | Reading aloud | The gaze scanning speed (excluding regressive saccades) was slower in PD patients. Saccade frequency was lower in PD. |
| Watanabe et al.52 | PD, on medication if taking (29) Age-matched HC (19) | Reading aloud | In horizontal reading PD patients exhibited slower velocity and increased fixations. In vertical reading PD patients had slower average velocity in X and Y directions. |
From the studies involving silent reading, most observed that PD patients displayed significantly longer fixations than HC.45–48 Tsitsi et al.47 reported that longer fixation durations were specific to PD patients with cognitive decline. Additionally, UPDRS-III scores were positively correlated with fixation durations and the number of fixations per second. Similarly, Waldthaler et al.48 observed that worse cognitive scores were associated with eye movement abnormalities, such as increased number of regressive saccades and longer mean fixation durations. Reading speed significantly decreased with disease duration.48 Stock et al.45 found that fixation durations significantly shortened when patients were on-medication.
Other significant findings include longer forward saccade durations49 and increased regressive saccades resulting in fewer words read per minute48 in PD compared to HC. One case study50 involving reading aloud met our inclusion criteria and reported findings consistent with the larger studies outlined above. Reading speed was approximately 50% slower than that of an age- and language-matched HC. This significantly slower reading speed may have been attributable to a smaller staircase eye movement pattern composed of many small saccades, as well as more saccades per line of text and smaller saccadic amplitudes. Furthermore, as above, the PD patient displayed significantly longer fixation durations and more regressive saccades. Finally, Tsitsi et al.47 observed sex-specific oculomotor deficits during silent reading, as male PD patients significantly differed from male HC, while female PD patients did not differ from female HC (although sex-deficits are not consistently found; see Stock et al.45).
In contrast, Watanabe et al.52 observed no differences in the time taken to complete a reading aloud task between Japanese speaking PD patients and HC. This is potentially because easily readable texts requiring minimal comprehension processing were selected, such as the alphabet and a well-known story. Terao et al.51 similarly observed no differences in reading aloud speed across a range of texts varying in complexity and readability. Watanabe et al.52 detected significant differences between the ratio of fixations and saccades, as well as eye movement velocities particularly in the vertical direction. Machine learning algorithms were able to distinguish between PD and HC with 82.4% accuracy using vertical eye movements during vertical text reading. Terao et al.51 further observed significant oculomotor differences between PD and HC, including decreased saccade frequency, regardless of text difficulty or readability. For both PD and HC, the location of gaze was approximately three words ahead of the uttered word position with the gaze of PD patients occasionally lagging behind their uttered word position. Moreover, scanning speed, referring to both saccadic amplitude and frequency, was significantly reduced in PD. To determine how far scanning preceded the utterance position, reading speed was subtracted from scanning speed and this was found to be significantly reduced in PD relative to HC.
Finally, Park46 recorded eye movements while participants read texts both silently and orally. Similarly to the studies outlined above,45–48 PD patients exhibited significantly longer fixation durations compared to HC during both silent and oral reading of sentences. Regardless of the number of stressed syllables, the first fixation duration on target words was significantly longer in PD than in HC. PD patients with longer disease duration had significantly longer first fixation durations compared to those with shorter disease durations. Participants also completed a delayed reading block, whereby they were required to silently read a sentence before orally reading it. Fixation durations were significantly longer in PD for both immediate and delayed readingconditions.
Overall, increased fixation duration during silent reading in PD patients appears to be a replicable finding. Whilst this finding was less consistent during aloud reading, this could be due to differences between the languages of the texts, as the languages using Latin-script alphabets (English and Spanish) observed increased fixation durations in PD,46,50 whereas increased fixation durations were not observed in PD during reading of Japanese logographic and syllabic texts.51,52 Cognitive decline47,48 and disease duration46,48 may also be associated with eye movement deficits during reading in PD. Importantly, the effects of medication on eye movements during reading in PD must be explored further, as medication status might be confounding the present literature.45
Eye movement behavior during real-life simulations and everyday tasks
Only four studies were identified as including a real-life simulation or an everyday task, including a real-life simulation of reach-to-eat behaviors53,54 and video-watching tasks55,56 (see Table 4 for a summary of the main results).
Table 4
Summary of main results of studies investigating eye movement behaviors during real-life simulations and everyday tasks All comparisons are between people with PD and HC. PD, Parkinson’s disease; HC, healthy controls; SVM-RFE, support vector machine with recursive feature elimination; *saccadic peak velocity, inter-saccade interval, saccade duration, saccade amplitude
| Study | Groups (n) | Task | Main results |
| Sacrey et al.53 | Mild (8) and advanced (7) PD, on medication Age-matched HC (15) Young HC (11) | Reach-to-eat | Advanced PD took longer to move after fixating the food target. After grasping, advanced PD took longer than mild PD to look away from the food target. Music normalized fixation durations. |
| Sacrey et al.54 | PD, tested ON and OFF medication (8) Age-matched HC (8) | Reach-to-eat, with and without background music | Without music: PD OFF took longer to look away after grasping than PD ON and HC. With music: PD OFF exhibited longer engage latency than PD ON and HC. PD ON and OFF took longer to initiate movement towards the target after visual fixation than HC. |
| Tseng et al.55 | PD, on medication (14) Age-matched HC (24) Young HC (18) | 1-minute video clip watching | SVM-RFE achieved 90% accuracy differentiating between PD and age-matched HC on 4 oculomotor features*. |
| Habibi et al.56 | PD, varying medication status (27) Age-matched HC (132) | 10 one-minute videos with clips changing every 2–4 s | PD exhibited a stronger center bias. Macro-saccade rate was lower in PD. Fixation duration was longer in PD. Saccadic amplitude was smaller in PD. |
During the reach-to-eat task, Sacrey et al.54 recorded eye movements while participants reached towards and grasped a food item which was withdrawn to the mouth and eaten. This study attempted to maintain a quasi-naturalistic testing environment in the participant’s home by having the experimenter engage in casual conversation with participants. Additionally, participants were unaware that their eye movements were being recorded. HC fixated on the food target prior to initiating the reach movement and stopped fixating when they grasped the food object. In contrast, PD patients off medication took significantly longer to disengage from fixating the food target than both HC and on-medication state PD patients. Playing music selected by the participant in the background was found to significantly decrease the disengagement latency in PD. These findings are consistent with an earlier study by the same group. Sacrey et al.53 utilized the same paradigm, but in a lab-based setting and with on-medication state PD patients only. They found that advanced PD patients fixated significantly longer than mild PD patients, HC, and young HC on the food object before reaching and withdrawing. Music normalized eye movements in advanced PD patients.
