Association Between Menopausal Hormone Therapy and Risk for Parkinson’s Disease
Abstract
Background:
The relationship between menopausal hormone therapy (MHT) and risk of Parkinson’s disease (PD) remains controversial.
Objective:
This nationwide population-based cohort study investigated the association between MHT and PD development.
Methods:
Data from the National Health Insurance System of South Korea from 2007 to 2020 were used. The MHT group included women who underwent MHT for the first time between 2011–2014, while the non-MHT group included women who visited a healthcare provider for menopause during the same period but never received hormonal therapy. We used propensity score matching (1 : 1) to adjust for potential confounders, and Cox regression models to assess the association between MHT and PD.
Results:
We selected 303,260 female participants (n = 151,630 per MHT and non-MHT groups). The median age of the participants was 50 (48–54) years, and the follow-up period lasted 7.9 (6.9–8.9) years. Cox regression analysis revealed an increased risk of PD with MHT (hazard ratio [HR] 1.377, 95% confidence interval [CI] 1.184–1.602), particularly with tibolone (HR 1.554, 95% CI 1.297–1.861) and estrogen alone (HR 1.465, 95% CI 1.054–2.036). Tibolone and estrogen alone were linked to PD within three years; however, no association was observed after three years. In contrast, the use of combined estrogen-progesterone was linked to a higher risk of PD, which increased with the duration of MHT (HR 1.885, 95% CI 1.218–2.918 for over five years).
Conclusions:
This study demonstrated that the MHT is closely associated with the risk of PD in a regimen- and duration-specific manner.
INTRODUCTION
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, which is characterized by the progressive loss of dopaminergic neurons in the substantia nigra in the midbrain and the presence of Lewy bodies because of aggregation of misfolded α-synuclein. Dysregulation of the cellular clearing system plays a crucial role in the accumulation of misfolded α-synuclein [1].
Epidemiologic studies have shown that PD is 1.5–2-fold more prevalent in men than in women [2, 3]. Preclinical studies have consistently shown a neuroprotective effect of estrogen on nigrostriatal dopaminergic neurons via anti-inflammatory and anti-oxidative activity [4–6]. Similarly, several case-control studies and clinical trials have shown the potential neuroprotective effect of estrogen in patients with PD [7–10]. In terms of epidemiological studies, although some previous studies showed that postmenopausal estrogen therapy is associated with a reduced risk of PD in women, several other studies reported contradictory results that do not support the neuroprotective effect of estrogen [11] or even suggest the possible detrimental effects of estrogen in PD [12–14]. Some studies emphasized the importance of the total length of lifetime estrogen exposure or the formula of hormone replacement therapy (i.e., estrogen alone or estrogen with progesterone) [12, 13].
In this study, we evaluated the association between the development of PD and the type of menopausal hormone therapy (MHT), including estrogen alone, combined estrogen/progesterone, and tibolone in in the Korean population using a nationwide population-based cohort from the Korean Health Insurance Review & Assessment Service (HIRA) database. Furthermore, we investigated the effect of the duration of each MHT type and demographic variables on the association between MHT and the risk of PD.
MATERIALS AND METHODS
Data source
In South Korea, individuals are required to be enrolled in the National Health Insurance System (NHIS) to access healthcare benefits [15]. The HIRA is a national agency that oversees healthcare expenses and quality, and all healthcare providers must participate in the program.1 This study utilized a dataset of the HIRA in South Korea from 2007 to 2020. The cohort dataset comprised an encrypted version of each patient’s original identification number and information on their age, sex, prescription medications, and diagnoses. Diagnoses were represented by codes following the International Classification of Diseases, Tenth Revision (ICD-10).
Study population
The research project recruited women aged 40 years and above who initially visited a healthcare provider for menopause (N95.1) between January 1, 2011, and December 31, 2014. Females who had used a single menopausal hormone (e.g., estrogen-progesterone or estrogen alone or tibolone) for less than six months were not included in the analysis. The MHT group included women aged≥40 years who underwent MHT for the first time between January 1, 2011, and December 31, 2014. In contrast, the non-MHT group included women aged≥40 years who had visited a healthcare provider for menopause between January 1, 2011, and December 31, 2014, but had never been prescribed menopausal hormones in the same period.
Both groups excluded females diagnosed with cancer before the 180th day from the first study entry date, those prescribed more than one menopausal hormone, and those diagnosed with neurodegenerative disorders (ICD-10 codes F00, F01, F02, F03, G30, G31, G20, and G23.1) before the 180th day from the first study entry date. The propensity score for the predicted probability of MHT use in each participant was estimated with a logistic regression model, using calendar year at inclusion, age at inclusion, socioeconomic status [SES], Charlson Comorbidity Index [CCI], region of residence, hypertension, diabetes, hyperlipidemia, uterine fibroids, endometriosis, previous hysterectomy, and previous adnexal surgery. For a 1 : 1 matching, a nearest neighbor matching algorithm with a caliper size of 1 was used to select the final study participants who were monitored until December 31, 2020.
Study outcome
In this study, PD was defined as a diagnosis of PD (G20) along with the prescription of PD medications (levodopa, pramipexole, ropinirole, selegiline, rasagiline, entacapone, amantadine, and anticholinergics) in women participants. To minimize the enrollment of patients with atypical or secondary parkinsonism, we excluded those who had been diagnosed with both atypical or secondary parkinsonism (ICD-10 codes G21, G22, G23, G25, and G26) and PD (G20) from further analysis.
Exposure
The analysis included various types of MHT, such as tibolone, estradiol hemihydrate (EH)/drospirenone (DRSP), EH/norethisterone acetate (NETA), EH/dydrogesterone (DYD), estradiol valerate (EV)/cyproterone acetate (CPA), EV/medroxyprogesterone acetate (MPA), EV/NETA, conjugated equine estrogen (CEE), EV, EH, micronized progesterone (MP), MPA, DYD, transdermal EH, and transdermal estradiol.
To conduct a subgroup analysis, we divided MHT into three categories: tibolone, estrogen-progesterone combinations (EH/DRSP, EH/NETA, EH/DYD, EV/CPA, EV/MPA, EV/NETA, CEE/MP or MPA or DYD, EV/MP or MPA or DYD, EH/MP or MPA or DYD), and estrogen alone (CEE, EV, EH, and transdermal estrogen). Additionally, the MHT was categorized based on whether it contained oral estrogen or progesterone components.
