Non-Pharmacological Interventions for Depression and Anxiety in Parkinson’s Disease
Abstract
Non-pharmacological interventions, including cognitive-behavioral therapy (CBT), non-invasive brain stimulation (NIBS), electroconvulsive therapy (ECT), light therapy (LT), and physical rehabilitation/exercise, have shown promise as effective approaches to treat symptoms of depression and anxiety in individuals with Parkinson’s disease (PD). In this narrative literature overview, we discuss the state-of-the-art regarding these treatment options and address future perspectives for clinical practice and research. Non-pharmacological interventions hold promise to treat depression and anxiety in PD. There is meta-analytic evidence for the efficacy of CBT, NIBS, ECT, LT, and exercise on improving depressive symptoms. For the treatment of anxiety symptoms, CBT shows large effects but scientific evidence of other non-pharmacological interventions is limited. Importantly, these treatments are safe interventions with no or mild side-effects. More research is needed to tailor treatment to the individuals’ needs and combined interventions may provide synergistic effects.We conclude that non-pharmacological interventions should be considered as alternative or augmentative treatments to pharmacological and neurosurgical approaches for the treatment of depression and anxiety in individuals with PD.
| Practical take home messages |
| Non-pharmacological interventions hold promise to treat depression and anxiety in PD however, for anxiety the scientific evidence is limited |
| The neural correlates for these intervention effects are still largely unknown. |
| Cognitive behavioral therapy (CBT) |
| CBT is probably effective for the treatment of anxiety and depression in PD, but disease-specific treatment protocols are needed for it to better fit PD-specific anxiety and depression symptoms |
| Internet-based CBT may improve the feasibility of intensive treatment and CBT may be combined with other treatment modalities to improve its efficacy |
| Electroconvulsive therapy (ECT) |
| ECT is indicated for treatment resistant depression in PD |
| Non-invasive brain stimulation (NIBS, e.g., repetitive transcranial magnetic stimulation, transcranial direct current stimulation) |
| NIBS can be considered as a possible non-invasive intervention for depression in PD, the evidence for anxiety is less clear. |
| Light Therapy |
| LT is an interesting treatment option for depression in PD as it can be performed at home and has minimal adverse effects, no evidence for anxiety is currently available. |
| Physical rehabilitation / exercise |
| Any type of exercise (mild, moderate, or high intensity) is likely to have a positive effect on mood in PD, resulting from possible neuroplasticity mechanisms. |
INTRODUCTION
People living with Parkinson’s disease (PD) typically present with motor symptoms, but depressive and anxiety disorders are common and highly disabling neuropsychiatric symptoms in PD, with an estimated prevalence of up to 50% [1]. These symptoms are cited among the most troubling for people with PD and are associated with significant decreased quality of life [1]. Also, depression, with or without comorbid anxiety symptoms, is a prodromal marker of PD [2], manifesting often as first symptom of PD many years before motor symptoms appear. The relatively larger prevalence of anxiety and depression in PD may be explained by PD-related aberrations in brain circuits that are dependent on dopamine, serotonin and noradrenaline; specifically related to the limbic cortico-striato-thalamo-cortical circuit and the ‘fear’ circuit (with a major role of the amygdala) [3, 4]. Management of depression and anxiety by optimizing dopaminergic treatment can be effective in case the affective dysregulation is related to suboptimal dopaminergic supplementation. The use of selective serotonin reuptake inhibitors can be considered, as it has shown effectiveness [5]. However, pharmacological options are not always well tolerated, may have suboptimal efficacy and undesirable side effects, and can be complex to implement due to polypharmacy [6]. Therefore, it is important to consider non-pharmacological treatments that may provide an alternative for or augment current pharmacological and neurosurgical treatment options. The objective of this paper is to provide clinicians and researchers with an up-to-date comprehensive review of the current state-of-the-art in key non-pharmacological interventions. We focus on cognitive-behavioral therapy, non-invasive brain stimulation, electroconvulsive therapy, light therapy, and use of physical rehabilitation/exercise for treatment of depression and anxiety in PD. We highlight promising results, hypothesized mechanisms of action, and future directions.
COGNITIVE-BEHAVIORAL THERAPIES
Psychological therapy options are regarded as the gold standard for the treatment of depressive and anxiety disorders. Cognitive behavioral therapy (CBT) is an umbrella term for the set of therapeutic methods that finds its roots in the conditioning theories. It focuses on improving psychological well-being by challenging negative thoughts and related dysfunctional behavior (such as avoidance). Recently, acceptance and commitment therapy (ACT), a ‘third-generation’ CBT method, has specifically received attention for the treatment of depressive and anxiety disorders in individuals with chronic illness. ACT, based on the relational frame theory, aims to strengthen two axes of psychological flexibility: mindfulness and acceptance processes, and behavioral change and committed action to personal values (see, e.g., [7]).
The neural correlates of CBT for the treatment of anxiety and depression are still largely unknown, in PD but also in the general population. A hypothesized mechanism of action of CBT is that CBT may strengthen brain circuits that are, for example, involved in cognitive control (frontoparietal network), emotion regulation and response inhibition (cortico-striato-thalamo-cortical circuits). One study in PD found evidence for normalization of disrupted connectivity in the limbic and fear circuits, in addition to improved cognitive control by enhanced connectivity within and between the central executive and salience resting-state networks, following CBT for the treatment of anxiety symptoms [8]. More research is needed to gain a better understanding of the effect of CBT on brain circuitry.
In the general adult population, CBT is regarded as an evidence-based intervention for depressive disorders and anxiety disorders including panic disorder, generalized anxiety disorder and social anxiety disorder [9]. The effect sizes of CBT for the treatment of depression and anxiety disorders in the general population are small to moderate (as compared with usual care) [10]. Moreover, the effects are durable [11] and, for most disorders, equal to those of taking antidepressant medication [12]. While psychological interventions for elderly individuals were long thought to be ineffective, nowadays this belief has been refuted [13, 14]. Also, cognitive impairment is often viewed by clinicians as a contra-indication for the use of CBT, but there is meta-analytic—albeit uncertain—evidence that with adaptations, CBT can improve mental health in cognitively impaired individuals [15].
In PD, the use of CBT for the treatment of depressive and anxiety disorders is increasingly common in clinical practice. In general, CBT has shown large, positive effects for the treatment of anxiety symptoms (Standardized mean difference (SMD) –0.85, 95% Confidence Interval (CI) –1.12 to –0.58, p < 0.001, I2 = 0%), and large but heterogeneous effects for the treatment of depression compared with waiting-list or treatment as usual control groups (SMD –0.83, 95% CI –1.26 to –0.40, p < 0.001, I2 = 55%) [16]. CBT, including mindfulness-based methods like ACT, can additionally improve psychological distress in persons living with neurologic diseases including PD [17]. There are individual studies suggesting durability of CBT effects in PD [18, 19] but more evidence from randomized clinical trials (RCTs) is needed, specifically given the progressive neurodegenerative nature of the disease. Remission rates of depressive and anxiety disorders after CBT have rarely been studied in PD to date.
