Orthostatic Hypotension in Multiple System Atrophy: Related Factors and Disease Prognosis
Abstract
Background:
Multiple system atrophy (MSA) is a rare neurodegenerative disease characterized by Parkinsonism, ataxia, and autonomic nervous failure. Orthostatic hypotension (OH) is the main feature of central vascular autonomic failure in MSA.
Objective:
The study aimed elucidate the effects of OH on cognitive function, disease milestones, and survival.
Methods:
A total of 444 patients with clinically established MSA were enrolled. Mild and severe OH were defined as a decrease in systolic blood pressure (SBP)/diastolic blood pressure (DBP) >20/10 mmHg and SBP/DBP ≥30/15 mmHg, respectively.
Results:
In this study, 215 MSA patients presented without OH, 88 had mild OH, and 141 had severe OH. The proportion of MSA-C in the severe OH subgroup was significantly higher than that in the subgroup without OH (95/46 vs. 113/102, p = 0.021). The UMSARS I score and the frequency of supine hypertension (SH) in patients with OH were significantly higher than those in patients without OH (16.22 vs. 16.89 vs. 14.60, p < 0.001; 77/64 vs. 29/59 vs. 32/183, p < 0.001). Factors related to the severity of OH included sex (OR, 0.65; p = 0.031), onset age (OR, 0.98; p = 0.029), and SH (OR, 0.21; p < 0.001). The median survival time of patients with severe OH was significantly lower than that of patients without OH (6.79 vs. 8.13 years, p = 0.001). Consistently, Cox survival analysis found that compared with patients without OH, patients with severe OH had a significantly increased risk of death (OR, 2.22; p < 0.001).
Conclusion:
Our large cohort study of MSA provides additional evidence for the negative impact of severe OH on survival.
INTRODUCTION
Multiple system atrophy (MSA) is a rare neurodegenerative disease in adults that affects the substantia nigra striatum system, cerebellum, pons, and inferior olive, as well as the brain stem and spinal cord nucleus, which are involved in autonomic function [1]. Its clinical features mainly include autonomic nervous failure, parkinsonism and ataxia [2], and it is classified into parkinsonian (MSA-P) and cerebellar (MSA-C) subtypes according to the motor phenotype. MSA progresses rapidly, with severe disability usually occurring within 5-6 years of symptom onset and death within 10 years [2]. Currently, there is no effective treatment.
Orthostatic hypotension (OH) is the main characteristic of cardiovascular autonomic failure in patients with MSA. Almost 50%of patients with MSA develop OH symptoms at an early stage [3], and it is often accompanied by supine and nocturnal hypertension [4]. One study showed that OH is more common in MSA-C than in MSA-P [5]. A study on 349 MSA patients revealed that the severity of OH was significantly associated with the severity of the disease, orthostatic symptoms and supine hypertension (SH) but was not associated with the subtype of MSA [6]. Some previous studies found that the presence of OH was associated with lower survival [7–9]. However, one study showed that the effect of OH on survival varied according to sex, and the decrease in diastolic blood pressure in the upright position only significantly increased the risk of death in males [10]. Additionally, one study found that MSA patients with OH performed worse in multiple cognitive task tests and had a higher percentage of deteriorating cognitive status at 12 months of follow-up than those without OH [11]. However, in contrast, another study found no significant difference in cognitive function between MSA patients with or without OH [12].
The current study aimed to describe the frequency and magnitude of OH in different subtypes in a large-sample cohort of MSA patients recruited from a Chinese population and clarify the effects of different magnitudes of OH on cognitive function, disease milestones and survival.
MATERIALS AND METHODS
Study population
A total of 444 patients with a diagnosis of clinically established MSA according to the consensus criteria in 2008 [13] were enrolled at the Department of Neurology, West China Hospital, Sichuan University between January 2014 and December 2020. All patients underwent brain magnetic resonance imaging (MRI) scanning and other supplementary tests, such as spinal cerebellar ataxia gene tests (SCA1, 2, 3, 6, 7), tumor marker tests and immunological tests, to exclude other neurological diseases. In addition, we also excluded stroke or brain injury patients. All participants signed written informed consent before recruitment, and this study was approved by the Ethics Committee of West China Hospital, Sichuan University.
Clinical assessment
The neurologist collected clinical information from all participants at baseline face-to-face interviews, including age, sex, age of onset, education, disease duration, onset symptoms, and patients’ supine and orthostatic blood pressure (BP). Supine and orthostatic BP is measured by the patient’s BP in the supine position for 10 min, then let the patient stand, and measure the BP at the first, third, fifth, and tenth minutes. OH was defined as a decrease in systolic BP (SBP) ≥20 mmHg or diastolic BP (DBP) ≥10 mmHg within 3 min from supine to standing position [14]. In the study, mild OH was defined as a decrease in SBP between 20–30 mmHg or DBP between 10–15 mmHg in 3 min, and severe OH was defined as a decrease in SBP ≥30 mmHg or DBP ≥15 mmHg in 3 min. SH was defined as SBP ≥140 mmHg or DBP ≥90 mmHg after at least 10 min of rest in the supine position [15]. Delayed OH was defined as a decrease in SBP ≥20 mmHg or DBP ≥10 mmHg in 10 min.
