Pharmacological Treatment of Tremor in Parkinson’s Disease Revisited
Abstract
The pathophysiology of Parkinson’s disease (PD) tremor remains incompletely understood and there is a lack of clinical trials specifically addressing its pharmacological treatment. Levodopa is the most efficacious drug for most patients and should be used as primary approach to control troublesome tremor. While the efficacy of oral dopamine agonists on PD tremor has been demonstrated in controlled trials, there is no evidence of greater antitremor efficacy compared to levodopa. The magnitude of the antitremor effect of anticholinergics is generally lower than that of levodopa. Due to their adverse effects, anticholinergics have a limited role in selected young and cognitively intact patients. Propranolol may improve resting and action tremor and may be considered as an adjunct in patients with insufficient tremor response to levodopa and this also applies to clozapine, despite its unfavorable adverse effect profile. Treating motor fluctuations with MAO-B and COMT inhibitors, dopamine agonists, amantadine, or on-demand treatments such as subcutaneous or sublingual apomorphine and inhaled levodopa as well as with continuous infusions of levodopa or apomorphine will improve off period tremor episodes. For patients with drug-refractory PD tremor despite levodopa optimization deep brain stimulation and focused ultrasound are first-line considerations. Surgery can also be highly effective for the treatment medication-refractory tremor in selected patients without motor fluctuations. The present review highlights the clinical essentials of parkinsonian tremor, critically examines available trial data on the effects of medication and surgical approaches and provides guidance for the choice of treatments to control PD tremor in clinical practice.
INTRODUCTION
The presence of classical asymmetric 4-6-Hz resting tremor has remained an important criterion for a clinical diagnosis of Parkinson’s disease (PD) ever since its first meticulous description by James Parkinson in 1817. Tremor is also one of the most obvious clinical signs of PD and can be distressing even when its severity or impact on motor function is limited. The pathophysiology of PD tremor remains incompletely understood and, in contrast to bradykinesia and rigidity, its severity is not related to the overall degree of nigro-striatal dopaminergic denervation [1–3]. The response of tremor to levodopa is often perceived as less consistent than that of bradykinesia and rigidity, although underdosing of levodopa or non-adherence are important causes of poor tremor control in clinical practice [4]. In a subgroup of patients, PD tremor can be genuinely resistant to dopaminergic or other drug therapies, and this scenario is an important indication for deep brain stimulation (DBS) surgery and, more recently, alternatively for focused ultrasound thalamotomy [5]. However, ‘drug-resistance’ of PD tremor has never been formally defined and the efficacy of different classes of PD medication specifically on tremor has not been reviewed in a comprehensive manner. The present review will highlight the clinical essentials of PD tremor including diagnostic pitfalls, summarize current knowledge on pathophysiology, critically examine available studies providing controlled data on medication effects on tremor in PD and finally provide guidance for the choice of drug regimens to control PD tremor in clinical practice.
METHODS
We reviewed English-written articles and abstracts published in PubMed between 1967 and August 2022 using the keywords ‘Parkinson’s disease’ AND ‘tremor’ AND ‘treatment’ or ‘randomized clinical trial’, ‘clinical trial’, ‘dopa’, ‘levodopa’, ‘MAO B inhibitor’, ‘selegiline’, ‘rasagiline’, ‘safinamide’, ‘COMT inhibitor’, ‘tolcapone’, ‘entacapone’, ‘opicapone’, ‘dopamine agonist’, ‘pramipexole’, ‘ropinirole’, ‘rotigotine’, ‘piribedil’, ‘pergolide’, ‘apomorphine’, ‘anticholinergic’, ‘trihexiphenidyl’, ‘biperiden’, ‘beta blocker’, ‘propranolol’, ‘clozapine’, ‘adenosine receptor antagonist’, ‘istradefylline’, ‘zonisamide’, ‘cannabinoid’, ‘cannabidiol’, ‘tetrahydrocannabinol’, ‘botulinum toxine’, ‘deep brain stimulation’, ‘subthalamic nucleus stimulation’, ‘thalamic stimulation’, ‘pallidal stimulation’, ‘thalamotomy’, or ‘focussed ultrasound’. Using similar key words we reviewed ongoing trials on the https://clinicaltrials.gov/ website. Previously published reviews on the treatment of PD tremor were scanned for additional references. Abstracts from the International Congress of Movement Disorders and Parkinson’s Disease from the years 2020 and 2021 were searched for abstracts covering new treatment trials of PD tremor.
Randomized, placebo-controlled trials were included for further review. For drugs without evidence from randomized controlled studies, lower quality studies including post-hoc analyses from randomized trials, open label trials and single dose studies, were also reviewed. Additionally, relevant literature on the phenomenology and pathophysiology of PD tremor published up to August 2022 was reviewed.
CLINICAL CLASSIFICATION AND PHENOMENOLOGY OF TREMORS IN PD
Tremor is defined as an involuntary rhythmic oscillating movement of a body part [6], usually as a result of alternating contractions of agonistic and antagonistic muscles. The classical tremor presentation in PD is a 4–6 Hz asymmetric resting tremor of the distal extremities (Supplementary Video 1), which may be provoked or exacerbated by actively moving other body parts (e.g., hand tremor during walking), cognitive tasks, or stress [6, 7]. Cranial resting tremor presentations may involve the head, lips, tongue, or chin, but are less common (Supplementary Video 2) [8]. Classical resting tremor is one of the cardinal motor features of PD. Resting tremor is a presenting feature in approximately 70% of patients [8, 9] and was recorded in about 77% of patients at some stage during the course of PD in a clinicopathological study [9]. Ephemeral episodes of tremor may be observed in some people years before the onset of classical motor signs and a diagnosis of PD [10].
Resting tremor commonly abates with voluntary movement of the affected body part (e.g., lifting an affected hand) but can re-occur on posturing or during movement after a delay (Supplementary Video 3). This re-emergent tremor is in the same frequency range as resting tremor and the severity of the tremor in the two situations is usually correlated [11, 12]. The average latency for a resting tremor to re-emerge with tonic muscle activation is around 9 seconds, with a broad interindividual range from 1 to more than 30 seconds [11]. Tremor may also affect the legs during standing, where it has been called ‘pseudo-orthostatic’ [13] (in contrast to the separate entity orthostatic tremor, which has a much higher frequency).
Areas of phenotypic overlap between PD and essential tremor (ET) include the occurrence of higher frequency action tremors in PD. In patients with ET who also exhibit resting tremor, a helpful sign in the differential diagnosis from PD tremor is the lack of upper extremity resting tremor suppression with postural muscle activation [14]. However, it is possible to have a similar postural tremor that arises in the context of PD which is apparently not ET. A study has shown that around 20% of patients with tremulous PD exhibited postural tremor of a higher frequency than re-emergent tremor and without amplitude suppression [15]. Isolated asymmetric postural tremor, without resting tremor, can also occur as an early sign of PD and may be a source of diagnostic confusion [11, 16]. On the other hand, PD emerges in some patients with long-standing ET and some researchers have argued that PD risk is increased in ET [17, 18]. However, in many of these cases, the latency between the onset of ET and PD symptoms was in the range of only a few years. The initial sign in most patients with a putative history of ET is resting tremor, and the side of the body with predominant PD tremor usually matches that with predominant ET tremor [17]. These facts cast doubt on the accuracy of the initial diagnosis of ET in some of these patients. Nevertheless, a substantial overlap between PD and ET can be expected based on the high prevalence of both conditions. Many PD patients develop a kinetic tremor of low amplitude during grip and lift maneuvers which can contribute to impaired dexterity [19]. Action tremor with higher frequency than resting tremor responds less well to dopaminergic treatment than classical resting tremor or re-emergent tremor [15, 20], and there is ample evidence from neurophysiological studies that resting tremor and action tremor in PD are subserved by different neuronal networks ([15, 21], see below).
The contribution of tremor to the progression of disability in PD appears to be different from the other cardinal motor features. While tremor progression from the initially affected limb to the other extremities is commonly observed during the first years of disease [8], the average rate of worsening of resting tremor appears to be slower than the worsening of bradykinesia, rigidity, and axial symptoms [22]. Tremor can even be greater on the side opposite the one affected by more severe bradykinesia, a clinical constellation denoted as “wrong-sided tremor” [23, 24]. Whereas there is a clear correlation between the degree of bradykinesia and rigidity in PD, neither the severity of resting tremor nor that of action tremor are related to bradykinesia or rigidity scores [25]. The latter is supported by studies investigating the dimensionality of the UPDRS motor section where tremor ratings represented an independent factor without significant correlation to the symptom factors rigidity, bradykinesia, axial symptoms/gait, and speech/hypomimia [26].
These specific aspects in the clinical expression of tremor compared to bradykinesia, rigidity, postural instability, and gait disorder have been used as the basis for empirical definitions of PD-subtypes, including rigid-akinetic or postural instability/gait difficulty type and tremor-dominant PD [27]. ‘Benign-tremulous’ parkinsonism has been empirically defined as a variant of PD marked by a predominance of resting tremor, often accompanied by action tremor, very slow progression without the development of gait impairment over at least 8 years’ disease duration and a lack of cognitive decline [28, 29]. However, subjects who initially meet criteria for benign tremulous PD nearly always develop common features of advanced PD during the final stages of their disease course [29]. According to a clinico-pathological study, the slower clinical progression in benign tremulous PD correlates with less severe neuronal loss in the substantia nigra as compared to the general PD population [29]. Similarly, postmortem studies found less marked dopamine nerve cell loss and less severe striatal dopamine loss in tremor-dominant than in other clinical subtypes of PD [30, 31]. However, it is conceivable that the overall milder dopaminergic deficit in tremor-dominant versus other subtypes of PD may be an artefact due to the earlier recognition and diagnosis of tremor-dominant PD because tremor is such an obvious clinical sign that is commonly associated with PD.
The response of tremor to levodopa and other antiparkinsonian drugs is less consistent than that of bradykinesia and rigidity [32]. Whereas some patients may show an excellent response (for an illustrative example, see Supplementary Video 4a, b), others do not respond to standard doses of levodopa and end up with “treatment-resistant” tremor. The variability of drug response as well as the temporal variability and alterations in severity of tremor during the course of PD remain poorly understood. In an open observational study of PD patients with severe resting tremor off medication, those with longer disease duration and more severe motor impairment, in particular those with more severe bradykinesia and rigidity, showed a better tremor response to levodopa than patients predominantly affected by tremor [33]. The correlation between clinician-rated, semiquantitative measures of tremor and patient-experienced tremor intensity is moderate at best [34]. In general, patients tend to overestimate the severity of resting tremor [35]. PD resting tremor is amplified by cognitive stress. A recent study using accelerometry showed that cognitive co-activation reduced the response of resting tremor to levodopa [36] and patients’ subjective experience of the effect of levodopa on tremor during cognitive coactivation is more closely related to objective measures of tremor improvement than during relaxation [34]. This suggests that patients may evaluate the antitremor effect of levodopa mainly based on its ability to reduce tremor during stress.
Improved methods of PD tremor assessment including long-term measurements using wearable sensors may be needed to better understand treatment effects.
