Factors Associated with Preferred Place of Care and Death in Patients with Parkinson’s Disease: A Cross-Sectional Study
Abstract
Background:
A significant proportion of people with Parkinson’s disease (PwPD) die in hospital settings. Although one could presume that most PwPD would favor being cared for and die at home, there is currently no evidence to support this assumption.
Objective:
We aimed at exploring PwPD’s preferences for place of end-of-life care and place of death, along with associated factors.
Methods:
A cross-sectional study was conducted to investigate PwPD’s end-of life wishes regarding their preferred place of care and preferred place of death. Using different approaches within a generalized linear model framework, we additionally explored factors possibly associated with preferences for home care and home death.
Results:
Although most PwPD wished to be cared for and die at home, about one-third reported feeling indifferent about their place of death. Preferred home care was associated with the preference for home death. Furthermore, a preference for dying at home was more likely among PwPD’s with informal care support and spiritual/religious affiliation, but less likely if they preferred institutional care towards the end of life.
Conclusions:
The variation in responses regarding the preferred place of care and place of death highlights the need to distinguish between the concepts when discussing end-of-life care. However, it is worth noting that the majority of PwPD preferred care and death at home. The factors identified in relation to preferred place of care and death provide an initial understanding of PwPD decision-making, but call for further research to confirm our findings, explore causality and identify additional influencing factors.
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder that is associated with higher disability and mortality in advanced stages [1]. A World Health Organization policy brief emphasizes the need for action in the provision of services and management of PD at different levels of health care systems, including palliative care [2]. The challenges are not only the rapidly increasing incidence [3] but also accumulating evidence that current standards fail to address critical elements of care for people with Parkinson’s disease (PwPD), resulting in under-recognition and under-treatment of symptoms, high rates of nursing home placements as well as hospital admissions towards the end of life and hospital deaths [4].
While hospital admissions may provide a sense of safety for some PwPD [5], there is an apparent discrepancy with statistics in other populations which show that most people wish to be cared for and die in the home environment [6, 7]. In addition, a meta-analysis has demonstrated a significant and growing association between a non-cancer diagnosis and a mismatch between preferred (pPOD) and actual place of death (POD) [8]. In this context, a study of patients receiving specialized palliative care showed that sharing preferences for end-of-life care led to patients being able to assert their preference for the POD. Furthermore, patients whose pPOD was not known were more likely to be admitted to hospital for end-of-life care [9].
To date, little evidence exists on the preferred place of care (pPOC) and pPOD for PwPD, although such research would be essential for the development of appropriate services [10]. The aim of this study was to examine the end-of-life preferences of individuals with PwPD in relation to their pPOC and pPOD. Additionally, the study sought to identify the factors associated with a preference for home care and home death. It was hypothesized that the majority of PwPD would express a preference for home care and home death, and that consistent with previous research on diverse populations [11] our study will show links between individuals’ preferences and a range of socio-demographic and clinical factors.
MATERIALS AND METHODS
This cross-sectional study forms part of a wider research project investigating the preferences of PwPD regarding end-of-life care. This includes not only preferences for pPOC and pPOD, but also aspects such as end-of-life communication behavior and recording of end-of-life wishes.
Study design
Our survey comprised a questionnaire to record participants’ pPOC and pPOD. It consisted of closed-ended questions with occasional opportunities for free-text responses if the predefined answers were not suitable (for an English translation, cf. Supplementary Material). The items of the questionnaire were developed in a two-stage process. First, a group of physicians and nurses with different backgrounds reviewed the questionnaire developed by A.P. Prior to distribution, cognitive interviews were conducted with five PD patients to confirm face and content validity and to assess issues related to feasibility such as comprehensibility of the questions and acceptance [12]. The two cognitive interview techniques think-aloud and verbal probing [13] served to gain insights into the thought processes of the respondents. This provided valuable information regarding potential problems with questionnaire items, resulting in minor revisions being made to the questionnaire.
Study population and recruitment
The study was originally planned as a bi-center study in two different regions of Germany, but due to local restrictions caused by the SARS-CoV-19 pandemic, one center had to drop out. As a result, all participants were recruited from the Department of Neurology at the University Hospital of Marburg between June 2021 and September 2022.
In Germany, the care insurance system provides financial support to individuals in need of care, encompassing various care options such as home care, assisted living, and nursing home care. The objective of this comprehensive system is to ensure that individuals receive appropriate care that aligns with their preferences. Additionally, end-of-life care is facilitated by integrated health and social systems, with palliative care available in hospitals, hospices, and at home. The catchment area of the University Hospital of Marburg boasts a well-developed infrastructure that includes long-term care facilities, inpatient hospices, palliative care units, community nursing services, and specialist outpatient palliative care. However, there is currently no specialized neuro-palliative services in place.
To uphold ethical principles and maintain the reliability of the data collected, we excluded individuals with severe depression and cognitive impairment. These exclusions were deemed necessary to enhance the validity of our research findings. Detailed eligibility criteria for study participation are shown in Table 1. All patients who met the eligibility criteria were registered as potential study participants and asked about their willingness to participate in the study by a researcher who was not involved in the clinical care of patients.
