The Use of Liquid Sinemet in Routine Clinical Practice of Advanced Parkinson’s Disease: A Comparison of Available Options
Abstract
Background:
Tablet formulations of Parkinson’s disease (PD) medications may become ineffective at managing motor fluctuations in advanced PD. The liquid formulation, levodopa carbidopa ascorbic acid solution, or LCAS, is an effective and inexpensive treatment for motor fluctuations however it remains underutilized.
Objective:
We compared the efficacy of LCAS with tablet formulations and Duodopa jejunal infusion through routine inpatient management using hourly functional status measures, the Timed Up and Go Test (TUG). The TUG differentiates between ‘off’ and ‘on’ states and quantifies motor fluctuations.
Methods:
Experienced nurses used the TUG times and functional observations recorded hourly throughout the waking day to optimize the LCAS hourly dose and the Duodopa flow rate over several days. When patients were stabilized on each of the interventions, the TUG measures were then recorded to compare the outcomes of the interventions.
Results:
Twenty-six participants had TUG times recorded while on one or more of the formulations: 19 had TUG times recorded on tablets, 23 on LCAS and 10 on Duodopa. TUG times on LCAS and Duodopa were significantly faster compared to tablets (p < 0.0001, p = 0.001 respectively). Severity of dyskinesia was not significantly different between formulations (p = 0.35). Daily dose for the three formulations and the hourly doses for LCAS and Duodopa did not differ significantly (p = 0.37, p = 0.19 respectively).
Conclusion:
This report demonstrated the efficacy of LCAS for improving motor complications and its equivalency with Duodopa jejunal infusion.
INTRODUCTION
Advanced Parkinson’s disease (PD) is characterized by unpredictable motor responses to levodopa which impact on the person’s quality of life and functional independence [1]. Motor fluctuations develop due to striatal denervation, delayed gastric transport to deliver the levodopa to the jejunum, and excessive peripheral degradation of levodopa prior to reaching the brain. The resultant variability of the plasma levels of levodopa presents clinically as the wearing “off” effect and/or random “on-off” fluctuations along with troublesome dyskinesias. There are multiple other factors that make optimization of the dosing schedule for levodopa difficult. Typically, people with PD are managed medically as outpatients and with infrequent reviews. Doctors rely on the patient’s self-reporting regarding their response to their current PD medication regimen however patients find it difficult to self-monitor and adjust dosage accordingly. Added to this, levodopa tablets have limited capacity to be fractionated when trying to enter the therapeutic window and COMT inhibition is limited with conventional agents. Inpatient management can overcome some of these difficulties as it enables close monitoring, dose to dose adjustments according to need and education of the patient in self-management of their PD medication regime.
Apomorphine subcutaneous infusion and Duodopa jejunal infusion are proven pharmaceutical options that can provide consistency of motor response [2, 3]. However, both are invasive, expensive, and not always suitable when other medical conditions are present, such as gastro-intestinal dysfunction and neuropsychological problems. One formulation that is not widely recognized or used, even though studies in the 1990s reported on its efficacy, is levodopa carbidopa ascorbic acid solution or LCAS [4–8]. LCAS is administered orally and is typically taken hourly throughout the person’s waking day. LCAS has the potential to overcome motor fluctuations in advanced PD as levodopa in solution rapidly passes out of the stomach and is more reliably absorbed in the jejunum. When taken at hourly intervals, the plasma levodopa levels can be stabilized due to the consistency in circulating levodopa [5]. This formulation can also be micro-titrated from dose to dose enabling the optimization of motor response without significant dyskinesia. LCAS is easy to administer, it is non-invasive and does not require medical devices such as pumps to administer and is low cost. To maximize the benefit of LCAS it needs to be administered in an inpatient setting where experienced nurses can monitor the patient’s clinical status hourly and adjust the LCAS dose to minimize dyskinesia and maximize mobility [7].
The primary objective of this study was to explore the efficacy of LCAS on motor complications by comparing the person’s mobility times on LCAS to that on tablet formulations and Duodopa gel performed on an inpatient basis but assessed on each intervention post community experience. The Timed Up and Go test (TUG) was used to measure mobility [9]. To the best of our knowledge the efficacy of LCAS compared to Duodopa has not been previously investigated.
METHODS
Participants
This prospective study, using routine clinical practice, monitored a group of patients in their transition through management options for advanced PD. It was not practical in a real world setting to randomize the two treatment options as each participant decided on their preferred treatment after participating in a trial of LCAS. Although participants would agree to trial LCAS they would not necessarily agree to Duodopa jejunal infusion, making randomization impractical. Patients were recruited from the Monash Health Comprehensive Parkinson Care program by a specialist PD neurologist and specialist PD geriatrician. Inclusion criteria were: 1) motor fluctuations that could not be adequately controlled using tablet formulations of PD medications; 2) agreement to trial LCAS as an inpatient. Exclusion criteria were: 1) a diagnosis of dementia, psychosis or other neuropsychological problems that might interfere with their ability to titrate LCAS doses; 2) the absence of a caregiver if help was required to administer the LCAS.
