Can Digital Mobility Assessment Enhance the Clinical Assessment of Disease Severity in Parkinson’s Disease?
Abstract
Background:
Real-world walking speed (RWS) measured using wearable devices has the potential to complement the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS III) for motor assessment in Parkinson’s disease (PD).
Objective:
Explore cross-sectional and longitudinal differences in RWS between PD and older adults (OAs), and whether RWS was related to motor disease severity cross-sectionally, and if MDS-UPDRS III was related to RWS, longitudinally.
Methods:
88 PD and 111 OA participants from ICICLE-GAIT (UK) were included. RWS was evaluated using an accelerometer at four time points. RWS was aggregated within walking bout (WB) duration thresholds. Between-group-comparisons in RWS between PD and OAs were conducted cross-sectionally, and longitudinally with mixed effects models (MEMs). Cross-sectional association between RWS and MDS-UPDRS III was explored using linear regression, and longitudinal association explored with MEMs.
Results:
RWS was significantly lower in PD (1.04 m/s) in comparison to OAs (1.10 m/s) cross-sectionally. RWS significantly decreased over time for both cohorts and decline was more rapid in PD by 0.02 m/s per year. Significant negative relationship between RWS and the MDS-UPDRS III only existed at a specific WB threshold (30 to 60 s, β= – 3.94 points, p = 0.047). MDS-UPDRS III increased significantly by 1.84 points per year, which was not related to change in RWS.
Conclusion:
Digital mobility assessment of gait may add unique information to quantify disease progression remotely, but further validation in research and clinical settings is needed.
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurological disorder characterized by the cardinal motor symptoms of tremor, rigidity, and bradykinesia [1, 2]. The presence of these motor symptoms manifest as mobility impairments which are detrimental to health and quality of life [3]. Measuring and monitoring the impact of motor severity upon mobility in PD is challenging due to its heterogeneous nature. The Movement Disorder Society– Unified Parkinson’s Disease Rating Scale Part III (MDS-UPDRS III) is the clinical standard to rate motor severity [4]. However, the assessment is conducted episodically in person, is time consuming to administer, and may not reflect the fluctuating nature of PD.
Remote monitoring solutions may exist in the form of home-based smartphone assessments [5], which are based upon semi-structured activities that have their respective advantages. In contrast, walking speed and a battery of clinically relevant gait characteristics (collectively referred to as digital mobility outcomes, (DMOs)) [6] can be measured quantitatively and continuously in the real-world using digital health technology such as body worn sensors. Recent work has explored quantitative assessment of gait to complement clinical assessment of motor severity in PD. Gait impairment appears early, even in the prodromal period, and deteriorates over time [7–10]. Changes in discrete DMOs translate to impaired motor and cognitive function, and increased fall risk [11, 12]. These tools could be used to complement the existing clinical assessment of motor symptom severity in PD [13, 14], addressing some of the limitations of existing scales [6]. Early work demonstrates possible clinical utility. For example, real-world walking speed (RWS) may be sensitive to discriminating PD from older adults (OAs) [7], is able to quantify PD motor symptoms [15] and fall risk [16], and is responsive to medication state (ON/OFF) [17].
Despite the promise, widespread adoption of real-world gait as a clinical mobility endpoint has not yet reached the clinic or clinical trials. To achieve this, comprehensive technical and clinical validation is required [6, 18] to establish what information RWS (or other DMOs) can provide that complement existing clinical assessment. Specifically, whether RWS is sensitive to the presence and progression of PD independent of typical ageing [8]. While walking speed is related to motor disease severity in controlled, supervised testing [19–23], the relationship between RWS and MDS-UPDRS III is yet to be explored [23].
The aims of this study were to cross-sectionally characterize RWS in people with PD compared to a cohort of OAs without PD, and to determine whether RWS changes in PD more rapidly. We also aimed to explore the cross-sectional and longitudinal relationships between RWS and motor disease severity (using MDS-UPDRS III) in PD.
METHODS
Participants
This study was a combined cross-sectional and longitudinal study. Participants were recruited from the Incidence of Cognitive Impairment with Longitudinal Evaluation – GAIT (ICICLE-GAIT) study [7, 8, 24, 25]. The main objective of ICICLE-GAIT was to examine the utility of gait, as a surrogate marker of cognitive decline and falls in early PD. Recruitment took place between June 2009 and December 2011. Participants were diagnosed with idiopathic PD according to the UK Parkinson’s Disease Brain Bank criteria [26] by a movement disorders specialist and diagnosis was confirmed at each follow-up visit. Baseline exclusion criteria comprised: significant memory impairment (Mini-Mental State Exam (MMSE)<24) or a diagnosis of Parkinson’s disease dementia [27]; dementia with Lewy bodies; drug-induced parkinsonism; “vascular” parkinsonism; atypical parkinsonian disorders; poor command of English; or presence of any neurological (other than idiopathic PD), orthopedic, or cardiovascular conditions that severely impacted mobility. OAs had to be at least 60 years of age, walk independently without a walking aid, and have no substantial cognitive impairment or mood or movement disorder. Participants underwent clinical and real-world assessment at 18-, 36-, 54-, and 72-months following baseline assessment. Across all time points, we included 88 individual PD participants, from a total of 120, and 111 people from 184 OAs for whom data was available. ICICLE-GAIT was undertaken in accordance with the Declaration of Helsinki and was granted ethical approval from the Newcastle and North Tyneside Research Ethics Committee (Ref: 09/H0906/82). All participants provided written informed consent prior to assessment.
Demographical and clinical measures
Motor symptom severity was evaluated using the MDS-UPDRS part III (0–108) and H&Y stage (I– V). Participants were tested ‘ON’ medication, defined as within 1 h after PD medication.
Real-world gait assessment protocol
Real-world walking was monitored over seven consecutive days at each assessment as part of the ICICLE-GAIT study. Data from the 36-month assessment was chosen for the cross-sectional analysis as it provided the largest sample size (Table 1). Some participants were not assessed at 18 months, due to changes in the device used for monitoring. Longitudinal analysis included data from all time points. Each participant wore a tri-axial device (Axivity AX3, York, UK) (23.0×32.5×7.6 mm; weight: 11 grams, data collected at 100 Hz, range±8 g) and was asked to continue their normal routine. The device was attached over the fifth lumbar vertebra (L5) with a hydrogel adhesive (PALStickies, PAL Technologies, Glasgow, UK) and covered with Hypafixtrademark bandage. After seven days, participants removed the device and posted it back to the researcher [7].
