Colonoscopy and Subsequent Risk of Parkinson’s Disease
Abstract
Background:
Parkinson’s disease (PD) is caused by the misfolding and aggregation of α-synuclein in neurons into toxic oligomers and fibrils that have prion-like properties allowing them to infect healthy neurons and to be transmitted to animal models of PD by injection or oral exposure. Given α-synuclein fibrils’ potential transmission on the gut-brain axis, α-synuclein may be transmitted through colonoscopy procedures.
Objective:
This study examines a possible association between colonoscopy and PD.
Methods:
Longitudinal health insurance data of 250,000 individuals aged 50+ from 2004–2019 was analyzed. Cox proportional hazard and competing risk models with death as a competing event were estimated to calculate the risk of PD. Colonoscopy was categorized as never receiving colonoscopy, colorectal cancer (CRC) screening without or with biopsy, destruction or excision (BDE), and diagnostic colonoscopy without or with BDE.
Results:
We identified 6,422 new cases of PD among 221,582 individuals. The Cox model revealed a significantly increased risk of PD for patients who ever had a diagnostic colonoscopy without or with BDE (HR = 1.31; 95% CI: [1.23–1.40]; HR = 1.32 [1.22–1.42]) after adjustment for age and sex. After controlling for covariates and death, persons who ever underwent CRC screening had a 40% reduced risk of PD (CRHR = 0.60 [0.54–0.67]), while persons who underwent diagnostic colonoscopy had a 20% reduced risk of PD (CRHR = 0.81 [0.75–0.88]).
Conclusions:
Colonoscopy does not increase the risk of PD, after adjusting for death and covariates. Individuals who underwent only CRC screening had the lowest risk of PD, which may be a result of a more health-conscious lifestyle.
INTRODUCTION
Parkinson’s disease (PD) is the second most frequent neurodegenerative disease after Alzheimer’s disease and one of the central events in its pathogenesis is the misfolding and aggregation of α-synuclein, a protein that is expressed in neurons of the central and peripheral nervous system [1]. Pathologic α-synuclein aggregates have prion-like properties and can spread within the nervous system by transmission from diseased to healthy neurons, where they seed de novo aggregation by recruiting α-synuclein monomers into growing α-synuclein aggregates [2]. Postmortem studies in PD patients revealed that pathologic α-synuclein propagates along relatively large distances within the central nervous system [3], and, surprisingly, also between the enteric and central nervous system along the vagal nerve, the so-called neural gut-brain-axis [4]. This allows the experimental transmission of pathologic α-synuclein aggregates to animal models of PD, including wild-type rodents and non-human primates, by injection of α-synuclein fibrils into the brain or gut, or by oral exposure [5–8]. In PD patients, pathologic α-synuclein can be present in gut-associated tissues, such as the submucosal and myenteric plexuses of the enteric nervous system, and in stool, including those of prodromal PD patients without obvious symptoms [9, 10]. Because of its prion-like properties, iatrogenic transmission of pathologic α-synuclein to humans is a threat that has not been sufficiently investigated [11–13]. Since 2002, insured individuals are eligible for a colorectal cancer (CRC) screening from the age of 55 two times every ten years [14]. Pathologic α-synuclein aggregates can adhere to various surfaces made of plastic, glass, stainless steel, or aluminum and exhibit remarkable stability and resistance to inactivation, which is why they could be transmitted after adhesion to an endoscope during colonoscopy [15–17]. Importantly, endoscopes cannot be autoclaved, and alternative sterilization methods can be insufficient to fully inactivate pathogens, which can be transmitted and cause infections when endoscopes are reused [18–20]. To assess the possible transmission of pathologic α-synuclein seeding activity from one person to another via endoscopes, we investigated here whether colonoscopy shows an association with PD in later life.
MATERIALS AND METHODS
Data
A random sample of 250,000 individuals aged 50 and older was obtained from Germany’s largest statutory health insurance. Individuals were followed from the first quarter of 2004 to the last quarter of 2019. Health claims data include demographic information on age and sex as well as medical diagnoses coded by ICD-10 (10th revision of the International Statistical Classification of Diseases and Related Health) and treatments coded by EBM (Einheitlicher Bewertungsmaßstab –uniform assessment standard) in the outpatient sector and OPS (Operationen- und Prozedurenschlüssel –operation and procedure codes) of the inpatient sector for billing purposes.
Definition of Parkinson’s disease
PD was coded with ICD-10 code G20. Individuals with a differential diagnosis of parkinsonism were excluded from the analysis. Atypical parkinsonism included progressive supranuclear ophthalmoplegia (G23.1), multiple system atrophy (G23.2, G23.3), dementia with Lewy bodies (G31.82) and corticobasal degeneration (G31.0). A valid PD diagnosis was present if an individual received diagnoses in both the inpatient and outpatient sectors in the same quarter, if more than one diagnosis per individual was present or if the diagnosis of PD and death occurred simultaneously in the last quarter of observation over the follow-up period from 2004 to 2019. To only consider incident PD cases, individuals with a PD diagnosis in 2004 and 2005 were excluded. This validation strategy employed in a previous paper, using the same data, was found to be effective [21]. For the analyses, patients were followed from 2006 until a valid PD diagnosis, death, censoring or the last quarter of 2018.
Colonoscopy
In Germany, colonoscopy procedures can be characterized into two broad types: CRC screening colonoscopy and diagnostic colonoscopy. CRC screenings are performed without indication as regular examinations or in patients with a family history of CRC. Diagnostic colonoscopies are only performed in case of relevant complaints or pathological findings [22–24]. During both procedures an additional biopsy, destruction, excision, laser vaporization or argon plasma coagulation (BDE) of tissue may be performed. We differentiated between the following groups: never any colonoscopy, CRC screening, diagnostic colonoscopy, CRC screening with BDE, and diagnostic colonoscopy with BDE. The corresponding EBM and OPS codes can be found in the appendix (Supplementary Tables 1 and 2).
In the administrative process, CRC screening or diagnostic colonoscopy with a concurrent BDE are billed at the same time. In this case the procedure is counted only as a CRC screening or diagnostic colonoscopy with BDE in the analysis to avoid double counting of CRC screening or diagnostic colonoscopy and to ensure exclusive categories.
Colonoscopy was coded as a time-dependent variable. An internal hierarchy of the colonoscopy procedures was established in which a diagnostic colonoscopy with BDE is considered the most severe procedure to be expected in terms of a possible transmission of PD [25–27]. Furthermore, individuals were also coded as diagnostic colonoscopy with BDE if they had a diagnostic colonoscopy followed by a CRC screening with BDE. A person remained in the state of having had a diagnostic colonoscopy with BDE, even if that person subsequently had a CRC screening without or with BDE or a diagnostic colonoscopy, in order to maintain the level of internal hierarchy.
