Effects of Continuous Dopaminergic Stimulation on Parkinson’s Disease Gait: A Longitudinal Prospective Study with Levodopa Intestinal Gel Infusion
Abstract
Background:
Gait issues, including reduced speed, stride length and freezing of gait (FoG), are disabling in advanced phases of Parkinson’s disease (PD), and their treatment is challenging. Levodopa/carbidopa intestinal gel (LCIG) can improve these symptoms in PD patients with suboptimal control of motor fluctuations, but it is unclear if continuous dopaminergic stimulation can further improve gait issues, independently from reducing Off-time.
Objective:
To analyze before (T0) and after 3 (T1) and 6 (T2) months of LCIG initiation: a) the objective improvement of gait and balance; b) the improvement of FoG severity; c) the improvement of motor complications and their correlation with changes in gait parameters and FoG severity.
Methods:
This prospective, longitudinal 6-months study analyzed quantitative gait parameters using wearable inertial sensors, FoG with the New Freezing of Gait Questionnaire (NFoG-Q), and motor complications, as per the MDS-UPDRS part IV scores.
Results:
Gait speed and stride length increased and duration of Timed up and Go and of sit-to-stand transition was significantly reduced comparing T0 with T2, but not between T0-T1. NFoG-Q score decreased significantly from 19.3±4.6 (T0) to 11.8±7.9 (T1) and 8.4±7.6 (T2) (T1-T0 p = 0.018; T2-T0 p < 0.001). Improvement of MDS-UPDRS-IV (T0-T2, p = 0.002, T0-T1 p = 0.024) was not correlated with improvement of gait parameters and NFoG-Q from T0 to T2. LEDD did not change significantly after LCIG initiation.
Conclusion:
Continuous dopaminergic stimulation provided by LCIG infusion progressively ameliorates gait and alleviates FoG in PD patients over time, independently from improvement of motor fluctuations and without increase of daily dosage of dopaminergic therapy.
INTRODUCTION
Gait impairment is a common feature of Parkinson’s disease (PD), with a progressive worsening during the disease course [1]. Specifically, in the advanced PD phases we observe a progression in severity of gait impairment, such as low gait speed, reduced step length, and impaired rhythmicity, as well as the onset of episodic gait impairment, such as freezing of gait (FoG) [2, 3]. Gait parameters like gait speed and stride length present a good response to dopaminergic therapy for a long time in the disease course, but this response is gradually reduced over time. FoG can have a more unpredictable response to dopaminergic therapy, as it may occur even in the presence of a good control of cardinal motor features of PD [4–6]. Gait impairment can be highly disabling, and it is one of the main risk of falls in advanced PD patients. In fact, a higher risk of falls was observed in PD patients with slower walking speed, lower cadence, shorter strides, and the strict association of falls with episodic gait impairments like FoG is well known [7, 8].
Literature evidence indicates that continuous infusion of Levodopa/carbidopa intestinal gel (LCIG) delivered via a percutaneous endoscopic gastrostomy with a jejunal extension (PEG-J) may improve gait and FoG in PD patients with suboptimal control of motor fluctuations, also when FoG episodes seem refractory to oral dopaminergic therapy [9–11]. LCIG benefit on PD motor symptoms is mainly explained by a more stable plasma concentration of levodopa [12], as chronic disease and protracted oral levodopa administration cause loss of striatal dopamine nerve terminals, short levodopa half-life, delayed gastric emptying and abnormal intestinal drug absorption [13, 14]. It has also been postulated that more stable plasma concentration of levodopa lead to a more physiological CNS delivery of dopamine, with the possibility of reducing not only symptom fluctuations but also the degree of axial symptoms that become progressively ‘resistant’ to pulsatile dopaminergic administration [15, 16]. However, this latter hypothesis has not been proven so far. To date, it is unclear whether the effect of a continuous dopaminergic stimulation can improve gait impairment independently from the reduction of motor fluctuations.
In this context, we performed a 6-month prospective, observational study on the effect of LCIG on gait impairment by analyzing (a) quantitative gait and balance parameters measured by wearable inertial sensors, (b) FoG severity evaluated by the NFoG questionnaire, (c) motor complications and their correlation with changes in gait parameters and FoG severity, before LCIG initiation (T0) and after 3 (T1) and 6 months (T2) of continuous infusion.
METHODS
Study population
All consenting PD patients with disabling motor fluctuations or dyskinesia who were eligible for treatment with LCIG, presenting with a PD phenotype characterized by prominent gait impairment were consecutively recruited from the Movement Disorder Unit of the University of Turin (Italy), between October 2020 and October 2022.
Patients were screened according to the following inclusion criteria: suffering from idiopathic PD fulfilling Movement Disorder Society criteria [17]; a Hoehn and Yahr (H&Y) score≤3 in ON therapeutic condition [18]; motor fluctuations or dyskinesia despite best medical treatment; presenting a Postural Instability Gait disorder phenotype (PIGD) as per validated formula [19]; having a recent history of FoG, according to a score of 1 on Question 1 of the New Freezing of Gait Questionnaire (NFoG-Q) [20]. Exclusion criteria were a diagnosis of dementia supported by a Montreal Cognitive Assessment (MoCA) score < 21 [21]; inability to walk independently for 10 meters in therapeutic ON condition; gastric abnormalities incompatible with PEG-J placement; current or past treatment with other advanced PD therapies, including deep brain stimulation or subcutaneous apomorphine.
