Postmortem Cerebellar Volume Is Not Reduced in Essential Tremor: A Comparison with Multiple System Atrophy and Controls
Abstract
Background:
Essential tremor (ET) is a common movement disorder in which cerebellar microscopic and volume alterations have been repeatedly reported although with disagreement between studies. However, pronounced heterogeneity was found with regard to cerebellar volume alterations.
Objective:
This study aimed to assess postmortem cerebellar volume in subjects with or without ET, as compared with subjects with multiple system atrophy (MSA), a well-established cerebellar neurodegeneration.
Methods:
Cases with ET (n = 29), MSA (n = 7), and non-demented control cases without any movement disorder (n = 22) were selected from the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND), a longitudinal clinicopathological study with annual research-dedicated clinical assessments by neuropsychologists, subspecialist movement disorders, and cognitive/behavioral neurologists, with comprehensive neuropathological examinations after death. Group comparisons were controlled for common age-related neurodegenerative and cerebrovascular pathologies. Cerebellar volumes were calculated using digital images of slices taken at the time of autopsy, immediately after brain removal and before fixation.
Results:
Cerebellar volume was not reduced in ET subjects compared to controls. The two groups did not differ in terms of incidental cerebrovascular and Alzheimer’s disease neuropathology. In contrast, cerebellar volume was significantly reduced in subjects with MSA when compared to ET and control subjects.
Conclusion:
In a well-characterized cohort, postmortem cerebellar volume measurements suggest that there are no volume alterations in ET when compared to controls, in contrast to significant cerebellar atrophy in subjects with MSA.
INTRODUCTION
Essential tremor (ET) is one of the most common movement disorders and can significantly impact the quality of life of affected patients [1]. ET is characterized by an involuntary bilateral action tremor principally of hands and arms that can also affect the head, jaw, and the voice, appearing with various clinical presentations [2, 3]. To date, the etiology and pathophysiology of ET is still poorly understood, and the extent of neurodegeneration underlying ET remains a matter of debate [4–7].
A dysfunction of the corticothalamo-olivo-cerebellar pathways and GABAergic neurotransmission as well as cerebellar degeneration have been hypothesized as causes of ET [8, 9]. To date, the cerebellum is the brain area that has been most frequently investigated in ET. Neuropathological findings have repeatedly implicated the cerebellum, with studies reporting cerebellar dendritic changes and Purkinje cell loss, increased numbers of Purkinje cell torpedoes and diverse other alterations of these cells as well as cerebellar gliosis, though these findings have not been consistent between laboratories [7, 10–19]. The Purkinje cell counting studies, in particular, have not been accompanied by volume determinations, have not adequately sampled cerebellar subregions, and have not used unbiased morphometric methods [20]. Similarly, magnetic resonance spectroscopy (MRS) studies of biochemical markers of Purkinje cells have had conflicting results [21]. Additionally, subject selection for these studies has not sufficiently excluded possibly confounding clinical and autopsy findings, including other movement disorders and diverse neurodegenerative and cerebrovascular neuropathologies that are common in elderly persons [22].
Conventionally-accepted cerebellar neurodegenerations, such as multiple system atrophy and spinocerebellar ataxias, result in cerebellar volume loss [23–26], but published MRI studies on cerebellar volume in ET are as conflicting as are the Purkinje cell studies. While some studies have reported reductions of total cerebellar gray matter volume and changes in specific lobules of the cerebellum [27–35], others have reported no differences [36–42]. Recent meta-analyses revealed pronounced heterogeneity between studies with regard to the presence or absence of volume alterations and its localization within the cerebellum, suggesting considerable clinical heterogeneity of study subjects, small subject numbers, and methodological imaging differences [43–45]. Altogether, these results indicate that voxel-based morphometry does not reliably discriminate between ET subjects and controls [44]. In contrast, these methods have reliably identified cerebellar volume loss in multiple system atrophy (MSA), a rare synucleinopathy characterized by autonomic dysfunction combined with parkinsonian and cerebellar features.
To the best of our knowledge, postmortem cerebellar volume has never been investigated in subjects with ET. Postmortem investigations have an advantage over in vivo studies in that they allow for the exclusion or control of confounding common age-related neurodegenerative and cerebrovascular pathologies. Therefore, in an attempt to determine whether there are cerebellar volume changes in ET, we assessed postmortem cerebellar volumes in ET, MSA, and control subjects derived from a longitudinal clinicopathological study.
MATERIALS AND METHODS
Subject selection
Subjects included in this study were enrolled in the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND) and Brain and Body Donation Program (BBDP; www.brainandbodydonationprogram.org) [46]. Subjects are annually assessed with standardized research-dedicated clinical assessments by cognitive/behavioral neurologists, movement disorders neurologists, and neuropsychologists. The cognitive behavioral assessment included the National Institute on Aging Uniform Data Set while the movement disorders evaluation included the Unified Parkinson’s Disease Rating Scale (UPDRS), tremor rating scale (Fahn-Tolosa-Martin scale), restless leg syndrome rating scale, Mayo Sleep Questionnaire, and assessment of other involuntary movements (dystonia, myoclonus). Subjects signed informed consent approved by the BSHRI Institutional Review Board and had agreed to have an autopsy with brain donation for research purposes.
