Neuropsychiatric Symptoms and Caregiver Stress in Parkinson’s Disease with Cognitive Impairment, Alzheimer’s Disease, and Frontotemporal Dementia
Abstract
Background:
A better understanding of factors associated with caregiver burden might facilitate the construction of coping strategies to improve their clinical outcomes and the comprehensive care model for dementia.
Objective:
To investigate the cognitive and neuropsychiatric domains that contribute to caregiver burden in three types of neurodegenerative disorders: Parkinson’s disease (PD), Alzheimer’s disease (AD), and frontotemporal disease (FTD).
Methods:
Eight hundred and fourteen patients and their caregivers were invited to participate; among them, 235 had PD with cognitive impairment; 429 had AD, and 150 had FTD. The evaluation protocol included the Neuropsychiatric Inventory (NPI), the Mini-Mental State Examination, the Chinese Version Verbal Learning Test, the modified Trail Making Test B, semantic fluency, and a geriatric depression score. Statistical comparisons of the cognitive tests, NPI total scores, and caregiver burden among the three diagnosed types of dementia, matched for a Clinical Dementia Rating (CDR) of 0.5 or 1, were performed, and multivariate linear regression models were used to evaluate the parameter significance.
Results:
Caregivers for patients with PD and FTD showed significant burden increments when the CDR scores changes from 0.5 to 1. For CDR = 0.5, the PD group had significantly lower caregiver burdens than the AD group, but the NPI total scores were significantly higher. Factors related to caregiver burden were the presence of delusion among all diagnosis groups, while the impact of NPI total scores related to caregiver burden was the highest in FTD, followed by AD and PD.
Conclusions:
At the mild to moderate stages, our results suggested different degrees of significance in terms of the cognitive test scores or NPI subdomains for predicting caregiver stress among the three types of dementia.
INTRODUCTION
The percentage of individuals in Taiwan aged≥65 years increased from 4.1% in 1980 to 10.7% in 2010. This increase in the aging population led to an increase in the prevalence of dementia [1]. Alzheimer’s disease (AD), Parkinson’s disease (PD), and frontotemporal disease (FTD) are among the three most common neurodegenerative entities leading to dementia [2, 3]. With the advance of neuroimaging and molecular biomarkers, more patients are receiving a clinical diagnosis at the mild cognitive impairment (MCI) phase. The heterogeneity and subtypes specific to the MCI phases in PD and AD warrant differences in treatment strategies. MCI due to AD represents an earlier disease stage prior to dementia [4, 5]. Similarly, PD-MCI is associated with increased risks of developing dementia in as many as 80% of PD patients [6].
In common degenerative disorders, an understanding of the factors correlated with caregiver burden may help to improve coping strategies. Compared with professionals, family members of dementia patients often experience greater levels of distress related to reduced quality of life, mood changes, sleep disorders, and cardiovascular morbidity and mortality [7–9]. In a demographic analysis, older caregivers, assistants without payment, or those who have been caregivers for longer periods, had poor health status, and female caregivers with lower income or without support were found to have more depressive symptoms [10]. Under such circumstances, a proper dementia care model should focus not only on the patients but also on the caregivers. In suburban Taiwan, the care of dementia patients is often provided by family members who stay with the patients [10]. Proper support leading to better management of neuropsychiatric disturbances may help restore cognitive abilities in these patients and also relieve the burden on caregivers.
Given the same dementia subtype, behavior related to caregiver burden may vary greatly among countries or cultures, where the caregiver burden will be higher when the degenerative disorders progress faster [11]. In the literature, a higher caregiver burden among caregivers to patients with behavioral variant FTD (bvFTD) was reported compared with those caring for AD patients [12, 13]. However, a constellation of factors such as dementia severity, relationships between caregivers and patients, or the caregiver’s level of depression may moderate each other [14, 15]. It is still being investigated whether the greater burden in bvFTD is related to the diagnosis per se or indirectly related due to the younger age of onset, behavioral patterns, or the stage at diagnosis. A direct comparison of dementia subtypes, matched for possible confounding factors, may help to delineate such relationships.
