Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2023 Update
Abstract
Background:
Since 2020, annual reports on the clinical development of new drug-based therapies for the neurodegenerative condition of Parkinson’s disease (PD) have been generated. These reviews have followed the progress of both “symptomatic treatments” (ST – improves/reduces symptoms of the condition) and “disease modifying treatments” (DMT - attempts to delay/slow progression by addressing the underlying biology of PD). Additional efforts have been made to further categorize these experimental treatments based on their mechanisms of action and class of drug.
Methods:
A dataset of clinical trials for drug therapies in PD was obtained using trial data downloaded from the ClinicalTrials.gov online registry. A breakdown analysis of all the studies that were active as of January 31st, 2023, was conducted.
Results:
There was a total of 139 clinical trials registered on the ClinicalTrials.gov website as active (with 35 trials newly registered since our last report). Of these trials, 76 (55%) were considered ST and 63 (45%) were designated DMT. Similar to previous years, approximately a third of the studies were in Phase 1 (n = 47; 34%), half (n = 72, 52%) were in Phase 2 and there were 20 (14%) studies in Phase 3. Novel therapies again represented the most dominant group of experimental treatments in this year’s report with 58 (42%) trials testing new agents. Repurposed drugs are present in a third (n = 49, 35%) of trials, with reformulations and new claims representing 19% and 4% of studies, respectively.
Conclusions:
Our fourth annual review of active clinical trials evaluating ST and DMT therapeutics for PD demonstrates that the drug development pipeline is dynamic and evolving. The slow progress and lack of agents transitioning from Phase 2 to Phase 3 is concerning, but collective efforts by various stakeholders are being made to accelerate the clinical trial process, with the aim of bringing new therapies to the PD community sooner.
ABBREVIATIONS
AAV | Adeno-associated virus |
DA-ST | Dopaminergic symptomatic therapy |
DMT | Disease Modifying Therapies |
EJS-ACT PD | The Edmond J. Safra Accelerating Clinical Treatments for Parkinson’s Disease |
GCase | Glucocerebrosidase |
GDNF | Glial cell-derived neurotrophic factor |
GIT | Gastrointestinal Tract |
GLP-1R | Glucagon-like peptide 1 receptor |
LD/CD | Levodopa and carbidopa |
LID | Levodopa-induced dyskinesia |
LRRK2 | Leucine-rich repeat kinase 2 |
MOA | Mechanisms of Action |
NMDA | N-methyl-D-aspartate |
NMS | Non-motor symptoms |
Non DA-ST | Non-dopaminergic symptomatic therapy |
PD | Parkinson’s Disease |
ST | Symptomatic Therapy |
WHO | World Health Organization |
INTRODUCTION
In June 2022, the World Health Organization released a technical brief entitled “Parkinson disease: a public health approach”, noting that the global impact of Parkinson’s disease (PD) is “increasing faster than for any other neurological disorder” [1]. The report highlighted a doubling of PD prevalence over the past 25 years, with over 5.8 million disability-adjusted life years in 2019 - an increase of 81% since 2000. Importantly, the report states that “an urgent public health response is necessary to meet the health and social requirements of people with PD and to improve functioning, quality of life and prevent disability as global longevity increases. A pressing need for effective preventive actions is also needed to slow the rising incidence of PD before the burden and costs of treatment overwhelm country healthservices”.
To confront this future strain on society, the PD research community is making significant efforts to provide not only better treatments and quality of life for PD patients and their families, but also to identify those at the earliest stages of the disease process, even before symptoms manifest. Recent data from the Parkinson’s Progression Markers Initiative, a large longitudinal PD patient cohort supports the use of so-called “seeding amplification assays” for the protein alpha-synuclein as a possible PD biochemical biomarker and early biological diagnostic tool [2]. With the development of such tools, there is growing attention on attempts to treat PD prior to onset of symptoms, as highlighted by community events like the recent “Planning for Prevention of Parkinson: A Trial Design Symposium and Workshop” [3]. In parallel, some groups are now actively planning early “prevention” therapeutic trials, such as The Michael J. Fox Foundation for Parkinson’s Research’s “Path to Prevention” platform trial [4]. In addition, there are ongoing projects seeking to accelerate clinical development of new disease modifying therapeutics for those already diagnosed with PD, such as The Edmond J. Safra Accelerating Clinical Treatments for Parkinson’s Disease (EJS-ACT PD) initiative, which is setting up a multi-arm, multi-stage platform [5]. With such efforts underway, we are now hopefully looking at a future where PD will be relegated to the medical history books.
A better understanding of subtypes of PD is also allowing for improved patient stratification in clinical trials, which will hopefully increase our chances of clinical trial success [6]. Some of the initial attempts at patient stratification have come from more than two decades of research focused on genetic variations, which have not only provided new insights into the biology potentially underlying PD but also pointed towards new classes of experimental therapies that are now being clinically tested (and are discussed in this report).
With encouraging progress being made in the clinical development of new therapies for PD, it is a useful process to regularly examine the landscape of these efforts to better understand the broader trends and provide the research and patient communities with a useful resource. Similar to annual reviews of experimental therapeutics in clinical trials for Alzheimer’s [7], since 2020 we have generated a report on the drug development pipeline for PD [8–10]. This current and fourth report adds to that growing collection of data and is intended to highlight areas of progress and hopefully stimulate greater awareness and involvement in the clinical trialprocess.
METHODS
The methods employed in this analysis closely resemble those used in our previous reports [10], with some minor adjustments. A diagram of the workflow is presented in Fig. 1.
Fig. 1
A schematic outlining the data collection and analysis.