From AZSAND/BBDP autopsies, a total of 29 subjects that had been clinically diagnosed with ET, as well as 21 control cases, were included in this study. Subjects were diagnosed with ET if they had postural or kinetic tremor of the hands or forearms without identifiable secondary cause or other exclusion criteria (e.g., prominent unilateral tremor, rigidity, or bradykinesia) [2]. To exclude the potential effect of other neurodegenerative disease, only non-demented subjects were included. Of the whole AZSAND/BBDP database, 237 cases had a clinical diagnosis of ET at final clinicopathological conference, of these 106 cases (44.5%) had dementia with frequent comorbid diseases including Alzheimer’s disease (AD) dementia (80%), vascular dementia (25%), Parkinsonism not otherwise specified (NOS) (19%), dementia with Lewy bodies (16%), dementia-NOS (11%), Parkinson’s disease (9%), and progressive supranuclear palsy (PSP) (10%). Moreover, subjects clinically diagnosed with parkinsonism of any type, restless leg syndrome, periodic limb movements of sleep, REM sleep behavior disorder, or any other movement disorders were specifically excluded. Also excluded were subjects with a history of alcoholism, metastatic cancer, seizure disorder, or having had medications for seizure disorders and alcoholism. Neuropathological exclusions applied to controls and ET groups included CNS alpha-synuclein pathology, PSP-type tauopathy, any large cerebral infarcts, and any cerebellar or infratentorial infarcts greater than microscopic in size. The rate of incidental Lewy body disease (ILBD) was 22. 1% in ET cases while this percentage was 24.2% in control cases.
For comparison with a conventional cerebellar neurodegeneration, we included 7 cases that had a final clinicopathological diagnosis of MSA. Of these, 6 subjects were clinically diagnosed as MSA-P with predominantly parkinsonian features, while one case was not clearly either MSA-P or MSA-C. Of these, 3 subjects had dementia. Immunohistochemistry for phosphorylated alpha-synuclein pathology was observed in the form of cytoplasmic glial inclusions as previously described in the neuropathology of MSA while no Lewy-type synucleinopathy was observed [47].
Neuropathological investigation
Brain pathology assessment was performed on all cases according to standard protocols including assessment of cortex, basal ganglia, brainstem, and cerebellum and included assessment of cerebrovascular pathologies such as gross or microscopic infarcts, semi quantitative gradings of white matter rarefaction in frontal, temporal, parietal and occipital cortex, and circle of Willis atherosclerosis. Different stains are performed [46] and neuropathological assessment includes assignment of the AD Braak neurofibrillary (NF) stage [48], semi-quantitative amyloid plaque densities in frontal, temporal, and parietal neocortex as well as hippocampal CA1 region and entorhinal region, the Thal amyloid phase for Aβ plaque brain distribution [49], the CERAD neuritic plaque density score [50], and alpha-synuclein Unified Stage pathology using an immunohistochemical method [51].
Cerebellar volume measurement
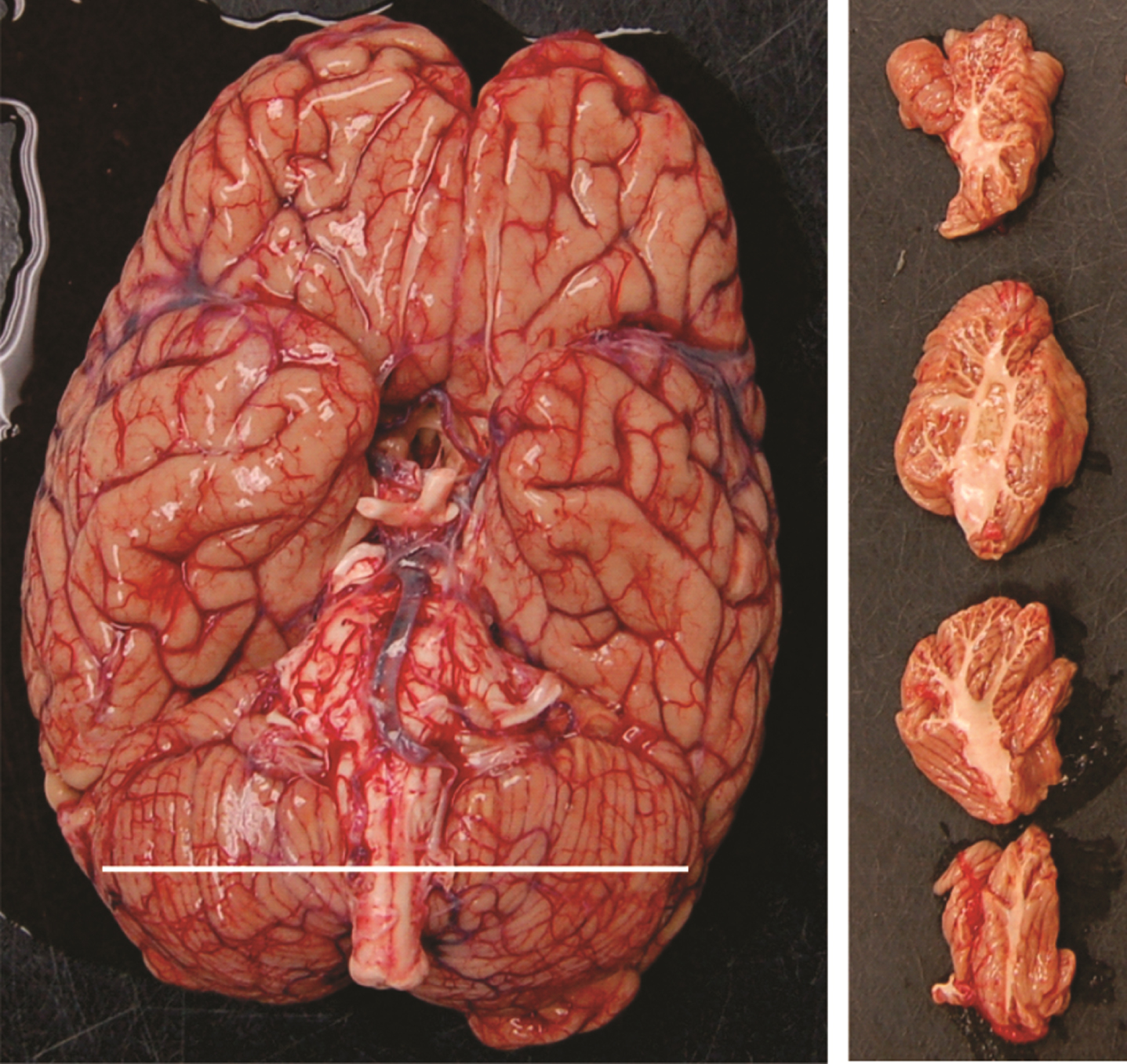
As part of the standard BBDP protocol, images of the brain, including images of the cerebellum, are taken at the time of autopsy after brain removal and before fixation. Available images (Fig. 1) included ventral digital images of the brain including the cerebellum in situ as well as images of parasagittal slices of the cerebellum for each cerebellar hemisphere (4 slices for each cerebellar hemisphere). These digital images were used to estimate the cerebellar volume using the software AxioVision 4.8 (https://carl-zeiss-vision-axiovision-viewer.software.informer.com/4.8/). This software allows, by tracing the contour of each slice, a measurement of the cross-sectional area of each cerebellar slice in pixels with subsequent conversion to cm2 using for calibration a ruler placed in the same image. The mean cross-sectional area of the 4 slices is then multiplied by the width of the hemisphere in the ventral brain image to obtain cerebellar hemispheric volume in cm3. Following this, the left and right hemisphere volumes are added to obtain the total cerebellar volume.