Factors related to caregiver stress can be complex because the clinical diagnosis, co-morbidities, neuropsychiatric presentations, cognitive profiles, or disease stages may interact [16]. Clarification of their inter-relationship may help to construct a comprehensive patient-centered family-oriented healthcare strategy. To date, limited information is available in the Asian population on the domains of Neuropsychiatric Inventory (NPI) and cognitive profiles related to caregiver stress among the common degenerative spectra. Since the usage of NPI for evaluating behavioral symptoms as well as caregiver burden has been well documented in AD [17], PD-dementia (PDD) [18], FTD [19], and in patients receiving antipsychotic treatment [20], this hospital-based study was aimed toward reporting the NPI subdomains and standardized cognitive test results [21] in three neurodegenerative subtypes to explore the clinical weightings among factors related to caregiver stress.
MATERIALS AND METHODS
Study design
A double-center observational study enrolled study participants treated at the Cognition and Aging Center at Kaohsiung Chang Gung Memorial Hospital and the Department of Neurology at Taipei Veteran General Hospital. The study protocol complied with the ethical standards established in the Declaration of Helsinki, and the protocol received approval from the Institutional Review Board (IRB) at both hospitals (IRB number: CORPG8N0041).
Inclusion and exclusion criteria
A total of 814 subjects were included after the consensus of a panel composed of neurologists, neuropsychologists, neuroradiologists, and experts in nuclear medicine [16, 22–25]. The NPI and cognitive tests were performed for all participants in the diagnostic phases, referring to patients who were drug naïve for acetylcholine esterase or antipsychotic medications.
The AD patients met the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th edition [26] and the probable AD criteria of the National Institute of Neurologic and Communicative Disorders and the Stroke-Alzheimer’s Disease and Related Disorders Association [27]. The clinical diagnosis of AD was supported by the results of the amyloid neuroimaging if the clinical diagnosis was not reached by the multi-disciplinary team [28]. The criteria for MCI due to AD was confirmed by the clinical core features and the neuropsychological tests [4].
The diagnosis of FTD and the three clinical subtypes were based on the Neary criteria [29], i.e., bvFTD, semantic dementia (SD), and progressive non-fluent aphasia (PNFA). The core features of SD included impaired confrontation naming and impaired single-word comprehension [30], while the PNFA met the diagnostic features of agrammatism in language production with effortful, halting speech with inconsistent speech sound errors and distortions.
PD-MCI and PDD were diagnosed by the Movement Disorder Society task force criteria [6, 31]. From the literature review, non-amnestic, single-domain impairment (i.e., any single non-memory domain) is the most common subtype of PD-MCI [6]. In this study, both single-domain and multi-domain PD-MCI were included. Because the caregiver burden and dementia functional scores are highly related and to make the functional severities for comparing the characteristics in PD-MCI and AD-MCI uniform, only patients with Clinical Dementia Rating (CDR) scores of 0.5 were included for analysis. For the dementia group, only CDR = 1 were eligible for enrollment. The patients with PD-MCI or PDD had Hoehn and Yahr stage 0–2. The rationale for the motor scale ranges was to control the influence of increased motor disability on caregiver stress. The exclusion criteria were a history of clinical stroke, neuroimaging evidence of territorial infarction, or autoimmune or systemic inflammatory diseases.
Caregiver burden, NPI and cognitive profiles
We used the 12-item version of the NPI [32] that divided mental/behavioral phenomena into 12 domains and the caregiver stress was accessed by the NPI-D scale [17]. Only caregivers who stayed with the patients represented the informants. All of the 12 NPI domains were rated on symptom frequency from 1 (occasional) to 4 (very frequent), symptom severity from 1 (mild) to 3 (severe), and caregiver burden from 0 (none) to 5 (extreme). The NPI-total score (ranging from 0 to 144) represented the sum of the subscale variables (frequency x severity). The sum of the caregiver burden in each domain of the NPI-D was also calculated (ranging from 0 to 60).
