Adherence to Non-Pharmacological Interventions in Parkinson’s Disease: A Rapid Evidence Assessment of the Literature
Abstract
Background:
Low adherence to non-pharmacological interventions can impact treatment effectiveness. Yet, there is limited information on adherence barriers and facilitators to non-pharmacological interventions in Parkinson’s disease (PD).
Objective:
1) To examine the quality of adherence reporting and 2) to identify key determinants of adherence to PD non-pharmacological interventions.
Methods:
A rapid evidence assessment was conducted, following PRISMA guidelines, that included controlled studies of exercise, physiotherapy, occupational therapy, speech-language therapy with explicit reporting of ‘adherence’ OR ‘compliance’, published in the last 15 years. Data extracted included: adherence rates, adherence outcomes, and factors associated with adherence. A collaborative thematic analysis was conducted to identify determinants of adherence.
Results:
The search yielded 2,445 articles of which 114 met criteria for full screening with 45 studies meeting all inclusion criteria. High quality adherence data that aligned with the intervention goals were reported by 22.22%(N = 10) of studies, with the majority reporting attendance/attrition rates only 51.11%(N = 23). Four major themes (34 subthemes) emerged: disease and health, personal, program design, and system and environmental.
Conclusions:
There has been limited progress in the quality of adherence reporting in PD non-pharmacological interventions over the last decade. Acknowledging this limitation, key determinants of adherence included: alignment with personal beliefs, attitudes, and expectations; the demands of the intervention and worsening disease symptoms and personal/time obligations; and accessibility and safety concerns. Program design elements found to facilitate adherence included: opportunities for social engagement and in-person offerings linked to higher levels of interventionist support, performative feedback, and social reinforcement.
INTRODUCTION
Non-pharmacological interventions play a crucial role in managing Parkinson’s disease (PD) symptoms, with exercise and rehabilitation programs such as physiotherapy (PT), occupational therapy (OT), and speech-language therapy (SLT) demonstrating effectiveness in improving symptoms [1, 2] and possibly slowing motor decline [3]. Numerous meta-analyses and systematic reviews provide evidence that exercise and rehabilitation programs improve balance [4, 5], mobility [5, 6], speech [7], swallowing [8], and activities of daily living [9]. These benefits invariably lead to an improved quality of life [10].
Adherence, which can be defined as the faithful enactment of a prescribed exercise or rehabilitation program, is a critical consideration when assessing intervention effectiveness [11]. Although the terms ‘adherence’ and ‘compliance’ differ qualitatively, they are often used interchangeably in the literature [12]. Low adherence rates signal possible issues with feasibility and indicate that there may be difficulties implementing an otherwise efficacious intervention into a real-world clinical context [11]. Importantly, a greater understanding of factors that contribute to adherence can facilitate better clinical decision-making regarding in whom and under what conditions an intervention’s expected benefits can be realized [11]. Although the literature on adherence to pharmacological therapies in PD is extensive, previous work highlights the paucity of systematic evidence regarding the determinants of adherence to non-pharmacological intervention [13–16].
We considered evidence from clinical trials that reported adherence data published over the last 15 years. Interpretation of these studies was complemented by findings from qualitative studies, systematic analyses, and expert opinion. This review aims to bridge gaps in the understanding of adherence to non-pharmacological interventions by:
1. Examining adherence data quality from recent exercise and rehabilitation clinical trials.
2. Exploring specific factors impacting adherence through an interdisciplinary perspective.
3. Suggesting strategies to improve non-pharmacological intervention adherence in PD and to strengthen future exercise and rehabilitation clinical trials.
This review aims to equip healthcare professionals and researchers with the knowledge to bolster the effectiveness of exercise and rehabilitation programs through improved adherence.
METHODS
We conducted a rapid evidence assessment with the search terms constructed in accordance with the Patient/Population, Intervention, and Outcomes (PICO) guidelines [17]. A rapid evidence assessment is a form of literature review that provides a structured search and data extraction but lacks the comprehensiveness of a systematic review [18]. This review methodology is suitable for obtaining a comprehensive understanding of the amount and caliber of evidence concerning a narrower issue than is typical for a systematic review, assisting in making programming choices through the provision of evidence, and facilitating the initiation of additional studies by pinpointing areas lacking evidence [19]. In consultation with a research librarian, we selected this methodology to align with the narrow focus of our research question that could be addressed with a more constrained data extraction compared to a typical systematic review [19]. The following electronic databases were searched to identify potential studies: Medline, EMBASE, Scopus, CINAHL, Cochrane. The search strategy (Supplementary Tables 1 and 2) was combined using “AND” for different groups, and the appropriate synonyms to the PICO guidelines were combined with “OR”. There was a 15-year limit set on the publication dates. This date range is consistent with Bloem et al. (2015) who, in their review, identified the increase in high-quality non-pharmacological trial publications as of 2013 [20]. In our literature search, we observed a similar inflection in the search results with no eligible studies identified between 2008 and 2012 followed by an increase in those meeting our criteria, suggesting that this date range captured relevant data to address our research questions. Included studies were those that examined factors influencing adherence with non-pharmacological therapies, encompassing rehabilitation techniques (PT, OT, SLT) and exercise, employing various research designs (qualitative, quantitative, mixed methods) including descriptive, quasi-experimental, and involving participants diagnosed with PD. Excluded studies were those not published in English, lacking full-text access, focusing on pharmaceutical drug trials, involving grey literature, examining instruments/surgical trials (e.g., deep brain stimulation, transcranial magnetic stimulation), lacking specific measurement or quantification of compliance to the intervention, or pertaining to non-human participants. All studies were assessed by at least two people. After the full-text review and extraction, a collaborative thematic analysis was conducted according to Richards and Hemphill [21] to extract relevant themes and subthemes of factors affecting adherence to non-pharmacological therapies in PD. Three coders (AR, JL, NA) completed the primary thematic analysis individually and then collectively finalized coding assignment and thematic labels [21]. A fourth researcher (IC) provided methodological expertise and collaborated in resolving labeling or coding issues. Data are available by request.
RESULTS
Literature search results
The electronic search results are presented in a PRISMA flow chart (Fig. 1). The studies included in this review (N = 45), and their key characteristics (including study design, adherence outcomes, and adherence data) are reported in Table 1. In cases where the adherence data were reported in a secondary analysis, the citations for the linked primary clinical trial results are indicated in Table 1. When two studies reported on the same dataset, as in Canning et al. (2015) and Allen et al. (2015), unique adherence factors from each article were extracted with redundant factors considered only once in the thematic analysis [22, 23]. Overall, the quality of adherence data was lacking based on published adherence reporting standards [24]. Many studies (51.11%, N = 23) reported only attendance or study attrition as their primary adherence outcome. Far fewer studies (24.44%, N = 11) reported a more comprehensive perspective of adherence specific to the program goals. Several studies (24.44%, N = 11) did not describe their adherence outcome. Adherence was reported as high (85%or greater) in 46.66%of studies (N = 21), with 33.33%of studies (N = 15) reporting adherence rates between 60 and 85%, and with 6.67%of studies reporting adherence rates of 60%or lower (Table 1). No study reported adherence rates of less than 50%, and 13.33%of studies (N = 6) did not report adherence rates.
Fig. 1
PRISMA Flow Diagram that shows the systematic process the authors followed to include papers captured by our search strategy.