Table 1
Eligibility criteria for study participation
| Inclusion criteria | Exclusion criteria |
| Patients of any gender with a clinical diagnosis of PD according to MDS criteria [42] | Patients for whom the clinical team indicated that they were too weak and/or psychologically too stressed to participate in the study |
| Sufficient German language comprehension | Incapacitated persons, minors, and persons institutionalized by court or administrative order |
| Ability to give informed consent | Presence of life-threatening or severe psychiatric illnesses |
| ∘ BDI-II > 19 points | |
| ∘ MoCA < 18 points | |
| ∘ Psychotic symptomsrequiring inpatient treatmentor affecting capacity to act | |
| Hearing or visual impairment of a severity that interferes with testing |
Data collection
After patients’ informed consent, S.F. and J.K. conducted the interviews and clinical assessments. The final questionnaire was read aloud to all participants and the interviewer filled in the questionnaire manually according to the answers given but was also available for any queries or comprehension problems. Clinical assessments, including Hoehn & Yahr stage, Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [14], Beck’s Depression Inventory II (BDI-II) [15], Montreal Cognitive Assessment (MoCA) [16], the age-adjusted Charlson Comorbidity Index (CCI) [17] and the Parkinson’s Disease Questionnaire (PDQ-39) [18] were administered during the interview, if not already done as part of the clinical routine. In instances where the clinical data were available, information was retrieved from the patient’s file.
Analyses
All statistical analyses were performed using R [version 4.12]. Prior to the study, we conducted a sample size calculation based on the calculation for odds ratios of 2.0 (equivalent to small effect sizes), which assumed a sample size of 94 and conservative estimates of small proportions of 0.25 with 95% confidence to provide a power of 80% [19, 20].
Descriptive analyses were performed on socio-demographic and clinical variables and data on end-of-life preferences. Due to the robust intercorrelation among measures of disease severity, principal component analysis (PCA) was employed in our study to mitigate the correlation between UPDRS Part III scores, Hoehn & Yahr stages, and Levodopa equivalent daily dosage (LEDD). The resulting principal component (PCA1) was subjected to further analysis as a proxy for disease severity. For an additional analysis of possible predictors of the dependent variables, i) preferred home care and ii) preferred home death, these were dichotomized and subjected to separate generalized linear models (GLMs). In addition, independent categorical variables were refactored where appropriate (e.g., German vs. non-German patients, etc.) resulting in the following predictors:
1) Gender
2) Age
3) Age at diagnosis
4) Disease duration
5) German nationality
6) Married
7) Religious/spiritual affiliation
8) Informal nursing support
9) Rurality
10) Professional education
11) Existence advance directive
12) Existence power of attorney
13) Palliative care knowledge
14) Hospice knowledge
15) Preferred place of care (for preferred home death), preferred place of death (for preferred home care)
16) Age-adjusted Charlson Comorbidity index
17) PDQ-39 score
18) BDI score
19) MoCA score
20) Disease severity.
All regression analyses were performed using the caret package [21]. We first estimated coefficients for the respective “full-model” with 20 predictors in a GLM using a binomial link function for the dichotomized dependent variables. Secondly, stepwise linear regression in both directions was used to identify important predictors of the two dependent variables out of all 20 possible independent variables. At each step, variables were subtracted based on the resulting Akaike Information Criterion (AIC) as a metric. To assess the model’s performance, 80% of the entire dataset was used (“training dataset”) and repeated ten-fold cross-validation ensured the robustness of performance results. The predictions of the two models were subsequently compared with the remaining 20% of data (“test dataset”) using accuracy, area under the curve (AUC) and logLoss as metrics. In a third approach, reduction of model complexity was tested using a regularized GLM with an ElasticNet approach [22]. This method has been extensively described, but briefly aims to identify coefficients minimising the sum of error squares by applying a penalty to these coefficients. ElasticNet has been suggested to be superior to other approaches in reducing the complexity of a model [23]. As before, the goodness of fit of the trained model (80% of the dataset) was tested on the remaining 20%, with robustness being sought through repeated 10-fold cross-validation. Values for accuracy, AUC, and logLoss served as metric to compare two reduced models to baseline. Finally, to obtain exploratory confidence intervals on the resulting coefficients, we chose a bootstrapping approach with 1000 resampling steps, using the identical methods as before. For the stepwise regression approach and the penalized model, the resulting coefficients were extracted to obtain their descriptive statistics.
The entire datasets and all performed analyses are available at https://github.com/dpedrosac/EOL_Parkinson.
Ethics
The study was approved by the institutional review board of the Philipps-University Marburg (reference number 88/21).
RESULTS
A total of 136 patients were approached, 27 could not be included because the neuropsychiatric tests showed that they did not meet the eligibility criteria, 9 because of a BDI score that was too high, 18 because of a MoCA score that was too low. Two patients could not take part in the survey because of insufficient knowledge of German, and 13 refused because they felt unable to participate for health reasons. 94 patients with a mean age of 65.0±8.7 years (29.8% female) finally consented to participate in this study. For detailed demographics and patient characteristics cf. Table 2.