The 30-item Mini-Mental State Examination (MMSE) and CDR scale were assessed. Verbal episodic memory was assessed using the Chinese Version Verbal Learning Test (CVVLT) [33]. The sum of the first four learning trials (CVVLT-total) represented the overall retaining ability of the episodic memory and the 10 min recalls (CVVLT-10 min) represented delayed recall ability. The ability to perform five arithmetic calculations was assessed, while frontal lobe function was assessed using digit forward and backward span, verbal fluency, and Modified Trails B tests [34] (correct numbers and time [maximum = 120 s]). Visuo-spatial abilities were assessed by the drawing of pentagons.
Statistical analysis
Statistical significance for intergroup differences was assessed by a Chi-square test for categorical variables or by an analysis of variance with a post-hoc analysis for continuous variables, as appropriate. The Pearson correlation analysis was used to explore the relationships between the NPI total score or the NPI burden and the continuous variables (i.e. demographic or cognitive data). A multivariate linear regression analysis was performed to evaluate the influence of the independent variables on NPI burden or total scores. Only factors showing significant correlations with the dependent variable were entered into the regression analysis model. The statistical analysis was performed using SPSS Statistics 12.0 (SPSS Inc., Chicago, IL, USA). A p value < 0.01 was considered statistically significant.
RESULTS
Baseline characteristics of the study patients
A total of 814 patients completed the study (Table 1). The demographic data and neuropsychiatric tests performance for the three degenerative disorders are listed in Table 1. Among the diagnostic groups, patients with FTD had the lowest scores on the cognitive tests and the highest in the NPI scores and caregiver distress levels. The results may be related to the sample inhomogeneity in the CDR scores. Patients with AD (CDR = 0.5, n = 316, 73.65%; CDR = 1, n = 113) and the PD groups (CDR = 0.5, n = 202, 85.96%; CDR = 1, n = 33) showed a higher proportion of patients with CDR = 0.5 compared with FTD (CDR = 0.5, n = 15, 10%; CDR = 1, n = 135).
Table 1
Demographical characteristics and neuropsychiatric tests in the three diagnostic groups
| Clinical diagnosis | FTD | AD | PD |
| Case numbers | 150 | 429 | 235 |
| Age (y) | 65.1 (7.0) * | 72.7 (8.8) § | 64.4 (11.0) |
| Education (y) | 9.9 (5.3) | 7.0 (5.0) *, § | 8.8 (5.0) # |
| Sex (male/female) | 76/74* | 191/238 | 134/101§ |
| Mini-Mental State Examination | 15.5 (7.4) *, # | 20.4(6.1) § | 24.5 (5.0) |
| case numbers of CDR 0.5 and CDR 1 | n = 15; n = 135 | n = 316; n = 113 | n = 202; n = 33 |
| Memory test | |||
| CVVLT-total | 12.8 (7.9) *, # | 18.5 (7.3) § | 22.7 (6.4) |
| CVVLT-10 min recalls | 1.6 (2.3) *, # | 3.2 (3.1) § | 5.4 (2.7) |
| Frontal-executive test | |||
| Digit forward | 6.8 (1.6) *, # | 7.2 (1.4) | 7.2 (1.6) |
| Digit backward | 3.2 (1.3) # | 3.1 (1.5) § | 3.8 (1.5) |
| Modified Trails B tests time (s) | 105.0 (28.2) # | 100.4 (31.9) § | 88.1 (34.8) |
| Modified Trails B tests scores | 7.2 (4.8) # | 6.4 (5.3) § | 9.2 (5.1) |
| Verbal fluency | 6.2 (5.1) *, # | 10.6 (4.8) § | 13.0 (4.8) |
| Calculations | 2.7 (1.7) *, # | 3.5 (1.5) § | 4.0 (1.2) |
| Visuospatial-Pentagone | 0.5 (0.5) *, # | 0.6 (0.5) | 0.7 (0.5) |
| Geriatric depression score | 3.6 (3.3) | 3.8 (3.5) | 5.0 (3.9) # , § |
| NPI total scores | 19.3 (18.1) *, # , § | 6.5 (9.5) § | 8.7 (10.2) |
| NPI caregiver burden | 9.7 (9.8) *, # , § | 2.6 (5.0) | 0.9 (3.0) |
Data are presented as mean (standard deviation) or number (percentage; %) CVVLT, Chinese version verbal learning test; NPI, Neuropsychiatric Inventory; CDR, Clinical Dementia Rating; FTD, frontotemporal disease; AD, Alzheimer’s disease; PD, Parkinson’s disease. *p < 0.05 FTD compared with AD; #p < 0.05 FTD compared with PDD; §p < 0.05 AD compared with PD.
