Cost-Effectiveness Analyses of Non-Pharmacological and Non-Surgical Interventions in Idiopathic Parkinson’s Disease: A Systematic Review
Abstract
Background:
Interest in non-pharmacological/non-surgical interventions to treat Parkinson’s disease (PD) has substantially increased. Although a few health-economic studies have been conducted, summary information on the cost-effectiveness is still scarce.
Objective:
To give an overview of cost-effectiveness analyses (CEA) focusing on non-pharmacological/non-surgical interventions in PD patients.
Methods:
A systematic literature search was conducted in five databases. Studies were included that provided cost-effectiveness analysis (CEA) or cost-utility analysis (CUA) of non-pharmacological/non-surgical interventions in PD patients. Study quality was assessed with the Drummond and CHEERS 2022 checklists, respectively for economic evaluation.
Results:
N = 9 studies published between 2012–2023 were identified. Most studies undertook a CUA (n = 5); n = 3 reported a combination of CEA and CUA, and n = 1 a pure CEA. Most studies (n = 6) examined physical exercise. The CEA studies identified additional costs of 170€ –660€ for the improvement of one single unit of a clinical outcome and savings of 18.40€ –22.80€ per score gained as measured with established instruments. The four studies that found significant quality of life benefits show large variations in the incremental cost effectiveness ratio (ICER) of 3,220€ –214,226€ per quality-adjusted life year (QALY); notably interventions were heterogenous regarding content and intensity.
Conclusions:
Despite increasing numbers of non-pharmacological/non-surgical intervention trials in PD patients, health-economic evaluations are rare. The examined intervention types and health-economic results vary greatly. Together with the heterogeneity of the health-economic studies these factors limit the conclusions that can be drawn. Further research and a standardization of methods is needed to allow decision makers to make meaningful interpretations, and to allocate scarce resources.
INTRODUCTION
Neurological disorders including Parkinson’s disease (PD) are the third most frequent cause of disability and early death in the EU [1]. According to the 2016 Global Burden of Diseases study neurological disorders were the primary cause of disability-adjusted life-years, and the total number of disability-adjusted life-years allocatable to neurological disorders in the EU was 21.0 million.
Despite optimal pharmacological adjustment, the majority of PD patients experience a broad range of quality of life (QoL)-limiting motor (e.g., bradykinesia, rigor, tremor, gait impairment) and non-motor symptoms (e.g., hyposmia, cognitive impairment, depression) [2, 3]. This places a huge burden on the affected patients, their families, and caregivers.
PD therapy guidelines comprise pharmacotherapy and deep brain stimulation (DBS) [4, 5]. Furthermore, non-pharmacological or non-surgical approaches have attracted increasing interest as part of the therapy regime. They comprise, e.g., physiotherapy, physical activity, occupational therapy, speech therapy, and cognitive and behavioral approaches [4]. Evidence for the efficacy of these interventions is growing [4–10].
However, a so far unresolved question is whether treatment options are cost-effective from a health-economic perspective (i.e., the degree to which an intervention is effective in relation to its costs). As policy makers and healthcare providers are concerned with resource allocation decisions, health-economic evaluations of effective interventions are of high relevance [11]. Health-economic evaluations can be conducted from different perspectives (e.g., payer or societal perspective). Depending on the perspective, different types of cost data need to be collected. The broader the perspective (e.g., societal), the broader the collection of cost data (e.g., through collecting data on welfare loss due to caregiver abstinence from work).
For example, a study with early PD patients comparing costs and utilities before and after starting drug treatment demonstrated that it improved utilities, and an Incremental Cost Effectiveness Ratio (ICER) of € 45,259 per quality adjusted life year (QALY) was shown [12]. Notably, a systematic review from 2019 [13] including 26 pharmacological trials concluded that health-economic studies are still rare and more studies are needed to clearly define the cost-effectiveness of these treatments. Even less data is available on health-economic evaluations of non-pharmacological interventions in PD. Afentou et al. [14] conducted a systematic review covering health-economic evaluation of pharmacological and non-pharmacological PD treatments. Within the non-pharmacological/non-surgical studies, eight studies were included covering physical exercise and occupational therapies. The authors summarized that among early-stage treatments Tai Chi dominated all physical interventions but its cost-effectiveness should be further explored in relation to its duration, intensity, and frequency. Notably, the literature search covered studies published between 2010 and 2018, so that older studies might have been missed, and an update of studies lasting until 2023 is reasonable in this rapidly developing research field. A systematic review addressing specifically health-economic evaluations of non-pharmacological interventions in PD is missing so far.
Against this background, the aim of this systematic review is to give an updated overview of cost-effectiveness analyses (CEA) focusing specifically on non-pharmacological or non-surgical interventions in PD patients, to evaluate the quality of the identified studies, and to evaluate their economic outcomes. Following the “PICO” format [15], this systematic review focuses on idiopathic PD patients (P = population), treated with a non-pharmacological and non-surgical intervention (I = intervention), compared with usual care and/or active control groups (C = comparison), and its cost-effectiveness (O = outcome).
MATERIALS AND METHODS
The systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [16]. It is registered in the International Prospective Register of Systematic Reviews (PROSPERO; CRD42020136015). In this registration the original, broad search strategy was lined out. However, after precise full-text examination non-comparative studies were discarded due to a lack of homogeneity and, thus, the impossibility to make comparisons between the health-economic results. Therefore, it was decided for this review to focus on the comparative study designs CEA or cost utility analyses (CUA).
Keywords, databases, and review process
To identify eligible studies, we searched the databases MEDLINE Ovid, EMBASE, PsycINFO, CENTRAL, and the Centre for Reviews and Dissemination up to July 21, 2023 (including two update literature searches in January 2022 and July 2023). English and German articles were included. Additionally, the included studies in previously published systematic reviews [8, 17–29] were identified via hand-search and checked for eligibility.