Covariates
A selection of relevant comorbidities of PD was obtained from the most recent article by Schrag et al. [28]. Time-dependent index variables were established to give an overview of related diseases or conditions. The index variables were categorized as “Never”, “1 morbidity ever” or “2 or more morbidities ever”. Relevant comorbidities of PD are sleep disorders (restless legs syndrome (G25.80, G25.81), hypersomnia (F51.1, G47.1), insomnia (F51.0, G47.0), other sleep disorders (F51.2, F51.8, F51.9, G47.2, G47.9), parasomnia (F51.3–F51.5, F47.8), sleep apnea (G47.3)), gastrointestinal disorders (duodenal ulcer (K26), gastric ulcer (K25), gastritis (K29.0–K29.9), gastroesophageal reflux disease (K21.0, K21.9), gastrojejunal ulcer (K28), peptic ulcer (K27), Crohn’s disease (K50.0, K50.1, K50.8, K50.9)), metabolic and cardiovascular disorders (diabetes mellitus type 1 (E10), diabetes mellitus type 2 (E11), hypercholesterinemia (E78), hypertension (I10)) and other comorbidities (osteoarthritis (M15–M19), seropositive inflammatory arthritis (M05), bipolar disorder (F31), schizophrenia (F20), epilepsy (G40), migraine (G43)). Crohn’s disease of the colon is included in Crohn’s disease, although it can also be understood as a reason for colonoscopy [29]. Behavioral risk factors were considered separately, including alcohol abuse (F10.1, F10.2), nicotine abuse (F17.1, F17.2) and traumatic brain injury (TBI) (S06.0–S06.3) [28]. A positive association between hearing impairment and subsequent PD was previously found by Simonet et al. [30] and was included in the analyses (H90.0–H90.8, H91.0–H91.3, H91.8, H91.9, H94.0).To overcome the problem of confounding by indication, we adjusted our regression models for diseases that may act as indications for a colonoscopy but also occur in the disease course of persons with PD in later life. Therefore, the following diseases and conditions are included in the analysis as reasons for colonoscopy: Malignant neoplasm of colon (C18), diverticular disease of large intestine (K57.2, K57.3), functional diarrhea (K59.1), neurogenic bowel (K59.2), unspecified functional intestinal disorder (K59.9), acute abdomen (R10.0), hemorrhage of anus and rectum (K62.5), melaena (K92.1) and irritable bowel syndrome (K58.1–K58.8) [31, 22, 32, 33]. Since constipation (K59.0) and ulcerative colitis (K51) are reasons for a colonoscopy and prodromal symptoms of PD at the same time [28, 34, 35] they were handled separately.
Statistical analysis
Analyses were performed from 2006 to 2018 (13 years). The incidence rate of PD per 1,000 person-years and the mortality rate per 1,000 person-years were calculated for type of colonoscopy, age, sex, reasons for colonoscopy and comorbidities of PD by dividing the number of new PD cases and deaths by person-years at risk. An extended Kaplan-Meier plot was computed to reveal the survival probability without PD for all types of colonoscopy over a 13-year period. Cox proportional hazard models and Fine and Gray competing risk models were estimated for the risk of PD, adjusted for type of colonoscopy, age, sex and additionally for reasons for colonoscopy and comorbidities of PD. Cox proportional hazard models were also estimated for the risk of death. The proportional hazard assumption was verified graphically by scaled Schoenfeld residual plots (Supplementary Figures 1–6). Age was added as a polynomial function centered around age 78, as this age is the most common age for incident PD cases in the underlying sample. In a sensitivity analysis, we conducted a subgroup analysis for the fully adjusted competing risk model by age group and sex. Age was categorized into individuals below 75 years and those 75 years and above at baseline. For the analyses, Stata 17 was used for data management and RStudio for statistical analyses, using the packages “haven”, “survival”, “survminer” and “epiR”.
RESULTS
The analysis sample consisted of 221,582 individuals, resulting in a total of 2,108,378 person-years from 2006 to 2018. During this time 6,422 incident PD cases occurred and 88,008 individuals died. 68.8% of all person-years were spent with never having had a colonoscopy, while 7.6%, 14.0%, 1.2%, and 8.4% were spent with having had at least one CRC screening, diagnostic colonoscopy, CRC screening with biopsy, destruction, excision, laser vaporization or argon plasma coagulation (BDE) and diagnostic colonoscopy with BDE, respectively (Table 1).
Table 1
Incidence rate of Parkinson’s disease (PD) and death rate
| % Person-years | % PD cases | Incidence rate of PD per 1000 person-years | 95% CI of PD incidence rate | % Deaths | Death rate per 1000 person-years | 95% CI of death rate | ||
| Total | 100(n = 2,108,378) | 100 (n = 6,422) | 3.1 | 3.0–3.1 | 100(n = 88,008) | 41.7 | 41.4–42.0 | |
| Colonoscopy | Never | 68.8 | 64.2 | 2.8 | 2.8–2.9 | 67.0 | 40.6 | 40.3–41.0 |
| CRC screening without BDE | 7.6 | 5.6 | 2.3 | 2.0–2.5 | 2.5 | 13.7 | 13.1–14.3 | |
| Diagnostic colonoscopy without BDE | 14.0 | 17.3 | 3.8 | 3.6–4.0 | 15.3 | 45.6 | 44.8–46.4 | |
| CRC screening with BDE | 1.2 | 1.0 | 2.5 | 1.9–3.2 | 0.5 | 17.4 | 15.8–19.1 | |
| Diagnostic colonoscopy with BDE | 8.4 | 11.8 | 4.3 | 4.0–4.6 | 14.7 | 73.4 | 72.1–74.6 | |
| Age group | 50–54 | 2.4 | 0.2 | 0.3 | 0.2–0.5 | 0.4 | 6.5 | 5.8–7.2 |
| 55–59 | 9.2 | 1.4 | 0.5 | 0.4–0.6 | 1.8 | 8.3 | 7.9–8.7 | |
| 60–64 | 14.1 | 4.1 | 0.9 | 0.8–1.0 | 4.3 | 12.7 | 12.3–13.1 | |
| 65–69 | 16.8 | 9.3 | 1.7 | 1.6–1.8 | 7.0 | 17.3 | 16.9–17.8 | |
| 70–74 | 18.0 | 18.5 | 3.1 | 3.0–3.3 | 10.8 | 25.2 | 24.7–25.7 | |
| 75–79 | 16.9 | 24.4 | 4.4 | 4.2–4.6 | 16.2 | 40.0 | 39.3–40.7 | |
| 80–84 | 12.3 | 23.1 | 5.7 | 5.5–6.0 | 19.6 | 66.8 | 65.8–67.8 | |
| 85–89 | 7.1 | 14.0 | 6.1 | 5.7–6.5 | 20.3 | 119.8 | 118.1–121.6 | |