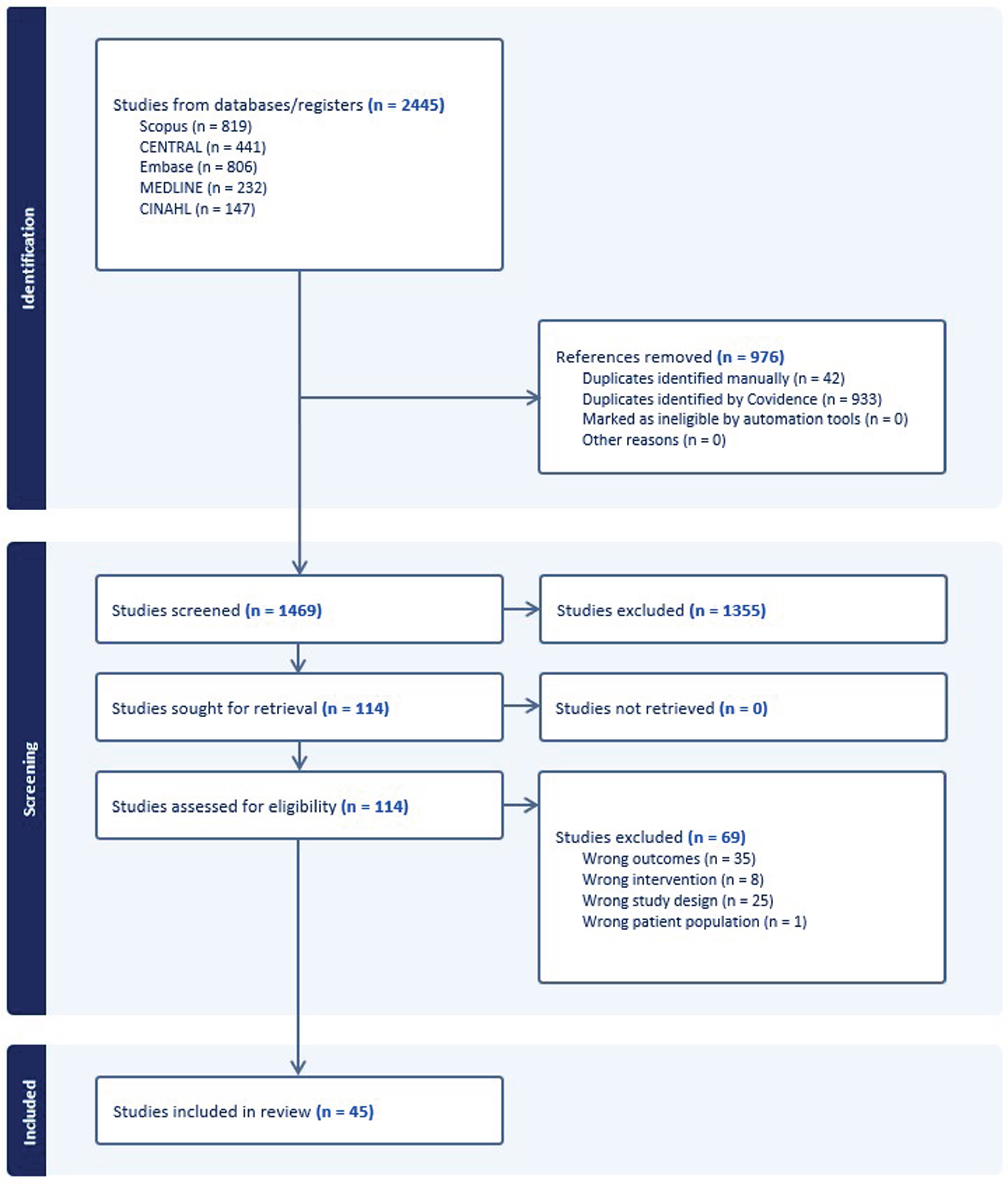
Table 1
Summary of included studies
| Study design | Intervention &comparison(s) | Sample size at baseline (# female) | Measurement of adherence | Adherence indicator(s) | %Adherence (Adjusted for 100%Maximum) | |
| Allen et al., 2015 (Australia)h [23] | RCTs | Weight-bearing exercise group for falls prevention (no comparison) | 108 (45) | Physiotherapist recorded supervision, logbook | %of prescribed sessions where any prescribed exercise was recorded as completed | 68% |
| Allen et al., 2017 (Australia) [35] | RCT | Interactive videogame for arm and hand exercise vs. standard care | 38 (15) | Participant logbook | %of prescribed sessions | 97% |
| Bega et al., 2016 (USA) [77] | Prospective RCT | Iyengar yoga (downtown vs. suburban campus) vs. resistance training (downtown vs. suburban campus) | 14 (3)a | Attendance at classes, scheduled phone calls | At least 70%of participants attending at least 75%of classes | 76% |
| Bek et al., 2021 (United Kingdom)b [47] | Pilot RCT | Home training exercise to improve functional hand movements vs. no intervention | 10 (1)a | Home training diaries, app | Not reported | 148.3%(non-adjusted)c |
| Canning et al., 2012 (Australia) [37] | Pilot RCT | Treadmill training vs. control (gym program) | 20 (9) | Semi-supervised, consultation, logbooks, post-intervention questionnaire | %of prescribed treadmill walking sessions completed | 78% |
| Canning et al., 2015 (Australia) [22] | RCT | Progressive balance and lower limb strengthening exercises vs. standard care for falls | 231 (96) | Home exercise logs and class records kept by the physical therapist | Completion of prescribed exercise sessions | 72%(exercise group) |
| Demonceau et al., 2017 (Belgium)g [32] | Pseudo-RCT | Aerobic or strength training vs. standard care | 46 (14)a | Supervisors monitored compliance with instructions | Mean sessions completed at the prescribed intensity | Aerobic: 30.1±5.1 Strength: 27.8±4.9 |
| Duncan et al., 2012 (USA) [27] | RCT | Argentine Tango vs. control | 62 (22) | Not reported | Attrition from study | Tango group: 50%Control group: 36.67% |
| Ellis et al., 2019 (USA)g [64] | Pilot RCT | mHealth-supported exercise vs. exercise | 51 (23) | mHealth: mobile health application Control: paper calendars | Daily records of steps taken, exercises performed | Not reported |
| Fernandez-Gonzalez et al., 2019 (Spain)d [61] | Feasibility RCT | Interactive video game for upper limb rehabilitation vs. standard intervention | 23 (12)a | Not reported | Attendance rate for therapy sessions | 100% |
| Flynn et al., 2021 (Australia) [75] | Pilot RCT | Centre-based exercise program vs. home-based with telehealth | 60 (15) | Centre-based: recorded by physiotherapist Home-based: recorded by participants using app or on paper | %of exercise sessions attempted | Centre-based: 93%Home-based: 84% |
| Jäggi et al., 2023 (Switzerland)d [33] | Pilot RCT | Interactive video game vs. control for fall risk factors | 40 (13) | Not reported | %of completed training sessions | 96.5% |
| Kalyani et al., 2019 (Australia)d [70] | Quasi-experimental parallel group pre-test post-test study | Dance group vs. standard care on psychological symptoms and quality of life | 33 (20) | Not reported | 20 or more sessions attended | Dance group: 92.89%attendance |
| Khalil et al., 2017 (Jordan) [57] | Randomized pilot study | Home exercise program vs. standard care | 30 (11) | Exercise diary, weekly phone call | %of completed exercise sessions | 77% |
| King et al., 2015 (USA) [58] | RCT | Home exercise program vs. individual PT vs. group PT interventions | 58 (34)a | Physical therapist, participant record | %of assigned exercise sessions in which exercise occurred | Group: 95%Individual: 97%Home: 85% |
| King et al., 2020 (USA)b [72] | Randomized crossover trial | Education vs. exercise interventions | 46e | Education: participant logbook Exercise: attendance records | Not reported | Education: 80%Exercise: 90% |
| Lai et al., 2020 (USA) [31] | Mixed-methods pilot study | Tele-coach assisted vs. self-regulated exercise groups | 20 (6) | Semi-structured qualitative interviews | Attendance rate | Tele-coach assisted: 99.2%Self-regulated: 63.3% |
| Langer et al., 2021 (Austria) [63] | RCT | Sport climbing vs. physical training | 48 (18) | Weekly phone call, training logs | Course participation (?) | 99% |
| Li et al., 2014 (USA)b [54] | RCT | Tai chi vs. resistance training vs. stretching exercises | 195 (73) | Participant self-report of continuing exercise | Not reported | Completed assigned intervention: 90%Provided complete data: 95% |
| Mak &Wong-Yu, 2021 (China)d [65] | RCT | Community-based exercise vs. upper limb training for motor symptoms and function | 64 (44) | Not reported | 6-month class attendance and average weekly exercise duration | 95%(both groups) |
| Martin et al., 2015 (New Zealand)b [50] | RCT (feasibility) | Immediate cueing program vs. delayed cueing program for falls management | 21 (8) | Weekly phone calls, anonymous questionnaire | Not reported | 83%e |
| McGinley et al., 2012 (Australia) [69] | RCT | Progressive strength training vs. movement strategy training vs. control (“lifeskills”) | 210 | Recorded on a home exercise sheet by a therapist | Adherence: Consistency of participant attendance at the intervention/control sessions. Compliance: Progression of exercises within each of the two intervention groups as evidenced by therapy record | Adherence PST: 90%attended 6-8 sessions, 4%attending < 5 sessions. MST: 93%attended 6–8 sessions, 3%attending < 5 sessions LS: 78%attended 6-8sessions, 9%attending < 5 sessions Compliance 89%were able to complete all 7 exercises within 2-hour sessions |
| McKee et al., 2021 (USA) [51] | Pragmatic feasibility study | High-cadence cycling (no comparison) | 27 (8) | Record of attendance over 24 offered sessions | # completed at least 80%of classes and finished program/# at baseline | 58% |
| Monteiro et al., 2016 (Brazil)b,d [34] | RCT | Nordic walking vs. free walking on functional parameters | 33 (13) | Not reported | Not reported | 90% |
| Moratelli et al., 2021 (Brazil)d [78] | RCT | Binary dance rhythm vs. quaternary dance rhythm on non-motor symptoms | 31 (9)a | Not reported | %of prescribed sessions completed | 84.3% |
| Park et al., 2014 (USA)b,g [66] | Randomized pilot study | Early-start exercise program vs. delayed-start exercise program | 31 (11) | Attendance taken at sessions, home exercise diary | Not reported | Not reported |
| Pastore-Wapp et al., 2023 (Switzerland)d [62] | Randomized feasibility study | Combined intermittent theta-burst stimulation and video-game based dexterity training vs sham stimulation and video game training | 9 (5) | Not reported | Ratio of number of sessions performed and planned number of sessions | 100% |
| Pickering et al., 2013 (United Kingdom)i [14] | Secondary analysis of RCT | Home-based exercise programme to reduce falls (no control) | 70 (32) | Self-report diary, reviewed by therapist | Ratio of reported to prescribed repetitions carried out | 79% |
| Pohl et al., 2013 (Sweden) [45] | Randomized feasibility study | Ronnie Gardiner Rhythm and Music Method vs. control group (no intervention specified) | 18 (10) | Practitioner records | Participation in RGRM therapy sessions | 93% |
