Freezing of Gait in Parkinson’s Disease: Implications for Dual-Task Walking
Abstract
Background:
The simultaneous completion of multiple tasks (dual-tasking, DT) often leads to poorer task performance (DT cost, DTC). People with Parkinson’s disease (PwPD) exhibit difficulty with DT, and DTC may be particularly pronounced in PwPD with freezing of gait (FOG).
Objective:
This study assessed the relationship between FOG status and DTC during gait.
Methods:
Gait parameters were collected using inertial sensors in 106 PwPD (off-medication), including definite-freezers (dFOG; n = 25), possible-freezers (pFOG; n = 16), and non-freezers (nFOG; n = 65) during single (ST)-and DT walking.
Results:
PwPD with dFOG had larger (worse) DTC than nFOG for foot-strike angle, stride length, toe-off angle, variability of foot-strike angle, and arm range of motion (ROM). After accounting for covariates, DTC for toe-off angle and stride length remained worse in PwPD who freeze. Worse cognition predicted larger DTC for stride length, gait cycle duration, gait speed, and step duration across groups. Men had larger DTC compared to women for gait speed, variability in foot-strike angle, stride length, and arm ROM. Increased variability in gait speed DTC was associated with increased disease severity.
Conclusion:
These findings provide additional support that PwPD who freeze may rely on greater cortical control for the execution of specific gait metrics. The results also underscore the importance of considering cognition when assessing DT ability in PwPD.
INTRODUCTION
Dual tasking (DT) involves the simultaneous completion of multiple tasks and typically yields poorer performance on one or both tasks compared to when each is performed in isolation [1, 2]. The magnitude of performance deterioration with the addition of a secondary task is often defined as dual-task cost (DTC).
DT is ubiquitous in daily life, and attentional control (which includes DT capacity) has been related to falls in people with Parkinson’s disease (PwPD) [3, 4]. Given the relevance of DT, considerable work has investigated DTC during gait in PwPD. This work indicates that PwPD exhibit greater DTC on gait speed than neurotypical peers [5–10], and worse cognition and/or disease severity may contribute to DTC [11].
DT affects some aspects of walking more in PwPD than neurotypical controls. For instance, changes in the DTCs for arm range of motion, foot strike angle, turn velocity, and turn duration discriminated PwPD from controls when performed with a concurrent verbal fluency task [12]. Among these changes, the arm range of motion DTC was the best discriminative gait metric between PwPD and healthy controls [12]. However, the relationship between freezing of gait (FOG) status and DTC has yet to be fully explored.
PwPD with FOG (FOG+) have reduced gait automaticity, as evaluated by increased prefrontal executive-attentional activity while walking [13, 14], and show more significant cognitive impairments [15–18], including frontal-executive dysfunction [18–20]. Given the impacts of FOG on cognition and the inherent importance of DT ability, it is relevant to characterize DTC in PwPD who experience FOG, as this subpopulation of PD often has a reduced quality of life [21], increased risk of falling, and decreased independence [22] compared to non-freezers (FOG-). However, few studies have explored DTC during gait in people with FOG, showing mixed findings. For example, compared to FOG-, FOG+ exhibit higher DTC on cadence [23], stride length, and stride velocity [7, 24]. The effects of DTC on turning have been less consistent. For instance, FOG+ turn with an increased cadence [7] and at a slower speed [24] than FOG-. However, no differences are observed in turning DTC metrics between FOG+ and FOG- [24]. Further, the designation of freezing status can be challenging. Certain PwPD exhibit freezing in laboratory settings and not at home or, conversely, report freezing at home but do not in the laboratory. Characterizing DTCs across these FOG groups (e.g., non-freezer, possible freezer, definite freezer) may provide a more thorough description of the effects of FOG on this critical outcome. Finally, as noted above [11], cognition may impact DTC, which is common in FOG+, but it is unknown whether cognition moderates the impact of FOG on DTC. In sum, while early work indicates possible effects of FOG status on DTC, additional work with 1) larger sample sizes, 2) inclusion of varying FOG groups, and 3) cognitive status included in the statistical model will facilitate a better understanding of the relationship between FOG and DTC.
Therefore, we conducted an exploratory analysis to determine the relationship between FOG status and DTC across a broad spectrum of gait and turning outcomes, controlling for covariates, such as cognitive function, sex, and disease severity, and characterizing FOG status as non-freezer, possible freezer, and definite freezers. Establishing these relationships is an important first step toward identifying targetable and salient DT outcomes for future clinical research examining cognitive-motor interference in PwPD and FOG. These findings may also enhance the efficiency of clinical rehabilitation by tailoring interventions for PwPD with FOG, taking into account their specific cognitive and gait impairments.
METHODS
Participants
One hundred and six individuals with idiopathic PD were included in the study. Portions of these data have been included in previous publications [12, 24]. However, the research questions examining the relationship between freezing status and DTC are novel.
Participants with PD were classified as definite freezers (dFOG; n = 25), possible freezers (pFOG; n = 16), or non-freezers (nFOG; n = 65) according to the New Freezing of Gait Questionnaire (NFOG-Q) [25] and clinical observation (walking in a single-task condition without a concurrent cognitive task). Specifically, pFOG was defined when freezing was confirmed from either the NFOG-Q or clinical observation, but not both. dFOG was defined as having freezing in the NFOG-Q and clinical observation. nFOG noted no FOG on the NFOG-Q and no FOG was observed via clinical observation. Participants met the following inclusion criteria: 1) age between 50–90 years; 2) a diagnosis of idiopathic PD from a movement disorders neurologist according to the United Kingdom Parkinson’s Disease Society Brain Bank criteria [26]; 3) absence of any significant musculoskeletal or peripheral disorders that altered balance or gait; 4) the ability to stand and walk without assistance; and 5) absence of recent medication changes (six weeks of stable medications). Participants were excluded if they: 1) could not follow directions; 2) had any additional neurological or musculoskeletal conditions that affected gait or balance; 3) had deep brain stimulation (DBS). Also, participants studied in this analysis were part of a larger clinical trial involving magnetic resonance imaging (MRI). Therefore, participants with MRI contraindications were excluded. Participants were examined in the OFF state after at least 12 hours of antiparkinsonian medication wash-out. The Oregon Health & Science University Institutional Review Board (#4131) and the joint OHSU and Veterans Affairs Portland Health Care System Institutional Review Board (#8979) approved the experimental protocol. Before participating, participants gave their signed, informed consent.
Experimental protocol
Participants’ demographic and clinical characteristics were recorded (Table 2), followed by functional and cognitive testing. The Mini Balance Evaluation System Test (Mini-BESTest) [27] and the Activities Specific Balance Confidence Scale (ABC scale) [28] evaluated mobility and balance. The Montreal Cognitive Assessment (MoCA) [29] assessed global cognition. The motor portion of the Movement Disorders Society’s (MDS-revised) Unified Parkinson’s Disease Rating Scale (MDS-UPDRS part III) assessed disease severity, and the Hoehn and Yahr Rating Scale assessed disease stage [30, 31].
