Parkinson’s Disease Stigma Questionnaire (PDStigmaQuest): Development and Pilot Study of a Questionnaire for Stigma in Patients with Idiopathic Parkinson’s Disease
Abstract
Background:
Stigma is significant in Parkinson’s disease (PD). However, no specific tool is available to assess stigma in PD comprehensively.
Objective:
This pilot study aimed to develop and test a stigma questionnaire specific to PD patients (PDStigmaQuest).
Methods:
Based on a literature review, clinical experience, expert consensus, and patients’ feedback, we developed the preliminary, patient-completed PDStigmaQuest in German language. It included 28 items covering five stigma domains: uncomfortableness, anticipated stigma, hiding, experienced stigma, and internalized stigma. In this pilot study, 81 participants (PD patients, healthy controls, caregivers, and health professionals) were included to investigate the acceptability, feasibility, comprehensibility, and psychometric properties of the PDStigmaQuest.
Results:
The PDStigmaQuest showed 0.3% missing data points for PD patients and 0.4% for controls, suggesting high data quality. Moderate floor effects, but no ceiling effects were found. In the item analysis, most items met the standard criteria of item difficulty, item variance, and item-total correlation. Cronbach’s alpha was > 0.7 for four of five domains. PD patients’ domain scores were significantly higher than healthy controls’ for uncomfortableness, anticipated stigma, and internalized stigma. Feedback to the questionnaire was predominantly positive.
Conclusion:
Our results indicate that the PDStigmaQuest is a feasible, comprehensive, and relevant tool to assess stigma in PD and helps to understand the construct of stigma in PD further. Based on our results, the preliminary version of the PDStigmaQuest was modified and is currently validated in a larger population of PD patients for use in clinical and research settings.
INTRODUCTION
Idiopathic Parkinson’s disease (PD) is characterized by a wide range of both motor and non-motor symptoms (NMS) [1–3]. The disease and many of the associated symptoms, e.g., impaired gait, facial masking, and drooling of saliva, can be associated with the experience of stigmatization in everyday life [4–6]. The term “stigma” was first defined as a rather undesirable attribute distinguishing a person from others, leading to being devalued and discredited by others [7]. Nowadays, stigma is most commonly understood as the “co-occurrence of its components— labeling, stereotyping, separation, status loss, and discrimination” [8].
In the past, several concepts of stigma were proposed [9–12]. Fox et al. (2018) established a stigma framework defining three stigma mechanisms most important to the stigmatized’s perspective, namely: anticipated stigma, experienced stigma, and internalized stigma. Anticipated stigma refers to the degree to which a person expects to be stigmatized, regardless of whether he or she is stigmatized or not [9, 13]. Experienced stigma refers to the actual experiences of stereotypes, prejudice, labeling, separation, and discrimination [8, 9, 14]. For example, PD patients report others mislabeling them as drunk or commenting about their “mad” facial expressions [5, 6, 15]. Internalized stigma is defined as “the extent to which people endorse the negative beliefs and feelings associated with the stigmatized identity for the self”, e.g., feelings of being different [9, 16].
Importantly, stigma in PD appears to be a determinant factor for patients’ quality of life and severity of different NMS, including depression, anxiety, or apathy [17–21]. Stigma can cause patients rather to stay at home and experience frustration and isolation [5, 22].
Although stigma is of great importance in PD, there is no specific tool to characterize stigma in PD patients. To our knowledge, only generic stigma measures for chronic illnesses [23–26] or a four-item stigma subscale of the PD Questionnaire (PDQ-39) [27], a frequently used quality of life questionnaire, have been applied in the past to assess stigma in PD patients. These measures are not comprehensive enough to measure the complex construct of disease-specific stigma in PD.
Therefore, our main objective was to develop a stigma questionnaire to be completed by PD patients directly addressing their stigma, considering core constructs and stigma mechanisms based on the current literature and clinical experience [9]. In this pilot study, we report the acceptability, feasibility, comprehensibility, and psychometric properties of the so-called Parkinson’s Disease Stigma Questionnaire (PDStigmaQuest).
MATERIALS AND METHODS
Development of the PDStigmaQuest
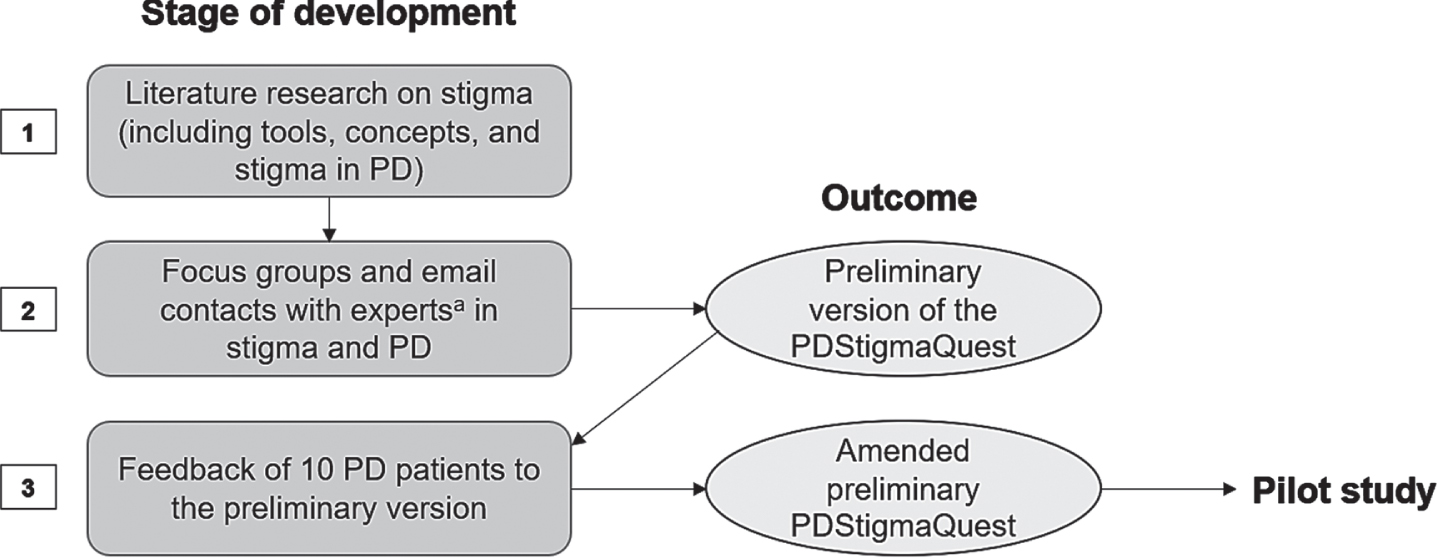
A preliminary version of the questionnaire was developed based on the current literature as well as clinical experience through in-person focus groups and email contacts involving health professionals and researchers with expertise in PD and stigma (from June 2021– September 2021). Neurologists, clinical and research fellows, psychologists, study nurses, occupational therapists, speech therapists, and physiotherapists were involved in the development to establish a holistic picture of stigma in PD. In the next step, the version was discussed with 10 PD patients to ensure comprehensibility and acceptability by patients. Thereafter, based on the patients’ feedback, the preliminary version was amended. The full process of development can be seen in Fig. 1.