The Unified Multiple System Atrophy Rating Scale (UMSARS) was used to assess disease severity in patients [16]. UMSARS-I (12 items) assessed patient-reported functional disability, UMSARS-II (14 items) assessed motor impairment based on a clinical examination, and the disease milestones included dependence on wheelchairs and death. The Montreal Cognitive Assessment (MoCA) and Frontal Assessment Battery (FAB) were used to assess overall cognitive and frontal function, respectively [17, 18]. The optimal cutoff score for screening for mild cognitive impairment (MCI) was 19 points for individuals with less than 6 years of education, 22 points for individuals with 7–12 years of education, and 24 points for individuals with more than 12 years of education [19]. In the study, the cutoff score for impaired frontal lobe function was defined as an overall score more than 1.5 standard deviations below the healthy control mean FAB score in a previous study [20]. The patients were followed up by telephone or face-to-face each year.
Statistical analysis
All data are expressed as the mean (standard deviation), ratio, OR (95%CI), or HR (95%CI). The Kruskal–Wallis test and chi-square test were used to analyze differences in the continuous and categorical variables, respectively, among the different subgroups, and an ordinal logistic regression model was used to explore the factors related to OH in MSA. Multivariate Cox regression analysis and Kaplan–Meier survival analysis were used to compare wheelchair dependence and death in MSA patients with different magnitudes of OH. Cox proportional hazard regression analysis adjusted for age of onset, sex and course of disease, clinical type, total UMSARS, SH (yes vs. no), urinary incontinence (yes vs. no), history of hypertension (yes vs. no), and anti-OH treatment. All data analysis was performed using SPSS 22.0 (IBM, Chicago, IL). A p value <0.05 was considered to indicate statistical significance.
RESULTS
Demographics and clinical characteristics
A total of 444 patients with a clinically established diagnosis of MSA were enrolled, including 215 without OH, 88 with mild OH, and 141 with severe OH. Among the 444 MSA patients, a total of 26 patients experienced delayed OH, including two patients with mild OH and 23 patients with severe OH. The proportion of female patients in the severe OH subgroup was significantly lower than that in the other two subgroups (53/88 vs. 46/42 vs. 109/106, p = 0.028), and the proportion of MSA-C in the severe OH subgroup was significantly higher than that in the patients without OH (95/46 vs. 113/102, p = 0.005). MSA patients with OH, regardless of mild or severe OH, had significantly greater UMSARS I score than those without OH (16.22±5.58 vs. 16.89±5.87 vs. 14.60±5.46, p = 0.000). There were no significant differences in the rest of the UMSARS scores among the three subgroups. In addition, there was a significant difference in the frequency of SH (yes vs. no) and delayed OH among the three subgroups (77/64 vs. 29/59 vs. 32/183, p = 0.000), with the highest frequency in the severe OH subgroup (54.6%). In terms of cognition, there were no significant differences in MoCA and FAB scores among the three subgroups (Table 1).
Table 1
Demographics and clinical characteristics in different magnitude of OH groups
| No OH | Mild OH | Severe OH | p | |
| N = 215 | N = 88 | N = 141 | ||
| Sex (Female/male) | 109/106 | 46/42 | 53/88 | 0.028 |
| Age, mean (SD), y | 60.01 (8.63) | 59.70 (8.87) | 58.85 (8.71) | 0.401 |
| Age of onset, mean (SD), y | 57.86 (8.48) | 57.46 (8.56) | 56.41 (8.70) | 0.306 |
| Disease duration, mean (SD), y | 2.15 (1.06) | 2.24 (1.06) | 2.44 (1.17) | 0.080 |
| Education, mean (SD), y | 9.43 (3.73) | 9.35 (3.90) | 9.50 (3.75) | 0.969 |
| Clinical type (MSA-C vs. MSA-P) | 113 vs. 102 | 51 vs.37 | 95 vs. 46 | 0.021 |
| UMSARS I, mean (SD) | 14.60 (5.46) | 16.22 (5.58) | 16.89 (5.87) | <0.001 |
| UMSARS II, mean (SD) | 17.32 (6.16) | 17.94 (6.51) | 18.11 (6.60) | 0.629 |
| UMSARS total, mean (SD) | 31.91 (10.76) | 34.16 (11.45) | 35.00 (11.44) | 0.056 |
| UMSARS IV, mean (SD) | 1.96 (0.85) | 1.97 (0.92) | 2.17 (0.92) | 0.095 |
| Urinary incontinence (Yes/No) | 135/80 | 55/33 | 98/43 | 0.377 |
| Delayed OH (Yes/No) | 0/215 | 23/65 | 3/138 | <0.001 |
| Supine hypertension (Yes/No) | 32/183 | 29/59 | 77/64 | <0.001 |
| History of hypertension (Yes/No) | 40/175 | 13/75 | 19/122 | 0.403 |
| Anti-OH treatment | 0/215 | 0/88 | 4/137 | 0.021 |
| MoCA, mean (SD) | 21.70 (4.78) | 21.92 (5.11) | 20.83 (5.44) | 0.242 |
| FAB, mean (SD) | 14.44 (2.78) | 14.70 (2.77) | 14.09 (2.99) | 0.294 |
| Wheelchair, median (95%CI), y | 5.04 (4.82–5.27) | 4.93 (4.35–5.50) | 5.37 (5.14–5.61) | 0.358 |
| Survival time, median (95%CI), y | 8.13 (7.44–8.81)* | 6.76 (6.10–7.42) | 6.79 (6.14–7.44)* | 0.003 |
OH, orthostatic hypotension; MSA, multiple system atrophy; MSA-C, MSA-cerebellar; MSA-P, MSA-parkinsonian; UMSARS, Unified MSA Rating Scale; MoCA, The Montreal Cognitive Assessment; MCI, mild cognitive impairment; FAB, Frontal Assessment Battery. *There was a significant difference between the two groups.