Treatment timeline
All participants admitted to the trial were on either an optimized tablet regime prior to admission, but were experiencing sporadic responses, or had previously been stabilized on LCAS and had found the hourly intake intolerable and had decided to accept Duodopa jejunal infusion as a better option. On admission the original treatment regime remained unchanged for three days while the motor control was documented. When a new regime was established the motor control was again documented for three days. Documentation of motor control therefore consisted of the three established treatment options of oral tablets, LCAS and Duodopa jejunal infusion, however the number of participants in each group varied according to the choices they made based on their treatment experiences.
Ethics and consent
All participants received usual clinical care during the time they were active in this study. The study was approved by Monash Health Human Research Ethics Committee (HREC reference number 16051L). All participants provided written informed consent. All methods were carried out in accordance with relevant guidelines and regulations.
Intervention
LCAS
Participants were admitted to a PD specialist inpatient unit at Monash Health where LCAS was introduced under the supervision of PD specialist doctors and nursing staff. The LCAS solution was prepared each morning by nursing staff. Ten levodopa/carbidopa 100/25 mg tablets were dissolved in one liter of water along with ascorbate (2000 mg/L) [4]. Ascorbate inclusion reduced the pH to enable solubility at room temperature for up to 3 days [10]. One milliliter of LCAS was equivalent to 1 mg of levodopa/carbidopa.
The PD neurologist/geriatrician determined the LCAS dosage range for each patient. PD specialist nurses titrated the hourly doses of LCAS in consultation with the patient. The first and last doses were administered at the same time the patient took their first and last tablet formulations. Tablet formulations were continued in the late evening to manage motor function overnight. Each dose of LCAS was based on the individual’s motor response to the prior dose. The patient’s TUG times, extra movements and other troublesome symptoms, such as dystonia, were used to decide if the dose was to be adjusted. Dosages were adjusted over several days until motor response was optimized and motor fluctuations were minimized. Once the optimal LCAS dosage was achieved, participants and their carers trialed administering LCAS under the supervision of nursing staff. Nurses provided education to patients and carers on selecting the optimal LCAS dose. Patients were then discharged home on LCAS with support from the program’s community nurse as required.
Duodopa
Participants who elected to change from LCAS to Duodopa were admitted to the PD inpatient unit at a later date. They were monitored for 1 to 3 days before being transferred to an acute hospital for the surgical insertion of the PEG-J tube. Once medically stable, they were transferred back to the PD inpatient unit. Duodopa was commenced with the initial dose based on the individual’s usual LCAS dose. The patient’s motor response, using TUG times and severity of any extra movements, to the Duodopa was monitored over several days and the flow rate and bolus dose adjusted accordingly.
Assessments/outcomes
Assessments were conducted at Test 1, Test 2, and Test 3. Demographic data collected included sex, age, disease duration, disease stage using the Hoehn and Yahr Stage scale when ‘on’, non-motor symptoms using the Non-Motor Symptom Questionnaire (NMS-Quest) [11], comorbidities, falls history and social living situation. Assessments on tablets and on LCAS reflected the prior community experiences on those forms of medications prior to the respective admission.
The primary outcome measure was the TUG, a simple, easy to use and practical clinical test to measure functional mobility throughout the medication cycle in people with PD. The TUG has high inter and intra-rater reliability, moderate to good validity when contrasted to walking and balance tests, it is highly correlated to gait velocity and sensitive enough to demonstrate change between motor states in PD [9, 12, 13]. PD trained nurses recorded the patient’s TUG times hourly throughout their waking day to capture mobility fluctuations over several medication cycles. The nurse recorded the time it took the participant to stand up from a chair, walk 3 meters, turn then walk back to the chair and sit down. If the participant was unable to perform the TUG independently, with or without a walking aid, the trial was recorded as ‘unable’. The presence of dyskinesias or tremor was noted and severity and body part affected recorded at each hourly test. Severity was rated 0 if not present, 1 if mild and present with muscle activation only, 2 if mild and present at rest and not interfering with voluntary movement, 3 if moderate causing interference with voluntary movement and 4 severe [14]. Visual information that described the motor status was also noted for each TUG measure by the attendant nurse.
Secondary outcome measures included patient reported outcome measures and observer rated outcome tools validated in PD populations. The outcome tools included the PD specific health related quality of life (HRQOL) tool the PDQ39, the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) parts I–IV, the Schwab and England (S&E) Activities of Daily Living tool, the Geriatric Depression Scale (GDS), and the Hoehn and Yahr (H&Y) disease stage scale. The MDS-UPDRS part III (motor function) and Hoehn and Yahr scales were administered during the participant’s ‘on’ time. The person’s self-report on the duration and severity of their dyskinesias and the duration, severity and complexity of their ‘off’ times was captured using part IV of the MDS-UPDRS.