The participants then performed cognitive and walking tasks in single-task (ST) and dual-task (DT) conditions. The ST condition was always completed first, followed by the DT condition. The cognitive ST was a letter recitation task that involved reciting every other letter of the alphabet for one minute. This task is commonly used to assess cognitive performance and attentional control in individuals, particularly those with neurological conditions [12, 32]. During this task, participants sat quietly and were asked to recite every other letter of the alphabet in order, beginning with the first letter. They were instructed to continue this process for one minute without stopping or repeating any letters. When participants reached the letter Z, they continued with the B. The number of correct responses was then recorded as a measure of cognitive performance, with higher scores indicating better attentional control and working memory. The walking ST condition was a 2-minute walk at a normal, comfortable speed without a cognitive task. Participants walked back and forth between two lines 7.62 meters apart, turning 180 degrees at each end. In the DT condition, participants were instructed to walk for 1 minute while simultaneously performing the letter recitation cognitive task. Walking performance was instrumented using 8 inertial sensors (Opals, APDM, Wearable Technologies, a Clario Company, Portland, OR, USA) that include triaxial accelerometers, gyroscopes, and a magnetometer recording at 128 Hz, attached to the participants at the sternum, lumbar spine, bilaterally on the wrists, shanks, and feet with Velcrostraps.
Data analysis and outcomes
The primary outcome was DTC, an index of interference (% worsening) when simultaneously performing the walking and cognitive task. Cognitive task performance was assessed based on the precision of the responses (that is, the total number of correct responses over the 1-minute bout while either walking [DT] or seated [ST]). Objective measures of walking performance were selected based on prior knowledge of the test-retest reliability, validity, and discriminative ability in separating PwPD from healthy controls [12, 33–38]. Briefly, 26 objective gait measures (including mean and standard deviations) across 4 mobility domains (upper body, lower body, turning, and variability) [34] were extracted using Mobility Lab software (Mobility Lab v2, APDM, Portland, OR, USA) [35, 37, 38]. DTC of the number of correct responses was used to assess cognitive performance. Details concerning Mobility Lab’s algorithms to identify gait and turning events have been previously reported [12, 33, 37]. The list of gait metrics included in this analysis and their definition are provided in Table 1. When performing the walking and cognitive tasks simultaneously, DTC was calculated for each gait metric as follows[39, 40]:
Dual-task costs [%] = 100 * (single-task metric – dual-task metric) / single-task metric.
Table 1
Definition of gait and turning outcomes
| Domain | Objective Measure | Unit | Definition |
| Lower Body | Foot Strike Angle | degree | Average angle of the foot at the point of initial contact |
| Lower Body | Stride Length | meters | Distance between two consecutive heel strikes |
| Lower Body | Toe Off Angle | degree | Average angle of the foot at the point of push off |
| Lower Body | Gait Speed | meter/second | The forward speed of the subject |
| Lower Body | Gait Cycle Duration | seconds | Duration of a complete gait cycle |
| Lower Body | Step Duration | seconds | Duration from one foot fall to other foot fall |
| Lower Body | Double Support Time | % of gait cycle | Percentage of a gait cycle that both feet are on the ground |
| Turning | Turn Duration | seconds | Duration of a 180-degree turn |
| Turning | Turn Velocity | degrees/second | Peak (95%) angular velocity of trunk during turning |
| Upper Body | Arm ROM | degree | Average of range of motion of both arms during arm-swing |
| Upper Body | Trunk Coronal ROM | degree | Average range of motion of trunk (coronal: in frontal plane, sagittal: in sagittal plane, transverse: in horizontal plane) |
| Upper Body | Trunk Transverse ROM | degree | |
| Upper Body | Trunk Sagittal ROM | degree | |
| Variability | Foot Strike Angle SD | SD | Variability is the measure of standard deviation calculated for each gait metric |
| Variability | Arm ROM SD | ||
| Variability | Trunk Transverse ROM SD | ||
| Variability | Trunk Sagittal ROM SD | ||
| Variability | Gait Speed SD | ||
| Variability | Stride Length SD | ||
| Variability | Turn Duration SD | ||
| Variability | Trunk Coronal ROM SD | ||
| Variability | Turn Velocity SD | ||
| Variability | Step Duration SD | ||
| Variability | Toe Off Angle SD | ||
| Variability | Double Support Time SD | ||
| Variability | Gait Cycle Duration SD | ||
| Cognitive | # correct recitals | The cognitive exercise involved reciting every other letter of the alphabet for one minute before the walking assessment for single task and during the period of walking trial for the dual task |
SD is the standard deviation. Variability is the measure of standard deviation calculated for each gait metric.
In some cases (e.g., variability outcomes), the valence was flipped to ensure that for all gait outcomes, positive values indicate that performance improved with the addition of a secondary task, and negative values reflect worse performance with a secondary task [12].
Statistical analysis
For this exploratory analysis, two sequential approaches were performed to determine the effect of FOG on DTC during gait. First, a one-way ANOVA assessed whether freezing status (dFOG, pFOG, nFOG) impacted DTC of the 26 gait outcomes. Then, a 2-way, mixed-model repeated measures ANOVA was performed to more precisely estimate the effect of FOG on DTC outcomes during gait. This model assessed the impact of group (dFOG, pFOG, nFOG), task (ST vs. DT performance), and group-by-task interactions for each gait outcome. We also included sex, cognition (measured via MoCA), and disease severity (measured via MDS-UPDRS Part III) as covariates in these models. Covariates were selected to include outcomes that directly measured either disease severity or outcomes that have previously been shown to potentially impact DTC (outside of FOG status) in people with PD. Post-hoc tests were conducted to clarify across-group effects. In instances where significant covariate (sex, disease severity, MoCA) by task interactions were observed, follow-up analyses including paired-sample t-tests (for task-by-sex interactions) and bivariate correlations (for task-by-disease severity and task-by-MoCA interactions) were conducted to determine the specific relationship between outcomes. As noted above, this report was exploratory in nature and meant to provide an initial characterization of which DTC outcomes may be impacted by FOG. Therefore, p-values were not corrected for multiple comparisons. Statistical significance was set at p < 0.05. All analyses were performed in IBM SPSS (v.27).
RESULTS
Group characteristics
Demographic and group characteristics are presented in Table 2. Disease duration, Hoehn & Yahr scale, MDS-UPDRS III, postural instability and gait disorder sub-score, MiniBESTest, ABC, N-FOG-Q, and freezing ratio were significantly different across groups, with outcomes worst in the dFOG group. Age and MoCA were not significantly different across groups.
