Rapid Voluntary Blinking as a Clinical Marker of Parkinson’s Disease
Abstract
Reduced spontaneous blinking is a recognized Parkinson’s disease (PD) feature. In contrast, voluntary blinking has been less studied and might serve as a measurable marker of facial bradykinesia. We tested 31 PD patients and 31 controls. Participants were filmed during conversation and a rapid blinking task. Both tasks were videorecorded to count the number of blinks per second. PD patients had lower blink rates. Rapid blinking accurately discriminated between groups with 77% sensitivity and 71% specificity. To conclude, rapid blinking may be a simple and quantifiable task of facial bradykinesia.
Plain Language Summary
Decreased blinking without conscious effort is a well-known characteristic of Parkinson’s disease (PD). However, voluntary blinking, which is blinking on purpose, has not been studied as much and could be a sign of slower facial movements. We studied a group of people with PD and another one without the disease. We recorded videos of them talking and doing a task where they blinked quickly. Then, we counted how many times they blinked per second in each video. We found that people with PD blinked less often. The rapid blinking task accurately distinguished between those with PD and those without it, being correct about 77% of the time for spotting PD and 71% for spotting non-PD. In conclusion, the rapid blinking task could be a simple and measurable way to identify slower facial movements in PD.
INTRODUCTION
Bradykinesia is one of the cardinal features of Parkinson’s disease (PD). It is defined as a reduction in the speed and amplitude of repetitive motor tasks. Previous studies have confirmed a close link between blink rates and PD. PD patients exhibited lower blink rates when compared to controls with this reduction in blink rate also being seen in early untreated PD [1–4]. This reduction in blink rate could be considered to be a surrogate marker of facial bradykinesia and hence hypomimia [1, 5, 6]. Although blinking is an unconscious task under natural circumstances (i.e., spontaneous blinking), it can also be performed consciously. This is the case of voluntary rapid blinking. Similar to the finger tapping task, which is used to assess appendicular bradykinesia in patients with PD, rapid voluntary blinking could be used to test for facial bradykinesia. A study by Agostino et al. showed that when PD patients are asked to blink rapidly, a significantly prolonged pause between the opening and closing phases is observed, suggesting an impairment in switching between voluntary lid movements [7]. Furthermore it is known that motor disturbances in PD are more pronounced during high-speed repetitive movements, [8–10] which could be the case of voluntary rapid blinking.
Blinking can also be influenced by other factors such as age, emotions, degree of concentration and the awareness of being observed, which is known as Hawthorne effect [3, 11–14]. Given that rapid blinking is considered a voluntary task, it could be argued that it might be less affected by other factors, such as degree of concentration, than spontaneous blinking.
This study aims to explore whether rapid blinking could be used as a quantifiable marker of facial hypomimia in comparison with the most commonly used spontaneous blinking.
METHODS
Voluntary rapid blinking was captured by asking participants to look straight ahead and blink as fast as possible for 10 seconds while being recorded. Footage was slowed down using the app Filmora Wondershare (https://filmora.wondershare.com/) to count the number of blinks per task. Spontaneous blinking was measured during informal conversation for one minute. To capture blinking under natural circumstances, conversation was focused on ordinary topics such as future plans and hobbies. Spontaneous blink rate was calculated by counting the number of blinks during one minute of footage. Footage was reviewed twice by a single examiner. If a discrepancy was noted in the number of blinks counted between the two reviews, then footage was reviewed again until these two numbers matched. The extent of eyelid displacement during blinking varies between sex and age, therefore blinks were not strictly defined by a specific percentage eyelid closure [15]. A blink was defined as a brief bilateral closure of the eyes away from the normal resting position with subsequent reopening of eyes.
Participants with PD were consecutively recruited from the Movement Disorder clinic at the Royal London Hospital. All patients fulfilled the Queen Square Brain Bank criteria for PD [16, 17]. Most healthy controls were relatives of PD patients. A small group of controls was recruited from the PREDICT-PD study. Exclusion criteria included any comorbidities that could interfere with the performance of the task, such as blepharospasm and any ophthalmological condition. Controls were excluded if they had any known neurological disorder or ophthalmological condition.
To avoid patients coming to the hospital another day, both tests were performed the same day of recruitment. Thus, it was not possible to adjust the assessment to their dopaminergic medication timings.
Ethical approval was granted by South-west-Central Bristol Research Ethics Committee (18/SW/0255). All participants gave written consent to take part in the study before performing the test. A previous version of the ethics protocol for the East London Parkinson’s project was amended to include video recording as part of the clinical assessment.