Caregiver burdens related to CDR severities in AD and FTD but not in PD
To investigate whether dementia severity interferes with the level of caregiver distress, we compared the differences in caregiver stress between CDR = 0.5 and CDR = 1 in each diagnosis. The caregiver burden score was significantly higher in CDR = 1 compared with CDR = 0.5 in AD (CDR: 0.5 vs. 1.0:1.97 vs, 4.74, p < 0.001), and FTD (CDR = 0.5 vs. 1.0:2.09 vs. 10.27, p < 0.001), but not in PD (CDR = 0.5 vs. 1.0:0.64 vs. 2.45, p = 0.064).
Changes in caregiver stress by different diagnosis groups
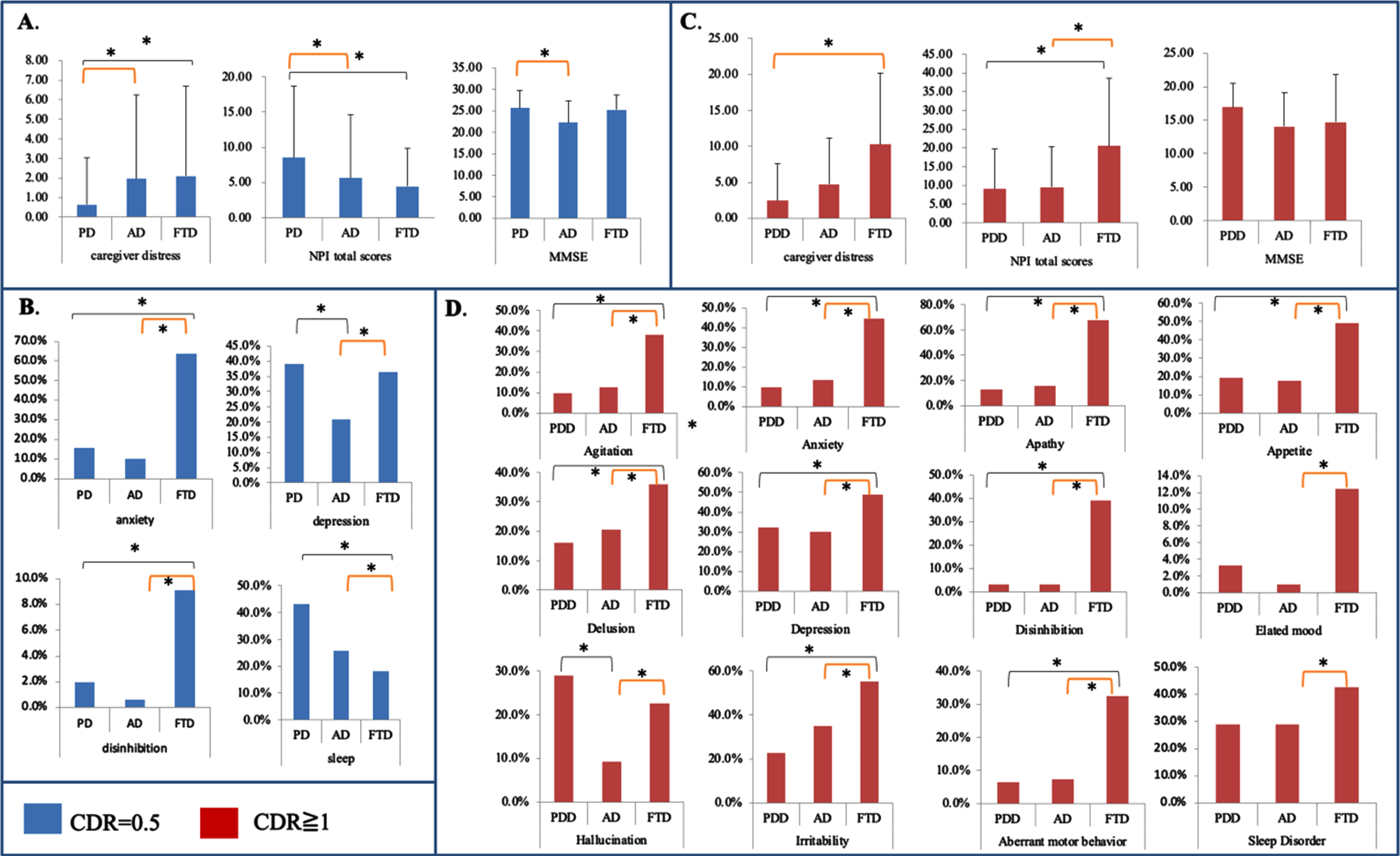
While stratifying by the same CDR scores, we further compared the differences in the caregiver burden scores between the three diagnosis groups (Fig. 1A and B, CDR = 0.5; Fig. 1C and D, CDR = 1). For CDR = 0.5, although the PD-MCI group had the highest total NPI score, the caregiver distress was the lowest. Patients with AD-MCI and FTD had comparable caregiver burdens (Fig. 1A). In the NPI subdomain analysis, patients with PD-MCI had the highest percentage of depression and sleep disorders, and the FTD group had the highest percentage of anxiety and disinhibition (Fig. 1B). Meanwhile, the caregiver stress in PD-MCI was related only to the frequency (r = 0.379, p < 0.01) but not the severity (r = 0.211, p = 0.27) or frequency*Severity scores (r = 0.3, p = 0.11).
For CDR = 1, while the MMSE scores were comparable among the three diagnosis groups, patients with FTD had the highest total NPI scores and caregiver distress scores (Fig. 1C). The FTD patients also had the highest percentage of the NPI subdomains (Fig. 1D) except in the hallucination domain.
Fig. 1
Different diagnosis groups in caregiver stress, Neuropsychiatric Inventory (NPI), and Mini-Mental State Examination (MMSE) stratified by the same Clinical Dementia Rating (CDR) score. The blue bar represents CDR 0.5 and the red bar is CDR = 1. PD, Parkinson’s disease; AD, Alzheimer’s disease; FTD, frontotemporal disease; PDD, Parkinson’s disease dementia. *p < 0.05.
Relationships between the cognitive tests scores and caregiver burden among the three diagnosis groups