Table 2
Demographic and group characteristics
| nFOG (n = 65) | pFOG (n = 16) | dFOG (n = 25) | ANOVA-F | P | ηp2 | |
| Sex (F | M) | 26 | 39 | 4 | 12 | 7 | 18 | |||
| Age | 68.8 (8) | 67.5 (6.3) | 69.88 (8.1) | 0.45 | 0.63 | 0.009 |
| Disease Duration | 4.8 (4) | 6.7 (3.6) | 9.4 (5.8) | 9.43 | <0.001 | 0.15 |
| Total LEDD | 601.1 | 642.8 | 1111.7 | 2.02 | 0.13 | 0.04 |
| Hoehn &Yahr | 2.1 (0.5) | 2.1 (0.3) | 2.6 (0.8) | 7.02 | 0.001 | 0.12 |
| MDS-UPDRS III | 36.5 (11.4) | 41.6 (9.9) | 51.4 (13.4) | 14.64 | <0.001 | 0.22 |
| PIGD | 3.9 (2.5) | 4.9 (1.6) | 7.6 (3.5) | 17.18 | <0.001 | 0.25 |
| MiniBEST | 19.4 (4.3) | 19.4 (4.5) | 14.8 (5.5) | 9.73 | <0.001 | 0.16 |
| ABC Scale | 85.9 (12.9) | 78.2 (17) | 72 (13.3) | 9.32 | <0.001 | 0.16 |
| MoCA | 26.0 (3.0) | 25.4 (2.9) | 25.4 (4.1) | 0.42 | 0.65 | 0.008 |
| nFOG-Q Total Score | NA | 7.2 (6.9) | 14.4 (5.7) | 132.8 | <0.001 | 0.72 |
| Freezing ratio | 0.68 (0.8) | 0.87 (0.6) | 3.43 (6.9) | 6.17 | 0.003 | 0.11 |
F, female; M, male; LEDD, Levodopa equivalent daily dose; nFOG-Q, New Freezing of Gait Questionnaire. The values represent the group means with standard deviation in the brackets. Eta-squared (ηp2) represents the effect size for the ANOVA. Bolded outcomes were significantly different across groups.
Effect of freezing status on DTC
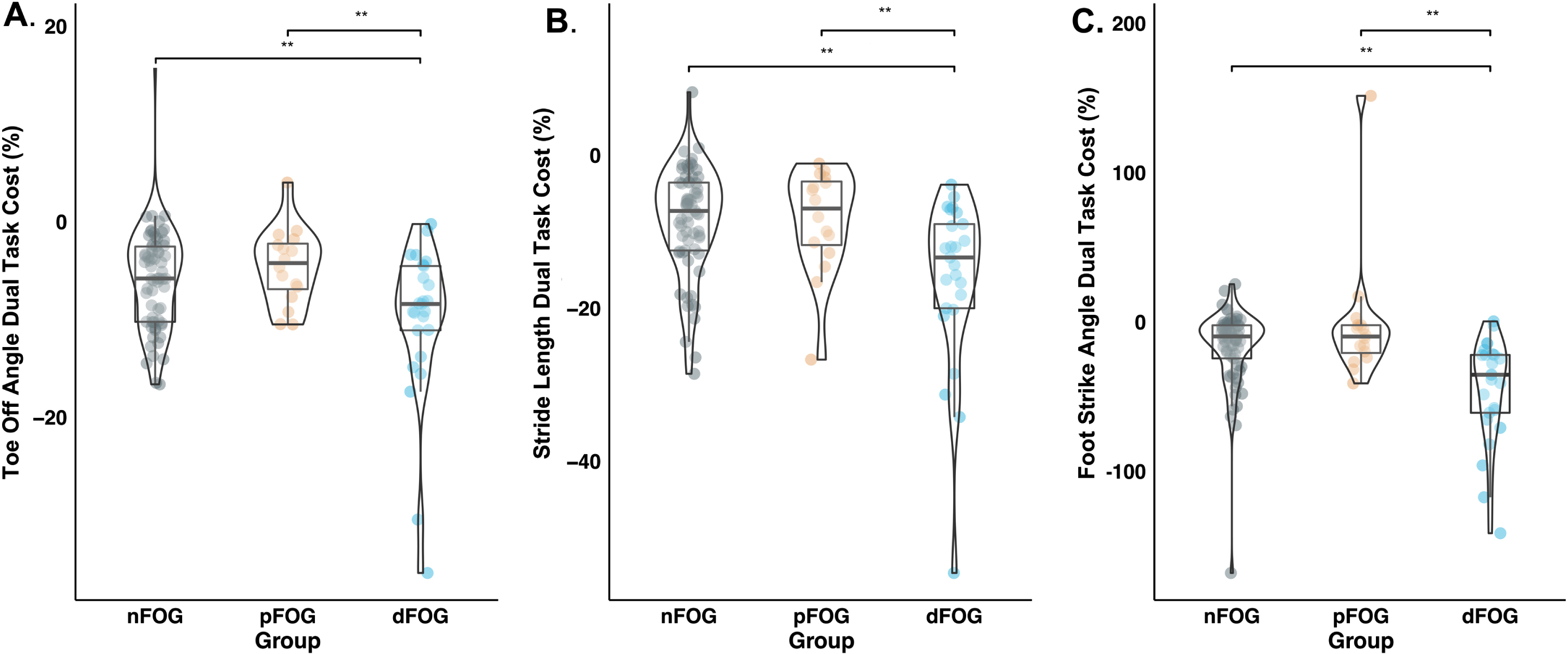
Figure 1 shows the eta-squared effect sizes of the FOG group for the 26 gait DTC measures (values provided in Supplementary Table 1). FOG status had the most robust and statistically significant effect on the DTCs for foot-strike angle (ηp2 = 0.17, p < 0.01) and stride length (ηp2 = 0.14, p < 0.01). FOG status had a medium effect on toe-off angle (ηp2 = 0.10, p < 0.01), variability of foot-strike angle (ηp2 = 0.07, p = 0.02), and arm range of motion (ηp2 = 0.07, p = 0.03). Post-hoc tests indicated that for foot-strike angle, toe-off angle, and stride length, dFOG exhibited larger (worse) DTC than pFOG and nFOG (Fig. 2A-C, Supplementary Table 2). For arm ROM and foot-strike variability, dFOG showed larger DTC than nFOG but not pFOG (see Supplementary Figure 1 and Supplementary Table 2). Cognitive DTCs were not significantly different across groups (Fig. 1).
Fig. 1
Forest Plot showing the effect sizes (Eta Squared values) of freezing status on dual-task cost measures in descending order. The horizontal lines indicate the confidence interval. Significant effect sizes are marked with solid dots. The values on the right indicate the eta squared value. Vertical dashed lines represent medium and large effect sizes. The color code represents the gait domain. ROM, range of motion; SD, standard deviation.
