Motor Memory Consolidation Deficits in Parkinson’s Disease: A Systematic Review with Meta-Analysis
Abstract
Background:
The ability to encode and consolidate motor memories is essential for persons with Parkinson’s disease (PD), who usually experience a progressive loss of motor function. Deficits in memory encoding, usually expressed as poorer rates of skill improvement during motor practice, have been reported in these patients. Whether motor memory consolidation (i.e., motor skill retention) is also impaired is unknown.
Objective:
To determine whether motor memory consolidation is impaired in PD compared to neurologically intact individuals.
Methods:
We conducted a pre-registered systematic review (PROSPERO: CRD42020222433) following PRISMA guidelines that included 46 studies.
Results:
Meta-analyses revealed that persons with PD have deficits in retaining motor skills (SMD = –0.17; 95% CI = –0.32, –0.02; p = 0.0225). However, these deficits are task-specific, affecting sensory motor (SMD = –0.31; 95% CI –0.47, –0.15; p = 0.0002) and visuomotor adaptation (SMD = –1.55; 95% CI = –2.32, –0.79; p = 0.0001) tasks, but not sequential fine motor (SMD = 0.17; 95% CI = –0.05, 0.39; p = 0.1292) and gross motor tasks (SMD = 0.04; 95% CI = –0.25, 0.33; p = 0.7771). Importantly, deficits became non-significant when augmented feedback during practice was provided, and additional motor practice sessions reduced deficits in sensory motor tasks. Meta-regression analyses confirmed that deficits were independent of performance during encoding, as well as disease duration and severity.
Conclusion:
Our results align with the neurodegenerative models of PD progression and motor learning frameworks and emphasize the importance of developing targeted interventions to enhance motor memory consolidation in PD.
INTRODUCTION
Parkinson’s disease (PD) is one of the most common neurodegenerative disorders and the number of persons with this clinical condition is expected to double by 2040 [1]. PD is a complex, heterogeneous, and progressive disorder, characterized by several motor and non-motor symptoms but its diagnosis is based on the onset of the cardinal motor features of the disease [2]. Pharmacological treatments are the first line of action to manage the motor symptoms of the disease but with time they tend to progressively lose efficacy [3]. As a result, patients with PD experience a relentless deterioration, leading to major motor dysfunctions, and eventually loss of autonomy [4]. Implementing non-pharmacological interventions to maintain the functional independence of these patients is thus important [5].
Motor rehabilitation helps patients with PD maintain the motor skills needed to function independently [6]. Motor learning, defined as the ability to acquire, adapt, and retain long-term skilled movements [7] is the base of motor rehabilitation [8]. Motor learning comprises motor memory encoding (i.e., acquisition) and consolidation (i.e., retention). Encoding, the on-line process during which sensory and motor information is acquired through deliberate or structured motor practice, is usually characterized by fast gains in skill performance during the initial phases of practice, followed by slower improvements (i.e., automatization) in later phases [9, 10]. Consolidation, in contrast, is the result of an off-line process lasting from several minutes to days, during which the sensory and motor information is used to form more stable and long-lasting motor memories that became less susceptible to interference [11–13]. Motor memory consolidation is inferred through either change in skill performance measured during retention tests, by the ability to generalize the acquired skills to other tasks assessed with transfer tests, or through savings (i.e., a more rapid rate of relearning at retention). Retention is clinically important because it reflects the permanent ability of the patients to perform a motor skill and not transient improvements in skill performance [7, 14].
Previous studies investigating deficits in motor memory consolidation in PD have reported inconsistent results [15–17]. Furthermore, the extent to which the task nature, amount of practice, type of feedback provided during motor practice, progression of the disease, and effect of antiparkinsonian medications moderate the capacity to consolidate motor skills has yet to be determined. This review, conducted following PRISMA guidelines [18] and registered in PROSPERO (CRD42020222433) [19], aimed to summarize the evidence regarding deficits in motor memory consolidation in people with PD relative to neurologically intact (NI) individuals. Determining to what extent people with PD have deficits in consolidation could stimulate the design of more targeted and effective motor rehabilitation therapies.
Table 1
Task nature classification
| Sensory Motor Tasks (SMTs) are acquired by learning novel movement kinematics and/or dynamics (e.g., muscle forces and joint coordination). These new motor routines are created by gathering sensory information using cognitive strategies, which are particularly important during the initial phase of learning. The main motor learning outcomes of SMTs are usually movement- (e.g., reaction and movement time) or accuracy-related (e.g., root mean square error-RMSE). SMTs have been shown to mainly engage the basal ganglia (i.e., striatum), cerebellum, and primary motor cortex as well as a core motor network [40] composed of several brain structures that are commonly involved during any form of motor learning. The visuomotor tracking tasks, pursuit rotary and ballistic motor tasks, as well as mirror-drawing and mirror-reversal adaptation tasks are considered SMTs [13, 25, 26, 40, 47]. |
| Sequential Fine Motor Tasks (SQTs) have minimal motor demands relative to other motor tasks and stress the sequential learning of motor behavior [40]. These tasks usually require pressing buttons/keys using fingers of one of both hands in sequential order to, for example, reproduce (as quickly and accurately as possible) and/or discover (a) given sequence(s). These simple actions constitute a group of discrete movements that together form a more complex movement acquired through stimulus-response mapping [26]. The main motor learning outcomes of SQTs are usually reaction time and accuracy, which are often combined to create a performance index. SQTs have been shown to engage brain regions and motor networks similar to those activated during SMTs but require a much higher involvement of cortical regions such as the left dorsal premotor cortex, supplementary motor cortex, and superior parietal cortex [40]. These cortical regions are even more engaged when the SQTs have to be learned explicitly [40]. Importantly, SQTs that implement higher-order sequence(s) also activate the medial temporal lobe, which effectively supports the higher temporal component of the task [48, 49]. Sequential movement using, for example, a response keypad, keyboard, or generic buttons, such as the serial reaction time task and its variations, as well as the m × n task are considered SQTs [25, 26, 50]. |
| Visuomotor Adaptation Tasks (VATs) require the learner to modify an already well-practiced motor behavior to respond to visual perturbations to maintain or regain the same levels of performance as before the introduction of the visual distortion. The motor system achieves this goal by updating existing motor programs rather than building entirely new motor routines. The main motor learning outcome of VATs is generally measured in form of directional error, defined as the angular difference between the target/goal movement and the actual movement. However, VATs can also involve changes in movement kinematics (e.g., reaction time and peak velocity). VATs have been shown to engage the cerebellum, basal ganglia (i.e., striatum), primary motor cortex, and fronto-parietal lobules as well as other sensorimotor-related cortical areas. Visuomotor tasks requiring a response to a visual perturbation are categorized as VATs [26, 51–54]. |
| Gross Motor Tasks (GMTs) involve the whole body and require the learner to maintain a stable position while performing different types of postural and balance tasks. Based on their characteristics and requirements [38], these postural/balance tasks are categorized as static, dynamic and reactive. The main motor learning outcomes of GMTs vary considerably, but normally they measure time in balance, reaction time, variables associated with CoP/CoM displacement (e.g., CoP velocity), and the root mean square error (RMSE), i.e., in whole-body tracking tasks. GMTs have been shown to engage the brainstem, cerebellum, basal ganglia, thalamus, and several cortical areas within the temporal, parietal and frontal lobes [38, 42] as well as different sensory systems (i.e., somatosensory, vestibular and visual systems) [55]. The frontal regions seem to play a critical role in GMTs, particularly in dynamic balance tasks, possibly supporting the development of task-specific strategies [56]. Gross postural/balance tasks involving the whole body were categorized as GMTs. |
| Speech Motor Tasks (SPTs) require the learner to build new motor commands to either produce novel nonsensical sequences of syllables or nonword phoneme sequences or to adapt already mastered speech motor schemes to efficiently respond to perturbations introduced in form of altered (auditory and/or somatosensory) feedback. SPTs can be classified as speech motor sequence learning or sensorimotor speech adaptation. Given that the (main) goal of speech is to produce acoustic or auditory signals that convey specific information [57], the main motor learning outcomes investigated in SPT studies are generally utterance duration, error rate (i.e., accuracy), reaction time [58], sequencing errors [45], voice amplitude, fundamental frequency, and articulatory movements (e.g., lip speed) [57]. SPTs have been shown to engage several areas of the brain associated with working memory, speech motor planning, as well as the basal-ganglia-thalamocortical loop, and the cerebellum. SPTs seem to be associated with the integrity of structural connectivity between the motor and sensory brain regions [44, 46, 59, 60]. |
CoM, center of mass; CoP, center of pressure.
METHODS
Eligibility criteria
The eligibility criteria of the studies included in the review were operationalized with the PECOS (population, exposure, comparator, outcomes, study design) framework [20, 21]. Population: participants with PD without other neurological comorbidities and not receiving deep brain stimulation [15]. Exposure: having idiopathic PD. Comparator: NI individuals of similar age. Outcomes: skill retention and transfer measured≥1 h following the end of practice to capture long-term change [7, 12]. Study design: observational studies with a PD and a NI group or interventional studies with a group of both PD patients and NI individuals who did not receive the intervention.
Search strategy
Two authors performed independently the electronic search on electronic databases (Web of Science, PubMed, Scopus, PsycINFO, SPORT Discuss) and screened the reference lists of relevant reviews [15–17, 22–32] as well as articles reviewed at the full-text level (see study selection section). The electronic search was neither language nor date restricted, but it was limited to peer-reviewed articles. The primary search was performed using the following three main terms and their variations: “Parkinson’s disease” (population), “healthy control” (comparator), and “motor learning” (outcome), combined with Boolean operators, and can be found in Supplementary Material 1. The final search was completed on December 2, 2021.
Study selection and data extraction
Two authors screened the list of titles and abstracts of articles retrieved in the search and selected potentially relevant articles for a more detailed review at full-text level. Following the screening of the articles, both authors held a meeting to compare their results. Disagreements were resolved by discussion including a third author.
Two authors extracted the following data from the studies: study design, number and characteristics of participants, characteristics of the motor task, as well as the outcomes and the endpoints used to assess motor learning. Means and standard deviations (SDs) of motor skill acquisition (i.e., encoding) and retention/transfer (i.e., consolidation) test scores were extracted. Subsequently, both authors compared their data to confirm that they were entered correctly.
When an article did not provide means and SDs and this information could be inferred from figures, data were extracted using a web-based tool (https://WebPlotDigitizer/). When this was not possible, the authors of the study were contacted. If data could still not be obtained, the study was not included in the quantitative meta-analysis and results were reported qualitatively.
Methodological quality assessment
Risk of bias at the study level was assessed by two authors that used the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [33]. This tool, which comprises 14 items, has shown good validity to assess the methodological quality of observational studies [34]. Given the design of the studies included in the review, items 10 (exposure repeatedly measured over time) and 12 (assessors blinded to the exposure) were scored as “not applicable” and not considered for evaluating study quality [33] (Supplementary Material 2). The two authors rated each item as “yes”, “no”, or “not reported”. All responses other than “yes” indicate a risk of bias. The number of “yes” responses was used to calculate a percentage score (i.e., number of “yes”/12 * 100) and categorize studies as “good” (≥90%), “fair” (≥70, but < 90), or “poor” (<70%) [33]. Sources of bias and heterogeneity were investigated with funnel plots and Egger’s regression tests [35–37].
Main analysis and influence of moderators
We grouped studies that assessed motor memory consolidation (i.e., skill retention) following a single session of practice or after extended practice (≥2 sessions) because retention tests following single and extended practice reflect different stages of the motor memory formation process [11]. Subgroup meta-analyses investigated whether the task nature influenced motor memory consolidation. To this end, studies were classified following well-established motor learning classifications [11, 13, 25, 26, 38–47] as sensory motor (SMT), sequential fine motor (SQT), visuomotor adaptation (VAT), gross motor (GMT), and speech motor (SPT) tasks (Table 1). The influence of augmented feedback [14, 15] was assessed by grouping studies based on whether extrinsic feedback was provided or not, and if so, which type: knowledge of results, performance, or both [14].
When at least 10 effect sizes were available [61], meta-regression was conducted to investigate the influence of different moderators and the interactions among them (e.g., disease duration * disease severity). Moderators included different features of PD [15] such as duration (i.e., years since diagnosis), severity of the disease (i.e., Hoehn and Yahr Score [62] and the motor score of the Unified Parkinson’s Disease Rating Scale (UPDRS) Part III Motor Examination) [63–65]. The moderating effect of the methodological quality of the studies (Supplementary Material 3) and, when possible, the effect of anti-parkinsonian medication (“on” vs. “off”), were also investigated (Supplementary Material 4).
Considering that differences in motor memory encoding (i.e., acquisition) between PD and NI groups [15, 23, 27, 32] could influence consolidation (i.e., retention), we also investigated its potential moderating effect using meta-regression and grouping studies that showed a significant improvement in skill performance during practice in favor of either NI individuals or PD patients, or that showed no difference between groups. We also explored deficits in skill transfer, which are reported separately in Supplementary Material 5.
When a study used different variations of the same motor task that still required similar motor and cognitive demands and it was possible to calculate multiple effect sizes, we pooled them together by creating a composite Z-score [66]. By contrast, when variations in the experimental conditions (e.g., blocked vs. random practice) were substantial, and thus potentially affecting the rate of encoding and/or consolidation [14, 67], we did not create a composite Z-score and treated the different conditions separately (Supplementary Material 6). All analyses were conducted using the primary outcome of the motor tasks.
Data analysis
Analyses were performed with R (https://www.r-project.org; version 4.0.3) using the packages meta, metafor, ggplot2, and robvis [61]. Data entered for each group included mean differences and pooled SD (SDpooled) for different endpoints, as well as the number of participants in each group. Data were analyzed as continuous variables using a random-effects model, the restricted maximum likelihood (REML), and the “Hedges” procedure method, to calculate the standardized mean difference (SMD) with a 95% confidence interval (CI) [61, 68].
To assess consolidation, we calculated the group mean difference in skill retention using end of practice and retention test scores (retention score –end of practice score) [14]. If end of practice scores could not be obtained, we used the scores of the retention test performed immediately after the end of acquisition [69]. Positive scores in the group mean difference in skill retention reflect successful consolidation. Similarly, skill acquisition (i.e., encoding) was calculated using the mean difference between scores obtained at baseline and the end of practice, either on a single session or multiple sessions (i.e., extended practice).
