Delivering Multidisciplinary Rehabilitation Care in Parkinson’s Disease: An International Consensus Statement
Abstract
Background:
Parkinson’s disease (PD) is a complex neurodegenerative disorder impacting everyday function and quality of life. Rehabilitation plays a crucial role in improving symptoms, function, and quality of life and reducing disability, particularly given the lack of disease-modifying agents and limitations of medications and surgical therapies. However, rehabilitative care is under-recognized and under-utilized in PD and often only utilized in later disease stages, despite research and guidelines demonstrating its positive effects. Currently, there is a lack of consensus regarding fundamental topics related to rehabilitative services in PD.
Objective:
The goal of the international Parkinson’s Foundation Rehabilitation Medicine Task Force was to develop a consensus statement regarding the incorporation of rehabilitation in PD care.
Methods:
The Task Force, comprised of international multidisciplinary experts in PD and rehabilitation and people directly affected by PD, met virtually to discuss topics such as rehabilitative services, existing therapy guidelines and rehabilitation literature in PD, and gaps and needs. A systematic, interactive, and iterative process was used to develop consensus-based statements on core components of PD rehabilitation and discipline-specific interventions.
Results:
The expert-based consensus statement outlines key tenets of rehabilitative care including its multidisciplinary approach and discipline-specific guidance for occupational therapy, physical therapy, speech language pathology/therapy, and psychology/neuropsychology across all PD stages.
Conclusions:
Rehabilitative interventions should be an essential component in the comprehensive treatment of PD, from diagnosis to advanced disease. Greater education and awareness of the benefits of rehabilitative services for people with PD and their care partners, and further evidence-based and scientific study are encouraged.
INTRODUCTION
Parkinson’s disease (PD) is a complex and progressive neurodegenerative condition occurring in over 10 million people worldwide and has both motor and non-motor symptoms that can affect functional abilities and quality of life [1–5]. Rehabilitative interventions play a crucial role in PD care and improving health outcomes, with growing evidence demonstrating benefit for motor and non-motor functioning, activities of daily living (ADL), and quality of life [6–10]. Several guidelines and quality standards propose incorporation of occupational therapy (OT), physical therapy/physiotherapy (PT), and speech and language pathology/therapy (SLP) in PD care, as well as exercise and physical activity [11–13]. Several research studies support multidisciplinary rehabilitative care to utilize basal ganglia circuitry, neuroplasticity, goal-based practice, and task-specific motor and cognitive training [14–22]. Despite these guidelines and increasingly recognized benefits of OT, PT, and SLP for PD, rehabilitation therapy referrals and utilization rates are low in many countries, even in expert specialty centers [23–26]. Data from the Parkinson’s Foundation Quality Improvement Initiative, reflecting over 5,000 participants from 4 countries, revealed that less than 1/3 of PD patients were referred for rehabilitation services, with rates of 30.4%, 8.0%, and 7.5% for PT, OT, and SLP, respectively [24]. Referral to rehabilitation specialists more often occurs late in the disease when complications such as falls or dysphagia occur [24], thereby missing critical opportunities to proactively limit or prevent functional decline even early in the course of PD.
At present, consensus is lacking regarding many fundamental topics and key points related to rehabilitative services in PD, ranging from the composition and integration of the rehabilitation team to the optimal setting, timing and delivery needed to inform best practices. While there are individual discipline-specific guidelines (e.g., OT, PT, and SLP) and papers on organizing multidisciplinary care, there are no integrated international guidelines outlining key principles of multidisciplinary rehabilitative care and practices in PD [17, 27, 28]. This lack of guidance limits the appropriate inclusion of rehabilitative services in PD and the provision of optimal care for people living with PD. Developing a unified understanding of the role of multidisciplinary rehabilitative care in PD represents a key step in improving patient outcomes.
This paper aims to address these challenges by providing a consensus statement based on expert opinion, best available evidence, and a priori processes as an initial step toward international guidance and frameworks for comprehensive, integrated rehabilitative care-delivery models in PD. In this regard, consensus statements (in contrast to clinical practice guidelines, which are recommendations based on systematic reviews of evidence and assessment of benefits and harms of alternative care options [29]) are particularly helpful when there is variability in clinical practices and evidence, and opportunities to improve quality of care. To date, clinical practice guidelines for rehabilitation in PD typically focus on individual disciplines (i.e., PT, OT, or SLP) separately, rather than in an integrated, inter-related approach, and recommendations for timing, content, and delivery vary by geographical region (e.g., United States, Europe, United Kingdom, Canada) and organization (e.g., American Academy of Neurology and professional rehabilitation organizations) [11, 12, 30–34]. Furthermore, despite these practice guidelines, rehabilitation services for PD have limited utilization and are inconsistently organized and implemented [23, 24, 26]. Thus, the objective of our work was to achieve consensus among experts in multidisciplinary rehabilitation and PD regarding fundamental components of rehabilitative care for PD and optimal interventions for rehabilitation therapies most often encountered in PD.
METHODS
Purpose and overview
The Parkinson’s Foundation established a Rehabilitation Task Force to address the lack of consensus regarding use of and guidance for rehabilitation care in PD. The Task Force utilized a systematic approach to develop an international consensus statement regarding multidisciplinary rehabilitation in PD with the following steps: 1) defining goals and timeline, 2) conducting a literature search and narrative review, 3) defining scope of the consensus statement, 4) discussing the literature and drafting the consensus statement, 5) developing and refining the proposed statement using a systematic, interactive, and iterative process, and 6) manuscript development.
Task Force and Steering Committee
The Task Force was comprised of international PD clinical, research, and rehabilitation healthcare experts, a person with PD, and care partner of a person with PD. Multiple professional disciplines were represented, specifically including movement disorders neurology, neuropsychology, nursing, OT, physiatry, PT, rehabilitation science (the field of research related to rehabilitation, e.g., its scientific basis, delivery models, implementation, among other topics), and SLP (see Supplementary Material for Task Force members). In the context of this initiative, the term “expert” was defined as “healthcare professional, researcher, or academic with expertise in the treatment, service provision, research or analysis of multidisciplinary rehabilitation in PD or a person with first-hand lived experience with PD.” The panel was identified by the Parkinson’s Foundation project leads and Rehabilitation Task Force co-chairs based on their recognized clinical experience in PD across the disease spectrum, scientific research and scholarship contributions to the field of PD and rehabilitation, and lived-experience.
All members were considered to have relevant knowledge and experiences to share and equally weighted voices in the Task Force, reflecting the teamwork and multidisciplinary nature typical of the field of rehabilitation. An expert panel of approximately 15 participants was planned, in keeping with our aim of having representation across disciplines and international regions, gender balance, and previously recommended panel sizes [35, 36].
The Steering Committee was comprised of the Rehabilitation Task Force co-chairs and Parkinson’s Foundation project leads, along with external healthcare consultants (Avalere, based in Washington, D.C.), who provided oversight and input for the project. The consultants assisted with preparing discussion topics, workshop materials, and iterative feedback; facilitating group discussions to ensure participation from all members, listening for any dissenting opinions or areas for additional discussion, and helping achieve consensus; and distributing, receiving, and reviewing votes on the statements for consensus agreement.
Preparation for Task Force discussions
In preparation for the group discussions, a narrative review of the literature was performed, thereby reviewing published systematic reviews, meta-analyses, guidelines, and seminal articles regarding evidence for rehabilitative interventions in PD from 2002–2022. The purpose of the review was to provide a broad overview on the topic of rehabilitation in PD with critical, comprehensive, objective, and current knowledge and to help establish a framework for the Task Force and context for the proposed consensus statement. As the Task Force paper was not intended to be a clinical practice guideline, our methodology did not include a formal systematic review of PD rehabilitation literature, though it incorporated existing systematic reviews on this topic into its review and group discussions [8, 28–47]. Several primary PD guidelines were reviewed specifically for their reference to rehabilitative therapies [11, 12, 37–41]. Limited emphasis on rehabilitation in existing guidelines, along with variability in the types of rehabilitation disciplines included and PD symptoms and treatments emphasized, further reinforced the rationale for our objective of developing a collective perspective on comprehensive, multidisciplinary rehabilitation approaches for PD.
Consensus-building process
Members participated in virtual workshops between February and October 2021 to discuss the current evidence for rehabilitation in PD and topics such as optimal interventions, team composition, and discipline-specific rehabilitation considerations. Workshops were used to develop the consensus statements regarding rehabilitative care and interventions for OT, PT, SLP, and psychology/neuropsychology (Fig. 1).
Fig. 1
Task Force process.
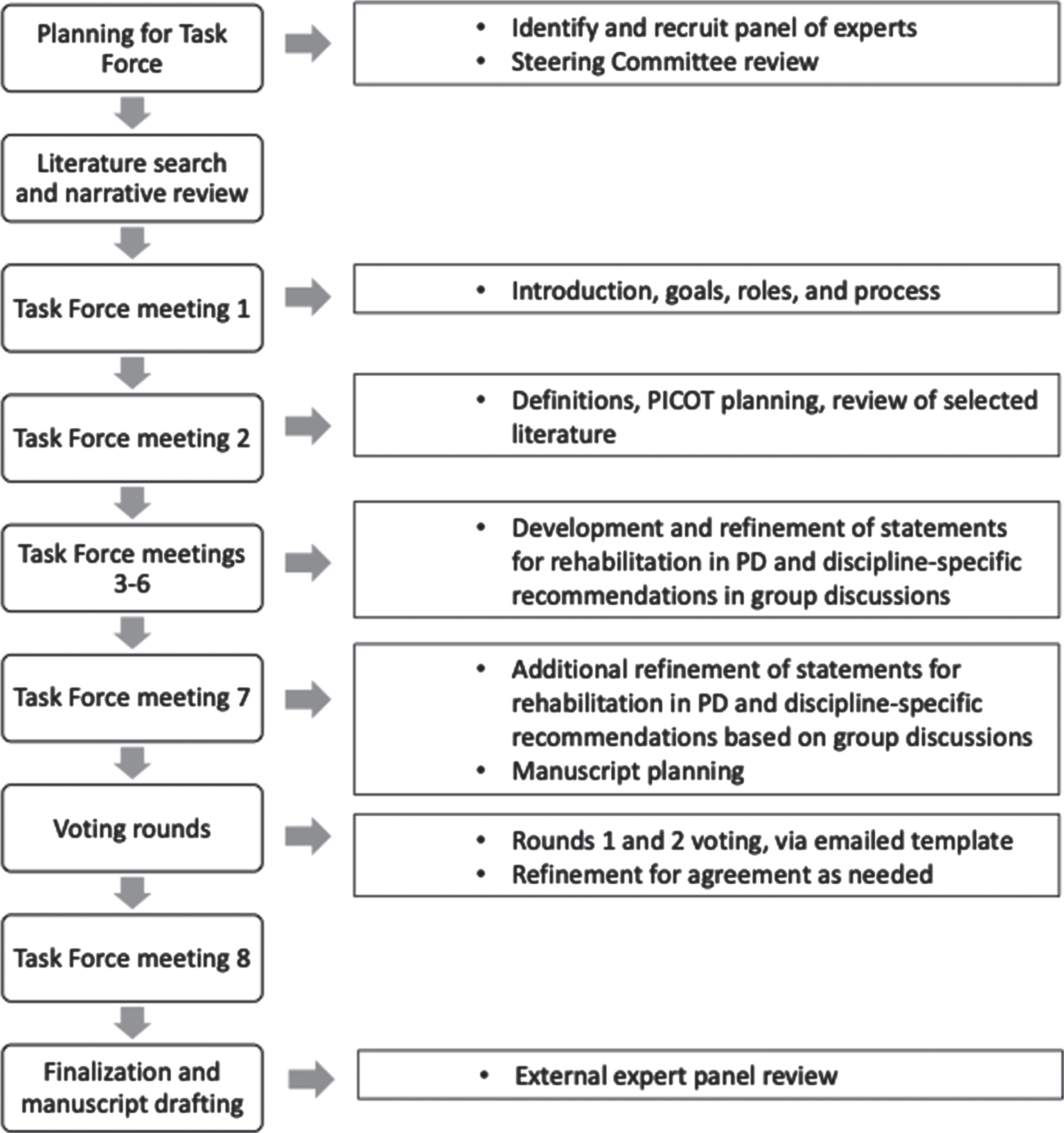
The Task Force defined the scope and parameters of the consensus statement using the PICOTS framework (i.e., Population, Interventions, Comparisons, Outcomes, Timing, and Settings) [42]. As such, the following parameters for the work were identified: Population (PD, all stages), Interventions (rehabilitation services), Comparisons/Co-interventions (other care services such as medications, surgery, education, and supportive services often utilized in PD), Outcomes (functional outcomes, patient goals, quality of life, symptomatic therapies), Timing (all stages of PD, including at diagnosis and early PD), Settings (multiple locations including hospital, outpatient, and others).
The Task Force achieved consensus on items using a structured and iterative process of developing and refining key statements, incorporating the evidence reviewed and collective expert opinion via virtual face-face conversations and email correspondences. We collected and aggregated information from the expert panel through multiple iterations and feedback. These discussions were organized and guided by the Steering Committee, including the healthcare consultants who provided oversight and encouraged and solicited participation from all members. This process enabled the group to maintain effective communication and open expression of opinions, dialogue and critique of topics, and response to iterative feedback.
The proposed tenets and recommendations were voted on anonymously by Task Force members using an emailed voting template, to which each member responded individually to the healthcare consultants so that independence and anonymity was respected. The voting for the discipline-specific interventions for OT, PT, SLP, and psychology/neuropsychology additionally included the physician co-chairs of the Steering Committee to capture their perspectives on the rehabilitation disciplines. The voting template format consisted of an excel spreadsheet listing the proposed items, request to “mark yes or no for agreement” for each item, and opportunity to provide commentary. Votes were tallied and noted as achieving consensus when at least 80% approved. As percentages for agreement vary in the literature, we wanted to ensure majority consensus in our international panel where wide variations in practice might occur, and thereby adopted a more conservative approach [43–45]. Any dissenting opinions were discussed during virtual workshops and resolved with majority agreement. For analysis, we calculated counts and proportions for the voting. Taking a conservative approach, we counted missing data for individual items as a “no” vote. Building on the iterative process, feedback and discussion, two rounds of voting were performed.
RESULTS
Task Force and Steering Committee composition
The Task Force members represented movement disorders neurology (n = 3), neuropsychology (n = 2), nursing (n = 1), OT (n = 2), physiatry (n = 1), PT (n = 2), rehabilitation science (n = 1), SLP (n = 2), person with PD (n = 1, also a PT), and care partner (n = 1). The Steering Committee included a movement disorders neurologist with neurorehabilitation expertise and a physiatrist (Task Force co-chairs), along with Parkinson’s Foundation staff of whom 2 also had professional experience in OT and nursing. The total group included 12 females and 9 males, residing across 7 countries (United States n = 12, Australia n = 2, Italy n = 2, United Kingdom n = 2, Canada n = 1, France n = 1, Netherlands n = 1 participant). Of the Task Force members, 79% had over 20 years of experience in their discipline and 66% reported over 20 years focused on rehabilitation.
Definitions and terminology
Building on fundamental concepts of rehabilitation from the World Health Organization and British Society of Rehabilitation Medicine [46, 47], the Task Force proposes that rehabilitation in the context of PD be defined as a process of active changes through interventions designed to optimize physical, psychological, social, cognitive and behavioral functioning for individuals at all stages of PD.
Throughout this statement, the Task Force utilizes a schema delineating four clinical stages of PD in order to capture a functional point of view, adapted from Thomas and MacMahon [48, 49] as: “Upon Diagnosis, Early-Stage, Mid-Stage, and Advanced-Stage PD.” While other PD staging methods exist (e.g., Hoehn and Yahr) [50], they focus mostly on motor aspects of PD. Incorporating both motor and functional features of PD into rehabilitative therapy is essential to our approach. The Task Force also recognizes that various terminology, such as “multidisciplinary,” “interdisciplinary,” “integrated,” is often used interchangeably in the literature to describe similar yet nuanced concepts (Table 1) [51]. For consistency in this article, multidisciplinary is used to describe the integrated care model of the rehabilitation team.
Table 1
Key definitions and terminology
| Multidisciplinary Team: people from different disciplines working together, each drawing on their disciplinary knowledge. The different disciplines typically provide evaluation and management in different locations and at separate times [28]. |
| Interdisciplinary Team: sharing/integrating knowledge and methods from different disciplines, using a real synthesis of approaches. The different disciplines typically provide evaluation and management together, in the same location and same time [28]. |
| Integrated Care: care approach to strengthen people-centered health systems through the promotion of the comprehensive delivery of quality services across the life course; it is designed according to the multidimensional needs of the population and the individual and is delivered by a coordinated multi- or interdisciplinary team of providers working across settings and levels of care [28]. |
| Person-centered care: care approaches and practices that see the person as a whole with many levels of needs and goals [216]. |
| Comprehensive care: equitable and timely access to specialists, throughout all stages of the disease, utilizing a person-centered, multidisciplinary team approach to symptom management to improve health and quality of life [217]. |
Statements and guidance for rehabilitation in PD
The consensus-based statements presented here address fundamental components of rehabilitative care for PD, including A) key principles of rehabilitative care; B) composition of the rehabilitation care team; C) rehabilitation care planning; D) timing and settings for rehabilitative services; and E) education and training of rehabilitation professionals for PD (Table 2). These statements help establish paradigms for high-quality rehabilitative care for PD. Additionally, the Task Force proposes optimal rehabilitative interventions across four rehabilitation therapy disciplines: OT, PT, SLP, and psychology/neuropsychology (Tables 3–6). The Task Force also highlights several emerging rehabilitative therapies with examples provided in the tables, but given their current state of evidence-base, the Task Force does not offer formal recommendations for these areas.