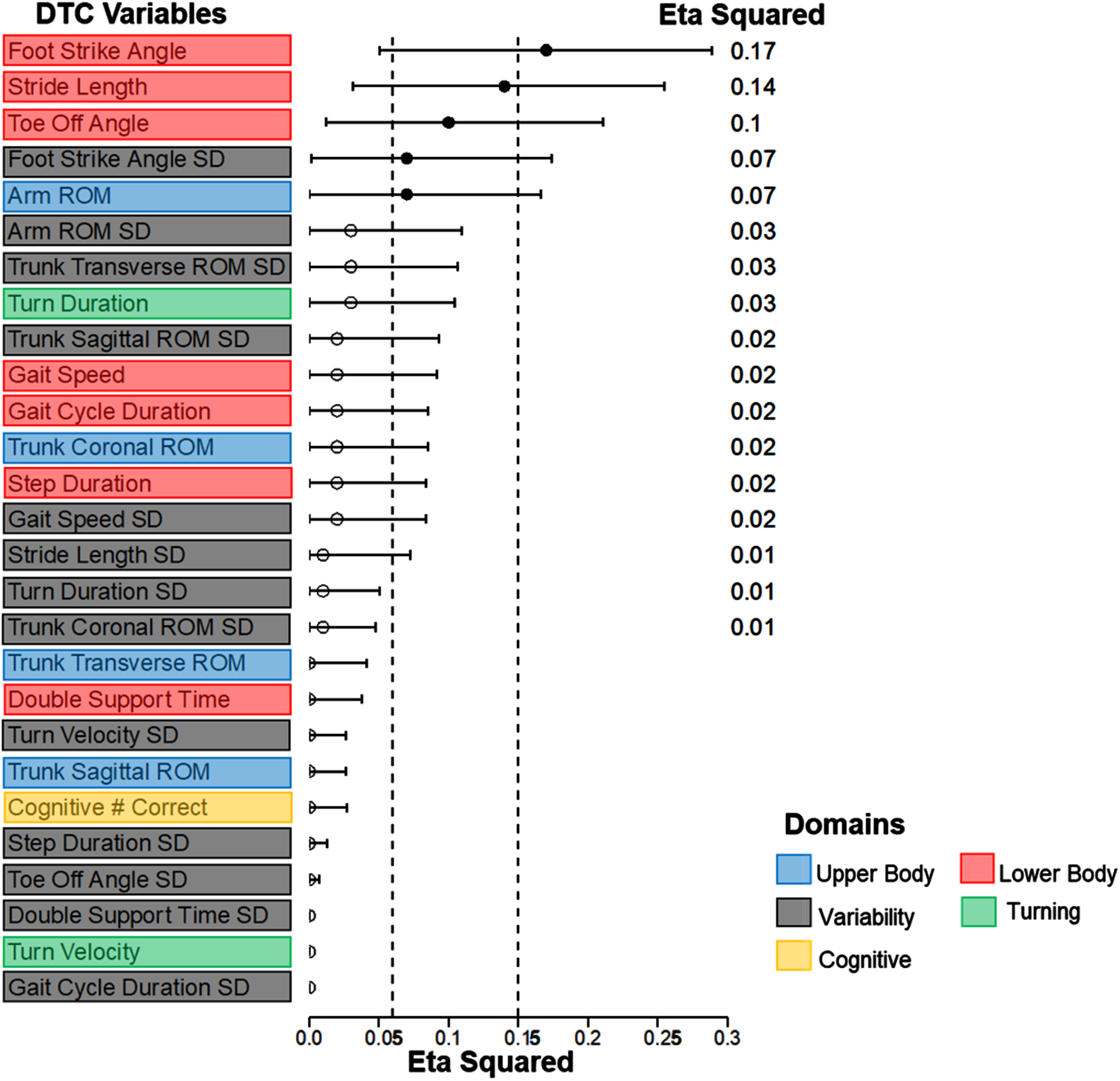
Fig. 2
Distribution of dual-task cost (DTC) metrics of A) Toe-Off Angle, B) Stride Length, and C) Foot-Strike Angle across Freezing groups (nFOG-nonfreezers, pFOG-potential freezers, and dFOG-definite freezers). The violin plot shows the distribution of DTC. Boxplot shows the median and interquartile range. Asterisks indicate significant differences in post-hoc paired comparisons (**p<0.01).
Effect of freezing status on single- and dual-task gait with covariates
Two-way ANOVAs established interaction effects between task (ST versus DT) and group (nFOG, pFOG, and dFOG) with cognition, sex, and disease severity entered as covariates. This analysis revealed a statistically significant group-by-task interaction for toe-off angle (ηp2 = 0.07, p = 0.01; Table 3). Consistent with results from the one-way ANOVA analysis, post-hoc t-tests indicated that for toe-off angle and stride length, dFOG groups exhibited a significantly larger decline from ST to DT performance than pFOG and nFOG groups (Supplementary Table 2).
Table 3
Effect of freezing status on single- and dual-task gait with covariates
| Variable | Domain | Task | Task*Group | Task*MoCA | Task*Sex | Task*MDS-UPDRS-III | ||||||||||
| F | Sig. | ηp2 | F | Sig. | ηp2 | F | Sig. | ηp2 | F | Sig. | ηp2 | F | Sig. | ηp2 | ||
| Double Support | Lower Body | 8.251 | 0.005 | 0.076 | 0.095 | 0.910 | 0.002 | 5.158 | 0.025 | 0.049 | 1.394 | 0.241 | 0.014 | 0.090 | 0.764 | 0.001 |
| Double Support SD | Variability | 0.100 | 0.753 | 0.001 | 0.064 | 0.938 | 0.001 | 0.002 | 0.964 | 0.000 | 3.616 | 0.060 | 0.035 | 0.043 | 0.836 | 0.000 |
| Gait Cycle Duration | Lower Body | 8.752 | 0.004 | 0.080 | 1.981 | 0.143 | 0.038 | 8.285 | 0.005 | 0.077 | 1.907 | 0.170 | 0.019 | 1.011 | 0.317 | 0.010 |
| Gait Cycle Duration SD | Variability | 5.114 | 0.026 | 0.049 | 0.536 | 0.587 | 0.011 | 4.665 | 0.033 | 0.045 | 2.543 | 0.114 | 0.025 | 0.493 | 0.484 | 0.005 |
| Gait Speed | Lower Body | 22.827 | 0.000 | 0.186 | 0.757 | 0.472 | 0.015 | 7.794 | 0.006 | 0.072 | 8.075 | 0.005 | 0.075 | 1.378 | 0.243 | 0.014 |
| Gait Speed SD | Variability | 1.061 | 0.306 | 0.010 | 0.159 | 0.853 | 0.003 | 0.010 | 0.919 | 0.000 | 2.941 | 0.089 | 0.029 | 7.023 | 0.009 | 0.066 |
| Foot Strike | Lower Body | 2.565 | 0.112 | 0.025 | 1.717 | 0.185 | 0.033 | 0.127 | 0.722 | 0.001 | 4.114 | 0.045 | 0.040 | 0.061 | 0.805 | 0.001 |
| Foot Strike SD | Variability | 1.159 | 0.284 | 0.011 | 2.059 | 0.133 | 0.040 | 0.004 | 0.953 | 0.000 | 6.114 | 0.015 | 0.058 | 2.920 | 0.091 | 0.028 |
| Toe Off* | Lower Body | 10.015 | 0.002 | 0.091 | 4.226 | 0.017 | 0.078 | 2.396 | 0.125 | 0.023 | 0.717 | 0.399 | 0.007 | 1.376 | 0.244 | 0.014 |
| Toe Off SD | Variability | 7.318 | 0.008 | 0.068 | 0.060 | 0.942 | 0.001 | 6.702 | 0.011 | 0.063 | 1.315 | 0.254 | 0.013 | 0.023 | 0.879 | 0.000 |
| Step Duration | Lower Body | 8.592 | 0.004 | 0.079 | 1.925 | 0.151 | 0.037 | 8.283 | 0.005 | 0.076 | 1.767 | 0.187 | 0.017 | 1.061 | 0.305 | 0.011 |
| Step Duration SD | Variability | 3.591 | 0.061 | 0.035 | 0.455 | 0.636 | 0.009 | 3.146 | 0.079 | 0.030 | 2.723 | 0.102 | 0.027 | 0.584 | 0.447 | 0.006 |