Variables
We gathered data on age, SES, and area of residence from the health insurance records in the medical study. They classified women who were insured under “medical aid” as having a low SES. We calculated CCI scores using ICD-10 codes from one year before the study until the participation date [16]. When the relevant ICD-10 codes were recorded three or more times, we confirmed a diagnosis of hypertension, diabetes mellitus, dyslipidemia, uterine fibroids, and endometriosis. We used surgical codes to collect information on hysterectomies and adnexal surgeries prior to the date of study entry.
Statistical analysis
In this study, R (version 3.5.1) was used for statistical analysis. Categorical variables are reported as numbers with percentages and continuous variables as median values with the 25th and 75th percentiles. The significance level was set at p < 0.05.
The chi-square test and t-test (parametric) or Mann-Whitney U-test (non-parametric) were used for categorical and continuous variables, respectively, before propensity score matching. After propensity score matching, the Cochran-Mantel-Haenszel test was used for categorical variables and the paired t-test (parametric) or Wilcoxon signed-rank test (non-parametric) was used for continuous variables. The standardized mean difference was used to determine the similarity between the two groups after propensity score matching.
The incidence of PD was calculated by dividing the number of cases by 100,000 person-years. The log-rank test was used to compare the Kaplan-Meier plots between groups. The PD risk of the MHT was analyzed using Cox proportional hazards regression models with time-dependent covariates. The Schoenfeld residual test was used to measure Cox proportional hazards assumption violations. If the proportional hazards assumption was violated, a Schoenfeld residual plot was used to determine the cutoff value for dividing the periods.
The start date for both group was the date when the first menopausal diagnosis code (N95.1) was confirmed. To reduce immortality bias, we calculated the time from the first diagnosis of menopause to the first prescription of MHT in the MHT group and applied this to the non-MHT group. The event date was defined as the date when PD was first confirmed, and the last observation date (censoring) was the last recorded date, including death, for any reason identified in our data.
The subgroup analysis calculated the risk of PD based on the duration of MHT. The duration of MHT use in the MHT group was compared to the duration of follow-up in the non-MHT group, ensuring that the follow-up duration was at least as long as the duration of MHT use. Cochran-Armitage tests were performed to explore the dose-response relationship. In addition, subgroup analyses were conducted for age, SES, area of residence, vascular risk factors (hypertension, diabetes mellitus, and dyslipidemia), gynecological disorders (uterine fibrinoids and endometriosis), and gynecological surgical history (hysterectomy and adnexal surgery) to explore potentially susceptible groups for PD onset.
Only women with a CCI score of 0 were analyzed using Cox regression models with a time-dependent covariate as a sensitivity test. The amount of missing data was handled using pairwise deletion if it was less than 10%, and multiple imputations if it was greater than 10%. However, further processing was not necessary, because no missing data were found.
RESULTS
Characteristics of study participants
The HIRA database contained 452,124 postmenopausal women aged > 40 years between January 2011 and December 2014. This study enrolled 303,260 female participants, with 151,630 allocated to the MHT and non-MHT groups, as shown in Fig. 1. The median age of the study participants was 50 (48–54) years, and the follow-up period lasted for 7.9 (6.9–8.9) years, as presented in Table 1. The standardized mean difference for all variables between the two groups was < 0.2. The detailed characteristics of the study participants are presented in Table 1, and their characteristics before propensity score matching are presented in Supplementary Table 1.
