A Coalition to Advance Treatments for Parkinson’s Disease, Dementia with Lewy Bodies, and Related Disorders
Abstract
Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) share underlying neuropathology. Despite overlapping biology, therapeutic development has been approached separately for these clinical syndromes and there remains no treatment to slow, stop or prevent progression of clinical symptoms and development disability for people living with PD or DLB. Recent advances in biomarker tools, however, have paved new paths for biologic definition and staging of PD and DLB under a shared research framework. Patient-centered research funding organizations see the opportunity for a novel biological staging system for PD and DLB to accelerate and increase success of therapeutic development for the patient communities they serve. Amid growing momentum in the field to develop biological definitions for these neurodegenerative diseases, 7 international nonprofit organizations focused on PD and DLB came together to drive multistakeholder discussion and input on a biological staging system for research. The impact of these convenings to date can be seen in changes incorporated into a proposed biological staging system and growing alignment within the field to rapidly apply new scientific knowledge and biomarker tools to inform clinical trial design. In working together, likeminded nonprofit partners who were initially catalyzed by the significant potential for a biological staging system also realized the power of a shared voice in calling the field to action and have since worked together to establish a coalition to advance precompetitive progress and reduce hurdles to developing better treatments for PD, DLB and biologically related disorders.
Plain Language Summary
Disease-focused nonprofit organizations serve to speed new treatments for patients through research funding and advocacy. In the Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) fields, several international nonprofit organizations came together to facilitate multistakeholder input on a new biological staging system for research. Stakeholders gathered included researchers, clinicians, drug developers, regulatory agencies, additional nonprofits, and people affected by PD and DLB. This example, fueled by a shared perspective that new drug development tools will improve clinical trials and get better treatments to patients sooner, serves as a model for continued collaborations across the PD and DLB fields. A new, international coalition of nonprofit organizations has emerged to support advancement of treatments to slow, stop, and one day prevent PD, DLB and related disorders, in part, by facilitating future multistakeholder collaborations.
INTRODUCTION
Parkinson’s disease (PD) and dementia with Lewy bodies (DLB) are associated with neuronal pathologic alpha-synuclein aggregation but present as divergent clinical syndromes with predominantly motor versus cognitive manifestations. Despite common pathology, PD and DLB currently have distinct routes for therapeutic development. In the PD and DLB fields, there is a rapidly evolving landscape of disease-targeted treatments moving into the clinical pipeline.1 These novel therapies build on emerging understanding of biologies that lead to clinical symptoms and functional impairment in patients. Disease-targeted therapies that affect underlying pathophysiology offer promise in disrupting the progressive course of both PD and DLB. Such advances stand to transform treatment options for these debilitating neurodegenerative diseases. The combination of innovation and accelerated pace of drug development offers new and continued hope for patients and families. However, drug development tools enabling informed clinical development lag far behind scientific advances to identify and develop disease-targeted therapies. The path for successfully advancing such treatments through pivotal trials remains murky at best and insurmountable at worst. Challenges in defining appropriate target populations for clinical trials, measuring efficacy against the backdrop of existing therapies, and lack of sensitive, clinically meaningful endpoints risk the sustained investment from pharmaceutical companies needed to bring novel disease-targeted therapies forward into late-stage trials and ultimately to patients.
Patient-centered research funding organizations sit at the nexus of scientific, drug development, and patient communities. Dedicated to accelerating new medicines for and with patients, these organizations are uniquely positioned to drive collaborations that address fieldwide challenges in drug development. In the PD and DLB fields, global nonprofit organizations have watched numerous clinical trials aimed at slowing disease progression fail to meet primary efficacy endpoints. These individual disappointments alongside growing trends with companies moving to prioritize other disease indications or exit their investments in neuroscience have led to increased concerns about viable options for successful drug development for the most disabling diseases with rising global prevalence. There is an escalating need for the community of people living with these diseases to be heard with a sense of urgency.
Patient-focused drug development has elevated the importance of the patient voice and the role of nonprofit organizations to serve as change agents. Forward-thinking revolutions in biomedical science have historically been championed by expert thought leaders, eventually trickling down to but rarely consulting end users: patients. However, that is not the only path for driving scientific change. Examples of powerful patient activism fundamentally transforming medical research and drug development, such as the HIV/AIDS advocacy movement,2 offer a blueprint for new types of change champions. Approaches for holding space for active solicitation of widespread input—including perspectives from industry, regulatory, and patient stakeholders often excluded from academic-led advancements—serve as a better model for advancing PD and DLB therapeutic development. Motivated solely by the promise of faster progress for patients, nonprofit charity organizations are well-suited to orchestrate inclusive methods for forming and furthering new scientific frameworks.
