Digital Intervention Promoting Physical Activity in People Newly Diagnosed with Parkinson’s Disease: Feasibility and Acceptability of the Knowledge, Exercise-Efficacy and Participation (KEEP) Intervention
Abstract
Background:
Exercise promotion interventions for people with Parkinson’s disease (PD) are often offered on a face-to-face basis, follow a generic “one-size-fit-all” approach, and are not typically delivered at diagnosis. Considering PD’s heterogenous nature, the existing evidence on the merits of exercise on symptom management and the expressed wishes of people living with PD for access to timely and tailored evidence-based information, there is a demand for interventions that are easily accessible, scalable and co-designed with people living with PD.
Objective:
Evaluate the feasibility and acceptability of a co-designed digital intervention promoting exercise and physical activity, in people newly diagnosed with PD.
Methods:
Thirty people living with PD for less than one year participated in an assessor-blinded randomized feasibility trial from June 2022 to April 2023. The intervention group received the 8-week Knowledge, Exercise Efficacy and Participation (KEEP) intervention comprising 6 interactive digital modules and 4 online live group discussions facilitated by a specialist physiotherapist. Assessments were performed at baseline, post intervention and at 6-month follow up.
Results:
Thirty participants were recruited to target with a 64% recruitment rate (30/47). All but one participant completed the 6-month follow-up assessment. There was high retention (97%), module completion (91%), and online discussion attendance (88%). Outcome measure collection was feasible, including accelerometer data with a daily average wear time of 23.9 hours (SD:0.295).
Conclusions:
The KEEP intervention was feasible and acceptable in people newly diagnosed with PD. A larger trial is needed to assess intervention efficacy and correlation between knowledge, self-efficacy, and activity levels.
Plain Language Summary
Exercise and physical activity have been found beneficial in managing both motor and non-motor symptoms in people living with Parkinson’s. But there aren’t many programs available right after diagnosis that focus on exercise and explain why it’s important for managing PD symptoms and how to exercise with PD. Most existing programs use a one-size-fit-all approach and don’t give personalized information. In this study, researchers wanted to see if people who were recently diagnosed with PD would join a study promoting exercise through an online program. This program included educational information and live online group discussions with both people living with Parkinson’s and a specialist physiotherapist. The program was developed together with people living with Parkinson’s and healthcare professionals to ensure that it better suited the needs of people newly diagnosed with PD. Thirty people took part in the study. They were randomly put into two groups: one received the online program, while the other group continued with their usual care. Participants filled out questionnaires and wore a wrist accelerometer for seven days to track their physical activity levels. The researchers found that most participants stayed in the study (97%), completed the program modules (91%), and attended the live discussions (88%) and wore the accelerometers for 23.9 hours a day on average. Overall, the study showed that the program was doable and well-received. Participants said they had a better understanding on the benefits of exercise in PD. However, a larger study is needed to see if the program helps increase activity levels.
INTRODUCTION
While increasingly recognized as a multisystem neurodegenerative condition,1 Parkinson’s disease (PD) is primarily identified by its cardinal motor symptoms of bradykinesia, rigidity, tremor, and postural instability.2 Concurrently, prevalent non-motor symptoms such as cognitive impairment, sleep disorders, anxiety, and depression contribute to a significant impact on both physical and social function, ultimately reducing the overall quality of life as the disease progresses.3
Exercise and physical activity are accepted as fundamental components in the management of PD with direct impact on motor symptoms like gait and balance,4,5 and non-motor symptoms such as constipation, sleep, and depression.6,7 Physical activity and exercise promotion should commence at the time of diagnosis8 in order to address known barriers that might hinder someone’s participation in exercise.9–11 This is crucial considering the mounting evidence which suggest that benefits are greater when exercise and physical activity are introduced early in the condition’s trajectory.12,13
However, there is a scarcity of interventions aimed at promoting the role of physical activity in the management of PD. Existing interventions are delivered to people who have been living with PD for a mean of five years, adopt a standardized approach, and provide the same content and a uniform, linear learning path for all participants without personalization based on individuals abilities or exercise habits.14 This approach lacks the person-centric ethos and design called by individuals with PD and hinders their ability to implement the information effectively to meet their unique needs.15
The time of diagnosis poses significant challenges for people with Parkinson’s (PwP),16 yet access to interventions and resources that promote physical activity and exercise self-management are lacking due to the pressure in the healthcare system17,18—this is more evident around the time of diagnosis.14,17–19 Empowering PwP by providing knowledge and skills to manage their condition actively20 is paramount and addresses one of their top unmet needs for interventions that focus on improving quality of life through lifestyle modifications like diet and exercise.21,22 Additionally, PwP report that being active offers a great sense of empowerment and control,10,11 as they are actively participating in the management of their condition, rather than being passive recipients of care.
The 2020 pandemic led to a rapid expansion of the use of digital therapeutics.23 This is believed to have improved the continuity of healthcare services in clinically vulnerable groups, and to have also lifted some of the persistent barriers to accessing services, including the availability of transport to hospital facilities, travel costs, mobility restrictions as well as the lack of specialist professionals and services in rural areas.24 Adding to these benefits, digital interventions and the use of technology allow for personalization of content, which translates into a tailored, meaningful, and empowering experience for the user.25 Although, since the pandemic, there has been an increasing number of research studies adopting digital therapeutics ranging from home-based exercise training26 to self-care in PD27, digital interventions remain underutilized,28 including educational interventions that promote physical activity in PD.
To bridge these gaps, a digital intervention was co-designed with people living with PD and healthcare professionals (HCPs). This intervention promotes physical activity and adapts the educational content according to participants’ baseline activity levels, needs and comorbidities. Participatory methods, which place the person at the center reflecting their needs and preferences,29,30 were utilized in the design and development phase. This study aims to assess the feasibility and acceptability of the co-designed digital intervention.
MATERIALS AND METHODS
Study design
This was an assessor blinded, randomized controlled feasibility study. It is reported in compliance with the CONSORT reporting checklist for pilot and feasibility trials.31 The study was registered at ClinicalTrials.gov (NCT05253040) and received ethical approval by the Health Research Authority and Care Research Wales, reference 22/EE/0063.
Setting
The study was conducted at Cambridge University Hospitals NHS Foundation Trust and Cambridgeshire and Peterborough NHS Foundation Trust.
Participants
Potential participants were eligible for inclusion if they had received an idiopathic PD diagnosis within the last 12 months; lived in the Cambridgeshire area; had access to a computer, tablet or phone that was connected to the internet; had no acute illness or history of other neurological conditions; and had no clinical diagnosis of dementia. Potential participants were excluded if they had received or participated in a structured NHS or private PD-specific education program with or without exercise classes in the previous 12 months.
Sample size
As this is a feasibility study, a formal sample size calculation to test efficacy was not performed. Instead, the study aimed to recruit a total of 30 individuals. This number was guided by recommendations indicating that feasibility and pilot studies typically have sample sizes ranging from 24 to 50 individuals.32,33
Recruitment and randomization
Potential participants were identified either by PD consultants in the clinic or by the PD specialist nurse team both during routine clinic and clinical caseload review. A study advert was also placed in the neurology and PD-Nurse community clinics and in the Parkinson’s Clinic at the John Van Geest Centre for Brain Repair at the University of Cambridge. The advertisement requested individuals who met the inclusion criteria and were interested in participating to contact the research team directly.
The study Primary Investigator (PI) conducted telephone screenings with potential participants, offering additional study information, and inviting eligible individuals to clinic appointments. At the appointment, participants provided written informed consent to participate in the study and completed the baseline assessment conducted by a PD specialist physiotherapist.
Participants were randomized (by an independent researcher) to either intervention or control group with a computer-generated random number sequence. Participants were informed of the study arm allocation after the baseline assessment was completed. Participants and the PI were unblinded to the study arm allocation at this point, but the assessor remained blinded for the duration of the study. The study measurements were performed between June 2022 to April 2023.