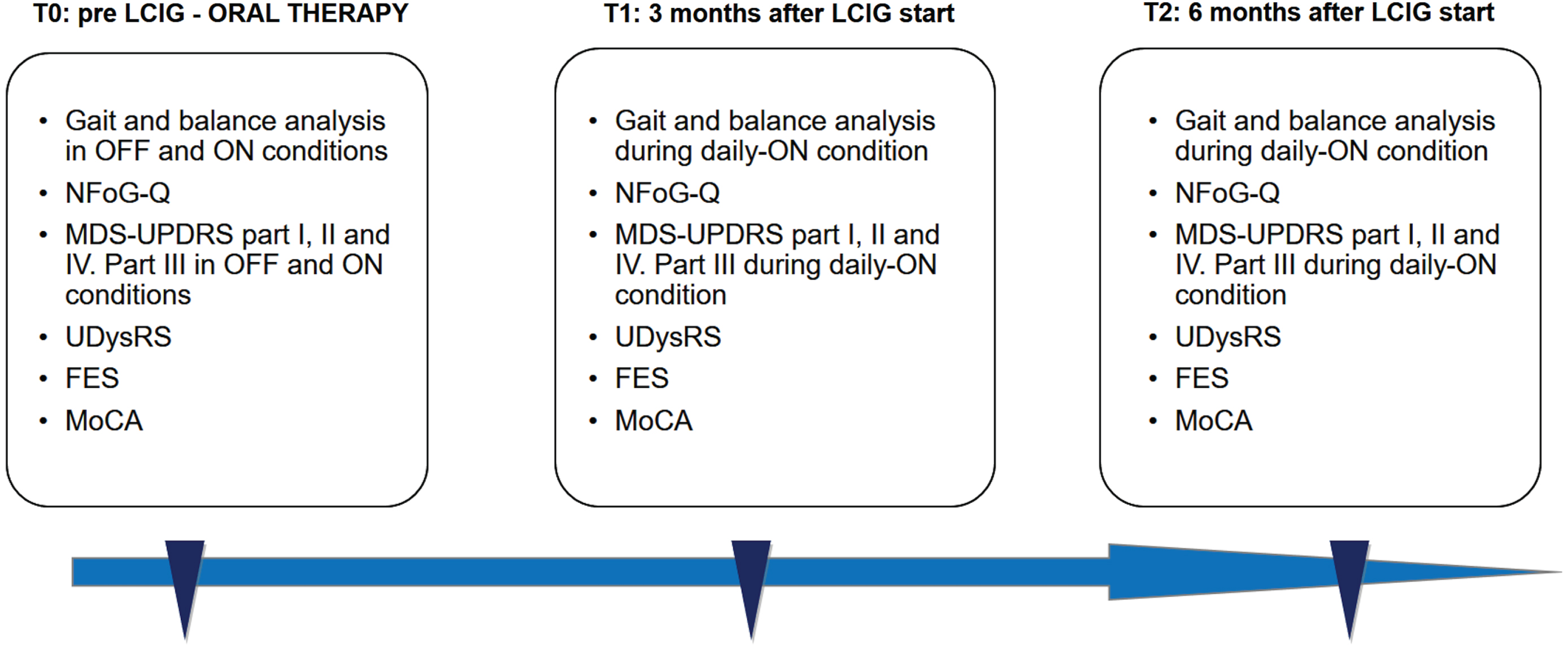
All data were collected at baseline (T0), before PEG-J implant, and 3 (T1) and 6 (T2) months after initiation of LCIG treatment. At baseline, patients were evaluated both in the practically defined OFF (following an overnight withdrawal of antiparkinsonian medications, at least 12 hours) and ON condition (45 minutes after the administration of 1.5 X the usual levodopa morning dose). At T1 and T2, patients were evaluated in daily-ON condition, during regular LCIG infusion, like previously reported [9] (Fig. 1).
Fig. 1
Flow chart of study, with the assessment conducted at each visit. NFoG-Q, New Freezing of Gait Questionnaire; MoCA, Montreal Cognitive Assessment; FES, Falls Efficacy Scale; MDS-UPDRS, Movement Disorder Society –Unified Parkinson’s Disease Rating Scale; UDysRS, Unified Dyskinesia Rating Scale; LCIG, Levodopa-Carbidopa Intestinal Gel.
Endpoints
Our primary endpoint was to evaluate the objective improvement of gait parameters 3 and 6 months after LCIG initiation using a quantitative, instrumental evaluation of gait by wearable inertial sensors. Secondary endpoints were the evaluation of FoG and motor fluctuations severity improvement 3 and 6 months after LCIG initiation using the NFoG-Q and MDS-UPDRS-IV scores, as well as the correlation between MDS-UPDRS-IV changes and the changes of gait parameters and NFoG-Q. The reduction of falls and changes in motor symptoms as per MDS-UPDRS-III and dyskinesia as per the Unified Dyskinesia Rating Scale (UDysRS), were also analyzed.
Procedures
Digital gait and balance analysis
All patients underwent instrumental evaluation of gait and balance parameters using wearable inertial sensors (Opal, APDM’s Mobility Lab system) placed at multiple points in the upper and lower body (two sensors attached on the feet, two at outer surface of the thighs, one at right hip joint, one on the sternum, and one at lumbar level) [22]. The Opal sensor includes a tri-axial accelerometer, gyroscope, and magnetometer with a sampling rate of 128 Hz. A range of spatio-temporal gait characteristics are available as automatic output from sensors and processed using manufacturer provided software with validated algorithm [23]. Only data directly available from the Mobility Lab software were considered as study outcomes. The tasks consisted of a battery of standardized motion tests, conducted in the usual outpatient examination room:
• 2 minute walking test (2MWT): this test was conducted in adequate space to allow a 2 minute walk, back and forth in a straight line and performing tight turnings, at a comfortable pace and with a distance of 10 meters per lap. This test measures full body gait, asymmetry, variability and turning. We considered the following outputs: the gait speed (m/s), the double support (% gait cycle time GCT, the percentage of the gait cycle in which both feet are on the ground), the Stride Length (m), and the Step Duration (s).
• Timed Up and Go (TUG) test, with subjects asked to stand up from a chair, walk 3 m straight, turn around, walk back, and sit down, at a comfortable pace. This is a test for measure of postural transitions. We considered the following outputs: the TUG duration (s), the turn duration (s), the turn velocity (degree/s), the Sit to stand duration (s), the Stand to sit duration (s).
• 360° Turn Test, with patients required to start from standing position, make a turn 360° clockwise, and as soon as they return to the initial position, 360° counterclockwise. This is a measure of dynamic balance. The parameters considered were the Turn duration (s) and the Turn velocity (degree/s).
• Sway test, with patients asked to remain in balance during an upright standing position for 30 s, with arms at rest and eyes closed. This is a test for evaluation of quiet stance balance. The parameters considered were the sway area (m2/s4, the area of an ellipse covering 95% of the sway angle in both the coronal and sagittal planes), and the Root mean square (RMS) area (m/s2, RMS of the sway angle in both the coronal and sagittal planes).
Gait and balance evaluations were performed in two conditions, the practically defined OFF condition (following an overnight withdrawal of antiparkinsonian medications) and the ON condition (after the administration of 1.5 X the usual levodopa morning dose); at T1 and T2 follow-up the assessment was carried out in daily-ON condition (during LCIG infusion), as previously reported [9].
FoG characterization and other clinical evaluations
The FoG experience in daily life of the patients was quantified using the New Freezing of Gait Questionnaire (NFoG-Q), a self-reported questionnaire assessing the clinical aspects of freezing (frequency and duration) and its impact on quality of life in the previous month [20].
Patients were also evaluated for PD symptoms and their fluctuations by a movement disorder expert as per the MDS-UPDRS Parts I–II–IV, further characterization of dyskinesia as per the Unified Dyskinesia Rating Scale (UDysRS), PD stage as per the Hoehn and Yahr (H& Y) score [18], cognitive abilities as per the the MoCA (Montreal Cognitive Assessment) [21], levodopa equivalent daily dose (LEDD) calculated according to a validated conversion table [24], number of falls in the past month [25] and fear of falls as per Falls Efficacy Scale [26]. These evaluations were conducted at each visit.
Clinical motor evaluation based on MDS-UPDRS part III at baseline was carried out at baseline (in OFF and ON condition), and at T1 and T2 (in daily ON condition).
FoG was further categorized in four different subtypes (OFF-type, Pseudo-ON, Unresponsive, and True-ON) based on the score of MDS-UPDRS item-3.11 in medication ON and OFF conditions at baseline evaluation, like previously reported [9].