Fig. 1
Flowchart representation of female participant selection into MHT and non-MHT group from HIRA national health claim data. HIRA, health insurance review & assessment service; MHT, menopausal hormone therapy.
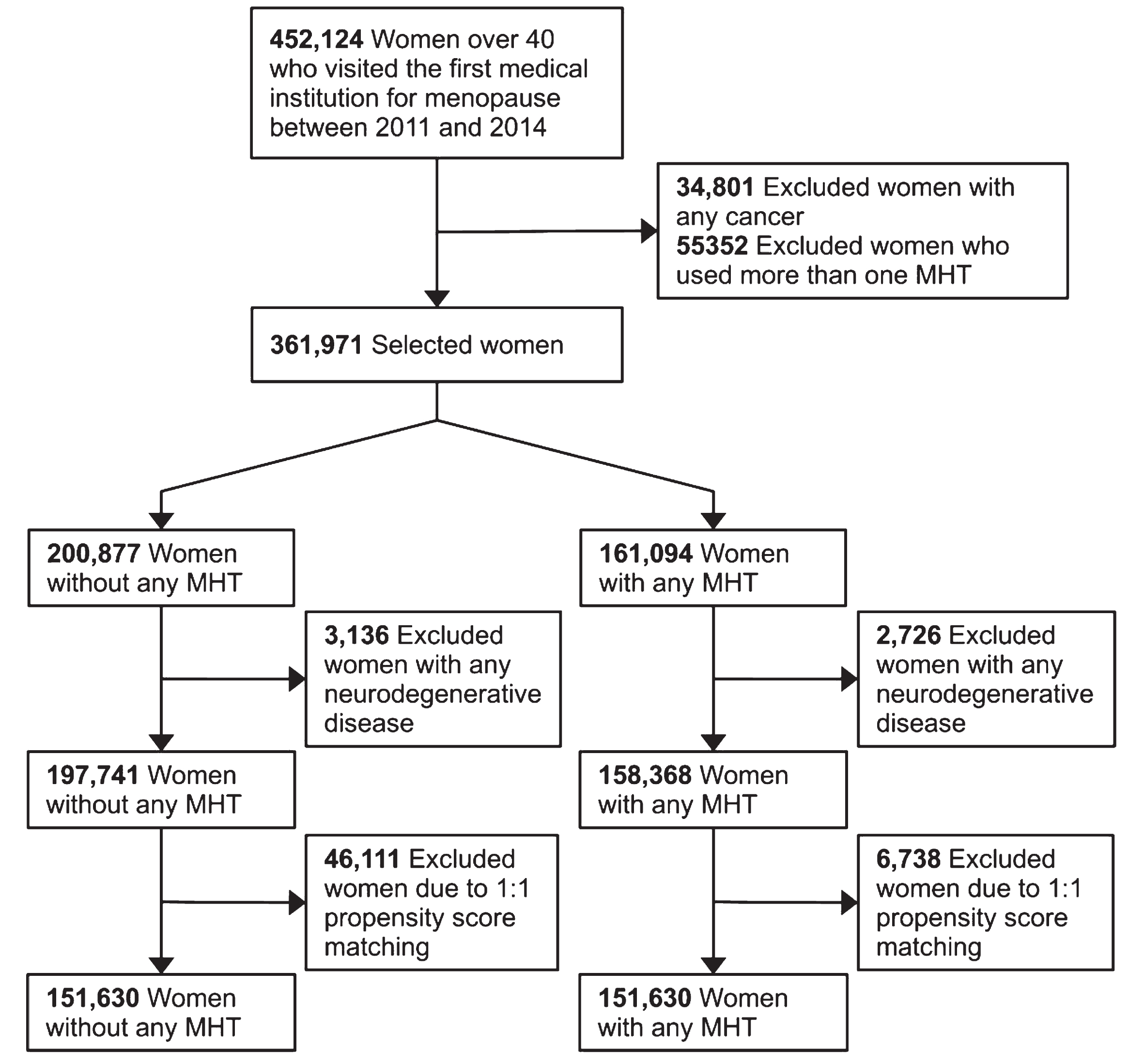
Table 1
Comparison of characteristics of women in MHT and non-MHT groups in the study after propensity score matching
| Non-MHT | MHT | Total | p | Standardized mean difference | Missing, % | |
| Number of participants | 151,630 | 151,630 | 303,260 | |||
| Median age (y) | 51 [48–54] | 50 [48–53] | 50 [48–54] | <0.001 | 0.075 | |
| Follow-up period (y) | 7.9 [6.8–8.9] | 8 [6.9–8.9] | 7.9 [6.9–8.9] | <0.001 | 0.063 | |
| Calendar year at inclusion | <0.001 | 0.007 | 0 | |||
| 2011 | 40,849 (26.9) | 40,608 (26.8) | 81,457 (26.9) | |||
| 2012 | 40,217 (26.5) | 40,574 (26.8) | 80,791 (26.6) | |||
| 2013 | 36,653 (31.1) | 36,806 (31.2) | 73,459 (31.2) | |||
| 2014 | 33,911 (22.4) | 33,642 (22.2) | 67,553 (22.3) | |||
| Age at inclusion (y) | <0.001 | 0.006 | 0 | |||
| 40∼49 | 62,012 (40.9) | 61,676 (40.7) | 123,688 (40.8) | |||
| 50∼59 | 81,867 (54) | 82,307 (54.3) | 164,174 (54.1) | |||
| 60∼69 | 7,611 (5) | 7,506 (5) | 15,117 (5) | |||
| 70∼ | 140 (0.1) | 141 (0.1) | 281 (0.1) | |||
| SES | <0.001 | 0.004 | 0 | |||
| Mid∼high SES | 147,677 (97.4) | 147,583 (97.3) | 295,260 (97.4) | |||
| Low SES | 3,953 (2.6) | 4,047 (2.7) | 8,000 (2.6) | |||
| Region | 0.039 | 0.007 | 0 | |||
| Urban area | 74,750 (49.3) | 74,183 (48.9) | 148,933 (49.1) | |||
| Rural area | 76,880 (50.7) | 77,447 (51.1) | 154,327 (50.9) | |||
| CCI | <0.001 | 0.017 | 0 | |||
| 0 | 108,130 (71.3) | 107,136 (70.7) | 215,266 (71) | |||
| 1 | 27,030 (17.8) | 27,982 (18.5) | 55,012 (18.1) | |||
| ≥2 | 16,470 (10.9) | 16,512 (10.9) | 32,982 (10.9) | |||
| Hypertension | <0.001 | 0.011 | 0 | |||
| No | 117,981 (77.8) | 117,297 (77.4) | 235,278 (77.6) | |||
| Yes | 33,649 (22.2) | 34,333 (22.6) | 67,982 (22.4) | |||
| DM | <0.001 | 0.007 | 0 | |||
| No | 134,591 (88.8) | 134,264 (88.5) | 268,855 (88.7) | |||
| Yes | 17,039 (11.2) | 17,366 (11.5) | 34,405 (11.3) | |||
| Dyslipidemia | <0.001 | 0.019 | 0 | |||
| No | 110,177 (72.7) | 108,864 (71.8) | 219,041 (72.2) | |||
| Yes | 41,453 (27.3) | 42,766 (28.2) | 84,219 (27.8) | |||
| Uterine fibroids | 0.007 | 0.009 | 0 | |||
| No | 127,636 (84.2) | 128,127 (84.5) | 255,763 (84.3) | |||
| Yes | 23,994 (15.8) | 23,503 (15.5) | 47,497 (15.7) | |||
| Endometriosis | <0.001 | 0.004 | 0 | |||
| No | 145,435 (95.9) | 145,313 (95.8) | 290,748 (95.9) | |||
| Yes | 6,195 (4.1) | 6,317 (4.2) | 12,512 (4.1) | |||
| Previous hysterectomy | <0.001 | 0.004 | 0 | |||
| No | 145,642 (96.1) | 145,515 (96) | 291,157 (96) | |||
| Yes | 5,988 (3.9) | 6,115 (4) | 12,103 (4) | |||
| Previous adnexal surgery | 0.001 | 0.003 | 0 | |||
| No | 148,692 (98.1) | 148,632 (98) | 297,324 (98) | |||
| Yes | 2,938 (1.9) | 2,998 (2) | 5,936 (2) |
CCI, Charlson comorbidity index; DM, diabetes mellitus; MHT, menopausal hormone therapy; SES, socioeconomic status. The data are presented as either percentages (in parentheses) or as medians with interquartile ranges [25th percentile, 75th percentile].
Detailed characteristics of the MHT group, including the duration of MHT use for women, are shown in Table 2. This illustrates that 31.8% of women used MHT for less than one year, 33.6% for 1–2.9 years, 15.2% for 3–4.9 years, and 19.4% for more than five years. Additionally, the median duration of MHT use among women in the MHT group was 22 (10–50) months. Supplementary Table 2 shows a comparison between the different MHT groups.