PATHOPHYSIOLOGY OF PD TREMOR AND TARGETS FOR DRUG THERAPY
Neurophysiological studies have identified multiple sites of oscillatory activity both within and outside the basal ganglia [37]. In PD patients and primates with experimental parkinsonism, neurons in the striatum, globus pallidus, and subthalamic nucleus discharge in oscillatory bursts at the tremor frequency or at double or triple the tremor frequency but these oscillations are not consistently coherent with simultaneously recorded tremor. This is in contrast to a high tremor synchronicity of neurons in the thalamic ventral intermediate nucleus (VIM) [38]. The VIM is part of the cerebello-thalamo-cortical circuit whereas the ventral anterior nucleus represents the target of basal ganglia output to the thalamus. Synchronization of basal ganglia neuronal activity in the beta range (15–30 Hz) is closely related to bradykinesia and rigidity but not to tremor [39]). In contrast, tremor is related to oscillatory activity in the motor cortex and cerebellum [40], suggesting that the cerebello-thalamo-cortical circuit may be essential for the expression of resting tremor [41]. According to the “dimmer-switch model” of parkinsonian resting tremor, the initiator of tremor episodes is localized in the basal ganglia and triggers tremor-related activity in the cerebello-thalamo-cortical circuit, which determines tremor intensity. According to this model, both circuits converge in the motor cortex [41] although it is now clear that there are subcortical connections between cerebellum and basal ganglia [42]. Loss of dopaminergic projections to the internal globus pallidus and thalamus appear to be critical determinants of tremor control [43].
The cerebello-thalamo-cortical pathway appears to play a larger role in the generation of postural tremor than in resting tremor in PD [44, 45]. Transcranial magnetic stimulation (TMS) over the cerebellum resets postural tremor (specifically shown for re-emergent tremor by Helmich et al. [45]) but not resting tremor. In these studies, TMS over the motor cortex resets both resting and postural tremor.
Neurons in the different basal ganglia nuclei and thalamus can have 3 different types of behavior, theta (or tremor) frequency, beta frequency, or no clear oscillatory behavior [46, 47]. Neurons can change their behavior; thus, when there is more tremor activity, there will be less beta activity. This phenomenon can be observed with local field potentials as well, leading to observations such as the emergence of resting tremor being associated with attenuation of beta band oscillations in the subthalamic nucleus [48]. According to the dimmer-switch model, it is a change in the basal ganglia that initiates the tremor activity, but the cellular mechanism of such a change is not known. In line with this hypothesis, tremor maintenance was associated with beta power suppression as well as subthalamic and cortical power increases around the individual tremor frequency [49], suggesting that the subthalamic nucleus may be a critical hub for both onset and maintenance of resting tremor.
Current invasive therapies of PD tremor such as DBS or focused ultrasound (FUS) target critical nodes in tremor-related oscillatory networks like the VIM or subthalamic nucleus. In contrast, the exact sites of action of pharmacological therapies of PD tremor are less clear. Dopaminergic striatal denervation is a prerequisite for the emergence of parkinsonian resting tremor and other cardinal motor features. However, in contrast to bradykinesia, the severity of parkinsonian tremor is poorly correlated to indices of dopaminergic deficiency like the degree of nigral cell loss, striatal dopamine loss, or the decrease of the dopamine metabolite homovanillic acid in cerebrospinal fluid [50–52]. Likewise, fluorodopa PET and dopamine transporter (DAT) SPECT imaging studies found a pronounced inverse correlation of striatal uptake with bradykinesia, a moderate inverse correlation with rigidity and axial symptoms, but no correlation with tremor [1–3]. The lack of correlation with striatal DAT binding applies to resting as well as to action tremor in PD [3].
Such discrepancies with the obvious anti-tremor efficacy of dopaminergic drugs in a majority of patients may be the result of differences between the pattern of dopaminergic degeneration underlying bradykinesia and tremor in PD. A small post mortem study observed more severe DA cell loss in the retrorubral field A8 in tremulous vs. non-tremulous PD [53]. Dopaminergic degeneration of the retrorubral field may contribute to the generation of tremor via more recently described projections to the subthalamic nucleus, globus pallidus or thalamus [54].
Dopaminergic dysfunction in the globus pallidus may indeed play a role in PD tremor as suggested by some, but not all studies. In a SPECT study involving 16 tremor-dominant and 19 non-tremor PD patients, the side-to-side difference in resting tremor severity correlated with the side-to-side difference in pallidal DAT binding, which the authors took as an argument that differences in pallidal dopamine function may trigger PD tremor [55]. However, subsequently a large study involving 382 de novo patients from the PPMI cohort found no correlation of pallidal DAT uptake with resting tremor amplitude or constancy in tremor-dominant PD and in the total cohort of PD subjects, making differences in pallidal dopamine a less likely determinant of PD tremor [56]. Alternatively, direct dopaminergic projections to components of the cerebello-thalamo-cortical circuit, in particular the VIM as thalamic relay of this pathway, have been hypothesized to be involved in the control of PD resting tremor [43].
In addition to loss of nigral dopaminergic neurons, PD is characterized by a degeneration of serotonergic and noradrenergic projections originating in the midbrain raphe nuclei and locus coeruleus and alterations in these non-dopaminergic systems have been put forward as possible contributors to the development of tremor. A postmortem study found no significant difference in dorsal raphe cell counts between tremulous and rigid-akinetic PD but less severe cell loss in the locus coeruleus in tremulous vs. rigid-akinetic PD [30]. Studies of serotonin transporter (SERT) availability in the brainstem, striatum, and thalamus found lower SERT levels in these regions in tremor-dominant vs. other subtypes of PD, though the absolute differences were quite small [57, 58]. In the Loane et al. (2013) study, the decrease in SERT correlated with action-postural tremor, but not resting tremor [57]. In line with these SERT imaging studies, a 11C-WAY 100635 PET study found an inverse correlation of raphe serotonin 5HT1A receptor binding with resting tremor and total (resting plus action) tremor [59]. These findings do not correlate with the absence of an established effect of serotonergic agents on PD tremor in clinical trials [60–62]. Agonism at 5HT1A and antagonism at 5HT2A receptors, however, may contribute to the antitremor effects of clozapine [63], suggesting that new drugs acting on these receptors may be potential targets for anti-tremor drug development [64].
The anti-tremor effect of anticholinergics in PD has been traditionally explained by a potential compensation of a striatal imbalance of reduced dopamine and increased cholinergic neurotransmission [65]. However, pathology and imaging studies rather point towards a reduction of cholinergic markers in PD and, according to a recent PET imaging study, parkinsonian tremor is correlated to reduced cholinergic nerve terminal function in the putamen and the cerebellar vermis [66]. Cholinergic interneurons make up only 1–2% of all striatal neurons but project widely throughout the striatum. The large aspiny cholinergic interneurons tonically interact with most neuronal components of the striatum via synaptic and non-synaptic transmission and thus have substantial impact on striatal microcircuitry function [67]. In mice, dopamine deficiency has been shown to reduce striatal cholinergic activity, but dopamine release from remaining nerve terminals increased the availability of acetylcholine, which may contribute to the imbalance and the expression of parkinsonian motor features [68]. In addition, dysfunction of cholinergic projection systems including the pedunculopontine-laterodorsal tegmental complex probably contributes to the development of non-motor and motor features from the earliest stages of PD [66]. Although it is unclear if these mechanisms also contribute to the development of PD tremor, the clinical efficacy of anticholinergic drugs and the effect of antimuscarinics [69] in experimental models as well as ample evidence of a clinical effect in PD patients [70] all support a role of the cholinergic system in PD tremor.
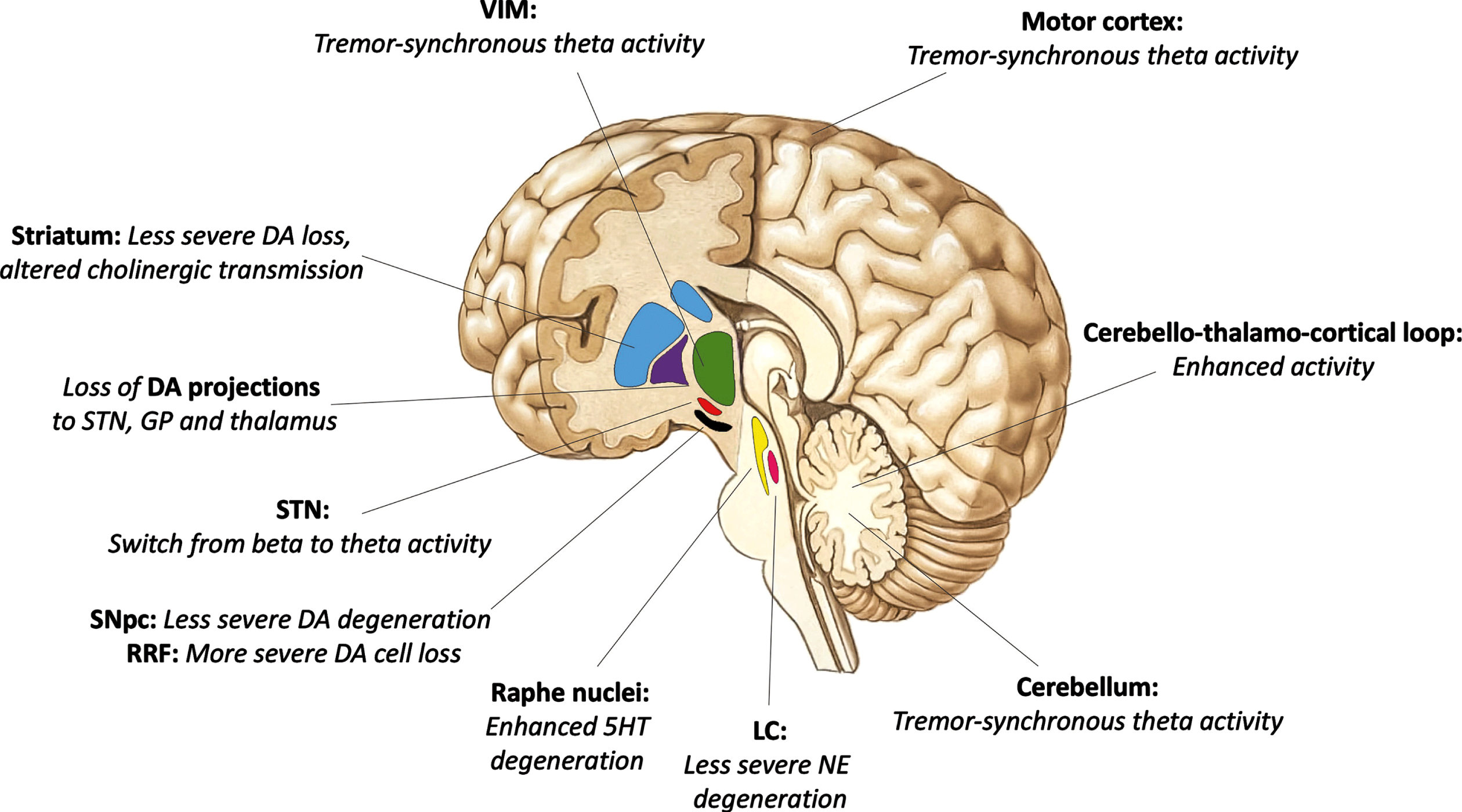
Anatomical regions involved in the pathophysiology of PD tremor and specifics of neurotransmission differentiating the tremor-dominant variant from other motor subtypes of PD are illustrated in Fig. 1.
Fig. 1
Anatomical regions (bold) involved in the pathogenesis of parkinsonian tremor. Distinctive changes in neurotransmission differentiating the tremor-dominant variant from other motor subtypes of Parkinson’s disease in italics. DA, dopamine; STN, subthalamic nucleus; VIM, ventral intermediate nucleus of the thalamus; SNpc, substantia nigra pars compacta; RRF, retrorubral field; GP, globus pallidus; 5-HT, serotonin; NE, norepinephrine; LC, locus coeruleus.
DRUGS TARGETING PD TREMOR: EVIDENCE FROM TRIALS
Few drug trials in PD have specifically focused on tremor. The available trial literature and relevant references are summarized in the Supplementary Material.