Statistical analysis
Data normality was assessed using the Anderson-Darling test. For normalized data, mean and standard deviation (SD) were calculated. Blinking rates were compared using Welch’s t test. Receiver operating characteristic curves (ROC) were plotted to determine optimal sensitivity and specificity values to differentiate between PD patients and controls. Statistical analysis was conducted using GraphPad Prism version 9.3.1 and STATA v.13 (StataCorp, College Station, TX).
RESULTS
Sixty-two participants were tested (31 PD cases and 31 controls). Spontaneous blinking was not available in one control. Both groups were matched in terms of sex (male-female ratio 18 : 13 in both groups) and were comparable in terms of age (PD group: 68 years (SD, 8) vs. controls: 66 years (SD, 10); p = 0.571). The average disease duration since PD diagnosis was 6 years (SD, 5). None of the PD cases had atypical parkinsonian signs on examination. Of note, five patients had axial symptoms (falls, freezing and swallowing difficulties) and seven were diagnosed with mild cognitive impairment.
Patients with PD performed the rapid blinking task more slowly (PD cases: 3.4 blinks/s (SD, 1.5) vs. controls: 4.3 blinks/s (SD, 1.0), p = 0.003) and had lower spontaneous blinking rates (PD cases: 0.42 blinks/s (SD, 0.28) vs. controls: 0.61 blinks/s (SD, 0.30), p = 0.020) than controls (Fig. 1).
Fig. 1
ROC curve of rapid blinking in red (AUC = 0.73, 95% CI 0.60–0.86) and spontaneous blinking in blue (AUC = 0.69, 95% CI 0.56–0.82).
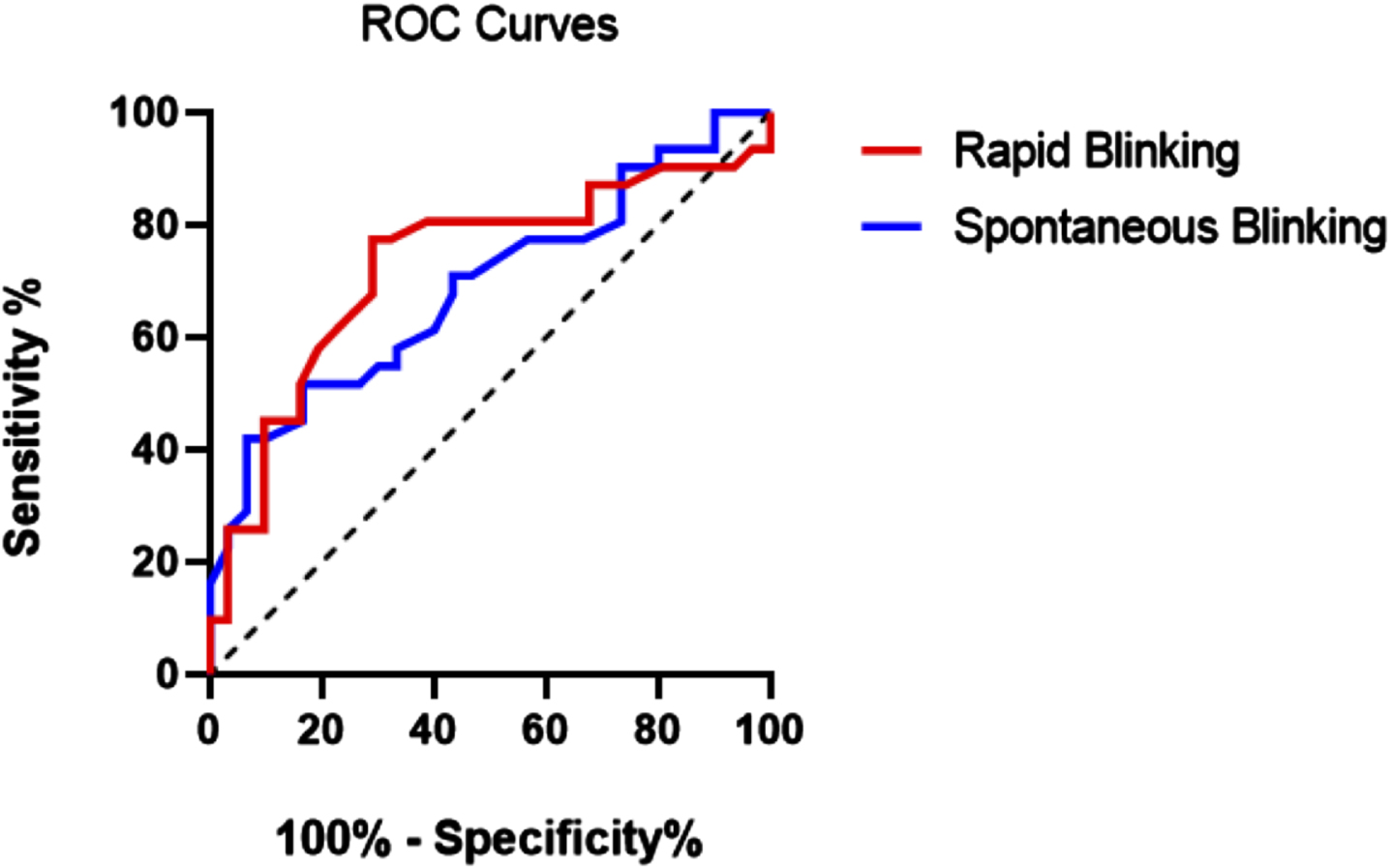
Rapid blinking had a greater ability to discriminate PD patients from controls (AUC, 0.73; 95% CI 0.60–0.86) compared with spontaneous blinking (AUC, 0.69; 95% CI 0.56–0.82). A cut off score of less than 4.15 blinks/s provided the best performance of the rapid blinking test with 77.4% sensitivity for 71% specificity. In contrast, spontaneous blinking had a lower sensitivity (61.3%) and specificity (60%) than rapid blinking. The combination of both tests did not improve discrimination to a great extent (AUC, 0.74; 95% CI 0.61–0.86), with a lower sensitivity (67.7%) for a slightly higher specificity (73.3%) than rapid blinking (Table 1 and Fig. 1).
Table 1
ROC analysis of rapid blinking and spontaneous blinking
| Cut off | Sensitivity | Specificity | |
| (blinks/s) | (%) | (%) | |
| Rapid blinking | < 3.55 | 58.1 | 80.7 |
| (AUC = 0.73, | < 4.15 | 77.4 | 71.0 |
| 95% CI: 0.60–0.86) | < 4.35 | 80.7 | 61.3 |
| Spontaneous blinking | < 0.39 | 54.8 | 70.0 |
| (AUC = 0.69, | < 0.46 | 61.3 | 60.0 |
| 95% CI: 0.56–0.82) | < 0.52 | 71.0 | 56.7 |