Associated risk factors for OH
In the ordinal logistic regression model, the factors related to the magnitude of OH included sex, age of onset, clinical subtype and SH (yes vs. no) (Table 2). Compared with MSA patients without OH and with mild OH, the patients with severe OH had a significantly lower proportion of female sex (OR, 0.65; 95%CI, 0.45–0.96; p = 0.031), younger age of onset (OR, 0.98; 95%CI, 0.95–1.00; p = 0.029), and lower proportion of patients without SH (OR, 0.21; 95%CI, 0.14–0.31; p < 0.001).
Table 2
Factors related to the magnitude of OH in MSA
| OR (95%CI) | p | |
| Sex (Female vs. Male) | 0.65 (0.45–0.96) | 0.031 |
| Age of onset | 0.98 (0.95–1.00) | 0.029 |
| Disease duration | 1.11 (0.92–1.34) | 0.272 |
| Clinical type (MSA-C vs. MSA-P) | 1.51 (1.02–2.24) | 0.038 |
| UMSARS total | 1.01 (0.98–1.03) | 0.601 |
| UMSARS IV | 1.20 (0.89–1.62) | 0.236 |
| Urinary incontinence (Yes vs. No) | 0.82 (0.54–1.23) | 0.335 |
| Supine hypertension (No vs. Yes) | 0.21 (0.14–0.31) | <0.001 |
| MoCA (Normal vs. MCI) | 0.86 (0.56–1.33) | 0.497 |
| FAB (Normal vs. Impairment) | 0.95 (0.63–1.44) | 0.814 |
Ordinal logistic regression: patients were classified in three categories according to the magnitude of OH as clinically defined; 0: No significant OH, 1: mild OH, 2: severe OH. MSA, Multiple system atrophy; OH, orthostatic hypotension; UMSARS, Unified MSA Rating Scale; MoCA, The Montreal Cognitive Assessment; MCI, mild cognitive impairment; FAB, Frontal Assessment Battery.
OH and clinical milestones
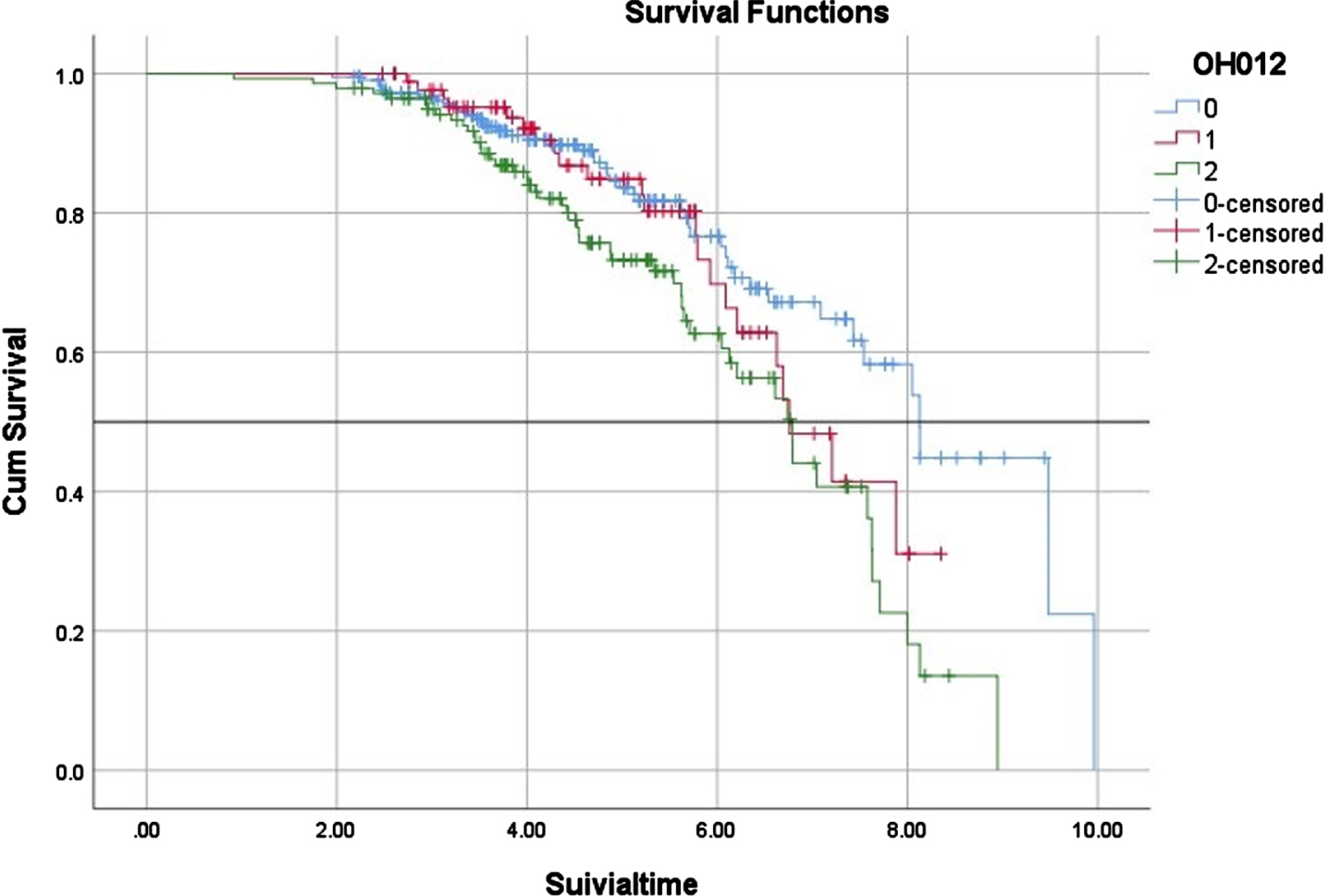
In terms of clinical milestones, we found no significant difference in the time of wheelchair occurrence among the three subgroups (Table 1, Fig. 1). However, in terms of survival, the patients with severe OH (6.79 years (95%CI, 6.14–7.44)) had a significantly shorter median survival time than that of patients without OH (8.13 years (95%CI, 7.44–8.81) (Table 1, Fig. 2). Although there was no significant difference between MSA patients with mild OH and those without OH (6.76 vs. 8.13 years, p = 0.294), patients with mild OH tended to have a worse prognosis, which was more significant after 6 years of survival (Fig. 2). The median survival time of patients with mild OH was not significantly different from that of patients with severe OH (6.76 vs. 6.79 years, p = 0.134). Cox proportional hazard regression analysis was consistently adjusted for age of onset, sex, course of disease, clinical subtype, total UMSARS, SH (yes vs. no), urinary incontinence (yes vs. no), history of hypertension (yes vs. no), and anti-OH treatment. Compared with MSA patients without OH, patients with severe OH had a significantly increased risk of death (OR, 2.22; 95%CI, 1.43–3.47; p < 0.001), while patients with mild OH had no significant difference in the risk of death (OR, 1.37; 95%CI, 0.81–2.31; p = 0.248) (Table 3).