Regulatory authorities issued a call for the PD and DLB fields to develop a biologic definition of disease that could be applied to a staging system for clinical trials,3 similar to frameworks in Alzheimer’s disease4 and Huntington’s disease.5 The field broadly acknowledges that neurodegenerative disease pathology precedes clinical diagnosis and drives progressive symptoms throughout the disease continuum. However, lack of operational biological definitions and approaches for staging biology alongside progressive clinical impairment in PD and DLB limits regulatory-acceptable clinical trial design for treatments aimed at slowing, stopping, or preventing development of disability.
Research-focused nonprofit organizations for PD and DLB each heard regulators’ calls to action. In parallel, these organizations observed the research field accelerate independent efforts to biologically define PD and related clinical syndromes. Recent success of PD patient-centered organizations in advancing field-enabling tools3 highlighted strategies and tactics to facilitate open, multistakeholder discourse toward alignment on a novel biological staging paradigm to optimize regulatory-acceptable clinical trial designs.
THE POWER OF CONVENING
A biological definition and staging framework anchored in contemporary understanding of disease biology and inclusive of broad clinical manifestations of underlying pathology is a major step forward for the PD and DLB fields.6 Both PD and DLB share a common underlying pathology of neuronal alpha-synuclein aggregation traditionally identified at autopsy. With the advancement of biomarker tools to detect these signatures in living humans, the landscape has evolved at a rapid pace. A biologically anchored staging framework could enable (1) regulatory-acceptable operational definitions of trial populations including biomarker-defined populations, (2) testing interventions at the earliest stages of disease including before the onset of clinical symptoms or any disability, and (3) stage-appropriate selection of trial endpoints across the disease continuum. Importantly, biological staging frameworks for other diseases have led to treatment breakthroughs for patients. For example, the biological classification of Alzheimer’s disease in living people combined with a staging system for biomarker and symptom severity4 has led to optimized clinical trial designs and, recently, FDA approved treatments such as lecanemab and donanemab that slow clinical symptoms.7,8 Similarly, a biological staging system for type 1 diabetes established a scientific roadmap for intervention prior to symptom onset9 and has yielded the first FDA approved treatment to delay the onset of type 1 diabetes.10 Nonprofit organizations saw an opportunity to accelerate similar success in bringing transformative treatments to patients by facilitating dialogue and input toward a biological staging system for therapeutic development in PD and DLB.
Global nonprofits that recently convened a precompetitive forum advancing tools for clinical trials in PD3 established plans to co-host a multistakeholder roundtable to discuss contemporary efforts to update biological definitions of PD and propose a staging system. As plans for the PD Staging Roundtable took shape, additional nonprofits serving PD and DLB communities raised their hand to co-sponsor the event and lent their voice to pivotal discussions on biological staging. The roundtable, which took place on April 13, 2023 in Washington, DC, was ultimately supported by 7 international nonprofit organizations (Panel 1). The shared vision of nonprofit partners was to facilitate transparent dialog among diverse stakeholders. To that end, roundtable attendees included international representatives from:
• 9 Patient-centered nonprofit organizations, including Aligning Science Across Parkinson’s and Parkinson’s Foundation alongside meeting conveners and funders;
• National Institutes of Health;
• US Food and Drug Administration (FDA);
• Academia, including 5 presidents (current and past) of the International Parkinson and Movement Disorder Society and experts from disease areas beyond PD and DLB;
• 7 Pharmaceutical companies; and
• Individuals affected by PD and DLB including patients and family members
The presence of people with lived experience (Boxes 1–5) and regulators served to differentiate the workshop from meetings previously convened in other disease areas to outline roadmaps for biological staging of neurodegenerative diseases.
VOICE OF THE PATIENT PERSPECTIVES
Box 1. Peter DiBiaso, MHSA| Parkinson’s advocate
Following several years of persistent right foot stiffness and a moderate right hand tremor, at the age of 49 I was diagnosed as having symptoms “suggestive” of early onset Parkinson’s Disease (EOPD). Despite my disbelief and denial of how this could be happening to me; particularly after competing in literally hundreds of triathlons including the IronMan, 10 marathons and adventure races over the past 30 years. It was further ironic that my entire professional career has been spent in roles focused on clinical research operations and biopharmaceutical drug development.
Eventually my short-lived grief turned into action, and, with encouragement from my wife Vicky, we signed up for a Parkinson’s fund-raising opportunity the New York City (NYC) marathon. This led to yet another NYC marathon the next year and culminated with the summitting of Mount Kilimanjaro; all while raising vital funds for continued PD research and development.