Supplementary analysis: long-term follow up
Patients available at long-term time-point (more than 12 months since LCIG start) were called back for a last evaluation, conducted in the morning, replacing the morning dose of LCIG therapy with oral levodopa (1.5 X the usual levodopa morning dose), to perform the same digital gait and balance analysis. The NFoG-Q and number of falls in the past month were also collected. This last analysis was performed to verify the possible benefit of a long-term continuous dopaminergic therapy on gait due to possible motor network reorganization.
Statistical analysis
Descriptive statistics (mean±SD) was used for continuous variables while frequency for categorical data. The nonparametric Friedman test was used to compare data collected from three time points, followed by post-hoc pairwise comparisons with Bonferroni correction for multiple comparisons. Significant differences among the conditions were determined based on adjusted significance levels. The same analysis was conducted also for comparison with the evaluation conducted at long-term follow-up (T3). The Wilcoxon rank sum test was applied for comparisons of continuous variables whenever evaluated in two time points only.
To explore the potential correlation between the change in motor complications and the changes in NFoG-Q and in gait and balance parameters, we calculated the percentage improvement between baseline (T0) and last follow-up (T2) assessments. As an example, the improvement in NFoG-Q was determined using the formula: ΔNFoG-Q = (NFOG-Q at T2 –NFoG-Q at T0)/NFoG-Q at T0. For gait and balance measures, we considered in the analysis only outcome measures that significantly improved from T0 to T2. These correlations were analyzed using the Spearman’s rank correlation test.
All employed tests were two-tailed, and a p-value<0.05 was considered as statistically significant.
Data were analyzed using the Statistical Package for the Social Sciences (SPSS 28). The study conforms to World Medical Association Declaration of Helsinki principles and was approved by the local institutional review board “A.O.U. Città della Salute e della Scienza di Torino –A.O. Ordine Mauriziano –A.S.L. Città di Torino” (protocol number 0106090), and all patients gave their written informed consent to participate in the study.
RESULTS
Twelve PD patients were included in the study, and all of them completed the three study phases (T0, T1 and T2). Mean age, disease duration, and H& Y stage in ON condition were 66.9±9 years, 11.1±2.1 years, and 2.4±0.9, respectively. Demographic, clinical, and therapeutic data of the cohort are detailed in Table 1.
Table 1
Patients’ baseline demographic and clinical features
| Sex (males/females) | 7/5 (58.3% /42.7%) |
| Age at onset of disease (y) | 55.5±7.1 (39–63) |
| Age at LCIG start (y) | 66.9±9 (45–80) |
| Disease duration at LCIG start (y) | 11.1±2.1 (6–16) |
| Motor fluctuations duration (y) | 4.1±2.2 (2–10) |
| Therapy at baseline | |
| Levodopa | 12 (100% ) |
| Dopamine agonists | 5 (41.7% ) |
| MAO-B inhibitors | 4 (33.3% ) |
| COMT inhibitors | 3 (25% ) |
| COMT inhibitors + MAO-B inhibitors | 1 (8.33% ) |
| Antipsychotics | 1 (8.33% ) |
Results are reported as average±standard deviation (range) or absolute values (percentage), as appropriate. LCIG, Levodopa-Carbidopa Intestinal Gel; COMT, Catechol-O-methyltransferase; MAO-B, Monoamine oxidase-B.
All patients presented a TD/PIGD score≤0.9 and experienced FoG at baseline. 9 patients presented at baseline evaluation OFF-type FoG and 3 patients a pseudo-ON FoG. Continuous LCIG infusion was maintained for an average of 12.7±2.2 h in daytime; LEDD remained stable after LCIG initiation, with a slight, not significant increase from 1275.4±374.1 mg at baseline to 1284.1±430.5 mg at T1, and 1317±426.6 mg at T2 (p = 0.779).
At baseline, 7 patients were treated also with dopamine agonists (DAs) and/or monoamine oxidase-B inhibitors (iMAO-B) and/or cathecol-o-methyl-transferase inhibitors (iCOMT) (Table 1). After LCIG initiation, 4 patients continued on DAs, and 1 on MAO-B; moreover, 2 patients started amantadine for better control of dyskinesia at dosage of 100 mg once daily between T1 and T2. No patients presented a FoG unresponsive to the levodopa challenge test conducted at T0.
Improvement of gait parameters