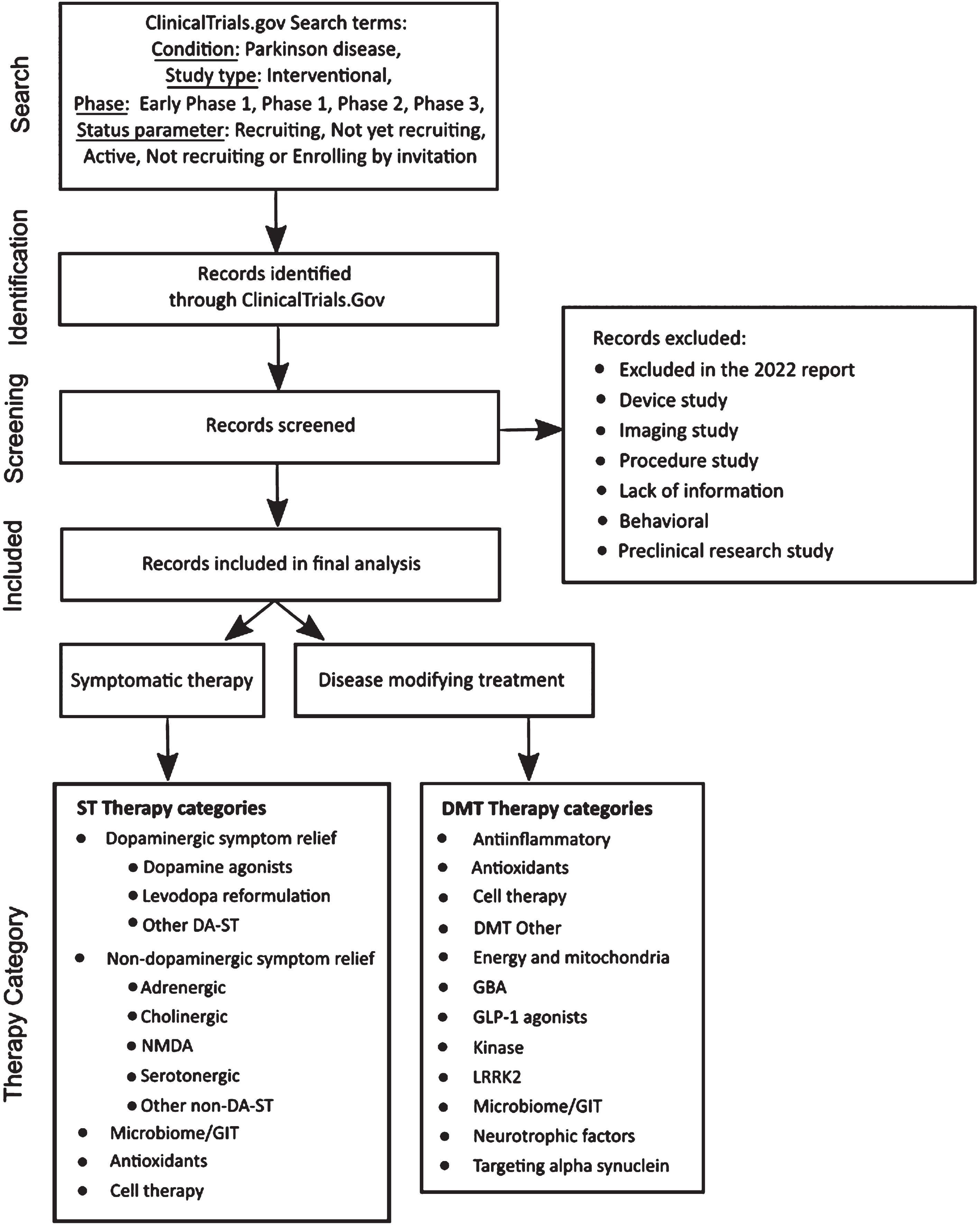
Trial data sourcing
Clinical trial data was downloaded from ClinicalTrials.gov on January 31, 2023, based on the following search criteria:
• Condition: Parkinson disease
• Study type: Interventional
• Phase: Early Phase 1, Phase 1, Phase 2, Phase 3
• Status parameter: “Recruiting”, “Not yet recruiting”, “Active, not recruiting”, or “Enrolling by invitation”.
To simplify the reporting, we grouped trials designated as “Early Phase 1” with Phase 1 trials. Moreover, we grouped trials designated as “Phase 1/Phase 2” trials with Phase 1 trials and “Phase 2/Phase 3” trials with Phase 2 trials. In previous reports we have also analyzed data collected from the World Health Organisation (WHO) International Clinical Trials Registry Platform [11]. However, due to lack of consistent and comparable data and our experience that ClinicalTrials.gov contributed the vast majority of available trial information (overlapping with many other registries), we decided to not include additional WHO registry data in our analyses this year.
Trial and therapeutic categorization
As of January 31, 2023, we identified and downloaded records of 179 active interventional clinical trials meeting our search criteria from the ClinicalTrials.gov website. Given our focused interest for this report is on pharmacological and biological agents, we excluded trials evaluating medical devices, biomarkers, or behavioral and other lifestyle interventions. We also excluded trials that in our evaluation lacked reliable supporting public information available online. This filtering process excluded 40 trials from the dataset, 30 of which were also excluded in our 2022 report [10].
We next reviewed and classified trials as either “symptomatic treatments” (ST - improves or reduces symptoms of the condition) or “disease modifying treatments” (DMT - attempts to delay or slow progression by targeting the underlying biology of PD). We then assigned trials to categories using an iterative process that considered the mechanism of action (MOA) and target of the therapeutic agents being tested. Our approach to therapeutic categorization has evolved over the four years of our pipeline reporting as we aim to improve the analysis. With increasing diversity now seen in the pipeline and in order to provide a richer view of the clinical trial landscape, we have created additional subcategories as well as pulled out more specific targetclarity.
We classified the therapeutic agent in each trial using a detailed coding framework (see Fig. 1). First, we classified agents as either a novel (i.e., not yet approved) or existing compound. We further classified existing compounds as either a “repurposed” agent, a “reformulation”, or a “new claim”. Repurposed compounds are those already approved by regulatory authorities for use in a different disease or condition that are now being evaluated for use in PD. Reformulated compounds involve drugs already in use for PD that have the potential to be delivered in alternate ways, such as subcutaneously or as an inhalant. Compounds seeking a new claim involve testing of drugs already approved for treating specific PD symptoms for use in treatment of additional PD symptoms.
The categories used in this year’s analysis are:
• ‘Dopaminergic symptom relief therapies’ (MOA) applies to ST agents that either restore, replace or mimic the neurotransmitter dopamine, and include the following new subcategories:
– DA agonist
– Levodopa (LD) reformulation
– Other DA-ST
• ‘Non-dopaminergic symptom relief therapies’ (MOA) applies to ST agents that target neurotransmitter systems other than dopamine and include:
– Adrenergic
– Cholinergic
– NMDA
– Serotonergic
– Other non-DA-ST
• ‘Anti-inflammatory’ (MOA) applies to agents seeking to reduce inflammatory processes.
• ‘Antioxidants’ (MOA) are agents primarily focused on reducing oxidative stress.
• ‘Cell therapy’ (MOA) are trials including either intracerebral cell transplantation or peripheral delivery of cells.
• ‘Energy and mitochondria’ (MOA) includes agents seeking to stimulate improvements in mitochondrial function.
• ‘GBA’ (MOA) agents are focused on enhancing the activity of glucocerebrosidase(GCase).
• ‘GLP-1 agonists’ (MOA) are a specific class of drugs activating the glucagon-like peptide-1 receptor.
• ‘Kinase inhibitors’ (MOA) includes agents blocking specific kinase activity.
• “LRRK2’ (target) are agents seeking to reduce or inhibit the activity of LRRK2. In previous reports LRRK2 was captured under ‘Kinase inhibitors’.
• ‘Microbiome/GIT’ (target) are agents specifically targeting the activity of the gastrointestinal tract.
• ‘Neurotrophic factors’ (MOA) is assigned to therapies involving the delivery of growth factors such as GDNF or CDNF.