Data analysis
All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA). Continuous outcomes were assessed for normality and log transformed where appropriate. To assess the effect of test on outcomes after accounting for repeat measures, data were analyzed using the PROC MIXED procedure in SAS with each patient treated as a random effect. Models were fitted using main effect for test for each outcome with results reported as least square means and standard errors. Post-hoc comparisons were performed using Bonferroni adjustment for multiple comparisons. The effect of test on the primary outcome was further assessed by adjusting for age and Hoehn and Yahr stage. The regression estimates for log-transformed data were re-transformed back to the original scale and results reported as geometric means and 95% confidence intervals [15]. All observed data were considered for analysis, with the mixed-effects models assuming non-informative dropout such that the probability of dropout may depend on a participant’s previous response but not on current or future responses. The association of test with dyskinesia and tremor was determined using ordinal logistic regression. All calculated p values were two-tailed and a p < 0.05 indicated statistical significance.
RESULTS
Characteristics
Twenty-six participants participated in this study. The 22 men and 4 women had a mean age of 72.3 (SD 8.8) years, a mean disease duration of 11.6 (SD 5.0) years and a mean Mini-Mental State Examination score 26.5 (SD 2.9). The median Hoehn and Yahr stage when ‘on’ was 3 (IQR 2,3) with 9 (36%) having mild PD (stages 1 and 2) and 16 (64%) having moderate to severe PD (stages 3 and 4). The NMS-Quest showed the minimum number of non-motor symptoms reported was 3 and the maximum 24, with mean (SE) of 14.38 (1.25). All participants had other medical conditions besides PD, ranging from 1 to 7 comorbidities. Most participants lived at home with a spouse/partner, 22 (85%), 1 (4%) lived alone and 3 (11%) lived in supported accommodation. Falls in the last 12 months were common, reported by 19 (73%).
Mobility scores
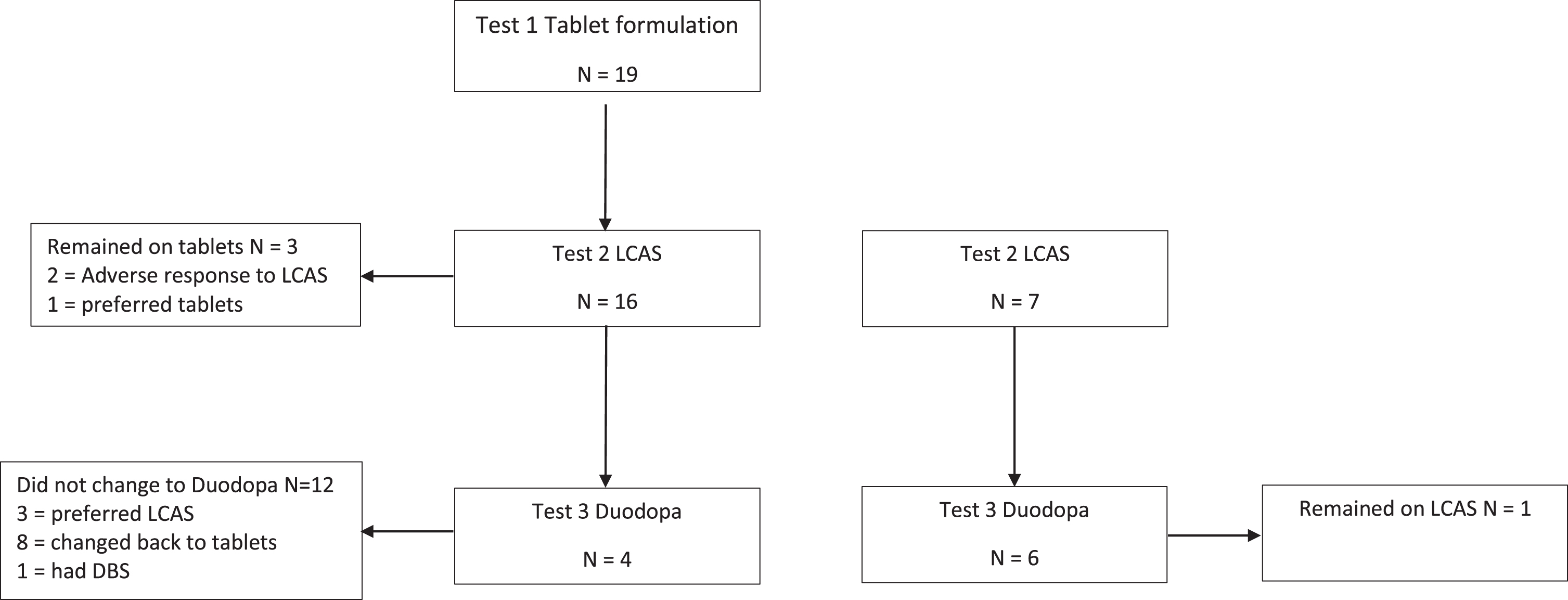
Nineteen participants had their TUG times recorded and secondary outcomes measured when on a stable dose of tablet formulation of levodopa (Test 1). All 19 participants commenced a trial of LCAS, with 16 progressing to a stable dose of LCAS and three reverting to tablet formulations. These three participants had their tablet formulations optimized before being discharged home. TUG and secondary outcomes were recorded for the 16 participants (Test 2). An additional 7 participants were admitted to the trial from the community, while on LCAS and their TUG and clinical measures were added to that obtained from the 16 participants that were recently transferred onto LCAS from oral tablets in the Test 2 data. Of the 23 participants included in Test 2, 10 changed to Duodopa (Test 3). A description of the flow of participants through the three test periods is described in Fig. 1. Thus TUG measures comprised 19 participants on oral tablets, 23 participants on LCAS and 10 participants on Duodopa.