There is room for improvement in the psychological treatment of depression and anxiety in PD. First, disease-specific treatment protocols are important to account for PD-specific anxiety and depression symptoms, that can be related to, e.g., fear of falling, wearing-off related stress responses, and feelings of shame. In PD, depression and anxiety symptoms do not seem to fit the classification system of the DSM-5. Anxiety roughly can consist of episodes without comorbid depressive symptoms, or more persistently with comorbid depression [20]. PD-specific symptoms of anxiety are reportedly related to psychological distress due to the diagnosis or (general and wearing-off related) symptoms of PD, but also social anxiety due to PD-related symptoms, fear of losing control, persistent worrying, feelings of inner unrest, panic episodes and fear of falling [21]. Depression, in turn, can present in an anxious-depressive subtype, or without comorbid anxiety [22]. Moreover, apathy is more common in PD patients with a depression than in patients with major depressive disorder (MDD) [23].
Interventions should be adapted to optimize efficacy, for example by constructing specific interoceptive and imaginal exposure interventions to cope with anxiety related to wearing-off. Some small studies have already shown efficacy of such disease-specific CBT protocols [18, 24]. However, PD-specific consensus protocols are needed to work towards best-practice methods. Second, internet-based CBT may enhance the feasibility of intensive psychological treatment, as it may not always be feasible for individuals with PD to have weekly face-to-face therapy sessions due to mobility issues. Studies have already shown promising results of internet-based interventions (see, e.g., [25]). Third, combination interventions may prove more efficacious than use of psychological therapy alone. For example, CBT may be combined with physical therapy for the treatment of wearing-off related affective symptoms [24] or non-invasive brain stimulation may add to the effects of CBT [26]. More knowledge about the neural correlates of CBT in PD will additionally improve the efficacy of combining CBT with non-invasive brain stimulation.
NON-INVASIVE BRAIN STIMULATION
In recent years, non-invasive brain stimulation (NIBS) techniques, such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), have emerged as potential non-pharmacological interventions for managing motor and non-motor symptoms in PD [26, 27], including cognitive decline and affective symptoms such as depression and anxiety. By targeting specific cortical areas that act as an ‘entry point’ to relevant brain circuits, rTMS and tDCS may normalize cortical excitability, modulate activity in inter-connected brain regions, improve brain network connectivity and promote neuroplasticity [28, 29]. The mechanisms underlying its therapeutic effects in depression and anxiety relate to the modulation of specifically those dysfunctional neural circuits associated with mood and anxiety regulation, and cognitive control.
Repetitive transcranial magnetic stimulation
rTMS involves the application of electromagnetic pulses to specific regions of the brain to modulate brain circuit function. In the context of PD, initial studies focused on improving motor functioning, targeting the primary motor cortex (M1), resulting in enhanced dopamine release in striatal areas [30, 31]. rTMS has also shown promise as a treatment for cognitive decline and affective symptoms, such as depression and anxiety [32, 33]. Various targets (primary motor cortex, pre-supplementary motor area, and left or right dorsolateral prefrontal cortex (DLPFC)) and stimulation protocols have been used, with high-frequency (5–10 Hz) rTMS targeting the left DLPFC most frequently. A recent meta-analysis by Chen et al. [34] on the effects of rTMS for depression in PD (12 studies, including 511 patients) showed that rTMS (mainly 5 Hz rTMS to the left DLPFC) gives a significant reduction in severity of depression when compared to sham stimulation (SMD –0.621, CI –0.964 to –0.278), with effect sizes similar to use of antidepressant medication. An accelerated form of magnetic stimulation concerns theta-burst stimulation (TBS). A recent meta-analysis by Cheng et al. [35] on the effects of TBS in PD (both targeting M1, aimed to improve motor function; and the DLPFC, aimed to treat depression) included only 2 RCTs on DLPFC intermittent TBS stimulation with severity of depressive symptoms as primary outcome measure [36, 37] and showed an antidepressant effect in one [36] but not the other trial. The evidence regarding the efficacy of rTMS for anxiety in individuals with PD is relatively limited compared to depression. A more recent meta-analysis by Zheng et al. [26], based on 29 randomized controlled trials on rTMS in both depression and anxiety in PD (including only 5 studies reporting on the effects on anxiety), showed a significant (and clinically relevant) rTMS effect on depressive symptoms (SMD –0.75, CI –0.99 to –0.50), but not anxiety (SMD –0.29, CI –0.76 to 0.17,) or apathy (SMD –0.05, CI –0.39 to 0.50), when compared to sham stimulation. When rTMS was used as adjuvant to other pharmacological or non-pharmacological treatments, rTMS showed a significant added value on both depressive symptoms and anxiety as compared to these other treatments alone.
Transcranial direct current stimulation
The mobile alternative NIBS technique, tDCS, involves delivering a low-intensity direct current through scalp electrodes to modulate cortical excitability. This technique can either increase (anodal stimulation) or decrease (cathodal stimulation) neuronal activity in targeted brain regions. tDCS is considered safe, well-tolerated, and relatively inexpensive, making it an attractive treatment option for individuals with PD and comorbid psychiatric symptoms [38, 39]. Based on a meta-analysis in healthy adults, tDCS results in significant reduction in symptoms of depression and anxiety [40]. In PD, tDCS has been mainly studied in the context of motor function and cognitive rehabilitation [39]. Regarding its application for the treatment of depression and anxiety in PD, anodal stimulation of the left DLPFC (in an open-labeled feasibility study) was associated with improvements in depressive symptoms [41]. However, further research is needed to establish its efficacy and optimal stimulation parameters for depression and anxiety treatment in PD.
In summary, while rTMS and tDCS hold promise as non-invasive, non-pharmacological treatments for depression and anxiety in PD, several limitations should be considered. First, the studies on the efficacy of tDCS in the treatment of depression and anxiety are scarce. Second, the optimal stimulation parameters of NIBS, such as target, intensity, frequency, and duration, have not been definitively established. Third, the long-term effects and the potential for relapse after discontinuation of treatment require further investigation. Fourth, large-scale randomized controlled trials are needed to provide cost-effectiveness and safety. Fifth, the field will profit from the current innovations towards personalized stimulation protocols and home-based treatments.
ELECTROCONVULSIVE THERAPY
Electroconvulsive therapy (ECT) is the most effective treatment for severe and treatment-resistant depression, by the induction of seizures through electrical brain stimulation under short-term anesthesia [42]. The working mechanism of ECT remains unclear, but recent studies suggest neuroplastic changes induced by ECT. Schurgers and colleagues showed that ECT-induced reduction in depression severity is found to be related to increased mRNA expression of brain-derived neurotrophic factor (BDNF) and related genes, which correlated with changes in DNA methylation of these genes and increased serum BDNF protein levels after treatment [43]. The dentate gyrus of the hippocampus is especially relevant for the neuroplastic effects of ECT and shows a very region-specific volume increase after ECT [44].
Although ECT has been employed for decades in adult and geriatric populations [45], including PD patients with depression, its effectiveness in individuals with PD is still under-researched [46]. A systematic review on the effects of ECT in PD by Borisovskaya et al. [47] included 43 studies, encompassing one retrospective case study, two retrospective chart reviews, 27 single case reports, and 13 case series. This overview showed that more than 93% of patients experienced an improvement in their depression symptoms and among those for whom motor function was assessed, 83% reported an improvement in motor symptoms along with their depression. Although the often feared negative effects on cognition were limited to a small subgroup, higher occurrence of adverse effects occurred, mainly delirium and transient confusion, probably related to anesthesia, which sometimes led to treatment discontinuation. Considering that autonomic dysregulation and falls are common in PD, ECT may potentially exacerbate issues like urine retention and fall rates [47].
