Overnight Distribution of REM Sleep Features in People with Parkinson’s Disease (PD) and Non-PD Controls
Abstract
Background:
Rapid eye movement (REM) sleep behavior disorder (RBD) is a leading predictor of Parkinson’s disease (PD). Diagnosis is performed in the sleep laboratory by detecting pathological REM sleep without atonia (RSWA). The evidence on the overnight distribution of RSWA% is conflicting.
Objective:
To investigate the temporal distribution of the number of ocular movements per REM sleep minute (REM density), and RSWA% in people with PD and non-PD controls.
Methods:
All participants underwent a single overnight evaluation in a sleep laboratory. Clinical evaluation was performed on a separate day. REM density and RSWA% were compared between PD and controls both across four sleep periods and individual REM cycles.
Results:
A total of 51 participants with recorded RSWA in polysomnography laboratory were included, 28 with PD aged 64±9 years with a disease duration of 3.3±2.9 years, and 23 controls aged 55±8 years. People with PD had lower REM density and higher RSWA% compared to controls. As expected, REM density was higher towards the morning. In contrast, RSWA% was equally distributed across the night, for both PD and controls.
Conclusions:
PD pathology affects REM sleep features, but not the overnight distribution of those features. While REM density increased towards the end of the night, RSWA% was equally distributed across the night for both PD and controls. Our findings have clinical implications for diagnosing RBD, as quantification of RSWA% in any sleep cycle is sufficient for reliably evaluating total RSWA% and reduced REM density may be a marker of PD.
INTRODUCTION
Rapid eye movement (REM) sleep behavior disorder (RBD) is a sleep parasomnia characterized by REM-related abnormal muscle activity, also known as REM sleep without atonia (RSWA), accompanied by dream enactment behaviors [1, 2]. RBD is the strongest prodromal marker of PD and other synucleinopathies with a phenoconversion rate of 68% at ten years of follow-up [3] and 80% at 15 years [4]. A clinical history of dream enactment could suggest probable RBD, yet a formal diagnosis of RBD requires detection of RSWA on a video polysomnographic recording (PSG). As dream enactment behaviors may not be present every night [5], RSWA is an important PSG signature of RBD, with relatively low night-to-night variability [1, 6, 7]. The Sleep Innsbruck Barcelona (SINBAR) group guidelines are the most acceptable approach to quantify RSWA [8]. Optimal montage for RSWA detection is based on EMG signals from the chin (mentalis muscle) and both upper (flexor digitorum superficialis) and lower (tibialis anterior) extremities [8, 9] collected during an overnight test in a sleep laboratory.
It is well established that REM sleep is more frequent in the second half of the sleep period. However, clinical PSG setups include early-than-usual morning awakenings, potentially leading to a reduced amount of REM sleep, and limiting an accurate habitual state. As the night progresses two natural processes occur, an increase in REM cycles length and an increase in REM density, a measure of the frequency of rapid eye movements (REMs) during REM sleep, calculated as the number of ocular movements per minute [10–13]. Nevertheless, the overnight distribution of REM sleep features in people with PD is not well understood. Arnaldi et al. demonstrated that REM cycle length increased only in healthy controls but not in people with PD [11]. Although PD patients may present with reduced REM density [14] compared to healthy controls, the overnight distribution of REM density was proposed to be comparable to that of healthy controls [11, 14]. Additionally, there is contradicting evidence on the overnight distribution of RSWA. Arnaldi and colleagues reported similar RSWA distribution across REM sleep cycles, as calculated from mentalis muscle activity, collected from 10 idiopathic RBD patients, 10 RBD + PD, 10 PD patients, and 10 controls [11]. Contrarily, Sasai-Sakuma and colleagues evaluated RSWA% distribution across four two-hour-long sleep periods [15]. Participants with incidental RSWA findings below the accepted 16.3% cut-off values for RBD diagnosis, showed a similar distribution of RSWA%, measured from the mentalis and tibialis anterior muscles, across the night [15]. Surprisingly, higher percentages of RSWA were present during later REM sleep periods among seven participants with RSWA above the cut-off value [15]. The above-mentioned studies present different methodologies and both included relatively small samples of participants with different characteristics which may account for the discrepancies in results, therefore, additional research is needed.
This study aimed to further investigate the temporal distribution of REM sleep features and to compare them between people with PD and controls. We combined both previously described methods [11, 15], comparing RSWA% and REM density between sleep periods as well as individual REM cycles. We hypothesized that for PD and controls, REM density will be higher in the last sleep period, compared to the first sleep period. In contrast, we hypothesized that RSWA% will be equally distributed across the night. Furthermore, we hypothesized that for those presenting RSWA% above RBD cut-off criteria, RSWA% will be higher towards the end of the night.
METHODS
Participants
Participants were recruited from the Laboratory for Early Markers of Neurodegeneration (LEMON), Neurological Institute, Tel Aviv Sourasky Medical Center (TASMC), Israel. All participants underwent overnight PSG between January 2020 and December 2022. Inclusion criteria were detection of any RSWA during sleep, presentation of at least 2 REM cycles longer than 3 min during PSG, and diagnosis of PD based on the MDS criteria [16]. Non-PD controls were defined as individuals with incidental RSWA between 40 and 80 years of age, without prior knowledge or complaints of RBD. Participants were excluded if they had cognitive dysfunction as observed using the Montreal Cognitive Assessment (MoCA) score of < 21, a history of other major neurological or psychiatric disorders or other forms of Parkinsonism (e.g., PSP or MSA), taking selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitor (SNRIs), hypnotics, antipsychotics and anxiolytics which may affect REM sleep and RSWA. As the study explored muscle activity during REM sleep, we excluded from the analysis participants that had moderate-severe obstructive sleep apnea measured by apnea-hypopnea index (AHI > 15) or extensive periodic limb movement (PLMS > 30), as those disorders could interfere with EMG signals during REM sleep.
The study was approved by Tel Aviv Sourasky Medical Center Ethical Committee, in accordance with the Helsinki agreement, and all the participants provided informed written consent before participation. On the day of the sleep assessment, all participants were instructed to avoid any sleep medication and restrict the consumption of caffeine and alcohol. PD patients were instructed to continue taking their normal schedule of dopaminergic treatment.
Demographic information about the participants, as well as cognitive evaluation measured by MoCA, were collected during a separate visit that occurred within one day of the PSG. All participants were asked to fill out the Epworth Sleepiness Scale (ESS) [17] and a 10-item RBD questionnaire RBDQ [18]. The MDS-UPDRS scale was evaluated in the ON medication state, approximately 1 h after taking their medication. The assessment was performed by a certified movement disorders specialist. Bradykinesia, rigidity, and tremor subscales were calculated based on standard criteria, averaging the relevant items of MDS-UPDRS part III [19–21]. PD medications were collected and converted into Levodopa equivalent dose (LEDD) based on standard criteria [22].
