Can Physical Exercise Be Considered as a Promising Enhancer of Global Cognition in People with Parkinson’s Disease? Results of a Systematic Review and Meta-Analysis
Abstract
Background:
Physical exercise interventions are known to improve quality of life, motor and non-motor symptoms in people with Parkinson’s disease (PD). However, systematic reviews and meta-analyses on cognitive outcomes are rare.
Objective:
To perform a systematic review and meta-analysis of physical exercise intervention effects compared with passive and active control groups (CGs) on global cognition in people with PD.
Methods:
A literature search was performed for randomized controlled trials (RCTs) on physical exercise interventions in PD using nine databases. We included RCTs reporting global cognition outcomes. A meta-analysis was performed using random-effects models and standardized mean differences (SMDs) with 95% confidence intervals (CIs). Bias was assessed with the revised Cochrane Risk of Bias tool and the certainty of evidence was rated using the GRADE approach.
Results:
Seventeen studies (ten with passive, seven with active CGs) were included in the systematic review. Exercise interventions varied considerably between studies. The meta-analysis included nine studies with 236 people with PD (seven with passive, two with active CGs). The SMD was 0.33 (95% CI 0.00; 0.65) demonstrating a small effect (p = 0.05) in favor of physical exercise. Compared with passive CGs, physical exercise had a small non-significant effect (SMD = 0.22, 95% CI –0.14;0.58, p = 0.24). Compared with active CGs, physical exercise had a medium significant effect (SMD = 0.72, 95% CI 0.12;1.33, p = 0.02).
Conclusions:
Physical exercise may increase global cognition in people with PD, but the evidence is very uncertain. Further large-scale RCTs are needed to confirm this finding and to identify the most effective type of physical exercise for improving cognition.
INTRODUCTION
Cognitive dysfunction is a frequent and highly debilitating symptom of Parkinson’s disease (PD). The prevalence of mild cognitive impairment in PD (PD-MCI) is reported to be 25–30%, with 10–20% of people with newly diagnosed PD fulfilling the PD-MCI diagnostic criteria [1]. A recent meta-analysis indicated that the global pooled PD dementia (PDD) prevalence was 26.3% [2], and evidence suggests that at least 75% of people with PD who survive for more than 10 years will develop PDD [3]. Data from another meta-analysis showed that 25% of people with PD and normal cognition converted to PD-MCI within 3 years and that 20% of those with PD-MCI converted to PDD [4]. Moreover, even if cognitive dysfunction is subclinical and cannot be detected using neuropsychological assessments, changes referred to as subjective cognitive impairment have been reported in 28.1% of individuals with newly diagnosed PD [5]. Although non-amnestic PD-MCI (particularly executive dysfunction) may be more frequent than amnestic PD-MCI, memory is also frequently affected, and all other cognitive domains including attention, visuocognition, and language may also have deteriorated [6]. In addition, cognitive dysfunction in PD is associated with individual, societal, and health-economic consequences. It has a negative impact on quality of life for people with PD and their families and leads to greater caregiver burden, a higher probability of nursing home admission, and increased mortality rates [7].
The burden of cognitive dysfunction in PD underlines the need for effective prevention and treatment strategies. While the acetylcholinesterase inhibitor rivastigmine is approved for PDD and memantine is under investigation [8], there are currently no pharmacotherapies to either treat PD-MCI or prevent cognitive decline in people with PD who have normal cognition or subjective cognitive impairment. However, non-pharmacological interventions have been identified as promising approaches for treatment and prevention, and key stakeholders, including people with PD and healthcare professionals, have identified physical exercise and cognitive interventions as particular research priorities [9].
While meta-analyses have provided robust evidence for the positive effects of cognitive training on global cognition and other cognitive outcomes [10, 11], less evidence is available on the effects of physical exercise interventions. Data suggest that physical exercise enhances neuroplasticity and brain health in both motor and cognitive circuits in people with PD [12]. In addition, a systematic review of studies in people with PD suggested that physical exercise may lead to changes in various markers of neuroplasticity, as indicated by improvements in brain function and structure [13]. Accordingly, randomized controlled trials (RCTs) that investigated the effects of treadmill training [14], aerobic exercise [15], and dance [16] reported cognitive benefits in people with PD. However, few systematic reviews of physical exercise interventions in PD have considered cognition as an outcome, and these studies focused on specific types of physical exercise interventions [17–19]. One recent comprehensive network meta-analysis of the effects of physical exercise in PD included cognition as an outcome but did not differentiate between global cognition or any cognitive (sub)domain [20]. The authors concluded that physical exercise (resistance training in particular) may benefit cognition. However, this study did not differentiate between effects in studies with passive versus active control groups (CGs). Such an analysis would allow more specific conclusions to be made on the possible effects of physical exercise.
Consequently, this study aimed to use established Cochrane standards to systematically review evidence on the effects of physical exercise interventions compared with passive and active CGs on cognition in people with PD and, where possible, to perform a meta-analysis. We intended to consider both global cognition and further cognitive (sub-)domains. However, due to its relevance for research and routine clinical practice and the high heterogeneity of considered cognitive (sub-)domains and assessment instruments, we set a focus on global cognition operationalized by cognitive screening instruments in people with PD. We hypothesized that physical exercise interventions have a positive impact on global cognition in people with PD.
MATERIALS AND METHODS
This study is a follow-up to the Cochrane systematic review and network meta-analysis comparing the effects of different physical exercise interventions on the severity of motor signs, quality of life, freezing of gait, and functional mobility in people with PD [21]. In the study reported here, we focused on cognition as an outcome. This systematic review was pre-registered in the international database of prospectively registered systematic reviews in health and social care (PROSPERO; CRD42021262162) and adheres to the PRISMA guidelines for reporting systematic reviews and meta-analyses [22].
Search strategy
The search methods for this review were described in detail in the Cochrane review [21]. An updated search with no language restrictions was conducted by an experienced information specialist on May 10, 2022, using the databases CENTRAL, EMBASE, CINAHL, MEDLINE, PEDro, SPORTDiscus, REHABDATA, and the trial registries clinicaltrials.gov and the International Clinical Trials Registry Platform (ICTRP). Supplementary Table 1 outlines the full search strategy for MEDLINE Ovid as an example.
Eligibility criteria
Study design
We considered RCTs, including cross-over randomized trials, examining the effects of physical exercise interventions compared with passive and/or active CGs on cognition in people with PD for this review. Only full-text articles published in peer-reviewed journals were included. However, conference abstracts and trial registers, if available, were incorporated to provide further information regarding the included trials.
Participants
Studies that included adults (≥18 years) of all sexes with a confirmed clinical diagnosis of idiopathic PD were eligible; no specific PD diagnostic criteria had to be reported. There were no restrictions on PD duration or severity, dopaminergic medication status, or cognitive state; i.e., people without cognitive dysfunction and those with PD-MCI or PDD were considered. Studies involving people with atypical parkinsonism or Lewy body dementia were excluded.
Intervention and control groups
Trials examining the effects of various structured physical exercise interventions composed of at least five supervised sessions were eligible for this review. There was no focus on a specific type of physical exercise. Thus, interventions using different training methods and devices that were delivered in either individual or group settings were considered. Home-based interventions were only eligible if supervision by a physical exercise instructor was provided. Hence, the interventions considered for this review included aqua-based training, dance, endurance training, flexibility training, gait/balance/functional training, exergaming approaches, Lee Silverman Voice Treatment (LSVT) BIG, mind-body training, multi-domain training, and strength/resistance training [21]. When different non-pharmacological approaches were combined within a single intervention program (e.g., physical exercise and cognitive training), physical exercise had to be the main component of the intervention. If a study intervention included motor as well as cognitive components, two authors (AKF, ME, RG, NC) with expertise in physical as well as cognitive interventions discussed the trials and its match regarding the eligibilty criteria until consensus was reached.
Studies comparing exercise interventions with either passive or active CGs were included. Passive CGs were defined as those receiving no structured intervention, i.e., usual care, no intervention, or wait-list control. Active CGs were defined as those with structured non-pharmacological treatment approaches (e.g., health education, communication training, or leisure activities) [21]. However, studies comparing two interventions of the same type (i.e., two physical exercise approaches) were not considered for this review as they could not be included in the meta-analysis.
Outcomes
We searched for studies that included at least one standardized and validated paper-and-pencil or digital neuropsychological instrument to assess cognition. We then categorized the cognitive domains assessed based on the instruments used (i.e., global cognition, memory, executive function, working memory, attention, visuocognition, and language) according to an established compendium of neuropsychological tests [23] and the authors’ neuropsychological expertise. Only studies reporting global cognition were included in the meta-analysis. In addition, only short-term effects were integrated (i.e., assessments conducted≤6 weeks post-intervention) due to limited data and high heterogeneity in the timing of follow-up assessments.
Study selection and data extraction
One review author (ME) initially screened and removed titles that did not meet the inclusion criteria. Then, two review authors (AKF, ME, RG, or NC) independently screened the titles and abstracts and reviewed the full-text articles for eligibility. Data extraction was then performed by the same authors using a standardized data extraction form and all extracted data was double-checked. If discrepancies were found, the two authors discussed the issue or consulted another author to reach a consensus. If data were missing, the first and senior authors of the study in question were contacted via e-mail. Reminder e-mails were sent if necessary. The extracted data consisted of general study information (author/s, publication date, country), study characteristics (trial design, number of trial arms), patient characteristics (baseline sociodemographic and clinical data including severity of motor signs and cognitive state, number of people with PD recruited/allocated/evaluated), information about the intervention (type, dose, frequency, and length) and CG (type, dose, frequency, and length), and cognitive outcomes (instruments used, assessment timings, type of analysis).
Risk of bias
Methodological quality was analyzed independently by two authors (AKF, ME, RG, or NC) for all studies included in the meta-analysis using the revised Cochrane Risk of Bias tool (RoB 2) for RCTs [24]. If discrepancies were found, the two authors discussed the issue or consulted another author until a consensus was reached. Five RoB domains were addressed that can affect RCT results including bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported results. Based on answers to signaling questions for each RoB domain, the tool assesses the risk as ‘low’, ‘high’, or ‘some concern’.
