Striatal Serotonin 4 Receptor is Increased in Experimental Parkinsonism and Dyskinesia
Abstract
Alterations of serotonin type 4 receptor levels are linked to mood disorders and cognitive deficits in several conditions. However, few studies have investigated 5-HT4R alterations in movement disorders. We wondered whether striatal 5-HT4R expression is altered in experimental parkinsonism. We used a brain bank tissue from a rat and a macaque model of Parkinson’s disease (PD). We then investigated its in vivo PET imaging regulation in a cohort of macaques. Dopaminergic depletion increases striatal 5-HT4R in the two models, further augmented after dyskinesia-inducing L-Dopa. Pending confirmation in PD patients, the 5-HT4R might offer a therapeutic target for dampening PD’s symptoms.
INTRODUCTION
Parkinson’s disease (PD) is characterized by the loss of dopaminergic (DA) neurons in the substantia nigra, leading to cardinal motor symptoms, bradykinesia, akinesia, rigidity, resting tremor and postural abnormalities [1]. The neurodegenerative process also affects the serotonergic (5-HT) neurons in raphe nuclei [2]. Strong links have been established between the alteration of the presynaptic 5-HT system (5-HT transporter, 5-HT1A/2A receptors) and manifestations of tremor, levodopa-induced dyskinesias (LIDs) and neuropsychiatric symptoms [3, 4].
Beyond the presynaptic 5-HT system, there is a growing interest towards the post-synaptic serotonin 4 receptor (5-HT4R) [5, 6]. This G-protein coupled receptor is widely distributed in the body and highly expressed in the brain, especially in the basal ganglia. Its activation modulates food intake [7] and supports pro-cognitive, anxiolytic and antidepressant effects [8, 9]. 5-HT4R agonists treat chronic idiopathic constipation in humans [10] and improve memory [11]. 5-HT4R expression is knowingly altered in abnormal food intake, mood disorders and cognitive deficits [12–14].
Surprisingly few studies have focused on the 5-HT4R in PD, while the myriad of PD non-motor symptoms encompasses such manifestations [15, 16]. As a first step, we wondered whether the striatal 5-HT4R is increased after DA depletion and L-Dopa supplementation using an existing brain bank tissue from a rat and a non-human primate (NHP) model of PD. We then investigated its in vivo PET imaging regulation in a second cohort of NHPs.
MATERIALS AND METHODS
Animals
Experiments were carried out in accordance with European Communities Council Directive of November 24, 1986 (86/609/EEC) revised in 2010 (2010/63/UE) and were approved by the local ethical committees. Following the three Rs (Reduction, Refinement, and Replacement) for animal experimentation, we first used existing well-validated brain collections featuring parkinsonian and dyskinetic rats [17] and Macaca mulatta NHPs [18]. Briefly, rats were rendered hemiparkinsonian following unilateral injection of 6-hydroxydopamine (12μg) into the substantia nigra pars compacta, rendered dyskinetic by a 10 days-treatment with L-Dopa (25 mg/kg twice daily), and sacrificed 6 weeks after dopaminergic lesion [17]. Macaca mulatta NHPs were rendered parkinsonian by daily injection of 1-methyl 4-phenyl 1,2,3,6-tetrahydropyridine (MPTP at 0.2 mg/kg/day) until stabilization of parkinsonian symptoms, dyskinetic by a 3-months oral L-Dopa treatment (20 mg/kg twice daily), and were sacrificed 6 months after onset of DA lesion [18]. For both species, behavioral analysis and lesion characteristics were published in detail [17, 18]. However, parkinsonian and dyskinetic measures obtained for rats and macaques are indicated in Supplementary Table 1. These measures have been acquired through the use of specific, well-known tests and scales (the rotational behavior and the scoring of axial, orolingual and forelimb dyskinesia [19] for rats; the PD disability score [20], the monkey clinical assessment scale [21] and the NHP dyskinesia rating scale [22] for macaques). In vivo molecular imaging was conducted on a novel macaque cohort, the experimental details of which are given below.