Although, I write you this not as a clinical professional but as a patient advocate who has witnessed firsthand the numerous successes and scientific advances over the last decade although, despite our commitments, I realize much work remains. A critical step towards finding a cure and new drug development is the ability to utilize evidence-based medicine to refine a targeted approach and robust study design to optimize clinical protocols. These new research efforts have been guided by a multi-disciplinary, diverse and globally represented team and formally cited as Biological Definition of Neuronal alpha-Synuclein Disease: Towards an Integrated Staging System for Research.3 Simply put, from the patient’s perspective we now have an opportunity to provide shared definitions and a common language irrespective of our geographies and disease progression. This enhanced approach to treatment will no doubt change as we learn more, and, while not perfect, this enables us to have a shared baseline to work from. Staging will serve as a vital tool to patients and researchers and, along with the recent biomarker success, provides firm footing by which we all can grow that much closer to a cure. The patient is waiting!
Box 2. Alison Handler, PharmD, RPh| Parkinson’s advocate
The burden of Parkinson’s Disease weighs heavily on my mind. As a patient advocate for 5 family members including my father living with PD, I see the daily challenges they face along with their primary caregivers. My pharmacist training only emphasizes the lack of treatment options available for PD patients, especially anything that treats the disease beyond symptom management. The knowledge that I gained from participating in the groundbreaking Parkinson’s Progression Markers Initiative (PPMI) study informed me that I have one the genetic mutations that may increase my risk for developing the disease. Yet so many people are not even aware of the importance of participating in clinical research such as this. I take all of this into consideration and worry about my two boys and who may be next in our own family.
The proposed staging system gives me hope that a more robust definition of the disease will allow for clinical trials that enroll more diverse and earlier in disease patients so that we can identify disease modifying treatments to either prevent or significantly alter the course of disease progression. We know this is possible in other therapeutic areas and I am excited to see this progress being made in the field of Parkinson’s research as well.
Box 3. Soania Mathur, MD| Parkinson’s advocate
Living with Parkinson’s disease is a challenge – one that affects those of us diagnosed with this incurable illness not only physically, but mentally, emotionally, and socially. It is progressive, unfaltering and the continuous decline into disability is what our community fears most. Developing effective treatments to slow down this neurodegeneration remains a painful, unmet need.
Since my diagnosis 25 years ago, I have seen much progress made in the research field, yet disease modification remains elusive. Progress rests in our ability to better define this disease, to measure it, to objectively identify it, to develop validated markers. Classification and staging systems based on biology, not clinical parameters alone are an exciting step towards progress. This whole concept of defining PD biologically by neuropathologic change or biomarkers and treating the clinical symptoms of Parkinsonism as just that, symptoms and signs of the disease rather than the definition of the disease, promises to enhance our understanding of this illness because clinical symptoms alone do not predict with certainty, the underlying molecular pathology.
Using new classification and staging systems will allow researchers to subdivide the general Parkinson’s population with all its inherent variability into cohorts of patients that have similar pathologic processes involved in their disease. Being able to include specific patients with specific molecular underpinnings thereby stratifying patients for clinical trials, is potentially game changing. It will really impact the way we do research. And the development of successful therapies begins with successful research efforts. I would measure that success in the development of treatments capable of stopping or slowing what is currently an inevitable decline and loss of independence, improving quality of life for those living with PD and ideally preventing those at risk from embarking on this journey in the first place.
Box 4. John Seibyl, MD | Parkinson’s researcher and advocate
Parkinson’s disease has been my quiet nightmare. I wake up in the morning each day thinking about the specific challenges that will be posed to my compromised motor capabilities; whether some new incompetence will be unveiled as the slowly inexorable disease marches along. I’m taunted by the sobering realization that this day, despite the challenges, is as good as it gets, and likely as good as it will ever be in the future. Yet, if I have nightmares, I can also have dreams.
Mostly, I dream of a treatment that eliminates the aberrant alpha synuclein from my brain, or at least stops its toxic spread. I boldly dream of preventing the onset of motor syndromes in those coming along behind me. And I dream that the remarkable and under-appreciated caregivers receive better, more accurate prognostic information and expectations about their charges’ changing functional capabilities to enlighten and support their care................. None of these are readily possible without a biological understanding of the disease that is Parkinson’s, rather than its eponymal syndromes. Otherwise, for me, there’s little chance of waking from this nightmare.