Fig. 1
Ventral view of a brain demonstrating the cerebellum width and parasagittal slices of the cerebellum.
Statistical analysis
Data analyses were performed using SPSS software (IBM SPSS Statistics 23.0). The principal focus was to compare ET cases to control cases. Kolmogorov–Smirnov tests were performed to test for normality. Two-tailed Mann–Whitney U tests, t-tests and Chi-square tests were used as appropriate for group comparisons. Spearman correlations were used to assess any correlations between cerebellar volume and clinical or neuropathological characteristics. Cerebellar volume was compared between the 3 diagnostic groups using the non-parametric Kruskall-Wallis test. Further, logistic regression analyses, adjusting for age, sex, and neuropathological levels of AD, were done to assess the ability of the cerebellar volume to predict the diagnosis (Control/ET) as dependent variable. Alpha of 0.05 was chosen as the cut-off criterion for statistical significance.
Data availability statement
Data from this study and ET cases from AZSAND/BBDP will be made available upon request to the authors.
RESULTS
Table 1 report demographic, neuropathological, and clinical characteristics of the studied cases. There was no age difference when comparing control cases (88.8±6.0) to ET cases (87.7±6.3) (t = 0.655; p = 0.516) and the proportion of women (13/22) in the control group was not significantly different from the ET group (14/29): (χ 2 = 0.443; p = 0.059). ET mean disease duration was 11.7±10.5 years and ranged from 1.4 to 48.8 years. The level of AD pathology was not different between groups with regards to Braak NF stage, summary regional amyloid plaque density, and Thal amyloid phase. No significant differences were observed for semi quantitative gradings of circle of Willis atherosclerosis (U = 223.5; p = 0.056), cerebral white matter rarefaction total score (U = 285.0; p = 0.7), and number of cerebral, deep nuclei, and infratentorial infarcts (all p > 0.05). No differences between groups were observed in Mini-Mental State Examination (MMSE) scores (t = 1.593; p = 0.118) but UPDRS motor scores were significantly higher in cases with ET (t = –4.421; p < 0.001) when compared to controls. MSA cases were younger (U = 16.0; p < 0.001) and had a significantly lower MMSE score (U = 49.5; p = 0.024), but no differences were found for brain weight (U = 116.5; p = 0.141) or postmortem interval (U = 108.8; p = 0.095).
Table 1
Demographics, postmortem, and clinical characteristics of subjects for each group
| ET | Control | MSA | |
| Nb. of cases | 29 | 22 | 7 |
| Age at death (y) | 87.7±6.3 | 88.8±6.0 | 71.6±7.8 |
| Sex (W/M) | 14/15 | 13/9 | 1/6 |
| MMSE | 27.9±1.9 | 28.6±1.4 | 23.6±5.6 |
| UPDRS motor score | 11.1±6.3 | 4.3±4.1 | 10.7±9.6 |
| Duration of ET (y) | 11.7±10.5 | – | – |
| APOE ɛ4 % | 9.1 | 10.3 | 42.8 |
| PMI (h) | 3.1±0.8 | 3.5±2.8 | 3.5±0.8 |
| Brain weight | 1176.4±128.0 | 1166.0±104.2 | 1109.7±62.6 |
| Braak NF stage (Median and range) | 4.0 (1–4) | 4.0 (3–4) | 3.0 (1–5) |
| Thal phase (Median and range) | 0 (0–5) | 1.5 (0–4) | 1.0 (0–3) |
| Plaque density (Median and range) | 1.0 (0–3) | 1.0 (0–3) | – |
| CWA (Median and range) | 2.0 (0–3) | 1.0 (0–3) | – |
| CWMR (Median and range) | 3.0 (0–12) | 3.0 (0–8) | – |
Data are presented as means and standard deviation of the mean or median and range when stated. ET, essential tremor; MSA, multiple system atrophy; UPDRS, last Unified Parkinson’s Disease Rating Scale motor score (part 3 motor score); MMSE, last Mini-Mental State Examination score; PMI, postmortem interval (interval between death and brain removal); NF, neurofibrillary; CWA, circle of Willis atherosclerosis; CWMR, cerebral white matter rarefaction; W, women; M, men.