Postmortem studies
Brain collections were issued from 15 rats (4 control, 5 hemiparkinsonian, 6 dyskinetic) and 11 macaques (4 controls, 4 parkinsonian, 3 dyskinetic). For each animal, four sections at the level of the posterior striatum were processed for immunohistochemistry (see detailed protocol in [23]) with the following antibodies: anti-5-HT4 receptor (5-HT4R) 1/100 rabbit polyclonal from Merck (Merck, Molsheim, France) (catalog number S0195), anti-FosB/ΔFosB 1/200 rabbit polyclonal from Tebu-Bio (catalog number SC-7203; Tebu-Bio, Le Perray en Yvelines, France). The specificity of the immunostaining was assessed by omission of the primary antibodies from the protocol. At the end of the protocol, sections were examined with a light microscope using a computerized image analyzer (Mercator, ExploraNova, La Rochelle, France). Striatal 5-HT4R expression levels were analyzed under blinded conditions relative to the animal by optical density measurements using Image J software.
In vivo molecular imaging studies
Six adult male macaques (Macaca fascicularis) were used for in vivo molecular imaging. Monkeys weighed between 4 and 9 kg and were aged between 4 and 6 years. They were kept under standard conditions (12 h light cycles, 23°C, and 50% humidity). They were rendered parkinsonian by systemic intoxication with MPTP (0.4 mg/kg) (MPTP from Sigma-Aldrich, Saint-Quentin-Fallavier, France). MPTP injections were stopped once Parkinsonian symptoms were established, as previously described [24]. The six monkeys were scanned before (baseline) and two months after MPTP intoxication (post-MPTP) with [11C]PE2I and [11C]SB207145, which bind to the dopaminergic transporter [25] and the 5-HT4 receptor [26, 27], respectively. PET (positron emission tomography) and MRI (magnetic resonance imaging) acquisitions were performed at the imaging center (CERMEP, Lyon, France) under anesthesia (atropine 0.05 mg/kg intramuscularly followed 15 min later by zoletil 15 mg/kg intramuscularly). Anatomical MRI acquisition consisted of a 3D T1-weighted sequence using a 1.5-T AvantoFit scanner (Siemens). The anatomical volume covered the whole brain with 176 planes of 0.6 mm cubic voxels. PET imaging was performed using a Siemens Biograph mCT/S64 scanner. The Biograph mCT had a spatial transverse resolution of 4.4 mm. Attenuation was obtained using a 1 min low-dose CT scan acquired before emission. Dynamic acquisition started with the intravenous injection of the radiotracer, synthesized in the cyclotron unit at CERMEP, and lasted 90 min for SB207145 scans and 70 min for PE2I scans. PET emission images were corrected for attenuation, random and scatter and reconstructed using the Siemens ultraHD PET algorithm with 12 iterations, 21 subsets and a zoom factor of 8. Reconstructed volumes were 109 slices (2.027 mm thickness, 256×256 matrices of 0.398×0.398 mm2 voxels), and consisted in multi-frames of increasing durations ([11C]SB207145:4×30 s, 6×60 s, 9×180 s, 11×300 s; [11C]PE2I: 4×30 s, 4×60 s, 8×180 s, 8×300). Individual PET images were registered to their corresponding individual anatomical MRI, which was registered to the Macaca fascicularis MRI template [28]. Transformations from native PET to individual MRI and individual MRI to template were then concatenated to provide direct (and inverse) affine transformations from PET native spaces to the template space. PET data were analyzed by tracer kinetic modelling at a voxel-based level. The parameters computed were the non-displaceable binding potential (BPND) of [11C]SB207145 and of [11C]PE2I using a simplified reference tissue model. The cerebellum (excluding the vermis) was considered as the reference region for the modelisation. Regional values of BPND were extracted from parametric maps using MAXPROB atlas as described in [24].
Statistical analysis
All statistical analyses were performed using GraphPad Prism software. PET imaging and immunohistochemical data were analyzed using non-parametric Mann–Whitney tests with p < 0.05. Histograms represent mean±SEM.