Fig. 1
Development process of the PDStigmaQuest tested in the pilot study. Based on literature research, focus groups and email contacts with experts in stigma and PD as well as input from patients with PD, a preliminary PDStigmaQuest was developed in German language to be tested in the pilot study. aThe experts were health professionals and researchers, namely neurologists, clinical and research fellows, psychologists, study nurses, occupational therapists, speech therapists, and physiotherapists. PD, Parkinson’s disease; PDStigmaQuest, Parkinson’s Disease Stigma Questionnaire.
After this process, the German-language PDStigmaQuest as a self-report measure consisted of 28 items in five domains: 1) uncomfortableness (3 items), 2) anticipated stigma (4 items), 3) hiding (4 items), 4) experienced stigma (12 items), and 5) internalized stigma (5 items). Besides the mechanisms defined in the stigma framework by Fox et al. [9], we included domains for uncomfortableness and hiding. These mechanisms were sometimes seen as part of internalized stigma [16]. We decided to address them separately because they do not necessarily result from negative beliefs and feelings of others but can also occur before experiencing stigma in public. The first item of domain one— uncomfortableness concerning different PD symptoms— included sub-items to evaluate the different symptoms separately.
All items were rated on a five-point Likert scale from 0 (never) to 4 (always). For some items, the response option “not applicable” could be chosen, e.g., if a certain symptom was not experienced or items referring to work when patients with PD were retired. The questionnaire also included reverse-scored items to control for response bias and avoid negative wording [28].
A time frame of the “past four weeks” was chosen as for more extended periods, the answers could be influenced by an impaired memory performance of PD patients [29]. Importantly, many self-reported questionnaires routinely applied in PD refer to the last four weeks or the last month, allowing for comparability [27, 30–32]. At the beginning of the questionnaire, it should be indicated whether the patient or a caregiver or both filled in the questionnaire. At the end of the questionnaire, an optional section for additional comments or ideas was provided to allow participants in the pilot study to comment directly on the questionnaire.
Study design and participants
This was a single-center and cross-sectional pilot study.
Four groups of participants were included: 1) patients with a diagnosis of idiopathic PD according to UK Brain Bank criteria [33], 2) non-spousal healthy controls, 3) caregivers of PD patients, and 4) health professionals with expertise in PD.
Exclusion criteria for all groups were moderate or severe medical conditions other than PD that could have interfered with the ability to complete the study, significant cognitive impairment or insufficient knowledge of the German language based on the judgment of the examining health professional, impaired hearing and/or sight which interfered with the study participation, age under 18 or above 90, and inability to consent. Additional exclusion criteria for patients were PD of non-idiopathic form or other clinically relevant neurological diseases besides PD. Exclusion criteria for other groups were additionally diagnosis of PD, dementia or other neurological or psychiatric disorders, and health professionals without experience in PD or older than 70 years.
All participants were included between March 2022 and June 2022.
Ethical aspects
All participants gave written informed consent. The study was performed under the principles of the Declaration of Helsinki. The local ethics committee approved the study protocols (vote: 21-1385; German Clinical Trials Register: DRKS00025513).
Procedures and materials
After being informed about the purpose of the study and having provided signed informed consent, all participants were asked about sociodemographic data. PD patients were additionally asked about their disease history and therapy. After that, PD patients and healthy controls completed the PDStigmaQuest, followed by a feedback questionnaire. Healthy controls were asked to answer items in the PDStigmaQuest only if they did not include phrases directly referring to the disease as these items did not apply to them, e.g., “I try to hide my Parkinson’s symptoms from others”. The following 10 items should be answered by healthy controls: item 1 (uncomfortableness with symptoms), 7 (fear of being seen as mentally impaired), 12 (decisions taken by others), 18 (being interrupted), 20 (being taken seriously), 22 (others acting as feeling uncomfortable in the presence of the patient/control), 24 (feeling worth as much as others), 25 (feeling like a burden to others), 27 (feeling useless), and 28 (self-respect). In contrast to patients and healthy controls, caregivers and health professionals were only asked to read the PDStigmaQuest carefully and after that, to fill in the feedback questionnaire. It covered mainly closed-ended questions about length, comprehensibility, embarrassment, difficulty of answering, item length, and additional comments. The health professionals’ version of the feedback questionnaire additionally contained questions about the (practical) relevance, comprehensiveness of included symptoms, extensiveness, time interval, and item relevance.
Sample size
Recommended sample sizes for initial scale development including assessment of item performance usually range from 24 to 40 representatives from the population of interest [34, 35], in our case, PD patients. For investigating the comprehensibility of instructions, item wording, or administration, only a sample of 10 per group could be sufficient [34]. Therefore, ≥ 10 representatives of the other groups were included. Due to the diversity of health professionals working with PD patients, ≥ 20 health professionals were included.