• ‘Targeting alpha-synuclein’ (target) covers molecules specifically focused on preventing alpha-synuclein aggregation, or disaggregation of existing complexes. In previous reports, ‘Immunotherapy’ was a distinct category, but it has now been folded into ‘Targeting alpha-synuclein’.
• ‘Other DMT’ (MOA) is assigned to trials whose therapy has a MOA that did not match another category.
For ST trials, we sought to classify by specific symptom, e.g., motor symptoms, dyskinesia, and cognitive impairment. When not available in the trial registration, the allocation of the symptom descriptor was primarily based on sponsor-provided information and, if ambiguity remained, the consensus of the authors of this report.
RESULTS
Of the 139 trials in the dataset (Supplemental file), 103 (74%) were represented in our 2022 review [10]. The remaining 36 trials were new studies that have not been tracked in prior reports. one of which had been registered in time for our 2022 paper but was excluded due to insufficient information available at the time (UCB0022, NCT04867642). When the nature and phase status of the 139 studies were reviewed, 76 (55%) were classified as ST trials and the other 63 (45%) were designated DMT (Table 1). Slightly more than half (52%) of all trials were in Phase 2 testing stages, followed by Phase 1 (34%) and Phase 3 (14%) (Table 1). These trials represented a variety of unique agents (Fig. 2).
Table 1
Trials categorized as ST or DMT and by phase
| ST AND DMT | ||||
| PHASE | ST | DMT | TOTAL NUMBER OF TRIALS | % OF TOTAL |
| 1 | 22 | 25 | 47 | 33.8% |
| 2 | 40 | 32 | 72 | 51.8% |
| 3 | 14 | 6 | 20 | 14.4% |
| TOTAL | 76 | 63 | 139 | 100.0% |
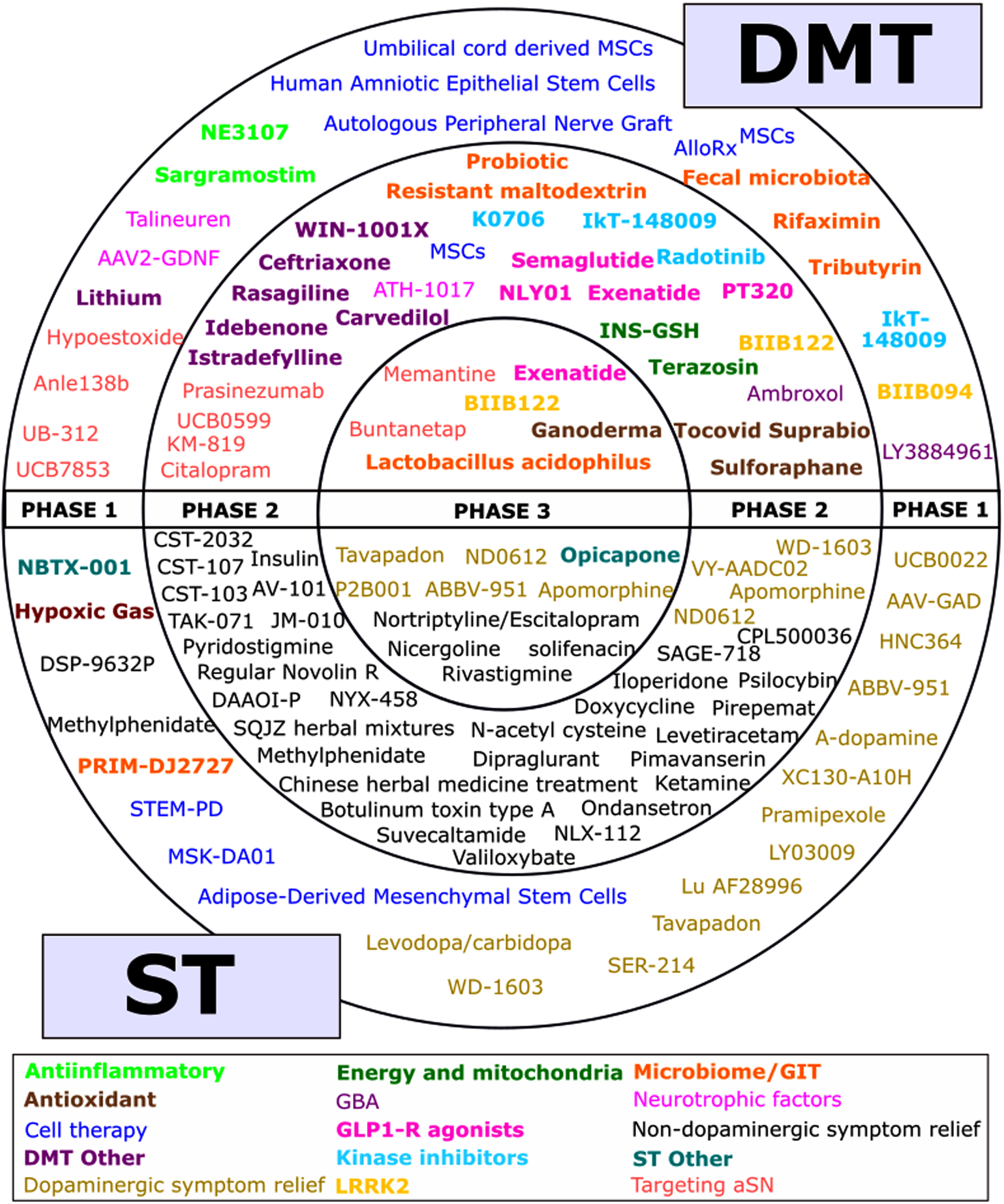
Fig. 2
A schematic of all of the agents in active clinical trials for PD, registered on ClinicalTrials.gov as of the 31st January 2023.
While the total number of trials (139) is the lowest since we started our annual analyses, and the proportion of DMT (45.3%) the highest, both the total number of trials and the proportions of ST and DMT have been similar over the years (Table 2). The number of ST trials in Phase 3 has decreased from 25 to 14, although the 6 DMT trials in Phase 3 is the highest number yet.
Table 2
Trials categorized by ST or DMT, 2020-2023
| 2020 | 2021 | 2022 | 2023 | |||||
| ST | 88 | 60.7% | 83 | 58.5% | 92 | 62.6% | 76 | 54.7% |
| DMT | 57 | 39.3% | 59 | 41.5% | 55 | 37.4% | 63 | 45.3% |
| TOTAL | 145 | 142 | 147 | 139 | ||||
The agents being tested in each phase are presented in Fig. 2. This figure shows the variety of therapies and categories being tested, but not the number of actual trials as there were many cases where the named therapy was tested in more than one trial (for example, there are six trials of Cerevel’s tavapadon in the 2023 dataset).