Table 2
Participant’s characteristics
| n = 94 | |
| Male (%) | 66 (70.2) |
| Age (mean (SD)) | 65.0 (8.7) |
| Disease duration, y (mean (SD)) | 10.5 (5.9) |
| Marital status (%) | |
| Single | 13 (13.8) |
| Married/registered civil partnership | 58 (61.7) |
| Divorced/dissolved registered civil partnership | 14 (14.9) |
| Widowed | 9 (9.6) |
| Professional education (%) | |
| None | 5 (5.3) |
| Vocational training (“Ausbildung”) | 50 (53.2) |
| Technical college (“Fachhochschule”) | 8 (8.5) |
| University | 31 (33.0) |
| Religious/spiritual affiliation (%) | 59 (62.8) |
| Catholic | 35 (37.2) |
| Protestant | 20 (21.3) |
| Other | 4 (4.3) |
| Living situation (%) | |
| Own household | 59 (62.8) |
| With children/relatives | 30 (31.9) |
| Assisted living | 2 (2.1) |
| Nursing home/senior residence | 3 (3.2) |
| Number of household members (mean (SD)) | 2.0 (0.9) |
| Nursing support (%) | |
| None | 48 (51.1) |
| Family carer | 30 (31.9) |
| Community nursing service | 12 (12.8) |
| Personal carer | 4 (4.3) |
| Residential location (%) | |
| City (>100,000 inhabitants) | 10 (12.0) |
| Middle town (20,000–99,999 inhabitants) | 32 (38.6) |
| Small town (5,000–19,999 inhabitants) | 21 (25.3) |
| Rural town/municipality (<5,000 inhabitants) | 20 (24.1) |
| LEDD (mean (SD)) | 728.5 (486.6) |
| Hoehn &Yahr (%) | |
| 1 | 3 (3.2) |
| 1.5 | 41 (43.6) |
| 2 | 21 (22.3) |
| 2.5 | 22 (23.4) |
| 3 | 5 (5.3) |
| 4 | 1 (1.1) |
| 5 | 1 (1.1) |
| PDQ39 score (mean (SD)) | 25.5 (13.7) |
| MDS-UPDRS score (mean (SD)) | 56.3 (31.1) |
| BDI score (mean (SD)) | 8.5 (4.6) |
| MoCA score (mean (SD)) | 25.9 (3.2) |
| CCI score (mean (SD)) | 2.5 (1.5) |
| Advance directive = yes (%) | 58 (61.7) |
| Power of attorney = yes (%) | 54 (57.4) |
| Palliative care knowledge = yes (%) | 81 (86.2) |
| Hospice knowledge = yes (%) | 85 (90.4) |
LEDD, Levodopa equivalent daily dosage; PDQ-39, Parkinson’s Disease questionnaire; MDS-UPDRS, Unified Parkinson’s Disease Rating Scale; BSI, Beck’s Depression Inventory II; MoCA, Montreal Cognitive Assessment; CCI, (age-adjusted) Charlson Comorbidity Index.
Preferred place of care and preferred place of death
The pPOC at the end of life was the subject’s home for 60 participants (63.8%), a relative’s home for 2 (2.1%), a nursing home for 14 (14.9%), and an inpatient hospice for 5 (5.3%). A single participant (1.1%) expressed a preference for hospital care and none specifically for a palliative care unit. The POC was deemed not important for 9 participants (9.6%).
As for the pPOD, 41 participants (43.6%) specified their own household as their preferred location, while 4 (4.3%) referred to the home of a relative, 6 (6.4%) preferred a nursing home, and 8 preferred an inpatient hospice (8.5%). One participant stated a preference for dying in a hospital (1.1%), but no one expressed a desire to die in a palliative care unit. Meanwhile, 31 participants (33.0%) felt that the POD was not important to them.
Factors associated with preferred home care and preferred home death