Data analysis
Review Manager (RevMan Version 5.4; Cochrane Collaboration) was used to perform the meta-analysis of the effect of physical exercise versus passive or active CGs on post-intervention global cognition as well as to create a forest plot. Data required for the meta-analysis were post-intervention means, standard deviations (SDs), and the number of people with PD included in the intervention and CG arms of each trial [25]. If the study reported only standard errors or confidence intervals (CIs), SDs for group means were determined according to recommendations from the Cochrane Handbook for Systematic Reviews of Interventions [25]. If a trial included multiple physical exercise treatment arms, these arms were combined and compared with the CG. In studies involving both intention-to-treat (ITT) and per-protocol (PP) analyses, only the ITT data were used in the meta-analysis. In trials reporting no ITT data, PP data were utilized. If data were missing, the study’s corresponding author was contacted via e-mail and, if necessary, reminder e-mails were sent.
One meta-analysis was performed that included two subgroup analyses, one to compare physical exercise intervention with passive CGs and the second to compare intervention with active CGs. We used a random-effects model with inverse variances to calculate standardized mean differences (SMDs) because of the diverse PD populations and different instruments used to assess global cognition, and because the studies were expected to have different effect sizes. For the statistical analyses, SMDs and 95% CIs were computed to compare effect sizes between intervention groups and CGs. Effects from 0.2 to <0.5 were categorized as small, effects from 0.5 to <0.8 were categorized as medium, and effects≥0.8 were categorized as large [26]. The alpha level was set at 0.05 for all analyses. The p-value from the Chi2 test, generalized I2 statistic, and Tau2 were used to address the heterogeneity and inconsistency of the included studies. We interpreted the heterogeneity of the I2 statistic as recommended in the Cochrane Handbook for Systematic Reviews of Interventions [25], whereby 0–40% indicated unimportant/low heterogeneity, 30–60% indicated moderate heterogeneity, 50–90% indicated substantial heterogeneity, and 75–100% indicated considerable heterogeneity. Additionally, a sensitivity analysis was conducted using a fixed-effects model and results from the random- and fixed-effects analyses were compared to test the robustness of the effects. A funnel plot was generated to identify potential publication bias.
Certainty of evidence
The GRADE approach [27] was used to assess the certainty of evidence showing the effect of physical exercise interventions on improving global cognition. This approach considers the following five dimensions: study limitations (RoB), unexplained heterogeneity and inconsistency of effect, imprecision, indirectness, and publication bias. The evidence from RCTs was initially rated as high quality and we then downgraded the evidence quality when important limitations were identified. The GRADE system uses the following criteria for assigning grades of evidence: high (we are very confident that the true effect lies close to that of the estimate of the effect); moderate (we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different); low (our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect); and very low (we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect). The formulation of a statement on the certainty of evidence and the creation of a summary of findings table followed the GRADE recommendations by Santesso et al. [28].
RESULTS
In total, 3,481 records were identified during the updated database searches. After screening, 3,218 records were excluded, leaving 263 full-text articles to be assessed for eligibility. Of these, 246 articles were excluded, resulting in the inclusion of 17 studies in this systematic review. Ten studies compared an exercise intervention with a passive CG, and seven studies used active CGs. Nine studies were included in the meta-analysis, of which seven compared physical exercise with a passive CG and two used an active CG. Figure 1 shows the PRISMA flow diagram and Table 1 presents the characteristics of all studies included in this systematic review.
Fig. 1
PRISMA flow diagram.
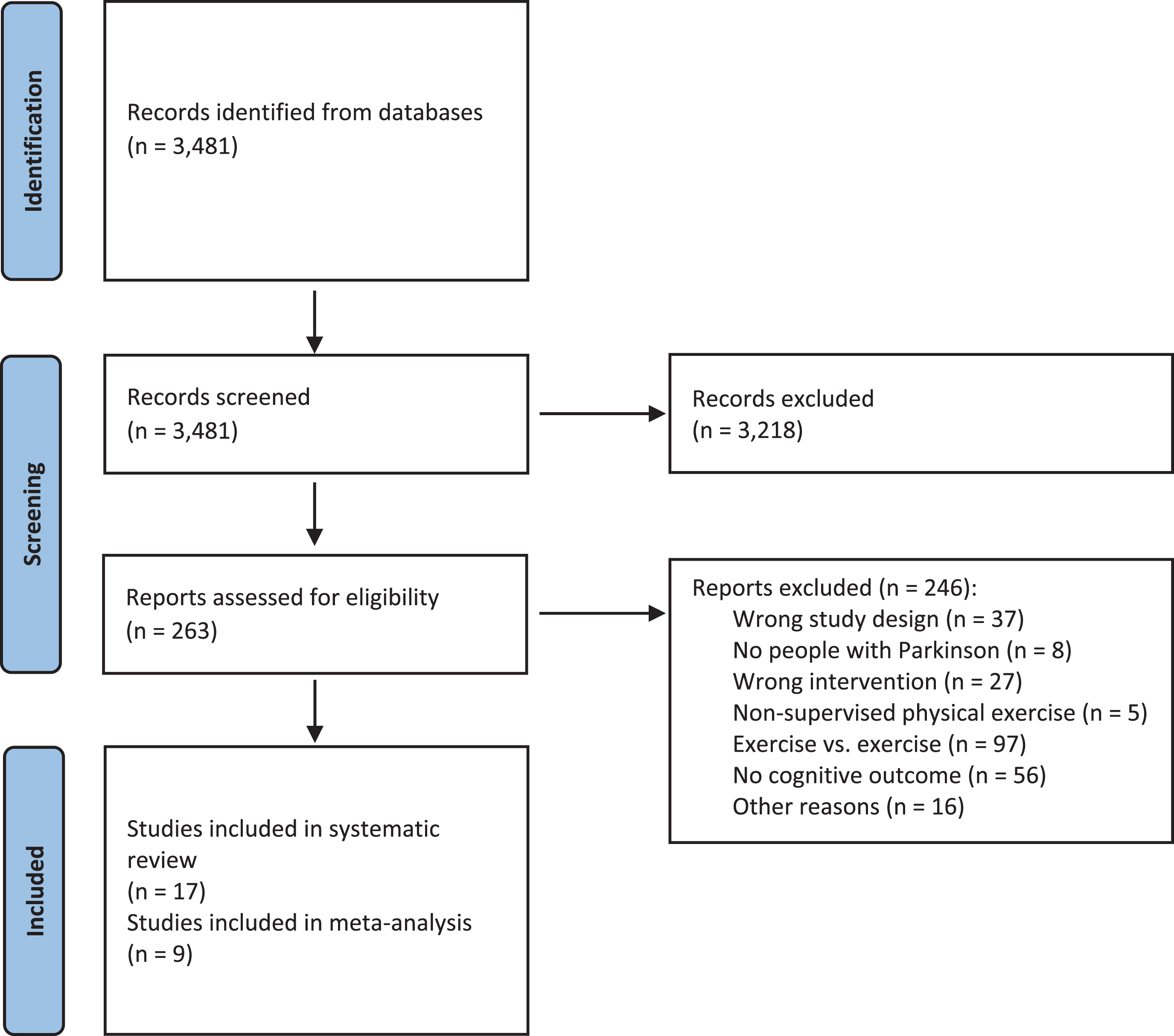
Table 1
Study characteristics
| StudyStudy designParticipants (N) Country | Sample size (n); Sex ratio (F:M) | Age (years) | H&Y; UPDRS-motor; LEDD; duration of disease (years) | Cognitive eligibility criteria | Cognitive screening results at baseline | Intervention and control group(s) | Frequency and durationTotal treatment minutes | Cognitive outcome domains; measurement time points |
| Physical exercise intervention vs. passive control group (N = 10) | ||||||||
| Altmann 2016 [15]RCTRandomized: N = 37Analyzed: N = 30USA | Arm 1: n = 11; NR | Arm 1: 62.8±8.6 | Arm 1: I–III; 23.7±8.7; NR; NR | Exclusion: MCI &dementia (MMSE < 25 points) | Arm 1: MDRS 141.3±1.7 | Arm 1: Aerobic exercise/treadmill training (start: 50% maximal HR; increased by 5% per week to 75% HR) | 20–45 min3×/week16 weeks1,560 min | •Global cognition, executive function, working memory, attention, language•Pre- and post-intervention |
| Arm 2: n = 9; NR | Arm 2: 63.3±7.3 | Arm 2: II–III; 17.5±6.4; NR; NR | Arm 2: MDRS 141.3±2.1 | Arm 2: Stretch-balance group (most performed while sitting) | ||||
| Arm 3: n = 10; NR | Arm 3: 67.8±9.8 | Arm 3: I–III; 20.6±11.5; NR; NR | Arm 3: MDRS 139.9±4.0 | Arm 3: Passive CG | N/A | |||
| Avenali 2021 [34]RCTRandomized: N = 40Analyzed: N = 34Italy | Arm 1: n = 15; 8 : 7 | Arm 1: 73.2±7.1 | Arm 1: 2.5±0.5; 33.7±10.2; NR; 9.7±5.4 | Inclusion: PD-MCI according to Litvan criteria | Arm 1: MoCA 18.8±3.0 | Arm 1: Individualized physical therapy including different exercise modalities (e.g., aerobic exercises, treadmill training) | 60 min6×/week4 weeks1,440 min | •Global cognition, executive function, attention, fluid intelligence•Pre- and post-intervention; 6-month FU |
| Arm 2: n = 19; 5 : 14 | Arm 2: 71.6±6.0 | Arm 2: 2.3±0.6; 34.1±4.0; NR; 9.5±1.7 | Arm 2: MoCA 18.0±1.5 | Arm 2: Passive CG | N/A | |||
| Cheung 2018 [29]Delayed-start RCTRandomized: N = 20Analyzed: N = 20USA | Arm 1: n = 10; NR | Arm 1: 63.5±8.5 | Arm 1: 2.0±0.8; 25.6±6.9; NR; range: 1–15 years | Exclusion: “significant cognitive impairment” (MMSE < 26 points) | Arm 1: MoCA 26.9±2.2 | Arm 1: Hatha yoga program including yoga postures, breathing techniques &mindfulness meditation | 60 min2×/week12 weeks1,440 min | •Global cognition•Pre- and post-intervention; 6-month FU |
| Arm 2: n = 10; NR | Arm 2: 65.8±6.6 | Arm 2: 2.0±0.8; 24.4±7.2; NR; range: 1–10 years | Arm 2: MoCA 26.1±2.4 | Arm 2: Wait-list CG | N/A | |||
| de Oliveira 2017 [33]RCTRandomized: N = 24Analyzed: N = 23Brazil | Arm 1: n = 7; 3 : 4 | Arm 1: 74.9±5.1 | Arm 1: 2.3±0.7; NR; NR; 4.4±2.8 | Excluded: “dementia of any kind” according to MMSE cutoff points followed by neurologist diagnosis | Arm 1: MMSE 25.1±2.8 | Arm 1: Individualized multi-domain exercise associated with cognitive tasks (Borg scale scores between 11 [fairly light] and 14 [somewhat hard]) | 60 min2×/week24 weeks2,880 min | •Executive function, fluid intelligence•Pre- and post-intervention |
| Arm 2: n = 8; 2 : 6 | Arm 2: 74.5±9.4 | Arm 2: 2.1±0.7; NR; NR; 4.3±1.5 | Arm 2: MMSE 26.3±2.9 | Arm 2: Group multi-domain exercise associated with cognitive tasks (Borg scale scores between 11 [fairly light] and 14 [somewhat hard]) | ||||
