Is There an Association Between Parkinson’s Disease and Periodontitis? A Systematic Review and Meta-Analysis
Abstract
Background:
Multiple observational studies have yielded controversial results regarding the association between Parkinson’s disease (PD) and periodontitis.
Objective:
This systematic review and meta-analysis was conducted to ascertain their bidirectional relationship.
Methods:
A literature search for relevant studies was performed in PubMed, EMBASE, the Cochrane Library, and Web of Science databases from inception to December 19, 2022. Effect sizes (ES) with 95% confidence intervals were pooled under the random-effects model. Then, leave-one-out sensitivity analysis and contour-enhanced funnel plot were applied to assess the stability of the results.
Results:
A total of 34 studies and 24 studies were included for systematic review and quantitative meta-analysis, respectively. Pooled ES indicated that periodontitis was not significantly associated with PD risk (HR = 1.13, 95% CI 0.88–1.45, n = 3; OR = 1.94, 95% CI 0.55–6.90, n = 7), while the Mendelian randomization study revealed no association between PD and periodontitis risk (coefficient [B] = –0.0001, standard error = 0.0001, p = 0.19). Furthermore, PD patients exhibited higher levels of periodontal pocket depth (SMD = 1.10, 95% CI 0.53–1.67), clinical attachment level (SMD = 1.40, 95% CI 0.55–2.26), plaque index (SMD = 0.81, 95% CI 0.22–1.39), and Oral Health Impact Profile-14 score (SMD = 0.91, 95% CI 0.33–1.49) compared to healthy controls.
Conclusions:
Our meta-analysis identified no bidirectional association between PD risk and periodontitis risk, though the prevalence of periodontitis and poorer oral status was higher in PD patients.
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disorder manifested by bradykinesia, resting tremor, rigidity, and postural instability, affecting millions of elderly adults globally. Between 1990 and 2016, the age-standardized prevalence, disability-adjusted life-years rates and mortality of PD have skyrocketed in almost every region worldwide, increasing the heavy global financial burden [1, 2]. Despite the specific pathogenesis remaining elusive, inflammatory and immunological factors have been suggested to participate in its underlying mechanisms [3, 4].
Periodontitis is a chronic inflammatory disease of the oral cavity that is triggered by the formation of dental plaque biofilm [5]. Advanced periodontitis is a leading cause of loss of teeth and supporting structures, affecting approximately 10–15% of the global population [6]. According to the data from the 2009–2012 National Health and Nutrition Examination Survey, its overall prevalence among Americans over 30 years was estimated to be as high as 46% [7, 8]. It has been observed that the prevalence of total periodontitis rises from the 30–34 age group to the aged 65+ age group [7]. The progression of age, coupled with reduced dexterity, results in the accumulation of dental plaque, leading to more frequent and severe periodontal inflammation [9], which may elevate the longitudinal risk of periodontitis. Moreover, periodontitis has been reported to be correlated with several systemic diseases, such as diabetes mellitus, cardiovascular diseases [10], Alzheimer’s disease (AD) [11], and cancer [12].
Various articles have reported the high prevalence of periodontitis and poor oral health in individuals with PD. For instance, a nested case-control study reported that the prevalence of periodontal diseases attained 48.73% in a cohort of 4765 PD patients [13]. In a cross-sectional case-control study, periodontitis was detected in 98 out of 104 PD patients [14]. Several factors could potentially explain the link between the prevalence or risk of periodontitis and PD. As is well documented, polypharmacy, cognitive impairment, motor dysfunction, and the subsequent deducing dental care elevate the risk of dental caries and periodontal disorders [15]. Sialorrhea, a commonly observed symptom of PD, is also hypothesized to promote periodontitis and other oral diseases, attributable to hypersalivation altering the composition of oral microbiota (like K. oralis) in PD cases and facilitating local inflammation in the oral cavity [16]. Additionally, the prevalence of sialorrhea has been reported to increase with age and disease duration in PD patients [17], insinuating that it may mediate the impact of age and PD duration on periodontitis risk. Similarly, gender is also considered a key factor in the development of sialorrhea, thereby influencing periodontitis risk, which can be supported by the finding that males with PD were twice more likely to develop sialorrhea compared to females [18]. Hyposmia, characterized by a reduced sense of smell in PD patients [19], which deteriorates significantly with age [20] and is often accompanied by taste dysfunction, contributes to prolonged mealtimes and inadequate chewing [15], thus the increasing risk of periodontal diseases. Moreover, since female gender might be a contributing factor for dysphagia [15, 21], another plausible explanation for the effect of gender on periodontitis risk is that dysphagia may result in acid reflux, which could trigger perimylolysis tooth erosion and potentially increase the periodontitis risk [22]. In addition, Fukayo et al. [23] reported that female PD patients brushed their teeth more frequently than male patients and tended to have a lower decayed, missing and filled teeth (DMFT) index than the male group, indicating that the alterations in dental care behavior may also be a reason for gender influencing periodontitis risk.
Despite numerous articles investigating the association between PD and periodontitis, their findings have been contradictory. While certain large-sample observational studies have noted that periodontal inflammatory disease is correlated with an elevated risk of developing PD [24, 25], other studies have yielded conflicting results. For example, a Mendelian randomization (MR) study revealed no convincing genetic association between the two diseases [26]. Meanwhile, the bidirectional relationship between PD and periodontitis has not been systematically assessed.
Therefore, we sought to systematically review studies focusing on PD and periodontitis and perform a meta-analysis to comprehensively investigate the bidirectional association between the two diseases and summarize the oral status of PD patients.
METHODS
Literature search
A systematic literature search of PubMed, EMBASE, the Cochrane Library, and Web of Science was performed for studies published until December 19, 2022 using the following keywords: Parkinson’s disease, parkinsonism, periodontitis, and periodontal disease. The detailed search strategies for PubMed search are provided in the Supplementary Material.
Eligibility criteria
Studies were considered eligible for inclusion if they investigated the relationship between PD and periodontitis or the oral status of patients. Besides, we also reviewed the reference lists of the related articles and screened for potentially relevant conference abstracts. The exclusion criteria were as follows: 1) reviews, case reports, letters, comments, or other types of articles; 2) studies of animal or cell models; 3) studies without quantitative data. Regarding duplicate publications reporting the same cohort, the study with the largest sample size was included.
Data extraction
Two reviewers (Y.Q.C. and K.L) independently extracted the following data from included studies: 1) study characteristics (the first author, publication year, study design); 2) population parameters (sample size, country, age, gender); 3) assessment of PD and periodontitis or oral status (scale of diagnosis, disease duration, clinical parameter values); 4) hazard ratios (HR), odds ratios (OR), standardized mean differences (SMD), and their corresponding 95% confidence intervals (CI). If both unadjusted and adjusted effect sizes were available, data on the latter were collected.
Any discrepancies were resolved by arbitration with a third reviewer (D.H.Y.).
Quality assessment
The methodological quality of all eligible studies in the systematic review was independently assessed by Y.Q.C. and K.L. The Newcastle Ottawa Scale (NOS), consisting of three parameters of quality: selection, comparability and exposure or outcome assessment, was used to assess the quality of the cohort studies and case-control studies. Meanwhile, the quality of cross-sectional studies was assessed using the Joanna Briggs Institute (JBI) checklist. The quality assessment of the MR study was based on the STROBE-MR Checklist [27].
Outcomes
The co-primary outcomes of our study were the risk of PD in patients with periodontitis and the risk of periodontitis in PD patients. Secondary outcomes were periodontal clinical parameters values including periodontal pocket depth (PPD), clinical attachment level (CAL), plaque index (PI), and the percentage of bleeding on probing sites (BoP %), all of which examined the prevalence of periodontitis in PD cases. Besides, based on the enrolled studies, we also pooled other oral hygiene parameters to assess the oral status and oral health of PD patients.
Statistical analysis
We performed a quantitative meta-analysis of the studies included in the systematic review to merge results for the outcomes reported with the same metrics in more than two studies. All statistical analyses were conducted via R software (version 4.1.0). Effect sizes (ES) were calculated as hazard ratios (HR), odds ratios (OR), and standardized mean differences (SMD), along with their corresponding 95% confidence intervals (CIs). Heterogeneity was determined with the Cochran Q statistic and the I2 statistic, with the significance level set at a 2-tailed value of p < 0.05 and I2 >50% indicating significant heterogeneity. Given the presence of heterogeneity, the study estimates were combined under the random-effects model. Leave-one-out sensitivity analysis was carried out to assess the stability of the combined results. Contour-enhanced funnel plot [28] and the Duvall and Tweedle trim-and-fill approach [8] were used to evaluate potential publication bias.