When comparing right (U = 315.0; p = 0.9), left (U = 309.0; p = 0.9), and total cerebellar volume (U = 311.0; p = 0.9) between ET cases and controls, no group differences were found (Fig. 2; Table 2).
Fig. 2
Mean total cerebellar volume in ET when compared to controls and MSA cases.
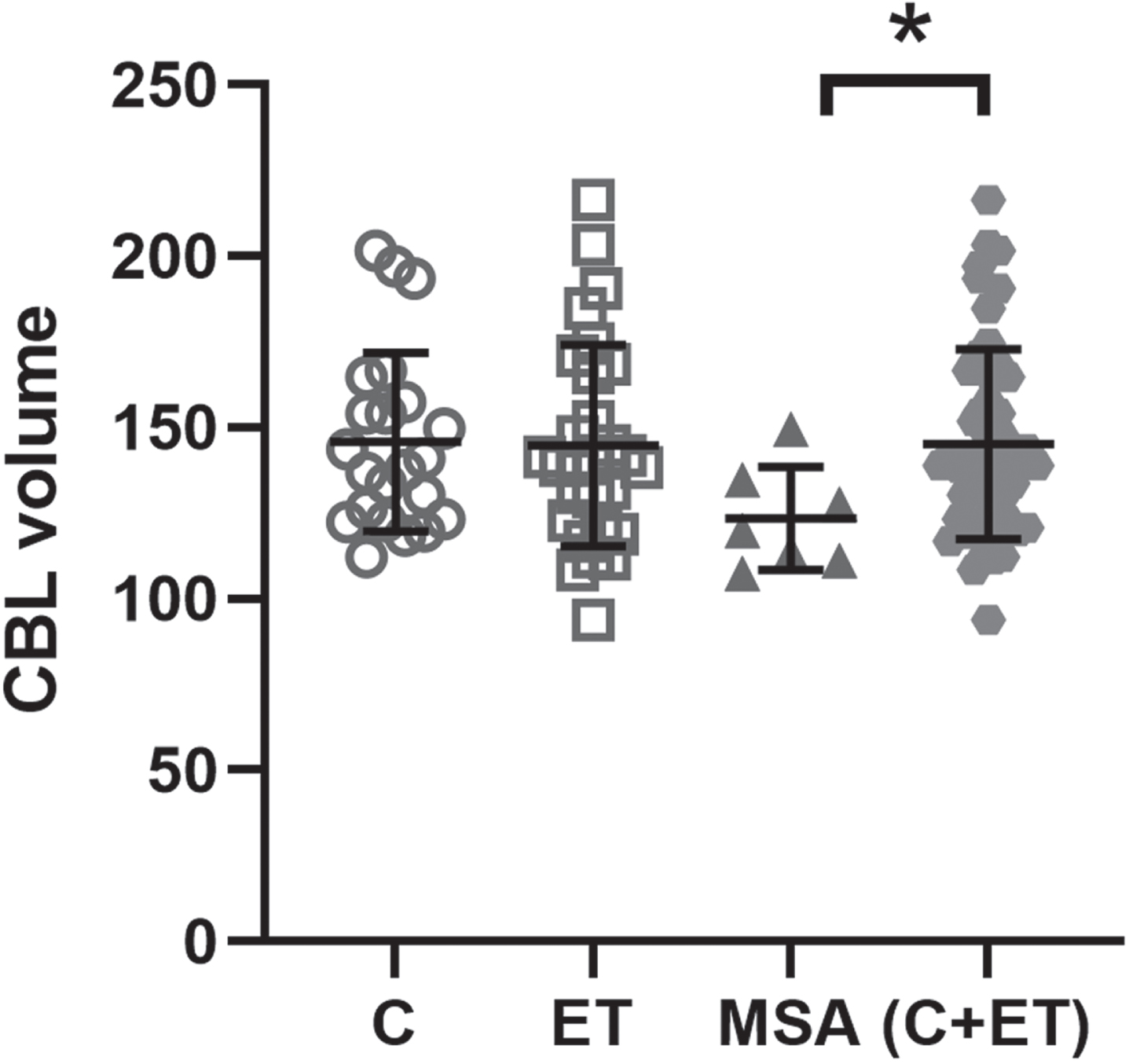
Table 2
Mean cerebellar volumes in each group
| ET | Control | MSA | |
| Total volume | 144.7±29.4 | 145.7±26.0 | 123.4±15.0 |
| In women (n) | 152.64±31.2 (14) | 143.44±25.7 (13) | 149.5±0 (1) |
| In men (n) | 136.14±25.6 (15) | 147.3±27.1 (19) | 119.0±10.5 (6) |
| Left hemi volume | 72.2±14.0 | 73.3±13.3 | 62.8±9.4 |
| Right hemi volume | 72.9±16.3 | 72.5±13.8 | 60.6±6.4 |
Data are presented as means and standard deviation of the mean. ET, essential tremor; MSA, multiple system atrophy; n, number of cases. Volumes are reported in cm3.
Sex comparison showed that the cerebellar volume was not different between all men and women (U = 280.0; p = 0.4), no differences were observed when considering only the control group (U = 52.0; p = 0.7), or the ET group (U = 74.0; p = 0.4). No differences were observed between control women and ET women (U = 53.0; p = 0.4) or between control and ET men (U = 71.0; p = 0.4).