Table 3
Association between magnitude of OH and the occurrence of wheelchair and death in MSA patients
| Model 1 | p | Model 2 | p | Model 3 | p | Model 4 | p | ||
| Wheelchair | Mild OH | 1.37 (0.96–1.95) | 0.080 | 1.29 (0.91–1.84) | 0.154 | 1.32 (0.92–1.90) | 0.137 | 1.35 (0.94–1.95) | 0.108 |
| Severe OH | 1.22 (0.89–1.68) | 0.225 | 1.16 (0.84–1.60) | 0.369 | 1.19 (0.85–1.66) | 0.322 | 1.25 (0.88–1.76) | 0.214 | |
| Death | Mild OH | 1.46 (0.87–2.45) | 0.151 | 1.43 (0.86–2.40) | 0.172 | 1.38 (0.82–2.34) | 0.231 | 1.37 (0.81–2.31) | 0.248 |
| Severe OH | 2.56 (1.71–3.91) | <0.001 | 2.44 (1.61–3.70) | <0.001 | 2.32 (1.50–3.60) | <0.001 | 2.22 (1.43–3.47) | <0.001 |
Cox proportional hazard regression: patients were classified in three categories according to the magnitude of OH as clinically defined; The data is expressed as HR (95%CI). Model 1: Adjusted for age of onset, sex, course of disease, and clinical type; Model 2: Model 1+ UMSARS total; Model 3: Model 2+ supine hypertension (No/Yes); Model 4: Model 3+ Urinary incontinence (Yes/No), history of hypertension (Yes/No), and anti-OH treatment (Yes/No).
Fig. 1
Time of wheelchair dependence in different OH groups in MSA patients. 0: No significant OH, 1: mild OH, 2: severe OH.
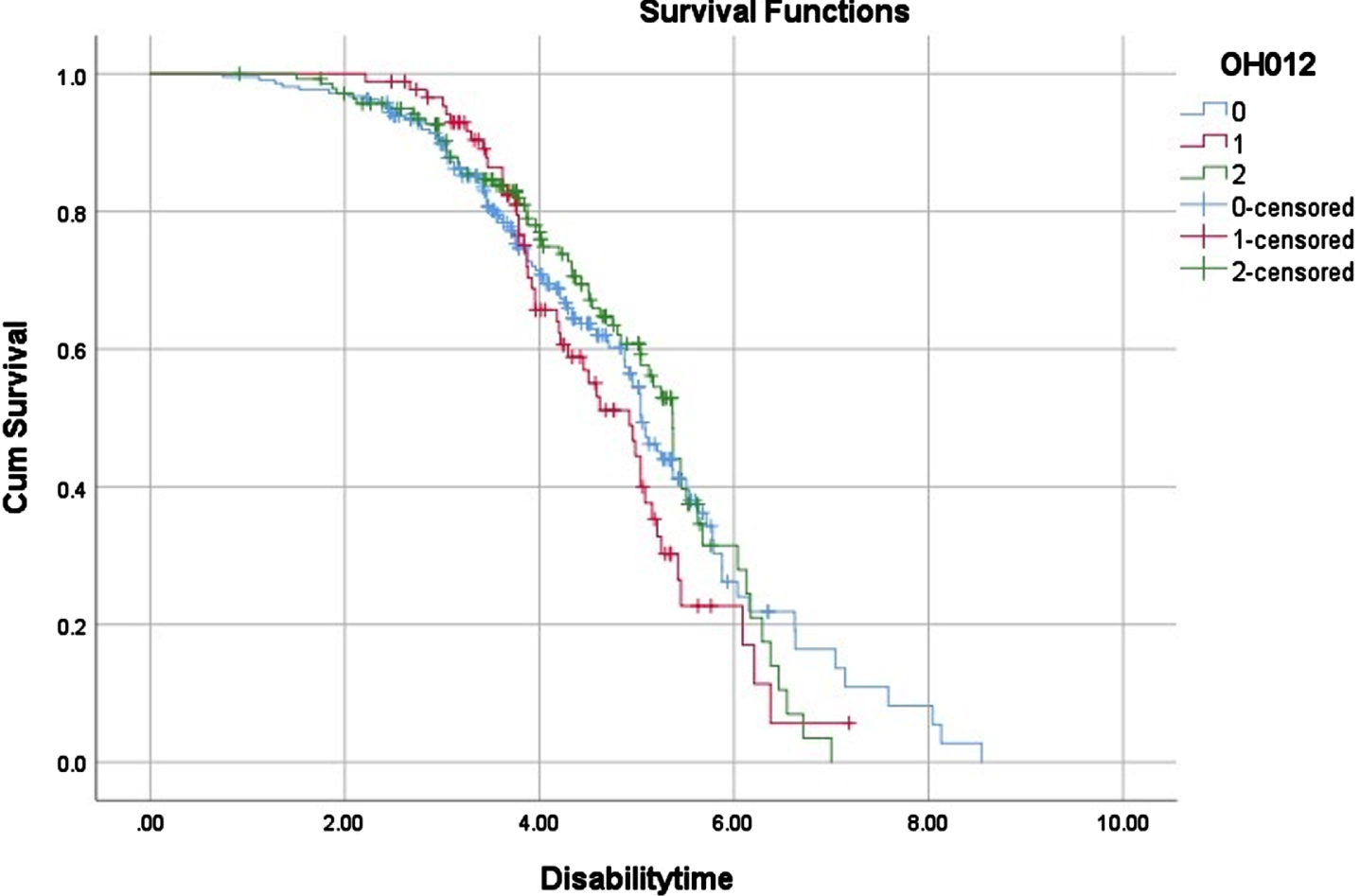
Fig. 2
Survival time in different OH groups in MSA patients. 0: No significant OH, 1: mild OH, 2: severe OH.