Both video-watching tasks required participants to simply watch a series of video clips without goal-directed instructions. Habibi et al.56 had participants watch ten one-minute video clips. Whilst both PD and HC displayed a strong center gaze distribution bias, this was significantly greater in PD patients. In addition, PD patients made significantly fewer macro-saccades and exhibited significantly longer fixation durations than HC. When considering the axial distribution of micro- and macro-saccades, PD patients exhibited significantly fewer and smaller horizontal and vertical macro-saccades compared to HC. After a clip change, both PD and HC displayed a reduction in macro-saccade frequency and amplitude followed by a rebound. However, both the macro-saccadic amplitude and macro-saccadic frequency of the rebound were significantly reduced in PD compared to HC. Whilst HC experienced a suppression of micro-saccade rate persisting for approximately 500 ms, the magnitude of this suppression was significantly reduced in PD.
In contrast, Tseng et al.55 had participants watch 20 minutes of video comprising 2–4 s clips of unrelated scenes to minimize predictability and maximize attention. Oculomotor deficits were identified in PD, including significantly smaller saccadic amplitudes and shorter saccadic durations, as well as abnormalities in peak saccadic velocity and inter-saccadic intervals. Machine learning was used to classify participants as either PD or HC by identifying the most useful distinguishing features using a support vector machine-with recursive feature elimination (SVM-RFE). Classification accuracy was 89.6% based upon the five features identified by the SVM-RFE as being the most discriminative with these relating to saccadic intervals, peak saccadic velocity, and saccadic duration. When the model considered only oculomotor features, classification was 86.4% accurate. Finally, while eye movements indicative of bottom-up attentional processes (e.g., eye movement influenced by stimulus saliency) did not differ between PD and HC, eye movements associated with weaker top-down attentional control (e.g., how much time spent looking at the video stimulus) were observed in PD. When the model considered only eye movements associated with top-down attentional processes, classification was 74.6% accurate.
To conclude, both video-watching tasks observed smaller saccadic amplitudes.55,56 Additionally, while there is little similarity between video-watching and reach-to-eat tasks, longer fixation durations were observed in both tasks.54,56
Free viewing of static images
Similar to free-viewing of dynamic video clips, seven papers were categorized as including a task involving free unrestricted viewing of static images without any concurrent goal-directed task57–63 (see Table 5 for main results).
Table 5
Summary of main results from papers utilizing free viewing paradigms. All comparisons are between people with PD and HC. PD, Parkinson’s disease; HC, healthy controls; PDnP, Parkinson’s disease non-pareidolia type; PDP, Parkinson’s disease pareidolia type; ROI, region of interest; DBS, deep brain stimulation; STN, subthalamic nucleus
| Study | Groups (n) | Task | Main results |
| Fischer et al.57 | PD, on medication, bilateral STN DBS-ON (unilateral and bilateral) and -OFF stimulation conditions (17) Age-matched HC (17) | Free viewing of naturalistic images | Mean saccade length was shorter in DBS-OFF compared to HC and DBS-ON. |
| Zhang et al.58 | PD, on medication (37) Age-matched HC (39) | Free viewing of a landscape picture | PD patients made fewer saccades. PD patients explored a narrower portion of the images. |
| Matsumoto et al.59 | PD, on medication (18) Age-matched HC (18) | Free viewing of four images of varying complexities (a cube, 2 overlapping pentagons, a house and the Rey-Osterrieth complex figure) | Fixations were distributed across a significantly smaller and narrower area in PD. Fewer saccades were made by PD for less complex pictures. Saccadic amplitudes were smaller in PD. |
| Dietz et al.60 | PD, on medication (14) Near age-matched HC (12) | Free viewing of images from the International Affective Picture System. | PD patients made fewer fixations when viewing pleasant or neutral pictures. PD patients exhibited shorter scan paths for pleasant compared to unpleasant stimuli. |
| Revankar et al.61 | PD, on medication: (21), separated into PD non-pareidolia type (PDnP, 11) and PD pareidolia type (PDP, 10) Aged-matched HC (12) | Free viewing of noise pareidolia test pictures where 20 faces had been replaced by Mooney faces with a shadow-effect | Fixation durations were longer in PD. PD made more ROI visits. PDP exhibited longer fixation durations and made more ROI visits than PDnP. |
| Buhmann et al.62 | PD, on medication (22) Age-matched HC (22) | Free viewing of photographs | No significant results. |
| Crutcher et al.63 | PD (4) Aged-matched HC (15) Medication status unreported. | Visual paired comparison task | No significant results. |
Both Buhmann et al.62 and Crutcher et al.63 observed no significant differences between PD and HC during free viewing of photographs and a visual paired comparison task involving simple black-and-white clipart images, respectively. However, several studies utilizing naturalistic photographs did observe significant differences between PD and HC. Zhang et al.58 observed that PD patients made fewer saccades than HC and explored a narrower portion of the images, particularly in the horizontal direction. In addition, Fischer et al.57 evaluated oculomotor patterns in PD patients during free viewing of images of natural or urban scenes with deep brain stimulation (DBS) and left-side symptom onset. Four DBS conditions were tested: OFF-stimulation, bilateral subthalamic nucleus stimulation, and left and right unilateral monopolar stimulation of the most ventral DBS electrode contacts. The mean length of saccades OFF-stimulation was significantly smaller than HC and bilateral ON-stimulation. Mean saccade lengths during unilateral monopolar stimulation conditions were between those observed in bilateral ON- and OFF-stimulation conditions. During all stimulation conditions, PD patients exhibited a mean horizontal fixation position and exploration pattern that was shifted rightwards compared to HC. Hence, PD patients with left-side symptoms onset displayed a rightward exploration bias in comparison to HC across DBS conditions.