Table 2
Tenets and recommendations for rehabilitative care in PD
| A. Key principles of rehabilitative care for PD |
| A1. Rehabilitative services play an integral role in the comprehensive, integrated model of PD care and should be multidisciplinary and available along the continuum of PD from diagnosis to end of life. |
| A2. Rehabilitative care in PD should be considered in the context of other PD treatments as it can be adjunctive to medication and surgical therapies in PD, or for some, at different time points, may be the primary therapeutic intervention. |
| A3. Rehabilitation goals should be person-centered and developed in collaboration with the person living with PD and care partners, as appropriate. The process of setting rehabilitation goals is dynamic, requiring assessment and re-evaluation along the continuum of PD. Rehabilitation goals are directed at optimizing physical, psychological, social, cognitive, and behavioral functioning (e.g., activities, work, hobbies, and other) and outcomes such as functional abilities, participation in activities, independence, and quality of life for persons at all stages of PD. |
| A4. Rehabilitative care is grounded in underlying neurobiological mechanisms and includes scientifically driven and personalized interventions. |
| B. Team structure for PD rehabilitative care |
| B1. The rehabilitation team should ideally include care managers; preferably physician specialists in neurology, physical medicine and rehabilitation (e.g., physiatry), or geriatrics; as well as PD nurse specialists and professionals in occupational therapy, physical therapy, speech language pathology/therapy, psychology/neuropsychology, and social work. At times, the core team may also include other specialists, practitioners for complementary modalities, or rehabilitation scientists, but must be flexible in the combination depending on the needs of the person with PD and on the availability of and access to various rehabilitation professionals (see Fig. 2). |
| B2. Persons living with PD, their care partners and family should be the central focus and considered integral and active members of the rehabilitation team. |
| B3. All treating professionals responsible for managing, coordinating, and/or delivering rehabilitative care for persons with PD should have appropriate knowledge, experience, and training regarding PD in their respective disciplines. |
| B4. The rehabilitation team should have a coordinating person(s) to oversee and facilitate the delivery of rehabilitative services across all relevant disciplines. Depending on the clinical setting or situation, the coordinating rehabilitation professional may be anyone from the core rehabilitation team (i.e., a physiatrist, neurologist, movement disorder specialist, geriatrician, and/or other health professional (e.g., occupational therapist, physical therapist, speech language pathologist, psychologists, neuropsychologists, social worker), or other relevant professional (e.g., case manager). |
| B5. The rehabilitation team members must demonstrate continuity and team connectedness in care by having timely, effective, clear, integrated, and cross-collaborative interactions and communications within the rehabilitation team, with other healthcare providers, and with people with PD and their care partners. |
| C. Care planning for PD rehabilitation |
| C1. Rehabilitation team members should provide a tailored, meaningful, person-centered approach when developing treatment plans for persons with PD. Care plans need to account for the goals, needs, preferences, values, and learning style of the person with PD. They should also account for the ability of the person with PD to readily access needed services, such as geographic location of medical offices, insurance coverage, language and culture, and other factors that could limit care utilization. |
| C2. Rehabilitation providers should involve the care partner of a person with PD, where appropriate, for management, education, and training, as well as take into account the psychosocial and physical needs of the care partner and the person with PD. It is essential that providers understand the person with PD’s and care partner’s health literacy, comprehension, and cognition, and their communications need to be tailored appropriately. |
| C3. Rehabilitation providers, persons with PD, and care partners, should work together using a shared decision-making approach. |
| C4. Rehabilitation providers should teach persons with PD and their care partners self-management strategies at early PD stages and on an ongoing basis throughout the disease course. |
| D. Timing and settings for rehabilitative services |
| D1. Rehabilitative services should be implemented across all stages of disease and integrated with other interventions (e.g., medications, surgery), from early stages (which can be as soon as the time of diagnosis) to advanced disease. Services implemented should involve all or any combination of the rehabilitation team disciplines according to the specific changing needs of the person with PD across all stages of the disease. |
| D2. Early referral to rehabilitative services should be recommended to individuals as soon as they are diagnosed with PD and should continue with regular modifications throughout disease progression as needed. |
| D3. Rehabilitation providers should conduct rehabilitation assessments at first presentation of PD to establish baseline status, with input from the multidisciplinary rehabilitation team, to determine which interventions should be initiated and set rehabilitation goals to optimize function, quality of life, promote a healthy lifestyle, and prevent functional decline. |
| D4. Rehabilitation providers should conduct serial and follow up rehabilitation assessments at regular intervals throughout disease progression (e.g., approximately every 6 months, designated timeframes, and when needed) to determine changes over time, identify new features or concerns about the diagnosis, set new therapy goals, prevent disease-related complications, address patient and care partner needs, and optimize function and quality of life. |
| D5. Rehabilitative services can be provided in a variety of settings including: outpatient therapy locations, day rehabilitation programs, inpatient hospital programs, community-based programs, at home, and long-term care facilities, as well as in single-discipline (e.g., physical therapy alone) or multidiscipline (e.g., physical therapy and occupational therapy) settings that take into account differences in patient needs and resource availability. |
| E. Education and training required for rehabilitation of people with PD |
| E1. The Parkinson’s community (i.e., people with PD, care partners, professionals, advocacy organizations) should have greater awareness and education about rehabilitative services, including the importance and impact of rehabilitative services, different types of rehabilitative services, resources available, and identification of symptoms throughout the PD journey that would prompt assessments or changes in treatment plans. |
| E2. The healthcare professional community should have greater awareness and education about rehabilitative services, including the importance and impact of rehabilitative services, different types of rehabilitative services, referral pathways, resources available, and early identification of symptoms throughout the PD journey that would prompt assessments or changes in treatment plans. |
| E3. Education and training for healthcare professionals providing PD specific rehabilitation should be developed and implemented by clinicians and other subject matter experts practicing in these areas, with consideration of the role of continuing education and incorporation of evidence-base standards and practice guidelines, where applicable. |
| E4. Education and training regarding teamwork, communication, and inter-professional interactions also should be provided to clinicians supporting rehabilitative services for persons with PD. |
Table 3
Optimal rehabilitation assessments and interventions for occupational therapy
| Upon Diagnosis | Early-Stage PD | Mid-Stage PD | Advanced PD | |
| Evaluations | •Baseline assessment of ADL/IADL, functional abilities, and social/or recreational activities, employment needs, fine motor control, functional cognition, fatigue, upper extremity function, and vision •Care partner and person with PD’s risk impressions on home and driving safety | •Regular re-assessment of ADL/IADL, functional abilities, and social/ or recreational activities, employment needs, fine motor control, cognition, fatigue, upper extremity function, and vision •Regular re-assessment of motor and non-motor functions and OT needs •Driving screen and evaluation- clinical and/or behind the wheel assessment •Screen for durable medical equipment and home safety for falls prevention •Home modification assessment | •Regular re-assessment of ADL/IADL, functional abilities, and social/ or recreational activities, employment needs, fine motor control, cognition, fatigue, upper extremity function, and vision •Regular re-assessment of motor and non-motor functions and OT needs •Follow-up driving screen and evaluation-clinical and/or behind the wheel assessment and discussion regarding alternative strategies for transportation as necessary (may coordinate with social work and others on team) •Care partner burden/strain •Environment modifications for visual dysfunction, balance and safety | •Regular re-assessment of ADL/IADL, functional abilities, and social/ or recreational activities, fine motor control, cognition, fatigue, upper extremity function, and vision •Regular re-assessment of motor and non-motor functions and OT needs •Occupational deprivation •Safety with domestic equipment (e.g., firearms, power tools, small appliances) •Community safety (e.g., wandering) |
| Management | •Time pressure management •Fatigue management (including exercise plan) •Strategies for non-motor symptoms: e.g., sleep hygiene, stress management, mindfulness, exercise | Same as interventions for Upon Diagnosis plus: •Medication management •Collaboration with speech-language pathologist to determine if dietary modifications should dysphagia occur and adaptive equipment and compensatory strategies to support self-feeding are needed for either oral eating or manual coordination reasons •Collaboration with PT to address exercise plan for managing fatigue •Daily schedule management •Sleep hygiene | Same as interventions for Early Stage plus: •Structured daily routine •Toileting schedule •Collaboration with SLP to determine dietary modification and adaptive equipment and compensatory strategies to support self-feeding as needed •Collaboration with PT to address safety with functional mobility strategies to support daily activities | Same as interventions for Mid-Stage plus: •Management of urinary dysfunctions •Posture, seating, and positioning, skin pressure care |
| Trainings | •Metacognitive and cognitive strategies training (e.g., training in the use of attentional strategies, auditory, visual, and sensory cueing, action observation, motor imagery techniques)* •Exercise modalities to support function and engagement in meaningful activity (e.g., LSVT BIG™) | •Task/goal-oriented training for ADL, IADL (e.g., meal preparation, home management, financial management), handwriting, fine motor coordination (e.g., attentional strategies, auditory, visual, and sensory cueing, action observation, motor imagery techniques) •Optimal transfer from floor strategies to reduce injury, fear and improve self-management | •Environmental adaptations •Cognitive strategies for visual dysfunction •Optimal assistance strategies for care partners (cueing as opposed to physical assistance) •Functional mobility training/consider motor and non-motor fluctuations & on/off states | •Utilize a tailored mix of cognitive and sensory strategies, assistive devices and adaptations, advice about support services - as desired/required, and goal oriented cognitive rehabilitation •Compensatory strategies to optimize comfort and well-being •Cognitive stimulation program/ tailored activities for best quality ‘leisure time’ •Care partner training (e.g., transfer techniques, manual handling) •Assist with end-of-life planning |
| Examples of Assistive Technology or Devices | •Education and demonstration about person-centered use of technology and devices to support and enhance daily living | Same as interventions for Upon Diagnosis plus: •Medication organizers •Calendar/memory aids •Hand & grab bars - steps, stairs, bathroom, toilet •Night light •Technology/Aids, (e.g., writing stylus for touch screen devices, mounting device/ holders/stands), familiarization with accessibility features on phone/computer (e.g., text size/display, dictation, reminders, touch screen, word prediction, speed control, hands-free/voice control, voice over/screen reader), introduction to application software (e.g., tremor regulation, speech to text), adaptive hardware (e.g., mouse, keyboard) •Personal/home safety alert systems •Environmental controls/home automation | Same as interventions for Early Stage plus: •Adaptive clothing (e.g., magnetic fasteners/Velcro) •Dressing aids (e.g., shoehorn, elastic laces, zipper pull) •Adaptive utensils/cups/straws •Electric can/jar opener •Electric supplies, (e.g., toothbrush, razor) •Home equipment needs, (e.g., bedside commode or urinal, shower chair/tub transfer bench, hand-held shower system, anti-slip bathroom mat, bed rail/ transfer pole, toilet safety frame, blow dryer stand) •External visual aids (e.g., reminder signs, visual targets) •Collaboration with PT to determine appropriate mobility aids •Recreation/Leisure aides, (e.g., large print books, magnifiers for reading, audio books/podcasts, card holder) | Same as interventions for Mid-Stage plus: •Companion wheelchair •Powered lift recliner chair •Rolling shower chair •Standing aids |
| Examples of Complementary Modalities/ Community Exercise | •Education in appropriate community and home exercise options, individual or group settings •Tai Chi, yoga, dance •Relaxation, mindfulness •Massage, acupuncture for pain or stiffness •Forced amplitude cycling •Nordic walking •Hobbies and leisure activities, (e.g., reading, gardening, music, arts and crafts) •Nutrition | Same as interventions for Upon Diagnosis plus: •Cognitive Behavioral Therapy •Partnered and unpartnered dance •Time in nature/outdoors •Self-management groups and community engagement opportunities | Same as interventions for Early Stage plus: •Support groups/socialization •Group exercise •Music (cueing and leisure) •Pelvic floor therapy | Same as interventions for Mid-Stage plus: •Socialization •Massage •Pet/animal companion |
ADL: activities of daily living; IADL: instrumental activities of daily living; LSVT: Lee Silverman Voice Treatment. *Meaningful activities include physical, social, and leisure activities that are tailored to the person’s needs and preferences. May include activities of daily living (ADL) or leisure activities, may be structured or spontaneous, and may provide emotional, creative, intellectual, and spiritual stimulation.[216]. NOTE: The assessments and interventions listed in this table are not a comprehensive list of all strategies used in PD. These are examples that may be used and tailored to a person with PD’s specific needs and goals. Not all treatment approaches listed in this table will be applicable for every individual. Furthermore, there may be items listed in later stages that may also be applicable upon diagnosis or in earlier stages, depending on the individual.
Table 4
Optimal rehabilitation assessments and interventions for physical therapy
| Upon Diagnosis | Early-Stage PD | Mid-Stage PD | Advanced PD | |
| Evaluations | •Baseline assessment of motor function, fall risk, and home exercise program | •Regular re-assessment of motor function, fall risk, and home exercise program •Screen for home equipment, work accommodations, walking aides, if needed | •Regular re-assessment of motor function, fall risk, home exercise program, and PT needs •Evaluation for ambulation devices •Home safety •Home mobility needs (e.g., bed, transfers) •Home equipment needs | •Regular re-assessment of motor function, fall risk, home exercise program, and PT needs •Ambulation devices •Wheelchair and positioning •Home safety Home mobility needs (e.g., bed, transfers) •Home equipment needs •Care partner education and needs |
| Management | •Exercise* prescription tailored to the individual’s profile and preferences •Moderate to high intensity aerobic exercise (e.g., treadmill walking, brisk overground walking, stationary cycling) •Progressive resistance exercise (e.g., free weights, weight machines, body weight) •Agility/balance Exercise (Tai Chi, dance, multicomponent balance exercises) •Stretching/flexibility Exercises | Same as interventions for Upon Diagnosis plus: •Amplitude-based exercise •Progress/modify exercise program and promote long-term adherence | Same as interventions for Early Stage plus: •Exercise program with assistance, if needed •Management of motor fluctuations and complications | Same as interventions for Mid-Stage plus: •Exercise program with assistance •Management of increased postural deformities |
| Trainings | •Home exercise program •Home and work set-up needs, as appropriate | •Gait training / Compensatory strategies as needed (e.g., external cueing: rhythmic auditory stimulus) •Pelvic floor training | •Falls prevention program (with OT) •Gait training / Compensatory strategies (e.g., external cueing, internal cueing, weight shifting for gait initiation, wide arcs for turning) •Dual-task training Strategies for freezing of gait •Respiratory training •Pelvic floor training •Functional mobility training / consider motor and non-motor fluctuations &on/off states | •Falls prevention program •Instruction in compensatory strategies •Respiratory training •Care partner training and/or home care •Functional mobility training / consider motor and non-motor fluctuations on/off states •Pelvic floor training |
| Examples of Assistive Technology or Devices | •Wearable devices to track physical activity &exercise | •Wearable devices to track physical activity &exercise | •Mobility aids (e.g., cane, rollator, laser/metronome mounted to cane) •Home equipment needs (e.g., raised toilet seat, tub bench, shower chair, grab bars in shower/tub; bed rail) •Auditory aids (e.g., metronome, music) to facilitate improved quality of walking or for freezing of gait •External visual aids (e.g., reminder signs, visual targets, laser pointer, lines on the floor for freezing of gait) •Wearable devices to track physical activity &exercise | •Mobility aids (e.g., cane, rollator, laser/metronome mounted to cane) •Home equipment needs (e.g., raised toilet seat, tub bench, shower chair, grab bars in shower/tub; bed rail) •Wheelchair (companion W/C for community outings) •Auditory cueing (e.g., metronome, music) to facilitate improved quality of walking or for freezing of gait •Visual Cueing (e.g., laser pointer, lines on the floor) for freezing of gait. •Wearable devices to track physical activity &exercise |
| Examples of Complementary Modalities/Community Exercises | •Education in appropriate community and home exercise options, individual or group sessions •Tai Chi, yoga, dance, aquatic exercise •Forced amplitude or stationary cycling •Nordic walking •Non-contact boxing •Relaxation training •Mindfulness training •Massage | Same as interventions for Upon Diagnosis | Same as interventions for Upon Diagnosis | •Relaxation training •Mindfulness training |
*Exercise defined as: “A subcategory of physical activity that is planned, structured, repetitive, and purposive in the sense that the improvement or maintenance of one or more components of physical fitness is the objective” [13]. NOTE: The assessments and interventions listed in this table are not a comprehensive list of all strategies used in PD. These are examples that may be used and tailored to a person with PD’s specific needs and goals. Not all treatment approaches listed in this table will be applicable for every individual. Furthermore, there may be items listed in later stages that may also be applicable upon diagnosis or in earlier stages, depending on the individual.