DISCUSSION
Our cohort study identified the factors associated with OH in patients with MSA and the relationship between OH and the prognosis of patients with MSA. First, we found that the severe OH subgroup was more likely to be male, have the MSA-C subtype and have SH than MSA patients with mild OH and without OH. In addition, patients with OH, regardless of mild or severe OH, had significantly higher UMSARS I score than those without OH. Second, we found that sex, age of onset and SH were related to the magnitude of OH. Finally, compared with patients without OH, patients with severe OH had a significantly increased risk of death, while patients with mild OH showed no significant difference in risk of death.
Neurogenic OH is due to the decreased ability of the sympathetic nerve to release norepinephrine and the inability to raise peripheral vascular resistance while standing [21, 22], which is common in MSA. OH in MSA is associated with the loss of preganglionic sympathetic neurons in the mesolateral spinal column [23], as well as the loss of catecholaminergic neurons in the ventrolateral medulla [24].
In our MSA cohort, male patients with MSA were likely to have severe OH, which was consistent with a previous study [6] and may be related to the more frequent use of α-blockers with antihypertensive effects for urinary diseases such as benign prostatic hyperplasia in males. In addition, MSA-C patients were likely to have severe OH, which was consistent with a previous study [5] but inconsistent with another study [6]. There were differences in pathology between MSA-C and MSA-P patients [25]. Previous studies found that exercise-induced cardiovascular abnormalities are more obvious in patients with MSA-C, indicating that the cerebellum or brainstem autonomic nerve pathways in patients with MSA-C are severely damaged compared with those in MSA-P patients [26].
We found that the incidence of SH in MSA patients with severe OH, mild OH and without OH was significantly different and increased with the appearance and severity of OH, among which the severe OH subgroup had the highest incidence (54.6%). A previous study also reported a significant correlation between the level of OH and the severity and frequency of SH [6], while another study reported that the frequency (49%) and severity of SH increased significantly in MSA patients with OH [27]. Both OH and SH occur as a result of autonomic nervous system failure, impaired baroreflex pathways, and the inability of the cardiovascular system to regulate blood pressure in response to postural changes. Since the central autonomic pathway is the primarily affected location in MSA patients, the postganglionic sympathetic nerve remains intact [28]. Thus, efferent norepinephrine fibers are preserved, and plasma norepinephrine is normal or only slightly reduced [29]. SH in MSA patients may be driven by residual but unregulated sympathetic activity [30]. In addition, some long-acting pressor drugs for the treatment of OH, such as fludrocortisone, can induce SH. Therefore, when treating patients with both OH and SH, it should be noted that drug treatment should selectively improve OH but not increase the risk of SH. Midodrine treatment combined with avoidance of the supine position for 3-4 hours after each dose can reduce the risk of SH. Servo-controlled abdominal venous compression with an automated inflatable binder is as effective as standard midodrine administration [31], and new therapies such as tomoxetine and droxidopa may also have a lower risk of SH exacerbation [32] [33]. The high incidence of SH in MSA patients with severe OH suggests that we should give more attention to this complication when treating MSA patients with severe OH.
In terms of cognition, we found no difference in the total MoCA score among the different OH subgroups. Our finding was also supported by a previous study showing that MSA patients with and without OH have similar cognitive levels through Mini-Mental State Examination (MMSE) assessments [12]. A recent study showed that early OH (rather than symptom severity) increased the risk of cognitive impairment in MSA patients (HR = 0.59; 95%CI, 0.42–0.83) [34]. The underlying mechanism of the relationship between OH and cognitive impairment remains unclear, and there are two proposed hypotheses: 1) diffuse neuropathological theory, that is, OH and cognitive impairment occur simultaneously due to diffuse cerebral and peripheral deposition of α-synuclein [35]; and 2) brain hypoperfusion mediated by OH may impair cognitive function [36]. No between-subgroup differences in the MoCA scores were found in our cohort, which may be explained by the following reasons: enrolled MSA patients in the current study were at the early stage of the disease (duration of approximately 2 years); we did not consider the onset time of OH of patients; and MoCA, as a global cognition assessment tool, may not be sensitive in the early stage of the disease.