RESULTS
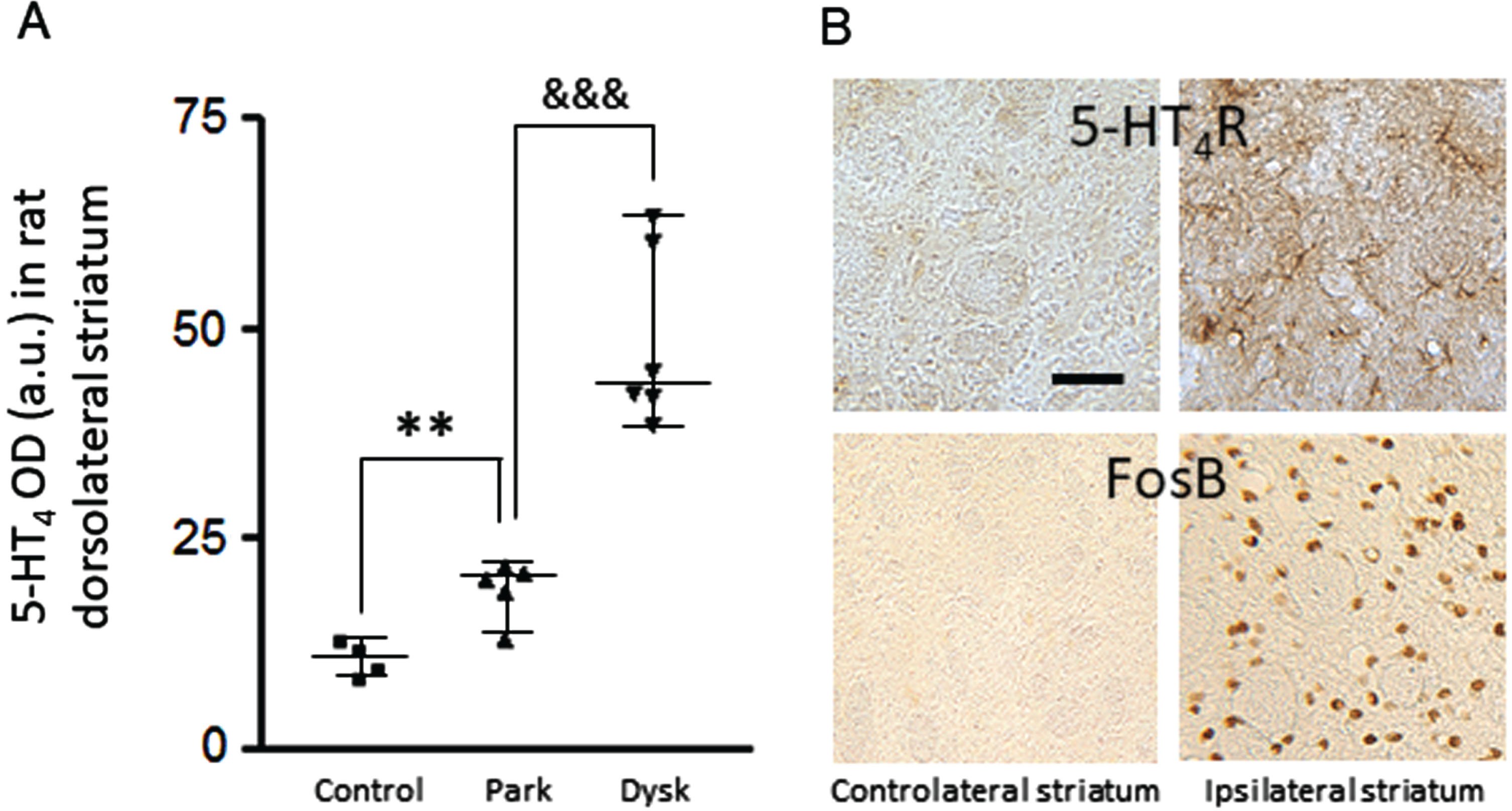
5-HT4R upregulated in the dorsolateral striatum of dopamine-depleted 6-hydroxydopamine (6-OHDA) rats (Fig. 1A). An even greater upregulation was induced by L-Dopa chronic exposure of these unilaterally lesioned rats (Fig. 1A, B). The neuropil was densely labelled, revealing some dendritic branches and varicosities (Fig. 1B). Of note is the observation of a spatial coincidence between the striatal upregulation of 5-HT4R and the striatal increase in FosB/ΔFosB, a transcriptional factor critically involved in L-Dopa-induced dyskinesia pathophysiology [29] (Fig. 1B).