Intervention for promoting physical activity based on the COM-B model
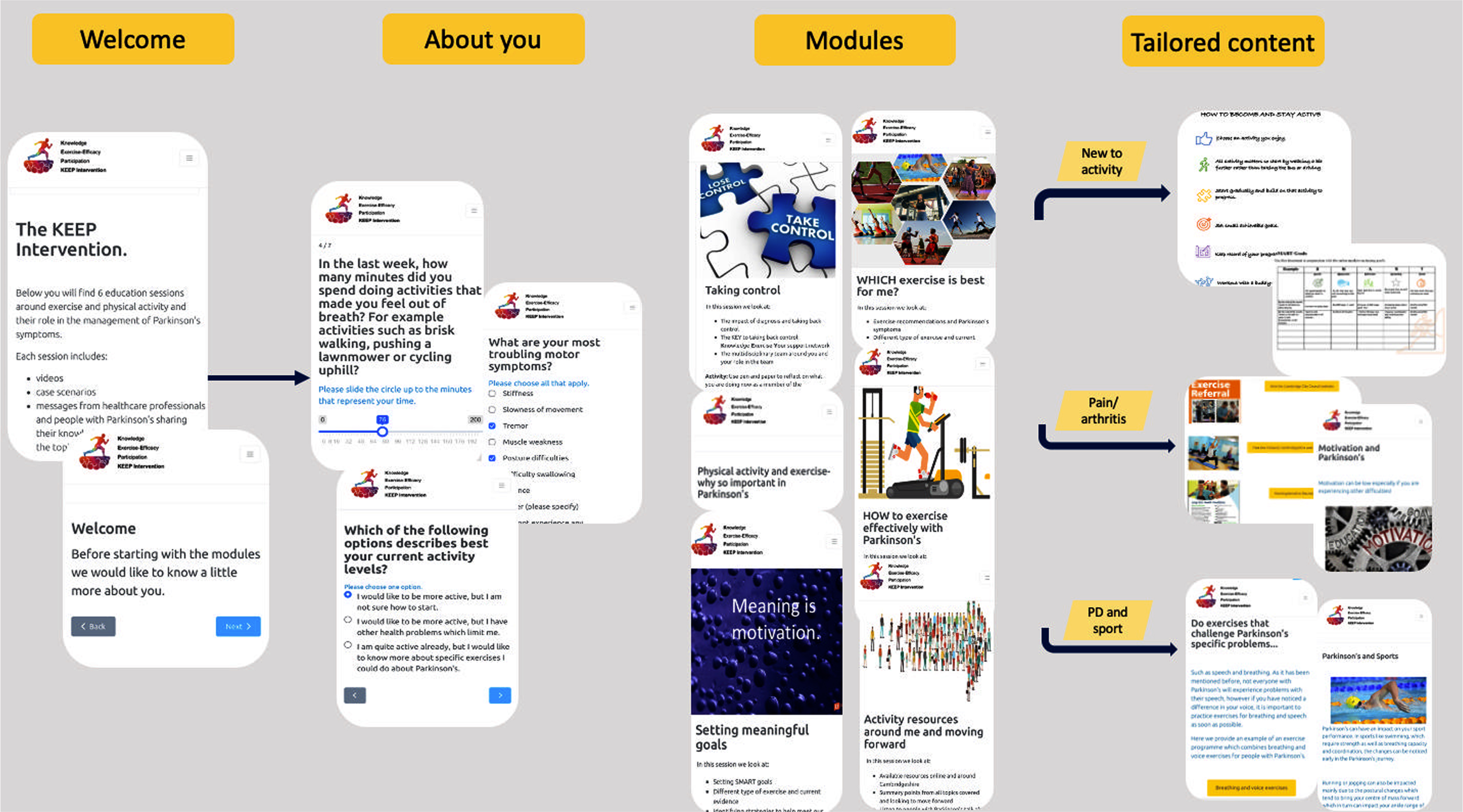
The Knowledge Exercise Efficacy and Participation (KEEP) intervention utilizes an innovative blended learning format and comprises six online modules with content that is tailored to people who are newly diagnosed. Depending on participants’ activity levels and goals, participants access specific exercise and physical activity content. For instance, those who are new to exercise are guided through the significance of starting out, offering information and activity examples to get started. For those already engaged in exercise and sports, the emphasis shifts to the importance of different exercise types and frequencies, with tailored advice and exercises for various sports. Individuals exercising with limitations due to comorbidities are provided with content addressing how to exercise with discomfort, along with modified exercise examples. In addition to the self-directed modules, participants engage in four online group discussions facilitated by a specialist physiotherapist accommodating up to eight newly diagnosed PwP per session.
The KEEP intervention was co-designed with PwP and HCPs, including a neurologist, a geriatrician, a physiotherapist and a speech and language therapist to represent the multidisciplinary skills required for the management of PD.20 The co-design entailed three iterative phases: phase one explored the experiences and needs around physical activity and exercise promotion. This phase entailed the triangulation of findings from a mixed-methods study which included surveys with both PwP34 and HCPs,19 and semi-structure interviews with PwP. The triangulation of data revealed high agreement (71%) between PwP and HCPs on the core topics to be included in the intervention. The second phase involved focus group discussions with six PwP and six HCPs. This phase enabled corroboration of findings from the triangulation, informed the structure, content and delivery method of the intervention, which was co-developed through the Experienced-Based-Co-design (EBCD) and participatory action research methodology,30,35,36 incorporating the experiences, preferences and needs of PwP as well as the views of HCPs around physical activity promotion. The validation phase included participants from the focus group discussions as well as PPI members to review the completed intervention, identify areas for further development and assess content and face validity (Fig. 1). The intervention drew upon the theoretical framework of COM-B model of behavior.37 The model describes a behavior in terms of three categories necessary for the behavior to be performed: capability, opportunity and motivation.37
Fig. 1
Co-design approach to develop KEEP and emergent themes.
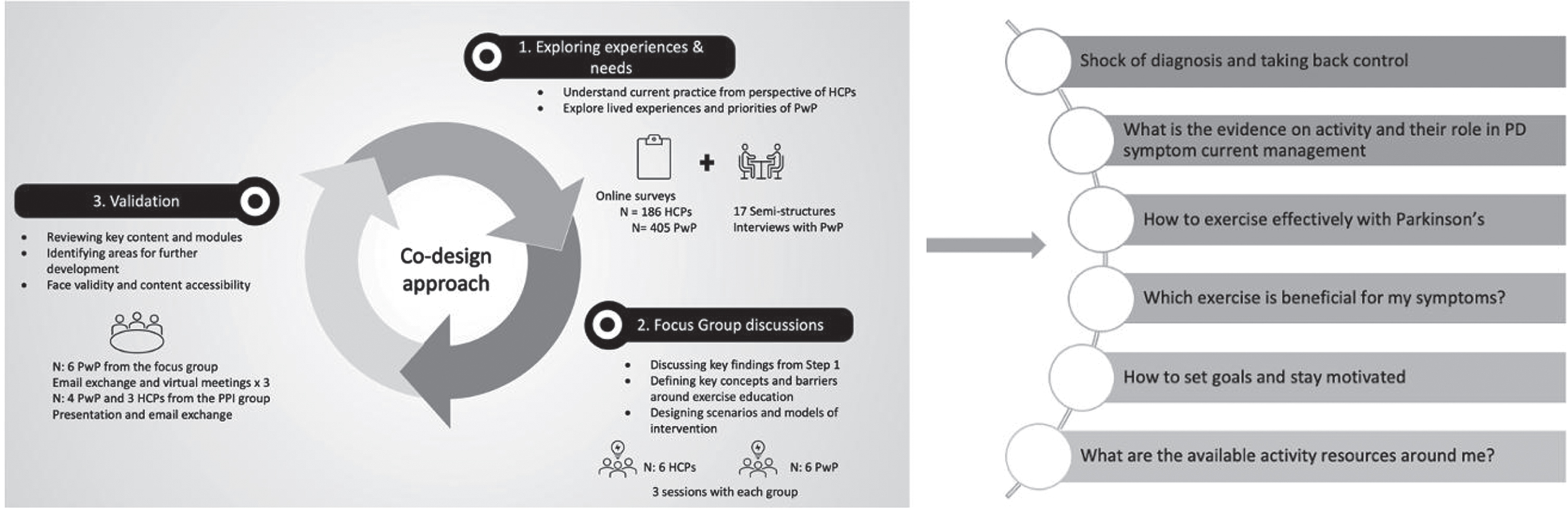
A full description of the intervention in line with the Template for Intervention Description and Replication (TIDieR) guidelines38 is presented in Supplementary Table 1. The online modules were hosted on the Mantal Platform (http://www.mantal.co.uk), a cloud-based software utilized in many research studies. The duration of the KEEP intervention was 8 weeks, however, participants had access to the online modules for up to one year after the end of the intervention (Fig. 2).
Fig. 2
The KEEP intervention participant flow in the online platform. The intervention includes multimedia education about exercise and physical activity and their importance in Parkinson’s disease (PD), delivered by healthcare professionals specializing in PD and people living with Parkinson’s. The content is tailored to the participant and based on their answers during the profile set up. Interactive functions include multiple choice questions, goal setting, and activity diary which are also provided for participants to download.
Control group
Participants randomized to the control group received a Parkinson’s UK booklet which comprised general advice on physical activity. Participants in the control group received the KEEP intervention after completion of the study (online modules only).
Training
A specialist physiotherapist was recruited to facilitate the online group discussions. The facilitator received training regarding the theory underpinning the KEEP intervention and its aims (TIDiER checklist).
Feasibility and acceptability
To determine the feasibility of the study, several key parameters were assessed. These included recruitment, follow-up, and dropout rates as well as feasibility of data collection, including the use of accelerometer monitors to assess activity levels in the PD population. Recruitment rate was determined by calculating the percentage of individuals who consented to participate in the study out of those who expressed interest. Attrition rate was estimated by analyzing the overall dropout rate and differences between the control and intervention groups post-intervention and at the 6-month follow-up. The feasibility of data collection procedures was determined by the percentage of data completeness. The feasibility of using accelerometer monitors was determined by compliance in wearing the device (hours per day). The aim was to assess the feasibility and acceptability of using accelerometers in this population by looking at the overall wear-time rather than specific time periods.