Dopaminergic agents
Levodopa
Levodopa is the most efficacious drug for the treatment of motor symptoms of PD [71, 72] and this includes the symptomatic control of PD tremor, although published data from controlled trials of levodopa have rarely reported differential effects on the different cardinal motor features. In his seminal paper on the efficacy of levodopa George Cotzias noted that “the sequence in which the signs of Parkinsonism responded seemed to be the following: first akinesia, then rigidity and finally tremor.” [73]. He also noted that tremor sometimes emerged after improvement of rigidity but tended to respond to higher doses of levodopa. Overall, all motor symptoms including tremor improved [73]. The first double-blind, placebo-controlled trial of levodopa reported a similar long-term effect of levodopa on bradykinesia, rigidity, and tremor [74]. A recently published post-hoc analysis of the randomized-controlled LEAP-study [75] also reported similar effect sizes of levodopa for the three cardinal motor domains bradykinesia, tremor, and rigidity in early PD (see Supplementary Material for further details and studies).
The beneficial effect of levodopa on PD tremor applies to both resting and postural tremor [76, 77], although none of the studies clarified if the postural tremor studied was re-emergent tremor or a higher frequency postural tremor. The antitremor effect of levodopa and apomorphine appear to be at least as good as, if not better than that of anticholinergics [78–80]. Levodopa may be superior to propranolol in suppressing postural tremor [77], although this may reflect levodopa’s good effect on re-emergent and not on high-frequency postural tremor. The proportion of patients with PD tremor resistant or poorly responsive to levodopa and the dose-response relationship of tremor have never been investigated. Furthermore, the effect of higher levodopa doses or higher single levodopa doses on poorly responsive tremor has not been formally studied.
MAO-B and COMT inhibitors
Post hoc analysis of the pivotal rasagiline trials suggests that MAO-B inhibitors and the COMT inhibitor entacapone added to levodopa may lead to an improvement of tremor [81]. This is in line with the established efficacy of both COMT and MAO-B inhibitor adjunct therapy in improving motor symptoms and also with clinical experience, showing that an increase of levodopa dose or adjunct therapy with enzyme inhibitors can lead to an improvement of previously suboptimal tremorcontrol.
Dopamine agonists
The effect of dopamine agonists on tremor poorly responsive to standard levodopa treatment was investigated in two randomized controlled trials involving pramipexole and pergolide [82, 83]. In addition, the effect of ropinirole on PD tremor was studied by post hoc analysis of three pivotal trials [84]. Overall, these studies confirm the effect of oral dopamine agonists on PD tremor when taken as monotherapy as well as when administered as adjunct to levodopa. Since the dopamine agonist trials in patients with insufficiently controlled tremor on levodopa [82, 83] did not have a control arm of increased levodopa dose they do not allow conclusions about possible higher efficacy of agonists compared to levodopa. Such enhanced agonist efficacy would seem unlikely given the consistent evidence from comparative randomized controlled trials showing a weaker overall effect of oral dopamine agonists on motor symptoms compared to levodopa [85].
Apomorphine is the only antiparkinsonian agent with an effect size on motor symptoms that is equivalent to that of levodopa ([86] for review). This includes similar anti-tremor effects compared to levodopa as shown in single-dose challenge studies [79, 87].
Non-dopaminergic agents
Anticholinergics
Anticholinergics were the earliest pharmacological agents developed for the treatment of PD. For historical reasons, most studies documenting their efficacy do not match current standards for randomized controlled trials but consistently show improvements of parkinsonism both as monotherapy and as adjuncts to levodopa [71]. Single dose comparative studies with dopaminergic agents have documented a stronger effect on tremor than on other motor symptoms. However, the effect size of anticholinergics on PD tremor did not exceed the effect of levodopa or apomorphine [79] and may be even lower. The clinical utility of anticholinergics in PD is limited by their poor tolerability: Due to their peripheral antimuscarinic action, they are contra-indicated in narrow-angle glaucoma and tachycardia. They may cause blurred vision due to accommodation impairment, urinary retention, dry mouth, and impaired sweating. Their central nervous adverse effects limit their use, in particular in cognitively impaired and elderly patients in whom they may worsen cognition and induce confusional states, although such adverse effects may also occur in cognitively intact patients [70]. In addition, a negative impact on gait has been described [88] and withdrawal may lead to deterioration of motor symptoms [70]. This unfavorable adverse effect profile calls for restrained use of anticholinergics in PD. In health care settings where other choices are available, anticholinergics should only be considered in young and cognitively intact tremor-dominant patients with insufficient tremor control on optimized dopaminergic treatment, and clinicians should actively monitor cognitive tolerability during treatment.
Amantadine
Amantadine is thought to exerts its effect on PD motor symptoms by blocking NMDA receptors, which modulate glutamatergic-dopaminergic interactions in the basal ganglia. Nowadays, amantadine is mainly used for the treatment of motor complications, in particular dyskinesias. Most studies in PD patients without motor complications were conducted in the early 1970 s and do not meet current methodological standards. Systematic reviews by the Movement Disorder and Parkinson’s Disease Society classified amantadine only as likely efficacious in symptomatic monotherapy or adjunct therapy of stable PD [63, 72]. Available evidence from older clinical studies points towards a weak effect on PD tremor, with an effect size markedly below that of levodopa and anticholinergics [89, 90]. However, the recent finding of a significant off time reduction with controlled-release amantadine [91] makes it likely that off period tremor responds to amantadine.
Beta blockers
Several small studies point towards a possible effect of propranolol and other beta-adrenoceptor antagonists (beta blockers) on tremor in PD, although evidence from adequately powered randomized controlled trials is lacking [92]. Adjunct therapy with beta blockers has been suggested to improve both resting and postural tremor in PD [89]. The mechanism underlying this effect is not entirely clear but may involve effects on beta2 receptors on central tremor-oscillators and an indirect effect on stress-related adrenergic activation, which commonly enhances PD tremor [36, 93]. In addition, effects of beta blocking agents on action tremor in PD could also be mediated by peripheral effects akin to those in ET [94]. The most common side effects include hypotension, bradycardia, fatigue and erectile dysfunction and their use is contraindicated in patients with higher-degree AV-conduction blocks and severe obstructive pulmonarydisease [95].
Benzodiazepines
Based on very limited evidence, clonazepam does not appear to be useful in the treatment of PD. Although a short-term effect on stress-enhanced tremor components is conceivable, this class carries a well-known risk of drug dependence. There is no evidence for an antitremor effect of primidone in PD [89].
Clozapine
Clozapine is a first-line option for the treatment of severe PD psychosis. It was studied as an antitremor drug in several open-label and two randomized, controlled studies [96, 97], demonstrating an improvement of PD resting and action tremor. Moreover, a randomized study of the efficacy of clozapine in PD psychosis demonstrated an improvement of tremor as compared to placebo [98]. Open-label studies have suggested that this beneficial effect also occurs in PD tremor resistant to other medications, including anticholinergics [99–101]. An open-label observation over more than a year found no evidence for the development of tolerance to the antitremor effect [97]. Clozapine may have other beneficial motor effects in PD including the improvement of dystonic symptoms and of levodopa-induced dyskinesias [102, 103].
The clinical utility of clozapine for tremor is limited by its risk to induce agranulocytosis and other side effects including sedation [104]. Its use as an antitremor agent in PD is off-label. Provided that mandatory leukocyte monitoring regulations are strictly followed, clozapine can be considered in patients intolerant to other antitremor medications and not eligible for tremor surgery. Slow dose titration (starting at 6.25 or 12.5 mg), single dosing at bedtime or prescribing a higher dose of clozapine before sleep and a very low dose in the morning may improve tolerability in patients responding with sedation or orthostatic hypotension.
Cannabinoids
A number of patient surveys and open-label studies suggest that smoked cannabis and oral cannabinoids (tetrahydrocannabinol, cannabidiol or mixed preparations) may improve PD tremor [105]. A randomized crossover trial compared the effect of a single dose of cannabidiol (CBD) and placebo on anxiety and PD tremor induced by a simulated public speaking test. CBD attenuated anxiety and tremor amplitude, suggesting that CBD may be a beneficial in PD patients with anxiety-related tremor [106]. Another randomized controlled trial found a beneficial effect of the synthetic cannabinoid nabilone on PD non-motor symptoms, in particular anxiety and sleep problems [107]. According to a recently published patient survey, substantially more PD patients observed an improvement of tremor with tetrahydrocannabinol (THC) or mixed preparations than with CBD [108]. Cannabinoids may be a class of drugs with the potential to ameliorate PD via the improvement of stress and anxiety, which are common problems in PD. Evidence for a direct antitremor effect is, however, lacking and the dose range for the treatment of PD tremor is unknown. Since CBD lacks psychotropic effects, it is of particular interest in the PD population and warrants further study. In real life, many CBD products contain various levels of THC and other contaminants, and even highly purified CBD preparations are associated with adverse events when dosed adequately [109]. Based on the low level of evidence, cannabinoids cannot be recommended for the treatment of PD tremor outside of clinical trials at present.
Botulinum toxin
Botulinum toxin A (BoNT-A) is occasionally used to treat sialorrhea or dystonic symptoms in PD [110, 111]. Its effect on parkinsonian upper limb tremor was investigated in open-label studies and one randomized, placebo-controlled study ([112], see Supplementary Material). These studies suggest that BoNT-A injections can lead to an improvement of disabling upper limb tremor in PD and that this may be associated with clinically meaningful functional improvement. BoNT-A treatment of the upper limbs carries a risk of relevant (but transient) weakness, which can be controlled by using targeted injection techniques and cautious dosing, especially for hand and finger extensors. This type of treatment is time-consuming and requires a high level of physician expertise. There is insufficient evidence to conclude on the efficacy and usefulness of BoNT-A injections as a treatment for jaw and chin tremor in PD (see Supplementary Material).
TRANSLATING EVIDENCE FROM TRIALS INTO CLINICAL PRACTICE
When initiating treatment, therapy of PD tremor goes hand in hand with the treatment of other motor symptoms. The choice of initial medication depends on clinical factors like age, cognitive status, comorbidities and severity of motor symptoms, and the side effect profile of a given therapeutic approach. A summary of pharmacological and surgical treatment options for PD tremor is presented in Table 1. Practical recommendations for the management of PD tremor are summarized below and presented in Figs. 2 and 3.