Polysomnographic sleep recording (PSG)
All participants were invited for a single overnight video polysomnography (vPSG) at the Institute for Sleep Medicine, TASMC, Israel. A standard PSG montage was used. The EMG electrodes were attached based on SINBAR guidelines [9], located on the mentalis muscle (chin), flexor digitorum superficialis (arm), and tibialis anterior (leg), bilaterally. All recordings lasted at least 6 h. vPSG was collected using a commercially available system (PSG Embla NDx, Natus). PSG was scored offline and analyzed by a board-certified sleep medicine physician blinded to the PD diagnosis according to the American Association of Sleep Medicine (AASM) guidelines [23].
Analysis of REM sleep without atonia (RSWA)
EMG signals were extracted from electrodes located on the mentalis muscle, the flexor digitorum superficialis, and the tibialis anterior muscles [8, 9]. Although RSWA could be measured solely in mentalis muscle, we opted to include the more comprehensive montage which also includes EMG signals from both upper extremities (right and left flexor digitorum superficialis) [24].
Both tonic and phasic muscle activity were visually scored according to previously described methods [9]. REM without atonia events were marked in case of increased muscle activity with more than two times background amplitude and phasic activity as events longer than 0.1 s, according to SINBAR guidelines [8, 9]. The overall percentage of muscle activity (RSWA%) across the entire night was calculated as the proportion of REM sleep epochs meeting RSWA criteria from all REM epochs [24]. To calculate the distribution of RSWA signal across different parts of the night, a more granular approach was applied. RSWA% was calculated as the sum of any phasic or tonic muscle activity divided by the REM length for each REM cycle separately and compared between cycles. Two REM segments were considered as two separate REM cycles based on the following definition: 1) they were separated by the N3 stage of sleep, or 2) they were separated by at least 30 min of N2, N1 stages of sleep or wake. In the rest of the cases, two REM segments were considered as one (fragmented) REM cycle. REM cycles shorter than seven 30-s-epochs i.e., 3.5 min, were excluded from the analysis and from the calculation of total RSWA% [14].
Detection of rapid eye movements during REM sleep
Rapid eye movements (REMs) were scored manually by a researcher blinded to the participant group. Rapid eye movement was determined if the signal extracted from the eye electrode exhibited a rapid time course shorter than 500 ms [25], appeared simultaneously on both left and right EOG channels, and was detectable above the background noise, regardless of its amplitude [26, 27]. REM density was calculated as the number of REMs per minute out of total REM sleep or within an individual REM sleep cycle.
Overnight distribution
To examine the overnight distribution of RSWA% and REM density, the whole night was divided into 4 sleep periods, according to a previously described method [15]: first sleep period (starting within 2 h from sleep onset), second sleep period (starting within 2–4 h), third sleep period (starting within 4–6 h), and fourth sleep period (starting after 6 h). The REM cycles were assigned to one of the four sleep periods based on their onset from sleep onset. RSWA% was calculated within each sleep period as the sum of RSWA divided by REM length.
Statistical analyses
Participants were divided into two groups according to the acceptable RBD cut-off value for SINBAR montage of 27% for 30-s epochs reflecting ‘any’ muscle activity: 1) participants having total RSWA% above the cut-off, and 2) participants with RSWA% below the cut-off. The overnight distribution of RSWA% was first compared between people with PD and controls, followed by a comparison of above vs. below cut-off groups (regardless of PD status). In the case of the PD vs. controls comparison, age was added as a covariate.
To account for the discrepancy in the number of REM cycles, we used a generalized linear mixed model for RSWA% with sleep periods (1st, 2nd, 3rd, and 4th) as a within-subject variable and a group (PD vs. controls or above vs. below RBD cut-off) as a between-subject variable. As a complementary analysis, we also compared the RSWA% of the REM sleep cycles themselves. This was explored using a generalized linear mixed model for RSWA% with REM cycle (1st, 2nd, and last) as a within-subject variable and a group (PD vs. controls or above vs. below RBD cut-off) as a between-subject variable. To avoid data duplication, in the case of only two REM cycles, the 2nd cycle was considered only as the last cycle and was excluded from the 2nd cycle calculation.
The overnight distributions of REM density and REM cycle duration were compared between PD and controls both across the four sleep periods and individual REM cycles using the above-described approach. In all the analyses, if a significant group by sleep period/REM cycle interaction was found, post hoc analysis was conducted in each group using the Friedman test adjusted with Bonferroni’s correction for multiple comparisons. Additionally, the REM density and RSWA% of PD patients were correlated with clinically relevant variables using Spearman correlation.
RESULTS
Clinical, demographic, and PSG results
A total of 51 participants, 28 people with PD and 23 controls were included in this study. Clinical, demographic, and PSG data are presented in Table 1. Compared to controls, people with PD were significantly older, had lower REM density (see Fig. 1), higher overall RSWA%, a higher score of RBDQ and ESS, and higher values of PLMS.
Table 1
Clinical, demographic, and polysomnography data for 28 people with PD and 23 non-PD controls presenting with RSWA during overnight sleep laboratory evaluation. Data are presented as mean±standard deviation [min – max]
| PD (n = 28) | Controls (n = 23) | p | |
| Age (y) | 64.7±8.5 [40–78] | 57.4±9.2 [45–75] | 0.009 |
| Gender (M/F) | 20/8 | 13/10 | ns |
| PD duration | 3.1±2.8 [0–12] | – | – |
| Above RBD cut off (n) | 12 | 3 | |
| Dream enactment behaviors (n) | 10 | 3 | |
| LEDD | 328±307 [0–1240] | – | – |
| MoCA | 25.5±2.5 [21–30] | 26.1±2.5 [22–30] | ns |
| ESS | 7.9±4.2 [1–18] | 5.5±4.3 [1–17] | 0.017 |
| RBDQ | 5.2±3.3 [1–12] | 3.0±2.6 [0–10] | 0.009 |
| Sleep period (h) | 7.2±0.6 [5.7–8.8] | 7.1±0.5 [6.1–8.2] | ns |
| Total sleep time (h) | 5.9±0.9 [4.6–7.8] | 5.9±0.8 [4.7–7.5] | ns |
| Sleep latency (min) | 14.2±19.2 [0.8–88.0] | 14.5±17.4 [1.1–73.3] | ns |
| AHI | 5.2±3.9 [0–14.7] | 5.7±4.4 [0–14.8] | ns |
| PLMS | 6.7±8.1 [0–28.0] | 2.9±6.6 [0–27.2] | 0.043 |
| REM duration (min) | 75±31 [33–155] | 65±26 [28–110] | ns |
| REM latency (min) | 93±52 [6–214] | 101±46 [43–218] | ns |
| REM cycles (n) | 3.2±1.1 [2–6] | 3.4±0.8 [2–5] | ns |
| RSWA% | 25.5±19.0 [2.5–71.2] | 12.6±10.6 [1.4–45.4] | 0.014 |
| REM density (n/min) | 4.6±2.2 [1.0–10.2] | 8.3±3.9 [2.5–15.7] | 0.001 |
| Sleep efficiency (%) | 79.8±9.2 [62.3–95.6] | 81.2±9.1 [61.5–97.7] | ns |
| N1 (%) | 8.3±5.5 [2.6–21.2] | 6.7±2.7 [2.7–14.8] | ns |
| N2 (%) | 44.1±9.5 [25.5–62.1] | 48.6±8.9 [27.2–62.6] | ns |
| N3 (%) | 26.7±9.5 [10.1–47.1] | 26.5±9.1 [11.9–49.9] | ns |
| REM (%) | 20.9±7.2 [9.9–43.0] | 18.3±6.4 [8.0–29.2] | ns |
| WASO (min) | 77±41 [17–173] | 69±40 [7–170] | ns |
| Arousal index | 6.1±3.0 [2.0–14.0] | 8.0±4.1 [1.4–17.0] | ns |
LEDD, levodopa equivalent dose; ESS, Epworth Sleepiness Scale; RBDQ, REM sleep behavior questionnaire; AHI, apnea-hypopnea index; PLMS, periodic limb movement in sleep; WASO, wake after sleep onset; ns, nonsignificant.