Uptake rate, attendance and completion of online modules were assessed to determine acceptability of intervention. Moreover, a post intervention survey was completed by participants to assess the acceptability of the intervention. The acceptability survey comprised open-ended questions and Likert- type questions.
Performance and participant-reported measures
Demographic data including age, sex, ethnicity, years in education, marital and employment status were collected. Performance-based and participant-reported outcome measures were utilized. As is appropriate in complex interventions,39 multiple health status variables were included to assess feasibility of data collection. Performance-based outcome measures included: 1) the Unified Parkinson’s Disease Rating Scale40 (UPDRS) motor examination part 3, which is the gold-standard scale for PD assessment; 2) the Mini-BESTest,41 which is a 14-item scale that assesses dynamic gait and balance components, and has high inter-rater reliability and high test-retest reliability when testing individuals with PD;42 3) the Five Time Sit To Stand (5TSTS), which is used to assess functional lower extremity strength, balance and risk of falls in older adults43 and has been used in PD populations with high interrater and test-retest reliability.44 The performance-based outcome measures were completed by a PD specialist physiotherapist at baseline and 6 months post intervention.
Participant-reported measures included: the Geriatric Depression Scale45 (GDS) which assesses depression in the older population; the Apathy Evaluation Scale46 (AES) which is a standardized and validated self-rating instrument to evaluate apathy, the Oxford Participation and Activities Questionnaire47 (Ox-PAQ) which assesses participation and activity in patients with long-term conditions; the Self-Efficacy for exercise scale48 (SEE) which assesses a person self-efficacy to exercise under different circumstances; the Multidimensional Outcomes Expectations for Exercise Scale49 (MOEES) measuring outcome expectations about the benefits of regular exercise and PA; the Gait-Specific Attentional Profile scale50 (GSAP) which assesses a series of distinct psychological factors that are known to be associated with conservative gait behaviors, often observed in anxious older adults. These outcome measures are used and validated in the PD population.
To assess specific knowledge about the role of exercise and PA in the management of PD, the Knowledge Exercise and Physical Activity (KEPA) in PD was designed by the research team with input from the Patient and Public Involvement (PPI) members, specifically to assess knowledge around the role of exercise and physical activity in PD management. The PPI members were PwP, who joined academics and clinicians who had experience and expertise in PD as members of the advisory group for the study.
Physical activity was also reported by participants both subjectively by completing the Recent Physical Activity Questionnaire51 (RPAQ) recalling physical activity in the last 4 weeks, and objectively with an accelerometer device. The GENEactiv Original (Activinsights Ltd., Cambridgeshire, UK) is a triaxial wrist-worn device with no external display that is small (36 cm×30 cm×12 cm), lightweight (16 g), and waterproof. The device was set to record at a sampling frequency of 75 Hz. Wrist-worn accelerometers have previously been shown to be a valid measure of physical activity and sedentary time and are commonly used in PwP.52 Participants were asked to wear the accelerometer continuously for seven days, on the non-tremor side or their non-dominant wrist if tremor was present in both arms, to reduce the likelihood of activity overestimation.53 The accelerometer and participant-reported outcomes were assessed at baseline, post-intervention and at the 6-month follow up (Table 1). For the post-intervention assessment, participant reported measures were collected bypost.
Table 1
Outcome measures and corresponding data collection timepoints
| Outcome measure | Baseline (In clinic) | Post-intervention (Remote) | 6-month post (In clinic) |
| MiniBESTest | Y | – | Y |
| UPDRS III | Y | – | Y |
| TUG | Y | – | Y |
| GDS | Y | Y | Y |
| Ox-PAQ | Y | Y | Y |
| SEE | Y | Y | Y |
| AES | Y | Y | Y |
| KEPA PD | Y | Y | Y |
| Accelerometer &RPAQ | Y | Y | Y |
| MOEES | Y | Y | Y |
| G-SAP | Y | Y | Y |
| AoI questionnaire* | – | Y | – |
Mini-BESTest, Balance Evaluation Systems Test; UPDRS III, Unified Parkinson’s Disease Rating Scale, part 3; 5TSTS, 5 times Sit to Stand; GDS, Geriatric Depression Scale; OX-PAQ, The Oxford Participation Questionnaire; SEE, Self-Efficacy for Exercise; AES, Apathy Evaluation Scale; KEPA PD, Knowledge on Exercise and Physical Activity in PD questionnaire; RPAQ, Recent Physical Activity Questionnaire; MEOES, Multifactorial Outcome Expectation for Exercise Scale; GSAP, Gait-Specific Attentional Profile; AoI questionnaire, Acceptability of Intervention questionnaire. *intervention group only.
Data analysis
Descriptive statistics are used to summarize participant demographic characteristics, performance and participant reported outcomes, and feasibility and acceptability outcomes. These are presented as mean with standard deviation (±SD) and median with interquartile range (IQR) for non-normally distributed variables or count with percentage (%) for categorical data. The responses from the open-ended questions on the acceptability of intervention survey, are presented as individual statements. To ensure homogeneity in the data captured via the accelerometers, day 1 and day 7 were removed to account for travel time to the clinic and posting day.
RESULTS
Feasibility
Recruitment and retention
From June to September 2022, 89 potential participants were screened and 47 were deemed eligible for the study. Clinicians reported main reasons for not meeting eligibility criteria were not having idiopathic PD diagnosis (n = 25, 42%), having a diagnosis of dementia (n = 10, 16%) and living with PD for more than one year (n = 6, 1%). Telephone screening was conducted with 47 eligible participants of whom, 17 declined to participate in the study. The main reasons for declining participation in the study were not having access or not being able to use the internet (n = 4), and transport issues (e.g., willingness to travel to the clinic or inability to drive; n = 4). Refer to Fig. 3. Recruitment to target (30 participants) was achieved within the predefined 4-month timeframe, with a 64% recruitment rate. Table 2 presents the demographic and clinical characteristics of participants at baseline. Twenty-nine of 30 participants completed the 6-month follow up assessment giving the study a 97% retention rate.
Fig. 3
Flow diagram of feasibility study for full details.
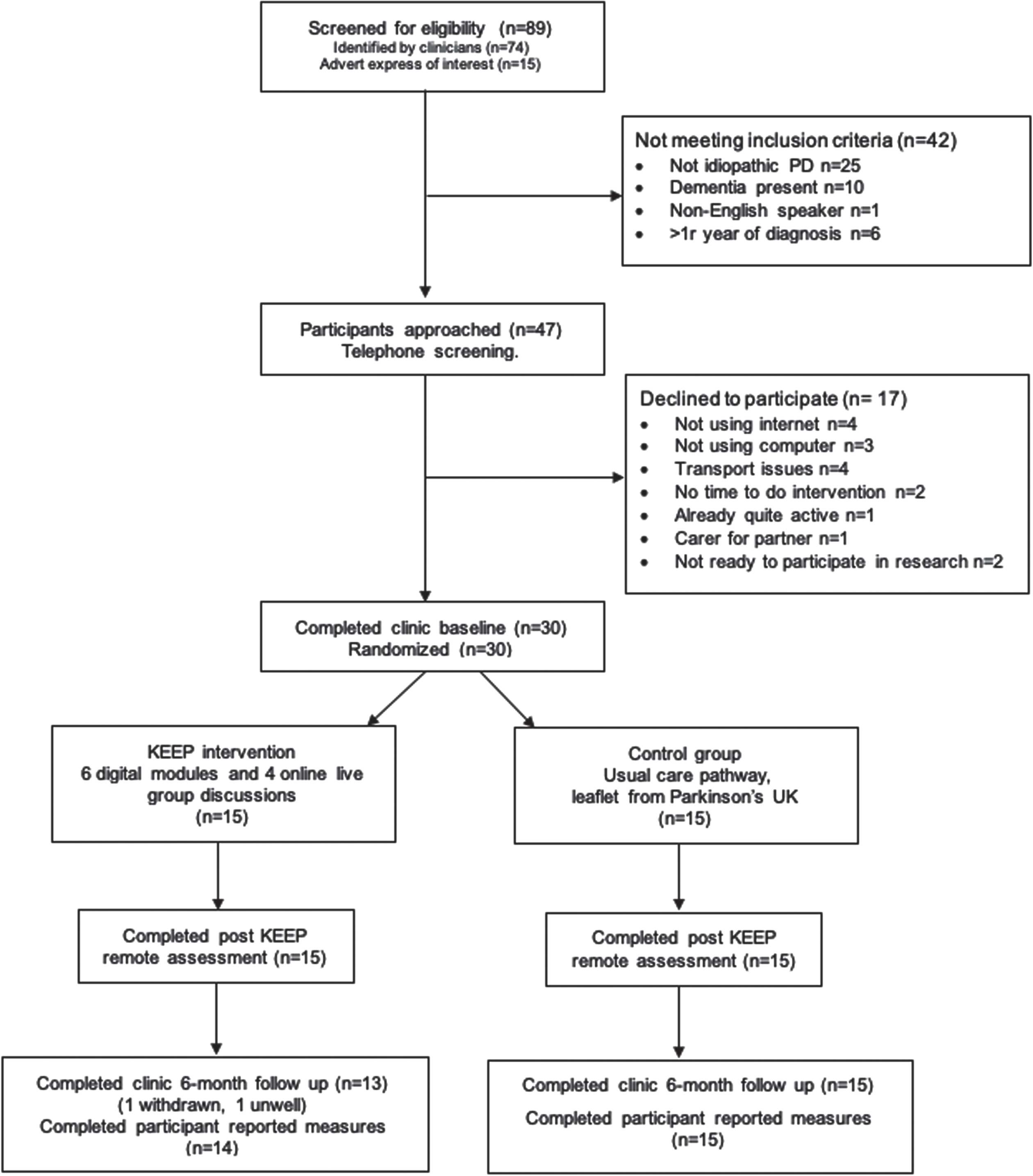
Table 2
Participants’ demographic and clinical characteristics at baseline
| Variables | All (n = 30) | Intervention (n = 15) | Control (n = 15) |
| Age | 67.3 (±10.8) | 70.27 (±5.23) | 64.40 (±13.99) |
| Male | 23 (76.7%) | 11 (73.3%) | 12 (80.0%) |
| White British | 26 (86.7%) | 13 (86.7%) | 13 (86.7%) |
| Years in education | 15.0 (±3.9) | 14.1 (±4) | 15.9 (±3.8) |
| Married/partnership | 25 (73.3%) | 15 (100%) | 10 (60.0%) |
| Employed | 10 (33.3%) | 5 (33.3%) | 5 (33.3%) |
| Retired | 15 (50.0%) | 10 (66.7%) | 5 (4%) |
| Unemployed | 5 (16.7%) | 0 | 5 (33.3%) |
| H&Y 1 | 7 (23%) | 4 (26.6%) | 3 (20%) |
| H&Y 2 | 9 (30%) | 6 (40%) | 3 (20%) |
| H&Y 3 | 13 (43%) | 5 (33.3%) | 8 (53.3%) |
| H&Y 4 | 1(0.03%) | 1 (6.6%) | |
| On PD medication | 28/30 (93%) | 14/15 (93%) | 14/15 (93%) |
| Number of comorbidities | 1.0 (±1.1) | 1.3 (±1.4) | 0.7 (±0.7) |
| Number of falls | 0.7 (±1.6) | 0.5 (±0.6) | 0.9 (±2.1) |
*Numbers are reported as mean (SD) or n (%). H& Y, Hoehn & Yahr Scale; PD, Parkinson’s disease.