Table 5
Optimal rehabilitation assessments and interventions for speech language pathology
| Upon Diagnosis | Early-Stage PD | Mid-Stage PD | Advanced PD | |
| Evaluations | •Baseline assessment of speech function, swallowing, cognitive function, and communication •Oro-facial muscle examination •Swallowing assessment with timed water swallow test or instrumented examination if appropriate •Request person with PD to make and save own speech video recordings [to review with SLP clinician] •Language domain evaluation | •Swallowing assessment with timed water swallow test or instrumented examination if appropriate •Regular re-assessment of speech, swallowing, cognitive and communication function •Request person with PD to make and save own speech video recordings [to review with SLP clinician] •Referral to ENT/ otolaryngology at any point if vocal fold pathology is suspected | •Regular re-assessment of speech, swallowing, cognitive and communication function | •Regular re-assessment of speech, swallowing, cognitive and communication function •Swallowing assessment and safety management advice •Medication swallowing assessment |
| Management | Carry out baseline assessment of communication and swallowing and record measures such as: •Vocal loudness average in conversational speech •Pitch range •Maximum phonation time •Intelligibility rating •Timed water swallow test (such as 150mls) | •Swallow safety management advice including head posture (chin tuck) •Strategies to improve the safety and efficiency of swallowing (e.g., expiratory muscle strength training, EMST) •Saliva management •Consider attention to effort therapy programs (e.g., Lee Silverman Voice Treatment (LSVT), Pitch Limiting Voice Treatment (PLVT) •Facial expression, lip and tongue exercise •Cognitive exercises (may link to OT and psychology/neuropsychology sections) •Advise to participate in group activities to encourage communication | •Strategies to improve the safety and efficiency of swallowing to minimize the risk of aspiration (e.g., expiratory muscle strength training (EMST), video-assisted swallowing therapy (VAST) and compensatory techniques) •Consider intensive speech program including LSVT, PLVT •Strategies to improve speech and communication, such as attention to effort therapies •Maintenance activities to optimize speech/voice function (e.g., reading aloud, singing groups, poetry) •Facial expression, lip and tongue agility exercise •Advise on regular oral hygiene and maintenance of dental appointments to guard against oral infections •Advise to participate in group activities to encourage communication •Cognitive exercises (may link to OT and psychology/neuropsychology sections) | •Use of cueing to aid safe swallow •Consider instrumental examination of swallowing using videofluoroscopic examination of swallowing or fiberoptic endoscopic examination of swallowing •Consideration of non-oral feeding support •Discussion with medical team of alternative medications for those unable to swallow pills safely •Facial expression, lip and tongue exercise •Advise on regular oral hygiene and maintenance of dental appointments to guard against oral infections •Advise to maintain social participation, storytelling and chatting •Cognitive exercises (may link to OT and psychology/neuropsychology sections) •Collaboration with dietician / nutritionist / OT/ PT to aid swallow management and avoidance of malnutrition, dehydration, and aspiration |
| Trainings | •Home exercise program for speech, swallowing, and cognition | •Home exercise program for speech, swallowing, and cognition | •Conversational care partner training to teach strategies such as cueing to facilitate the learned technique for producing more intelligible speech | •Care partner training for evolving communication needs |
| Examples of Assistive Technology or Devices | •Education about speech assistive devices and technology | •Vocal loudness monitoring apps and vocal loudness feedback. •Text to speech apps on phones and tablets | •Vocal loudness monitoring apps and vocal loudness feedback. •Swallow reminder buzzers/apps to aid saliva management | •Assessment and trial of alternative and augmentative communication equipment to supplement speech and optimize communication (alphabet and picture charts, communication apps with key words and phrases spoken out, text to speech apps on phones and tablets, pacing boards) |
| Examples of Complementary Modalities/ Community Exercises | •Music Therapy •Rhythm Therapy •Theatre/acting •Choir / singing •Relaxation •Breathing Therapy•Poetry reading •Book Groups •Pilates and Yoga for breath and voice control •Nutrition | Same as modalities for Upon Diagnosis | Same as modalities for Upon Diagnosis | Same as modalities for Upon Diagnosis |
NOTE: The assessments and interventions listed in this table are not a comprehensive list of all strategies used in PD. These are examples that may be used and tailored to a person with PD’s specific needs and goals. Not all treatment approaches listed in this table will be applicable for every individual. Furthermore, there may be items listed in later stages that may also be applicable upon diagnosis or in earlier stages, depending on the individual.
Table 6
Optimal rehabilitation assessments and interventions for psychology/neuropsychology
| Upon Diagnosis | Early-Stage PD | Mid-Stage PD | Advanced PD | |
| Evaluations | •Baseline assessment of cognitive and behavioral function •Assessment of psychosocial needs and support system •Assessment of care partner needs | •Regular re-assessment of cognitive and behavioral function, psychosocial needs of the person with PD and their care partners | •Regular re-assessment of cognitive and behavioral function, psychosocial needs of the person with PD and their care partners | •Regular re-assessment of cognitive and behavioral function, psychosocial needs of the person with PD and their care partners |
| Management and Training with specific interventions | ||||
| Cognition-Oriented Interventions | COGNITIVE TRAINING (PD with intact cognition or PD with mild cognitive impairment, MCI) Intervention focus: Structured, standardized tasks to train individuals on cognitive processes and abilities Context: Structured environment (at home or in laboratory) •Heterogeneous approaches: numerosity (single vs group-based training), level of support (therapist-facilitated vs. self-guided vs. hybrid) tools (paper and pencil vs PC-based, non-adaptive vs adaptive). Cognitive strategy instruction: •method of loci, visual imagery, memory cards | COGNITIVE TRAINING or COGNITIVE REHABILITATION (PD with MCI to early-stage dementia) Intervention focus: Cognitive abilities, unstructured tasks required to perform everyday (instrumental and basic) activities •cognitive training •cognitive oriented physical therapy Context: the person’s natural environment Approaches: Individualized goal-oriented and holistic approaches to target restrictions in everyday life functioning. Cognitive strategy instruction (specific): •method of loci, visual imagery, memory cards | Overlap exists with early-stage PD (heterogeneous intact to PD MCI to early-stage dementia) •Treatments should be tailored to PD cognitive stage. •If intact cognition to early PDD, consider treatment as in the early-stage PD •If moderate to severe dementia, consider treatment as in the Advanced PD stage | COGNITIVE STIMULATION (moderate to severe dementia) Intervention focus: Non-specific, cognitive and social stimulation focused on basic functioning: •Spatial/temporal orientation •Global cognitive status •Self-reported quality of life/well-being improvement Context: at home clinic or community settings •orientation global cognitive status self-reported quality of life well-being improvement Approaches: mostly provided in a small group (7-8) twice a week, or individualized approach Cognitive strategy instruction: •reminiscence therapy, reality orientation, multisensory stimulation, personalized psychosocial intervention |
| Behavioral therapies | •Cognitive behavioral therapy for depression and anxiety •Acceptance and Commitment Therapy | •Cognitive behavioral therapy for depression and anxiety •Compassion Focused therapy | Same as interventions for Early-Stage PD plus (adaptations for level of cognitive, motor functioning): •Psychoeducation •Environmental and behavioral modifications •Strategy training Also, •Psychosocial strategies to address other behavioral aspects, (e.g., psychosis, Impulse control behaviors, agitation, apathy, including care partner support) •Modify home environment for simplicity and reduction of excess stimuli •Cognitive behavioral therapy targeted for specific behavior | Same as interventions for Mid-Stage PD plus (adaptations for level of cognitive and motor functioning, increased focus on caregiver and cognitive fluctuations): •Psychoeducation •Environmental and behavioral modifications •Strategy training Also, •Psychosocial strategies to address other behavioral aspects, (e.g., psychosis, Impulse control behaviors, agitation, apathy, including care partner support) •Cognitive behavioral therapy targeted for specific behavior |
| Examples of Assistive Technology or Devices | •Virtual reality training •Computer-based cognitive training •Telerehabilitation: therapist facilitated, self-guided, hybrid. •Non-invasive stimulation (e.g., TMS, tDCS) •Biofeedback devices •Communication aides/assistive technology | •Virtual reality training •Computer-based cognitive training •Telerehabilitation: therapist facilitated, self-guided, hybrid. •Non-invasive stimulation (e.g., TMS, tDCS) •Biofeedback devices •Communication aides/assistive technology | •Virtual reality training •Computer-based cognitive training •Telerehabilitation: therapist facilitated, self-guided, hybrid. •Non-invasive stimulation (e.g., TMS, tDCS) •Biofeedback devices •Communication aides/assistive technology •Compensatory strategies | •Communication aides/assistive technology •Compensatory strategies and cues •Ecologic tools (e.g., paper, whiteboard, food, objects, colors, money, pictures) •Environmental adaptations (e.g., ‘Prosthetic environment,’ ‘smart house technology,’ reduced stimuli design |
| Examples of Complementary Modalities/ Community Exercises | •Mindfulness, relaxation, yoga •Music and arts therapy | Same as interventions for Upon Diagnosis plus: •Relaxation therapy (autogenic training, breathing techniques) | Same as interventions for Early-Stage PD with adaptations for level of cognitive, motor, and other functioning | Same as interventions for Early-Stage PD with adaptations for level of cognitive, motor, and other functioning •Care partner involvement |
NOTE: The assessments and interventions listed in this table are not a comprehensive list of all strategies used in PD. These are examples that may be used and tailored to a person with PD’s specific needs and goals. Not all treatment approaches listed in this table will be applicable for every individual. Furthermore, there may be items listed in later stages that may also be applicable upon diagnosis or in earlier stages, depending on the individual.
All Task Force members (n = 16) completed voting rounds for the statements regarding fundamental components of rehabilitative care. In round one, there was > 80% consensus on all items, except one, and comments provided. With feedback and discussion, the statements were revised and refined in their ordering and wording (e.g., to reflect an international audience, reduce repetition, etc.). After revisions, they received unanimous (100%) agreement. For the discipline-specific interventions, voting was completed by 15 of 18 group members and met with > 80% agreement on the suggested OT, PT, SLP, and psychology/neuropsychology strategies.
Fundamental components of rehabilitative care for PD
Rehabilitative care in PD is grounded in several key tenets: the recognition of the complexity and heterogeneity of PD symptoms and progression; the importance of an individually tailored, comprehensive, and collaborative therapeutic approach; and its role, alongside medical, surgical, experimental, and other strategies often utilized in PD, but also as monotherapy at times [12]. Given this complexity, rehabilitation for PD should involve the person with PD and care partners (if present) throughout the process, thereby adopting a person-centered approach and shared decision-making model to promote symptom management and self-efficacy [28, 38–40, 52].
To ensure a comprehensive approach, the rehabilitation care team should include multiple disciplines working together in a coordinated fashion, along with the person with PD and care partners. The clinical team should have knowledge and expertise specific to PD and rehabilitation. Composition and structure of the rehabilitation team may vary across the world depending on patient needs related to their motor and non-motor symptoms, stages of disease, and individual goals and priorities; different practice settings; local or national health systems; and availability of trained personnel, but at its core may include those listed alphabetically in Fig. 2.
Fig. 2
Core Rehabilitation Care Team and Other Relevant Healthcare Professionals for PD.
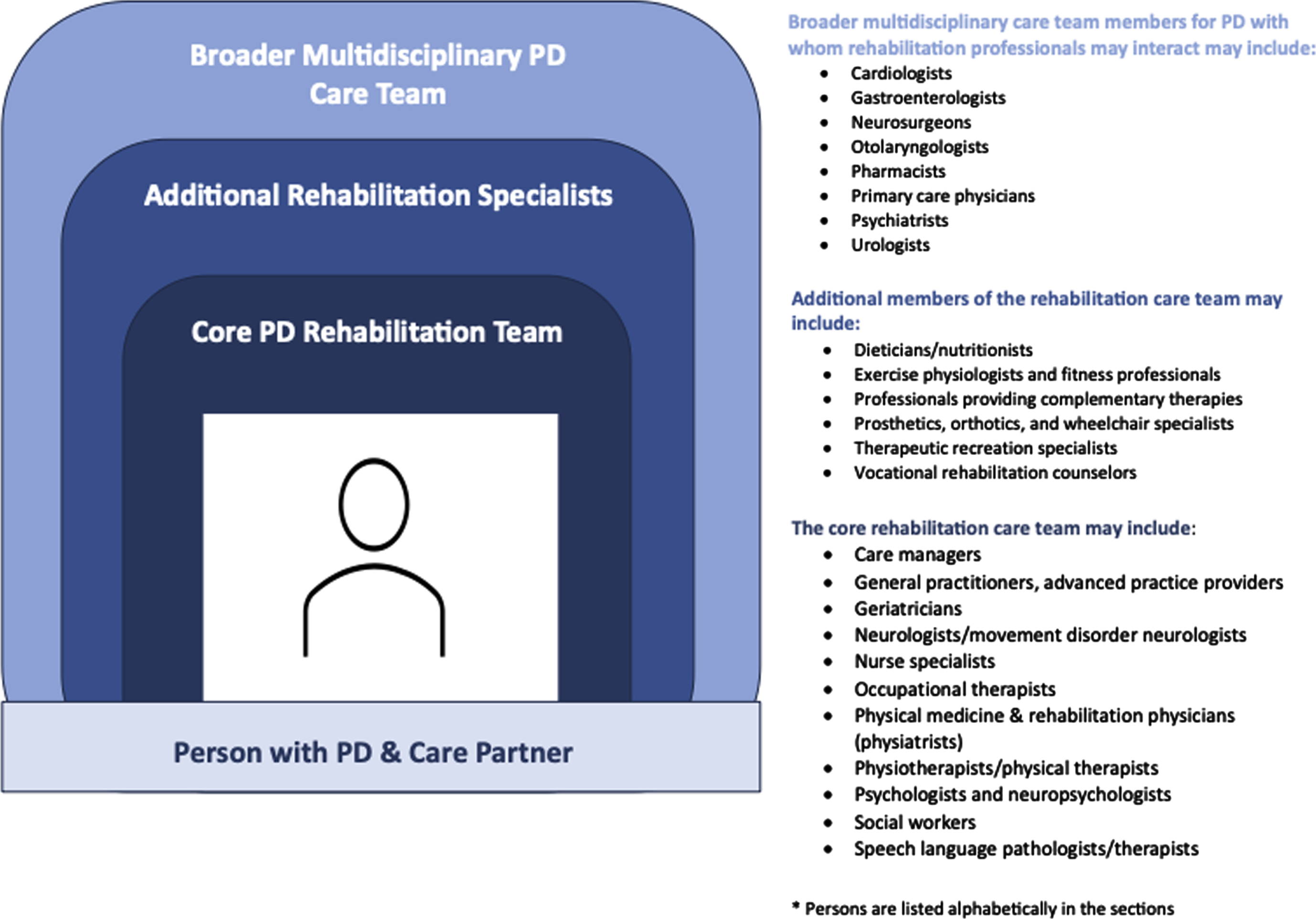
The rehabilitation team is anticipated to interact with other healthcare professionals of the PD multidisciplinary care team (e.g., cardiologists, gastroenterologists, neurosurgeons, pharmacists, psychiatrists), among others [27]. To date, the optimal combination of these multidisciplinary rehabilitation and other healthcare professionals in PD care is unknown [41] but should be tailored to each individual’s specific needs.
Care coordination and communication among the rehabilitation team, other PD-related multidisciplinary team members, the person living with PD, and care partners are critical to support an optimal approach to rehabilitation care [27, 28, 37, 53]. In some settings, the physiatrist plays a key role in coordinating and working with the multidisciplinary rehabilitation care team in PD; this teamwork may occur in different rehabilitation care settings (e.g., inpatient rehabilitation hospital, outpatient, or day rehabilitation) and may vary across geographical locations [26, 54]. However, in other locations and settings, this care coordination may be spearheaded by other team members such as movement disorder neurologists, geriatricians, nurses, case managers, social workers, or rehabilitation therapists.
A unifying principle of rehabilitative care is the integration of the multidisciplinary team. This integration may be facilitated on a practice level by scheduled and regular multidisciplinary rehabilitative team meetings in outpatient, inpatient rounding, and/or case conference settings [27, 55, 56]. Examples from interprofessional, multidisciplinary rehabilitation care for stroke and other neurological conditions may provide insights for team-based opportunities for people with PD [57–59]. Additionally, the inclusion of people with lived experience and shared decision making into team settings is an actively growing area in PD [60, 61]. Ideally, clinical activities are supported by and combined with educational and research-related activities (e.g., interprofessional journal clubs, seminars, and projects involving various rehabilitative care team members, clinicians, and scientists working together and co-leading, where appropriate). These settings may enrich interactive discussions among team members regarding topics such as evidence-based and cost-effective interventions for integrated care and bridging clinical care and research. Clinical-research-patient partnerships may help advance the scientific and evidence-base of rehabilitation therapies, delivery models, and implementation in clinical settings. Successful multidisciplinary integration requires knowledge and skill building related to teamwork and communication.
Rehabilitative care planning should emphasize the needs and goals of the person with PD, develop strategies for self-management, and include care partners where appropriate [26]. Furthermore, the Task Force recommends that rehabilitative interventions occur across the continuum of PD, from the time of diagnosis through advanced disease stages. Baseline rehabilitation assessments and serial follow up are recommended with adjustments to goals as a person’s needs change in order to enable timely provision of continuous care in the context of a progressive disease [62].