| 90+ | 3.4 | 5.0 | 4.5 | 4.0–5.1 | 19.7 | 243.9 | 240.3–247.6 | |
| Sex | Male | 41.7 | 46.4 | 3.4 | 3.3–3.5 | 43.0 | 43.1 | 42.6–43.5 |
| Female | 58.3 | 53.6 | 2.8 | 2.7–2.9 | 57.0 | 40.8 | 40.4–41.2 | |
| Reason for colon. and prodromal symptom/ risk of PD | Constipation: Yes | 12.3 | 27.8 | 6.9 | 6.6–7.2 | 32.6 | 110.3 | 31.9–32.4 |
| Constipation: No | 87.7 | 72.2 | 2.5 | 2.4–2.6 | 67.4 | 32.1 | 109.0–111.6 | |
| Ulcerative Colitis: Yes | 0.6 | 0.8 | 4.2 | 3.2–5.5 | 0.9 | 61.3 | 57.0–65.7 | |
| Ulcerative Colitis: No | 99.4 | 99.2 | 3.0 | 3.0–3.1 | 99.1 | 41.6 | 41.4–41.9 | |
| Reason for colonoscopy | 0 | 81.5 | 76.0 | 2.8 | 2.8–2.9 | 73.3 | 37.5 | 37.3–37.8 |
| 1 | 15.3 | 19.2 | 3.8 | 3.6–4.0 | 20.6 | 56.5 | 55.7–57.3 | |
| 2 or more | 3.3 | 4.8 | 4.5 | 4.0–5.0 | 6.1 | 77.6 | 75.5–79.7 | |
| Gastrointestinal disorders | 0 | 59.4 | 49.8 | 2.6 | 2.5–2.7 | 47.4 | 33.3 | 33.0–33.7 |
| 1 | 23.0 | 27.7 | 3.7 | 3.5–3.8 | 28.9 | 52.4 | 51.8–53.1 | |
| 2 or more | 17.6 | 22.5 | 3.9 | 3.7–4.1 | 23.6 | 56.2 | 55.4–56.9 | |
| Sleep disorders | 0 | 77.8 | 65.0 | 2.6 | 2.5–2.6 | 70.2 | 37.6 | 37.3–37.9 |
| 1 | 17.0 | 24.5 | 4.4 | 4.2–4.6 | 22.5 | 55.3 | 54.5–56.1 | |
| 2 or more | 5.2 | 10.5 | 6.1 | 5.7–6.6 | 7.4 | 59.1 | 57.7–60.6 | |
| Metabolic and cardiovascular disorders | 0 | 13.0 | 4.5 | 1.1 | 0.9–1.2 | 6.1 | 19.6 | 19.1–20.1 |
| 1 | 26.8 | 22.1 | 2.5 | 2.4–2.6 | 22.7 | 35.3 | 34.8–35.8 | |
| 2 or more | 60.2 | 73.4 | 3.7 | 3.6–3.8 | 71.2 | 49.4 | 49.0–49.8 | |
| Other comorbidities | 0 | 38.0 | 26.6 | 2.1 | 2.0–2.2 | 33.5 | 36.8 | 36.4–37.2 |
| 1 | 50.9 | 56.7 | 3.4 | 3.3–3.5 | 52.8 | 43.3 | 42.9–43.7 | |
| 2 or more | 11.1 | 16.7 | 4.6 | 4.3–4.9 | 13.7 | 51.4 | 50.5–52.3 | |
| Behavioral risk factors | Alcohol abuse: Yes | 2.2 | 2.8 | 3.8 | 3.3–4.4 | 4.3 | 79.0 | 76.5–81.6 |
| Alcohol abuse: No | 97.8 | 97.2 | 3.0 | 3.0–3.1 | 95.7 | 40.9 | 40.6–41.2 | |
| Nicotine abuse: Yes | 2.9 | 3.5 | 3.7 | 3.2–4.2 | 5.7 | 82.2 | 80.0–84.5 | |
| Nicotine abuse: No | 97.1 | 96.5 | 3.0 | 3.0–3.1 | 94.3 | 40.5 | 40.3–40.8 | |
| TBI: Yes | 2.8 | 6.9 | 7.4 | 6.7–8.1 | 8.0 | 117.7 | 115.0–120.5 | |
| TBI: No | 97.2 | 93.1 | 2.9 | 2.8–3.0 | 92.0 | 39.5 | 39.3–39.8 | |
| Hearing impairment | Yes | 62.1 | 47.6 | 3.8 | 3.7–4.0 | 43.6 | 37.9 | 37.6–38.2 |
| No | 37.9 | 52.4 | 2.6 | 2.5–2.7 | 56.4 | 48.0 | 47.6–48.5 |
Incidence rate of PD and death rate. PD, Parkinson’s disease; CI, confidence interval; CRC, colorectal cancer; BDE, biopsy, destruction, polyp removal, laser vaporization or argon plasma coagulation; TBI, traumatic brain injury. n = 221,582. Source: AOK 2004–2019.
Incidence rates
The incidence rate of PD was 2.8 cases per 1,000 person-years (95% confidence interval: [2.8–2.9]) in people who never had a colonoscopy, whereas the incidence was significantly lower in people who had a CRC screening (2.3 [2.0–2.5]), as the confidence intervals did not overlap (Table 1). The incidence rate with additional BDE (2.5 [1.9–3.2]) was also lower, but not significantly. The incidence rate was significantly higher in persons undergoing a diagnostic colonoscopy without BDE (3.8 [3.6–4.0]) and with BDE (4.3 [4.0–4.6]). Across age groups the incidence rate of PD increased. At ages 85–89 the incidence rate reached the maximum (6.1 [5.7–6.5]) and significantly decreased for individuals aged 90 and older (4.5 [4.0–5.0]). Men (3.4 [3.3–3.5]) showed a significant higher incidence rate of PD than women (2.8 [2.7–2.9]). Persons diagnosed with constipation had a significantly higher incidence rate (6.9 [6.6–7.2]) than persons not diagnosed with constipation (2.5 [2.4–2.6]). This is similar for individuals diagnosed with (4.2 [3.2–5.5]) and without ulcerative colitis (3.0 [3.0–3.1]). The higher the number of diagnosed reasons for a colonoscopy, sleep disorders, gastrointestinal disorders, metabolic and cardiovascular disorders or other comorbidities an individual was, the higher was the incidence rate of PD. Individuals with a diagnosis of alcohol abuse (3.8 [3.3–4.4]), nicotine abuse (3.7 [3.2–4.2]), or TBI (7.4 [6.7–8.1]) had significantly higher incidence rates of PD than those without a diagnosis (3.0 [3.0–3.1], 3.0 [3.0–3.1], 2.9 [2.8–3.0], respectively). Additionally, we found a higher incidence rate in individuals with hearing impairment (3.8 [3.7–4.0]) compared to those without (2.6 [2.5–2.7]).
Death rates
Mortality was lowest among individuals who had a CRC screening without BDE (13.7 cases per 1,000 person-years; 95% confidence interval: [13.1–14.3]) and who had a CRC screening with BDE (17.4 [15.8–19.1]) (Table 1). Individuals who never had a colonoscopy (40.6 [40.3–41.0]), who had diagnostic colonoscopy without BDE (45.6 [44.8–46.4]) and especially those who had diagnostic colonoscopy with BDE (73.4 [72.1–74.6]) showed significant higher death rates. The higher the age was, the higher was the death rate. The death rate for persons aged 90 years and older was 243.9 cases per 1,000 person-years [240.3–247.6]. Mortality was significantly higher in men (43.1 [42.6–43.5]) than in women (40.8 [40.4–41.2]). Individuals with constipation (110.3 [109.0–111.6]) had a higher death rate than individuals without constipation (32.1 [31.9–32.4]). The same applies for individuals diagnosed with ulcerative colitis (61.3 [57.0–65.7]) compared to persons not diagnosed with ulcerative colitis (41.6 [41.3–41.9]). The higher the number of diagnosed reasons for colonoscopy, gastrointestinal disorders, sleep disorders, metabolic and cardiovascular disorders or other comorbidities of PD was, the higher was the death rate. Individuals diagnosed with alcohol abuse, nicotine abuse or TBI had higher death rates than those without such diagnoses. The death rate was higher in individuals without hearing impairment than in those with hearing impairment.