During the 2MWT, we observed a significant improvement in gait speed (p = 0.028), ranging from 0.67±0.27 m/s at T0 to 0.72±0.15 m/s at T1 and 0.77±0.2 m/s at T2 (post-hoc analysis significant for T2 vs. T0; p = 0.024), and stride length (p = 0.013), ranging from 0.81±0.3 m at T0 to 0.85±0.2 m at T1 and 0.91±0.2 m at T2 (post-hoc analysis significant for T2 vs. T0; p = 0.013). Step duration and double support did not change significantly during the 2MWT (Table 2; Fig. 2).
Table 2
Patients’ clinical and kinematic prospective assessment
| T0 –Baseline | T1 –3 months | T2 –6 months | p | |
| MDS-UPDRS I | 13.5±3.8 | 14.3±4.2 | 13.5±2.6 | 0.662 |
| MDS-UPDRS II | 19.1±6.4 | 17.5±5.6 | 16.1±4.5 | 0.457 |
| MDS-UPDRS III | 49.2±16.4 (OFF) | |||
| 19.4±8.8 (ON) | 22.5±12 | 23.2±7.4 | 0.273 | |
| Hoehn and Yahr stage | 2.9±1.0 (OFF) | |||
| 2.4±0.9 (ON) | 2.2±0.4 | 2.4±0.5 | 0.368 | |
| MDS-UPDRS IV | 12.6±2.9*,∧ | 7.8±2.2∧ | 7.2±2.1* | < 0.001 |
| MDS-UPDRS IV dyskinesia (items 4.1–4.2) | 3.5±1.9 | 2.2±2.2 | 3.0±2.3 | 0.176 |
| MDS-UPDRS IV motor fluctuations (items 4.3 –4.4 –4.5) | 7.3±1.9*,∧ | 4.8±1.2∧ | 3.8±2.0* | 0.002 |
| UDysRS –Total score | 33.6±14.3*,∧ | 19.2±11.7∧ | 18.6±13.5* | 0.003 |
| LEDD (mg) | 1275.4±374.1 | 1284.1±430.5 | 1317±426.6 | 0.779 |
| LCIG total daily dosage (mg) | 49.3±15.4 | 50.6±16.9 | 0.109 | |
| – | – | – | ||
| Hours per day | (12.7±2.2) | (12.7±2.2) | ||
| N-FoG-Q –Total score | 19.3±4.6*,∧ | 11.8±7.9∧ | 8.4±7.6* | < 0.001 |
| FES | 28.8±12.2 | 28.3±12.2 | 26.1±7.8 | 0.517 |
| Number of falls (last month) | 0.9±1.0 | 0.25±0.87 | 0.17±0.39 | 0.009 |
| MoCA | 24.1±3.7 | 25±3.4 | 23.9±3.6 | 0.856 |
| 2MWT –Gait speed (m/s) Normative 1.04 —1.64 m/s | 0.67±0.27* | 0.72±0.15 | 0.77±0.2* | 0.028 |
| 2MWT –Step duration (s)Normative 0.450 —0.580 s | 0.63±0.09 | 0.61±0.1 | 0.60±0.08 | 0.096 |
| 2MWT –Stride Length (m) Normative 1.11 —1.66 m | 0.81±0.24* | 0.85±0.15 | 0.91±0.2* | 0.013 |
| 2MWT –Double support (% GCT) Normative 12.4 —24.6 % | 27.2±7.3 | 24.7±4.8 | 25.3±6.2 | 0.779 |
| TUG –Duration (s) Normative 6.28 —11.6 s | 20.1±8.4* | 16.9±3.1 | 16.9±6.1* | 0.039 |
| TUG –Turn velocity (degree/s) Normative 158 —322 degree/s | 138.5±41.6 | 143.7±26.8 | 147.1±38.9 | 0.517 |
| TUG –Turn duration (s) Normative 1.42 —2.53 s | 2.73±0.9 | 2.61±0.50 | 2.49±0.59 | 0.587 |
| TUG –Sit To Stand duration (s) Normative 0.69 —1.27 s | 1.17±0.17* | 1.09±0.25 | 0.96±0.25* | 0.017 |
| 360° –Turn duration (s) Normative 1.86 —3.80 s | 4.69±1.54 | 4.7±1.07 | 4.37±1.13 | 0.273 |
| 360° –Turn velocity (degree/s) Normative 146 —359 degree/s | 128.3±65.1 | 130.1±44.1 | 136.7±23.9 | 0.436 |
| Sway –Area (m2/s4) Normative 0.248 —1.59 m2/s4 | 8.86±6.66 | 8.33±6.66 | 8.21±5.68 | 0.517 |
| RMS Sway (m/s2) Normative 0.231 —0.661 m/s2 | 1.23±0.59 | 1.17±0.39 | 1.18±0.36 | 0.754 |
Results are reported as mean±standard deviation. Normative values reported on Mobility lab software are reported for each gait or balance evaluation conducted. Statistical difference at post-hoc analysis between two evaluations is reported with *(if between assessment at T0 and T2) or ∧(if between assessment at T0 and T1). MDS-UPDRS, Movement Disorder Society –Unified Parkinson’s Disease Rating Scale; UDysRS, Unified Dyskinesia Rating Scale; LEDD, Levodopa Equivalent Daily Dose; N-FoG-Q, New Freezing of Gait Questionnaire; MoCA, Montreal Cognitive Assessment; FES, Falls Efficacy Scale; LCIG, Levodopa-Carbidopa Intestinal Gel; 2MWT, 2 Minute Walking Test; TUG, Timed Up and GO; GCT, Gait Cycle Time; RMS, Root Mean Square.
Fig. 2
Kinematic parameters significantly improved after LCIG initiation. Data are reported as mean values of all 12 patients, and the bars represent the standard deviation. Only data with statistical significance at the Friedman test are graphically reported. Individual scores are also reported for each assessment. Baseline assessment (T0) was conducted in the ON condition (45 minutes after the administration of 1.5 X the usual levodopa morning dose). At T1 and T2, the patients were evaluated in daily-ON condition, during regular LCIG infusion. Statistical difference at post-hoc analysis between two assessments is reported with *.
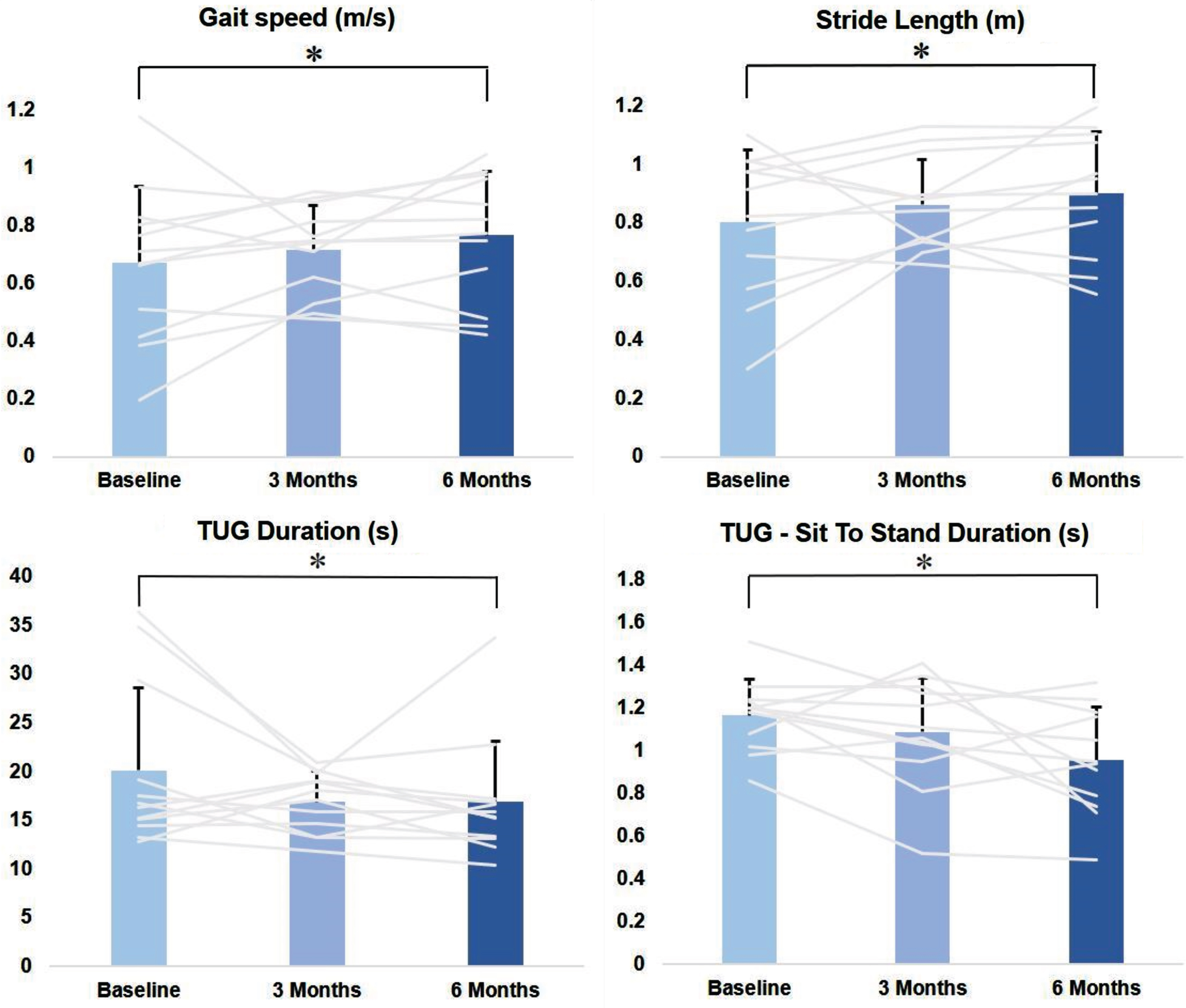
During the TUG test, we observed a significant decrease in the total test duration (p = 0.039), ranging from 20.1±8.4 s at T0 to 16.9±3.1 s at T1 and 16.9±6.1 s at T2 (post-hoc analysis significant for T2 vs. T0; p = 0.043) (Fig. 3), and in the Sit to Stand duration (p = 0.017), ranging from 1.17±0.17 s at T0 to 1.09±0.25 s at T1, to 0.96±0.25 s at T2 (post-hoc analysis significant for T2 vs. T0; p = 0.013). Turn velocity and turn duration did not change significantly (Table 2; Fig. 2).