Table 1
Interventions against PD tremor
| Intervention | Mechanism of action | Level of evidence* | Comments |
| Dopaminergig agents | |||
| Levodopa | striatal DA replacement | +++ | marked antitremor effect in majority of pts |
| Selegiline | MAO-B inhibition | + | low antitremor effect |
| Rasagiline | MAO-B inhibition | + | low antitremor effect, option to improve tremor associated with motor fluctuations |
| Safinamide | MAO-B inhibition | EO | low antitremor effect, option to improve tremor associated with motor fluctuations |
| Tolcapone | COMT inhibition | EO | antitremor effect via levodopa enhancement |
| Entacapone | COMT inhibition | + | antitremor effect via levodopa enhancement |
| Opicapone | COMT inhibition | EO | antitremor effect via levodopa enhancement |
| Pramipexole | Direct D2/D3 receptor agonism | ++ | overall motor effect (including tremor) weaker than levodopa |
| Ropinirole | Direct D2/D3 receptor agonism | +++ | overall motor effect (including tremor) weaker than levodopa |
| Piribedil | Direct D2/D3 receptor agonism | ++ | overall motor effect (including tremor) weaker than levodopa |
| Rotigotine | Direct D1/D2/D3 receptor agonism | + | overall motor effect (including tremor) weaker than levodopa |
| Apomorphine | Direct D1/D2/D3 receptor agonism | + | antitremor effect similar to levodopa |
| Non-dopaminergig agents | |||
| Trihexiphenidyl | Muscarinic ACh receptor antagonism | ++ | moderate antitremor effect, use only in young and cognitively intact pts |
| Bornaprin | Muscarinic ACh receptor antagonism | ++ | moderate antitremor effect, use only in young and cognitively intact pts |
| Methixene | Muscarinic ACh receptor antagonism | ++ | moderate antitremor effect, use only in young and cognitively intact pts |
| Benztropine | Muscarinic ACh receptor antagonism | ++ | moderate antitremor effect, use only in young and cognitively intact pts |
| Amantadine | Glutamate NMDA receptor antagonism | ++ | low antitremor effect, option to improve tremor associated with motor fluctuations |
| Propranolol | Non-selective adrenergic β-receptor antagonism | ++ | moderate antitremor effect |
| Oxprenolol | Non-selective adrenergic β-receptor antagonism | ++ | moderate antitremor effect |
| Clozapine | 5HT1A receptor agonism, 5HT2A/5HT2C receptor antagonism | ++ | may improve tremor resistant to DAergic medication, unfavorable AE profile requiring safety monitoring |
| D2/D4 receptor antagonism, actions on other receptors | |||
| Cannabidiol | CB1/CB2 receptor partial agonism | + | investigational |
| Botulinum toxin A | peripheral/reduction of muscle strength | ++ | treatment option in selected patients |
| Surgical treatments | |||
| STN DBS | modulation of basal ganglia output | +++ | marked antitremor effect (includes levodopa resistant tremor) |
| VIM DBS | electrical modulation of oscillatory VIM activity | +++ | marked antitremor effect (includes levodopa resistant tremor) |
| VIM FUS | ablation of VIM oscillatory neuronal activity | +++ | marked antitremor effect (includes levodopa resistant tremor) |
| RF thalamotomy | ablation of VIM or VOA oscillatory neuronal activity | ++ | marked antitremor effect (includes levodopa resistant tremor) |
| GK thalamotomy | ablation of VIM or VOA oscillatory neuronal activity | + | generally marked antitremor effect (includes levodopa resistant tremor) |
Levels of evidence: +++efficacy shown in at least one high quality randomized controlled trial. ++efficacy shown in at least one low-quality randomized controlled trial. +efficacy shown at least in one randomized single-dose study, one case-control study. or one post-hoc analysis of randomized trials. EO expert opinion level of evidence. D1, D2, D3 receptor, dopamin D1, D2, D3 receptor; ACh, acetylcholine; NMDA, N-methyl-D-aspartate, 5-HT, 5-hydroxytryptamine; CB receptor, cannabinoid receptor; STN, subthalamic nucleus; DBS, deep brain stimulation; VIM, ventral intermediate nucleus of the thalamus; FUS, high-intensity focussed ultrasound; RF, radiofrequency; GK, gamma knife; VOA, ventral oral anterior nucleus of the thalamus; AE, adverse events.
Fig. 2
Sequential treatment options in PD patients with insufficient tremor response to different baseline regimens. PD, Parkinson’s disease; ICD, impulse control disorders; L-dopa, levodopa; DA, dopamine; rx, risk; STN, subthalamic nucleus; DBS, deep brain stimulation; VIM, ventral intermediate nucleus of the thalamus; FUS, high-intensity focused ultrasound; pts, patients; motor compl., motor complications; inh., inhibitor; tx, treatment; MAO-B, monoamine oxidase B; COMT, catechol-O-methyl-transferase.
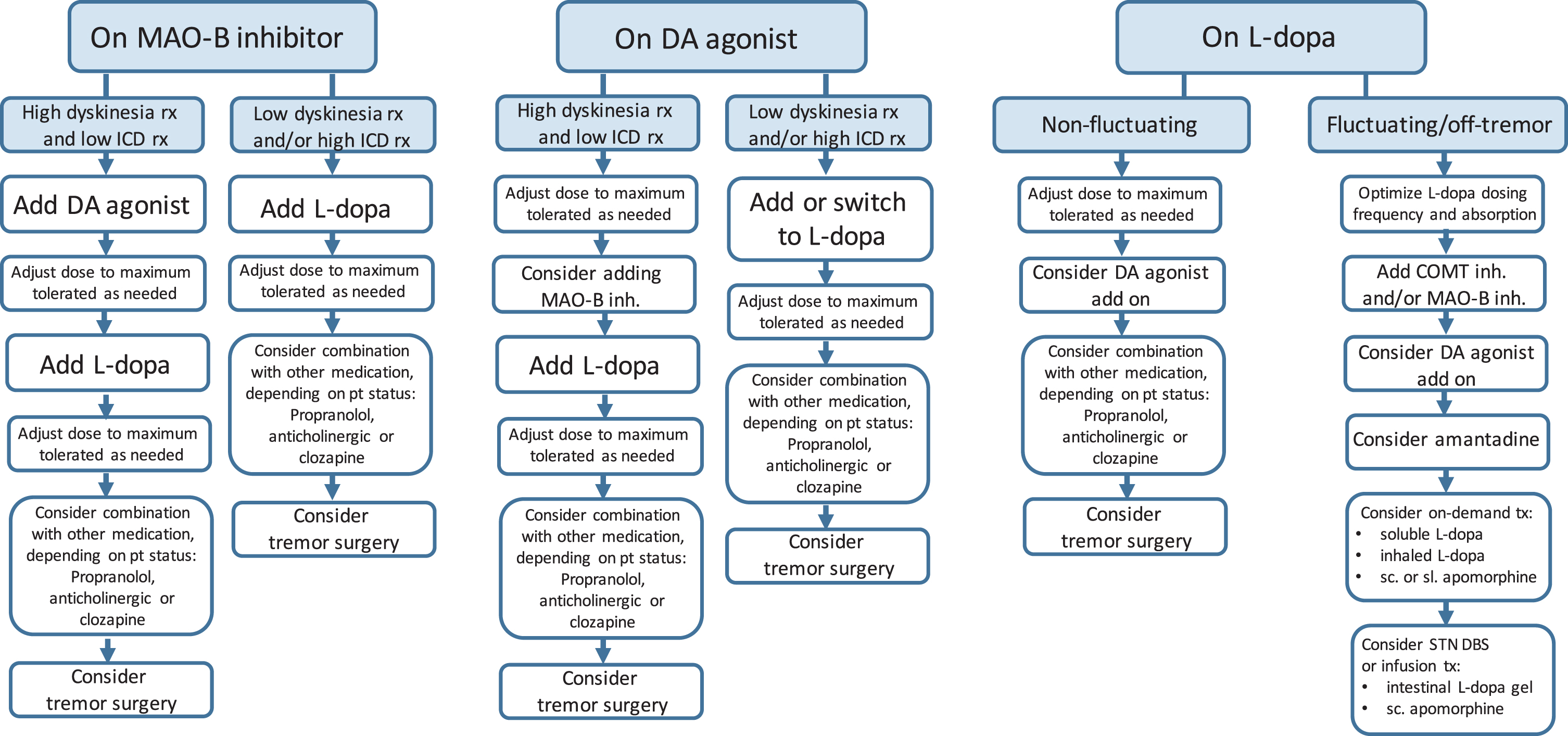
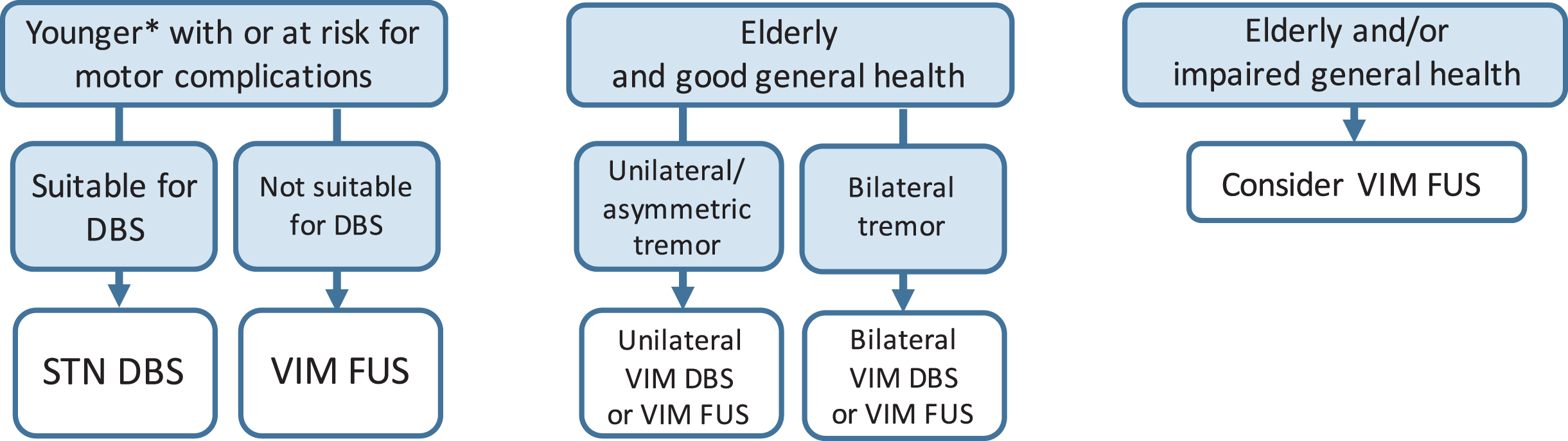
Fig. 3
Surgical options in-drug refractory PD tremor. *Age limit for STN DBS in patients with established motor complications 70–75 years. Note that VIM FUS in PD is usually performed unilaterally, resulting in unilateral tremor reduction in the contralateral hemibody. PD, Parkinson’s disease; rx, risk; STN, subthalamic nucleus; DBS, deep brain stimulation; GPi, globus pallidus interna; VIM, ventral intermediate nucleus of the thalamus; FUS, high-intensity focused ultrasound.
DRUG SELECTION AND DOSING PRINCIPLES
The role of levodopa as the ‘gold-standard’ of symptomatic efficacy relates to all cardinal motor features of PD including tremor. If classical resting tremor does not respond to an adequate trial of levodopa, it is unlikely to respond to other oral dopaminergic agents. Levodopa also appears superior to propranolol in suppressing postural tremor.
The proportion of patients with PD tremor resistant or poorly responsive to levodopa has never been formally studied nor have guidelines been formulated on how to dose levodopa and to assess its antitremor effect in order to define lack of response. In the recently revised diagnostic criteria for MSA— a condition where poor or absent response of parkinsonism to levodopa is a key diagnostic feature [113] - daily levodopa doses of up to 1000 mg over at least 2 months are required to confirm lack of responsiveness, which may not be easily applicable or advisable in patients with early PD. Nonetheless, common clinical experience suggests that poorly responsive tremor can be improved by increasing the total daily dose of levodopa or optimizing its delivery by avoiding administration close to mealtimes, by using enzyme inhibitors (MAO-B in stable patients, or COMT inhibitors in fluctuating PD, which may be combined with MAO-B inhibitors) and dispersible formulations where available or, in fluctuating patients, by using non-oral routes of delivery of levodopa or apomorphine.
Peripheral pharmacokinetic factors such as impaired swallowing, altered gastric motility and bacterial overgrowth leading to impaired absorption of levodopa in the small intestine may contribute to levodopa “pseudoresistance”, problems which might be overcome by bypassing the oral route by direct intestinal administration of levodopa in gel formulations (with or without combined entacapone), or by subcutaneous delivery of apomorphine (or levodopa when this becomes available). Although ‘levodopa resistant tremor’ has occasionally been listed as a potential decision node towards DBS [114], in patients with impaired levodopa absorption, parenteral administration of levodopa or apomorphine may still be efficacious. The effect of these continuous infusions in patients with ‘resistant tremor’ has never been formally studied.