| Stride Length* | Lower Body | 12.650 | 0.001 | 0.112 | 2.967 | 0.056 | 0.056 | 3.685 | 0.058 | 0.036 | 6.963 | 0.010 | 0.065 | 0.162 | 0.689 | 0.002 |
| Stride Length SD | Variability | 8.723 | 0.004 | 0.080 | 0.014 | 0.986 | 0.000 | 6.214 | 0.014 | 0.059 | 0.563 | 0.455 | 0.006 | 1.946 | 0.166 | 0.019 |
| Trunk Coronal | Upper Body | 1.530 | 0.219 | 0.015 | 0.490 | 0.614 | 0.010 | 1.102 | 0.296 | 0.011 | 0.000 | 0.994 | 0.000 | 0.346 | 0.558 | 0.003 |
| Trunk Coronal SD | Variability | 1.239 | 0.268 | 0.012 | 0.287 | 0.751 | 0.006 | 0.861 | 0.356 | 0.009 | 0.323 | 0.571 | 0.003 | 0.136 | 0.713 | 0.001 |
| Trunk Sagittal | Upper Body | 0.022 | 0.882 | 0.000 | 0.094 | 0.910 | 0.002 | 0.012 | 0.912 | 0.000 | 0.394 | 0.532 | 0.004 | 0.275 | 0.601 | 0.003 |
| Trunk Sagittal SD | Variability | 0.435 | 0.511 | 0.004 | 0.570 | 0.568 | 0.011 | 0.296 | 0.588 | 0.003 | 0.023 | 0.880 | 0.000 | 0.399 | 0.529 | 0.004 |
| Trunk Transverse | Upper Body | 0.685 | 0.410 | 0.007 | 0.095 | 0.910 | 0.002 | 0.385 | 0.537 | 0.004 | 0.761 | 0.385 | 0.008 | 0.680 | 0.411 | 0.007 |
| Trunk Transverse SD | Variability | 0.775 | 0.381 | 0.008 | 1.538 | 0.220 | 0.030 | 0.281 | 0.597 | 0.003 | 0.691 | 0.408 | 0.007 | 0.190 | 0.664 | 0.002 |
| Arm ROM | Upper Body | 0.072 | 0.789 | 0.001 | 1.069 | 0.347 | 0.021 | 1.878 | 0.174 | 0.018 | 7.116 | 0.009 | 0.066 | 0.263 | 0.609 | 0.003 |
| Arm ROM SD | Variability | 0.200 | 0.656 | 0.002 | 0.496 | 0.610 | 0.010 | 0.246 | 0.621 | 0.002 | 2.069 | 0.153 | 0.020 | 0.765 | 0.384 | 0.008 |
| Turn Velocity | Turning | 4.758 | 0.032 | 0.046 | 0.342 | 0.711 | 0.007 | 1.219 | 0.272 | 0.012 | 1.227 | 0.271 | 0.012 | 0.838 | 0.362 | 0.008 |
| Turn Velocity SD | Variability | 0.397 | 0.530 | 0.004 | 0.246 | 0.782 | 0.005 | 0.293 | 0.589 | 0.003 | 1.760 | 0.188 | 0.017 | 1.133 | 0.290 | 0.011 |
| Turn Duration | Turning | 0.004 | 0.952 | 0.000 | 0.622 | 0.539 | 0.012 | 2.081 | 0.152 | 0.021 | 3.786 | 0.055 | 0.037 | 2.733 | 0.101 | 0.027 |
| Turn Duration SD | Variability | 0.593 | 0.443 | 0.006 | 0.206 | 0.814 | 0.004 | 0.049 | 0.826 | 0.000 | 0.044 | 0.835 | 0.000 | 1.597 | 0.209 | 0.016 |
| Cognitive # Correct | Cognitive | 3.441 | 0.067 | 0.033 | 0.099 | 0.906 | 0.002 | 2.796 | 0.098 | 0.027 | 4.604 | 0.034 | 0.044 | 0.075 | 0.785 | 7E-04 |
2-way, mixed-model repeated measures ANOVA was also performed to estimate the effect of FOG more precisely on DTC outcomes during gait. ηp2, partial eta squared.
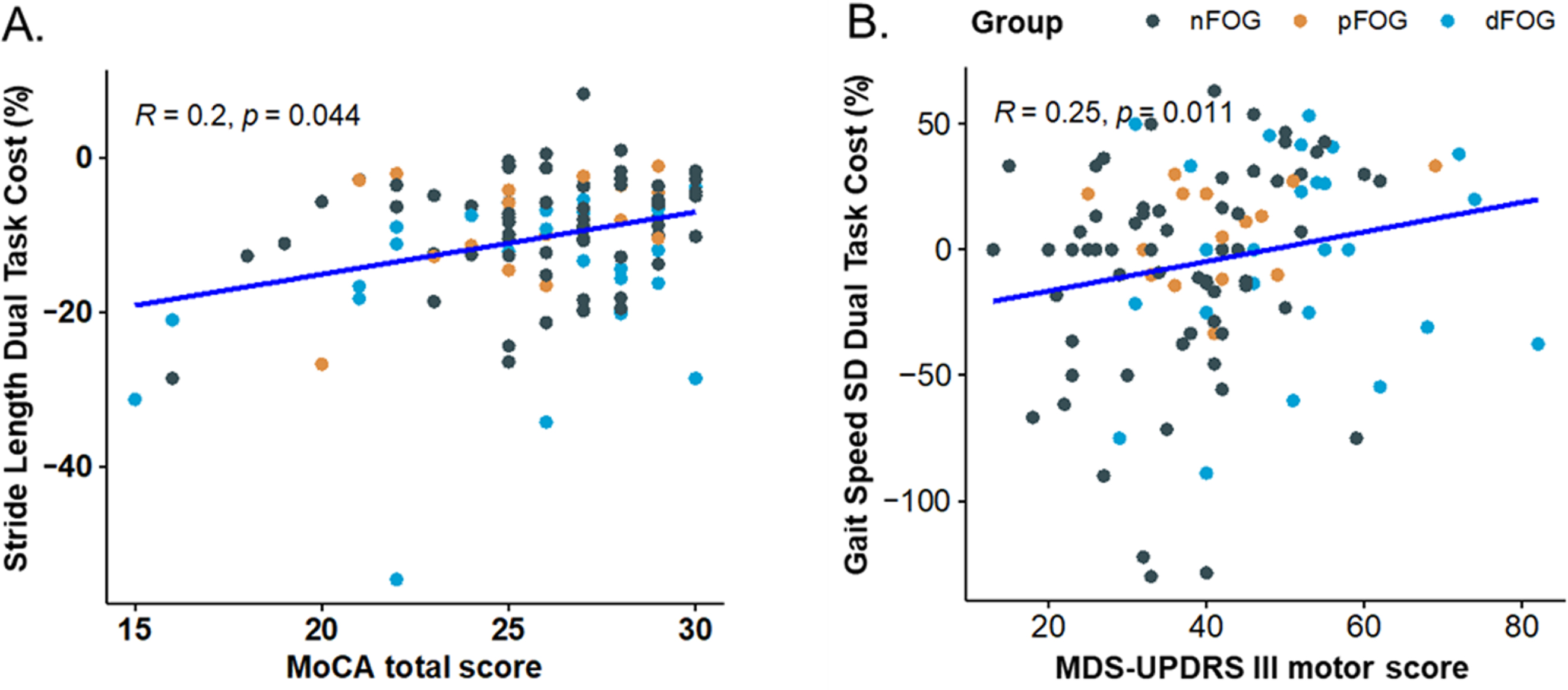
Statistically significant interactions between task and cognition were observed in 7 of 26 gait measures: double-support time, gait-cycle duration, variability of gait-cycle duration, gait speed, variability in toe-off angle, step duration, and stride-length variability (Table 3). Post-hoc Spearman correlations indicated that worse cognition (smaller MoCA) was significantly associated with more negative (worse) DTC for stride length (R = 0.20, p = 0.04), gait-cycle duration (R = 0.21, p = 0.02), gait speed (R = 0.23, p = 0.01), and step duration (R = 0.22, p = 0.02). For double-support time, gait-cycle duration variability, toe-off variability, and stride-length variability, worse DTC was also related to worse cognition, albeit not at a statistically significant threshold (0.17 > r>0.11; 0.07 < ps<0.24).