Fig. 3
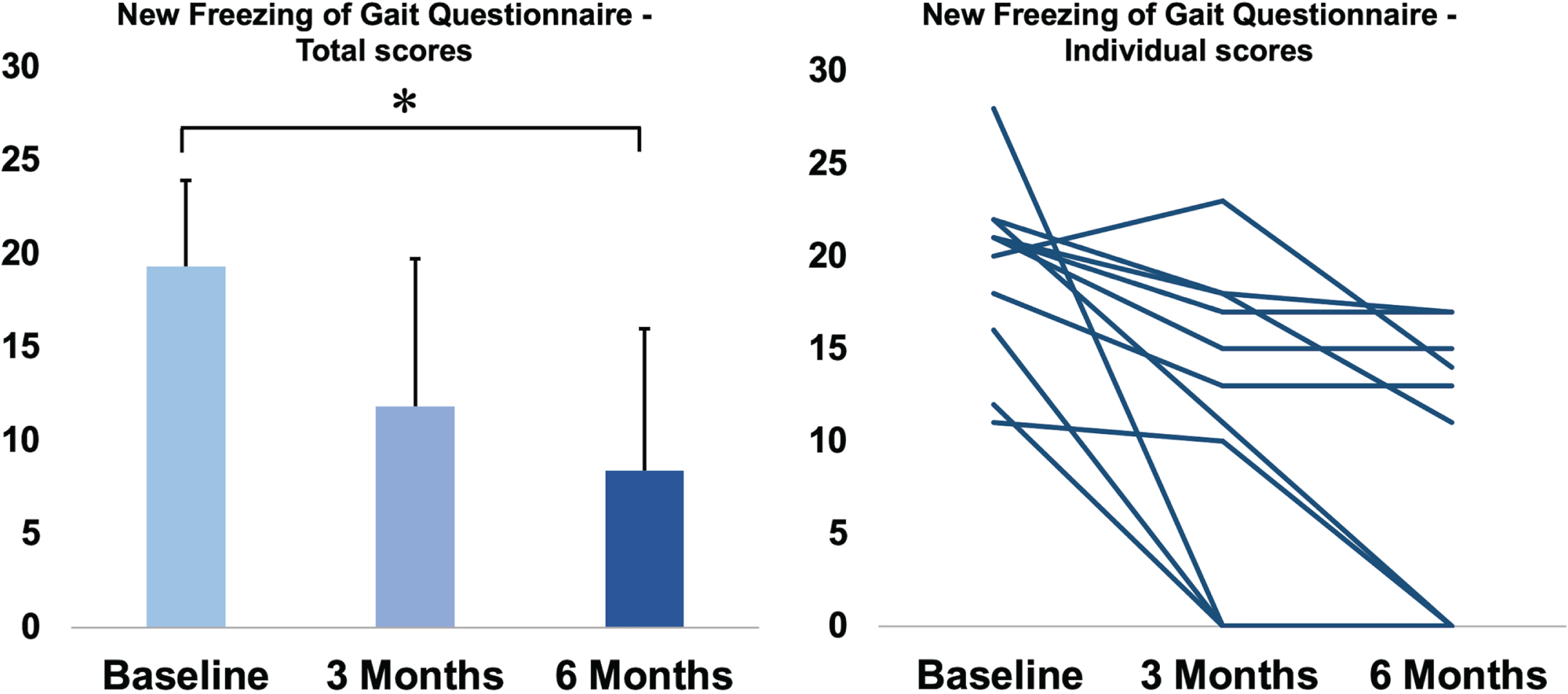
New Freezing of Gait Questionnaire total score in the three assessments. On the left side, data of NFoG questionnaire total score are reported as mean values of all 12 patients, and the bars represent the standard deviation. Individual scores are reported on the right side of the figure. Statistical difference at post-hoc analysis between two assessments is reported with *.
The turn duration (p = 0.273) and the turn velocity (p = 0.436) did not change significantly during the 360° turn test (Table 2; Fig. 3), as well as the sway area (p = 0.517) and the RMS sway (p = 0.754) obtained during the sway test (Table 2).
Improvement of FoG
The severity of FoG showed a significant improvement after LCIG start (p < 0.001), with NFoG-Q score ranging from 19.3±4.6 at T0 to 11.8±7.9 at T1 and 8.4±7.6 at T2 (post-hoc analysis significant for T1 vs. T0 -p = 0.018-, and T2 vs. T0 -p < 0.001-) (Fig. 3).
Considering the possible confounding factor given from Amantadine start between T1 and T2 and its effect on FoG [27], we repeated our analysis excluding the two patients who started this therapy, with similar results. NFoG-Q score ranged from 18.2±3.9 at T0 to 12.4±7.5 at T1 and 8.4±7.4 at T2 (post-hoc analysis significant for T2 vs. T0; p = 0.001).
Motor symptoms, falls, and motor complications
PD motor symptoms did not significantly differ during the study, with MDS-UPDRS part III total score ranging from 19.4±8.8 at T0, to 22.5±12 at T1 and 23.2±7.4 at T2 (p = 0.273). The number of falls of the previous month gradually decreased during follow-up, ranging from 0.9±1.0 at T0, to 0.25±0.87 at T1 and 0.17±0.39 at T2 (p = 0.009) (Table 2).
Motor complications significantly improved after LCIG initiation (p < 0.001), with MDS-UPDRS IV scores ranging from 12.6±2.9 at T0, to 7.8±2.2 at T1, and 7.2±2.1 at T2 (post-hoc analysis significant for T2 vs. T0 -p<0.001-, and T1 vs. T0 -p = 0.007-), with a significant reduction also for the subsection of motor fluctuations (sum of score 3-4-5 of MDS-UPDRS IV; 7.3±1.9 at T0, 4.8±1.2 at T1, 3.8±2 at T2, p = 0.002; T1 vs. T0 p = 0.043; T2 vs. T0 p = 0.001) but not of dyskinesia (sum of score 1–2 of MDS-UPDRS, p = 0.176) (Table 2). However, the total score of the UDysRS improved significantly after LCIG (33.6±14.3 at T0, 19.2±11.7 at T1, 18.6±13.5 at T2; p = 0.003; T1 vs. T0 p = 0.018; T2 vs. T0 p = 0.007).