The use of oral non-ergot dopamine agonists to improve PD tremor should follow the same principles as have been formulated by international guidelines for their use as monotherapy or levodopa adjuncts in early and advanced PD [115–117]. Similarly to levodopa, dopamine agonists likely improve not only resting but also action tremor. Evidence from available studies does not allow conclusions on the effect size of dopamine agonists specifically on PD tremor. The overall effect of oral dopamine agonists on motor symptoms is weaker than the effect of levodopa [85, 117], and there is no evidence to suggest superior antitremor efficacy. Dopamine agonist treatment carries a substantial risk of inducing impulse control disorders and excessive daytime sleepiness, but initial agonist monotherapy remains an option for patients at high risk to develop motor complications [115–117]. The goal of delaying motor complications and a potentially additional antitremor effect should always be weighed against their adverse effect profile. Conversely, in selected patients with poorly responsive PD tremor tolerating dopamine agonists without adverse events, adequate doses should be tried, up to the maximum approved dose, under close monitoring of tolerability.
Adding anticholinergics should only be considered in young and cognitively intact patients with tremor poorly controlled by optimized dopaminergic treatment, provided no contraindications exist and weighing their potential to worsen cognition even in initially cognitively intact patients. Propranolol can sometimes lead to a meaningful improvement of resting and action tremor and is a treatment option for PD tremor not well controlled by dopaminergic drugs unless there are contraindications. Clozapine can improve resting and action tremor in PD, but its use is limited by its unfavorable side effect profile. Cannabinoids may have the potential to ameliorate PD via the improvement of stress and anxiety but evidence for a direct antitremor effect is lacking. Since CBD lacks psychotropic effects, its potential effect on PD tremor warrants further study. At present, cannabinoids cannot be generally recommended for the treatment of PD tremor outside of clinical studies. BoNT-A can lead to an improvement of disabling upper limb tremor in individual PD patients. Using a targeted injection technique and cautious dosing, the risk of muscle weakness can be kept low.
If tremor is a disabling part of motor fluctuations, the treatment target is reduction in off-time and oral options include increasing levodopa dose frequency, use of extended-release formulations of levodopa and adjunct therapy with MAO-B or COMT inhibitors, oral dopamine agonists, amantadine and, depending on the health care system, other drugs such as zonisamide or istradefylline [72]. Such approaches can be supplemented by on-demand therapies to terminate off periods including dispersible levodopa formulations, subcutaneous or sublingual administration of apomorphine or inhaled levodopa. Device-aided treatments like DBS or infusion therapies should be strongly considered in all fluctuating patients refractory to these non-invasive measures. DBS and focused ultrasound have an additional potential role in treating resistant and disabling PD tremor in patients without motor fluctuations.
SURGICAL AND ABLATIVE APPROACHES TO PD TREMOR
Surgical treatment should be offered to all patients with confirmed drug resistant tremor but also for patients intolerant to drug treatment at effective doses. With focused ultrasound thalamotomy now becoming an alternative to DBS and to traditional forms of lesional surgery, the number of patients eligible for tremor surgery is increasing and now also includes the very elderly and patients with substantial comorbidities. The beneficial effect of bilateral subthalamic nucleus (STN) stimulation in controlling motor symptoms and improving quality of life in PD has been well documented in randomized controlled studies [118]. A non-randomized study in fluctuating PD patients with substantial off-tremor found that STN stimulation led to an 82% improvement of resting and a 78% improvement of action tremor during the “medication off” condition [119]. A randomized study that included a high number of patients with tremor as the reason for considering surgery found a similar impact of STN stimulation on quality of life in this subgroup as in patients with fluctuations and/or dyskinesias as the main reason for surgery [120]. Long-term studies demonstrate that the marked improvement of off period tremor by STN stimulation is maintained at 5 years and beyond [121]. Finally, a double-blind, sham-controlled study found a 61% improvement of tremor in the “medication off” condition by STN stimulation at 3 months post surgery [122]. Open-label 1 year follow up of the stimulated patients showed an 82% improvement of tremor [122].
Among the principal motor features of PD— tremor, bradykinesia, rigidity, balance, and gait, tremor appears to be the symptom most responsive to STN stimulation, independent of its response to medication [118, 119, 122].
The globus pallidus internus (GPi) is an alternative target for the treatment of patients with medication refractory motor complications. A retrospective study found a comparable improvement of PD resting and action tremor following 1 year of GPi and STN stimulation [123]. Ten-year follow up of a large randomized study comparing GPi and STN stimulation showed a sustained response of PD tremor to stimulation of both targets [124]. However, the overall motor effects of pallidal stimulation are somewhat smaller than those of STN stimulation and in contrast to STN surgery, GPi stimulation does not allow for a sustained reduction in PD medication [118].
Several non-randomized studies document the beneficial effect of thalamic VIM stimulation on PD tremor [125, 126]. Whereas unilateral VIM stimulation ameliorates contralateral limb tremor, bilateral stimulation is needed for improving bilateral and axial PD tremor [126]. Long-term follow up found a sustained >60% improvement of PD tremor with VIM stimulation beyond 10 years [127].
Since VIM stimulation effectively reduces tremor but does not alter other motor features of PD, STN stimulation is preferred in PD tremor patients suffering from fluctuations and dyskinesias and in those with a future risk of developing motor complications. However, due to its lower risk of cognitive and other adverse events, thalamic surgery is a good alternative for elderly PD patients with medication resistant tremor. Advances in DBS technology including directional stimulation, the application of short pulse widths and alternative targets including the dentato-rubro-thalamic tract and the posterior subthalamic area may further improve the risk-benefit ratio of DBS in PD tremor [128].
Lesional surgery was the main approach to the treatment of medication refractory tremor from the 1960 s to the 1980 s. Radiofrequency (RF) thalamotomy requires brain penetration with a RF probe for thermocoagulation. This has been shown to improve contralateral limb tremor but is associated with a relatively high rate of adverse events including paresis, dysarthria, and gait ataxia, which may be transient but can persist in a minority of patients [129]. A randomized controlled study comparing RF thalamotomy and VIM stimulation found a better functional outcome and fewer adverse events with stimulation [130]. The superiority of VIM stimulation in terms of tremor control is owed to the fact that DBS surgery can be safely performed bilaterally, whereas RF thalamotomy, due to its higher risk of side effects, is usually restricted to unilateral lesions [129].
Gamma knife thalamotomy is another treatment option for unilateral or asymmetrical limb tremor. This technique does not require brain penetration but lacks intraoperative clinical feedback. Improvements in tremor become apparent only months after treatment. In addition, some patients undergoing radiosurgery develop adverse events months to years after treatment [129].
Magnetic resonance guided high-intensity FUS thalamotomy is the most novel surgical modality for the treatment of medication refractory tremor. FUS combines high-resolution imaging for targeting with incisionless surgery using transcranial delivery of ultrasound energy. Advantages of the technique include intraoperative monitoring of tremor and side effects during the application of progressive doses of ultrasound energy and magnetic resonance thermometry to verify target temperatures and location, finally allowing for the application of a permanent lesion with optimal efficacy and minimal side effects [129]. The antitremor effect of FUS thalamotomy in PD was documented in a randomized, sham-controlled study which showed a 62% improvement of contralateral hand tremor (on medication) in the FUS group vs. a 22% improvement in the sham-treated group at 3 months [131]. Adverse events in this and a number of non-randomized series of FUS in patients with medication refractory PD tremor included paresthesias, dygeusia, gait ataxia, and transient paresis [129]. A decay of benefit over time, similar to VIM stimulation, has been observed in some PD patients [132]. Long-term follow up (max. 5 years, median 3 years) of 26 tremor-dominant PD patients with good to excellent initial tremor response to thalamic FUS found overall good long-term tolerability, a complete loss of effect in 2, a partial return of tremor in 8 and a persisting excellent antitremor effect in the remainder of the patients [133]. Initial experience regarding staged bilateral FUS thalamotomy, particularly in patients with essential tremor, has been published. However, bilateral treatments are still considered experimental [134] and PD patients may not be good candidates for bilateral FUS for their intrinsic high risk of balance and gait problems.
Other targets for FUS in PD patients, in addition to VIM, are under investigation and include GPi, the pallidothalamic tract, and STN [129]. Patients may not be eligible for FUS treatment because of a low skull bone ratio, skull deformities, and contraindications to magnetic resonance imaging. The method is incisionless but not non-invasive, and anticoagulants and platelet inhibitors have to be withheld for the procedure [134].
SUMMARY
The treatment of tremor in PD follows the same principles as that of the other levodopa-responsive motor features. This means that for each patient, the individually adequate levodopa dose needs to be determined, making an increase in levodopa the fundamental strategy in the majority of patients. In younger patients with stable PD, an additional treatment goal may be to delay motor complications by using levodopa-sparing drugs, if they are well tolerated and achieve sufficientimprovement.
Tremor differs from bradykinesia and rigidity in its pathophysiology and in its correlation to disease duration and nigrostriatal neurodegeneration, which may explain why in some patients, tremor does not respond equally well to dopamine replacement treatment as other motor symptoms. In addition, tremor may be exacerbated by mental stress, which likely contributes to social stigmatization [135] and to the subjective perception of tremor severity.
Treatment resistance has not been formally defined for PD tremor but if it is perceived as persistently troublesome, the primary approach should be optimizing levodopa treatment. This includes factors that improve bioavailability, such as ensuring intake on an empty stomach, adding dispersible formulations, blocking the degrading enzymes MAO-B and COMT, and, most importantly, increasing the levodopa dose based on each patient's needs.
There is no evidence that any other class of drugs has an effect that would exceed that of levodopa although very rarely, individual patients may show a better response to a dopamine agonist than to levodopa. When considering dopamine agonists, their higher adverse event rate compared to levodopa needs to be taken into account. Adding anticholinergics may be considered in patients with tremor poorly controlled by optimized dopaminergic treatment but this should be limited to young and cognitively intact patients and requires monitoring for central and peripheral anticholinergic side effects. Propranolol may improve resting and action tremor in individual patients without contraindications for beta blockers. Clozapine is a treatment option for patients intolerant to other antitremor medications and not eligible for tremor surgery, provided that regular leukocyte monitoring is performed.
When patients report troublesome tremor, it is important to determine if this is part of motor fluctuations, in which case control of fluctuations becomes the treatment goal. This may include the use of levodopa or apomorphine infusion, or on-demand treatments such as apomorphine injections or sublingual strips for off periods not otherwise well controlled. All these interventions may help off period tremor. Surgical options and MRI-guided focused ultrasound can improve all off symptoms including tremor, and patients with refractory tremor may benefit from DBS even if they do not fluctuate. However, a substantial proportion of PD patients with troublesome tremor do not have access to tremor surgery or are not willing to take the risk of surgical tremor treatment despite an overall good risk-to-benefit ratio of DBS and thalamic FUS, highlighting the need of optimal medical treatment.
In summary, while studies are needed that specifically investigate the effects of interventions on PD tremor and its subtypes, and new treatments are emerging, such as focused ultrasound, the most important approach that should be offered to patients with PD tremor that appears refractory is an adequately dosed levodopa treatment trial.
ACKNOWLEDGMENTS
Publication of this work was supported by the Forschungsförderungsverein Wilhelminenspital (FWFW). We thank Markus Bürger for excellent assistance in the preparation of the illustrations.