Databases were searched using a combination of keywords (Supplementary Table 1). Keywords were clustered into three categories: study participants, intervention types, and health-economic evaluations. Based on previous research on evidence-based medicine recommendations for treating motor and non-motor symptoms [6, 30], we identified seven different categories of non-pharmacological interventions covering cognitive interventions including transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS); music, art and drama; psychotherapy; language and speech therapy; physical activity; gaming; and other interventions such as occupational therapy.
Titles and abstracts were screened according to the eligibility criteria using the software Covidence [31]. Afterwards, the full-text articles of the studies were then evaluated with regard to the final decision on inclusion in the systematic review. The process was independently done by two authors (DHN, AKF). In case of uncertainty a third author (EK) was consulted to discuss critical studies until consensus was reached on the abstract as well as full-text level.
Eligibility criteria
Study designs and publication type
Health-economic evaluations (including both trial-based and modeling studies) for non-pharmacological or non-surgical interventions in idiopathic PD patients were included. Studies that just stated that the intervention is cost-effective without providing an evaluation were not included.
Reviews and meta-analysis were excluded as well as editorials, letters to the editors, comments, conference posters and further conference contributions, study protocols, books and book chapters.
Study participants
Eligible patients were individuals of all sexes 50 years or older with a clinical diagnosis of idiopathic PD. Studies with patients having a diagnosis of atypical, genetic, or secondary Parkinsonism were excluded.
Types of interventions
Single and combined non-pharmacological/non-surgical approaches were considered. Additionally, interventions for both patients and relatives were included.
Patient-centered care approaches, nursing/care interventions/networks following a health services research approach which were included in the previously published review by Afentou et al. [14], studies targeting the evaluation of diagnostic instruments (e.g., neuroimaging techniques, test materials, experimental designs), and interventions only provided to PD relatives were excluded [32, 33].
Types of health-economic evaluations
Two types of health economic analyses were considered:
• CEA, i.e., comparing the incremental costs of the competing interventions and their incremental clinical outcome effects in natural units (e.g., points of blood pressure reduction) [34].
• CUA, as a variant of a CEA, i.e., comparing the incremental costs of a program to the incremental health improvement measured typically in QALY as a generic measure [34].
Health-economic outcomes
CEA and its variant CUA relate costs and benefits (outcomes) of interventions by dividing the difference in costs by the difference of the intervention effects. These ratios are called ICER (for CEA) and Incremental cost-utility ratio (ICUR; for CUA). ICER and ICUR can be used as guidance as to how cost-effective an intervention is deemed in relationship to its comparator (e.g., usual care). For example, if the new intervention is both more effective and costs less, the new intervention is considered to be dominant. If the new intervention is more effective but also costs more, the cost-effectiveness is determined by the willingness-to-pay of the chosen perspective (i.e., society, health politics or the Statutory Health Insurance). An evaluation on the cost-effectiveness of an intervention depends on increases realized in the outcome, e.g., PDQ-39 changes. This means that it is possible to provide information about how much money needs to be invested to gain a change in a specific clinical outcome.
CEA uses clinical effects to express benefits that are revealed as ICERs [35]. An ICER summarizes the costs per unit in a measurement instrument of the intervention compared with control groups. An ICER is derived by dividing the difference in total costs between the competing interventions (incremental cost) by the difference in a specific measure of health effect or outcome (incremental effect) [36].
In contrast, CUA uses generic measures such as QALYs derived from standardized questionnaires (e.g., EQ-5D).
To ensure comparability, the monetary values given in the included studies in different currencies were recalculated in Euro and inflated to 2022 by using the CCEMG-EPPI-Centre Cost Converter software (version 1.6).
Data extraction and synthesis
The following information was extracted from every included study: general information (i.e., authors and publication year, country, sample size), intervention details (i.e., intervention type, duration and frequency, comparator) and detailed information of the health-economic analysis (i.e., health-economic analysis type, perspective, costs included, health-economic outcomes). Standardized data selection forms were employed by two reviewers (DHN and AKF). In case of ambiguities, an external expert was asked for advice (DM, see Acknowledgments).
A formal meta-analysis could not be conducted due to the heterogenous intervention types and outcomes.
Quality assessment
For the assessment of methodological study quality in terms of study design, data collection and analysis of the included studies, the 10-item Drummond checklist for economic evaluations [34] and the 28-item Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist [37] were used. To ensure objectivity, two reviewers (DHN and AKF) independently evaluated the studies. In case of conflicts, an external expert was asked for advice (DM, see Acknowledgments).