Correlations between gait and motor complication changes
No significant correlations were found between the improvement of MDS-UPDRS IV and the improvement of gait parameters ( Supplementary Table 1). Specifically, we observed no correlation between T2 vs. T0 changes of MDS-UPDRS IV score and changes of gait speed (rho =–0.214, p = 0.505), stride length (rho=–0.266, p = 0.403), TUG duration (rho =–0.305, p = 0.336), TUG Sit to Stand duration (rho = 0.137, p =–0.455), and change of 360° Turn duration (rho =–0.214, p = 0.505).
Also, we found no correlation between T2 vs. T0 improvement of MDS-UPDRS IV and NFoG-Q scores (rho = 0.154, p = 0.560).
Finally, we found no significant correlation between the T2 vs. T0 changes of the motor fluctuations subsection of the MDS-UPDRS IV and of the gait parameters and NFoG-Q scores ( Supplementary Table 1).
Supplementary analysis: long-term follow up
Nine of twelve patients were evaluated at the long-term follow-up analysis (T3), with an average duration of LCIG therapy of 26.3±7.6 months (range 14–38 months). Of the three drop-outs, one patient discontinued LCIG after 24 months due to cognitive decline and difficulty with pump maintenance, one patient was institutionalized 36 months after LCIG start, and one patient refused to conduct another evaluation while turning off the pump.
The NFoG-Q score (total score = 13.1±10.5) was still better compared to baseline (p = 0.002) (T3 vs. T0 p = 0.045). Falls were lower than baseline (only one patients presented a fall in the previous month at T3), although the analysis did not reached statistical significance (p = 0.052). Regarding gait analysis, patients performed better or similar gait performances than baseline, and significant improvement at post-hoc analysis between T3 and T0 were observed for stride length (p = 0.014) (T3 vs. T0 p = 0.036) and TUG total duration (p = 0.040) (T3 vs. T0 p = 0.045) ( Supplementary Table 2).
DISCUSSION
In this longitudinal, objective assessment of gait and FoG changes in PD patients treated with LCIG infusion therapy, we found that the main spatio-temporal parameters of gait and FoG severity significantly improved after LCIG initiation, with a progressive improvement over 6 months. Interestingly, motor complications, and specifically motor fluctuations, also improved with LCIG as expected; however, their improvement was not correlated with the improvement of gait impairment. The progressive amelioration of gait and FoG from T0 to T1 (i.e., 3 months after LCIG start) and T2 (i.e., 6 months after LCIG start) and the lack of correlation with the reduction of daily Off time, endorses the hypothesis of continuous dopaminergic stimulation providing a specific improvement of gait impairment, independently from the improvement of motor fluctuations [9–11].
Several studies suggest that long-term pulsatile levodopa treatment induces alteration in postsynaptic dopaminergic receptors [28, 29], favoring the appearance of gait and balance disturbances and therapy-resistant FoG. In a recent study conducted by our group on advanced PD patients treated with LCIG and presenting FoG episodes despite good control of motor cardinal symptoms, we hypothesized the possibility of a higher threshold for the improvement of axial motor symptoms, demonstrating an objective improvement in FoG with higher levodopa doses [22]. The current study primarily focused on the potential of transition to a continuous dopaminergic stimulation, as opposed to pulsatile treatment, to overcome the activation of these aberrant motor circuits [30]. It is interesting to note that in the present study the greatest improvement was observed at the longest follow-up, despite the absence of a significant increase of LEDD between T0, T1 and T2. This observation strengthens the hypothesis that gait disturbances are induced, at least in part, by aberrant synaptic plasticity that need longer time to be modulated, as already postulated for Deep Brain Stimulation [30, 31]. To support this pathophysiological hypothesis, the re-evaluation at a long-term time-point (mean of about 26 months from LCIG start), confirmed the improvement of FoG when compared with baseline. It is also relevant to note that the gait analysis at T3, conducted in the morning with an oral levodopa dose, showed similar or even better parameters than baseline, with significant improvement of the stride length and the TUG test duration. These results provide new insight into how long-term motor disabilities might be partially restored rather than just masked by continuous dopaminergic delivery, endorsing the hypothesis of a beneficial effect of continuous dopaminergic delivery which goes beyond the simple stabilization of levodopa plasma levels. These findings, despite limited by the small sample size, might be useful to support indications for infusion therapies in selected patients, especially considering new available strategies like levodopa subcutaneous infusion. This kind of indication would be even more relevant when considering the possibility of a lower risk of falls likely provided by the reduction of FoG severity and improvement of gait parameters; unfortunately, despite the reduced number of falls over follow-up in our cohort, the statistical analysis of our data did not prove a significant change in the number of falls. It is possible that this result is impacted by the low number of falls at baseline along with a small sample size, thus reducing the power to disclose a group-level statistical difference. Given the strength of correlation between falls and hospitalization and mortality in PD [25], further longitudinal studies with larger sample size are warranted to verify the possible beneficial impact of LCIG or other infusion therapies on falls.
We did not observe an improvement in global motor performance assessed by MDS-UPDRS III in ON condition at the three timepoints, which indicates an improvement of the gait parameters also independent from the control of cardinal symptoms [4, 5, 32]. As expected, the MDS-UPDRS part IV score improved significantly after LCIG initiation [33], except for the dyskinesia susbcore. However, the limited sensitivity of this subsection for dyskinesia assessment is well known, and in fact we observed a significant improvement over time of dyskinesia, when assessed by the UDysRS scale [34, 35].
Differently from previous studies on the role of LCIG on axial symptoms, our study present the innovation of a prospective, longitudinal design and the adoption of motion sensors for gait and balance assessment, allowing an objective and quantitative analysis of gait impairment. Interestingly, both constant and episodic gait impairments improved. The implementation of APDM Mobility Lab for gait and balance assessment in PD has been previously validated, and it was also proposed as a potential tool to monitor the progression of the disease [23, 36], especially for the quick assembly and data processing of these small sensors, able to provide outcomes that can be used directly by the clinician without the need for raw data reprocessing or analysis. Endorsing the usefulness of this assessment, a recent study conducted with sensor-based gait analysis during subcutaneous apomorphine titration confirmed that gait parameters as gait speed and stride length can support clinical assessment of individual PD patients during dopaminergic treatment [37]. Moreover, the implementation of technology in PD assessment has been endorsed in recent MDS consensus statement [38], for more sophisticated characterization of patients’ function, better tailoring of symptomatic therapy, and consequently improved health care outcomes. Finally, we performed a follow-up including two different observations within 6 months since LCIG start. This design allowed us to disclose a progressive improvement of gait over time, while limiting an excessive time-span since the LCIG start, reasonably allowing us to avoid a significant influence of disease progression on gait.