Rehabilitative care may occur in a variety of settings depending on geographical and cultural diversity, available trained personnel, resources, and patient needs. Providing optimal PD rehabilitative care likely necessitates addressing potential barriers such as access, insurance coverage, availability of trained personnel, social and cultural factors, and other elements that may limit referrals and care utilization.
Education about the role of rehabilitative interventions, types of providers, and access to care is needed for the PD patient community and the healthcare professional community. For healthcare professionals, PD-specific education and training in rehabilitation disciplines is paramount. For example, the ParkinsonNet concept has proven that PT delivered by specialized therapists in PD leads to better quality of care and lower complication rates compared to PT delivered by general therapists [63]. Developing communication skills and effective teamwork among clinicians is also essential [64].
Guidance for discipline-specific rehabilitative interventions
Based on the available evidence-base and its expert opinion, the Task Force presents what it considers optimal rehabilitative interventions across the stages of PD for the disciplines of OT, PT, SLP, and psychology/neuropsychology. The Task Force chose to focus on these disciplines for this document given current literature supporting their roles in PD and their generally greater availability and accessibility to the PD community across diverse practice settings and delivery models, even though often under-utilized, at present [23, 24, 56]. To date, the largest body of evidence exists for PT, and PT is more commonly represented among rehabilitation services used in PD compared to OT and SLP [23, 24]; however, for this document, we have chosen to present the rehabilitation disciplines in alphabetical order as OT, PT, and SLP, followed by psychology/neuropsychology. We acknowledge the important roles of other healthcare disciplines in rehabilitative care for PD, and future recommendations may highlight professionals such as care managers, dieticians/nutritionists, exercise physiology, nurse specialists, social workers, and therapeutic recreation, among others, as their evidence bases increase [53]. The interventions proposed for OT, PT, SLP, and psychology/neuropsychology are intended to be utilized in an integrated but flexible fashion that allows for cross-disciplinary collaboration to address specific needs and goals in PD, especially as these may change in a chronic, progressive condition. While beyond the scope of this work to depict, there are many examples where the rehabilitative team can communicate and work across different disciplines (e.g., SLP and OT for cognition and communication skills in everyday activities; SLP and nutrition regarding dysphagia; OT and PT for dual-tasking and navigating home or other environments; psychology and PT for motivation and exercise).
Regardless of discipline, rehabilitative care professionals should conduct PD-related baseline assessments upon diagnosis and at regular re-evaluations over the disease course and accordingly adjust interventions for different needs and goals [65], ensure collaborative teamwork and person-centered care to tailor interventions and enhance self-management strategies, and coordinate with multidisciplinary team members, as appropriate, to establish and deliver care plans. For the disciplines discussed here, assessments and interventions may occur in a variety of settings, including outpatient therapy, inpatient rehabilitation, day programs, home therapy, skilled nursing facilities, and via telehealth.
Occupational therapy
Occupational therapists help people restore, improve, and maintain skills and abilities to participate in meaningful activities [66]. For people living with PD, occupational therapists foster improvement, safety and independence in self-care, daily tasks, and functional mobility to support continued engagement in activities related to various environments such as the home, work, and community [65]. Occupational therapists play a role in educating and supporting care partners to safely and appropriately assist the person with PD in daily tasks while being mindful of their own safety and well-being [67]. Occupation-based performance assessments should be led by an occupational therapist skilled in the use of PD-specific intervention approaches [68]. The occupational therapist should collaborate with the person with PD and care partner to prioritize goals that are meaningful and relevant, select outcome measures that inform interventions, and support engagement in the therapy process. An individualized, goal-oriented approach—where OT intervention is focused on a specific set of personally meaningful goals—has been shown to yield greater improvement for people with PD compared to use of a generalized approach [69]. The role of OT in PD is supported by the American Occupational Therapy Association clinical practice guidelines [34] and systematic reviews with moderate to strong evidence for OT-related interventions for activities of daily living, instrumental activities of daily living, handwriting, physical activity, but lower evidence to date for cognitive rehabilitation [70–72].
Several categories of OT-related interventions for people with PD have been identified: 1) exercise or physical activity; 2) environmental cues, stimuli, and objects; and 3) self-management and cognitive behavioral strategies [34, 73]. Occupational therapy strategies may include a combination of personalized adaptation of tasks and daily routines, cues and compensatory strategy training, adaptations to the physical environment, and incorporation of concepts of motivation, reward, and learning [67].
Emerging areas of intervention in occupational areas include sleep, activities of daily living (ADL), instrumental activities of daily living (IADL), leisure, and social activities [34]. Systematic reviews identify several beneficial interventions for ADL such as multidisciplinary inpatient rehabilitation [16, 21, 74, 75], group multimodal exercise programs [74, 76–78], and home-based hand exercise programs [74, 79]. Cognitive behavioral therapy (CBT) focused on sleep hygiene and behaviors [80, 81], resistance training, multimodal exercise, and mindfulness meditation combined with exercise can be used to improve sleep [74, 82–84]. Evidence supports interventions for IADL including education, mentoring, social support, and behavioral change techniques to increase activity levels and multimodal exercise programs that promote IADL participation, function, and social engagement [34, 85–89]. Handwriting may improve with use of intensive amplitude training with visual targets [90, 91], hand exercises, and writing activities [92]. Dual-task training can be used to forge stronger connectivity between two task-specific networks to enable smoother performance of selected tasks such as carrying items while walking [93] which can link OT with PT interventions. Goal-oriented cognitive rehabilitation for dementia associated with PD was found to be feasible, with improved quality of life including care partners [1, 94]. As PD progresses, sensory and cognitive coping strategies may wane in effect, and goals may shift. Participation in work/employment, driving, and other home or hobby endeavors may change with PD progression, and OTs may play a role in these areas.
Physical therapy
Physical therapists are experts in human movement who diagnose, evaluate, and treat individuals to improve their ability to move, manage pain, and prevent or manage disability [95]. For people with PD, physical therapists play a role in areas such as physical movement, gait and balance, exercise interventions, daily activities, among others. The initial visit to a physical therapist may occur before or at the onset of medication initiation and may coincide with initial visits to other members of the rehabilitation team, including occupational therapists and speech language pathologist/therapist. The role of the PT in PD is supported by clinical practice guidelines [72, 96] and systematic reviews that include Class I and Class II randomized clinical trials. PT can improve gait, balance, and falls in early and moderate stages of PD, though additional evidence base in advanced PD is needed. Recently published PT clinical practice guidelines offer the highest strength of recommendation for aerobic exercise, resistance training, balance training, community-based exercise, gait training, task-specific training, and integrated care [96].
The components of PT intervention incorporate a variety of forms of physical and cognitive exercise and strategy training [8, 11, 97]. Moderate to high-intensity aerobic exercise has been shown to reduce the severity of motor symptoms, increase oxygen consumption, and improve function [96, 98–100]. These benefits have been demonstrated in early (including de novo) PD, suggesting the importance of early engagement in aerobic exercise [99, 100]. Treadmill training and cycling yield similar results, thereby, suggesting that aerobic exercise benefits cross a variety of modalities [99, 100]. Resistance training has been shown to reduce bradykinesia and improve force production, cognition and function [101, 102]. Balance training (e.g., Tai Chi, dance, virtual reality, motor-cognitive training, multicomponent balance exercises) is a critical element of exercise in PD [103–106]. Evidence reveals improvements in balance outcomes with balance training, although the impact on falls is mixed [107–109]. Studies suggest that people in moderate (i.e., early fallers) rather than later stages of PD are more likely to respond to balance training, thereby reducing fall frequency [107, 108, 110, 111]. These findings support the initiation of early balance training to mitigate falls and secondary complicating conditions.
Strategy training in PT improves function in PD [112, 113]. For example, gait training encompassing cueing (e.g., metronome, music, instructional cues to “take long steps,” and vibrotactile feedback), strategies to overcome freezing of gait, and dual-task training (e.g., cognitive-motor interference) have been shown to improve a variety of gait outcomes, particularly when tailored to the individual’s needs [93, 114–119]. Exercises and strategy training also improve walking, turning, and general mobility such as chair and bed mobility [97, 110, 120]. Many of these interventions encompass task-based or skill practice which needs to be implemented with sufficient dosing (e.g., high repetitions) to optimize motor learning [22]. Therefore, training should be initiated early in the disease process as persons with PD may require more time to achieve learning. In the later stages, explicit learning methods and cueing may enhance learning [121]. Context-specificity should be considered by matching the learning environment as closely as possible to the daily functional context to optimize learning [22].
Motor-cognitive approaches in PD, or “cognitive oriented physical therapy,” reflect techniques that incorporate dual-task training or multitasking, or use cognitive strategies in order to cope with motor disability. Principles related to the complex interplay between basal ganglia-cortical and cerebellar networks suggest that rehabilitative interventions geared to engage cognition may indeed harness motor learning schema leading to broad clinical, motor and functional benefits [122, 123].
Though evidence is limited, rehabilitation and exercise may improve postural deformities in PD, particularly if initiated early [124]. Increased exercise and physical activity have been shown to improve non-motor symptoms including depression, anxiety, cognition, and sleep [125], though more evidence is needed. Incorporating behavioral change and self-management strategies into PT interventions is important to sustain engagement in exercise with the goal of optimizing long-term functional outcomes.
Speech and language pathology/therapy
Speech and language pathologists/therapists are experts in communication who evaluate and treat communication issues, including speech sounds and production, volume, language, swallowing, and cognitive aspects. For people with PD, speech language pathologists/therapists play a role in optimizing speech production, voice function, dysphagia, language and cognition, and for some, saliva management [126, 127]. In their roles, speech language pathologists/therapists may readily interface with other rehabilitation team members such as dieticians/nutritionists, otolaryngologists, and occupational therapists. As speech language pathologists/therapists may work with people regarding cognitive-communication functions, coordination, and communication, collaboration with neuropsychologists is paramount. The role of SLP is supported by systematic review for improving hypokinetic dysarthria and dysphagia, though greater evidence base is needed in the literature [12, 128–131].
SLP therapy in PD utilizes strategies to improve voice and speech, particularly hypophonia; communication, such as attention to effort therapies [132]; and safety and efficiency of swallowing to minimize risk of aspiration such as expiratory muscle strength training (EMST). Meta-analyses and Cochrane reports on voice, speech, and language therapies highlight challenges of small sample sizes and lack of randomized control trials [133, 134]. Nonetheless, SLP interventions are selected on individualized needs and use techniques such as breathing control, vocal training, speech control training, articulation therapy, or even singing [135, 136]. As pharmacological and neurosurgical treatments may have variable and sometimes adverse, unpredictable effects on improving speech production, SLP evaluation and interventions play an important adjunctive role in these contexts [137]. Regular SLP outcome measures should be obtained for vocal loudness in conversational speech, pitch range, maximum phonation time, intelligibility rating, and for swallowing. Intensive speech programs with amplitude-based training should be considered, including the Lee Silverman Voice Treatment (LSVT®), which has the largest evidence-base to date [138, 139], pitch-limiting voice treatment, or SPEAK OUT! [140, 141]. Singing provides another modality to enhance voice and help people with PD engage socially and in leisure activities [142, 143]. Use of volume monitoring apps, audio recordings, and other technologies can provide ways to self-monitor voice and speech production, volume, and facial expression and thereby receive external feedback and cues for adjustments [144–148]. Referrals for augmentative alternative communication technology to supplement speech and optimize communication (e.g., alphabet and picture charts, communication apps with key words and phrases spoken out, text-to-speech apps, pacing boards) may be required if patients experience greater decline in communication ability.
Although dysphagia is highly prevalent in PD, it is often under-reported, and may lead to aspiration pneumonia and other consequences [116, 149–151]. It requires regular clinical assessment and potentially instrumental examinations such as video fluoroscopy or fibreoptic endoscopic examinations, if available. Treatment involves education regarding ways to optimize swallowing safety with food texture modifications, postural changes when eating or drinking, and exercises including LSVT [152], EMST [153], and chin-tuck against resistance [135, 154–157]. Speech language pathologists/therapists may play a role in managing drooling, particularly as oral medications to reduce sialorrhea may have adverse effects or contraindications in PD, and also in improving facial expression [12, 158–160].
Psychology/neuropsychology
Psychologists and neuropsychologists play a role in the assessment and management of cognitive, mood, and behavioral symptoms in PD (e.g., cognitive decline, depression, anxiety, apathy, psychosis, impulse control behaviors, fatigue, and sleep disturbances), and the impact of these issues on relationships, independence, work, and quality of life for the person with PD and care partner. Therapeutic strategies used by psychologists/neuropsychologists may overlap with other disciplines (e.g., social work, SLP, OT, vocational rehab) and may occur simultaneously or separately with medical and surgical interventions. Evidence for the role of psychology and neuropsychology in PD is growing but available evidence on effectiveness of interventions is heterogeneous due to the broad nature of types of symptoms and interventions, small number of studies, and limited robustness of studies at present [161]. Cognitive rehabilitation interventions may improve global cognition, executive function, and memory, but more randomized controlled trials are needed [162–164].
Optimal rehabilitative interventions for neuropsychiatric symptoms should focus on symptom screening, regular assessments, and management including psychoeducation and support for people with PD and their care partners. The screening and assessment for neuropsychiatric symptoms may involve brief screenings (e.g., bedside tests) or more comprehensive neuropsychological test batteries, depending on the needs of a patient, practices, and availability of neuropsychological services.
Management for depression may involve CBT, which has been noted as efficacious for depression whether delivered via in-person, telephone-based, or telehealth video [165–170]. While few randomized controlled studies of CBT for anxiety in PD exist, preliminary investigations report reductions in anxiety symptoms [165, 169, 170].
Cognitive rehabilitation refers to a wide range of interventions focused on improving cognitive function or compensating for cognitive deficits by using individualized programs of specific skill training [171]. Evidence suggests that cognitive training (CT), physical exercise, and the combination of both can improve certain cognitive functions in PD [164, 172–174]. CT may be most effective for those with mild or moderate cognitive dysfunction rather than severe deficits. Evidence based on randomized controlled trials (7 RCTs, 272 pts), though limited, indicates efficacy on working memory, executive functioning, and processing speed domains [174]. CT can be performed using pen-and-pencil or computer-based exercises in individual or small group settings. Tailored “game-like” tasks (videogames and virtual reality) have the advantages of providing immediate feedback, motivating engagement, and being performed at home, which can increase frequency of training and reduce drop-out [175, 176].
In the setting of dementia, the clinical outcomes of a cognitive rehabilitation program should focus on maintaining cognitive competence, ADL, mood, and coping strategies; lessening social and psychological disability; and reducing the experience of ‘burden’ and stress in families. When considering disease-specific characteristics, effective cognitive programs need to be tailored to the PD stages, severity of cognitive dysfunction (i.e., dementia or mild cognitive impairment), and type of cognitive characteristics (e.g., executive function vs. memory) [177].
Emerging rehabilitation therapies in PD
Complementary medicine therapies, community exercise training, and other modalities have increased applications for PD [6, 178]. Examples include mind and body practices (e.g., massage, meditation) [179], dance therapies [180–182], aquatic exercise [78, 183], boxing [141], Nordic walking [184], acupuncture [185], relaxation techniques and mindfulness [185, 186], Tai Chi [187], yoga [188], music therapy [189], art therapy [190], and theater [191]. There are few evidence-based recommendations for the effectiveness of many of these modalities, though this is a growing area of study [192]. However, for some, the evidence supports integration into rehabilitative medicine for PD [6]. In particular, dance (e.g., Irish set dancing, Argentine tango, ballet, ballroom dancing) may improve mobility, gait, balance, fall rates, ADL, and quality of life [180, 182, 193, 194]. Tai Chi has been found to increase mobility, balance, and reduce falls [105, 195, 196]. Aquatic exercise programs also may improve mobility, gait and balance, ADL, and quality of life [78, 183, 195, 197, 198]. With increasing use of these complementary modalities, knowledge of PD symptoms, stages, and safety issues are critical for those professionals involved.
In recent years, the use of technology in PD and rehabilitation has grown including assistive technology (e.g., adaptive technology and devices), wearable sensors, home monitoring, and virtual or augmented reality techniques [199]. Objective assessment and monitoring of motor symptoms across large PD populations and in home or other environments are feasible [200]. Adaptive technology and mobile applications in PD demonstrate promising ways to improve speech, communication, and motor functionality [201]. Assessments with assistive technology in standardized settings are well validated, but studies often had small sizes with significant variability in outcomes and results reported, [202] and controlled trials of adaptive technology and devices are limited to date. Virtual reality techniques have been employed in PD as exergames, immersive environments, and other strategies for promoting physical activity, improving balance and motor function, reducing freezing of gait, and enhancing manual dexterity. Systematic reviews of virtual reality techniques, however, suggest variable methodological quality of studies, thereby limiting conclusions regarding effectiveness and safety for PD rehabilitation at present. While promising, rigorous and robust randomized controlled trials with larger sample sizes are needed [203, 204]. Given the emerging and broad nature of these therapies and interventions, the Task Force does not offer guidance for these interventions at the present time.