Data analysis
Descriptive statistics for demographic characteristics were calculated. Levodopa equivalent daily dose (LEDD) was calculated according to the formula of Tomlinson et al. (2010) [36]. The score of item 1 (uncomfortableness with symptoms) was calculated as follows: For every participant, scores of the applicable symptoms were summed and divided by the number of applicable symptoms. This way, the score of item 1 containing sub-items was comparable to other items’ scores. The domain score and total score were calculated similarly: For every participant, scores of the applicable items in a domain or in total were summed, divided by the maximum achievable score on applicable items, respectively, and multiplied by 100. Thus, the maximum score for the domains and the total score was 100.
Data quality was explored by the proportion of missing data points. For patients, acceptability was investigated through floor and ceiling effects measured as the percentage of extreme values (standard value≤15%) [37].
In order to compare PD patients’ and healthy controls’ scores, new domain scores were calculated only with items answered by PD patients and controls. In the domain hiding, no items applied to healthy controls. Mann-Whitney U tests were conducted to identify differences in the domain scores. Tests were corrected for multiple comparisons according to the Benjamini-Hochberg procedure.
For patients only, an item analysis was conducted to determine item difficulty, item variance, and corrected item-total correlation to evaluate the items’ psychometric properties. Item difficulty does not refer to the difficulty of answering the item but is defined as the quotient of the mean score on that item and the maximum achievable score on the same item, multiplied by 100 [38]. Items with a medium difficulty of 50 can differentiate the best between people with a low and people with a high level of the measured characteristic. As we intended to also differentiate between patients with levels of stigma at the extremes (e.g., differentiate between two patients with low stigma levels), we also intended to include items with item difficulties of 5-20 and 80-95 [38]. The variance of an item should be high and the item-total correlation should be≥0.3 [38, 39]. For item selection, all three values, content-related considerations, and reliability should be considered.
For patients, a preliminary internal consistency analysis of the stigma domains was conducted (Cronbach’s alpha, standard value≥0.7) [38, 40]. Including the option “not applicable” in some items resulted in systematic data loss for internal consistency analysis. Therefore, only for the internal consistency analysis, items answered as not applicable were coded as zeros [41]. For this study, the method was considered acceptable because an item not applicable to the patient also means that the patient does not experience the stigma described by the item.
The feedback questionnaires of all participants were analyzed descriptively to consider criticism and suggestions for improving the preliminary version of the PDStigmaQuest.
All analyses were conducted using Statistical Package for Social Science (SPSS version 28.0). p values < 0.05 were considered statistically significant.
RESULTS
Demographic characteristics
In total, 27 PD patients (33.3% female) with a mean age of 63.9 (±5.7) years, a mean disease duration of 11.4 (±5.0) years, and a mean LEDD of 828.1 mg/d (±358.3) were included. Their average years of education were 13.9 (±3.4). Patients were married (63.0%), divorced (22.2%), or widowed (14.8%), and mostly retired (77.8%) or (self-)employed (18.5%). Nine patients (33.3%) were undergoing deep brain stimulation.
Twenty-two healthy controls (45.5% female; mean age of 62.0 (±11.2) years) were included. Age and gender did not differ significantly compared to patients (p > 0.05). Mean years of education were 16.8 (±2.5) and controls were mainly married (63.6%) or divorced (18.2%) and (self-)employed (63.6%) or retired (31.8%).
Besides, 10 caregivers of PD patients (70.0% female) with a mean age of 55.8 (±13.1) years were included, as well as 22 health professionals with expertise in PD (77.3% female; 11 neurologists, three psychologists, two study nurses, two speech therapists, two physiotherapists, two occupational therapists) with a mean age of 35.4 (±9.0) years.
PDStigmaQuest scores, acceptability, and data quality
Descriptive statistics of PDStigmaQuest scores and acceptability parameters for PD patients are shown in Table 1. All questionnaires were completed by the patient without the help of caregivers. In PD patients, moderate floor effects were found, while ceiling effects were absent. Overall, there were 5/1512 (0.3%) missing data points for PD patients and 3/792 (0.4%) for controls.
Table 1
Distribution and acceptability of PDStigmaQuest domain scores in percentage (%) for patients with Parkinson’s disease
| M | SD | Min | Max | Floor effect | Ceiling effect | Applicable Items | |
| Uncomfortableness | 32.0 | 19.2 | 0 | 68.7 | 3.7 | 0 | 100.0 |
| Anticipated Stigma | 27.2 | 20.8 | 0 | 75.0 | 18.5 | 0 | 87.0 |
| Hiding | 17.6 | 20.1 | 0 | 65.6 | 37.0 | 0 | 100.0 |
| Experienced Stigma | 22.1 | 14.5 | 0 | 54.6 | 3.7 | 0 | 94.1 |
| Internalized Stigma | 21.7 | 16.1 | 0 | 50.0 | 22.2 | 0 | 100.0 |
| Total Score | 24.6 | 14.8 | 0.4 | 54.7 | 0 | 0 | 96.2 |
PDStigmaQuest, Parkinson’s Disease Stigma Questionnaire. For every patient, scores of the applicable items of a domain were summed, divided by the respective maximum achievable score in a domain, and multiplied by 100.