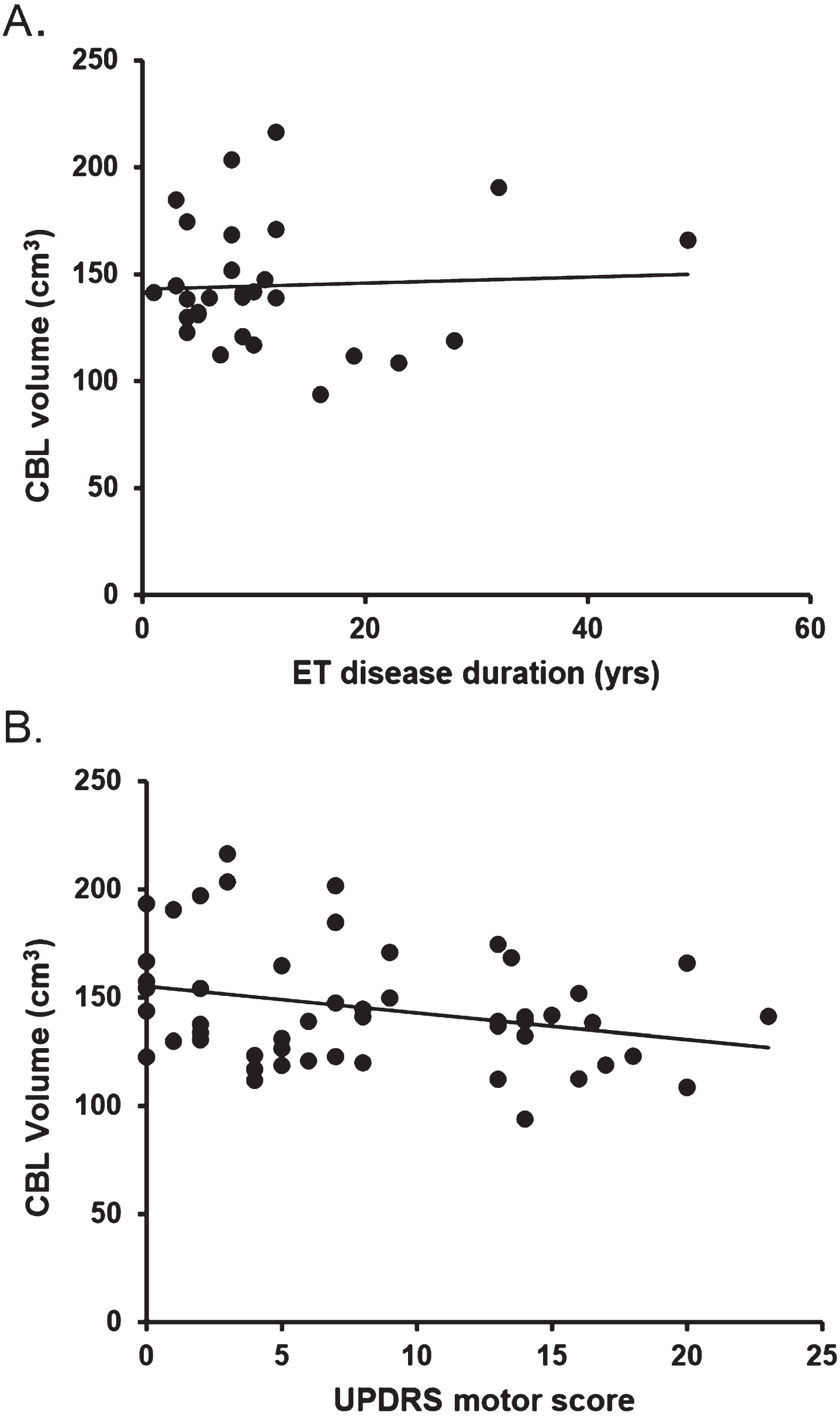
Total CBL volume correlated with brain weight (Rho = 0.555; p < 0.001) but did not correlate with age (Rho = –0.155; p = 0.274), ET disease duration (ET group only; Rho = –0.096; p = 0.618), MMSE (Rho = 0.071; p = 0.625), UPDRS score (Rho = –0.238; p = 0.092), semi quantitative gradings of circle of Willis atherosclerosis (Rho = 0.057; p = 0.690), or cerebral white matter rarefaction (Rho = –0.130; p = 0.370) (Fig. 3).
Fig. 3
Absence of correlation between total cerebellar volume and ET disease duration (A) as well as UPDRS motor score (B).
Logistic regression models showed that cerebellar volume did not significantly predict the diagnosis of ET versus control, either with covariates for age and sex [χ 2(3) = 1.304; p = 0.728; R2 = 0.025] or with age, sex, Braak AD stages, Thal amyloid phase, and summary amyloid plaque density [χ 2(6) = 5.184; p = 0.520; R2 = 0.116].
When comparing the 3 groups for total cerebellar volume (Fig. 2), no significant overall group differences were found [H(2) = 5.098, p = 0.078). For subsequent analysis, controls and ET cases were grouped together to compare with the MSA cases. In this comparison, MSA cases had significantly reduced total cerebellar volume (U = 85.0; p = 0.024) as compared to the combined ET and control groups (Fig. 2).
DISCUSSION
This study investigated the postmortem cerebellar volume, using photographs taken at the time of autopsy, in subjects that were clinically diagnosed with ET in comparison to control cases without dementia or any movement disorder. Our main result demonstrates that the postmortem cerebellar volume is not reduced in ET subjects when compared to controls. In contrast, cerebellar volume was found to be reduced in subjects with MSA when compared to the combined ET subjects and controls.