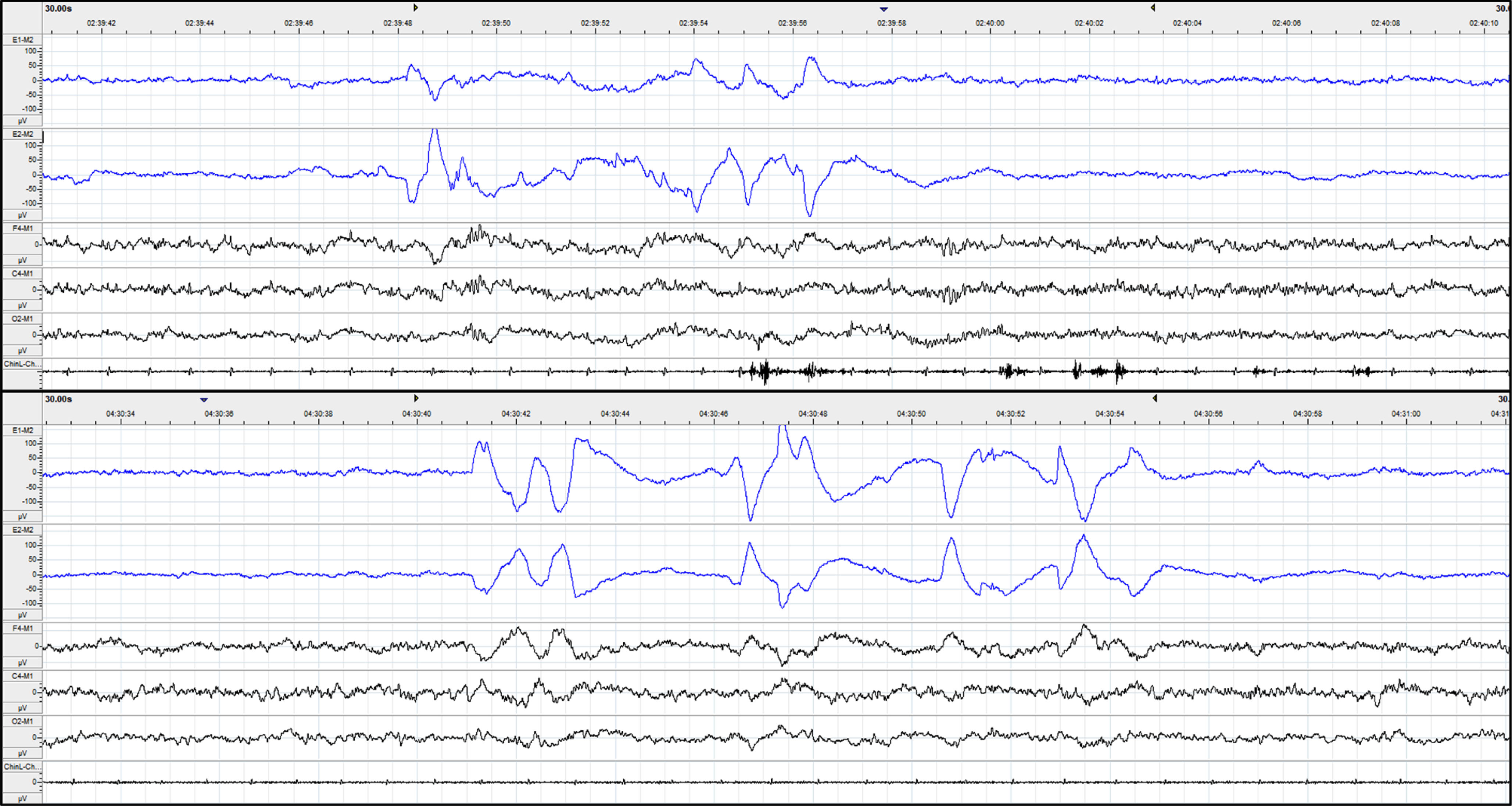
Fig. 1
Raw data of 30-second epoch of REM sleep for PD (upper) and controls (lower). The first two channels marked in blue are left and right EOG, followed by frontal (F4), central (C4), and occipital (O2) EEG channels. The last channel is the chin EMG measured from the mentalis muscle. Note the much higher REM density of controls, compared to PD. Across the whole night, controls had a REM density of 7.5, compared to a REM density of 4.3 in a PD patient.
For the whole cohort, participants, on average, slept 5.9±0.9 h. RSWA ranged from 1.4% to 71.2% out of the total REM sleep time. RSWA was detected in 164 out of 167 REM cycles (98%). Fifteen participants presented with RSWA above the RBD cut-off value (PD = 12, controls = 3). During the vPSG, ten people with PD and three controls demonstrated typical dream enactment behaviors, including movements, talking, and vocalization during REM sleep with a confirmed diagnosis of RBD. Of the three controls with dream enactment, only one had RSWA% above the RBD cut-off and could be considered clinical RBD while the other two did not present with high RSWA. The other two control participants with RSWA above the cut-off for RBD did not show any signs of dream enactment.