RESULTS
Literature search
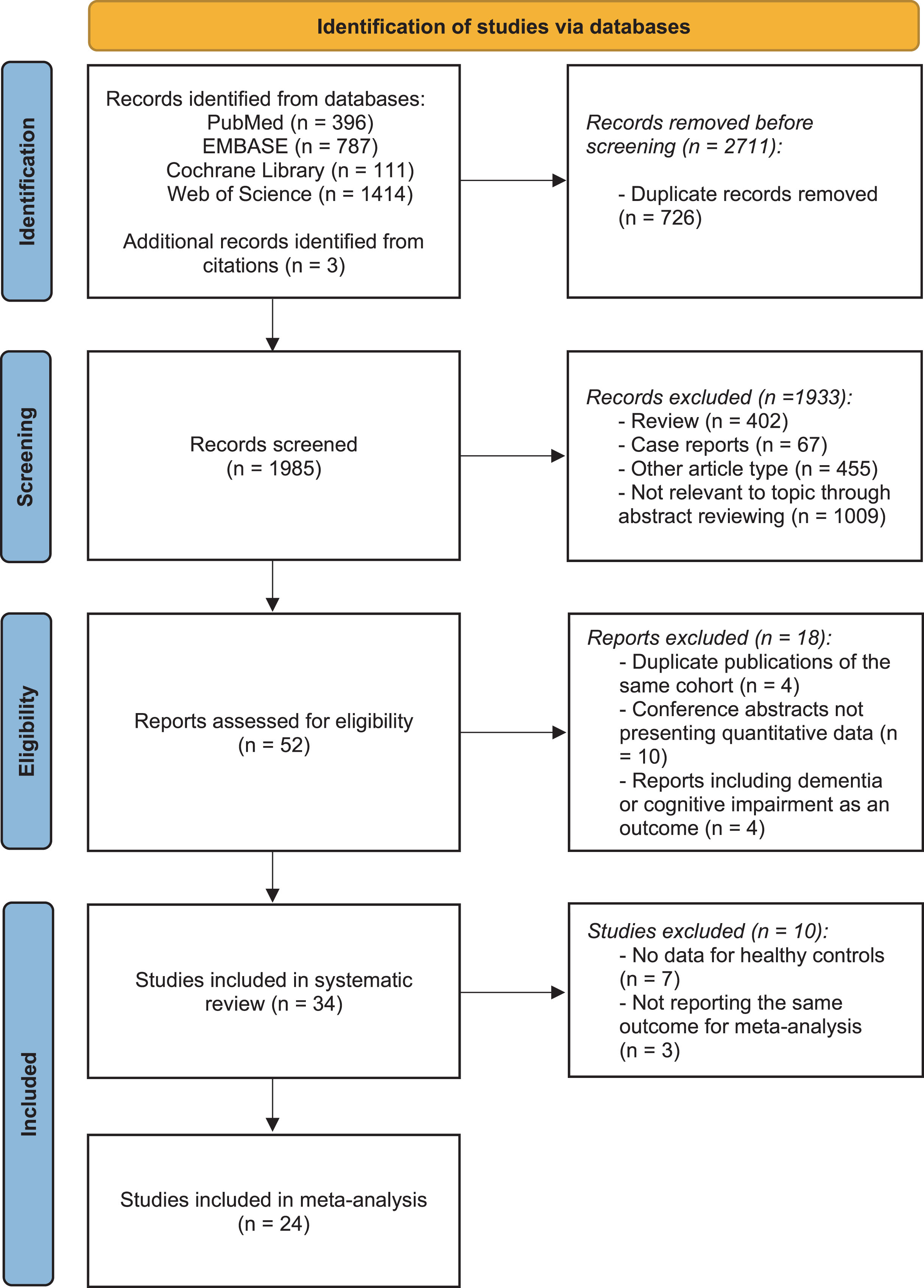
The literature search yielded 2,708 studies, and three additional publications were identified by reviewing reference lists. After excluding duplicates, a total of 1,985 unique titles and abstracts were screened. Subsequently, the full text of 52 potentially eligible articles was reviewed. Following a thorough assessment, 18 studies were excluded: four studies using the same data from another publication, four studies reporting dementia or cognitive impairment as the outcome, and ten conference abstracts did not report quantitative data. Therefore, 34 studies were included in the systematic review. Concerning studies with data on control groups, a quantitative meta-analysis was performed on 24 studies (involving 7,720,793 participants) reporting the same outcomes. The process of study identification is illustrated in the PRISMA flow chart [29] in Fig. 1.
Fig. 1
PRISMA flow diagram delineating study identification.
Study characteristics and quality assessment
The characteristics of 34 studies included in the systematic review are summarized in Table 1. The included studies comprised six cohort studies, sixteen case-control studies, eleven cross-sectional studies, and an MR study. The majority of the studies assessed the severity of PD using Hoehn and Yahr (H&Y) stage, United Parkinson’s Disease Rating Scale (UPDRS), or Movement Disorder Society-sponsored United Parkinson’s Disease Rating Scale (MDS-UPDRS). However, the diagnostic criteria and clinical periodontal examination protocols for periodontitis and oral status varied and were inconsistent among the studies. Due to a lack of data on healthy controls and the heterogeneity of outcomes, three cohort studies, fifteen case-control studies, five cross-sectional studies, and the MR study were eventually included in the meta-analysis.
Table 1
Characteristics of included studies in the systematic review
| Study | Year | Country | Study Design | Groups | Sample Size | Age | Sex | Assessment of Parkinson’s Disease | Assessment of Periodontitis or Oral Status |
| (Mean±SD) | (M/F) | ||||||||
| Botelho et al. [34] | 2020 | Portugal | Cohort | PD with PeriodontitisPD without Periodontitis | 2017 | 61.6±13.853.1±14.6 | 10/105/12 | PD diagnosis: cases reporting the use of Benztropine, Carbidopa, Levodopa and other antiparkinsonian medication | 1. Periodontitis diagnosis: 2 or more sites with CAL ≥3 mm and a PPD ≥4 mm or one site with PPD ≥5 mm; 2. Number of missing teeth |
| Jeong et al. [25] | 2021 | Korea | Cohort | PeriodontitisHealthy Control | 903,0635,922,621 | 55.47±9.9754.21±10.54 | 485,941/417,1222,941,386/2,981,235 | PD diagnosis:ICD-10 code for PD (G20) | Periodontitis diagnosis:ICD-10 code for Periodontitis |
| Ledwon et al. [48] | 2020 | Austria | Cohort | PD with periodontal carePD Control | 6132 | 70.8±8.469.8±8.0 | 34/2721/11 | 1. PD diagnosis: patients receiving antiparkinsonian drug2. UPDRS-III | 1. Number of teeth2. PI3. BoP% 4. PDD5. Tooth mobility |
| Liu et al. [49] | 2013 | Taiwan of China | Cohort | PeriodontitisHealthy Control | 53,351266,755 | NA | NA | 1. PD diagnosis: ICD-9-CM code 3322. HR of risk of PD | Periodontitis diagnosis: ICD-9-CM code 523.4 |
| Liu et al. [31] | 2017 | Sweden | Cohort | PDNon-PD | 30719,868 | 55.5±13.943.4±18.1 | 175/1329,781/10,087 | PD diagnosis: (ICD-7 code: 350; ICD-8 code: 342.00; ICD-9 code: 332.0; ICD-10 code: G20) | 1. Number of existing teeth2. Dental plaque3.The presence of oral mucosal lesions |
| Woo et al. [50] | 2020 | Korea | Cohort | PeriodontitisHealthy Control | 30,580122,585 | 67.8±9.369.1±9.3 | 98,434/54,731 | 1. PD diagnosis: (ICD-10 G20; G21-26) 2. HR of risk of PD | 1. Periodontitis diagnosis: (ICD-10 K052-056) 2. Number of tooth loss |
| Botelho et al. [26] | 2021 | Portugal | Mendelian Randomization | PeriodontitisHealthy ControlPDHealthy Control | 1,8172,21520,184397,324 | NA | NA | OR of risk of PD | OR of risk of periodontitis |
| Auerbacher et al. [51] | 2022 | Germany | Case control | PDHealthy Control | 2430 | 73±962.5±12 | 14/1011/19 | NA | 1. DMFT2. Number of missed teeth3. Number of decayed teeth4. Presence of edentulism |
| Bakke et al. [52] | 2011 | Denmark | Case control | PDHealthy Control | 1515 | 68.47±6.26NA | 6/96/9 | 1. Hoehn &Yahr Stage2. UPDRS-III3. PD duration | 1. OHIP2. OHI3.Nordic Orofacial Test-Screening |
| Balash et al. [53] | 2014 | Israel | Case control | PDHealthy Control | 9693 | 68.1±7.166.6±8.4 | NA | NA | OR of risk of periodontitis |
| Barbe et al. [54] | 2017 | Germany | Case control | PDHealthy Control | 3030 | 69.3±8.069.3±7.9 | 17/1317/13 | 1. MDS-UPDRS-II2. PD duration | 1. Periodontitis diagnosis: Community Periodontal Index of Treatment Needs2. OHIP-143. PI4.Xerostomia |
| Cicciù et al. [55] | 2012 | Italy | Case control | PDHealthy Control | 4545 | NA | 17/2810/35 | Hoehn &Yahr Stage | 1. PDD2. Number of missed teeth3. Number of decayed teeth |
| Einarsdóttir et al. [56] | 2009 | Iceland | Case control | PDHealthy Control | 6755 | (<60) n = 40 (60- 0) n = 42 (>70) n = 40 | 39/2821/34 | NA | 1. Periodontitis diagnosis: PPD ≥4 mm2. OR of risk of periodontitis3. Presence of gingivitis4. DMFT5. Number of missed teeth |
| Fleury et al. [16] | 2021 | Switzerland | Case control | PDHealthy Control | 2020 | 63.34±5.5163.90±7.26 | 9/119/11 | 1. Hoehn &Yahr Stage2. PD duration | 1. PPD2. PI3. BoP% |
| Fukayo et al. [23] | 2003 | Japan | Case control | PDHealthy Control | 31104 | (60–69) n = 12 (≥70) n = 19 (60–69) n = 76 (≥70) n = 28 | 17/1461/43 | Hoehn &Yahr Stage | DMFT |
| Garcia-de-la-Fuente et al. [14] | 2022 | Spain | Case control | PDHealthy Control | 104106 | 66.2±9.359.2±14.1 | 66/3837/69 | NA | 1. Presence of periodontitis2. PI3.CAL4. DMFT5. Community Periodontal Index of Treatment Needs6. Number of decayed teeth7. Number of missed teeth8. Presence of xerostomia |
| Hanaoka et al. [57] | 2009 | Japan | Case control | PDControls with mild neurological disorders | 8968 | 72.1±5.069.0±5.8 | 38/5126/42 | Hoehn &Yahr Stage | Periodontitis diagnosis: PPD ≥4 mm |
| Kennedy et al. [43] | 1994 | USA | Case control | PD craving sweetsPD not craving sweetsHealthy Control | 141414 | 65.7±6.267.6±9.264.1±6.8 | 9/57/76/8 | 1. Hoehn &Yahr Stage2. PD duration | 1. Presence of mucositis2. Presence of gingivitis3. DMFT |
| Müller et al. [42] | 2011 | Germany | Case control | PDHealthy Control | 10175 | 66.2±10.571.0±11.5 | 55/4635/40 | 1. Hoehn &Yahr Stage2. UPDRS-I3. UPDRS-II4. UPDRS-III5. UPDRS-IV | 1. CAL2. PDD3. PI4. OHI5. Number of missed teeth |
| Nakayama et al. [58] | 2004 | Japan | Case control | PDHealthy Control | 104191 | (60–69) n = 38 (≥70) n = 66 (60–69) n = 121 (≥70) n = 70 | 44/6078/113 | Hoehn &Yahr Stage | 1. Tooth movement2. Presence of edentulism |
| Persson et al. [59] | 1992 | Sweden | Case control | PDHistorical Control in 1911-1913 | 30585 | 73.0±7.3NA | 17/13279/302 | PD duration | 1. BoP% 2. Mean number of teeth |
| Schwarz et al. [60] | 2006 | Germany | Case control | PDHealthy Control | 7085 | 64.5±6.862.0±6.3 | 39/3141/44 | NA | CPITN (Community Periodontal Index for Treatment Needs) |
| van Stiphout et al. [61] | 2018 | Netherlands | Case control | PDHealthy Control | 7474 | 70.2±8.867.9±10.1 | 48/2639/35 | 1. Hoehn &Yahr Stage2. PD duration | 1. Tooth movement2. Presence of edentulism3. Much dental plaque |