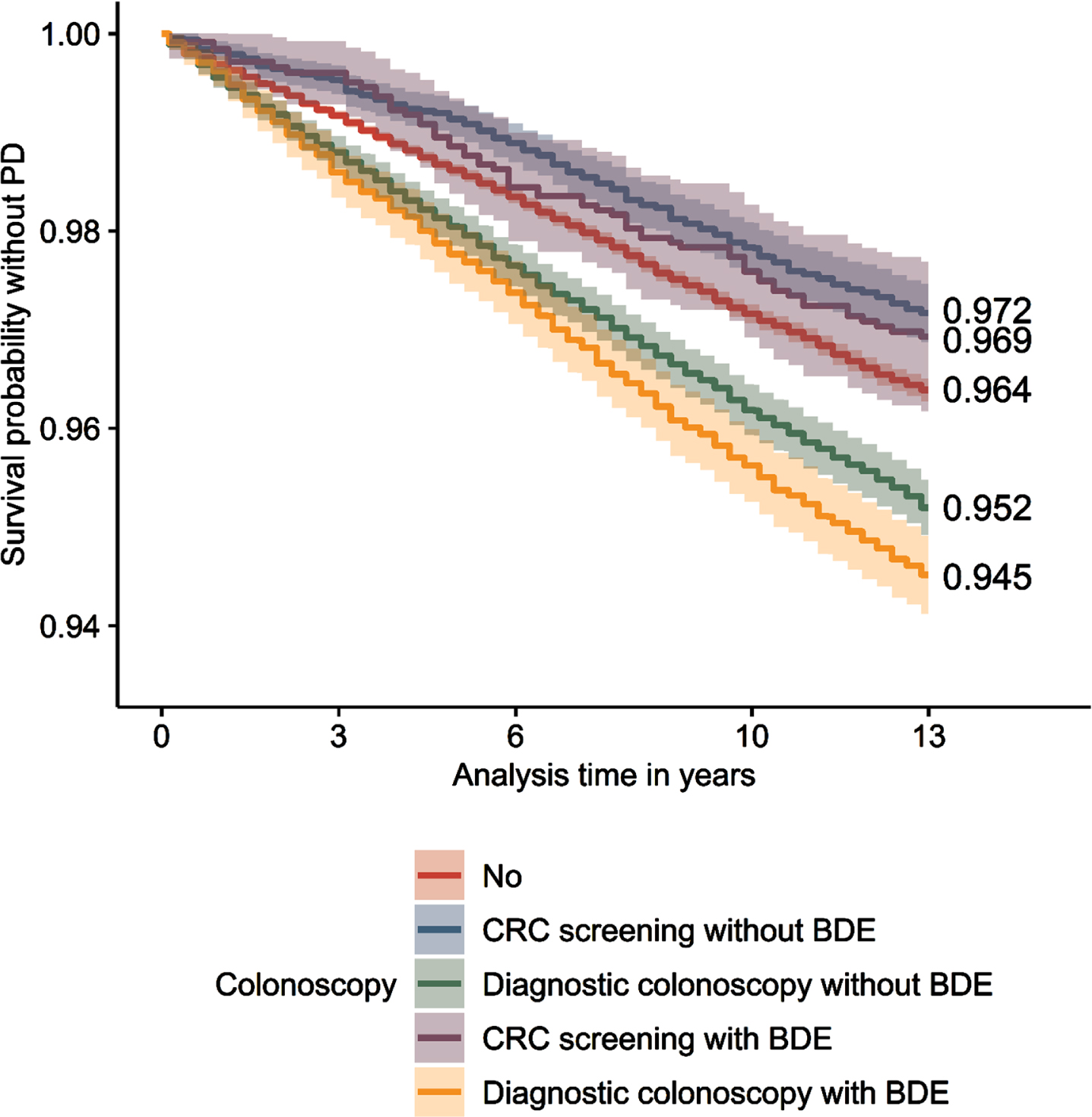
Extended Kaplan-Meier estimator
After 13 years of observation, 94.5% (survival probability without PD; 95% confidence interval: [94.1–94.9%]) of individuals who underwent a diagnostic colonoscopy with BDE were free of PD, whereas 95.2% [94.9–95.5%] of individuals who underwent a diagnostic colonoscopy without BDE were free of PD (Fig. 1). 96,4% [96.3–96.5%] of individuals who never had a colonoscopy were free of PD, while individuals who had a CRC screening with BDE showed a percentage of 96.9% [96.2–97.7%] and without BDE of 97.2% [96.9–97.5%].
Fig.1
Extended Kaplan-Meier curves. Survival probability without PD for all types of colonoscopy. PD, Parkinson’s disease; CRC, colorectal cancer; BDE, biopsy, destruction, polyp removal, laser vaporization or argon plasma coagulation. n = 221,582. Source: AOK 2004–2019.
Model results
In Model 1, individuals who had a diagnostic colonoscopy without BDE and with BDE had a significant increase of PD incidence compared to individuals who never had a colonoscopy, after only adjusting for age and sex (Table 2). However, these effects disappeared when adjusting for covariates: In the fully adjusted Cox model (Model 2), the effect of diagnostic colonoscopy without (HR = 1.03; 95% confidence interval: [0.95–1.11]) and with BDE (HR = 1.01 [0.93–1.10]) was no longer significant. The significant increase of PD incidence among persons with diagnostic colonoscopy without and with BDE can be fully explained by reasons for colonoscopy, gastrointestinal disorders, sleep disorders, metabolic and cardiovascular disorders, other comorbidities of PD, alcohol abuse, nicotine abuse, TBI and hearing impairment. Individuals who had CRC screening now showed a significant reduction of PD incidence (HR = 0.86 [0.77–0.96]) after controlling for all covariates. There was a significant positive effect of age with the linear term (HR = 1.05 [1.05–1.06]) and a negative association (HR = 0.997 [0.996–0.997]) with the quadratic term, shaping an increasing risk of PD, followed by a deceleration in the rate of increase. Females showed a 39% (HR = 0.61 [0.58–0.64]) lower risk of PD than males.
Table 2
Model results for the risk of Parkinson’s disease (PD)
| Risk of PD | Model 1: Cox model | Model 2: Cox model | Model 3: Competing risk model | Model 4: Competing risk model | |||||
| HR* | p | HR**[95% CI] | p | CRHR*[95% CI] | p | CRHR**[95% CI] | p | ||
| Colonoscopy (Ref. never) | |||||||||
| CRC screening without BDE | 0.94 [0.85–1.05] | 0.292 | 0.86 [0.77–0.96] | 0.007 | 0.61 [0.54–0.68] | < 0.001 | 0.60 [0.54–0.67] | < 0.001 | |
| Diagnostic colonoscopy without BDE | 1.31 [1.23–1.40] | < 0.001 | 1.03 [0.95–1.11] | 0.525 | 0.87 [0.81–0.93] | < 0.001 | 0.81 [0.75–0.88] | < 0.001 | |
| CRC screening with BDE | 0.93 [0.72–1.19] | 0.550 | 0.85 [0.66–1.10] | 0.221 | 0.63 [0.49–0.82] | 0.001 | 0.63 [0.49–0.81] | 0.001 | |
| Diagnostic colonoscopy with BDE | 1.32 [1.22–1.42] | < 0.001 | 1.01 [0.93–1.10] | 0.806 | 0.83 [0.77–0.89] | < 0.001 | 0.77 [0.70–0.84] | < 0.001 | |
| Demographics | |||||||||
| Age | 1.06 [1.06–1.07] | < 0.001 | 1.05 [1.05–1.06] | < 0.001 | 1.04 [1.04–1.05] | < 0.001 | 1.04 [1.03–1.04] | < 0.001 | |
| Age2 | 0.997 [0.997–0.997] | < 0.001 | 0.997 [0.996–0.997] | < 0.001 | 0.997 [0.997–0.998] | < 0.001 | 0.997 [0.997–0.997] | < 0.001 | |
| Sex (Ref. male) | Female | 0.67 [0.64–0.70] | < 0.001 | 0.61 [0.58–0.64] | < 0.001 | 0.72 [0.68–0.75] | < 0.001 | 0.68 [0.64–0.71] | < 0.001 |
| Reason for colonoscopy and prodromal symptom/risk for PD | |||||||||
| Constipation (Ref. no) | 1.84 [1.74–1.95] | < 0.001 | 1.63 [1.53–1.73] | < 0.001 | |||||
| Ulcerative colitis (Ref. no) | 1.10 [0.84–1.45] | 0.479 | 1.08 [0.82–1.42] | 0.572 | |||||
| Reasons for colonoscopy(Ref. no) | 1 | 0.95 [0.88–1.02] | 0.125 | 0.91 [0.84–0.97] | 0.008 | ||||
| 2 or more | 0.92 [0.81–1.04] | 0.203 | 0.86 [0.76–0.97] | 0.017 | |||||
| Gastrointestinal disorders (Ref. no) | 1 | 1.15 [1.08–1.22] | < 0.001 | 1.04 [0.98–1.11] | 0.175 | ||||
| 2 or more | 1.11 [1.03–1.19] | 0.004 | 0.91 [0.85–0.98] | 0.010 | |||||
| Sleep disorders (Ref. no) | 1 | 1.46 [1.37–1.55] | < 0.001 | 1.27 [1.19–1.35] | < 0.001 | ||||
| 2 or more | 1.88 [1.72–2.05] | < 0.001 | 1.38 [1.27–1.50] | < 0.001 | |||||
| Metabolic and cardiovascular disorders (Ref. no) | 1 | 1.53 [1.35–1.74] | < 0.001 | 1.53 [1.34–1.74] | < 0.001 | ||||
| 2 or more | 1.79 [1.58–2.02] | < 0.001 | 1.47 [1.30–1.66] | < 0.001 | |||||
| Other comorbidities (Ref. no) | 1 | 1.21 [1.14–1.29] | < 0.001 | 1.00 [0.94–1.06] | 0.884 | ||||
| 2 or more | 1.64 [1.51–1.78] | < 0.001 | 1.18 [1.09–1.28] | < 0.001 | |||||
| Behavioral risk factors | |||||||||
| Alcohol abuse (Ref. no) | 1.18 [1.00–1.40] | 0.052 | 0.96 [0.81–1.14] | 0.670 | |||||
| Nicotine abuse (Ref. no) | 1.31 [1.13–1.53] | < 0.001 | 1.17 (1.00–1.36 | 0.048 | |||||
| TBI (Ref. no) | 1.80 [1.63–1.99] | < 0.001 | 1.48 (1.34–1.63 | < 0.001 | |||||
| Hearing impairment (Ref. no) | 1.04 [0.99–1.09] | 0.156 | 0.85 [0.81–0.89] | < 0.001 | |||||
Cox and competing risk regression models. Competing risk: Death. HR, hazard ratio; CRHR, competing risk hazard ratio; *adjusted for colonoscopy, age, and sex; **adjusted for colonoscopy, age, sex, reasons for colonoscopy, and comorbidities of PD; CRC, colorectal cancer; BDE, biopsy, destruction, polyp removal, laser vaporization or argon plasma coagulation; TBI, traumatic brain injury. n = 221,582. Source: AOK 2004–2019.