Fig. 1
Flow of 26 patients in the LCAS study.
TUG times for Test 1, Test 2, and Test 3 are reported in Table 1. There was evidence of a test effect on TUG (p < 0.0001). Bonferroni adjustment indicated TUG times were significantly slower on tablets compared to LCAS (ratio 1.27, CI 1.16 to 1.38, p < 0.0001) and Duodopa (ratio 1.29, CI 1.14 to 1.46, p = 0.001). Differences in TUG times on LCAS compared to Duodopa were not significant (ratio 1.02, CI 0.92 to 1.13, p = 1.0). The association remained significant after adjusting for age and Hoehn and Yahr (Table 1). Estimates for dyskinesia and tremor found no significant association with test condition (p = 0.35, p = 0.07 respectively).
Table 1
Geometric means for TUG times (sec) at each test
| Test | Geometric mean | 95% CI | p |
| TUG (s) | |||
| Unadjusted | |||
| Test 1 | 16.99 | 15.05 to 19.19 | <0.001 |
| Test 2 | 13.42 | 11.93 to 15.11 | |
| Test 3 | 13.14 | 11.42 to 15.13 | |
| Adjusted* | |||
| Test 1 | 16.83 | 15.17 to 18.67 | |
| Test 2 | 13.30 | 12.09 to 14.62 | 0.002 |
| Test 3 | 13.46 | 11.86 to 15.28 |
Geometric mean is the average time in seconds after logarithmic transformation. p value is for the overall test effect. *Adjusted for age and Hoehn and Yahr; Test 1, on tablet formulations; Test 2, on LCAS; Test 3, on Duodopa. TUG, Timed Up and Go Test.
Estimates of levodopa dose equivalency (LED) were calculated for each participant and means calculated for each of the 3 test conditions. The average daily dose for tablets was 1802.5 (Std Err 167.0) gms, 1918.3 (Std Err 159.9) gms for LCAS and 2080.8 (Std Err 201.2) gms for Duodopa. There was no evidence of a test effect on LED (p = 0.37).
The mean hourly dose for LCAS and Duodopa were calculated once the doses had been stabilized. The average hourly dose for LCAS was 106.9 (Std Err 7.26) mg and 102.7 (Std Err 7.58) mg for Duodopa. There was no evidence of a test effect on hourly dose (p = 0.19).
Secondary outcomes
There was evidence of a test effect for the PDQ39 summary index score (p = 0.003) and domains and for MDS-UPDRS Part 1 (p = 0.010), Part II (p = 0.002), and Part IV (p = 0.006) scores. Bonferroni adjusted p values are reported in Table 2. Self-report of the motor complications in each of the 3 test conditions showed the duration of OFF time was worse on tablets compared to Duodopa (p = 0.002) and the functional impact of the fluctuations was lowest on Duodopa compared to tablets (p < 0.001) and LCAS (p = 0.008) (Table 3).