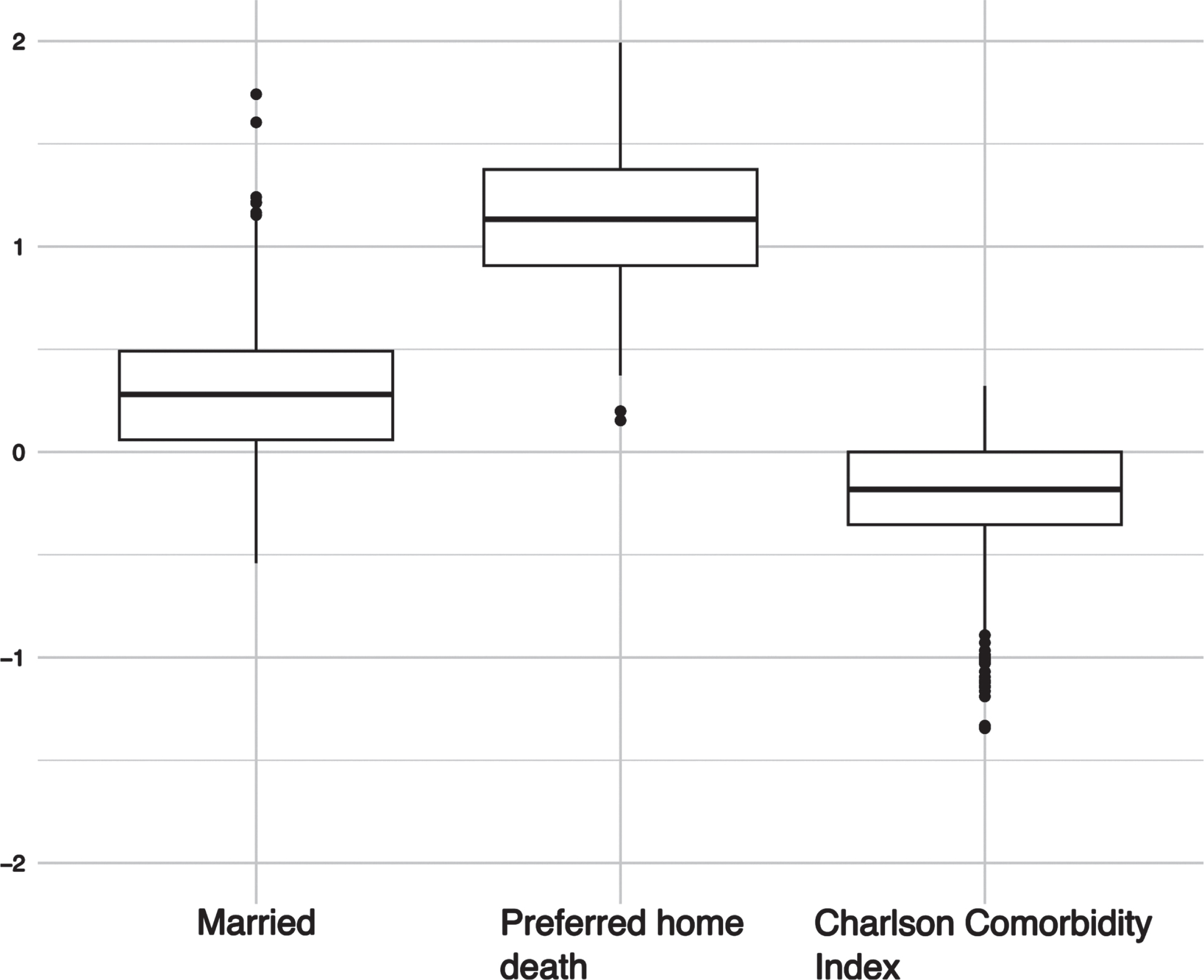
In comparison to the full model, the reduced models exhibited higher values for AUC and accuracy, which suggest superior performance, as well as reduced complexity for a “better fit”. This was further supported in the case of the stepwise regression, where the model had the lowest Aikake information criteria (AIC), and for all models, lower logLoss values were observed when applied to the respective test datasets. This applied for both pPOC and pPOD (see Fig. 1). For pPOC, the stepwise reduction resulted in higher odds of affirming a preference to be taken care of at home when the pPOD was at home (cf. Table 3) as well. The penalized regression model corroborated these results (cf. Table 4). The results obtained from the bootstrap analysis suggest that the coefficients for the preferred place of death (pPOD) at home remained predictive for individuals who expressed a preference for home care in over 99% of the cases where the data was shuffled. Furthermore, the rate of significant coefficients for lower comorbidity indices and being married were found to be 75.8% and 81.5% respectively (see Fig. 2). For pPOD, the models were a little more substantial. In the stepwise regression, we were able to identify the following significant predictors of affirming the wish to die at home: lower age, religious/spiritual affiliation, receiving informal support, higher CCI score, and lower PDQ39 score. Participants who preferred to be cared for in an institutional setting were less likely to express a preference for dying at home (cf. Table 5).
Table 3
Results for the stepwise reduced model for preferred home care
| Predictor | Estimate | SE | z-value | Pr(> |z|) |
| Intercept | 1.04 | 0.32 | 3.23 | 0.00 |
| Preferred home death | 1.47 | 0.36 | 4.14 | 3.47e-5 |
SE, standard error, Pr(> |z|) = p-value associated with the z-value.
Table 4
Results for the ElasticNet model for preferred home care
| Predictor | Coef |
| Intercept | 0.74 |
| Married | 0.09 |
| Preferred home death | 0.84 |
| Charlson Comorbidity index | –0.01 |
Table 5
Results for the step wise reduced model for preferred home death
| Predictor | Estimate | SE | z-value | Pr(> |z|) |
| Intercept | –4.03 | 435.34 | –0.01 | 0.99 |
| Age | –2.03 | 0.79 | –2.58 | 0.01 |
| Religious/spiritual affiliation | 0.77 | 0.36 | 2.17 | 0.03 |
| Informal nursing support | 0.87 | 0.38 | 2.26 | 0.02 |
| Preferred institutional care | –8.66 | 841.88 | –0.01 | 0.99 |
| Charlson Comorbidity index | 1.72 | 0.80 | 2.15 | 0.03 |
| PDQ 39 score | –1.02 | 0.44 | –2.29 | 0.02 |
SE, standard error, Pr(> |z|) = p-value associated with the z-value.
Fig. 1
Comparing full model, stepwise reduced and regularized model for the predictor a) home care and b) home death. Higher values for accuracy and AUC (area under the curve) and lower values for logLoss indicate better performance.
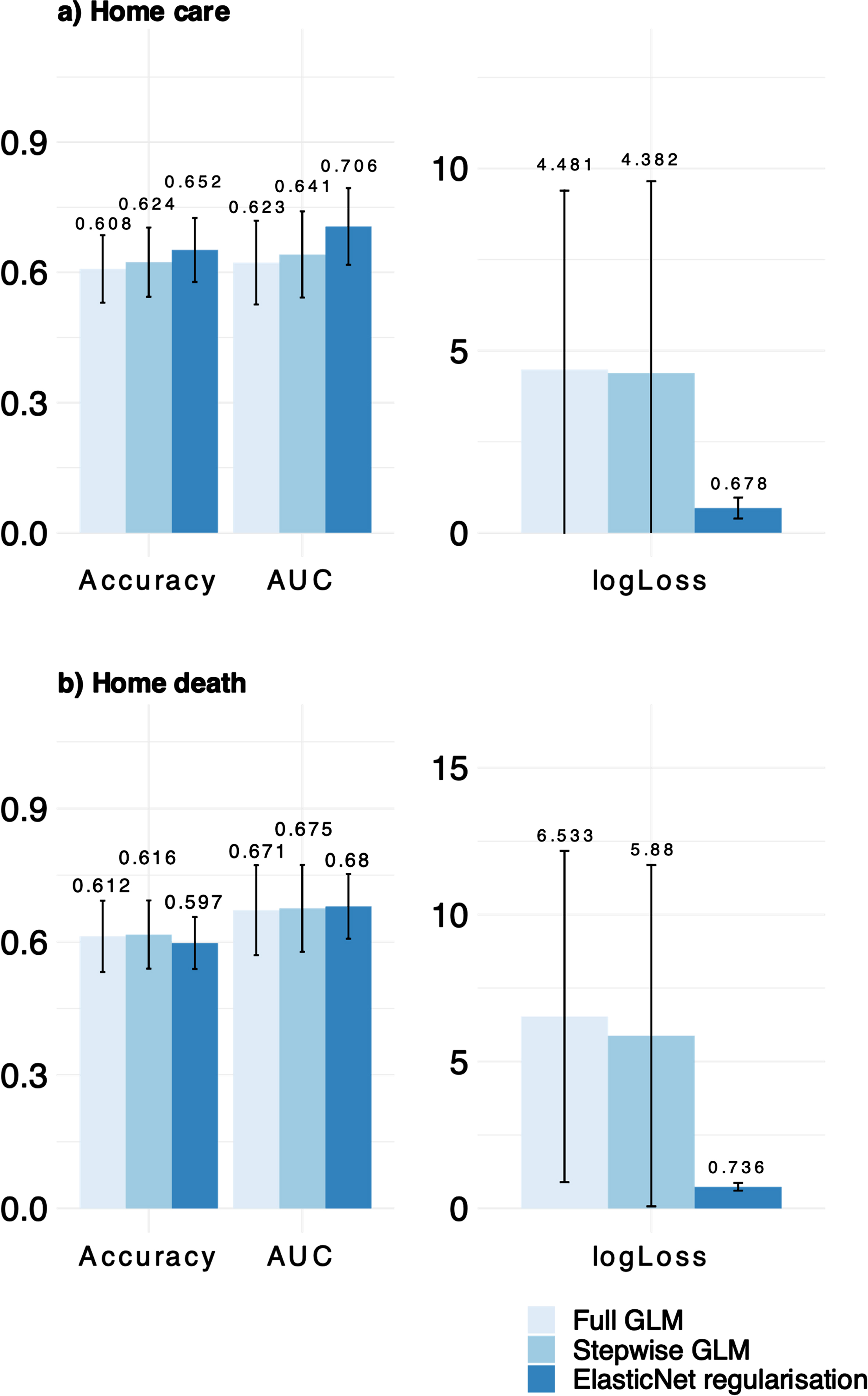
Fig. 2
Bootstrapped coefficients for the penalized regression model (ElasticNet) for the dependent variable home care.