Age and educational levels were not related to total NPI scores or caregiver burden (Table 2). The cognitive test scores showed inverse correlations with total NPI scores, but the domains affected were different among the diagnoses. In AD, the caregiver burden was significantly correlated with memory and executive scores, while the caregiver burden in the FTD and PD groups was related to the frontal-executive test.
Table 2
Correlations between Cognitive test scores and NPI total scores (or Caregiver Burden)
| Frontotemporal disease | Alzheimer’s disease | Parkinson’s disease | ||||
| NPI-total | burden | NPI-total | burden | NPI-total | burden | |
| Age (y) | 0.07 | 0.11 | 0.06 | 0.09 | –0.10 | 0.07 |
| Education (y) | –0.07 | –0.15 | –0.10 | 0.00 | –0.08 | –0.09 |
| Mini-Mental State Examination | –0.15 | –0.14 | –0.12* | –0.20* | –0.05 | –0.17* |
| Gender | 0.544 | 0.432 | 0.72 | –0.975 | 0.849 | 0.510 |
| Memory test | ||||||
| CVVLT-total | –0.03 | –0.07 | –0.05 | –0.13* | –0.02 | –0.06 |
| CVVLT-10 min recalls | –0.09 | –0.07 | –0.01 | –0.16* | 0.00 | –0.08 |
| Frontal-executive test | ||||||
| Digit forward | –0.17 | –0.18* | –0.11* | –0.05 | –0.05 | –0.07 |
| Digit backward | –0.11 | –0.19 | –0.13* | –0.03 | –0.07 | –0.13 |
| Modified Trails B tests time (s) | 0.17 | 0.14 | 0.15* | 0.12* | 0.03 | 0.09 |
| Modified Trails B tests scores | –0.17 | –0.17 | –0.14* | –0.12* | –0.05 | –0.19* |
| Verbal fluency | –0.04 | –0.03 | –0.08 | –0.09 | –0.04 | –0.11 |
| Calculations | –0.22* | –0.20* | –0.11* | –0.08 | –0.07 | –0.10 |
| Visuospatial-Pentagone | –0.25* | –0.20* | –0.10 | –0.05 | –0.10 | –0.06 |
| Geriatric depression score | 0.04 | 0.10 | 0.29* | 0.08 | 0.36* | 0.02 |
| All patients: NPI-total score | – | 0.80* | – | 0.3* | – | 0.16* |
| CDR = 0.5: NPI-total score | – | –0.113 | – | 0.292 ** | – | 0.222* |
| CDR = 1: NPI-total score | – | 0.803 ** | – | 0.3 ** | – | 0.163 |
NPI, Neuropsychiatric Inventory; NPI-total, sum of 12 domains in the NPI (frequency x severity); burden, sum of caregiver burden in 12 domains in the NPI. * p < 0.05; numbers indicate Pearson Correlation Coefficient or Mean difference (after Gender).