Table 2
Clinical outcomes with estimate of average differences between Test 1 and 2, Test 1 and 3, Test 2 and 3
| Variable | Test | Mean | Tests compared | Mean diff | p |
| (Std Error) | (Std Error) | ||||
| PDQ39_SI | 1 | 41.99 (3.32) | 1 versus 2 | 8.83 (2.88) | 0.017* |
| 2 | 33.16 (3.26) | 1 versus 3 | 14.10 (3.89) | 0.005* | |
| 3 | 27.89 (4.03) | 2 versus 3 | 5.27 (3.42) | 0.414 | |
| PDQ39_Mobility | 1 | 59.04 (5.05) | 1 versus 2 | 7.66 (4.97) | 0.413 |
| 2 | 51.38 (4.91) | 1 versus 3 | 13.80 (6.67) | 0.151 | |
| 3 | 45.24 (6.36) | 2 versus 3 | 6.14 (5.95) | 0.939 | |
| PDQ39_ADL | 1 | 49.11 (5.91) | 1 versus 2 | 6.56 (5.55) | 0.748 |
| 2 | 42.55 (5.77) | 1 versus 3 | 16.43 (7.47) | 0.116 | |
| 3 | 32.68 (7.34) | 2 versus 3 | 9.87 (6.62) | 0.451 | |
| PDQ39_Emotional well being | 1 | 45.85 (4.61) | 1 versus 2 | 11.37 (4.24) | 0.041* |
| 2 | 34.48 (4.50) | 1 versus 3 | 18.17 (5.71) | 0.013* | |
| 3 | 27.68 (5.68) | 2 versus 3 | 6.81 (5.05) | 0.573 | |
| PDQ39_Stigma | 1 | 27.97 (4.27) | 1 versus 2 | 5.08 (4.48) | 0.806 |
| 2 | 22.89 (4.14) | 1 versus 3 | 16.83 (5.97) | 0.030* | |
| 3 | 11.15 (5.50) | 2 versus 3 | 11.74 (5.38) | 0.120 | |
| PDQ39_Social | 1 | 29.34 (5.25) | 1 versus 2 | 13.80 (6.17) | 0.107 |
| 2 | 15.55 (5.04) | 1 versus 3 | 20.08 (8.08) | 0.063 | |
| 3 | 9.27 (7.01) | 2 versus 3 | 6.28 (7.46) | 1.000 | |
| PDQ39_Cognition | 1 | 41.37 (5.18) | 1 versus 2 | 3.96 (3.61) | 0.854 |
| 2 | 37.41 (5.12) | 1 versus 3 | 11.83 (4.91) | 0.074 | |
| 3 | 29.53 (5.93) | 2 versus 3 | 7.88 (4.25) | 0.232 | |
| PDQ39_Communication | 1 | 38.20 (4.10) | 1 versus 2 | 11.99 (3.86) | 0.015* |
| 2 | 26.21 (4.00) | 1 versus 3 | 8.17 (5.20) | 0.390 | |
| 3 | 30.03 (5.09) | 2 versus 3 | –3.82 (4.61) | 1.000 | |
| PDQ39_Discomfort | 1 | 43.36 (3.90) | 1 versus 2 | 6.33 (3.88) | 0.351 |
| 2 | 37.02 (3.80) | 1 versus 3 | 3.09 (5.20) | 1.000 | |
| 3 | 40.27 (4.94) | 2 versus 3 | –3.24 (4.65) | 1.000 | |
| MDS-UPDRS_Part I_nM_EDL | 1 | 15.12 (1.49) | 1 versus 2 | 3.60 (1.18) | 0.019* |
| 2 | 11.53 (1.43) | 1 versus 3 | 4.90 (1.65) | 0.022* | |
| 3 | 10.22 (1.77) | 2 versus 3 | 1.30 (1.42) | 1.000 | |
| MDS-UPDRS_Part II_M_EDL | 1 | 22.16 (1.58) | 1 versus 2 | 4.72 (1.24) | 0.003* |
| 2 | 17.44 (1.51) | 1 versus 3 | 5.72 (1.65) | 0.007* | |
| 3 | 16.43 (1.80) | 2 versus 3 | 1.01 (1.42) | 1.000 | |
| MDS-UPDRS_Part III_motor exam | 1 | 35.79 (3.39) | 1 versus 2 | 5.90 (3.75) | 0.390 |
| 2 | 29.88 (3.12) | 1 versus 3 | 4.37 (4.86) | 1.000 | |
| 3 | 31.42 (4.25) | 2 versus 3 | –1.53 (4.38) | 1.000 | |
| MDS-UPDRS_Part IV_Complications | 1 | 10.80 (0.89) | 1 versus 2 | 1.78 (0.97) | 0.246 |
| 2 | 9.02 (0.82) | 1 versus 3 | 4.71 (1.30) | 0.005* | |
| 3 | 6.09 (1.16) | 2 versus 3 | 2.93 (1.18) | 0.067 | |
| Schwab & England | 1 | 61.55 (4.38) | 1 versus 2 | –2.94 (3.36) | 1.000 |
| 2 | 64.49 (4.36) | 1 versus 3 | –1.13 (4.46) | 1.000 | |
| 3 | 62.68 (5.10) | 2 versus 3 | 1.81 (3.86) | 1.000 | |
| GDS | 1 | 6.77 (0.92) | 1 versus 2 | 2.62 (1.08) | 0.070 |
| 2 | 4.14 (0.88) | 1 versus 3 | 2.98 (1.41) | 0.139 | |
| 3 | 3.78 (1.23 | 2 versus 3 | 0.36 (1.30) | 1.000 | |
| HY | 1 | 2.75 (0.19) | 1 versus 2 | 0.10 (0.18) | 1.000 |
| 2 | 2.65 (0.17) | 1 versus 3 | 0.09 (0.24) | 1.000 | |
| 3 | 2.67 (0.22) | 2 versus 3 | –0.02 (0.21) | 1.000 |
Post-hoc comparisons were performed using Bonferroni adjustment for multiple comparisons. Std Error, standard error; Mean diff, mean difference. *difference is significant. PDQ 39-SI, Parkinson’s disease questionnaire 39 Summary Index; MDS-UPDRS, Movement Disorder Society Unified Parkinson’s Disease Rating Scale; MDS-UPDRS_Part I_nM_EDL, MDS-UPDRS non motor experiences of daily living; MDS-UPDRS_Part II-M-EDL, MDS-UPDRS motor experiences of daily living; GDS, Geriatric Depression Scale: HY, Hoehn and Yahr.