A p-value of less than 0.05 indicated statistical significance for an overall effect. Negative values represented worse skill consolidation for persons with PD in comparison with NI individuals. Heterogeneity was assessed with the I2 index, which was categorized as: low (0% to < 40%), moderate (≥40% to < 60%), substantial (≥60% to < 75%), or large (≥75%) [66] and its statistical significance was assessed with the Cochran’s Q test [61].
For meta-regression, we implemented the steps outlined by Harrer et al. (2019) [61] and followed the guidelines provided by Veroniki and colleagues [70]. Multicollinearity among predictors (r≥0.8) [61] was investigated using correlation matrices. To confirm the robustness of the meta-regression results and verify their true significance, we conducted permutation tests as described by Harrer et al. (2019) [61]. Permutation tests are a resampling method used to adjust the p-value of the meta-regression and thus control for type I error, which can be inflated when heterogeneity is present [71].
RESULTS
Articles retrieved
The stages of the search and review processes with the main reasons for exclusion can be found in Supplementary Material 7. The electronic search yielded 9,427 records but 59 additional studies from previous reviews were added. After removing duplicates, 4,237 abstracts were screened with 184 studies reviewed at full-text level. Ninety-five studies were excluded because they did not have a retention test, or the latter was assessed less than one hour after the end of practice (see eligibility criteria). Nineteen studies were excluded because they did not use an appropriate motor task/method to assess motor learning and thirteen studies used a study design that did not meet the inclusion criteria. Two studies used deep brain stimulation, one study included a control group with neurological conditions, and another study had a control group with an age that differed significantly from the PD group. Four studies were excluded due to the lack of a control/PD group and four studies were excluded for other reasons (e.g., duplicated data). After identifying 45 studies, one additional study [72] found in the reference list of studies reviewed was added. The review included a total of 46 studies but since it was not possible to obtain means and SDs from six studies [72–77], whose results are reported qualitatively, the meta-analyses included 40 studies [78–117].
Characteristics of the studies
A detailed summary of the 46 studies included in the review is reported in Table 2. Overall, data from 652 persons living with PD and 620 NI individuals acting as control were included. Of these participants, 550 were males and 423 were females, while the sex of 299 participants was not reported. The studies investigated mainly older adults with mean ages ranging from 52 to 74. Disease severity, which was not reported in eight studies [72, 78, 90, 92, 96, 106, 110, 113], ranged from I to IV on the Hoehn & Yahr scale. The mean overall scores of the motor evaluation conducted with the UPDRS part III, which was reported in 22 studies [75, 78, 80, 85, 87, 88, 90, 92, 93, 95, 97–99, 101–103, 105, 106, 108, 109, 116, 117], ranged from 8 to 33.4, indicating that the severity of motor symptoms of patients ranged from mild to moderate [65, 118]. Disease duration, which was reported in all but seven studies [81, 95, 96, 107, 109, 110, 113], ranged from 1.3 to 11.1 years. Only three investigations [83, 85, 88] manipulated (e.g., compared “on” vs. “off”) medication status, while two studies [87, 104] tested patients “off” medication (Table 2). Six studies did not report and/or evaluate cognitive functioning [72, 82, 83, 86, 98, 116]. In all, except five studies [74, 84, 85, 113, 119] that reported small differences in cognition between groups, PD and NI participants had similar cognitive status. None of the studies that explored associations between cognitive scores and skill acquisition or retention found significant correlations [74, 84, 97, 103, 107].
Forty studies [73–78, 80, 82–104, 106–110, 113–117] assessed consolidation after a single session of practice, while 14 studies [72, 76, 78, 79, 81, 84, 85, 100, 102, 105, 107, 108, 111, 112] investigated consolidation after extended practice (≥2 sessions). Regarding the nature of the task, 25 studies used SMTs [72, 76, 78, 79, 83, 84, 87, 93, 95, 97–102, 104–107, 109, 111–114, 119]; 10 studies SQTs [73, 74, 77, 80, 88–91, 96, 115]; three studies VATs [75, 92, 94]; eight studies GMTs [81, 82, 85, 86, 103, 108, 112, 116], and one study SPTs [117]. Nineteen studies [75, 79, 83, 88–90, 94, 95, 98, 101, 102, 104, 109–115] provided feedback in the form of knowledge of results (e.g., numeric score), three [87, 92, 106] as knowledge of performance (e.g., movement trajectory), and four [81, 93, 100, 112] combined these two types of feedback. Of the remaining studies, five studies [73, 78, 86, 105, 108] did not provide feedback and 16 [72, 74, 76, 77, 80, 82, 84, 85, 91, 96, 97, 99, 103, 107, 116, 117] did not explicitly state if feedback was provided or not.
Methodological quality
Details of the methodological quality assessment can be found in Supplementary Material 8. The percentage score and quality rating of each study are reported in Table 2. The mean±SD percentage score was 77.5±11.6% with 31 studies rated as “fair”, five as “good” and 10 as “poor”. The most common methodological flaws were the lack of both a sample size justification and information regarding the number of participants who were excluded from participation because they did not meet the inclusion criteria. The visual inspection of the funnel plots (Supplementary Material 9) and the results from Egger’s regressions (Supplementary Material 10) including studies of the meta-analyses, suggested no, or minimal, presence of heterogeneity and risk of biases at the study level. Finally, meta-regressions using the methodological quality as a covariate showed non-significant results, suggesting that low quality studies did not inflate between-group differences in skill retention after single (p = 0.1750) (Supplementary Material 3) or extended practice (p = 0.6850).
Consolidation after a single practice session
Overall, 17 [75, 76, 87, 92, 94, 97–99, 101, 102, 106, 107, 109, 110, 113, 114, 117] of the 40 studies that investigated motor memory consolidation after a single practice session (42.5%), reported that persons with PD had poorer skill retention than the control group. These 17 studies employed SMTs, VATs, and SPTs (Table 2). The remaining 23 studies, most of which implemented SQTs and GMTs, revealed no significant differences in consolidation between groups [73, 74, 77, 78, 80, 82–86, 88–91, 93, 95, 96, 100, 103, 104, 108, 115, 116].
When pooled together in the meta-analysis, data from the 35 studies [78, 80, 82–104, 106–110, 113–117] (47 effect sizes and 1187 participants) investigating motor memory consolidation after a single practice session showed a small significant effect in favor of NI individuals (SMD = –0.17; 95% CI = –0.32, –0.02; p = 0.0225; N = 47; I2 = 39.6%). Heterogeneity was low but statistically significant (Q-test: p = 0.0034). Sub-group analyses revealed a significant moderating effect of the task nature (p < 0.0001) (Fig. 1) and type of feedback (p = 0.0238) (Table 3). Specifically, people with PD showed worse poorer consolidation in both SMTs (SMD = –0.31; 95% CI –0.47, –0.15; N = 25; I2 = 27.4%; p = 0.0002) and VATs (SMD = –1.55; 95% CI = –2.32, –0.79; N = 3; I2 = 0%; p = 0.0001) but not in SQTs (SMD = 0.17; 95% CI = –0.05, 0.39; N = 10; I2 = 0%; p = 0.1292) or GMTs (SMD = 0.04; 95% CI = –0.25, 0.33; N = 8; I2 = 0%; p = 0.7771). The only study that investigated SPTs showed a large effect in favor of the NI group (SMD = –1.28; 95% CI = –2.07, –0.50; p = 0.0013). Sub-group analyses pertaining to feedback are reported in Table 3.
The results of the meta-regression exploring the influence of disease duration and severity, as well as their interactions are reported in Supplementary Material 3. None of these analyses yielded significant results, suggesting that disease duration and severity did not affect motor memory consolidation following a single session of practice. Similarly, consolidation did not seem to be moderated by improvements in skill performance during acquisition (Supplementary Material 3). Due to the small number of studies, we could not establish direct comparisons to investigate potential moderating effects of medication status (“on” vs. “off”). However, sensitivity analyses revealed that studies that manipulated medication status did not influence the results of the meta-analyses (Supplementary Material 4).
Table 2
Study characteristics
| Author, year (Design) | Participants characteristics | NIH | Med | Motor Task | Main Findings | ||||
| PD | NI | Task | FB | Outcomes | Ret. Int. | ||||
| ß Agostino et al., 2004 (Observational) | N = 9 M/F = 7/2 A = 64.4±6.3 H&Y = NRUPDRS = 15.3±4.0 DD = 7.56±3.13 | N = 7M/F = 5/2A = 62.1±6.6 | Fair 75.0% | On | SMT | NP | Total movement Duration*Total pause durationInaccuracy index | 1 hour post single and extended practice | Persons with PD had similar retention to the NI group after single and following extended motor practice.The PD group had a similar acquisition rate to, but lower performance than, the NI group. |
| ß Behrman et al., 2000 [79](Observational) | N = 15 M/F = 10/5A = 74±7 H&Y = 2.6±0.5 UPDRS = NR DD = 7±4 | N = 15 M/F = 10/5A = 73±7 | Good – 91.7% | On | SMT | KR | Reaction time*Pre-motor timeMotor timeMovement time | 48 hours post extended practice | Persons with PD had similar retention to the NI group following extended motor practice.The PD group had a similar acquisition rate and performance level to the NI group. |
| Dan et al., 2015 [80](Observational) | N = 24 M/F = 11/13 A = 57.7±8.8 H&Y = 1.1±0.2 UPDRS = 9.4±3.2 DD = 1.6±1.0 | N = 29 M/F = 13/16 A = 61.5±7.4 | Fair – 83.3% | Drug-naïve | SQT | NR | Performance index*SpeedAccuracy | 24 hours | Persons with PD had similar retention to the NI group.The PD group had a similar acquisition rate and performance level to the NI group. |
| Dan et al., 2015 [80](Observational) | N = 18 M/F = 10/8 A = 59.4±7.7 H&Y = 1.2±0.2 UPDRS = 9.6±3.5 DD = 1.3±1.0 | N = 29 M/F = 13/16 A = 61.5±7.4 | Fair – 83.3% | Drug-naïve | SQT | NR | Performance index*SpeedAccuracy | 24 hours | Persons with PD had similar retention to the NI group.The PD group had a lower acquisition rate and performance level than the NI group. |
| ß Dantas et al., 2018 [81](Observational) | N = 8 M/F = 4/4 A = 65.6±11.8 H&Y = 2.3±0.7 UPDRS = NR DD = NR | N = 11 M/F = 6/5 A = 70.0±7.7 | Fair – 83.3% | On | GMT | B | Performance score* | 30 days post extended practice | Persons with PD had similar retention to the NI group following extended motor practice.The PD group had similar acquisition rates to the NI group in all but two tasks. |