In our MSA cohort, there was no relationship between different magnitudes of OH and patients’ motor symptoms and overall disability (UMSARS II and IV scores). However, patients with OH had significantly higher UMSARS I scores than patients without OH, which may be because four items of the UMSARS I (12 items) were related to orthostatic symptoms, resulting in a significantly greater score. In terms of clinical milestones, we did not find that different magnitudes of OH were associated with wheelchair dependence, but we found that MSA patients with severe OH had a significantly higher risk of death than those without OH, while there was no significant difference in the risk of death between patients with mild OH and patients without OH. Several previous studies have found that early symptoms of autonomic nervous failure are associated with lower survival in MSA patients [37–39]. However, another study showed that the severity of OH assessed in the 10-min standing trial was not significantly associated with mortality in patients with MSA, but a higher risk of death was observed for patients with daytime SBP variability (HR 3.66, p < 0.01) and those who underwent OH treatment (HR: 2.13, p = 0.02) [40]. Therefore, that study proposed that antihypotensive treatment itself may have a direct negative impact on the survival of MSA patients with OH [40], and most previously published randomized placebo-controlled trials evaluating the effect of antihypotensive drugs on a limited number of MSA patients, lasting only a few weeks at most, lack information on the safety and efficacy of their long-term use [41–43]. Such a conclusion was inconsistent with our finding that patients with severe OH had a significantly increased risk of death, but our cohort was not adjusted for the use of antihypotensive drugs in those patients. A reduction in catecholaminergic neurons in the rostral ventrolateral medulla induced sympathetic vasomotor failure in patients with MSA [44], and dysfunction of the medulla serotonergic system, which regulates the cardiovascular system, may be the cause of early sudden death in MSA patients [45]. Additionally, severe OH is often accompanied by SH, both of which may lead to left ventricular hypertrophy and diastolic dysfunction, which may increase the patient’s risk of future cardiovascular events [46]. OH may also increase the risk of falls and syncope, with the risk of recurrent falls being approximately 2.5 times higher in elderly patients with OH than in older adults without OH [47, 48], thus increasing the risk of fractures and other potentially life-shortening traumas.
Therefore, the mortality of MSA patients with severe OH was significantly increased, and it was caused by OH itself, increased BP variability due to the use of antihypotensive drugs, or the combination of the two; therefore, this topic warrants more rigorous clinical research.
Our study has some limitations. First, only cross-sectional data were available for the assessment of patients’ symptoms and cognition, and the sample size of follow-up data was too small for statistical calculations. Second, detailed OH data, such as the onset time of OH in the course of MSA, was not collected. Finally, we did not specifically record and adjust for the use of antihypotensive drugs in MSA patients after baseline.
Conclusion
Our large cohort study of patients with MSA showed that there was no significant difference in survival between MSA patients with mild OH and those without OH, but severe OH had a significant negative effect on survival. It is necessary to give more clinical attention to the presence of OH in patients with MSA. In addition, antihypotensive drugs for OH in MSA should be selected carefully by considering both the increased occurrence of SH and also the long-term safety associated with the treatment.
ACKNOWLEDGMENTS
The authors thank all the participants of the study.
FUNDING
This article was supported by the Sichuan Science and Technology Program (Grant No. 2022ZDZX0023).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
Anonymized data will be shared upon request with any qualified investigator.
REFERENCES
[1] | Poewe W , Stankovic I , Halliday G , Meissner WG , Wenning GK , Pellecchia MT , Seppi K , Palma JA , Kaufmann H ((2022) ) Multiple system atrophy. Nat Rev Dis Primers 8: , 56. |
[2] | Fanciulli A , Wenning GK ((2015) ) Multiple-system atrophy. N Engl J Med 372: , 249–263. |
[3] | Frongillo D , Stocchi F , Buccolini P , Stecconi P , Viselli F , Ruggieri S , Cannata D ((1995) ) Ambulatory blood pressure monitoring and cardiovascular function tests in multiple system atrophy. Fundam Clin Pharmacol 9: , 187–196. |
[4] | Goldstein DS , Pechnik S , Holmes C , Eldadah B , Sharabi Y ((2003) ) Association between supine hypertension and orthostatic hypotension in autonomic failure. Hypertension 42: , 136–142. |
[5] | Wenning GK , Granata R , Krismer F , Dürr S , Seppi K , Poewe W , Bleasdale-Barr K , Mathias CJ ((2012) ) Orthostatic hypotension is differentially associated with the cerebellar versus the parkinsonian variant of multiple system atrophy: A comparative study. Cerebellum 11: , 223–226. |