| Barbe et al. [62] | 2017 | Germany | Cross-sectional | PD | 100 | 71.0±8.7 | 72/28 | 1. MDS-UPDRS-II2. PD duration | OHIP-14 |
| Gardner et al. [63] | 2013 | USA | Cross-sectional | PDHealthy Control | 620393 | 72.2±9.2NA | 373/247NA | UPDRS-II | 1. Prevalence of xerostomia2. Rating of dental health |
| Gopalakrishnan et al. [64] | 2021 | India | Cross-sectional | PD | 50 | (30–49) n = 12 (50–59) n = 13 (≥60) n = 25 | 41/9 | NA | 1. Prevalence of periodontitis and gingivitis2. Prevalence of xerostomia |
| John et al. [30] | 2021 | India | Cross-sectional | PDHealthy Control | 3242 | 58.41±10.6254.36±9.72 | 18/1422/20 | 1. Hoehn &Yahr Stage2. PD duration | 1. Periodontitis diagnosis: CAL score ≥3 mm2. PDD3. OHI4. DMFT |
| Lyra et al. [65] | 2020 | Portugal | Cross-sectional | PD | 28 | 72.3±8.1 | 23/5 | 1. Hoehn &Yahr Stage2. MDS-UPDRS | 1. Periodontitis diagnosis: interdental CAL ≥2 non-adjacent teeth, or Buccal or Oral CAL ≥3 mm with PDD >3 mm is detectable at ≥2 teeth2. OHIP-143. PI |
| Lyra et al. [35] | 2022 | Portugal | Cross-sectional | PD with PeriodontitisPD without Periodontitis | 2328 | 60.04±14.3965.36±14.79 | 13/1014/14 | PD diagnosis: cases reporting the use of specific PD medications | 1. Periodontitis diagnosis: positive self-report on one of several oral health-related questions2. Number of missed teeth |
| Pradeep et al. [66] | 2015 | India | Cross-sectional | PDHealthy Control | 4546 | 64.5±9.163.9±13.1 | 30/1528/18 | Hoehn &Yahr Stage | 1. BoP% 2. CAL3. PDD |
| Purisinsith et al. [67] | 2021 | Thailand | Cross-sectional | PD | 274 | NA | 144/133 | NA | OHIP-14 |
| Ribeiro et al. [68] | 2016 | Brazil | Cross-sectional | PDHealthy Control | 1720 | 69.41±4.6572.00±5.69 | 9/810/10 | 1. PD diagnosis: patients receiving levodopa treatment2. PD duration | 1. DMFT2. Visual plaque index3. Number of decayed teeth |
| Silva et al. [69] | 2015 | Brazil | Cross-sectional | PD | 59 | 65.4±8.7 | 30/29 | 1. Hoehn &Yahr Stage2. PD duration | OHIP-14 |
| Verhoeff et al. [70] | 2022 | Netherlands | Cross-sectional | PDHealthy Control | 341411 | 65.5±8.462.6±5.3 | 60/63 (There were missing data) 209/202 | 1. MDS-UPDRS-II2. PD duration | OHIP-14 |
PD, Parkinson’s disease; ES, effect size; HR, hazard ratio; OR, odds ratio; PDD, periodontal pocket depth; CAL, clinical attachment level; PI, plaque index; BoP%, the percentage of bleeding on probing sites; OHI, oral hygiene index; OHIP-14, Oral Health Impact Profile-14; DMFT, decayed, missing, and filled teeth index; UPDRS, United Parkinson’s Disease Rating Scale; MDS-UPDRS, Movement Disorder Society-sponsored United Parkinson’s Disease Rating Scale.
Table 2 presents the detailed quality score of each included study. All six cohort studies and the majority of the case-control studies (13 out of 16 studies) were deemed to be of high methodological quality (low risk of bias), with a NOS score of ≥7 (Table 2A, B). Likewise, the ten cross-sectional studies were also of high quality, according to the JBI checklists (Table 2C). Additionally, the detailed checklist items of the Mendelian randomization study according to the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) Checklist are listed in Table 2D.
Table 2
Methodological quality assessment of included studies
| A. Quality assessment of cohort studies based on the Newcastle–Ottawa Scale (NOS). | ||||||||||
| Study | Year | Overall | Selection (4★) | Comparability (2★) | Outcome (3★) | |||||
| score | Representativeness | Selection of | Ascertainment | Outcome of | Comparability | Assessment | Sufficient | Adequacy | ||
| of the exposed | the control | of exposure | interest not | of cohorts | of outcome | follow-up | of follow | |||
| cohort | cohort | present at start | up cohorts | |||||||
| Botelho et al. [34] | 2020 | 7 | ★ | ★ | ★ | ★ | ★ | ★ | ★ | |
| Jeong et al. [25] | 2021 | 9 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ |
| Ledwon et al. [48] | 2020 | 7 | ★ | ★ | ★ | ★★ | ★ | ★ | ||
| Liu et al. [49] | 2013 | 9 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ |
| Liu et al. [31] | 2017 | 8 | ★ | ★ | ★ | ★★ | ★ | ★ | ★ | |
| Woo et al. [50] | 2020 | 9 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ |
| B. Quality assessment of case-control studies based on the Newcastle–Ottawa Scale (NOS). | ||||||||||
| Study | Year | Overall | Selection (4★) | Comparability (2★) | Exposure (3★) | |||||
| score | Adequate | Representativeness | Selection | Definition | Comparability of cases | Ascertainment | Same method of | Non- | ||
| case | of the cases | of controls | of controls | and controls on the basis | of exposure | ascertainment for | Response | |||
| definition | of the design or analysis | cases and controls | rate | |||||||
| Auerbacher et al. [51] | 2022 | 7 | ★ | ★ | ★ | ★★ | ★ | ★ | ||
| Bakke et al. [52] | 2011 | 7 | ★ | ★ | ★ | ★ | ★★ | ★ | ||
| Balash et al. [53] | 2014 | 7 | ★ | ★ | ★ | ★★ | ★ | ★ | ||
| Barbe et al. [54] | 2017 | 9 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | ★ |
| Cicciù et al. [55] | 2012 | 7 | ★ | ★ | ★ | ★ | ★★ | ★ | ||
| Einarsdóttir et al. [56] | 2009 | 7 | ★ | ★ | ★ | ★★ | ★ | ★ | ||
| Fleury et al. [16] | 2021 | 8 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | |
| Fukayo et al. [23] | 2003 | 6 | ★ | ★ | ★ | ★ | ★ | ★ | ||
| Garcia-de-la-Fuente et al. [14] | 2022 | 7 | ★ | ★ | ★ | ★★ | ★ | ★ | ||
| Hanaoka et al. [57] | 2009 | 7 | ★ | ★ | ★ | ★★ | ★ | ★ | ||
| Kennedy et al. [43] | 1994 | 8 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | |
| Müller et al. [42] | 2011 | 8 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | |
| Nakayama et al. [58] | 2004 | 7 | ★ | ★ | ★ | ★ | ★★ | ★ | ||
| Persson et al. [59] | 1992 | 6 | ★ | ★ | ★ | ★ | ★ | ★ | ||
| Schwarz et al. [60] | 2006 | 6 | ★ | ★ | ★ | ★★ | ★ | |||
| van Stiphout et al. [61] | 2018 | 8 | ★ | ★ | ★ | ★ | ★★ | ★ | ★ | |
| C. Quality assessment of cross-sectional studies based on Joanna Briggs Institute (JBI) checklists. | |||||||||||
| Study | Year | Overall score | Q1* | Q2* | Q3* | Q4* | Q5* | Q6* | Q7* | Q8* | Q9* |
| Barbe et al. [62] | 2017 | 8 | Y | Y | Y | Y | Y | Y | Y | Y | U |
| Gardner et al. [63] | 2013 | 6 | Y | U | Y | N | Y | Y | Y | Y | U |
| Gopalakrishnan et al. [64] | 2021 | 5 | Y | U | Y | Y | Y | U | Y | N | U |
| John et al. [30] | 2021 | 8 | Y | Y | Y | Y | Y | Y | Y | Y | U |
| Lyra et al. [65] | 2020 | 8 | Y | Y | N | Y | Y | Y | Y | Y | Y |
| Lyra et al. [35] | 2022 | 7 | Y | Y | N | Y | Y | Y | N | Y | Y |
| Pradeep et al. [66] | 2015 | 9 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Purisinsith et al. [67] | 2021 | 6 | Y | Y | Y | N | Y | U | Y | Y | U |
| Ribeiro et al. [68] | 2016 | 7 | Y | Y | N | Y | Y | Y | Y | Y | U |
| Silva et al. [69] | 2015 | 8 | Y | Y | Y | Y | Y | Y | Y | N | Y |
| Verhoeff et al. [70] | 2022 | 9 | Y | Y | Y | Y | Y | Y | Y | Y | Y |
Y, Yes; N, No; U, Unclear. *Q1: Was the sample frame appropriate to address the target population? *Q2: Were study participants sampled in an appropriate way? *Q3: Was the sample size adequate? *Q4: Were the study subjects and the setting described in detail? *Q5: Was the data analysis conducted with sufficient coverage of the identified sample? *Q6: Were valid methods used for the identification of the condition? *Q7: Was the condition measured in a standard, reliable way for all participants? *Q8: Was there appropriate statistical analysis? *Q9: Was the response rate adequate, and if not, was the low response rate managed appropriately?