Table 3
Part IV MDs-UPDRS motor complications
| Variable | Test | Mean | Tests compared | Mean diff | p |
| (Std Error) | (Std Error) | ||||
| MDS -UPDRS 4_1_dyskinesias duration | 1 | 1.63 (0.30) | 1 versus 2 | –0.34 (0.35) | 1.000 |
| 2 | 1.98 (0.27) | 1 versus 3 | –1.06 (0.45) | 0.088 | |
| 3 | 2.69 (0.38) | 2 versus 3 | –0.71 (0.41) | 0.299 | |
| MDS-UPDRS 4_2_impact dyskinesias | 1 | 1.30 (0.24) | 1 versus 2 | 0.69 (0.30) | 0.099 |
| 2 | 0.62 (0.22) | 1 versus 3 | 0.95 (0.38) | 0.062 | |
| 3 | 0.35 (0.31) | 2 versus 3 | 0.26 (0.35) | 1.000 | |
| MDS-UPDRS 4_3_time OFF | 1 | 1.88 (0.18) | 1 versus 2 | 0.57 (0.23) | 0.064 |
| 2 | 1.31 (0.16) | 1 versus 3 | 1.20 (0.30) | 0.002* | |
| 3 | 0.69 (0.25) | 2 versus 3 | 0.63 (0.28) | 0.108 | |
| MDS-UPDRS 4_4_impact fluctuations | 1 | 3.24 (0.25) | 1 versus 2 | 0.91 (0.32) | 0.033* |
| 2 | 2.34 (0.22) | 1 versus 3 | 2.26 (0.41) | <0.001* | |
| 3 | 0.98 (0.34) | 2 versus 3 | 1.35 (0.40) | 0.008* | |
| MDS-UPDRS 4_5_complexity fluctuations | 1 | 2.00 (0.30) | 1 versus 2 | –0.13 (0.37) | 1.000 |
| 2 | 2.13 (0.27) | 1 versus 3 | 0.58 (0.48) | 0.737 | |
| 3 | 1.42 (0.41) | 2 versus 3 | 0.71 (0.45) | 0.399 | |
| MDS-UPDRS 4_6_painful off dystonia | 1 | 0.69 (0.26) | 1 versus 2 | 0.02 (0.29) | 1.000 |
| 2 | 0.67 (0.24) | 1 versus 3 | 0.47 (0.37) | 0.651 | |
| 3 | (0.22 (0.33) | 2 versus 3 | 0.46 (0.33) | 0.552 |
Std Error, standard error; Mean diff, mean difference. *difference is significant. MDS-UPDRS, Movement Disorder Society Unified Parkinson’s Disease Rating Scale.
DISCUSSION
In this prospective study, TUG scores improved significantly on both LCAS and Duodopa treatments compared to tablet treatment. The lower mean TUG times and the lower variance of times in the LCAS condition supports the efficacy of LCAS in the treatment of bradykinesia and motor fluctuations in advanced PD. The finding for the efficacy of LCAS is supported by previous studies [4, 5, 7]. No significant difference was found in TUG times between the LCAS and the Duodopa conditions, suggesting that LCAS provided similar benefit in eliminating fluctuations as Duodopa. Participants’ self-report on motor complications, MDS-UPDRS Part IV, suggests they perceived fewer complications with Duodopa compared to tablets. Motor complications decreased on LCAS compared to tablets but failed to reach significance. The perceived impact of fluctuations on function and social activities (question 4.4) was lower on both LCAS and Duodopa, not previously reported. This benefit of LCAS was also supported in the secondary outcome measures of PDQ39 (SI), MDS - UPDRS Parts I and II.
Optimizing PD medication regimens to manage motor fluctuations can be challenging. It is routine practice for most people with PD to see their doctor in the outpatient setting. The doctor sees their patient over a narrow therapeutic window due to short consultation times. They rely on the patient’s self-report to determine how long the benefits of the PD medications last, when motor fluctuations occur within the medication cycle, which symptoms re-emerge when ‘off’ and presenting complications. Patient diaries are commonly inaccurate due to recall bias, reporting errors and poor compliance, thereby limiting their usefulness [16]. Relying on the patient to self-monitor can lead to under or over treatment of symptoms.
This current study introduced LCAS and Duodopa in the in-patient setting. In our experience inpatient management allows for continual monitoring of responses to adjustments of medications ensuring optimal outcomes for the patient. Similar methods for introducing LCAS have been previously reported [7]. A previous Duodopa study [17] demonstrated the benefits of in-patient management with the control group demonstrating a significant reduction in off time despite being reported as having had an optimized regime as an outpatient.