A more recent meta-analysis aimed to assess the impact of ECT on motor and non-motor symptoms in PD [46]. Among a total of 14 studies (n = 129), there was one RCT, nine prospective observational studies, and four retrospective studies. ECT had a significant positive impact on motor manifestations in a subpopulation of PD without psychiatric symptoms (SMD 1.18, CI 0.87–1.49). ECT significantly improved depression (SMD 1.33, CI 0.42–2.24) and psychosis (SMD 1.64, CI 0.90–2.38) and was found to be effective in relieving the wearing-off phenomenon (SMD 0.71, CI 0.28–1.14) without causing any worsening of cognitive function (even small improvement, probably related to improved mood).
In summary, despite the scarce literature on controlled studies on the efficacy, durability, and side effects of ECT in PD, ECT can be considered a safe and fast-acting treatment for severe depression in PD when other methods do not sufficiently reduce the burden of depression. However, strategies to deal with transient post-ECT delirium/confusion and risk of relapse after treatment discontinuation warrant further research.
LIGHT THERAPY
In individuals with PD, the circadian rhythm can become disrupted due to various factors, contributing to depressive symptoms and sleep disorders [48, 49]. The circadian rhythm is generated by the ‘circadian pacemaker’, located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Its rhythm is entrained to the 24-hour day-night cycle by “zeitgebers”, of which light is the most important one for humans. Light excites specialized melanopsin containing ganglion cells in the retina (melanocytes), that project a “daytime” signal towards the SCN via the retino-hypothalamic tract.
Individuals with PD show decreased neuroplasticity and altered neurophysiology of the SCN neuronal network as well as neurodegeneration of dopaminergic cells in the SCN [50], making individuals with PD more prone to circadian rhythm disturbances. Moreover, treatment with levodopa or dopaminergic agonists can alter the rhythmic expression of CLOCK genes (genes involved in the temporal regulation of the transcriptome), inducing changes in the circadian rhythm [50]. Finally, various PD-related symptoms, like excessive daytimes sleepiness and sleep-disrupting motor and non-motor symptoms, can negatively impact the sleep-wake cycle, leading to further perturbation of the circadian rhythm [48]. Wrist-worn actigraphy shows a phase advance of the sleep–wake cycle in later stage PD and a decrease in the amplitude of the circadian rhythm, due to the presence of greater nocturnal activity and lower daytime activity [51]. A disturbed circadian rhythm in individuals with PD is also expressed in the timing of core body temperature rhythms and secretion patterns of melatonin, the ‘sleep hormone’ [52–55]. As serotonin, noradrenalin and dopamine all have a circadian rhythm in their release, synthesis-related enzymes and the expression and activity of their receptors, it is conceivable that a disturbed circadian rhythm can have a negative impact on both PD-related motor and non-motor symptoms [49, 55].
Light therapy (LT) involves the daily rhythmic exposure to light-emitting devices, restoring the circadian rhythm by stimulating the SCN via melanocytes in the retina. As LT has few contraindications and side-effects, it is an attractive treatment option. LT has been used to treat mood and circadian rhythm sleep disorders for many years, and has received increasing scientific attention in PD research over the past decade. A recent meta-analysis by Sun et al [56] on the efficacy and safety of LT for both motor and non-motor symptoms of PD shows positive effects on depression in PD [56]. This meta-analysis included four RCTs with a total of 234 participants with PD. The included studies compared different phototherapy devices, including light boxes, portable head-mounted devices and fluorescent tubes, to placebo devices with the same appearance. All trials assessed the effect on depressive symptoms, using the Beck Depression Inventory (BDI) and Hamilton Depression Rating Scale [57–60]. The meta-analysis demonstrated a significant improvement in depression in the LT group as compared to the control group (SMD –0.27, CI –0.52 to –0.02, P = 0.04, high quality of evidence; [56]). Moreover, two studies evaluated the effectiveness of LT in sleep involving 122 participants [57, 60], demonstrated a statistically significant improvement of sleep (Mean Difference 3.45, CI 0.12 to 6.78, P = 0.04, high quality of evidence; n = 122, [56]). The three trials assessing anxiety (n = 141) found no significant improvement in the LT group [56–58, 60]. None of the reviewed studies reported any significant adverse effects, only mild and transient adverse effects like minor eye discomfort and headache. Limitations of the meta-analysis include the limited number of RCTs, some of them with small sample sizes, and the use of different light devices and treatment regimens.
In conclusion, LT can restore the disrupted circadian rhythm in individuals with PD, resulting in a decrease in depressive symptoms and positive effect on sleep. However, there is no evidence, to date, for the efficacy of LT for the treatment of anxiety symptoms. As LT has minimal adverse effects and can be performed at home, it is an interesting treatment option for depression in PD.
PHYSICAL REHABILITATION/EXERCISE
Physical rehabilitation/exercise is expected to affect neurostructural and chemical processes in PD. Studies suggest co-morbid depression or anxiety in PD correlates with abnormalities in expression of inflammatory cytokines, receptor availability, neurotrophin level, brain volume and connectivity, dopamine, norepinephrine, and serotonin availability [61]. It has been hypothesized that exercise may exert influence on each of these structural and neurochemical processes [61, 62]. Preliminary evidence from human studies in people with PD without depression or anxiety suggests physical rehabilitation/exercise might increase D2/3 dopamine receptor availability [63, 64], increase endogenous release of anti-inflammatory cytokines (including interleukin-10, a valid immunological marker for severity of non-motor features in PD and critical for microglia and astroglia activation) [65], trigger volumetric changes [66, 67], improve functional connectivity [68], enhance dorsal striatal dopamine release [64, 69], and alter neurotrophin levels (including BDNF) [66, 70–72]. Only two studies reported on exercise-induced changes in BDNF in patients with PD with co-morbid depression or anxiety. Sajatovich and colleagues [73] studied group aerobic/resistance exercise in PD with co-morbid depression, and showed improved Montgomery-Asberg Depression Rating Scale (p < 0.001) associated with a 3-fold increase in plasma BDNF (p < 0.001). Anxiety did not change. Landers and colleagues showed in their trial, comparing “high-intensity exercise boot camp” and usual care, that plasma BDNF and interleukin 10/tumor necrosis factor-α ratio improved; however, BDI scores did not [74].
In the adult healthy population, physical exercise (defined as “subcategory of physical activity that is planned, structured, and repetitive with the primary purpose of improvement or maintenance of physical fitness, physical performance, or health”) is moderately effective in improving depression (SMD –0.62, CI –0.81 to –0.42, 35 studies, N = 1353, [75]). In individuals with PD, a large range of physical rehabilitation and exercise interventions (e.g., aqua-based training, gait/balance/functional training, multi-domain physical training, dance, mind-body training (e.g., tai chi or yoga), endurance training, strength/resistance training have shown positive effects on both motor symptoms and quality of life as shown in a recent Cochrane network meta-analysis (156 studies, 7,939 participants [76]). More intensive exercise is suggested to be more effective, as shown by a large RCT on treadmill training in PD [77].