PDStigmaQuest scores: comparison of PD patients and healthy controls
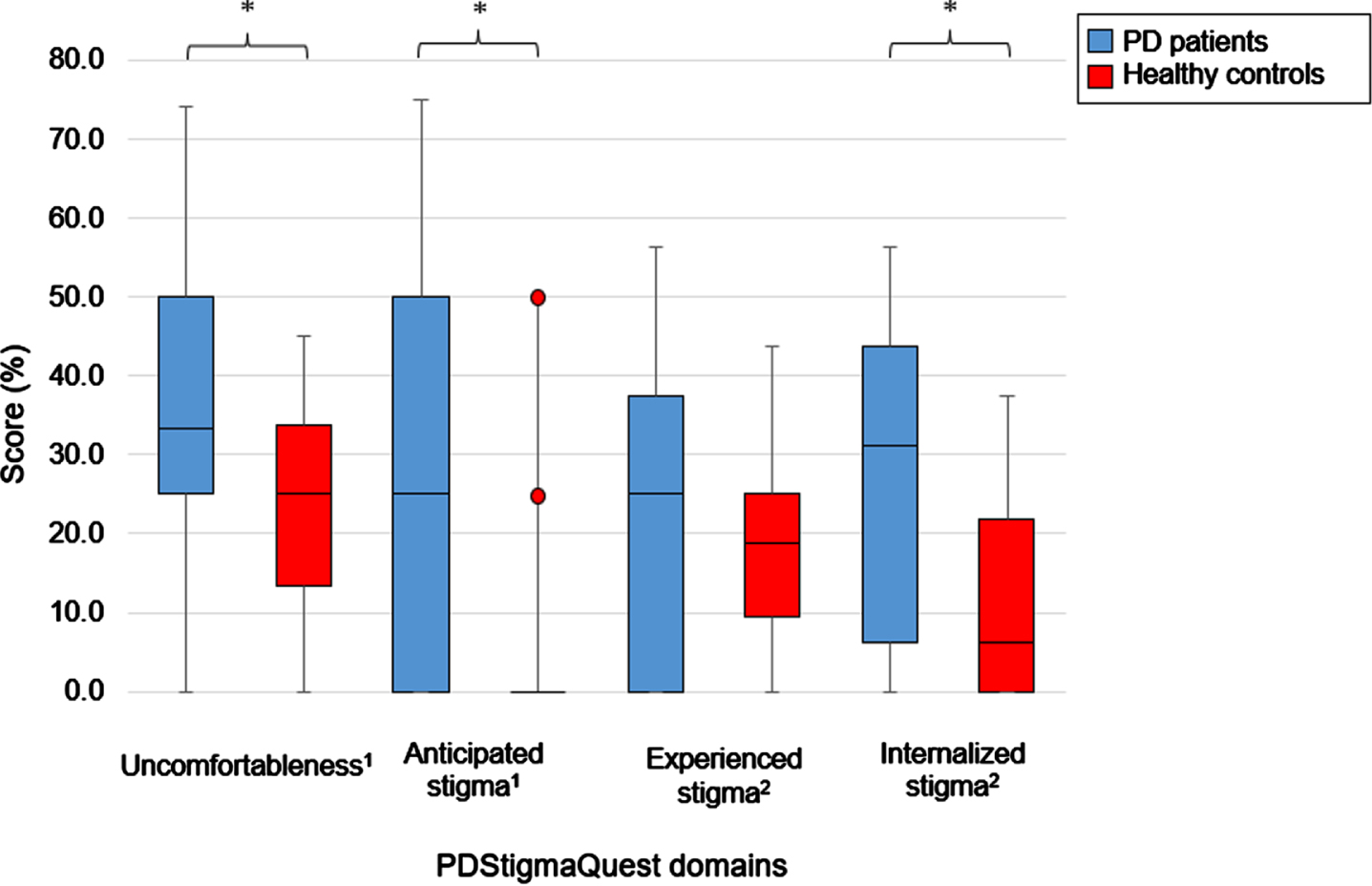
For domain scores calculated only with items answered by PD patients and healthy controls, descriptive statistics are presented separately in Fig. 2. Domain scores were significantly higher in PD patients for the domains uncomfortableness (p = 0.035), anticipated stigma (p = 0.032), and internalized stigma (p = 0.032). In the domain hiding, no items applied to healthy controls; therefore, no comparison was made.
Fig. 2
Descriptive statistics of items answered by patients with Parkinson’s disease and healthy controls in the different PDStigmaQuest domains. Domain scores are presented as percentage of the maximum domain score. Mann-Whitney U tests were calculated between patients with PD and controls. Red dots represent outliners by healthy controls. In the domain hiding, no comparison was made as no items applied to healthy controls. No boxplot can be seen for the domain anticipated stigma in healthy controls as all values in the box were 0. 11 item; 24 items; *p < 0.05. PD, Parkinson’s disease; PDStigmaQuest, Parkinson’s Disease Stigma Questionnaire.
Item analysis
For PD patients, item difficulty, item variance, and corrected item-total correlation are shown in Table 2. Items with item difficulties of 5-20 (indicating lower stigma levels) were item 2 (uncomfortableness with appearance), 9 (concealing PD), 10 (hiding treatment), 11a (speaking openly with family and close friends about PD), 14 (others making fun of the patient), 15a (rejection by friends), 17 (others avoiding looking at the patient), 20 (being taken seriously), 22 (others acting uncomfortable), and 26 (feeling responsible for PD). Only item 15b (rejection by family members) had an item difficulty < 5. Items with a variance near 0 (< 0.5) were item 14 (others making fun of the patient), 15b (rejection by family members), and 17 (others avoiding looking at the patient). Items with item-total correlations < 0.3 were item 17 (others avoiding looking at the patient), 19 (being observed), 23 (respect by others), and 24 (feeling worth as much as others).