The relationships between the NPI scores and caregiver burden were significantly correlated in all diagnosis groups (correlation coefficient: FTD > AD > PD). There were dissociations between diagnosis and caregiver-NPI relationships in that FTD showed significance only in the dementia stage, while PD showed it in the CDR = 0.5 stage.
Factors predicting caregiver burden among the three diagnosis groups were further analyzed using the multivariate linear regression models (Supplementary Table 1). The results suggested that caregiver burden in FTD and PD was only related to the total NPI scores. In AD, the MMSE, delay recalls, and NPI total scores were all significantly related to the caregiver burden scores.
Relationships between cognitive test performance and NPI total scores among the three diagnosis groups
Factors predicting total NPI scores are shown in Supplementary Table 2. None of the selected cognitive tests significantly predicted the total NPI scores among the three groups. For AD and PD, the NPI scores were related to the geriatric depression scores.
NPI subdomains and caregiver burden stratified by dementia severity
Since the functional severities using the CDR revealed different impacts on the relationships between total NPI scores and caregiver burden, a correlation analysis between NPI caregiver burden and each subdomain score was first conducted, followed by a multivariate regression analysis to evaluate their independent roles on the caregiver burden (Table 5). The results suggested that in AD and PD, the presence of irritability was related to greater caregiver burden at the MCI stage. For the dementia stage of CDR = 1, the presence of delusions was related to a greater caregiver burden in all three groups, and the presence of hallucinations in the AD and FTD groups was also related to a greater caregiver burden.
Comparisons of neuropsychiatric scores among the FTD subtypes
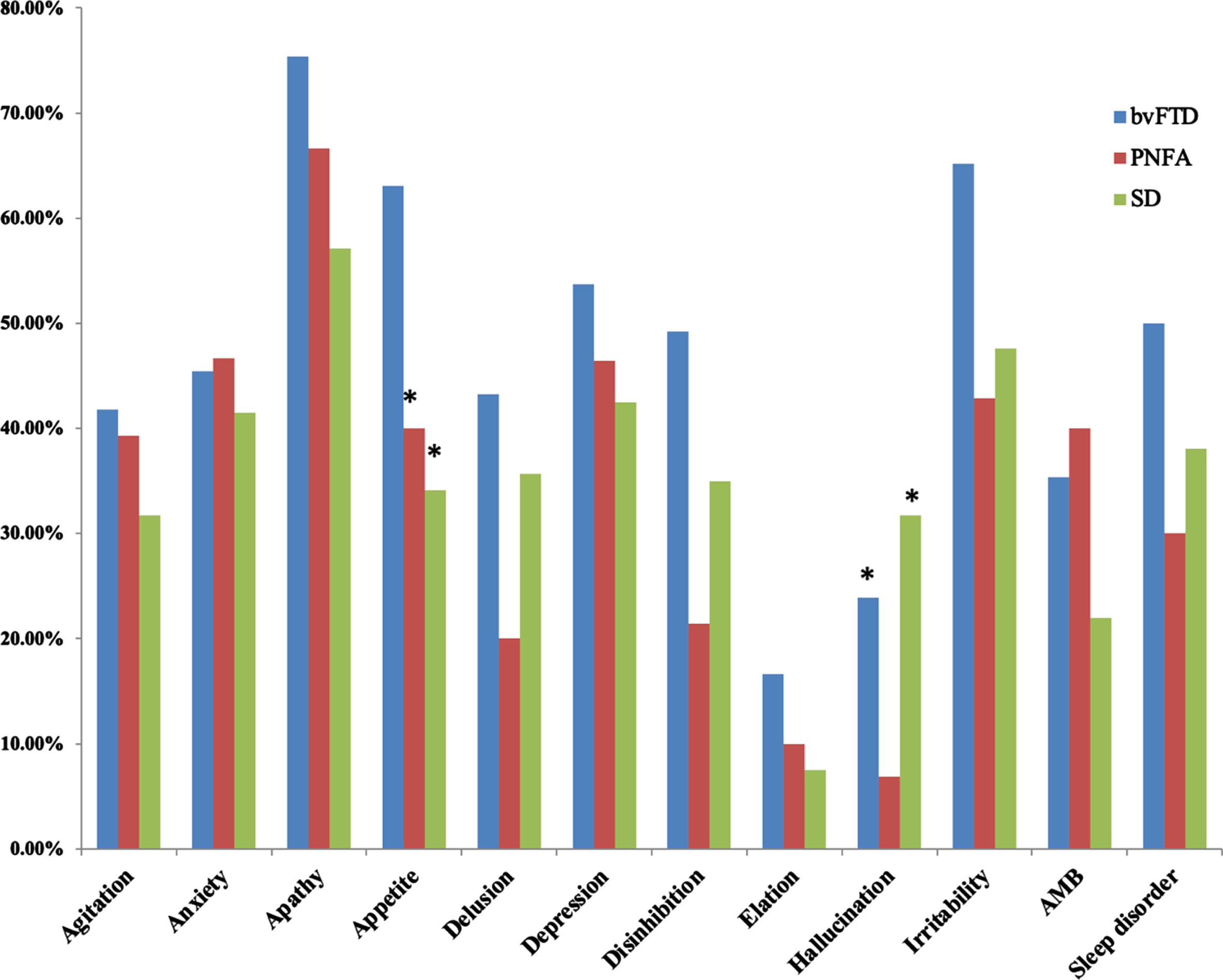