| Ridgel et al., 2016 (USA) [71] | RCT | Group vs. Self-guided exercise therapy | 30 (11) | Group-based: recorded by research assistant Self-guided: weekly phone call | Number of exercise sessions over the 12-week exercise intervention | 80%overall retention Group-based: 20.7/36 sessions Self-guided: 22/36 sessions |
| Rosenfeldt et al., 2022 (USA) [52] | Pragmatic observational study | Community-based high-intensity cycling exercise program | 49(19) | Exercise-monitoring systems; web-based or mobile application | Attendance for each session | 53.1%of all available sessions attended |
| Rosenfeldt et al., 2022 (USA) [52] | Pragmatic observational study | Community-based high-intensity cycling exercise program | 41 (16) | Web-based monitoring system mobile application, fitness and cadence monitor | Attendance and participation in weekly cycling sessions | 65%attendance |
| Rowsell et al., 2020 (United Kingdom)b,g,j [55] | Longitudinal qualitative study, part of RCT | Tailored physiotherapy intervention | 42 (18) | Semi-structured interviews | Not reported | Not reported |
| Sackley et al., 2018 (United Kingdom) [46] | Pilot RCT | LSVT vs. SLT vs. control | 89 (20) | Intervention record forms | Proportion of participants who completed the intervention as per protocol | 73%completed LSVT as per protocol |
| Shanahan et al., 2017 (Ireland) [29] | RCT | Irish set dancing vs. control | 41 (15)a | Home exercise diary | Attendance taken at sessions | Attendance: 93.5%Diary compliance: 71.46% |
| Spina et al., 2021 (Italy)d,b,g [76] | RCT | Robotic balance training vs. conventional balance training on postural instability | 22 (9) | Not reported | Not reported | Not reported |
| Strouwen., 2017 (Belgium, Netherlands)b [30] | RCT | Consecutive dual-task training vs. integrated dual task training | 121 (33) | Training diary | %completed more than 80%of sessions | Consecutive: 84.6%Integrated: 82.1% |
| Sturkenboom et al., 2016 (Netherlands)k [56] | Mixed methods w/RCT | Home-based occupational therapy intervention | 259e | Patients &caregivers: questionnaire Therapists: case notes | (# of steps performed)/12*100% | Complete adherence reached in 46%of cases |
| Terrens et al., 2020 (Australia)d [28] | Single-blind pilot study | Halliwick-style of aquatic PT vs. traditional aquatic PT vs. land-based PT for falls and balance dysfunction | 30 (6) | Not reported | Number of sessions each participant attended in the intervention period, as a %of total sessions offered | 89% |
| Vanbellingen et al., 2017 (Switzerland, Netherlands)b [36] | RCT | Home-based dexterity vs. Thera-band program | 103 (40) | Diary, phone calls | Not reported | Dexterity group: 88%Control group: 84%Average: 86% |
| van der Kolk et al., 2019 (Netherlands)d,b,g [60] | RCT | Aerobic exercise vs. stretching for motor symptoms | 130 (50) | Not reported | Not reported | Not reported |
| Vasconcellos et al., 2021 (Brazil)d [68] | RCT | Telerehabilitation-based trunk exercise vs. upper and lower limb exercise | 28 (10) | Not reported | Non-adherence: failure to perform protocol for more than 3 consecutive days, non-response to telephone contact for more than 4 consecutive days | 67% |
| Vorasoot et al., 2020 (Thailand)b [53] | RCT | Handwriting exercise vs. control for fine manual motor function; | 46 (21) | Handwriting practice book returned after 4 weeks | Not reported | 100%follow-up ratef |
| Wu et al., 2021 (Japan) [74] | RCT | Home-based exercise on motor, non-motor symptoms and health-related quality of life vs. control | 98 (42) | Self-reported diary, weekly telephone checks | Compliance: exercise > / = 150 min/week | 55.10% |
| Yang et al., 2017 (China) [67] | Pilot RCT | Group-based vs. individual-based tai chi training on nonmotor symptoms | 36 (16)a | Diary | Home exercise compliance rate (%days): (number of days of home exercise participation)*(100%/ total number of days) | Group-based: mean 64.84%Individual-based: mean 51.17% |
aDemographics for the analysis set were reported, baseline demographic data for the full cohort were not reported. bQuality of adherence or compliance not reported. cAdjusted adherence or compliance rate not reported. dMeasurement of adherence or compliance not recorded. eBreakdown of participants by sex not reported. fOnly long-term adherence data reported. gAdherence or compliance rate not reported. hCorresponding primary trial: Canning et al. (2015) [41]. iCorresponding primary trial: https://doi.org/10.1136/jnnp.2006.099333. jCorresponding primary trial: https://doi.org/10.1136/jnnp-2018-319448. kCorresponding primary trial: https://doi.org/10.1016/S1474-4422(14)70055-9.
Thematic synthesis
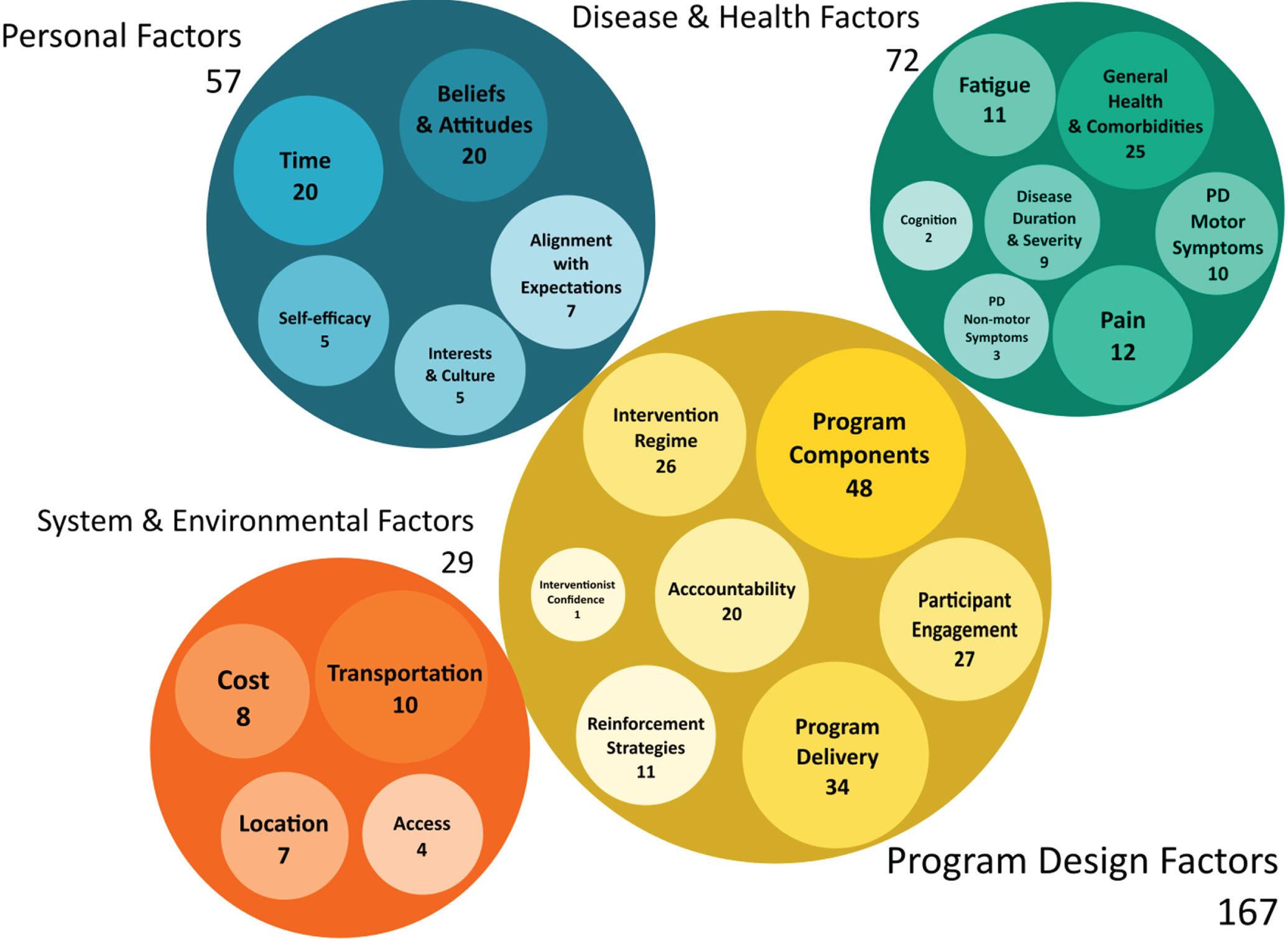
We identified four major themes: Disease and Health Factors, Personal Factors, Program Design Factors, and System and Environmental Factors, along with several subthemes, that affected adherence in the literature reviewed (Fig. 2, Supplementary Table 3).
Fig. 2
Thematic Analysis of the Determinants of Adherence. Bubble graph of the major themes (large circles) and subthemes (smaller embedded circles) from the thematic analysis of data extracted from the included articles. The size of each bubble represents the frequency of a theme derived from the thematic analysis. The frequencies are reported within each bubble.
Disease and health factors
Individuals with PD tend to be less active in daily life compared to healthy aging controls, which can impact exercise and rehabilitation outcomes [25]. Motor symptoms and physical limitations associated with PD, such as bradykinesia, rigidity, and gait problems, can make it challenging for people with PD to adhere to exercise and rehabilitation programs [26]. Disease progression and sudden health complications, including falls or the onset of new comorbidities, were reported as reasons why participants did not complete exercise or rehabilitation programs as prescribed [27–30]. Moreover, many studies emphasized the detrimental role of overall health, including neuropsychiatric symptoms, like depression and anxiety, on adherence. Recent clinical trials identified physical challenges, especially the exacerbation of pain during exercise [23, 28, 31–34] and fatigue [28, 30, 35–37] as common culprits for diminished adherence. Allen et al. (2015) made a significant observation: people experiencing lesser pain and an improved sense of physical well-being, as gauged by the SF-12 physical composite score, were notably more adherent to a balance-focused exercise program [23]. Acute or intensifying fatigue is not solely a concern for physical rehabilitation. In a qualitative study of lived experiences with SLT, some individuals reported fatigue and exacerbated symptoms after therapy [38]. This observation was corroborated by results from a superiority trial conducted by Richardson et al. (2022), who suggested a link between high-intensity voice therapy, heightened cognitive load, and fatigue [39]. Another dimension to consider is dyskinesias, with one study reporting that they negatively impacted adherence in an exergaming program [33].