An interaction between task and sex was observed in 6 gait outcomes: gait speed, foot-strike angle, foot-strike angle variability, stride length, arm range of motion, and turn duration (Table 2). Post-hoc t-tests showed that men had more negative (worse) DTC than women for gait speed (t = 2.71, p < 0.01), foot-strike angle variability (t = 1.98, p = 0.05), stride length (t = 2.39, p = 0.01) and arm ROM (t = 2.83, p < 0.01).
An interaction between task and disease severity (measured by MDS-UPDRS III) was observed only for gait speed variability. The post-hoc Spearman correlation between DTC of gait-speed variability and MDS-UPDRS III revealed a significant association (R = 0.25, p = 0.01), such that increased motor severity predicted increased DTC of gait speed variability (Fig. 3B). All 2-way ANOVA analyses are presented in Table 3.
Fig. 3
Spearman correlation between (A) Stride Length Dual Task Cost (% change) and MoCA total score, (B) Gait Speed variability and MDS-UPDRS III motor score for all subjects. Freezing groups: nFoG (no freezing), pFoG (possible freezing), and dFoG (definite freezing) are represented with diamonds, stars, & circles, respectively.
DISCUSSION
Adding a secondary cognitive task during gait typically results in changes in performance on each task, forcing attentional resource allocation between two tasks. The resultant DTCs provide insights into the interplay between automatic and cortical control of gait. In this exploratory analysis, we observed that PwPD and FOG had more pronounced DTC of several lower-body (foot-strike angle, stride length, toe-off angle) and upper-body (arm ROM) outcomes. The effect of freezing status was observed for toe-off angle after accounting for global cognition (measured by the MoCA), disease severity, and sex. Furthermore, MoCA scores impacted the DTC of several gait measures. Across most findings, it was observed that definite freezers (those who presented with both self-reported and in-lab freezing events) exhibited the most pronounced DTC compared to possible freezers and non-freezers. Future replication studies will be necessary to confirm or contradict current findings and more fully understand the relationship between FOG and DTC. However, these results illustrate the potential relationship between DTC and freezing status and further underscore the impact of cognition on DT ability in PwPD. Furthermore, knowledge of task-specific interference during gait may help identify: 1) those most susceptible to DT walking deficits in everyday life and 2) optimal candidates for DT training.
We extend previous findings in several ways. First, we report on a larger sample stratified into 3 subgroups to represent FOG more thoroughly. Further, we performed analyses that account for potential covariates of sex, cognition, and disease severity, which allowed us to determine how these variables may also impact DTC. This is particularly important due to the potential interactions among these outcomes (e.g., cognition and FOG status). Lastly, we investigated these relationships across a comprehensive set of gait outcomes. These analyses yielded several notable findings.
First, consistent with previous work [7, 12, 24], we observed that FOG status impacted several gait DTC measures in PwPD. In particular, even after controlling for cognition, sex, and disease severity, larger DTC in toe-off angle was observed between FOG+and FOG-. The lack of differences in turning DTCs between freezing groups was somewhat surprising. PwPD negotiate turns slower than neurotypical controls [12], and FOG+tend to turn with more steps [7] at a slower speed [24]. However, our findings agree with recent work showing that turning DTCs were unaffected by FOG [24]. Second, we observed that impaired cognition, as measured by the MoCA, was associated with larger DTC for several gait outcomes. Previous results relating DTC and cognition in PwPD have been mixed. For example, poorer cognition, including executive function and attention, has previously been linked to larger DTCs in gait speed and step time variability [11, 19], while other work shows no such relationship [41]. The partially conflicting evidence between current and some [41] previous work may be due to several reasons. First, it is possible that our relatively large sample was better powered to detect such differences. Alternatively, the large number of variables, or the more granular description of freezing status (nFOG, pFOG, dFOG), used in the current study may have provided increased sensitivity. Finally, it is possible that different secondary tasks used during DT resulted in inconsistent findings. Although PwPD and FOG commonly show cognitive impairments across multiple domains [18], the selection of secondary cognitive tasks in DT paradigms may have important implications for the DTCs observed and their relationship to “cognition.” Indeed, across studies (including the current report), varying secondary tasks were used: serial subtraction [24, 41], auditory Stroop [11], listing alternating letters of the alphabet (current task), and both motor and an attentional/memory task (remembering the number of tones presented; [19]). Further, these DTCs were correlated to either executive function tasks [11, 19] or global cognition, as measured by the MoCA [41]. Given the variability in both choice of secondary task and cognitive measures, it is perhaps unsurprising that the interference across these studies was variably related to cognition. Given the heterogeneous presentation of cognitive impairment, selecting an appropriate task for DTCs in PwPD and FOG is ambiguous and a complete discussion of this topic is outside the scope of the current manuscript. Sufficed to say, it is crucial to consider both the nature of cognitive deficits and the selection of concurrent cognitive tasks in the interpretation of results. Further, systematic studies will be necessary to determine whether interference via different secondary tasks is variably related to cognitive outcomes in PD who freeze. Research exploring this question shows that various cognitive tasks demonstrate similar dual-task effects (e.g., auditory Stroop task, backward digit span) during walking [42] but that complex attention and memory tasks may be more sensitive in eliciting DT impairments [10]. Additionally, no significant differences in MoCA were observed between freezing groups. A possible explanation for the influence of cognition on DTC but the absence of global cognitive differences between freezing groups is that the MoCA lacks sensitivity to detect subtle changes in specific cognitive domains, such as the executive function task used in this report.
We also observed that females exhibited less DTC than males with PD across several gait metrics. This partially conflicts with previous findings indicating that neurotypical females may exhibit increased DTC on walking speed compared to males [43, 44]. The rationale for this discrepancy is unclear. However, variable secondary tasks may provide insight into this across-study variance, as the previous work used an arithmetic task, while the current study used alternative letters. Second, previous studies [43, 44] were completed in neurotypical adults rather than PwPD, potentially influencing the sex-DTC relationship. Furthermore, due in part to the different groups studied, it is notable that the relative number of males and females differed across groups in current and previous studies. Specifically, 35% of participants in the current study were female, and 65% were male. However, in [44], these ratios were flipped (69% female, 31% male). Finally, in the previous study, the duration of a 3-meter Timed-up-and go-task was used as the mobility task. In contrast, the current report used a 2-minute, naturally paced walk, with turns analyzed separately. This longer task may have provided a different (or perhaps more robust) gait characterization than the shorter, 3-meter walk. In sum, the general finding of reduced DTC in females with PD compared to males in the current study highlights the importance of considering primary and secondary task choices when conducting across-group DTC experiments.