Among patients of FTD with CDR = 1, we further compared whether the diagnosis of FTD subtypes affected the caregiver burden scores (Table 3). The analysis showed no significant in-group differences in the NPI caregiver burden or the total scores. In the cognitive domains, however, semantic dementia showed significantly lower scores in the MMSE, memory tests, digit forward, and verbal fluency compared with bvFTD. Meanwhile, the PNFA patients also had lower MMSE scores and total memory test scores compared to the bvFTD patients. In contrast with the cognitive test, the bvFTD patients had the highest presentation of NPI subdomains, especially the subdomains of anxiety, hallucination, and aberrant motor behavior (Fig. 2).
Table 3
Neuropsychiatric data in frontotemporal dementia subtypes
| bvFTD (n = 67) | PNFA (n = 27) | SeD (n = 27) | ||||
| Mean | SD | Mean | SD | Mean | SD | |
| NPI Caregiver burden | 11.9 | 9.5 | 7.4 | 8.5 | 9.5 | 10.9 |
| NPI total scores | 24.5 | 18.3 | 16.0 | 17.2 | 16.9 | 17.5 |
| MMSE | 17.2 | 6.4 | 13.6 a | 7.3 | 11.6 b | 6.7 |
| Memory test | ||||||
| CVVLT-total | 16.0 | 6.6 | 10.5 a | 8.7 | 7.9 b | 7.0 |
| CVVLT-10 min recalls | 1.9 | 2.3 | 1.6 | 2.6 | 0.3 b,c | 0.7 |
| Frontal-executive test | ||||||
| Digit forward | 7.1 | 1.4 | 6.4 | 2.0 | 6.1 b | 1.7 |
| Digit backwards | 3.1 | 1.1 | 3.4 | 1.4 | 2.7 | 1.3 |
| MTBT Time (s) | 104.4 | 27.3 | 116.7 | 12.7 | 105.9 | 29.5 |
| MTBT scores | 7.4 | 4.7 | 6.1 | 4.9 | 7.3 | 5.3 |
| Verbal Fluency | 7.6 | 4.9 | 5.1 | 4.0 | 3.5 b | 4.2 |
| Pentagone | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Geriatric depression score | 3.4 | 3.3 | 3.0 | 3.2 | 4.2 | 3.5 |
NPI, Neuropsychiatric Inventory; SD, standard deviation; MTBT, Modified Trails B tests; CVVLT, Chinese version verbal learning test; MMSE, Mini-Mental State Examination; bvFTD, behavior variant frontotemporal dementia; PNFA, progressive non-fluent aphasia; SeD, semantic dementia. All the FTD patients have a clinical dementia rating = 1. ap < 0.05 between bvFTD and PNFA; bp < 0.05 between bvFTD and SeD; cp < 0.05 between SeD and PNFA.