Trials by therapeutic category
A breakdown of trials by therapeutic classification (Table 3) revealed that dopaminergic symptom relief therapies (including agonists, levodopa reformulations and other dopaminergic therapies) represent nearly 21% of active trials, although this was a decrease from the proportion (26%) seen in our 2022 report [10]. Of the new subcategories used in the current report for dopaminergic symptom relief therapies (see Methods for details), ‘DA agonists’ represented the largest category (10.1%) with ‘LD reformulation’ (5.8%), and ‘Other DA-ST’ (5.0%) contributing roughly equally to the remaining total. Dopamine agonists thus represented half of dopaminergic symptom relief trials.
Table 3
Trials by therapeutic category
| NUMBER OF TRIALS BY THERAPEUTIC CATEGORY | ||||||||
| Category | Phase 1 | Phase 2 | Phase 3 | TOTAL | ||||
| Anti-inflammatories | 3 | 6.4% | 0 | 0.0% | 0 | 0.0% | 3 | 2.2% |
| Antioxidants | 1 | 2.1% | 2 | 2.8% | 1 | 5.0% | 4 | 2.9% |
| Cell therapy | 10 | 21.3% | 2 | 2.8% | 0 | 0.0% | 12 | 8.6% |
| Dopaminergic symptom relief - DA agonist | 7 | 14.9% | 1 | 1.4% | 6 | 30.0% | 14 | 10.1% |
| Dopaminergic symptom relief - LD reformulation | 3 | 6.4% | 2 | 2.8% | 3 | 15.0% | 8 | 5.8% |
| Dopaminergic symptom relief - other | 5 | 10.6% | 1 | 1.4% | 1 | 5.0% | 7 | 5.0% |
| Energy and mitochondria | 0 | 0.0% | 3 | 4.2% | 0 | 0.0% | 3 | 2.2% |
| GBA | 1 | 2.1% | 2 | 2.8% | 0 | 0.0% | 3 | 2.2% |
| GLP-1 agonists | 0 | 0.0% | 4 | 5.6% | 1 | 5.0% | 5 | 3.6% |
| Kinase inhibitors | 1 | 2.1% | 3 | 4.2% | 0 | 0.0% | 4 | 2.9% |
| LRRK2 | 1 | 2.1% | 1 | 1.4% | 1 | 5.0% | 3 | 2.2% |
| Microbiome/GIT | 4 | 8.5% | 3 | 4.2% | 1 | 5.0% | 8 | 5.8% |
| Neurotrophic factors | 2 | 4.3% | 1 | 1.4% | 0 | 0.0% | 3 | 1.4% |
| Non-dopaminergic symptom relief - Adrenergic | 0 | 2.1% | 3 | 4.2% | 1 | 5.0% | 4 | 2.9% |
| Non-dopaminergic symptom relief - Cholinergic | 0 | 0.0% | 3 | 2.8% | 2 | 10.0% | 5 | 3.6% |
| Non-dopaminergic symptom relief - NMDA | 0 | 0.0% | 7 | 9.9% | 0 | 0.0% | 7 | 5.0% |
| Non-dopaminergic symptom relief - Serotoninergic | 1 | 2.1% | 8 | 11.3% | 1 | 5.0% | 10 | 7.2% |
| Non-dopaminergic symptom relief - other | 1 | 2.1% | 12 | 16.9% | 0 | 0.0% | 13 | 9.4% |
| Targeting alpha- synuclein | 6 | 12.8% | 6 | 8.5% | 2 | 10.0% | 14 | 10.1% |
| Other DMT | 1 | 2.1% | 8 | 11.3% | 0 | 0.0% | 9 | 6.5% |
| TOTAL | 47 | 100% | 72 | 100% | 20 | 100% | 139 | 100% |
‘Non-dopaminergic symptom relief’ trials now represent the largest proportion of active trials in our current tracking (39/139 active trials; 28%). Using our new subcategories to characterize trials by relevant targeted neurotransmitter system, we see trials testing agents targeting a range of systems including ‘adrenergic’, ‘cholinergic’, ‘serotonergic’ and ‘glutamatergic’ signaling. In exploring DMT active trials, those testing agents targeting alpha-synuclein remain a large presence in the pipeline(Table 3).
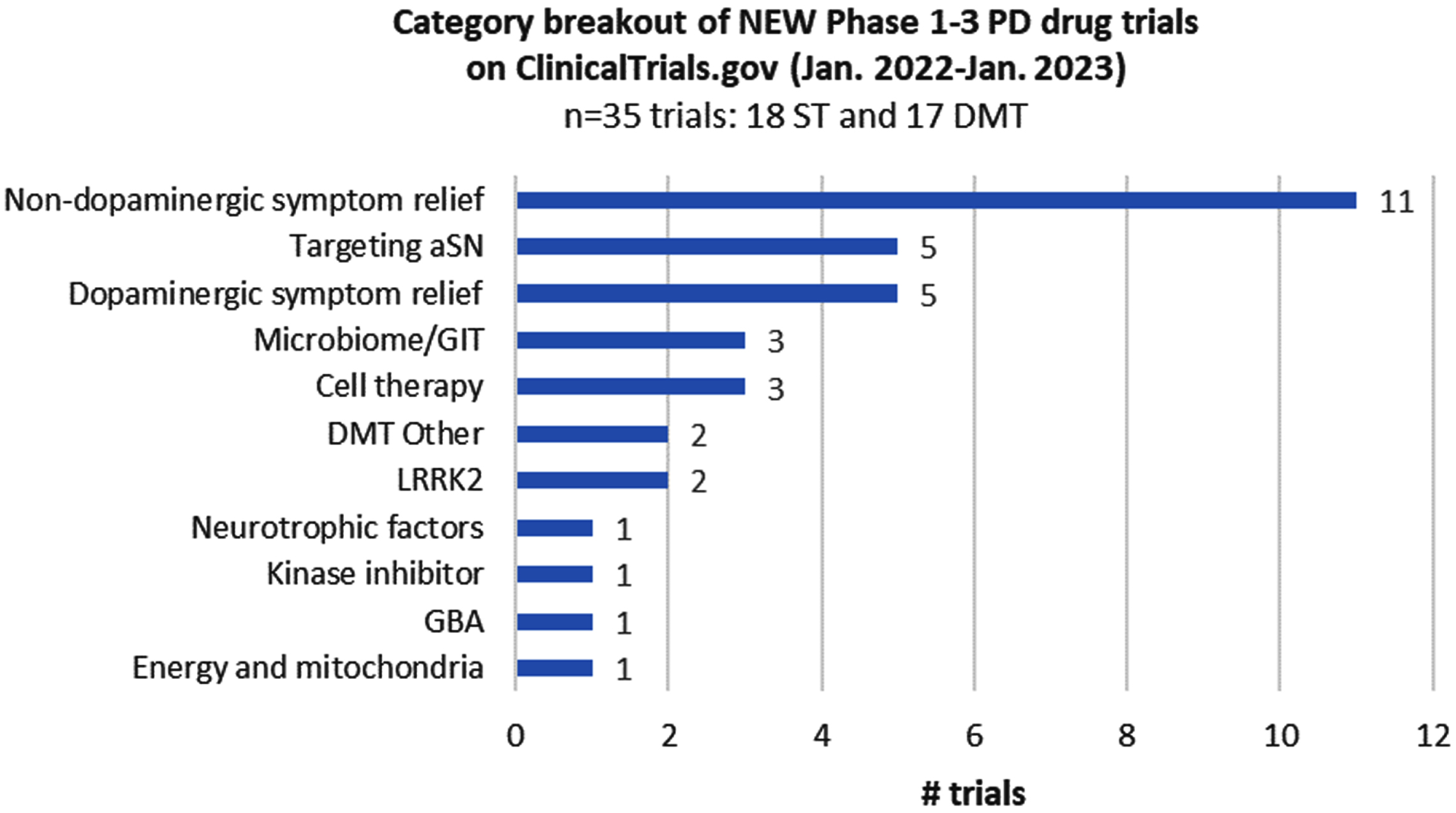
4.2Newly registered trials
Of the 139 trials in the dataset, 35 were registered on ClinicalTrials.gov within the last year. They include 18 ST and 17 DMT trials, with those targeting non-dopaminergic symptom relief representing the largest therapeutic subcategory (Fig. 3). Most new trials launched at Phase 1 (13/35) or Phase 2 (18/35) stages, although it was exciting to see four trials entering Phase 3, including three DMT trials (for Lactobacillus, BIIB122, and buntanetap).