DISCUSSION
Robust literature supports the benefits of rehabilitative interventions in PD for motor and non-motor symptoms, functional abilities, and quality of life. However, to date, many healthcare professionals and people with PD remain unaware of the potential impact of rehabilitative services and evidence-based approaches to rehabilitation interventions in PD [23, 24, 26], thereby limiting their use and availability in the PD community. Studies reveal that up to 57% of people with PD are never referred to PT in their lifetime [205], with similar rates regarding referrals to SLP [206]. Furthermore, utilization of rehabilitation services is more likely to occur in later stages of PD despite mounting evidence of benefits from earlier engagement in therapies [24], even at the time of diagnosis, and regarding proactive strategies that impact symptoms and quality of life [26, 207, 208]. Unless contraindicated, physical activity and exercise also should be encouraged given their beneficial effects [89]. In many cases, rehabilitative services are only offered once in the course of the disease. However, given the progressive nature of PD and the subsequent changes in individual’s goals and needs across these different PD stages, rehabilitative care should be offered regularly throughout the disease course with repeat assessments and interventions adapted to changes in a person’s condition or needs [24]. As such, this international consensus statement begins to address these gaps and serves as a first step in raising global awareness of the role of comprehensive rehabilitation programs in PD.
The field of rehabilitation is inherently multidisciplinary, uses a coordinated and integrated team approach, and focuses on goal-oriented, person-centered, and individualized approaches to improve function and quality of life. These elements are essential for PD care, especially due to its complexity, heterogeneity, and chronicity [28, 52, 209]. Indeed, giving patients greater control over their situation through patient-provider collaboration and shared-decision making has recognized benefits in PD [38, 210, 211]. Person-centered, goal-directed interventions have been shown to be more effective than usual care across the continuum of PD [69, 94]. The Task Force consensus statements reflect these key elements of rehabilitative care and services in PD.
Regarding the multidisciplinary rehabilitation team, the Task Force emphasizes the importance of including healthcare professionals who demonstrate appropriate knowledge and expertise in PD and rehabilitation. Education and training in these areas, along with teamwork and coordination of care, are areas for continued growth opportunities. Considerable debate remains as to the optimal organization and management of rehabilitation care models and their effects in PD. For example, questions remain regarding the optimal combination(s) of rehabilitation professionals, outcome measures, and practice settings, among other aspects. In addition, the timing and regularity of assessments and interventions, as well as their “dose” or intensity, are not fully elucidated. In one model, called the secondary prevention or “dental” model in PT as proposed by Ellis and colleagues, a person with PD consults with a PT with PD-related expertise at the point of diagnosis but since the mode and intensity of exercise and PT requires adaptation to the individual’s needs as PD symptoms change over time, patients are recommended to have regular visits with the PT every 6 months or annually, much like dental care [207]. These topics represent areas for future research and consensus statements.
The Task Force consensus statements on optimal rehabilitation assessments and interventions for OT, PT, SLP, and psychology/neuropsychology complement a recent 2022 World Health Organization (WHO) report of workable avenues for action regarding the global burden of PD [212]. Specifically, the WHO report highlighted the need for inclusive rehabilitative treatments tailored to the individual and unique needs of the person with PD, for increased advocacy and awareness for all health care workers involved in PD care including neurologists, non-neurologist physicians, and allied health team members, and for greater physical activity throughout the course of PD along with the importance of the caregiver role. This further supports the need to accelerate multi-disciplinary research, in line with the key recommendations of the Task Force consensus statement.
Strengths of the Task Force consensus statement include the large international, multidisciplinary group of experts in PD and rehabilitation, use of systematized methodology, and active engagement of individuals with first-hand personal experience as integral Task Force members. In addition, the proposed discipline-specific assessments and interventions may be used in combination with previously developed mono-disciplinary guidelines, thereby expanding rehabilitative care opportunities in PD [11, 31, 34, 96]. Limitations include that due to our scope of work, the Task Force did not focus on other elements of rehabilitative care, e.g., its theoretical basis, temporal applicability, dosing schedules, and intervention combinations, though future research may address. We emphasized rehabilitation therapies (i.e., OT, PT, SLP, and psychology/neuropsychology) that are most commonly utilized, recommended, or available, even though these remain under-utilized in PD treatment; however, future recommendations could incorporate additional disciplines working in rehabilitation, such as case managers, dieticians, nurses, orthotists, social workers, physiatrists, among others. To date, available guidelines on rehabilitation in PD do not include many rehabilitation team members beyond OT, PT and SLP or reflect a variety of comprehensive care settings. While the task force composition represented 7 countries and different healthcare systems, future work may incorporate additional countries and regions to enhance knowledge sharing and greater generalization. Our inclusion of people with lived experience of PD as equal stakeholders in the Task Force represents an important advance towards person-centered care [60, 61], but we acknowledge that including even greater numbers of voices and perspectives from people with PD and care partners would be informative. Future studies are needed to address optimal ways to deliver rehabilitative care and measure its effects of clinical symptoms, disease progression, and underlying neurobiological aspects of PD. Lastly, our overall approach was based on a systematic and iterative process involving a collaborative process with group participation and feedback. As such, the group members’ identities were known to each other, and the statements were crafted with group contributions, iterative feedback, and refinement in our discussions. We employed safeguards, however, to minimize bias, groupthink, and unbalanced group dynamics with the oversight of the Steering committee and third-party healthcare consultants. Moreover, given the vast background and experience of the Task Force in the field of rehabilitation which emphasizes multidisciplinary collaboration and teamwork, this type of group and teamwork flowed naturally to allow all members to participate openly. We acknowledge that the lack of a formal Delphi process may be a methodological limitation, though also recognize that literature supports various adaptations and reporting of Delphi methods [35, 36, 213–215].
In conclusion, rehabilitative interventions are critical in the complete treatment of PD, along with medical, surgical, and other PD-related interventions. It is hoped this consensus statement will raise awareness of the importance of multidisciplinary rehabilitation in PD and motivate professionals to include evidence-based rehabilitation interventions in their multidisciplinary care of persons with PD.
ACKNOWLEDGMENTS
The authors would like to thank the Avalere Health team (Michelle Bruno, Starr Webb, and Mallory Yung) for their helpful guidance and facilitation of the Task Force consensus procedures and their assistance editing the manuscript. The authors also thank the following external manuscript reviewers for their valuable input and contributions: Stanley Fahn, MD (USA), Genko Oyama, MD (Japan), Meg Morris, PT (Australia), Ingrid Sturkenboom, OT (Netherlands), Lindsey Heidrick, SLP (USA), Rob Skelly, MD (United Kingdom), Lisa Cone, Parkinson’s advocate (USA).
FUNDING
The authors have no funding to report.
CONFLICT OF INTEREST
All Task Force members received honorarium from the Parkinson’s Foundation for their participation in the Task Force initiative unless noted.
Dr. Goldman has received grant/research support from Acadia Pharmaceuticals, American Parkinson’s Disease Foundation, Lewy Body Dementia Association, Michael J. Fox Foundation, and Parkinson’s Foundation; she has received honoraria from the International Parkinson and Movement Disorder Society and Parkinson’s Foundation.
Dr. Ellis receives funding paid to Boston University from NIH and NSF to support research. She also receives funding paid to Boston University from Parkinson’s Foundation and the American Parkinson Disease Association to support education and outreach activities. Dr. Ellis participates on various committees for the Parkinson’s Foundation, American Parkinson Disease Association, and the Michael J. Fox Foundation, and the Movement Disorder Society; all roles are unpaid.
Dr. Hirsch receives grant and other funding paid to Wake Forest School of Medicine including from: Wake Forest Clinical and Translational Science Institute, Dutch Brain Foundation, and Dutch Parkinson Patient Association. He is a board member on the Scientific Program Planning Board of the International Association of Parkinsonism and Related Disorders, and on the Editorial Board of the Journal of Parkinsonism and Related Disorders. He is also a member of the advisory board of the Parkinson Association of the Carolinas; this role is unpaid. Dr. Hirsch declined honorarium for the Task Force initiative.
Ms. Johnson received honoraria from the Parkinson’s Foundation.
Dr. Biundo has received honoraria from Bial Pharmaceuticals. She is supported by the Ministry of Health under grant number GR-2016-02361986.
Ms. St. Clair received honoraria from the Parkinson’s Foundation.
Dr. York serves on the Michael J. Fox Foundation steering committee Registry for the Advancement of Deep Brain Stimulation in Parkinson’s Disease.
Todaro, Yarab, and Wallock – Parkinson’s Foundation, NY
All other authors have no conflict of interest to report.
DATA AVAILABILITY
Data supporting this study are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230117.
REFERENCES
[1] | Bloem BR , Okun MS , Klein C ((2021) ) Parkinson’s disease. Lancet 397: , 2284–2303. |
[2] | DeMaagd G , Philip A ((2015) ) Parkinson’s disease and its management: Part 1: Disease entity, risk factors, pathophysiology, clinical presentation, and diagnosis. P T 40: , 504–532. |
[3] | Deuschl G , Beghi E , Fazekas F , Varga T , Christoforidi KA , Sipido E , Bassetti CL , Vos T , Feigin VL ((2020) ) The burden of neurological diseases in Europe: An analysis for the Global Burden of Disease Study 2017. Lancet Public Health 5: , e551–e567. |
[4] | Kaji R ((2019) ) Global burden of neurological diseases highlights stroke. Nat Rev Neurol 15: , 371–372. |
[5] | Marras C , Beck JC , Bower JH , Roberts E , Ritz B , Ross GW , Abbott RD , Savica R , Van Den Eeden SK , Willis AW , Tanner CM ((2018) ) Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis 4: , 21. |
[6] | Alves Da Rocha P , McClelland J , Morris ME ((2015) ) Complementary physical therapies for movement disorders in Parkinson’s disease: A systematic review. Eur J Phys Rehabil Med 51: , 693–704. |
[7] | Atkinson-Clement C , Sadat J , Pinto S ((2015) ) Behavioral treatments for speech in Parkinson’s disease: Meta-analyses and review of the literature. Neurodegener Dis Manag 5: , 233–248. |
[8] | Radder DLM , Lígia Silva de Lima A , Domingos J , Keus SHJ , van Nimwegen M , Bloem BR , de Vries NM ((2020) ) Physiotherapy in Parkinson’s disease: A meta-analysis of present treatment modalities. Neurorehabil Neural Repair 34: , 871–880. |
[9] | Goodwin VA , Richards SH , Taylor RS , Taylor AH , Campbell JL ((2008) ) The effectiveness of exercise interventions for people with Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 23: , 631–640. |
[10] | Sturkenboom IH , Graff MJ , Hendriks JC , Veenhuizen Y , Munneke M , Bloem BR , Nijhuis-van der Sanden MW ((2014) ) Efficacy of occupational therapy for patients with Parkinson’s disease: A randomised controlled trial. Lancet Neurol 13: , 557–566. |
[11] | Keus S , Munneke M , Graziano M , Paltamaa J , Pelosin E , Domingos J , Bruhlmann S , Ramaswamy B , Prins J , Struiksma C , Rochester L , Nieuwboer A , Bloem B ((2014) ) European Physiotherapy Guideline for Parkinson’s Disease. KNGF/ParkinsonNet, the Netherlands. |
[12] | National Institute for Health and Care Excellence, Parkinson’s disease in adults: Diagnosis and management (NG71), https://www.nice.org.uk/guidance/ng71/resources/parkinsons-disease-in-adults-pdf-1837629189061, Accessed September 4, 2021. |
[13] | Rimmer JH , Chen MD , McCubbin JA , Drum C , Peterson J ((2010) ) Exercise intervention research on persons with disabilities: What we know and where we need to go. Am J Phys Med Rehabil 89: , 249–263. |
[14] | Cohen N , Manor Y , Green Y , Tahel G , Badichi I , Ben-Or G , Shtainshlaifer N , Shiffer A , Gabso-Rajuan M , Kurtzman H , Shtraifler L , Furst T , Shtein S , Shulman J , Hyute A , Levin I , Inbar N , Ariela H , Peled R , Gheriani N , Ezra A , Messer S , Geva N , Giladi N , Gurevich T ((2021) ) Multidisciplinary intensive outpatient rehabilitation program for patients with moderate-to-advanced Parkinson’s disease. Neurorehabilitation 49: , 47–55. |
[15] | Frazzitta G , Bertotti G , Riboldazzi G , Turla M , Uccellini D , Boveri N , Guaglio G , Perini M , Comi C , Balbi P , Maestri R ((2012) ) Effectiveness of intensive inpatient rehabilitation treatment on disease progression in parkinsonian patients: A randomized controlled trial with 1-year follow-up. Neurorehabil Neural Repair 26: , 144–150. |
[16] | Frazzitta G , Maestri R , Bertotti G , Riboldazzi G , Boveri N , Perini M , Uccellini D , Turla M , Comi C , Pezzoli G , Ghilardi MF ((2015) ) Intensive rehabilitation treatment in early Parkinson’s disease: A randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair 29: , 123–131. |
[17] | Ferrazzoli D , Ortelli P , Iansek R , Volpe D ((2022) ) Rehabilitation in movement disorders: From basic mechanisms to clinical strategies. Handb Clin Neurol 184: , 341–355. |
[18] | Hirsch MA , Farley BG ((2009) ) Exercise and neuroplasticity in persons living with Parkinson’s disease. Eur J Phys Rehabil Med 45: , 215–229. |
[19] | van Wegen E , Hirsch M , Huiskamp M , Kwakkel G ((2014) ) Harnessing cueing training for neuroplasticity in Parkinson’s disease. Topics Geri Rehab 30: , 46–57. |
[20] | Ferrazzoli D , Ortelli P , Madeo G , Giladi N , Petzinger GM , Frazzitta G ((2018) ) Basal ganglia and beyond: The interplay between motor and cognitive aspects in Parkinson’s disease rehabilitation. Neurosci Biobehav Rev 90: , 294–308. |
[21] | Ferrazzoli D , Ortelli P , Zivi I , Cian V , Urso E , Ghilardi MF , Maestri R , Frazzitta G ((2018) ) Efficacy of intensive multidisciplinary rehabilitation in Parkinson’s disease: A randomised controlled study. J Neurol Neurosurg Psychiatry 89: , 828–835. |