Mobility, a key factor impacting on quality of life [18], is an important outcome when optimizing medications. The TUG was used to capture the effect of the differing formulations on mobility. Prior studies that reported on the efficacy of LCAS used a range of rating scales as their primary outcome, such as the UPDRS [5] and the Colombia Rating Scale [19]. Most tools used in prior studies investigating the efficacy of LCAS required the tester to be skilled in administering them and were time consuming to perform, particularly when repeated numerous times over a day [4, 5, 7]. The advantage of the TUG compared to the above tools is it is easy and quick to administer, practical to administer hourly, it does not require formal training to use and only requires a stop watch and chair. The TUG also has high interrater reliability [9]. The TUG measures daily fluctuations by averaging TUG times over the 10 hour day, the higher the average the more severe the fluctuations. The TUG used in the context of falls prediction has not been found as robust as the four meter walk test; however we utilized this measure in a different context. Other studies have found it correlated strongly with gait speed and that it is sensitive enough to document functional change in people with PD [9, 12]. The recorded value of the TUG is reflective of multiple gait disturbances, such as hypokinesia, festination, motor blocks and turning hesitations, however, the contribution from each disturbance can only be ascertained by the observation notes made by the nurse who performed the test. We did not use this information as it was not relevant to our main aim. However, improvement in any of these gait disturbances would have been reflected in the TUG measure.
The benefit of LCAS lies in its solubility. It is not subject to hold up in the stomach due to the acidity provided by the vitamin C, thereby providing reliability of motor benefit. The concentration of 1 mg/ml also enables the titration of the dose to minimize extra movements. In our experience most patients have a therapeutic window of about 5 mg and the initial titrations focus on adjusting the dose to minimize the dyskinesia. This was further demonstrated by the insignificant changes to the dyskinesia measures despite the improvements in the TUG. However, the participant’s choice in determining their preferred treatment option is of tantamount importance and certainly this was demonstrated in our cohort as not all participants chose LCAS and similarly not all participants chose Duodopa. The ongoing use of LCAS has similarly been shown to be not acceptable to all participants with a fallout rate of 40% [7]. It also needs to be reaffirmed that although our TUG measures were performed during the participants’ inpatient stay, all three treatment options were continued and maintained in the community setting once established. The community setting was overseen by our community nurse and our outreach program.
The comparison of LCAS and Duodopa hourly rates of intake and infusions were similar suggesting that the basis of benefit was the same as both are dependent on the levodopa/carbidopa ratio.
This study attests to the benefit of LCAS in the management of advanced PD similar to Duodopa jejunal infusion. The advantage of LCAS is its ease of use and low cost. The disadvantages are the need to titrate the doses and to swallow, at times, large volumes on an hourly basis which might make it unsuitable for patients with swallowing problems or heart failure. However, compared to the invasiveness and costs of Duodopa it represents a viable alternative and warrants a larger trial to confirm these findings.
From a practical clinical perspective LCAS can be used to determine the likely benefit of Duodopa without the invasiveness of the nasogastric phase. A trial of LCAS under observation would quickly determine if unpredictable “off” times were improved without significant dyskinesia. LCAS can also be readily introduced when problems with an existing Duodopa system disrupt delivery of the gel. The change to LCAS at the same flow rate and bolus, delivered orally or through the gastric port, would maintain the patient’s motor function until the problem was resolved.
The efficacy of LCAS demonstrated in this report rests with inpatient management, movement charting and experienced nursing support. We have previously demonstrated the benefit of such an approach to demonstrate improvement for COMT inhibition failure with entacapone with the use of combined slow release and quick release forms of levodopa, utilizing tablet forms of medication [20]. Such approaches are rarely used but our findings do suggest a review given the expensive and invasive alternatives.
There are several limitations to this study that need to be acknowledged. The small sample size limits the generalizability of the findings to the broader PD population however it should be noted that key outcome measures were found to be significant. Future research using a larger sample size is recommended. The non-randomization of participants meant there is the potential for bias and other confounding variables that may impact on the results were not controlled for. However, the motor monitoring was performed by blinded nurses and the patient flow was pragmatic in context and indicative of common clinical practice. Participants were not formally assessed in the community, but the persistence of the benefits post discharge was documented by the program’s community nurse who supported them. Future research is needed to measure these outcomes in the community rather than on admission to the inpatient ward as performed in this current study.
In summary this report demonstrated the benefit of LCAS in advanced PD as well as the equivalence to Duodopa in reducing “off” times without significant changes in dyskinesia. It may also act as an interim for patients waiting for transition onto Duodopa.
ACKNOWLEDGMENTS
The authors wish to acknowledge the patients for their willingness to participate in this study. We also wish to thank the carers who assisted those they cared for in getting to appointments and in returning required documentation.