| Arm 3: n = 8; 5 : 3 | Arm 3: 68.4±6.3 | Arm 3: 1.9±0.6; NR; NR; 4.0±1.1 | Arm 3: MMSE 24.9±2.4 | Arm 3: Passive CG | N/A | |||
| Harvey 2019 [30]Delayed-start RCTRandomized: N = 20Analyzed: N = 18UK | Arm 1: n = 10; 6 : 4 | Arm 1: 68.0±7.8 | Arm 1: I–III; NR; NR; NR | None | Arm 1: MoCA 22.9±3.6 | Arm 1: High-intensity interval training on Speed flex machines (≥85% maximal HR; adapted according to individual performance) | 45 min3×/week12 weeks1,620 min | •Global cognition•Pre- and post-intervention; 6-week FU |
| Arm 2: n = 10; 6 : 4 | Arm 2: 69.0±6.0 | Arm 2: I–III; NR; NR; NR | Arm 2: MoCA 25.1±3.5 | Arm 2: Wait-list CG | N/A | |||
| Pohl 2013 [31]RCTRandomized: N = 18Analyzed: N = 16Sweden | Arm 1: n = 12; NR | Total: 68.2±5.1 | Total: 2.4±0.7; NR; NR; 8.8±3.8 | None | NR | Arm 1: Ronnie Gardiner Method (group-based) | 60 min2×/week6 weeks720 min | •Memory, executive function, attention, visuocognition, language•Pre- and post-intervention |
| Arm 2: n = 6; NR | NR | Arm 2: Passive CG | N/A | |||||
| Pohl 2020 [36]RCTRandomized: N = 51Analyzed: N = 46Sweden | Arm 1: n = 26; 7 : 19 | Arm 1: 69.7±7.0 | Arm 1: I–III; 34.0±12.9; 727.7±327.3; 6.0±4.4 | None | Arm 1: MoCA 25.5±2.8 | Arm 1: Ronnie Gardiner Method (group-based) | 60 min2×/week12 weeks1,440 min | •Global cognition, memory, executive function, attention•Pre- and post-intervention; 3-month FU |
| Arm 2: n = 20; 7 : 13 | Arm 2: 70.4±6.0 | Arm 2: I–III; 28.6±10.4; 690.0±231.0; 6.8±3.6 | Arm 2: MoCA 25.0±3.3 | Arm 2: Passive CG | N/A | |||
| Silveira 2018 [32]RCTRandomized: N = 76Analyzed: N = 58Canada | Arm 1: n = 22; 3 : 18 | Arm 1: 70.6±9.3 | Arm 1: NR; 25.4±8.1; 710.63±425.70; 6.0±5.1 | None | Arm 1: MoCA 25.2±4.5 | Arm 1: Aerobic (i.e., ergometer) training (start: 40–50% maximal HR; increased to 60–70% HR) | 60 min3×/week12 weeks2,160 min | •Memory, executive function, working memory, attention, visuocognition, language•Pre- and post-intervention |
| Arm 2: n = 21; 9 : 12 | Arm 2: 69.8±8.3 | Arm 2: NR; 27.6±9.7; 482.91±342.67; 6.1±4.2 | Arm 2: MoCA 24.6±4.0 | Arm 2: Goal-based multimodal training (PD SAFExTM); progressively increase of the difficulty level | ||||
| Arm 3: n = 15; 4 : 11 | Arm 3: 67.6±8.3 | Arm 3: NR; 21.8±9.2; 759.70±560.19; 5.6±5.7 | Arm 3: MoCA 25.8±5.0 | Arm 3: Passive CG | N/A | |||
| Solla 2019 [16]RCTRandomized: N = 20Analysed: N = 19Italy | Arm 1: n = 10; 4 : 6 | Arm 1: 67.8±5.9 | Arm 1: 2.1±0.6; 13.7±7.2; 481.1±213.1; 4.4±4.5 | Inclusion: MMSE≥24 points | Arm 1: MoCA 25.0±4.0 | Arm 1: Sardinian folk dance | 90 min2×/week12 weeks2,160 min | •Global cognition•Pre- and post-intervention |
| Arm 2: n = 10; 3 : 7 | Arm 2: 67.1±6.3 | Arm 2: 2.3±0.4; 14.7±7.0; 487.5±198.5; 5.0±2.9 | Arm 2: MoCA 25.7±2.8 | Arm 2: Passive CG | N/A | |||
| Youm 2020 [35]RCTRandomized: N = 23Analyzed: N = 17Korea | Arm 1: n = 10; 4 : 6 | Arm 1: 68.0±6.8 | Arm 1: 2.4±0.3; 40.4±10.9; 561.0±274.6; 6.4±3.6 | Inclusion: MMSE > 24 points | Arm 1: MMSE 26.6±2.8 | Arm 1: Progressive trunk resistance and stretching exercise program (RPE was increased every 3 weeks from 2–3 to 5–6) | 60–90 min3×/week12 weeks2,700 min | •Global cognition•Pre- and post-intervention |
| Arm 2: n = 7; 3 : 4 | Arm 2: 72.1±6.0 | Arm 2: 2.3±0.4; 44.4±8.8; 852.9±564.4; 8.0±4.0 | Arm 2: MMSE 27.6±1.3 | Arm 2: Passive CG | N/A | |||
| Physical exercise intervention vs. active control group (N = 7) | ||||||||
| Albrecht 2021 [42]RCTRandomized: N = 95Analyzed: N = 65Sweden | Arm 1: n = 34; 14 : 20 | Arm 1: 70.3±5.8 | Arm 1: 2.1±0.3; 31.6±12.9; 610.50±355.83; 5.7±4.6 | Inclusion: MoCA≥21 points | Arm 1: MoCA 26.1±2.6 | Arm 1: Group-based balance and gait training (HiBalance) + home exercise; progressively increase of the difficulty level | 60 min2×/week10 weeks& 60 min homework 1×/week1,800 min | •Memory, executive functions, working memory, attention•Pre- and post-intervention |
| Arm 2: n = 31; 11 : 20 | Arm 2: 70.5±6.1 | Arm 2: 2.2±0.4; 28.0±10.0; 458.30±293.26; 4.5±3.5 | Arm 2: MoCA 25.9±2.4 | Arm 2: Group-based speech and communication training (HiCommunication) + home exercise; progressively increase of the difficulty level | ||||
| Gobbi 2021 [40]RCTRandomized: N = 152Analyzed: N = 107Brazil | Arm 1: n = 42; 19 : 23 | Arm 1: 68.8±9.5 | Arm 1: 1.7±0.4; 25.4±8.8; 557.9±413.3; 6.3±4.5 | Exclusion: “Cognitive impairment assessed by the MMSE” | Arm 1: MMSE 28.2±1.5 | Arm 1: Multimodal program | 60 min2×/week32 weeks3,840 min | •Global cognition, memory, executive function, working memory, attention, visuocognition•Pre- and post-intervention; intermediate assessment after 4 of 8 intervention months |
| Arm 2: n = 33; 16 : 17 | Arm 2: 69.8±7.5 | Arm 2: 1.7±0.5; 23.7±9.9; 552.3±385.2; 5.5±2.7 | Arm 2: MMSE 28.0±2.1 | Arm 2: Functional mobility program (conduct of tasks at individual maximum) | ||||
| Arm 3: n = 36; 21 : 15 | Arm 3: 68.9±7.6 | Arm 3: 1.7±0.5; 24.5±8.9; 605.8±376.1; 6.0±3.5 | Arm 3: MMSE 27.7±2.0 | Arm 3: Mental/leisure program | ||||
| Hasegawa 2020 [37]Cross-over RCTRandomized: N = 94Analyzed: N = 86USA | Total: n = 86; 28 : 58 | Total: 68.8±7.6 | Total: I-IV; 42.3±12.2; NR; 6.5±5.0 | None | Total: MoCA 25.6±3.5 | Arm 1: Agility Boot Camp with Cognitive Challenges (ABC-C) intervention; progressively increase of the difficulty level | 90 min3×/week6 weeks1,620 min | •Global cognition•Pre- and post-intervention |
| Arm 2: Health education | 90 min1×/week6 weeks640 min | |||||||
| Johansson 2020 [38]RCTRandomized: N = 13Analyzed: N = 12Sweden | Arm 1: n = 7; 1 : 6 | Arm 1 (Median, min–max): 72.0 (60–78) | Arm 1 (Median, min–max): 2 (2–3); 35.0 (24–46); 700 (380–920); 10.0 (3–13) | Inclusion: MoCA≥21 points | Arm 1 (Median, min–max): MoCA 27.0 (26–30) | Arm 1: Group-based balance and gait training (HiBalance) + home exercise; progressively increase of the difficulty level | 60 min2×/week10 weeks& 60 min homework 1×/week1,800 min | •Memory, executive function, working memory, attention, visuocognition•Pre- and post-intervention |
| Arm 2: n = 6; 3 : 3 | Arm 2 (Median, min–max): 67.5 (63–70) | Arm 2 (Median, min–max): 2.5 (2–3); 32.5 (22–52); 765.5 (525–1171); 7.0 (3–11) | Arm 2 (Median, min–max): MoCA 26.5 (21–28) | Arm 2: Group-based speech and communication training (HiCommunication) + home exercise; progressively increase of the difficulty level | ||||
| Michels 2018 [39]RCTRandomized: N = 13Analyzed: N = 13USA | Arm 1: n = 9; NR | Arm 1: 66.4±NR | Arm 1: 2.1±0.3; 27.6±11.6; NR; NR | Exclusion: “significant cognitive impairment” (MoCA < 24 points) | Arm 1: MoCA 27.0±2.2 | Arm 1: Individualized dance therapy; adapted to individuals’ capacity | 60 min1×/week10 weeks600 min | •Global cognition•Pre- and post-intervention |
| Arm 2: n = 4; NR | Arm 2: 75.5±NR | Arm 2: 2.5±1.0; 40.8±8.7; NR; NR | Arm 2: MoCA 25.3±1.5 | Arm 2: Traditional talk therapy support group | ||||
| Picelli (2016) [14]RCTRandomized: N = 17Analyzed: N = 17Italy | Arm 1: n = 9; 4 : 5 | Arm 1: 71.2±9.2 | Arm 1: III; NR; NR; 11.2±5.6 | Inclusion: MMSE < 24 points | Arm 1 (Median, IQR): MoCA 24.0 (18.5–27.0) | Arm 1: Treadmill training (increase of speed 1.0 km/h up to 2.0 km/h in each session) | 45 min3×/week4 weeks540 min | •Global cognition, executive function, working memory, attention•Pre- and post-intervention |
| Arm 2: n = 8; 4 : 4 | Arm 2: 71.6±7.2 | Arm 2: III; NR; NR; 10.8±4.1 | Arm 2 (Median, IQR): MoCA 23.0 (20.25–26.0) | Arm 2: Regular social interactions according to a lifestyle program | ||||