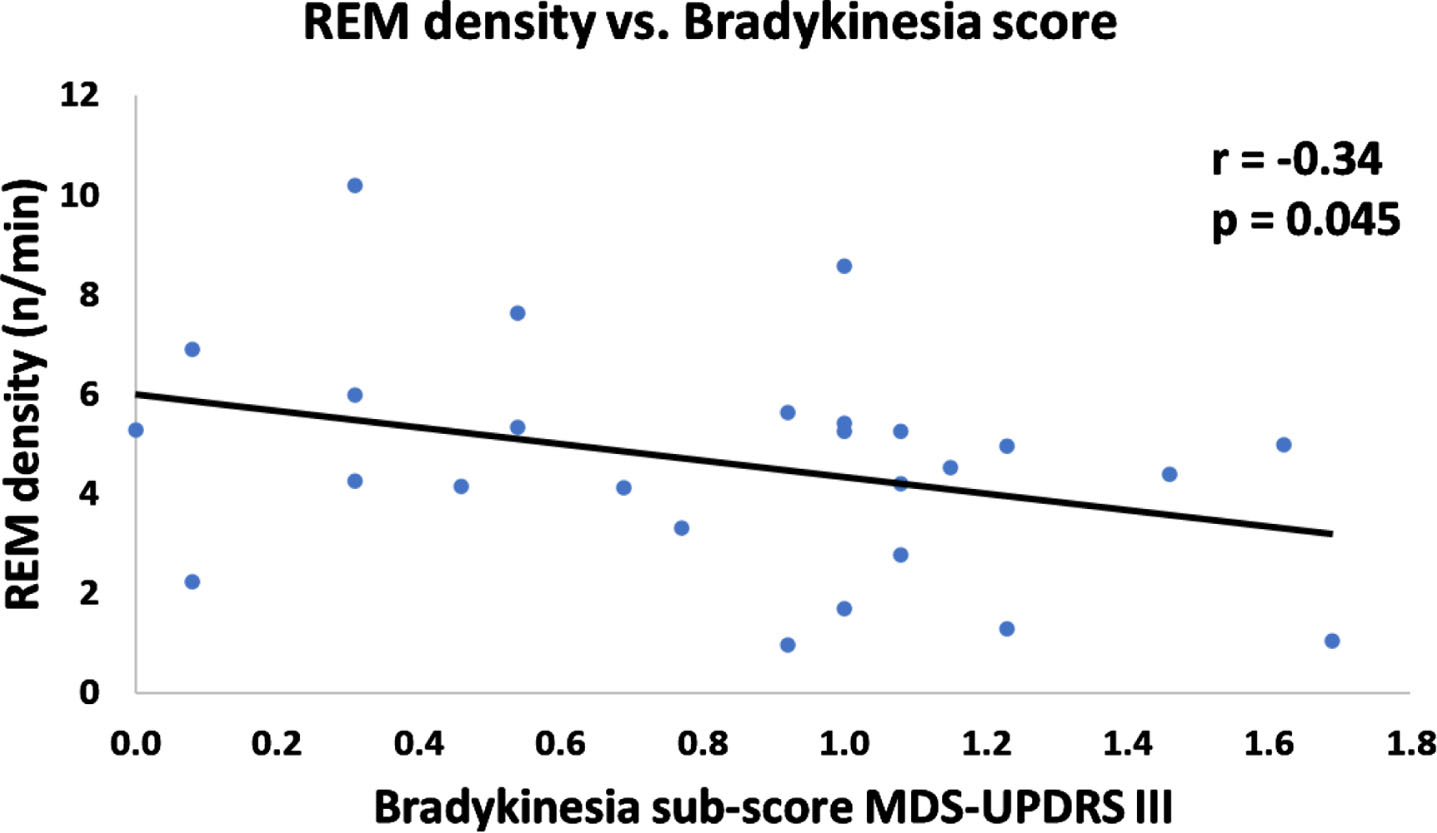
REM density of PD patients negatively correlated with MDS-UPDRS III bradykinesia subscale index (Fig. 2). There was no significant correlation between REM density and disease duration, age, LEDD, MDS-UPDRS III, rigidity subscale, tremor subscale, and overall RSWA%. Additionally, for people with PD, there was no difference in REM density between those above and below RBD cut-off (Mann– Whitney U = 84, n1 = 11, n2 = 15, p = 0.94; median values and range of 5.0 (6.7) and 4.5 (9.1), respectively). Within the PD group, RSWA% did not correlate with any of the clinical features of age, disease duration, LEDD, MDS-UPDRS III total score, and MDS-UPDRS III sub-scores.
Fig. 2
Scatterplot of REM density vs. Bradykinesia sub-score of MDS-UPDRS III. REM density of PD patients negatively correlated with bradykinesia sub-score of MDS-UPDRS III (one-tailed Spearman r =-0.34, p = 0.045).
Overnight distribution across the 4 sleep periods (Fig. 3)
Fig. 3
Boxplots of overnight distribution of RSWA% (A, B) and REM density (C) across four sleep periods. A) RSWA% compared between people with PD and controls. B) RSWA% compared between above vs. below RBD cut-off groups. C) REM density compared between people with PD and controls.
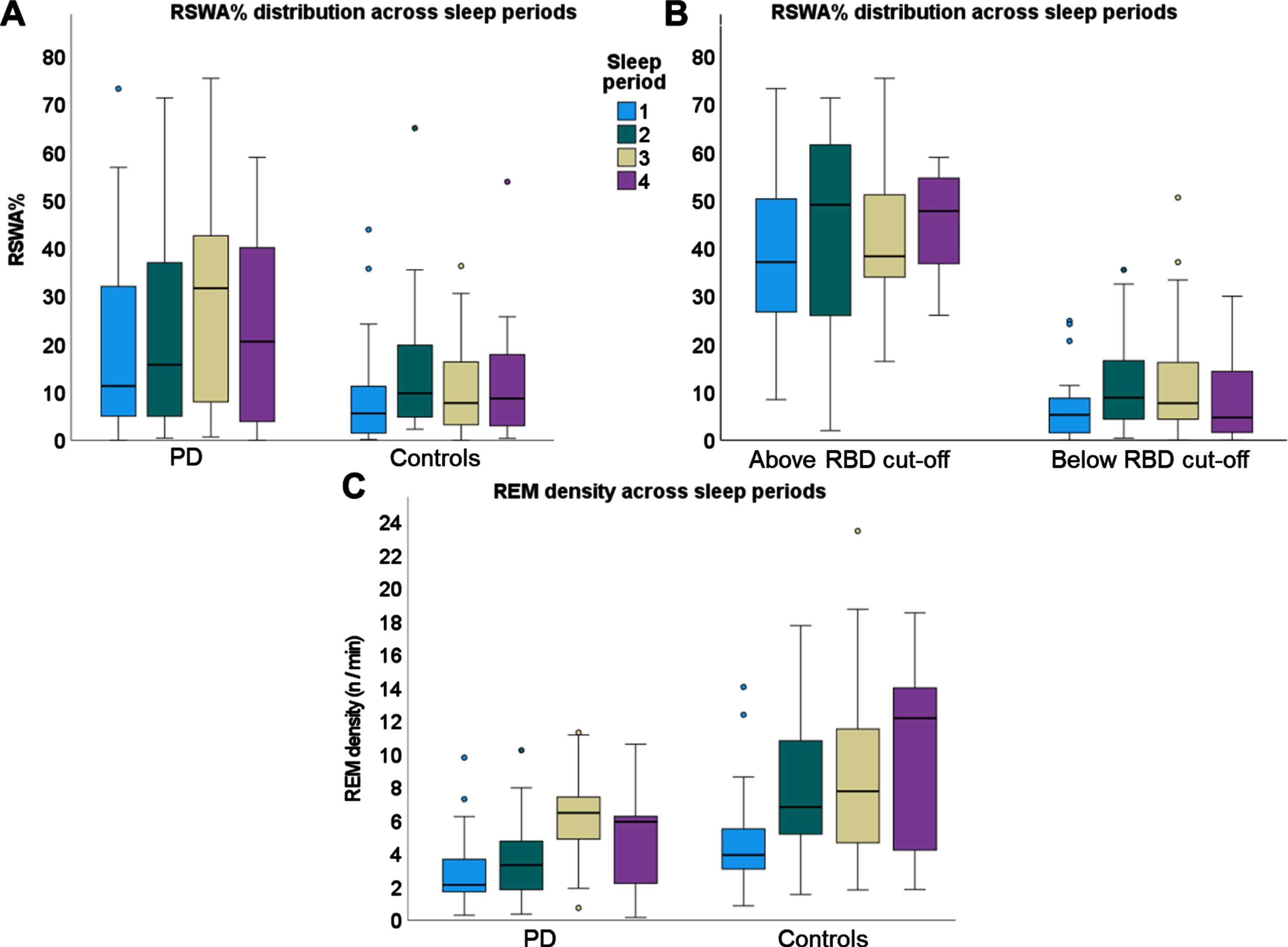
Only 14 out of 51 participants had REM sleep spread across the 4 sleep periods. Forty-one participants had REM sleep during the 1st sleep period, 44 participants had REM during the 2nd sleep period, 41 participants had REM during the 3rd sleep period, and only 26 participants had REM during the 4th sleep period.