Fig. 3
Category breakout of new trials registered since the last report.
Trials by therapeutic novelty
Next, we analysed the number of trials by the novelty of the therapeutic agent being tested (novel, repurposed, reformulation, and new claim). Similar to our prior reporting, there was relatively little change in the distribution with most (58/139; 42%) active trials testing novel therapies (Table 4). Testing of repurposed agents also continued to be an emphasis within the PD pipeline, representing roughly a third of trials (49/139; 35%).
Table 4
Trials by therapeutic type and phase
| NUMBER OF TRIALS BY PROJECT TYPE AND PHASE | |||||
| PHASE | NOVEL | REPURPOSED | REFORMULATION | NEW CLAIM | TOTAL |
| 1 | 22 | 10 | 14 | 1 | 47 |
| 2 | 30 | 33 | 5 | 4 | 72 |
| 3 | 6 | 6 | 7 | 1 | 20 |
| TOTAL | 58 | 49 | 26 | 6 | 139 |
| % | 41.7% | 35.3% | 18.7% | 4.3% | |
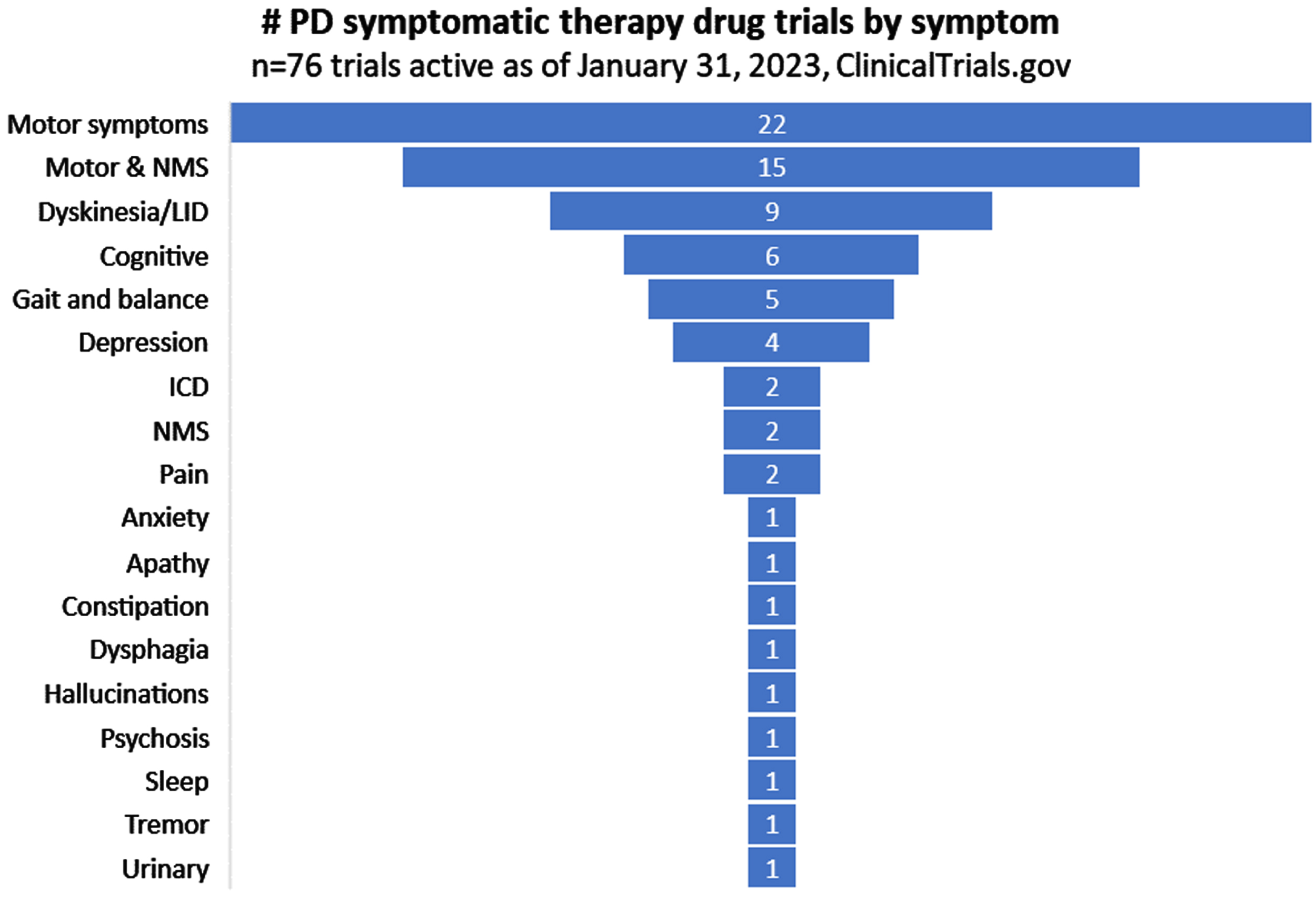
Trials by symptom type
Of the 76 active trials currently evaluating symptomatic therapies for PD, those addressing motor symptoms represented the largest proportion (22/76; 29%; Fig. 4). A number of trials suggested potential impact for a combination of “motor and non-motor symptoms (NMS)”. With that caveat in mind, it is worth noting that some symptoms which feature strongly in patient surveys as a source of concern are poorly represented. One of the most frequently reported symptoms of PD that survey respondents wish to improve at earlier stages of disease duration is tremor [12], but only one study (suvecaltamide) in our database was specifically targeting this feature of PD (Fig. 4).
Fig. 4
Symptomatic focus of active PD drug trials.