Regarding its effects on anxiety and depression, a regular exercise regimen is associated with reduced prevalence of depression in PD [78]. However, a limited amount of studies have specifically focused on depression and/or anxiety as main target for exercise interventions in PD. A review by Feller et al. [79] reported significant overall effects of exercise training compared with usual care on depression (SMD –0.49, CI –0.74 to –0.24; 14 RCTs; N = 961). This corresponds to a 2.47-point change in BDI score, which is clinically relevant. General exercise was used in 18 of the included studies (51%) as their experimental intervention. Of the remainder, 7 (21%) used a form of aerobic exercise, 4 (12%) dance, 4 (12%) a combination of yoga and qigong, 1 (2%) Lee Silverman Voice Treatment therapy, and 1 (2%) Ai Chi in water. Subgroup analysis showed that only general exercise (SMD –0.61, CI –0.99 to –0.23, 8 RCTs, N = 573) and dance (SMD –1.22, CI –2.29 to –0.15, 2 RCTs, N = 41) significantly improved depression, which is consistent with an earlier systematic review [80]. However, the quality of evidence was judged as low, so future high-quality studies are needed.
The evidence regarding exercise for the treatment of anxiety in PD is much less convincing. Abuoaf et al. [81] recently reviewed 5 RCTs (N = 328) and concluded that there is not enough high quality evidence in favor of a positive effect of exercise on anxiety in PD. They identified only two controlled studies [82, 83] that compared exercise to usual care. One study found significantly reduced Beck Anxiety Inventory scores after clinic-based endurance training (n = 35, 24 weeks, 2 times a week, 40 minutes): The exercise group reduced anxiety by 32.8%, while usual care reduced anxiety by only 6.6% [83]. Lower dose home-based exercise (n = 98, 8 weeks, 3 times a week, 30–50 minutes) was not effective for reducing anxiety [82]. There is a need for high quality RCTs with anxiety as primary outcome, as well as large sample sizes across the spectrum of disease stages, with longer periods of follow-up.
Overall, the current state-of-the-art scientific evidence suggests that exercise interventions, possibly combined with other non-pharmacologic interventions, can be effective in reducing depression symptoms in individuals with PD, who do not display contraindications to exercise. The effect of exercise on anxiety has been less studied.
FUTURE DIRECTIONS
Evidence for non-pharmacological interventions for PD with co-morbid depression is promising; however, for anxiety in PD the evidence is limited (due to lack of proper RCTs, Fig. 1). Thus, current application of treatment modalities for depression and anxiety in PD in daily clinical practice is predominantly based on knowledge from the general population and expert opinion. Further research with adequately powered, well controlled, longer term follow-up RCTs is needed to determine the optimal dose, modality, type, duration, intensity, and frequency for interventions targeting affective symptoms in individuals with PD, and to elucidate the underlying mechanisms. Standardization of mood scales may help reduce heterogeneity in outcome measurement. In addition, there have been rapid ongoing developments in artificial intelligence (AI) technology and wearable technology (e.g., body worn sensors) for health care and clinical use. Such wearable AI offers numerous advantages for individualizing diagnoses, treatment and monitoring for management of depression and anxiety in individuals with PD [84, 85].
Fig. 1
Non-pharmacological interventions for depression and anxiety in PD. Cognitive Behavioral Therapy (CBT), Electroconvulsive Therapy (ECT), Non-Invasive Brain Stimulation (NIBS, e.g., (r)TMS, tDCS), Light Therapy (LT); Exercise (Physical Activity). Solid lines: at least moderate evidence for an association, rough dashed lines (LT/NIBS/Exercise –anxiety): limited or no evidence, fine dashed line (ECT/LT –anxiety): not studied/not indicated.
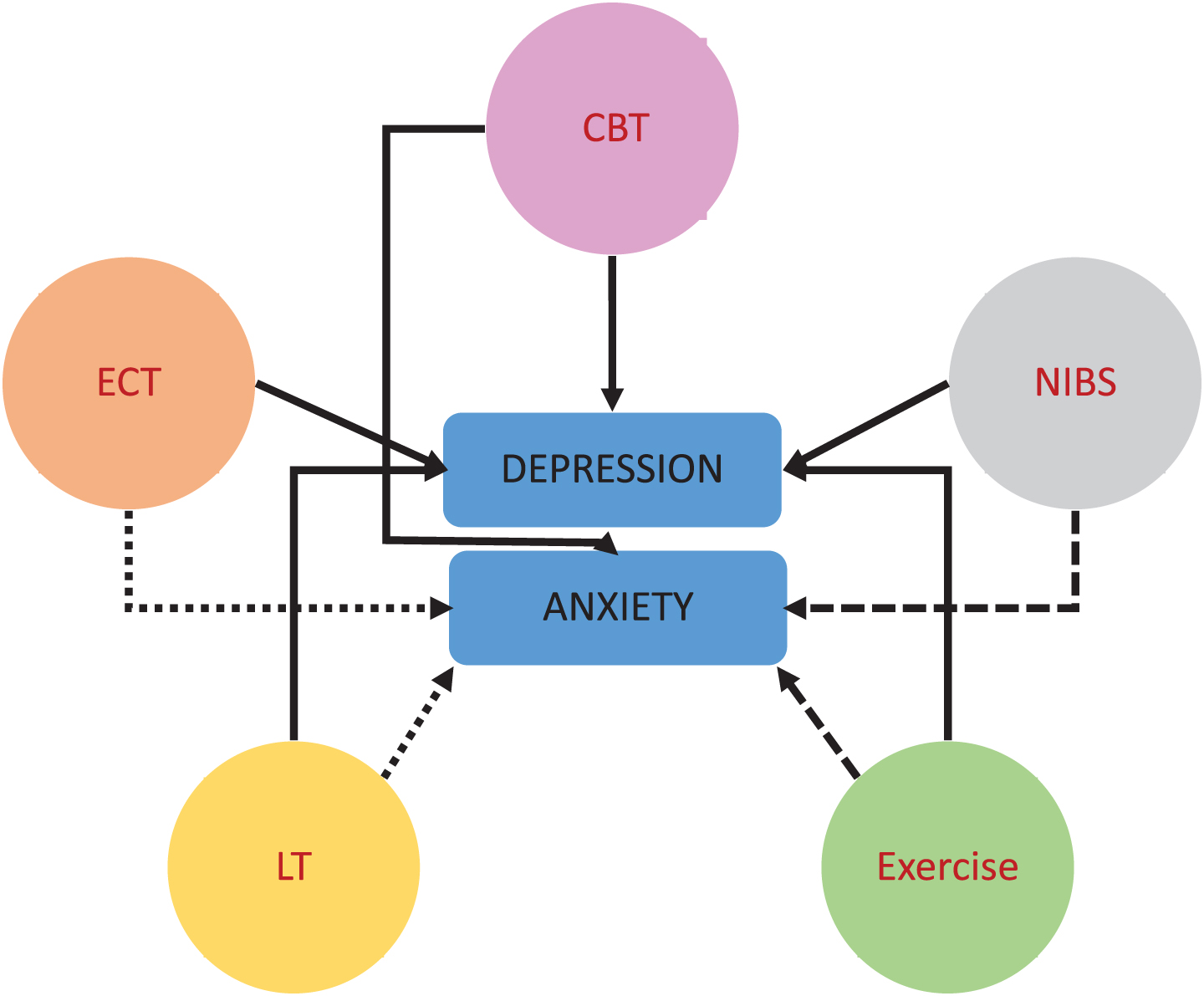
There is a need for PD-specific treatment protocols (e.g., for the use of CBT, ECT NIBS, LT, exercise), and knowledge on what type of non-pharmacological treatment is most effective. It is also noteworthy that more emphasis is needed on the interplay between gender, exercise, depression and anxiety in the rehabilitation of patients with PD and on studies in which people with PD are engaged as true partners or colleagues in their care and research on depression or anxiety [86, 87], in which people living with PD administer non-pharmacological treatments or are even involved in the research as patient-scientists, rather than merely as “subjects” or “objects of the research” [87]. It is plausible that non-pharmacological shared decision making or participatory research approaches may favorably affect anxiety in PD [88]; however, research has rarely examined this. This knowledge may in the end be used to develop evidence-based guidelines for treating anxiety and depressive disorders in PD.