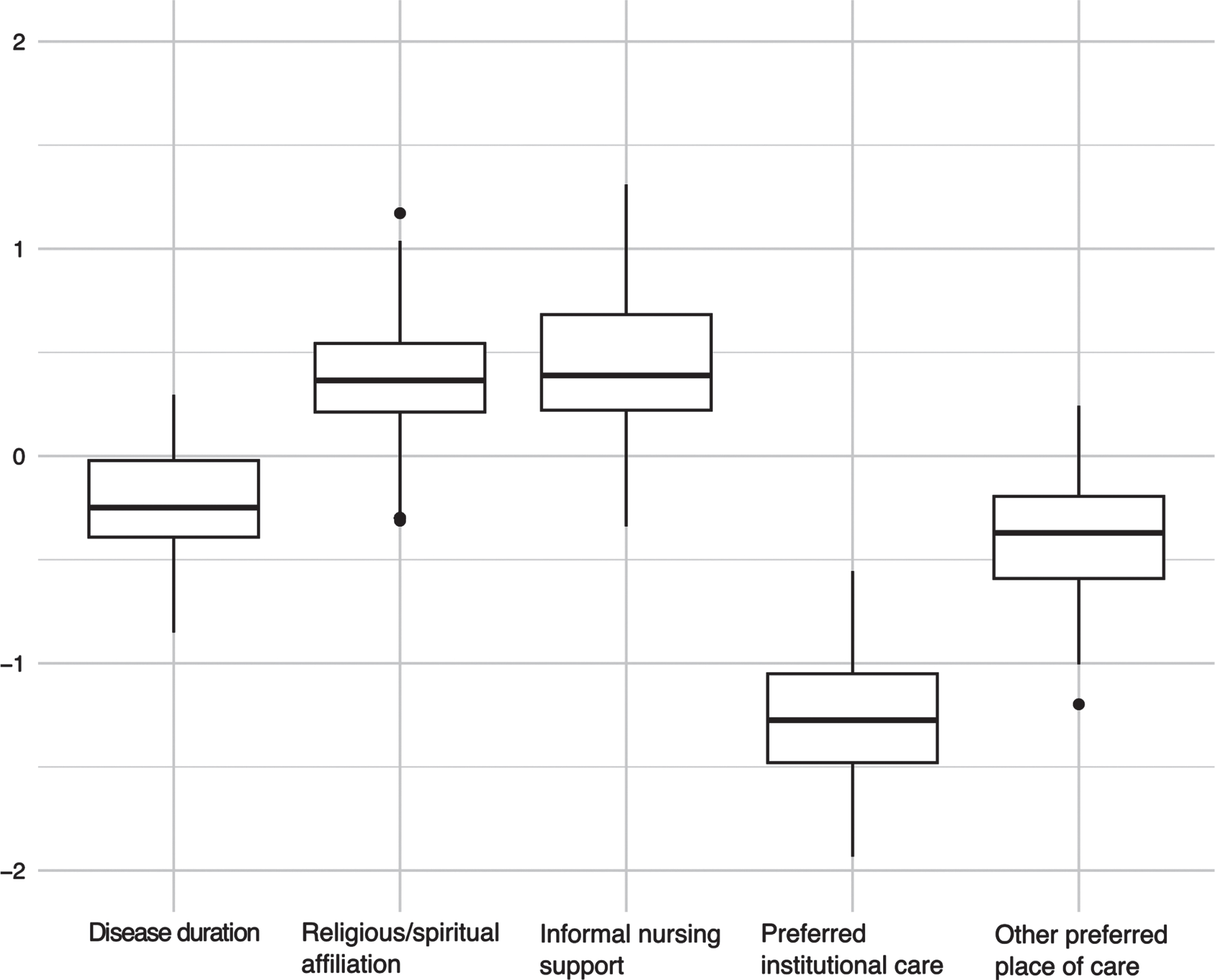
In the alternative model applying shrinkage via ElasticNet we could identify place of care at an institution or at other places as predictive for the wish to die at home (in the bootstrapped data this coefficient remained in 99.7% and 95.3% of cases respectively) but also religious affiliation (93.3%) and receiving informal nursing support (89.2%) as predictive for preferring home death (cf. Table 6 and Fig. 3).