| D. Quality assessment of the mendelian randomization study based on the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization (STROBE-MR) Checklist. | ||||
| Study | Year | Tool for quality assessment | Checklist item | Result of quality assessment |
| Botelho et al. [26] | 2021 | STROBE-MR Checklist | Title and Abstract | 1. Have indicated MR as the design. |
| 1. Title and Abstract | 2. Explain plausible potential causal relationship between exposure (PD and periodontitis) and outcome (risk of periodontitis and risk of PD). | |||
| Introduction | 3. Have stated prespecified causal hypotheses. | |||
| 2. Background 3. Objectives | 4. Describe eligibility criteria and the sources of participants, selection of genetic variants. | |||
| 5. Have stated 3 main IV assumptions for main analysis. | ||||
| Methods | 6. Utilize inverse-variance weighted method, weighted median and MR-Egger as MR effect estimation methods. | |||
| 4. Study design and data source | 7. Compute F-statistic and use MR-Egger method to assess the assumption. | |||
| 5. Assumptions | 8. Use the leave-one-out analysis. | |||
| 6. Statistical methods | 9. Use R (v 3.6.1) through MRPRESSO and TwoSampleMR packages. | |||
| 7. Assessment of assumptions | 10. Calculate Pseudo R2 and F-statistic to justify of the similarity of the genetic variant–exposure associations. | |||
| 8. Sensitivity analyses and additional analyses | ||||
| 9. Software and preregistration Results | ||||
| Results | 11. Report MR estimates of the relationship between PD and periodontitis by odds ratio and visualize the results through forest plot. | |||
| 10. Descriptive data 11. Main results | 12. Calculate I2 and Q statistic to assess the heterogeneity across genetic variants. | |||
| 12. Assessment of assumptions | 13. Present a bidirectional MR and perform leave-one-out analyses. | |||
| 13. Sensitivity analyses and additional analyses | 14. Have summarized key results. | |||
| Discussion | ||||
| 14. Key results | 15. Have discussed limitations of the study and imprecision of assumption. | |||
| 15. Limitations | 16. Have provided a cautious overall interpretation of results. | |||
| 16. Interpretation | ||||
| 17. Generalizability | ||||
| 17. None. |
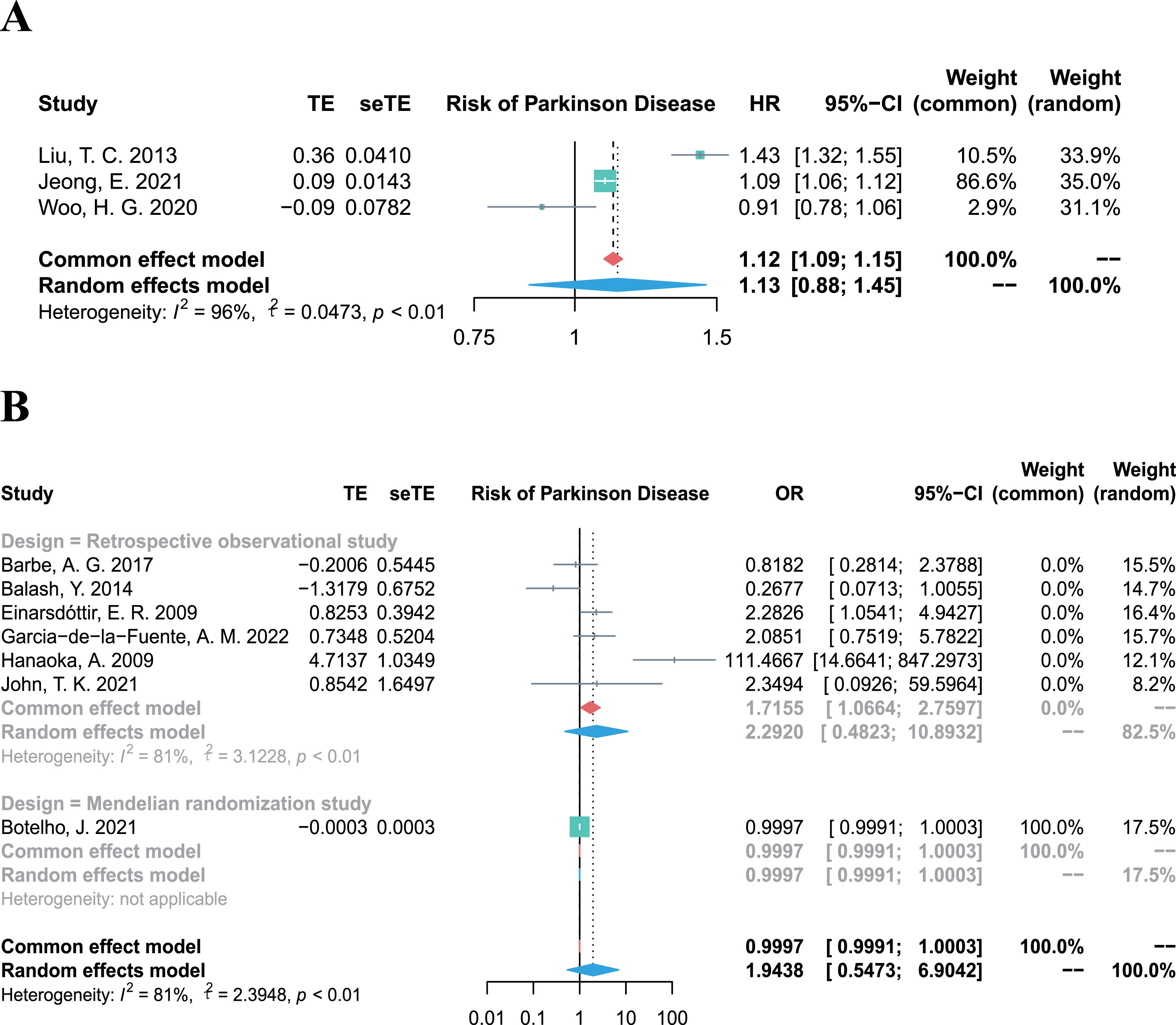
Association between periodontitis and risk of PD
The overall HR for the 3 cohort studies (involving 7,298,955 participants) was 1.13 (95% CI 0.88–1.45, p = 0.3436, I2 = 96%; Fig. 2A), demonstrating substantial evidence that periodontitis is not associated with the incidence of PD. In line the results of cohort studies, the combined OR, including 4,844 participants from six retrospective studies and one Mendelian randomization study [26] also showed an overall null effect (OR 1.94, 95% CI 0.55–6.90, p = 0.3040, I2 = 81%; Fig. 2B) for the association between periodontitis and the risk of PD.
Fig. 2
Forest plots of meta-analysis for the association between periodontitis and risk of Parkinson’s disease, combining the HR of three cohort studies (A), and combining the OR of six retrospective studies (B).
Association between PD and risk of periodontitis
The bidirectional Mendelian randomization study was the only study that investigated the relationship between PD and the risk of periodontitis, and found no significant genetic association between them when analyzing the data from genome-wide association studies involving 417,508 participants (coefficient [B] = –0.0001, standard error [SE] = 0.0001, p = 0.19) [26].