Table 1
Clinical and demographic information of the ICICLE-GAIT cohort at 18-, 36-, 54-, and 72-months assessment timepoints
| 18 months | 36 months | 54 months | 72 months | |||||
| Group | PD | OA | PD | OA | PD | OA | PD | OA |
| n | 43 | 51 | 62 | 94 | 59 | 49 | 49 | 43 |
| Age (y) | 69±10 | 70±7 | 69±10 | 72±6 | 68±9 | 73±8 | 71±9 | 72±6 |
| Sex (Male / Female) | 31 / 12 | 27 / 24 | 40 / 22 | 44 / 50 | 39 / 20 | 28 / 24 | 35 / 14 | 26 / 17 |
| Height (meters) | 1.69±0.88 | 1.69±0.08 | 1.69±0.08 | 1.68±0.09 | 1.68±0.8 | 1.70±0.09 | 1.67±0.09 | 1.70±0.08 |
| Body Mass (kg) | 79±15 | 81±15 | 79±17 | 77±13 | 76±15 | 81±13 | 77±14* | 84±13 |
| MDS-UPDRS III (points) | 33±11 | - | 38±12.4 | - | 39.1±12.6 | - | 40.9±13.8 | - |
| Disease duration (y) | 7.90±4.69 | - | 8.77±4.02 | - | 10.36±4.31 | - | 12.01±4.5 | - |
| Hoehn and Yahr Stage | ||||||||
| I, n (%) | 5 (11%) | - | 1 (1%) | - | 1 (2%) | - | 0 (0%) | - |
| II, n (%) | 40 (85%) | - | 57 (90%) | - | 51 (86%) | - | 35 (70%) | - |
| III, n (%) | 2 (4%) | - | 6 (9%) | - | 7 (12%) | - | 12 (24%) | - |
| IV, n (%) | 0 (0%) | - | 0 (0%) | - | 0 (0%) | - | 3 (6%) | - |
| LEDD (mg/day) | 395±206 | - | 515±256 | - | 663±294 | - | 720±312 | - |
Data presented as mean±standard deviation. Bold highlight indicates significant difference between PD and OAs at specific time point. ‘-‘describes an empty field, due to data availability. MDS-UPDRS III = Movement Disorder Society – Unified Parkinson’s Disease Rating Scale – Part III. LEDD = Levodopa equivalent daily dosage.
We took a conservative approach and used a threshold of three steps (minimum bout length) to define a walking bout (WB) with a minimum resting period of 2.5 s between bouts [28]. Only participants with >3 days of collected data were included. Furthermore, we excluded all WBs <10 s from any analysis. This is because activity within these very short durations does not always reflect gait and previous research has shown that DMOs evaluated in shorter bouts are less accurate and are less able to discriminate between PD and OAs [7].
Real-world walking speed (RWS) estimation
RWS was calculated from the tri-axial raw accelerometer data from both devices using bespoke validated algorithms in MATLAB® R2018a (MathWorks, California, United States) [7]. The accelerometer data was first segmented into WBs as detailed in previous work [7]. Initial contact and final contact gait events were then estimated, which enabled calculation of step duration and step length, where RWS was defined as the ratio of step length to step duration [28]. RWS was quantified as the weekly mean, where we first calculated mean RWS within each WB and then calculated the mean RWS from all bouts in each day [7, 29].
Statistical analysis
Analyses are shown below corresponding to each study aim. Statistical analysis was completed using R (R Foundation for statistical computing, V4.02, Austria). For the linear regression and mixed effects models (MEMs), the estimate of association is a regression coefficient. Specifically, the β should be interpreted as a reduction in x points of the MDS-UPDS III per each 0.1 m/s increase in RWS.
1) Cross-sectional comparison of RWS in PD and OA
RWS within each WB duration threshold underwent assessment for normality, utilizing Shapiro-Wilkes testing. Subsequently, we applied either the T-test (parametric) or Wilcoxon-H test (non-parametric) as appropriate, to determine whether the weekly mean of RWS at each WB duration was significantly different between 62 PD participants and 94 OAs.
2) Longitudinal changes in RWS in PD compared to OA
Mixed effects linear models (MEMs) (‘lmer’ function in ‘lme4’ package) [30] were used to investigate change in RWS and MDS-UPDRS III in 88 PD participants, and change in RWS in 111 OAs. MEMs allow flexibility when dealing with the missing data and are in accordance with the Food and Drug Administration guidelines for dealing with missing data [31]. We included the assessment timepoint (in years), alongside sex and baseline age as fixed effects, and HY stage as an additional fixed effect for PD to account for potential confounding. To establish whether the annual rate of change in RWS, across the study duration, differed between OAs and PD participants, we modelled a group and time point of assessment interaction term, alongside sex, and baseline age with a random intercept for participant. Performance was assessed by calculating conditional R2, marginal R2 and confidence intervals. Conditional R2 considers the combined explanatory power of both fixed and random effects. Goodness of fit for the models was achieved by reviewing residuals, Q-Q plots with tests of dispersion, distribution and outliers, and residual vs. predicted plots.
3) Cross sectional relationship between RWS and MDS-UPDRS-III
Bivariate correlations and linear effects models (LEMs) (‘lm’ function in ‘lme4’ R package) [32] were applied to investigate the cross-sectional relationship between RWS and MDS-UPDRS III score. In the LEMs, we included sex and age as fixed effects. Model performance was assessed by adjusted R2 and confidence intervals. Diagnosis of goodness of fit for the LEMs was achieved by reviewing residuals vs. fitted, Q-Q, scale location and Cook’s distance plots. We identified an outlier in the analysis, thus we replicated the analysis with and without the outlier and it did not impact the findings, so the participant was included. Finally, a secondary analysis was performed on discrete thresholds of WB duration in both datasets (10 to 30 s, 30 to 60 s, >60 s) as defined in previous research [7, 29]. In the comparison of WB duration, we did not adjust for multiple comparisons, due to the exploratory nature of the analysis.