The RSWA% was higher for the PD compared to controls (F(1,143) = 4.2, p = 0.04). There was no main effect for the sleep period (F(3, 143) = 2.1, p = 0.10) with a trend for group by sleep period interaction (F)3, 143) = 2.3, p = 0.08). RSWA% was higher for the RBD group compared to those with RSWA that did not meet the cut-off diagnosis of RBD (F(1,144) = 73.3, p < 0.001). There was no main effect for the difference in sleep period (F(3, 144) = 1.7, p = 0.17) nor the group by sleep period interaction (F)3, 144) = 0.2, p = 0.86).
The REM density was lower for the PD compared to controls (F(1,129) = 5.4, p = 0.02). There was a main effect for the sleep period (F(3, 129) = 12.9, p < 0.001). Post-hoc analysis revealed that REM density within the first sleep period was significantly lower than the REM density within the 3rd and 4th sleep periods (p < 0.001 and p = 0.025, respectively). There was no group by sleep period interaction (F)3, 129) = 1.9, p = 0.13).
REM sleep features across 1st, 2nd, and last REM cycles (Fig. 4)
Fig. 4
Boxplots of overnight distribution of REM-related features across 1st, 2nd, and last REM cycles. A) RSWA% compared between people with PD and controls. B) RSWA% compared between above vs. below RBD cut-off groups. C) REM density compared between people with PD and controls. D) REM cycle duration compared between PD and controls.
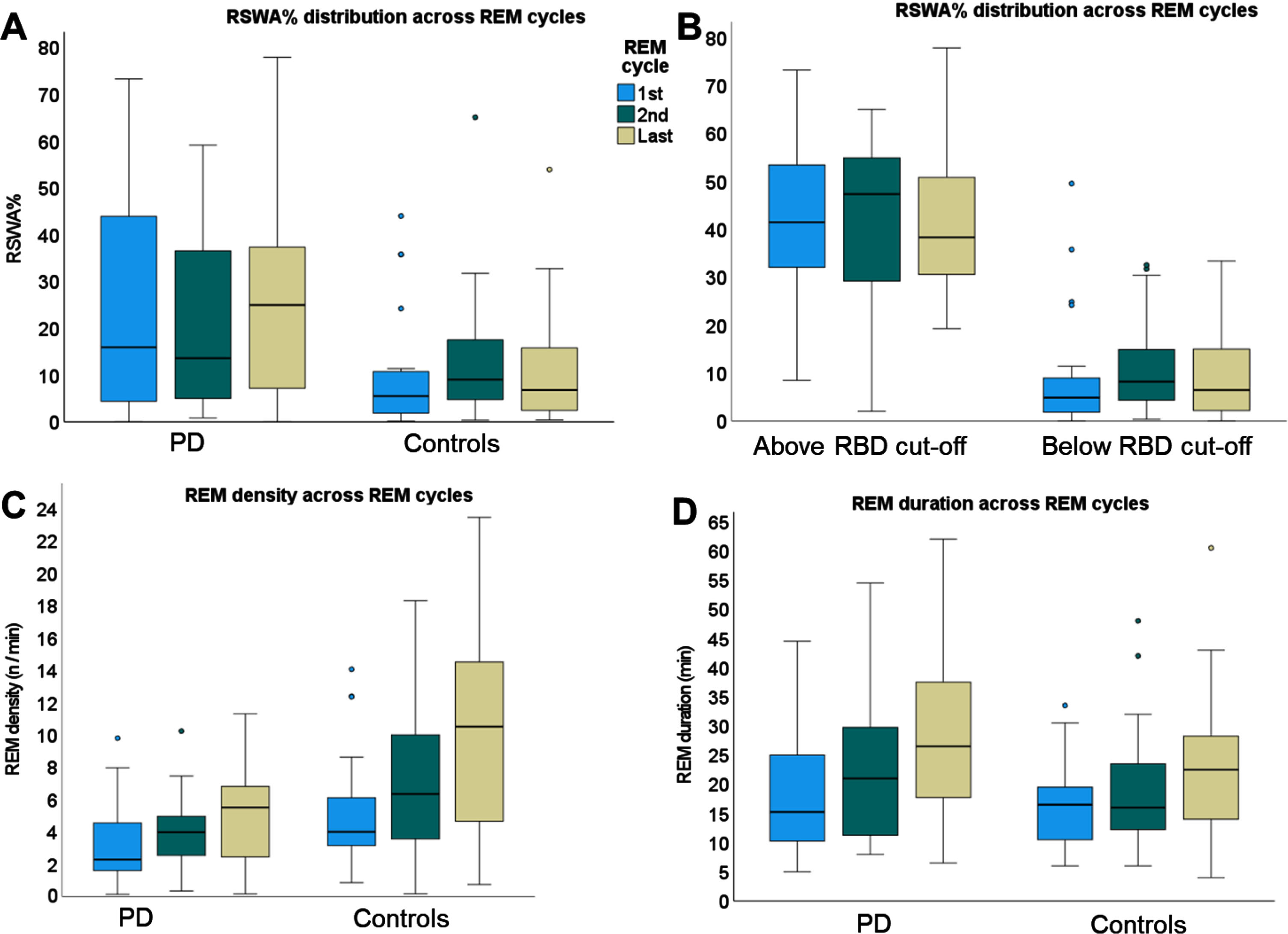
A total of 39 participants had more than 2 REM cycles, with 12 participants having only 2 REM cycles. The RSWA% was higher for the PD compared to controls (F(1,134) = 4.0, p = 0.048). There was no main effect for the REM cycle (F(2, 134) = 0.2, p = 0.85) nor the group by REM cycle interaction (F)2, 134) = 1.6, p = 0.22). The RSWA% was higher for the above cut-off group compared to the below cut-off group (F(1,135) = 61.1, p < 0.001). There were no main effect for REM cycle (F(2,135) = 0.2, p = 0.98) and no group by cycle interaction (F(2,135) = 0.3, p = 0.74).