[22] | Nieuwboer A , Rochester L , Müncks L , Swinnen SP ((2009) ) Motor learning in Parkinson’s disease: Limitations and potential for rehabilitation. Parkinsonism Relat Disord 15: (Suppl 3), S53–58. |
[23] | Fullard ME , Thibault DP , Hill A , Fox J , Bhatti DE , Burack MA , Dahodwala N , Haberfeld E , Kern DS , Klepitskava OS , Urrea-Mendoza E , Myers P , Nutt J , Rafferty MR , Schwalb JM , Shulman LM , Willis AW ((2017) ) Utilization of rehabilitation therapy services in Parkinson disease in the United States. Neurology 89: , 1162–1169. |
[24] | Roberts AC , Rafferty MR , Wu SS , Miao G , Cubillos F , Simuni T ((2021) ) Patterns and predictors of referrals to allied health services for individuals with Parkinson’s disease: A Parkinson’s foundation (PF) QII study. Parkinsonism Relat Disord 83: , 115–122. |
[25] | Seo HG , Park SJ , Seo J , Byun SJ , Oh B-M ((2018) ) Rehabilitation therapy utilization in patients with Parkinson’s disease in Korea. Parkinsons Dis 2018: , 9475415. |
[26] | Rafferty MR , Nettnin E , Goldman JG , MacDonald J ((2021) ) Frameworks for Parkinson’s disease rehabilitation addressing when, what, and how. Curr Neurol Neurosci Rep 21: , 12. |
[27] | Radder DLM , Nonnekes J , van Nimwegen M , Eggers C , Abbruzzese G , Alves G , Browner N , Chaudhuri KR , Ebersbach G , Ferreira JJ , Fleisher JE , Fletcher P , Frazzitta G , Giladi N , Guttman M , Iansek R , Khandhar S , Klucken J , Lafontaine AL , Marras C , Nutt J , Okun MS , Parashos SA , Munneke M , Bloem BR ((2020) ) Recommendations for the organization of multidisciplinary clinical care teams in Parkinson’s disease. J Parkinsons Dis 10: , 1087–1098. |
[28] | Rajan R , Brennan L , Bloem BR , Dahodwala N , Gardner J , Goldman JG , Grimes DA , Iansek R , Kovács N , McGinley J , Parashos SA , Piemonte MEP , Eggers C ((2020) ) Integrated care in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 35: , 1509–1531. |
[29] | Institute of Medicine ((2011) ) Clinical Practice Guidelines We Can Trust. The National Academies Press., Washington, DC. |
[30] | Kalf H , Swart Bd , Bonnier-Baars M , Kanters J , Hofman M , Kocken J , Miltenburg M , Bloem B , Munneke M ((2011) ) Guidelines for speech-language therapy in Parkinson’s disease. ParkinsonNet/NPF, Nijmegen, The Netherlands/Miami, FL, USA. |
[31] | Sturkenboom I , Thijssen M , Gons-van Elsacker J , Jansen I , Maasdam A , Schulten M , Vijver-Visser D , Steultjens E , Bloem B , Munneke M ((2011) ) Guidelines for occupational therapy in Parkinson’s disease rehabilitation. ParkinsonNet/NPF, Nijmegen, The Netherlands/Miami, FL, USA. |
[32] | Chou KL , Martello J , Atem J , Elrod M , Foster ER , Freshwater K , Gunzler SA , Kim H , Mahajan A , Sarva H , Stebbins GT , Lee E , Yang L ((2021) ) Quality improvement in neurology: 2020 Parkinson disease quality measurement set update. Neurology 97: , 239–245. |
[33] | Grimes D , Fitzpatrick M , Gordon J , Miyasaki J , Fon EA , Schlossmacher M , Suchowersky O , Rajput A , Lafontaine AL , Mestre T , Appel-Cresswell S , Kalia SK , Schoffer K , Zurowski M , Postuma RB , Udow S , Fox S , Barbeau P , Hutton B ((2019) ) Canadian guideline for Parkinson disease. CMAJ 191: , E989–e1004. |
[34] | Wood J , Henderson W , Foster ER ((2022) ) Occupational therapy practice guidelines for people with Parkinson’s disease. Am J Occup Ther 76: , 7603397010. |
[35] | Hsu C-C , Sandford BA ((2007) ) The Delphi technique: Making sense of consensus. Pract Assess Res Eval 12: , 10. |
[36] | Okoli C , Pawlowski SD ((2004) ) The Delphi method as a research tool: An example, design considerations and applications. Inf Manag 42: , 15–29. |
[37] | Bloem BR , Henderson EJ , Dorsey ER , Okun MS , Okubadejo N , Chan P , Andrejack J , Darweesh SKL , Munneke M ((2020) ) Integrated and patient-centred management of Parkinson’s disease: A network model for reshaping chronic neurological care. Lancet Neurol 19: , 623–634. |
[38] | Grosset KA , Grosset DG ((2005) ) Patient-perceived involvement and satisfaction in Parkinson’s disease: Effect on therapy decisions and quality of life. Mov Disord 20: , 616–619. |
[39] | Hirsch MA , Sanjak M , Englert D , Iyer S , Quinlan MM ((2014) ) Parkinson patients as partners in care. Parkinsonism Relat Disord 20: (Suppl 1), S174–179. |
[40] | Hirsch MA , Simpson AM ((2013) ) Why bother with shared decision-making in Parkinson’s disease? Parkinsonism Relat Disord 19: , 928–929. |
[41] | Post B , van der Eijk M , Munneke M , Bloem BR ((2011) ) Multidisciplinary care for Parkinson’s disease: Not if, but how!. Pract Neurol 11: , 58–61. |
[42] | Agency for Healthcare Research and Quality (AHRQ) ((2011) ) The Effective Health Care Program Stakeholder Guide, Appendix D: Research Questions & PICO(TS). https://effectivehealthcare.ahrq.gov/products/stakeholders-engagement-others/research-2011 |
[43] | Diamond IR , Grant RC , Feldman BM , Pencharz PB , Ling SC , Moore AM , Wales PW ((2014) ) Defining consensus: A systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol 67: , 401–409. |
[44] | Jünger S , Payne SA , Brine J , Radbruch L , Brearley SG ((2017) ) Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: Recommendations based on a methodological systematic review. Palliat Med 31: , 684–706. |
[45] | Antonini A , Stoessl AJ , Kleinman LS , Skalicky AM , Marshall TS , Sail KR , Onuk K , Odin PLA ((2018) ) Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: A multi-country Delphi-panel approach. Curr Med Res Opin 34: , 2063–2073. |
[46] | World Health Organization, Rehabilitation, https://www.who.int/news-room/fact-sheets/detail/rehabilitation#:~:text=Rehabilitation%20is%20defined%20as%20%E2%80%9Ca,in%20interaction%20with%20their%20environment%E2%80%9D, Accessed December 1, 2022. |
[47] | Royal College of Physicians and British Society of Rehabilitation Medicine ((2003) ) Rehabilitation following acquired brain injury: National clinical guidelines (Turner-Stokes L, ed). RCP, BSRM, London. https://www.headway.org.uk/media/3320/bsrm-rehabilitation-following-acquired-brain-injury.pdf |
[48] | MacMahon DG , Thomas S ((1998) ) Practical approach to quality of life in Parkinson’s disease: The nurse’s role. J Neurol 245: (Suppl 1), S19–22. |
[49] | Thomas S , MacMahon D ((2004) ) Parkinson’s disease, palliative care and older people: Part 1. Nurs Older People 16: , 22–26. |
[50] | Hoehn MM , Yahr MD ((1967) ) Parkinsonism: Onset, progression and mortality. Neurology 17: , 427–442. |
[51] | Thomas S , MacMahon D , Henry S ((1999) ) Moving and Shaping-the Future: Commissioning Services for People with Parkinson’s Disease. Parkinson’s Disease Society, London. |
[52] | Buetow SA , Martínez-Martín P , Hirsch MA , Okun MS ((2016) ) Beyond patient-centered care: Person-centered care for Parkinson’s disease. NPJ Parkinsons Dis 2: , 16019. |
[53] | Radder DLM , de Vries NM , Riksen NP , Diamond SJ , Gross D , Gold DR , Heesakkers J , Henderson E , Hommel ALAJ , Lennaerts HH , Busch J , Dorsey RE , Andrejack J , Bloem BR ((2019) ) Multidisciplinary care for people with Parkinson’s disease: The new kids on the block!. Expert Rev Neurother 19: , 145–157. |
[54] | Khan U , Stoff L , Yahuaca JD , Bhagavat B , Toledo S , Goldman JG , Simuni T , Rafferty M ((2020) ) Evaluation of an interdisciplinary screening program for people with Parkinson disease and movement disorders. Arch Rehabil Res Clin Transl 2: , 100067. |
[55] | Giladi N , Manor Y , Hilel A , Gurevich T ((2014) ) Interdisciplinary teamwork for the treatment of people with Parkinson’s disease and their families. Curr Neurol Neurosci Rep 14: , 493. |
[56] | Pearson C , Hartzman A , Munevar D , Feeney M , Dolhun R , Todaro V , Rosenfeld S , Willis A , Beck JC ((2023) ) Care access and utilization among medicare beneficiaries living with Parkinson’s disease. NPJ Parkinsons Dis 9: , 108. |
[57] | Ritsma BR , Gariscsak PJ , Vyas A , Chan-Nguyen S , Appireddy R ((2023) ) The virtual family conference in stroke rehabilitation: Education, preparation, and transition planning. Clin Rehabil 37: , 1099–1110. |
[58] | Kushner DS , Strasser DC ((2020) ) Stroke Inpatient Rehabilitation Team Conferences: Leadership and structure improve patient outcomes. J Stroke Cerebrovasc Dis 29: , 104622. |
[59] | Roelofsen EE , Lankhoorst GJ , Bouter LM ((2000) ) Team conferences in paediatric rehabilitation: organization, satisfaction and aspects eligible for improvement. Int J Rehabil Res 23: , 227–232. |
[60] | Riggare S , Unruh KT ((2015) ) Patients organise and train doctors to provide better care. BMJ 351: , h6318. |
[61] | van der Eijk M , Nijhuis FA , Faber MJ , Bloem BR ((2013) ) Moving from physician-centered care towards patient-centered care for Parkinson’s disease patients. Parkinsonism Relat Disord 19: , 923–927. |
[62] | Riggare S , Höglund PJ , Hvitfeldt Forsberg H , Eftimovska E , Svenningsson P , Hägglund M ((2019) ) Patients are doing it for themselves: A survey on disease-specific knowledge acquisition among people with Parkinson’s disease in Sweden. Health Informatics J 25: , 91–105. |
[63] | Ypinga J , de Vries N , Boonen L , Koolman X , Munneke M , Zwinderman A , Bloem B ((2018) ) Effectiveness and costs of specialised physiotherapy given via ParkinsonNet: A retrospective analysis of medical claims data. Lancet Neurol 17: , 153–161. |
[64] | Bosch B , Mansell H ((2015) ) Interprofessional collaboration in health care: Lessons to be learned from competitive sports. Can Pharm J (Ott) 148: , 176–179. |
[65] | Aragon A , Kings J ((2018) ) Occupational Therapy for People with Parkinson’s. The Royal College of Occupational Therapists, London. |
[66] | American Occupational Therapy Association ((2015) ), Occupational Therapy’s Role with Health Promotion. https://www.aota.org/∼/media/corporate/files/aboutot/professionals/whatisot/hw/facts/factsheet_healthpromotion.pdf |
[67] | Radder DLM , Sturkenboom IH , van Nimwegen M , Keus SH , Bloem BR , de Vries NM ((2017) ) Physical therapy and occupational therapy in Parkinson’s disease. Int J Neurosci 127: , 930–943. |
[68] | Jansa J , Aragon A ((2015) ) Living with Parkinson’s and the emerging role of occupational therapy. Parkinsons Dis 2015: , 196303. |
[69] | Cabrera-Martos I , Ortiz-Rubio A , Torres-Sanchez I , Rodriguez-Torres J , Lopez-Lopez L , Valenza MC ((2019) ) A randomized controlled study of whether setting specific goals improves the effectiveness of therapy in people with Parkinson’s disease. Clin Rehabil 33: , 465–472. |
[70] | Tofani M , Ranieri A , Fabbrini G , Berardi A , Pelosin E , Valente D , Fabbrini A , Costanzo M , Galeoto G ((2020) ) Efficacy of occupational therapy interventions on quality of life in patients with Parkinson’s disease: A systematic review and meta-analysis. Mov Disord Clin Pract 7: , 891–901. |
[71] | Foster ER , Carson LG , Archer J , Hunter EG ((2021) ) Occupational therapy interventions for instrumental activities of daily living for adults with Parkinson’s disease: A systematic review. Am J Occup Ther 75: , 7503190030p1–7503190030p24. |
[72] | Saba RA , Maia DP , Cardoso FEC , Borges V , LA FA , Ferraz HB , Barbosa ER , Rieder CRM , da Silva DJ , Chien HF , Capato T , Rosso AL , Souza Lima CF , Bezerra JMF , Nicaretta D , Povoas Barsottini OG , Godeiro-Júnior C , Broseghini Barcelos L , Cury RG , Spitz M , Azevedo Silva SMC , Della Colletta MV ((2022) ) Guidelines for Parkinson’s disease treatment: Consensus from the Movement Disorders Scientific Department of the Brazilian Academy of Neurology - motor symptoms. Arq Neuropsiquiatr 80: , 316–329. |
[73] | Foster ER , Bedekar M , Tickle-Degnen L ((2014) ) Systematic review of the effectiveness of occupational therapy-related interventions for people with Parkinson’s disease. Am J Occup Ther 68: , 39–49. |
[74] | Doucet BM , Franc I , Hunter EG ((2021) ) Interventions within the scope of occupational therapy to improve activities of daily living, rest, and sleep in people with Parkinson’s disease: A systematic review. Am J Occup Ther 75: . |
[75] | Monticone M , Ambrosini E , Laurini A , Rocca B , Foti C ((2015) ) In-patient multidisciplinary rehabilitation for Parkinson’s disease: A randomized controlled trial. Mov Disord 30: , 1050–1058. |
[76] | Schenkman M , Hall DA , Barón AE , Schwartz RS , Mettler P , Kohrt WM ((2012) ) Exercise for people in early- or mid-stage Parkinson disease: A 16-month randomized controlled trial. Phys Ther 92: , 1395–1410. |
[77] | Wallén MB , Hagströmer M , Conradsson D , Sorjonen K , Franzén E ((2018) ) Long-term effects of highly challenging balance training in Parkinson’s disease-a randomized controlled trial. Clin Rehabil 32: , 1520–1529. |
[78] | Volpe D , Giantin MG , Maestri R , Frazzitta G ((2014) ) Comparing the effects of hydrotherapy and land-based therapy on balance in patients with Parkinson’s disease: A randomized controlled pilot study. Clin Rehabil 28: , 1210–1217. |
[79] | Vanbellingen T , Ottiger B , Maaijwee N , Pflugshaupt T , Bohlhalter S , Müri RM , Nef T , Cazzoli D , Nyffeler T ((2017) ) Spatial neglect predicts upper limb use in the activities of daily living. Cerebrovasc Dis 44: , 122–127. |
[80] | Advocat J , Enticott J , Vandenberg B , Hassed C , Hester J , Russell G ((2016) ) The effects of a mindfulness-based lifestyle program for adults with Parkinson’s disease: A mixed methods, wait list controlled randomised control study. BMC Neurol 16: , 166. |
[81] | Son HG , Choi EO ((2018) ) The effects of mindfulness meditation-based complex exercise program on motor and non-motor symptoms, and quality of life in patients with Parkinson’s disease. Asian Nurs Res (Korean Soc Nurs Sci) 12: , P145–153. |
[82] | Li G , Huang P , Cui SS , Tan YY , He YC , Shen X , Jiang QY , Huang P , He GY , Li BY , Li YX , Xu J , Wang Z , Chen SD ((2022) ) Mechanisms of motor symptom improvement by long-term Tai Chi training in Parkinson’s disease patients. Transl Neurodegener 11: , 6. |
[83] | Nascimento CM , Ayan C , Cancela JM , Gobbi LT , Gobbi S , Stella F ((2014) ) Effect of a multimodal exercise program on sleep disturbances and instrumental activities of daily living performance on Parkinson’s and Alzheimer’s disease patients. Geriatr Gerontol Int 14: , 259–266. |
[84] | Silva-Batista C , Corcos DM , Barroso R , David FJ , Kanegusuku H , Forjaz C , DE Mello MT , Roschel H , Tricoli V , Ugrinowitsch C ((2016) ) Resistance training with instability for patients with Parkinson’s disease. Med Sci Sports Exerc 48: , 1678–1687. |
[85] | Demonceau M , Maquet D , Jidovtseff B , Donneau AF , Bury T , Croisier JL , Crielaard JM , Rodriguez de la Cruz C , Delvaux V , Garraux G ((2017) ) Effects of twelve weeks of aerobic or strength training in addition to standard care in Parkinson’s disease: A controlled study. Eur J Phys Rehabil Med 53: , 184–200. |
[86] | Goodwin VA , Richards SH , Henley W , Ewings P , Taylor AH , Campbell JL ((2011) ) An exercise intervention to prevent falls in people with Parkinson’s disease: A pragmatic randomised controlled trial. J Neurol Neurosurg Psychiatry 82: , 1232–1238. |
[87] | Reuter I , Mehnert S , Leone P , Kaps M , Oechsner M , Engelhardt M ((2011) ) Effects of a flexibility and relaxation programme, walking, and nordic walking on Parkinson’s disease. J Aging Res 2011: , 232473. |
[88] | Ridgel AL , Phillips RS , Walter BL , Discenzo FM , Loparo KA ((2015) ) Dynamic high-cadence cycling improves motor symptoms in Parkinson’s disease. Front Neurol 6: , 194. |