Fig. 1
Striatal upregulation of 5-HT4R after dopamine depletion and L-Dopa exposure in the 6-OHDA lesioned rat. A) Histogram represents optical density measurements of 5-HT4R (in arbitrary units) in the ipsilateral dorsolateral striatum for the 3 experimental groups. **p < 0.01 versus control; &&&p < 0.001 versus parkinsonian. B) Photomicrographs at high magnification (x16) of coronal sections of a dyskinetic rat, exemplifying spatio-temporal concomitance of 5-HT4R and FosB increases in the ipsilateral dorsolateral striatum. Scale bar on B is 50μm. OD, optical density, Park, parkinsonian; Dysk, dyskinetic.
Such 5-HT4R upregulation was also observed in the gold-standard experimental model of PD, namely the MPTP-treated macaque NHP (Fig. 2A). Chronic (3 months) L-Dopa supplementation at therapeutic doses led to LID manifestations and to further upregulation of striatal 5-HT4R levels (Fig. 2A).
Fig. 2
Striatal upregulation of 5-HT4R after dopamine depletion and L-Dopa exposure in MPTP-treated macaques. A) Histogram represents optical density measurements of 5-HT4R (in arbitrary units) in the posterior putamen for the 3 experimental groups. *p < 0.05 versus control; &p < 0.05 versus parkinsonian. B) [11C]PE2I PET averaged images (in color) on coronal planes at the level of the posterior caudate and putamen for each condition. C) Histogram represents 11C-PE2I BPND in the posterior putamen for each condition. D) [11C]SB207145 PET averaged images (in color) on coronal planes at the level of the posterior caudate and putamen for each condition. E) Histogram represents 11C-SB207145 BPND in the posterior putamen for each condition. *p < 0.05, **p < 0.01 versus baseline. Color represents the level of BPND using the cerebellum as the reference region (red indicates high whereas bleu indicates low BPND on each scale). a.u., arbitrary units; BPND, non-displaceable binding potential; Dysk, dyskinetic; OD, optical density; Park, parkinsonian.
![Striatal upregulation of 5-HT4R after dopamine depletion and L-Dopa exposure in MPTP-treated macaques. A) Histogram represents optical density measurements of 5-HT4R (in arbitrary units) in the posterior putamen for the 3 experimental groups. *p < 0.05 versus control; &p < 0.05 versus parkinsonian. B) [11C]PE2I PET averaged images (in color) on coronal planes at the level of the posterior caudate and putamen for each condition. C) Histogram represents 11C-PE2I BPND in the posterior putamen for each condition. D) [11C]SB207145 PET averaged images (in color) on coronal planes at the level of the posterior caudate and putamen for each condition. E) Histogram represents 11C-SB207145 BPND in the posterior putamen for each condition. *p < 0.05, **p < 0.01 versus baseline. Color represents the level of BPND using the cerebellum as the reference region (red indicates high whereas bleu indicates low BPND on each scale). a.u., arbitrary units; BPND, non-displaceable binding potential; Dysk, dyskinetic; OD, optical density; Park, parkinsonian.](https://content.iospress.com:443/media/jpd/2024/14-2/jpd-14-2-jpd230331/jpd-14-jpd230331-g002.jpg)
Given the translational value of Parkinsonian macaques, we then longitudinally investigated 5-HT4R in vivo binding by PET imaging before (control situation) and after MPTP intoxication (Parkinsonian situation). The extent of nigrostriatal lesion was documented using a clinical-grade radiotracer specific to the dopamine transporter, the [11C]PE2I (Fig. 2B, C). We then ran [11C]SB207245, a highly specific radiotracer of 5-HT4R [30]. [11C]SB207145 BPND was increased after parkinsonism induction in all striatal areas (Fig. 2D), notably in the posterior motor putamen (Fig. 2E).