| Silva-Batista 2018 [41]RCTRandomized: N = 39Analyzed: N = 39Brazil | Arm 1: n = 13; 3 : 10 | Arm 1: 64.1±9.1 | Arm 1: 2.5±0.5; 43.7±13.4; 835.8±287.0; 9.6±3.9 | Inclusion: “not having cognitive impairment” (MMSE < 23 points) | Arm 1: MoCA 21.8±4.3 | Arm 1: Resistance training; training load progressed from high-volume low-intensity to low-volume high intensityloads throughout the 12 weeks | 50 min2×/week12 weeks1,200 min | •Global cognition•Pre- and post-intervention |
| Arm 2: n = 13; 3 : 10 | Arm 2: 64.2±10.6 | Arm 2: 2.5±0.4; 45.1±8.2; 875.9±223.4; 10.5±4.1 | Arm 2: MoCA 20.8±3.2 | Arm 2: Resistance training with instability; trainingload progressed from high-volume low-intensity to low-volume high intensityloads throughout the 12 weeks | ||||
| Arm 3: n = 13; 4 : 9 | Arm 3: 64.2±8.3 | Arm 3: 2.5±0.4; 43.4±8.6; 796.7±151.3; 10.7±6.1 | Arm 3: MoCA 22.7±5.7 | Arm 3: Bingo &PD-associated education | 60 min1×/week12 weeks720 min | |||
CG, control group; F, female; FU, follow-up; H&Y, Hoehn and Yahr; HR, heart rate; IQR, interquartile range; LEDD, Levodopa equivalent daily dose; M, male; MCI, mild cognitive impairment; MDRS, Mattis Dementia Rating Scale; MMSE, Mini-Mental Status Examination; MoCA, Montreal Cognitive Assessment; NR, not reported; PD, Parkinson’s disease; RCT, randomized controlled trial; RPE, rating of perceived exertion; UPDRS, Unified Parkinson’s Disease Rating Scale.
Characteristics of studies with passive CGs
Two of the 10 studies [29, 30] were RCTs with delayed-start designs, while the other trials were standard RCTs. The number of participants in studies with passive CGs ranged from 18 [31] to 76 [32]. The mean age of participants in the intervention arms ranged from 62.8 [15] to 74.9 years [33], with an average age of 63–70 years. Six studies included more male than female participants and three studies did not report the sex ratio [15, 29, 31]. All studies reported on clinical diagnoses of their study participants; in four studies PD diagnoses according to UK Brain Bank Criteria were available for the included participants [15, 30, 34, 35]. Disease severity was mild to moderate in all trials, with mean Hoehn and Yahr stages ranging from 1.9 [33] to 2.5 [34]. Only four studies reported the Levodopa equivalent daily dose (LEDD) of the included participants [16, 32, 35, 36] ranging from 481.1 [16] to 852.9 mg [36]; in seven studies a stable PD medication status of the people with PD was required as a precondition for study participation [15, 16, 29, 30, 31, 34, 36]. Mean disease duration ranged from 4.0 [33] to 9.7 years [34].
Four studies did not define any cognitive inclusion or exclusion criteria for the study population [30–32, 36], while the study by Avenali et al. [34] only considered people with PD-MCI. In four studies, cognitive dysfunction was defined as an exclusion criterion using Mini-Mental State Examination (MMSE) cut-off scores from <24 to 26 points [15, 16, 29, 33, 35]. The relatively high cognitive performance level of the included individuals was also reflected in the mean total baseline cognitive screening scores, except in the study by Avenali et al. [34]. These authors included people with PD who had clearer signs of cognitive dysfunction, with mean Montreal Cognitive Assessment (MoCA) scores of 18.8±3.0 and 18.0±1.5 for the intervention and control arms, respectively.
In three of 10 studies, two physical exercise study arms were compared with one passive control arm [15, 32, 33]. All other studies compared a single experimental arm with one passive CG. In five studies, the intervention arms involved multimodal exercise. These included stretch-balance groups [15], physical therapy [34], “multidomain exercise” associated with cognitive tasks with intensity levels ranging from “fairly light” to “somewhat hard” [33], goal-based multimodal training (PD-SAFExTM) [32], and a combination of resistance and stretching elements with a progressively increase of the difficulty level [35]. Three studies examined the effects of endurance training with moderate to vigorous exercise intensities that were increased throughout the intervention period [15, 30, 32], while three investigated the impact of a dance intervention [16, 31, 36]. Finally, one study examined the effects of a mindfulness-based approach using Hatha yoga [29].
The total duration of the exercise ranged from 720 [31] to 2,880 minutes [33]. Treatment duration ranged from 4 [34] to 24 weeks [33], with a frequency ranging from twice per week [16, 31, 33, 36] to six times per week [34]. Session duration ranged from 20–45 minutes [15] to 90 minutes [16, 35].
All studies assessed cognitive function pre- and post-intervention. Four studies included additional follow-up assessments at 6 weeks [30] to 6 months [29, 34] post-intervention. Global cognition was measured in seven of 10 studies [15, 16, 29, 30, 34–36], mostly using the MoCA, but also the MMSE and the Mattis Dementia Rating Scale (MDRS). Six studies used various instruments to operationalize other cognitive domains, including memory (three studies: [31, 32, 36]), executive function (six studies: [15, 31, 32–34, 36]), working memory (two studies: [15, 32]), fluid intelligence (two studies: [33, 34]), attention (five studies: [15, 31, 32, 34, 36]), visuocognition (two studies: [31, 32]), and language (three studies: [15, 31, 32]) (for details see Supplementary Table 2). Since all studies used a passive comparator, people in these control arms received treatment as usual.
Characteristics of studies with active CGs
One of the seven studies with an active CG used a cross-over RCT design [37]; all other studies were standard RCTs. The number of participants in studies with active CGs ranged from 13 [38, 39] to 152 [40]. The mean age of participants in the intervention arms ranged from 64.1 [41] to 75.5 years [39]. Five studies included more male than female participants and one study did not report the sex ratio [39]. All studies reported on clinical diagnoses of their study participants; in three studies PD diagnoses according to UK Brain Bank Criteria were available for the included participants [14, 40, 41]. Disease severity was mild to moderate in all trials, with mean Hoehn and Yahr stages ranging from 1.7 [40] to 2.5 [39, 41]. Only four studies reported the Levodopa equivalent daily dose (LEDD) of the included participants [38, 40, 41, 42] ranging from 458.3 [42] to 875.9 mg [41]; in four studies a stable PD medication status of the people with PD was required as a precondition for study participation [14, 37, 39, 42]. Mean disease duration ranged from 4.5 [42] to 11.2 years [14].
One study did not define any cognitive inclusion or exclusion criteria for the study population [37]. All other studies included people with PD with no cognitive dysfunction or MCI, which was defined, for example, by cut-off scores from cognitive screening instruments. Cognitive status was reflected by baseline scores from cognitive screening, which ranged from 20.8 [41] to 27.0 [39] on the MoCA, and from 27.7 to 28.2 on the MMSE in the intervention and control arms of the RCT conducted by Gobbi et al. [40].
In two of the seven studies, two physical exercise study arms were compared with one active control arm [40, 41], all other studies used a single experimental arm and one active CG. Three studies assessed the effects of balance and gait training [14, 38, 42] and two investigated the benefits of multimodal training [37, 40]. Dance [39] and resistance training [41] were each investigated in one study. Most studies reported on a progressively increase of the difficulty level with each intervention session [39] or throughout the intervention period [37, 38, 41, 42].