4) Longitudinal relationship between RWS and MDS-UPDRS III
We applied MEMs with data from 88 PD participants to investigate the longitudinal relationship between RWS and MDS-UPDRS III score. For our fixed effects, we included RWS, HY stage at each assessment point, sex, baseline age and an RWS*assessment time point interaction term. We also modelled a random intercept for the participants. Model performance was assessed as per the characterizing longitudinal RWS analysis (analysis 2 above).
RESULTS
Demographic and clinical data, as well as RWS aggregated across all bouts, are shown in Table 1.
1) Cross-sectional comparison of RWS in PD and OA
RWS was significantly different between PD and OAs at each time point and WB duration, excluding >60 s at the 54-month time point. For both cohorts the largest number of available WBs for analysis existed in short durations (10 to 30 s) and the lowest number of WBs were within long WB durations (>60 s). Differences between PD and OAs in the number of WBs undertaken per day, was dependent upon the time point and WB duration (Table 2).
Table 2
Characterization of Real-world walking speed (RWS) and the number of Walking Bouts (WBs) recorded per day across the study duration in people with PD and OAs. *36 months RWS data was utilized used as time point for cross-sectional analysis. For longitudinal analysis, we included RWS data from all time points (18 to 72 months)
| RWS (m/s) | ||||||||
| WB duration (s) | 18 months | *36 months | 54 months | 72 months | ||||
| PD | OA | PD | OA | PD | OA | PD | OA | |
| All >10 | 1.03±0.09 | 1.10±0.09 | 1.04±0.09 | 1.10±0.09 | 1.02±0.09 | 1.07±0.07 | 0.99±0.07 | 1.06±0.07 |
| 10 to 30 | 1.00±0.08 | 1.05±0.06 | 0.99±0.77 | 1.05±0.66 | 0.97±0.07 | 1.02±0.06 | 0.96±0.07 | 1.02±0.06 |
| 30 to 60 | 1.04±0.08 | 1.10±0.08 | 1.03±0.76 | 1.08±0.07 | 1.02±0.08 | 1.07 0.06 | 1.00±0.07 | 1.07±0.06 |
| >60 | 1.05±0.12 | 1.16±0.15 | 1.07±0.12 | 1.15±0.13 | 1.09±0.12 | 1.13±0.11 | 1.04±0.11 | 1.11±0.13 |
| Walking bouts per day (number) | ||||||||
| WB duration (s) | 18 months | 36 months | 54 months | 72 months | ||||
| PD | OA | PD | OA | PD | OA | PD | OA | |
| All >10 | 583±202 | 617±208 | 625±225 | 629±189 | 574±195 | 622±195 | 600±199 | 609±183 |
| 10 to 30 | 183±71 | 206±71 | 192±72 | 208±66 | 174±65 | 203±68 | 190±67 | 205±65 |
| 30 to 60 | 34±16 | 45±2 | 38±20 | 45±18 | 33±17 | 44±18 | 37±19 | 47±19 |
| >60 | 19±12 | 24±13 | 21±14 | 24±12 | 19±12 | 25±13 | 19±11 | 22±11 |
PD, Parkinson’s disease; OA, older adults; RWS, real-world walking speed; WB, walking bout. We report the mean and standard deviation (SD), for both RWS and walking bouts per day across each time point of the study duration in people with PD and OAs. If value highlighted in bold, indicates statistically significant difference between PD and OA at that time point.
Cross-sectionally at 36 months, RWS was significantly lower in PD comparison to OAs (1.035 m/s vs. 1.097 m/s, p = 0.007) at all WBs and within each WB duration threshold (Table 2 – 36 months).
2) Longitudinal changes in RWS in PD compared to OA
Longitudinally, RWS significantly slowed in PD by 0.021 m/s (or 2 cm/s) per year (p = 0.014) and in OAs by 0.011 m/s (or 1 cm/s) per year (p = <0.001), when aggregated within WBs >10 s (Table 3). When analyzing RWS calculated within each WB threshold RWS slowed significantly at each WB duration, excluding long WBs (>60 s) in PD (Table 3). Rate of decline in RWS was larger in PD in comparison to OAs, at each WB duration threshold, excluding >60 s where we observed no difference with OAs (Table 3).
Table 3
Annual decline in RWS recorded in PD and Oas and the difference in annual decline between PD and Oas across the study duration, from 18 months to 72 months
| Annual Decline of RWS | Difference in annual decline of RWS | ||
| WB duration (s) | OA (β, 95% CI, P, R2) | PD (β, 95% CI, P, R2) | (β, 95% CI, P, R2) |
| All >10 | – 0.011, – 0.017, – 0.006 m/s,<0.001, 72% | – 0.021, – 0.037, – 0.004 m/s, 0.014, 55% | – 0.017, – 0.030, – 0.005 m/s, 0.006*, 39% |
| 10 to 30 | – 0.008, – 0.013, – 0.003 m/s, 0.001, 69% | – 0.013, – 0.020, – 0.006 m/s,<0.001, 71% | – 0.007, – 0.013, – 0.000 m/s, 0.036*, 67% |
| 30 to 60 | – 0.009, – 0.015, – 0.004 m/s, 0.001, 61% | – 0.014, – 0.021, – 0.008 m/s,<0.001, 76% | – 0.007, – 0.014, – 0.001 m/s, 0.035*, 64% |
| >60 | – 0.013, – 0.021, – 0.005 m/s, 0.001, 73% | – 0.004, – 0.014, 0.006 m/s, 0.466, 71% | – 0.000, (– 0.011, 0.011 m/s, 0.938, 69%) |
PD, Parkinson’s disease; OA, older adults; RWS, real-world walking speed; WB, walking bout. Estimated from a mixed linear regression model including 186 RWS measures from 85 PD participants and 240 RWS measures from 111 OA participants with age, sex and HY stage (in PD only), as covariates. Subject also a random effect to account for the correlation of measures of the same subject and a interaction term between follow up time (in years) and group (PD or OA). ‘*” – indicates significant difference in annual decline of RWS between PD and OAs.