Feasibility of data collection
Participants completed the face-to-face clinic assessment with no complication or any adverse events. All 30 participants completed the performance and participant reported outcome measures at baseline. 28 of 30 participants attended the 6-month post intervention assessment in clinic with the PD Specialist Physiotherapist. Table 3 provides a descriptive summary for all the performance and participant reported outcomes.
Table 3
Descriptive summaries for performance and participant reported outcomes at baseline, post intervention and at 6-month follow up including median and interquartile range
| Intervention group (15) | Control group (15) | |||||
| Variables | Baseline | Post intervention | 6-month follow up | Baseline | Post intervention | 6-month follow-up |
| Physical activity enmo | 13.10 (11.80–19.26) | 15.01 (12.15–18.13) | 13.13 (12.2–17.37) | 17.83 (12.02–23.03) | 17.48 (10.39–20.40) | 17.38 (10.53–21.29) |
| Physical activity PAEE | 17.07 (13.90–27.99) | 26.40 (16.26–37.24) | 23.73 (14.27–37.91) | 20.51 (15.68–30.51) | 14.60 (12.28–26.62) | 19.66 (8.67–31.76) |
| Apathy | 34 (26.5–41.5) | 34 (28–42) | 30.5 (25.25–37.75) | 31 (26.5–35.5) | 31 (28.5–35) | 30 (27.5–34.5) |
| Self-efficacy | 56 (49–68) | 40 (37.5–63.5) | 65 (53.75–78.25) | 64 (52.50–74) | 56 (51.5–69.5) | 66 (50–76) |
| Knowledge score KEPA | 5 (3.5–6) | 6 (5–7) | 7 (6–7.75) | 5 (3.5–5.5) | 5 (4–6) | 5 (5–6.5) |
| MOEES Physical outcome expectation | 25 (24–27) | 26 (24–27.50) | 24.5 (23.25–27) | 27 (24–29) | 27 (24–28) | 26 (24–29.5) |
| MOEES Social outcome expectation | 13 (11.50–15) | 11 (11–15) | 13 (11–14) | 13 (11.50–16) | 13 (12–14.5) | 13 (11–14.5) |
| MOEES Self-evaluative outcome | 20 (19–21.5) | 20 (19.50–21) | 20 (19–22) | 20 (18.50–23) | 21 (20–23) | 20 (19.5–23) |
| OXPAQ Routine activities | 21.43 (8.04–32.14) | 23.21 (9.82–30.36) | 21.43 (9.82–29.91) | 14.29 (9.82–33.04) | 8.93 (5.36–28.57) | 10.71 (4.46–25) |
| OXPAQ Emotional well-being | 30 (17.5–42.5) | 50 (25–55) | 48.75 (17.5 –55) | 35 (22.5–45) | 25 (12.5–50) | 25 (12.5–50) |
| OXPAQ Social engagement | 12.50 (6.25–37.5) | 18.75 (6.25–40.62) | 15.62 (6.25–25) | 12.50 (3.12–18.75) | 12.50 (6.25–18.75) | 18.75 (6.25–25) |
| GSAP Somatic anxiety | 4 (3–5) | 4 (2.5–6) | 5 (3.25–6.75) | 4 (3–6) | 4 (3–5.5) | 4 (3.5–6) |
| GSAP Conscious movement processing | 7 (5.5–11) | 9 (6–11.5) | 9 (7–11) | 9 (7.5–12) | 8 (6.5–12.5) | 8 (7–10) |
| GSAP Task irrelevant thoughts | 4 (3–5.5) | 4 (3–6) | 6 (3–7.75) | 6 (4–7.5) | 5 (3–6.5) | 4 (3–5.5) |
| GSAP Processing inefficiencies | 4 (3–5) | 3 (3–5) | 4 (3–5) | 3 (2–5) | 4 (2–4) | 3 (2–4) |
| GDS | 3 (2–7) | 3 (1–5) | 3 (2–5.5) | 2 (1–5) | 2 (0–4) | 2 (1–4) |
| 5TSTS | 13.34 (10.18–14.39) | – | 11.48 (9.46–14.12) | 12.37 (10.45–13.72) | – | 11.42 (9.73–14.88) |
| UPDRS III | 30 (27–36) | – | 36.5 (30.5–39.75) | 38 (33.50–41) | – | 41.5 (33.75–50) |
| MiniBesTest | 23 (20.5–25) | – | 23.5 (21.25–25) | 22 (20.5–25.5) | – | 22 (19.5–24.75) |
Mini-BESTest, Balance Evaluation Systems Test; UPDRS III, Unified Parkinson’s Disease Rating Scale, part 3; TUG, Time up and go; 5TSTS, 5 times Sit to Stand; GDS, Geriatric Depression Scale; OX-PAQ, The Oxford Participation Questionnaire; SEE, Self-Efficacy for Exercise; AES, Apathy Evaluation Scale; KEPA PD, Knowledge on Exercise and Physical Activity in PD questionnaire; PAEE, Physical Activity Energy Expenditure: calculated by self-report data from the Recent Physical Activity Questionnaire; To compute PAEE from the RPAQ, reported time spent on activities was multiplied by the metabolic cost of each activity (in metabolic equivalents, METs) obtained from the physical activity compendium72,73 minus one MET for resting metabolic rate, to provide activity-specific PAEE estimates;51 MEOES, Multifactorial Outcome Expectation for Exercise Scale; GSAP, Gait-Specific Attentional Profile; AoI questionnaire, Acceptability of Intervention questionnaire.
One participant withdrew from the study after the intervention had started due to bereavement and one was unwell to travel and attend the clinic session but consented to wear the accelerometer and complete the participant reported measures. Data completion rate was over 96% for the participant-reported measures at all three timepoints (Supplementary Table 2). All measures were feasible to collect both in clinic and by post. There was no missing data due to postage delivery issues.