Table 6
Results for the ElasticNet model for preferred home death
| Predictor | Coef. |
| Intercept | –0.34 |
| Religious/spiritual affiliation | 0.08 |
| Informal nursing support | 0.02 |
| Preferred institutional care | –0.80 |
| Preferred care at other place | –0.07 |
Fig. 3
Bootstrapped coefficients for the penalized regression model (ElasticNet) for the dependent variable home death.
DISCUSSION
To the best of our knowledge, this is the first study to systematically investigate PwPD’s preferred place of end-of-life care and place of death. Three main conclusions may be drawn from our research: 1) home was reported as the most common pPOC and pPOD, but 2) many PwPD did not have a strong opinion about their pPOD, with around one in three stating that it did not matter to them, and, 3) we identified factors associated with pPOC and pPOD that inspire further research on this topic.
The question of preferred place of death seems almost trivial as many preliminary studies with patients with chronic diseases indicated that people would like to spend the final stages of life and death at home [6, 24]. In line with our findings, this advocates for policies for PwPD that ensure patients’ preferred place of care and death are prioritized by promoting home care services with expertise in end-of-life care. Noteworthy in this context, our results suggest that participants’ pPOC is associated with, but does not correspond to, where they would like to die. Accordingly, pPOC and pPOD need to be understood as two independent and not synonymous aspects that have to be considered in end-of-life planning [25]. Further studies exploring factors relevant to such decision-making processes will help to support PwPD in decisional empowerment but also to identify potential barriers to fulfilling patients’ wishes.
While localization may have a substantial impact on the quality of end-of-life care [26, 27], a comprehensive analysis of the literature concerning the healthcare quality indicators pPOC and pPOD suggests that patients may face challenges in articulating their explicit preferences [28]. This indecision may be due to uncertainty about the illness, the carer’s perspective, and the services available at regional or local level [29]. In our study, as many as 33% of the participants stated that POD was not important to them possibly indicating that quality in end-of-life care transcends the issue of POD [28]. Future research projects should be dedicated to identifying other criteria of good end-of-life care, in addition to POD, that are relevant to PwPD, in order to further improve end-of-life experiences across all care settings.
Although the results for pPOC and pPOD were not always congruent, our findings did corroborate the value of uninterrupted care at the end of life for a significant number of PwPD [30]. According to all models used, a preference for home death was associated with a preference for home care supporting the notion of continuity of care as a crucial element of optimal PD care [31]. Our results help to advocate for early integration of well-designed home care models for PwPD with advanced disease [32], but also for the provision of informational continuity when relational continuity is not achievable due to end-of-life transitions.
Community-dwelling PwPD report feelings of being a burden to relatives [33], having unmet palliative care needs [34], and uncertainty about available support services [35]. The reduced models rendered additional evidence for the importance of marital status and multimorbidity with respect to the pPOC. In other words, PwPD who are in committed relationships are more inclined to prefer home-based care and this could indicate the belief that the informal care system will operate effectively when required. Furthermore, patients with more comorbidities were less likely to express a wish to be cared for at home. Common consequences of multimorbidity are disability and functional decline, which may be perceived as more challenging to manage at home towards the end of life [36]. Home care programs for PwPD, particularly those with multimorbidity, may have the potential to reduce healthcare utilization, resulting in good patient satisfaction and lower costs [37].
All models agreed on two additional factors associated with preferred home death, 1) receiving informal nursing support and 2) religious/spiritual affiliation. Stable and supportive family structures assisting patients in their care have been shown to be associated with a wish to die at home [38] and may be linked to a sense that dying at home is achievable. Contrarily, a lack of support at home could lead to a decision against home death, so that patients with extended family support are more likely to die at home [39]. Concerns about safety related to inadequate or lack of family support may be one of the underlying reasons [5], so that one may speculate about a need to furnish informal carers with targeted support, so as to prevent any interference with the patient’s wishes. Further research on the causal links is, however, warranted. Additionally, it may be necessary to apprise patients without a personal care network about options available for formal home care until death, subject to their end-of-life wishes. Besides care provision, religious/spiritual affiliation was associated with a wish to die in the home environment, which may also be rooted in an increased level of trust in healthcare providers [40], self-management performance [41] and perceived security [5].
Strengths and limitations
Our explorative research was conducted at a single center in Germany, which may somewhat limit the applicability to patients from other geographical regions. Future research with greater sample sizes and outside of Germany may confirm our results but also identify additional factors, including cultural and health system-specific characteristics of the pPOC and pPOD. Participants living in nursing homes and assisted living facilities were intentionally included to enrich diversity, and offer insights into the unique preferences within these care settings. This enhances real-world relevance, allows for a holistic exploration of influencing factors, and aligns with ethical considerations. However, under-representation may have occurred most likely due to eligibility criteria excluding many PwPD in advanced stages of disease, limiting generalizability. Moreover, our study has the limitations of a cross-sectional study; a longitudinal design could have provided additional information on the consistency of wishes. A mixed-methods approach would have allowed additional exploration of determinants, such as expectations, that are difficult to capture in a strictly quantitative research design. To better understand the decision-making processes in this context, further qualitative work is needed. Finally, our study lacks evidence on the congruence of pPOC and pPOD with the actual place of care and death, which could have provided deeper insights for service improvement and development.
Conclusion
Although the majority of PwPD expressed a preference to be cared for and die at home, the discrepancies in the responses show that pPOC and pPOD are two different concepts that need to be discussed with patients when discussing end-of-life care plans. The factors associated with pPOC and pPOD offer initial insights into the decision-making of PwPD. Further research is required to validate our findings, investigate causality and identify additional factors that may influence the decision-making process.