Area Under the Curve (AUC), CI (Confidence Intervals), cut off scores of blink rates and corresponding sensitivities and specificities for PD.
DISCUSSION
In this present study, we demonstrated that patients with PD performed the rapid blinking test more slowly and had lower spontaneous blinking rates than controls. These findings are in agreement with a previous study carried out by Alarcón and colleagues who found reduced rapid blinking rates in patients with PD [18]. Similarly, Fitzpatrick and collaborators found that spontaneous blinking was also reduced in PD patients during normal conversation [3]. They suggested an optimal cut off score of 20 blinks per minute during conversation to distinguish PD patients from controls, which yielded 65% sensitivity for 83% specificity. In our study, a slightly higher optimal cut off (27,6 blinks/min, which corresponds to 0.46 blinks/s) had a similar sensitivity (61.3%) but lower specificity (60%).
To our knowledge, this is the first study comparing the ability of voluntary and involuntary blinking in distinguishing PD patients from controls. We found that rapid blinking had a somewhat higher discriminating ability than spontaneous blinking in identifying patients with PD. Rapid blinking is a voluntary movement, therefore it requires cortical preparation prior to movement initiation [19]. The Supplementary Motor Area (SMA) is essential to activate voluntary actions [19]. It receives information from output nuclei of the basal ganglia (Gpi) [19, 20]. In PD, there is excessive inhibition of the SMA, which causes difficulties in initiating voluntary movements such as rapid blinking [20]. Further, rapid blinking involves a repeated planning, initiation, execution and switching between motor tasks (eyelid opening and closing). Repetitive motor tasks are particularly difficult in PD [9, 10, 21]. Apart from having a decrement in speed [8–10, 21], patients with PD have a limited ability to initiate and switch between tasks as part of sequential (repetitive) movement [8–10, 21]. In voluntary rapid blinking this phenomenon is represented as a prolonged pause between eye opening and eye closing [5, 7, 22]. In contrast, spontaneous blinking is an involuntary movement and therefore it does not require cortical preparation by the SMA. It does not involve deliberate planning, initiation, execution and switching between movements as happens with rapid blinking. For all these reasons, voluntary rapid blinking could be considered a potential motor marker in PD. The rapid blinking test presents an opportunity to quantify blinking rate in a standardized manner, which could be validated in patients with PD and subsequently utilized in clinical settings as a supportive diagnostic marker.