DISCUSSION
This study shows that dopaminergic depletion is sufficient to induce a striatal upregulation of the 5-HT4R, and that this increase is potentiated, and concomitant with FosB/ΔFosB, following L-Dopa supplementation causing dyskinesias.
5-HT4R distribution in the brain is highly conserved across species [31, 32]. Very few studies investigated 5-HT4R expression regulation so far. Experimentally, they were performed only in rodents, i.e., rats and guinea pigs. In the 6-OHDA-injured rat, Compan and colleagues (1996) showed a 59% increase in 5-HT4R binding in the caudal part of the caudate-putamen [33]. A recent in situ hybridization study, therefore measuring mRNA transcripts and not receptors themselves, did not detect changes in striatal 5-HT4R mRNA levels after dopaminergic lesion or after chronic L-Dopa treatment [34]. These results suggest that the 5-HT4R expression must be functionally investigated with direct binding or immunohistochemistry, as 5-HT4R displays a commonly observed decoupling between the transcript abundance and the protein expression.
Only two studies, from the same lab in 1995, report tritiated radioligand binding studies of the 5-HT4R in postmortem human brain homogenates. Peculiarly, the authors did not report a difference in putaminal 5-HT4 binding levels between control and Parkinsonian subjects although the mean binding values were increased [35, 36]. The low power of the studies associated to the lack of spatial resolution due to the homogenization of tissues as opposed to ligand binding or immunostaining of brain sections should account for this difference. The trend is however similar.
In conclusion, the 5-HT4R is over-expressed in the putamen both after DA depletion and DA dyskinesiogenic supplementation. This suggests that two distinct mechanisms are involved: firstly, a post-injury compensatory mechanism, and secondly, a LID-driven maladaptive plasticity mechanism involving FosB/ΔFosB, which may in turn regulate the transcription of 5-HT4R. Indeed, ΔFosB likely binds to the 5-HT4R [37]. Although we do not know, at this stage, whether 5-HT4R is involved in motor disorders or is due to compensatory mechanisms, this work raises the broader question of the role of the 5-HT4R in the pathophysiology of PD, with possible implications on the pathophysiology of cognitive deficits or mood disorders to which this receptor has been linked in other pathologies. Future PET imaging studies in humans urgently need to confirm (or infirm) these preclinical results since the 5-HT4R upregulation might offer a therapeutic target for dampening PD’s motor symptomatology. However, the clinical use of 5-HT4 antagonists could prove tricky given the lack of available pharmacological agents and the high risk of inducing non-targeted side effects, particularly due to the expression of these receptors outside the brain, such as in the gastrointestinal tract or the heart [5, 38]. Also, given that constipation [39, 40] and non-motor disorders [41, 42] are reportedly improved by 5-HT4R agonists, the neurologists may have to opt for different pharmacological options to treat these non-motor symptoms alongside motor disorders.
ACKNOWLEDGMENTS
We thank Professor Gitte Moos Knudsen and her team for help in developing the [11C]SB207145 radiotracer synthesis. We thank E. Ecuer and F. Francioly for animal care, the CERMEP for radiochemistry and imaging acquisitions. The Université de Lyon and the Centre National de la Recherche Scientifique provided infrastructural support. The authors thank the Fondation de France (Grant N° 00096651) and the Agence Nationale de la Recherche (ANR-20-CE17-0039) for supporting this study.
FUNDING
This work was funded by the Fondation de France and the Agence Nationale de la Recherche.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
Erwan Bezard is an Editorial Board Member of this journal but was not involved in the peer-review process of this article nor had access to any information regarding its peer-review.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230331.