3) Cross sectional relationship between RWS and MDS-UPDRS-III
At the 36-month time point of the ICICLE-GAIT dataset, there was no significant association with MDS-UPDRS III score with RWS at all WBs (β= 1.25 [95% CI = – 4.29, 1.78] points, p = 0.412).
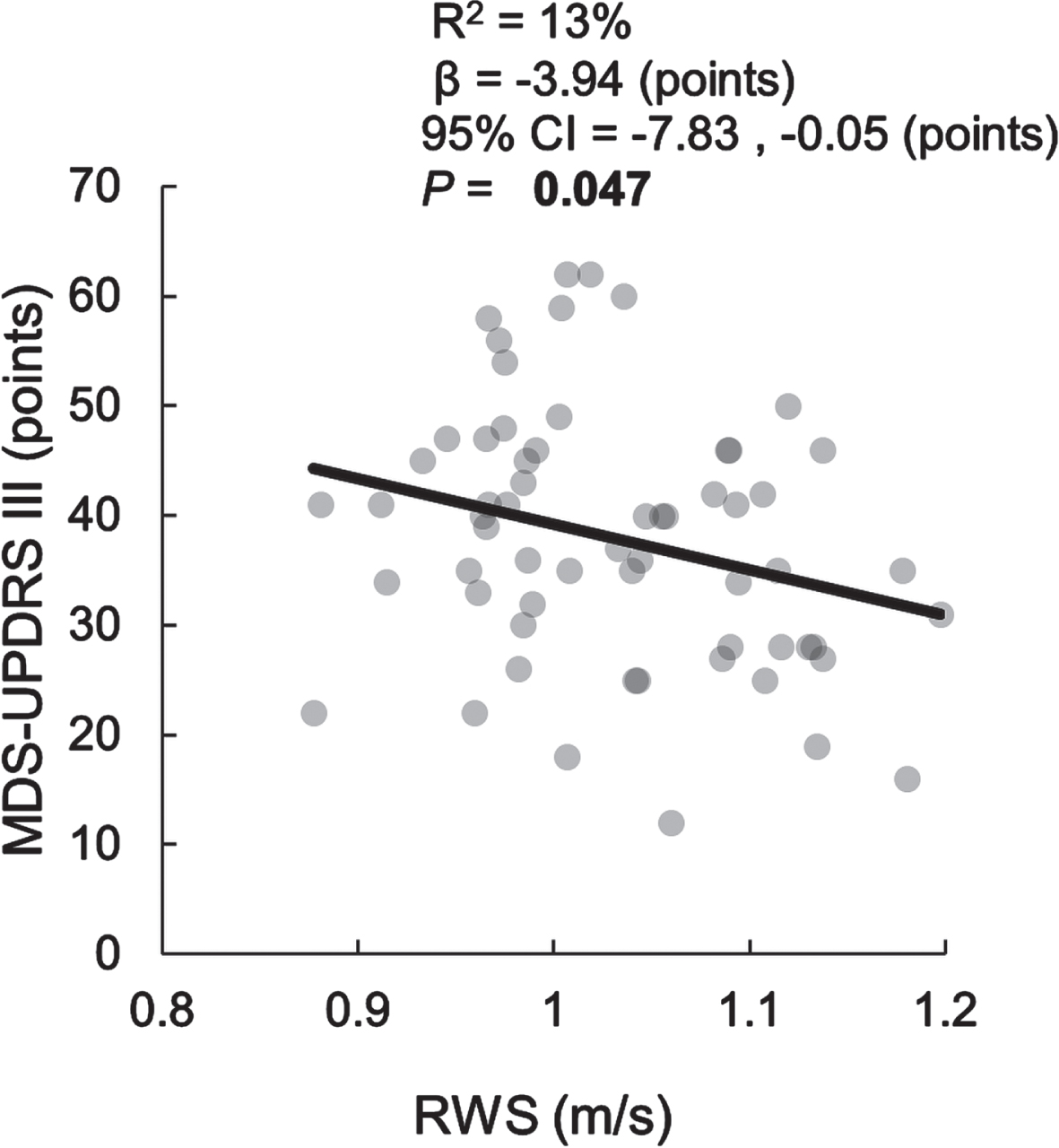
However, when calculating RWS within WB thresholds, we found a significant negative association with the MDS-UPDRS III at WBs between 30 to 60 s (β= – 3.94, [95% CI = – 7.83, – 0.05] points, p = 0.047) (Fig. 1).
Fig. 1
Cross-sectional relationship between MDS-UPDRS III and RWS for Walking Bouts between 30 and 60 seconds.
We did not observe any association between RWS and MDS-UPDRS III at ‘All WBs’ (β= – 1.36, [95% CI = – 4.71, 2.03] points, p = 0.42), or any WB duration, in the independent dataset.
4) Longitudinal relationship between RWS and MDS-UPDRS III
MDS-UPDRS III scores significantly increased by 1.86 [95% CI = 1.11, 2.61] points per year across the study duration. However, there was no association between change in RWS with changes in MDS-UPDRS III at all WBs and each other WB duration threshold (Table 4).
Table 4
Relationship between decline inRWS and change inMDS-UPDRS III score in PD participants for all WBs pooled and for each WB threshold. Adjusted for age, sex, and HY stage, according to WB duration
| WB duration (s) | Association of change in RWS with change in MDS-UPDRS III (β, 95% CI, P, Cd. R2) |
| WBs > 10 | 0.323, (–0.203, 0.850), 0.229, 70% |
| 10 to 30 | –0.223, (–1.121, 0.764), 0.657, 69% |
| 30 to 60 | –0.076, (–1.046, 0.888) 0.872, 69% |
| >60 & 0.141 | (–0.506, 0.789), 0.668, 71% |
DISCUSSION
This study provides a comprehensive exploration of RWS to understand whether it can provide information that is complementary to the clinical assessment of mobility, motor symptom severity, and progression in PD. Cross-sectionally, RWS was significantly slower in PD compared to OAs across a range of different WBs. RWS decreased in both cohorts and the reduction was generally more rapid in PD compared to OAs. Significant cross-sectional associations between motor symptom severity (MDS-UPDRS III) and RWS were seen for medium length WBs. MDS-UPDRS III scores increased annually; however, change in MDS-UPDRS III scores were not significantly associated with change in RWS longitudinally. Therefore, our findings highlight that remote monitoring may add complementary additional information to improve the clinical assessment of PD.