[6] | Pavy-Le Traon A , Piedvache A , Perez-Lloret S , Calandra-Buonaura G , Cochen-De Cock V , Colosimo C , Cortelli P , Debs R , Duerr S , Fanciulli A , Foubert-Samier A , Gerdelat A , Gurevich T , Krismer F , Poewe W , Tison F , Tranchant C , Wenning G , Rascol O , Meissner WG ((2016) ) New insights into orthostatic hypotension in multiple system atrophy: A European multicentre cohort study. J Neurol Neurosurg Psychiatry 87: , 554–561. |
[7] | Cao B , Zhang L , Zou Y , Wei Q , Ou R , Chen Y , Shang HF ((2018) ) Survival analysis and prognostic nomogram model for multiple system atrophy. Parkinsonism Relat Disord 54: , 68–73. |
[8] | Foubert-Samier A , Pavy-Le Traon A , Guillet F , Le-Goff M , Helmer C , Tison F , Rascol O , Proust-Lima C , Meissner WG ((2020) ) Disease progression and prognostic factors in multiple system atrophy: A prospective cohort study. Neurobiol Dis 139: , 104813. |
[9] | Grimaldi S , Boucekine M , Witjas T , Fluchere F , Azulay JP , Guedj E , Eusebio A ((2021) ) Early atypical signs and insula hypometabolism predict survival in multiple system atrophy. J Neurol Neurosurg Psychiatry 92: , 881–889. |
[10] | Gurevich T , Merkin L , Rozenberg A , Fisher A , Atanasova Mishkova-Serafimova E , Klepikov D , Giladi N , Peretz C ((2021) ) Interrelationships between survival, sex, and blood pressure in patients with multiple system atrophy. Neuroepidemiology 55: , 56–61. |
[11] | Cuoco S , Carotenuto I , Cappiello A , Scannapieco S , Russillo MC , Andreozzi V , Forino L , Amboni M , Picillo M , Erro R , Barone P , Pellecchia MT ((2021) ) Relationship between orthostatic hypotension and cognitive functions in multiple system atrophy: A longitudinal study. Front Neurol 12: , 711358. |
[12] | Eschlböck S , Delazer M , Krismer F , Bodner T , Fanciulli A , Heim B , Heras Garvin A , Kaindlstorfer C , Karner E , Mair K , Rabensteiner C , Raccagni C , Seppi K , Poewe W , Wenning GK ((2020) ) Cognition in multiple system atrophy: A single-center cohort study. Ann Clin Transl Neurol 7: , 219–228. |
[13] | Gilman S , Wenning GK , Low PA , Brooks DJ , Mathias CJ , Trojanowski JQ , Wood NW , Colosimo C , Dürr A , Fowler CJ , Kaufmann H , Klockgether T , Lees A , Poewe W , Quinn N , Revesz T , Robertson D , Sandroni P , Seppi K , Vidailhet M ((2008) ) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71: , 670–676. |
[14] | Wenning GK , Stankovic I , Vignatelli L , Fanciulli A , Calandra-Buonaura G , Seppi K , Palma JA , Meissner WG , Krismer F , Berg D , Cortelli P , Freeman R , Halliday G , Höglinger G , Lang A , Ling H , Litvan I , Low P , Miki Y , Panicker J , Pellecchia MT , Quinn N , Sakakibara R , Stamelou M , Tolosa E , Tsuji S , Warner T , Poewe W , Kaufmann H ((2022) ) The Movement Disorder Society Criteria for the Diagnosis of Multiple System Atrophy. Mov Disord 37: , 1131–1148. |
[15] | Fanciulli A , Jordan J , Biaggioni I , Calandra-Buonaura G , Cheshire WP , Cortelli P , Eschlboeck S , Grassi G , Hilz MJ , Kaufmann H , Lahrmann H , Mancia G , Mayer G , Norcliffe-Kaufmann L , Pavy-Le Traon A , Raj SR , Robertson D , Rocha I , Struhal W , Thijs R , Tsioufis KP , van Dijk JG , Wenning GK ((2018) ) Consensus statement on the definition of neurogenic supine hypertension in cardiovascular autonomic failure by the American Autonomic Society (AAS) and the European Federation of Autonomic Societies (EFAS): Endorsed by the European Academy of Neurology (EAN) and the European Society of Hypertension (ESH). Clin Auton Res 28: , 355–362. |
[16] | Wenning GK , Tison F , Seppi K , Sampaio C , Diem A , Yekhlef F , Ghorayeb I , Ory F , Galitzky M , Scaravilli T , Bozi M , Colosimo C , Gilman S , Shults CW , Quinn NP , Rascol O , Poewe W ((2004) ) Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS). Mov Disord 19: , 1391–1402. |
[17] | Royall DR ((2001) ) The FAB: A frontal assessment battery at bedside. Neurology 57: , 565. |
[18] | Nasreddine ZS , Phillips NA , Bédirian V , Charbonneau S , Whitehead V , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: , 695–699. |
[19] | Chen KL , Xu Y , Chu AQ , Ding D , Liang XN , Nasreddine ZS , Dong Q , Hong Z , Zhao QH , Guo QH ((2016) ) Validation of the chinese version of montreal cognitive assessment for basic screening mild cognitive impairment. J Am Geriatr Soc 64: , e285–e290. |
[20] | Cao B , Zhao B , Wei QQ , Chen K , Yang J , Ou R , Wu Y , Shang HF ((2015) ) The global cognition, frontal lobe dysfunction and behavior changes in Chinese patients with multiple system atrophy. PLoS One 10: , e0139773. |
[21] | Kaufmann H , Biaggioni I ((2003) ) Autonomic failure in neurodegenerative disorders. Semin Neurol 23: , 351–363. |
[22] | Goldstein DS , Holmes CS , Dendi R , Bruce SR , Li ST ((2002) ) Orthostatic hypotension from sympathetic denervation in Parkinson’s disease. Neurology 58: , 1247–1255. |
[23] | Oppenheimer DR ((1980) ) Lateral horn cells in progressive autonomic failure. J Neurol Sci 46: , 393–404. |
[24] | Benarroch EE , Schmeichel AM , Parisi JE ((2000) ) Involvement of the ventrolateral medulla in parkinsonism with autonomic failure. Neurology 54: , 963–968. |
[25] | Fecek C , Nagalli S Shy drager syndrome. In StatPearls, StatPearls Publishing LLC, Treasure Island (FL). |