The REM density was lower for the PD compared to controls (F(1,123) = 6.8, p = 0.01). There was a main effect for the REM cycle (F(2, 123) = 13.8, p < 0.001). Post-hoc analysis revealed that REM density within the first REM cycle was significantly lower than the REM density within the last REM cycle (p < 0.002). There was a trend for group by sleep period interaction (F)2,123) = 2.7, p = 0.07) where REM density of controls increased by 6.5 REMs/min from the first to the last cycle (p = 0.018), while PD had a more moderate increase of 2.2 REMs/min from first to the last cycle (p = 0.043).
There was no difference in REM cycle length between PD and controls (F(1,133) = 1.3, p = 0.26). There was a main effect for the REM length (F(2, 133) = 7.1, p = 0.001), with 1st REM being shorter than the last REM (p = 0.003). There was no group by REM length interaction (F)2,133) = 0.3, p = 0.74).
DISCUSSION
The goal of the present study was to evaluate the temporal distribution of REM sleep features across the night and to compare them between people with PD and controls. Compared to controls, people with PD had lower REM density and higher RSWA%. For both groups, REM cycle length and REM density increased across the night. Importantly, RSWA% was distributed similarly across the night for all participants, leading to a conclusion that an accurate assessment of RSWA% can be sufficiently determined by examining the REM sleep in the first part of the night.
Sleep disturbances are a prevalent non-motor symptom of PD, with two-thirds of patients with PD reporting poor sleep quality [28]. Indeed, in this study, PD patients reported higher scores on the ESS and RBDQ questionnaires. Nevertheless, there was no difference in sleep macroarchitecture parameters between PD and controls, except for a slightly higher PLMS score and higher RSWA%. Within the PD group, RSWA% did not correlate with age, disease duration, LEDD, and MDS-UPDRS III total score. Our findings diverge from the outcomes of the meta-analysis, which indicated that advanced age and longer disease duration are associated with an increased risk of RBD in PD [29] or with disease duration [30] and progression [31]. Differences in results could be partially explained by our relatively homogeneous group of patients all of which had RSWA.
Interestingly, in the microarchitecture features, people with PD had overall lower REM density, supporting previous findings [14]. Exploration of differences in REM density between participants above and below the RBD cut-off showed no difference between the groups. REM density increased towards the end of the night, in line with previous research [11–13]. This increase occurred both for controls and PD. Within the PD group, REM density negatively correlated with the bradykinesia subscale of the MDS-UPDRS part III. Rapid eye movements, a phasic REM sleep activity, have been associated with the activation of the motor cortex during sleep [32]. One possible explanation for the reduced REM density in PD may be reduced cortical activation during REM sleep, which parallels the reduced cortical activation observed during wakefulness in individuals with bradykinesia [14, 33, 34]. The similarity between the two phenomena and the negative correlation between REM density and the bradykinesia score suggests a potential common underlying mechanism. Another possible explanation for the reduced REM density in PD may be due to saccadic deficits in PD patients observed during wake, suggested as alternations in oculomotor control [35–38]. Interestingly, a previous report in non-diagnosed individuals showed higher REM density in isolated RSWA compared to controls [39] raising several questions regarding the neurodegenerative process and its role on REM density. Future longitudinal studies should explore why the increase in REM density observed in RSWA may later decrease once phenoconverted to a neurodegenerative disorder and whether REM density is correlated with biological markers of disease (i.e., alpha-synuclein deposits or DaT SPECT imaging) [10].
Contrary to the previous report, REM cycle length increased from the first to the last REM cycle for both groups [11]. These results suggest that PD patients in this study have preserved underlying circadian regulation. However, contrary to the increased REM density towards the end of the night, RSWA% was similarly distributed across the night, supporting most of the previous results [11, 15]. Different temporal distributions of these two phenomena could be explained by different underlying mechanisms. REMs are a natural phenomenon, generated by ponto-genicular-occipital waves [40, 41], and following homeostatic regulation, i.e., REM density is highest when the sleep pressure is low [27]. Conversely, RSWA is a pathological state of dysfunction of neurons in the brain stem [1]. The equal distribution of RSWA% across the night suggests that RSWA pathology is potentially independent from homeostatic and/or circadian regulation.
Unlike previous results reporting higher RSWA% during later sleep periods in people with RSWA% above the RBD cut-off value [15], the present study found similar RSWA% distribution across the night for both the above and below cut-off groups. The discrepancy in the results could be explained by methodological differences and different participant populations. First, this study included both controls and people with PD and eight out of 15 of the above cut-off group demonstrated typical dream enactment behaviors, while Sasai-Sakuma et al. analyzed healthy participants with incidental RSWA findings. The inclusion of diagnosed patients with PD provided an insight into the integration of RSWA from prodromal to disease. The similar % RSWA across the night could reflect the more advanced neurodegenerative process. This should be further explored in future studies. Additionally, in our study, the RBD cut-off values were determined by ‘any’ EMG activity of the mentalis, flexor digitorum superficialis, and tibialis anterior muscles, while Sasai-Sakuma et al. used the RBD cut-off value based on phasic EMG activity in mentalis muscle. When evaluating the night-to-night variability of RSWA%, they reported a good between-night agreement for tonic, but not for phasic excessive chin muscle activity [6]. Importantly, REM-sleep-related behavioral events occur predominantly in the upper limbs [42], with some evidence suggesting that 65% of events would be missed if the upper limbs were not recorded [43]. Speculatively, higher phasic EMG activity in the mentalis muscle toward the end of the night in the above RBD cut-off group could be obscured by an equal overnight distribution of RSWA% in the upper limbs muscle, but this claim needs to be further validated.
In this study, we combined two previously reported methods to assess the distribution of RSWA% across the four sleep periods and within individual REM cycles [11, 15]. Comparing RSWA% between sleep periods provides a good allocation of REM across the night, yet some sleep periods remain without any REM. On the other hand, comparing directly between REM cycles within each participant and across groups requires controlling for a similar number of REM cycles and therefore may introduce selection bias. The similar results of these two analyses reinforce our findings of similar distribution of RSWA% across the night. Nonetheless, our study has several limitations, mainly the relatively small sample size. In addition, we did not assess the effect of dopaminergic medication dosage or intake time on the distribution of REM sleep features and more so on REM density. However, by excluding participants with sleep disorders, potentially interfering with muscle signals during REM sleep such as high AHI and PLMS indices and excluding people taking SSRI we ensured that our sample reflects the true findings of RSWA%. Future studies should explore the effect of medication, associated with reduced bradykinesia, with REM density.
In conclusion, PD pathology affects REM sleep features, i.e., decreased REM density and increased RSWA%, but does not affect the overnight distribution of those features. While REM density increased towards the end of the night, RSWA% was similarly distributed across the night for both PD vs. controls and above vs. below RBD cut-off. These findings suggest that identification of RSWA in any sleep period is sufficient for a clinical diagnosis. This finding has clinical implications for diagnosing RBD, especially in the following situations: 1) People presenting with REM sleep only in the first part of the night; 2) People with OSA undergoing split-night PSG; and 3) People undergoing clinical PSG with early-than-usual awakenings, thus missing the REM most rich sleep period.