When the death of individuals was considered as the competing risk in the Fine and Gray competing risk models, individuals who underwent any type of colonoscopy had a significantly lower risk of PD than individuals who never underwent a colonoscopy, after adjusting for age and sex (Model 3). In the fully adjusted competing risk model (Model 4), the significant reduction of PD incidence remained for any type of colonoscopy as compared to no colonoscopy. Adjustment for reasons for colonoscopy, gastrointestinal disorders, sleep disorders, metabolic and cardiovascular disorders, other comorbidities of PD, alcohol abuse, nicotine abuse, TBI, and hearing impairment reduced the negative effect of the types of colonoscopy, but only to a small extent. Individuals who had a diagnostic colonoscopy without BDE had a 19% (CRHR = 0.81 [0.75–0.88]) and with BDE a 33% (CRHR = 0.77 [0.70–0.84]) reduction of PD incidence. A CRC screening without BDE lead to a reduction of PD incidence of 40% (CRHR = 0.60 [0.54–0.67] and with BDE of 37% (CRHC = 0.63 [0.49–0.81]), when adjusted for all covariates and death. Subgroup analysis of the fully adjusted competing risk model by age group and sex revealed no significant associations between types of colonoscopy and PD due to the reduction of subsample size (Supplementary Material 3).
When death was treated as the only outcome (Table 3), individuals with a CRC screening without and with BDE had significantly lower risks of death compared to those who never had a colonoscopy, adjusted for age and sex (Model 5). Conversely, individuals with a diagnostic colonoscopy with and without BDE showed increased risks of death. After controlling for all covariates (Model 6), individuals who had diagnostic colonoscopy with BDE (HR = 1.13 [1.11–1.16]) still had a significantly higher risk of death, but the magnitude was reduced. However, individuals who only had diagnostic colonoscopy without BDE now showed a 10% (HR = 0.90 [0.88–0.92]) lower risk of death, compared to individuals having never had a colonoscopy. The effect on the risk of death remained for those who had CRC screening, even with BDE.
Table 3
Model results for the risk of death
| Risk of death | Model 5: Cox model | Model 6: Cox model | |||
| HR* | p | HR**[95% CI] | p | ||
| Colonoscopy (Ref. never) | |||||
| CRC screening without BDE | 0.49 [0.46–0.51] | < 0.001 | 0.46 [0.44–0.48] | < 0.001 | |
| Diagnostic colonoscopy without BDE | 1.16 [1.14–1.18] | < 0.001 | 0.90 [0.88–0.92] | < 0.001 | |
| CRC screening with BDE | 0.55 [0.50–0.61] | < 0.001 | 0.52 [0.47–0.57] | < 0.001 | |
| Diagnostic colonoscopy with BDE | 1.57 [1.53–1.60] | < 0.001 | 1.13 [1.11–1.16] | < 0.001 | |
| Demographics | |||||
| Age | 1.11 [1.11–1.11] | < 0.001 | 1.10 [1.10–1.11] | < 0.001 | |
| Age2 | 1.001 [1.001–1.001] | < 0.001 | 1.001 [1.001–1.001] | < 0.001 | |
| Sex | Female (Ref. male) | 0.63 [0.62–0.64] | < 0.001 | 0.63 [0.62–0.64] | < 0.001 |
| Reason for colonoscopy and prodromal symptom/risk for PD | |||||
| Constipation (Ref. no) | 1.94 [1.91–1.97] | < 0.001 | |||
| Ulcerative colitis (Ref. no) | 1.09 [1.01–1.17] | 0.022 | |||
| Reasons for colonoscopy (Ref. no) | 1 | 1.12 [1.10–1.14] | < 0.001 | ||
| 2 or more | 1.21 [1.18–1.25] | < 0.001 | |||
| Gastrointestinal disorders (Ref. no) | 1 | 1.31 [1.29–1.33] | < 0.001 | ||
| 2 or more | 1.33 [1.30–1.35] | < 0.001 | |||
| Sleep disorders (Ref. no) | 1 | 1.15 [1.13–1.17] | < 0.001 | ||
| 2 or more | 1.10 [1.07–1.12] | < 0.001 | |||
| Metabolic and cardiovascular disorders (Ref. no) | 1 | 1.14 [1.11–1.18] | < 0.001 | ||
| 2 or more | 1.35 [1.31–1.39] | < 0.001 | |||
| Other comorbidities (Ref. no) | 1 | 0.83 [0.82–0.84] | < 0.001 | ||
| 2 or more | 0.99 [0.99–1.01] | 0.302 | |||
| Hearing impairment (Ref. no) | 0.78 [0.76–0.79] | < 0.001 | |||
| Behavioral risk factors | |||||
| Alcohol abuse (Ref. no) | 1.60 [1.54–1.67] | < 0.001 | |||
| Nicotine abuse (Ref. no) | 2.30 [2.22–2.38] | < 0.001 | |||
| TBI (Ref. no) | 1.63 [1.59–1.67] | < 0.001 | |||
Cox regression models. HR, hazard ratio; *adjusted for colonoscopy, age, and sex; **adjusted for colonoscopy, age, sex, reasons for colonoscopy and comorbidities of PD; CRC, colorectal cancer; BDE, biopsy, destruction, polyp removal, laser vaporization or argon plasma coagulation; TBI, traumatic brain injury. n = 221,582. Source: AOK 2004–2019.
DISCUSSION
This is to our knowledge the first investigation of a possible association between colonoscopy procedures and the risk of PD, based on a large sample of insured individuals in Germany. We found a significantly associated reduction of PD incidence for persons who underwent any type of colonoscopy compared to those who had never undergone a colonoscopy adjusted for age, sex, mortality, relevant comorbidities, prodromal symptoms, and risk factors for PD.
The initially increased risk of PD in persons who had a diagnostic colonoscopy with or without BDE disappeared after controlling for reasons for colonoscopy and comorbidities of PD. We conclude that the seen association between colonoscopy and PD in the descriptive part of the results (e.g., Kaplan-Meier plot, Fig. 1) is misleading, because the association is confounded by indication, i.e., by factors such as underlying reasons for colonoscopy or by prodromal symptoms of PD. When death was additionally considered, individuals who had any type of colonoscopy even showed a significant reduction of PD incidence compared to individuals who never had a colonoscopy. This change may be explained by higher death rates among PD patients who had a diagnostic colonoscopy. Berry et al. emphasize the importance of considering death as the competing risk in studies of older adults, as the risk of disease may be overestimated when mortality is high [36]. In line with this, we found a higher risk of mortality for individuals who underwent diagnostic colonoscopy with BDE. During colonoscopies perforations or bleeding may occur and may lead to premature death [37, 38], which may partly explain the higher risk of all-cause mortality for this group. In a meta-analysis estimating the overall lifetime gains across cancer screening procedures, Bretthauer et al. found a significant gain in lifetime only for individuals who underwent a partial colonoscopy compared with those who did not [37]. This is in line with the lower mortality risks for individuals who underwent CRC screening and diagnostic colonoscopy in our study.