Box 5. Angela Taylor | DLB advocate
After my father was diagnosed with DLB, I was shocked to learn there were no treatments that were FDA-approved to treat DLB. Despite being a common cause of dementia, DLB research is decades behind more well-established diseases like PD and Alzheimer’s disease. While momentum is now building, it’s been enormously frustrating to watch as countless clinical trials have been done in Alzheimer’s disease and PD over the last 20 years compared to only handful of DLB clinical trials.22
Despite the shared α-synuclein pathophysiology in DLB and PD, clinical trials of anti-synuclein therapies are currently only being done in PD or other diseases, leaving DLB families without access to these investigational medications. The NSD-ISS will fill an enormous gap and enable trials to enroll participants from either end of the Lewy body disorders clinical spectrum. This new framework will foster exciting, new collaborations between the DLB and PD fields, accelerate our understanding of these disorders, and bring new hope especially to DLB families like mine.
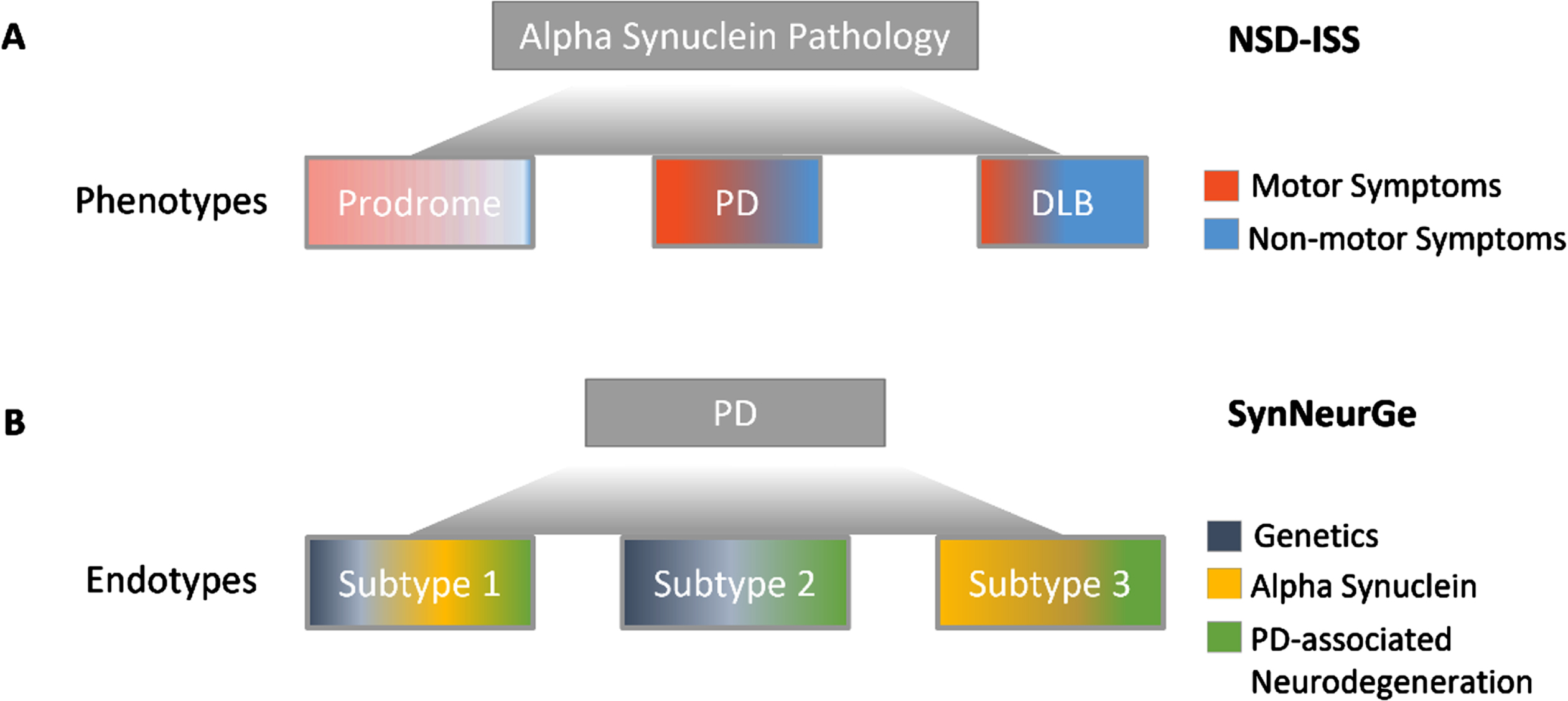
Presentations and discussion at the roundtable included 2 independent, contemporary initiatives proposing a biologic definition of disease—the neuronal alpha-synuclein disease integrated staging system (NSD-ISS) and SynNeurGe criteria—which have since been published.11,12 Both frameworks, presented in draft form at the roundtable, highlight the readiness of the field to use a biologic definition of disease for research and, importantly, clinical trial design.13 Interestingly, the NSD-ISS and SynNeurGe frameworks diverge in their conceptual approach to biologically defining disease. The NSD-ISS homes in on a single molecular pathology, neuronal alpha-synuclein, associated with diverse clinical phenotypes. The SynNeurGe criteria anchors to the clinical PD phenotype and classifies disease endotypes with distinct pathophysiological mechanisms (Fig. 1). Detailed comparison of the two approaches is beyond the scope of this manuscript. This paper largely focuses on the NSD-ISS with the aim of highlighting the process for development with an emphasis on the role of patient-centered nonprofits in convening multi-stakeholder input to advance a new research framework.