1) Cross-sectional comparison of RWS in PD and OA
RWS was significantly slower in PD in comparison to OAs, which is in agreement with previous research in the same cohort [7], plus the work of others [33]. This finding further validates how a slower RWS corresponds to real-world mobility impairments that occur in PD and are separate or interact with age-related changes. The challenges of modulating RWS to safely navigate complex real-world environments are likely exacerbated by presence of motor symptoms and fluctuations. Thus, real-world mobility measures such as RWS have potential to capture novel insights of PD in comparison to supervised assessments of capacity [34]. RWS reflects a complex measure of real-world mobility that has been assessed across a variety of WBs that differ in their duration, context, and purpose. For example, short WBs may capture more demanding activities such as obstacle negotiation, change in direction, gait initiation and termination. In contrast, longer WBs that require greater physical endurance may reflect steady-state gait and a more consistent gait pattern.
2) Longitudinal changes in RWS in PD compared to OA
RWS slowed in both PD and OAs over six years in the ICICLE-GAIT study. We found that RWS significantly reduced by 0.02 m/s more per year (all WBs) in PD compared to OAs. While there is lack of agreement of what constitutes a clinically meaningful difference in real-world DMOs, in a distribution-based analysis a change in supervised walking speed of 0.06 m/s has been shown to be a meaningful change in PD [35]. However, we would expect meaningful changes in RWS to be more sensitive and dependent upon WB duration [7, 17, 36]. Our findings were in contrast to our work in the same cohort in a laboratory setting [8] where the rate of walking speed decline did not significantly differ between OAs and PD. Thus, supervised laboratory assessments [37] may be a less sensitive measure of more rapid PD-specific deterioration of real-world mobility, reflected by differences in RWS.
Interestingly, we did not observe differences in rate of decline in RWS at long WBs. As time (and disease severity) progressed, the ability to walk for extended periods becomes more challenging and the number of longer walking bouts decreased. Long WBs reflect more optimal walking, where individuals may achieve performance close to that observed in supervised laboratory assessments, so this further supports the view that supervised laboratory assessment may be less sensitive to discrete changes in mobility. This is in agreement with previous research that found only the maximum values of RWS correlated with supervised walking speed [17] and further demonstrates how RWS can provide novel information to existing mobility assessment.
3) Cross sectional relationship between RWS and MDS-UPDRS-III
When considering all WBs >10 s, we found no cross-sectional association between RWS and motor severity in either dataset. This is in contrast to previous studies that found associations of the MDS-UPDRS III with laboratory walking speed; thus both measures were assessed in similar, supervised setting [19–22]. A The MDS-UPDRS III score assesses a wide range of symptoms within a brief clinical visit, which does include a gait-item in its assessment, which makes up a small proportion of the overall score (4 out of 108 points). In contrast, RWS reflects different contexts of mobility dependent upon the WB duration that it is estimated from. This is supported by our finding that RWS of medium length WBs (30–60 s duration) were associated with greater motor disease severity, in contrast to other WB durations. Short to medium length WBs may contain prolonged periods of navigating the household environment, or perhaps intermittent periods of outdoor walking which provide the optimal balance between periods of straight walking, while maintaining some challenge to motor control. These additional explorations of WBs are helpful as they may represent different contexts of mobility, and thus WB duration may moderate the relationship with RWS and disease severity [22, 38]. From our results, a faster RWS of 0.1 m/s was associated with less severe motor disease (equating to four points on the MDS-UPDRS III), which is between the range of minimally and moderate important difference of 2.7 to 5.2 points that has been previously reported [39].
4) Longitudinal relationship between RWS and MDS-UPDRS III
MDS-UPDRS III scores increased by 1.86 points per year, which suggests that after two years our cohort experienced a change above the threshold of minimally clinically important change [39], although note the reference was using a previous version of the UPDRS III (rated out of 108, [40]). Alongside increasing MDS-UPDRS III scores, RWS increased per year; however, we did not find an association between the two measures. This is not necessarily surprising, given that we compared changes in RWS, a complex measure of real-world mobility, with changes in the MDS-UPDRS III, a large composite score that assesses many upper and lower body signs, some of which are not directly related to gait (tremor, speech, etc.). Previous studies conducted in supervised, laboratory setting have found associations between walking speed and MDS-UPDRS III [14, 35]. Interestingly Hass et al. [35] found that a 0.02 m/s change in walking speed was associated with the minimally important change in MDS-UPDRS III score as reported by Shulman et al. [39]. However, they assessed walking speed across a short distance and duration, and participants were optimally medicated. MDS-UPDRS III scores have been demonstrated to reflect slower rates of progression within unmedicated compared to medicated groups [14]. Thus, RWS may only be associated with motor severity when assessed in a similar medication state [17]. Despite the lack of statistical association, both MDS-UPDRS III and RWS changed independently over time, which suggests that RWS may be able to capture additional insights into the impact of progression upon real-world mobility, that is not currently captured by the MDS-UPDRS III.
Clinical implications and future research
RWS could be deployed to remotely monitor aspects of PD which are not currently captured in routine clinical assessments. Such information would allow clinicians to target and manage aspects of mobility disability that are of utmost importance to people with PD, such as preservation of their walking ability [41, 42]. The ability to objectively evaluate patients remotely has significant advantages for both clinical research and clinical management. We anticipated that the relationship between RWS and the MDS-UPDRS III would be moderate at best due to the diverse nature of the clinical scale. The results support this, but also demonstrate that RWS is sensitive to change over time and thus may offer a supportive tool to monitor motor function remotely in PD. Walking in particular is challenging to manage and highlighted as of key importance by people with PD. The ability to detect change over time therefore makes this an important complimentary feature. Future research should explore the influence of longitudinal increases in medication dosage [8] and change in cognition [43] upon RWS.
Capturing longitudinal real-world data presents a number of technical and logistical challenges which are being addressed. Efforts are ongoing to improve the validity of outcomes, with more advanced wearable devices that contain gyroscope sensors, which enable the enhanced validity of measurement and repeat analyses, in larger cohorts [44]. In addition, the methods of data aggregation and summary metrics explored in this study offer a starting point, where future research could explore the optimal combination of aggregation values and summary metrics to capture RWS (such as extreme values etc.). Assessment of RWS in independent cohort studies would corroborate these findings.