Association between PD and prevalence of periodontitis
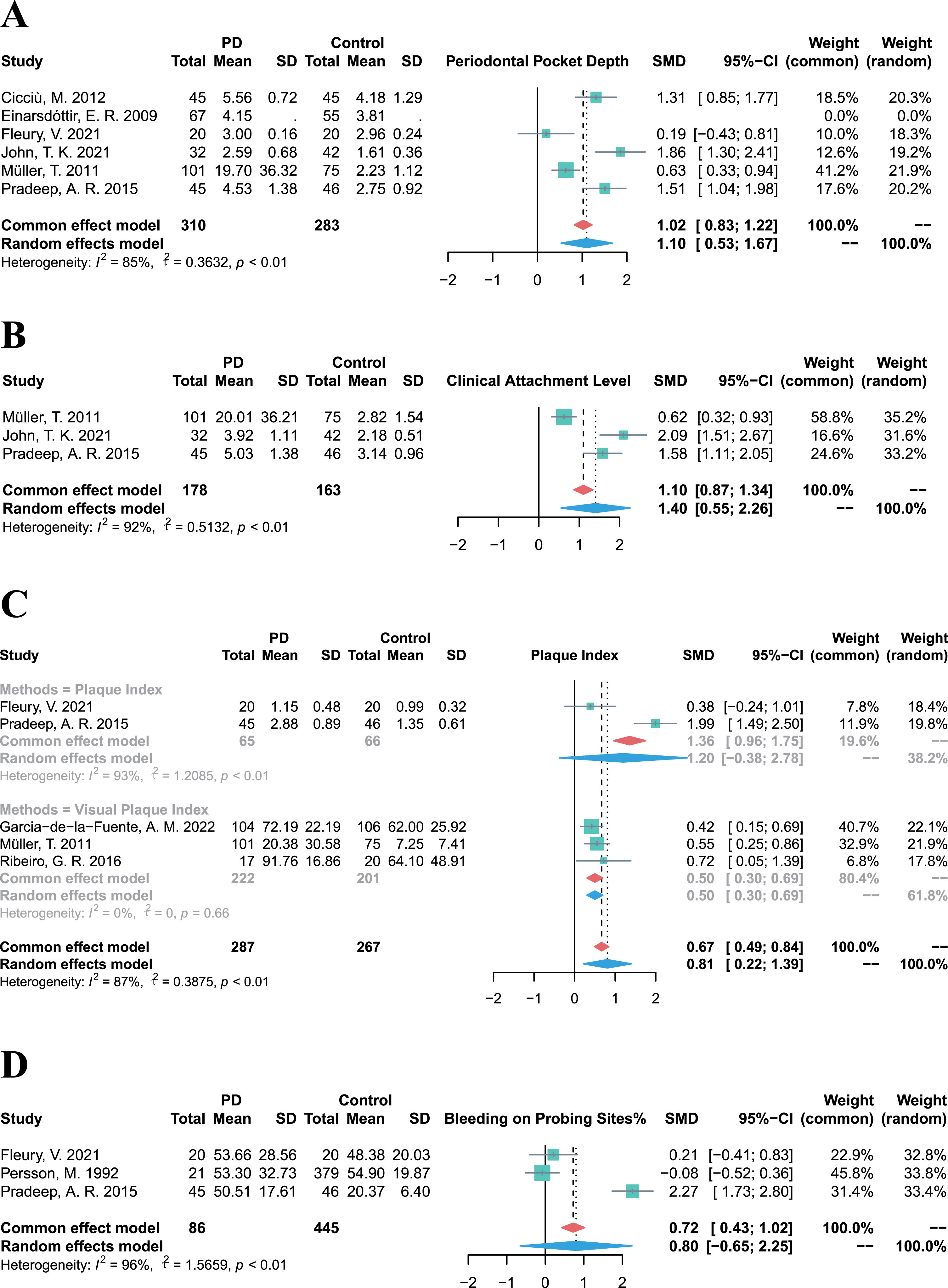
Owing to the diverse and heterogeneous outcomes in the included studies, periodontal clinical parameter values were integrated to evaluate the prevalence of periodontitis and the periodontal status of PD patients. The pooled PPD in PD patients was significantly higher than that in control groups (SMD = 1.10, 95% CI 0.53–1.67, p = 0.0002, I2 = 85%; Fig. 3A). Similarly, the CAL of PD patients was significantly higher (SMD = 1.40, 95% CI 0.55–2.26, p = 0.0013, I2 = 92%; Fig. 3B) compared to control groups, and the PI of PD patients was also significantly higher (SMD = 0.81, 95% CI 0.22–1.39, p = 0.0072, I2 = 87%; Fig. 3C). Besides, although the BoP % was higher in PD groups than that in control groups, there was high heterogeneity and no statistically significant difference (SMD = 0.80, 95% CI –0.65–2.25, p = 0.2803, I2 = 96%; Fig. 3D). Above all, PD patients exhibited higher values of periodontal clinical parameters, indicating a higher prevalence of periodontitis among PD cases.
Fig. 3
Forest plots of meta-analysis for PPD (A), CAL (B), PI (C), and BoP% (D) in patients with Parkinson’s disease.
Oral status in PD
Nevertheless, considering PD patients are likely to present poor oral hygiene in clinical practice, we also conducted meta-analysis on additional clinical parameters of oral hygiene status and summarized the oral manifestation of PD patients in Table 3. PD patients experienced an unfavorable oral health-related quality of life, as evidenced by significantly higher scores on the Oral Health Impact Profile (OHIP-14) in the PD group (SMD = 0.91, 95% CI 0.33–1.49, p = 0.0022, I2 = 80%). Compared to controls, PD patients had a higher prevalence of xerostomia (OR = 3.60, 95% CI 2.79–4.65, p < 0.0001, I2 = 26%), in accordance with their declining salivary flow rate (SMD = –0.43, 95% CI –0.80– –0.05, p = 0.0260, I2 = 76%). While PD patients suffered from significantly higher tooth mobility (OR = 6.67, 95% CI 1.73–25.68, p = 0.0058, I2 = 69%), they also had a tendency to experience edentulism (OR = 1.44, 95% CI 0.65–3.20, p = 0.3644, I2 = 73%) and exhibit a higher number of loss teeth (SMD = 0.34, 95% CI –0.26–0.94, p = 0.2644, I2 = 89%), decayed teeth (SMD = 0.28, 95% CI –0.09–0.65, p = 0.1417, I2 = 81%), and a higher DMFT index (SMD = 0.10, 95% CI –0.50–0.70, p = 0.7397, I2 = 91%), although these differences were not statistically significant. Overall, the prevalence of poorer oral health tended to be higher in PD patients. The relevant forest plots are displayed in the Supplementary Material.
Table 3
Overview of the oral status and dental manifestation of patients with Parkinson’s disease
| Outcomes | No. of studies | No. of participants | ES (95% CI) | p | Heterogeneity |
| (I2, p for Cochran Q) | |||||
| OHIP-14 | 3 | 842 | SMD: 0.91 (0.33, 1.49) | 0.0022 | I2 = 80%, p < 0.01 |
| Xerostomia | 4 | 1431 | OR: 3.60 (2.79, 4.65) | <0.0001 | I2 = 26%, p = 0.26 |
| Salivary flow rate | 6 | 570 | SMD: –0.43 (–0.80, –0.05) | 0.0260 | I2 = 76%, p < 0.01 |
| Tooth mobility | 3 | 433 | OR: 6.67 (1.73, 25.68) | 0.0058 | I2 = 69%, p = 0.04 |
| Oral hygiene index | 3 | 280 | SMD: 1.35 (–0.06, 2.75) | 0.0598 | I2 = 95%, p < 0.01 |
| Edentulism | 7 | 1336 | OR: 1.44 (0.65, 3.20) | 0.3644 | I2 = 73%, p < 0.01 |
| DMFT | 7 | 674 | SMD: 0.10 (–0.50, 0.70) | 0.7397 | I2 = 91%, p < 0.01 |
| Tooth loss | 6 | 689 | SMD: 0.34 (–0.26, 0.94) | 0.2644 | I2 = 89%, p < 0.01 |
| Decayed teeth | 7 | 1124 | SMD: 0.28 (–0.09, 0.65) | 0.1417 | I2 = 81%, p < 0.01 |
ES, effect size; SMD, standard mean difference; OR, odds ratio; CI, confidence interval; OHIP-14, Oral Health Impact Profile-14; DMFT, decayed, missing, and filled teeth index.
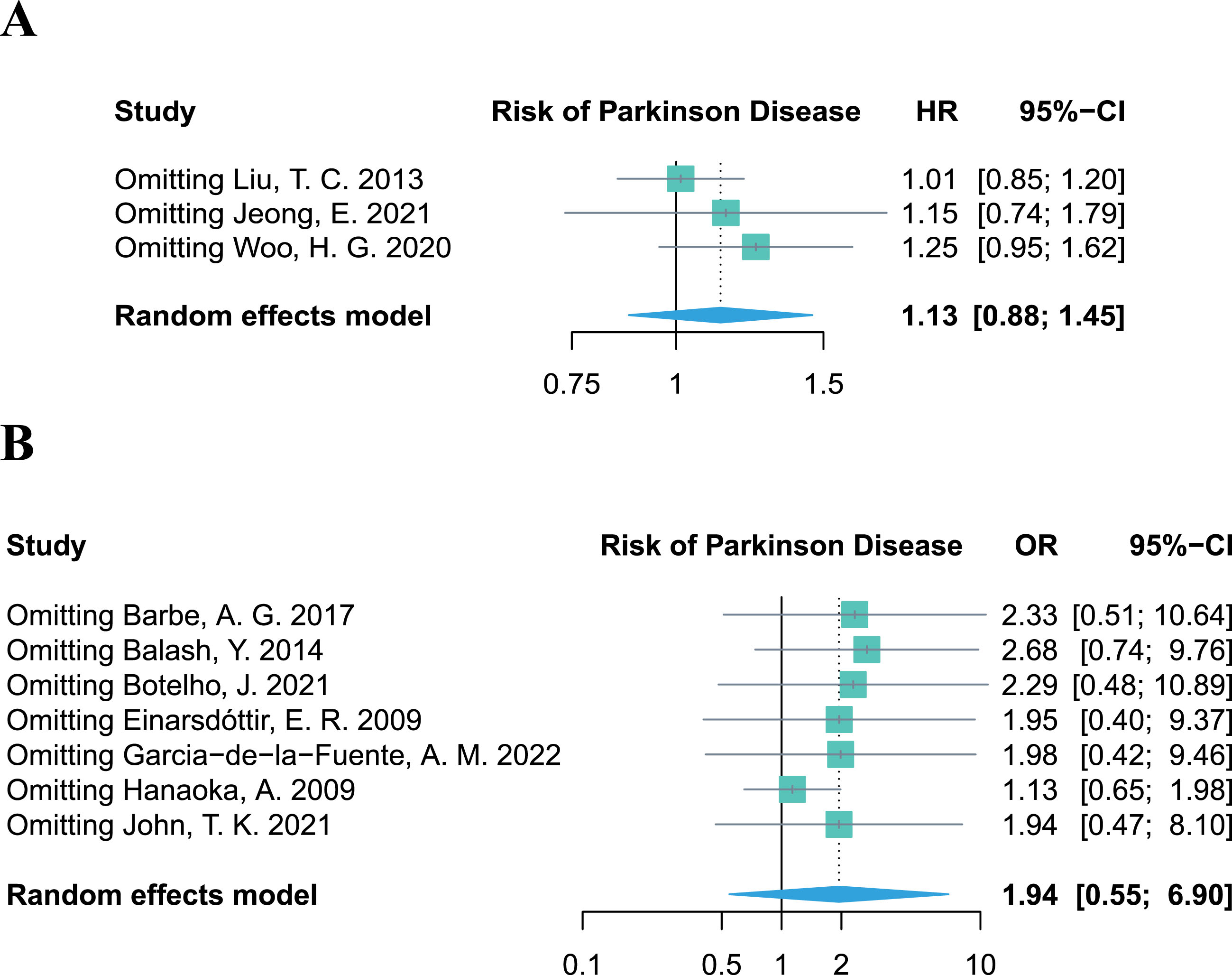
Sensitivity analysis
The leave-one-out sensitivity analysis revealed that no single study altered the pooled effect of HR and OR (Fig. 4A, B), proving the robustness of our results, which demonstrated no significant association between periodontitis and the risk of PD. Additional results of the sensitivity analyses on other outcomes (PPD, CAL, PI, and BoP%) are provided in the Supplementary Material.