This study has some relevant limitations. First, the sample size was relatively small, albeit similar to previous studies aimed at finding therapeutic effect on FoG. This is mainly explained by the Covid-19 pandemic limitations that resulted in enrollment delays and reduced the number of patients admitted to advanced therapies [39]. However, we performed a deep characterization of each patient, and the relatively small sample size did not interfere with our possibility to disclose significant improvement in the main outcome measures. A second limitation of the study is the absence of an objective measurement of FoG using motion sensors. Research is ongoing to determine the best algorithm for an automatic detection of FoG [40], and algorithm for FoG evaluation directly from the APDM were also reported [41]. Nevertheless, this phenomenon exhibits a great variability during a single clinical assessment, and at the current time reliable software with automated home analysis is not yet available [42, 43]. Thus, we opted for an evaluation of FoG based on a validated, patient-centered questionnaire able to capture a more comprehensive change of FoG frequency and severity over time. On the other hand, a possible shortcoming could be the poor usability of the NFoG-Q as a relevant outcome measure; nevertheless, the difference between baseline and T2 scores exceeded the limit of 9.95 (35.5%), recently suggested as threshold necessary to overcome the measurement error of the tool, and indicating the clinical relevance of our findings for the treatment of FoG in advanced PD [44]. Finally, the open label study design and the absence of a control group need to be considered as another limitation of the study.
These limitations notwithstanding, our study indicates that LCIG therapy can effectively improve continuous and episodic gait impairment in advanced PD patients independently from the improvement of motor fluctuations. The significant improvements observed after six months of therapy with a stable dosage of dopaminergic therapy, highlight the potential of LCIG as a valuable treatment option for gait issues in PD. This aspect is even more interesting if considering the recent approval of other forms of continuous levodopa delivery (i.e., subcutaneous levodopa infusion). Further investigations are warranted to unravel the complex pathophysiology underlying these improvements and to explore long-term trends. Moreover, the integration of new technologies holds promise for enhancing precision and individualization in the management of axial PD symptoms.
ACKNOWLEDGMENTS
We thank the patients and their families for participating in this study.
FUNDING
Funding of the study was provided by AbbVie srl.
CONFLICT OF INTEREST
The authors report no conflicts of interest directly in connection with this article.
GI, EM, and MB report no disclosures. CAA has received speech honoraria from Zambon, Bial, Lusofarmaco. CL has received speech honoraria from Lusofarmaco. AR and MGR have received speech honoraria from Zambon. LL has received speech honoraria from Medtronic, Bial, Zambon and AbbVie. MZ has received speech honoraria from Medtronic, Bial, and AbbVie.
DATA AVAILABILITY
The data that support the findings of this study are available in anonymized dataset from the corresponding author, upon reasonable request.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-240003.
REFERENCES
[1] | Mirelman A , Bonato P , Camicioli R , Ellis TD , Giladi N , Hamilton JL , Hass CJ , Hausdorff JM , Pelosin E , Almeida QJ ((2019) ) Gait impairments in Parkinson’s disease. Lancet Neurol 18: , 697–708. |
[2] | Nutt JG , Bloem BR , Giladi N ((2011) ) Freezing of gait: Moving forward on a mysterious clinical phenomenon. Lancet Neurol 10: , 734–744. |
[3] | Giladi N , Horak FB , Hausdorff JM ((2013) ) Classification of gait disturbances: Distinguishing between continuous and episodic changes. Mov Disord 28: , 1469–1473. |
[4] | Nieuwboer A , Giladi N ((2013) ) Characterizing freezing of gait in Parkinson’s disease: Models of an episodic phenomenon. Mov Disord 28: , 1509–1519. |
[5] | Heremans E , Nieuwboer A , Vercruysse S ((2013) ) Freezing of gait in Parkinson’s disease: Where are we now? . Curr Neurol Neurosci Rep 13: , 350. |
[6] | Espay AJ , Fasano A , van Nuenen BF , Payne MM , Snijders AH , Bloem BR ((2012) ) “On” state freezing of gait in Parkinson disease: A paradoxical levodopa-induced complication. Neurology 78: , 4547–4457. |
[7] | Creaby MW , Cole MH ((2018) ) Gait characteristics and falls in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat Disord 57: , 1–8. |
[8] | Okuma Y ((2014) ) Freezing of gait and falls in Parkinson’s disease . J Parkinsons Dis 4: , 255–260. |
[9] | Zibetti M , Angrisano S , Dematteis F , Artusi CA , Romagnolo A , Merola A , Lopiano L ((2018) ) Effects of intestinal Levodopa infusion on freezing of gait in Parkinson disease. J Neurol Sci 385: , 105–108. |
[10] | Rispoli V , Golfrè Andreasi N , Penna G , Preda F , Contini E , Sensi M ((2018) ) Levodopa/carbidopa intestinal gel infusion therapy: Focus on gait and balance. Mov Disord Clin Pract 5: , 542–545. |
[11] | Cossu G , Ricchi V , Pilleri M , Mancini F , Murgia D , Ricchieri G , Mereu A , Melis M , Antonini A ((2015) ) Levodopa-carbidopa intrajejunal gel in advanced Parkinson disease with “on” freezing of gait. Neurol Sci 36: , 1683–1686. |
[12] | Nyholm D , Odin P , Johansson A , Chatamra K , Locke C , Dutta S , Othman AA ((2013) ) Pharmacokinetics of levodopa, carbidopa, and 3-O-methyldopa following 16-hour jejunal infusion of levodopa-carbidopa intestinal gel in advanced Parkinson’s disease patients. AAPS J 15: , 316–323. |
[13] | Marsden CD ((1994) ) Problems with long-term levodopa therapy for Parkinson’s disease. Clin Neuropharmacol 17: (Suppl 2), S32–S44. |
[14] | Schapira AH , Emre M , Jenner P , Poewe W ((2009) ) Levodopa in the treatment of Parkinson’s disease. Eur J Neurol 16: , 982–989. |
[15] | Fabbri M , Pongmala C , Artusi CA , Romagnolo A , Rizzone MG , Zibetti M , Lopiano L ((2019) ) Long-term effect of levodopa-carbidopa intestinal gel on axial signs in Parkinson’s disease. Acta Neurol Scand 140: , 157–161. |
[16] | Standaert DG , Aldred J , Anca-Herschkovitsch M , Bourgeois P , Cubo E , Davis TL , Iansek R , Kovács N , Pontieri FE , Siddiqui MS , Simu M , Bergmann L , Kukreja P , Robieson WZ , Chaudhuri KR ((2021) ) DUOGLOBE: One-year outcomes in a real-world study of levodopa carbidopa intestinal gel for Parkinson’s disease. Mov Disord Clin Pract 8: , 1061–1074. |