The total duration of the exercise ranged from 540 [14] to 3,840 minutes [40]. Treatment duration ranged from 4 [14] to 32 weeks [40], with frequencies ranging from once per week [39] to three times per week [14, 37]. Session duration was 45 [14] to 90 minutes [37].
All studies assessed cognitive function pre- and post-intervention. One study also assessed mid-intervention effects after 16 weeks of a 32-week intervention [40]. No studies conducted follow-up assessments. Global cognition was measured in five of seven studies [14, 37, 39–41]; four used the MoCA, and one [40] used the MMSE. Four studies used various test instruments to test other cognitive functions, including memory (three studies: [38, 40, 42]), executive function as well as working memory and attention (four studies: [14, 38, 40, 42]), fluid intelligence (one study: [40]), visuocognition (two studies: [38, 40]). Language was not assessed in any study (for details see Supplementary Table 2).
All studies used an active comparator. Various approaches were used in the active CGs, including speech and communication training [38, 42], a mental/leisure program [40], health education [37], a talk therapy support group [39], a lifestyle program [14], and bingo and PD-associated education [41].
Meta-analysis of the effects of physical exercise on global cognition
The meta-analysis of the short-term effects of physical exercise versus CGs on global cognition included nine studies with a total of 236 people with PD (Fig. 2). The SMD was 0.33 (95% CI 0.00–0.65) demonstrating a small effect (p = 0.05) in favor of physical exercise. Heterogeneity was low (I2 = 29%). The sensitivity analysis, which used a fixed-effects model, confirmed the robustness of this result (Supplementary Figure 1). Thus, physical interventions may have a positive impact on global cognition in people with PD, but the evidence is very uncertain.
Fig. 2
Short-term effects of physical exercise interventions vs. passive and active control groups on global cognition. MDRS, Mattis Dementia Rating Scale; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment.
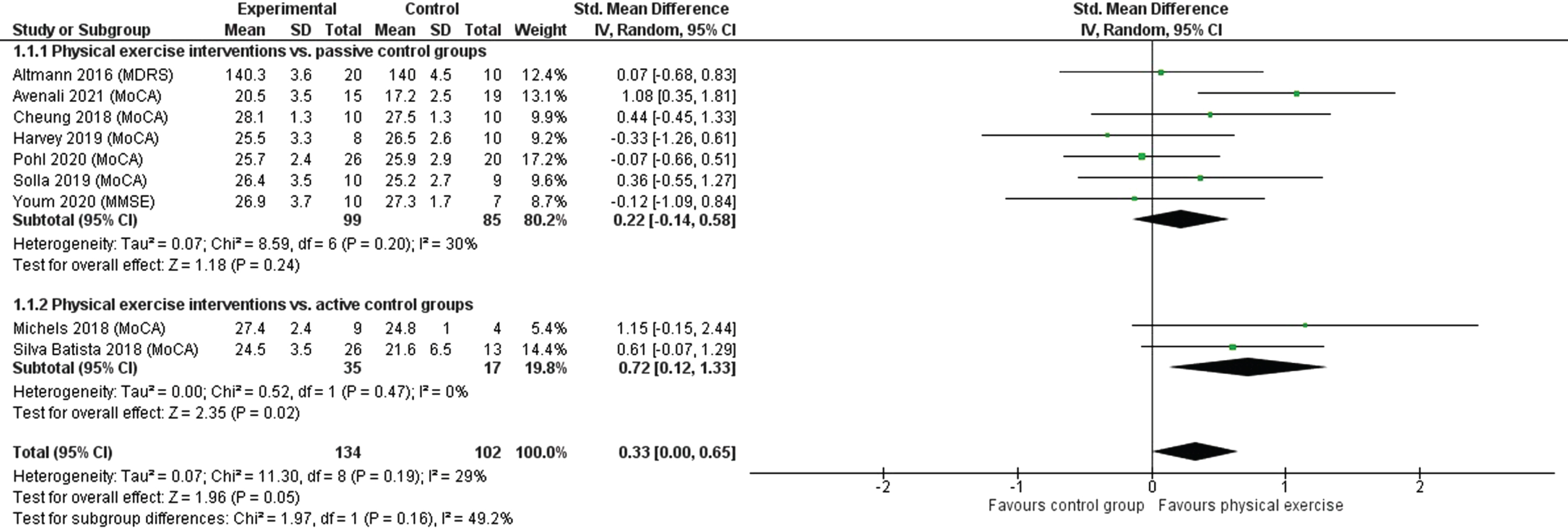
Subgroup analyses examining different types of CGs revealed that physical exercise had a small, non-significant effect (p = 0.24) compared with passive CGs. The analysis included seven studies and 184 people with PD (SMD = 0.22; 95% CI 0.14–0.58). Heterogeneity was again low (I2 = 30%). The comparison of physical exercise with active CGs included two studies with 52 people with PD and showed that exercise had a medium significant effect (p = 0.02). The SMD was 0.72 (95% CI 0.12–1.33) and no heterogeneity was detected (I2 = 0%). Both subgroup analyses showed robustness when comparing the random- and fixed-effects models in a sensitivity analysis (Supplementary Figure 1). When converting the SMD into mean differences (MD) in MoCA scores (range 0–30 points with higher scores indicating better global cognition), a clinically meaningful difference, which has been defined as ≥1.73 points [43], was demonstrated (MD = 1.87; 95% CI 0.31–3.46).
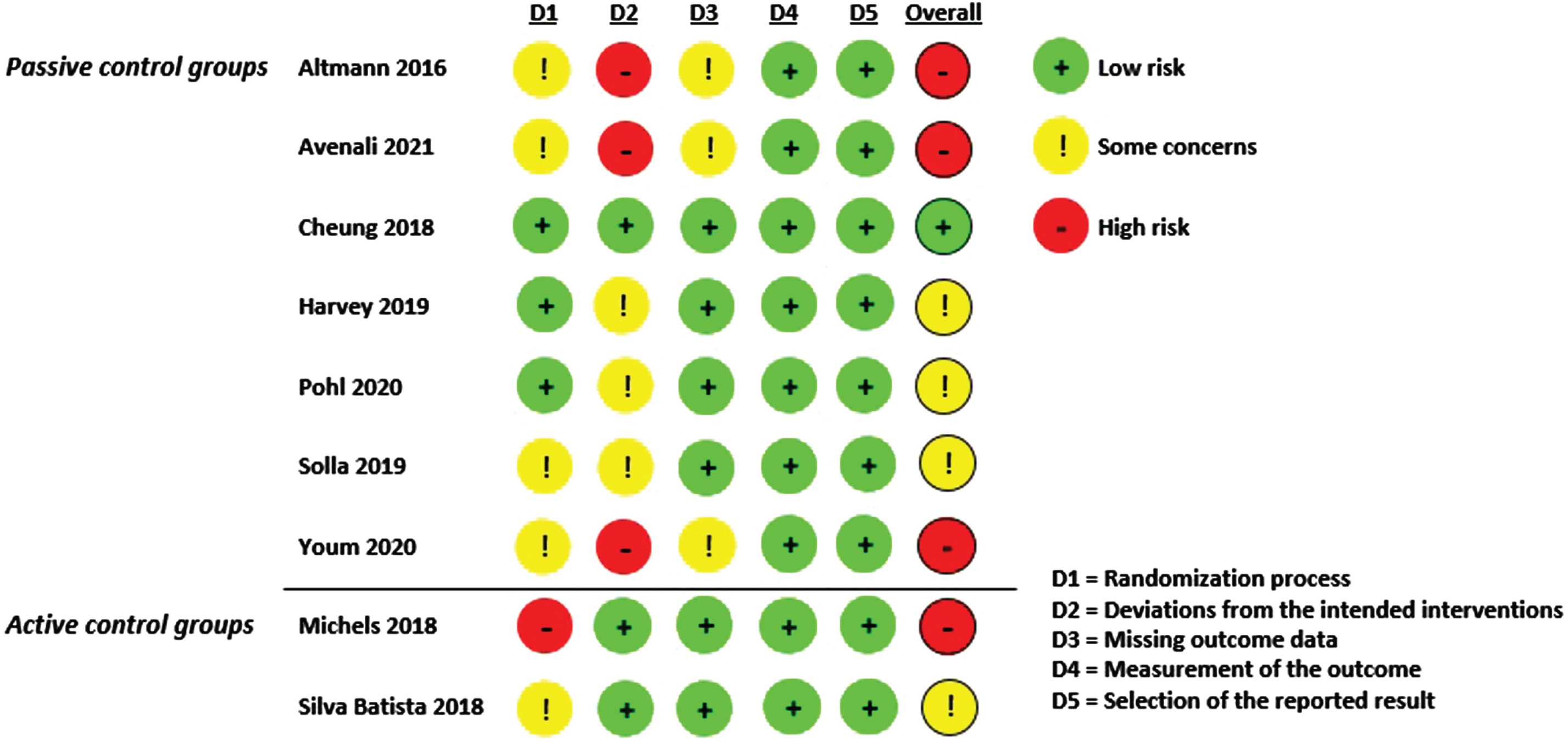
Risk of bias
Evaluation of the studies for bias using the RoB 2.0 tool is shown in Fig. 4 and Supplementary Figure 2. Four studies were judged to have a high risk of bias [15, 34, 35, 39] and four caused some concerns for bias [16, 30, 36, 41]. One [29] of the nine was judged to have a low risk of bias. In three cases, the high risk
of bias was driven by deviations from the intended interventions due to high dropout rates (>10%) and the lack of ITT analyses [15, 34, 35]. These findings led to some concerns about the risk of bias due to missing outcomes data. Three studies did not report an ITT analysis, but since dropout rates were ≤10% there was only some concern for risk of bias [16, 30, 36]. In six studies, the reporting of the randomization process and the allocation concealment was inappropriate [15, 16, 34, 35, 39, 41]; however, only one study had baseline differences between the intervention and control arms that suggested a randomization problem [39]. No risk of bias in terms of measurement of the outcome (standardized and validated cognitive screening instruments were used to operationalize global cognition in all studies) or selection of the reported result was identified in any of the nine studies included in the meta-analysis.