Even though no study has previously investigated postmortem cerebellar volume in ET subjects, these negative results concur with some in vivo reports using VBM measurements from MRI scans [36–42]. Nonetheless, the literature on cerebellar atrophy in ET is controversial and several other studies have reported volume changes in total cerebellar volume or in specific cerebellar lobules [27–33, 43]. However, recent meta-analyses demonstrate high heterogeneity in gray matter alterations and highlighted the clinical variability and the consequent lack of reliable and robust findings with regards to ET cerebellar volume changes [43–45].
Our results do not support the hypothesis of atrophy in the cerebellum in ET subjects. Multiple postmortem studies, like in vivo MRI studies, have had conflicting results regarding the presence of cerebellar neurodegeneration in ET, including whether or not there are alterations or loss of Purkinje cells [4, 6–8, 10, 12, 14, 15, 19, 52]. Nevertheless, it is possible that changes in Purkinje cells or other cerebellar cellular constituents may be pathogenic but yet not affect total cerebellar volume.
We acknowledge some limitations of this study. First, measurements of the cerebellar volume were done at autopsy on only 4 slices of each cerebellar hemisphere and are therefore unlikely to be as accurate as VBM done with MRI during life. Future postmortem studies could more accurately assess cerebellar volume by measuring the volume of water displaced. We did not attempt to estimate volumes of cerebellar subregions and therefore could have missed significant differences in any of these. To some extent we validated our methods by showing that we could detect a significant volumetric cerebellar change in subjects with MSA, a conventional cerebellar disorder known to be associated with reduced cerebellar volume. Moreover, to specifically assess the potential effect of ET on cerebellar volume without the influence of other neurodegenerative diseases that can affect the brain, we included only non-demented subjects and future studies could also extend this work to investigate ET subjects with dementia. We further excluded subjects with Lewy-type synucleinopathy as while this is a pathological finding that has been reported in ET, it is not specific to ET and incidental Lewy-type synucleinopathy has been found at similar frequency in brains of controls that had died without any neurological disease [51]. Indeed, we report 22% of ET cases with ILBD, comparably to 25% reported in a recent publication from a large ET cohort [53], while we similarly observed ILBD at autopsy in 24% of non-demented age-matched controls cases that do not present any parkinsonism or other tremor clinically. Although we do not expect to find any differences between ET with or without Lewy-type synucleinopathy, future studies could specifically address this comparison. Despite these limitations, this is the first study to compare postmortem cerebellar volumes in ET and control subjects. A major strength of our study was our ability to exclude many confounding clinical and neuropathological conditions that previous studies have not addressed.
In conclusion, our results demonstrate that the postmortem cerebellar volume is not reduced in subjects who were clinically diagnosed with ET when compared to controls without dementia or movement disorder. In contrast, cerebellar volume was reduced, as expected, in MSA subjects when compared to ET subjects and controls.
ACKNOWLEDGMENTS
The Arizona Study of Aging and Neurodegenerative Disorders and Brain and Body Donation Program has been supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 and P30AG072980, Arizona Alzheimer’s Disease Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium), and the Michael J. Fox Foundation for Parkinson’s Research.
The authors thank the autopsy personnel who helped contribute clinical data and postmortem brains from study subjects. They also thank the donors who were recruited for this study as well as their families.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Louis ED , Ferreira JJ ((2010) ) How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord 25: , 534–541. |
[2] | Bhatia KP , Bain P , Bajaj N , Elble RJ , Hallett M , Louis ED , Raethjen J , Stamelou M , Testa CM , Deuschl G ; Tremor Task Force of the International Parkinson and Movement Disorder Society ((2018) ) Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 33: , 75–87. |
[3] | Louis ED , Bares M , Benito-Leon J , Fahn S , Frucht SJ , Jankovic J , Ondo WG , Pal PK , Tan EK ((2020) ) Essential tremor-plus: A controversial new concept. Lancet Neurol 19: , 266–270. |
[4] | Rajput AH , Adler CH , Shill HA , Rajput A ((2012) ) Essential tremor is not a neurodegenerative disease. Neurodegener Dis Manag 2: , 259–268. |
[5] | Benito-León J ((2014) ) Essential tremor: A neurodegenerative disease? Tremor Other Hyperkinet Mov (N Y) 4: , 252. |
[6] | Deuschl G , Elble R ((2009) ) Essential tremor–neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord 24: , 2033–2041. |