RESULTS
Inclusion of studies
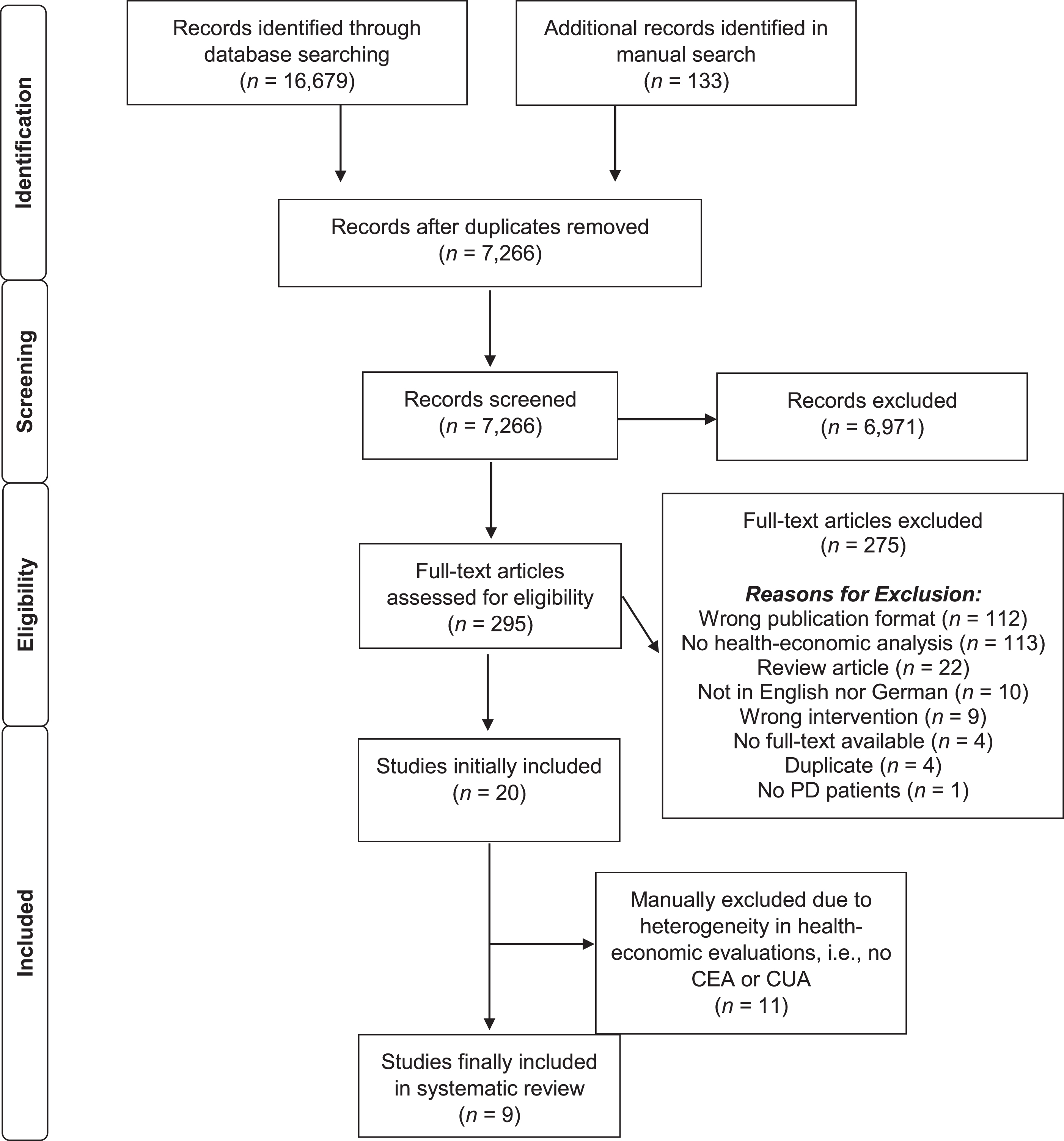
The systematic database literature search led to a total of 16,679 identified publications; additional 133 publications were collected through manual search. After removing duplicates, a total of 7,266 publications were screened. Potentially eligible full-text papers (N = 295) were retrieved for further consideration, of which 275 were excluded. Health-economic evaluations of eligible interventions were identified in 20 studies. Eleven studies were further excluded because of methodological heterogeneity, i.e., these studies did not include a CEA or CUA. Finally, nine studies were included in this systematic review (see Fig. 1 for the PRISMA flow chart and including reasons for exclusion).
Fig. 1
PRISMA flow diagram of the study selection process. CEA, cost-effectiveness analysis; CUA, cost-utility analysis; PD, Parkinson’s disease.
Study designs
All included studies (N = 9; Tables 1–3) were health-economic evaluations along-side a randomized controlled trial. The majority of these studies (4) were conducted in the UK [38–41], the others were conducted in Sweden [42], France [43], Australia [44], USA [45], or in the Netherlands [46]. Studies were published between 2012 and 2022.
Table 1
Key characteristics of studies including pure cost-effectiveness analyses
| Author (year; country) Sample size | Intervention (duration, frequency) | Comparator(s) | Economicstudy design (perspective) | Costs included | Health-economic results, ICER (value in Euro € inflated to 2022) |
| Canivet [43] (2016, F) N = 120 | Psychosocial support program and standard neurological care (12 months, quarterly 1 individual and 3 group sessions) | Usual care (12 months) | CEA (cost per score point gained on the UPDRS II and UPDRS III scale (Payer) | Intervention-related: Travel expenses Non-intervention- related: Medication use, health service use | ICER: |
| –22,80€ / per score point gained on the UPDRS II | |||||
| –18,40€ / per score point gained on the UPDRS III |
CEA, Cost-effectiveness analysis; ICER, Incremental cost-effectiveness ratio; UPDRS, Unified Parkinson’s Disease Rating Scale.