Utilizing a different type of naturalistic stimuli, Matsumoto et al.59 presented participants with line drawings of varying complexities (a cube, two overlapping pentagons, a house and the Rey-Osterrieth complex figure). Like Zhang et al.58 PD patients made significantly fewer saccades and fixated longer than HC. However, this was only true for the simpler images, such as the cube and overlapping pentagons. Saccadic amplitudes increased with image complexity across both groups with PD exhibiting significantly smaller saccadic amplitudes than HC overall.
Revankar et al.61 tested on-medication PD patients on a noise pareidolia test, which examines the propensity to impose meaning onto stimuli where there is none. PD patients were divided into those with and without a propensity for pareidolia with susceptible PD patients fixating significantly longer on areas of interest than non-susceptible PD patients who, in turn, fixated longer than HC. Susceptible PD patients also dwelled longer than non-susceptible PD patients. However, this difference was only observed for ambiguous face stimuli, as opposed to clearer face stimuli.
Finally, Dietz et al.60 measured eye movements as an index for emotional processing during free viewing of pleasant, unpleasant, and neutral images. Both PD and HC made more fixations when viewing emotionally evocative stimuli. However, PD patients made significantly fewer fixations when viewing pleasant and neutral images and displayed shorter scan paths than HC. The scan paths of PD patients were significantly shorter for pleasant compared to unpleasant stimuli in contrast to HC who exhibited equivalent scan path lengths across valanced stimuli.
Overall, despite the range of stimuli utilized across these free viewing tasks, several consistent findings were observed, including longer fixation durations,58,59,61 fewer saccades,58,59 and reduced exploration of the presented images57–59 in PD compared to HC.
Facial processing
Finally, five of the included studies analyzed eye movements in PD during processing of facial stimuli displaying different emotions.64–68 The following studies utilized standardized facial stimuli (e.g., Ekman’s faces) and experimental procedures, allowing for easier comparison of results between studies than those outlined above (see Table 6 for a summary of the main results).
Table 6
Summary of main results from papers utilizing facial processing paradigms. All comparisons are between people with PD and HC. PD, Parkinson’s disease; HC, Healthy controls; CN, cognitively normal; RVF, right visual field
| Study | Group (n) | Task | Main results |
| Clark et al.64 | PD, on medication (16) Age-matched HC (20) | Emotion recognition task using stimuli from Ekman database | PD fixated on the RVF of fearful faces more than HC. HC women fixated less on fearful faces than PD women. |
| Polet et al.65 | PD (20) Age-matched HC (22) Medication state unreported. | Emotion recognition task using stimuli from Reading of the Mind in the Eyes test and Ekman database | No significant results between PD and HC. |
| Bek et al.66 | PD, on medication where applicable (18) HC, significantly older than PD (10) | Facial processing using stimuli from the Amsterdam Dynamic Facial Expression Set. | Reduced fixation rates towards the eyes of dynamic faces expressing sadness, anger, and disgust compared to static faces in HC, but not PD. Facial motion improved emotion recognition in HC, but not PD. |
| Waldthaler et al.67 | PD-CN, on medication (12) Age and sex matched HC (12) | Emotion recognition from Radboud faces | Scanned area reduced in PD-CN compared to HC. Effect of valence on fixation duration in PD. |
| Ciccarelli et al.68 | PD, on medication (30) Age-matched HC (20) | Emotion recognition task using stimuli from Ekman database | HC, but not PD, modified scanning strategy to focus on eyes of faces displaying negative emotions. |
Regarding where PD patients look when viewing facial stimuli, Ciccarelli et al.68 observed that gaze duration across top and bottom facial regions was the same across facial expressions for PD patients. In contrast, HC gazed significantly longer and displayed increased fixations to the top region of faces expressing negative emotions, suggesting that HC, but not PD, modify their visual scanning strategy according to the valance of the presented emotional expression (but see Clark et al.64). Ciccarelli et al.68 observed that a higher number of fixations on the bottom region of facial stimuli was associated with a higher number of errors on the Stroop test. Furthermore, Clark et al.64 found that PD patients made a significantly greater number of fixations toward the right visual hemifield of fearful faces. This rightward fixation bias was present regardless of whether PD patients had left- or right-side symptom onset. Finally, Waldthaler et al.67 found that PD patients spent significantly less time fixating on sad and angry faces compared to happy faces and had a reduced scanning area compared to HC. Oculomotor deficits may account for the observed impairment in recognizing emotions in PD compared to HC64,65,68. However, Polet et al.65 found that, while PD patients were impaired in emotion recognition compared to HC, there were no significant differences in eye movements. The only study we could find in the literature to present dynamic facial expressions was by Bek et al.66 Results showed reduced fixation rates towards the eyes of dynamic faces expressing sadness, anger, and disgust compared to static faces in HC, but not PD. Additionally, Bek et al.66 found that facial motion improved emotion recognition in HC, but not PD, suggesting that PD patients are impaired in effectively using motion cues to recognize emotional expressions.