Non-motor symptoms, including cognitive impairment and apathy were also raised as potential barriers to adherence [14, 33, 36, 40–42]. Cognitive deficits in areas such as attention, memory, and executive dysfunction can influence the ability to follow instructions and actively participate in exercise and rehabilitation programs. Reflecting this, current PD dysphagia guidelines suggest cautious use of certain swallowing strategies and exercises for people with significant cognitive impairment [43].
One evident gap that emerged from our review is that most contemporary exercise and rehabilitation programs cater to people exhibiting mild to moderate symptoms, often sidelining those with dementia, pronounced mood disorders, sensory impairments, or more severe motor symptoms [35, 44, 45]. Interestingly, Canning et al., (2015) reported no significant difference in adherence between their high and low motor severity groups [22]. Despite this, the scarcity of adherence data for those with more severe motor and non-motor symptoms creates challenges in assessing treatment effectiveness across the spectrum of PD presentations.
Personal factors
A significant number of studies (44.4%, N = 20) pointed out time constraints and family/work obligations as primary barriers to adherence, often leading to early study withdrawal. This is consistent with prior reviews reporting that people with PD often face difficulties in maintaining motivation and exercise routines because of medical and life obligations [15]. Developed using a participatory research framework, the ACTION-PD trial provides an example of how interventions can optimize adherence by catering to the unique challenges presented by the disease while offering participants input on their training activities and intervention schedule [46].
The alignment of the intervention with personal beliefs/attitudes, interests/culture, and expectations was found to be important in 55.6%(N = 25) of studies. In keeping with person-centered models of care [47, 48], researchers often cited personal alignment as an important determinant of adherence [27, 29, 35, 49–55]. As evidence, participants in Shanahan et al. (2017) were motivated to adhere to a balance and mobility program due to the cultural significance of its Irish dance components [29]. Similarly, one intervention included Arabic language materials into their intervention, motivated by their observation that the lack of linguistic and cultural alignment contributed to low adherence and uptake [56]. The congruence between participants’ expectations of exercise and rehabilitation programs, and their confidence in completing these interventions, also stood out. In one cycling exercise program, researchers highlighted that participants’ beliefs in the efficacy of exercise, coupled with their beliefs in the ability to exercise despite their disabilities, boosted adherence [51]. Yet, when intervention outcomes did not match participant expectations or their belief in their ability to complete the program (self-efficacy), adherence was impacted negatively [31, 54, 57]. Perhaps not surprisingly, King et al. (2015) concluded that high self-efficacy, measured by a standardized scale, contributed to high adherence in their exercise program [57]. Others reported that participants were more likely to abandon or not follow interventions as prescribed, if their expectations regarding the amount of physical effort were not aligned with the actual intervention requirements [54].
Program design factors
Characteristics of the intervention design featured prominently across studies and emerged as the major consideration in our thematic analysis, revealing subthemes around intervention regime, program delivery mode, individual program components, accountability, reinforcement strategies, and engagement (Fig. 2). Key recommendations for intervention design to minimize adherence barriers are summarized in Box 1.
Box 1. Summary of key strategies that facilitate adherence
| •Adaptable program elements that align with participant interests, personal demands, and abilities and that can be shifted over time to meet participant needs. |
| •Flexible timing and location of program offerings. |
| •Opportunities for social engagement and social reinforcement learning. |
| •Engaging and fun activities. |
| •Strong participant-interventionist relationships. |
| •Supervised programs that provide clear models and instructions, physical support, confidence, and behavior reinforcement/performative feedback. |
| •In-person sessions when feasible, or hybrid opportunities for remote + in-person sessions. When designing remote interventions, consider other key factors such as social reinforcement, participant-therapist relationships, safety, and accountability. |
| •Goal setting and self-management principles. |
| •Systematic education about the intervention including the theoretical framework, expected benefits, and when appropriate how outcomes will be measured. |
| •Digital health tools (both low-tech and high-tech) for providing instruction, self-monitoring, social reinforcement, and accountability. Reduce adherence barriers by providing (or loaning) required technology resources and technology literacy training. |
| •Robust and knowledgeable support system: existing care partners, peer supports, interventionists, and professional support personnel. |
Intervention regime. The number of exercises/activities, intensity/challenge, frequency, and duration can impact adherence, mirroring observations from pharmacological therapies [58]. A notable 46.67%(N = 21) of studies pinpointed regime intensity as a key determinant of adherence. Intensive and challenging programs can yield lower adherence in some people, especially if they induce pain or fatigue [22, 35, 45, 59], but for others may optimize adherence [35, 46, 60, 61]. Langer et al. (2021) found that the inherent challenges of rock-climbing spurred adherence due to a perceived high level of intrinsic reward for some people [62]. Another study noted that some younger participants’ preferences for strenuous exercises, like those with weighted vests, facilitated adherence [54]. However, for others, more intensive exercise programs may be less ideal, reducing their commitment to the program and thus lowering adherence row [54]. Noted in the previous section, intensive programs and those with rigid schedules may interact with personal demands to affect adherence. Our review found that programs with flexible schedules and components can facilitate adherence by mitigating conflicts with personal obligations, symptom variability, and medical appointments [22, 32, 33, 45, 63, 64]. Also, activities that required little external support, minimal equipment, and offered flexibility in terms of space and time promoted adherence [64].
Program delivery mode and program components. Service delivery mode and specific program components strongly influenced adherence. Duncan and Earhart (2012) compared attrition rates between programs of similar length and found that more infrequent visits and the option of home exercises contributed to reduced attrition [27]. While both individual and group modes showed high adherence, group settings were able to capitalize on community support and peer encouragement, thus enhancing social reinforcement and subsequent adherence [51, 65, 66]. Group interventions also can be a valuable environment for peer-modeled behaviors and norm-setting, which may support adherence. Some remotely delivered programs boosted adherence by overcoming access issues [67]. However, despite this advantage technological barriers reduced adherence for some [30, 31, 35]. In-person exercise and rehabilitation programs appeared to better support adherence due to enhanced opportunities for social engagement [27, 29, 68], relationship-building with interventionists [14, 56, 68], physical support and modeling for safe performance of activities [69, 70], and closer interventionist supervision [33, 63, 68]. Authors also identified the requirement of off-medication assessments as a possible reason for study withdrawal over the trial duration, which has implications for designing future clinical trials and exercise programs [27].
Digital aids typically promoted adherence. Digital health tools, which monitored and provided activity feedback, positively impacted adherence [31, 64]. Even simpler solutions, like a DVD, supported adherence by providing a visual model for performing exercises [56]. Several studies highlighted the importance of leveraging these technologies to facilitate remote monitoring, real-time feedback, and personalized guidance to promote adherence and engagement in future studies [6, 46, 64].
Other components leading to higher adherence included educational modules about the intervention itself [70], self-management tools including motivational interviewing and goal setting [40], and embedded coaching strategies for enhancing self-efficacy and confidence [31, 71]. Consistent with prior work [72], this review underscores the importance of pairing interventions with comprehensive education and self-management content that can help foster adherence by synchronizing participant expectations with likely outcomes, thereby shifting their perspectives and attitudes around exercise and rehabilitation [15, 36, 63, 64]. Studies in this review provide insights on how such content promotes adherence, in part, by increasing understanding of the intervention, thus enabling skilled and safe exercise execution and increased perceived mastery [15, 36, 63, 64].
Accountability. In this review, 35.6%(N = 16) of studies indicated that integrating accountability strategies bolstered adherence. Such strategies may be especially vital for individuals with low motivation, diminished self-efficacy, or complex health conditions [31, 56]. A common adherence barrier was participants ‘forgetting’ to complete activities. Examples of countermeasures reported as effective include phone reminders for intervention activities [55, 73], the use of digital health tools and wearables [64], daily exercise tracking diaries [14], and personalized reminders and encouragement from interventionists [64].
Reinforcement strategies. Both social reinforcement learning and interventionist reinforcement of positive behaviors contributed substantially to increased program adherence. Social reinforcement through group intervention designs [57, 74] and peer feedback [54, 70] allowed participants to benchmark their performance against other participants, which emerged as a key determinant of adherence. For some, group interventions set off a positive behavior change cycle starting with a sense of ‘competitiveness’ that was a driver for higher adherence, which in turn yielded increased positive reinforcement from other group members and interventionists, and subsequently further strengthened adherence [66]. Positive and frequent verbal feedback from the interventionist also increased motivation and perceived support, which was associated with improved adherence [55, 63, 73].
Engagement. Most (44.4%,N = 20) studies endorsed a factor we labeled as ‘engagement’ as a key to higher adherence. Enjoyable activities, or having ‘fun’, was associated with higher adherence [29, 35, 49, 60, 62, 75]. Park et al. (2014) attributed engagement with other participants, and the resultant optimism and improved ‘attitude’ about the intervention, to a group delivery mode [65]. Also, Kalyani (2019) found higher adherence in dance classes may be due to a sense of community belonging [69]. Notably, 28.9%(N = 13) of studies attributed positive adherence contributions from social engagement with other participants in group exercise and rehabilitation settings [44, 54, 56, 65, 68–70, 74, 76, 77]. Taken on whole, findings regarding the positive effects of social engagement and social reinforcement on adherence are consistent with social cognitive theory and its role in health behaviour changes in PD [78].