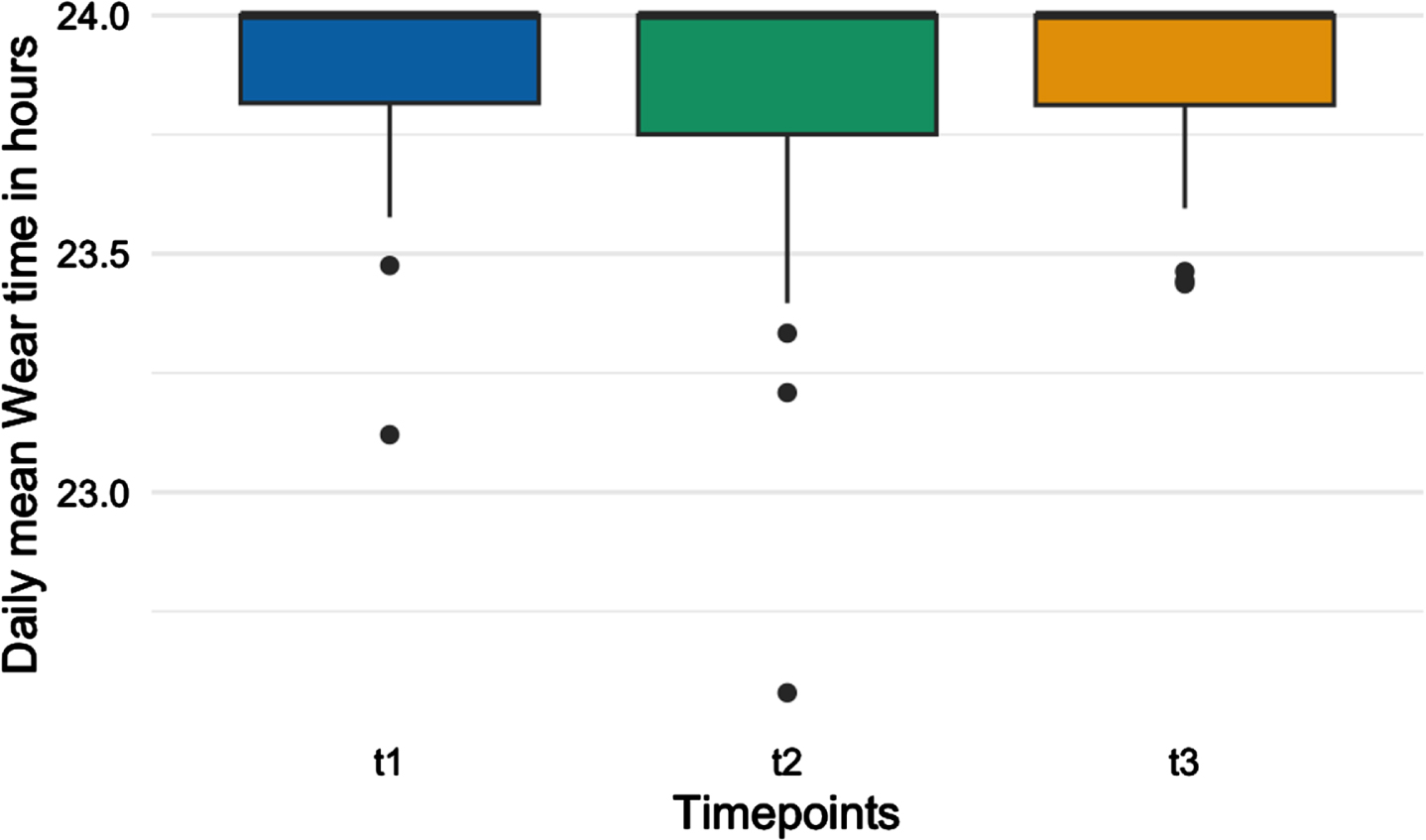
At baseline, 26 wore the accelerometer for 6 out of 6 days and 4 wore it for 5 out of 6, with a daily mean wear time of 23.9 hours (SD:0.234). At post intervention follow-up, daily mean wear time was 23.8 hours (SD: 0.484), and at the 6-month follow up daily mean wear time was 23.9 hours (SD:03.43 (Fig. 4).
Fig. 4
Box plot of average daily wear time of accelerometers during the study period.
Intervention acceptability and adherence
Attendance and completion rate of modules was recorded on the Mantal platform. Thirteen participants completed the intervention as intended (86.6 %), one withdrew after 3 sessions due to family bereavement and one could not attend the sessions due to prebooked family holidays. Table 4 presents the completed modules for each participant. Nine out of 13 (69%) participants completed all six modules (100%), and the rest completed at least 3/6 modules. The completion rate for the self-directed online modules was 91%.
Table 4
Intervention uptake
| ID | First access | Last access | Taking control | Physical Activity and Exercise – WHY so important in Parkinson’s? | WHICH exercise is best for me? | How to exercise effectively Option 3 I am an exerciser | How to exercise effectively Option 2 I have comorbidities | How to exercise effectively Option 1 I am new to exercise | Setting meaningful goals | Resources around me and moving forward | Online Self-dire ted modules Completion rate | Online group sessions attended |
| P1 | 15/07 | 27/08 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6/6 | 4/4 | ||
| P2 | 15/07 | 08/09 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6/6 | 2/4 | ||
| P3 | 21/07 | 08/08 | ✓ | ✓ | ✓ | ■ | ■ | ■ | ■ | ■ | ||
| P4 | 21/07 | 07/09 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6/6 | 4/4 | ||
| P5 | 28/07 | 07/09 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6/6 | 4/4 | ||
| P6 | 28/07 | 08/09 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6/6 | 4/4 | ||
| P7 | 29/07 | 24/08 | ✓ | ✓ | ✓ | ✓ | x | x | 4/6 | 4/4 | ||
| P8 | 04/09 | 31/10 | ✓ | ✓ | ✓ | ✓ | x | ✓ | 5/6 | 3/4 | ||
| P9 | 18/09 | 01/11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6/6 | 4/4 | ||
| P10 | 18/09 | 16/10 | ✓ | ✓ | ✓ | x | x | x | 3/6 | 3/4 | ||
| P11 | 19/09 | 17/10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6/6 | 4/4 | |
| P12 | 20/09 | 18/10 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6/6 | 4/4 | ||
| P13 | 22/09 | 01/11 | ✓ | ✓ | ✓ | ✓ | ✓ | x | 5/6 | 4/4 | ||
| P14 | 26/09 | 01/11 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | 6/6 | 2/4 |
*Data collected from Mantal platform.
The attendance rate for the online group sessions was 88%. Nine participants (69%) attended all four online group discussion sessions, 2 participants (15%) attended two sessions and 3 participants (23%) attended two out of four sessions. Reasons given for not attending the online group sessions were hospital appointments and being unwell.
The acceptability of the intervention was measured with the online survey which was completed by 12 out of 13 participants (92% response rate). 83% of participants strongly agreed or somewhat agreed they found it easy to register and use the online platform. 67% of participants somewhat disagreed or strongly disagreed that the sessions were difficult to understand with 25% of participants somewhat agreeing that the sessions were difficult to understand. 92% participants somewhat to strongly agreed they enjoyed reading the online sessions. 83% of participants somewhat to strongly agreed that the online sessions explained the reasons why exercise and PA is important in the management of PD and that the sessions were a great source of information to allow them choose activities specific to their symptoms and preference (Fig. 5).
Fig. 5
Likert responses from participant on the acceptability of the KEEP intervention.
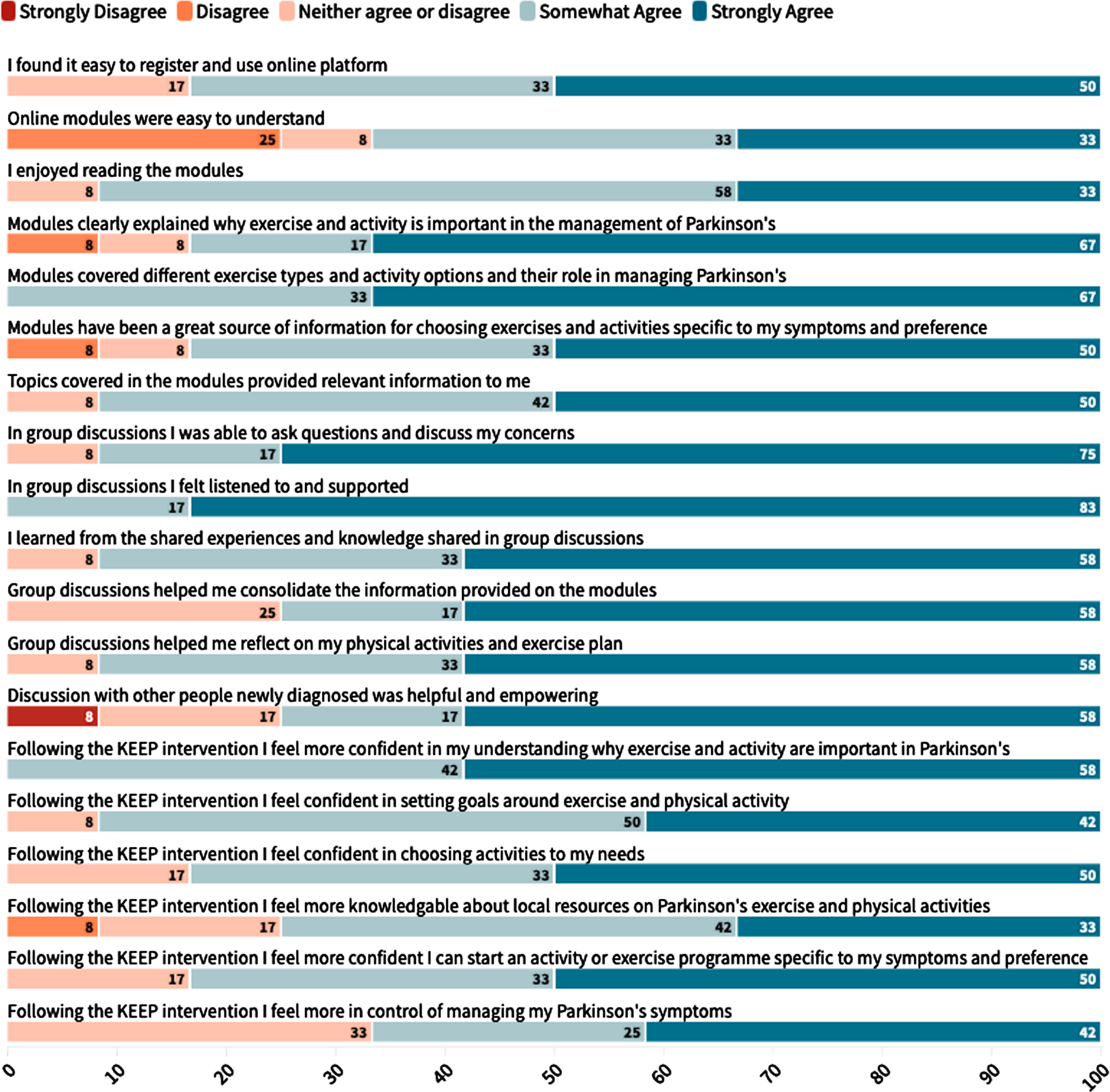
All participants reported having learned from the experiences and knowledge shared during the group discussions. They reported the intervention was helpful as it brought together the evidence but also reinforced the importance of setting goals. Specifically, when asked whether they had experienced any unexpected benefits, one participant mentioned:
“The huge link between PD and exercise and the benefits it can bring. The need to set small but significant goals to keep myself engaged with exercise, because I’m not a person who naturally engages with exercise.”