DISCUSSION
To our knowledge, this is the first review comprehensively summarizing the current literature on naturalistic eye movements in PD. Such eye movement tasks may allow us to detect more subtle changes in eye movement behaviors in PD than standard laboratory tasks, as they are often more comfortable for patients and involve familiar stimuli andbehaviors.23
A quality assessment of included papers revealed a mean risk of bias score of 25/34. A large range of risk of bias scores were observed (range = 16–31), indicating that bias may have influenced included studies to differing degrees. Here, bias refers to factors that could impact the observations and conclusions drawn by individual studies. Risk of bias is assessed using a comprehensive checklist (see Supplementary Material) with lower scores indicating a higher risk of bias. The most common sources of bias identified were failure to describe the characteristics of participants lost to exclusion, failure to consider those lost to exclusion in analyses, no justification of sample size, and failure to consider potential confounds in the analyses (see the Supplementary Material for risk of bias item ratings for each included study). Therefore, a certain level of caution should be taken when interpreting the results of included studies. Future work should aim to minimize these common potential sources of bias.
This systematic review has identified several findings across the literature. Firstly, longer fixation durations in PD were observed across a range of different naturalistic eye movement tasks, in particular during reading tasks.45–48,50 Longer fixation durations were also observed in other paradigms such as video-watching,56 goal-directed paradigms43 and free viewing.61 PD patients have been reported to spend less time exploring the periphery of presented stimuli and explored a narrower portion of presented stimuli during video watching,56 free viewing58,59 and facial processing.67 Finally, PD patients also displayed smaller saccadic amplitudes across a range of tasks, including reading,50 video-watching,55,56 free viewing,59 and a goal-directed clock matching task.43
Nevertheless, several inconsistencies were found in the literature. For example, an inconsistent effect of emotional valence on eye movements was observed, dependent on whether patients were viewing facial or non-facial stimuli.60,66,67 Furthermore, while Tsitsi et al.47 observed sex-specific differences in eye movements during reading, Stock et al.45 did not. Importantly, these were the only two reading studies to analyze potential sex differences. Confounding differences between PD groups in disease duration, medication status, motor symptom severity, cognitive status, and others may partially explain any inconsistent findings, but the complex interactions between these may mask effects arising directly from affected neural circuits from indirect effects caused by complications (see Beylergil et al.39; Archibald et al.43; Tsitsi et al.47; Waldthaler et al.48; Sacrey et al.53; Zhang et al.58).
Regarding the definition of a naturalistic eye movement task prescribed to here, a clear distinction must be made between lab-based tasks employing naturalistic stimuli and naturalistic tasks that participants engage with in everyday life. Of the 30 included studies in this review, arguably none employed a completely naturalistic paradigm. Future studies could utilize digital technology to collect a large amount of quantitative naturalistic eye movement data remotely in patient’s home environments, as participants engage in everyday tasks (see Sacrey et al.54; Adams et al69).
We hypothesized that volitional eye movements might be more affected than automatic eye movements. In line with this hypothesis, Tseng et al.55 found that eye movements indicative of top-down attentional processes were more affected than saliency-based eye movements indicative of bottom-up attentional processes. These results suggest that eye movements relying on the oculomotor loop alone are relatively unaffected compared to eye movements relying on prefrontal loop involvement.19 However, this was the only study out of all reviewed to explicitly separate volitional from automatic eye movements. It is apparent from the summarized literature that dissociating volitional from automatic eye movements is particularly difficult during naturalistic eye movement tasks compared to standard laboratory eye movement tasks. Future research should aim to distinguish volitional and automatic eye movements during naturalistic eye movement tasks to inform the role of cognition in these tasks. For example, Tokushige et al.70 successfully utilized naturalistic eye movement tasks and DBS to distinguish volitional and automatic eye movements. In line with our hypothesis and findings by Antoniades et al.19 volitional internally guided saccades involving top-down attentional control were more affected by DBS stimulation than automatic externally guided saccades reliant on bottom-up attentional processes.
Another important dissociation that needs to be addressed by in future research is whether eye movement abnormalities are driven by cognitive deficits, motor deficits, or a combination of both. As it stands, many studies included in this review did not ascertain baseline cognitive and motor function nor did they correlate eye movement parameters with cognitive and motor function. Therefore, we cannot ascertain whether cognitive or motor deficits are driving the current findings. Additional measures may therefore be needed to complement eye-tracking data. For example, functional magnetic resonance imaging could be used to explore prefrontal cortical activity during specific eye movement behaviors. However, many naturalistic tasks cannot be performed inside a magnetic resonance imaging scanner. Whilst functional near-infrared spectroscopy measures the hemodynamic response in a smaller area, patients are less restricted and would therefore be able to undertake more naturalistic tasks. Furthermore, electroencephalography signatures associated with specific cognitive processes would allow for better temporal resolution than either functional magnetic resonance spectroscopy or functional near-infrared spectroscopy. Electroencephalography and functional near-infrared spectroscopy offer a unique opportunity to utilize portable neuroimaging devices in conjunction with portable eye-tracking devices. Finally, DBS allows us to directly manipulate neural circuits to generate and inform hypotheses regarding the involvement of specific brain areas in eye movement behaviors (see Antoniades et al.19; Tokushige et al.70). DBS was utilized in only one of the included studies.57
Methodological heterogeneity among the included studies is a significant limiting factor in the interpretation of the presented results precluding attempts at direct comparison. In general, standardized stimuli were used most consistently in studies of facial emotion recognition with two of the three studies using Ekman facial stimuli finding abnormal scanning patterns in PD compared to HC.64,68 From the current literature, the diagnostic and prognostic potential of naturalistic eye movement evaluation in PD cannot be ascertained and must be investigated further. To do this, we advocate for the introduction of standardized stimuli (e.g., standardized image banks for visual search tasks, standardized texts for reading tasks, stimulus position) and protocols (e.g., standardization of outcome measures, timing of stimulus presentation, the number and arrangement of trials in blocks) for different domains of naturalistic eye movement tasks, see, e.g., Antoniades et al.71 Standardization will allow for more valid comparisons of results to identify and determine the reliability of potential diagnostic and prognostic naturalistic oculomotor biomarkers for PD.