Although research demonstrates that female sex is associated with a higher risk of depression in PD [23], there is a lack of literature on sex-associated differential effects of non-pharmacological interventions in PD. In the general population, a meta-analysis showed no association between sex and the effect of CBT for the treatment of depression [13]. In contrast, TMS may show larger antidepressant effects in females with MDD [89]. Research on PD response to exercise rarely fully considers sex effects (for review, see Subramanian [90]).While some sex specific recommendations for exercise in PD have been proposed, these are not related to mood outcomes [91]. Overall, knowledge about possible differential sex response in PD is still in its early infancy. Robust, longitudinal and controlled trials are needed to scientifically evaluate possible sex differences in response to non-pharmacological interventions for anxiety and depression in PD.
Besides the mentioned main intervention approaches, novel treatment approaches may offer further benefits for individuals with PD experiencing depression or anxiety symptomatology. For example, there may be potential synergistic effects of NIBS and behavioral interventions (e.g., NIBS combined with cognitive training [92]; or exercise [93]). Also, multimodal body awareness training combining physical therapy/exercise, ACT, and CBT components has shown benefit on emotional wellbeing [17, 24]. Other promising approaches may be high intensity interval training [94], acupuncture [95], and vagal nerve stimulation [96], but this needs further study.
CONCLUSION
Overall, it can be concluded that non-pharmacological, non-invasive interventions offer promising avenues for treating anxiety and depression in individuals with PD and may be safe and potentially effective alternative or augmentative treatments to pharmacological and neurosurgical approaches. Effective treatment options for depressive and anxiety disorders in the general population seem to translate well to PD, regarding, e.g., side-effects (BLT, NIBS, ECT) and feasibility (CBT, physical exercise), but more research is needed to tailor these treatment options towards PD-specific affective symptoms.
ACKNOWLEDGMENTS
The authors have no acknowledgments to report.
FUNDING
This work was supported in part by the Dutch Parkinson Patient Association (ParkinsonVereniging, grant number: 2019-R05), ParkinsonNL (PR0184), the Dutch Brain Foundation (Hersenstichting, grant number DR2019-00311) and ZonMw (grant number: 10390052210003).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Weintraub D ((2020) ) Management of psychiatric disorders in Parkinson’s disease: Neurotherapeutics-movement disorders therapeutics. Neurotherapeutics 17: , 1511–1524. |
[2] | Heinzel S , Berg D , Gasser T , Chen H , Yao C , Postuma RB , MDS Task Force on the Definition of Parkinson’s Disease ((2019) ) Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord 34: , 1464–1470. |
[3] | Carey G , Görmezo?lu M , de Jong JJA , Hofman PAM , Backes WH , Dujardin K , Leentjens AFG ((2021) ) Neuroimaging of anxiety in Parkinson’s disease: A systematic review. Mov Disord 36: , 327–339. |
[4] | Vriend C , Pattij T , van der Werf YD , Voorn P , Booij J , Rutten S , Berendse HW , van den Heuvel OA ((2014) ) Depression and impulse control disorders in Parkinson’s disease: Two sides of the same coin? . Neurosci Biobehav Rev 38: , 60–71. |
[5] | Zhuo C , Xue R , Luo L , Ji F , Tian H , Qu H , Lin X , Jiang R , Tao R ((2017) ) Efficacy of antidepressive medication for depression in Parkinson disease: A network meta-analysis. Medicine (Baltimore) 96: , e6698. |
[6] | Pontone GM , Mills KA ((2021) ) Optimal treatment of depression and anxiety in Parkinson’s disease. Am J Geriatr Psychiatry 29: , 530–540. |
[7] | Hayes SC , Luoma JB , Bond FW , Masuda A , Lillis J ((2006) ) Acceptance and commitment therapy: Model, processes and outcomes. Behav Res Ther 44: , 1–25. |
[8] | Carey G , Lopes R , Moonen AJH , Mulders AEP , de Jong JJA , Kuchcinski G , Defebvre L , Kuijf ML , Dujardin K , Leentjens AFG ((2023) ) Cognitive behavioral therapy for anxiety in Parkinson’s disease induces functional brain changes. J Parkinsons Dis 13: , 93–103. |
[9] | NICE, Mental health, behavioural and neurodevelopmental conditions.https://www.nice.org.uk/guidance/conditionsand-diseases/mental-health-behavioural-and-neurodevelopmental-conditions |
[10] | Cuijpers P , Cristea IA , Karyotaki E , Reijnders M , Huibers MJ ((2016) ) How effective are cognitive behavior therapies for major depression and anxiety disorders? A meta-analytic update of the evidence. World Psychiatry 15: , 245–258. |
[11] | Van Dis EA , Van Veen SC , Hagenaars MA , Batelaan NM , Bockting CL , Van Den Heuvel RM , Engelhard IM ((2020) ) Long-term outcomes of cognitive behavioral therapy for anxiety-related disorders: A systematic review and meta-analysis. JAMA Psychiatry 77: , 265–273. |
[12] | Cuijpers P , Sijbrandij M , Koole SL , Andersson G , Beekman AT , Reynolds Iii CF ((2013) ) The efficacy of psychotherapy and pharmacotherapy in treating depressive and anxiety disorders: A meta-analytic update of the evidence. World Psychiatry 12: , 137–148. |
[13] | Cuijpers P , Karyotaki E , Reijnders M , Huibers MJ ((2018) ) Who benefits from psychotherapies for adult depression? A meta-analytic update of the evidence. Cogn Behav Ther 47: , 91–106. |
[14] | Saunders R , Buckman JE , Stott J , Leibowitz J , Aguirre E , John A , Pilling S ((2021) ) Older adults respond better to psychological therapy than working-age adults: Evidence from a large sample of mental health service attendees. J Affect Disord 294: , 85–93. |
[15] | Orgeta V , Leung P , del-Pino-Casado R , Qazi A , Orrell M , Spector AE , Methley AM ((2022) ) Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Syst Rev 4: , CD009125. |
[16] | Hong CT , Tan S , Huang TW ((2021) ) Psychotherapy for the treatment of anxiety and depression in patients with Parkinson disease: A meta-analysis of randomized controlled trials. J Am Med Direct Assoc 22: , 2289–2295. |
[17] | Ghielen I , Rutten S , Boeschoten RE , Houniet-de Gier M , van Wegen EE , van den Heuvel OA , Cuijpers P ((2019) ) The effects of cognitive behavioral and mindfulness-based therapies on psychological distress in patients with multiple sclerosis, Parkinson’s disease and Huntington’s disease: Two meta-analyses. J Psychosom Res 122: , 43–51. |