Table 2
Item analysis for patients with Parkinson’s disease
| Item | n | Item difficulty | Item variance | Item-total correlation | |
| Domain 1: Uncomfortableness | |||||
| Uncomfortableness with … | |||||
| 1 | PD symptoms | 27 | 35.33 | 0.51 | 0.54 |
| 2 | PD treatmenta | 27 | 14.81 | 0.64 | 0.89 |
| 3 | PD appearance | 26 | 45.19 | 1.60 | 0.56 |
| Domain 2: Anticipated Stigma | |||||
| 4 | Reactions to PD | 27 | 24.07 | 1.11 | 0.80 |
| 5 | Devaluation at worka | 13 | 25.00 | 1.00 | 0.43 |
| 6 | Reactions to disease progression | 27 | 37.04 | 1.18 | 0.56 |
| 7 | Being seen as mentally impaired | 27 | 21.30 | 0.90 | 0.93 |
| Domain 3: Hiding | |||||
| 8 | Hiding of PD symptoms | 27 | 25.00 | 1.62 | 0.53 |
| 9 | Concealing PD | 27 | 12.04 | 0.88 | 0.82 |
| 10 | Hiding of PD treatmenta | 27 | 12.04 | 0.80 | 0.85 |
| 11a | Speaking openly with family and close friends about PDb | 27 | 14.81 | 1.10 | 0.75 |
| 11b | Speaking openly with others (except for family and close friends) about PDb | 27 | 27.78 | 1.49 | 0.75 |
| Domain 4: Experienced Stigma | |||||
| 12 | Decisions taken on behalf of patient by others | 27 | 27.78 | 1.03 | 0.50 |
| 13 | Unfair treatment at worka | 10 | 27.50 | 2.32 | 0.82 |
| 14 | Others making fun of the patient | 27 | 10.19 | 0.48 | 0.32 |
| 15a | Rejection by friends | 27 | 17.59 | 1.06 | 0.44 |
| 15b | Rejection by family members | 27 | 3.70 | 0.21 | 0.51 |
| 16 | Behavior of others after learning about PD treatmentab | 24 | 21.88 | 1.25 | 0.50 |
| 17 | Others avoiding looking at the patient | 26 | 10.58 | 0.33 | 0.05 |
| 18 | Being interrupted | 27 | 34.26 | 1.63 | 0.71 |
| 19 | Being observed | 26 | 33.65 | 0.96 | 0.26 |
| 20 | Being taken seriouslyb | 26 | 16.35 | 1.04 | 0.70 |
| 21 | Being invited less often | 26 | 22.12 | 0.91 | 0.74 |
| 22 | Others acting as feeling uncomfortable in the presence of the patient | 27 | 14.81 | 0.71 | 0.85 |
| 23 | Respect by othersb | 27 | 23.15 | 1.61 | 0.07 |
| Domain 5: Internalized Stigma | |||||
| 24 | Feeling worth as much as othersb | 27 | 22.22 | 1.33 | 0.03 |
| 25 | Feeling like a burden to others | 27 | 34.26 | 1.63 | 0.52 |
| 26 | Feeling of being responsible for PD | 27 | 6.48 | 0.51 | 0.76 |
| 27 | Feeling useless | 27 | 20.37 | 1.16 | 0.76 |
| 28 | Self-respectb | 27 | 25.00 | 1.46 | 0.34 |
PD, Parkinson’s Disease. In bold are item difficulties > 20, item variances ≥ 0.5, and item-total correlations ≥ 0.3, representing preferable item characteristics. aitem with response option “not applicable”. breverse-scored item.
Internal consistency
Cronbach’s alpha was 0.72 for domain uncomfortableness, 0.71 for anticipated stigma, 0.77 for hiding, 0.77 for experienced stigma, and 0.52 for internalized stigma.
Feedback to the preliminary version of the PDStigmaQuest
Frequencies of responses to the closed-ended feedback questions about the questionnaire are shown in Table 3. Positive responses were at least 60% for each question. In PD patients, healthy controls, and caregivers, responses indicating negative feedback regarding the questionnaire were > 20% for questions referring to the questionnaire’s length and difficulty of answering specific questions. In health professionals, negative responses were > 20% for questions referring to questionnaire’s length, ease of understanding the items, adding or deleting symptoms in item 1, time interval, and lower level of relevance for specific items. Qualitative data (i.e., participants’ suggestions for improving the questionnaire) were considered in the modification process of the PDStigmaQuest.
Table 3
Responses (%) to the closed-ended feedback questions to the preliminary PDStigmaQuest
| PD patients | Healthy controls | Caregivers | Health professionals | ||
| 1. Did you find the questionnaire too long? | No | 85.2 | 95.5 | 60.0 | 68.2 |
| Yes | 11.1 | 4.5 | 40.0 | 31.8 | |
| NR | 3.7 | 0 | 0 | 0 | |
| 2. Were the questions easy to understand? | No | 11.1 | 4.5 | 20.0 | 27.3 |
| Yes | 88.9 | 95.5 | 80.0 | 72.7 | |
| NR | 0 | 0 | 0 | 0 | |
| 3. Did you have difficulties with some questions formulated in the present and others in the past?a | No | 96.3 | 100.0 | – | – |
| Yes | 3.7 | 0 | – | – | |
| NR | 0 | 0 | – | – | |
| 4. Did you find any question(s) embarrassing? | No | 92.6 | 90.9 | 90.0 | 90.9 |
| Yes | 7.4 | 9.1 | 10.0 | 9.1 | |
| NR | 0 | 0 | 0 | 0 | |
| 5. Did you find any question(s) difficult to answer? | No | 74.1 | 86.4 | 70.0 | 77.3 |
| Yes | 25.9 | 13.6 | 20.0 | 13.6 | |
| NR | 0 | 0 | 10.0 | 9.1 | |
| 6. Did you find any question(s) too long? | No | 96.3 | 100.0 | 90.0 | 86.4 |
| Yes | 3.7 | 0 | 10.0 | 9.1 | |
| NR | 0 | 0 | 0 | 4.5 | |
| 7. Do you find the questionnaire relevant?b | No | – | – | – | 4.5 |
| Yes | – | – | – | 95.5 | |
| NR | – | – | – | 0 | |
| 8. Does the questionnaire help you to better understand your PD patients’ current condition?b | No | – | – | – | 9.1 |
| Yes | – | – | – | 86.4 | |
| NR | – | – | – | 4.5 | |
| 9. Do you find that symptoms in question 1 should be deleted and/or others added?b | No | – | – | – | 63.6 |
| Yes | – | – | – | 31.8 | |
| NR | – | – | – | 4.5 | |
| 10. Do you find the questionnaire comprehensive enough?b | No | – | – | – | 4.5 |
| Yes | – | – | – | 95.5 | |
| NR | – | – | – | 0 | |
| 11. Do you find the chosen time interval of four weeks reasonable for evaluating the different statements?b | No | – | – | – | 31.8 |
| Yes | – | – | – | 63.6 | |
| NR | – | – | – | 4.5 | |
| 12. Did you find any question(s) less relevant for the patients’ stigma?b | No | – | – | – | 68.2 |
| Yes | – | – | – | 27.3 | |
| NR | – | – | – | 4.5 |
NR, No Response; PD, Parkinson’s disease; PDStigmaQuest, Parkinson’s Disease Stigma Questionnaire. Amended from [42]. Responses indicating positive feedback about the questionnaire are in bold. aquestions only for participants filling in the questionnaire. badditional questions for health professionals.