Fig. 4
Leave-one-out sensitivity analysis of studies focusing on periodontitis and the risk of Parkinson’s disease.
Publication bias assessment
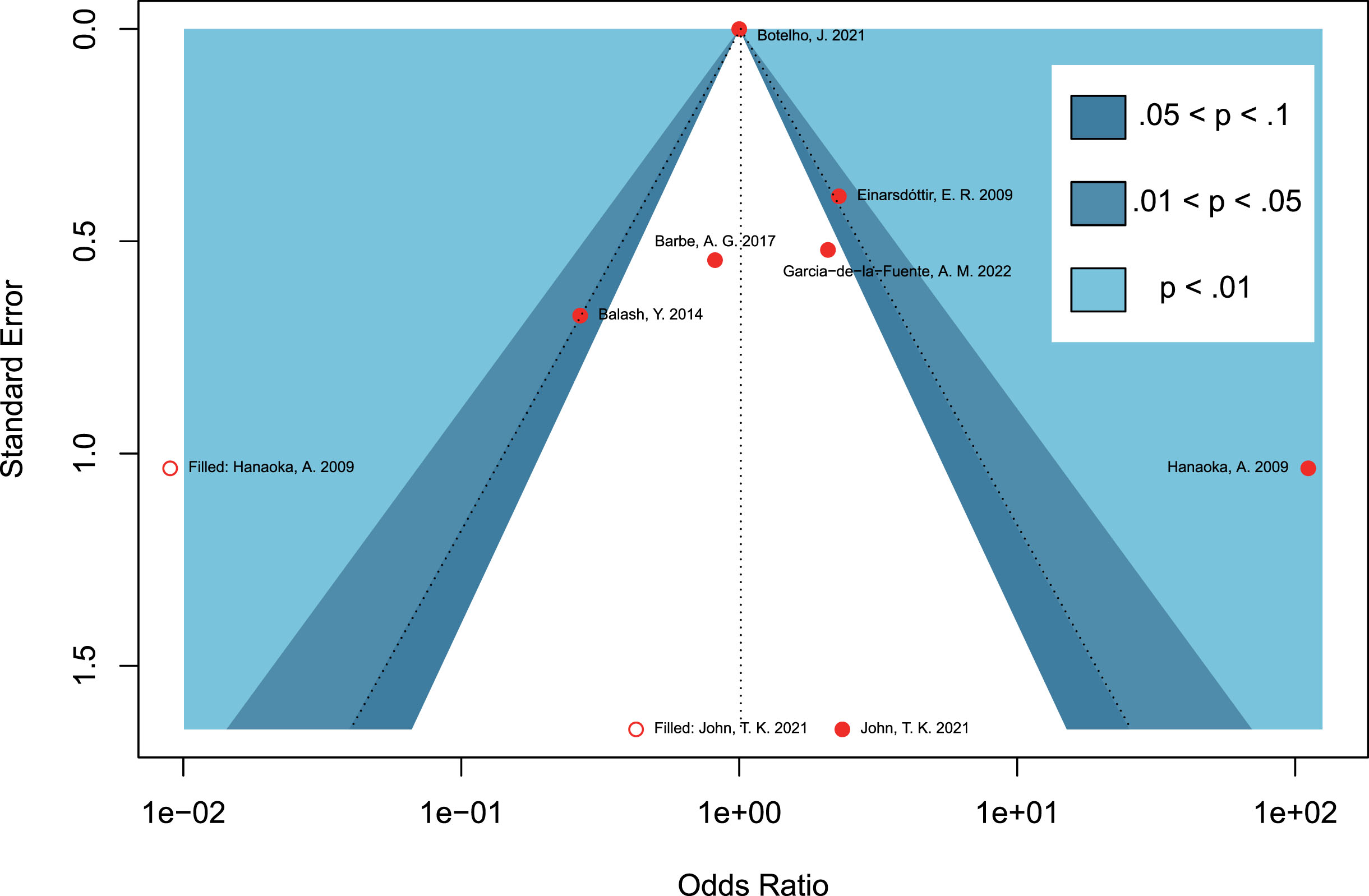
Due to the limited number of included studies for each outcome in the meta-analysis, a contour-enhanced funnel plot was only performed for the seven retrospective studies regarding periodontitis and the risk of PD. Asymmetry of the funnel plot was observed. In the contour-enhanced funnel plot (Fig. 5), the hollow dots filled by trim-and-fill approach depicted that one filled study was situated in sky-blue areas indicating high statistical significance (p < 0.01), whereas another filled study was located in white areas of no statistical significance. This discrepancy demonstrates that the potential publication bias may stem from the study conducted by John et al. [30]. However, even after excluding this study in the leave-one-out sensitivity analysis, the combined OR remained statistically insignificant (OR = 1.94, 95% CI 0.47–8.10, p = 0.3614; Fig. 4B), which further supports the stability of our results.
Fig. 5
Contour-enhanced funnel plots assessing the potential publication bias on studies concerning periodontitis and the risk of Parkinson’s disease.
DISCUSSION
The principal findings of our meta-analysis imply that patients with periodontitis do not tend to have a higher risk of developing PD, and that PD patients might not confer a higher risk of periodontitis. Nonetheless, it was observed that PD patients presented a higher prevalence of periodontitis and other periodontal diseases, as well as poorer oral hygiene.
Our findings, which demonstrate no association between periodontitis and PD risk, are in agreement with a prospective cohort study recruiting 15,528 patients [31]. This study illustrated that the risk of PD was not significantly associated with dental plaque whether in males (pooled HR = 1.23, 95% CI 0.81–1.86, p = 0.3309, I2 = 0%) or in females (pooled HR = 0.86, 95% CI 0.56–1.31, p = 0.4788, I2 = 0%). However, due to the heterogeneity of the outcomes (the presence of dental plaque but not confirmed periodontitis), this study was only included for the systematic review but not in the quantitative meta-analysis.
Previous studies have stressed the significant role of periodontitis in the pathogenesis of PD, demonstrating that periodontitis triggers the systemic translocation of bacteremia and inflammatory mediators, resulting in the breakdown of the blood-brain barrier and activation of microglia, eventually driving in necrosis and apoptosis of dopaminergic neurons and contributing to the mechanism of PD [32, 33].
In contrast to these viewpoints, we posit that the subsequent inflammatory processes may play a dominant role in the progression of PD, rather than periodontitis itself, with periodontitis serving as one of the numerous initiating factors of inflammation. Our hypotheses are supported by the following evidence.
As was reported in one of the included studies [16], although there were no differences in dental or periodontal parameters between 20 PD patients and 20 healthy controls, the elevated pro-inflammatory cytokine levels, such as interleukine-1β and tumor necrosis factor-α, were still observed in the gingival crevicular fluid of PD cases compared with controls. This reflected the presence of active peripheral inflammatory responses in the oral cavity of PD patients, irrespective of the presence of periodontitis, thus partially corroborating our assumptions.
Furthermore, a study [34] enrolling 37 PD patients reported an increase in the number of various inflammatory cells, including leukocytes, basophils, and segmented neutrophils, in PD cases complicated by periodontitis. Intriguingly, another study involving 51 PD participants described the levels of circulating high-sensitive C-reactive protein were increased in the periodontitis group, whereas the lymphocyte levels were elevated in the group without periodontitis [35]. However, these studies did not include a periodontitis control group without PD, which prevented the investigation of whether the rising levels of inflammatory cells and parameters were primarily attributed to PD or periodontitis itself. Thus, further studies are required to clarify the mediating role played by inflammatory factors in these two diseases.
Apart from that, previous research has emphasized the essential role of inflammation in the development of PD. It has been suggested that systemic inflammation events could induce neuronal death and contribute to the progression of neurodegenerative diseases [36]. Specifically, activated glial and peripheral immune cells could mediate neuroinflammatory processes which are deleterious to neurodegeneration and stimulates the death of dopaminergic cells [3]. Besides, inflammation appears to be the bridges connecting several chronic diseases with PD. For example, there are shared inflammation dysregulated pathways linking diabetes with PD [37]. Additionally, chronic kidney disease promotes PD development by exacerbating inflammation and oxidative stress via excessive activation of immune response caused by metabolic toxins [38]. Hence, further studies should be undertaken to investigate the extent to which inflammation plays a role in the progression of PD.
Intriguingly, it is worthwhile noting that our results regarding causal inference between PD and the periodontitis risk were primarily inferred from the MR study, since it was the sole study in the literature examining their association. However, MR study could only assist in confirming their weak association with genetic liability. As documented in the MR study, MR may be powerless in the presence of a strong environmental interaction and may not be able to elucidate the influence of a disease on the occurrence of another disease during a specific period of lifetime, thereby resulting in potentially inaccurate conclusions [26]. In line with this perspective, the result of the lack of association between PD and the risk of periodontitis should be interpreted with caution. Considering the higher prevalence of periodontitis and worse oral cavity health among PD patients, we speculate that it is the several environmental factors that mediate the spurious weak association between PD and periodontitis risk, and that these factors cannot be evaluated at the genetic level using an MR study. For instance, motor deficits in PD patients might be an influencing environmental factor. Indeed, the manifestation of motor impairment, hypokinesia, and involuntary movements in PD patients hinder their capability to maintain their daily oral hygiene, ultimately giving rise to poor oral health prognosis and deterioration in their quality of life [39]. Meanwhile, insufficient dental management induced by cognitive impairment may also be an environmental factor placing PD patients at risk for dental caries and periodontal diseases [15], as neglect of oral cavity disorders by PD cases, their caregivers and their professional physician has been reported [40]. Furthermore, other common symptoms of PD patients, like hyposmia, taste dysfunction [19], dysphagia [21], and masticatory disturbance [41], would contribute to longer meal times and more inadequate mastication, inducing a reduction in food intake quantity and quality, thereby causing nutritional imbalance and deterioration of oral health disorders in PD [15]. Last but not least, fluctuations in eating habits, such as preference for soft sticky foods [42] or sweets [43], would also conduce to the formation of cariogenic environments and subsequently lead to the occurrence of periodontal diseases [15]. In a nutshell, the aforementioned non-negligible environmental factors may potentially account for the high prevalence of periodontal diseases and poor oral health of PD patients, and more studies are necessitated to explore their effect.