Table 2
Key characteristics of studies including pure cost-utility analyses
| Author (year; country) Sample size | Intervention (duration, frequency) | Comparator(s) | Economicstudy design (perspective) | Costs included | Health-economic results, ICER (value in Euro € inflated to2022) |
| Bogosian [38] (2022, UK) N = 60 | Mindfulness-based Intervention (8 weeks, 1 session/week) | Usual care | CUA (Payer) | Intervention-related: Intervention costs (no specification given) Non-intervention-related: - | ICER: 31.010 € / QALY |
| Xin [39] (2020, UK) N = 474 | Physiotherapy intervention for fall prevention (PDSAFE) (6 months, 12 sessions) | Usual care (plus a DVD) | CUA (Payer) | Intervention-related: Training, travel expenses, equipment, consumables Non-intervention-related: - | ICER: 152.240 € / QALY |
| Clarke [40] (2016, UK) N = 762 | Physiotherapy and occupational therapy (8 weeks, frequency N/S) | No therapy | CUA (Payer) | Intervention-related: Intervention costs Non-intervention-related: Medication use, health service use | ICER:4.680€ / QALY |
| Sturkenboom [46] (2015, NL) N = 191 | Occupational therapy (10 weeks, frequency N/S) | Usual care | CUA (Societal) | Intervention-related: Intervention costs, implementation costs | ICER: non-significant differences in costs and QALYs* |
| Non-intervention-related: Health service use, absence from work and informal care hours | *When society is willing to pay € 20.000 per QALY gained, the net benefit of the intervention for the caregiver is positive, with a probability of 95%. | ||||
| Fletcher [41] (2012, UK) N = 130 | Exercise intervention (10 weeks, 1 day/week) | Usual care | CUA (Payer) | Intervention-related: Training, travel expenses, equipment, consumables | ICER: dominates; i.e., less costly and clinically more effective |
| Non-intervention-related: Medication use, health service use |
CUA, Cost-utility analysis; ICER, Incremental cost-effectiveness ratio; N/S, not specified; QALY, Quality-adjusted life year.
Table 3
Key characteristics of studies including cost-effectiveness and cost-utility analyses
| Author (year; country) Sample size | Intervention(duration,frequency) | Comparator(s) | Economicstudy design (perspective) | Costs included | Health-economic results, ICER (value in Euro € inflated to2022) |
| Joseph [43] (2019, SE) N = 100 | Balance training program (HiBalance) (10 weeks, 3 sessions/week) | Usual care | CEA (cost per one unit increase in the Mini-BESTest (Balance performance) | Intervention-related: Time, training, travel expenses, equipment, consumables Non-intervention-related: – | ICER: 660 € /one unit increasein the Mini-BESTest(Balance performance) |
| CEA (cost per unit increase of gait velocity) | ICER: 170€ / per unit increase of gait velocity | ||||
| CUA (Payer) | ICER: 33.170€ / QALY | ||||
| Farag [44] (2016, AU) N = 231 | Falls prevention exercise program (6 months, 1 monthly exercise class and 2–4 home visits) | Usual care (plus a falls prevention booklet) (7 weeks, 3 days/week) | CEA (cost per fall prevented) CEA (cost per extra person avoiding mobility deterioration) CUA (Payer) | Intervention-related: Intervention costs, implementation costs, travel expenses Non-intervention-related: Medication use, health service use | ICER: 360€ / fall prevented 6.051€ / per extra person avoiding mobility deterioration 214.230€ / QALY |
| Li &Harmer [45] (2015, US) N = 176 | Tai Ji Quan (6 months, 2 times weekly) | C1: Stretching training C2: Resistance training (6 months, 2 times weekly) | CEA (cost per fall prevented) CUA (Payer) | Intervention-related: Intervention costs, implementation costs Non-intervention-related: Medication use, health service use, participant travel time | ICER: dominates; i.e., less costly and clinically more effective 3.220€ / QALY dominates |
C, Control group; CEA, Cost-effectiveness analysis; CUA, Cost-utility analysis; ICER, Incremental cost-effectiveness ratio.
Patient population
In total, N = 2244 PD patients were included in the studies. Mean age of the study samples ranged from 60 years [38] to 77 years [45]. Equal or more male than female patients were included (between 50% [38] and 65% [40] male patients), except for one study (30% male patients) [45]. All patients had a confirmed clinical PD diagnosis, whereas in 4 studies the UK Brain Bank Clinical Diagnosis Criteria was used as an inclusion criterion [38, 40, 43, 45]. Hoehn and Yahr (H&Y) stages ranged between I and V, whereby stage V was only defined as eligibility criteria in two studies [40, 46], and Bogosian et al. [38] did not report any H&Y stage at all. Mean disease duration ranged between 2.1 years [38] and 8.6 years [41].
Intervention and control groups
From the nine studies, six studies conducted a physical intervention. Four of these studies conducted physiotherapy [39, 41, 42, 44], one study combined physiotherapy with occupational therapy [40], and one study conducted Tai Ji Quan [45]. One study examined a mindfulness-based intervention [38], another one an education (psychosocial support) program [43]. One study exclusively examined occupational therapy [46].
Study duration varied from 2 to 12 months. Most studies (N = 5) conducted an intervention period of 2 and 2.5 months, respectively [38, 40–42, 46]. Three studies examined an intervention period of six months [39, 44, 45], and one study an intervention period of 12 months [43]. The studies varied substantially with regard to intervention intensity. Four studies conducted a weekly intervention with at least one contact per week [38, 41, 42, 45]. Three studies delivered their interventions on a monthly basis with at least one contact per month [39, 41, 43]. One study which used a “real life” setting in which the prescriptions for physiotherapy and occupational therapy were variable and reported a mean number of four therapy sessions, with a mean time per session of 58 min and a mean therapy duration of 8 weeks [40]. Finally, in one study, patients received 10 weeks of intervention with a maximum of 16 h [46].
Concerning the comparators, one study used two active control groups of comparable intensity in form of a stretching and a resistance training program, respectively [45], in one study patients received a DVD on PD and one advice session after trial completion [39], while all other studies (n = 7) used a passive control group with patients receiving treatment as usual (TAU) [38–44, 46].
Intervention efficacy
Within the physical interventions, Xin et al. [39] reported no fall reduction after physiotherapy as compared to an active control group, but reduction of fall rates among those with moderate disease severity. Fletcher et al. [41] did not demonstrate a statistically significant difference in falls after exercise vs. TAU, but between-group differences for balance, falls, and recreational physical activity levels. The balance training program compared to TAU in a study by Joseph et al. [42] was found to be effective in improving balance and gait velocity. The falls prevention exercise program examined by Farag et al. [44] revealed no significant benefit compared to TAU in the frequency of falls, but in mobility and QoL. QoL was also improved in a study of Clarke et al. [40] who compared physiotherapy combined with occupational therapy to TAU. However, they did not find a significant impact on activities of daily living. Finally, Tai Ji Quan was demonstrated to outperform the active control groups in maximum excursion, directional control, and further secondary functional outcomes [45]. The falls’ incidence was lowered in comparison to stretching but not to resistance training.