| QDoyon et al. 1998 [73](Observational) | N = 15 M/F = 8/7 A = 56.7±6.7 H&Y = range 1– 3 UPDRS = NR DD = range 6–21 | N = 15 M/F = 7/8 A = 54.3±8.1 | Fair – 83.3% | On | SQT | NP | Reaction time*Accuracy | 12 months | Persons with PD had similar retention and acquisition rate to the NI group. |
| Foreman et al., 2013(Observational) | N = 7 M/F = 7/0 A = 68.7±9.2 H&Y = range 1– 3 UPDRS = NR DD = 4.11±2.31 | N = 7 M/F = 5/2 A = 70.5±11.9 | Good – 91.7% | On | GMT | NR | Reaction time*CoP velocityCoM-CoPHeel position coefficient of variation | 48 hours and 1 week | Persons with PD had similar retention and acquisition rate to the NI group. |
| Q Gawrys et al., 2008 [74](Observational) | N = 19 (16Δ) M/F = NRA = 57.0±10.7 H&Y = 1.9±0.6 UPDRS = NR DD = 4.5±0.5 | N = 21 (20Δ) M/F = NRA = 55.7±9.1 | Fair – 83.3% | On | SQT | NR | Mean reaction Time* | 1 and 24 hours | Persons with PD had similar retention to the NI group.The PD group had a similar acquisition rate to, but lower performance than, the NI group. |
| Hadj-Bouziane, et al., 2013 [83](Observational) | N = 16 (8On/8Off) M/F = 11/5 A = 55.2±7.9 H&Y(on) = 1.9±0.4 H&Y(off) = 3.25±0.6UPDRS = NR DD = 11.1±2.0 | N = 9 M/F = 5/4 A = 54.1±8.59 | Fair – 83.3% | On/Off | SMT | KR | Trial per set*ErrorsResponse timeRepetition and search errorStrategy score | 3– 5 hours | Persons with PD had similar retention to the NI group.The PD group had a lower acquisition rate and performance than the NI group.Medication status (i.e., acquisition-retention: On– Off / Off– On) did not modulate motor learning. |
| ß Harrington et al., 1990 [84] (Observational) | N = 20 M/F = 16/4 A = 65.8±6 H&Y = 2.1±0.9 UPDRS = NR DD = 6.2±7 | N = 20 M/F = 9/11 A = 66.7±9 | Fair – 83.3% | On / Drug-naïve | SMT | NR | Mean time on target* | 24 hours post single and extended practice | Persons with PD had similar retention to the NI group after a single and following extended motor practice.The PD group had similar performance but a lower learning rate than the NI group following extended motor practice. |
| ß Hayes et al., 2015 [85](Observational) | N = 9 M/F = 8/1 A = 71.1±7.1 H&Y = 1.9±1.2 UPDRS = 17.8±5.9 DD = 7.52±3.19 | N = 10 M/F = 3/7 A = 71.0±8.7 | Good – 91.7% | Off | GMT | NR | Root mean square error* of the CoP | 24 and post single and 48 hours post extended practice | Persons with PD while Off medication had similar retention and acquisition rate to the NI group. |
| ß Hayes et al., 2015 [85](Observational) | N = 10 M/F = 9/1 A = 68.0±9.1 H&Y = 1.9±1.2 UPDRS = 13.3±6.7 DD = 4.21±2.19 | N = 10 M/F = 3/7 A = 71.0±8.7 | Good – 91.7% | On | GMT | NR | Root mean square error* of the CoP | 24 post single and 48 hours post extended practice | Persons with PD while On medication had similar retention and acquisition rate to the NI group. |
| Q Isaias et al., 2011(Observational) | N = 7 M/F = 1/6 A = range 39– 57 H&Y = 2 UPDRS = range 6– 20 DD<5y | N = 8 M/F = 3/5 A = range 42– 66 | Fair – 75.0% | Drug-naïve | VAT | NR | Adaptation (%)*Curvature | 3 weeks | Persons with PD had poorer transfer than the NI group.The PD group had a similar acquisition rate and performance to the NI group. |
| Jessop et al., 2006 [86](Observational) | N = 10 M/F = 6/4 A = 71.1±10.3 H&Y = 2.5±0.5 UPDRS = NR DD = 5.4±3.9 | N = 10 M/F = 6/4 A = 71.5±10.3 | Fair – 83.3% | On | GMT | NP | Movement velocity*Endpoint excursionDirectional control | 24 hours and 1 week | Persons with PD had similar retention to the NI group.The PD group had a similar acquisition rate to, but lower performance than, the NI group. |
| Kawashima et al., 2018 [87] (Observational) | N = 8 M/F = 5/3 A = 65.9±5.6 H&Y = 1.6±0.5 UPDRS = 10.3±5.4 DD = 4.0±2.5 | N = 8 M/F = 5/3 A = 68.7±2.8 | Fair – 83.3% | Off | SMT | KP | Mean peak acceleration* | 14 days | Persons with PD had lower retention than the NI group.The PD group had a lower acquisition rate and performance than the NI group. |
| Lahlou et al., 2021 [88] (Observational) | N = 23 (13Δ) M/F = 15/8 A = 63.2±7.02 H&Y = 2.09±0.29 UPDRS = 23.2±7.96 DD = 6.83±3.24 | N = 23 (13Δ) M/F = 10/13 A = 62.2±7.52 | Poor – 58.3% | On | SQT | KR | Performance index*SpeedAccuracy | 48 hours | Persons with PD had similar retention and acquisition rate to the NI group. |
| Lahlou et al., 2021 [88] (Observational) | N = 25 (13Δ) M/F = 13/12 A = 62.0±7.60 H&Y = 2.13±0.34 UPDRS = 25.7±8.76 DD = 6.6±3.93 | N = 23 (13Δ) M/F = 10/13 A = 62.2±7.52 | Poor – 58.3% | Off | SQT | KR | Performance index*SpeedAccuracy | 48 hours | Persons with PD had similar retention and acquisition rate to the NI group. |
| Lee et al., 2016 [91](Observational) | N = 10 M/F = 6/4 A = 64.6±10.1 H&Y = 2.3±0.5 UPDRS = NR DD = 5.8±4.4 | N = 10 M/F = 6/4 A = 6 4.0±12.7 | Fair – 83.3% | On | SQT | NR | Total time accuracy cost*Response time accuracyMovement time accuracy cost | 24 hours | Persons with PD had similar retention to the NI group.The PD group had a similar acquisition rate to, but lower performance than, the NI group. |
| Lee et al., 2018 [89] (Interventional) | N = 9 M/F = 6/3 A = 64.6±10.6 H&Y = 2.2±0.4 UPDRS = NR DD = 6.0±4.7 | N = 9 M/F = 5/4 A = 66.8±9.8 | Good – 91.7% | On | SQT | KR | Total time accuracy cost*Response time accuracyMovement time accuracy cost | 24 hours | Persons with PD had similar retention and acquisition rate to the NI group. |
| Lee et al., 2019 [90] (Observational) | N = 16 M/F = 9/7 A = 68.13±6.34 H&Y = NR UPDRS = 21.44±7.75 DD = 7.72±4.84 | N = 15 M/F = 7/8 A = 68.4±5.08 | Fair – 75.0% | On | SQT | KR | Total time accuracy cost*Response time accuracyMovement time accuracy cost | 24 hours | Persons with PD and freezing of gate had similar retention and acquisition rate to the NI group. |
| Lee et al., 2019 [90] (Observational) | N = 15 M/F = 7/8 A = 64.67±4.42 H&Y = NR UPDRS = 13.33±5.95 DD = 3.37±2.75 | N = 15 M/F = 7/8 A = 68.4±5.08 | Fair – 75.0% | On | SQT | KR | Total time accuracy cost*Response time accuracyMovement time accuracy cost | 24 hours | Persons with PD without freezing of gate had similar retention and acquisition rate to the NI group. |
| Leow et al., 2012 [92] (Observational) | N = 8 M/F = 4/4 A = 66±8 H&Y = NR UPDRSMDS = 28±13 DD = 8.3±6.9 | N = 8 M/F = 0/8 A = 69±9 | Poor – 58.3% | On | VAT | KP | Directional error* | 24 hours | Persons with PD had poorer retention (i.e., saving) than the NI group.The PD group had a similar adaptation rate (i.e., acquisition) to the NI group. |
| Lin et al., 2007 [93] (Observational) | N = 10 M/F = 9/1 A = 62.2±15.81 H&Y = 2.1±0.16 UPDRS = 28.3±10.4 DD≤3 | N = 10 M/F = 8/2 A = 61.9±11.70 | Fair – 83.3% | On | SMT | B | Root mean square error* | 24 hours | Persons with PD had better retention than the NI group while practicing under blocked schedule.The PD group had a similar acquisition rate to, but lower performance than, the NI group. |
| Lin et al., 2007 [93] (Observational) | N = 10 M/F = 6/4 A = 67.9±7.91 H&Y = 2.0±0.00 UPDRS = 29.1±10.92 DD≤3 | N = 10 M/F = 4/6 A = 61.2±9.80 | Fair – 83.3% | On | SMT | B | Root mean square error* | 24 hours | Persons with PD had lower retention than the NI group while practicing under random schedule.The PD group had a similar acquisition rate to, but lower performance that, the NI group. |
| Marinelli et al., 2009 [94] (Observational) | N = 11 (6Δ) M/F = 8/3 A = 57.9±7.3 H&Y = range 1– 2 UPDRS = NR DD = 2.1±3.1 | N = 11 (6Δ) M/F = 7/4 A = 54.2±8.6 | Fair – 75.0% | Drug-naïve | VAT | KR | Directional error*Onset timeTiming errorMovement time | 24 hours | Persons with PD had poorer retention than the NI group.The PD group had a similar adaptation rate (i.e., acquisition) to the NI group. |
| Marinelli et al., 2009 [94] (Observational) | N = 5 M/F = 4/1 A = 60.0±7.4 H&Y = range 2– 2.5 UPDRS = NR DD = 8.4±4.5 | N = 5 M/F = 1/4 A = 61.0±12.0 | Fair – 75.0% | On | VAT | KR | Directional error*Reaction timeMovement time | 48 hours | Persons with PD had poorer retention than the NI group.The PD group had a similar adaptation rate (i.e., acquisition) to the NI group. |
| Marinelli et al., 2017 [95] (Observational) | N = 11 M/F = 9/2 A = 64.8±3.4 H&Y = range 1– 2 UPDRS = 15±2 DD = NR | N = 11 M/F = NRA = 64.4±1.8 | Fair – 83.3% | Drug-näive | SMT | KR | Correct anticipated movements* | 24 hours | Persons with PD had similar retention to, but a lower acquisition rate than, the NI group. |
| Mochizuki-Kawai et al., 2010 [96] (Observational) | N = 10 M/F = 4/6 A = 66.3±9.3 H&Y = NR UPDRS = NR DD = NR | N = 12 M/F = 4/8 A = 62.3±7.0 | Fair – 75.0% | On/Drug-näive | SQT | NR | Errors to reach learning criterion*Mean number of correctly recalled sets | 1 month | Persons with PD had similar retention and acquisition rate to the NI group. |
| Nackaerts et al., 2020 [97] (Observational) | N = 10 M/F = 4/6A = 67.5±6.2H&Y = 2.2±0.42UPDRSMDS = 25.8±10.9DD = 5.9±4.0 | N = 10 M/F = 6/4A = 63.6±6.7 | Fair – 83.3% | On | SMT | NR | Movement time*Euclidean distanceCoefficient of variationPerformance index | 24 hours | Persons with PD had lower retention than, but a similar acquisition rate to, the NI group. |
| Nelson et al., 2017 [98] (Observational) | N = 11 M/F = 10/1 A = 59.1±5.8 H&Y = 2.0±0.2 UPDRS = 20.9±8.5 DD = 5.0±2.1 | N = 13 M/F = 7/6 A = 57.5±8.2 | Fair – 83.3% | On | SMT | KR | Normalized hand-path area*Reaction timeAmplitude to peak velocity | 24 hours | Persons with PD had poorer retention than, but a similar acquisition rate to, the NI group. |
| Nicastro et al., 2018 [99] (Observational) | N = 9 M/F = 7/2 A = 65.1±9.8 H&Y = 1.6±0.7 UPDRSMDS = 15.0±10.4 DD = 1.0±0.3 | N = 10 M/F = 7/3 A = 60.3±5.4 | Good – 100% | On | SMT | NR | Performance index*Error rateTime per trial | 24 hours | Persons with PD had poorer retention than, but a similar acquisition rate to, the NI group. |
| Q - ß Nutt et al., 2000 [72] (Observational) | N = 5 M/F = 2/3 A = 65±9 H&Y = NR UPDRS = NR DD = 2.1±1.7 | N = 14 M/F = 5/9 A = 63±12 | Poor – 50.0% | Drug-naïve | SMT | NR | Tapping speed | ∼9 hours post extended practice | Persons with PD had similar retention to the NI group following extended practice.The PD group had a similar acquisition rate to, but lower performance than, the NI group. |
| ß Onla-or et al., 2008 [100] (Observational) | N = 10 M/F = NR A = 61.7±9.5 H&Y = 2.5±0.7 UPDRS = NR DD = 10.2±5.8 | N = 10 M/F = NR A = 60.0±10.9 | Fair – 83.3% | On | SMT | B | Root mean square error* | 24 hours post single and extended practice | Persons with PD had similar retention and acquisition rate to the NI group after a single session of motor practice under a random schedule. |
| ß Onla-or et al., 2008 [100] (Observational) | N = 10 M/F = NR A = 61.4±9.4 H&Y = 2.6±0.6 UPDRS = NR DD = 7.3±4.2 | N = 10 M/F = NR A = 66.0±3.2 | Fair – 83.3% | On | SMT | B | Root mean square error* | 24 hours post single and extended practice | Persons with PD had a similar acquisition rate to, but better retention than, the NI group after a single session of practice under a blocked schedule.The PD group had lower retention than, but a similar acquisition rate to, the NI group following extended practice under a blocked schedule. |