Fig. 1
Divergent conceptual approaches to biologically defining disease. A) The NSD-ISS anchors to alpha-synuclein pathology. NSD-ISS offers a framework for therapeutic development across clinical syndromes associated with detectable neuronal alpha-synuclein biomarkers but does not apply to all individuals with clinically diagnosed PD or DLB. B) The SynNeurGe criteria anchors to PD clinical diagnostic criteria and offers classification of all currently known endotypes.
Following the initial in-person roundtable, a related virtual symposium to socialize and solicit questions and feedback on the draft NSD-ISS was promoted by nonprofit partners. The symposium, hosted on April 18, 2023, reached over 550 individuals across 6 continents from academia, industry, patient communities, nonprofit organizations, and government including representatives from FDA, the European Medicines Agency, and the Medicines and Healthcare products Regulatory Agency. Given the volume of registrants, the symposium was hosted as a Zoom webinar, which allowed participants to provide input via private comments, which were provided to the NSD-ISS writing group and nonprofit partners. Subsequently, nonprofits that supported the roundtable along with additional partners issued a call for public comment on a pre-publication manuscript.11 The manuscript was made available online and shared widely with the research and clinical communities for review and comment prior to finalization and submission to a peer-reviewed journal.
No single individual, entity, or organization can solve challenges in clarifying the clinical development, regulatory, and commercial path to getting better treatments to patients. Thus, it becomes the responsibility of many to contribute to new solutions. Active solicitation of widespread input on novel proposals for scientific change is increasingly possible through virtual platforms and partnerships among organizations with diverse networks. Notably, a draft proposal for new Alzheimer’s disease diagnostic criteria was presented at the 2023 Alzheimer’s Association International Conference and subsequently posted for public comment. The workgroup proposing revisions to the 2018 National Institute on Aging-Alzheimer’s Association (NIA-AA) Alzheimer’s research framework leveraged the public conference and online platform of the Alzheimer’s Association to engage broad community input. While such processes can be complex, politically challenging, and time consuming, they yield a stronger product. In the case of the NSD-ISS, this framework now incorporates stakeholder input with differing points of view and provides a foundation for strategic research collaborations in the future thanks to the efforts of the growing coalition of nonprofit charity organizations. Examples of changes incorporated into the NSD-ISS framework and concept manuscript based on public feedback include refinement of genetic anchors for staging (e.g., Stage 0 is now restricted to SNCA variants), highlighting anticipated scientific advances that will expand biologic anchors (e.g., opportunity for additional data to drive D+ toward broader biomarkers of neurodegeneration, greater attention to skin-based biomarkers of S+), removal of preliminary clinical anchors of functional impairment (Stages 3–6), and changes to nomenclature to avoid alienating stakeholders (e.g., change from PD to NSD, revision of genetic risk from Stage –1 to RH and RL). Importantly, the NSD-ISS is also now positioned as a research framework, consistent with multistakeholder input.
A VISION FOR PROGRESS POWERED BY COLLECTIVE VOICES
This early impact of PD and DLB focused nonprofit organizations fortified a shared interest in collaboration to enhance resources for improving therapeutic development. The nonprofits that initially came together to support the PD Staging Roundtable (Panel 1) have since established an international coalition with growing membership. Each organization within this new coalition realizes its influence and impact in accelerating transformative treatments for PD and DLB can be amplified through a harmonized, global advocacy voice. The coalition will continue convening the field including industry, regulators and people affected by PD and DLB for multistakeholder input and alignment on emerging priority topics related to therapeutic development and will drive new efforts such as patient education to accelerate treatments that slow, stop, or prevent disease-related disability. Our aims include advancing tools and resources to build efficiencies for clinical development of disease modifying treatments and informing how the PD and DLB patient communities at large will receive and contribute to more precise tools, like the NSD-ISS, that help accelerate therapeutic development.