DISCUSSION
The present study aimed to develop and test the comprehensibility, feasibility, and psychometric properties of a self-completed questionnaire addressing stigma in PD patients. Our results demonstrate that the PDStigmaQuest is a feasible and comprehensive tool easily applied to measure stigma in a general PD population.
Acceptability and data quality
The PDStigmaQuest showed only 0.3% missing data points for PD patients and 0.4% for controls, suggesting high data quality. Moderate floor effects were found, which could be explained by the fact that some patients stated not caring about others’ opinions. In future studies, it would be essential to capture personality traits like neuroticism, as it was found that this trait is associated with stigma in PD [43]. People with a low level of neuroticism worry less and could therefore experience lower levels of stigma [44]. Notably, the floor effects were only found for three of five domains (i.e., anticipated stigma, hiding, and internalized stigma) and not for the total score. Furthermore, ceiling effects were absent, indicating an appropriate acceptability of the PDStigmaQuest.
PDStigmaQuest scores: comparison of PD patients and healthy controls
PD patients’ domain scores were significantly higher for the domains uncomfortableness, anticipated stigma, and internalized stigma. For the domain experienced stigma, PD patients’ domain scores were higher than those of healthy controls, however, not reaching statistical significance. Explorative post-hoc analyses for the specific items of this domain showed that only the item 12 score (decisions taken by others) was significantly higher in PD patients (p = 0.028). These results were somehow expected as items 18 (being interrupted) and 20 (being taken seriously) cover aspects that also older people in general might experience. Furthermore, item 22 (others acting as feeling uncomfortable) might be more common among PD patients in earlier stages of the disease and, therefore, not highly represented in our cohort with advanced stage of PD (mean disease duration of 11.4 years). This aspect warrants further investigation.
Differences in the domain hiding could not be explored as this domain addresses hiding aspects of the disease and therefore represents experiences that healthy controls do not experience.
Item analysis
Item analysis revealed that the majority of items met standard criteria. Only three items showed relatively low scores for more than one of the three criteria item difficulty, item variance, and item-total correlation: item 14 (others making fun of the patient), 15b (rejection by family members), and 17 (others avoiding looking at the patient). Items’ difficulty and variance indicated that these experienced stigma aspects were very rare. Particular attention was paid to these items in the modification process.
Internal consistency
Internal consistency results showed good internal consistency for four of five domains. Only the domain internalized stigma showed a Cronbach’s alpha < 0.7. This result can be explained by negative correlations between items 24 (feeling worth as much as others) and 26 (feeling responsible for PD). Both items showed high floor effects. Item 26 showed an item difficulty of 6.48. Nevertheless, we decided to keep this item as we assume that it will apply to PD populations in other countries [45].
Feedback to the preliminary version of the PDStigmaQuest
Feedback to the preliminary PDStigmaQuest was predominantly positive. Comments especially led to modifying symptoms in item 1 (uncomfortableness with PD symptoms) and rewording of items. Special attention was paid to repetitive feedback. As critical comments on the time interval were highly contradictory, we decided to keep the chosen time interval of four weeks.
Modification of the PDStigmaQuest
Based on the results of the statistical analysis and participants’ comments on the questionnaire, the PDStigmaQuest was modified as follows: 1) at the beginning of the questionnaire, it should only be indicated whether the questionnaire was filled in alone or with help, 2) instead of offering the “not applicable” option for items referring to work, we added an optional section for these items, 3) response option “not applicable” was only offered for item 1 regarding uncomfortableness with symptoms and therefore reworded to “symptom not applicable”, 4) some symptoms in item 1 were deleted, reworded, or summarized, 5) three items were deleted: item 10 (hiding of treatment), item 16 (behavior of others after learning about PD treatment), and item 17 (others avoiding looking at the patient), 6) items 11a and 11b (speaking openly about the disease) as well as 15a and 15b (rejection by others) were summarized to one item respectively, 7) eight items were reworded, 8) the order of the items was changed in the way that reverse-scored items were well distributed across the questionnaire.
Limitations
This pilot study has some limitations. Firstly, a relatively high proportion (33.3%) of patients undergoing deep brain stimulation could influence the transferability of our results to the general PD population. However, disease duration, LEDD, PD domain, and total scores did not differ between patients with and without deep brain stimulation. Further, the PDStigmaQuest is intended as a questionnaire for PD patients with and without invasive therapies. Therefore, we consider including PD patients with deep brain stimulation is necessary and justified. Secondly, only 18.5% of patients were (self-)employed and could answer the items referring to work. Therefore, the study’s results regarding these items were cautiously interpreted when modifying the questionnaire. Thirdly, comparing PD patients and controls about the domains of uncomfortableness and anticipated stigma included only one item for each domain answered by both groups. However, other items in the domains, including e.g., “because of my PD symptoms”, differed by their very nature between PD patients and controls since controls cannot have these experiences at all.
Conclusions
In conclusion, the results of this pilot study indicate that the German version of the PDStigmaQuest is a feasible, comprehensive, and relevant tool to assess stigma in PD patients. Based on the results, the preliminary version of the PDStigmaQuest was modified and is currently formally validated for further use in clinical and research settings in German and English language. To our knowledge, this is the first specific stigma tool in PD and will allow to comprehensively measure the prevalence and severity of stigma in PD patients and reveal its association with other clinical parameters like motor and NMS and quality of life.