| Pendt et al., 2011 [102] (Observational) | N = 19 M/F = NR A = 63.9±8.8 H&Y = range 1.5– 3 UPDRS = 26.0±9.0 DD = 6.8±5.7 | N = 19 M/F = NR A = 64.8±10.1 | Fair – 83.3% | On | SMT | KR | Performance index*ToleranceNoise reductionCovariationTiming of release | 24 hours | Persons with PD had lower retention than the NI group.The PD group had a similar acquisition rate to, but lower performance than, the NI group. |
| ß Pendt et al., 2011 [102] (Observational) | N = 6 M/F = NR A = 62±11.3 H&Y = range 2– 3 UPDRS = 24±6.7 DD = 5±0.9 | N = 7 M/F = NR A = 68±9.1 | Fair – 83.3% | On | SMT | KR | Performance index*ToleranceNoise reductionCovariationTiming of release | 7– 9 months post extended practice | Persons with PD had lower retention than the NI group following extended practice. The PD group had a similar acquisition rate to, but lower performance than, the NI group. |
| Pendt et al., 2012 [101] (Observational) | N = 12 M/F = NR A = 63.5±11.5 H&Y = range 2– 4 UPDRS = 32.4±7.8 DD = 6±2.9 | N = 16 M/F = NR A = 64.6±9.6 | Fair – 83.3% | On | SMT | KR | Performance index*Timeshift | 24 hours | Persons with PD had lower retention than, but a similar acquisition rate to, the NI group. |
| Peterson et al., 2016 [103] (Observational) | N = 15 M/F = 12/3 A = 66.34±6.02 H&Y = 2.00±0.38 UPDRS = 25.4±13.8 DD = 6.38±4.75 | N = 12 M/F = 6/6 A = 68.04±6.61 | Fair – 83.3% | On | GMT | NR | CoM displacement* Margin of stabilityStep lengthStep latencyNumber of stepsEMG onset | 24 hours | Persons with PD had similar retention and acquisition rate to the NI group. |
| Platz et al., 1998 [104] (Observational) | N = 7 M/F = 3/4 A = 65.9±8.3 H&Y = 2.5±0.5 UPDRS = NR DD = 7.6±2.4 | N = 7 M/F = 3/4 A = 62.1±13.3 | Fair – 83.3% | Off | SMT | KR | Movement time* AccelerationDecelerationAccuracy | 1 hour | Persons with PD had similar retention to the NI group while practicing under a “cue” condition.The PD group had a similar acquisition rate to, but lower performance than, the NI group. |
| Platz et al., 1998 [104] (Observational) | N = 8 M/F = 5/3 A = 62.0±14.6 H&Y = 2.0±0.75 UPDRS = NR DD = 4.3±1.8 | N = 8 M/F = 5/3 A = 60.8±15.2 | Fair – 83.3% | Off | SMT | KR | Movement time* AccelerationDecelerationAccuracy | 1 hour | Persons with PD had better retention than the NI group while practicing under an “uncue” condition.The PD group had a similar acquisition rate to, but lower performance than, the NI group. |
| ß Rostami et al., 2009 [105] (Observational) | N = 9 M/F = 7/2 A = 63.8±4.8 H&Y = 2.0±0.0 UPDRS = 16.8±1.6 DD = 9.8±1.5 | N = 9 M/F = 7/2 A = 64.5±5.2 | Fair – 75.0% | On | SMT | NP | Mean reaction time* | 1 week post extended practice | Persons with PD had similar retention and acquisition rate to, but lower performance than, the NI group. |
| Roy et al., 2015 [106] (Observational) | N = 18 M/F = 10/8 A = 67.3±6.6 H&Y = NR UPDRS = 24.0±6.5# DD = 4.6±3.4 | N = 18 M/F = 10/8 A = 70.8±6.8 | Fair – 75.0% | On | SMT | KP | Completion time*AccuracyRecall accuracy | 3 weeks | Persons with PD had poorer retention than the NI group. The PD group had a similar acquisition rate to the NI group. |
| ß Sato et al., 2014 [107] (Observational) | N = 12 M/F = 4/8 A = 63.7±5.1 H&Y = 1.0±0.0 UPDRS = NR DD = NR | N = 12 M/F = 4/8 A = 61.3±6.8 | Fair – 83.3% | On | SMT | NR | Mean reaction time* | 24 hours post single and extended practice | Persons with PD had lower retention than the NI group after single and following extended motor practice.The PD group had a similar acquisition rate to, but lower performance than, the NI group during single and extended practice. |
| ß Sehm et al., 2014 [108] (Observational) | N = 20 M/F = 11/9 A = 62.9±7.1 H&Y = 2.1±0.4 UPDRS = 21.9±9.5 DD = 4.3±3.2 | N = 16 M/F = 9/7 A = 64.9±6.8 | Fair – 83.3% | On | GMT | NP | Total time on target (balance)* | 1 week and 20 months post extended practice | Persons with PD had similar retention to, but a lower acquisition rate and performance than, the NI group after a single motor practice session.Persons with PD had greater retention, but a lower acquisition rate and performance, than the NI group following extended motor practice. |
| Sidaway et al., 2016 [109] (Observational) | N = 14 M/F = NR A = 62.1±7.7 H&Y = range 1– 3 UPDRS = 19.14±NR DD = NR | N = 14 M/F = NR A = 63.2±6.6 | Fair – 83.3% | On | SMT | KR | Movement time*Errors | 24 hours and 1 week | Persons with PD had poorer retention than the NI group, particularly when the practice was performed under random schedule rather than blocked practice schedule.Persons with PD had a similar acquisition rate and performance to the NI group. |
| Smiley-Oyen et al., 2002 [119] (Observational) | N = 8 M/F = 5/3 A = 62±8.8 H&Y = NR UPDRS = NR DD = NR | N = 8 M/F = 4/4 A = 65±7.6 | Poor – 50.0% | On | SMT | KR | Movement time*Reaction timeFlight/Contact time ErrorOthers | 24 hours | Persons with PD had lower retention than the NI group.The PD group had a similar acquisition rate to, but lower performance that, the NI group. |
| Smiley-Oyen et al., 2003 [113] (Observational) | N = 9 M/F = 6/3 A = 61.3±8.40 H&Y = NR UPDRS = NR DD = NR | N = 9 M/F = 5/4 A = 64.8±7.3 | Poor – 58.3% | On | SMT | KR | Variable error*Movement distance | 24 hours | Persons with PD had lower retention than the NI group.The PD group had a similar acquisition rate to the NI group. |
| ß Smiley-Oyen et al., 2006 [112] (Observational) | N = 7 M/F = 4/3 A = 66.1±6.4 H&Y = 1.6±0.53 UPDRS = NR DD = 5.6±5.85 | N = 7 M/F = 4/3 A = 66.4±4.5 | Poor – 58.3% | On | GMT &SMT | B&KR | Total time*Errors | 48 h post extended practice and 3 weeks post extended practice | GMT: Persons with PD had similar retention and acquisition rate to, but lower performance than, the NI group.SMT: Persons with PD had similar retention and acquisition rate to, but lower performance than, the NI group. |
| ß Smiley-Oyen et al., 2012 (Observational) | N = 7 M/F = 4/3 A = 66.1±6.4 H&Y = 1.6±0.53 UPDRS = NR DD = 5.6±5.85 | N = 7 M/F = 4/3 A = 66.4±4.5 | Poor – 58.3% | On | SMT | KR | Movement time* Peak velocityTime-to-peak velocityCoefficient of variation | 48 h post extended practice and 3 weeks post extended practice | Persons with PD had similar retention to the NI group.The PD group had a similar acquisition rate to, but lower performance than, the NI group. |
| Swinnen et al., 2000 [114] (Observational) | N = 13 M/F = 9/4 A = 68.2±9.19 H&Y = 2.5±0.78 UPDRS = NR DD = 7.5±6.13 | N = 13 M/F = NR A = 67.5±7.42 | Poor – 66.7% | On | SMT | KR | Cycle duration*AmplitudeCross-correlation between limbsDrift | 24 hours | Persons with PD had poorer retention than the NI group.Although persons with PD had a higher acquisition rate, they had lower performance than the NI group. |
| Terpening et al., 2013 [115] (Observational) | N = 40 M/F = 29/11 A = 63.6±7.6 H&Y = 1.7±0.5 UPDRS = NR DD = 4.1±4.4 | N = 20 M/F = 8/12 A = 66.1±9.5 | Fair – 83.3% | On | SQT | KR | Number of correct sequenceErrors | 12 hours | Persons with PD had similar retention and acquisition rate to the NI group. |
| Q - ß Thomas-Antérion et al., 1996 [76] (Observational) | N = 24 M/F = 13/11 A = range 40– 75 H&Y = 2.0±0.8 UPDRS = NR DD = range 0.5– 10 | N = 90 (54Δ) M/F = 45/45 A = age-matched | Fair – 75.0% | On/Drug- naïve | SMT | NR | Percentage of learning*Completion times | 24/72 hours post single and extended practice | Persons with PD had poorer retention than the NI group after a single and following extended practice. |
| Van Ooteghem et al., 2017 [116] (Observational) | N = 11 M/F = 4/7 A = 68±4 H&Y = 2.2±0.34 UPDRS = 33.4±12.2 DD = 6.7±3.1 | N = 11 M/F = 3/8 A = 68±6.4 | Poor – 58.3% | On | GMT | NR | Mean CoM gain* Mean CoM phase | 24 hours | Persons with PD had similar retention and acquisition rate to the NI group. |
| Q Werheid et al., 2003(Observational) | N = 7 M/F = 5/2 A = 58.7±7.8 H&Y = 1.5±0.3 UPDRS = NR DD = 2.7±1.0 | N = 7 M/F = 2/5 A = 52.9±5.5 | Fair – 83.3% | On | SQT | NR | Reaction time*Accuracy | 1 hour | Persons with PD had similar retention to the NI group.The PD group had a similar acquisition rate to, but lower performance than, the NI group. |
| Whitfield et al., 2017 [117] (Observational) | N = 16 M/F = 10/6 A = 66.43±6.82 H&Y = 2.2±0.58 UPDRSMDS = 35.4±12.5 DD = 7.2±5.71 | N = 15 M/F = 6/9 A = 64.96±8.25 | Poor – 66.7% | On | SPT | NR | Standardized duration*Accuracy | 24/48 hours | Persons with PD had poorer retention than the NI group.The PD group had a similar acquisition rate to the NI group. |
*Primary outcome; ßretention following extended practice; Δsubsample used in the analyses; Qdata for meta-analysis unavailable; A, age; B, both FB; CoM, center of mass; CoP, center of pressure; DD, disease duration (years); EMG, electromyography; FB, feedback; GMT, gross motor task; H&Y, Hoehn and Yahr scale; KP, knowledge of performance; KR, knowledge of result; M/F, male/female; Med, medication status during practice: on/off; N, number; NI, neurologically intact; NIH, study quality; NP, not provided; NR, not reported; PD, Parkinson’s disease; Ret. Int., retention interval; SMT, sensory motor task; SPT, speech motor task; SQT, sequential fine motor task; UPDRS, Unified Parkinson’s Disease Rating Scale part III; UPDRSMDS, Movement Disorder Society-UPDRS part III; VAT, visuomotor adaptation task.
Fig. 1
Motor memory consolidation after a single session of practice grouped by the task nature. CI, confidence interval; NI, neurologically intact individuals; PD, people with Parkinson’s disease; SD, standard deviation; SMD, standard mean difference. Negative values represented worse skill consolidation for persons with PD in comparison with NI individuals. The solid vertical black line represents an SMD of 0. The dashed vertical grey line represents the overall Random Effect Model.
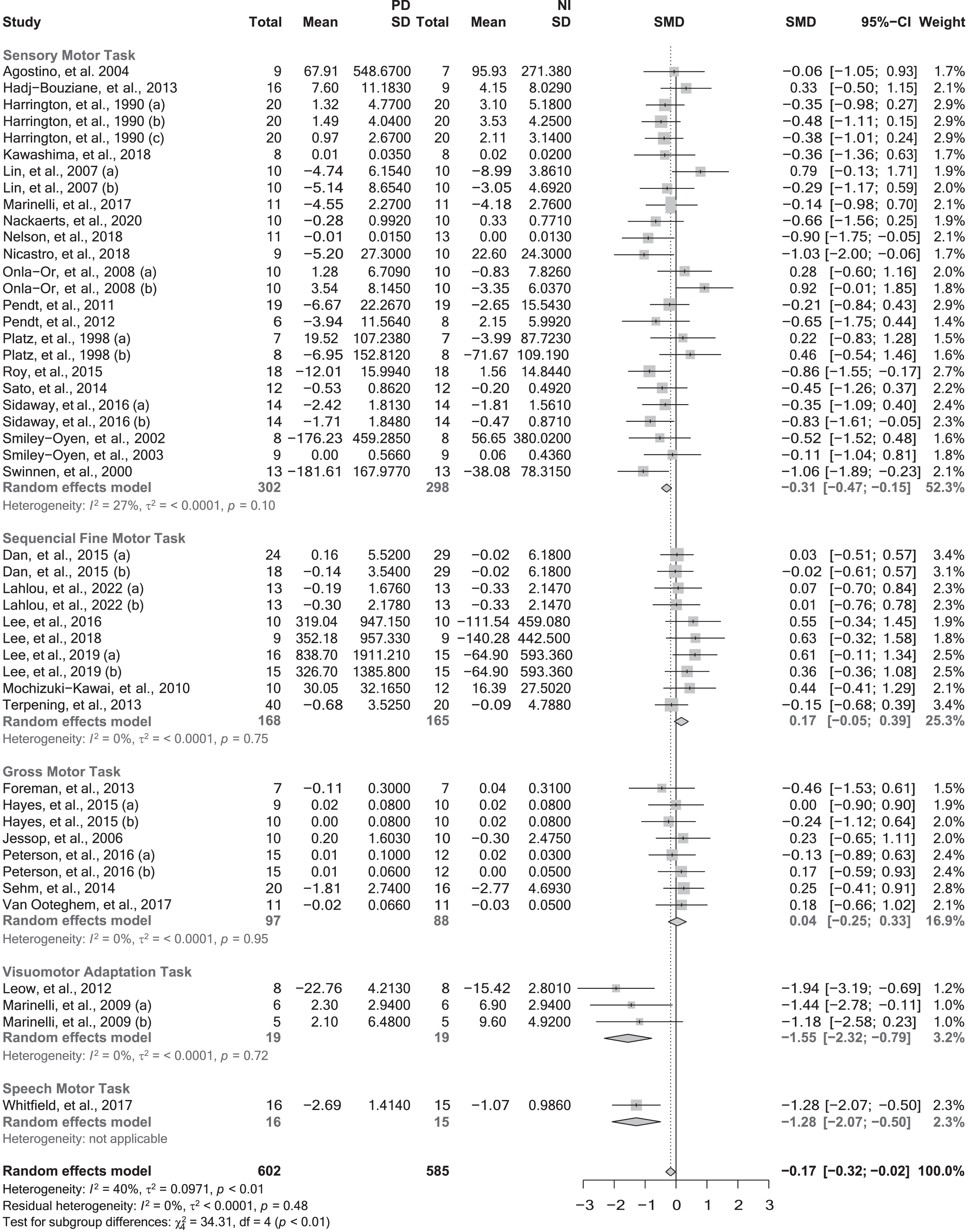
Table 3
Sub-group analysis for motor memory consolidation after a single session of practice
| N | SMD | 95% -CI | p | I2 | P | |
| Feedback | 0.0238 | |||||
| • Not reported | 17 | –0.23 | –0.43, –0.03 | 0.0276 | 23.3% | |
| • Not provided | 3 | –0.18 | –0.29, 0.64 | 0.4596 | 0.0% | |
| • K. of results | 20 | –0.18 | –0.41, 0.05 | 0.1337 | 37.3% | |
| • K. of performance | 3 | –0.96 | –1.69, –0.22 | 0.0105 | 47.2% | |
| • Both types of feedback | 4 | 0.41 | –0.13, 0.95 | 0.1369 | 30.2% |
CI, confidence interval; I2, heterogeneity; K, knowledge; N, number of effect sizes; SMD, standardized mean difference.
Consolidation after extended practice
Only studies employing SMTs and GMTs investigated the effect of extensive practice on motor memory consolidation in PD. Therefore, the results pertaining to extended practice cannot be generalized to other types of motor tasks. Only two [76, 107] of the 14 studies that investigated consolidation following extended practice (14.3%) found that persons with PD had poorer skill retention than the control group. Except for one study [108] that observed better retention in persons with PD using a GMT, the remaining studies reported no significant differences in consolidation between groups, regardless of whether SMTs [72, 78, 79, 84, 100, 102, 105, 111, 112] or GMTs [81, 85, 112] were implemented.