[26] | Smith GD , Mathias CJ ((1996) ) Differences in cardiovascular responses to supine exercise and to standing after exercise in two clinical subgroups of Shy-Drager syndrome (multiple system atrophy). J Neurol Neurosurg Psychiatry 61: , 297–303. |
[27] | Fanciulli A , Göbel G , Ndayisaba JP , Granata R , Duerr S , Strano S , Colosimo C , Poewe W , Pontieri FE , Wenning GK ((2016) ) Supine hypertension in Parkinson’s disease and multiple system atrophy. Clin Auton Res 26: , 97–105. |
[28] | Coon EA , Cutsforth-Gregory JK , Benarroch EE ((2018) ) Neuropathology of autonomic dysfunction in synucleinopathies. Mov Disord 33: , 349–358. |
[29] | Goldstein DS , Polinsky RJ , Garty M , Robertson D , Brown RT , Biaggioni I , Stull R , Kopin IJ ((1989) ) Patterns of plasma levels of catechols in neurogenic orthostatic hypotension. Ann Neurol 26: , 558–563. |
[30] | Shannon JR , Jordan J , Diedrich A , Pohar B , Black BK , Robertson D , Biaggioni I ((2000) ) Sympathetically mediated hypertension in autonomic failure. Circulation 101: , 2710–2715. |
[31] | Okamoto LE , Diedrich A , Baudenbacher FJ , Harder R , Whitfield JS , Iqbal F , Gamboa A , Shibao CA , Black BK , Raj SR , Robertson D , Biaggioni I ((2016) ) Efficacy of servo-controlled splanchnic venous compression in the treatment of orthostatic hypotension: A randomized comon with midodrine. Hypertension 68: , 418–426 paris. |
[32] | Ramirez CE , Okamoto LE , Arnold AC , Gamboa A , Diedrich A , Choi L , Raj SR , Robertson D , Biaggioni I , Shibao CA ((2014) ) Efficacy of atomoxetine versus midodrine for the treatment of orthostatic hypotension in autonomic failure. Hypertension 64: , 1235–1240. |
[33] | Biaggioni I , Arthur Hewitt L , Rowse GJ , Kaufmann H ((2017) ) Integrated analysis of droxidopa trials for neurogenic orthostatic hypotension. BMC Neurol 17: , 90. |
[34] | Barrio IR , Miki Y , Jaunmuktane ZT , Warner T , De Pablo-Fernandez E ((2023) ) Association between orthostatic hypotension and dementia in patients with Parkinson disease and multiple system atrophy. Neurology 100: , e998–e1008. |
[35] | Idiaquez J , Roman GC ((2011) ) Autonomic dysfunction in neurodegenerative dementias. J Neurol Sci 305: , 22–27. |
[36] | Fanciulli A , Strano S , Colosimo C , Caltagirone C , Spalletta G , Pontieri FE ((2013) ) The potential prognostic role of cardiovascular autonomic failure in α-synucleinopathies. Eur J Neurol 20: , 231–235. |
[37] | O’Sullivan SS , Massey LA , Williams DR , Silveira-Moriyama L , Kempster PA , Holton JL , Revesz T , Lees AJ ((2008) ) Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 131: , 1362–1372. |
[38] | Tada M , Onodera O , Tada M , Ozawa T , Piao YS , Kakita A , Takahashi H , Nishizawa M ((2007) ) Early development of autonomic dysfunction may predict poor prognosis in patients with multiple system atrophy. Arch Neurol 64: , 256–260. |
[39] | Coon EA , Sletten DM , Suarez MD , Mandrekar JN , Ahlskog JE , Bower JH , Matsumoto JY , Silber MH , Benarroch EE , Fealey RD , Sandroni P , Low PA , Singer W ((2015) ) Clinical features and autonomic testing predict survival in multiple system atrophy. Brain 138: , 3623–3631. |
[40] | Pavy-Le Traon A , Foubert-Samier A , Ory-Magne F , Fabbri M , Senard JM , Meissner WG , Rascol O , Amar J ((2022) ) Ambulatory blood pressure and drug treatment for orthostatic hypotension as predictors of mortality in patients with multiple system atrophy. Eur J Neurol 29: , 1025–1034. |
[41] | Biaggioni I , Freeman R , Mathias CJ , Low P , Arthur Hewitt L , Kaufmann H ((2015) ) Randomized withdrawal study of patients with symptomatic neurogenic orthostatic hypotension responsive to droxidopa. Hypertension 65: , 101–107. |
[42] | Izcovich A , Malla CG , Manzotti M , Catalano HN , Guyatt G ((2014) ) Midodrine for orthostatic hypotension and recurrent reflex syncope: A systematic review. Neurology 83: , 1170–1177. |
[43] | Parsaik AK , Singh B , Altayar O , Mascarenhas SS , Singh SK , Erwin PJ , Murad MH ((2013) ) Midodrine for orthostatic hypotension: A systematic review and meta-analysis of clinical trials. J Gen Intern Med 28: , 1496–1503. |
[44] | Benarroch EE , Smithson IL , Low PA , Parisi JE ((1998) ) Depletion of catecholaminergic neurons of the rostral ventrolateral medulla in multiple systems atrophy with autonomic failure. Ann Neurol 43: , 156–163. |
[45] | Tada M , Kakita A , Toyoshima Y , Onodera O , Ozawa T , Morita T , Nishizawa M , Takahashi H ((2009) ) Depletion of medullary serotonergic neurons in patients with multiple system atrophy who succumbed to sudden death. Brain 132: , 1810–1819. |
[46] | Magnusson M , Holm H , Bachus E , Nilsson P , Leosdottir M , Melander O , Jujic A , Fedorowski A ((2016) ) Orthostatic hypotension and cardiac changes after long-term follow-up. Am J Hypertens 29: , 847–852. |
[47] | Ooi WL , Hossain M , Lipsitz LA ((2000) ) The association between orthostatic hypotension and recurrent falls in nursing home residents. Am J Med 108: , 106–111. |
[48] | Gangavati A , Hajjar I , Quach L , Jones RN , Kiely DK , Gagnon P , Lipsitz LA ((2011) ) Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: The maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc 59: , 383–389. |