CONFLICT OF INTEREST
W. Pirker has received travel grants from AbbVie, AOP Orphan, Boehringer Ingelheim, Grünenthal, Medtronic, Merz and Stada. He has received lecturing honoraria and/or consultancy fees from AbbVie, Bial, AOP Orphan, Boehringer Ingelheim, GE, Grünenthal, Medtronic, Merz, Stada and UCB, but has no owner interest in any pharmaceutical company.
R. Katzenschlager has received research support from Acorda, Biotie, Britannia, Stada and Zambon, and financial compensation for consulting and speaking from AbbVie, AOP Pharma, Bial, Britannia, Ever Pharma, Merz, Neuroderm, Novartis, Stada, UCB and Zambon.
M. Hallett is an inventor of a patent held by NIH for the H-coil for magnetic stimulation for which he receives license fee payments from Brainsway.
W. Poewe reports consultancy and lecture fees in relation to clinical drug development programmes for PD from AC Immune, Alterity, AbbVie, Affiris, BIAL, Biogen, Britannia, Lilly, Lundbeck, Merz, Neuroderm, Neurocrine, Roche, Sunovion, Stada, Takeda, UCB and Zambon.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-225060.
REFERENCES
[1] | Vingerhoets FJ , Schulzer M , Calne DB , Snow BJ ((1997) ) Which clinical sign of Parkinson’s disease best reflects the nigrostriatal lesion? Ann Neurol 41: , 58–64. |
[2] | Benamer HT , Patterson J , Wyper DJ , Hadley DM , Macphee GJ , Grosset DG ((2000) ) Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord 15: , 692–698. |
[3] | Pirker W ((2003) ) Correlation of dopamine transporter imaging with parkinsonian motor handicap: How close is it? Mov Disord 18: (Suppl 7), S43–51. |
[4] | Nonnekes J , Timmer MH , de Vries NM , Rascol O , Helmich RC , Bloem BR ((2016) ) Unmasking levodopa resistance in Parkinson’s disease. Mov Disord 31: , 1602–1609. |
[5] | Lin F , Wu D , Yu J , Weng H , Chen L , Meng F , Chen Y , Ye Q , Cai G ((2021) ) Comparison of efficacy of deep brain stimulation and focused ultrasound in parkinsonian tremor: A systematic review and network meta-analysis. J Neurol Neurosurg Psychiatry. 10.1136/jnnp-2020-323656. |
[6] | Deuschl G , Bain P , Brin M ((1998) ) Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. MovDisord 13: (Suppl 3), 2–23. |
[7] | Bhatia KP , Bain P , Bajaj N , Elble RJ , Hallett M , Louis ED , Raethjen J , Stamelou M , Testa CM , Deuschl G ((2018) ) Consensus Statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 33: , 75–87. |
[8] | Pasquini J , Ceravolo R , Qamhawi Z , Lee JY , Deuschl G , Brooks DJ , Bonuccelli U , Pavese N ((2018) ) Progression of tremor in early stages of Parkinson’s disease: A clinical and neuroimaging study. Brain 141: , 811–821. |
[9] | Hughes AJ , Daniel SE , Blankson S , Lees AJ ((1993) ) A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol 50: , 140–148. |
[10] | Fearon C , Lees AJ , McKinley JJ , McCarthy A , Smyth S , Farrell M , Lynch T ((2021) ) On the emergence of tremor in prodromal Parkinson’s disease. J Parkinsons Dis 11: , 261–269. |
[11] | Jankovic J , Schwartz KS , Ondo W ((1999) ) Re-emergent tremor of Parkinson’s disease. J Neurol Neurosurg Psychiatry 67: , 646–650. |
[12] | Belvisi D , Conte A , Cutrona C , Costanzo M , Ferrazzano G , Fabbrini G , Berardelli A ((2018) ) Re-emergent tremor in Parkinson’s disease: The effect of dopaminergic treatment. Eur J Neurol 25: , 799–804. |
[13] | Hassan A , Caviness J ((2019) ) Slow orthostatic tremor: Review of the current evidence. Tremor Other Hyperkinet Mov (N Y) 9: .10.7916/tohm.v0.721. |
[14] | Papengut F , Raethjen J , Binder A , Deuschl G ((2013) ) Rest tremorsuppression may separate essential from parkinsonian rest tremor. Parkinsonism Relat Disord 19: , 693–697. |
[15] | Dirkx MF , Zach H , Bloem BR , Hallett M , Helmich RC ((2018) ) The nature of postural tremor in Parkinson disease, Neurology 90: , e1095–e1103. |
[16] | Louis ED , Pullman SL , Eidelberg D , Dhawan V ((2008) ) Re-emergent tremor without accompanying rest tremor in Parkinson’s disease. Can J Neurol Sci 35: , 513–515. |
[17] | Minen MT , Louis ED ((2008) ) Emergence of Parkinson’s disease in essential tremor: A study of the clinical correlates in 53 patients. Mov Disord 23: , 1602–1605. |
[18] | Fekete R , Jankovic J ((2011) ) Revisiting the relationship between essential tremor and Parkinson’s disease. Mov Disord 26: , 391–398. |
[19] | Wenzelburger R , Raethjen J , Loffler K , Stolze H , Illert M , Deuschl G ((2000) ) Kinetic tremor in a reach-to-grasp movement in Parkinson’s disease. Mov Disord 15: , 1084–1094. |
[20] | Raethjen J , Pohle S , Govindan RB , Morsnowski A , Wenzelburger R , Deuschl G ((2005) ) Parkinsonian action tremor: Interference with object manipulation and lacking levodopa response. Exp Neurol 194: , 151–160. |
[21] | Hallett M , Deuschl G ((2010) ) Are we making progress in the understanding of tremor in Parkinson’s disease? Ann Neurol 68: , 780–781. |
[22] | Louis ED , Tang MX , Cote L , Alfaro B , Mejia H , Marder K ((1999) ) Progression of parkinsonian signs in Parkinson disease. Arch Neurol 56: , 334–337. |
[23] | Koh SB , Kwon DY , Seo WK , Kim JH , Kim JH , Lee SH , Oh K , Kim BJ , Park KW ((2010) ) Dissociation of cardinal motor signs in Parkinson’s disease patients. Eur Neurol 63: , 307–310. |
[24] | Kaasinen V ((2016) ) Ipsilateral deficits of dopaminergic neurotransmission in Parkinson’s disease. Ann Clin Transl Neurol 3: , 21–26. |
[25] | Louis ED , Levy G , Cote LJ , Mejia H , Fahn S , Marder K ((2001) ) Clinical correlates of action tremor in Parkinson disease. Arch Neurol 58: , 1630–1634. |
[26] | Stochl J , Boomsma A , Ruzicka E , Brozova H , Blahus P ((2008) ) On the structure of motor symptoms of Parkinson’s disease. Mov Disord 23: , 1307–1312. |
[27] | Tolosa E , Garrido A , Scholz SW , Poewe W ((2021) ) Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol 20: , 385–397. |
[28] | Josephs KA , Matsumoto JY , Ahlskog JE ((2006) ) Benign tremulous parkinsonism. Arch Neurol 63: , 354–357. |
[29] | Selikhova M , Kempster PA , Revesz T , Holton JL , Lees AJ ((2013) ) Neuropathological findings in benign tremulous parkinsonism. Mov Disord 28: , 145–152. |
[30] | Paulus W , Jellinger K ((1991) ) The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol 50: , 743–755. |
[31] | Rajput AH , Sitte HH , Rajput A , Fenton ME , Pifl C , Hornykiewicz O ((2008) ) Globus pallidus dopamine and Parkinson motor subtypes: Clinical and brain biochemical correlation. Neurology 70: , 1403–1410. |
[32] | Fishman PS ((2008) ) Paradoxical aspects of parkinsonian tremor. Mov Disord 23: , 168–173. |
[33] | Sung YH , Chung SJ , Kim SR , Lee MC ((2008) ) Factors predicting response to dopaminergic treatment for resting tremor of Parkinson’s disease. Mov Disord 23: , 137–140. |
[34] | Zach H , Dirkx M , Pasman JW , Bloem BR , Helmich RC ((2017) ) The patient’s perspective: The effect of levodopa on Parkinson symptoms. Parkinsonism Relat Disord 35: , 48–54. |
[35] | Parveen S ((2016) ) Comparison of self and proxy ratings for motor performance of individuals with Parkinson disease. Brain Cogn 103: , 62–69. |
[36] | Zach H , Dirkx MF , Pasman JW , Bloem BR , Helmich RC ((2017) ) Cognitive stress reduces the effect of levodopa on Parkinson’s resting tremor. CNS Neurosci Ther 23: , 209–215. |
[37] | Deuschl G , Raethjen J , Baron R , Lindemann M , Wilms H , Krack P ((2000) ) The pathophysiology of parkinsonian tremor: A review, J Neurol 247: (Suppl 5), V33–48. |
[38] | Zaidel A , Arkadir D , Israel Z , Bergman H ((2009) ) Akineto-rigid vs. tremor syndromes in Parkinsonism. Curr Opin Neurol 22: , 387–393. |
[39] | Kuhn AA , Tsui A , Aziz T , Ray N , Brucke C , Kupsch A , Schneider GH , Brown P ((2009) ) Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol 215: , 380–387. |
[40] | Timmermann L , Gross J , Dirks M , Volkmann J , Freund HJ , Schnitzler A ((2003) ) The cerebral oscillatory network of parkinsonian resting tremor. Brain 126: , 199–212. |
[41] | Helmich RC , Hallett M , Deuschl G , Toni I , Bloem BR ((2012) ) Cerebral causes and consequences of parkinsonian resting tremor: A tale of two circuits? Brain 135: , 3206–3226. |
[42] | Bostan AC , Dum RP , Strick PL ((2010) ) The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci U S A 107: , 8452–8456. |
[43] | Dirkx MF , den Ouden HE , Aarts E , Timmer MH , Bloem BR , Toni I , Helmich RC ((2017) ) Dopamine controls Parkinson’s tremor by inhibiting the cerebellar thalamus. Brain 140: , 721–734. |
[44] | Ni Z , Pinto AD , Lang AE , Chen R ((2010) ) Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann Neurol 68: , 816–824. |
[45] | Helmich RC , Van den Berg KRE , Panyakaew P , Cho HJ , Osterholt T , McGurrin P , Shamim EA , Popa T , Haubenberger D , Hallett M ((2021) ) Cerebello-cortical control of tremor rhythm and amplitude in Parkinson’s disease. Mov Disord 36: , 1727–1729. |
[46] | Du G , Zhuang P , Hallett M , Zhang YQ , Li JY , Li YJ ((2018) ) Properties of oscillatory neuronal activity in the basal ganglia and thalamus in patients with Parkinson’s disease. Transl Neurodegener 7: , 17. |
[47] | Meng D , Zhuang P , Hallett M , Zhang Y , Li J , Hu Y , Li Y ((2020) ) Characteristics of oscillatory pallidal neurons in patients with Parkinson’s disease. J Neurol Sci 410: , 116661. |
[48] | Shreve LA , Velisar A , Malekmohammadi M , Koop MM , Trager M , Quinn EJ , Hill BC , Blumenfeld Z , Kilbane C , Mantovani A , Henderson JM , Bronte-Stewart H ((2017) ) Subthalamic oscillations and phase amplitude coupling are greater in the more affected hemisphere in Parkinson’s disease. Clin Neurophysiol 128: , 128–137. |
[49] | Hirschmann J , Abbasi O , Storzer L , Butz M , Hartmann CJ , Wojtecki L , Schnitzler A ((2019) ) Longitudinal recordings reveal transient increase of alpha/low-beta power in the subthalamic nucleus associated with the onset of parkinsonian rest tremor. Front Neurol 10: , 145. |