Another participant found the intervention empowering as it allowed them to reflect on their perceptions of their own capabilities around exercise:
“Made me aware of what I am capable of.”
While one participant discussed the impact that participation in the intervention had on their motivation and overall well-being:
“Made me feel more positive, that I can do something to help my Parkinson’s as well as medication. I have improved my fitness and strength which I probably wouldn’t have done had I not been motivated by being on the trial. I still have some way to go before I’m where I want to be, but I’m working on it.”
Finally, 11 of 12 participants reported to have made a change in their everyday activities as a result of the intervention. Some reported having increased their activity levels by joining a gym or doing more exercise classes:
“Took up free gym membership offered by Everybody Active and classes there”.
Other participants are implementing the knowledge they gained in the intervention by improving posture or adjusting their exercise program:
“Yes, repeating exercises during short periods, several times a day rather than a single long session” (Supplementary Table 3).
Performance and participant recorded outcome measures
Median knowledge score in the intervention group increased in the intervention group from 5 (IQR: 3.5–6) to 6 (IQR: 5–7) post-KEEP and 7 (IQR: 6–7.75) at the 6-month follow up but remained relatively unchanged in the control group. Outcome expectations (MOEES) showed minor changes, with the intervention group maintaining stable physical and social outcome expectations from 25 (IQR: 24–27) to 26 (IQR: 24–27.50), and 24.5 (IQR: 23.25–27) at the 6-month timepoint. The control group showed similar stability in physical and social outcome expectations.
In the intervention group, objective physical activity (ENMO) increased from a baseline median of 13.10 mg (milli gravitational units) (IQR: 11.80–19.26) to 15.01 mg (IQR: 12.15–18.13) post-KEEP, then slightly decreased to 13.13 mg (IQR: 12.20–17.37) at the 6-month follow-up, while the control group showed a general decrease from 17.83 mg (IQR: 12.02–23.03) at baseline to 17.38 mg (IQR: 10.53–21.29) at the 6-month follow up. Self-efficacy in the intervention group decreased from 56 (IQR: 49–68) to 40 (IQR: 37.5–63.5) post-KEEP but improved to 65 (IQR: 53.75–78.25) at the 6-month follow-up, while the control group showed slight fluctuations from 64 (IQR: 52.50–74) to 56 (IQR: 51.5–69.5), and increased to 66 (IQR: 50–76) at 6-months follow up. Depression (GDS), physical function (5TSTS), motor symptoms (UPDRS III), and dynamic balance and gait (MiniBesTest) measures also showed minimal changes across both groups. Summary of the performance and patient reported outcome measures are presented in Table 3.
DISCUSSION
This study demonstrated that the co-designed digital intervention promoting physical activity was feasible and acceptable in people living with PD for less than one year, with high acceptability and retention rates. Additionally, the completeness of data collection was high at all three timepoints indicating feasibility in collecting them both in person and by post. The chosen outcome measures were selected to reflect the potential impact of the intervention on various factors such as knowledge, exercise outcome expectations, and exercise efficacy, while also considering known barriers to physical activity participation such as apathy, depression, and anxiety. Utilizing many outcome measures has allowed a better understanding on the measures to use in future definite trial; however, it is understood that the large number of measures could become burdensome for some participants. Although in this study participants were able to complete them with no input from the research team, in future studies, involving and soliciting PwP and their perspectives on the most appropriate outcome measures to capture changes in physical activity behavior would be valuable. The use of accelerometers to objectively measure activity levels was also found to be feasible with high daily mean wear time at all three timepoints. Participants were asked to wear the accelerometers continuously so that all activity and inactivity is captured and so avoid introducing any recording bias between days or participants, for example, not wearing it for a full day. In large-scale population-based studies it has been shown that participants find wearing the monitor 24 hours acceptable and that it reduces the chance of diurnal bias.54 In a future effectiveness trial, the accelerometers could be used to assess change in time spent in different intensities of activity across each 24-hour period.
Results from the acceptability survey confirm participants were able to register in the digital platform, use it and complete the modules with no accessibility issues being reported. Acceptability of intervention was also high, with 92% reporting they enjoyed reading the modules, and that modules provided information relevant to them. All the participants somewhat to strongly agreed they felt more confident in their understanding of the benefits of physical activity in the management of PD. In the open-ended questions, 92% of participants reported to have made a change in their everyday activities following their participation in the intervention. Participants reported to have incorporated regular exercise sessions, joined exercise classes, purchased exercise equipment, and even taken advantage of free gym memberships available locally for PwP. Additionally, they mentioned to be paying more attention to their posture and the type and duration of exercise they do to ensure they engage in all types of exercises, for example doing more aerobic exercises.
This feasibility study corroborates findings of other studies showing that digital interventions are feasible and acceptable in people with neurodegenerative conditions.55,56 However, during recruitment four participants declined participation due to lack of access to a laptop or internet. While acknowledging the benefits of digital interventions, it is paramount to consider factors such as digital accessibility, health and digital literacy given these factors are known to influence engagement with digital platforms.57 Adopting co-design methods can ensure these areas are discussed inclusively as early as possible in the design and development of interventions.58
Health literacy was a topic of discussion during the co-design process. The intervention modules were specifically designed to present information in a clear, lay language while also providing additional resources, links to research studies, and further material for participants who wished to explore the topics in greater depth. This approach aimed to accommodate varying levels of health literacy and ensure that participants could engage meaningfully with the intervention content. A step-by step guide was produced to aid participants with the creation and logging in to their account to access the online sessions. These insights from the co-design process were very meaningful.
The study findings support previous observational studies, which report that understanding the effects of exercise on PD symptom management and possessing exercise self-efficacy (i.e., confidence in one’s ability to engage in exercise) are pivotal motivators for people with PD to participate in physical activity.9–11,59 The need for non-pharmacological interventions that complement the gold-standard pharmacological approach in the management of PD symptoms is well recognized,60 as is the necessity to work collaboratively with PwP in the design and development of interventions that aligns with their needs.61 This study represents a co-designed initiative which equips PwP with the knowledge and skills necessary to enable physical activity participation and maintenance as early as the time of diagnosis. Currently, there is a lack of standardized approach to promoting physical activity and exercise interventions.62 This lack of standardization poses challenges in ensuring equitable access to physical activity promotion, irrespective of the geographical location or socioeconomic background of PwP. Moreover, physical activity interventions at the time of diagnosis are scarce. A recent survey exploring physical activity promotion from the perspective of healthcare professionals in the UK showed that PwP are referred to AHPs when difficulties with mobility and balance occur,19 pointing to a reactive approach in PD management. In a neurodegenerative condition such as PD, there is a need for a shift from the tertiary prevention approach to a secondary prevention approach, where the focus is on health promotion rather than disability prevention.63,64
Exercise knowledge, engagement, confidence, and competence, collectively form the concept of physical literacy.65 The KEEP intervention aimed to promote exercise and PA participation through improving physical literacy and exercise efficacy in people newly diagnosed with PD. Participants were invited to learn more about the Parkinson’s symptoms and how exercise and PA can support their management. The intervention was adapted to their baseline activity levels, their goals and comorbidities, which were assessed via a questionnaire at the beginning of the intervention. To our knowledge, this is the first study to provide a personalized learning path, enhancing the experience and empowering participants by delivering information that is both useful and directly applicable to their individual needs.66
Association between physical literacy and physical activity participation has been researched in adults and older people as well as in people with diabetes and suggests a positive association between the two.65 This relationship has not however been explored in the PD population14,67 and further methodologically robust research studies need to explore this relationship. This study used a robust randomized design to provide insights on the potential impact of the intervention on previously identified drivers of physical activity behavior among individuals living with PD, and the role of specific and tailored education in facilitating behavior change. Previous investigations of this topic have primarily relied on observational10,11 and qualitative studies.68
The acceptability survey highlighted the valuable role of group discussions in fostering mutual learning and support among participants. While group interventions are recognized for their benefits in alleviating isolation and facilitating learning through the exchange and sharing of experiences,69 the challenge of lower participation rates due to fixed-time sessions was evident. This underscores the need for intervention designs that prioritize flexibility (i.e., offering participants a range of session times to choose from) allowing participants to access and integrate interventions into their daily activities more flexibly. Furthermore, this study demonstrated the feasibility of data collection both in person and by post. This is a positive indication that remote data collection methods are viable alternatives to in-person visit, which may reduce the burden on participants, particularly for those who may face challenges with transportation or have mobility limitations.