Compared to other colonoscopy procedures, individuals who had CRC screening with and without BDE had lower risks of PD. It is reasonable to assume that screening measures are mainly used by individuals who follow a health-conscious lifestyle and therefore have an overall lower risk of PD and any other disease. Little is known about health behaviors and the risk of PD, but studies suggest that more health-conscious individuals are more likely to seek preventive examinations and have a healthier lifestyle in general [39, 40]. This suggestion may be supported by previous studies showing that healthy lifestyle factors reduce the risk of disability and all-cause mortality [41, 42]. Further, a higher use of CRC screening can be found in people with a high socioeconomic status (SES) compared to people with a low SES [43, 44], confirming that individuals with a higher SES have a more health-conscious lifestyle [45]. However, there is strong evidence of higher incidence and risk of PD for individuals with higher SES compared to those with lower SES [46–48]. This may be due to the fact that individuals who are more self-observant and attend medical services more frequently are more likely to be diagnosed with PD earlier than those who do not attend regular health checks Still, it remains unclear how the positive association between SES and the use of CRC screening and the positive association between SES and the risk of PD interact and shape the association between CRC screening and the risk of PD. In our study we were not able to control for SES, but we included a number of comorbidities that correlate with SES (see limitation section). Higher regional deprivation and exposure to pesticides and herbicides increase the risk of PD [49–51]. Concurrently, the more deprived the living of a person, the lower is the use of CRC screening [52, 53].
In line with previous studies, we found a positive association between constipation and a risk increase of PD [28, 54]. Constipation as a prodromal symptom of PD [22, 33] may be a reason for having a diagnostic colonoscopy, which may explain the loss of significance for diagnostic colonoscopy in the fully adjusted model (Model 2). No significant effect on the risk of PD could be found in individuals with ulcerative colitis which is in line with previous findings [55], despite ulcerative colitis is considered as another possible prodromal symptom of PD [35]. In the fully adjusted competing risk model, we found no significant association between alcohol abuse and PD, but a significant increase of PD incidence in individuals diagnosed with nicotine abuse und TBI. While the association found between TBI and PD is consistent with previous studies [56], we cannot confirm a significant association between alcohol abuse and PD [57]. For nicotine abuse, we even find the opposite direction [28, 58], with nicotine abuse leading to an increased risk of PD. This may be due to the fact that only harmful tobacco use and nicotine dependence are coded in the data, which tends to affect heavy smokers but not occasional smokers, for whom the neuroprotective aspects of nicotine in relation to PD risk may outweigh the general harmful effects of nicotine. The associations may also be altered by the age range of the individuals being studied, as only those aged 50 and above were followed. In addition, few person-years were spent with a diagnosis of alcohol abuse, nicotine abuse or TBI, which may further bias the effect sizes. We found a significant reduction in the incidence of PD among individuals with hearing impairment after adjusting for death, whereas a previous study found a positive association when death was not taken into account [30].
Strengths and limitations
A major advantage of health claims data is the low sample attrition and the absence of recall bias due to the billing character of the data. There is no selection bias by health care or self-selection. Regardless of the current health status, all individuals can be included in the study. Properties of administrative data result in left and right censoring, indicating the presence of incomplete health information prior to the start of observation, as well as missing information due to study drop-out and end of observation. Therefore, an underestimation of both new-onset PD cases and the use of colonoscopy must be considered, also with regard to the limited follow-up of 13 years given the prodromal phase of PD, which may last from a few years to several decades [3, 59].
The study population is not representative for the whole elderly German population. Most of the German residents have a compulsory health insurance, but especially a small number of individuals with a higher income own a private health insurance. Therefore, the underlying population has a lower socioeconomic status, which may result in an unhealthier population in general [60]. It is reasonable that the lower SES is also attributable to an underestimation of CRC screenings in the analysis [43, 44]. Although SES, regional deprivation and environmental factors could not be included because of the billing character of the data, the consideration of comorbidities provide some insight into the lifestyle of insured individuals, as more disadvantaged individuals have poorer health [61]. The diagnosis of diseases is based on ICD-10 codes and the treatment is based in EBM and OPS codes. Because of missing diagnoses or incorrect coding of diagnosis and treatment in general, the results may be biased. A change of diagnosis over time does not necessarily mean a change of the disease, therefore time-dependent variables and a validation strategy for incident PD cases were introduced [21]. Because we begin our observation in 2004, it is possible that individuals who had a colonoscopy before 2004 were erroneously coded as never having had a colonoscopy. This may also lead to underestimated effect sizes. In addition, unambiguous classification of colonoscopy indication is a challenge. A patient’s medical history is not always available. Practitioners may not be aware of previous polyp removals, which may lead to misclassification [62]. Individuals with a family history of CRC are usually screened more often and at an earlier age [24]. This means the group of individuals that underwent a CRC screening may be heterogeneous, as individuals with a higher risk of CRC and individuals with a health-conscious lifestyle are included. This could lead to distorted effect sizes. A sensitivity analysis for the risk of PD was performed restricted to individuals who ever had any colonoscopy. There was no significant difference between the types of colonoscopy. One explanation for this is the possibility of a biopsy during a rectoscopy, sigmoidoscopy or a partial colonoscopy with a flexible instrument in the outpatient sector. Therefore, CRC screening without BDE and CRC screening with BDE may not be exclusive categories. Still, both groups differ significantly from the group of no colonoscopy in the included models. As the data do not contain any clinical information, it was not possible to differentiate between different phenotypes of incident PD cases, particularly for those who underwent colonoscopy versus those who did not. Based on the hypothesis of this study, it may be reasonable to expect gut-first PD phenotypes among incident PD cases who underwent colonoscopy with and without BDE. Future studies could investigate this.
Conclusion and implication
Our findings show that the risk of PD is not increased after colonoscopy, suggesting that iatrogenic transmission of pathologic α-synuclein does not occur during colonoscopy. At present, there is no justification for patients to forgo CRC screening due to concerns about a higher risk of PD. However, it is essential to conduct further research to address the highlighted impact of confounding by indication (e.g., constipation) and differences in mortality among different risk groups. These factors may lead to an overestimation of PD risk in individuals facing higher mortality rates.
ACKNOWLEDGMENTS
We are grateful to the Scientific Research Institute of the AOK, WIdO, for providing the data. This study received no funding. Anna-Victoria Holtz gratefully acknowledges the resources provided by the International Max Planck Research School for Population, Health and Data Science (IMPRS-PHDS).
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The scientific research institute of the AOK (WIdO) has strict rules regarding data sharing because of the fact that health claims data are a sensitive data source and have ethical restrictions imposed due to concerns regarding privacy. Anonymized data are available to all interested researchers upon request. Interested individuals or an institution who wish to request access to the health claims data of the AOK may contact the WIdO (webpage: http://www.wido.de/, mail: ).
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-240017.