Across the included reading studies, texts of a range of languages were utilized, including German, Swedish, Spanish, English, and Japanese. A deficit in vertical eye movements during vertical reading was uniquely observed by Watanabe et al.52 in vertically orientated Japanese texts. Indeed, Watanabe et al.52 found that machine learning algorithms were able to distinguish PD and HC with 82.4% accuracy during vertical reading. Hence, some eye movement deficits may only be detectable under specific conditions that are not universally common. As previous literature has demonstrated cross-cultural variations in eye movements,72–75 it is important to explore eye movements across the global community to aid accurate diagnoses of specific subpopulations.75 Furthermore, important differences in eye movement behaviors, such as longer fixations, occur when one reads a text in their non-native language.76
Aside from variability between stimuli, it has previously been reported that equipment produces significant effects on eye-tracking measurements despite calibration procedures.9 The studies reported in the present systematic review used a range of eye trackers, including fixed and head-mounted devices. The most popular device was the EyeLink 1000 video oculometer used in 14 studies. With many products already available or under active development, it will be important to establish the effects of equipment on results obtained in naturalistic tasks. Studies should be designed to evaluate this in patients where equipment calibration may have different requirements than for use with healthy controls. Additionally, future studies should keep source data (e.g., video recordings) where possible, so that bias resulting from different movement detection and classification algorithms can be investigated. Furthermore, while eye-tracking is widely available and would be relatively easy to implement within clinical practice, future work must ascertain which equipment is more suitable for accurate detection to make reliable suggestions for clinical practice.
The effects of medication on naturalistic eye movement behaviors in PD is a particularly important question that has been largely neglected in the current literature. Of the reviewed studies, only three included off-medication patients as a separate group.45,48,54 Two of these studies found that antiparkinsonian medication reduced the magnitude of observed differences between PD and HC.45,54 Habibi et al.56 pooled results from on- and off-state patients, complicating interpretation of results. Dopamine replacement does not restore normal electrophysiological activity within the basal ganglia.22,77 Furthermore, previous studies have found differential effects of levodopa on non-naturalistic eye movements, in particular a deterioration in the execution of visually guided saccades.78,79 Further study in off-medication and medication-naïve patients will allow the utility of naturalistic eye movements in the initial diagnosis of PD to be explored and counter the potential masking effect of medication. Studies comparing different classes of antiparkinsonian medications in more naturalistic contexts may also help to optimize future management for individual patients based on their functional goals.
Whilst the present review excluded papers in which only patients who had PD with comorbid mild cognitive impairment or PD dementia were studied, cognitive impairment is common in PD with a prevalence of around 40%.80,81 A thorough discussion of the effects of cognitive status on eye movement behaviors in PD is beyond the scope of this work but should be addressed in a future review. Several of the included studies explored the effects of cognitive impairment in PD on eye movements.43,45,47,52,63,67 In most other included studies, mild cognitive impairment was not an exclusion criterion and so results were pooled with those of cognitively healthy patients. A number of these studies reported positive findings, meaning that this should be a priority for research in this field.
Finally, in certain cases parkinsonian disorders may be hard to distinguish leading to misdiagnosis.82 This can be a challenge for clinical management given their different underlying mechanisms, prognosis and potential therapeutic targets. Several attempts have been made to ascertain reliable differential biomarkers that can accurately distinguish these conditions during the early stages of the disease course (e.g., Saeed et al.83). Several studies included in this systematic review explored eye movement abnormalities in both patients with progressive supranuclear palsy and multiple system atrophy during naturalistic reading51,52 and video-watching tasks.56 These studies revealed important differences in the naturalistic eye movements between PD, progressive supranuclear palsy, and multiple system atrophy that may have differential diagnostic potential. For example, vertical saccades,52 as well as macro- and micro-saccades,56 were particularly affected in progressive supranuclear palsy compared to both multiple system atrophy and PD.56 However, machine learning algorithms showed weakness in distinguishing PD from progressive supranuclear palsy using eye movements during vertical reading.52
In summary, the reviewed literature has revealed some differences in naturalistic eye movements between PD and healthy aging, including longer fixation durations, reduced saccadic amplitudes, and different scanning patterns. We encourage future work to build upon the studies summarized here by considering the effects of disease duration, cognitive status, motor symptom severity, and medication status on naturalistic eye movements. Whilst noticeable patterns were observed, the wide range of methodologies covered in this review makes comparison between and replication of studies difficult. Longer fixation durations and abnormal scanning patterns are unique observations of naturalistic eye movement tasks due to the unique potential of these tasks to provoke these eye movements. Used in conjunction with traditional paradigms, naturalistic eye movement abnormalities may provide additional complimentary discriminative power that may better illuminate potential underlying cognitive mechanisms. However, we are currently unable to determine whether naturalistic eye movement tasks allow for better or worse discrimination between PD and HC compared to traditional laboratory paradigms, which are generally more standardized. Furthermore, due to a lack of standardized outcomes across naturalistic eye movement tasks, it is difficult to determine whether naturalistic paradigms reveal more oculomotor abnormalities in comparison to traditional paradigms, despite abnormalities being observed across all categories of naturalistic eye movement tasks summarized. We therefore encourage future work to aim for standardization of naturalistic eye movement protocols (e.g., by using or producing standardized stimuli and procedures i.e., Antoniades et al.71). If standardization is achieved, we believe that the rich and ecologically valid data acquired during these tasks, in particular via a remote and personalized set up, would allow for improved discrimination between PD and HC compared to traditional laboratory tasks. Improved ecological validity may arise from these familiar eye movements being more comfortable and less likely to induce fatigue, therefore improving our interpretation of both motor and cognitive mechanisms in PD and the potential future diagnostic utility of these stereotyped eye movements.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
Both MRR and MP receive support from the NIHR Applied Research Collaboration ARC North West Coast and Alzheimer’s Society and are funded through a Post-Doctoral Fellowship. MG and JH have been funded by research grant support provided by UCB and the National Institute for Health Research Oxford Biomedical Research Centre. CAA was supported by the National Institute for Health Research Oxford Biomedical Research Centre. CAA has received research grant support from UCB Pharma and MSD Laboratories. The views expressed are those of the authors and not necessarily those of the funders, NHS or Department of Health and Social Care.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-240092.