ACKNOWLEDGMENTS
The authors would like to thank the participants of the study for their time and effort and thank Chen Zanzuri and Reut Rital for their assistance with PSG data collection.
FUNDING
This project was supported by the USA MED Research Acquisition Activity, Department of Defence awarding office, through the FY19 Award Mechanism: Parkinson’s Research Program Investigator-Initiated under Award No. W81XWH2010468. The funding agency was not involved in design, acquisition, interpretation or the findings or the writing of this manuscript. A.D. is a PhD student partially supported by the Israeli Scholarship Education Foundation (ISEF) for PhD excellence in academic and social leadership.
CONFLICT OF INTEREST
AD, SO, SK, DW, RT report no COI pertaining to the current project.
NG reports no conflict of interest pertaining to the manuscript. He discloses counseling for: Neuroderm, Intec Pharma, Teva, Genzyme-Sanofi, Biogen, Lysosomal Therapeutics, Denali, Cellanis, GaitBetter, Vibrant and Sionara; he holds shares or options in Lysosomal Therapeutics, Cellanis, GaitBetter and Vibrant; he received royalties from Lysosomal Therapeutics; received honorarium from UCB, Teva, Novartis, Abbvie, Genzyme-Sanofie, Neuroderm, Bial, Shire, MDS. He received grant support from MJFF and Biogen.
AT reports receiving research grants from MJFF, honoraria from Abbvie, and consulting fees from Capsida. AT reports no conflict of interest pertaining to the manuscript.
AM reports receiving a grant from the USA MED Research Acquisition Activity, Department of Defense to support this research as well as grants from MJFF and JPND. She does not have any COI pertaining to this study. AM is an Editorial Board member of this journal but was not involved in the peer-review process nor had access to any information regarding its peer review.
DATA AVAILABILITY
The data supporting the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
[1] | Dauvilliers Y , Schenck CH , Postuma RB , Iranzo A , Luppi PH , Plazzi G , Montplaisir J , Boeve B ((2018) ) REM sleep behaviour disorder. Nat Rev Dis Primers 4: , 19. |
[2] | Sateia MJ ((2014) ) International Classification of Sleep Disorders-Third Edition. Chest 146: , 1387–1394. |
[3] | Zhang H , Iranzo A , Högl B , Arnulf I , Ferini-Strambi L , Manni R , Miyamoto T , Oertel WH , Dauvilliers Y , Ju Y , Puligheddu M , Sonka K , Pelletier A , Montplaisir JY , Stefani A , Ibrahim A , Frauscher B , Leu-Semenescu S , Zucconi M , Terzaghi M , Miyamoto M , Janzen A , Figorilli M , Fantini ML , Postuma RB ((2022) ) Risk factors for phenoconversion in rapid eye movement sleep behavior disorder. Ann Neurol 91: , 404–416. |
[4] | Postuma RB , Iranzo A , Hu M , Högl B , Boeve BF , Manni R , Oertel WH , Arnulf I , Ferini-Strambi L , Puligheddu M , Antelmi E , Cochen De Cock V , Arnaldi D , Mollenhauer B , Videnovic A , Sonka K , Jung KY , Kunz D , Dauvilliers Y , Provini F , Lewis SJ , Buskova J , Pavlova M , Heidbreder A , Montplaisir JY , Santamaria J , Barber TR , Stefani A , St Louis EK , Terzaghi M , Janzen A , Leu-Semenescu S , Plazzi G , Nobili F , Sixel-Doering F , Dusek P , Bes F , Cortelli P , Ehgoetz Martens K , Gagnon JF , Gaig C , Zucconi M , Trenkwalder C , Gan-Or Z , Lo C , Rolinski M , Mahlknecht P , Holzknecht E , Boeve AR , Teigen LN , Toscano G , Mayer G , Morbelli S , Dawson B , Pelletier A ((2019) ) Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 142: , 744–759. |
[5] | Neikrug AB , Ancoli-Israel S ((2012) ) Diagnostic tools for REM sleep behavior disorder. Sleep Med Rev 16: , 415–429. |
[6] | Cygan F , Oudiette D , Leclair-Visonneau L , Leu-Semenescu S , Arnulf I ((2010) ) Night-to-night variability of muscle tone, movements, and vocalizations in patients with REM sleep behavior disorder. J Clin Sleep Med 6: , 551–555. |
[7] | Ferri R , Marelli S , Cosentino FII , Rundo F , Ferini-Strambi L , Zucconi M ((2013) ) Night-to-night variability of automatic quantitative parameters of the chin EMG amplitude (atonia index) in REM sleep behavior disorder. J Clin Sleep Med 9: , 253–258. |
[8] | Cesari M , Heidbreder A , St. Louis EK , Sixel-Döring F , Bliwise DL , Baldelli L , Bes F , Fantini ML , Iranzo A , Knudsen-Heier S , Mayer G , McCarter S , Nepozitek J , Pavlova M , Provini F , Santamaria J , Sunwoo J-S , Videnovic A , Högl B , Jennum P , Christensen JAE , Stefani A ((2022) ) Video-polysomnography procedures for diagnosis of rapid eye movement sleep behavior disorder (RBD) and the identification of its prodromal stages: Guidelines from the International RBD Study Group.zsab. Sleep 45: , 257. |
[9] | Iranzo A , Frauscher B , Santos H , Gschliesser V , Ratti L , Falkenstetter T , Stürner C , Salamero M , Tolosa E , Poewe W , Santamaria J , Högl B ((2011) ) Usefulness of the SINBAR electromyographic montage to detect the motor and vocal manifestations occurring in REM sleep behavior disorder. Sleep Med 12: , 284–288. |
[10] | Saleh C , Diederich NJ , Schroeder LA , Schmidt M , Rüegg S , Khatami R ((2022) ) Time to reconsider REM density in sleep research. Clin Neurophysiol 137: , 63–65. |
[11] | Arnaldi D , Latimier A , Leu-Semenescu S , Vidailhet M , Arnulf I ((2016) ) Loss of REM sleep features across nighttime in REM sleep behavior disorder. Sleep Med 17: , 134–137. |
[12] | Peters KR , Ray LB , Fogel S , Smith V , Smith CT ((2014) ) Age differences in the variability and distribution of sleep spindle and rapid eye movement densities. PLoS One 9: , e91047. |
[13] | Darchia N , Campbell IG , Palagini L , Feinberg I ((2004) ) Rapid eye movement density shows trends across REM periods but is uncorrelated with NREM delta in young and elderly human subjects. Brain Res Bull 63: , 433–438. |
[14] | Schroeder LA , Rufra O , Sauvageot N , Fays F , Pieri V , Diederich NJ ((2016) ) Reduced rapid eye movement density in Parkinson disease: A polysomnography-based case-control study. Sleep 39: , 2133–2139. |