REFERENCES
[1] | Rodriguez-Oroz MC , Jahanshahi M , Krack P , Litvan I , Macias R , Bezard E , Obeso JA ((2009) ) Initial clinical manifestations of Parkinson’s disease: Features and pathophysiological mechanisms. Lancet Neurol 8: , 1128–1139. |
[2] | Pagano G , Niccolini F , Fusar-Poli P , Politis M ((2017) ) Serotonin transporter in Parkinson’s disease: A meta-analysis of positron emission tomography studies. Ann Neurol 81: , 171–180. |
[3] | Maillet A , Krack P , Lhommée E , Météreau E , Klinger H , Favre E , Le Bars D , Schmitt E , Bichon A , Pelissier P , Fraix V , Castrioto A , Sgambato-Faure V , Broussolle E , Tremblay L , Thobois S ((2016) ) The prominentrole of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain 139: (Pt 9), 2486–2502. |
[4] | Pagano G , Niccolini F , Politis M ((2018) ) The serotonergic system in Parkinson’s patients with dyskinesia: Evidence from imaging studies. J Neural Transm 125: , 1217–1223. |
[5] | Rebholz H , Friedman E , Castello J ((2018) ) Alterations of expression of the serotonin 5-HT4 receptor in brain disorders. Int J Mol Sci 19: , 3581. |
[6] | Roux CM , Leger M , Freret T ((2021) ) Memory disorders related to hippocampal function: The interest of 5-HT4Rs targeting. Int J Mol Sci 22: , 12082. |
[7] | Jean A , Conductier G , Manrique C , Bouras C , Berta P , Hen R , Charnay Y , Bockaert J , Compan V ((2007) ) Anorexia induced by activation of serotonin 5-HT4 receptors is mediated by increases in CART in the nucleus accumbens. Proc Natl Acad Sci U S A 104: , 16335–16340. |
[8] | Hatat B , Yahiaoui S , Lecoutey C , Davis A , Freret T , Boulouard M , Claeysen S , Rochais C , Dallemagne P ((2019) ) A novel invivo anti-amnesic agent, specially designed to express both acetylcholinesterase (AChE) inhibitory, serotonergic subtype 4 receptor (5-HT4R) agonist and serotonergic subtype 6 receptor (5-HT6R) inverse agonist activities, with a potential interest against Alzheimer’s disease. Front Aging Neurosci 11: , 148. |
[9] | Karayol R , Medrihan L , Warner-Schmidt JL , Fait BW , Rao MN , Holzner EB , Greengard P , Heintz N , Schmidt EF ((2021) ) Serotonin receptor 4 in the hippocampus modulates mood and anxiety. Mol Psychiatry 26: , 2334–2349. |
[10] | Mahajan R ((2019) ) Prucalopride: A recently approved drug by the Food and Drug Administration for chronic idiopathic constipation. Int J Appl Basic Med Res 9: , 1–2. |
[11] | de Cates AN , Wright LC , Martens MAG , Gibson D , Türkmen C , Filippini N , Cowen PJ , Harmer CJ , Murphy SE ((2021) ) Déjà-vu? Neural and behavioural effects of the 5-HT4 receptor agonist, prucalopride, in ahippocampal-dependent memory task. Transl Psychiatry 11: , 497. |
[12] | Haahr ME , Rasmussen PM , Madsen K , Marner L , Ratner C , Gillings N , Baaré WF , Knudsen GM ((2012) ) Obesity isassociated with high serotonin 4 receptor availability in the brain reward circuitry. Neuroimage 61: , 884–888. |
[13] | Madsen K , Torstensen E , Holst KK , Haahr ME , Knorr U , Frokjaer VG , Brandt-Larsen M , Iversen P , Fisher PM , KnudsenGM ((2014) ) Familial risk for major depression is associated with lower striatal 5-HT4 receptor binding. Int J Neuropsychopharmacol 18: , 1–7. |
[14] | Köhler-Forsberg K , Dam VH , Ozenne B , Sankar A , Beliveau V , Landman EB , Larsen SV , Poulsen AS , Ip C-T , Jørgensen A , Meyer M , Stenbæk DS , Eiberg HRL , Madsen J , Svarer C , Jørgensen MB , Frokjaer VG , Knudsen GM ((2023) ) Serotonin 4 receptor brain binding in major depressive disorder and association with memory dysfunction. JAMA Psychiatry 80: , 296–304. |