[18] | Moonen AJ , Mulders AE , Defebvre L , Duits A , Flinois B , Köhler S , Leentjens AF ((2021) ) Cognitive behavioral therapy for anxiety in Parkinson’s disease: A randomized controlled trial. Mov Disord 36: , 2539–2548. |
[19] | Dissanayaka NN , Pye D , Mitchell LK , Byrne GJ , O’Sullivan JD , Marsh R , Pachana NA ((2017) ) Cognitive behavior therapy for anxiety in Parkinson’s disease: Outcomes for patients and caregivers. Clin Gerontol 40: , 159–171. |
[20] | Starkstein SE , Dragovic M , Dujardin K , Marsh L , Martinez-Martin P , Pontone GM , Richard IH , Weintraub D , Leentjens AF ((2014) ) Anxiety has specific syndromal profiles in Parkinson disease: A data-driven approach. Am J Geriatr Psychiatry 22: , 1410–1417. |
[21] | Dissanayaka NN , Forbes EJ , Perepezko K , Leentjens AFG , Dobkin RD , Dujardin K , Pontone GM ((2022) ) Phenomenology of atypical anxiety disorders in Parkinson’s disease: A systematic review. Am J Geriatr Psychiatry 30: , 1026–1050. |
[22] | Brown RG , Landau S , Hindle JV , Playfer J , Samuel M , Wilson KC , Hurt CS , Anderson RJ , Carnell J , Dickinson L , Gibson G , van Schaick R , Sellwood K , Thomas BA , Burn DJ ((2011) ) Depression and anxiety related subtypes in Parkinson’s disease. J Neurol Neurosurg Psychiatry 82: , 803–809. |
[23] | Buoli M , Caldiroli A , Altamura AC ((2016) ) Psychiatric conditions in Parkinson disease: A comparison with classical psychiatric disorders. J Geriatr Psychiatry Neurol 29: , 72–91. |
[24] | Ghielen I , van Wegen EE , Rutten S , de Goede CJ , Houniet-de Gier M , Collette EH , van den Heuvel OA ((2017) ) Body awareness training in the treatment of wearing-off related anxiety in patients with Parkinson’s disease: Results from a pilot randomized controlled trial. J Psychosom Res 103: , 1–8. |
[25] | Dobkin RD , Mann SL , Weintraub D , Rodriguez KM , Miller RB , St.Hill L , Interian A ((2021) ) Innovating Parkinson’s care: A randomized controlled trial of telemedicine depression treatment. Mov Disord 36: , 2549–2558. |
[26] | Zheng HB , Liu B , Shen J , Xie F , Ji QM , Zhu XY ((2022) ) Non-invasive brain stimulation for treating psychiatric symptoms in Parkinson’s disease: A systematic review and meta-analysis. J Clin Neurosci 106: , 83–90. |
[27] | Zhang X , Jing F , Liu Y , Tang J , Hua X , Zhu J , Tuo H , Lin Q , Gao P , Liu W ((2023) ) Personalized stimulation protocols and home-based treatments. Front Aging Neurosci 14: , 1065126. |
[28] | Jannati A , Oberman LM , Rotenberg A , Pascual-Leone A ((2023) ) Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology 48: , 191–208. |
[29] | Chan MMY , Yau SSY , Han YMY ((2021) ) The neurobiology of prefrontal transcranial direct current stimulation (tDCS) in promoting brain plasticity: A systematic review and meta-analyses of human and rodent studies. Neurosci Biobehav Rev 125: , 392–416. |
[30] | Strafella AP , Paus T , Barrett J , Dagher A ((2001) ) Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 21: , RC157. |
[31] | Strafella AP , Paus T , Fraraccio M , Dagher A ((2003) ) Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain 126: , 2609–2615. |
[32] | Fregni F , Santos CM , Myczkowski ML , Rigolino R , Gallucci-Neto J , Barbosa ER , Valente KD , Pascual-Leone A , Marcolin MA ((2004) ) Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 75: , 1171–1174. |
[33] | Pal E , Nagy F , Aschermann Z , Balazs E , Kovacs N ((2010) ) The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson’s disease: A randomized, double-blind, placebo-controlled study. Mov Disord 25: , 2311–2317. |
[34] | Chen J , He P , Zhang Y , Gao Y , Qiu Y , Li Y , Zhang Q , Wang L , Huang Z , Zhao J , Nie K , Wang L ((2021) ) Non-pharmacological treatment for Parkinson disease patients with depression: A meta-analysis of repetitive transcranial magnetic stimulation and cognitive-behavioral treatment. Int J Neurosci 131: , 411–424. |
[35] | Cheng B , Zhu T , Zhao W , Sun L , Shen Y , Xiao W , Zhang S ((2022) ) Effect of theta burst stimulation-patterned rTMS on motor and nonmotor dysfunction of Parkinson’s disease: A systematic review and metaanalysis. Front Neurol 12: , 762100. |
[36] | Benninger DH , Berman BD , Houdayer E , Pal N , Luckenbaugh DA , Schneider L , Miranda S , Hallett M ((2011) ) Intermittent theta-burst transcranial magnetic stimulation for treatment of Parkinson disease. Neurology 76: , 601–609. |
[37] | Trung J , Hanganu A , Jobert S , Degroot C , Mejia-Constain B , Kibreab M , Bruneau MA , Lafontaine AL , Strafella A , Monchi O ((2019) ) Transcranial magnetic stimulation improves cognition over time in Parkinson’s disease. Parkinsonism Relat Disord 66: , 3–8. |
[38] | Lefaucheur JP , Antal A , Ayache SS , Benninger DH , Brunelin J , Cogiamanian F , Cotelli M , De Ridder D , Ferrucci R , Langguth B , Marangolo P , Mylius V , Nitsche MA , Padberg F , Palm U , Poulet E , Priori A , Rossi S , Schecklmann M , Vanneste S , Ziemann U , Garcia-Larrea L , Paulus W ((2017) ) Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 128: , 56–92. |
[39] | Fregni F , El-Hagrassy MM , Pacheco-Barrios K , Carvalho S , Leite J , Simis M , Brunelin J , Nakamura-Palacios EM , Marangolo P , Venkatasubramanian G , San-Juan D , Caumo W , Bikson M , Brunoni AR , Neuromodulation Center Working Group ((2021) ) Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol 24: , 256–313. |
[40] | Cheng YC , Kuo PH , Su MI , Huang WL ((2022) ) The efficacy of non-invasive, non-convulsive electrical neuromodulation on depression, anxiety and sleep disturbance: A systematic review and meta-analysis. Psychol Med 52: , 801–812. |
[41] | Hadoush H , Alqudah A , Banihani SA , Al-Jarrah M , Amro A , Aldajah S ((2021) ) Melatonin serum level, sleep functions, and depression level after bilateral anodal transcranial direct current stimulation in patients with Parkinson’s disease: A feasibility study. Sleep Sci 14: , 25–30. |
[42] | Chikatimalla R , Dasaradhan T , Koneti J , Cherukuri SP , Kalluru R , Gadde S ((2022) ) Depression in Parkinson’s disease: A narrative review. Cureus 14: , e27750. |
[43] | Schurgers G , Walter S , Pishva E , Guloksuz S , Peerbooms O , Incio LR , Arts BMG , Kenis G , Rutten BPF ((2022) ) Longitudinal alterations in mRNA expression of the BDNF neurotrophin signaling cascade in blood correlate with changes in depression scores in patients undergoing electroconvulsive therapy. Eur Neuropsychopharmacol 63: , 60–70. |