The association between the risk of periodontitis and another degenerative disease, AD, has yielded inconsistent conclusions as well. In addition to a longitudinal study determining no statistical differences in several periodontal parameters between AD patients and healthy controls during 3 years of follow-up [44], no significant association between AD and periodontitis risk (RR = 1.4, 95% CI 0.9–2.1) was also reported by another study including 174 AD patients [45]. However, a population-based cohort study recruiting 8,640 individuals demonstrated an inverse association between AD and periodontitis risk (HR = 1.667, 95% CI 1.244–2.232, p = 0.0004) [46]. Hence, the relationship between AD and the risk of periodontitis also remains paradoxical and requires further investigation. Likewise, since our result on the weak association between PD and periodontitis risk may be biased and unreliable in our study, their casual association remains to be further investigated and clarified by additional well-designed and large-scale observational studies.
Our study possesses several strengths. To the best of our knowledge, this is the first systematic review and meta-analysis to comprehensively evaluate the bidirectional association between periodontitis and PD. Moreover, integrating the evidence from a Mendelian randomization study enhances the robustness and persuasiveness of our findings, since MR is widely recognized as providing higher-quality evidence compared to conventional observational studies by utilizing genetic variation as an instrumental variable to detect causality. Furthermore, contrary to previous theories, our intriguing findings provide no compelling evidence to support a bidirectional correlation between the incidence of the two diseases, neither in clinical manifestation nor in genetic liability [26], which may challenge traditional hypotheses concerning their potential relationship. Lastly, our study demonstrated that PD patients indeed suffer from poor oral hygiene, which has implications for guiding the management of dental diseases and improving oral care among PD patients.
Some potential limitations of our study should also be acknowledged. First of all, high heterogeneity was detected in our results, which can be chiefly attributed to variations in diagnostic criteria for periodontitis and measurements of outcomes. The lack of consensus on the definitions of periodontitis, discrepancies in periodontal examination methods, and the heterogeneity in dental conditions pose challenges in estimating the actual prevalence of periodontitis [47] while these factors were also an important source of high heterogeneity in our results and the asymmetry of the funnel plot. Thus, caution should be exercised in interpreting our results due to the existing heterogeneity. However, sensitivity analysis and contour-enhanced funnel plots supported the stability of our findings, reinforcing the credibility of our results. Secondly, as previously mentioned, the weak association between PD and the risk of periodontitis may be unreliable and should be interpreted with caution. This is primarily due to only one MR study addressing this topic while large-scale cohort studies focusing on the risk of periodontitis in PD patients with long-term follow-up are scarce, which also prevented an accurate reviewing and assessment of the incidence of periodontitis in PD cases. Finally, despite the limited number of included studies, grey literature databases comprising unpublished articles were not searched, given the uncertainty in their level of evidence quality. Despite these limitations, our study comprehensively evaluated the association between PD and periodontitis and provide profiles of the oral status of PD patients, which could aid dentists and specialist physicians in formulating considerate dental care strategies for PD patients to optimize their overall quality of life.
Conclusion
Our meta-analysis demonstrated that there might be no bidirectional association between periodontitis risk and PD risk, while PD patients tend to suffer from poor oral health.
ACKNOWLEDGMENTS
We appreciate the efforts of all the authors for this article. We also thank Home for Researchers editorial team (www.home-for-researchers.com) for language editing service.
FUNDING
This study was supported by the National Natural Science Foundation of China (No. 82302081) and the Fundamental Research Funds for the Central Universities (No. 226-2023-0067).
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The datasets generated during and/or analyzed during the current study are available upon reasonable request from the corresponding author.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230059.
REFERENCES
[1] | GBD 2016 Parkinson’ s Disease Collaborators ((2018) ) Global, regional, and national burden of Parkinson’s disease, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17: , 939–953. |
[2] | GBD 2016 Neurology Collaborators ((2019) ) Global, regional, and national burden of neurological disorders, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18: , 459–480. |
[3] | Hirsch EC , Hunot S ((2009) ) Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol 8: , 382–397. |
[4] | Tansey MG , Romero-Ramos M ((2019) ) Immune system responses in Parkinson’s disease: Early and dynamic. Eur J Neurosci 49: , 364–383. |
[5] | Darveau RP ((2010) ) Periodontitis: A polymicrobial disruption of host homeostasis. Nat Rev Microbiol 8: , 481–490. |
[6] | Kinane DF , Stathopoulou PG , Papapanou PN ((2017) ) Periodontal diseases. Nat Rev Dis Primers 3: , 17038. |
[7] | Papapanou PN , Susin C ((2017) ) Periodontitis epidemiology: Is periodontitis under-recognized, over-diagnosed, or both? Periodontol 2000 75: , 45–51. |
[8] | Duval S , Tweedie R ((2000) ) Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56: , 455–463. |
[9] | Persson GR ((2018) ) Periodontal complications with age. Periodontol 2000 78: , 185–194. |
[10] | Liccardo D , Cannavo A , Spagnuolo G , Ferrara N , Cittadini A , Rengo C , Rengo G ((2019) ) Periodontal disease: A risk factor for diabetes and cardiovascular disease. Int J Mol Sci 20: , 1414. |
[11] | Leira Y , Dominguez C , Seoane J , Seoane-Romero J , Pias-Peleteiro JM , Takkouche B , Blanco J , Aldrey JM ((2017) ) Is periodontal disease associated with Alzheimer’s disease? A systematic review with meta-analysis. Neuroepidemiology 48: , 21–31. |
[12] | Nwizu N , Wactawski-Wende J , Genco RJ ((2020) ) Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontol 2000 83: , 213–233. |
[13] | Chen CK , Huang JY , Wu YT , Chang YC ((2018) ) Dental scaling decreases the risk of Parkinson’s disease: A nationwide population-based nested case-control study. Int J Environ Res Public Health 15: , 1587. |
[14] | Garcia-de-la-Fuente AM , Lafuente-Ibanez-de-Mendoza I , Lartitegui-Sebastian MJ , Marichalar-Mendia X , Echebarria-Goikouria MA , Aguirre-Urizar JM ((2022) ) Facts and controversies regarding oral health in Parkinson’s disease: A case-control study in Spanish patients. Medicina Oral Patologia Oral Cirugia Bucal 27: , E419–E425. |
[15] | Auffret M , Meuric V , Boyer E , Bonnaure-Mallet M , Verin M ((2021) ) Oral health disorders in Parkinson’s disease: More than meets the eye. J Parkinsons Dis 11: , 1507–1535. |
[16] | Fleury V , Zekeridou A , Lazarevic V , Gaïa N , Giannopoulou C , Genton L , Cancela J , Girard M , Goldstein R , Bally JF , Mombelli A , Schrenzel J , Burkhard PR ((2021) ) Oral dysbiosis and inflammation in Parkinson’s disease. J Parkinsons Dis 11: , 619–631. |
[17] | van Wamelen DJ , Leta V , Johnson J , Ocampo CL , Podlewska AM , Rukavina K , Rizos A , Martinez-Martin P , Chaudhuri KR ((2020) ) Drooling in Parkinson’s disease: Prevalence and progression from the non-motor international longitudinal study. Dysphagia 35: , 955–961. |
[18] | Rana AQ , Yousuf MS , Awan N , Fattah A ((2012) ) Impact of progression of Parkinson’s disease on drooling in various ethnic groups. Eur Neurol 67: , 312–314. |
[19] | Shah M , Deeb J , Fernando M , Noyce A , Visentin E , Findley LJ , Hawkes CH ((2009) ) Abnormality of taste and smell in Parkinson’s disease. Parkinsonism Relat Disord 15: , 232–237. |
[20] | Doty RL ((2012) ) Olfactory dysfunction in Parkinson disease. Nat Rev Neurol 8: , 329–339. |
[21] | Argolo N , Nobrega AC ((2013) ) Dysphagia complaint and gender in Parkinson’s disease. Eur J Neurol 20: , e42. |
[22] | DeBowes SL , Tolle SL , Bruhn AM ((2013) ) Parkinson’s disease: Considerations for dental hygienists. Int J Dent Hyg 11: , 15–21. |
[23] | Fukayo S , Nonaka K , Shimizu T , Yano E ((2003) ) Oral health of patients with Parkinson’s disease: Factors related to their better dental status. Tohoku J Exp Med 201: , 171–179. |
[24] | Chen CK , Wu YT , Chang YC ((2017) ) Periodontal inflammatory disease is associated with the risk of Parkinson’s disease: A population-based retrospective matched-cohort study. , e. PeerJ 5: , 3647. |
[25] | Jeong E , Park JB , Park YG ((2021) ) Evaluation of the association between periodontitis and risk of Parkinson’s disease: A nationwide retrospective cohort study. Sci Rep 11: , 16594. |