[89] | van Nimwegen M , Speelman AD , Overeem S , van de Warrenburg BP , Smulders K , Dontje ML , Borm GF , Backx FJ , Bloem BR , Munneke M ((2013) ) Promotion of physical activity and fitness in sedentary patients with Parkinson’s disease: Randomised controlled trial. BMJ 346: , f576. |
[90] | Nackaerts E , Nieuwboer A , Broeder S , Smits-Engelsman BC , Swinnen SP , Vandenberghe W , Heremans E ((2016) ) Opposite effects of visual cueing during writing-like movements of different amplitudes in Parkinson’s disease. Neurorehabil Neural Repair 30: , 431–439. |
[91] | Ziliotto A , Cersosimo MG , Micheli FE ((2015) ) Handwriting rehabilitation in Parkinson disease: A pilot study. Ann Rehabil Med 39: , 586–591. |
[92] | Collett J , Franssen M , Winward C , Izadi H , Meaney A , Mahmoud W , Bogdanovic M , Tims M , Wade D , Dawes H ((2017) ) A long-term self-managed handwriting intervention for people with Parkinson’s disease: Results from the control group of a phase II randomized controlled trial. Clin Rehabil 31: , 1636–1645. |
[93] | Strouwen C , Molenaar E , Munks L , Keus SHJ , Zijlmans JCM , Vandenberghe W , Bloem BR , Nieuwboer A ((2017) ) Training dual tasks together or apart in Parkinson’s disease: Results from the DUALITY trial. Mov Disord 32: , 1201–1210. |
[94] | Hindle JV , Watermeyer TJ , Roberts J , Brand A , Hoare Z , Martyr A , Clare L ((2018) ) Goal-orientated cognitive rehabilitation for dementias associated with Parkinson’s disease-A pilot randomised controlled trial. Int J Geriatr Psychiatry 33: , 718–728. |
[95] | American Physical Therapy Association, APTA Fit for Practice: Movement, https://www.apta.org/fit-for-practice/movement. |
[96] | Osborne JA , Botkin R , Colon-Semenza C , DeAngelis TR , Gallardo OG , Kosakowski H , Martello J , Pradhan S , Rafferty M , Readinger JL , Whitt AL , Ellis TD ((2022) ) Physical therapist management of Parkinson disease: A clinical practice guideline from the American Physical Therapy Association. Phys Ther 102: , pzab302. |
[97] | Mak MK , Wong-Yu IS , Shen X , Chung CL ((2017) ) Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat Rev Neurol 13: , 689–703. |
[98] | Lamotte G , Rafferty MR , Prodoehl J , Kohrt WM , Comella CL , Simuni T , Corcos DM ((2015) ) Effects of endurance exercise training on the motor and non-motor features of Parkinson’s disease: A review. J Parkinsons Dis 5: , 993. |
[99] | Schenkman M , Moore CG , Kohrt WM , Hall DA , Delitto A , Comella CL , Josbeno DA , Christiansen CL , Berman BD , Kluger BM , Melanson EL , Jain S , Robichaud JA , Poon C , Corcos DM ((2018) ) Effect of high-intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurol 75: , 219–226. |
[100] | van der Kolk NM , de Vries NM , Kessels RPC , Joosten H , Zwinderman AH , Post B , Bloem BR ((2019) ) Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol 18: , 998–1008. |
[101] | Chung CL , Thilarajah S , Tan D ((2016) ) Effectiveness of resistance training on muscle strength and physical function in people with Parkinson’s disease: A systematic review and meta-analysis. Clin Rehabil 30: , 11–23. |
[102] | Corcos DM , Robichaud JA , David FJ , Leurgans SE , Vaillancourt DE , Poon C , Rafferty MR , Kohrt WM , Comella CL ((2013) ) A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord 28: , 1230–1240. |
[103] | Jung SH , Hasegawa N , Mancini M , King LA , Carlson-Kuhta P , Smulders K , Peterson DS , Barlow N , Harker G , Morris R , Lapidus J , Nutt JG , Horak FB ((2020) ) Effects of the agility boot camp with cognitive challenge (ABC-C) exercise program for Parkinson’s disease. NPJ Parkinsons Dis 6: , 31. |
[104] | Kalyani HHN , Sullivan K , Moyle G , Brauer S , Jeffrey ER , Roeder L , Berndt S , Kerr G ((2019) ) Effects of dance on gait, cognition, and dual-tasking in Parkinson’s disease: A systematic review and meta-analysis. J Parkinsons Dis 9: , 335–349. |
[105] | Li F , Harmer P , Fitzgerald K , Eckstrom E , Stock R , Galver J , Maddalozzo G , Batya SS ((2012) ) Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med 366: , 511–519. |
[106] | Mirelman A , Rochester L , Maidan I , Del Din S , Alcock L , Nieuwhof F , Rikkert MO , Bloem BR , Pelosin E , Avanzino L , Abbruzzese G , Dockx K , Bekkers E , Giladi N , Nieuwboer A , Hausdorff JM ((2016) ) Addition of a non-immersive virtual reality component to treadmill training to reduce fall risk in older adults (V-TIME): A randomised controlled trial. Lancet 388: , 1170–1182. |
[107] | Ashburn A , Pickering R , McIntosh E , Hulbert S , Rochester L , Roberts HC , Nieuwboer A , Kunkel D , Goodwin VA , Lamb SE , Ballinger C , Seymour KC ((2019) ) Exercise- and strategy-based physiotherapy-delivered intervention for preventing repeat falls in people with Parkinson’s: The PDSAFE RCT. Health Technol Assess 23: , 1–150. |
[108] | Canning CG , Sherrington C , Lord SR , Close JC , Heritier S , Heller GZ , Howard K , Allen NE , Latt MD , Murray SM , O’Rourke SD , Paul SS , Song J , Fung VS ((2015) ) Exercise for falls prevention in Parkinson disease: A randomized controlled trial. Neurology 84: , 304–312. |
[109] | Morris ME , Menz HB , McGinley JL , Watts JJ , Huxham FE , Murphy AT , Danoudis ME , Iansek R ((2015) ) A randomized controlled trial to reduce falls in people with Parkinson’s disease. Neurorehabil Neural Repair 29: , 777–785. |
[110] | Shen X , Wong-Yu IS , Mak MK ((2016) ) Effects of exercise on falls, balance, and gait ability in Parkinson’s disease: A meta-analysis. Neurorehabil Neural Repair 30: , 512–527. |
[111] | Allen NE , Canning CG , Almeida LRS , Bloem BR , Keus SH , Lofgren N , Nieuwboer A , Verheyden GS , Yamato TP , Sherrington C ((2022) ) Interventions for preventing falls in Parkinson’s disease. Cochrane Database Syst Rev 6: , CD011574. |
[112] | Morris ME , Iansek R , Kirkwood B ((2009) ) A randomized controlled trial of movement strategies compared with exercise for people with Parkinson’s disease. Mov Disord 24: , 64–71. |
[113] | Ortelli P , Ferrazzoli D , Bera R , Caremani L , Giladi N , Maestri R , Frazzitta G ((2018) ) Effectiveness of a goal-based intensive rehabilitation in parkinsonian patients in advanced stages of disease. J Parkinsons Dis 8: , 113–119. |
[114] | Forte R , Tocci N , De Vito G ((2021) ) The impact of exercise intervention with rhythmic auditory stimulation to improve gait and mobility in Parkinson disease: An umbrella review. Brain Sci 11: , 685. |
[115] | Gilat M , Ginis P , Zoetewei D , De Vleeschhauwer J , Hulzinga F , D’Cruz N , Nieuwboer A ((2021) ) A systematic review on exercise and training-based interventions for freezing of gait in Parkinson’s disease. NPJ Parkinsons Dis 7: , 81. |
[116] | Cosentino C , Baccini M , Putzolu M , Ristori D , Avanzino L , Pelosin E ((2020) ) Effectiveness of physiotherapy on freezing of gait in Parkinson’s disease: A systematic review and meta-analyses. Mov Disord 35: , 523–536. |
[117] | Capato TTC , de Vries NM , IntHout J , Ramjith J , Barbosa ER , Nonnekes J , Bloem BR ((2020) ) Multimodal balance training supported by rhythmic auditory stimuli in Parkinson disease: Effects in freezers and nonfreezers. Phys Ther 100: , 2023–2034. |
[118] | Capato TTC , Nonnekes J , de Vries NM , IntHout J , Barbosa ER , Bloem BR ((2020) ) Effects of multimodal balance training supported by rhythmical auditory stimuli in people with advanced stages of Parkinson’s disease: A pilot randomized clinical trial. J Neurol Sci 418: , 117086. |
[119] | Nieuwboer A , Kwakkel G , Rochester L , Jones D , van Wegen E , Willems AM , Chavret F , Hetherington V , Baker K , Lim I ((2007) ) Cueing training in the home improves gait-related mobility in Parkinson’s disease: The RESCUE trial. J Neurol Neurosurg Psychiatry 78: , 134–140. |
[120] | Choi HY , Cho KH , Jin C , Lee J , Kim TH , Jung WS , Moon SK , Ko CN , Cho SY , Jeon CY , Choi TY , Lee MS , Lee SH , Chung EK , Kwon S ((2020) ) Exercise therapies for Parkinson’s disease: A systematic review and meta-analysis. Parkinsons Dis 2020: , 2565320. |
[121] | Rochester L , Baker K , Hetherington V , Jones D , Willems AM , Kwakkel G , Van Wegen E , Lim I , Nieuwboer A ((2010) ) Evidence for motor learning in Parkinson’s disease: Acquisition, automaticity and retention of cued gait performance after training with external rhythmical cues. Brain Res 1319: , 103–111. |
[122] | Ferrazzoli D , Ortelli P , Cucca A , Bakdounes L , Canesi M , Volpe D ((2020) ) Motor-cognitive approach and aerobic training: A synergism for rehabilitative intervention in Parkinson’s disease. Neurodegener Dis Manag 10: , 41–55. |
[123] | Petzinger GM , Fisher BE , McEwen S , Beeler JA , Walsh JP , Jakowec MW ((2013) ) Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol 12: , 716–726. |
[124] | Debû B , De Oliveira Godeiro C , Lino JC , Moro E ((2018) ) Managing gait, balance, and posture in Parkinson’s disease. Curr Neurol Neurosci Rep 18: , 23. |
[125] | Cusso ME , Donald KJ , Khoo TK ((2016) ) The impact of physical activity on non-motor symptoms in Parkinson’s disease: A systematic review. Front Med (Lausanne) 3: , 35. |
[126] | Luchesi K , De Toledo I , Mourão L ((2017) ) Dysphagia in Parkinson’s disease: Prevalence, impact and management challenges. J Otolaryngol ENT Res 6: , 00176. |
[127] | Pflug C , Bihler M , Emich K , Niessen A , Nienstedt JC , Flügel T , Koseki JC , Plaetke R , Hidding U , Gerloff C , Buhmann C ((2018) ) Critical dysphagia is common in Parkinson disease and occurs even in early stages: A prospective cohort study. Dysphagia 33: , 41–50. |
[128] | Schindler A , Pizzorni N , Cereda E , Cosentino G , Avenali M , Montomoli C , Abbruzzese G , Antonini A , Barbiera F , Benazzo M , Benarroch E , Bertino G , Clavè P , Cortelli P , Eleopra R , Ferrari C , Hamdy S , Huckabee ML , Lopiano L , Marchese-Ragona R , Masiero S , Michou E , Occhini A , Pacchetti C , Pfeiffer RF , Restivo DA , Rondanelli M , Ruoppolo G , Sandrini G , Schapira A , Stocchi F , Tolosa E , Valentino F , Zamboni M , Zangaglia R , Zappia M , Tassorelli C , Alfonsi E ((2021) ) Consensus on the treatment of dysphagia in Parkinson’s disease. J Neurol Sci 430: , 120008. |
[129] | Kim JY , Kim H ((2023) ) Effects of behavioural swallowing therapy in patients with Parkinson’s disease: A systematic review. Int J Speech Lang Pathol 25: , 269–280. |
[130] | Muñoz-Vigueras N , Prados-Román E , Valenza MC , Granados-Santiago M , Cabrera-Martos I , Rodríguez-Torres J , Torres-Sánchez I ((2021) ) Speech and language therapy treatment on hypokinetic dysarthria in Parkinson disease: Systematic review and meta-analysis. Clin Rehabil 35: , 639–655. |
[131] | Yuan F , Guo X , Wei X , Xie F , Zheng J , Huang Y , Huang Z , Chang Z , Li H , Guo Y , Chen J , Guo J , Tang B , Deng B , Wang Q ((2020) ) Lee Silverman Voice Treatment for dysarthria in patients with Parkinson’s disease: A systematic review and meta-analysis. Eur J Neurol 27: , 1957–1970. |
[132] | Behrman A , Cody J , Chitnis S , Elandary S ((2022) ) Dysarthria treatment for Parkinson’s disease: One-year follow-up of SPEAK OUT!(®) with the LOUD Crowd(®). Logoped Phoniatr Vocol 47: , 271–278. |
[133] | Herd CP , Tomlinson CL , Deane KH , Brady MC , Smith CH , Sackley CM , Clarke CE ((2012) ) Speech and language therapy versus placebo or no intervention for speech problems in Parkinson’s disease. Cochrane Database Syst Rev, CD002812. |
[134] | Whurr R , Lorch MP , Nye C ((1992) ) A meta-analysis of studies carried out between 1946 and 1988 concerned with the efficacy of speech and language therapy treatment for aphasic patients. Eur J Disord Commun 27: , 1–17. |
[135] | Atkinson-Clement C , Letanneux A , Baille G , Cuartero MC , Véron-Delor L , Robieux C , Berthelot M , Robert D , Azulay JP , Defebvre L , Ferreira J , Eusebio A , Moreau C , Pinto S ((2019) ) Psychosocial impact of dysarthria: The patient-reported outcome as part of the clinical management. Neurodegener Dis 19: , 12–21. |
[136] | Johnson JA ((2017) ) Speech, voice, and communication. Int Rev Neurobiol 134: , 1189–1205. |
[137] | Pinto S , Ozsancak C , Tripoliti E , Thobois S , Limousin-Dowsey P , Auzou P ((2004) ) Treatments for dysarthria in Parkinson’s disease. Lancet Neurol 3: , 547–556. |
[138] | Ramig L , Halpern A , Spielman J , Fox C , Freeman K ((2018) ) Speech treatment in Parkinson’s disease: Randomized controlled trial (RCT). Mov Disord 33: , 1777–1791. |
[139] | Ramig LO , Sapir S , Countryman S , Pawlas AA , O’Brien C , Hoehn M , Thompson LL ((2001) ) Intensive voice treatment (LSVT) for patients with Parkinson’s disease: A 2 year follow up. J Neurol Neurosurg Psychiatry 71: , 493–498. |
[140] | Behrman A , Cody J , Elandary S , Flom P , Chitnis S ((2020) ) The effect of SPEAK OUT! and The LOUD Crowd on dysarthria due to Parkinson’s disease. Am J Speech Lang Pathol 29: , 1448–1465. |
[141] | Morris ME , Ellis TD , Jazayeri D , Heng H , Thomson A , Balasundaram AP , Slade SC ((2019) ) Boxing for Parkinson’s disease: Has implementation accelerated beyond current evidence? Front Neurol 10: , 1222. |
[142] | Moreau C , Pinto S ((2019) ) Misconceptions about speech impairment in Parkinson’s disease. Mov Disord 34: , 1471–1475. |
[143] | Monroe P , Halaki M , Kumfor F , Ballard KJ ((2020) ) The effects of choral singing on communication impairments in acquired brain injury: A systematic review. Int J Lang Commun Disord 55: , 303–319. |
[144] | Maas JJL , De Vries NM , Bloem BR , Kalf JG ((2022) ) Design of the PERSPECTIVE study: PERsonalized SPEeCh Therapy for actIVE conversation in Parkinson’s disease (randomized controlled trial). Trials 23: , 274. |
[145] | Nolan P , Hoskins S , Johnson J , Powell V , Chaudhuri KR , Eglin R ((2012) ) Implicit theory manipulations affecting efficacy of a smartphone application aiding speech therapy for Parkinson’s patients. Stud Health Technol Inform 181: , 138–142. |
[146] | Orozco-Arroyave JR , Vásquez-Correa JC , Klumpp P , Pérez-Toro PA , Escobar-Grisales D , Roth N , Ríos-Urrego CD , Strauss M , Carvajal-Castaño HA , Bayerl S , Castrillón-Osorio LR , Arias-Vergara T , Künderle A , López-Pabón FO , Parra-Gallego LF , Eskofier B , Gómez-Gómez LF , Schuster M , Nöth E ((2020) ) Apkinson: The smartphone application for telemonitoring Parkinson’s patients through speech, gait and hands movement. Neurodegener Dis Manag 10: , 137–157. |
[147] | Patel RR , Awan SN , Barkmeier-Kraemer J , Courey M , Deliyski D , Eadie T , Paul D , Švec JG , Hillman R ((2018) ) Recommended protocols for instrumental assessment of voice: American Speech-Language-Hearing Association Expert Panel to develop a protocol for instrumental assessment of vocal function. Am J Speech Lang Pathol 27: , 887–905. |
[148] | Rusz J , Tykalova T , Ramig LO , Tripoliti E ((2021) ) Guidelines for speech recording and acoustic analyses in dysarthrias of movement disorders. Mov Disord 36: , 803–814. |
[149] | Akbar U , Dham B , He Y , Hack N , Wu S , Troche M , Tighe P , Nelson E , Friedman JH , Okun MS ((2015) ) Incidence and mortality trends of aspiration pneumonia in Parkinson’s disease in the United States, 1979-2010. Parkinsonism Relat Disord 21: , 1082–1086. |
[150] | Cosentino G , Avenali M , Schindler A , Pizzorni N , Montomoli C , Abbruzzese G , Antonini A , Barbiera F , Benazzo M , Benarroch EE , Bertino G , Cereda E , Clavè P , Cortelli P , Eleopra R , Ferrari C , Hamdy S , Huckabee ML , Lopiano L , Marchese Ragona R , Masiero S , Michou E , Occhini A , Pacchetti C , Pfeiffer RF , Restivo DA , Rondanelli M , Ruoppolo G , Sandrini G , Schapira AHV , Stocchi F , Tolosa E , Valentino F , Zamboni M , Zangaglia R , Zappia M , Tassorelli C , Alfonsi E ((2022) ) A multinational consensus on dysphagia in Parkinson’s disease: Screening, diagnosis and prognostic value. J Neurol 269: , 1335–1352. |