REFERENCES
1. | Ou Z , Pan J , Tang S , et al. Global trends in the incidence, prevalence, and years lived with disability of Parkinson’s disease in 204 countries/territories from 1990 to 2019. Front Public Health (2021) ; 9: : 776847. |
2. | Dorsey ER , Elbaz A , Nichols E , et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol (2018) ; 17: : 939–953. |
3. | Morris HR , Spillantini MG , Sue CM , et al. The pathogenesis of Parkinson’s disease. Lancet (2024) ; 403: : 293–304. |
4. | Wakabayashi K , Tanji K , Odagiri S , et al. The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol Neurobiol (2013) ; 47: : 495–508. |
5. | Kouli A , Torsney KM , Kuan W-L . Parkinson’s disease: etiology, neuropathology, and pathogenesis. Exon Publ (2018) ; 3–26. |
6. | Opwonya J , Doan DNT , Kim SG , et al. Saccadic eye movement in mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Neuropsychol Rev (2022) ; 32: : 193–227. |
7. | Sun YR , Beylergil SB , Gupta P , et al. Monitoring eye movement in patients with Parkinson’s disease: what can it tell us? Eye Brain (2023) ; 15: : 101–112. |
8. | Srivastava A , Sharma R , Sood SK , et al. Saccadic eye movements in Parkinson’s disease. Indian J Ophthalmol (2014) ; 62: : 538–544. |
9. | Chambers JM , Prescott TJ . Response times for visually guided saccades in persons with Parkinson’s disease: A meta-analytic review. Neuropsychologia (2010) ; 48: : 887–899. |
10. | Waldthaler J , Stock L , Student J , et al. Antisaccades in Parkinson’s disease: a meta-analysis. Neuropsychol Rev (2021) ; 31: : 628–642. |
11. | Antoniades CA , Spering M . Eye movements in Parkinson’s disease: from neurophysiological mechanisms to diagnostic tools. Trends Neurosci (2024) ; 47: : 71–83. |
12. | Antoniades CA , Demeyere N , Kennard C , et al. Antisaccades and executive dysfunction in early drug-naive Parkinson’s disease: The discovery study. Mov Disord (2015) ; 30: : 843–847. |
13. | Hallett PE . Primary and secondary saccades to goals defined by instructions. Vision Res (1978) ; 18: : 1279–1296. |
14. | Munoz DP , Everling S . Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci (2004) ; 5: : 218–228. |
15. | Zhou H , Wang X , Ma D , et al. The differential diagnostic value of a battery of oculomotor evaluation in Parkinson’s disease and multiple system atrophy. Brain Behav (2021) ; 11: : e02184. |
16. | Crawford TJ , Henderson L , Kennard C . Abnormalities of nonvisually guided eye movements in Parkinson’s Disease. Brain (1989) ; 112: : 1573–1586. |
17. | Beylergil SB , Murray J , Noecker AM , et al. Effects of subthalamic deep brain stimulation on fixational eye movements in Parkinson’s disease. J Comput Neurosci (2021) ; 49: : 345–356. |
18. | Alexander GE , DeLong MR , Strick PL . Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci (1986) ; 9: : 357–381. |
19. | Antoniades CA , Rebelo P , Kennard C , et al. Pallidal deep brain stimulation improves higher control of the oculomotor system in Parkinson’s disease. J Neurosci (2015) ; 35: : 13043–13052. |
20. | Guthrie M , Leblois A , Garenne A , et al. Interaction between cognitive and motor cortico-basal ganglia loops during decision making: a computational study. J Neurophysiol (2013) ; 109: : 3025–3040. |
21. | Adam EM , Brown EN , Kopell N , et al. Deep brain stimulation in the subthalamic nucleus for Parkinson’s disease can restore dynamics of striatal networks. Proc Natl Acad Sci (2022) ; 119: : e2120808119. |
22. | Singh A , Liang L , Kaneoke Y , et al. Dopamine regulates distinctively the activity patterns of striatal output neurons in advanced parkinsonian primates. J Neurophysiol (2015) ; 113: : 1533–1544. |
23. | Readman MR , Polden M , Gibbs MC , et al. The potential of naturalistic eye movement tasks in the diagnosis of Alzheimer’s disease: a review. Brain Sci (2021) ; 11: : 1503. |
24. | Seligman SC and Giovannetti T. The potential utility of eye movements in the detection and characterization of everyday functional difficulties in mild cognitive impairment. Neuropsychol Rev (2015) ; 25: : 199–215. |
25. | Pretegiani E and Optican LM. Eye movements in Parkinson’s disease and inherited parkinsonian syndromes. Front Neurol (2017) ; 8: : 592. |
26. | Topor M , Pickering J , Mendes AB , et al. Non-Interventional, Reproducible, and Open Systematic Review (NIRO-SR) guidelines v1. Open Sci Framew (2020) ; 31: : 222. |
27. | Kohl C , McIntosh EJ , Unger S , et al. Online tools supporting the conduct and reporting of systematic reviews and systematic maps: a case study on CADIMA and review of existing tools. Environ Evid (2018) ; 7: : 8. |
28. | Kohl C , McIntosh EJ , Unger S , et al. Correction to: Online tools supporting the conduct and reporting of systematic reviews and systematic maps: a case study on CADIMA and review of existing tools. Environ Evid (2018) ; 7: : 12. |
29. | Jung I , Kim J-S . Abnormal eye movements in parkinsonism and movement disorders. J Mov Disord (2019) ; 12: : 1–13. |