Strengths and Limitations
This is the first study to assess feasibility and acceptability of a co-designed intervention that promotes physical activity in newly diagnosed PwP and provides access to AHPs within the first year of diagnosis. The study might have introduced some barriers in participation given it was available only to participants with access to the internet and mobile device or computer, yet it had high recruitment and retention rates.
The study had a strong recruitment strategy and considered factors such as socioeconomic status, educational background, and geographic location of participants, as these variables can significantly influence recruitment into studies as well as health-related behaviors and outcomes.70,71 One recruitment cite was in the center of Cambridge while the PD nurse community clinics were based throughout Cambridgeshire reaching a more diverse population to recruit from. Despite this, recruitment from ethnic minority groups was low and thus, caution should be taken when generalizing the findings beyond the specific context of the sites the study was run at. Physical activity was assessed both subjectively and objectively. Assessing physical activity via subjective questionnaires are subject to self-report-bias and social desirability bias–they were, however, used in combination with objective measures and were utilized to give an indication of what activities had changed, rather than to quantify absolute change.
Conclusion
The study findings suggests that a co-designed digital intervention promoting physical activity is feasible and acceptable in people who are newly diagnosed with PD. Measuring physical activity levels objectively with accelerometer monitors is also feasible and holds significant potential in assessing the effectiveness of interventions, mitigating the self-report bias commonly encountered in subjective measures of physical activity. This type of intervention appears ideal for promoting the role of non-pharmacological interventions early in PD management, fostering the establishment of physical activity habits from the outset. The results of this study also add to the existing literature on the positive role of knowledge and exercise efficacy in being active and support the further assessment of these motivators in larger studies to understand their association and active role in promoting physical activity participation for PwP.
ACKNOWLEDGMENTS
The authors would like to thank all the participants who participated in this study. Moreover, we are thankful to the John Van Geest Centre for Brain Repair at the University of Cambridge for their assistance with recruitment. We would also like to thank the British Geriatric Society Movement Disorder Special Interest Group as well as Addenbrooke’s Charitable Trust (ACT) for the research grants awarded to support this work. ACT research grants and patient benefit grants can help where the project is likely to deliver a patient benefit and is over and above core NHS funding requirements.
FUNDING
LA and LL, University of Cambridge, are supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration East of England (NIHR ARC EoE) at Cambridge and Peterborough NHS Foundation Trust. KR is supported by the NIHR Cambridge Biomedical Research Centre (NIHR203312). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
PH is supported by Homerton College and the Health Foundation’s grant to the University of Cambridge for The Healthcare Improvement Studies Institute (THIS Institute). THIS Institute is supported by the Health Foundation, an independent charity committed to bringing about better health and healthcare for people in the UK.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-240071.
REFERENCES
1. | Costa HN , Esteves AR , Empadinhas N , et al. Parkinson’s disease: a multisystem disorder. Neurosci Bull (2023) ; 39: : 113–124. |
2. | Armstrong MJ and Okun MS. Diagnosis and treatment of Parkinson disease: a review. JAMA (2020) ; 323: : 548–560. |
3. | Soh S-E , McGinley JL , Watts JJ , et al. Determinants of health-related quality of life in people with Parkinson’s disease: a path analysis. Qual Life Res (2013) ; 22: : 1543–1553. |
4. | Jin X , Wang L , Liu S , et al. The impact of mind-body exercises on motor function, depressive symptoms, and quality of life in Parkinson’s disease: a systematic review and meta-analysis. Int J Environ Res Public Health (2019) ; 17: : 31. |
5. | Bhalsing KS , Abbas MM and Tan LCS. Role of physical activity in Parkinson’s disease. Ann Indian Acad Neurol (2018) ; 21: : 242–249. |
6. | Amara AW and Memon AA. Effects of exercise on non-motor symptoms in Parkinson’s disease. Clin Ther (2018) ; 40: : 8–15. |
7. | Cruise KE , Bucks RS , Loftus AM , et al. Exercise and Parkinson’s: benefits for cognition and quality of life. Acta Neurol Scand (2011) ; 123: : 13–19. |
8. | National Institute for Health and Care Excellence. Guidelines for Parkinson’s disease in adults. 2017. |
9. | Ellis T , Boudreau JK , Deangelis TR , et al. Barriers to exercise in people with Parkinson disease. Phys Ther (2013) ; 93: : 628–636. |
10. | Afshari M , Yang A and Bega D. Motivators and barriers to exercise in Parkinson’s disease. J Parkinsons Dis (2017) ; 7: : 703–711. |
11. | Schootemeijer S , van der Kolk NM , Ellis T , et al. Barriers and motivators to engage in exercise for persons with Parkinson’s disease. J Parkinsons Dis (2020) ; 10: : 1293–1299. |
12. | Ellis TD , Colón-Semenza C , DeAngelis TR , et al. Evidence for early and regular physical therapy and exercise in Parkinson’s disease. Semin Neurol (2021) ; 41: : 189–205. |
13. | Tsukita K , Sakamaki-Tsukita H and Takahashi R. Long-term effect of regular physical activity and exercise habits in patients with early Parkinson disease. Neurology (2022) ; 98: : e859–e87. |
14. | Alushi L , Alexander J , Jones J , et al. A systematic review on physical health education interventions for people with Parkinson’s disease: content, impact, and implementation considerations across the Parkinson’s trajectory. J Parkinsons Dis (2022) ; 12: : 1389–1407. |
15. | Hellqvist C , Bertero C , Hagell P , et al. Effects of self-management education for persons with Parkinson’s disease and their care partners: A qualitative observational study in clinical care. Nurs Health Sci (2020) ; 22: : 741–748. |
16. | Schrag A , Modi S , Hotham S , et al. Patient experiences of receiving a diagnosis of Parkinson’s disease. J Neurol (2018) ; 265: : 1151–1157. |
17. | Shah R , Read J , Davies N , et al. People with Parkinson’s perspectives and experiences of self-management: Qualitative findings from a UK study. PLoS One (2022) ; 17: : e0273428. |
18. | Zaman MS , Ghahari S and McColl MA. Barriers to accessing healthcare services for people with Parkinson’s disease: a scoping review. J Parkinsons Dis (2021) ; 11: : 1537–1553. |
19. | Agley L , Hartley P and Lafortune L. Exercise and physical activity promotion for people newly diagnosed with Parkinson’s disease: a UK survey exploring current practice and the views of healthcare professionals. Physiotherapy (2024) ; 122: : 17–26. |
20. | Bloem BR , Okun MS and Klein C. Parkinson’s disease. Lancet (2021) ; 397: : 2284–2303. |
21. | Andrejack J and Mathur S. What people with Parkinson’s disease want. J Parkinsons Dis (2020) ; 10: : S5–S10. |
22. | Vlaanderen FP , Rompen L , Munneke M , et al. The voice of the Parkinson customer. J Parkinsons Dis (2019) ; 9: : 197–201. |
23. | Mann DM , Chen J , Chunara R , et al. COVID-19 transforms health care through telemedicine: Evidence from the field. J Am Med Inform Assoc (2020) ; 27: : 1132–1135. |
24. | Kruse CS , Krowski N , Rodriguez B , et al. Telehealth and patient satisfaction: a systematic review and narrative analysis. BMJ Open (2017) ; 7: : e016242. |
25. | Dang A , Arora D and Rane P. Role of digital therapeutics and the changing future of healthcare. J Family Med Prim Care (2020) ; 9: : 2207–2213. |
26. | Grosjean S , Ciocca J-L , Gauthier-Beaupré A , et al. Co-designing a digital companion with people living with Parkinson’s to support self-care in a personalized way: The eCARE-PD Study. Digital Health (2022) ; 8: : 20552076221081695. |
27. | Putzolu M , Manzini V , Gambaro M , et al. Home-based exercise training by using a smartphone app in patients with Parkinson’s disease: a feasibility study. Front Neurol (2023) ; 14: : 1205386. |
28. | Ellis TD and Earhart GM. Digital therapeutics in Parkinson’s disease: practical applications and future potential. J Parkinsons Dis (2021) ; 11: : S95–S101. |