Panel 1. Nonprofit organizations working in collaboration with additional stakeholders to advance drug development tools for PD and DLB
Critical Path for Parkinson’s Consortium within the Critical Path Institute
Cure Parkinson’s
Lewy Body Dementia Association
Michael J. Fox Foundation for Parkinson’s Research
Parkinson Canada
Parkinson’s UK
Shake It Up Australia Foundation
Panel 2. Research opportunities to refine and advance utility of the NSD-ISS.
ASSAY DEVELOPMENT – Advancing alpha-synuclein SAA toward quantification, reliable measurement in blood and other matrices, and scalability will ensure inclusivity, enable tools for tracking biological disease progression, and, eventually, offer utility for general clinical care;
COMPLEMENTARY STAGING SYSTEMS – Biological characterization of individuals with clinical diagnoses of PD, DLB, and related disorders who do not fit NSD staging will inform smarter therapeutic development for those patients;
DATA STANDARDS – A core, standard set of clinical and biomarker assessments for cohorts across PD and DLB research, harmonized by standardized protocols, and collected under consents that permit data and biosample sharing will accelerate future therapeutic innovation.
ENDPOINTS – Precompetitive development of stage and domain appropriate measures will advance novel, sensitive endpoints in future clinical trials;
FRAMEWORK VALIDATION – Independent validation the NSD-ISS in clinical cohorts will inform future iterations of the framework. Cohorts with varying eligibility criteria will clarify outstanding questions in the NSD-ISS, particularly regarding sequencing of dopaminergic deficit and functional impairment in DLB, and will ensure applicability to a broad population;
MULTIMODAL BIOMARKERS AND CO-PATHOLOGIES – Further testing of emerging biomarkers will increase field confidence to implement these tools in clinical trials and will expand biomarker anchors included in future iterations of the NSD-ISS. Candidate neuroimaging, biofluid, and tissue biomarkers for PD are well summarized by Höglinger et al.12 Examination of co-pathologies including amyloid, tau and other neurodegenerative biomarkers in individuals with clinical diagnoses of PD and DLB is also critical as there are likely multiple contributors to disease.18,21 Increased understanding of disease endotypes could accelerate development of combination therapies that offer increased clinical benefit to patients;
REPRESENTATIVENESS – Inclusive recruitment strategies and engagement of representative cohorts are needed to understand how well new biological staging and biological classification paradigms apply to the general population; and
Filling knowledge gaps
Given involvement of U.S. regulators in shaping the NSD-ISS, we hope this framework will offer sponsors a regulatory-acceptable framework for defining target populations for clinical trials, ensuring biologically-targeted treatments are tested in volunteers most likely to benefit based on their biomarker signature. Currently, there are more than 15 clinical trials targeting pathologic alpha-synuclein,1,14 yet without biomarkers to identify study participants with pathologic alpha-synuclein trials are likely to fail.15,16 The NSD-ISS offers a simple framework for clinical development plans including biomarker enrichment schemes and selection of preclinical individuals with biomarker signatures of disease,17,18 widening the window for therapeutic intervention and reducing individual sponsor risk and burden of operationally defining such target populations. This is particularly valuable for trials aiming to intervene before clinical diagnosis of PD or DLB as regulators do not endorse the use of the term ‘prodromal’ to describe this population because there is no established, universal definition that is consistently understood within the neurology community. Necessary but anticipated scientific advancements in alpha-synuclein biomarker measures will enable feasible, scalable mechanisms to identify such populations for future trials. The framework is also likely to increase industry appetite to test alpha-synuclein targeted therapies in DLB patients broadly left out of these trials today (Box 5).
As research funders and patient advocates, coalition members have an opportunity to direct resources that enable the field to build additional data needed to examine and modify the NSD-ISS across PD and DLB populations and, ultimately, to incorporate new biomarkers (including biomarkers of relevant co-pathologies) and clinical measures of disease into future iterations of the research framework. The growing coalition also serves to support similar methods for developing adapted or parallel biological staging systems for PD and DLB patients who do not meet NSD-ISS criteria (e.g., alpha-synuclein biomarker negative patients). Such efforts combined with alignment with global regulatory agencies can de-risk future clinical trials for PD and DLB patients through streamlined regulatory review compared to the traditional individual sponsor-led paths.
The NSD-ISS also offers a strategic framework to highlight critical knowledge gaps and guide new research (Panel 2). Conveniently, current limitations and necessary future directions for evolving the NSD-ISS have been clearly articulated by the many informal referees who engaged in public forums facilitated by the forming coalition. These outstanding questions offer a roadmap to focus aligned international funding on a research agenda to advance broad utility and adoption in the field. Key priorities for future research are summarized in Panel 2.