Target enrollment for trials in the dataset
A trial’s target enrollment is provided by the sponsor within the ClinicalTrials.gov registry entry. Table 5 presents the total of the participants required by phase and then further broken down by average per trial and median, minimum and maximum (Table 5).
Table 5
Enrollment table for Active Phase 1-3 drug trials
| # of trials | Target enrollment | Average/trial | median | min | max | |
| Phase 1 | 47 | 1,591 | 34 | 20 | 3 | 120 |
| Phase 2 | 72 | 9,091 | 126 | 67 | 10 | 732 |
| Phase 3 | 20 | 6,005 | 300 | 310 | 50 | 600 |
| Total | 139 | 16,687 |
Completed DMT trials
In general, DMT trials have a longer duration than ST studies due to the time required to observe any effects. Of the 31 DMT trials in our 2022 analysis that were due to complete by the end of 2022 [10], 39% of the Phase 1 trials (7/18) and 23% (3/13) of the Phase 2 trials had completed by the time of the current 2023 data download (Table 6). 18/33 (55%) DMT trials listed as due to complete in 2023 in our current dataset (Table 7) were expected to complete by the end of 2022 in our previous report [10], indicating that there have been delays to trials over the last year. There were 3 DMT trials that were due to complete in 2022 that have been delayed by more than a year; radotinib (NCT04691661), carvedilol (NCT04218968) and sargramostin (NCT05677633).
Table 6
Disease modifying clinical trials that have completed since the McFarthing et al (2022) report
| Phase 1 | Phase 2 |
| Glycerol Phenylbutyrate | Deferiprone |
| NCT02046434 | NCT02655315 |
| Exenatide | ANAVEX2-73 |
| NC03456687 | NCT04575259 |
| NNI-362 | Liraglutide |
| NCT04074837 | NCT02953665 |
| AAV2-GDNF | |
| NCT01621581 | |
| MEDI1341 | |
| NCT04449484 | |
| Ursodeoxycholic acid | |
| NCT02967250 | |
| Buntanetap/Posiphen | |
| NCT04524351 |
Trials due to complete in 2023
There are 89 trials (64%) in the dataset with a completion date before the end of 2023 (33 Phase 1 trials, 44 Phase 2 trials and 12 Phase 3 trials). Of these, 37% are classified as disease modifying, of which 14 are in Phase 1, 16 in Phase 2 and 3 are in Phase 3 (Table 7). Therefore, it is not clear if some of these studies may simply be delayed rather than scheduled tocomplete.
Those trials in italics have estimated completion dates before 2023 but are still categorized as active in our current reporting and thus may not have been updated by the trial sponsors.
Table 7
Disease modifying clinical trials listed in ClinicalTrials.gov with estimated completion dates before the end of 2023
| Phase 1 | Phase 2 | Phase 3 |
| Anle138b | Idebenone | Memantine |
| Human Amniotic Epithelial Stem Cells | NLY01 | Buntanetap |
| hAESCs treatment | K0706 | Ganoderma/Lingzhi |
| Lithium | ATH-1017 | |
| UB-312 | Carvedilol | |
| BIIB094 | Exenatide | |
| Rifaximin | IkT-148009 | |
| Talineuren | Ambroxol | |
| Anle138b | MSC | |
| Mesenchymal stem cells | Ceftriaxone | |
| IkT-148009 | Idebenone | |
| Injection of Umbilical cord derived MSCs | PT320 | |
| Hypoestoxide | Rasagiline | |
| NE3107 | WIN-1001X | |
| Autologous mesenchymal stem cells | ||
| Sulforaphane |
Those trials in italics have estimated completion dates before 2023 but are still categorized as active in our current reporting and thus may not have been updated by the trial sponsors.
DISCUSSION
This fourth annual update of the clinical development therapeutic pipeline for PD presents a relative stability in overall trial activity (total number of trials) since our first report in 2020. Other features of the pipeline, such as therapeutic novelty (new or existing treatments) also appear relatively stable; while there are some signals that the proportions of ST vs DMT trials may be shifting, more data will be required to determine if this is a trend. Given the length of time that trials for PD can take, especially those for DMTs, this relative stability may be expected. However, we should not ignore that a global pandemic occurred in the middle of this period, which speaks volumes to the remarkable resilience and determination of the PD community in their efforts to maintain momentum in identifying and evaluating new therapies.
Another positive feature of the pipeline is the continued diversity of therapeutic options being explored in terms of differing targets, mechanisms and drug delivery systems. Of note, the variety of symptomatic relief trials is exemplified not only by those targeting dopaminergic and other neurotransmitter systems but also by the ‘non-dopaminergic symptom relief (other)’ category, which spans varied treatments such as suvecaltamide (Jazz Pharmaceuticals), an inhibitor of T-type calcium channels, to the repurposing of levetiracetam, an anti-epileptic drug, by Queensland University. Similarly, with DMT trials addressing a range of gene and pathogenic pathway-defined mechanisms, the ‘DMT Other’ category includes trials exploring approaches such as low dose lithium (Buffalo University) to istradefylline (Kiowa Kirin), an adenosine A2A receptor antagonist drug currently used as an adjunct treatment for symptom relief in PD.
While diversity is seen in many areas of the pipeline, it is interesting to see where opportunities still exist given the state of research. For example, inflammation is thought to play an important role in the pathogenesis of PD, and there are three trials in this category, but all in Phase 1, Biovie’s NE-3107 and two trials on sargramostim (leukine) at the University of Nebraska. It should be noted that some agents designated to different categories have anti-inflammatory potential as secondary properties (for example, GLP-1 receptor agonists). There is a Phase 2 trial of the anti-inflammatory, azathioprine, under way at Cambridge University that is due to report in 2024; as it is not registered on ClinicalTrials.gov but rather on ISRCTN [13], we did not include it in our analysis. The pharmaceutical company Roche is testing a novel anti-inflammatory agent – a NLRP3 inflammasome inhibitor called selnoflast – in a clinical trial also registered on ISRCTN [14]. Trials targeting other mechanisms such as oxidative stress (four trials) and mitochondrial dysfunction (three trials) also indicates more opportunity for greater presence in the pipeline given their continued strong research interest.
Cell therapy trials fall into two groups, those aiming to surgically implant stem cells into relevant brain regions; and those using stem cells delivered intravenously. The rationale behind the latter group is that such stem cells are the source of molecules that may influence the course of disease progression, and thus were classified as DMTs. The implanted cells aim to restore dopaminergic function and were therefore classified as ST. Most of these trials are at an early stage in Phase 1. Many of the cell therapy trials currently active were listed in our 2020 report underlining the long duration of cell therapy trials.
Our analysis of dopaminergic therapies shows a continued emphasis on DA agonists and levodopa reformulation. These include Cerevel’s tavapadon, a D1/D5 receptor partial agonist and UCB0022, a positive allosteric modulator of the D1 receptor, as well as approaches to sub-cutaneously deliver levodopa/carbidopa such as Abbvie’s ABBV-951 and Neuroderm’s ND0612. A gene therapy approach to target dopaminergic pathways is represented by Voyager Therapeutics’ AAV coding for amino acid decarboxylase.