Finally, continuous real-world gait outcomes such as RWS also may capture important additional information relating to fluctuating nature of disease, and further work is warranted to explore this topic. Further work is also required to establish whether relationships exist between RWS and additional clinical measures, such as MDS-UPDRS Part II or falls status and to establish the influence of changes in medication and cognition upon RWS. Real-world context is critical to our interpretation of RWS, as in the present study we inferred the context based upon only the WB duration. Specific types of real-world environments could also influence RWS, where inclusion of environmental data would improve our understanding of real-world data.
Limitations
Due to a lack of variation in H&Y stages, we did not include analysis of H&Y in this study (the majority were H&Y stage II); future studies with a greater variability in H&Y stages would be helpful to determine generalizability across disease stage and assess known groups’ validity. Participants were relatively early PD in both cohorts, and we did not have information on motor fluctuations. Compared to laboratory-based research, this study was relatively low in number. Future studies in larger cohorts of participants are needed. The possibility of a type two error was not directly explored, and this should be addressed in future studies.
Future research is also needed to understand whether the statistical power and possibility of a type two error is reduced due to the reduced number of datapoints at long WBs in particular. PD motor disease symptoms are associated with gait abnormalities such as a reduced stride length and step time, alongside a slower walking speed [11]. It could be speculated that these gait variables may present more sensitive representations of motor disease severity; therefore, other real-world DMOs could be evaluated in future research. Further optimization of algorithms and utilization of additional sensors (such as gyroscopes) could improve the relationships we report.
Conclusion
Assessment of real-world mobility using real-world walking speed as an exemplar shows potential to compliment monitoring of mobility in PD which is an important feature with clinical and research utility [41, 42]. Ongoing multidisciplinary efforts (such as Mobilise-D) [45] between academic, industrial and clinical partners are underway to address existing challenges and facilitate wide scale adoption of real-world mobility monitoring [6].
ACKNOWLEDGMENTS
The authors would like to thank all the participants and assessors of the ICICLE and ICICLE-GAIT study, Dr Rachael Lawson, and Dr Rosie Morris for their support.
FUNDING
The ICICLE-Gait study was supported by Parkinson’s UK (J-0802, G-1301) and by the National Institute for Health Research (NIHR) Newcastle Biomedical Research Centre (BRC) based at Newcastle Upon Tyne Hospital NHS Foundation Trust and Newcastle University. The work was also supported by the NIHR/Wellcome Trust Clinical Research Facility (CRF) infrastructure at Newcastle upon Tyne Hospitals NHS Foundation Trust. All opinions are those of the authors and not the funders.
This work is also supported by the Innovative Medicines Initiative 2 Joint Undertaking (IMI2 JU) project Mobilise-D that has received funding from the Innovative Medicines Initiative 2 Joint Undertaking (JU) under grant agreement No. 820820. This JU receives support from the European Union’s Horizon 2020 research and innovation program and the European Federation of Pharmaceutical Industries and Associations (EFPIA). Content in this publication reflects the authors’ view and neither IMI nor the European Union, EFPIA, or any Associated Partners are responsible for any use that may be made of the information contained herein.
CONFLICT OF INTEREST
Luca Palmerini is co-founder and owns shares of mHealth Technologies s.r.l. Authors report no other conflicts. Dr Daniela Berg is an Editorial Board Member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review.
DATA AVAILABILITY
The data supporting these findings is available on request from the corresponding author.
REFERENCES
[1] | Jankovic J ((2008) ) Parkinson’s disease: Clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79: , 368–376. |
[2] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W , Obeso J , Marek K , Litvan I , Lang AE , Halliday G , Goetz CG , Gasser T , Dubois B , Chan P , Bloem BR , Adler CH , Deuschl G ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30: , 1591–1601. |
[3] | Bechtold U , Stauder N , Fieder M ((2021) ) Let’s walk it: Mobility and the perceived quality of life in older adults. Int J Environ Res Public Health 18: , 11515. |
[4] | Goetz CG , Tilley BC , Shaftman SR , Stebbins GT , Fahn S , Martinez-Martin P , Poewe W , Sampaio C , Stern MB , Dodel R , Dubois B , Holloway R , Jankovic J , Kulisevsky J , Lang AE , Lees A , Leurgans S , LeWitt PA , Nyenhuis D , Olanow CW , Rascol O , Schrag A , Teresi JA , van Hilten JJ , La Pelle N , Movement Disorder Society UPDRS Revision Task Force ((2008) ) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 23: , 2129–2170. |
[5] | Arora S , Baig F , Lo C , Barber TR , Lawton MA , Zhan A , Rolinski M , Ruffmann C , Klein JC , Rumbold J , Louvel A , Zaiwalla Z , Lennox G , Quinnell T , Dennis G , Wade-Martins R , Ben-Shlomo Y , Little MA , Hu MT ((2018) ) Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder, controls, and PD. Neurology 91: , e1528–e1538. |