Khalil et al. (2017) and Pickering et al. (2013) also highlight the important role of a positive participant-interventionist relationship in adherence, specifically motivating participation and facilitating the successful uptake of information [14, 56]. Although mentioned in a single study, the level of interventionists’ engagement may also be a factor to consider in optimizing adherence. McGinley et al. (2012) found that interventionists’ confidence in and engagement with the program contributed to participant adherence [68]. Beyond the primary interventionist, facilitating engagement with care partners and support staff was also a successful adherence strategy employed by some programs [54, 56, 70]. Having peer and professional support staff to assist with exercises and intervention activities was viewed positively and encouraged a sense of safety and confidence that bolstered adherence [69].
System and environmental factors
Accessibility and logistical barriers are central in influencing therapy adherence. The proximity, safety (including considerations for individuals with mobility and cognitive challenges), and ease of access to locations where programs are hosted stand as important determinants of adherence [32, 51, 76]. Home-based programs, while perceived as a solution to transportation challenges, may present their own set of complexities. In the current review, challenges were observed with unsupervised, home-delivered exercise programs where adherence waned [67]. Real-world challenges, such as the equipment expense or the need for appropriate spaces at home, can limit adherence to some home exercise programs [35]. Regardless of delivery mode, the participant’s support network, often family care partners, played a key role in adherence [54–56, 65, 66, 70].
People with PD also may face challenges due to limited access to and cost of both general and specialized exercise facilities [62, 79]. Fernandez-Gonzalez et al. (2019) identified that having an intervention be low-cost is a pivotal factor for increased adherence, but to fully appreciate its magnitude, it is essential to recognize the broader economic disparities that often exacerbate this challenge [60]. Insurance inadequacies further magnify these barriers, potentially preventing many from starting therapeutic interventions or continuing with them in the future [6, 80].
In the current review, community-delivered programs generally experienced high adherence [30, 56, 66, 69, 70]. However, previous studies have highlighted access challenges to outpatient, community-based rehabilitation interventions in PD [79]. This paucity was also identified as a barrier to adherence by authors of studies in the current review [50, 51]. Compounding these system level barriers, previous research exposed difficulties in securing referrals to rehabilitation services, particularly to OT and SLT [81]. Accessibility barriers to adherence may be further exaggerated by sex, race, and geographical health disparities in PD care [82, 83]. This gap is evident in the current review, in which the included studies were largely conducted in high income countries, were male biased (only 38.64%female participants, Table 1), and included primarily participants from non-racialized groups.
Technology integrated programs showcase potential for increasing access to care, with many reporting satisfactory adherence rates [31, 75]. However, successful adherence to these digital interventions and tools hinges on consistent device availability, quality, and reliable internet access [6, 30, 31, 40]. While a significant proportion of the global populace accesses the internet, there’s a pronounced need for high-speed connectivity, especially for telehealth initiatives [84]. For some older adults, and those in economically constrained or remote regions, cost and technological literacy can also be significant systems-level barriers to improving adherence via technology solutions [85, 86].
DISCUSSION
Limited progress in the reporting of adherence data has been made over the last decade since Allen et al. (2012) published their review on adherence to exercise and motor interventions in PD [13]. Although our methodology differs, this rapid evidence assessment complements prior work from Allen et al. (2012) and Schootemeijer et al. (2020) in a few key areas [13, 15]. First, aligned with a focus on the quality of adherence data and the barriers/facilitators to adherence the search terms differed, requiring the terms ‘adherence’ or ‘compliance’ to be present in the abstract, text, or MeSH terms. Second, consistent with best practices in adherence measurement [24], we considered adherence broadly, examining outcomes beyond study dropout and attrition rates. Lastly, we expanded this review to include rehabilitation and exercise programs across all major rehabilitation disciplines, inclusive of PT, OT, and SLT.
Despite methodological differences, several themes emerged from the current review that parallel those noted in in prior studies [13, 78]. Principally, the evolving nature of PD, characterized by progressive symptom changes and medication adjustments, frequently posed barriers to intervention participation, highlighting the imperative for interventions to dynamically adapt and accommodate these fluctuations over time. A particular concern highlighted by this review, and reinforced by other reviews and qualitative studies, is that increased discomfort and fatigue reduces study adherence [13–15, 38]. To mitigate this barrier, several studies demonstrated how personalization of difficulty level and intervention components can support adherence and thus improve treatment efficacy [14, 23, 31, 39, 54, 64, 69]. Like others, the current review also underscored the importance of interventions with the logistical flexibility to accommodate a variety of personal factors that may interfere with adherence, across the duration of the intervention [13, 15, 16, 27, 38].
One factor that rose to prominence in the current review, that has received less attention in prior work, is the importance of the alignment between the intervention and personal beliefs, values, and culture on facilitating adherence. Incorporating person-centered principles when developing interventions is one strategy for addressing these barriers. Grounded in Rogerian principles, the person-centered rehabilitation model proposes a holistic approach that positions the individual—with their beliefs, cultural identities, and expectations—at the core of an intervention [47, 48]. We found several studies that incorporated successfully goal setting, education modules, and self-management strategies to align participant expectations with the intervention goals [36, 63, 64, 68]. Although it did not meet the criteria for inclusion because of the study design, Danoudis and Iansek (2022) is worth highlighting here because of its approach to identifying individualized personal barriers to adherence, prior to participants starting the program, using standardized measures of self-efficacy and beliefs regarding exercise and health behavior changes [87].
Social reinforcement and social connectedness played a critical role in facilitating adherence to rehabilitation and exercise interventions. Opportunities for social engagement with other group members was not the only form of engagement found to enhance adherence. Physical, instructional, and cognitive support in performing activities; adherence reminders; and performative feedback from family care partners, support personnel, and interventionists were also reported to increase motivation and to promote adherence. A factor that did not emerge from the current review of exercise and rehabilitation literature, and thus was not captured in our thematic analysis, was emotions like frustration, embarrassment, shame, or low self-esteem, often stemming from the stigmatization of living with PD symptoms. This factor has been highlighted as a barrier to clinical management by prior narrative reviews on stigma [88].
System and environmental factors that featured prominently in the current review, such as financial costs, access challenges, and perceived safety risks, have also been identified previously as barriers to adherence in PD pharmacological and non-pharmacological interventions [15, 89]. A comprehensive strategy is imperative to address systems-level barriers and improve the effectiveness of exercise and rehabilitation interventions in individuals with PD. Such efforts should consider the safety of persons with PD living with motor and cognitive impairments. Collaborative efforts, especially between local authorities and healthcare providers, can enhance adherence by encompassing transportation provisions and accessible facilities. The telehealth avenue, promising as it seems, mandates a robust technological infrastructure. Increasing the availability of technology literacy programs, especially for older adults, is critical. In line with contemporary insights, allocating dedicated funds for technology and equipment in clinical trials can reduce economic barriers and promote equitable participation, which will thereby foster better adherence in clinical trials [90]. Economic barriers cannot be overlooked. Initiatives aimed at better insurance provisions for rehabilitation services and the exploration of affordable intervention models are vital.
In this review, we spotlight actionable design elements and strategies that, when addressed, may elevate adherence and, consequently, the overall efficacy of PD interventions. In Table 2, we provide overarching recommendations for optimizing adherence research in PD and hope to increase the likelihood of translating effective treatments into clinical practice.
Table 2
Summary recommendations for examining key determinants of adherence
| Leverage participatory research models |
| •Identifying potential sources of, and solutions for, adherence issues |
| •Optimizing alignment between the needs and perspectives of people with PD and the program objectives/approach |
| •Selecting adherence outcomes that take into account the perspectives of people with PD |
| Embrace person-centered care principles |
| •Fostering alignment with personal interests, culture, beliefs, and attitudes |
| •Addressing logistical and family/work obligations barriers through flexible components, delivery models, and scheduling options |
| •Building adaptive interventions informed by the lived experiences of people with PD and their support network |
| Delve deeper into adherence factors |
| •Not a one-size-fits-all approach. Adherence outcomes should be tailored to the goals of the intervention and the context in which the intervention occurs |
| •Moving beyond binary measures to quantify adherence along a continuum can help capture levels of adherence and reveal nuanced predictors of treatment compliance |
| •Requires a multi-faceted measurement approach that may include self-report and clinical observations, interviews, checklists, manual and/or digital behavior monitoring. Participant burden should be considered when selecting adherence measurement tools. |
| •Should consider differences in symptom presentation, disease impact and health service utilization for women, trans-people, and racialized groups; people across socioeconomic and geographic communities; and people of different ages and disease severities. |
| Adopt uniform reporting guidelines |
| •Taking inspiration from the publication of the recent MDS guidelines for reporting pharmacological adherence, the field should develop PD-specific guidelines for reporting non-pharmacological intervention adherence data. |
| •Adherence outcomes should be specified prior to starting the clinical trial |
| •Transparent adherence reporting should include the rates of adherence by outcome for both control (when applicable) and intervention groups, a description of the assessment approach/tools used, adherence sub-analyses for treatment responders and non-responders. Combining qualitative and quantitative approaches is particularly powerful. |
Limitations
This review, while insightful, has several limitations. While the use of rapid review methodologies may lack the comprehensiveness of a traditional systematic review, it has been suggested that for more focused questions, they generate similar results to systematic review methodologies [91]. Our data range omitted clinical trials reporting adherence data prior to 2008, which may have impacted our thematic analysis. However, we note that within our lookback period the earliest study meeting our eligibility criteria was published in 2012. The inconsistent and variable reporting of adherence data across studies often required us to extract themes from anecdotal observations and comments provided by authors, which might lack a strong quantitative or qualitative basis. The stringent inclusion criteria applied in this review, necessitating explicit references to ‘adherence’ or ‘compliance’, could have excluded relevant clinical trials that discussed attrition rates but that did not delve into specific discussion of adherence or compliance. This possibly constrains the comprehensiveness of this review.