Genetic (and pathology)-linked PD is the focus of much research and has driven a robust drug development effort. A leading focus in the pipeline continues to be in targeting alpha-synuclein pathology, removal of which is theorized to offer similar disease-slowing benefits to what is now being reported with related therapeutics recently approved for Alzheimer’s disease [15].
The previously used immunotherapy category, which contained trials for antibodies to alpha-synuclein has been moved to the targeting alpha-synuclein category to give a full focus to those projects with alpha synuclein as a primary target. There are five trials for antibody-based projects representing three agents – prasinezumab, (Roche), UB-312 (Vaxxinity) and UCB7853 (UCB). A further Phase 3 at Wayne State University is using memantine to prevent cell to cell transmission of alpha-synuclein. Other ongoing trials are aiming to prevent the aggregation of alpha-synuclein using small molecule agents, e.g., Anle 138b from Modag. One of the highlights of this category is the Phase 3 study of buntanetap from Annovis Bio. This agent reduces the production of alpha-synuclein thereby reducing aggregation [16]. The treatment time is 6 months, a relatively short duration for a DMT trial, giving the prospect of results becoming available early in 2024.
While much of the focus has been seen in attempts to directly target alpha-synuclein pathology, focus on drugs to target GBA and LRRK2-associated PD is also evident although to a lesser degree. A leading example is BIIB122, a LRRK2 inhibitor under development by Biogen and Denali, currently in Phase 3 testing. However, since our cut-off date for this year’s report, additional trials targeting gene-defined pathogenic pathways have been launched. These include additional trials testing ambroxol for its ability to increase activity of glucocerebrosidase, the product of the GBA gene have been reported, including a Phase 3 DMT registered by University College London (ASPRO-PD; NCT05778617) and a study in a Dutch cohort (DUPARG-AMBROXOL; NCT05830396). In addition, Bial has recently launched a Phase 2 trial of BIA 28-6156 in GBA-PD (ACTIVATE; NCT05819359) while Neuron23 is now assessing its LRRK2 kinase inhibitor NEU-723 in a Phase 1 trial (NCT05633745).
Following the move of LRRK2 inhibition to a separate category, the remaining four projects in the ‘Kinase inhibitors’ group are all targeted at the c-Abelson kinase. While prior trials testing the repurposed c-Abl kinase inhibitor nilotinib were largely unsupportive of continued use of that drug [17, 18], it is clear that there remains interest in further assessing targeting c-Abl kinase as a possible treatment [19]. There are three Phase 2 studies in this category, K0706 from SPARC, IKT148009 from Inhibikase and radotinib from Il-Yang.
The role of the microbiome in the development of PD and gastrointestinal dysfunction is gaining attention due to the putative role of the gut-brain axis in PD. Trials targeting the microbiome/GIT (gastro-intestinal tract) focus on both ST and DMT approaches including testing an antibiotic (rifaximin), fecal microbiota transfer and probiotics. There is also a study looking at the influence of brain small chain fatty acid enhancement via tributyrin supplementation (mimicking the natural small chain fatty acid production in the gut).
An area of growing interest in the PD clinical pipeline has been the evaluation of GLP-1 receptor agonists. Following encouraging early trial results [20], multiple clinical trials have focused on GLP-1 receptor agonists, including repurposing of exenatide (currently in a Phase 3 trial in the UK) and lixisenatide as well as commercial efforts from Neuraly (NLY-01) and Peptron (PT-320).
Another area of interest that has been closely watched by the PD community has been clinical trials involving neurotrophic factors. There are four studies investigating neurotrophic factors in our dataset: sargramostim (Nebraska University), talineurin (Innomedica), ATH-1017 (an enhancer of hepatocyte growth factor activity from Athira Pharma), and an AAV vector-driven GDNF gene therapy agent from Brain Neurotherapy Bio.
The path to impact for a new medicine to treat PD can sometimes be tortuous, with long intervals where one clinical Phase ends with favourable results and the next one is yet to start. The potential offered by some of these approaches, which we have called “Inbetweeners”, could be overlooked if our view was restricted purely to only active registered trials on ClinicalTrials.gov. Other resources track such approaches, such as the Parkinson’s Hope List [21], and a recently available Clinical Pipeline Report from The Michael J. Fox Foundation for Parkinson’s Research [22]. Some programs of note that fall on our Inbetweeners list include Anavex’s blarcamesine (Anavex2-73), ENT-01 from Enterin (which is targeting constipation, psychosis and dementia) and PT-001 (low dose ketamine) for dyskinesia from Pharmather who suspended a Phase 2 study due to favourable results at an interim analysis and which is now planned to move to Phase 3. In addition, some interventional trials posted on ClinicalTrials.gov may not designate a phase and thus do not get captured in our search criteria. For example, there is a proof-of-concept study under way at Haukeland University Hospital testing nicotinamide riboside (NOPARK, NCT03568968). The structure of the trial looks very much like a Phase 2, with 400 participants in a randomized double-blind design; as the phase is officially listed as “not applicable”, it has not been included in our analysis.
Finally, it is important to acknowledge trials that have completed but not met their primary endpoints and to gain as much knowledge as we can from these studies. One such example was the FAIRPARK II Phase 2 trial of deferiprone in France [23], in which the recently diagnosed de novo participants on active drug actually fared worse than those on placebo. A better understanding of why some trials are not successful is important not only for the insights it can provide to our knowledge of PD, but also for encouraging more engagement in the clinical trial process. We encourage study sponsors to keep registry entries up to date and to publish results as soon as is practical. Better communication of study outcomes and learnings from the trials that have been conducted are key to avoid duplication of trial efforts and instill greater confidence in the trial process among potential participants. The number of participants required for the 139 interventional drug development trials in this report is almost 17,000. Additional participants are required for other studies not registered on ClinicalTrials.gov, and for the many observational studies under way that do not feature in our dataset. The contribution of the PD community is essential, and we acknowledge the value of their commitment and involvement.
AUTHOR CONTRIBUTIONS
KMcF, SB, GR, BF, LM, RF, RKW & SRWS performed the trial categorization & helped write/edit the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Prof Tanya Simuni of Northwestern University and Helen Matthews of Cure Parkinson’s for reading the manuscript and providing constructive feedback. The authors would also like to thank all of the trial participants and their families, and the researchers involved in the ongoing clinical research for PD.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-239901.