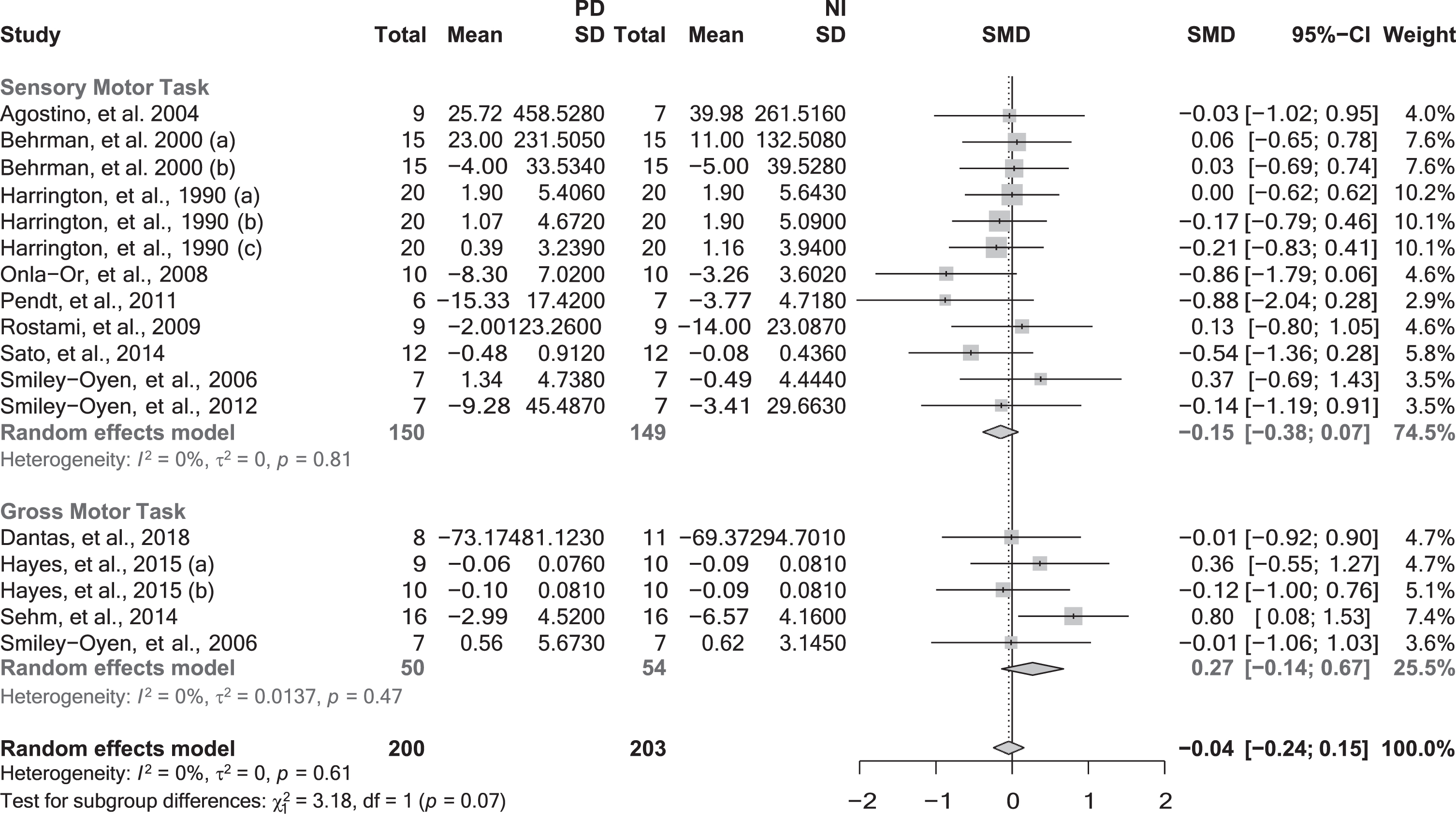
When pooled together in the meta-analysis, the data of 12 studies [78, 79, 81, 84, 85, 100, 102, 105, 107, 108, 111, 112] (17 effect sizes and 403 participants) that investigated motor memory consolidation following extended practice using SMTs and GMTs revealed no significant differences between groups (SMD = –0.04; 95% CI = –0.24, 0.15; p = 0.6565; N = 17; I2 = 0%). Similarly, subgroup analyses did not reveal any significant moderating effect regarding the task nature (p = 0.0745) (Fig. 2), or feedback provided (p = 0.9511).
Fig. 2
Motor memory consolidation after extended practice grouped by the task nature. CI, confidence interval; NI, neurologically intact individuals; PD, people with Parkinson’s disease; SD, standard deviation; SMD, standard mean difference. Negative values represented worse skill consolidation for persons with PD in comparison with NI individuals. The solid vertical black line represents an SMD of 0. The dashed vertical grey line represents the overall Random Effect Model.
The results of meta-regression analyses exploring the influence of disease duration (p = 0.1980) and severity (p = 0.7730), as well as their interaction (p = 0.2600), were non-significant, indicating that these factors did not moderate motor memory consolidation after extended practice. Importantly, performance during skill acquisition (i.e., encoding) did not appear to moderate retention either (p = 0.7162). Consistent with the results of single practice studies, sensitivity analyses showed that medication status did not influence the results of the meta-analyses including extended practice studies (Supplementary Material 4).
DISCUSSION
The results of this review confirm that, in comparison with NI individuals of similar age, persons with mild to moderate PD have deficits in the capacity to consolidate motor skills after a single practice session. These findings, which have important clinical implications, suggest that to maximize the long-term retention of motor skills, motor rehabilitation should not only focus on optimizing skill acquisition and thus encoding but also on ensuring an effective motor memory consolidation process [15, 120]. Deficits in motor memory consolidation, however, do not affect all types of motor tasks to the same extent. Differences between PD and NI individuals were found to be statistically significant only in the consolidation of motor skills acquired during STMs, VATs, and SPTs, although only one study investigated SPTs and thus these results should be interpreted cautiously. Patients with PD, in contrast, appear to have a more preserved capacity for efficiently consolidating, and thus retaining, skills acquired during SQTs and GMTs. Motor rehabilitation programs aimed at maintaining motor function in PD should target a broad range of tasks to ensure functional mobility in multiple activities of daily living [16, 121]. However, identifying task-specific deficits could inform clinicians to design more targeted interventions to optimize motor memory consolidation [6].
During the first symptomatic stages of PD [122, 123], neurodegenerative changes are localized within the basal ganglia and cortico-striatal motor networks while neocortical areas are less affected [124–126]. Subsequently, structural and functional signs of deterioration appear in the cerebellum [127, 128] and, to a lesser extent, in cortical areas such as parietal [129] and primary motor (M1) cortices [130, 131]. As the disease progresses, neurodegeneration changes within the basal ganglia spread across the striatum [126, 132], affecting non-motor cortico-striatal circuits [124, 125, 133] and the less affected contralateral side [123, 134]. It will be in the late stages of PD when neurodegeneration will eventually reach neocortical areas [122, 135]. Whereas the encoding and consolidation of SMTs involve mostly basal ganglia, cerebellum and cortical areas such as M1 [13, 40, 41], VATs engage primarily the striatum [26, 136], cerebellum, fronto-parietal lobules and M1 [13, 26, 40, 137–139] (Table 1). Neuroimaging studies have demonstrated that deficits in the consolidation of these types of motor tasks in PD are associated with alterations in parietal and cortico-striatal connectivity and dopamine uptake [75, 87, 98, 99, 140]. Deficits in the consolidation of motor skills acquired during the practice of SMTs and VATs in persons with mild to moderate PD could therefore be explained by the deterioration that these specific areas of the brain experience during the early symptomatic stages of the disease.
Given the broad implication of cortico-striatal networks in the acquisition of SQTs [13, 41, 141], the preserved capacity that patients with PD showed for successfully consolidating motor skills encoded during the practice of these motor tasks was unexpected. The reason for this preservation is unknown but it could be related to the capacity to activate neocortical areas that are consistently engaged during the practice of SQTs [40] and tend to be less affected by neurodegeneration in the early symptomatic stages of the disease [122]. Indeed, compared to SMTs and VATs, the performance of SQTs is characterized by greater activation of cortical areas such as the left dorsal premotor cortex, the supplementary motor cortex as well as the superior parietal cortex [40]. Increased participation of the cerebellum during motor practice could also contribute to the preservation of SQTs in people with PD [142–145], although this brain structure displays signs of structural and functional alterations already during the Hoehn & Yahr stage II–III of the disease [135, 146]. It is noteworthy that the activation of cortical and cerebellar regions is even more pronounced during the acquisition of explicit variants of the SQTs used in all the studies of the meta-analysis, in which participants are consciously aware of the repeating numerical sequence embedded in the motor task [40]. Importantly, these patterns of brain activation are not only present during acquisition but also during motor memory consolidation [147]. Clearly, more studies are needed to identify which mechanisms underlie the preserved capacity to retain SQTs shown by people with PD.
Deficits in the consolidation of SQTs could nevertheless become more pronounced when neurodegeneration progresses [148, 149] and thus alterations in motor automaticity mechanisms that are more relevant in the late phases of sequential motor learning start to emerge [150, 151]. In one study included in the review, participants performed a retention test of a SQT 10–18 months after initial practice [73] and only those patients whose disease severity worsened, transitioning from Hoehn and Yahr stage I to II [64] during the retention interval, showed significant deficits in skill retention, indicating poorer consolidation [73]. In addition, two studies of the review reported deficits only at the end of the second day of practice [80, 115], reinforcing the idea that deficits in motor skills practiced during SQTs become more pronounced in late phases of motor learning [9], when sequential finger movements should be performed automatically and with less attentional demand. The results of these studies suggest that while patients in the initial symptomatic stages of the disease can encode and consolidate sequential fine motor skills similarly to NI individuals, they can show deficits in the ability to automatize these motor skills [150, 151]. Cognitive strategies such as verbal instructions, cueing, and segmentation, could help those individuals to shift learning toward a more volitional mode of action to compensate for deficits in automaticity [121].
The performance of GMTs requires complex postural control strategies involving multiple brain structures such as the brainstem, cerebellum, basal ganglia, thalamus, and neocortical areas (e.g., sensory-motor cortex), as well as the integration of information from proprioceptive, vestibular, and visual systems [38, 55, 152]. We found that people with mild to moderate PD can improve the performance of GMTs and retain these gains similarly to NI individuals. This finding is encouraging because patients with PD suffer from gait disorders and postural stability problems [153] that aggravate despite pharmacological treatment [154, 155] and that lead to an increased risk of falls [156–160]. GMT-based training could be a good strategy to slow down the deterioration of these complex motor skills [6] as this type of training can potentially mitigate alterations in neuroplasticity commonly observed in patients with PD [161]. For example, step training increases intra-cortical inhibition measured with transcranial magnetic stimulation [162], an indirect marker of γ-aminobutyric acid (GABA) activity, which tends to be suppressed in people with PD [130, 163, 164]. Additionally, dynamic balance training has been shown to increase gray matter in the cerebellum, parietal and temporal lobes, as well as in the pre-motor cortex in patients with PD [108]. Taken together, these studies reinforce the importance of gait and postural control training in PD. Initiating these interventions as early as possible could potentially preserve neuroplasticity and slow down the progressive motor deterioration of the disease [6]. Nevertheless, additional research is needed to confirm whether our findings can be translated to different and more complex GTMs such as gait and can be generalized to real-world clinical scenarios [6, 16, 154, 165].
We used subgroup analyses and meta-regression techniques to explore factors moderating the capacity to consolidate motor skills in PD such as performance during acquisition, disease severity and duration, amount of practice and feedback provided. Although we cannot rule out the possibility that a ceiling effect in motor skill acquisition in NI individuals could have masked differences [14], the rate of skill improvement during acquisition was similar between groups. Regardless, acquisition performance and thus encoding did not appear to modulate the capacity to improve consolidation processes in patients with PD (Supplementary Material 3). Similarly, neither disease severity, disease duration, nor their interaction, had any significant influence on the observed deficits in motor memory consolidation, challenging previous studies indicating that these factors had detrimental effects on consolidation in people with PD [32, 73, 74, 76, 83, 84, 96, 97, 112, 116, 148, 149, 166, 167]. Discrepancies with previous studies could be explained by the fact that most studies included in our review recruited patients with mild-to-moderate PD. This possibly resulted in a very homogenous sample that limited the capacity of the meta-regression to capture moderating effects of disease severity or, to a lesser extent, disease duration on consolidation processes. Therefore, these results should be interpreted with caution, and further research is needed to determine whether the capacity to consolidate some specific motor skills is affected or worsens more rapidly as the disease progresses.
Importantly, subgroup analyses revealed that deficits in motor memory consolidation were reduced when more practice was afforded [16, 95]. With additional practice, patients with PD appear to be able to consolidate motor skills acquired during SMTs similarly to NI individuals [6, 16, 17, 168]. Extended motor practice possibly allows patients the time to encode sensory and motor information more effectively, compensating for some of the neural deficits that they display during motor learning [22, 29, 98]. This is consistent with studies showing that, compared to NI individuals, persons in the early stages of PD require greater neural activity during practice to achieve similar skill performance [22, 169–172]. However, extended motor practice alone, while being effective during the early phases of sensory motor learning, may not be sufficient during late phases that involve automatization [9, 151].
Augmented feedback provided as knowledge of results, alone or in combination with knowledge of performance, also improved motor memory consolidation [6, 15, 16, 173]. Persons with PD experience proprioceptive [174, 175] and central processing integration impairments [127, 129, 176] as well as attentional deficits [177]. Augmented feedback could therefore help these patients activate their cognitive reserve to use a more volitional mode of action [121] as well as to focus on the extrinsic information of the task during practice to enhance consolidation processes [173, 178, 179].
Limitations
It is important to acknowledge that, given their design, the results of the studies investigating consolidation following single practice cannot be easily translated to specific rehabilitation interventions in PD. They can, however, provide important information to guide the general principles of clinical practice [7]. It is further important to emphasize that most studies of the review included participants in the early symptomatic stages of PD. More studies are thus needed to determine if the task-specific deficits in motor memory consolidation found in this study remain present or are augmented in later stages of the disease. Given the limited number of studies available, it was not possible to conduct additional analyses investigating interactions between augmented feedback and task nature. Future studies should determine which forms, frequency, timing, and focus (internal vs. external) [179] of augmented feedback is more effective to improve consolidation processes across different (simple vs. complex) motor tasks [180].
Since only three studies manipulated medication status [83, 85, 88], we could not investigate the effects of antiparkinsonian medications on motor memory consolidation. Although our sensitivity analyses suggested that medication status did not affect consolidation (Supplementary Material 4), the limited data available suggests that assessing the influence of medication requires additional studies. The effects that antiparkinsonian medications can have on motor memory encoding and consolidation are complex and conflicting results have been reported [15, 28, 161, 169, 171, 181–183]. More investigations are needed to elucidate the effects of these medications on motor memory consolidation processes and whether their potential modulating effects are similar across different types of motor tasks at different stages of the disease. Finally, since in most studies, participants had similar cognitive levels, it was not possible to determine the influence of cognition deficits (or lack thereof) on consolidation [16] and whether certain motor tasks are more affected by cognitive deterioration in PD.