[7] | Shill HA , Adler CH , Beach TG ((2012) ) Pathology in essential tremor. Parkinsonism Relat Disord 18: , S135–S137. |
[8] | Louis ED , Faust PL ((2020) ) Essential tremor within the broader context of other forms of cerebellar degeneration. Cerebellum 19: , 879–896. |
[9] | Hopfner F , Helmich RC ((2018) ) The etiology of essential tremor: Genes versus environment. Parkinsonism Relat Disord 46: (Suppl 1), S92–S96. |
[10] | Shill HA , Adler CH , Sabbagh MN , Connor DJ , Caviness JN , Hentz JG , Beach TG ((2008) ) Pathologic findings in prospectively ascertained essential tremor subjects. Neurology 70: , 1452–1455. |
[11] | Symanski C , Shill HA , Dugger B , Hentz JG , Adler CH , Jacobson SA , Driver-Dunckley E , Beach TG ((2014) ) Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord 29: , 496–500. |
[12] | Louis ED , Faust PL , Vonsattel J-PG , Honig LS , Rajput A , Robinson CA , Rajput A , Pahwa R , Lyons KE , Ross GW , Borden S , Moskowitz CB , Lawton A , Hernandez N ((2007) ) Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain 130: , 3297–3307. |
[13] | Louis E , Vonsattel J , Honig L , Ross G , Lyons K , Pahwa R ((2006) ) Neuropathologic findings in essential tremor. Neurology 66: , 1756–1759. |
[14] | Louis ED , Vonsattel JP ((2008) ) The emerging neuropathology of essential tremor. Mov Disord 23: , 174–182. |
[15] | Louis ED , Faust PL , Ma KJ , Yu M , Cortes E , Vonsattel JP ((2011) ) Torpedoes in the cerebellar vermis in essential tremor cases vs. controls. Cerebellum 10: , 812–819. |
[16] | Axelrad JE , Louis ED , Honig LS , Flores I , Ross GW , Pahwa R , Lyons KE , Faust PL , Vonsattel JP ((2008) ) Reduced Purkinje cell number in essential tremor: A postmortem study. Arch Neurol 65: , 101–107. |
[17] | GGionco JT , Hartstone WG , Martuscello RT , Kuo S-H , Faust PL , Louis ED ((2021) ) Essential tremor versus “ET-plus”: A detailed postmortem study of cerebellar pathology. Cerebellum 20: , 904–912. |
[18] | Louis ED ((2016) ) Essential tremor: A common disorder of purkinje neurons? Neuroscientist 22: , 108–118. |
[19] | Rajput AH , Robinson CA , Rajput ML , Robinson SL , Rajput A ((2012) ) Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord 18: , 626–628. |
[20] | Andersen BB , Gundersen HJ , Pakkenberg B ((2003) ) Aging of the human cerebellum: A stereological study. J Comp Neurol 466: , 356–365. |
[21] | Buijink AWG , Prent N , Puts NA , Schrantee A , Potters WV , van Rootselaar AF ((2021) ) GABA, glutamate, and NAA levels in the deep cerebellar nuclei of essential tremor patients. Front Neurol 12: , 664735. |
[22] | Béliveau E , Tremblay C , Aubry-Lafontaine É , Paris-Robidas S , Delay C , Robinson C , Ferguson L , Rajput AH , Rajput A , Calon F ((2015) ) Accumulation of amyloid-β in the cerebellar cortex of essential tremor patients. Neurobiol Dis 82: , 397–408. |
[23] | Wan N , Chen Z , Wan L , Tang B , Jiang H ((2020) ) MR imaging of SCA3/MJD. Front Neurosci 14: , 749. |
[24] | Nigri A , Sarro L , Mongelli A , Pinardi C , Porcu L , Castaldo A , Ferraro S , Grisoli M , Bruzzone MG , Gellera C , Taroni F , Mariotti C , Nanetti L ((2020) ) Progression of cerebellar atrophy in spinocerebellar ataxia type 2 gene carriers: A longitudinal MRI study in preclinical and early disease stages. Front Neurol 11: , 616419. |
[25] | Gilman S , Wenning GK , Low PA , Brooks DJ , Mathias CJ , Trojanowski JQ , Wood NW , Colosimo C , Durr A , Fowler CJ , Kaufmann H , Klockgether T , Lees A , Poewe W , Quinn N , Revesz T , Robertson D , Sandroni P , Seppi K , Vidailhet M ((2008) ) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71: , 670–676. |
[26] | Campabadal A , Abos A , Segura B , Monte-Rubio G , Perez-Soriano A , Giraldo DM , Munoz E , Compta Y , Junque C , Marti MJ ((2022) ) Differentiation of multiple system atrophy subtypes by gray matter atrophy. J Neuroimaging 32: , 80–89. |
[27] | Benito-León J , Alvarez-Linera J , Hernández-Tamames JA , Alonso-Navarro H , Jiménez-Jiménez FJ , Louis ED ((2009) ) Brain structural changes in essential tremor: Voxel-based morphometry at 3-Tesla. J Neurol Sci 287: , 138–142. |
[28] | Bhalsing K , Upadhyay N , Kumar K , Saini J , Yadav R , Gupta A , Pal P ((2014) ) Association between cortical volume loss and cognitive impairments in essential tremor. Eur J Neurol 21: , 874–883. |
[29] | Gallea C , Popa T , Garcia-Lorenzo D , Valabregue R , Legrand AP , Marais L , Degos B , Hubsch C , Fernandez-Vidal S , Bardinet E , Roze E , Lehericy S , Vidailhet M , Meunier S ((2015) ) Intrinsic signature of essential tremor in the cerebello-frontal network. Brain 138: , 2920–2933. |
[30] | Bagepally BS , Bhatt MD , Chandran V , Saini J , Bharath RD , Vasudev MK , Prasad C , Yadav R , Pal PK ((2012) ) Decrease in cerebral and cerebellar gray matter in essential tremor: A voxel-based morphometric analysis under 3T MRI. J Neuroimaging 22: , 275–278. |
[31] | Cerasa A , Nistico R , Salsone M , Bono F , Salvino D , Morelli M , Arabia G , Quattrone A ((2014) ) Neuroanatomical correlates of dystonic tremor: A cross-sectional study. Parkinsonism Relat Disord 20: , 314–317. |