We also intend to establish robust communication flows and avenues for sharing information across international public, private, and government funders to increase synergies and reduce duplication in research funding toward successful development of transformative treatments. One critical way to reduce duplication is through data sharing. As research funders and conveners, coalition partners maintain a clear focus on driving open access resources and establishing platforms for data sharing to accelerate progress in therapeutic development. Critical Path Institute, a nonprofit neutral third party, offers several examples of how collaborative data sharing can advance regulatory science toward accelerating drug development across diseases of high unmet need.19,20
Education and awareness
Though initial applications of the NSD-ISS and SynNeurGe criteria are intended for research, the patient perspective must be centered if such systems are to be adopted into recruitment practices and ultimately inform label indications for future therapies.
Coalition partners are ideally positioned to socialize biomarker testing and biological staging for clinical research with patients and families in the PD and DLB communities. By informing and fielding questions from the communities we jointly serve, the coalition can identify educational needs and develop informational resources using shared messaging while adapting and contextualizing them for disease community and geographic relevance. At present, the NSD-ISS is not ready for use in routine clinical care. Nonetheless, we anticipate patients will have questions about what the research framework means for them and believe there is a critical role for the coalition to inform them and highlight actionable opportunities to participate in research. Common questions raised by informed community members to date include:
• Should my family and I get biomarker testing? If so, which test(s) is right for me, and how can I get access?
• I do not have [PD or DLB] but have a positive biomarker test(s). How likely am I to develop symptoms? How much time do I have before then?
• My biomarker status does not fit NSD definitions or staging, but I have [PD, DLB, or another clinical syndrome associated with alpha-synuclein pathology] – will there be a framework that helps guide development of precision medicines for me?
• How will I know what future treatments are right for me?
• Will the NSD-ISS change how I can get involved in clinical trials?
Community questions warrant thoughtful, dynamic, disease-community relevant answers developed through collaboration of experts with scientific, clinical, and lived experience with PD and DLB, which can and will readily be convened by global partners within the coalition.
The commitment to develop and disseminate shared messaging also avoids confusion in the patient community, as patients who turn to disease charities for information rarely ever seek only one source. Consistent communication across coalition partners deeply reinforces new concepts, facilitates broader awareness, and provides assurance to patients that nonprofits are working together on their behalf. Development of common messaging ensures that the community understands fundamental aspects of biological staging of disease and can be achieved by applying learning from other diseases such as Alzheimer’s disease, type 1 diabetes, and oncology. We similarly envision this patient-centered communication around biologic staging being a valuable resource for sponsors, clinical trial sites, and other stakeholders seeking to recruit and enroll volunteers into future trials creating a cycle of shared understanding and partnership between researchers and patients to speed clinical research.
SUMMARY AND A CALL TO ACTION
Our growing international coalition formed around the opportunity to speed therapeutic development through a new drug development tool informed by widespread community input. Our continued mission as a coalition is to facilitate multistakeholder efforts to advance therapeutic development that will change the trajectory of living with PD and DLB. The nascent coalition of nonprofit charities calls for continued inclusive scientific discourse, aligned and complementary research investments, and broad cooperation on our urgent journey to accelerate novel therapies.
Parkinson’s advocate Michael J. Fox said ‘The answers we want aren’t going to fall out of the sky. We have to get ladders and climb up and get them.’ Our coalition believes we must start climbing now because patients and families are waiting for better treatments.
ACKNOWLEDGMENTS
We appreciate FDA representative Dr. Michelle Campbell’s contributions to informing opportunities to use patient experience data to advance patient-focused drug development in PD, DLB and beyond. We are grateful to the community of research advocates who continually provide their perspectives as individuals affected by PD and DLB.
We also look forward to collaborating with core and affiliate member organizations of the new nonprofit coalition as we continue convening multi-stakeholder forums to meaningfully advance treatments aimed at slowing progression of PD, DLB, and biologically related conditions. Current member organizations include Alzheimer’s Association, American Parkinson Disease Association, Critical Path Institute, Cure Parkinson’s, Cure Parkinson’s New Zealand, CurePSP, Lewy Body Dementia Association, Michael J. Fox Foundation for Parkinson’s Research, Mission MSA, Parkinson Canada, Parkinson’s Europe, Parkinson’s Foundation, Parkinson’s UK, and Shake It Up Australia Foundation.