Publication bias
No evidence of publication bias was found, and the funnel plot appeared reasonably symmetrical (Fig. 3).
Fig. 3
Funnel plot: Physical exercise vs. passive and active control groups.
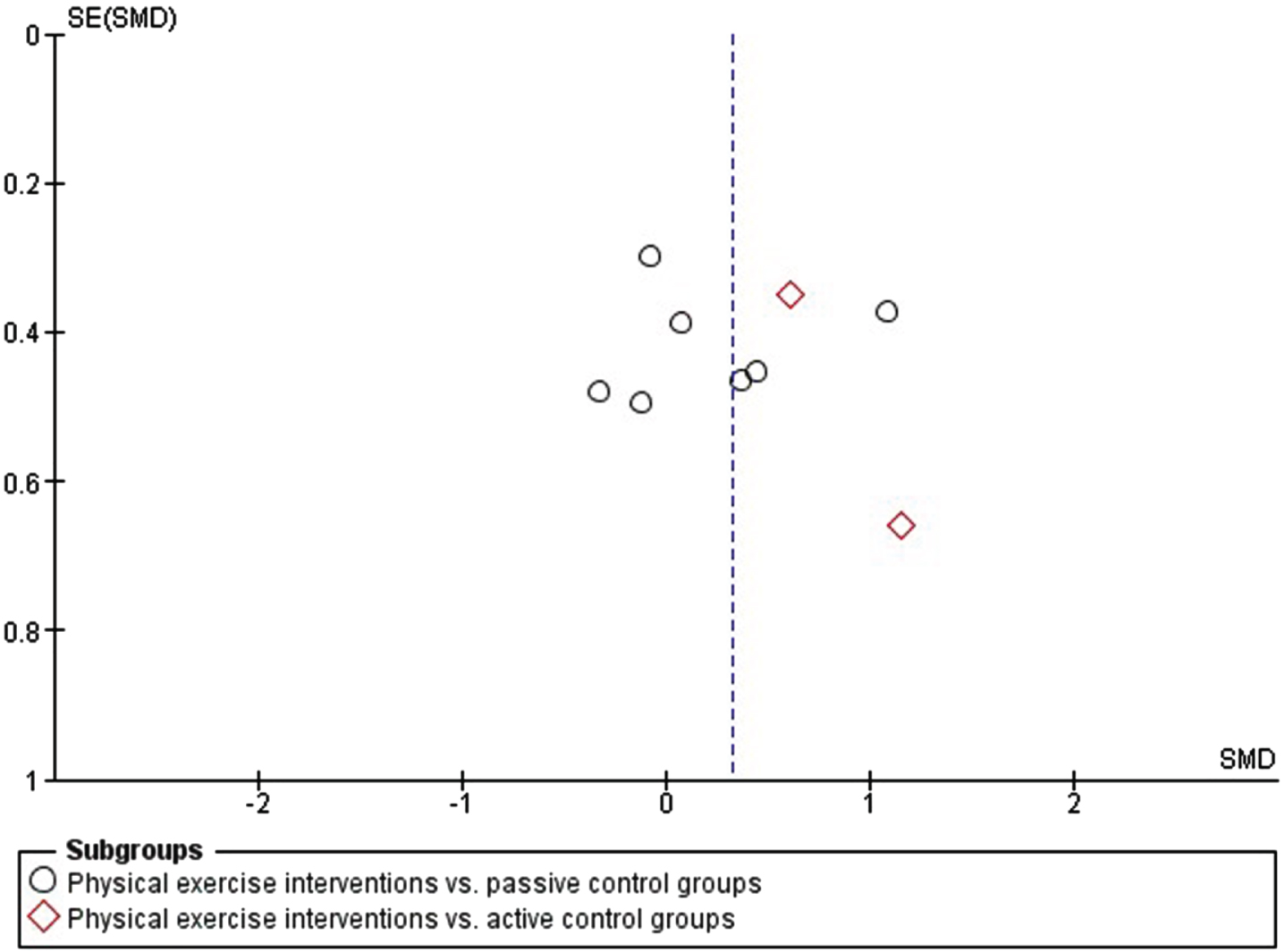
Fig. 4
Risk of bias summary.
Certainty of evidence
For both subgroup analyses (physical exercise interventions versus passive and active CGs, respectively) we assessed the short-term effects (<6 weeks post-intervention) on global cognition operationalized with standardized, validated cognitive screening instruments (MDRS, MMSE, and MoCA). Since all included studies were RCTs, we started the assessment with high certainty for evidence. As a high risk of bias was evident in three of seven studies with passive CGs, and in one of two studies with active CGs, the certainty of evidence was downgraded by one level for both comparisons. Although the I2-statistic revealed low heterogeneity for the comparison of physical exercise and passive CGs, the forest plot suggested substantial heterogeneity between study effects. Thus, we downgraded one level for inconsistency in the passive CG analysis. Due to particularly small study populations in the two subgroup analyses (ranging from 13 to 46 people with PD) we downgraded the certainty of evidence by two levels for both. No problems were observed regarding the indirectness of the effects or potential publication bias. Table 2 provides a summary of these findings.
Table 2
Summary of findings comparing physical exercise interventions with passive and active control groups including assessment of the certainty of evidence
| Patient or population: people with PD | ||||||
| Interventions: physical exercise interventions including endurance training, balance/gait/functional training, resistance training, dance, mind-body training, multimodal training approaches | ||||||
| Comparison: passive and active CGs | ||||||
| Outcomes: global cognition assessed with cognitive screening instruments (minimally clinically meaningful difference: 1.73 points for the MoCA total score [43]*) | ||||||
| Settings: not specified (i.e., outpatient and inpatient care; home-based) | ||||||
| Outcome | Anticipated absolute effects (95% CI) | Estimated absolute effects on global cognition (SMD and 95% CI) | Number of patients and studies | Quality of the evidence (GRADE) | Comments | |
| Risk in the CG** | Risk in the intervention group*** | |||||
| Global cognition, short-term effects (<6 weeks post-intervention) vs. passive CG assessed with the cognitive screening instruments MoCA, MMSE, and MDRS | m = 23.7 (5 studies) | MD = 0.57 (95% CI 0.36; 1.51) | SMD = 0.22 (95% CI 0.14; 0.58) | n = 1847 RCTs |  Very lowa,b,c Very lowa,b,c | Physical exercise interventions compared to passive CGs may increase global cognition in people with PD, but the evidence is very uncertain. |
| Global cognition, short-term effects (<6 weeks post-intervention) vs. active CG assessed with the cognitive screening instrument MoCA | m = 22.35 (2 studies) | MD = 1.87 (95% CI 0.31; 3.46) | SMD = 0.72 (95% CI 0.12; 1.33) | n = 522 RCTs |  Very lowa,c Very lowa,c | Physical exercise interventions compared to active CGs may increase global cognition in people with PD, but the evidence is very uncertain. |
CG: Control group; CI: Confidence interval; MD: Mean differences; MDRS: Mattis Dementia Rating Scale; MMSE: Mini-Mental State Examination; MoCA: Montreal Cognitive Assessment; PD: Parkinson’s disease; RCT: Randomized controlled trials; SMD: Standardized mean differences. *A difference of 1.73 points or larger in the MoCA total score is indicative for a clinically meaningful difference [43]. MoCA scores range between 0 and 30 points with higher scores indicating better global cognition. **Weighted mean (post-intervention) of the studies that measured global cognition with the MoCA. ***Scores were rescaled from SMDs to MD of the MoCA total score using a SD of 2.6 (pooled from the MoCA total score baseline SDs used in by Krishnan et al. [43]). Reasons for downgrading the quality of evidence (GRADE): aDowngraded by one level for risk of bias as the majority of studies was judged to be at high risk of bias (cf. Fig. 4). bDowngraded by one level for inconsistency as heterogeneity was present (cf. Fig. 2). cDowngraded by two levels for imprecision as sample sizes were small (ranging from 13 to 46 people with PD per study).
DISCUSSION
The aim of this study was to perform a systematic review and meta-analysis of the effects of physical exercise interventions on global cognition in people with PD. The main findings are as follows. (i) Few RCTs investigating physical exercise in people with PD have included cognitive outcomes (specifically global cognition); consequently, only 10 studies with passive CGs and seven with active CGs were included in our systematic review. Of these, seven and two studies, respectively, were included in the meta-analysis. (ii) The studies had high heterogeneity regarding type of intervention, exercise intensity, frequency and duration, and cognitive outcomes. (iii) The MoCA was the most frequently used instrument for assessing global cognition. (iv) Compared with passive CG, physical exercise interventions had a small non-significant effect on global cognition, while the comparison with active CGs showed a medium significant effect favoring physical exercise that was clinically meaningful. (v) According to GRADE guidance, physical exercise interventions may have a positive impact on global cognition in people with PD, but the evidence is very uncertain.
Cognitive outcomes are rarely considered in physical exercise intervention trials. In contrast, the Cochrane review by Ernst et al. [21] included 71 studies with data on the severity of motor signs and 55 studies with quality-of-life outcomes in a network meta-analysis. Here, an updated search including a further year of research that was not covered by the Cochrane review revealed only 17 studies for inclusion in the systematic review and only nine for the meta-analysis.
When comparing physical exercise interventions with passive CGs, the meta-analysis showed a small (but non-significant) effect on global cognition favoring physical exercise. However, there was high heterogeneity in the effects reported between studies. This might be linked to combining different physical exercise approaches with different exercise intensities as well as frequency and duration of the intervention conduct in this meta-analysis. Furthermore, the total number of participants in all seven trials was 184, suggesting that these studies were underpowered. Therefore, it is likely that findings from a future large-scale RCT could have a considerable impact on this result, suggesting that our results should be interpreted with caution.
The analysis of the effect of physical exercise interventions compared with active CGs was based on only two studies including a total of 52 people with PD. While this again shows that these trials were underpowered, it also highlights the striking effect size in these very small trials (large in one and moderate in the other). This suggests that future large-scale trials have a high probability of finding convincing effects.