[6] | Rochester L , Mazza C , Mueller A , Caulfield B , McCarthy M , Becker C , Miller R , Piraino P , Viceconti M , Dartee WP , Garcia-Aymerich J , Aydemir AA , Vereijken B , Arnera V , Ammour N , Jackson M , Hache T , Roubenoff R ((2020) ) A roadmap to inform development, validation and approval of digital mobility outcomes: The Mobilise-D Approach. Digit Biomark 4: , 13–27. |
[7] | Del Din S , Godfrey A , Galna B , Lord S , Rochester L ((2016) ) Free-living gait characteristics in ageing and Parkinson’s disease: Impact of environment and ambulatory bout length. J Neuroeng Rehabil 13: , 46. |
[8] | Wilson J , Alcock L , Yarnall AJ , Lord S , Lawson RA , Morris R , Taylor J-P , Burn DJ , Rochester L , Galna B ((2020) ) Gait progression over 6 years in Parkinson’s disease: Effects of age, medication, and pathology. Front Aging Neurosci 12: , 577435. |
[9] | Galna B , Lord S , Burn DJ , Rochester L ((2015) ) Progression of gait dysfunction in incident Parkinson’s disease: Impact of medication and phenotype. Mov Disord 30: , 359–67. |
[10] | Del Din S , Yarnall AJ , Barber TR , Lo C , Crabbe M , Rolinski M , Baig F , Hu MT , Rochester L ((2020) ) Continuous real-world gait monitoring in idiopathic REM sleep behavior disorder. J Parkinsons Dis 10: , 283–299. |
[11] | Zanardi APJ , da Silva ES , Costa RR , Passos-Monteiro E , Dos Santos IO , Kruel LFM , Peyre-Tartaruga LA ((2021) ) Gait parameters of Parkinson’s disease compared with healthy controls: A systematic review and meta-analysis. Sci Rep 11: , 752. |
[12] | Lord S , Galna B , Yarnall AJ , Coleman S , Burn D , Rochester L ((2016) ) Predicting first fall in newly diagnosed Parkinson’s disease: Insights from a fall-naïve cohort. Mov Disord 31: , 1829–1836. |
[13] | Morris R , Lord S , Lawson RA , Coleman S , Galna B , Duncan GW , Khoo TK , Yarnall AJ , Burn DJ , Rochester L ((2017) ) Gait rather than cognition predicts decline in specific cognitive domains in early Parkinson’s disease. J Gerontol A Biol Sci Med Sci 72: , 1656–1662. |
[14] | Holden SK , Finseth T , Sillau SH , Berman BD ((2018) ) Progression of MDS-UPDRS scores over five years in de novo Parkinson disease from the Parkinson’s Progression Markers Initiative Cohort. Mov Disord Clin Pract 5: , 47–53. |
[15] | Samà A , Pérez-López C , Rodríguez-Martín D , Català A , Moreno-Aróstegui JM , Cabestany J , de Mingo E , Rodríguez-Molinero A ((2017) ) Estimating bradykinesia severity in Parkinson’s disease by analysing gait through a waist-worn sensor. Comput Biol Medi 84: , 114–123. |
[16] | Del Din S , Galna B , Godfrey A , Bekkers EMJ , Pelosin E , Nieuwhof F , Mirelman A , Hausdorff JM , Rochester L ((2019) ) Analysis of free-living gait in older adults with and without Parkinson’s disease and with and without a history of falls: Identifying generic and disease-specific characteristics. J Gerontol A Biol Sci Med Sci 74: , 500–506. |
[17] | Corrà MF , Atrsaei A , Sardoreira A , Hansen C , Aminian K , Correia M , Vila-Chã N , Maetzler W , Maia L ((2021) ) Comparison of laboratory and daily-life gait speed assessment during ON and OFF states in Parkinson’s disease. Sensors (Basel) 21: , 3974. |
[18] | Del Din S , Kirk C , Yarnall AJ , Rochester L , Hausdorff JM ((2021) ) Body-worn sensors for remote monitoring of Parkinson’s disease motor symptoms: Vision, state of the art, and challenges ahead. J Parkinsons Dis 11: (s1), S35–S47. |
[19] | Schlachetzki JCM , Barth J , Marxreiter F , Gossler J , Kohl Z , Reinfelder S , Gassner H , Aminian K , Eskofier BM , Winkler J , Klucken J ((2017) ) Wearable sensors objectively measure gait parameters in Parkinson’s disease, PLoS One 12: , e0183989. |
[20] | Raccagni C , Gassner H , Eschlboeck S , Boesch S , Krismer F , Seppi K , Poewe W , Eskofier BM , Winkler J , Wenning G , Klucken J ((2018) ) Sensor-based gait analysis in atypical parkinsonian disorders, Brain Behav 8: , e00977. |
[21] | Hill EJ , Mangleburg CG , Alfradique-Dunham I , Ripperger B , Stillwell A , Saade H , Rao S , Fagbongbe O , von Coelln R , Tarakad A , Hunter C , Dawe RJ , Jankovic J , Shulman LM , Buchman AS , Shulman JM ((2021) ) Quantitative mobility measures complement the MDS-UPDRS for characterization of Parkinson’s disease heterogeneity. Parkinsonism Relat Disord 84: , 105–111. |
[22] | Galperin I , Hillel I , Del Din S , Bekkers EMJ , Nieuwboer A , Abbruzzese G , Avanzino L , Nieuwhof F , Bloem BR , Rochester L , Della Croce U , Cereatti A , Giladi N , Mirelman A , Hausdorff JM ((2019) ) Associations between daily-living physical activity and laboratory-based assessments of motor severity in patients with falls and Parkinson’s disease. Parkinsonism Relat Disord 62: , 85–90. |
[23] | Polhemus A , Ortiz LD , Brittain G , Chynkiamis N , Salis F , Gaßner H , Gross M , Kirk C , Rossanigo R , Taraldsen K , Balta D , Breuls S , Buttery S , Cardenas G , Endress C , Gugenhan J , Keogh A , Kluge F , Koch S , Micó-Amigo ME , Nerz C , Sieber C , Williams P , Bergquist R , de Basea MB , Buckley E , Hansen C , Mikolaizak AS , Schwickert L , Scott K , Stallforth S , van Uem J , Vereijken B , Cereatti A , Demeyer H , Hopkinson N , Maetzler W , Troosters T , Vogiatzis I , Yarnall A , Becker C , Garcia-Aymerich J , Leocani L , Mazzà C , Rochester L , Sharrack B , Frei A , Puhan M ((2021) ) Walking on common ground: A cross-disciplinary scoping review on the clinical utility of digital mobility outcomes. NPJ Digit Med 4: , 149. |
[24] | Rochester L , Galna B , Lord S , Burn D ((2014) ) The nature of dual-task interference during gait in incident Parkinson’s disease. Neuroscience 265: , 83–94. |