Table 2
The demographic and clinical features of women who utilized MHT are described in this study
| Median duration (mo) | Duration (y) | Total | ||||
| <1 | 1–2.9 | 3–4.9 | Over 5 | |||
| Total | 22 [10–50] | 48,192 (31.8) | 50,968 (33.6) | 23,104 (15.2) | 29,366 (19.4) | 151,630 (100) |
| Category 1 | ||||||
| Tibolone | 23 [11–52] | 21,496 (30.1) | 24,222 (33.9) | 11,226 (15.7) | 14,555 (20.4) | 71,499 (100) |
| E+P | 21 [10–48] | 20,946 (32.4) | 22,233 (34.4) | 9,625 (14.9) | 11,751 (18.2) | 64,555 (100) |
| E alone | 20 [9–50] | 5,750 (36.9) | 4,513 (29) | 2,253 (14.5) | 3,060 (19.6) | 15,576 (100) |
| Category 2 | 0 | |||||
| Tibolone | 23 [11–52] | 21,496 (30.1) | 24,222 (33.9) | 11,226 (15.7) | 14,555 (20.4) | 71,499 (100) |
| EH/DRSP | 23 [11–51] | 10,831 (30.4) | 12,134 (34.1) | 5,658 (15.9) | 7,006 (19.7) | 35,629 (100) |
| EH/NETA | 16 [9–35] | 1,789 (40) | 1,611 (36) | 603 (13.5) | 466 (10.4) | 4,469 (100) |
| EH/DYD | 23 [11–51.2] | 2,023 (31.2) | 2,187 (33.7) | 976 (15) | 1,302 (20.1) | 6,488 (100) |
| EV/CPA | 20 [10–47] | 5,059 (33) | 5,355 (35) | 2,106 (13.8) | 2,789 (18.2) | 15,309 (100) |
| EV/MPA | 15 [9–29] | 384 (40.6) | 389 (41.1) | 112 (11.8) | 61 (6.4) | 946 (100) |
| EV/NETA | 11 [8–19] | 74 (54.4) | 54 (39.7) | 8 (5.9) | 0 (0) | 136 (100) |
| CEE | 13 [7–34>] | 1,009 (49.9) | 535 (26.5) | 265 (13.1) | 213 (10.5) | 2,022 (100) |
| EV | 22 [10–54] | 4,446 (33.9) | 3,852 (29.4) | 1,968 (15) | 2,839 (21.7) | 13,105 (100) |
| EH | 10 [7–16] | 129 (64.5) | 48 (24) | 17 (8.5) | 6 (3) | 200 (100) |
| CEE/MP | 15 [9–29] | 181 (43.4) | 153 (36.7) | 49 (11.8) | 34 (8.2) | 417 (100) |
| CEE/MPA | 9 [7–14] | 83 (74.1) | 20 (17.9) | 5 (4.5) | 4 (3.6) | 112 (100) |
| CEE/DYD | 9 [7–13] | 24 (72.7) | 7 (21.2) | 2 (6.1) | 0 (0) | 33 (100) |
| EV/MP | 18 [10–40] | 153 (34.2) | 165 (36.9) | 68 (15.2) | 61 (13.6) | 447 (100) |
| EV/MPA | 10 [7–23] | 189 (61.4) | 70 (22.7) | 27 (8.8) | 22 (7.1) | 308 (100) |
| EV/DYD | 7 [7–10] | 27 (93.1) | 2 (6.9) | 0 (0) | 0 (0) | 29 (100) |
| EH/MP | 11 [8–18.2] | 125 (55.8) | 82 (36.6) | 11 (4.9) | 6 (2.7) | 224 (100) |
| EH/MPA | 15 [9.5–23.5] | 3 (42.9) | 4 (57.1) | 0 (0) | 0 (0) | 7 (100) |
| EH/DYD | 9 [9–9] | 1 (100) | 0 (0) | 0 (0) | 0 (0) | 1 (100) |
| TE | 9 [7–15] | 166 (66.7) | 78 (31.3) | 3 (1.2) | 2 (0.8) | 249 (100) |
CEE, conjugated equine estrogen; CPA, cyproterone acetate; DRSP, drospirenone; DYD, dydrogesterone; E, estrogen; EH, estradiol hemihydrate; EV, estradiol valerate; MHT, menopausal hormone therapy; MP, micronized progesterone; MPA, medroxyprogesterone acetate; NETA, norethisterone acetate; P, progesterone; TE,Transdermal estrogen. The data are presented as either percentages (in parentheses) or as medians with interquartile ranges [25th percentile, 75th percentile].
In the non-MHT group, there were 311 women diagnosed with PD, while in the MHT group, there were 363 women diagnosed with PD. When categorized by MHT type, 190 women were diagnosed with PD while using tibolone, 133 with combined estrogen-progesterone, and 40 with estrogen alone. The number of women diagnosed with PD by detailed MHT type is shown in Supplementary Table 3.
Table 3
Risk of Parkinson’s disease by the duration of MHT
| MHT Duration (years) | ||||||||
| <1a | 1 –2.9a | 3 –4.9a | Over 5a | |||||
| HR (95% CI) | p | HR (95% CI)a | p | HR (95% CI)a | p | HR (95% CI)a | p | |
| Non-MHT | 1 | 1 | 1 | 1 | ||||
| Total MHT | 1.607 (1.309–1.973) | <0.001 | 1.503 (1.218–1.855) | <0.001 | 1.165 (0.817–1.661) | 0.400 | 1.366 (0.972–1.918) | 0.072 |
| Category | ||||||||
| Tibolone | 2.07 (1.601–2.674) | <0.001 | 1.685 (1.286–2.207) | <0.001 | 1.049 (0.622–1.767) | 0.859 | 1.118 (0.678–1.843) | 0.661 |
| E+P | 1.087 (0.779–1.516) | 0.624 | 1.204 (0.877–1.652) | 0.251 | 1.411 (0.874–2.278) | 0.159 | 1.885 (1.218–2.918) | 0.004 |
| E alone | 1.876 (1.167–3.017) | 0.009 | 2.056 (1.224–3.454) | 0.006 | 0.67 (0.167–2.696) | 0.573 | 0.597 (0.148–2.406) | 0.468 |
CI, confidence interval; E, estrogen; HR, hazard ratio; MHT, menopausal hormone therapy; P, progesterone. aWomen in each MHT duration were compared to non-MHT women who were observed without Parkinson’s disease or death over that duration. For example, women who used MHT for 3–4.9 years were compared to non-MHT women with at least three years of observation.