REFERENCES
[1] | Spillantini MG , Schmidt ML , Lee VM , Trojanowski JQ , Jakes R , Goedert M ((1997) ) Alpha-synuclein in Lewy bodies. Nature 388: , 839–840. |
[2] | Woerman AL , Tamgüney G ((2022) ) Body-first Parkinson’s disease and variant Creutzfeldt-Jakob disease –similar or different? Neurobiol Dis 164: , 105625. |
[3] | Braak H , Del Tredici K , Rüb U , Vos de RAI , Jansen Steur ENH , Braak E ((2003) ) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24: , 197–211. |
[4] | Braak H , Vos de RAI , Bohl J , Del Tredici K ((2006) ) Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396: , 67–72. |
[5] | Luk KC , Kehm V , Carroll J , Zhang B , O’Brien P , Trojanowski JQ , Lee VM-Y ((2012) ) Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338: , 949–953. |
[6] | Kim S , Kwon S-H , Kam T-I , Panicker N , Karuppagounder SS , Lee S , Lee JH , Kim WR , Kook M , Foss CA , Shen C , Lee H , Kulkarni S , Pasricha PJ , Lee G , Pomper MG , Dawson VL , Dawson TM , Ko HS ((2019) ) Transneuronal propagation of pathologic α-synuclein from the gut to the brain models Parkinson’s disease. Neuron 103: , 627–641.e7. |
[7] | Arotcarena M-L , Dovero S , Prigent A , Bourdenx M , Camus S , Porras G , Thiolat M-L , Tasselli M , Aubert P , Kruse N , Mollenhauer B , Trigo Damas I , Estrada C , Garcia-Carrillo N , Vaikath NN , El-Agnaf OMA , Herrero MT , Vila M , Obeso JA , Derkinderen P , Dehay B , Bezard E ((2020) ) Bidirectional gut-to-brain and brain-to-gut propagation of synucleinopathy in non-human primates. Brain 143: , 1462–1475. |
[8] | Lohmann S , Bernis ME , Tachu BJ , Ziemski A , Grigoletto J , Tamgüney G ((2019) ) Oral and intravenous transmission of α-synuclein fibrils to mice. Acta Neuropathol 138: , 515–533. |
[9] | Stokholm MG , Danielsen EH , Hamilton-Dutoit SJ , Borghammer P ((2016) ) Pathological α-synuclein in gastrointestinal tissues fromprodromal Parkinson disease patients. Ann Neurol 79: , 940–949. |
[10] | Schaffrath A , Schleyken S , Seger A , Jergas H , Özdüzenciler P , Pils M , Blömeke L , Cousin A , Willbold J , Bujnicki T , Bannach O , Fink GR , Willbold D , Sommerauer M , Barbe MT , Tamgüney G ((2023) ) Patients with isolated REM-sleep behavior disorder have elevated levels of alpha-synuclein aggregates in stool. NPJ Parkinsons Dis 9: , 14. |
[11] | Ritchie DL , Barria MA ((2021) ) Prion diseases: A unique transmissible agent or a model for neurodegenerative diseases? Biomolecules 11: , 207. |
[12] | Jaunmuktane Z , Brandner S ((2020) ) Invited Review: The role of prion-like mechanisms in neurodegenerative diseases. Neuropathol Appl Neurobiol 46: , 522–545. |
[13] | Gomez-Gutierrez R , Morales R ((2020) ) The prion-like phenomenon in Alzheimer’s disease: Evidence of pathology transmission in humans. PLoS Pathog 16: , e1009004. |
[14] | Weber C , Blumenstein I (2021) Die Darmkrebsvorsorge wird volljährig –Bilanz und Ausblick. Hessisches Ärzteblatt, pp. 380-384. |
[15] | Bousset L , Brundin P , Böckmann A , Meier B , Melki R ((2016) ) An efficient procedure for removal and inactivation of alpha-synuclein assemblies from laboratory materials. J Parkinsons Dis 6: , 143–151. |
[16] | Woerman AL , Kazmi SA , Patel S , Freyman Y , Oehler A , Aoyagi A , Mordes DA , Halliday GM , Middleton LT , Gentleman SM , Olson SH , Prusiner SB ((2018) ) MSA prions exhibit remarkable stability and resistance to inactivation. Acta Neuropathol 135: , 49–63. |
[17] | Fenyi A , Coens A , Bellande T , Melki R , Bousset L ((2018) ) Assessment of the efficacy of different procedures that remove and disassemble alpha-synuclein, tau and A-beta fibrils from laboratory material and surfaces. Sci Rep 8: , 10788. |
[18] | Ofstead CL , Wetzler HP , Doyle EM , Rocco CK , Visrodia KH , Baron TH , Tosh PK ((2015) ) Persistent contamination on colonoscopes and gastroscopes detected by biologic cultures and rapid indicators despite reprocessing performed in accordance with guidelines. Am J Infect Control 43: , 794–801. |
[19] | Wang P , Xu T , Ngamruengphong S , Makary MA , Kalloo A , Hutfless S ((2018) ) Rates of infection after colonoscopy and osophagogastroduodenoscopy in ambulatory surgery centres in the USA. Gut 67: , 1626–1636. |
[20] | McCafferty CE , Aghajani MJ , Abi-Hanna D , Gosbell IB , Jensen SO ((2018) ) An update on gastrointestinal endoscopy-associated infections and their contributing factors. Ann Clin Microbiol Antimicrob 17: , 36. |
[21] | Nerius M , Fink A , Doblhammer G ((2017) ) Parkinson’s disease in Germany: Prevalence and incidence based on health claims data. Acta Neurol Scand 136: , 386–392. |
[22] | Rösch T , Zimmermann-Fraedrich K , Faiss S , Denzer U , Lerch MM , Moog G (2015) S2k Leitlinie Qualitätsanforderungen in der gatrointestinalen Endoskopie: Kapitel 4.5 Koloskopie, AWMF Register Nr. 021-022. |
[23] | IQWiG (2022) Leitliniensynopse zur organisierten Darmkrebsfrüherkennung, https://www.iqwig.de/download/s21-02_leitliniensynopse-zur-organisierten-darmkrebsfrueherkennung_rapid-report_v1-0.pdf. |
[24] | Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF (2019) S3- Leitlinie Kolorektales Karzinom, Langversion, http://www.leitlinienprogramm-onkologie.de/leitlinien/kolorektales-karzinom/. |
[25] | Bretthauer M , Løberg M , Wieszczy P , Kalager M , Emilsson L , Garborg K , Rupinski M , Dekker E , Spaander M , Bugajski M , Holme Ø , Zauber AG , Pilonis ND , Mroz A , Kuipers EJ , Shi J , Hernán MA , Adami H-O , Regula J , Hoff G , Kaminski MF ((2022) ) Effect of colonoscopy screening on risks of colorectal cancer and related death. N Engl J Med 387: , 1547–1556. |
[26] | Levin TR , Zhao W , Conell C , Seeff LC , Manninen DL , Shapiro JA , Schulman J ((2006) ) Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med 145: , 880–886. |
[27] | Moreno CC , Mittal PK , Sullivan PS , Rutherford R , Staley CA , Cardona K , Hawk NN , Dixon WT , Kitajima HD , Kang J , Small WC , Oshinski J , Votaw JR ((2016) ) Colorectal cancer initial diagnosis: Screening colonoscopy, diagnostic colonoscopy, or emergent surgery, and tumor stage and size at initial presentation. Clin Colorectal Cancer 15: , 67–73. |
[28] | Schrag A , Bohlken J , Dammertz L , Teipel S , Hermann W , Akmatov MK , Bätzing J , Holstiege J ((2023) ) Widening the spectrum of risk factors, comorbidities, and prodromal features of Parkinson disease. JAMA Neurol 80: , 161–171. |
[29] | Brudek T ((2019) ) Inflammatory bowel diseases and Parkinson’s disease. J Parkinsons Dis 9: , S331–S344. |
[30] | Simonet C , Bestwick J , Jitlal M , Waters S , Ben-Joseph A , Marshall CR , Dobson R , Marrium S , Robson J , Jacobs BM , Belete D , Lees AJ , Giovannoni G , Cuzick J , Schrag A , Noyce AJ ((2022) ) Assessment of risk factors and early presentations of Parkinson disease in primary care in a diverse UK population. JAMA Neurol 79: , 359–369. |