Study limitations
There are several limitations to note. First, the main aim of this study was exploratory to enable future research; thus, the results were not corrected for multiple comparisons. Further, due to the exploratory nature of this analysis, pre-planned power analyses were not conducted. Rather, this was a convenience sample aimed at generating preliminary data to explore the relationship between freezing status and DTC. Second, data for this study was collected with PwPD in the Off-levodopa medication state, so this may make it difficult to compare the results with other studies performed on medication. Third, we only focused on gait parameters and not balance. Future studies could focus on how balance may be affected by DT. Similarly, while inertial measurement unit (IMU)-based gait analysis provides important gait outcomes related to mobility impairment in PD, it is not comprehensive and cannot report detailed spatiotemporal gait outcomes concerning footfall pattern and center of pressure-derived gait data. Fourth, it is possible that individuals with higher scores on the verbal memory and digit span tasks of the MoCA were more likely to remember the correct alternate alphabet letters during the dual-task condition since they had already completed these tasks during the single-task condition. Fifth, while this study examined the potential moderating effects of cognition, sex, and symptom severity on DTC, numerous other factors, such as balance and medication, could impact DTCs in PwPD and FOG. Future studies should seek to elucidate the influence of these variables on the relationship between DT and FOG. Sixth, as no neurotypical control group was included in this study (focused on FOG), we cannot confirm any differences in DTC gait measures between PwPD and neurotypical controls. Please refer to Vitorio et al. (2021) [12] for a comprehensive analysis. Finally, while not included in the current analysis, it should be noted that gait is affected by other factors besides cognition such as behavioral and mood alterations like anxiety, fear of falling, and fall history [45].
Conclusions
This exploratory analysis identified several gait metrics in which DTC was altered across FOG status, such that PwPD and FOG exhibited worse gait DTC than those without FOG. This indicates that FOG+ may have a reduced ability to control certain gait metrics (e.g., arm swing and pace) while DT. Although more research is required to establish the relationship between FOG and DTC, these findings show the potential impact of freezing status on DTC and implicate cognitive impairment and sex as additional significant contributors. This study’s outcomes may aid in identifying those who are most vulnerable to DT walking deficits and highlighting potential candidates for DT gait training.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (Grant Number AG006457) and the Veterans Affairs (Grant Number RX001075).
CONFLICT OF INTEREST
OHSU and Fay B. Horak have a significant financial interest in APDM Wearable Technologies, a Clario company, that may have a commercial interest in the results of this research and technology. This potential conflict has been reviewed and managed by OHSU.
DATA AVAILABILITY
Data available based upon reasonable request from the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230063.
REFERENCES
[1] | Pashler H ((1984) ) Processing stages in overlapping tasks: Evidence for a central bottleneck. J Exp Psychol Hum Percept Perform 10: , 358–377. |
[2] | Tombu M , Jolicøeur P ((2003) ) A central capacity sharing model of dual-task performance. J Exp Psychol Hum Percept Perform 29: , 3–18. |
[3] | Allcock LM , Rowan EN , Steen IN , Wesnes K , Kenny RA , Burn DJ ((2009) ) Impaired attention predicts falling in Parkinson’s disease. Parkinsonism Relat Disord 15: , 110–115. |
[4] | Heinzel S , Maechtel M , Hasmann SE , Hobert MA , Heger T , Berg D , Maetzler W ((2016) ) Parkinsonism and Related Disorders Motor dual-tasking de fi cits predict falls in Parkinson’s disease: A prospective study. Parkinsonism Relat Disord 26: , 73–77. |
[5] | Belghali M , Chastan N , Davenne D , Decker LM ((2017) ) Improving dual-task walking paradigms to detect prodromal Parkinson’s and Alzheimer’s diseases. Front Neurol 8: , 207. |
[6] | Fino PC , Mancini M , Curtze C , Nutt JG , Horak FB ((2018) ) Gait stability has phase-dependent dual-task costs in Parkinson’s disease. Front Neurol 9: , 373. |
[7] | Spildooren J , Vercruysse S , Desloovere K , Vandenberghe W , Kerckhofs E , Nieuwboer A ((2010) ) Freezing of gait in Parkinson’s disease: The impact of dual-tasking and turning. Mov Disord 25: , 2563–2570. |
[8] | Yogev G , Giladi N , Peretz C , Springer S , Simon ES , Hausdorff JM ((2005) ) Dual tasking, gait rhythmicity, and Parkinson’s disease: Which aspects of gait are attention demanding? Eur J Neurosci 22: , 1248–1256. |
[9] | Yogev-Seligmann G , Giladi N , Gruendlinger L , Hausdorff JM ((2013) ) The contribution of postural control and bilateral coordination to the impact of dual tasking on gait. Exp Brain Res 226: , 81–93. |
[10] | Zirek E , Ersoz Huseyinsinoglu B , Tufekcioglu Z , Bilgic B , Hanagasi H ((2018) ) Which cognitive dual-task walking causes most interference on the Timed Up and Go test in Parkinson’s disease: A controlled study. Neurol Sci 39: , 2151–2157. |
[11] | Johansson H , Ekman U , Rennie L , Peterson DS , Leavy B , Franzén E ((2021) ) Dual-task effects during a motor-cognitive task in Parkinson’s Disease: Patterns of prioritization and the influence of cognitive status. Neurorehabil Neural Repair 35: , 356–366. |
[12] | Vitorio R , Hasegawa N , Carlson-Kuhta P , Nutt JG , Horak FB , Mancini M , Shah VV ((2021) ) Dual-task costs of quantitative gait parameters while walking and turning in people with Parkinson’s disease: Beyond gait speed. J Parkinsons Dis 11: , 653–664. |
[13] | Belluscio V , Stuart S , Bergamini E , Vannozzi G , Mancini M ((2019) ) The association between prefrontal cortex activity and turning behavior in people with and without freezing of gait. Neuroscience 416: , 168–176. |
[14] | Maidan I , Nieuwhof F , Bernad-Elazari H , Reelick MF , Bloem BR , Giladi N , Deutsch JE , Hausdorff JM , Claassen JAH , Mirelman A ((2016) ) The role of the frontal lobe in complex walking among patients with Parkinson’s disease and healthy older adults: An fNIRS study. Neurorehabil Neural Repair 30: , 963–971. |