[32] | Quattrone A , Cerasa A , Messina D , Nicoletti G , Hagberg G , Lemieux L , Novellino F , Lanza P , Arabia G , Salsone M ((2008) ) Essential head tremor is associated with cerebellar vermis atrophy: A volumetric and voxel-based morphometry MR imaging study. Am J Neuroradiol 29: , 1692–1697. |
[33] | Lin C-H , Chen C-M , Lu M-K , Tsai C-H , Chiou J-C , Liao J-R , Duann J-R ((2013) ) VBM reveals brain volume differences between Parkinson’s disease and essential tremor patients. Front Hum Neurosci 7: , 247. |
[34] | Dyke JP , Cameron E , Hernandez N , Dydak U , Louis ED ((2017) ) Gray matter density loss in essential tremor: A lobule by lobule analysis of the cerebellum. Cerebellum Ataxias 4: , 10. |
[35] | Ågren R , Awad A , Blomstedt P , Fytagoridis A ((2021) ) Voxel-based morphometry of cerebellar lobules in essential tremor. Front Aging Neurosci 13: , 667854. |
[36] | Daniels C , Peller M , Wolff S , Alfke K , Witt K , Gaser C , Jansen O , Siebner H , Deuschl G ((2006) ) Voxel-based morphometry shows no decreases in cerebellar gray matter volume in essential tremor. Neurology 67: , 1452–1456. |
[37] | Fang W , Lv F , Luo T , Cheng O , Liao W , Sheng K , Wang X , Wu F , Hu Y , Luo J , Yang QX , Zhang H ((2013) ) Abnormal regional homogeneity in patients with essential tremor revealed by resting-state functional MRI. PLoS One 8: , e69199. |
[38] | Nicoletti V , Cecchi P , Frosini D , Pesaresi I , Fabbri S , Diciotti S , Bonuccelli U , Cosottini M , Ceravolo R ((2015) ) Morphometric and functional MRI changes in essential tremor with and without resting tremor. J Neurol 262: , 719–728. |
[39] | Cameron E , Dyke JP , Hernandez N , Louis ED , Dydak U ((2018) ) Cerebral gray matter volume losses in essential tremor: A case-control study using high resolution tissue probability maps. Parkinsonism Relat Disord 51: , 85–90. |
[40] | Buijink A , Broersma M , Van Der Stouwe A , Sharifi S , Tijssen M , Speelman J , Maurits N , Van Rootselaar A ((2016) ) Cerebellar atrophy in cortical myoclonic tremor and not in hereditary essential tremor—a voxel-based morphometry study. Cerebellum 15: , 696–704. |
[41] | Klein JC , Lorenz B , Kang JS , Baudrexel S , Seifried C , van de Loo S , Steinmetz H , Deichmann R , Hilker R ((2011) ) Diffusion tensor imaging of white matter involvement in essential tremor. Hum Brain Mapp 32: , 896–904. |
[42] | Archer DB , Coombes SA , Chu WT , Chung JW , Burciu RG , Okun MS , Wagle Shukla A , Vaillancourt DE ((2018) ) A widespread visually-sensitive functional network relates to symptoms in essential tremor. Brain 141: , 472–485. |
[43] | Mavroudis I , Petrides F , Karantali E , Chatzikonstantinou S , McKenna J , Ciobica A , Iordache AC , Dobrin R , Trus C , Kazis D ((2021) ) A voxel-wise meta-analysis on the cerebellum in essential tremor. Medicina (Kaunas) 57: , 264. |
[44] | Luo R , Pan P , Xu Y , Chen L ((2019) ) No reliable gray matter changes in essential tremor. Neurol Sci 40: , 2051–2063. |
[45] | Han Q , Hou Y , Shang H ((2018) ) A voxel-wise meta-analysis of gray matter abnormalities in essential tremor. Front Neurol 9: , 495. |
[46] | Beach TG , Adler CH , Sue LI , Serrano G , Shill HA , Walker DG , Lue L , Roher AE , Dugger BN , Maarouf C , Birdsill AC , Intorcia A , Saxon-Labelle M , Pullen J , Scroggins A , Filon J , Scott S , Hoffman B , Garcia A , Caviness JN , Hentz JG , Driver-Dunckley E , Jacobson SA , Davis KJ , Belden CM , Long KE , Malek-Ahmadi M , Powell JJ , Gale LD , Nicholson LR , Caselli RJ , Woodruff BK , Rapscak SZ , Ahern GL , Shi J , Burke AD , Reiman EM , Sabbagh MN ((2015) ) Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 35: , 354–389. |
[47] | Koga S , Sekiya H , Kondru N , Ross OA , Dickson DW ((2021) ) Neuropathology and molecular diagnosis of synucleinopathies. Mol Neurodegener 16: , 83. |
[48] | Braak H , Alafuzoff I , Arzberger T , Kretzschmar H , Del Tredici K ((2006) ) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112: , 389–404. |
[49] | Thal DR , Rüb U , Orantes M , Braak H ((2002) ) Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58: , 1791–1800. |
[50] | Mirra SS , Heyman A , McKeel D , Sumi SM , Crain BJ , Brownlee LM , Vogel FS , Hughes JP , van Belle G , Berg L ((1991) ) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41: , 479–486. |
[51] | Beach TG , Adler CH , Lue L , Sue LI , Bachalakuri J , Henry-Watson J , Sasse J , Boyer S , Shirohi S , Brooks R , Eschbacher J , White CL , 3rd, Akiyama H , Caviness J , Shill HA , Connor DJ , Sabbagh MN , Walker DG ((2009) ) Unified staging system for Lewy body disorders: Correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117: , 613–634. |
[52] | Louis ED , Faust PL ((2020) ) Essential tremor: The most common form of cerebellar degeneration? Cerebellum Ataxias 7: , 12. |
[53] | Louis ED , Iglesias-Hernandez D , Hernandez NC , Flowers X , Kuo SH , Vonsattel JPG , Faust PL ((2022) ) Characterizing Lewy pathology in 231 essential tremor brains from the essential tremor centralized brain repository. J Neuropathol Exp Neurol 81: , 796–806. |