Comparison of risk of PD between the MHT and non-MHT groups
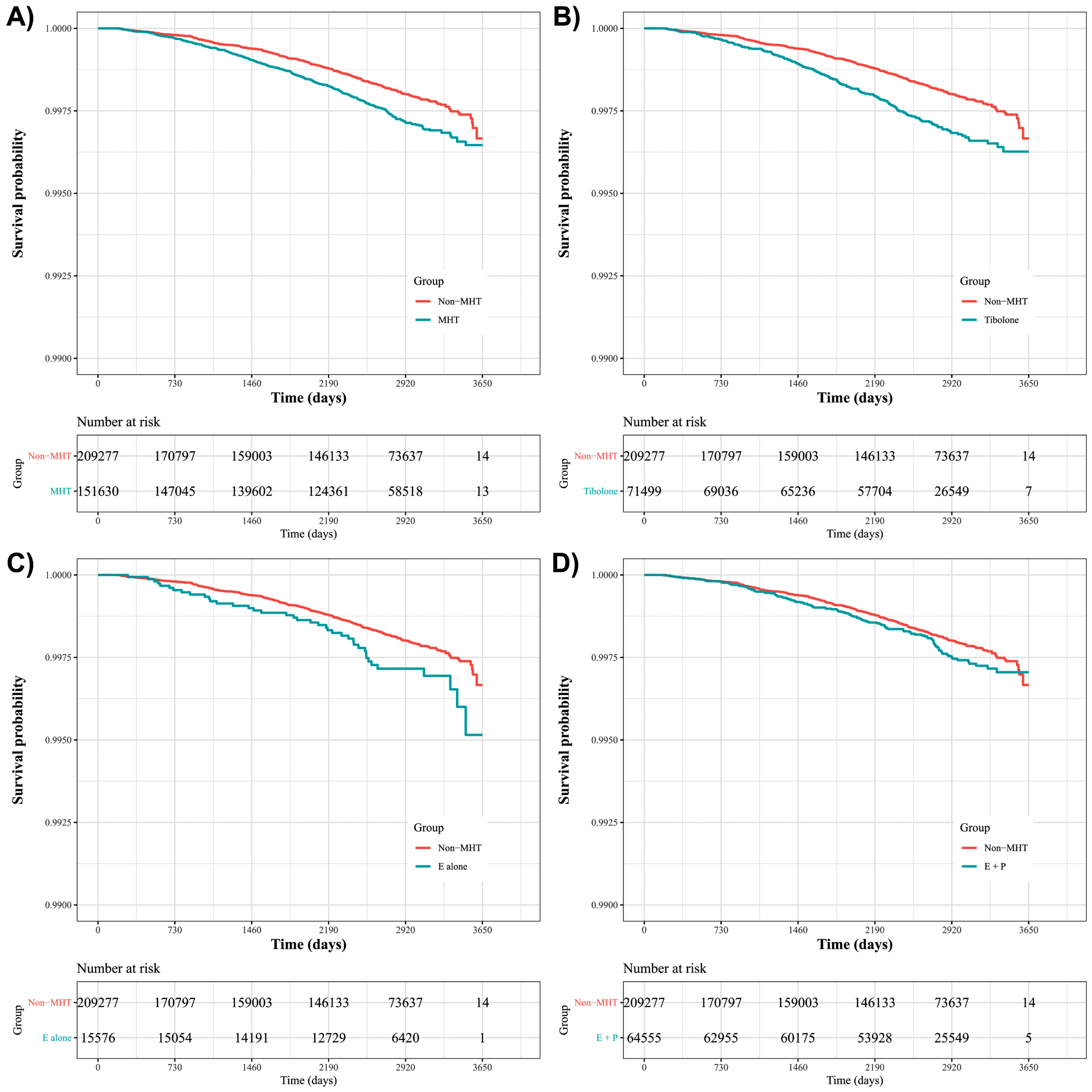
The Kaplan-Meier graph showed that MHT increased the risk of PD (plog - rank <0.001; Fig. 2A). When further analysis was performed after classifying the MHT into three categories, tibolone (plog - rank <0.001; Fig. 2B) and estrogen (plog - rank = 0.02; Fig. 2C) levels were associated with the risk of PD. Meanwhile, although the risk of PD seems to be different after 7 years between the combined estrogen-progesterone and non-MHT groups, this difference did not reach statistical significance (plog - rank = 0.100; Fig. 2D).
Fig. 2
Kaplan-Meier plotting of the risk of Parkinson’s disease in relation to menopausal hormone therapy use. A Kaplan-Meier plot is presented to compare MHT users versus non-users (A), with statistical significance assessed using a log-rank test (p < 0.001). In terms of each category of MHT, tibolone (B) and estrogen alone (C) were associated with an increased risk of PD (p < 0.001 and 0.02, respectively), whereas combined estrogen-progesterone was not associated with PD risk (p = 0.100). E, estrogen; MHT, menopausal hormone therapy; P, progesterone.
Cox proportional hazards analysis with time-dependent covariates showed an association between MHT and increased risk of PD (hazard ratio [HR] 1.377, 95% confidence interval [CI] 1.184–1.602, p < 0.001) (Supplementary Table 3). Combined estrogen-progesterone did not demonstrate any significant difference in the risk of PD (HR 1.167, 95% CI 0.952–1.429, p = 0.137), whereas tibolone (HR 1.554, 95% CI 1.297–1.861, p < 0.001) and estrogen alone (HR 1.465, 95% CI 1.054–2.036, p = 0.023) were found to be associated with an increased risk of PD compared to the non-MHT group. Further analyses of the detailed components of MHT are available in Supplementary Table 3.
The risk of PD based on the components of oral estrogen and progesterone, including combined estrogen-progesterone, is shown in Supplementary Figure 1. The analysis indicated that the CEE use (i.e., CEE, CEE/MP, CEE/MPA, and CEE/DYD use) was associated with a higher risk of PD (HR 2.297, 95% CI 1.259–4.192) compared to the non-MHT group. However, there was no significant difference in the risk of PD observed for progesterones (DRSP, NETA, DYD, CPA, MPA, and MP) and other estrogens (EV and EH), as shown in Supplementary Figure 2.
Effect of MHT use duration on PD risk
MHT use for less than three years increased the risk of PD, whereas the risk of PD was unaffected when women used MHT for longer than three years (Fig. 3). Table 3 presents results of the risk of PD based on the duration of each MHT type. Tibolone and estrogen alone were associated with PD with a treatment duration of less than one year (tibolone: HR 2.070, CI 1.601–2.674, p < 0.001; estrogen alone: HR 1.876, CI 1.167–3.017, p < 0.001) or 1–2.9 years (tibolone: HR 1.685, CI 1.286–2.207, p < 0.001; estrogen alone: HR 2.056, CI 1.224–3.454, p = 0.006), whereas no association was found after three years of tibolone or estrogen alone use.
Fig. 3
Risk of Parkinson’s disease stratified by duration of MHT use. A) Estrogen alone (p for trend:<0.001); B) Combined estrogen-progesterone (p for trend:<0.001); C) Tibolone (p for trend:<0.001); D) Total MHT (p for trend:<0.001). E, estrogen; MHT, menopausal hormone therapy; P, progesterone. The dark grey shading above the solid line represents the upper 95% confidence interval, and the dark grey shading below the solid line represents the lower 95% confidence interval.