Bogosian et al. [38] reported that a mindfulness intervention improved QoL, but no further outcomes compared to TAU. Participating in a psychosocial support program compared to TAU did not lead to QoL improvement but in the Unified Parkinson’s Disease Rating Scale Part I (UPDRS-I), which assesses cognition, mood, and behavior, in the UPDRS-II scale assessing activities of daily living, and in the UPDRS total score as an indicator of disease severity [43]. Finally, a home-based, individualized occupational therapy compared to TAU showed a significant improvement in self-perceived performance in daily activities [46].
Health-economic analyses and health-economic outcomes
Nine studies were included. A CEA was calculated in one study [43], a CUA was conducted in five studies [38–41, 46], and three studies employed both a CEA and a CUA [42, 44, 45]. Concerning the included costs, all studies (n = 9) examined intervention-related costs, i.e., costs directly linked to the delivery of the intervention including costs of receiving the intervention [38], cost of therapy sessions in terms of salaries, training, travel, equipment, and consumables [39], therapists’ time needed for intervention conduct [40], therapist's time needed for intervention conduct, costs of venue hire, equipment costs and travel costs for therapists [41], education of therapists, assessment costs and costs for equipment and facilities [42], price to be paid for the education program [43], costs for service delivery and travel [44], promotional costs, recruitment costs, and class delivery costs [45], intervention costs [46]. Furthermore, non-intervention-related costs were considered in the majority of studies (n = 7) including primary, secondary and social care services [39], hospital costs and medication use [40], hospital contacts and medication use [41], ambulatory care, laboratory tests, medication use and medical equipment [43], medication, hospital and health services costs [44], costs of medication use, physical therapy, and medical treatment of falls [45], healthcare use, and absence from work and informal care [46].
While only one of the nine studies [46] assessed health-economic evaluations from a societal perspective, the other eight studies considered a third-party payer’s perspective [38–45].
Results of health-economic analyses
Cost-effectiveness analyses (CEA)
A 10-week balance training program to improve balance performance compared with a passive control group demonstrated the following incremental ICERs: 660€ to increase one unit in the Mini Balance Evaluation Systems Test (Mini BESTest) and 170€ per 1 cm increase in a gait velocity instrument [42]. Within the 6-month minimally supervised falls prevention exercise program a price of 360€ per fall prevented and a price of 6,051€ per extra person avoiding mobility deterioration operationalized with the Short Physical Performance Battery Test (SPPB) was shown [44]. Comparing a 6-month Tai Ji Quan intervention with two active control groups, the Tai Ji Quan intervention was cheaper and more effective than the stretching control intervention, i.e., Tai Ji Quan showed an average reduction of 166€ per additional fall avoided; notably, also resistance training as a control group showed an average of 95€ per additional fall prevented [45]. Since resistance was more costly and less effective, the authors eliminated it from the subsequent analysis.
A psychosocial support program over 12 months combined with standard neurological treatment found that the intervention dominated usual care as it was less costly and more effective in the improvement of the outcomes UPDRS II and III. The saving per patient to realize an additional score point on the UPDRS-II and UPDRS III, respectively, was 22.80€ and 18.40€.
Cost-utility analyses (CUA)
Among the eight studies, which undertook a CUA, four studies found significant differences in the quality-of-life outcome [38, 42, 44, 45]. The ICER of these studies ranged between 3,220€ [45] and 214,226€ per QALY gained [44]. More specifically, data indicate that the costs to realize a QALY for PD patients are 214,226€ in case of a 6-months falls prevention exercise program [44], 33,174€ in case of a 10-week balance training program [42], and 31,010€ in case of a mindfulness-based intervention over 8-weeks [38]. A 6-month Tai Ji Quan intervention [45] with a QALY of 3,220€ was found to be less costly and more effective than the active control group receiving stretching training. Resistance training, the second active control group, yielded 1,174€ per participant per additional QALY gained, and, thus, Tai Ji Quan intervention was superior.
Four studies did not find significant differences between their intervention group and their control group in the QALY outcome [39–41, 46]. A 6-month physiotherapy intervention showed an insignificant QoL gain with large uncertainty which results in a ICER of 152,240€ / QALY [39]. The 8-week combination of physiotherapy and occupational therapy intervention yielded a slight, but insignificant QoL gain [40]. One gained QALY had a price of 4,680€. Although there was no statistically significant difference in QoL from baseline to follow-up in a 10-week exercise intervention, the intervention is presumably (over 80%) dominant, i.e., cheaper and more effective at a willingness to pay of £ 20,000 (23,630€) [41]. A 10-week occupational therapy intervention did not reach significant differences in costs and QALY [46]. However, the results were in favor of the intervention group. When society is willing to pay 20,000€ per QALY gained, the net benefit of the intervention for the caregiver is positive, with a probability of 95%. All studies calculated the QALYs using the correct country-specific data sets.
Quality assessment
The quality of the health-economic evaluations of the included studies was assessed using the Drummond’s quality assessment tool (Supplementary Table 2). The methodological quality of the included studies is moderately high considering that the health-economic evaluations were follow-up analyses of clinical studies. Thus, sample size calculations and power were not sufficient for health-economic analyses. All included publications posed a well-defined question, presented a detailed description of the considered comparators and provided a comprehensive discussion of the study results. One study considered only one incremental cost factor as the main difference between the groups [38]. All other included studies identified relevant costs. However, the measurement of costs varied between studies. The quality appraisal employing the CHEERS 2022 checklist [37] (Supplementary Table 3) yielded similar results.