[31] | Leifeld L , Germer C-T , Böhm S , Dumoulin FL , Frieling T , Kreis M , Meining A , Labenz J , Lock JF , Ritz J-P , Schreyer A , Kruis W ((2022) ) S3-Leitlinie Divertikelkrankheit/Divertikulitis –Gemeinsame Leitlinie der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS) und der Deutschen Gesellschaft für Allgemein- und Viszeralchirurgie (DGAV). Z Gastroenterol 60: , 613–688. |
[32] | Froehlich F , Pache I , Burnand B , Vader JP , Fried M , Beglinger C , Stalder G , Gyr K , Thorens J , Schneider C , Kosecoff J , Kolodny M , DuBois RW , Gonvers JJ , Brook RH ((1998) ) Performance of panel-based criteria to evaluate the appropriateness of colonoscopy: A prospective study. Gastrointest Endosc 48: , 128–136. |
[33] | Lieberman DA , Williams JL , Holub JL , Morris CD , Logan JR , Eisen GM , Carney P ((2014) ) Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc 80: , 133–143. |
[34] | Mollenhauer B , Sixel-Döring F , Storch A , Schneider C , Hilker R , Kalbe E ((2013) ) Früherkennung der Parkinson-Krankheit. Objektivierbare nichtmotorische Symptome und Biomarker. Nervenarzt 84: , 918–926. |
[35] | Zhu F , Li C , Gong J , Zhu W , Gu L , Li N ((2019) ) The risk of Parkinson’s disease in inflammatory bowel disease: A systematic review and meta-analysis. Dig Liver Dis 51: , 38–42. |
[36] | Berry SD , Ngo L , Samelson EJ , Kiel DP ((2010) ) Competing risk of death: An important consideration in studies of older adults. J Am Geriatr Soc 58: , 783–787. |
[37] | Bretthauer M , Wieszczy P , Løberg M , Kaminski MF , Werner TF , Helsingen LM , Mori Y , Holme Ø , Adami H-O , Kalager M ((2023) ) Estimated lifetime gained with cancer screening tests: A meta-analysis of randomized clinical trials. JAMA Intern Med 183: , 1196–1203. |
[38] | Penston J ((2011) ) Should we use total mortality rather than cancer specific mortality to judge cancer screening programmes? Yes. BMJ 343: , d6395. |
[39] | Vernon GM , Jenkins M ((1995) ) Health maintenance behaviors in advanced Parkinson’s disease. J Neurosci Nurs 27: , 229–235. |
[40] | Chen H , Zhang SM , Schwarzschild MA , Hernán MA , Ascherio A ((2005) ) Physical activity and the risk of Parkinson disease. Neurology 64: , 664–669. |
[41] | Chakravarty EF , Hubert HB , Krishnan E , Bruce BB , Lingala VB , Fries JF ((2012) ) Lifestyle risk factors predict disability and death in healthy aging adults. Am J Med 125: , 190–197. |
[42] | Loef M , Walach H ((2012) ) The combined effects of healthy lifestyle behaviors on all cause mortality: A systematic review and meta-analysis. Prev Med 55: , 163–170. |
[43] | Doubeni CA , Jambaulikar GD , Fouayzi H , Robinson SB , Gunter MJ , Field TS , Roblin DW , Fletcher RH ((2012) ) Neighborhood socioeconomic status and use of colonoscopy in an insured population–a retrospective cohort study. PLoS One 7: , e36392. |
[44] | Pruitt SL , Shim MJ , Mullen PD , Vernon SW , Amick BC ((2009) ) Association of area socioeconomic status and breast, cervical, and colorectal cancer screening: A systematic review. Cancer Epidemiol Biomarkers Prev 18: , 2579–2599. |
[45] | Marmot MG , Kogevinas M , Elston MA ((1987) ) Social/economic status and disease. Annu Rev Public Health 8: , 111–135. |
[46] | Lix LM , Hobson DE , Azimaee M , Leslie WD , Burchill C , Hobson S ((2010) ) Socioeconomic variations in the prevalence and incidence of Parkinson’s disease: A population-based analysis. J Epidemiol Community Health 64: , 335–340. |
[47] | Li X , Sundquist J , Sundquist K ((2009) ) Socioeconomic and occupational groups and Parkinson’s disease: A nationwide study based on hospitalizations in Sweden. Int Arch Occup Environ Health 82: , 235–241. |
[48] | Yang F , Johansson ALV , Pedersen NL , Fang F , Gatz M , Wirdefeldt K ((2016) ) Socioeconomic status in relation to Parkinson’s disease risk and mortality: A population-based prospective study. Medicine (Baltimore) 95: , e4337. |
[49] | Warner TT , Schapira AHV ((2003) ) Genetic and environmental factors in the cause of Parkinson’s disease. Ann Neurol 53 Suppl 3: , S16–23. |
[50] | Perrin L , Spinosi J , Chaperon L , Kab S , Moisan F , Ebaz A ((2021) ) Pesticides expenditures by farming type and incidence of Parkinson disease in farmers: A French nationwide study. Environ Res 197: , 111161. |
[51] | Hatcher JM , Pennell KD , Miller GW ((2008) ) Parkinson’s disease and pesticides: A toxicological perspective. Trends Pharmacol Sci 29: , 322–329. |
[52] | Klerk de CM , Gupta S , Dekker E , Essink-Bot ML ((2018) ) Socioeconomic and ethnic inequities within organised colorectal cancer screening programmes worldwide. Gut 67: , 679–687. |
[53] | Kurani SS , McCoy RG , Lampman MA , Doubeni CA , Finney Rutten LJ , Inselman JW , Giblon RE , Bunkers KS , Stroebel RJ , Rushlow D , Chawla SS , Shah ND ((2020) ) Association of neighborhood measures of social determinants of health with breast, cervical, and colorectal cancer screening rates in the US Midwest. JAMA Netw Open 3: , e200618. |
[54] | Jost WH , Schimrigk K ((1991) ) Constipation in Parkinson’s disease. Klin Wochenschr 69: , 906–909. |
[55] | Camacho-Soto A , Gross A , Searles Nielsen S , Dey N , Racette BA ((2018) ) Inflammatory bowel disease and risk of Parkinson’s disease in Medicare beneficiaries. Parkinsonism Relat Disord 50: , 23–28. |
[56] | Crane PK , Gibbons LE , Dams-O’Connor K , Trittschuh E , Leverenz JB , Keene CD , Sonnen J , Montine TJ , Bennett DA , Leurgans S , Schneider JA , Larson EB ((2016) ) Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol 73: , 1062–1069. |
[57] | Eriksson A-K , Löfving S , Callaghan RC , Allebeck P ((2013) ) Alcohol use disorders and risk of Parkinson’s disease: Findings from a Swedish national cohort study 1972-2008. BMC Neurol 13: , 190. |
[58] | Greenbaum L , Rigbi A , Lipshtat N , Cilia R , Tesei S , Asselta R , Djaldetti R , Goldwurm S , Lerer B ((2013) ) Association of nicotine dependence susceptibility gene, CHRNA5, with Parkinson’s disease age at onset: Gene and smoking status interaction. Parkinsonism Relat Disord 19: , 72–76. |
[59] | Hawkes CH ((2008) ) The prodromal phase of sporadic Parkinson’s disease: Does it exist and if so how long is it? Mov Disord 23: , 1799–1807. |
[60] | Hoffmann F , Icks A ((2012) ) Unterschiede in der Versichertenstruktur von Krankenkassen und deren Auswirkungen für die Versorgungsforschung: Ergebnisse des Bertelsmann-Gesundheitsmonitors. Gesundheitswesen 74: , 291–297. |
[61] | Adler NE , Ostrove JM ((1999) ) Socioeconomic status and health: What we know and what we don’t. Ann N Y Acad Sci 896: , 3–15. |
[62] | Singal AG , Gupta S , Lee J , Halm EA , Rutter CM , Corley D , Inadomi J ((2014) ) Importance of determining indication for colonoscopy: Implications for practice and policy original. Clin Gastroenterol Hepatol 12: , 1958–63.e1-3. |