[15] | Chaudhuri KR , Schapira AHV ((2009) ) Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol 8: , 464–474. |
[16] | Poewe W , Seppi K , Tanner CM , Halliday GM , Brundin P , Volkmann J , Schrag AE , Lang AE ((2017) ) Parkinson disease. Nat Rev Dis Primers 3: , 17013. |
[17] | El Atifi-Borel M , Buggia-Prevot V , Platet N , Benabid AL , Berger F , Sgambato-Faure V. ((2009) ) De novo and long-term l-Dopa induce both common and distinct striatal gene profiles in the hemiparkinsonian rat. Neurobiol Dis 34: , 340–350. |
[18] | Fridjonsdottir E , Shariatgorji R , Nilsson A , Vallianatou T , Odell LR , Schembri LS , Svenningsson P , Fernagut P-O , Crossman AR , Bezard E , Andrén PE ((2021) ) Mass spectrometry imaging identifies abnormally elevated brain l-DOPAlevels and extrastriatal monoaminergic dysregulation in l-DOPA-induced dyskinesia. Sci Adv 7: , eabe5948. |
[19] | Cenci MA , Lee CS , Bjorklund A ((1998) ) L-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci 10: , 2694–2706. |
[20] | Ko WKD , Pioli E , Li Q , McGuire S , Dufour A , Sherer TB , Bezard E , Facheris MF ((2014) ) Combined fenobam and amantadine treatment promotes robust antidyskinetic effects in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned primate model of Parkinson’s disease. Mov Disord 29: , 772–779. |
[21] | Schneider JS , Kovelowski CJ ((1990) ) Chronic exposure to low doses of MPTP. I. Cognitive deficits in motor asymptomatic monkeys. Brain Res 519: , 122–128. |
[22] | Fox SH , Johnston TH , Li Q , Brotchie J , Bézard E ((2012) ) A critique of available scales and presentation of thenon-human primate dyskinesia rating scale. Mov Disord 2: , 1373–1378. |
[23] | Duperrier S , Bortolozzi A , Sgambato V ((2022) ) Increased expression of alpha-, beta-, and gamma-synucleins in brainstem regions of a non-human primate model of Parkinson’s disease. Int J Mol Sci 23: , 8586. |
[24] | Beaudoin-Gobert M , Epinat J , Météreau E , Duperrier S , Neumane S , Ballanger B , Lavenne F , Liger F , Tourvielle C , Bonnefoi F , Costes N , Bars DL , Broussolle E , Thobois S , Tremblay L , Sgambato-Faure V ((2015) ) Behavioural impact of a double dopaminergic and serotonergic lesion in the non-human primate. Brain 138: (Pt 9), 2632–2647. |
[25] | Chalon S , Vercouillie J , Payoux P , Deloye JB , Malherbe C , Le Jeune F , Arlicot N , Salabert AS , Guilloteau D , Emond P , Ribeiro MJ ((2019) ) The story of the dopamine transporter PET tracer LBT-999: From conception to clinical use. Front Med (Lausanne) 6: , 90. |
[26] | Kornum BR , Lind NM , Gillings N , Marner L , Andersen F , Knudsen GM ((2009) ) Evaluation of the novel 5-HT4 receptor PET ligand [11C]SB207145 in the Göttingen minipig. J Cereb Blood Flow Metab 29: , 186–196. |
[27] | Marner L , Gillings N , Comley RA , Baaré WF , Rabiner EA , Wilson AA , Houle S , Hasselbalch SG , Svarer C , Gunn RN , Laruelle M , Knudsen GM ((2009) ) Kinetic modeling of 11C-SB207145 binding to 5-HT4 receptors in the human brain in vivo. J Nucl Med 50: , 900–908. |
[28] | Ballanger B , Tremblay L , Sgambato-Faure V , Beaudoin-Gobert M , Lavenne F , Le Bars D , Costes N ((2013) ) A multi-atlas based method for automated anatomical Macaca fascicularis brain MRI segmentation and PET kinetic extraction. Neuroimage, 77: , 26–43. |