FUNDING
The author CBS was supported by the Lions John Cockayne Memorial Fellowship Trust Fund. The funds were provided to enable and support the development of this clinician-driven research. The grant was made for the purpose of contributing to the costs of the research activity. The funding body had no role in the design of the study, in collection, analysis or interpretation of data, in writing the report or in decision to submit the article for publication.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
DATA AVAILABILITY
The data supporting the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
[1] | Schrag A , Quinn N ((2000) ) Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain 123: (Pt 11), 2297–2305. |
[2] | Katzenschlager R , Hughes A , Evans A , Manson AJ , Hoffman M , Swinn L , Watt H , Bhatia K , Quinn N , Lees AJ ((2005) ) Continuous subcutaneous apomorphine therapy improves dyskinesias in Parkinson’s disease: A prospective study using single-dose challenges. Mov Disord 20: , 151–157. |
[3] | Nyholm D , Nilsson Remahl AI , Dizdar N , Constantinescu R , Holmberg B , Jansson R , Aquilonius SM , Askmark H ((2005) ) Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease. Neurology 64: , 216–223. |
[4] | Kurth MC , Tetrud JW , Irwin I , Lyness WH , Langston JW ((1993) ) Oral levodopa/carbidopa solution versus tablets in Parkinson’s patients with severe fluctuations: A pilot study. Neurology 43: , 1036–1039. |
[5] | Pappert EJ , Goetz CG , Niederman F , Ling ZD , Stebbins GT , Carvey PM ((1996) ) Liquid levodopa/carbidopa produces significant improvement in motor function without dyskinesia exacerbation. Neurology 47: , 1493–1495. |
[6] | Kurth MC ((1997) ) Using liquid levodopa in the treatment of Parkinson’s disease. A practical guide. Drugs Aging 10: , 332–340. |
[7] | Yang HJ , Ehm G , Kim YE , Yun JY , Lee WW , Kim A , Kim HJ , Jeon B ((2017) ) Liquid levodopa-carbidopa in advanced Parkinson’s disease with motor complications. J Neurol Sci 377: , 6–11. |
[8] | Iansek R ((2004) ) Pharmacological management of Parkinson’s disease. J Pharm Pract Res 34: , 229–232. |
[9] | Morris S , Morris ME , Iansek R ((2001) ) Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys Ther 81: , 810–818. |
[10] | Pappert EJ , Buhrfiend C , Lipton JW , Carvey PM , Stebbins GT , Goetz CG ((1996) ) Levodopa stability in solution: Time course, environmental effects, and practical recommendations for clinical use. Mov Disord 11: , 24–26. |
[11] | Chaudhuri KR , Martinez-Martin P , Schapira AH , Stocchi F , Sethi K , Odin P , Brown RG , Koller W , Barone P , MacPhee G , Kelly L , Rabey M , MacMahon D , Thomas S , Ondo W , Rye D , Forbes A , Tluk S , Dhawan V , Bowron A , Williams AJ , Olanow CW ((2006) ) International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMSQuest study. Mov Disord 21: , 916–923. |
[12] | Mollinedo I , Ma Cancela J ((2020) ) Evaluation of the psychometric properties and clinical applications of the Timed Up and Go test in Parkinson disease: A systematic review. J Exerc Rehabil 16: , 302–312. |
[13] | Steffen TM , Hacker TA , Mollinger L ((2002) ) Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther 82: , 128–137. |
[14] | Goetz CG , Stebbins GT , Shale HM , Lang AE , Chernik DA , Chmura TA , Ahlskog JE , Dorflinger EE ((1994) ) Utility of an objective dyskinesia rating scale for Parkinson’s disease: Inter- and intrarater reliability assessment. Mov Disord 9: , 390–394. |
[15] | Olivier J , Johnson WD , Marshall GD ((2008) ) The logarithmic transformation and the geometric mean in reporting experimental IgE results: What are they and when and why to use them? Ann Allergy Asthma Immunol 100: , 333–337. |
[16] | Papapetropoulos SS ((2012) ) Patient diaries as a clinical endpoint in Parkinson’s disease clinical trials. CNS Neurosci Ther 18: , 380–387. |
[17] | Olanow CW , Kieburtz K , Odin P , Espay AJ , Standaert DG , Fernandez HH , Vanagunas A , Othman AA , Widnell KL , Robieson WZ , Pritchett Y , Chatamra K , Benesh J , Lenz RA , Antonini A , Group LHS ((2014) ) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: A randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13: , 141–149. |
[18] | Rahman S , Griffin HJ , Quinn NP , Jahanshahi M ((2008) ) Quality of life in Parkinson’s disease: The relative importance of the symptoms. Mov Disord 23: , 1428–1434. |
[19] | Metman LV , Hoff J , Mouradian MM , Chase TN ((1994) ) Fluctuations in plasma levodopa and motor responses with liquid and tablet levodopa/carbidopa. Mov Disord 9: , 463–465. |
[20] | Iansek R , Danoudis M ((2011) ) A single-blind cross over study investigating the efficacy of standard and controlled release levodopa in combination with entacapone in the treatment of end-of-dose effect in people with Parkinson’s disease. Parkinsonism Relat Disord 17: , 533–536. |