Hypomimia has also been found to be one of the first motor signs to emerge in PD [23]. Voluntary rapid blinking could be considered a challenging task that could potentially unmask compensatory mechanisms present in paucisymptomatic patients at early stages of the disease [24]. Several studies have utilized technology-based tools to measure hypomimia in the early stages of PD [25, 26]. Further research is warranted to investigate the role of rapid blinking as an early clinical marker in large at-risk cohorts. In this context, automatic video-based assessment tools may prove valuable for quantifying blinking rate and validating the test on a larger scale.
This present study has some limitations. We could not control antiparkinsonian medication timings given that recruitment and testing of participants was done on the same day. It is well known that levodopa increases spontaneous blinking [22, 27, 28] and improves the speed of voluntary movements such as rapid blinking [7, 9, 29]. Thus, dopaminergic medication might have mitigated differences between groups [22, 27, 28]. The possibility that participants modified their spontaneous blinking in response to their awareness of being observed can also not be excluded [14].
To conclude, rapid blinking test is an easy and inexpensive motor tool to assess facial hypomimia in PD. It could be used in conjunction with other biomarkers to help identify patients with PD.
ACKNOWLEDGMENTS
I want to thank Mariella Francis (University of Oxford) for helping with data analysis and Andy Ely for reviewing earlier drafts of the manuscripts.
FUNDING
Michaela Francis was funded by the Queen Mary University of London. No other specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
CONFLICT OF INTEREST
Alastair Noyce is an Editorial Board Member of this journal but was not involved in the peer-review process of this article nor had access to any information regarding its peer-review.
DATA AVAILABILITY
The data supporting the findings of this study are available within the article and/or its supplementary material.
REFERENCES
[1] | Karson CN , LeWitt PA , Calne DB , Wyatt RJ ((1982) ) Blink rates in parkinsonism. Ann Neurol 12: , 580–583. |
[2] | Biousse V , Skibell BC , Watts RL , Loupe DN , Drews-Botsch C , Newman NJ ((2004) ) Ophthalmologic features of Parkinson’s disease. Neurology 62: , 177–180. |
[3] | Fitzpatrick E , Hohl N , Silburn P , O’Gorman C , Broadley SA ((2012) ) Case-control study of blink rate in Parkinson’s disease under different conditions. J Neurol 259: , 739–744. |
[4] | Deuschl G , Goddemeier C ((1998) ) Spontaneous and reflex activity of facial muscles in dystonia, Parkinson’s disease, and in normal subjects. J Neurol Neurosurg Psychiatry 64: , 320–324. |
[5] | Bologna M , Fabbrini G , Marsili L , Defazio G , Thompson PD , Berardelli A ((2013) ) Facial bradykinesia. J Neurol Neurosurg Psychiatry 84: , 681–685. |
[6] | Jankovic J ((2008) ) Parkinson’s disease: Clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79: , 368–376. |
[7] | Agostino R , Bologna M , Dinapoli L , Gregori B , Fabbrini G , Accornero N , Berardelli A ((2008) ) Voluntary, spontaneous, and reflex blinking in Parkinson’s disease. Mov Disord 23: , 669–675. |
[8] | Benecke R , Rothwell JC , Dick JP , Day BL , Marsden CD ((1987) ) Disturbance of sequential movements in patients with Parkinson’s disease. Brain 110: (Pt 2), 361–379. |
[9] | Benecke R , Rothwell JC , Dick JP , Day BL , Marsden CD ((1987) ) Simple and complex movements off and on treatment in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 50: , 296–303. |
[10] | Pastor MA , Jahanshahi M , Artieda J , Obeso JA ((1992) ) Performance of repetitive wrist movements in Parkinson’s disease. Brain 115: (Pt 3), 875–891. |