AUTHOR CONTRIBUTIONS
A.P. is responsible for the conception of the study. All authors were involved in the questionnaire design. A.P., S.F. and J.K. conducted the cognitive interviews. S.F. and J.K. conducted the cross-sectional survey. A.P. and D.P. carried out the analysis of the data and drafted the manuscript. All authors were involved in the interpretation of the data and revision of the manuscript.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This work was supported by the funding received from the Parkinson’s Foundation.
CONFLICTS OF INTEREST
A.P., S.F., J.K., C.V. and C.W. have no conflicts of interest to report. S.L. has received honoraria as a speaker at symposia sponsored AbbVie, Bial, STADA, TEVA. D.P. has received honoraria as a speaker at symposia sponsored by Boston Scientific Corp., Medtronic, AbbVie Inc., Zambon, and Esteve Pharmaceuticals GmbH. He received payments as a consultant for Boston Scientific Corp and Bayer, and he received a scientific fellowship from Boston Scientific Corp. for a project entitled: “Sensor-based optimization of Deep Brain Stimulation settings in Parkinson’s disease” (COMPARE-DBS). Finally, David Pedrosa was reimbursed for travel expenses by Esteve Pharmaceuticals GmbH and Boston Scientific Corp for attending conferences.
DATA AVAILABILITY
All processed data of this study are available online. The sensible data have been pseudonymized. Furthermore, the analyses steps may be traced at https://github.com/dpedrosac/EOL_parkinson.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230311.
REFERENCES
[1] | Barer Y , Gurevich T , Chodick G , Giladi N , Gross R , Cohen R , Bergmann L , Jalundhwala YJ , Shalev V , Grabarnik-John M ((2022) ) Advanced-stage Parkinson’s disease: From identification to characterization using a nationwide database. Mov Disord Clin Pract 9: , 458–467. |
[2] | World Health Organization(2022) Parkinson Disease: A public health approach. Technical brief. |
[3] | Feigin VL , Nichols E , Alam T , Bannick MS , Beghi E , Blake N , Culpepper WJ , Dorsey ER , Elbaz A , Ellenbogen RG ((2019) ) Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: , 459–480. |
[4] | Kluger BM , Fox S , Timmons S , Katz M , Galifianakis NB , Subramanian I , Carter JH , Johnson MJ , Richfield EW , Bekelman D ((2017) ) Palliative care and Parkinson’s disease: Meeting summary and recommendations for clinical research. Parkinsonism Relat Disord 37: , 19–26. |
[5] | Pedrosa AJ , van Munster M , Timmermann L , Pedrosa DJ ((2023) ) Safety perception in patients with advanced idiopathic Parkinson’s Disease –a qualitative study. Front Aging Neurosci 15: , 1200143. |
[6] | Skorstengaard MH , Neergaard MA , Andreassen P , Brogaard T , Bendstrup E , Løkke A , Aagaard S , Wiggers H , Bech P , Jensen AB ((2017) ) Preferred place of care and death in terminally ill patients with lung and heart disease compared to cancer patients. J Palliat Med 20: , 1217–1224. |
[7] | Grote-Westrick M , Volbracht E ((2015) ) Palliativversorgung. Leistungsangebot entspricht (noch) nicht dem Bedarf–Ausbau erfordert klare ordnungspolitische Strategie. Bertelsmann Stiftung (Hg): Spotlight Gesundheit 10: . |
[8] | Billingham MJ , Billingham S-J ((2013) ) Congruence between preferred and actual place of death according to the presence of malignant or non-malignant disease: A systematic review and meta-analysis. BMJ Support Palliat Care02/03/2024 3: , 144–154. |
[9] | Ali M , Capel M , Jones G , Gazi T ((2019) ) The importance of identifying preferred place of death. BMJ Support Palliat Care 9: , 84–91. |
[10] | Snell K , Pennington S , Lee M , Walker R ((2009) ) The place of death in Parkinson’s disease. Age Ageing 38: , 617–619. |
[11] | Fereidouni A , Rassouli M , Salesi M , Ashrafizadeh H , Vahedian-Azimi A , Barasteh S ((2021) ) Preferred place of death in adult cancer patients: A systematic review and meta-analysis. Front Psychol 12: , 704590. |
[12] | Schildmann EK , Groeneveld EI , Denzel J , Brown A , Bernhardt F , Bailey K , Guo P , Ramsenthaler C , Lovell N , Higginson IJ ((2016) ) Discovering the hidden benefits of cognitive interviewing in two languages: The first phase of a validation study of the Integrated Palliative care Outcome Scale. Palliat Med 30: , 599–610. |
[13] | Murtagh FE , Addington-Hall JM , Higginson IJ ((2007) ) The value of cognitive interviewing techniques in palliative care research. Palliat Med 21: , 87–93. |
[14] | Martinez-Martin P , Rodriguez-Blazquez C , Alvarez-Sanchez M , Arakaki T , Bergareche-Yarza A , Chade A , Garretto N , Gershanik O , Kurtis MM , Martinez-Castrillo JC ((2013) ) Expanded and independent validation of the movement disorder society–unified Parkinson’s disease rating scale (MDS-UPDRS). J Neurol 260: , 228–236. |
[15] | Kühner C , Bürger C , Keller F , Hautzinger M ((2007) ) Reliability and validity of the Revised Beck Depression Inventory (BDI-II) results from German samples. Der Nervenarzt 78: , 651–656. |
[16] | Hoops S , Nazem S , Siderowf A , Duda J , Xie S , Stern M , Weintraub D ((2009) ) Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 73: , 1738–1745. |