[151] | Fabbri M , Coelho M , Abreu D , Guedes LC , Rosa MM , Godinho C , Cardoso R , Guimaraes I , Antonini A , Zibetti M , Lopiano L , Ferreira JJ ((2019) ) Dysphagia predicts poor outcome in late-stage Parkinson’s disease. Parkinsonism Relat Disord 64: , 73–81. |
[152] | Miles A , Jardine M , Johnston F , de Lisle M , Friary P , Allen J ((2017) ) Effect of Lee Silverman Voice Treatment (LSVT LOUD(R)) on swallowing and cough in Parkinson’s disease: A pilot study. J Neurol Sci 383: , 180–187. |
[153] | Claus I , Muhle P , Czechowski J , Ahring S , Labeit B , Suntrup-Krueger S , Wiendl H , Dziewas R , Warnecke T ((2021) ) Expiratory muscle strength training for therapy of pharyngeal dysphagia in Parkinson’s disease. Mov Disord 36: , 1815–1824. |
[154] | Miller N , Noble E , Jones D , Deane KH , Gibb C ((2011) ) Survey of speech and language therapy provision for people with Parkinson’s disease in the United Kingdom: Patients’ and carers’ perspectives. Int J Lang Commun Disord 46: , 179–188. |
[155] | Wolff L , Benge J ((2019) ) Everyday language difficulties in Parkinson’s disease: Caregiver description and relationship with cognition, activities of daily living, and motor disability. Am J Speech Lang Pathol 28: , 165–173. |
[156] | Zimmerman AS , Shune S , Smith KG , Estis JM , Garand KL ((2022) ) Comparison of patient-reported and caregiver-reported swallowing-related quality of life in Parkinson’s disease. Dysphagia 37: , 436–445. |
[157] | Park JS , Hwang NK ((2021) ) Chin tuck against resistance exercise for dysphagia rehabilitation: A systematic review. J Oral Rehabil 48: , 968–977. |
[158] | Leibner J , Ramjit A , Sedig L , Dai Y , Wu SS , Jacobson Ct , Okun MS , Rodriguez RL , Malaty IA , Fernandez HH ((2010) ) The impact of and the factors associated with drooling in Parkinson’s disease. Parkinsonism Relat Disord 16: , 475–477. |
[159] | Miller N , Walshe M , Walker RW ((2019) ) Sialorrhea in Parkinson’s disease: Prevalence, impact and management strategies. Res Rev Parkinsonism 9: , 17–28. |
[160] | Ricciardi L , Baggio P , Ricciardi D , Morabito B , Pomponi M , Bentivoglio AR , Bernabei R , Maestri R , Frazzitta G , Volpe D ((2016) ) Rehabilitation of hypomimia in Parkinson’s disease: A feasibility study of two different approaches. Neurol Sci 37: , 431–436. |
[161] | Kampling H , Brendel LK , Mittag O ((2019) ) (Neuro)psychological interventions for non-motor symptoms in the treatment of patients with Parkinson’s disease: A systematic umbrella review. Neuropsychol Rev 29: , 166–180. |
[162] | Sanchez-Luengos I , Balboa-Bandeira Y , Lucas-Jiménez O , Ojeda N , Peña J , Ibarretxe-Bilbao N ((2021) ) Effectiveness of cognitive rehabilitation in Parkinson’s disease: A systematic review and meta-analysis. J Pers Med 11: , 429. |
[163] | Giustiniani A , Maistrello L , Danesin L , Rigon E , Burgio F ((2022) ) Effects of cognitive rehabilitation in Parkinson disease: A meta-analysis. Neurol Sci 43: , 2323–2337. |
[164] | Orgeta V , McDonald KR , Poliakoff E , Hindle JV , Clare L , Leroi I ((2020) ) Cognitive training interventions for dementia and mild cognitive impairment in Parkinson’s disease. Cochrane Database Syst Rev 2: , CD011961. |
[165] | Calleo JS , Amspoker AB , Sarwar AI , Kunik ME , Jankovic J , Marsh L , York M , Stanley MA ((2015) ) A pilot study of a cognitive-behavioral treatment for anxiety and depression in patients with Parkinson disease. J Geriatr Psychiatry Neurol 28: , 210–217. |
[166] | Dobkin RD , Mann SL , Gara MA , Interian A , Rodriguez KM , Menza M ((2020) ) Telephone-based cognitive behavioral therapy for depression in Parkinson disease: A randomized controlled trial. Neurology 94: , e1764–e1773. |
[167] | Dobkin RD , Menza M , Allen LA , Gara MA , Mark MH , Tiu J , Bienfait KL , Friedman J ((2011) ) Cognitive-behavioral therapy for depression in Parkinson’s disease: A randomized, controlled trial. Am J Psychiatry 168: , 1066–1074. |
[168] | Dobkin RD , Menza M , Allen LA , Tiu J , Friedman J , Bienfait KL , Gara MA , Mark MH ((2011) ) Telephone-based cognitive-behavioral therapy for depression in Parkinson disease. J Geriatr Psychiatry Neurol 24: , 206–214. |
[169] | Egan SJ , Laidlaw K , Starkstein S ((2015) ) Cognitive behaviour therapy for depression and anxiety in Parkinson’s disease. J Parkinsons Dis 5: , 443–451. |
[170] | Reynolds GO , Saint-Hilaire M , Thomas CA , Barlow DH , Cronin-Golomb A ((2020) ) Cognitive-behavioral therapy for anxiety in Parkinson’s disease. Behav Modif 44: , 552–579. |
[171] | Cicerone KD , Goldin Y , Ganci K , Rosenbaum A , Wethe JV , Langenbahn DM , Malec JF , Bergquist TF , Kingsley K , Nagele D , Trexler L , Fraas M , Bogdanova Y , Harley JP ((2019) ) Evidence-based cognitive rehabilitation: Systematic review of the literature from 2009 through 2014. Arch Phys Med Rehabil 100: , 1515–1533. |
[172] | da Silva FC , Iop RDR , de Oliveira LC , Boll AM , de Alvarenga JGS , Gutierres Filho PJB , de Melo L , Xavier AJ , da Silva R ((2018) ) Effects of physical exercise programs on cognitive function in Parkinson’s disease patients: A systematic review of randomized controlled trials of the last 10 years. PLoS One 13: , e0193113. |
[173] | Hindle JV , Petrelli A , Clare L , Kalbe E ((2013) ) Nonpharmacological enhancement of cognitive function in Parkinson’s disease: A systematic review. Mov Disord 28: , 1034–1049. |
[174] | Leung IHK , Walton CC , Hallock H , Lewis SJG , Valenzuela M , Lampit A ((2015) ) Cognitive training in Parkinson disease: A systematic review and meta-analysis. Neurology 85: , 1843–1851. |
[175] | Barry G , Galna B , Rochester L ((2014) ) The role of exergaming in Parkinson’s disease rehabilitation: A systematic review of the evidence. J Neuroeng Rehabil 11: , 33. |
[176] | Gavelin HM , Domellof M , Leung I , Neely AS , Finke C , Lampit A ((2020) ) Computerised cognitive training in Parkinson’s disease: A protocol for a systematic review and updated meta-analysis. BMJ Open 10: , e040656. |
[177] | McCormick SA , Vatter S , Carter LA , Smith SJ , Orgeta V , Poliakoff E , Silverdale MA , Raw J , Ahearn DJ , Taylor C , Rodda J , Abdel-Ghany T , Kwapong B , Leroi I ((2019) ) Parkinson’s-adapted cognitive stimulation therapy: Feasibility and acceptability in Lewy body spectrum disorders. J Neurol 266: , 1756–1770. |
[178] | World Health Organization, Traditional, Complementary and Integrative Medicine, https://www.who.int/health-topics/traditional-complementary-and-integrative-medicine#tab=tab_1 |
[179] | Jin X , Wang L , Liu S , Zhu L , Loprinzi PD , Fan X ((2019) ) The impact of mind-body exercises on motor function, depressive symptoms, and quality of life in Parkinson’s disease: A systematic review and meta-analysis. Int J Environ Res Public Health 17: , 31. |
[180] | Hackney ME , Kantorovich S , Levin R , Earhart GM ((2007) ) Effects of tango on functional mobility in Parkinson’s disease: A preliminary study. J Neurol Phys Ther 31: , 173–179. |
[181] | Shanahan J , Morris ME , Bhriain ON , Saunders J , Clifford AM ((2015) ) Dance for people with Parkinson disease: What is the evidence telling us? Arch Phys Med Rehabil 96: , 141–153. |
[182] | Volpe D , Signorini M , Marchetto A , Lynch T , Morris ME ((2013) ) A comparison of Irish set dancing and exercises for people with Parkinson’s disease: A phase II feasibility study. BMC Geriatr 13: , 54. |
[183] | Vivas J , Arias P , Cudeiro J ((2011) ) Aquatic therapy versus conventional land-based therapy for Parkinson’s disease: An open-label pilot study. Arch Phys Med Rehabil 92: , 1202–1210. |
[184] | Cugusi L , Manca A , Dragone D , Deriu F , Solla P , Secci C , Monticone M , Mercuro G ((2017) ) Nordic walking for the management of people with Parkinson disease: A systematic review. PM R 9: , 1157–1166. |
[185] | Wen X , Li K , Wen H , Wang Q , Wu Z , Yao X , Jiao B , Sun P , Ge S , Wen C , Lu L ((2021) ) Acupuncture-related therapies for Parkinson’s disease: A meta-analysis and qualitative review. Front Aging Neurosci 13: , 676827. |
[186] | Pickut B , Vanneste S , Hirsch MA , Van Hecke W , Kerckhofs E , Mariën P , Parizel PM , Crosiers D , Cras P ((2015) ) Mindfulness training among individuals with Parkinson’s disease: Neurobehavioral effects. Parkinsons Dis 2015: , 816404. |
[187] | Kedzior KK , Kaplan I ((2019) ) Tai Chi and Parkinson’s disease (PD): A systematic overview of the scientific quality of the past systematic reviews. Complement Ther Med 46: , 144–152. |
[188] | Mailankody P , Varambally S , Thennarasu K , Pal PK ((2021) ) The rationale of yoga in Parkinson’s disease: A critical review. Neurol India 69: , 1165–1175. |
[189] | Machado Sotomayor MJ , Arufe-Giráldez V , Ruíz-Rico G , Navarro-Patón R ((2021) ) Music therapy and Parkinson’s disease: A systematic review from 2015-2020. Int J Environ Res Public Health 18: , 11618. |
[190] | Cucca A , Di Rocco A , Acosta I , Beheshti M , Berberian M , Bertisch HC , Droby A , Ettinger T , Hudson TE , Inglese M , Jung YJ , Mania DF , Quartarone A , Rizzo JR , Sharma K , Feigin A , Biagioni MC , Ghilardi MF ((2021) ) Art therapy for Parkinson’s disease. Parkinsonism Relat Disord 84: , 148–154. |
[191] | Mirabella G , De Vita P , Fragola M , Rampelli S , Lena F , Dilettuso F , Iacopini M , d’Avella R , Borgese MC , Mazzotta S , Lanni D , Grano M , Lubrani S , Modugno N ((2017) ) Theatre is a valid add-on therapeutic intervention for emotional rehabilitation of Parkinson’s disease patients. Parkinsons Dis 2017: , 7436725. |
[192] | Cho KH , Kim TH , Kwon S , Jung WS , Moon SK , Ko CN , Cho SY , Jeon CY , Lee SH , Choi TY , Jun JH , Choi J , Lee MS , Chung EK ((2018) ) Complementary and alternative medicine for idiopathic Parkinson’s disease: An evidence-based clinical practice guideline. Front Aging Neurosci 10: , 323. |
[193] | Marchant D , Sylvester JL , Earhart GM ((2010) ) Effects of a short duration, high dose contact improvisation dance workshop on Parkinson disease: A pilot study. Complement Ther Med 18: , 184–190. |
[194] | Duncan RP , Earhart GM ((2012) ) Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair 26: , 132–143. |
[195] | Amano S , Nocera JR , Vallabhajosula S , Juncos JL , Gregor RJ , Waddell DE , Wolf SL , Hass CJ ((2013) ) The effect of Tai Chi exercise on gait initiation and gait performance in persons with Parkinson’s disease. Parkinsonism Relat Disord 19: , 955–960. |
[196] | Li F , Harmer P , Fisher KJ , Xu J , Fitzgerald K , Vongjaturapat N ((2007) ) Tai Chi-based exercise for older adults with Parkinson’s disease: A pilot-program evaluation. J Aging Phys Act 15: , 139–151. |
[197] | Ayán C , Cancela JM ((2012) ) Effects of aquatic exercise on persons with Parkinson’s disease: A preliminary study. Sci Sports 27: , 300–304. |
[198] | Carroll LM , Morris ME , O’Connor WT , Volpe D , Salsberg J , Clifford AM ((2022) ) Evidence-based aquatic therapy guidelines for Parkinson’s disease: An international consensus study. J Parkinsons Dis 12: , 621–637. |
[199] | Puyjarinet F , Bégel V , Geny C , Driss V , Cuartero MC , De Cock VC , Pinto S , Dalla Bella S ((2022) ) At-home training with a rhythmic video game for improving orofacial, manual, and gait abilities in Parkinson’s disease: A pilot study. Front Neurosci 16: , 874032. |
[200] | Silva de Lima AL , Hahn T , Evers LJW , de Vries NM , Cohen E , Afek M , Bataille L , Daeschler M , Claes K , Boroojerdi B , Terricabras D , Little MA , Baldus H , Bloem BR , Faber MJ ((2017) ) Feasibility of large-scale deployment of multiple wearable sensors in Parkinson’s disease. PLoS One 12: , e0189161. |
[201] | Lee J , Yeom I , Chung ML , Kim Y , Yoo S , Kim E ((2022) ) Use of mobile apps for self-care in people with Parkinson disease: Systematic review. JMIR Mhealth Uhealth 10: , e33944. |
[202] | Evers LJ , Raykov YP , Krijthe JH , Silva de Lima AL , Badawy R , Claes K , Heskes TM , Little MA , Meinders MJ , Bloem BR ((2020) ) Real-life gait performance as a digital biomarker for motor fluctuations: The Parkinson@Home Validation Study. J Med Internet Res 22: , e19068. |
[203] | Chau B , Humbert S , Shou A ((2021) ) Systemic literature review of the use of virtual reality for rehabilitation in Parkinson disease. Fed Pract 38: , S20–s27. |
[204] | Lu Y , Ge Y , Chen W , Xing W , Wei L , Zhang C , Yang Y ((2022) ) The effectiveness of virtual reality for rehabilitation of Parkinson disease: An overview of systematic reviews with meta-analyses. Syst Rev 11: , 50. |
[205] | Nijkrake MJ , Keus SH , Oostendorp RA , Overeem S , Mulleners W , Bloem BR , Munneke M ((2009) ) Allied health care in Parkinson’s disease: Referral, consultation, and professional expertise. Mov Disord 24: , 282–286. |
[206] | Schalling E , Johansson K , Hartelius L ((2017) ) Speech and communication changes reported by people with Parkinson’s disease. Folia Phoniatr Logop 69: , 131–141. |
[207] | Ellis TD , Colon-Semenza C , DeAngelis TR , Thomas CA , Hilaire MS , Earhart GM , Dibble LE ((2021) ) Evidence for early and regular physical therapy and exercise in Parkinson’s disease. Semin Neurol 41: , 189–205. |
[208] | MacDonald J , Doyle L , Moore JL , Rafferty MR ((2021) ) Sustainment of proactive physical therapy for individuals with early-stage Parkinson’s disease: A quality improvement study over 4 years. Implement Sci Commun 2: , 111. |
[209] | van der Marck MA , Kalf JG , Sturkenboom IH , Nijkrake MJ , Munneke M , Bloem BR ((2009) ) Multidisciplinary care for patients with Parkinson’s disease. Parkinsonism Relat Disord 15 Suppl 3: , S219–223. |
[210] | Lee JM , Shine JM , Lewis SJ ((2015) ) What matters to people with Parkinson’s disease living in Australia? J Clin Neurosci 22: , 338–341. |
[211] | van den Heuvel L , Dorsey RR , Prainsack B , Post B , Stiggelbout AM , Meinders MJ , Bloem BR ((2020) ) Quadruple decision making for Parkinson’s disease patients: Combining expert opinion, patient preferences, scientific evidence, and big data approaches to reach precision medicine. J Parkinsons Dis 10: , 223–231. |
[212] | Schiess N , Cataldi R , Okun MS , Fothergill-Misbah N , Dorsey ER , Bloem BR , Barretto M , Bhidayasiri R , Brown R , Chishimba L , Chowdhary N , Coslov M , Cubo E , Di Rocco A , Dolhun R , Dowrick C , Fung VSC , Gershanik OS , Gifford L , Gordon J , Khalil H , Kühn AA , Lew S , Lim SY , Marano MM , Micallef J , Mokaya J , Moukheiber E , Nwabuobi L , Okubadejo N , Pal PK , Shah H , Shalash A , Sherer T , Siddiqui B , Thompson T , Ullrich A , Walker R , Dua T ((2022) ) Six action steps to address global disparities in Parkinson disease: A world health organization priority. JAMA Neurol 79: , 929–936. |
[213] | Niederberger M , Spranger J ((2020) ) Delphi technique in health sciences: A map. Front Public Health 8: , 457. |
[214] | Spranger J , Homberg A , Sonnberger M , Niederberger M ((2022) ) Reporting guidelines for Delphi techniques in health sciences: A methodological review. Z Evid Fortbild Qual Gesundhwes 172: , 1–11. |
[215] | Dalkey NC ((1969) ) The Delphi Method: An Experimental Study of Group Opinion, RAND Corporation, Santa Monica, CA. |
[216] | World Health Organization, WHO Global Strategy on Integrated People-Centered Health Services, 2016-2026. https://www.who.int/health-topics/integrated-people-centered-care. |
[217] | Andrejack J , Mathur S ((2020) ) What People with Parkinson’s Disease Want. J Parkinsons Dis 10: , S5–S10. |
[218] | National Institute for Health and Care Excellence (NICE) ((2013) ) Mental wellbeing of older people in care homes. https://www.nice.org.uk/guidance/qs50/resources/mental-wellbeing-of-older-people-in-care-homes-pdf-2098720457413 |