We could only identify a significant effect on cognition in the meta-analysis comparing physical exercise interventions with active CGs (but not passive CGs). This might be again linked to the high heterogeneity of physical exercise types, intensities, as well as frequencies and durations. One possible explanation might be that, for example, vigorous intensity endurance training or physical exercise approaches including specifically cognitive tasks might have a greater impact on cognitive outcomes than low or moderate intensity exercise approaches.
There is a large body of evidence demonstrating that physical exercise intervention can improve cognition in other target groups. For example, a recent systematic review and meta-analysis including 71 trials with 5,606 participants who were either healthy older individuals or people with MCI or dementia showed that all types of exercise helped people to increase or maintain global cognition [44]. It was found that resistance exercise was likely to be the most effective intervention in slowing the decrease in global cognition, memory, and executive function in people with cognitive dysfunction. Another systematic review and meta-analysis of 21 physical exercise studies in people with multiple sclerosis also reported positive effects on cognitive outcomes for this type of intervention [45]. The authors found a small but significant effect on overall cognition and memory. More generally, evidence both from animal and human studies demonstrates the broad effects of exercise on brain plasticity, brain health, and cognition in PD [46, 47]. Taken together, the evidence underlines the potential of physical exercise to target not only motor symptoms in PD [21] but also non-motor symptoms like cognition. Further research is needed.
Strengths and limitations
This is one of the first systematic reviews and meta-analyses to examine the effects of physical exercise intervention on cognition in people with PD. A strength of our study is the differentiation between passive and active comparators in the meta-analysis. A further strength is the high methodological quality following Cochrane standards and the GRADE approach. Furthermore, a broad literature search was conducted using nine scientific databases. Also, a detailed summary of cognitive outcomes in physical exercise intervention trials was performed (see Supplementary Table 2), which could serve as a database for decision-making processes regarding the choice of cognitive domains and test instruments in future studies.
Several limitations should be considered when interpreting the results of this study. One factor is the small number of studies of physical exercise interventions that included cognitive outcomes. These studies had small sample sizes and high heterogeneity in terms of physical exercise type, duration and frequency, and cognitive outcomes. Also, due to the small number of studies no conclusions can be drawn on any differential effects between the various physical exercise types (e.g., gait/balance vs. endurance vs. resistance training) and exercise intensities. Besides, there is still lack of evidence of exercise types that may have the potential to strengthen cognition (e.g., Tai chi [48]). Once sufficient evidence is available for the effects on cognition of different types of physical exercise for people with PD, network-meta-analyses will be able to inform about the superiority of specific intervention approaches. Since the study participants had mild-to-moderate PD, no conclusions can be drawn for people with advanced PD. The same holds for the cognitive state of the participants; conclusions for people with advanced cognitive dysfunctions are not possible. Additionally, most studies lacked a thorough characterization of the cognitive state, i.e., patients were not grouped according to PD-MCI, PDD, or no cognitive dysfunction following established diagnostic criteria [49, 50] and some studies had no cognitive eligibility criteria at all. Further, since the Cochrane Handbook for Systematic Reviews of Interventions [25] recommends the generation of forest plots for meta-analyses including ≥10 studies, our funnel plot of nine studies should be interpreted with caution.
Conclusion
Physical exercise interventions may increase global cognition in people with PD, but the evidence is very uncertain. Given the importance of cognitive dysfunction and dementia in people with PD and the considerable potential of physical exercise interventions to improve cognition, large-scale RCTs that include global cognition and other cognitive outcomes are needed. Future trials, meta-analyses, and network-meta-analyses should differentiate between types of physical exercise interventions, and analyze the best training regimes to improve cognition in people with PD.
ACKNOWLEDGMENTS
We thank all authors of the studies included in our systematic review who responded to our requests and provided data for our analysis.
FUNDING
This project was funded by the German Federal Ministry of Education and Research (grant no. 01KG1902).
CONFLICT OF INTEREST
AKF has received grants from the German Parkinson Society, the German Alzheimer’s Society, the German Parkinson Foundation, STADAPHARM GmbH and the General Joint Committee Germany as well as honoraria from Springer Medizin Verlag GmbH, Heidelberg, Germany; Springer-Verlag GmbH, Berlin; ProLog Wissen GmbH, Cologne, Germany; Seminar- und Fortbildungszentrum Rheine, Germany; LOGOMANIA, Fendt & Sax GbR, Munich, Germany; LOGUAN, Ulm, Germany; dbse.V., Moers, Germany; STADAPHARM GmbH, Bad Vilbel, Germany; NEUROPSY, St. Konrad, Austria; Multiple Sclerosis Society Vienna, Vienna, Austria; and Gossweiler Foundation, Bern, Switzerland. AKF is one of the authors of the cognitive intervention series “NEUROvitalis” but receives no corresponding honoraria.
EK has received grants from the German Ministry of Education and Research, General Joint Committee, Germany, the German Parkinson Society, and STADAPHARM GmbH; honoraria from AbbVie GmbH Germany; memodio GmbH Germany; license fees from Prolog GmbH, Germany. EK is one of the authors of the cognitive intervention series “NEUROvitalis” but receives no corresponding honoraria.
All other authors have no conflict of interest to report.
DATA AVAILABILITY
Data is available on request from the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230343
REFERENCES
[1] | Svenningsson P , Westman E , Ballard C , Aarsland D ((2012) ) Cognitive impairment in patients with Parkinson’s disease: Diagnosis, biomarkers, and treatment. Lancet Neurol 11: , 697–707. |
[2] | Severiano Sousa EC , Alarcão J , Pavão Martins I , Ferreira JJ ((2022) ) Frequency of dementia in Parkinson’s disease: A systematic review and meta-analysis. J Neurol Sci 432: , 120077. |
[3] | Aarsland D , Kurz MW ((2010) ) The epidemiology of dementia associated with Parkinson disease. J Neurol Sci 289: , 18–22. |
[4] | Saredakis D , Collins-Praino LE , Gutteridge DS , Stephan BCM , Keage HAD ((2019) ) Conversion to MCI and dementia in Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat Disord 65: , 20–31. |
[5] | Yang N , Ju Y , Ren J , Wang H , Li P , Ning H , Tao J , Liu W ((2022) ) Prevalence and affective correlates of subjective cognitive decline in patients with de novo Parkinson’s disease. Acta Neurol Scand 146: , 276–282. |
[6] | Kalbe E , Rehberg SP , Heber I , Kronenbuerger M , Schulz JB , Storch A , Linse K , Schneider C , Gräber S , Liepelt-Scarfone I , Berg D , Dams J , Balzer-Geldsetzer M , Hilker R , Oberschmidt C , Witt K , Schmidt N , Mollenhauer B , Trenkwalder C , Spottke A , Roeske S , Wittchen HU , Riedel O , Dodel R ((2016) ) Subtypes of mild cognitive impairment in patients with Parkinson’s disease: Evidence from the LANDSCAPE study. J Neurol Neurosurg Psychiatry 87: , 1099–1105. |
[7] | Aarsland D , Batzu L , Halliday GM , Geurtsen GJ , Ballard C , Ray Chaudhuri K , Weintraub D ((2021) ) Parkinson disease-associated cognitive impairment. Nat Rev Dis Primers 7: , 47. |
[8] | Sun C , Armstrong M ((2021) ) Treatment of Parkinson’s disease with cognitive impairment: Current approaches and future directions. Behav Sci (Basel) 11: , 54. |
[9] | Bogosian A , Rixon L , Hurt CS ((2020) ) Prioritising target non-pharmacological interventions for research in Parkinson’s disease: Achieving consensus from key stakeholders. Res Involv Engagem 6: , 35. |
[10] | Gavelin HM , Domellöf ME , Leung I , Neely AS , Launder NH , Nategh L , Finke C , Lampit A ((2022) ) Computerized cognitive training in Parkinson’s disease: A systematic review and meta-analysis. Ageing Res Rev 80: , 101671. |
[11] | Leung IH , Walton CC , Hallock H , Lewis SJ , Valenzuela M , Lampit A ((2015) ) Cognitive training in Parkinson disease: A systematic review and meta-analysis. Neurology 85: , 1843–1851. |
[12] | Petzinger GM , Fisher BE , McEwen S , Beeler JA , Walsh JP , Jakowec MW ((2013) ) Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol 12: , 716–726. |
[13] | Johansson H , Hagströmer M , Grooten WJA , Franzén E ((2020) ) Exercise-induced neuroplasticity in Parkinson’s disease: A metasynthesis of the literature. Neural Plast 2020: , 8961493. |
[14] | Picelli A , Varalta V , Melotti C , Zatezalo V , Fonte C , Amato S , Saltuari L , Santamato A , Fiore P , Smania N ((2016) ) Effects of treadmill training on cognitive and motor features of patients with mild to moderate Parkinson’s disease: A pilot, single-blind, randomized controlled trial. Funct Neurol 31: , 25–31. |