DISCUSSION
This systematic review examined health-economic evaluations of non-pharmacological and non-surgical interventions in PD patients. In total, the review included 9 RCTs and 2,244 participants. Only studies that undertook a CEA and/or CUA were included. The main findings are that (i) only nine studies eligible for our review were identified; (ii) the majority of studies included physical exercise interventions (n = 5) while studies with other intervention types (i.e., cognitive training, music/art/drama, language and speech therapy, and gaming) were not represented; (iii) most studies undertook a CUA (n = 5) or a combination of CEA and CUA (n = 3), while one study used a pure CEA; (iv) CEA identified costs of between 170€ and 660€ for improvements of single units in the different clinical outcomes and savings of between 18.40€ and 22.80€ per score gained; (v) CUA in the four studies that found significant benefits of the experimental intervention in QoL indicated that the ICER of QALY differs greatly and ranged between 3,220€ and 214,226€; (vi) the methodological quality of the included studies was high.
Compared to the only review considering health-economic outcomes of non-pharmacological studies in PD patients so far [14] which covered 8 studies but also included broader PD management concepts, our review has an overlap with six studies and integrated further three studies; both reviews demonstrate the high heterogeneity of studies in the field of health-economic evaluations of non-pharmacological interventions in PD, but physical exercise is the intervention type most frequently evaluated, and that this intervention might be cost-effective. Next to differences regarding intervention types, studies differed significantly in terms of the chosen payer perspectives and the defined cost categories, but also regarding country-specific available cost data.
In our review, four out of eight CUA studies demonstrated a significant increase in QoL, but the ICER for a QALY varied greatly. However, the question remains what amount decision-makers are willing to pay for a QALY gained. According to NICE an ICER between £ 20,000 (23,630€) and £ 30,000 (35,451€) per QALY are assumed to be acceptable, and in US cost-effectiveness ratios between $US 100,000 (98,407€) and $US 200,000 (196,814€) per QALY gained are considered reasonable [47]. Based on this thresholds, three out of four interventions could be regarded as cost-effective in our review [38, 42, 45]. However, there is an ongoing controversy among health-economics of how to set an adequate cost-effectiveness threshold (e.g., £ 20,000–£ 30,000) for new interventions [48].
As far as the CEA are concerned, it is difficult to interpret the willingness-to-pay thresholds for the different outcomes (e.g., for 1 cm increase in gait velocity) due to the fact that no adequate other published health-economic evaluations are available to serve as comparators. To be used as a basis for health-economic decision-making, head-to-head comparisons of competing interventions and long-term follow-up studies with comparable outcomes are necessary [49]. These studies should include various types of non-pharmacological interventions potentially contributing to PD healthcare.
Strengths and limitations
To the best of our knowledge, this is the first systematic review focusing specifically on the health-economic evaluations of non-pharmacological and non-surgical interventions in PD patients. A further strength is that this study followed the Cochrane collaboration standards for conducting systematic reviews.
There are some limitations of this review. First, the findings of our systematic review yielded only a small number of studies which were highly heterogenous regarding interventions and parameters included in the health-economic evaluations. Furthermore, certain types of health-economic analyses which may be specifically helpful for decision-making in the healthcare sector, including analysis of cost-efficacy, are missing so far. Therefore, the data base is incomplete, and clear conclusions are limited. Another limiting aspect concerns the countries of the included studies. These were only conducted in western countries. Due to differences in cultural aspects, socio-economics, and healthcare policies, our findings have restricted generalizability to African, Asian or South American countries. The relevance of health-economic evaluations may be even higher in developing countries due to more resource constraints.
Implications for research
Future studies on non-pharmacological and non-surgical interventions in PD should not purely focus on clinical outcome measures, but include health-economic evaluations for short-term, but also long-term intervention effects to facilitate solid decision-making in PD healthcare. Within the scope of health-economic evaluations, more CEA are needed. The advantage of this type of analysis is that they enable to examine the association of the effects of the intervention (e.g., PROMS) with the costs of the intervention.
To increase the comparability between health-economic evaluations it is fundamental for researchers to follow consistent guidelines (cf. CHEERS statement [37] for reporting health-economic evaluations). Finally, it would be of high importance to define cost categories for different payer perspectives in order to guarantee the comparability of data for future meta-analytic approaches.
CONCLUSION
PD has a high economic burden for patients, payers, and the entire society. Health-economic evaluations are an essential pillar for decision-makers to compare competing interventions regarding costs and effects. Health-economic evaluations of non-pharmacological and non-surgical interventions in PD are promising, but still at a nascent stage, so that this important research gap will have to be faced in the future. Interdisciplinary research teams including experts for health-economics are necessary to promote this research field in terms of high-quality analyses; also, the education of clinical researchers would be of great merit. High-quality research could further be supported by guidelines specifically designed for health-economic evaluations of non-pharmacological interventions. Ultimately, growing evidence in the field has the potential to accelerate the clinical application of this important spectrum of interventions in PD healthcare.
ACKNOWLEDGMENTS
The authors thank Dirk Müller for his support during the whole process of conducting this systematic review.
FUNDING
This systematic review was financed by budget resources of the participating study sites.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Data is available on request from the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230213.