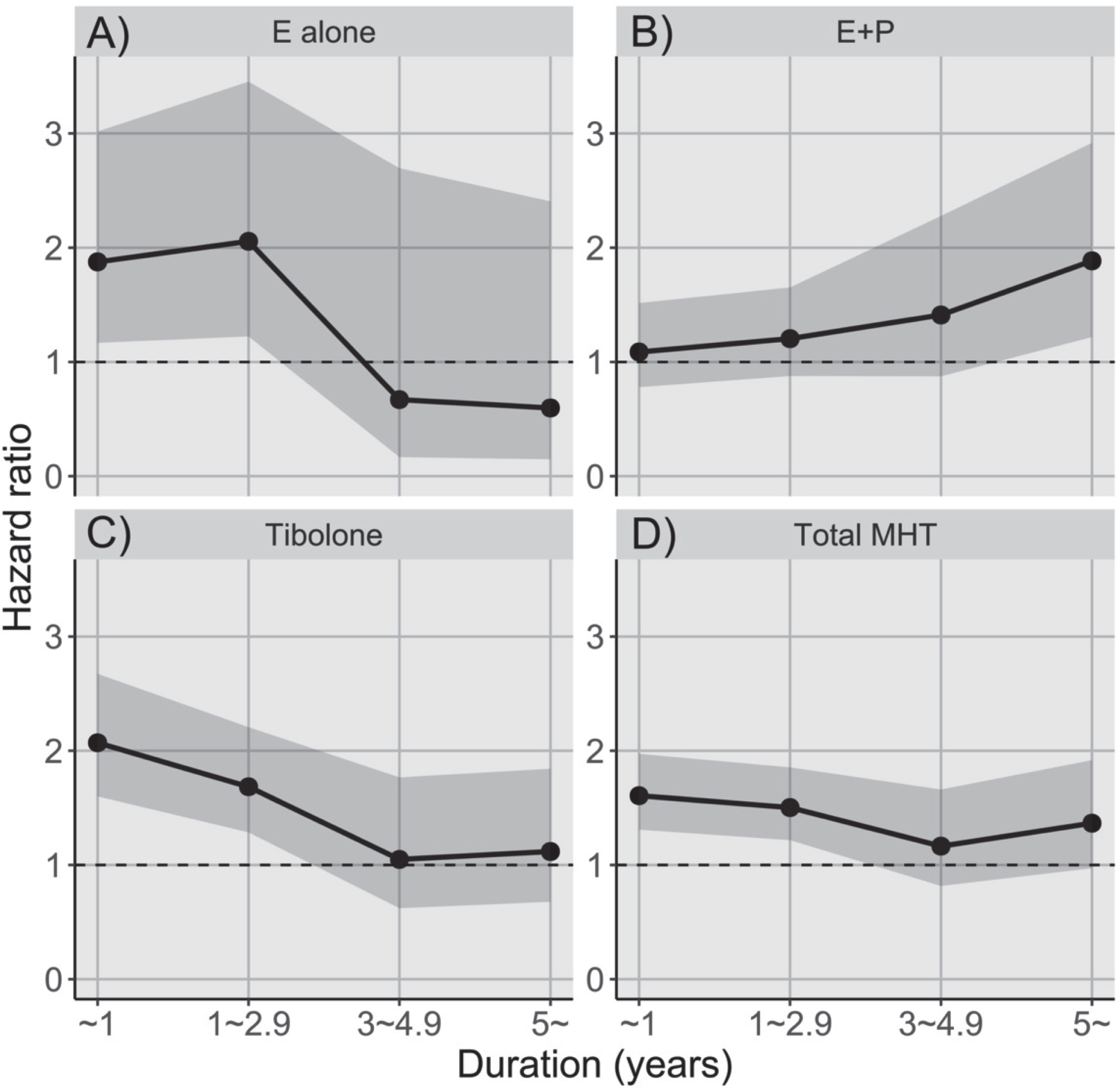
Conversely, combined estrogen-progesterone use was not associated with PD risk when patients were treated with MHT for less than five years, whereas there was a significant association between combined estrogen-progesterone use and PD risk in patients treated with MHT for > 5 years (HR 1.885, 95% CI 1.218–2.918, p = 0.004).
In addition, the Cochran-Armitage test showed a significant p for trend (p < 0.001) in combined estrogen-progesterone users, suggesting a dose-response relationship between combined estrogen-progesterone use and the risk of PD.
Subgroup analysis
Supplementary Table 4 presents the incidence rates of PD per 100,000 person-years for the variables in both groups. When we analyzed the interaction of the MHT with each variable, none of the variables were significant, except for region (Supplementary Figure 2). Analysis for each hormonal agent showed a similar trend (Supplementary Table 5). In a sensitivity analysis that included only women with a CCI score of 0, MHT increased the risk of PD (HR 1.401, 95% CI 1.152–1.704), and the analyses based on MHT classification yielded similar results as the primary outcomes.
DISCUSSION
This study investigated the association between MHT and the risk of PD. Our analysis showed that MHT is generally associated with the risk of PD; however, this association was MHT regimen- and duration-specific. MHT with tibolone or estrogen alone was associated with an increased risk of PD, whereas the trend of MHT facilitating PD disappeared with increasing duration. However, the longer the women were treated with combined estrogen-progesterone, the higher the risk of PD, which was consistent with the dose-response relationship.
In this study, we found that the association of MHT with tibolone or estrogen alone with an increased risk of PD incidence was robust for the short-term use of tibolone or estrogen alone (<3 years), whereas the long-term use of tibolone or estrogen alone was not significantly associated with the future risk of PD. Although in vivo and in vitro studies have provided evidence for the neuroprotective effects of estrogen in PD [17–19], clinical studies have shown conflicting results. Previous cohort studies have suggested that MHT with estrogen alone is associated with an increased risk of PD [14, 20]. In contrast, some case-control studies have shown that postmenopausal estrogen therapy is associated with a reduced risk of PD [7, 8]. When analyses were performed according to the duration of MHT in this study, MHT with tibolone or estrogen alone increased the risk of PD only in the short term. A meta-analysis conducted by Wang et al. showed a similar result [21].
The difference in the risk of PD according to MHT duration could be attributed to the well-known neuroprotective effects of estrogen; however, its effects on the dopaminergic system are controversial. Indeed, previous studies have shown that estrogen has anti-dopaminergic effects in the striatum [22, 23], which may unmask PD symptoms in individuals with preclinical PD. However, these anti-dopaminergic effects are counterbalanced by the neuroprotective effects of estrogen in women with prolonged use of estrogen alone. Also, the associations between MHT use and the risk of PD under 3 years may be due to reverse causation. For example, it may have been misinterpreted as menopausal symptoms at first, leading to hormone therapy; however, it later became evident that it could have been indicative of early symptoms of PD. In addition, regarding that Asian women may have different genetic susceptibilities to PD [24, 25], different genetic and ethnic background may account for the discrepancy between our results, which showed a higher risk in those taking estrogen treatment, and previous studies suggesting a protective effect of estrogen in PD [7–10].