Conclusion
Persons with PD who perform sensory motor skills and sequential movements daily need to constantly adapt pre-existing motor routines to cope with the motor dysfunctions arising during the disease [8, 31, 120]. Similarly, these individuals suffer from gait disorders and postural instability [156–160], as well as speech and voice disorders [184–186]. The results of this review confirm that people with mild to moderate PD have deficits in motor memory consolidation processes affecting primarily SMTs and VATs following a single practice session. Our results suggest that extended motor practice and augmented feedback might be valuable means to reduce deficits in the consolidation of functional skills, but more evidence is needed to confirm if these strategies are effective to improve different motor tasks in clinical practice. Overall, our findings underline the importance of developing targeted interventions to enhance motor memory processes to support long-term skill retention in PD.
ACKNOWLEDGMENTS
We would like to thank the authors of the papers included in the review who provided additional information: Dr. Bo Foreman, Dr. Dagmar Sternad, Dr. Lisa Maurer, Dr. Alfredo Berardelli, MSc Soraya Lahlou, Dr. Ya-Yun Alice Lee, Dr. Evelien Nackaerts, Dr. Lucio Marinelli, Dr. Ann Smiley-Oyen, Dr. David Wright, Dr. Julien Doyon, Dr. Nicolas Nicastro, Dr. Karen Van Ooteghem, Dr. Jason Whitfield, Dr. Carolee Winstein, and Dr. Leszek Kaczmarek. We would also like to thank Alina Andretzky for reviewing an early version of this manuscript.
FUNDING
The study has been supported by Parkinson’s Canada, Graduate Student Award Competition, and the Fonds de recherche du Québec - FRQS (doctoral scholarships). Marc Roig was supported with a Fonds de Recherche Santé Québec (FRQS) Salary Award (252967) and CIHR Projects Grant (02109PJT-468982-MOV-CFAA-244681). Simon Steib received funding from the German Foundation of Neurology. Jacopo Cristini received funding from Parkinson’s Canada, Graduate Student Award Competition (GSA-2021-0000000080), and FRQS (doctoral scholarship - 317490). Zohra Parwanta received funding from Graduate Student Award Competition, and FRQS (doctoral scholarship). Bernat De las Heras received funding from FRQS (doctoral scholarship). The funding sources did not have any involvement in the study (i.e., design, data analysis and interpretation) as well as in the decision to submit the manuscript for publication.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-230038.
Supplementary Material 1. Terms used for the electronic search.
Supplementary Material 2. The NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies.
Supplementary Material 3. Meta-regression analyses for motor memory consolidation after single practice.
Supplementary Material 4. Sensitivity analyses exploring the effect of medication.
Supplementary Material 5. Motor skill transfer after single practice.
Supplementary Material 6. Multiple effect sizes.
Supplementary Material 7. Flow diagram showing the flow of information through the different stages of the systematic review with meta-analysis.
Supplementary Material 8. NIH study quality assessment scores.
Supplementary Material 9. Funnels plots for motor memory consolidation after single and extended practice.
Supplementary Material 10. Results of Egger’s tests.
REFERENCES
[1] | Dorsey ER , Sherer T , Okun MS , Bloem BR ((2018) ) The emerging evidence of the Parkinson pandemic. J Parkinsons Dis 8: , S3–s8. |
[2] | Kalia LV , Lang AE ((2015) ) Parkinson’s disease. Lancet 386: , 896–912. |
[3] | Connolly BS , Lang AE ((2014) ) Pharmacological treatment of Parkinson disease: a review. JAMA 311: , 1670–1683. |
[4] | Aarsland D , Larsen JP , Tandberg E , Laake K ((2000) ) Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. J Am Geriatr Soc 48: , 938–942. |
[5] | Keus SHJ , Bloem BR , Hendriks EJM , Bredero-Cohen AB , Munneke M , on behalf of the Practice Recommendations Development Group ((2007) ) Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov Disord 22: , 451–460. |
[6] | Abbruzzese G , Marchese R , Avanzino L , Pelosin E ((2016) ) Rehabilitation for Parkinson’s disease: Current outlook and future challenges. Parkinsonism Relat Disord 22: , S60–.S64. |
[7] | Kantak SS , Winstein CJ ((2012) ) Learning-performance distinction and memory processes for motor skills: a focused review and perspective. Behav Brain Res 228: , 219–231. |
[8] | Wolpert DM , Diedrichsen J , Flanagan JR ((2011) ) Principles of sensorimotor learning. Nat Rev Neurosci 12: , 739–751. |
[9] | Karni A , Meyer G , Rey-Hipolito C , Jezzard P , Adams MM , Turner R , Ungerleider LG ((1998) ) The acquisition of skilled motor performance: Fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A 95: , 861–868. |
[10] | Ericsson KA , Harwell KW ((2019) ) Deliberate practice and proposed limits on the effects of practice on the acquisition of expert performance: Why the original definition matters and recommendations for future research. Front Psychol 10: , 2396. |
[11] | Dayan E , Cohen LG ((2011) ) Neuroplasticity subserving motor skill learning. Neuron 72: , 443–454. |
[12] | Dudai Y ((2004) ) The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol 55: , 51–86. |
[13] | Doyon J , Bellec P , Amsel R , Penhune V , Monchi O , Carrier J , Lehéricy S , Benali H ((2009) ) Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res 199: , 61–75. |
[14] | Schmidt RA , Lee TD , Winstein C , Wulf G , Zelaznik HN ((2018) ), Motor control and learning: A behavioral emphasis, Human Kinetics. |
[15] | Marinelli L , Quartarone A , Hallett M , Frazzitta G , Ghilardi M ((2017) ) The many facets of motor learning and their relevance for Parkinson’s disease. Clin Neurophysiol 128: . |
[16] | Nieuwboer A , Rochester L , Müncks L , Swinnen SP ((2009) ) Motor learning in Parkinson’s disease: limitations and potential for rehabilitation. Parkinsonism Relat Disord 15 Suppl 3: , 53–58. |
[17] | Olson M , Lockhart TE , Lieberman A ((2019) ) Motor learning deficits in Parkinson’s disease (PD) and their effect on training response in gait and balance: a narrative review. Front Neurol 10: , 62. |
[18] | Moher D , Liberati A , Tetzlaff J , Altman DG , Group P ((2009) ) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: , e1000097. |
[19] | Booth A , Clarke M , Dooley G , Ghersi D , Moher D , Petticrew M , Stewart L ((2012) ) The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev 1: , 2. |
[20] | Morgan RL , Thayer KA , Bero L , Bruce N , Falck-Ytter Y , Ghersi D , Guyatt G , Hooijmans C , Langendam M , Mandrioli D ((2016) ) GRADE: Assessing the quality of evidence in environmental and occupational health. Environ Int 92: , 611–616. |
[21] | Morgan RL , Whaley P , Thayer KA , Schünemann HJ ((2018) ) Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int 121: , 1027–1031. |
[22] | Aslan DH , Hernandez ME , Frechette ML , Gephart AT , Soloveychik IM , Sosnoff JJ ((2021) ) The neural underpinnings of motor learning in people with neurodegenerative diseases: A scoping review. Neurosci Biobehav Rev 131: , 882–898. |
[23] | Felix K , Gain K , Paiva E , Whitney K , Jenkins ME , Spaulding SJ ((2012) ) Upper extremity motor learning among individuals with Parkinson’s disease: a meta-analysis evaluating movement time in simple tasks. Parkinsons Dis 2012: , 589152. |
[24] | Hayes HA , Hunsaker N , Dibble LE ((2015) ) Implicit motor sequence learning in individuals with Parkinson disease: a meta-analysis. J Parkinsons Dis 5: , 549–560. |
[25] | Knowlton B , Siegel A , Moody T ((2017) ) Procedural learning in humans. In Learning and Memory: A Comprehensive Reference |
[26] | Krakauer JW , Hadjiosif AM , Xu J , Wong AL , Haith AM ((2019) ) Motor learning. Compr Physiol 9: , 613–663. |
[27] | Siegert RJ , Taylor KD , Weatherall M , Abernethy DA ((2006) ) Is implicit sequence learning impaired in Parkinson’s disease? A meta-analysis. Neuropsychol 20: , 490–495. |
[28] | Ruitenberg MF , Duthoo W , Santens P , Notebaert W , Abrahamse EL ((2015) ) Sequential movement skill in Parkinson’s disease: a state-of-the-art. Cortex 65: , 102–112. |
[29] | Nackaerts E , D’Cruz N , Dijkstra BW , Gilat M , Kramer T , Nieuwboer A ((2019) ) Towards understanding neural network signatures of motor skill learning in Parkinson’s disease and healthy aging. Brit J Radiol 92: , 20190071. |
[30] | Barry G , Galna B , Rochester L ((2014) ) The role of exergaming in Parkinson’s disease rehabilitation: a systematic review of the evidence. J Neuroeng Rehabilitation 11: , 33. |
[31] | Abbruzzese G , Trompetto C , Marinelli L ((2009) ) The rationale for motor learning in Parkinson’s disease. Eur J Phys Rehabil Med 45: , 209–214. |
[32] | Clark GM , Lum JA , Ullman MT ((2014) ) A meta-analysis and meta-regression of serial reaction time task performance in Parkinson’s disease. Neuropsychol 28: , 945–958. |
[33] | National Institutes of Health ((2019) ) Quality assessment tool for observational cohort and cross-sectional studies. National Heart Lung and Blood Institute. |
[34] | Ma L-L , Wang Y-Y , Yang Z-H , Huang D , Weng H , Zeng X-T ((2020) ) Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res 7: , 7. |
[35] | Page MJ , Higgins JPT , Sterne JAC ((2019) ) Assessing risk of bias due to missing results in a synthesis. Cochrane Handbook for Systematic Reviews of Interventions, Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane. pp. 349–374. |
[36] | Egger M , Smith GD , Schneider M , Minder C ((1997) ) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: , 629–634. |
[37] | Sterne JAC , Sutton AJ , Ioannidis JPA , Terrin N , Jones DR , Lau J , Carpenter J , Rücker G , Harbord RM , Schmid CH , Tetzlaff J , Deeks JJ , Peters J , Macaskill P , Schwarzer G , Duval S , Altman DG , Moher D , Higgins JPT ((2011) ) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343: , d4002. |
[38] | Dijkstra BW , Bekkers EM , Gilat M , de Rond V , Hardwick RM , Nieuwboer A ((2020) ) Functional neuroimaging of human postural control: A systematic review with meta-analysis. Neurosci Biobehav Rev 115: , 351–362. |
[39] | Doya K ((2000) ) Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr Opin Neurobiol 10: , 732–739. |
[40] | Hardwick RM , Rottschy C , Miall RC , Eickhoff SB ((2013) ) A quantitative meta-analysis and review of motor learning in the human brain. NeuroImage 67: , 283–297. |
[41] | Hikosaka O , Nakamura K , Sakai K , Nakahara H ((2002) ) Central mechanisms of motor skill learning. Curr Opin Neurobiol 12: , 217–222. |
[42] | Surgent OJ , Dadalko OI , Pickett KA , Travers BG ((2019) ) Balance and the brain: A review of structural brain correlates ofostural balance and balance training in humans. Gait Posture 71: , 245–252. |
[43] | Taylor JA , Ivry RB ((2012) ) The role of strategies in motor learning. Ann N Y Acad Sci 1251: , 1–12. |
[44] | Maas E , Robin DA , Austermann Hula SN , Freedman SE , Wulf G , Ballard KJ , Schmidt RA ((2008) ) Principles of motor learning in treatment of motor speech disorders. Am J Speech Lang Pathol 17: , 277–298. |
[45] | Masapollo M , Segawa JA , Beal DS , Tourville JA , Nieto-Castañón A , Heyne M , Frankford SA , Guenther FH ((2021) ) Behavioral and neural correlates of speech motor sequence learning in stuttering and neurotypical speakers: an fMRI investigation. Neurobiol Lang (Camb) 2: , 106–137. |
[46] | Segawa JA , Tourville JA , Beal DS , Guenther FH ((2015) ) The neural correlates of speech motor sequence learning. J Cogn Neurosci 27: , 819–831. |
[47] | Spampinato D , Celnik P ((2021) ) Multiple motor learning processes in humans: defining their neurophysiological bases. Neuroscientist 27: , 246–267. |
[48] | Poldrack RA , Rodriguez P ((2003) ) Sequence learning: what’s the hippocampus to do? Neuron 37: , 891–893. |
[49] | Robertson EM ((2007) ) The serial reaction time task: implicit motor skill learning? J Neurosci 27: , 10073–10075. |
[50] | Karni A , Meyer G , Jezzard P , Adams MM , Turner R , Ungerleider LG ((1995) ) Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: , 155–158. |
[51] | Krakauer JW ((2009) ) Motor learning and consolidation: the case of visuomotor rotation. Adv Exp Med Biol 629: , 405–421. |
[52] | Krakauer JW , Ghez C , Ghilardi MF ((2005) ) Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci 25: , 473–478. |
[53] | Krakauer JW , Pine ZM , Ghilardi MF , Ghez C ((2000) ) Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20: , 8916–8924. |
[54] | Krakauer JW , Shadmehr R ((2006) ) Consolidation of motor memory. Trends Neurosci 29: , 58–64. |
[55] | Ivanenko Y , Gurfinkel VS ((2018) ) Human postural control. Front Neurosci 12: , 171. |