29. | Bowen S , McSeveny K , Lockley E , et al. How was it for you? Experiences of participatory design in the UK health service. CoDesign (2013) ; 9: : 230–246. |
30. | Robert G . Participatory action research: using experience-based co-design to improve the quality of healthcare services. In: Ziebland S, Coulter A, Calabrese JD, et al. (eds) Understanding and Using Health Experiences: Improving patient care. Oxford University Press, (2013) , pp. 138–149. |
31. | Eldridge SM , Chan CL , Campbell MJ , et al. CONSORT statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud (2016) ; 2: : 64. |
32. | Julious SA . Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat (2005) ; 4: : 287–291. |
33. | Sim J and Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol (2012) ; 65: : 301–308. |
34. | Agley L , Hartley P and Lafortune L. Exploring the experiences, priorities and preferences of people living with Parkinson’s on exercise and physical activity promotion in the UK. PLoS One (2024) ; 19: : e0304223. |
35. | Zesiewicz TA and Hauser RA. Depression in patients with Parkinson’s disease. CNS Drugs (2000) ; 13: : 253–264. |
36. | Bate P and Robert G. Experience-based design: from redesigning the system around the patient to co-designing services with the patient. Qual Saf Health Care (2006) ; 15: : 307–310. |
37. | Michie S , Van Stralen MM and West R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implement Sci (2011) ; 6: : 42. |
38. | Hoffmann TC , Glasziou PP , Boutron I , et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ (2014) ; 348: : g1687. |
39. | Pitkala KH and Strandberg TE. Clinical trials in older people. Age Ageing (2022) ; 51: : afab282. |
40. | The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord (2003) ; 18: : 738–750. |
41. | Franchignoni F , Horak F , Godi M , et al. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med (2010) ; 42: : 323–331. |
42. | Leddy AL , Crowner BE and Earhart GM. Utility of the Mini-BESTest, BESTest, and BESTest sections for balance assessments in individuals with Parkinson disease. J Neurol Phys Ther (2011) ; 35: : 90–97. |
43. | Melo TAd , Duarte ACM , Bezerra TS , et al. The Five Times Sit-to-Stand Test: safety and reliability with older intensive care unit patients at discharge. Rev Bras Ter Intensiva (2019) ; 31: : 27–33. |
44. | Duncan RP , Leddy AL and Earhart GM. Five Times Sit-to-Stand Test performance in Parkinson’s disease. Arch Phys Med Rehabil (2011) ; 92: : 1431–1436. |
45. | Weintraub D , Oehlberg KA , Katz IR , et al. Test characteristics of the 15-item geriatric depression scale and hamilton depression rating scale in Parkinson disease. Am J Geriatr Psychiatry (2006) ; 14: : 169–175. |
46. | Santangelo G , Barone P , Cuoco S , et al. Apathy in untreated, de novo patients with Parkinson’s disease: validation study of Apathy Evaluation Scale. J Neurol (2014) ; 261: : 2319–2328. |
47. | Morley D , Dummett S , Kelly L , et al. Administering the routine activities domain of the oxford participation and activities questionnaire as a stand-alone scale: the Oxford routine activities measure. Patient Relat Outcome Meas (2018) ; 9: : 239–243. |
48. | Bandura A . Guide for constructing self-efficacy scales. In Pajares F and Urdan T (eds) Self-efficacy beliefs of adolescents. Vol. 5: . Greenwich, CT: Information Age Publishing, (2006) , pp. 307–337. |
49. | Wojcicki TR , White SM and McAuley E. Assessing outcome expectations in older adults: the multidimensional outcome expectations for exercise scale. J Gerontol B Psychol Sci Soc Sci (2009) ; 64B: : 33–40. |
50. | Young WR , Ellmers TJ , Kinrade NP , et al. Re-evaluating the measurement and influence of conscious movement processing on gait performance in older adults: Development of the Gait-Specific Attentional Profile. Gait Posture (2020) ; 81: : 73–77. |
51. | Besson H , Brage S , Jakes RW , et al. Estimating physical activity energy expenditure, sedentary time, and physical activity intensity by self-report in adults123. Am J Clin Nutr (2010) ; 91: : 106–114. |
52. | Wendel N , Macpherson CE , Webber K , et al. Accuracy of activity trackers in Parkinson disease: should we prescribe them? Phys Ther (2018) ; 98: : 705–714. |
53. | Kim DW , Hassett LM , Nguy V , et al. A comparison of activity monitor data from devices worn on the wrist and the waist in people with Parkinson’s disease. Mov Disord Clin Pract (2019) ; 6: : 693–699. |
54. | White T , Westgate K , Hollidge S , et al. Estimating energy expenditure from wrist and thigh accelerometry in free-living adults: a doubly labelled water study. Int J Obes (2019) ; 43: : 2333–2342. |
55. | Busse M , Playle R , Latchem-Hastings J , et al. A web-based life-style, exercise and activity intervention for people with progressive multiple sclerosis: Results of a single-arm feasibility study. Mult Scler Relat Disord (2022) ; 57: : 103388. |
56. | Quinn L , Macpherson C , Long K , et al. Promoting physical activity via telehealth in people with Parkinson disease: the path forward after the COVID-19 pandemic? Phys Ther (2020) ; 100: : 1730–1736. |
57. | Sounderajah V , Clarke J , Yalamanchili S , et al. A national survey assessing public readiness for digital health strategies against COVID-19 within the United Kingdom. Sci Rep (2021) ; 11: : 5958. |
58. | Wannheden C and Revenäs Å. How people with Parkinson’s disease and health care professionals wish to partner in care using eHealth: co-design study. J Med Internet Res (2020) ; 22: : e19195. |
59. | Ellis T , Cavanaugh JT , Earhart GM , et al. Factors associated with exercise behavior in people with Parkinson disease. Phys Ther (2011) ; 91: : 1838–1848. |
60. | Foltynie T , Bruno V , Fox S , et al. Medical, surgical, and physical treatments for Parkinson’s disease. Lancet (2024) ; 403: : 305–324. |
61. | Robert G , Cornwell J , Locock L , et al. Patients and staff as codesigners of healthcare services. BMJ (2015) ; 350: : g7714. |
62. | Tennigkeit J , Feige T , Haak M , et al. Structured care and self-management education for persons with Parkinson’s disease: why the first does not go without the second-systematic review, experiences and implementation concepts from Sweden and Germany. J Clin Med (2020) ; 9: : 1–34. |
63. | Rimmer JH . Health promotion for people with disabilities: the emerging paradigm shift from disability prevention to prevention of secondary conditions. Phys Ther (1999) ; 79: : 495–502. |
64. | Rimmer J and Lai B. Framing new pathways in transformative exercise for individuals with existing and newly acquired disability. Disabil Rehabil (2017) ; 39: : 173–180. |
65. | Cornish K , Fox G , Fyfe T , et al. Understanding physical literacy in the context of health: a rapid scoping review. BMC Public Health (2020) ; 20: : 1569. |
66. | Riggare S , Hoglund PJ , Hvitfeldt Forsberg H , et al. Patients are doing it for themselves: A survey on disease-specific knowledge acquisition among people with Parkinson’s disease in Sweden. Health Informatics J (2019) ; 25: : 91–105. |
67. | Dominick GM , Dunsiger SI , Pekmezi DW , et al. Health literacy predicts change in physical activity self-efficacy among sedentary Latinas. J Immigr Minor Health (2013) ; 15: : 533–539. |
68. | Quinn L , Busse M , Khalil H , et al. Client and therapist views on exercise programmes for early-mid stage Parkinson’s disease and Huntington’s disease. Disabil Rehabil (2010) ; 32: : 917–928. |
69. | Gidron Y . Group therapy/intervention. In: Gellman MD and Turner JR (eds) Encyclopedia of Behavioral Medicine. New York, NY: Springer New York, (2013) , pp. 880–881. |
70. | Fluharty ME , Pinto Pereira SM , Benzeval M , et al. Educational differentials in key domains of physical activity by ethnicity, age and sex: a cross-sectional study of over 40 000 participants in the UK household longitudinal study (2013-2015). BMJ Open (2020) ; 10: : e033318. |
71. | Ige-Elegbede J , Pilkington P , Gray S , et al. Barriers and facilitators of physical activity among adults and older adults from Black and Minority Ethnic groups in the UK: A systematic review of qualitative studies. Prev Med Rep (2019) ; 15: : 100952. |
72. | Ainsworth BE , Haskell WL , Herrmann SD , et al. Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc (2011) ; 43: : 1575–1581. |
73. | Ainsworth BE HW , Herrmann SD , Meckes N , et al. The Compendium of Physical Activities Tracking Guide. Healthy Lifestyles Research Center, College of Nursing & Health Innovation, Arizona State University. Retrieved [22/01/2024] from the World Wide Web. |