[17] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W , Obeso J , Marek K , Litvan I , Lang AE , Halliday G , Goetz CG , Gasser T , Dubois B , Chan P , Bloem BR , Adler CH , Deuschl G ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30: , 1591–1601. |
[18] | Hoehn MM , Yahr MD ((1967) ) Parkinsonism: Onset, progression and mortality. Neurology 17: , 427–442. |
[19] | Stebbins GT , Goetz CG , Burn DJ ((2013) ) How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: Comparison with the unified Parkinson’s disease rating scale. Mov Disord 28: , 668–670. |
[20] | Nieuwboer A , Rochester L , Herman T , Vandenberghe W , Emil GE , Thomaes T , Giladi N ((2009) ) Reliability of the new freezing of gait questionnaire: Agreement between patients with Parkinson’s disease and their carers. Gait Posture 30: , 459–463. |
[21] | Dalrymple-Alford JC , MacAskill MR , Nakas CT , Livingston L , Graham C , Crucian GP , Melzer TR , Kirwan J , Keenan R , Wells S , Porter RJ , Watts R , Anderson TJ ((2010) ) The MoCA: Well-suited screen for cognitive impairment in Parkinson disease. Neurology 75: , 1717–1725. |
[22] | Imbalzano G , Rinaldi D , Calandra-Buonaura G , Contin M , Amato F , Giannini G , Sambati L , Ledda C , Romagnolo A , Olmo G , Cortelli P , Zibetti M , Lopiano L , Artusi CA ((2023) ) How resistant are levodopa-resistant axial symptoms? Response of freezing, posture, and voice to increasing levodopa intestinal infusion rates in Parkinson disease. Eur J Neurol 30: , 96–106. |
[23] | Morris R , Stuart S , Mcbarron G , Fino PC , Mancini M , Curtze C ((2019) ) Validity of mobility lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiol Meas 40: , 095003. |
[24] | Schade S , Mollenhauer B , Trenkwalder C ((2020) ) Levodopa equivalent dose conversion factors: An updated proposal including opicapone and safinamide. Mov Disord Clin Pract 7: , 343–345. |
[25] | Fasano A , Canning CG , Hausdorff JM , Lord S , Rochester L ((2017) ) Falls in Parkinson’s disease: A complex and evolving picture. Mov Disord 32: , 1524–1536. |
[26] | Mehdizadeh M , Martinez-Martin P , Habibi SA , Fereshtehnejad SM , Abasi A , Niazi Khatoon J , Saneii SH , Taghizadeh G ((2019) ) Reliability and validity of fall efficacy scale-international in people with Parkinson’s disease during on-and off-drug phases. Parkinsons Dis 2019: , 6505237. |
[27] | Smulders K , Dale ML , Carlson-Kuhta P , Nutt JG , Horak FB ((2016) ) Pharmacological treatment in Parkinson’s disease: Effects on gait. Parkinsonism Relat Disord 31: , 3–13. |
[28] | Calabresi P , Ghiglieri V , Mazzocchetti P , Corbelli I , Picconi B ((2015) ) Levodopa-induced plasticity: A double-edged sword in Parkinson’s disease? . Philos Trans R Soc Lond B Biol Sci 370: , 20140184. |
[29] | Olanow CW , Calabresi P , Obeso JA ((2020) ) Continuous dopaminergic stimulation as a treatment for Parkinson’s disease: Current status and future opportunities. Mov Disord 35: , 1731–1744. |
[30] | Nonnekes J , Bereau M , Bloem BR ((2020) ) Freezing of gait and its levodopa paradox. JAMA Neurol 77: , 287–288. |
[31] | Castrioto A , Carnicella S , Fraix V , Chabardes S , Moro E , Krack P ((2017) ) Reversing dopaminergic sensitization. Mov Disord 32: , 1679–1683. |
[32] | Schaafsma JD , Giladi N , Balash Y , Bartels AL , Gurevich T , Hausdorff JM ((2003) ) Gait dynamics in Parkinson’s disease: Relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci 212: , 47–53. |
[33] | Antonini A , Poewe W , Chaudhuri KR , Jech R , Pickut B , PirtoŠek Z , Szasz J , Valldeoriola F , Winkler C , Bergmann L , Yegin A , Onuk K , Barch D , Odin P ; GLORIA study co-investigators ((2017) ) Levodopa-carbidopa intestinal gel in advanced Parkinson’s: Final results of the GLORIA registry. Parkinsonism Relat Disord 45: , 13–20. |
[34] | Fabbri M , Zibetti M , Calandra-Buonaura G , Contin M , Sambati L , Mohamed S , Romagnolo A , Berchialla P , Imbalzano G , Giannini G , Rizzone MG , Artusi CA , Cortelli P , Lopiano L ((2020) ) Levodopa/carbidopa intestinal gel long-term outcome in Parkinson’s disease: Focus on dyskinesia. Mov Disord Clin Pract 7: , 930–939. |
[35] | Goetz CG , Stebbins GT , Chung KA , Hauser RA , Miyasaki JM , Nicholas AP , Poewe W , Seppi K , Rascol O , Stacy MA , Nutt JG , Tanner CM , Urkowitz A , Jaglin JA , Ge S ((2013) ) Which dyskinesia scale best detects treatment response? . Mov Disord 28: , 341–346. |
[36] | Mancini M , Horak FB ((2016) ) Potential of APDM mobility lab for the monitoring of the progression of Parkinson’s disease. Expert Rev Med Devices 13: , 455–462. |
[37] | Marxreiter F , Gaßner H , Borozdina O , Barth J , Kohl Z , Schlachetzki JCM , Thun-Hohenstein C , Volc D , Eskofier BM , Winkler J , Klucken J ((2018) ) Sensor-based gait analysis of individualized improvement during apomorphine titration in Parkinson’s disease. J Neurol 265: , 2656–2665. |
[38] | Espay AJ , Hausdorff JM , Sánchez-Ferro Á , Klucken J , Merola A , Bonato P , Paul SS , Horak FB , Vizcarra JA , Mestre TA , Reilmann R , Nieuwboer A , Dorsey ER , Rochester L , Bloem BR , Maetzler W ; Movement Disorder Society Task Force on Technology ((2019) ) Movement Disorder Society Task Force on Technology. A roadmap for implementation of patient-centered digital outcome measures in Parkinson’s disease obtained using mobile health technologies. Mov Disord 34: , 657–663. |
[39] | Fearon C , Fasano A ((2021) ) Parkinson’s disease and the COVID-19 pandemic. J Parkinsons Dis 11: , 431–444. |
[40] | Pardoel S , Kofman J , Nantel J , Lemaire ED ((2019) ) Wearable-sensor-based detection and prediction of freezing of gait in Parkinson’s disease: A review. Sensors (Basel) 19: , 5141. |
[41] | Mancini M , Smulders K , Cohen RG , Horak FB , Giladi N , Nutt JG ((2017) ) The clinical significance of freezing while turning in Parkinson’s disease. Neuroscience 343: , 222–228. |
[42] | Warmerdam E , Hausdorff JM , Atrsaei A , Zhou Y , Mirelman A , Aminian K , Espay AJ , Hansen C , Evers LJW , Keller A , Lamoth C , Pilotto A , Rochester L , Schmidt G , Bloem BR , Maetzler W ((2020) ) Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol 19: , 462–470. |
[43] | Mancini M , Bloem BR , Horak FB , Lewis SJG , Nieuwboer A , Nonnekes J ((2019) ) Clinical and methodological challenges for assessing freezing of gait: Future perspectives. Mov Disord 34: , 783–790. |
[44] | Hulzinga F , Nieuwboer A , Dijkstra BW , Mancini M , Strouwen C , Bloem BR , Ginis P ((2020) ) The New Freezing of Gait Questionnaire: Unsuitable as an outcome in clinical trials? Mov Disord Clin Pract 7: , 199–205. |