Many studies relied heavily on self-reported data, which is susceptible to biases like recall and social desirability, an observation also highlighted by authors of studies in the review [23, 59]. Additionally, conclusions from the current review may be confounded by selection bias since those volunteering for exercise and rehabilitation studies may be predisposed toward higher adherence [73]. Furthermore, the focus of many studies on reporting ‘simple’ adherence measures, such as attrition or session attendance rates, provides an incomplete view of adherence, which can obscure understanding the broader impact of adherence on treatment effectiveness [11, 24].
Lastly, the dominance of studies from countries with developed healthcare systems and limited data from typically marginalized and underserved communities raises concerns about the generalizability of these findings. Adherence factors from these contexts may not reflect the challenges in medically underserved groups, possibly omitting key cultural, socioeconomic, and accessibility considerations vital for a holistic understanding of global adherence behaviors.
Summary
This rapid evidence assessment parallels the conclusions of Allen et al. (2012), emphasizing the limited research on adherence [13]. The suboptimal quality of adherence reporting can impact significantly treatment effectiveness research. Noted by others, this disparity is especially pronounced when juxtaposed with the vast literature on medication adherence in PD [14]. Factors, such as intervention costs, disease related factors, and therapy regime impact both pharmacological and non-pharmacological interventions [89]. However, unique adherence factors specific to non-pharmacological interventions warrant attention.
ACKNOWLEDGMENTS
We acknowledge Meagan Stanley for assistance in developing the search strategy; Mehvish Jamal, Emily Narayan, and Benjamin Katz for assistance with editing and article assessment.
FUNDING
Research reported herein was supported by the Canada Research Chairs program (AR) and the National Institute of Deafness and Communication Disorders of the National Institutes of Health award number 1R21DC017255 (AR). The content is solely the responsibility of the authors and does not represent the official views of the funding agencies.
CONFLICT OF INTEREST
AR receives honoraria from the World Parkinson Congress and Parkinson Canada and sits on the Research Advisory Board for Parkinson Canada. Her research is funded by the National Institutes of Health (USA), the Ontario Brain Institute, McKnight Brain Research Foundation, and the Canada Research Chairs program. JL, NA, and IC have no conflicts to report.
DATA AVAILABILITY
All data associated with this publication are available upon request of the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230266.
REFERENCES
[1] | Ellis T , Rochester L ((2018) ) Mobilizing Parkinson’s disease: The future of exercise. J Parkinsons Dis 8: , S95–S100. |
[2] | Zhen K , Zhang S , Tao X , Li G , Lv Y , Yu L ((2022) ) A systematic review and meta-analysis on effects of aerobic exercise in people with Parkinson’s disease. NPJ Park Dis 8: , 146. |
[3] | Li JA , Loevaas MB , Guan C , Goh L , Allen NE , Mak MKY , Lv J , Paul SS ((2023) ) Does exercise attenuate disease progression in people with Parkinson’s disease? A systematic review with meta-analyses. Neurorehabil Neural Repair 37: , 328–352. |
[4] | Allen NE , Sherrington C , Paul SS , Canning CG ((2011) ) Balance and falls in Parkinson’s disease: A meta-analysis of the effect of exercise and motor training. Mov Disord 26: , 1605–1615. |
[5] | Radder DLM , Lígia Silva de Lima A , Domingos J , Keus SHJ , van Nimwegen M , Bloem BR , de Vries NM ((2020) ) Physiotherapy in Parkinson’s disease: A meta-analysis of present treatment modalities. Neurorehabil Neural Repair 34: , 871–880. |
[6] | van der Kolk NM , King LA ((2013) ) Effects of exercise on mobility in people with Parkinson’s disease. Mov Disord 28: , 1587–1596. |
[7] | Muñoz-Vigueras N , Prados-Román E , Valenza MC , Granados-Santiago M , Cabrera-Martos I , Rodríguez-Torres J , Torres-Sánchez I ((2021) ) Speech and language therapy treatment on hypokinetic dysarthria in Parkinson disease: Systematic review and meta-analysis. Clin Rehabil 35: , 639–655. |
[8] | Athukorala RP , Jones RD , Sella O , Huckabee M-L ((2014) ) Skill training for swallowing rehabilitation in patients with Parkinson’s disease. Arch Phys Med Rehabil 95: , 1374–1382. |
[9] | Tofani M , Ranieri A , Fabbrini G , Berardi A , Pelosin E , Valente D , Fabbrini A , Costanzo M , Galeoto G ((2020) ) Efficacy of occupational therapy interventions on quality of life in patients with Parkinson’s disease: A systematic review and meta-analysis. Mov Disord Clin Pract 7: , 891–901. |
[10] | Baatile J , Langbein WE , Weaver F , Maloney C , Jost MB ((2000) ) Effect of exercise on perceived quality of life of individuals with Parkinson’s disease. J Rehabil Res Dev 37: , 529–534. |
[11] | Nagpal TS , Mottola MF , Barakat R , Prapavessis H ((2021) ) Adherence is a key factor for interpreting the results of exercise interventions. Physiotherapy 113: , 8–11. |
[12] | Mir T ((2023) ) Adherence versus compliance. HCA Healthc J Med 4: , 219–220. |
[13] | Allen NE , Sherrington C , Suriyarachchi GD , Paul SS , Song J , Canning CG ((2012) ) Exercise and motor training in people with Parkinson’s disease: A systematic review of participant characteristics, intervention delivery, retention rates, adherence, and adverse events in clinical trials. Parkinsons Dis 2012: , e854328. |
[14] | Pickering RM , Fitton C , Ballinger C , Fazakarley L , Ashburn A ((2013) ) Self reported adherence to a home-based exercise programme among people with Parkinson’s disease. Parkinsonism Relat Disord 19: , 66–71. |
[15] | Schootemeijer S , van der Kolk NM , Ellis T , Mirelman A , Nieuwboer A , Nieuwhof F , Schwarzschild MA , de Vries NM , Bloem BR ((2020) ) Barriers and motivators to engage in exercise for persons with Parkinson’s disease. J Parkinsons Dis 10: , 1293–1299. |
[16] | Crizzle AM , Newhouse IJ ((2012) ) Themes associated with exercise adherence in persons with Parkinson’s disease: A qualitative study. Occup Ther Health Care 26: , 174–186. |
[17] | Page P , Eardley W , Carr J , Chadwick D , Porter K ((2011) ) An Introduction to Clinical Research, OUP Oxford. |
[18] | Varker T , Forbes D , Dell L , Weston A , Merlin T , Hodson S , O’Donnell M ((2015) ) Rapid evidence assessment: Increasing the transparency of an emerging methodology . J Eval Clin Pract 21: , 1199–1204. |
[19] | Hamel C , Michaud A , Thuku M , Skidmore B , Stevens A , Nussbaumer-Streit B , Garritty C ((2021) ) Defining Rapid Reviews: A systematic scoping review and thematic analysis of definitions and defining characteristics of rapid reviews. J Clin Epidemiol 129: , 74–85. |
[20] | Bloem BR , De Vries NM , Ebersbach G ((2015) ) Nonpharmacological treatments for patients with Parkinson’s disease. Mov Disord 30: , 1504–1520. |
[21] | Richards KAR , Hemphill MA ((2018) ) A practical guide to collaborative qualitative data analysis. J Teach Phys Educ 37: , 225–231. |
[22] | Canning CG , Sherrington C , Lord SR , Close JCT , Heritier S , Heller GZ , Howard K , Allen NE , Latt MD , Murray SM , O’Rourke SD , Paul SS , Song J , Fung VSC ((2015) ) Exercise for falls prevention in Parkinson disease: A randomized controlled trial. Neurology 84: , 304–312. |
[23] | Allen NE , Song J , Paul SS , Sherrington C , Murray SM , O’Rourke SD , Lord SR , Fung VSC , Close JCT , Howard K , Canning CG ((2015) ) Predictors of adherence to a falls prevention exercise program for people with Parkinson’s disease. Mov Disord Clin Pract 2: , 395–401. |
[24] | Vitolins MZ , Rand CS , Rapp SR , Ribisl PM , Sevick MA ((2000) ) Measuring adherence to behavioral and medical interventions. Control Clin Trials 21: , S188–S194. |
[25] | van Nimwegen M , Speelman AD , Hofman-van Rossum EJM , Overeem S , Deeg DJH , Borm GF , van der Horst MHL , Bloem BR , Munneke M ((2011) ) Physical inactivity in Parkinson’s disease. J Neurol 258: , 2214–2221. |
[26] | Vellata C , Belli S , Balsamo F , Giordano A , Colombo R , Maggioni G ((2021) ) Effectiveness of telerehabilitation on motor impairments, non-motor symptoms and compliance in patients with Parkinson’s disease: A systematic review. Front Neurol 12: , 627999. |
[27] | Duncan RP , Earhart GM ((2012) ) Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair 26: , 132–143. |