REFERENCES
[1] | Deuschl G , Beghi E , Fazekas F , Varga T , Christoforidi KA , Sipido E , Bassetti CL ((2020) ) The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health 5: , e551–e567. |
[2] | Sprenger F , Poewe W ((2013) ) Management of motor and non-motor symptoms in Parkinson’s disease. CNS Drugs 27: , 259–272. |
[3] | Dowding CH , Shenton CL , Salek SS ((2006) ) A review of the health-related quality of life and economic impact of Parkinson’s disease. Drugs Aging 23: , 693–721. |
[4] | Bloem BR , de Vries NM , Ebersbach G ((2015) ) Nonpharmacological treatments for patients with Parkinson’s disease. Mov Disord 30: , 1504–1520. |
[5] | Tomlinson CL , Herd CP , Clarke CE , Meek C , Patel S , Stowe R , Deane KH , Shah L , Sackley CM , Wheatley K , Ives N ((2014) ) Physiotherapy for Parkinson’s disease: A comparison of techniques. Cochrane Database Syst Rev 6: , CD002815. |
[6] | Fox SH , Katzenschlager R , Lim SYB ((2018) ) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 3: , 1248–1266. |
[7] | Lauzé M , Daneault J-F , Duval C ((2016) ) The effects of physical activity in Parkinson’s disease: A review. J Parkinsons Dis 6: , 685–698. |
[8] | da Silva FC , Iop RDR , de Oliveira LC , Boll AM , de Alvarenga JGS , Gutierres Filho PJB , de Melo LMAB , Xavier AJ , da Silva R ((2018) ) Effects of physical exercise programs on cognitive function in Parkinson’s disease patients: A systematic review of randomized controlled trials of the last 10 years. PLoS One 13: , e0193113. |
[9] | Sousa NMF , Neri A , Brandi IV , Brucki SMD , Brucki SMD ((2021) ) Impact of cognitive intervention on cognitive symptoms and quality of life in idiopathic Parkinson’s disease: A randomized and controlled study. Dement Neuropsychol 15: , 51–59. |
[10] | Petrelli A , Kaesberg S , Barbe MT , Timmermann L , Fink GR , Kessler J , Kalbe E ((2014) ) Effects of cognitive training in Parkinson’s disease: A randomized controlled trial. Parkinsonism Relat Disord 11: , 1196–1202. |
[11] | Detsky AS , Laupacis A ((2007) ) Relevance of cost-effectiveness analysis to clinicians and policy makers. JAMA 298: , 221–224. |
[12] | Vossius C , Nilsen OB , Larsen JP ((2009) ) Health state values during the first year of drug treatment in early-stage Parkinson’s disease. Drugs Aging 26: , 973–980. |
[13] | Wang AS , Gunzler SA ((2019) ) Systematic review of the pharmacoeconomics of Parkinson disease medications. Expert Opin Pharmacother 20: , 1659–1670. |
[14] | Afentou N , Jarl J , Gerdtham UG , Saha S ((2019) ) Economic evaluation of interventions in Parkinson’s disease: A systematic literature review. Mov Disord Clin 6: , 282–290. |
[15] | Schardt C , Adams MB , Owens T , Keitz S , Fontelo P ((2007) ) Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 7: , 16. |
[16] | Moher D , Liberati A , Tetzlaff J , Altman DG ((2009) ) Group, Reprint— Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. Phys Ther 89: , 873–880. |
[17] | Leung IHK , Walton CC , Hallock H , Lewis SJG , Valenzuela M , Lampit A ((2015) ) Cognitive training in Parkinson disease A systematic review and meta-analysis. Neurology 85: , 1843–1851. |
[18] | Lawrence BJ , Gasson N , Bucks RS , Troeung L , Loftus AM ((2017) ) Cognitive training and noninvasive brain stimulation for cognition in Parkinson’s disease: A meta-analysis. Neurorehabil Neural Repair 31: , 597–608. |
[19] | Chou Y-h , Hickey PT , Sundman M , Song AW , Chen NK ((2015) ) Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson disease: A systematic review and meta-analysis. JAMA Neurol 72: , 432–440. |
[20] | Elsner B , Kugler J , Pohl M , Mehrholz J ((2016) ) Transcranial direct current stimulation (tDCS) for idiopathic Parkinson’s disease. Cochrane Database Syst Rev 18: , 7. |
[21] | Barnish J , Atkinson RA , Barran SM , Barnish MS ((2016) ) Potential benefit of singing for people with Parkinson’s disease: A systematic review. J Parkinsons Dis 6: , 473–484. |
[22] | Zhang S , Liu D , Ye D , Li H , Chen F ((2017) ) Can music-based movement therapy improve motor dysfunction in patients with Parkinson’s disease? Systematic review and meta-analysis. Neurol Sci 38: , 1629–1636. |
[23] | Yang S , Sajatovic M , Walter BL ((2012) ) Psychosocial interventions for depression and anxiety in Parkinson’s disease. J Geriatr Psychiatry Neurol 25: , 113–121. |
[24] | McDonnell MN , Rischbieth B , Schammer TT , Seaforth C , Shaw AJ , Phillips AC ((2018) ) Lee Silverman Voice Treatment (LSVT)-BIG to improve motor function in people with Parkinson’s disease: A systematic review and meta-analysis. Clin Rehabil 32: , 607–618. |
[25] | Xie CL , Wang XD , Chen J , Lin HZ , Chen YH , Pan JL ((2015) ) A systematic review and meta-analysis of cognitive behavioral and psychodynamic therapy for depression in Parkinson’s disease patients. Neurol Sci 36: , 833–843. |
[26] | Chung CLH , Thilarajah S , Tan D ((2016) ) Effectiveness of resistance training on muscle strength and physical function in people with Parkinson’s disease: A systematic review and meta-analysis. Clin Rehabil 30: , 11–23. |