[50] | Rinne JO , Rummukainen J , Paljarvi L , Rinne UK ((1989) ) Dementia in Parkinson’s disease is related to neuronal loss in the medial substantia nigra. Ann Neurol 26: , 47–50. |
[51] | Bernheimer H , Birkmayer W , Hornykiewicz O , Jellinger K , Seitelberger F ((1973) ) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20: , 415–455. |
[52] | Rinne UK , Sonninen V ((1972) ) Acid monoamine metabolites in the cerebrospinal fluid of patients with Parkinson’s disease. Neurology 22: , 62–67. |
[53] | Hirsch EC , Mouatt A , Faucheux B , Bonnet AM , Javoy-Agid F , Graybiel AM , Agid Y ((1992) ) Dopamine, tremor, and Parkinson’s disease. Lancet 340: , 125–126. |
[54] | Helmich RC ((2018) ) The cerebral basis of Parkinsonian tremor: Anetwork perspective. Mov Disord 33: , 219–231. |
[55] | Helmich RC , Janssen MJ , Oyen WJ , Bloem BR , Toni I ((2011) ) Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol 69: , 269–281. |
[56] | Lee JY , Lao-Kaim NP , Pasquini J , Deuschl G , Pavese N , Piccini P ((2018) ) Pallidal dopaminergic denervation and rest tremor in early Parkinson’s disease: PPMI cohort analysis. Parkinsonism Relat Disord 51: , 101–104. |
[57] | Loane C , Wu K , Bain P , Brooks DJ , Piccini P , Politis M ((2013) ) Serotonergic loss in motor circuitries correlates with severity of action-postural tremor in PD. Neurology 80: , 1850–1855. |
[58] | Qamhawi Z , Towey D , Shah B , Pagano G , Seibyl J , Marek K , Borghammer P , Brooks DJ , Pavese N ((2015) ) Clinical correlates of raphe serotonergic dysfunction in early Parkinson’s disease. Brain 138: , 2964–2973. |
[59] | Doder M , Rabiner EA , Turjanski N , Lees AJ , Brooks DJ , study CWP ((2003) ) Tremor in Parkinson’s disease and serotonergic dysfunction: An 11C-WAY 100635 PET study. Neurology 60: , 601–605. |
[60] | Sanson F , Schergna E , Semenzato D , Trevisan CP , Bizzarini M , Violante F , Santagostino I , Ravenna C , Maccarone G ((1986) ) [Therapeutic effects of trazodone in the treatment of tremor. Multicentric double-blind study]. Riv Neurol 56: , 358–364. |
[61] | Henderson J , Yiannikas C , Graham JS ((1992) ) Effect of ritanserin, a highly selective 5-HT2 receptor antagonist, on Parkinson’s disease. Clin Exp Neurol 29: , 277–282. |
[62] | Gordon PH , Pullman SL , Louis ED , Frucht SJ , Fahn S ((2002) ) Mirtazapine in Parkinsonian tremor. Parkinsonism Relat Disord 9: , 125–126. |
[63] | Fox SH ((2013) ) Non-dopaminergic treatments for motor control in Parkinson’s disease. Drugs 73: , 1405–1415. |
[64] | Ohno Y , Shimizu S , Tokudome K , Kunisawa N , Sasa M ((2015) ) New insight into the therapeutic role of the serotonergic system in Parkinson’s disease. Prog Neurobiol 134: , 104–121. |
[65] | Duvoisin RC ((1967) ) Cholinergic-anticholinergic antagonism in parkinsonism. Arch Neurol 17: , 124–136. |
[66] | Bohnen NI , Kanel P , Koeppe RA , Sanchez-Catasus CA , Frey KA , Scott P , Constantine GM , Albin RL , Muller M ((2021) ) Regional cerebral cholinergic nerve terminal integrity and cardinal motor features in Parkinson’s disease, Brain Commun 3: , fcab109. |
[67] | Ztaou S , Amalric M ((2019) ) Contribution of cholinergic interneurons to striatal pathophysiology in Parkinson’s disease. Neurochem Int 126: , 1–10. |
[68] | McKinley JW , Shi Z , Kawikova I , Hur M , Bamford IJ , Sudarsana Devi SP , Vahedipour A , Darvas M , Bamford NS ((2019) ) Dopamine deficiency reduces striatal cholinergic interneuron function in models of Parkinson’s disease, Neuron 103: , 1056–1072e1056. |
[69] | Betz AJ , McLaughlin PJ , Burgos M , Weber SM , Salamone JD ((2007) ) The muscarinic receptor antagonist tropicamide suppresses tremulous jaw movements in a rodent model of parkinsonian tremor: Possible role of M4 receptors. Psychopharmacology (Berl) 194: , 347–359. |
[70] | Katzenschlager R , Sampaio C , Costa J , Lees A ((2003) ) Anticholinergics for symptomatic management of Parkinson’s disease. Cochrane Database Syst Rev, CD003735. |
[71] | Goetz CG ((2002) ) Management of Parkinson’s disease: An evidence-based review . Mov Disord 17: (Suppl 4), S1–S166. |
[72] | F Fox SH , Katzenschlager R , Lim SY , Barton B , de Bie RMA , Seppi K , Coelho M , Sampaio C ; Movement Disorder Society Evidence-Based Medicine Committee ((2018) ) International Parkinson and Movement Disorder Society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33: , 1248–1266. |
[73] | Cotzias GC , Papavasiliou PS , Gellene R ((1969) ) Modification of Parkinsonism–chronic treatment with L-dopa. N Engl J Med 280: , 337–345. |
[74] | Yahr MD , Duvoisin RC , Schear MJ , Barrett RE , Hoehn MM ((1969) ) Treatment of parkinsonism with levodopa. Arch Neurol 21: , 343–354. |
[75] | Frequin HL , Schouten J , Verschuur CVM , Suwijn SR , Boel JA , Post B , Bloem BR , van Hilten JJ , van Laar T , Tissingh G , Munts AG , Dijk JM , Deuschl G , Lang A , Dijkgraaf MGW , de Haan RJ , de Bie RMA , Group LS ((2023) ) Levodopa response in patients with early Parkinson disease: Further observations of the LEAP study. Neurology 100: , e367–e376. |
[76] | Tedeschi G , Sasso E , Marshall RW , Bonavita V ((1990) ) Tremor in Parkinson disease: Acute response to oral levodopa. Ital J Neurol Sci 11: , 259–263. |
[77] | Henderson JM , Yiannikas C , Morris JG , Einstein R , Jackson D , Byth K ((1994) ) Postural tremor of Parkinson’s disease. Clin Neuropharmacol 17: , 277–285. |
[78] | Koller WC ((1986) ) Pharmacologic treatment of parkinsonian tremor. Arch Neurol 43: , 126–127. |
[79] | Schrag A , Schelosky L , Scholz U , Poewe W ((1999) ) Reduction of Parkinsonian signs in patients with Parkinson’s disease by dopaminergic versus anticholinergic single-dose challenges. Mov Disord 14: , 252–255. |
[80] | Sahoo LK , Holla VV , Batra D , Prasad S , Bhattacharya A , Kamble N , Yadav R , Pal PK ((2020) ) Comparison of effectiveness of trihexyphenidyl and levodopa on motor symptoms in Parkinson’s disease. J Neural Transm (Vienna) 127: , 1599–1606. |
[81] | Lew MF ((2013) ) Rasagiline treatment effects on parkinsonian tremor. Int J Neurosci 123: , 859–865. |
[82] | Pogarell O , Gasser T , van Hilten JJ , Spieker S , Pollentier S , Meier D , Oertel WH ((2002) ) Pramipexole in patients with Parkinson’s disease and marked drug resistant tremor: A randomised, double blind, placebo controlled multicentre study. J Neurol Neurosurg Psychiatry 72: , 713–720. |
[83] | Navan P , Findley LJ , Jeffs JA , Pearce RK , Bain PG ((2003) ) Randomized, double-blind, 3-month parallel study of the effects of pramipexole, pergolide, and placebo on Parkinsonian tremor. Mov Disord 18: , 1324–1331. |
[84] | Schrag A , Keens J , Warner J , Ropinirole Study G ((2002) ) Ropinirole for the treatment of tremor in early Parkinson’s disease. Eur J Neurol 9: , 253–257. |
[85] | Stowe RL , Ives NJ , Clarke C , van Hilten J , Ferreira J , Hawker RJ , Shah L , Wheatley K , Gray R ((2008) ) Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev CD006564. |
[86] | Carbone F , Djamshidian A , Seppi K , Poewe W ((2019) ) Apomorphine for Parkinson’s disease: Efficacy and safety of current and new formulations. CNS Drugs 33: , 905–918. |
[87] | Kempster PA , Frankel JP , Stern GM , Lees AJ ((1990) ) Comparison of motor response to apomorphine and levodopa in Parkinson’s disease. J Neurol Neurosurg Psychiatry 53: , 1004–1007. |
[88] | Rajan R , Saini A , Verma B , Choudhary N , Gupta A , Vishnu VY , Bhatia R , Singh MB , Srivastava AK , Srivastava MVP ((2020) ) Anticholinergics may carry significant cognitive and gait burden in Parkinson’s disease. Mov Disord Clin Pract 7: , 803–809. |
[89] | Koller WC , Herbster G ((1987) ) Adjuvant therapy of parkinsonian tremor. Arch Neurol 44: , 921–923. |
[90] | Parkes JD , Baxter RC , Marsden CD , Rees JE ((1974) ) Comparative trial of benzhexol, amantadine, and levodopa in the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry 37: , 422–426. |
[91] | Pahwa R , Tanner CM , Hauser RA , Isaacson SH , Nausieda PA , Truong DD , Agarwal P , Hull KL , Lyons KE , Johnson R , Stempien MJ ((2017) ) ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson disease (EASE LID Study): A randomized clinical trial. JAMA Neurol 74: , 941–949. |
[92] | Crosby NJ , Deane KH , Clarke CE ((2003) ) Beta-blocker therapy for tremor in Parkinson’s disease. Cochrane Database Syst Rev CD003361. |
[93] | Raethjen J , Austermann K , Witt K , Zeuner KE , Papengut F , Deuschl G ((2008) ) Provocation of Parkinsonian tremor. Mov Disord 23: , 1019–1023. |
[94] | Hopfner F , Deuschl G ((2020) ) Managing essential tremor. Neurotherapeutics 17: , 1603–1621. |
[95] | Tolosa E , Marti MJ , Katzenschlager R ((2015) ) Pharmacologic management of Parkinson’s disease. In Parkinson’s Disease and Movement Disorders, JankovicJ, TolosaE, eds. Wolters Kluver, Philadelphia, pp. 86–111. |
[96] | Friedman JH , Koller WC , Lannon MC , Busenbark K , Swanson-Hyland E , Smith D ((1997) ) Benztropine versus clozapine for the treatment of tremor in Parkinson’s disease. Neurology 48: , 1077–1081. |
[97] | Bonuccelli U , Ceravolo R , Salvetti S , D’Avino C , Del Dotto P , Rossi G , Murri L ((1997) ) Clozapine in Parkinson’s disease tremor. Effects of acute and chronic administration. Neurology 49: , 1587–1590. |
[98] | Parkinson Study G ((1999) ) Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. N Engl J Med 340: , 757–763. |
[99] | Friedman JH , Lannon MC ((1990) ) Clozapine-responsive tremor in Parkinson’s disease. Mov Disord 5: , 225–229. |
[100] | Fischer PA , Baas H , Hefner R ((1990) ) Treatment of parkinsonian tremor with clozapine. J Neural Transm Park Dis Dement Sect 2: , 233–238. |