[26] | Botelho J , Machado V , Mendes JJ , Mascarenhas P ((2021) ) Causal association between periodontitis and Parkinson’s disease: A bidirectional mendelian randomization study. Genes (Basel) 12: , 772. |
[27] | Skrivankova VW , Richmond RC , Woolf BAR , Yarmolinsky J , Davies NM , Swanson SA , VanderWeele TJ , Higgins JPT , Timpson NJ , Dimou N , Langenberg C , Golub RM , Loder EW , Gallo V , Tybjaerg-Hansen A , Davey Smith G , Egger M , Richards JB ((2021) ) Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 326: , 1614–1621. |
[28] | Peters JL , Sutton AJ , Jones DR , Abrams KR , Rushton L ((2008) ) Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 61: , 991–996. |
[29] | Page MJ , McKenzie JE , Bossuyt PM , Boutron I , Hoffmann TC , Mulrow CD , Shamseer L , Tetzlaff JM , Akl EA , Brennan SE , Chou R , Glanville J , Grimshaw JM , Hrobjartsson A , Lalu MM , Li T , Loder EW , Mayo-Wilson E , McDonald S , McGuinness LA , Stewart LA , Thomas J , Tricco AC , Welch VA , Whiting P , Moher D ((2021) ) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372: , n71. |
[30] | John TK , Vasanthy B , Madhavanpillai BR , Gomez MS , Kuriakose R ((2021) ) Does parkinsonism affect periodontal health? A cross-sectional study in a tertiary hospital. J Indian Soc Periodontol 25: , 538–543. |
[31] | Liu Z , Roosaar A , Axéll T , Ye W ((2017) ) Tobacco use, oral health, and risk of Parkinson’s disease. Am J Epidemiol 185: , 538–545. |
[32] | Alvarenga MOP , Frazao DR , de Matos IG , Bittencourt LO , Fagundes NCF , Rosing CK , Maia LC , Lima RR ((2021) ) Is there any association between neurodegenerative diseases and periodontitis? A systematic review. Front Aging Neurosci 13: , 651437. |
[33] | Kaur T , Uppoor A , Naik D ((2016) ) Parkinson’s disease and periodontitis - the missing link? A review. Gerodontology 33: , 434–438. |
[34] | Botelho J , Lyra P , Proença L , Godinho C , Mendes JJ , Machado V ((2020) ) Relationship between blood and standard biochemistry levels with periodontitis in Parkinson’s disease patients: Data from the NHANES 2011-2012. J Pers Med 10: , 69. |
[35] | Lyra P , Botelho J , Machado V , Rota S , Walker R , Staunton J , Proenca L , Chaudhuri KR , Mendes JJ ((2022) ) Self-reported periodontitis and C-reactive protein in Parkinson’s disease: A cross-sectional study of two American cohorts. NPJ Parkinsons Dis 8: , 40. |
[36] | Cunningham C , Wilcockson DC , Campion S , Lunnon K , Perry VH ((2005) ) Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci 25: , 9275–9284. |
[37] | Santiago JA , Potashkin JA ((2013) ) Shared dysregulated pathways lead to Parkinson’s disease and diabetes. Trends Mol Med 19: , 176–186. |
[38] | Melendez-Flores JD , Estrada-Bellmann I ((2021) ) Linking chronic kidney disease and Parkinson’s disease: A literature review. Metab Brain Dis 36: , 1–12. |
[39] | Newadkar U , Khairnar S , Dodamani A , Newadkar R ((2017) ) Oral health issues and challenges in Parkinson’s disease. Int J Nutr Pharmacol Neurol Dis 7: , 54–59. |
[40] | Zlotnik Y , Balash Y , Korczyn AD , Giladi N , Gurevich T ((2015) ) Disorders of the oral cavity in Parkinson’s disease and parkinsonian syndromes. Parkinsons Dis 2015: , 379482. |
[41] | Ribeiro GR , Campos CH , Rodrigues Garcia RCM ((2017) ) Parkinson’s disease impairs masticatory function. Clin Oral Investig 21: , 1149–1156. |
[42] | Müller T , Palluch R , Jackowski J ((2011) ) Caries and periodontal disease in patients with Parkinson’s disease. Spec Care Dentist 31: , 178–181. |
[43] | Kennedy MA , Rosen S , Paulson GW , Jolly DE , Beck FM ((1994) ) Relationship of oral microflora with oral health status in Parkinson’s disease. Spec Care Dentist 14: , 164–168. |
[44] | Ship JA , Puckett SA ((1994) ) Longitudinal study on oral health in subjects with Alzheimer’s disease. J Am Geriatr Soc 42: , 57–63. |
[45] | Syrjala AM , Ylostalo P , Ruoppi P , Komulainen K , Hartikainen S , Sulkava R , Knuuttila M ((2012) ) Dementia and oral health among subjects aged 75 years or older. Gerodontology 29: , 36–42. |
[46] | Ma KS , Hasturk H , Carreras I , Dedeoglu A , Veeravalli JJ , Huang JY , Kantarci A , Wei JC ((2022) ) Dementia and the risk of periodontitis: A population-based cohort study. J Dent Res 101: , 270–277. |
[47] | Holtfreter B , Albandar JM , Dietrich T , Dye BA , Eaton KA , Eke PI , Papapanou PN , Kocher T , Joint EU/USA Periodontal Epidemiology Working Group ((2015) ) Standards for reporting chronic periodontitis prevalence and severity in epidemiologic studies: Proposed standards from the Joint EU/USA Periodontal Epidemiology Working Group. J Clin Periodontol 42: , 407–412. |
[48] | Ledwon B , Miskiewicz A , Grabowska E , Kowalski J , Gorska R ((2020) ) The relationship between periodontal disease and motor impairment in the course of Parkinson’s disease. Postepy Higieny I Medycyny Doswiadczalnej 74: , 340–347. |
[49] | Liu TC , Sheu JJ , Lin HC , Jensen DA ((2013) ) Increased risk of parkinsonism following chronic periodontitis: A retrospective cohort study. Mov Disord 28: , 1307–1308. |
[50] | Woo HG , Chang Y , Lee JS , Song TJ ((2020) ) Association of tooth loss with new-onset Parkinson’s disease: A nationwide population-based cohort study. Parkinsons Dis 2020: , 4760512. |
[51] | Auerbacher M , Gebetsberger L , Kaisarly D , Schmidmaier R , Hickel R , Drey M ((2023) ) Oral health in patients with neurodegenerative and cerebrovascular disease: A retrospective study. Disabil Rehabil 45: , 2316–2324. |
[52] | Bakke M , Larsen SL , Lautrup C , Karlsborg M ((2011) ) Orofacial function and oral health in patients with Parkinson’s disease. Eur J Oral Sci 119: , 27–32. |
[53] | Balash Y , Peretz C , Rozenberg A , Zlotnik Y , Ezra A , Rabinovich A , Giladi N , Gurevich T ((2014) ) Chronic inflammatory gingival disease in patients with Parkinson’s disease. Mov Disord 29: , S336–S337. |
[54] | Barbe AG , Heinzler A , Derman S , Hellmich M , Timmermann L , Noack MJ ((2017) ) Hyposalivation and xerostomia among Parkinson’s disease patients and its impact on quality of life. Oral Dis 23: , 464–470. |
[55] | Cicciù M , Risitano G , Lo Giudice G , Bramanti E ((2012) ) Periodontal health and caries prevalence evaluation in patients affected by Parkinson’s disease. Parkinsons Dis 2012: , 541908. |
[56] | Einarsdóttir ER , Gunnsteinsdóttir H , Hallsdóttir MH , Sveinsson S , Jónsdóttir SR , Olafsson VG , Bragason TH , Saemundsson SR , Holbrook WP ((2009) ) Dental health of patients with Parkinson’s disease in Iceland. Spec Care Dentist 29: , 123–127. |
[57] | Hanaoka A , Kashihara K ((2009) ) Increased frequencies of caries, periodontal disease and tooth loss in patients with Parkinson’s disease. J Clin Neurosci 16: , 1279–1282. |
[58] | Nakayama Y , Washio M , Mori M ((2004) ) Oral health conditions in patients with Parkinson’s disease. J Epidemiol 14: , 143–150. |
[59] | Persson M , Osterberg T , Granérus AK , Karlsson S ((1992) ) Influence of Parkinson’s disease on oral health. Acta Odontol Scand 50: , 37–42. |
[60] | Schwarz J , Heimhilger E , Storch A ((2006) ) Increased periodontal pathology in Parkinson’s disease. J Neurol 253: , 608–611. |
[61] | van Stiphout MAE , Marinus J , van Hilten JJ , Lobbezoo F , de Baat C ((2018) ) Oral health of Parkinson’s disease patients: A case-control study. Parkinsons Dis 2018: , 9315285. |
[62] | Barbe AG , Bock N , Derman SH , Felsch M , Timmermann L , Noack MJ ((2017) ) Self-assessment of oral health, dental health care and oral health-related quality of life among Parkinson’s disease patients. Gerodontology 34: , 135–143. |
[63] | Gardner J , Kuyper D , Madden M , Wielinski C ((2013) ) Dental health of patients and carepartners in Parkinson’s disease. J Parkinsons Dis 3: , 90. |
[64] | Gopalakrishnan T , Mastan KMK , Mouli PEC , Guntuku NL , Priyadharshini A , Sangeetha GF ((2021) ) Evaluation of oral manifestations of patients with parkinson’s disease–an observational study. Indian J Forensic Med Toxicol 15: , 1424–1429. |
[65] | Lyra P , Machado V , Proença L , Domingos J , Godinho C , Mendes JJ , Botelho J ((2020) ) Parkinson’s disease, periodontitis and patient-related outcomes: A cross-sectional study. Medicina (Kaunas) 56: , 383. |
[66] | Pradeep AR , Singh SP , Martande SS , Raju AP , Rustagi T , Suke DK , Naik SB ((2015) ) Clinical evaluation of the periodontal health condition and oral health awareness in Parkinson’s disease patients. Gerodontology 32: , 100–106. |
[67] | Purisinsith S , Kanjanabuch P , Puapatanakul P , Phannajit J , Johnson DW , Robinson B , Kanjanabuch T ((2021) ) Oral health-related quality of life (OHRQoL) in PD: A preliminary result from Thailand PDOPPS. Nephrology 26: , 24–27. |
[68] | Ribeiro GR , Campos CH , Garcia RC ((2016) ) Oral health in elders with Parkinson’s disease. Braz Dent J 27: , 340–344. |
[69] | Silva PF , Biasotto-Gonzalez DA , Motta LJ , Silva SM , Ferrari RA , Fernandes KP , Bussadori SK ((2015) ) Impact in oral health and the prevalence of temporomandibular disorder in individuals with Parkinson’s disease. J Phys Ther Sci 27: , 887–891. |
[70] | Verhoeff MC , Lobbezoo F , van Leeuwen AM , Schuller AA , Koutris M ((2022) ) Oral health-related quality of life in patients with Parkinson’s disease. J Oral Rehabil 49: , 398–406. |