[15] | Chaudhary S , Kumaran SS , Kaloiya GS , Goyal V , Sagar R , Kalaivani M , Jaganathan NR , Mehta N , Srivastava A ((2020) ) Domain specific cognitive impairment in Parkinson’s patients with mild cognitive impairment. J Clin Neurosci 75: , 99–105. |
[16] | Lanni KE , Ross JM , Higginson CI , Dressler EM , Sigvardt KA , Zhang L , Malhado-Chang N , Disbrow EA ((2014) ) Perceived and performance-based executive dysfunction in Parkinson’s disease. J Clin Exp Neuropsychol 36: , 342–355. |
[17] | Morris R , Smulders K , Peterson DS , Mancini M , Carlson-Kuhta P , Nutt JG , Horak FB ((2020) ) Cognitive function in people with and without freezing of gait in Parkinson’s disease. NPJ Parkinsons Dis 6: , 9. |
[18] | Monaghan AS , Gordon E , Graham L , Hughes E , Peterson DS , Morris R ((2023) ) Cognition and freezing of gait in Parkinson’s disease: A systematic review and meta-analysis. Neurosci Biobehav Rev 147: , 105068. |
[19] | Lord S , Rochester L , Hetherington V , Allcock LM , Burn D ((2010) ) Executive dysfunction and attention contribute to gait interference in ‘off’ state Parkinson’s disease. Gait Posture 31: , 169–174. |
[20] | Pieruccini-Faria F , Jones JA , Almeida QJ ((2014) ) Motor planning in Parkinson’s disease patients experiencing freezing of gait: The influence of cognitive load when approaching obstacles. Brain Cogn 87: , 76–85. |
[21] | Walton CC , Shine JM , Hall JM , O’Callaghan C , Mowszowski L , Gilat M , Szeto JYY , Naismith SL , Lewis SJG ((2015) ) The major impact of freezing of gait on quality of life in Parkinson’s disease. J Neurol 262: , 108–115. |
[22] | Okuma Y ((2014) ) Freezing of gait and falls in Parkinson’s disease. J Parkinsons Dis 4: , 255–260. |
[23] | Camicioli R , Oken BS , Sexton G , Kaye JA , Nutt JG ((1998) ) Verbal fluency task affects gait in Parkinson’s disease with motor freezing. J Geriatr Psychiatry Neurol 11: , 181–185. |
[24] | de Souza Fortaleza AC , Mancini M , Carlson-Kuhta P , King LA , Nutt JG , Chagas EF , Freitas IFJ , Horak FB ((2017) ) Dual task interference on postural sway, postural transitions and gait in people with Parkinson’s disease and freezing of gait. Gait Posture 56: , 76–81. |
[25] | Nieuwboer A , Rochester L , Herman T , Vandenberghe W , Emil GE , Thomaes T , Giladi N ((2009) ) Reliability of the new freezing of gait questionnaire: Agreement between patients with Parkinson’s disease and their carers. Gait Posture 30: , 459–463. |
[26] | Hughes AJ , Daniel SE , Kilford L , Lees AJ ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: , 181–184. |
[27] | Franchignoni F , Horak F , Godi M , Nardone A , Giordano A ((2010) ) Using psychometric techniques to improve the Balance Evaluation Systems Test: The mini-BESTest. J Rehabil Med 42: , 323–331. |
[28] | Powell LE , Myers AM ((1995) ) The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci 50A: , M28–M34. |
[29] | Dalrymple-Alford JC , MacAskill MR , Nakas CT , Livingston L , Graham C , Crucian GP , Melzer TR , Kirwan J , Keenan R , Wells S , Porter RJ , Watts R , Anderson TJ ((2010) ) The MoCA. Neurology 75: , 1717–1725. |
[30] | Goetz CG , Tilley BC , Shaftman SR , Stebbins GT , Fahn S , Martinez-Martin P , Poewe W , Sampaio C , Stern MB , Dodel R , Dubois B , Holloway R , Jankovic J , Kulisevsky J , Lang AE , Lees A , Leurgans S , LeWitt PA , Nyenhuis D , Olanow CW , Rascol O , Schrag A , Teresi JA , van Hilten JJ , LaPelle N ((2008) ) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 23: , 2129–2170. |
[31] | Hoehn MM , Yahr MD ((1967) ) Parkinsonism. Neurology 17: , 427–427. |
[32] | Learmonth YC , Sandroff BM , Pilutti LA , Klaren RE , Ensari I , Riskin BJ , Holtzer R , Motl RW ((2014) ) Cognitive motor interference during walking in multiple sclerosis using an alternate-letter alphabet task. Arch Phys Med Rehabil 95: , 1498–1503. |
[33] | El-Gohary M , Pearson S , McNames J , Mancini M , Horak F , Mellone S , Chiari L ((2014) ) Continuous monitoring of turning in patients with movement disability. Sensors 14: , 356–369. |
[34] | Hasegawa N , Shah V V , Carlson-Kuhta P , Nutt JG , Horak FB , Mancini M ((2019) ) How to select balance measures sensitive to Parkinson’s disease from body-worn inertial sensors—separating the trees from the forest. Sensors 19: , 3320. |
[35] | Mancini M , King L , Salarian A , Holmstrom L , McNames J , Horak FB ((2011) ) Mobility lab to assess balance and gait with synchronized body-worn sensors. J Bioeng Biomed Sci Suppl 1: , 007. |
[36] | Mancini M , Horak FB ((2016) ) Potential of APDM mobility lab for the monitoring of the progression of Parkinson’s disease. Expert Rev Med Devices 13: , 455–462. |
[37] | Morris R , Stuart S , Mcbarron G , Fino PC , Mancini M , Curtze C ((2019) ) Validity of mobility lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease. Physiol Meas 40: , 095003. |
[38] | Washabaugh EP , Kalyanaraman T , Adamczyk PG , Claflin ES , Krishnan C ((2017) ) Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 55: , 87–93. |
[39] | Longhurst JK , Rider J V , Cummings JL , John SE , Poston B , Held Bradford EC , Landers MR ((2022) ) A novel way of measuring dual-task interference: The reliability and construct validity of the dual-task effect battery in neurodegenerative disease. Neurorehabil Neural Repair 36: , 346–359. |
[40] | Plummer P , Eskes G ((2015) ) Measuring treatment effects on dual-task performance: A framework for research and clinical practice. Front Hum Neurosci 9: , 225. |
[41] | Gaßner H , Marxreiter F , Steib S , Kohl Z , Schlachetzki JCM , Adler W , Eskofier BM , Pfeifer K , Winkler J , Klucken J ((2017) ) Gait and cognition in Parkinson’s disease: Cognitive impairment is inadequately reflected by gait performance during dual task. Front Neurol 8: , 550. |
[42] | Strouwen C , Molenaar EALM , Keus SHJ , Münks L , Heremans E , Vandenberghe W , Bloem BR , Nieuwboer A ((2016) ) Are factors related to dual-task performance in people with Parkinson’s disease dependent on the type of dual task? Parkinsonism Relat Disord 23: , 23–30. |
[43] | Agmon M , Armon G , Denesh S , Doumas M ((2018) ) The role of gender in the association between personality and task priority in older adults’ dual-tasking while walking. BMC Geriatr 18: , 1. |
[44] | Peterson DS ((2022) ) Effects of gender on dual-tasking and prioritization in older adults. Gait Posture 97: , 104–108. |
[45] | Paker N , Bugdayci D , Goksenoglu G , Demircioğlu DT , Kesiktas N , Ince N ((2015) ) Gait speed and related factors in Parkinson’s disease. J Phys Ther Sci 27: , 3675–3679. |