ACKNOWLEDGMENTS
We want to thank all subjects for participating in the study. VS and AS gratefully acknowledge being funded by the Advanced Cologne Clinician Scientist program of the Medical Faculty of the University of Cologne. GRF gratefully acknowledges support by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project-ID 431549029 – SFB 1451.
CONFLICT OF INTEREST
Vasilija Stopic is funded by the Advanced Cologne Clinician Scientist program of the Medical Faculty of the University of Cologne and will receive funding from the Prof. Klaus Thiemann Foundation.
Stefanie T. Jost was funded by the Prof. Klaus Thiemann Foundation.
Juan Carlos Baldermann was funded by the Else Kroener-Fresenius-Stiftung (grant number 2022 EKES.23) and receives funding by the German Research Foundation (project ID 431549029–C07, CRC-1451).
Jan Niklas Petry-Schmelzer is funded by the Cologne Clinician Scientist Program (CCSP)/ Faculty of Medicine/ University of Cologne; funded by the German Research Foundation (DFG, FI 773/15-1).
Gereon R. Fink serves as an editorial board member of Cortex, Neurological Research and Practice, NeuroImage: Clinical, Zeitschrift fur Neuropsychologie, and DGNeurologie; receives royalties from the publication of the books Funktionelle MRT in Psychiatrie und Neurologie, Neurologische Differentialdiagnose, and SOP Neurologie; received honoraria for speaking engagements from Bayer, Desitin, Ergo DKV, Forum fur medizinische Fortbildung FomF GmbH, GSK, Medica Academy Messe Dusseldorf, Medicbrain Healthcare, Novartis, Pfizer, and Sportarztebund NRW.
Till A. Dembek received personal fees from Medtronic, personal fees from Boston Scientific, outside the submitted work.
Haidar S. Dafsari was funded by the EU Joint Programme – Neurodegenerative Disease Research (JPND), the Prof. Klaus Thiemann Foundation in the German Society of Neurology, the Felgenhauer Foundation, the KoelnFortune program of the Medical Faculty of the University of Cologne, and has received honoraria by Boston Scientific, Medtronic, Bial, Kyowa Kirin, Abbvie, Everpharma, and Stadapharm.
Josef Kessler has no conflicts of interest to report.
Michael T. Barbe received speaker’s honoraria from Medtronic, Boston Scientific, Abbott (formerly St. Jude), GE Medical, UCB, Apothekerverband Koln e.V. and Bial as well as research funding from the Felgenhauer-Stiftung, Forschungspool Klinische Studien (University of Cologne), Horizon 2020 (Gondola), Medtronic (ODIS), and Boston Scientific and advisory honoraria for the IQWIG.
Anna Sauerbier is funded by the Gusyk program and the Advanced Cologne Clinician Scientist program of the Medical Faculty of the University of Cologne and has received funding from the Prof. Klaus Thiemann Foundation.
DATA AVAILABILITY
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
[1] | Sveinbjornsdottir S ((2016) ) The clinical symptoms of Parkinson’s disease. J Neurochem 139: , 318–324. |
[2] | Chaudhuri KR , Healy DG , Schapira AH ((2006) ) Non-motor symptoms of Parkinson’s disease: Diagnosis and management.. Lancet Neurol 5: , 235–245. |
[3] | Poewe W ((2008) ) Non-motor symptoms in Parkinson’s disease. Eur J Neurol 15: , 14–20. |
[4] | da Silva AG , Leal VP , da Silva PR , Freitas FC , Linhares MN , Walz R , Malloy-Diniz LF , Diaz AP , Palha AP ((2020) ) Difficulties in activities of daily living are associated with stigma in patients with Parkinson’s disease who are candidates for deep brain stimulation. J Bras Psiquiatr 42: , 190–194. |
[5] | Hermanns M ((2013) ) The invisible and visible stigmatization of Parkinson’s disease. J Nurse Pract 25: , 563–566. |
[6] | Ma H-I , Gunnery SD , Stevenson MT , Saint-Hilaire M , Thomas CA , Tickle-Degnen L ((2019) ) Experienced facial masking indirectly compromises quality of life through stigmatization of women and men with Parkinson’s disease. Stigma Health 4: , 462. |
[7] | Goffman E ((1963) ) Stigma: Notes on the management of spoiled identity. Simon & Shuster, New York. |
[8] | Link BG , Phelan JC ((2001) ) Conceptualizing stigma. Annu Rev Sociol 27: , 363–385. |
[9] | Fox AB , Earnshaw VA , Taverna EC , Vogt D ((2018) ) Conceptualizing and measuring mental illness stigma: The mental illness stigma framework and critical review of measures. Stigma Health 3: , 348. |
[10] | Jacoby A ((1994) ) Felt versus enacted stigma: A concept revisited: Evidence from a study of people with epilepsy in remission. Soc Sci Med 38: , 269–274. |
[11] | Rüsch N , Angermeyer MC , Corrigan PW ((2005) ) Mental illness stigma: Concepts, consequences, and initiatives to reduce stigma. Eur Psychiatry 20: , 529–539. |
[12] | Corrigan PW , Watson AC ((2002) ) Understanding the impact of stigma on people with mental illness. World Psychiatry 1: , 16. |
[13] | Quinn DM , Chaudoir, SR ((2009) ) Living with a concealable stigmatizedidentity: The impact of anticipated stigma, centrality, salience, and cultural stigma on psychological distress and health. J Pers Soc Psychol 97: , 634–651. |
[14] | Quinn DM , Earnshaw VA ((2011) ) Understanding concealable stigmatized identities: The role of identity in psychological, physical, and behavioral outcomes. Soc Issues Policy Rev 5: , 160–190. |
[15] | Maffoni M , Giardini A , Pierobon A , Ferrazzoli D , Frazzitta G ((2017) ) Stigma experienced by Parkinson’s disease patients: A descriptivereview of qualitative studies. Parkinsons Dis 2017: , 7203259. |