30. | Brooks SH , Klier EM , Red SD , et al. Slowed prosaccades and increased antisaccade errors as a potential behavioral biomarker of multiple system atrophy. Front Neurol (2017) ; 8: : 261. |
31. | Rivaud-Péchoux S , Vidailhet M , Gallouedec G , et al. Longitudinal ocular motor study in corticobasal degeneration and progressive supranuclear palsy. Neurology (2000) ; 54: : 1029–1032. |
32. | Cong S , Xiang C , Zhang S , et al. Prevalence and clinical aspects of depression in Parkinson’s disease: A systematic review and meta-analysis of 129 studies. Neurosci Biobehav Rev (2022) ; 141: : 104749. |
33. | Pontone GM , Dissanayaka N , Apostolova L , et al. Report from a multidisciplinary meeting on anxiety as a non-motor manifestation of Parkinson’s disease. NPJ Parkinson Dis (2019) ; 5: : 30. |
34. | Zhang J , Xu C-Y , Liu J . Meta-analysis on the prevalence of REM sleep behavior disorder symptoms in Parkinson’s disease. BMC Neurol (2017) ; 17: : 23. |
35. | Carvalho N , Laurent E , Noiret N , et al. Eye movement in unipolar and bipolar depression: a systematic review of the literature. Front Psychol (2015) ; 6: : 1809. |
36. | Page MJ , McKenzie JE , Bossuyt PM , et al. The PRISMA statement: An updated guideline for reporting systematic reviews. Int J Surg (2021) ; 88: : 105906. |
37. | Downs SH , Black N . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health (1998) ; 52: : 377–384. |
38. | Luo C , Yang Y , Liu Y , et al. Intention to COVID-19 vaccination and associated factors among health care workers: A systematic review and meta-analysis of cross-sectional studies. Am J Infect Control (2021) ; 49: : 1295–1304. |
39. | Beylergil SB , Kilbane C , Shaikh AG , et al. Eye movements in Parkinson’s disease during visual search. J Neurol Sci (2022) ; 440: : 120299. |
40. | Bek J , Gowen E , Vogt S , et al. Action observation and imitation in Parkinson’s disease: The influence of biological and non-biological stimuli. Neuropsychologia (2021) ; 150: : 107690. |
41. | Bek J , Gowen E , Vogt S , et al. Observation and imitation of object-directed hand movements in Parkinson’s disease. Sci Rep (2023) ; 13: : 18749. |
42. | Nagai K , Kaneko Y , Suzuki M , et al. Multimodal visual exploration disturbances in Parkinson’s disease detected with an infrared eye-movement assessment system. Neurosci Res (2020) ; 160: : 50–56. |
43. | Archibald NK , Hutton SB , Clarke MP , et al. Visual exploration in Parkinson’s disease and Parkinson’s disease dementia. Brain (2013) ; 136: : 739–750. |
44. | Aveni K , Ahmed J , Borovsky A , et al. Predictive language comprehension in Parkinson’s disease. PLoS One (2023) ; 18: : e0262504. |
45. | Stock L , Krüger-Zechlin C , Deeb Z , et al. Natural reading in Parkinson’s disease with and without mild cognitive impairment. Front Aging Neurosci (2020) ; 12: : 120. |
46. | Park E . The Effect of Lexical Stress on Eye Movements and Prosody Planning During Reading in Individuals with Parkinson’s Disease - ProQuest. PhD Thesis, University of Oklahoma, https://www.proquest.com/openview/ee4cbbc69751db4d0f186101b6aaa890/1?pq-origsite=gscholar&cbl=18750 ((2017) ). |
47. | Tsitsi P , Nilsson M , Seimyr GÖ , et al. Reading alterations in Parkinson’s disease indicate worse cognitive status. Mov Disord Clin Pract (2023) ; 10: : 579–585. |
48. | Waldthaler J , Tsitsi P , Seimyr GÖ , et al. Eye movements during reading in Parkinson’s disease: A pilot study. Mov Disord (2018) ; 33: : 1661–1662. |
49. | Kannan M . Development of an Eye Movement Based Predictive Model for Discrimination of Parkinson’s Disease from Other Parkinsonisms and Controls. Master’s Thesis, Virginia Commonwealth University, https://scholarscompass.vcu.edu/etd/6070 ((2019) ). |
50. | Yu CY , Lee T , Shariati MA , et al. Abnormal eye movement behavior during reading in Parkinson’s disease. Parkinsonism Relat Disord (2016) ; 32: : 130–132. |
51. | Terao Y , Tokushige S , Inomata-Terada S , et al. How do patients with Parkinson’s disease and cerebellar ataxia read aloud? -Eye–voice coordination in text reading. Front Neurosci (2023) ; 17: : 1202404. |
52. | Watanabe Y , Takeuchi S , Uehara K , et al. Clinical availability of eye movement during reading. Neurosci Res (2023) ; 195: : 52–61. |
53. | Sacrey L-AR , Clark CAM , Whishaw IQ . Music attenuates excessive visual guidance of skilled reaching in advanced but not mild Parkinson’s disease. PLoS One (2009) ; 4: : e6841. |
54. | Sacrey L-AR , Travis SG , Whishaw IQ . Drug treatment and familiar music aids an attention shift from vision to somatosensation in Parkinson’s disease on the reach-to-eat task. Behav Brain Res (2011) ; 217: : 391–398. |
55. | Tseng P-H , Cameron IGM , Pari G , et al. High-throughput classification of clinical populations from natural viewing eye movements. J Neurol (2013) ; 260: : 275–284. |
56. | Habibi M , Oertel WH , White BJ , et al. Eye tracking identifies biomarkers in α-synucleinopathies versus progressive supranuclear palsy. J Neurol (2022) ; 269: : 4920–4938. |