[11] | Doughty MJ ((2001) ) Consideration of three types of spontaneous eyeblink activity in normal humans: During reading and video display terminal use, in primary gaze, and while in conversation. Optom Vis Sci 78: , 712–725. |
[12] | Bentivoglio AR , Bressman SB , Cassetta E , Carretta D , Tonali P , Albanese A ((1997) ) Analysis of blink rate patterns in normal subjects. Mov Disord 12: , 1028–1034. |
[13] | Maffei A , Angrilli A ((2019) ) Spontaneous blink rate as an index of attention and emotion during film clips viewing. Physiol Behav 204: , 256–263. |
[14] | Shaafi Kabiri N , Brooks C , Comery T , Kelley ME , Fried P , Bhangu J , Thomas K ((2020) ) The Hawthorne Effect in eye-blinking: Awareness that one’s blinks are being counted alters blink behavior. Curr Eye Res 45: , 1380–1384. |
[15] | Sforza C , Rango M , Galante D , Bresolin N , Ferrario VF ((2008) ) Spontaneous blinking in healthy persons: An optoelectronic study of eyelid motion. Ophthalmic Physiol Opt 28: , 345–353. |
[16] | Hughes AJ , Daniel SE , Kilford L , Lees AJ ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: , 181–184. |
[17] | Clarke CE , Patel S , Ives N , Rick CE , Woolley R , Wheatley K , Walker MF , Zhu S , Kandiyali R , Yao G , Sackley CM , on behalf of the PD REHAB Collaborative Group ((2016) ) UK Parkinson’s Disease Society Brain Bank Diagnostic Criteria. Health Technology Assessment, No. 20.63. NIHR Journals Library, Southampton (UK). |
[18] | Alarcón F , Maldonado JC , Cañizares M , Molina J , Noyce AJ , Lees AJ ((2020) ) Motor dysfunction as a prodrome of Parkinson’s disease. J Parkinsons Dis 10: , 1067–1073. |
[19] | Kaneko K , Mito K , Makabe H , Takanokura M , Sakamoto K ((2004) ) Cortical potentials associated with voluntary, reflex, and spontaneous blinks as bilateral simultaneous eyelid movement. Electromyogr Clin Neurophysiol 44: , 455–462. |
[20] | Grafton ST ((2004) ) Contributions of functional imaging to understanding parkinsonian symptoms. Curr Opin Neurobiol 14: , 715–719. |
[21] | Agostino R , Berardelli A , Formica A , Accornero N , Manfredi M ((1992) ) Sequential arm movements in patients with Parkinson’s disease, Huntington’s disease and dystonia. Brain 115: (Pt 5), 1481–1495. |
[22] | Bologna M , Fasano A , Modugno N , Fabbrini G , Berardelli A ((2012) ) Effects of subthalamic nucleus deep brain stimulation and L-DOPA on blinking in Parkinson’s disease. Exp Neurol 235: , 265–272. |
[23] | Postuma RB , Lang AE , Gagnon JF , Pelletier A , Montplaisir JY ((2012) ) How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain 135: , 1860–1870. |
[24] | Maetzler W , Hausdorff JM ((2012) ) Motor signs in the prodromal phase of Parkinson’s disease. Mov Disord 27: , 627–633. |
[25] | Lim WS , Chiu SI , Wu MC , Tsai SF , Wang PH , Lin KP , Chen YM , Peng PL , Chen YY , Jang JR , Lin CH ((2022) ) An integrated biometric voice and facial features for early detection of Parkinson’s disease. NPJ Parkinsons Dis 8: , 145. |
[26] | Novotny M , Tykalova T , Ruzickova H , Ruzicka E , Dusek P , Rusz J ((2022) ) Automated video-based assessment of facial bradykinesia in de-novo Parkinson’s disease. NPJ Digit Med 5: , 98. |
[27] | Iwaki H , Sogo H , Morita H , Nishikawa N , Ando R , Miyaue N , Tada S , Yabe H , Nagai M , Nomoto M ((2019) ) Using spontaneous eye-blink rates to predict the motor status of patients with Parkinson’s disease. Intern Med 58: , 1417–1421. |
[28] | Karson CN ((1983) ) Spontaneous eye-blink rates and dopaminergic systems. Brain 106: (Pt 3), 643–653. |
[29] | Magrinelli F , Picelli A , Tocco P , Federico A , Roncari L , Smania N , Zanette G , Tamburin S ((2016) ) Pathophysiology of motor dysfunction in Parkinson’s disease as the rationale for drug treatment and rehabilitation. Parkinsons Dis 2016: , 9832839. |