[25] | Yarnall AJ , Breen DP , Duncan GW , Khoo TK , Coleman SY , Firbank MJ , Nombela C , Winder-Rhodes S , Evans JR , Rowe JB , Mollenhauer B , Kruse N , Hudson G , Chinnery PF , O’Brien JT , Robbins TW , Wesnes K , Brooks DJ , Barker RA , Burn DJ , ICICLE-PD Study Group ((2014) ) Characterizing mild cognitive impairment in incident Parkinson disease: The ICICLE-PD study. Neurology 82: , 308–316. |
[26] | Hughes A ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol 55: , 181–184. |
[27] | Emre M , Aarsland D , Brown R , Burn DJ , Duyckaerts C , Mizuno Y , Broe GA , Cummings J , Dickson DW , Gauthier S , Goldman J , Goetz C , Korczyn A , Lees A , Levy R , Litvan I , McKeith I , Olanow W , Poewe W , Quinn N , Sampaio C , Tolosa E , Dubois B ((2007) ) Clinical diagnostic criteria for dementia associated with Parkinson’s disease, Mov Disord 22: , 1689–1707; quiz 1837. |
[28] | Del Din S , Godfrey A , Rochester L ((2016) ) Validation of an accelerometer to quantify a comprehensive battery of gait characteristics in healthy older adults and Parkinson’s disease: Toward clinical and at home use. IEEE J Biomed Health Inform 20: , 838–847. |
[29] | Mc Ardle R , Del Din S , Donaghy P , Galna B , Thomas AJ , Rochester L ((2021) ) The impact of environment on gait assessment: Considerations from real-world gait analysis in dementia subtypes. Sensors (Basel) 21: , 813. |
[30] | Bates D ((2020) ) Package “lme4.”. |
[31] | Garcia TP , Marder K ((2017) ) Statistical approaches to longitudinal data analysis in neurodegenerative diseases: Huntington’s disease as a model. Curr Neurol Neurosci Rep 17: , 14. |
[32] | Pinheiro J ((2020) ) Package “nlme.”. |
[33] | Shah VV , McNames J , Mancini M , Carlson-Kuhta P , Spain RI , Nutt JG , El-Gohary M , Curtze C , Horak FB ((2020) ) Laboratory versus daily life gait characteristics in patients with multiple sclerosis, Parkinson’s disease, and matched controls. J Neuroeng Rehabil 17: , 159. |
[34] | Warmerdam E , Hausdorff JM , Atrsaei A , Zhou Y , Maetzler W ((2020) ) Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol 19: , 462–470. |
[35] | Hass CJ , Bishop M , Moscovich M , Stegemöller EL , Skinner J , Malaty IA , Wagle Shukla A , McFarland N , Okun MS ((2014) ) Defining the clinically meaningful difference in gait speed in persons with Parkinson disease. J Neurol Phys Ther 38: , 233–238. |
[36] | Hillel I , Gazit E , Nieuwboer A , Avanzino L , Rochester L , Cereatti A , Croce UD , Rikkert MO , Bloem BR , Pelosin E , Del Din S , Ginis P , Giladi N , Mirelman A , Hausdorff JM ((2019) ) Is every-day walking in older adults more analogous to dual-task walking or to usual walking? Elucidating the gaps between gait performance in the lab and during 24/7 monitoring. Eur Rev Aging Phys Act 16: , 6. |
[37] | Viceconti M , Tome M , Dartee W , Knezevic I , Hernandez Penna S , Mazzà C , Caulfield B , Garcia-Aymerich J , Becker C , Maetzler W , Troosters T , Sharrack B , Davico G , Corriol-Rohou S , Rochester L , the Mobilise-D Consortium ((2022) ) On the use of wearable sensors as mobility biomarkers in the marketing authorization of new drugs: A regulatory perspective. Front Med (Lausanne) 9: , 996903. |
[38] | Shah VV , McNames J , Harker G , Mancini M , Carlson-Kuhta P , Nutt JG , El-Gohary M , Curtze C , Horak FB ((2020) ) Effect of bout length on gait measures in people with and without Parkinson’s disease during daily life. Sensors (Basel) 20: , 5769. |
[39] | Shulman L ((2010) ) The clinically important difference on the Unified Parkinson’s Disease Rating Scale. JAMA Neurol 67: , 64–70. |
[40] | Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease ((2003) ) The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations. Mov Disord 18: , 738–750. |
[41] | Deane KHO , Flaherty H , Daley DJ , Pascoe R , Penhale B , Clarke CE , Sackley C , Storey S ((2014) ) Priority setting partnership to identify the top 10 research priorities for the management of Parkinson’s disease. BMJ Open 4: , e006434. |
[42] | Port RJ , Rumsby M , Brown G , Harrison IF , Amjad A , Bale CJ ((2021) ) People with Parkinson’s disease: What symptoms do they most want to improve and how does this change with disease duration? J Parkinsons Dis 11: , 715–724. |
[43] | Lawson RA , Williams-Gray CH , Camacho M , Duncan GW , Khoo TK , Breen DP , Barker RA , Rochester L , Burn DJ , Yarnall AJ ((2021) ) Which neuropsychological tests? Predicting cognitive decline and dementia in Parkinson’s disease in the ICICLE-PD Cohort. J Parkinsons Dis 11: , 1297–1308. |
[44] | Mikolaizak AS , Rochester L , Maetzler W , Sharrack B , Demeyer H , Mazzà C , Caulfield B , Garcia-Aymerich J , Vereijken B , Arnera V , Miller R , Piraino P , Ammour N , Gordon MF , Troosters T , Yarnall AJ , Alcock L , Gaßner H , Winkler J , Klucken J , Schlenstedt C , Watz H , Kirsten A-M , Vogiatzis I , Chynkiamis N , Hume E , Megaritis D , Nieuwboer A , Ginis P , Buckley E , Brittain G , Comi G , Leocani L , Helbostad JL , Johnsen LG , Taraldsen K , Blain H , Driss V , Frei A , Puhan MA , Polhemus A , Basea MB de , Gimeno E , Hopkinson NS , Buttery SC , Hausdorff JM , Mirelman A , Evers J , Neatrour I , Singleton D , Schwickert L , Becker C , Jansen C-P , clinical validation study (WP4)on behalf of Mobilise-D consortium ((2022) ) Connecting real-world digital mobility assessment to clinical outcomes for regulatory and clinical endorsement–the Mobilise-D study protocol, PLoSOne 17: , e0269615. |
[45] | Mobilise-D – Mobilise-D. https://www.mobilise-d.eu/ (accessed 16 November 2021). |