[44] | Nuninga JO , Mandl RCW , Boks MP , Bakker S , Somers M , Heringa SM , Nieuwdorp W , Hoogduin H , Kahn RS , Luijten P , Sommer IEC ((2020) ) Volume increase in the dentate gyrus after electroconvulsive therapy in depressed patients as measured with 7T. Mol Psychiatry 25: , 1559–1568. |
[45] | Kirov G , Jauhar S , Sienaert P , Kellner CH , McLoughlin DM ((2021) ) Electroconvulsive therapy for depression: 80 years of progress. Br J Psychiatry 219: , 594–597. |
[46] | Takamiya A , Seki M , Kudo S , Yoshizaki T , Nakahara J , Mimura M , Kishimoto T ((2021) ) Electroconvulsive therapy for Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 36: , 50–58. |
[47] | Borisovskaya A , Bryson WC , Buchholz J , Samii A , Borson S ((2016) ) Electroconvulsive therapy for depression in Parkinson’s disease: Systematic review of evidence and recommendations. Neurodegener Dis Manag 6: , 161–176. |
[48] | Rutten S, V C , van den Heuvel OA , Smit JH , Berendse HW , van der Werf D ((2012) ) Bright light therapy in Parkinson’s disease: An overview of the background and evidence. Parkinsons Dis 2012: , 767105. |
[49] | Videnovic A , Golombek D ((2017) ) Circadian dysregulation in Parkinson’s disease. Neurobiol Sleep Circadian Rhythms 2: , 53–58. |
[50] | Asadpoordezaki Z , Coogan AN , Henley BM ((2023) ) Chronobiology of Parkinson’s disease: Past, present and future. Eur J Neurosci 57: , 178–200. |
[51] | Obayashi K , Saeki K , Yamagami Y , Kurumatani N , Sugie K , Kataoka H ((2021) ) Circadian activity rhythm in Parkinson’s disease: Findings from the PHASE study. Sleep Med 85: , 8–14. |
[52] | Bordet R , D D , Brique S , Touitou Y , Guieu JD , Libersa C , Destèe A ((2003) ) Study of circadian melatonin secretion pattern at different stages of Parkinson’s disease. Clin Neuropharmacol 26: , 65–72. |
[53] | Raupach AK , Ehgoetz Martens KA , Memarian N , Zhong G , Matar E , Halliday GM , Grunstein R , Lewis S ((2020) ) Assessing the role of nocturnal core body temperature dysregulation as a biomarker of neurodegeneration. J Sleep Res 29: , e12939. |
[54] | Sanchez-Barcelo EJ , Rueda N , Mediavilla MD , Martinez-Cue C , Reiter RJ ((2017) ) Clinical uses of melatonin in neurological diseases and mental and behavioural disorders. Curr Med Chem 24: , 3851–3878. |
[55] | Videnovic A , Noble C , Reid KJ , Peng J , Turek FW , Marconi A , Rademaker AW , Simuni T , Zadikoff C , Zee PC ((2014) ) Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol 71: , 463–469. |
[56] | Sun W, Y J , Wu J , Ma H ((2022) ) Efficacy and safety of light therapy as a home treatment for motor and non-motor symptoms of Parkinson Disease: A meta-analysis. Med Sci Monit 28: , e935074. |
[57] | Raymackers JM , A M , Baey E , Vanneste M , Evrard F ((2019) ) Bright light therapy with a head-mounted device for anxiety, depression, sleepiness and fatigue in patients with Parkinson’s disease. Acta Neurol Belg 119: , 607–613. |
[58] | Rutten S,V C , Smit JH , Berendse HW , van Someren EJW , Hoogendoorn AW , Twisk JWR , van der Werf YD , van den Heuvel OA ((2019) ) Bright light therapy for depression in Parkinson disease: A randomized controlled trial. Neurology 92: , e1145–e1156. |
[59] | Videnovic A,K E , Wang W , Marconi A , Kuhta T , Zee PC ((2017) ) Timed light therapy for sleep and daytime sleepiness associated with Parkinson disease: A randomized clinical trial. JAMA Neurol 74: , 411–418. |
[60] | Willis GL , B J , Freelance CB ((2018) ) Polychromatic light exposure as a therapeutic in the treatment and management of Parkinson’s disease: A controlled exploratory trial. Front Neurol 9: , 741. |
[61] | Zhao JL , Jiang WT , Wang X , Cai ZD , Liu ZH , Liu GR ((2020) ) Exercise, brain plasticity, and depression. CNS Neurosci Ther 26: , 885–895. |
[62] | Anderson E , Shivakumar G ((2013) ) Effects of exercise and physical activity on anxiety. Front Psychiatry 4: , 27. |
[63] | Fisher BE , Li Q , Nacca A , Salem GJ , Song J , Yip J , Hui JS , Jakowec MW , Petzinger GM ((2013) ) Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson’s disease. Neuroreport 24: , 509–514. |
[64] | Sacheli MA , Neva JL , Lakhani B , Murray DK , Vafai N , Shahinfard E , English McCormick S , Dinelle K , Neilson N , McKenzie J , Schulzer M , McKenzie DC , Appel-Cresswell S , McKeown MJ , Boyd LA , Sossi V , Stoessl AJ ((2019) ) Exercise increases caudate dopamine release and ventral striatal activation in Parkinson’s disease. Mov Disord 34: , 1891–1900. |
[65] | Kwilasz AJ , Grace PM , Serbedzija P , Maier SF , Watkins LR , Malczynska-Sims P , Chalimoniuk M , Wronski Z , Marusiak J , Sulek A ((2015) ) The therapeutic potential of interleukin-10 in neuroimmune diseases. Neuropharmacology 69: , 55–69. |
[66] | Schaeffer E , Roeben B , Granert O , Hanert A , Liepelt-Scarfone I , Leks E , Otterbein S , Saraykin P , Busch JH , Synofzik M , Stransky E , Bartsch T , Berg D ((2022) ) Effects of exergaming on hippocampal volume and brain-derived neurotrophic factor levels in Parkinson’s disease. Eur J Neurol 29: , 441–449. |
[67] | Sehm B , Taubert M , Conde V , Weise D , Classen J , Dukart J , Draganski B , Villringer A , Ragert P ((2014) ) Structural brain plasticity in Parkinson’s disease induced by balance training. Neurobiol Aging 35: , 232–239. |
[68] | Johansson ME , Cameron IGM , Van der Kolk NM , de Vries NM , Klimars E , Toni I , Bloem BR , Helmich RC ((2022) ) Aerobic exercise alters brain function and structure in Parkinson’s disease: A randomized controlled trial. Ann Neurol 91: , 203–216. |
[69] | Petzinger GM , Holschneider DP , Fisher BE , McEwen S , Kintz N , Halliday M , Toy W , Walsh JW , Beeler J , Jakowec MW ((2015) ) The effects of exercise on dopamine neurotransmission in Parkinson’s disease: Targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast 1: , 29–39. |
[70] | Hirsch MA , van Wegen EEH , Newman MA , Heyn PC ((2018) ) Exercise-induced increase in brain-derived neurotrophic factor in human Parkinson’s disease: A systematic review and meta-analysis. Transl Neurodegener 7: , 7. |
[71] | Li JA , Loevaas MB , Guan C , Goh L , Allen NE , Mak MKY , Lv J , Paul SS ((2023) ) Does exercise attenuate disease progression in people with Parkinson’s disease? A systematic review with meta-analysis. Neurorehabil Neural Repair 37: , 328–352. |