[56] | Taubert M , Draganski B , Anwander A , Müller K , Horstmann A , Villringer A , Ragert P ((2010) ) Dynamic properties of human brain structure: learning-related changes in cortical areas and associated fiber connections. J Neurosci 30: , 11670–11677. |
[57] | Guenther FH ((2016) ) Neural control of speech, MIT Press. |
[58] | Sternberg S , Monsell S , Knoll RL , Wright CE ((1978) ) The latency and duration of rapid movement sequences: comparisons of speech and typewriting. In Information Processing in Motor Control and Learning, Stelmach GE, ed. Academic Press Inc., New York, pp. 117–152. |
[59] | Alm PA ((2021) ) The dopamine system and automatization of movement sequences: a review with relevance for speech and stuttering. Front Hum Neurosci 15: , 661880. |
[60] | Frank JS , Horak FB , Nutt J ((2000) ) Centrally initiated postural adjustments in parkinsonian patients on and off levodopa. J Neurophysiol 84: , 2440–2448. |
[61] | Harrer M , Cuijpers P , Furukawa TA , Ebert DD ((2021) ) doing meta-analysis with R: A hands-on guide, CRC. |
[62] | Goetz CG , Poewe W , Rascol O , Sampaio C , Stebbins GT , Counsell C , Giladi N , Holloway RG , Moore CG , Wenning GK , Yahr MD , Seidl L ((2004) ) Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations The Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Mov Disord 19: , 1020–1028. |
[63] | Goetz CG , Tilley BC , Shaftman SR , Stebbins GT , Fahn S , Martinez-Martin P , Poewe W , Sampaio C , Stern MB , Dodel R , Dubois B , Holloway R , Jankovic J , Kulisevsky J , Lang AE , Lees A , Leurgans S , LeWitt PA , Nyenhuis D , Olanow CW , Rascol O , Schrag A , Teresi JA , van Hilten JJ , LaPelle N ((2008) ) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 23: , 2129–2170. |
[64] | Hoehn MM , Yahr MD ((1967) ) Parkinsonism. Neurology 17: , 427. |
[65] | Hentz JG , Mehta SH , Shill HA , Driver-Dunckley E , Beach TG , Adler CH ((2015) ) Simplified conversion method for unified Parkinson’s disease rating scale motor examinations. Mov Disord 30: , 1967–1970. |
[66] | Higgins JP , Green S ((2011) ) Cochrane handbook for systematic reviews of interventions, John Wiley & Sons. |
[67] | Kantak SS , Sullivan KJ , Fisher BE , Knowlton BJ , Winstein CJ ((2010) ) Neural substrates of motor memory consolidation depend on practice structure. Nat Neurosci 13: , 923–925. |
[68] | Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021), John Wiley & Sons, www.training.cochrane.org/handbook. |
[69] | Roig M , Nordbrandt S , Geertsen SS , Nielsen JB ((2013) ) The effects of cardiovascular exercise on human memory: A review with meta-analysis. Neurosci Biobehav Rev 37: , 1645–1666. |
[70] | Veroniki AA , Jackson D , Viechtbauer W , Bender R , Bowden J , Knapp G , Kuss O , Higgins JPT , Langan D , Salanti G ((2016) ) Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 7: , 55–79. |
[71] | Higgins JPT , Thompson SG ((2004) ) Controlling the risk of spurious findings from meta-regression. Stat Med 23: , 1663–1682. |
[72] | Nutt JG , Lea ES , Van Houten L , Schuff RA , Sexton GJ ((2000) ) Determinants of tapping speed in normal control subjects and subjects with Parkinson’s disease: Differing effects of brief and continued practice. Mov Disord 15: , 843–849. |
[73] | Doyon J , Laforce R Jr , Bouchard G , Gaudreau D , Roy J , Poirier M , Bédard PJ , Bédard F , Ouchard JP ((1998) ) Role of the striatum, cerebellum and frontal lobes in the automatization of a repeated visuomotor sequence of movements. Neuropsychologia 36: , 625–641. |
[74] | Gawrys L , Szatkowska I , Jamrozik Z , Janik P , Friedman A , Kaczmarek L ((2008) ) Nonverbal deficits in explicit and implicit memory of Parkinson’s disease patients. Acta Neurobiol Exp 68: , 58–72. |
[75] | Isaias IU , Moisello C , Marotta G , Schiavella M , Canesi M , Perfetti B , Cavallari P , Pezzoli G , Ghilardi MF ((2011) ) Dopaminergic striatal innervation predicts interlimb transfer of a visuomotor skill. J Neurosci 31: , 14458–14462. |
[76] | ThomasAnterion C , Laurent B , FoyatierMichel N , Laporte S , Michel D ((1996) ) Procedural memory: Computer learning in control subjects and in Parkinson’s disease patients. Behav Neurol 9: , 127–134. |
[77] | Werheid K , Zysset S , Müller A , Reuter M , von Cramon DY ((2003) ) Rule learning in a serial reaction time task: an fMRI study on patients with early Parkinson’s disease. Brain Res Cogn Brain Res 16: , 273–284. |
[78] | Agostino R , Curra A , Soldati G , Dinapoli L , Chiacchiari L , Modugno N , Pierelli F , Berardelli A ((2004) ) Prolonged practice is of scarce benefit in improving motor performance in Parkinson’s disease. Mov Disord 19: , 1285–1293. |
[79] | Behrman AL , Cauraugh JH , Light KE ((2000) ) Practice as an intervention to improve speeded motor performance and motor learning in Parkinson’s disease. J Neurol Sci 174: , 127–136. |
[80] | Dan X , King BR , Doyon J , Chan P ((2015) ) Motor sequence learning and consolidation in unilateral de novo patients with Parkinson’s disease. PloS One 10: , e0134291. |
[81] | Dantas I , Leal J , Hilgert L , Allegretti A , Mendes F ((2018) ) Training healthy persons and individuals with Parkinson’s disease to use Xbox Kinect games: A preliminary study. Int J Ther Rehabil 25: , 280–290. |
[82] | Foreman KB , Sondrup S , Dromey C , Jarvis E , Nissen S , Dibble LE ((2013) ) The effects of practice on the concurrent performance of a speech and postural task in persons with Parkinson disease and healthy controls. Parkinsons Dis 2013: , 987621. |
[83] | Hadj-Bouziane F , Benatru I , Brovelli A , Klinger H , Thobois S , Broussolle E , Boussaoud D , Meunier M ((2013) ) Advanced Parkinson’s disease effect on goal-directed and habitual processes involved in visuomotor associative learning. Front Hum Neurosci 6: , 351. |
[84] | Harrington DL , Haaland KY , Yeo RA , Marder E ((1990) ) Procedural memory in Parkinson’s disease: impaired motor but not visuoperceptual learning. J Clin Exp Neuropsychol 12: , 323–339. |
[85] | Hayes HA , Hunsaker N ((2015) ) Does dopamine replacement medication affect postural sequence learning in Parkinson’s disease? Motor Control 19: , 325–340. |
[86] | Jessop RT , Horowicz C , Dibble LE ((2006) ) Motor learning and Parkinson disease: Refinement of movement velocity and endpoint excursion in a limits of stability balance task. Neurorehabil Neural Repair 20: , 459–467. |
[87] | Kawashima S , Ueki Y , Kato T , Ito K , Matsukawa N ((2018) ) Reduced striatal dopamine release during motor skill acquisition in Parkinson’s disease. PLoS One 13: , e0196661. |
[88] | Lahlou S , Gabitov E , Owen L , Shohamy D , Sharp M ((2022) ) Preserved motor memory in Parkinson’s disease. Neuropsychologia 167: , 108161. |
[89] | Lee YY , Fisher BE ((2018) ) Use of low-frequency repetitive transcranial magnetic stimulation to reduce context-dependent learning in people with Parkinson’s disease. Eur J Phys Rehabil 54: , 560–567. |
[90] | Lee YY , Tai CH , Fisher BE ((2019) ) Context-dependent behavior in Parkinson’s disease with freezing of gait. Neurorehabil Neural Repair 33: , 1040–1049. |
[91] | Lee Y-Y , Winstein CJ , Gordon J , Petzinger GM , Zelinski EM , Fisher BE ((2016) ) Context-dependent learning in people with Parkinson’s disease. J Mot Behav 48: , 240–248. |
[92] | Leow LA , Loftus AM , Hammond GR ((2012) ) Impaired savings despite intact initial learning of motor adaptation in Parkinson’s disease. Exp Brain Res 218: , 295–304. |
[93] | Lin C , Sullivan KJ , Wu AD , Kantak S , Winstein CJ ((2007) ) Effect of task practice order on motor skill learning in adults with Parkinson disease: a pilot study. Phys Ther 87: , 1120–1131. |
[94] | Marinelli L , Crupi D , Di Rocco A , Bove M , Eidelberg D , Abbruzzese G , Ghilardi MF ((2009) ) Learning and consolidation of visuo-motor adaptation in Parkinson’s disease. Parkinsonism Relat Disord 15: , 6–11. |
[95] | Marinelli L , Trompetto C , Canneva S , Mori L , Nobili F , Fattapposta F , Currà A , Abbruzzese G , Ghilardi MF ((2017) ) Learning “how to learn”: super declarative motor learning is impaired in Parkinson’s disease. Neural Plast 2017: , 3162087. |
[96] | Mochizuki-Kawai H , Mochizuki S , Kawamura M ((2010) ) A flexible sequential learning deficit in patients with Parkinson’s disease: a 2 x 8 button-press task. Exp Brain Res 202: , 147–153. |
[97] | Nackaerts E , Ginis P , Heremans E , Swinnen SP , Vandenberghe W , Nieuwboer A ((2020) ) Retention of touchscreen skills is compromised in Parkinson’s disease. Behav Brain Res 378: . |
[98] | Nelson AB , Moisello C , Lin J , Panday P , Ricci S , Canessa A , Di Rocco A , Quartarone A , Frazzitta G , Isaias IU , Tononi G , Cirelli C , Ghilardi MF ((2017) ) Beta oscillatory changes and retention of motor skills during practice in healthy subjects and in patients with Parkinson’s disease. Front Hum Neurosci 11: , 104. |
[99] | Nicastro N , Manuel AL , Garibotto V , Burkhard PR , Schnider A ((2018) ) Consolidation of a learned skill correlates with dopamine SPECT uptake in early Parkinson’s disease. J Clin Neurol 14: , 505–512. |
[100] | Onla-Or S , Winstein CJ ((2008) ) Determining the optimal challenge point for motor skill learning in adults with moderately severe Parkinson’s disease. Neurorehabil Neural Repair 22: , 385–395. |
[101] | Pendt LK , Maurer H , Muller H ((2012) ) The influence of movement initiation deficits on the quantification of retention in Parkinson’s disease. Front Hum Neurosci 6: , 226. |
[102] | Pendt LK , Reuter I , Muller H ((2011) ) Motor skill learning, retention, and control deficits in Parkinson’s disease. PLoS One 6: , e21669. |
[103] | Peterson DS , Dijkstra BW , Horak FB ((2016) ) Postural motor learning in people with Parkinson’s disease. J Neurol 263: , 1518–1529. |
[104] | Platz T , Brown RG , Marsden CD ((1998) ) Training improves the speed of aimed movements in Parkinson’s disease. Brain 121: , 505–514. |
[105] | Rostami HR , Ashayeri H ((2009) ) Effects of motor skill practice on reaction time and learning retention in Parkinson’s disease. Neurol India 57: , 768–771. |
[106] | Roy S , Park NW , Roy EA , Almeida QJ ((2015) ) Interaction of memory systems during acquisition of tool knowledge and skills in Parkinson’s disease. Neuropsychologia 66: , 55–66. |
[107] | Sato E , Onitsuka T , Ninomiya H , Nakamura I , Kanba S ((2014) ) Prism adaptation and perceptual skill learning deficits in early-stage Parkinson’s disease. Neuropsychobiology 70: , 165–172. |
[108] | Sehm B , Taubert M , Conde V , Weise D , Classen J , Dukart J , Draganski B , Villringer A , Ragert P ((2014) ) Structural brain plasticity in Parkinson’s disease induced by balance training. Neurobiol Aging 35: , 232–239. |
[109] | Sidaway B , Ala B , Baughman K , Glidden J , Cowie S , Peabody A , Roundy D , Spaulding J , Stephens R , Wright DL ((2016) ) Contextual interference can facilitate motor learning in older adults and in individuals with Parkinson’s disease. J Mot Behav 48: , 509–518. |
[110] | Simley-Oyen AL , Worringham CJ , Cross CL ((2002) ) Practice effects in three-dimensional sequential rapid aiming in Parkinson’s disease. Mov Disord 17: , 1196–1204. |
[111] | Smiley-Oyen AL , Hall SA , Lowry KA , Kerr JP ((2012) ) Effects of extensive practice on bradykinesia in Parkinson’s disease: improvement, retention and transfer. Motor Control 16: , 1–18. |
[112] | Smiley-Oyen AL , Lowry KA , Emerson QR ((2006) ) Learning and retention of movement sequences in Parkinson’s disease. Mov Disord 21: , 1078–1087. |
[113] | Smiley-Oyen AL , Worringham CJ , Cross CL ((2003) ) Motor learning processes in a movement-scaling task in olivopontocerebellar atrophy and Parkinson’s disease. Exp Brain Res 152: , 453–465. |
[114] | Swinnen SP , Steyvers M , Van den Bergh L , Stelmach GE ((2000) ) Motor learning and Parkinson’s disease: refinement of within-limb and between-limb coordination as a result of practice. Behav Brain Res 111: , 45–59. |
[115] | Terpening Z , Naismith S , Melehan K , Gittins C , Bolitho S , Lewis SJ ((2013) ) The contribution of nocturnal sleep to the consolidation of motor skill learning in healthy ageing and Parkinson’s disease. J Sleep Res 22: , 398–405. |
[116] | Van Ooteghem K , Frank JS , Horak FB ((2017) ) Postural motor learning in Parkinson’s disease: The effect of practice on continuous compensatory postural regulation. Gait Posture 57: , 299–304. |
[117] | Whitfield JA , Goberman AM ((2017) ) Speech motor sequence learning: acquisition and retention in Parkinson disease and normal aging. J Speech Lang Hear Res 60: , 1477–1492. |
[118] | Martínez-Martín P , Rodríguez-Blázquez C , Mario A , Arakaki T , Arillo VC , Chanáa P , Fernández W , Garretto N , Martínez-Castrillo JC , Rodríguez-Violante M , Serrano-Dueñas M , Ballesteros D , Rojo-Abuin JM , Chaudhuri KR , Merello M ((2015) ) Parkinson’s disease severity levels and MDS-Unified Parkinson’s Disease Rating Scale. Parkinsonism Relat Disord 21: , 50–54. |