[27] | Garcia-Ruiz PJ , Chaudhuri KR , Martinez-Martin P ((2014) ) Non-motor symptoms of Parkinson’s disease A review... from the past. J Neurol Sci 338: , 30–33. |
[28] | Foster ER , Bedekar M , Tickle-Degnen L ((2014) ) Systematic review of the effectiveness of occupational therapy–related interventions for people with Parkinson’s disease. Am J Occup Ther 68: , 39–49. |
[29] | McLean G , Lawrence M , Simpson R , Mercer SW ((2017) ) Mindfulness-based stress reduction in Parkinson’s disease: A systematic review. BMC Neurol 17: , 92. |
[30] | Seppi K , Ray Chaudhuri K , Coelho M , Fox SH , Katzenschlager R , PerezLloret S , Weintraub D , Sampaio C ((2019) ) Update on Non-Motor SymptomsStudy Group on behalf of the Movement Disorders SocietyEvidence-Based Medicine Committee, Update on treatments for nonmotorsymptoms of Parkinson’s disease— an evidence-based medicinereview. Mov Disord 34: , 180–198. |
[31] | Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org. |
[32] | Munneke M , Nijkrake MJ , Keus SH , Kwakkel G , Berendse HW , Roos RA , Borm GF , Adang EM , Overeem S , Bloem BR ((2010) ) Efficacy of community-based physiotherapy networks for patients with Parkinson’s disease: A cluster-randomised trial. Lancet Neurol 9: , 46–54. |
[33] | van der Marck MA , Munneke M , Mulleners W , Hoogerwaard EM , Borm GF , Overeem S , Bloem BR ((2013) ) Integrated multidisciplinary care in Parkinson’s disease: A non-randomised, controlled trial (IMPACT). Lancet Neurol 12: , 947–956. |
[34] | Drummond MF , Sculpher MJ , Claxton K , Stoddart GL , Torrance GW (2015) Methods for the economic evaluation of health care programmes, 4th ed. Oxford University Press. |
[35] | Sanders GD , Maciejewski ML , Basu A ((2019) ) Overview of cost-effectiveness analysis. JAMA 321: , 1400–1401. |
[36] | York Health Economics Consortium (2016) Incremental Cost-Effectiveness Ratio (ICER), https://yhec.co.uk/glossary/incremental-cost-effectiveness-ratio-icer/Accessed June 23, 2022. |
[37] | Husereau D , Drummond M , Augustovski F , de Bekker-Grob E , Briggs AH , Carswell C , Caulley L , Chaiyakunapruk N , Greenberg D , Loder E , Mauskopf J , Mullins CD , Petrou S , Pwu R-F , Staniszewska S ((2022) ) Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. Int J Technol Assess Health Care 38: , e13. |
[38] | Bogosian A , Hurt CS , Hindle JV , McCracken LM , Vasconcelos Sa EDA , Axell S , Tapper K , Stevens J , Hirani PS , Salhab M , Ye W , Cubi-Molla P ((2022) ) Acceptability and feasibility of a mindfulness intervention delivered via videoconferencing for people with Parkinson’s. J Geriatr Psychiatry Neurol 35: , 155–167. |
[39] | Xin Y , Ashburn A , Pickering RM , eymour KC , Hulbert S , Fitton C , Kunkel D , Marian I , Roberts HC , Lamb SE , Goodwin VA , Rochester L , McIntosh E ((2020) ) Cost-effectiveness of the PDSAFE personalised physiotherapy intervention for fall prevention in Parkinson’s: An economic evaluation alongside a randomised controlled trial. BMC Neurol 20: , 295. |
[40] | Clarke CE , Patel S , Ives N ((2016) ) Clinical effectiveness and cost-effectiveness of physiotherapy and occupational therapy versus no therapy in mild to moderate Parkinson’s disease: A large pragmatic randomised controlled trial (PD REHAB). Health Technol Assess 20: , 1–96. |
[41] | Fletcher E , Goodwin VA , Richards SH , Campbell JL , Taylor RS ((2012) ) An exercise intervention to prevent falls in Parkinson’s: An economic evaluation. BMC Health Serv Res 12: , 1–9. |
[42] | Joseph C , Brodin N , Leavy B , Hagströmer M , Löfgren N , Franzén E ((2019) ) Cost-effectiveness of the HiBalance training program for elderly with Parkinson’s disease: Analysis of data from a randomized controlled trial. Clin Rehabil 33: , 222–232. |
[43] | Canivet C , Costa N , Ory-Magne F ((2016) ) Clinical impact and cost-effectiveness of an education program for PD patients: A randomized controlled trial. PLoS One 11: , e0162646. |
[44] | Farag I , Sherrington C , Hayes A , Canning CG , Lord SR , Close Fung JCVS , Howard K ((2016) ) Economic evaluation of a falls prevention exercise program among people with Parkinson’s disease. Mov Disord 31: , 53–61. |
[45] | Li F , Harmer P ((2015) ) Peer reviewed: Economic evaluation of a Tai Ji Quan intervention to reduce falls in people with Parkinson disease, Oregon, 2008–2011. Prev Chronic Dis 12: , E120. |
[46] | Sturkenboom IH , Hendriks JC , Graff MJ , Adang EM , Munneke M , Nijhuis-van der Sanden MW , Bloem BR ((2015) ) Economic evaluation of occupational therapy in Parkinson’s disease: A randomized controlled trial. Mov Disord 30: , 1059–1067. |
[47] | McCabe C , Claxton K , Culyer AJ ((2008) ) The NICE cost-effectiveness threshold: What it is and what that means. Pharmacoeconomics 26: , 733–744. |
[48] | Gandjour A ((2020) ) Willingness to pay for new medicines: A step towards narrowing the gap between NICE and IQWiG. Res 20: , 343. |
[49] | Reese JP , Dams J , Winter Y , Balzer-Geldsetzer M , Oertel WH , Dodel R ((2012) ) Pharmacoeconomic considerations of treating patients with advanced Parkinson’s disease. Expert Opin on Pharmaco 13: , 939–958. |