REFERENCES
[1] | World Health Organization technical brief “Parkinson disease: Apublic health approach”; ISBN: 9789240050983; https://www.who.int/publications/i/item/9789240050983. |
[2] | Siderowf A , Concha-Marambio L , Lafontant DE , Farris CM , Ma Y , Urenia PA , Nguyen H , Alcalay RN , Chahine LM , Foroud T , Galasko D , Kieburtz K , Merchant K , Mollenhauer B , Poston KL , Seibyl J , Simuni T , Tanner CM , Weintraub D , Videnovic A , Choi SH , Kurth R , Caspell-Garcia C , Coffey CS , Frasier M , Oliveira LMA , Hutten SJ , Sherer T , Marek K , Soto C ; Parkinson’s Progression Markers Initiative ((2023) ) Assessmentof heterogeneity among participants in the Parkinson’s ProgressionMarkers Initiative cohort using α-synuclein seedamplification: A cross-sectional study. Lancet Neurol 22: (5), 407–417. |
[3] | Crotty GF , Keavney JL , Alcalay RN , Marek K , Marshall GA , Rosas HD , Schwarzschild MA ((2022) ) Planning for Prevention of ParkinsonDisease: Now Is the Time. Neurology 99: (7 Suppl 1), 1–9. |
[4] | Path to Prevention Platform Trial; https://www.ppmi-info.org/study-design/path-to-prevention-platform-trial. |
[5] | Foltynie T , Gandhi S , Gonzalez-Robles C , Zeissler ML , Mills G , Barker R , Carpenter J , Schrag A , Schapira A , Bandmann O , Mullin S , Duffen J , McFarthing K , Chataway J , Parmar M , Carroll C ; EJS ACT-PDConsortium ((2023) ) Towards a multi-arm multi-stage platform trial of disease modifying approaches in Parkinson’s disease. Brain. Online ahead of print 10.1093/brain/awad063. |
[6] | Frasier M , Fiske BK , Sherer TB ((2022) ) Precision medicine for Parkinson’s disease: The subtyping challenge. Front Aging Neurosci 14: , 1064057. |
[7] | Cummings J , Lee G , Nahed P , Kambar MEZN , Zhong K , Fonseca J , Taghva K ((2022) ) Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement (N Y) 8: (1), e12295. |
[8] | McFarthing K , Buff S , Rafaloff G , Dominey T , Wyse RK , Stott SRW ((2020) ) Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2020. J Parkinsons Dis 10: (3), 757–774. |
[9] | McFarthing K , Rafaloff G , Baptista MAS , Wyse RK , Stott SRW ((2021) ) Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2021 Update. J Parkinsons Dis 11: (3), 891–903. |
[10] | McFarthing K , Rafaloff G , Baptista M , Mursaleen L , Fuest R , Wyse RK , Stott SRW ((2022) ) Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2022 Update. J Parkinsons Dis 12: (4), 1073–1082. |
[11] | World Health Organisation (WHO) International Clinical Trials Registry Platform: https://www.who.int/clinical-trials-registry-platform/the-ictrp-search-portal. |
[12] | Port RJ , Rumsby M , Brown G , Harrison IF , Amjad A , Bale CJ ((2021) ) People with Parkinson’s Disease: What Symptoms Do They Most Want to Improve and How Does This Change with Disease Duration? J Parkinsons Dis 11: (2), 715–724. |
[13] | A trial investigating whether suppressing the immune system with azathioprine slows the progression of Parkinson’s disease; https://www.isrctn.com/ISRCTN14616801. |
[14] | A study to investigate the safety, tolerability, pharmacokinetics, and pharmacodynamics of RO7486967 in participants with early idiopathic Parkinson’s disease; https://www.isrctn.com/ISRCTN85338453. |
[15] | Reardon S ((2023) ) Alzheimer’s drug donanemab: What promising trial means for treatments. Nature. Online ahead of print 10.1038/d41586-023-01537-5. |
[16] | Fang C , Hernandez P , Liow K , Damiano E , Zetterberg H , Blennow K , Feng D , Chen M , Maccecchini M ((2023) ) Buntanetap, a NovelTranslational Inhibitor of Multiple Neurotoxic Proteins, Proves toBe Safe and Promising in Both Alzheimer’s and Parkinson’s Patients. J Prev Alzheimers Dis. 10: (1), 25–33. |
[17] | Pagan FL , Wilmarth B , Torres-Yaghi Y , Hebron ML , Mulki S , Ferrante D , Matar S , Ahn J , Moussa C ((2021) ) Long-Term Safety and Clinical Effects of Nilotinib in Parkinson’s Disease. Mov Disord 36: (3), 740–749. |
[18] | Simuni T , Fiske B , Merchant K , Coffey CS , Klingner E , Caspell-Garcia C , Lafontant DE , Matthews H , Wyse RK , Brundin P , Simon DK , Schwarzschild M , Weiner D , Adams J , Venuto C , Dawson TM , Baker L , Kostrzebski M , Ward T , Rafaloff G ; Parkinson Study Group NILO-PD Investigators and Collaborators ((2021) ) Efficacy of Nilotinib in Patients With Moderately Advanced Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol 78: (3), 312–320. |
[19] | Merchant K , Sullivan J ((2022) ) c-Abl Inhibitors as Disease-ModifyingTherapies for Parkinson’s Disease: Gaps and Opportunities. Mov Disord 37: (1), 3–5. |
[20] | Athauda D , Maclagan K , Skene SS , Bajwa-Joseph M , Letchford D , Chowdhury K , Hibbert S , Budnik N , Zampedri L , Dickson J , Li Y , Aviles-Olmos I , Warner TT , Limousin P , Lees AJ , Greig NH , Tebbs S , Foltynie T ((2017) ) Exenatide once weekly versus placebo in Parkinson’s disease: A randomised, double-blind, placebo-controlled trial. Lancet 390: (10103), 1664–1675. |
[21] | The Parkinson’s Hope List: bit.ly/ParkinsonsHopeList. |
[22] | The Michael J. Fox Foundation for Parkinson’s Research: https://doi.org/10.5281/zenodo.7853180. |
[23] | Devos D , Labreuche J , Rascol O , Corvol JC , Duhamel A , Guyon Delannoy P , Poewe W , Compta Y , Pavese N , Růžička E , Dušek P , Post B , Bloem BR , Berg D , Maetzler W , Otto M , Habert MO , Lehericy S , Ferreira J , Dodel R , Tranchant C , Eusebio A , Thobois S , Marques AR , Meissner WG , Ory-Magne F , Walter U , de Bie RMA , Gago M , Vilas D , Kulisevsky J , Januario C , Coelho MVS , Behnke S , Worth P , Seppi K , Ouk T , Potey C , Leclercq C , Viard R , Kuchcinski G , Lopes R , Pruvo JP , Pigny P , Garçon G , Simonin O , Carpentier J , Rolland AS , Nyholm D , Scherfler C , Mangin JF , Chupin M , Bordet R , Dexter DT , Fradette C , Spino M , Tricta F , Ayton S , Bush AI , Devedjian JC , Duce JA , Cabantchik I , Defebvre L , Deplanque D , Moreau C ; FAIRPARK-IIStudy Group ((2022) ) Trial of Deferiprone in Parkinson’s Disease. N Engl J Med 387: (22), 2045–2055. |