[28] | Terrens AF , Soh S-E , Morgan P ((2020) ) The safety and feasibility of a Halliwick style of aquatic physiotherapy for falls and balance dysfunction in people with Parkinson’s Disease: A single blind pilot trial. PLoS One 15: , e0236391. |
[29] | Shanahan J , Morris ME , Bhriain ON , Volpe D , Lynch T , Clifford AM ((2017) ) Dancing for Parkinson disease: A randomized trial of irish set dancing compared with usual care. Arch Phys Med Rehabil 98: , 1744–1751. |
[30] | Strouwen C , Molenaar EALM , Münks L , Keus SHJ , Zijlmans JCM , Vandenberghe W , Bloem BR , Nieuwboer A ((2017) ) Training dual tasks together or apart in Parkinson’s disease: Results from the DUALITY trial: Training Dual Tasks Together or Apart in PD. Mov Disord 32: , 1201–1210. |
[31] | Lai B , Bond K , Kim Y , Barstow B , Jovanov E , Bickel CS ((2020) ) Exploring the uptake and implementation of tele-monitored home-exercise programmes in adults with Parkinson’s disease: A mixed-methods pilot study. J Telemed Telecare 26: , 53–63. |
[32] | Demonceau M , Jidovtseff B , Donneau AF , Bury T , Croisier JL , Crielaard JM , Rodriguez De La Cruz C , Delvaux V , Garraux G ((2017) ) Effects of twelve weeks of aerobic or strength training in addition to standard care in Parkinson’s disease: A controlled study. Eur J Phys Rehabil Med 53: , 184–200. |
[33] | Jäggi S , Wachter A , Adcock M , de Bruin ED , Möller JC , Marks D , Schweinfurther R , Giannouli E ((2023) ) Feasibility and effects of cognitive-motor exergames on fall risk factors in typical and atypical Parkinson’s inpatients: A randomized controlled pilot study. Eur J Med Res 28: , 30. |
[34] | Monteiro EP , Franzoni LT , Cubillos DM , De Oliveira Fagundes A , Carvalho AR , Oliveira HB , Pantoja PD , Schuch FB , Rieder CR , Martinez FG , Peyré-Tartaruga LA ((2016) ) Effects of Nordic walking training on functional parameters in Parkinson’s disease: A randomized controlled clinical trial. Scand J Med Sci Sports 27: , 351–358. |
[35] | Allen NE , Song J , Paul SS , Smith S , O’Duffy J , Schmidt M , Love R , Sherrington C , Canning CG ((2017) ) An interactive videogame for arm and hand exercise in people with Parkinson’s disease: A randomized controlled trial. Parkinsonism Relat Disord 41: , 66–72. |
[36] | Vanbellingen T , Nyffeler T , Nigg J , Janssens J , Hoppe J , Nef T , Müri RM , Van Wegen EEH , Kwakkel G , Bohlhalter S ((2017) ) Home based training for dexterity in Parkinson’s disease: A randomized controlled trial. Parkinsonism Relat Disord 41: , 92–98. |
[37] | Canning CG , Allen NE , Dean CM , Goh L , Fung VS ((2012) ) Home-based treadmill training for individuals with Parkinson’s disease: A randomized controlled pilot trial. Clin Rehabil 26: , 817–826. |
[38] | Spurgeon L , Clarke CE , Sackley C ((2015) ) Subjective experiences of speech and language therapy in patients with Parkinson’s disease: A pilot study. Rehabil Res Pract 2015: , e839895. |
[39] | Richardson K , Huber JE , Kiefer B , Snyder S ((2022) ) Perception of physical demand, mental demand, and performance: A comparison of two voice interventions for Parkinson’s disease. Am J Speech Lang Pathol 31: , 1963–1978. |
[40] | Van De Weijer SCF , Duits AA , Bloem BR , De Vries NM , Kessels RPC , Köhler S , Tissingh G , Kuijf ML ((2020) ) Feasibility of a cognitive training game in Parkinson’s disease: The randomized Parkin’Play Study. Eur Neurol 83: , 426–432. |
[41] | Svaerke K , Faerk AK , Riis A , Stiegnitz Von Ehrenfels SEM , Mogensen J , Lokkegaard A ((2022) ) Effects of computer-based cognitive rehabilitation on attention, executive functions, and quality of life in patients with Parkinson’s disease: A randomized, controlled, single-blinded pilot study. Dement Geriatr Cogn Disord 50: , 519–528. |
[42] | Das J , Barry G , Walker R , Vitorio R , Morris R , Stuart S ((2023) ) The integration of technology into a home-based visuo-cognitive training intervention for people with Parkinson’s: Is the future digital? PLoS One 18: , e0285100. |
[43] | Schindler A , Pizzorni N , Cereda E , Cosentino G , Avenali M , Montomoli C , Abbruzzese G , Antonini A , Barbiera F , Benazzo M , Benarroch E , Bertino G , Clavè P , Cortelli P , Eleopra R , Ferrari C , Hamdy S , Huckabee M-L , Lopiano L , Marchese-Ragona R , Masiero S , Michou E , Occhini A , Pacchetti C , Pfeiffer RF , Restivo DA , Rondanelli M , Ruoppolo G , Sandrini G , Schapira A , Stocchi F , Tolosa E , Valentino F , Zamboni M , Zangaglia R , Zappia M , Tassorelli C , Alfonsi E ((2021) ) Consensus on the treatment of dysphagia in Parkinson’s disease. J Neurol Sci 430: , 120008. |
[44] | Pohl P , Dizdar N , Hallert E ((2013) ) The Ronnie Gardiner Rhythm and Music Method - A feasibility study in Parkinson’s disease. Disabil Rehabil 35: , 2197–2204. |
[45] | Sackley CM , Smith CH , Rick CE , Brady MC , Ives N , Patel S , Woolley R , Dowling F , Patel R , Roberts H , Jowett S , Wheatley K , Kelly D , Sands G , Clarke CE ((2018) ) Lee Silverman Voice Treatment versus standard speech and language therapy versus control in Parkinson’s disease: A pilot randomised controlled trial (PD COMM pilot). Pilot Feasibility Stud 4: , 30. |
[46] | Bek J , Holmes PS , Craig CE , Franklin ZC , Sullivan M , Webb J , Crawford TJ , Vogt S , Gowen E , Poliakoff E ((2021) ) Action Imagery and Observation in Neurorehabilitation for Parkinson’s Disease (ACTION-PD): Development of a user-informed home training intervention to improve functional hand movements. Parkinsons Dis 2021: , 4559519. |
[47] | Buetow SA , Martínez-Martín P , Hirsch MA , Okun MS ((2016) ) Beyond patient-centered care: Person-centered care for Parkinson’s disease. NPJ Parkinsons Dis 2: , 16019. |
[48] | Jesus TS , Papadimitriou C , Bright FA , Kayes NM , Pinho CS , Cott CA ((2022) ) Person-centered rehabilitation model: Framing the concept and practice of person-centered adult physical rehabilitation based on a scoping review and thematic analysis of the literature. Arch Phys Med Rehabil 103: , 106–120. |
[49] | Martin T , Weatherall M , Anderson TJ , MacAskill MR ((2015) ) A randomized controlled feasibility trial of a specific cueing program for falls management in persons with Parkinson disease and freezing of gait. J Neurol Phys Ther 39: , 179–184. |
[50] | McKee KE , Johnson RK , Chan J , Wills A ((2021) ) Implementation of high-cadence cycling for Parkinson’s disease in the community setting: A pragmatic feasibility study. Brain Behav 11: , e02053. |
[51] | Rosenfeldt AB , Miller Koop M , Penko AL , Alberts JL ((2022) ) Individuals with Parkinson disease are adherent to a high-intensity community-based cycling exercise program. J Neurol Phys Ther 46: , 73–80. |
[52] | Vorasoot N , Termsarasab P , Thadanipon K , Pulkes T ((2020) ) Effects of handwriting exercise on functional outcome in Parkinson disease: A randomized controlled trial. J Clin Neurosci 72: , 298–303. |
[53] | Li F , Harmer P , Liu Y , Eckstrom E , Fitzgerald K , Stock R , Chou L ((2014) ) A randomized controlled trial of patient-reported outcomes with tai chi exercise in Parkinson’s disease. Mov Disord 29: , 539–545. |
[54] | Rowsell A , Ashburn A , Fitton C , Goodwin VA , Hulbert S , Lamb SE , McIntosh E , Nieuwboer A , Pickering R , Rochester L , Chivers-Seymour K , Ballinger C ((2022) ) Participant expectations and experiences of a tailored physiotherapy intervention for people with Parkinson’s and a history of falls. Disabil Rehabil 44: , 727–735. |
[55] | Sturkenboom IH , Nijhuis-van Der Sanden MW , Graff MJ ((2016) ) A process evaluation of a home-based occupational therapy intervention for Parkinson’s patients and their caregivers performed alongside a randomized controlled trial. Clin Rehabil 30: , 1186–1199. |
[56] | Khalil H , Busse M , Quinn L , Nazzal M , Batyha W , Alkhazaleh S , Alomari MA ((2017) ) A pilot study of a minimally supervised home exercise and walking program for people with Parkinson’s disease in Jordan. Neurodegener Dis Manag 7: , 73–84. |
[57] | King LA , Wilhelm J , Chen Y , Blehm R , Nutt J , Chen Z , Serdar A , Horak FB ((2015) ) Does group, individual or home exercise best improve mobility for people with Parkinson’s disease? J Neurol Phys Ther 39: , 204–212. |
[58] | Daley DJ , Myint PK , Gray RJ , Deane KHO ((2012) ) Systematic review on factors associated with medication non-adherence in Parkinson’s disease. Parkinsonism Relat Disord 18: , 1053–1061. |
[59] | Van der Kolk NM , De Vries NM , Kessels RPC , Joosten H , Zwinderman AH , Post B , Bloem BR ((2019) ) Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol 18: , 998–1008. |