[119] | Smiley-Oyen AL , Worringham CJ , Cross CL ((2002) ) Practice effects in three-dimensional sequential rapid aiming in Parkinson’s disease. Mov Disord 17: , 1196–1204. |
[120] | Doyon J ((2008) ) Motor sequence learning and movement disorders. Curr Opin Neurol 21: , 478–483. |
[121] | Ferrazzoli D , Ortelli P , Madeo G , Giladi N , Petzinger GM , Frazzitta G ((2018) ) Basal ganglia and beyond: The interplay between motor and cognitive aspects in Parkinson’s disease rehabilitation. Neurosci Biobehav Rev 90: , 294–308. |
[122] | Hawkes CH , Del Tredici K , Braak H ((2010) ) A timeline for Parkinson’s disease. Parkinsonism Relat Disord 16: , 79–84. |
[123] | Blesa J , Foffani G , Dehay B , Bezard E , Obeso JA ((2022) ) Motor and non-motor circuit disturbances in early Parkinson disease: which happens first? Nat Rev Neurosci 23: , 115–128. |
[124] | Rodriguez-Oroz MC , Jahanshahi M , Krack P , Litvan I , Macias R , Bezard E , Obeso JA ((2009) ) Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol 8: , 1128–1139. |
[125] | Obeso JA , Rodríguez-Oroz MC , Benitez-Temino B , Blesa FJ , GuridiJ , Marin C , Rodriguez M ((2008) ) Functional organization of the basalganglia: therapeutic implications for Parkinson’s disease. Mov Disord 23 Suppl 3: , S548–559. |
[126] | Kordower JH , Olanow CW , Dodiya HB , Chu Y , Beach TG , Adler CH , Halliday GM , Bartus RT ((2013) ) Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 136: , 2419–2431. |
[127] | Schindlbeck KA , Eidelberg D ((2018) ) Network imaging biomarkers: insights and clinical applications in Parkinson’s disease. Lancet Neurol 17: , 629–640. |
[128] | Wu T , Hallett M ((2013) ) The cerebellum in Parkinson’s disease. Brain 136: , 696–709. |
[129] | Tahmasian M , Eickhoff SB , Giehl K , Schwartz F , Herz DM , Drzezga A , van Eimeren T , Laird AR , Fox PT , Khazaie H , Zarei M , Eggers C , Eickhoff CR ((2017) ) Resting-state functional reorganization in Parkinson’s disease: An activation likelihood estimation meta-analysis. Cortex 92: , 119–138. |
[130] | Ammann C , Dileone M , Pagge C , Catanzaro V , Mata-Marín D , Hernández-Fernádez F , Monje MHG , Sánchez-Ferro À , Fernández-Rodríguez B , Gasca-Salas C , Máñez-Miró JU , Martínez-Fernández R , Vela-Desojo L , Alonso-Frech F , Oliviero A , Obeso JA , Foffani G ((2020) ) Cortical disinhibition in Parkinson’s disease. Brain 143: , 3408–3421. |
[131] | Lindenbach D , Bishop C ((2013) ) Critical involvement of the motor cortex in the pathophysiology and treatment of Parkinson’s disease. Neurosci Biobehav Rev 37: , 2737–2750. |
[132] | Kish SJ , Shannak K , Hornykiewicz O ((1988) ) Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med 318: , 876–880. |
[133] | Kehagia AA , Barker RA , Robbins TW ((2013) ) Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neurodegener Dis 11: , 79–92. |
[134] | Pineda-Pardo JA , Sánchez-Ferro À , Monje MHG , Pavese N , Obeso JA ((2022) ) Onset pattern of nigrostriatal denervation in early Parkinson’s disease. Brain 145: , 1018–1028. |
[135] | Agosta F , Canu E , Stojković T , Pievani M , Tomić A , Sarro L , Dragašević N , Copetti M , Comi G , Kostić VS , Filippi M ((2013) ) The topography of brain damage at different stages of Parkinson’s disease. Hum Brain Mapp 34: , 2798–2807. |
[136] | Huang VS , Haith A , Mazzoni P , Krakauer JW ((2011) ) Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron 70: , 787–801. |
[137] | Huber R , Ghilardi MF , Massimini M , Tononi G ((2004) ) Local sleep and learning. Nature 430: , 78–81. |
[138] | Galea JM , Vazquez A , Pasricha N , Orban de Xivry J-J , Celnik P ((2011) ) Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex 21: , 1761–1770. |
[139] | Moisello C , Blanco D , Fontanesi C , Lin J , Biagioni M , Kumar P , Brys M , Loggini A , Marinelli L , Abbruzzese G , Quartarone A , Tononi G , Di Rocco A , Ghilardi MF ((2015) ) TMS enhances retention of a motor skill in Parkinson’s disease. Brain Stimul 8: , 224–230. |
[140] | Manuel AL , Nicastro N , Schnider A , Guggisberg AG ((2018) ) Resting-state connectivity after visuo-motor skill learning is inversely associated with offline consolidation in Parkinson’s disease and healthy controls. Cortex 106: , 237–247. |
[141] | Lehéricy S , Benali H , Van de Moortele P-F , Pélégrini-Issac M , Waechter T , Ugurbil K , Doyon J ((2005) ) Distinct basal ganglia territories are engaged in early and advanced motor sequence learning. Proc Natl Acad Sci U S A 102: , 12566–12571. |
[142] | Appel-Cresswell S , de la Fuente-Fernandez R , Galley S , McKeown MJ ((2010) ) Imaging of compensatory mechanisms in Parkinson’s disease. Curr Opin Neurol 23: , 407–412. |
[143] | Mentis MJ , Dhawan V , Feigin A , Delalot D , Zgaljardic D , Edwards C , Eidelberg D ((2003) ) Early stage Parkinson’s disease patients and normal volunteers: comparative mechanisms of sequence learning. Hum Brain Mapp 20: , 246–258. |
[144] | Mentis MJ , Dhawan V , Nakamura T , Ghilardi M , Feigin A , Edwards C , Ghez C , Eidelberg D ((2003) ) Enhancement of brain activation during trial-and-error sequence learning in early PD. Neurology 60: , 612–619. |
[145] | Simioni AC , Dagher A , Fellows LK ((2016) ) Compensatory striatal-cerebellar connectivity in mild-moderate Parkinson’s disease. Neuroimage Clin 10: , 54–62. |
[146] | O’Callaghan C , Hornberger M , Balsters JH , Halliday GM , Lewis SJG , Shine JM ((2016) ) Cerebellar atrophy in Parkinson’s disease and its implication for network connectivity. Brain 139: , 845–855. |
[147] | Sami S , Robertson EM , Miall RC ((2014) ) The time course of task-specific memory consolidation effects in resting state networks. J Neurosci 34: , 3982–3992. |
[148] | Carbon M , Reetz K , Ghilardi MF , Dhawan V , Eidelberg D ((2010) ) Early Parkinson’s disease: Longitudinal changes in brain activity during sequence learning. Neurobiol Dis 37: , 455–460. |
[149] | Carbon M , Felice Ghilardi M , Dhawan V , Eidelberg D ((2007) ) Correlates of movement initiation and velocity in Parkinson’s disease: A longitudinal PET study. Neuroimage 34: , 361–370. |
[150] | Redgrave P , Rodriguez M , Smith Y , Rodriguez-Oroz MC , Lehericy S , Bergman H , Agid Y , DeLong MR , Obeso JA ((2010) ) Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci 11: , 760–772. |
[151] | Wu T , Hallett M , Chan P ((2015) ) Motor automaticity in Parkinson’s disease. Neurobiol Dis 82: , 226–234. |
[152] | Schoneburg B , Mancini M , Horak F , Nutt JG ((2013) ) Framework for understanding balance dysfunction in Parkinson’s disease. Mov Disord 28: , 1474–1482. |
[153] | Kim SD , Allen NE , Canning CG , Fung VS ((2013) ) Postural instability in patients with Parkinson’s disease. CNS Drugs 27: , 97–112. |
[154] | Mak MK , Wong-Yu IS , Shen X , Chung CL ((2017) ) Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat Rev Neurol 13: , 689–703. |
[155] | Klawans HL ((1986) ) Individual manifestations of Parkinson’s disease after ten or more years of levodopa. Mov Disord 1: , 187–192. |
[156] | Allen NE , Schwarzel AK , Canning CG ((2013) ) Recurrent falls in Parkinson’s disease: a systematic review. Parkinsons Dis 2013: , 906274. |
[157] | Bloem BR , Grimbergen YA , Cramer M , Willemsen M , Zwinderman AH ((2001) ) Prospective assessment of falls in Parkinson’s disease. J Neurol 248: , 950–958. |
[158] | Boonstra TA , van der Kooij H , Munneke M , Bloem BR ((2008) ) Gait disorders and balance disturbances in Parkinson’s disease: clinical update and pathophysiology. Curr Opin Neurol 21: , 461–471. |
[159] | Canning CG , Paul SS , Nieuwboer A ((2014) ) Prevention of falls in Parkinson’s disease: a review of fall risk factors and the role of physical interventions. Neurodegener Dis Manag 4: , 203–221. |
[160] | Stolze H , Klebe S , Baecker C , Zechlin C , Friege L , Pohle S , Deuschl G ((2005) ) Prevalence of gait disorders in hospitalized neurological patients. Mov Disord 20: , 89–94. |
[161] | Zhuang X , Mazzoni P , Kang UJ ((2013) ) The role of neuroplasticity in dopaminergic therapy for Parkinson disease. Nat Rev Neurol 9: , 248–256. |
[162] | Liu H-H , Wang R-Y , Cheng S-J , Liao K-K , Zhou J-H , Yang Y-R ((2022) ) Balance training modulates cortical inhibition in individuals with Parkinson’s disease: a randomized controlled trial. Neurorehabil Neural Repair 36: , 613–620. |
[163] | Rothwell JC , Edwards MJ ((2013) ) Parkinson’s disease. Handb Clin Neurol 116: , 535–542. |
[164] | Blesa J , Trigo-Damas I , Dileone M , del Rey NL-G , Hernandez LF , Obeso JA ((2017) ) Compensatory mechanisms in Parkinson’s disease: Circuits adaptations and role in disease modification. Exp Neurol 298: , 148–161. |
[165] | Rennie L , Opheim A , Dietrichs E , Löfgren N , Franzén E ((2021) ) Highly challenging balance and gait training for individuals with Parkinson’s disease improves pace, rhythm and variability domains of gait–A secondary analysis from a randomized controlled trial. Clin Rehabil 35: , 200–212. |
[166] | Muslimovic D , Post B , Speelman JD , Schmand B ((2007) ) Motor procedural learning in Parkinson’s disease. Brain 130: , 2887–2897. |
[167] | Stephan MA , Meier B , Zaugg SW , Kaelin-Lang A ((2011) ) Motor sequence learning performance in Parkinson’s disease patients depends on the stage of disease. Brain Cogn 75: , 135–140. |
[168] | Ghilardi MF , Eidelberg D , Silvestri G , Ghez C ((2003) ) The differential effect of PD and normal aging on early explicit sequence learning. Neurology 60: , 1313–1319. |
[169] | Carbon M , Eidelberg D ((2006) ) Functional imaging of sequence learning in Parkinson’s disease. J Neurol Sci 248: , 72–77. |
[170] | Carbon M , Ma Y , Barnes A , Dhawan V , Chaly T , Ghilardi MF , Eidelberg D ((2004) ) Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in Parkinsonism. Neuroimage 21: , 1497–1507. |
[171] | Carbon M , Ghilardi MF , Feigin A , Fukuda M , Silvestri G , Mentis MJ , Ghez C , Moeller JR , Eidelberg D ((2003) ) Learning networks in health and Parkinson’s disease: Reproducibility and treatment effects. Hum Brain Mapp 19: , 197–211. |
[172] | Palmer SJ , Ng B , Abugharbieh R , Eigenraam L , McKeown MJ ((2009) ) Motor reserve and novel area recruitment: amplitude and spatial characteristics of compensation in Parkinson’s disease. Eur J Neurosci 29: , 2187–2196. |
[173] | Chiviacowsky S , Campos T , Domingues MR ((2010) ) Reduced frequency of knowledge of results enhances learning in persons with Parkinson’s disease. Front Psychol 1: , 226. |
[174] | Konczak J , Corcos DM , Horak F , Poizner H , Shapiro M , Tuite P , Volkmann J , Maschke M ((2009) ) Proprioception and motor control in Parkinson’s disease. J Mot Behav 41: , 543–552. |
[175] | Adamovich SV , Berkinblit MB , Hening W , Sage J , Poizner H ((2001) ) The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in Parkinson’s disease. Neurosci 104: , 1027–1041. |
[176] | Contreras-Vidal JL , Gold DR ((2004) ) Dynamic estimation of hand position is abnormal in Parkinson’s disease. Parkinsonism Relat Disord 10: , 501–506. |
[177] | Watson GS , Leverenz JB ((2010) ) Profile of cognitive impairment in Parkinson’s disease. Brain Pathol 20: , 640–645. |
[178] | Wulf G , Landers M , Lewthwaite R , Toöllner T ((2009) ) External focus instructions reduce postural instability in individuals with Parkinson disease. Phys Ther 89: , 162–168. |
[179] | Wulf G ((2013) ) Attentional focus and motor learning: a review of 15 years. Int Rev Sport Exerc Psychol 6: , 77–104. |
[180] | Wulf G , Shea CH ((2002) ) Principles derived from the study of simple skills do not generalize to complex skill learning. Psychon Bull Rev 9: , 185–211. |
[181] | Vaillancourt DE , Schonfeld D , Kwak Y , Bohnen NI , Seidler R ((2013) ) Dopamine overdose hypothesis: evidence and clinical implications. Mov Disord 28: , 1920–1929. |
[182] | Cools R , Barker RA , Sahakian BJ , Robbins TW ((2001) ) Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex 11: , 1136–1143. |
[183] | Ghilardi MF , Feigin AS , Battaglia F , Silvestri G , Mattis P , Eidelberg D , Di Rocco A ((2007) ) L-Dopa infusion does not improve explicit sequence learning in Parkinson’s disease. Parkinsonism Relat Disord 13: , 146–151. |
[184] | Miller N , Noble E , Jones D , Burn D ((2006) ) Life with communication changes in Parkinson’s disease. Age Ageing 35: , 235–239. |
[185] | Ramig LO , Fox C , Sapir S ((2008) ) Speech treatment for Parkinson’s disease. Expert Rev Neurother 8: , 297–309. |
[186] | Ramig L , Fox C , Sapir S ((2011) ) Speech and voice disorders in parkinson’s disease. In Parkinson’s Disease: Non-Motor and Non-Dopaminergic Features, Blackwell Publishing Ltd. |