[101] | Jansen EN ((1994) ) Clozapine in the treatment of tremor in Parkinson’s disease. Acta Neurol Scand 89: , 262–265. |
[102] | Trosch RM , Friedman JH , Lannon MC , Pahwa R , Smith D , Seeberger LC , O’Brien CF , LeWitt PA , Koller WC ((1998) ) Clozapine use in Parkinson’s disease: A retrospective analysis of a large multicentered clinical experience. Mov Disord 13: , 377–382. |
[103] | Durif F , Vidailhet M , Assal F , Roche C , Bonnet AM , Agid Y ((1997) ) Low-dose clozapine improves dyskinesias in Parkinson’s disease. Neurology 48: , 658–662. |
[104] | Yaw TK , Fox SH , Lang AE ((2016) ) Clozapine in parkinsonian rest tremor: A review of outcomes, adverse reactions, and possible mechanisms of action. Mov Disord Clin Pract 3: , 116–124. |
[105] | Urbi B , Corbett J , Hughes I , Owusu MA , Thorning S , Broadley SA , Sabet A , Heshmat S ((2022) ) Effects of cannabis in Parkinson’s disease: A systematic review and meta-analysis. J Parkinsons Dis 12: , 495–508. |
[106] | de Faria SM , de Morais Fabricio D , Tumas V , Castro PC , Ponti MA , Hallak JE , Zuardi AW , Crippa JAS , Chagas MHN ((2020) ) Effects of acute cannabidiol administration on anxiety and tremors induced by a Simulated Public Speaking Test in patients with Parkinson’s disease. J Psychopharmacol 34: , 189–196. |
[107] | Peball M , Krismer F , Knaus HG , Djamshidian A , Werkmann M , Carbone F , Ellmerer P , Heim B , Marini K , Valent D , Goebel G , Ulmer H , Stockner H , Wenning GK , Stolz R , Krejcy K , Poewe W , Seppi K , Collaborators of the Parkinson’s Disease Working Group Innsbruck ((2020) ) Non-motor symptoms in Parkinson’s disease are reduced by nabilone. Ann Neurol 88: , 712–722. |
[108] | Holden SK , Domen CH , Sillau S , Liu Y , Leehey MA ((2022) ) Higher risk, higher reward? Self-reported effects of real-world cannabis use in Parkinson’s disease. Mov Disord Clin Pract 9: , 340–350. |
[109] | Leehey MA , Liu Y , Hart F , Epstein C , Cook M , Sillau S , Klawitter J , Newman H , Sempio C , Forman L , Seeberger L , Klepitskaya O , Baud Z , Bainbridge J ((2020) ) Safety and tolerability of cannabidiol in Parkinson disease: An open label, dose-escalation study. Cannabis Cannabinoid Res 5: , 326–336. |
[110] | Mitchell SD , Sidiropoulos C ((2021) ) Therapeutic applications of botulinum neurotoxin for autonomic symptoms in Parkinson’s disease: An updated review. Toxins (Basel) 13: , 226. |
[111] | Jost WH ((2021) ) Use of botulinum neurotoxin in Parkinson’s disease: A critical appraisal. Toxins (Basel) 13: , 87. |
[112] | Mittal SO , Machado D , Richardson D , Dubey D , Jabbari B ((2017) ) Botulinum toxin in Parkinson disease tremor: A randomized,double-blind, placebo-controlled study with acustomized injection approach. Mayo Clin Proc 92: , 1359–1367. |
[113] | Wenning GK , Stankovic I , Vignatelli L , Fanciulli A , Calandra-Buonaura G , Seppi K , Palma JA , Meissner WG , Krismer F , Berg D , Cortelli P , Freeman R , Halliday G , Hoglinger G , Lang A , Ling H , Litvan I , Low P , Miki Y , Panicker J , Pellecchia MT , Quinn N , Sakakibara R , Stamelou M , Tolosa E , Tsuji S , Warner T , Poewe W , Kaufmann H ((2022) ) The Movement Disorder Society criteria for the diagnosis of multiple system atrophy. Mov Disord 37: , 1131–1148. |
[114] | Odin P , Ray Chaudhuri K , Slevin JT , Volkmann J , Dietrichs E , Martinez-Martin P , Krauss JK , Henriksen T , Katzenschlager R , Antonini A , Rascol O , Poewe W , National Steering Committees ((2015) ) Collective physician perspectives on non-oral medication approaches for the management of clinically relevant unresolved issues in Parkinson’s disease: Consensus from an international survey and discussion program. Parkinsonism Relat Disord 21: , 1133–1144. |
[115] | Ferreira JJ , Katzenschlager R , Bloem BR , Bonuccelli U , Burn D , Deuschl G , Dietrichs E , Fabbrini G , Friedman A , Kanovsky P , Kostic V , Nieuwboer A , Odin P , Poewe W , Rascol O , Sampaio C , Schupbach M , Tolosa E , Trenkwalder C , Schapira A , Berardelli A , Oertel WH ((2013) ) Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur J Neurol 20: , 5–15. |
[116] | NICE. (2017) Parkinson’s disease in adults: Diagnosis and management: Full guideline. www.nice.org.uk/guidance/ng71/evidence/full-guideline-pdf-4538466253 (accessed 26 October 2022). |
[117] | Pringsheim T , Day GS , Smith DB , Rae-Grant A , Licking N , Armstrong MJ , de Bie RMA , Roze E , Miyasaki JM , Hauser RA , Espay AJ , Martello JP , Gurwell JA , Billinghurst L , Sullivan K , Fitts MS , Cothros N , Hall DA , Rafferty M , Hagerbrant L , Hastings T , O’Brien MD , Silsbee H , Gronseth G , Lang AE , Guideline Subcommittee of the AAN ((2021) ) Dopaminergic therapy for motor symptoms in early Parkinson disease Practice Guideline Summary: A report of the AAN Guideline Subcommittee. Neurology 97: , 942–957. |
[118] | Mahlknecht P , Foltynie T , Limousin P , Poewe W ((2022) ) How does deep brain stimulation change the course of Parkinson’s disease? Mov Disord 37: , 1581–1592. |
[119] | Krack P , Benazzouz A , Pollak P , Limousin P , Piallat B , Hoffmann D , Xie J , Benabid AL ((1998) ) Treatment of tremor in Parkinson’s disease by subthalamic nucleus stimulation. Mov Disord 13: , 907–914. |
[120] | Williams A , Gill S , Varma T , Jenkinson C , Quinn N , Mitchell R , Scott R , Ives N , Rick C , Daniels J , Patel S , Wheatley K , PD SURG Collaborative Group ((2010) ) Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): A randomised, open-label trial. Lancet Neurol 9: , 581–591. |
[121] | Limousin P , Foltynie T ((2019) ) Long-term outcomes of deep brain stimulation in Parkinson disease. Nat Rev Neurol 15: , 234–242. |
[122] | Vitek JL , Jain R , Chen L , Troster AI , Schrock LE , House PA , Giroux ML , Hebb AO , Farris SM , Whiting DM , Leichliter TA , Ostrem JL , SanLuciano M , Galifianakis N , Verhagen Metman L , Sani S , Karl JA , Siddiqui MS , Tatter SB , Ul Haq I , Machado AG , Gostkowski M , Tagliati M , Mamelak AN , Okun MS , Foote KD , Moguel-Cobos G , Ponce FA , Pahwa R , Nazzaro JM , Buetefisch CM , Gross RE , Luca CC , Jagid JR , Revuelta GJ , Takacs I , Pourfar MH , Mogilner AY , Duker AP , Mandybur GT , Rosenow JM , Cooper SE , Park MC , Khandhar SM , Sedrak M , Phibbs FT , Pilitsis JG , Uitti RJ , Starr PA ((2020) ) Subthalamic nucleus deep brain stimulation with a multipleindependent constant current-controlled device in Parkinson’sdisease (INTREPID): A multicentre, double-blind, randomised, sham-controlled study. Lancet Neurol 19: , 491–501. |
[123] | Wong JK , Viswanathan VT , Nozile-Firth KS , Eisinger RS , Leone EL , Desai AM , Foote KD , Ramirez-Zamora A , Okun MS , Wagle Shukla A ((2020) ) STN versus GPi deep brain stimulation for action and rest tremor in Parkinson’s disease. Front Hum Neurosci 14: , 578615. |
[124] | Ostrem J , Luo P , Weaver F , Follett K , Galifianakis N , Lai E , Bronstein J , Duda J , Holloway K , Sarwar A , Brodsky M , Chung K , Spindler M , Reda D , Rothland J , Snodgrass A , Marks W ((2021) ) 10 year clinical outcomes of subthalamic nucleus versus pallidal deep brain stimulation for Parkinson’s disease: VA/NINDS CSP #468F (4312). Neurology 96: (15 Suppl), 4312. |
[125] | Benabid AL , Pollak P , Gervason C , Hoffmann D , Gao DM , Hommel M , Perret JE , de Rougemont J ((1991) ) Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 337: , 403–406. |
[126] | Limousin P , Speelman JD , Gielen F , Janssens M ((1999) ) Multicentre European study of thalamic stimulation in parkinsonian and essential tremor. J Neurol Neurosurg Psychiatry 66: , 289–296. |
[127] | Cury RG , Fraix V , Castrioto A , Perez Fernandez MA , Krack P , Chabardes S , Seigneuret E , Alho EJL , Benabid AL , Moro E ((2017) ) Thalamic deep brain stimulation for tremor in Parkinson disease, essential tremor, and dystonia. Neurology 89: , 1416–1423. |
[128] | Chandra V , Hilliard JD , Foote KD ((2022) ) Deep brain stimulation forthe treatment of tremor. J Neurol Sci 435: , 120190. |
[129] | Binder DK , Shah BB , Elias WJ ((2022) ) Focused ultrasound and other lesioning in the treatment of tremor. J Neurol Sci 435: , 120193. |
[130] | Schuurman PR , Bosch DA , Bossuyt PM , Bonsel GJ , van Someren EJ , de Bie RM , Merkus MP , Speelman JD ((2000) ) A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med 342: , 461–468. |
[131] | Bond AE , Shah BB , Huss DS , Dallapiazza RF , Warren A , Harrison MB , Sperling SA , Wang XQ , Gwinn R , Witt J , Ro S , Elias WJ ((2017) ) Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease: A randomized clinical trial. JAMA Neurol 74: , 1412–1418. |
[132] | Fasano A , De Vloo P , Llinas M , Hlasny E , Kucharczyk W , Hamani C , Lozano AM ((2018) ) Magnetic resonance imaging-guided focused ultrasound thalamotomy in Parkinson tremor: Reoperation after benefit decay. Mov Disord 33: , 848–849. |
[133] | Sinai A , Nassar M , Sprecher E , Constantinescu M , Zaaroor M , Schlesinger I ((2022) ) Focused ultrasound thalamotomy in tremor dominant Parkinson’s disease: Long-term results. J Parkinsons Dis 12: , 199–206. |
[134] | Stieglitz LH , Oertel MF , Accolla EA , Bally J , Bauer R , Baumann CR , Benninger D , Bohlhalter S , Buchele F , Hagele-Link S , Kagi G , Krack P , Kruger MT , Mahendran S , Moller JC , Mylius V , Piroth T , Werner B , Kaelin-Lang A ((2021) ) Consensus statement on high-intensity focused ultrasound for functional neurosurgery in Switzerland. Front Neurol 12: , 722762. |
[135] | Hanff AM , Leist AK , Fritz JV , Pauly C , Kruger R , Halek M , Consortium N-P ((2022) ) Determinants of self-stigma in people with Parkinson’s disease: A mixed methods scoping review. J Parkinsons Dis 12: , 509–522. |