In addition, postmenopausal symptoms overlap with the non-motor symptoms of PD [26, 27], individuals who have not yet been diagnosed with PD may have visited gynecological clinics with non-motor symptoms of PD mistaken for postmenopausal symptoms. Furthermore, estrogen intolerance in individuals with prodromal PD may provide a possible explanation that should be investigated in future studies. To the best of our knowledge, this is the first study to demonstrate an association between MHT with tibolone use and risk of PD. As tibolone acts as an agonist primarily at estrogen receptors [28], its similar results with estrogen alone are plausible.
Further analyses of the detailed components of the MHT showed that CEE was associated with a higher risk of PD, whereas other estrogen components (EH and EV) were not associated with PD risk. A previous study showed that esterified estrogen, a significant component of estrone, is associated with an increased PD risk [13]. The results of this study, focused on CEE composed of estrone exceeding 50%, are in line with the previous study. Future studies are warranted to investigate the effect of the estrone-to-estradiol ratio on the risk of PD.
Previous studies have shown inconsistent results regarding the association between the risk of PD and estrogen-progesterone use. Although the primary analysis did not show the relationship between estrogen-progesterone use and the risk of PD, further analysis according to exposure time demonstrated that the risk of PD associated with combined estrogen-progesterone use increases with prolonged use. Popat et al. showed that a combined estrogen-progestin regimen does not increase the risk of PD [29], whereas Lundin et al. reported that esterified estrogen use combined with progestin is associated with an increased risk of PD [13]. Simon et al. found that using progestin alone was associated with a 3-fold higher risk of PD [14]. This is the first study demonstrating the detrimental effect of estrogen-progesterone MHT on the risk of PD in a dose-response manner. Previous studies provided possible explanations for these findings. Several preclinical studies have shown that progesterone counteracts estrogen-mediated neuroprotection [30–32], which suggests that it may accelerate neurodegeneration. In addition, Strijks et al. showed that progesterone had possible anti-dopaminergic effects in postmenopausal women with PD [33]. Taken together, it is plausible that longer use of the estrogen-progesterone regimen as MHT increases PD risk.
There was no significant interaction in the subgroup analyses, except for residency status. MHT in women living in rural areas was associated with an increased risk of PD, whereas there was no relationship between MHT and risk of PD in women living in urban areas. This urban-rural disparity may be ascribed to differences in medical accessibility. As rural areas often face challenges related to access to healthcare services, including specialists and healthcare facilities, women who live in rural areas may visit clinics at a relatively advanced stage of PD, which facilitates early recognition and diagnosis of PD. In addition, environmental factors such as pesticides, which is a well-known risk factor for PD, may have contributed an interaction between MHT use and residency status [34].
This study has several limitations. First, as the identification of PD was based on National Health Insurance claims data, disease-specific outcomes, such as parkinsonian motor symptom severity assessed using the Unified Parkinson’s Disease Rating Scale, were unavailable. In addition, although we tried to precisely define the outcome of PD diagnosis by excluding the ICD codes of atypical or secondary parkinsonism and adding the condition of continuous dopaminergic drug use, possible misdiagnosis of PD may have occurred because of the characteristics of this study. Second, we did not have data on the exact date of menarche and menopause, which may be critical confounding factors because previous studies have shown that reproductive characteristics are associated with the risk of PD [12, 14, 35]. Our study also has limitations regarding exercise, caffeine, and smoking [36–39]. Third, as the population of this study was restricted to Koreans, replication studies on other ethnic populations are necessary.
In conclusion, the MHT is associated with the risk of PD in a regimen- and duration-specific manner. These results provide insights into the use of the MHT in the development of PD.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. RS-2023-00209580).
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021049270).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
According to HIRA’s privacy policy, raw data cannot be provided.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230230.
REFERENCES
[1] | Hijaz BA , Volpicelli-Daley LA ((2020) ) Initiation and propagation of α-synuclein aggregation in the nervous system. Mol Neurodegener 15: , 19. |
[2] | Van Den Eeden SK , Tanner CM , Bernstein AL , Fross RD , Leimpeter A , Bloch DA , Nelson LM ((2003) ) Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. Am J Epidemiol 157: , 1015–1022. |
[3] | Wooten G , Currie L , Bovbjerg V , Lee J , Patrie J ((2004) ) Are men at greater risk for Parkinson’s disease than women? . J Neurol Neurosurg Psychiatry 75: , 637–639. |
[4] | Morale MC , Serra PA , L’Episcopo F , Tirolo C , Caniglia S , Testa N , Gennuso F , Giaquinta G , Rocchitta G , Desole MS , Miele E , Marchetti B ((2006) ) Estrogen, neuroinflammation and neuroprotection in Parkinson’s disease: Glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience 138: , 869–878. |
[5] | Cyr M , Calon F , Morissette M , Di Paolo T ((2002) ) Estrogenic modulation of brain activity: Implications for schizophrenia and Parkinson’s disease. J Psychiatry Neurosci 27: , 12–27. |
[6] | Dluzen DE ((2000) ) Neuroprotective effects of estrogen upon the nigrostriatal dopaminergic system. J Neurocytol 29: , 387–399. |
[7] | Currie LJ , Harrison MB , Trugman JM , Bennett JP , Wooten GF ((2004) ) Postmenopausal estrogen use affects risk for Parkinson disease. Arch Neurol 61: , 886–888. |