[16] | Ritsher JB , Otilingam PG , Grajales M ((2003) ) Internalized stigma of mental illness: Psychometric properties of a new measure. Psychiatry Res 121: , 31–49. |
[17] | Ma H-I , Saint-Hilaire M , Thomas CA , Tickle-Degnen L ((2016) ) Stigma as a key determinant of health-related quality of life in Parkinson’s disease. Qual Life Res 25: , 3037–3045. |
[18] | Salazar RD , Weizenbaum E , Ellis TD , Earhart GM , Ford MP , Dibble LE , Cronin-Golomb A ((2019) ) Predictors of self-perceived stigma in Parkinson’s disease. Parkinsonism Relat Disord 60: , 76–80. |
[19] | Hou M , Mao X , Hou X , Li K ((2021) ) Stigma and associated correlates of elderly patients with Parkinson’s disease. Front Psychiatry 12: , 708960. |
[20] | Schrag A , Jahanshahi M , Quinn NP ((2001) ) What contributes to depression in Parkinson’s disease? Psychol Med 31: , 65–73. |
[21] | Skorvanek M , Rosenberger J , Minar M , Grofik M , Han V , Groothoff JW , Valkovic P , Gdovinova Z , van Dijk JP ((2015) ) Relationship between the non-motor items of the MDS– UPDRS and Quality of Life in patients with Parkinson’s disease. J Neurol Sci 353: , 87–91. |
[22] | Soleimani MA , Negarandeh R , Bastani F , Greysen R ((2014) ) Disrupted social connectedness in people with Parkinson’s disease. Br J Community Nurs 19: , 136–141. |
[23] | Earnshaw VA , Quinn DM , Kalichman SC , Park CL ((2013) ) Development and psychometric evaluation of the chronic illness anticipated stigma scale. J Behav Med 36: , 270–282. |
[24] | Fife BL , Wright ER ((2000) ) The dimensionality of stigma: A comparison of its impact on the self of persons with HIV/AIDS and cancer. J Health Soc Behav 41: , 50–67. |
[25] | Gershon RC , Lai JS , Bode R , Choi S , Moy C , Bleck T , Miller D , Peterman A , Cella D ((2012) ) Neuro-QOL: Quality of life item banks for adults with neurological disorders: Item development and calibrations based upon clinical and general population testing. Qual Life Res 21: , 475–486. |
[26] | Rao D , Choi SW , Victorson D , Bode R , Peterman A , Heinemann A , Cella D ((2009) ) Measuring stigma across neurological conditions: The development of the stigma scale for chronic illness (SSCI). Qual Life Res 18: , 585–595. |
[27] | Peto V , Jenkinson C , Fitzpatrick R , Greenhall R ((1995) ) The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res 4: , 241–248. |
[28] | Nunnally JC ((1994) ) Psychometric theory 3E, Tata McGraw-Hill Education. |
[29] | Watson GS , Leverenz JB ((2010) ) Profile of cognitive impairment in Parkinson’s disease. Brain Pathol 20: , 640–645. |
[30] | Probst CC , Winter LM , Möller B , Weber H , Weintraub D , Witt K , Deuschl G , Katzenschlager R , van Eimeren T ((2014) ) Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease (QUIP) and the QUIP-rating scale in a German speaking sample. J Neurol 261: , 936–942. |
[31] | Marin RS , Biedrzycki RC , Firinciogullari S ((1991) ) Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 38: , 143–162. |
[32] | Romenets SR , Wolfson C , Galatas C , Pelletier A , Altman R , Wadup L , Postuma R ((2012) ) Validation of the non-motor symptoms questionnaire (NMS-Quest). Parkinsonism Relat Disord 18: , 54–58. |
[33] | Hughes AJ , Daniel SE , Lees AJ ((2001) ) Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology 57: , 1497–1499. |
[34] | Hertzog MA ((2008) ) Considerations in determining sample size for pilot studies. Res Nurs Health 31: , 180–191. |
[35] | Johanson GA , Brooks GP ((2010) ) Initial scale development: Sample sizefor pilot studies. Educ Psychol Meas 70: , 394–400. |
[36] | Tomlinson CL , Stowe R , Patel S , Rick C , Gray R , Clarke CE ((2010) ) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25: , 2649–2653. |
[37] | McHorney CA , Tarlov AR ((1995) ) Individual-patient monitoring inclinical practice: Are available health status surveys adequate? . Qual Life Res 4: , 293–307. |
[38] | Moosbrugger H , Kelava A ((2012) ) Testtheorie und Fragebogenkonstruktion, Springer. |
[39] | Ferketich S ((1991) ) Focus on psychometrics. Aspects of item analysis. Res Nurs Health 14: , 165–168. |
[40] | Nunnally JC , Bernstein IH ((1994) ) Psychometric theory. MacGraw-Hill, New York. |
[41] | Bradley C , Todd C , Gorton T , Symonds E , Martin A , Plowright R ((1999) ) The development of an individualized questionnaire measure ofperceived impact of diabetes on quality of life: The ADDQoL. Qual Life Res 8: , 79–91. |
[42] | Martinez-MartinP, SchragA, WeintraubD, RizosA, Rodriguez-BlazquezC, ChaudhuriKR, IPMDS Non Motor PD Study Group ((2019) ) Pilot study of the International Parkinson and Movement Disorder Society-sponsored nonmotor rating scale (MDS-NMS). Mov Disord Clin Pract 6: , 227–234. |
[43] | Dubayova T , Nagyova I , Havlikova E , Rosenberger J , Gdovinova Z , Middel B , van Dijk JP , Groothoff JW ((2009) ) Neuroticism and extraversion in association with quality of life in patients with Parkinson’s disease. Qual Life Res 18: , 33–42. |
[44] | Eysenck HJ , Eysenck SGB ((1965) ) The Eysenck personality inventory. Br J Educ Stud 14: , 140. |
[45] | Pan S , Stutzbach J , Reichwein S , Lee BK , Dahodwala N ((2014) ) Knowledge and attitudes about Parkinson’s disease among a diverse group of older adults. J Cross Cult Gerontol 29: , 339–352. |