[29] | Beck G , Singh A , Zhang J , Potts LF , Woo JM , Park ES , Mochizuki H , Mouradian MM , Papa SM ((2019) ) Role of striatal ΔFosB in l-Dopa-induced dyskinesias of parkinsonian nonhuman primates. Proc Natl Acad Sci U S A 116: , 18664–18672. |
[30] | Gee AD , Martarello L , Passchier J , Wishart M , Parker C , Matthews J , Comley R , Hopper R , Gunn R ((2008) ) Synthesis and evaluation of [11C]SB207145 as the first in vivo serotonin 5-HT4 receptor radioligand for PET imaging in man. Curr Radiopharm 1: , 110–114. |
[31] | Bonaventure P , Hall H , Gommeren W , Cras P , Langlois X , Jurzak M , Leysen JE ((2000) ) Mapping of serotonin 5-HT(4) receptor mRNA and ligand binding sites in the post-mortem human brain. Synapse 36: , 35–46. |
[32] | Vilaró MT , Cortés R , Mengod G ((2005) ) Serotonin 5-HT4 receptors and their mRNAs in rat and guinea pig brain: Distribution and effects of neurotoxic lesions J Comp Neurol 484: , 418–439. |
[33] | Compan V , Daszuta A , Salin P , Sebben M , Bockaert J , Dumuis A ((1996) ) Lesion study of the distribution of serotonin 5-HT4 receptors in rat basal ganglia and hippocampus. Eur J Neurosci 8: , 2591–2598. |
[34] | Padovan-Neto FE , Patterson S , Voelkner NMF , Altwal F , Beverley JA , West AR , Steiner H ((2020) ) Selective regulation of 5-HT1B serotonin receptor expression in the striatum by dopamine depletion and repeated L-DOPA treatment: Relationship to L-DOPA-induced dyskinesias. Mol Neurobiol 57: , 736–751. |
[35] | Reynolds GP , Mason SL , Meldrum A , De Keczer S , Parnes H , Eglen RM , Wong EH ((1995) ) 5-Hydroxytryptamine (5-HT)4 receptors in post mortem human brain tissue: Distribution, pharmacology and effects of neurodegenerative diseases. Br J Pharmacol 114: , 993–998. |
[36] | Wong EHF , Reynolds GP , Bonhaus DW , Hsu S , Eglen RM ((1995) ) Characterization of [3H]GR 113808 binding to 5-HT4 receptors in brain tissues from patients with neurodegenerative disorders. Behav Brain Res 73: , 249–252. |
[37] | Yeh S-Y , Estill M , Lardner CK , Browne CL , Minier-Toribio A , Futamura R , Beach K , McManus CA , Xu S-Y , Zhang S , Heller EA , Shen L , Nestler EJ ((2023) ) Cell type-specific whole-genome landscape of ΔFOSB binding in the nucleus accumbens after chronic cocaine exposure. Biol Psychiatry 94: , 367–377. |
[38] | Hiroi T , Hayashi-Kobayashi N , Nagumo S , Ino M , Okawa Y , Aoba A , Matsui H ((2001) ) Identification and characterization of the human serotonin-4 receptor gene promoter. Biochem Biophys Res Commun 289: , 337–344. |
[39] | Mozaffari S , Nikfar S , Daniali M , Abdollahi M ((2020) ) The pharmacological management of constipation in patients with Parkinson’s disease: A much-needed relief. Expert Opin Pharmacother 21: , 701–707. |
[40] | Bassotti G , Satta PU , Berti G , Lai M , Villanacci V , Bellini M ((2022) ) Pharmacotherapeutic advances for chronic idiopathic constipation in adults. Expert Opin Pharmacother 23: , 2053–2078. |
[41] | Murphy SE , Wright LC , Browning M , Cowen PJ , Harmer CJ ((2020) ) A role for 5-HT4 receptors in human learning and memory. Psychol Med 50: , 2722–2730. |
[42] | de Cates AN , Wright LC , Martens MAG , Gibson D , Türkmen C , Filippini N , Cowen PJ , Harmer CJ , Murphy SE ((2021) ) Déjà-vu? Neural and behavioural effects of the 5-HT4 receptor agonist, prucalopride, in ahippocampal-dependent memory task. Transl Psychiatry 11: , 497. |