[8] | Martignoni E , Nappi RE , Citterio A , Calandrella D , Zangaglia R , Mancini F , Corengia E , Riboldazzi G , Polatti F , Nappi G ((2003) ) Reproductive life milestones in women with Parkinson’s disease. Funct Neurol 18: , 211–218. |
[9] | ((2011) ) A randomized pilot trial of estrogen replacement therapy in post-menopausal women with Parkinson’s disease. Parkinsonism Relat Disord 17: , 757–760. |
[10] | Saunders-Pullman R , Gordon-Elliott J , Parides M , Fahn S , Saunders HR , Bressman S ((1999) ) The effect of estrogen replacement on early Parkinson’s disease. Neurology 52: , 1417–1421. |
[11] | Rugbjerg K , Christensen J , Tjønneland A , Olsen JH ((2013) ) Exposure to estrogen and women’s risk for Parkinson’s disease: A prospective cohort study in Denmark. Parkinsonism Relat Disord 19: , 457–460. |
[12] | Liu R , Baird D , Park Y , Freedman ND , Huang X , Hollenbeck A , Blair A , Chen H ((2014) ) Female reproductive factors, menopausal hormone use, and Parkinson’s disease. Mov Disord 29: , 889–896. |
[13] | Lundin JI , Ton TG , LaCroix AZ , Longstreth WT , Franklin GM , Swanson PD , Smith-Weller T , Racette BA , Checkoway H ((2014) ) Formulations of hormone therapy and risk of Parkinson’s disease. Mov Disord 29: , 1631–1636. |
[14] | Simon KC , Chen H , Gao X , Schwarzschild MA , Ascherio A ((2009) ) Reproductive factors, exogenous estrogen use, and risk of Parkinson’s disease. Mov Disord 24: , 1359–1365. |
[15] | Kim L , Kim JA , Kim S ((2014) ) A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol Health 36: , e2014008. |
[16] | Quan H , Li B , Couris CM , Fushimi K , Graham P , Hider P , Januel JM , Sundararajan V ((2011) ) Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 173: , 676–682. |
[17] | Al Sweidi S , Sánchez MG , Bourque M , Morissette M , Dluzen D , Di Paolo T ((2012) ) Oestrogen receptors and signalling pathways: Implications for neuroprotective effects of sex steroids in Parkinson’s disease. J Neuroendocrinol 24: , 48–61. |
[18] | Morissette M , Al Sweidi S , Callier S , Di Paolo T ((2008) ) Estrogen and SERM neuroprotection in animal models of Parkinson’s disease. Mol Cell Endocrinol 290: , 60–69. |
[19] | Sawada H , Ibi M , Kihara T , Honda K , Nakamizo T , Kanki R , Nakanishi M , Sakka N , Akaike A , Shimohama S ((2002) ) Estradiol protects dopaminergic neurons in a MPP+Parkinson’s disease model. Neuropharmacology 42: , 1056–1064. |
[20] | Ascherio A , Weisskopf MG , O’Reilly EJ , McCullough ML , Calle EE , Rodriguez C , Thun MJ ((2004) ) Coffee consumption, gender, and Parkinson’s disease mortality in the cancer prevention study II cohort: The modifying effects of estrogen. Am J Epidemiol 160: , 977–984. |
[21] | Wang P , Li J , Qiu S , Wen H , Du J ((2015) ) Hormone replacement therapy and Parkinson’s disease risk in women: A meta-analysis of 14 observational studies. Neuropsychiatr Dis Treat 11: , 59–66. |
[22] | McDermott JL ((1993) ) Effects of estrogen upon dopamine release from the corpus striatum of young and aged female rats. Brain Res 606: , 118–125. |
[23] | Euvrard C , Oberlander C , Boissier JR ((1980) ) Antidopaminergic effect of estrogens at the striatal level. J Pharmacol Exp Ther 214: , 179–185. |
[24] | Abbas MM , Xu Z , Tan LCS ((2018) ) Epidemiology of Parkinson’s disease— east versus west. Mov Disord Clin Pract 5: , 14–28. |
[25] | Kang SH , Moon S-J , Kang M , Chung SJ , Cho GJ , Koh S-B ((2023) ) Incidence of Parkinson’s disease and modifiable risk factors in Korean population: A longitudinal follow-up study of a nationwide cohort. Front Aging Neurosci 15: . |
[26] | Thurston RC , Joffe H ((2011) ) Vasomotor symptoms and menopause: Findings from the Study of Women’s Health across the Nation. Obstet Gynecol Clin North Am 38: , 489–501. |
[27] | Akaogi Y , Asahina M , Yamanaka Y , Koyama Y , Hattori T ((2009) ) Sudomotor, skin vasomotor, and cardiovascular reflexes in 3 clinical forms of Lewy body disease. Neurology 73: , 59–65. |
[28] | Karsdal MA , Byrjalsen I , Leeming DJ , Christiansen C ((2008) ) Tibolone inhibits bone resorption without secondary positive effects on cartilage degradation. BMC Musculoskelet Disord 9: , 153. |
[29] | Popat RA , Van Den Eeden SK , Tanner CM , McGuire V , Bernstein AL , Bloch DA , Leimpeter A , Nelson LM ((2005) ) Effect of reproductive factors and postmenopausal hormone use on the risk of Parkinson disease. Neurology 65: , 383–390. |
[30] | Jayaraman A , Pike CJ ((2009) ) Progesterone attenuates oestrogen neuroprotection via downregulation of oestrogen receptor expression in cultured neurones. J Neuroendocrinol 21: , 77–81. |
[31] | Aguirre CC , Baudry M ((2009) ) Progesterone reverses 17β-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur J Neurosci 29: , 447–454. |
[32] | Aguirre C , Jayaraman A , Pike C , Baudry M ((2010) ) Progesterone inhibits estrogen-mediated neuroprotection against excitotoxicity by down-regulating estrogen receptor-β. J Neurochem 115: , 1277–1287. |
[33] | Strijks E , Kremer JA , Horstink MW ((1999) ) Effects of female sex steroids on Parkinson’s disease in postmenopausal women. Clin Neuropharmacol 22: , 93–97. |
[34] | Cha ES , Khang Y-H , Lee WJ ((2014) ) Mortality from and incidence of pesticide poisoning in South Korea: Findings from National Death and Health Utilization Data between 2006 and 2010. PLoS One 9: , e95299. |
[35] | Pesce G , Artaud F , Roze E , Degaey I , Portugal B , Nguyen TTH , Fournier A , Boutron-Ruault M-C , Severi G , Elbaz A , Canonico M ((2023) ) Reproductive characteristics, use of exogenous hormones and Parkinson disease in women from the E3N study. Brain 146: , 2535–2546. |
[36] | Hughes KC , Gao X , Molsberry S , Valeri L , Schwarzschild MA , Ascherio A ((2019) ) Physical activity and prodromal features of Parkinson disease. Neurology 93: , e2157–e2169. |
[37] | Chen H , Zhang SM , Schwarzschild MA , Hernán MA , Ascherio A ((2005) ) Physical activity and the risk of Parkinson disease. Neurology 64: , 664–669. |
[38] | Liu R , Guo X , Park Y , Huang X , Sinha R , Freedman ND , Hollenbeck AR , Blair A , Chen H ((2012) ) Caffeine intake, smoking, and risk of Parkinson disease in men and women. Am J Epidemiol 175: , 1200–1207. |
[39] | Noyce AJ , Bestwick JP , Silveira-Moriyama L , Hawkes CH , Giovannoni G , Lees AJ , Schrag A ((2012) ) Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol 72: , 893–901. |