[15] | Altmann LJ , Stegemöller E , Hazamy AA , Wilson JP , Bowers D , Okun MS , Hass CJ ((2016) ) Aerobic exercise improves mood, cognition, and language function in Parkinson’s disease: Results of a controlled study. J Int Neuropsychol Soc 22: , 878–889. |
[16] | Solla P , Cugusi L , Bertoli M , Cereatti A , Della Croce U , Pani D , Fadda L , Cannas A , Marrosu F , Defazio G , Mercuro G ((2019) ) Sardinian folk dance for individuals with Parkinson’s disease: A randomized controlled pilot trial. J Altern Complement Med 25: , 305–316. |
[17] | Gollan R , Ernst M , Lieker E , Caro-Valenzuela J , Monsef I , Dresen A , Roheger M , Skoetz N , Kalbe E , Folkerts AK ((2022) ) Effects of resistance training on motor- and non-motor symptoms in patients with Parkinson’s disease: A systematic review and meta-analysis. J Parkinsons Dis 12: , 1783–1806. |
[18] | Marotta N , Calafiore D , Curci C , Lippi L , Ammendolia V , Ferraro F , Invernizzi M , de Sire A ((2022) ) Integrating virtual reality and exergaming in cognitive rehabilitation of patients with Parkinson disease: A systematic review of randomized controlled trials. Eur J Phys Rehabil Med 58: , 818–826. |
[19] | Wang K , Li K , Zhang P , Ge S , Wen X , Wu Z , Yao X , Jiao B , Sun P , Lv P , Lu L ((2021) ) Mind-body exercises for non-motor symptoms of patients with Parkinson’s disease: A systematic review and meta-analysis. Front Aging Neurosci 13: , 770920. |
[20] | Yang Y , Wang G , Zhang S , Wang H , Zhou W , Ren F , Liang H , Wu D , Ji X , Hashimoto M , Wei J ((2022) ) Efficacy and evaluation of therapeutic exercises on adults with Parkinson’s disease: A systematic review and network meta-analysis. BMC Geriatr 22: , 813. |
[21] | Ernst M , Folkerts AK , Gollan R , Lieker E , Caro-Valenzuela J , Adams A , Cryns N , Monsef I , Dresen A , Roheger M , Eggers C , Skoetz N , Kalbe E ((2023) ) Physical exercise for people with Parkinson’s disease: A systematic review and network meta-analysis. Cochrane Database Syst Rev 1: , CD013856. |
[22] | Page MJ , McKenzie JE , Bossuyt PM , Boutron I , Hoffmann TC , Mulrow CD , Shamseer L , Tetzlaff JM , Akl EA , Brennan SE , Chou R , Glanville J , Grimshaw JM , Hróbjartsson A , Lalu MM , Li T , Loder EW , Mayo-Wilson E , McDonald S , McGuinness LA , Stewart LA , Thomas J , Tricco AC , Welch VA , Whiting P , Moher D ((2021) ) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372: , n71. |
[23] | Strauss E , Sherman EMS , Spreen O (2006) A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary (3rd ed.). Oxford University Press. |
[24] | Sterne JA , Savović J , Page MJ , Elbers RG , Blencowe NS , Boutron I , Cates CJ , Cheng HY , Corbett MS , Eldridge SM , Emberson JR , Hernán MA , Hopewell S , Hróbjartsson A , Junqueira DR , Jüni P , Kirkham JJ , Lasserson T , Li T , McAleenan A , Reeves BC , Shepperd S , Shrier I , Stewart LA , Tilling K , White IR , Whiting PF , Higgins JPT ((2019) ) RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366: , l4898. |
[25] | Higgins JPT , Thomas J , Chandler J , Cumpston M , Li T , Page MJ , Welch VA (2023) Cochrane Handbook for Systematic Reviews of Interventions (Version 6.4),https://training.cochrane.org/handbook.Last updated 22 August, 2023, Accessed October 2nd, 2023. |
[26] | Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences (Second Edition). Lawrence Erlbaum Associates, Hillsdale, NJ. |
[27] | Schünemann H , Brożek J , Guyatt G , Oxman A , editors (2013) GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. Updated October 2013. The GRADE Working Group. |
[28] | Santesso N , Glenton C , Dahm P , Garner P , Akl EA , Alper B , Brignardello-Petersen R , Carrasco-Labra A , De Beer H , Hultcrantz M , Kuijpers T , Meerpohl J , Morgan R , Mustafa R , Skoetz N , Sultan S , Wiysonge C , Guyatt G , Schünemann HJ GRADE Working Group ((2020) ) GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol 119: , 126–135. |
[29] | Cheung C , Bhimani R , Wyman JF , Konczak J , Zhang L , Mishra U , Terluk M , Kartha RV , Tuite P ((2018) ) Effects of yoga on oxidative stress, motor function, and non-motor symptoms in Parkinson’s disease: A pilot randomized controlled trial. Pilot Feasibility Stud 4: , 162. |
[30] | Harvey M , Weston KL , Gray WK , O’Callaghan A , Oates LL , Davidson R , Walker RW ((2019) ) High-intensity interval training in people with Parkinson’s disease: A randomized, controlled feasibility trial. Clin Rehabil 33: , 428–438. |
[31] | Pohl P , Dizdar N , Hallert E ((2013) ) The Ronnie Gardiner Rhythm and Music Method –A feasibility study in Parkinson’s disease. Disabil Rehabil 35: , 2197–204. |
[32] | Silveira CRA , Roy EA , Intzandt BN , Almeida QJ ((2018) ) Aerobic exercise is more effective than goal-based exercise for the treatment of cognition in Parkinson’s disease. Brain Cogn 122: , 1–8. |
[33] | de Oliveira RT , Felippe LA , Gobbi LTB , Barbieri FA , Christofoletti G ((2017) ) Benefits of exercise on the executive functions in people with Parkinson disease. J Phys Med Rehabil 96: , 301–306. |
[34] | Avenali M , Picascia M , Tassorelli C , Sinforiani E , Bernini S ((2021) ) Evaluation of the efficacy of physical therapy on cognitive decline at 6-month follow-up in Parkinson disease patients with mild cognitive impairment: A randomized controlled trial. Aging Clin Exp Res 33: , 3275–3284. |
[35] | Youm C , Kim Y , Noh B , Lee M , Kim J , Cheon SM ((2020) ) Impact of trunk resistance and stretching exercise on fall-related factors in patients with Parkinson’s disease: A randomized controlled pilot study. Sensors (Basel) 20: , 4106. |
[36] | Pohl P , Wressle E , Lundin F , Enthoven P , Dizdar N ((2020) ) Group-based music intervention in Parkinson’s disease –Findings from a mixed-methods study. Clin Rehabil 34: , 533–544. |
[37] | Hasegawa N , Shah VV , Harker G , Carlson-Kuhta P , Nutt JG , Lapidus JA , Jung SH , Barlow N , King LA , Horak FB , Mancini M ((2020) ) Responsiveness of objective vs. clinical balance domain outcomes for exercise intervention in Parkinson’s disease. Front Neurol 11: , 940. |
[38] | Johansson H , Freidle M , Ekman U , Schalling E , Leavy B , Svenningsson P , Hagströmer M , Franzén E ((2020) ) Feasibility aspects of exploring exercise-induced neuroplasticity in Parkinson’s disease: A pilot randomized controlled trial. Parkinsons Dis 2020: , 2410863. |
[39] | Michels K , Dubaz O , Hornthal E , Bega D ((2018) ) “Dance Therapy” as a psychotherapeutic movement intervention in Parkinson’s disease. Complement Ther Med 40: , 248–252. |
[40] | Gobbi LTB , Pelicioni PHS , Lahr J , Lirani-Silva E , Teixeira-Arroyo C , Santos PCRD ((2021) ) Effect of different types of exercises on psychological and cognitive features in people with Parkinson’s disease: A randomized controlled trial. Ann Phys Rehabil Med 64: , 101407. |
[41] | Silva-Batista C , Corcos DM , Kanegusuku H , Piemonte MEP , Gobbi LTB , de Lima-Pardini AC , de Mello MT , Forjaz CLM , Ugrinowitsch C ((2018) ) Balance and fear of falling in subjects with Parkinson’s disease is improved after exercises with motor complexity. Gait Posture 61: , 90–97. |
[42] | Albrecht F , Pereira JB , Mijalkov M , Freidle M , Johansson H , Ekman U , Westman E , Franzén E ((2021) ) Effects of a highly challenging balance training program on motor function and brain structure in Parkinson’s disease. J Parkinsons Dis 11: , 2057–2071. |
[43] | Krishnan K , Rossetti H , Hynan LS , Carter K , Falkowski J , Lacritz L , Cullum CM , Weiner M ((2017) ) Changes in Montreal Cognitive Assessment Scores over time. Assessment 24: , 772–777. |
[44] | Huang X , Zhao X , Li B , Cai Y , Zhang S , Wan Q , Yu F ((2022) ) Comparative efficacy of various exercise interventions on cognitive function in patients with mild cognitive impairment or dementia: A systematic review and network meta-analysis. J Sport Health Sci 11: , 212–223. |
[45] | Li G , You Q , Hou X , Zhang S , Du L , Lv Y , Yu L ((2023) ) The effect of exercise on cognitive function in people with multiple sclerosis: A systematic review and meta-analysis of randomized controlled trials. J Neurol 270: , 2908–2923. |
[46] | Johansson ME , Cameron IGM , Van der Kolk NM , de Vries NM , Klimars E , Toni I , Bloem BR , Helmich RC ((2022) ) Aerobic exercise alters brain function and structure in Parkinson’s disease: A randomized controlled trial. Ann Neurol 91: , 203–216. |
[47] | Petzinger GM , Fisher BE , Van Leeuwen JE , Vukovic M , Akopian G , Meshul CK , Holschneider DP , Nacca A , Walsh JP , Jakowec MW ((2010) ) Enhancing neuroplasticity in the basal ganglia: The role of exercise in Parkinson’s disease. Mov Disord 25 Suppl 1: , S141–145. |
[48] | Jasim N , Balakirishnan D , Zhang H , Steiner-Lim GZ , Karamacoska D , Yang GY ((2023) ) Effects and mechanisms of Tai Chi on mild cognitive impairment and early-stage dementia: A scoping review. Syst Rev 12: , 200. |
[49] | Litvan I , Goldman JG , Tröster AI , Schmand BA , Weintraub D , Petersen RC , Mollenhauer B , Adler CH , Marder K , Williams-Gray CH , Aarsland D , Kulisevsky J , Rodriguez-Oroz MC , Burn DJ , Barker RA , Emre M ((2012) ) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord 27: , 349–356. |
[50] | Emre M , Aarsland D , Brown R , Burn DJ , Duyckaerts C , Mizuno Y , Broe GA , Cummings J , Dickson DW , Gauthier S , Goldman J , Goetz C , Korczyn A , Lees A , Levy R , Litvan I , McKeith I , Olanow W , Poewe W , Quinn N , Sampaio C , Tolosa E , Dubois B ((2007) ) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22: , 1689–1707; quiz 1837. |