[17] | Charlson M , Szatrowski TP , Peterson J , Gold J ((1994) ) Validation of a combined comorbidity index. J Clin Epidemiol 47: , 1245–1251. |
[18] | Berger K , Broll S , Winkelmann J , Heberlein I , Müller T , Ries V ((1999) ) Untersuchung zur Reliabilität der deutschen Version des PDQ-39: Ein krankheitsspezifragebogen zur erfassung der lebensqualität von parkinson-patienten. Aktuelle Neurol 26: , 180–184 fischer. |
[19] | Hsieh F ((1989) ) Sample size tables for logistic regression. Stat Med 8: , 795–802. |
[20] | Chen H , Cohen P , Chen S ((2010) ) How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat Simul Comput 39: , 860–864. |
[21] | Kuhn M ((2008) ) Building predictive models in R using the caret package. J Stat Softw 28: , 1–26. |
[22] | Tay JK , Narasimhan B , Hastie T ((2023) ) Elastic net regularization paths for all generalized linear models. J Stat Softw 106: , 1–31. |
[23] | Morozova O , Levina O , Uusküla A , Heimer R ((2015) ) Comparison of subset selection methods in linear regression in the context of health-related quality of life and substance abuse in Russia. BMC Med Res Methodol 15: , 1–17. |
[24] | Klietz M , Tulke A , Müschen LH , Paracka L , Schrader C , Dressler DW , Wegner F ((2018) ) Impaired quality of life and need for palliative care in a German cohort of advanced Parkinson’s disease patients. Front Neurol 9: , 120. |
[25] | Agar M , Currow DC , Shelby-James TM , Plummer J , Sanderson C , Abernethy AP ((2008) ) Preference for place of care and place of death in palliative care: Are these different questions? . Palliat Med 22: , 787–795. |
[26] | Reyniers T , Houttekier D , Cohen J , Pasman HR , Deliens L ((2014) ) The acute hospital setting as a place of death and final care: A qualitative study on perspectives of family physicians, nurses and family carers. Health Place 27: , 77–83. |
[27] | Pottle J , Hiscock J , Neal RD , Poolman M ((2020) ) Dying at home of cancer: Whose needs are being met? The experience of family carers and healthcare professionals (a multiperspective qualitative study). BMJ Support Palliat Care 10: , e6–e6. |
[28] | Pollock K ((2015) ) Is home always the best and preferred place of death? BMJ 351: , h4855. |
[29] | Gerber K , Hayes B , Bryant C ((2019) ) ‘It all depends!’: A qualitative study of preferences for place of care and place of death in terminally ill patients and their family caregivers. Palliat Med 33: , 802–811. |
[30] | Stewart AL , Teno J , Patrick DL , Lynn J ((1999) ) The concept of quality of life of dying persons in the context of health care. J Pain Symptom Manage 17: , 93–108. |
[31] | Radder DL , Nonnekes J , Van Nimwegen M , Eggers C , Abbruzzese G , Alves G , Browner N , Chaudhuri K , Ebersbach G , Ferreira JJ ((2020) ) Recommendations for the organization of multidisciplinary clinical care teams in Parkinson’s disease. J Parkinsons Dis 10: , 1087–1098. |
[32] | Tarolli CG , Holloway RG ((2020) ) Palliative care and Parkinson’s disease: Outpatient needs and models of care over the disease trajectory. Ann Palliat Med 9: , S44–S51. |
[33] | Read J , Cable S , Löfqvist C , Iwarsson S , Bartl G , Schrag A ((2019) ) Experiences of health services and unmet care needs of people with late-stage Parkinson’s in England: A qualitative study. PLoS One 14: , e0226916. |
[34] | Boersma I , Jones J , Carter J , Bekelman D , Miyasaki J , Kutner J , Kluger B ((2016) ) Parkinson disease patients’ perspectives on palliative care needs: What are they telling us? . Neurol Clin Pract 6: , 209–219. |
[35] | Fox S , Cashell A , Kernohan WG , Lynch M , McGlade C , O’Brien T , O’Sullivan SS , Foley MJ , Timmons S ((2017) ) Palliative care for Parkinson’s disease: Patient and carer’s perspectives explored through qualitative interview. Palliat Med 31: , 634–641. |
[36] | Marengoni A , Angleman S , Melis R , Mangialasche F , Karp A , Garmen A , Meinow B , Fratiglioni L ((2011) ) Aging with multimorbidity: A systematic review of the literature. Ageing Res Rev 10: , 430–439. |
[37] | Vilà A , Villegas E , Cruanyes J , Delgado R , Sabaté RA , Ortega J , Araguás C , Humet C ((2015) ) Cost-effectiveness of a Barcelona home care program for individuals with multimorbidity. J Am Geriatr Soc 63: , 1017–1024. |
[38] | Lee A , Pang W ((1998) ) Preferred place of death–a local study of cancer patients and their relatives. Singapore Med J 39: , 447–450. |
[39] | Gomes B , Higginson IJ ((2006) ) Factors influencing death at home in terminally ill patients with cancer: Systematic review. BMJ 332: , 515–521. |
[40] | Benjamins MR ((2006) ) Religious influences on trust in physicians and the health care system. Int J Psychiatry Med 36: , 69–83. |
[41] | Lim KE , Kim SR , Sung YH , Oh S-Y , Kim MS , Chung SJ ((2020) ) Factors influencing self-management in Parkinson’s disease: A cross-sectional study. Geriatr Nurs 41: , 254–260. |
[42] | Postuma RB , Berg D , Adler CH , Bloem BR , Chan P , Deuschl G , Gasser T , Goetz CG , Halliday G , Joseph L ((2016) ) The new definition and diagnostic criteria of Parkinson’s disease. {Lancet Neurol 15: , 546–548. |