[60] | Fernández-González P , Carratalá-Tejada M , Monge-Pereira E , Collado-Vázquez S , Sánchez-Herrera Baeza P , Cuesta-Gómez A , Oña-Simbaña ED , Jardón-Huete A , Molina-Rueda F , Balaguer-Bernaldo De Quirós C , Miangolarra-Page JC , Cano-de La Cuerda R ((2019) ) Leap motion controlled video game-based therapy for upper limb rehabilitation in patients with Parkinson’s disease: A feasibility study. J Neuroeng Rehabil 16: , 133. |
[61] | Pastore-Wapp M , Kaufmann BC , Nyffeler T , Wapp S , Bohlhalter S , Vanbellingen T ((2023) ) Feasibility of a combined intermittent theta-burst stimulation and video game-based dexterity training in Parkinson’s disease. J Neuroeng Rehabil 20: , 2. |
[62] | Langer A , Hasenauer S , Flotz A , Gassner L , Pokan R , Dabnichki P , Wizany L , Gruber J , Roth D , Zimmel S , Treven M , Schmoeger M , Willinger U , Maetzler W , Zach H ((2021) ) A randomised controlled trial on effectiveness and feasibility of sport climbing in Parkinson’s disease. NPJ Parkinsons Dis 7: , 49. |
[63] | Ellis TD , Cavanaugh JT , DeAngelis T , Hendron K , Thomas CA , Saint-Hilaire M , Pencina K , Latham NK ((2019) ) Comparative effectiveness of mhealth-supported exercise compared with exercise alone for people with Parkinson disease: Randomized controlled pilot study. Phys Ther 99: , 203–216. |
[64] | Mak MKY , Wong-Yu ISK ((2021) ) Six-month community-based brisk walking and balance exercise alleviates motor symptoms and promotes functions in people with Parkinson’s disease: A randomized controlled trial. J Parkinsons Dis 11: , 1431–1441. |
[65] | Park A , Zid D , Russell J , Malone A , Rendon A , Wehr A , Li X ((2014) ) Effects of a formal exercise program on Parkinson’s disease: A pilot study using a delayed start design. Parkinsonism Relat Disord 20: , 106–111. |
[66] | Yang JH , Wang YQ , Ye SQ , Cheng YG , Chen Y , Feng XZ ((2017) ) The effects of group-based versus individual-based Tai Chi training on nonmotor symptoms in patients with mild to moderate Parkinson’s disease: A randomized controlled pilot trial. Parkinsons Dis 2017: , 8562867. |
[67] | Vasconcellos LSD , Silva RS , Pachêco TB , Nagem DA , Sousa CDO , Ribeiro TS ((2023) ) Telerehabilitation-based trunk exercise training for motor symptoms of individuals with Parkinson’s disease: A randomized controlled clinical trial. J Telemed Telecare 29: , 698–706. |
[68] | McGinley JL , Martin C , Huxham FE , Menz HB , Danoudis M , Murphy AT , Watts JJ , Iansek R , Morris ME ((2012) ) Feasibility, safety, and compliance in a randomized controlled trial of physical therapy for Parkinson’s disease. Parkinsons Dis 2012: , 795294. |
[69] | Kalyani HHN , Sullivan KA , Moyle G , Brauer S , Jeffrey ER , Kerr GK ((2019) ) Impacts of dance on cognition, psychological symptoms and quality of life in Parkinson’s disease. Neurorehabilitation 45: , 273–283. |
[70] | Ridgel AL , Walter BL , Tatsuoka C , Walter EM , Colón-Zimmermann K , Welter E , Sajatovic M ((2016) ) Enhanced exercise therapy in Parkinson’s disease: A comparative effectiveness trial. J Sci Med Sport 19: , 12–17. |
[71] | King LA , Mancini M , Smulders K , Harker G , Lapidus JA , Ramsey K , Carlson-Kuhta P , Fling BW , Nutt JG , Peterson DS , Horak FB ((2020) ) Cognitively challenging agility boot camp program for freezing of gait in Parkinson disease. Neurorehabil Neural Repair 34: , 417–427. |
[72] | Lorig KR , Sobel DS , Stewart AL , Brown BW , Bandura A , Ritter P , Gonzalez VM , Laurent DD , Holman HR ((1999) ) Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: A randomized trial. Med Care 37: , 5–14. |
[73] | Wu P , Lee M , Wu S , Ho H , Chang M , Lin H , Huang T ((2021) ) Effects of home-based exercise on motor, non-motor symptoms and health-related quality of life in Parkinson’s disease patients: A randomized controlled trial. Jpn J Nurs Sci 18: , e12418. |
[74] | Flynn A , Preston E , Dennis S , Canning CG , Allen NE ((2021) ) Home-based exercise monitored with telehealth is feasible and acceptable compared to centre-based exercise in Parkinson’s disease: A randomised pilot study. Clin Rehabil 35: , 728–739. |
[75] | Spina S , Facciorusso S , Cinone N , Armiento R , Picelli A , Avvantaggiato C , Ciritella C , Fiore P , Santamato A ((2021) ) Effectiveness of robotic balance training on postural instability in patients with mild Parkinson’s disease: A pilot, single blind, randomized controlled trial. J Rehabil Med 53: , jrm00154. |
[76] | Bega D , Stein J , Zadikoff C , Simuni T , Victorson D , Ring M , Jovanovic B , Corcos D ((2016) ) Yoga versus resistance training in mild to moderate severity Parkinson’s disease: A 12-week pilot study. J Yoga Phys Ther 6: , 1. |
[77] | Moratelli J , Alexandre KH , Boing L , Swarowsky A , Corrêa CL , Guimarães ACDA ((2021) ) Binary dance rhythm or Quaternary dance rhythm which has the greatest effect on non-motor symptoms of individuals with Parkinson’s disease? Complement Ther Clin Pract 43: , 101348. |
[78] | Ellis T , Motl RW ((2013) ) Physical activity behavior change in persons with neurologic disorders: Overview and examples from Parkinson disease and multiple sclerosis. J Neurol Phys Ther 37: , 85–90. |
[79] | Fullard ME , Thibault DP , Hill A , Fox J , Bhatti DE , Burack MA , Dahodwala N , Haberfeld E , Kern DS , Klepitskava OS , Urrea-Mendoza E , Myers P , Nutt J , Rafferty MR , Schwalb JM , Shulman LM , Willis AW ((2017) ) Utilization of rehabilitation therapy services in Parkinson disease in the United States. Neurology 89: , 1162–1169. |
[80] | Bloem BR , Rompen L , Vries de NM , Klink A , Munneke M , Jeurissen P ((2017) ) ParkinsonNet: A low-cost health care innovation with a systems approach from The Netherlands. Health Aff (Millwood) 36: , 1987–1996. |
[81] | Roberts AC , Rafferty MR , Wu SS , Miao G , Cubillos F , Simuni T , Marras C , Davis T , Dahodwala N , Neault M , Ramirez-Zamora A , Rafferty M , Malaty I , Parashos S , Kraakevik J , Simuni T , Dahodwala N , Jankovic J , Simon D , Pahwa R , Mills K , Way C , Morgan J , Pagan F , Hauser R , Davis T , Salins N , Gurevich T , Bloem B , Marras C , Singer C , Lafontaine AL , Feigin A , Miyasaki J , Litvan I ((2021) ) Patterns and predictors of referrals to allied health services for individuals with Parkinson’s disease: A Parkinson’s foundation (PF) QII study. Parkinsonism Relat Disord 83: , 115–122. |
[82] | Hobson DE , Lix LM , Azimaee M , Leslie WD , Burchill C , Hobson S ((2012) ) Healthcare utilization in patients with Parkinson’s disease: A population-based analysis. Parkinsonism Relat Disord 18: , 930–935. |
[83] | Fullard ME , Thibault DP , Todaro V , Foster S , Katz L , Morgan R , Kern DS , Schwalb JM , Mendoza EU , Dahodwala N , Shulman L , Willis AW ((2018) ) Sex disparities in health and health care utilization after Parkinson diagnosis: Rethinking PD associated disability. Parkinsonism Relat Disord 48: , 45–50. |
[84] | The State of broadband 2022: Accelerating broadband for new realities - UNESCO Digital Library. https://unesdoc.unesco.org/ark:/48223/pf0000383330 |
[85] | Rivera V , Aldridge MD , Ornstein K , Moody KA , Chun A ((2021) ) RESEARCHRacial and socioeconomic disparities in access to telehealth. J Am Geriatr Soc 69: , 44–45. |
[86] | Cortelyou-Ward K , Atkins DN , Noblin A , Rotarius T , White P , Carey C ((2020) ) Navigating the digital divide: Barriers to telehealth in rural areas. J Health Care Poor Underserved 31: , 1546–1556. |
[87] | Danoudis M , Iansek R ((2022) ) A long-term community gym program for people with Parkinson’s disease: A feasibility study of the Monash Health “Health and Fitness” model. Disabil Rehabil 44: , 7330–7338. |
[88] | Maffoni M , Giardini A , Pierobon A , Ferrazzoli D , Frazzitta G ((2017) ) Stigma experienced by Parkinson’s disease patients: A descriptive review of qualitative studies. Parkinsons Dis 2017: , 7203259. |
[89] | Gast A , Mathes T ((2019) ) Medication adherence influencing factors—an (updated) overview of systematic reviews. Syst Rev 8: , 112. |
[90] | Vaswani PA , Tropea TF , Dahodwala N ((2020) ) Overcoming barriers to Parkinson disease trial participation: Increasing diversity and novel designs for recruitment and retention. Neurotherapeutics 17: , 1724–1735. |
[91] | Watt A , Cameron A , Sturm L , Lathlean T , Babidge W , Blamey S , Facey K , Hailey D , Norderhaug I , Maddern G ((2008) ) Rapid versus full systematic reviews: Validity in clinical practice? ANZ J Surg 78: , 1037–1040. |