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
All authors are employed by nonprofit organizations participating in the coalition (Panel 1). CMK, SC, and YX are employed by The Michael J. Fox Foundation. AA and KKL are employed by Parkinson Canada. CC is employed by Shake It Up Australia Foundation. DTD is employed by Parkinson’s UK. KNF and AT are employed by Lewy Body Dementia Association. HM is employed by Cure Parkinson’s. DS is employed by Critical Path Institute.
REFERENCES
1. | McFarthing K , Buff S , Rafaloff G , et al. Parkinson’s disease drug therapies in the clinical trial pipeline: 2023 update. J Parkinsons Dis (2023) ; 13: : 427–439. |
2. | Manganiello M , Anderson M . Back to basics: HIV/AIDS advocacy as a model for catalyzing change, forces4quality.org/node/6968. (2011, accessed 20 July 2023). |
3. | O’Hanlon CE , Farmer CM , Ryan J , et al. Clinical outcome assessments and digital health technologies supporting clinical trial endpoints in early Parkinson’s disease: roundtable proceedings and roadmap for research, RAND Corporation. https://doi.org/10.7249/CFA2550-1. Posted 24 April 2023. |
4. | Jack CR , Bennett DA , Blennow K , et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement (2018) ; 14: : 535–562. |
5. | Tabrizi SJ , Schobel S , Gantman EC , et al. A biological classification of Huntington’s disease: the Integrated Staging System. Lancet Neurol (2022) ; 21: : 632–644. |
6. | Weintraub D . What’s in a name? The time has come to unify Parkinson’s disease and dementia with Lewy bodies. Mov Disord (2023) ; 38: : 1977–1981. |
7. | van Dyck CH , Swanson CJ , Aisen P , et al. Lecanemab in early Alzheimer’s disease. N Engl J Med (2023) ; 388: : 9–21. |
8. | Sims JR , Zimmer JA , Evans CD , et al. Donanemab in early symptomatic Alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA (2023) ; 330: : 512–527. |
9. | Insel RA , Dunne JL , Atkinson MA , et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care (2015) ; 38: : 1964–1974. |
10. | Carvalho T . FDA approves first drug to delay type 1 diabetes, NatureMedicine News Article. https://www.nature.com/articles/d41591-022-00115-y. Posted 15 December (2022) . |
11. | Simuni T , Chahine LM , Poston K , et al. Biological definition of neuronal alpha-synuclein disease: towards an integrated staging system for research. Lancet Neurol (2024) ; 23: : 178–190. |
12. | Höglinger GU , Adler CH , Berg D , et al. A biological classification of Parkinson’s disease: the SynNeurGe research criteria. Lancet Neurol (2024) ; 23: : 191–204. |
13. | Darweesh SKL , Sampaio C , Bloem B . Has the time come to redefine Parkinson’s disease? Lancet Neurol (2024) ; 23: : 130–133. |
14. | Grosso Jasutkar H , Oh SE , Mouradian MM . Therapeutics in the pipeline targeting α-synuclein for Parkinson’s disease. Pharmacol Rev (2022) ; 74: : 207–237. |
15. | Pagano G , Taylor KI , Anzures-Cabrera J , et al. Trial of prasinezumab in early-stage Parkinson’s disease. N Engl J Med (2022) ; 387: : 421–432. |
16. | Lang TE , Siderowf AD , Macklin EA , et al. Trial of cinpanemab in early Parkinson’s disease. N Engl J Med (2022) ; 387: : 408–420. |
17. | Siderowf A , Concha-Marambio L , Lafontant DE , et al. Assessment of heterogeneity among participants in the Parkinson’s Progression Markers Initiative cohort using α-synuclein seed amplification: a cross-sectional study. Lancet Neurol (2023) ; 22: : 407–417. |
18. | Palmqvist S , Rossi M , Hall S , et al. Cognitive effects of Lewy body pathology in clinically unimpaired individuals. Nat Med (2023) ; 29: : 1971–1978. |
19. | Karpen SR , Kael White J , Mullin AP , et al. Effective data sharing as a conduit for advancing medical product development. Ther Innov Regul Sci (2021) ; 55: : 591–600. |
20. | Thompson A , Parekh A . Value of data sharing to advance drug development: a regulatory perspective. Ther Innov Regul Sci (2021) ; 55: : 850–852. |
21. | Quadalti C , Palmqvist S , Hall S , et al. Clinical effects of Lewy body pathology in cognitively impaired individuals. Nat Med (2023) ; 29: : 1964–1970. |
22. | Tolea MI , Ezzeddine R , Camacho S , et al. Emerging drugs for dementia with Lewy bodies: a review of Phase II & III trials. Expert Opin Emerg Drugs (2023) ; 28: : 167–180. |