57. | Fischer P , Ossandón JP , Keyser J , et al. STN-DBS reduces saccadic hypometria but not visuospatial bias in Parkinson’s disease patients. Front Behav Neurosci (2016) ; 10: : 85. |
58. | Zhang Y , Yan A , Liu B , et al. Oculomotor performances are associated with motor and non-motor symptoms in Parkinson’s disease. Front Neurol (2018) ; 9: : 960. |
59. | Matsumoto H , Terao Y , Furubayashi T , et al. Small saccades restrict visual scanning area in Parkinson’s disease. Mov Disord (2011) ; 26: : 1619–1626. |
60. | Dietz J , Bradley MM , Okun MS , et al. Emotion and ocular responses in Parkinson’s disease. Neuropsychologia (2011) ; 49: : 3247–3253. |
61. | Revankar GS , Hattori N , Kajiyama Y , et al. Ocular fixations and presaccadic potentials to explain pareidolias in Parkinson’s disease. Brain Commun (2020) ; 2: : fcaa073. |
62. | Buhmann C , Kraft S , Hinkelmann K , et al. Visual attention and saccadic oculomotor control in Parkinson’s disease. Eur Neurol (2015) ; 73: : 283–293. |
63. | Crutcher MD , Calhoun-Haney R , Manzanares CM , et al. Eye tracking during a visual paired comparison task as a predictor of early dementia. Am J Alzheimers Dis Demen (2009) ; 24: : 258–266. |
64. | Clark US , Neargarder S , Cronin-Golomb A . Visual exploration of emotional facial expressions in Parkinson’s disease. Neuropsychologia (2010) ; 48: : 1901–1913. |
65. | Polet K , Hesse S , Morisot A , et al. Eye-gaze strategies during facial emotion recognition in neurodegenerative diseases and links with neuropsychiatric disorders. Cogn Behav Neurol (2022) ; 35: : 14. |
66. | Bek J , Poliakoff E , Lander K . Measuring emotion recognition by people with Parkinson’s disease using eye-tracking with dynamic facial expressions. J Neurosci Methods (2020) ; 331: : 108524. |
67. | Waldthaler J , Krüger-Zechlin C , Stock L , et al. New insights into facial emotion recognition in Parkinson’s disease with and without mild cognitive impairment from visual scanning patterns. Clin Park Relat Disord (2019) ; 1: : 102–108. |
68. | Ciccarelli N , Anzuino I , Pepe F , et al. The facial emotion recognition deficit in Parkinson’s disease: Implications of a visual scanning strategy. Neuropsychology (2022) ; 36: : 279–287. |
69. | Adams JL , Kangarloo T , Tracey B , et al. Using a smartwatch and smartphone to assess early Parkinson’s disease in the WATCH-PD study. NPJ Parkinsons Dis (2023) ; 9: : 1–10. |
70. | Tokushige S , Matsuda S , Oyama G , et al. Effect of subthalamic nucleus deep brain stimulation on visual scanning. Clin Neurophysiol (2018) ; 129: : 2421–2432. |
71. | Antoniades C , Ettinger U , Gaymard B , et al. An internationally standardised antisaccade protocol. Vision Res (2013) ; 84: : 1–5. |
72. | Alotaibi A , Underwood G and Smith AD. Cultural differences in attention: Eye movement evidence from a comparative visual search task. Conscious Cogn (2017) ; 55: : 254–265. |
73. | Knox PC , Amatya N , Jiang X , et al. Performance deficits in a voluntary saccade task in Chinese “Express Saccade Makers”. PLoS One (2012) ; 7: : e47688. |
74. | Polden M and Crawford TJ. Active visual inhibition is preserved in the presence of a distracter: A cross-cultural, ageing and dementia study. Cortex (2021) ; 142: : 169–185. |
75. | Xia X , Liu Y , Yu L , et al. Are there preferred viewing locations in Chinese reading? Evidence from eye-tracking and computer simulations. J Exp Psychol Learn Mem Cogn (2023) ; 49: : 607–625. |
76. | Conklin K , Alotaibi S , Pellicer-Sánchez A , et al. What eye-tracking tells us about reading-only and reading-while-listening in a first and second language. Second Lang Res (2020) ; 36: : 257–276. |
77. | Liang L , DeLong MR and Papa SM. Inversion of dopamine responses in striatal medium spiny neurons and involuntary movements. J Neurosci (2008) ; 28: : 7537–7547. |
78. | Lu Z , Buchanan T , Kennard C , et al. The effect of levodopa on saccades – Oxford Quantification in Parkinsonism study. Parkinsonism Relat Disord (2019) ; 68: : 49–56. |
79. | Munoz MJ , Reilly JL , Pal GD , et al. Medication adversely impacts visually-guided eye movements in Parkinson’s disease. Clin Neurophysiol (2022) ; 143: : 145–153. |
80. | Baiano C , Barone P , Trojano L , et al. Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: a meta-analysis. Mov Disord (2020) ; 35: : 45–54. |
81. | Duncan GW , Khoo TK , Yarnall AJ , et al. Health-related quality of life in early Parkinson’s disease: The impact of nonmotor symptoms. Mov Disord (2014) ; 29: : 195–202. |
82. | Talitckii A , Kovalenko E , Anikina A , et al. Avoiding misdiagnosis of Parkinson’s disease with the use of wearable sensors and artificial intelligence. IEEE Sens J (2021) ; 21: : 3738–3747. |
83. | Saeed U , Lang AE and Masellis M. Neuroimaging advances in Parkinson’s disease and atypical parkinsonian syndromes. Front Neurol (2020) ; 11: : 572976. |