[72] | Szymura J , Kubica J , Wiecek M , Pera J ((2020) ) The immunomodulatory effect of systematic exercise in older adults and people with Parkinson’s disease. J Clin Med 9: , 184. |
[73] | Sajatovich M , Ridgel AL , Walter EM , Color-Zimmermann K , Ramsey RK , Welter E , Gunzler SA , Whitney CM , Walter BL ((2017) ) A randomized trial of individual versus group-format exercise and self-management in individuals with Parkinson’s disease and comorbid depression. Patient Prefer Adherence 11: , 965–973. |
[74] | Landers MR , Navalta JW , Murtishaw AS , Kinney JW , Richardson SP ((2019) ) A high-intensity exercise boot camp for persons with Parkinson disease: A phase II, pragmatic, randomized clinical trial of feasibility, safety, signal of efficacy, and disease mechanisms. J Neurol Phys Ther 43: , 12–25. |
[75] | Cooney GM , Dwan K , Greig CA , Lawlor DA , Rimer J , Waugh FR , McMurdo M , Mead GE ((2013) ) Exercise for depression. Cochrane Database Syst Rev 2013: , CD004366. |
[76] | Ernst M , Folkerts AK , Gollan R , Lieker E , Caro-Valenzuela J , Adams A , Cryns N , Monsef I , Dresen A , Roheger M , Eggers C , Skoetz N , Kalbe E (2023) Physical exercise for people with Parkinson's disease: A systematic review and network meta-analysis. Cochrane Database Syst Rev 1, CD013856. |
[77] | Schenkman M , Moore CG , Kohrt WM , Hall DA , Delitto A , Comella CL , Josbeno DA , Christiansen CL , Berman BD , Kluger BM , Melanson EL , Jain S , Robichaud JA , Poon C , Corcos DM ((2018) ) Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol 75: , 219–226. |
[78] | Bonsavage N , Brown A , Davis T ((2022) ) Regular exercise regimen associated with reduced prevalence of depression in Parkinson’s disease. Mov Disord 37: , 367S366–S367. |
[79] | Feller D , Fox I , Gozzer P , Trentin F , Papola D ((2023) ) Exercise for depressive symptoms in Parkinson disease: A systematic review and meta-analysis of randomized controlled trials. Arch Phys Med Rehabil 104: , 331–339. |
[80] | Wang Y , Sun X , Li F , Li Q , Jin Y ((2022) ) Efficacy of non-pharmacological interventions for depression in individuals with Parkinson’s disease: A systematic review and network meta-analysis. Front Aging Neurosci 14: , 1050715. |
[81] | Abuoaf R , AlKaabi R , Mohamed Saleh A , Zerough U , Hartley T , van Niekerk SM , Khalil H , Morris LD ((2023) ) The effect of physical exercise on anxiety in people with Parkinson’s disease: A systematic review of randomized control trials. Neurorehabilitation 52: , 387–402. |
[82] | Wu PL , Lee M , Wu SL , Ho HH , Chang MH , Lin HS , Huang TT ((2021) ) Effects of home-based exercise on motor, non-motor symptoms and health-related quality of life in Parkinson’s disease patients: A randomized controlled trial. Jpn J Nurs Sci 18: e12418. |
[83] | Ferreira RM , Alves W , de Lima TA , Alves TGG , Filho P , Pimentel CP , Sousa EC , Cortinhas-Alves EA ((2018) ) The effect of resistance training on the anxiety symptoms and quality of life in elderly people with Parkinson’s disease: A randomized controlled trial. Arq Neuropsiquiatr 76: , 499–506. |
[84] | Abd-Alrazaq A , AlSaad R , Shuweihdi F , Ahmed A , Aziz S , Sheikh J ((2023) ) Systematic review and meta-analysis of performance of wearable artificial intelligence in detecting and predicting depression. NPJ Digit Med 6: , 84. |
[85] | Meinert E , Milne-Ives M , Chaudhuri KR , Harding T , Whipps J , Whipps S , Carroll C ((2022) ) The impact of a digital artificial intelligence system on the monitoring and self-management of nonmotor symptoms in people with Parkinson disease: Proposal for a phase 1 implementation study. JMIR Res Protoc 11: , e40317. |
[86] | Hirsch MA , Sanjak M , Englert D , Iyer SS , Quinlan MM ((2014) ) Parkinson patients as partners in care. Parkinsonism Relat Disord 20: , S174–S179. |
[87] | Buetow S , Martinez-Martin P , Hirsch MA , Okun M ((2016) ) Beyond patient-centred care: Person-centred care for Parkinson’s disease. NPJ Parkinsons Dis 2: , 16019. |
[88] | Grosset KA , Grosset DG ((2005) ) Patient-perceived involvement and satisfaction in Parkinson’s disease: Effect on therapy decisions and quality of life. Mov Disord 20: , 616–619. |
[89] | Kedzior KK , Azorina V , Reitz SK ((2014) ) More female patients and fewer stimuli per session are associated with the short-term antidepressant properties of repetitive transcranial magnetic stimulation (rTMS): A meta-analysis of 54 sham-controlled studies published between 1997-2013. Neuropsychiatr Dis Treat 10: , 727–756. |
[90] | Subramanian I , Mathur S , Oosterbaan A , Flanagan R , Keener AM , Moro E ((2022) ) Unmet needs of women living with Parkinson’s disease: Gaps and controversies. Mov Disord 37: , 444–455. |
[91] | Rigby BR , Davis RW ((2018) ) Should exercise be prescribed differently between women and men? An emphasis on women diagnosed with Parkinson’s disease. Front Physiol 9: , 1040. |
[92] | Manenti R , Cotelli MS , Cobelli C , Gobbi E , Brambilla M , Rusich D , Alberici A , Padovani A , Borroni B , Cotelli M ((2018) ) Transcranial direct current stimulation combined with cognitive training for the treatment of Parkinson disease: A randomized, placebo-controlled study. Brain Stimul 11: , 1251–1262. |
[93] | Beretta VS , ConceiÇão NR , Nóbrega-Sousa P , Orcioli-SilvaD , Dantas L , Gobbi LTB , Vitório R ((2020) ) Transcranial directcurrent stimulation combined with physical or cognitive training inpeople with Parkinson’s disease: A systematic review. JNeuroeng Rehabil 17: , 74. |
[94] | Gomes ESA , Van den Heuvel OA , Rietberg MB , De Groot V , Hirsch MA , Van de Berg WDJ , Jaspers RT , Vriend C , Vanbellingen T , Van Wegen EEH ((2023) ) (HIIT-The Track) High-intensity interval training for people with Parkinson’s disease: Individual response patterns of (non-)motor symptoms and blood-based biomarkers-a crossover single-case experimental design. Brain Sci 13: , 849. |
[95] | Li Q , Wu C , Wang X , Li Z , Hao X , Zhao L , Li M , Zhu M ((2022) ) Effect of acupuncture for non-motor symptoms in patients with Parkinson’s disease: A systematic review and meta-analysis. Front Aging Neurosci 14: , 995850. |
[96] | Sigurdsson HP , Hunter H , Alcock L , et al. ((2023) ) Safety and tolerability of adjunct non-invasive vagus nerve stimulation in people with Parkinson’s: A study protocol. BMC Neurol 23: , 58. |