[15] | Sasai-Sakuma T , Frauscher B , Mitterling T , Ehrmann L , Gabelia D , Brandauer E , Inoue Y , Poewe W , Högl B ((2014) ) Quantitative assessment of isolated rapid eye movement (REM) sleep without atonia without clinical REM sleep behavior disorder: Clinical and research implications. Sleep Med 15: , 1009–1015. |
[16] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W , Obeso J , Marek K , Litvan I , Lang AE , Halliday G , Goetz CG , Gasser T , Dubois B , Chan P , Bloem BR , Adler CH , Deuschl G ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30: , 1591–1601. |
[17] | Johns MW ((1991) ) A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep 14: , 540–545. |
[18] | Stiasny-Kolster K , Mayer G , Schäfer S , Möller JC , Heinzel-Gutenbrunner M , Oertel WH ((2007) ) The REM sleep behavior disorder screening questionnaire - A new diagnostic instrument. Mov Disord 22: , 2386–2393. |
[19] | Pal G , Goetz CG ((2013) ) Assessing bradykinesia in Parkinsonian disorders. Front Neurol 4: , 54. |
[20] | Krack P , Pollak P , Limousin P , Hoffmann D , Xie J , Benazzouz A , Benabid AL ((1998) ) Subthalamic nucleus or internal pallidal stimulation in young onset Parkinson’s disease. Brain 121: (Pt 3), 451–457. |
[21] | Horisawa S , Fukui A , Yamahata H , Tanaka Y , Kuwano A , Momosaki O , Iijima M , Nanke M , Kawamata T , Taira T ((2021) ) Unilateral pallidothalamic tractotomy for akinetic-rigid Parkinson’s disease: A prospective open-label study. J Neurosurg 135: , 799–805. |
[22] | Tomlinson CL , Stowe R , Patel S , Rick C , Gray R , Clarke CE ((2010) ) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25: , 2649–2653. |
[23] | Berry RB , Brooks R , Gamaldo CE , Harding SM , Lloyd RM , Marcus CL , Vaughn BV for the American Academy of Sleep Medicine ((2015) ) The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.2. www.aasmnet.org. American Academy of Sleep Medicine, Darien, IL. . |
[24] | St Louis EK , Boeve BF ((2017) ) REM sleep behavior disorder: Diagnosis, clinical implications, and future directions. Mayo Clin Proc 92: , 1723–1736. |
[25] | Leclair-Visonneau L , Oudiette D , Gaymard B , Leu-Semenescu S , Arnulf I ((2010) ) Do the eyes scan dream images during rapid eye movement sleep? Evidence from the rapid eye movement sleep behaviour disorder model. Brain 133: , 1737–1746. |
[26] | Hong CC , Gillin JC , Dow BM , Wu J , Buchsbaum MS ((1995) ) Localized and lateralized cerebral glucose metabolism associated with eye movements during REM sleep and wakefulness: A positron emission tomography (PET) study. Sleep 18: , 570–580. |
[27] | Khalsa SBS , Conroy DA , Duffy JF , Czeisler CA , Dijk DJ ((2002) ) Sleep- and circadian-dependent modulation of REM density. J Sleep Res 11: , 53–59. |
[28] | Bargiotas P , Schuepbach MWM , Bassetti CL ((2016) ) Sleep-wake disturbances in the premotor and early stage of Parkinson’s disease. Curr Opin Neurol 29: , 763–772. |
[29] | Zhang X , Sun X , Wang J , Tang L , Xie A ((2017) ) Prevalence of rapid eye movement sleep behavior disorder (RBD) in Parkinson’s disease: A meta and meta-regression analysis. Neurol Sci 38: , 163–170. |
[30] | Sringean J , Ambra S , Marini K , Bergmann M , Werkmann M , Holzknecht E , De Marzi R , Brandauer E , Hackner H , Djamshidian A , Stockner H , Gaig C , Iranzo A , Santamaria J , Tolosa E , Seppi K , Poewe W , Högl B ((2021) ) REM sleep behavior disorder and REM sleep without atonia are more frequent in advanced versus early Parkinson’s disease. Sleep 44: , zsab067. |
[31] | Figorilli M , Marques AR , Vidal T , Delaby L , Meloni M , Pereira B , Lambert C , Puligheddu M , Durif F , Fantini ML ((2020) ) Does REM sleep behavior disorder change in the progression of Parkinson’s disease? Sleep Med 68: , 190–198. |
[32] | De Carli F , Proserpio P , Morrone E , Sartori I , Ferrara M , Gibbs SA , De Gennaro L , Russo G Lo , Nobili L ((2016) ) Activation of the motor cortex during phasic rapid eye movement sleep. Ann Neurol 79: , 326–330. |
[33] | Chen R , Kumar S , Garg RR , Lang AE ((2001) ) Impairment of motor cortex activation and deactivation in Parkinson’s disease. Clin Neurophysiol 112: , 600–607. |
[34] | Wilhelm E , Quoilin C , Derosiere G , Paço S , Jeanjean A , Duque J ((2022) ) Corticospinal suppression underlying intact movement preparation fades in Parkinson’s disease. Mov Disord 37: , 2396–2406. |
[35] | Terao Y , Fukuda H , Ugawa Y , Hikosaka O ((2013) ) New perspectives on the pathophysiology of Parkinson’s disease as assessed by saccade performance: A clinical review. Clin Neurophysiol 124: , 1491–1506. |
[36] | Ekker MS , Janssen S , Seppi K , Poewe W , de Vries NM , Theelen T , Nonnekes J , Bloem BR ((2017) ) Ocular and visual disorders in Parkinson’s disease: Common but frequently overlooked. Parkinsonism Relat Disord 40: , 1–10. |
[37] | Pinkhardt EH , Kassubek J ((2011) ) Ocular motor abnormalities in Parkinsonian syndromes. Parkinsonism Relat Disord 17: , 223–230. |
[38] | Pinkhardt EH , Jürgens R , Lulé D , Heimrath J , Ludolph AC , Becker W , Kassubek J ((2012) ) Eye movement impairments in Parkinson’s disease: Possible role of extradopaminergic mechanisms. BMC Neurol 12: , 5. |
[39] | Dijkstra F , Viaene M , De Volder I , Fransen E , Cras P , Crosiers D ((2020) ) Polysomnographic phenotype of isolated REM sleep without atonia. Clin Neurophysiol 131: , 2508–2515. |
[40] | Ermis U , Krakow K , Voss U ((2010) ) Arousal thresholds during human tonic and phasic REM sleep: Phasic and tonic REM sleep. J Sleep Res 19: , 400–406. |
[41] | Yong MH , Fook-Chong S , Pavanni R , Lim LL , Tan EK ((2011) ) Case control polysomnographic studies of sleep disorders in Parkinson’s disease. PLoS One 6: , e22511. |
[42] | Bugalho P , Lampreia T , Miguel R , Mendonça M , Caetano A , Barbosa R ((2017) ) Characterization of motor events in REM sleep behavior disorder. J Neural Transm 124: , 1183–1186. |
[43] | Högl B , Stefani A , Videnovic A ((2018) ) Idiopathic REM sleep behaviour disorder and neurodegeneration - An update. Nat Rev Neurol 14: , 40–56. |