Fig. 2
Comparisons of neuropsychiatric inventory presentations in 3 subtypes of frontotemporal dementia. bvFTD, behavior variant frontotemporal dementia; PNFA, progressive non-fluent aphasia; SD, semantic dementia.
DISCUSSION
Major findings
The study explored factors related to caregiver burdens in three common degenerative diseases matched for dementia functional severity. There were three major findings. First, the caregiver burden was affected by the interactions between diagnosis and disease severity. The cognitive and neuropsychiatric domains also contributed different weightings. Overall, the total NPI score was the most important factor related to the caregiver burden among the three disease spectrums (Supplementary Table 1). Significant interactions in dementia severity and the diagnostic group were evident in the caregiver burden scores. The paradoxical findings of higher NPI scores but lower burdens in the PD-MCI compared with the AD-MCI suggested differences in mechanisms mediating caregiver stress (Fig. 1). Such interactions were also true for FTD in the dementia stage because the total NPI scores and burden were significantly higher based on equivalent cognitive test scores compared with patients with either AD or PD (Fig. 1). Second, the age and educational levels of the patients were not related to the caregiver burden. Although some cognitive domains showed significant correlations with caregiver burden in PD or FTD, the cognitive scores were not significant after entering into the regression model with NPI (Supplementary Table 1). In contrast, the delayed memory recall, MMSE scores, and NPI scores were related to caregiver burden in AD (Supplementary Table 1). Finally, the presence of any NPI symptom may not explain the related caregiver burden because the symptom and burden correlation was not consistent among the three diseases (Supplementary Table 3).
Caregiver burden in the PD spectrum and comparisons with AD
PD-MCI is defined by clinical, cognitive, and functional criteria [6]. Our patients with PD-MCI had higher total NPI scores, percentage of depression, and sleep disorders compared with AD-MCI, but the caregiver burdens were significantly lower. This dissociation may be explained partly by the NPI domains being affected in PD-MCI. PD-MCI caregivers experience elevated levels of caregiver distress related to physical health problems or lower economic loads compared with those with normal cognition [35]. Previously, retaining hallucination-specific insight has been accessed in patients with PD and the hallucination-specific insights were related to higher severity and MMSE scores [36]. The caregiver burden in hallucination was related to the frequency of hallucination rather than the severities in our PD-MCI. The retained insights of these PD-MCI patients in visual hallucination can explain the caregiver burden.
There is a paucity of research regarding caregiver outcomes in PD-MCI. Based on our results, we inferred several possible explanations. First, our PD-MCI analysis suggested the greatest weighting of irritability and hallucinations in predicting caregiver distress, which was in contrast to AD-MCI showing agitation, delusion, depression, and irritability (Supplementary Table 3). The differences in NPI clusters may have affected the degree of caregiver stress. The prevalence of delusion in AD varies [37], but studies in Taiwan have reported that delusions are the most distressing symptom for AD caregivers [38, 39]. In fact, symptoms of delusion in AD among ethnic Chinese have been reported more frequently than among Caucasians [40]. Another study in Hong Kong suggested high collinearity of delusional disorders with hallucinations, aggressiveness, and affective disturbances in elderly Chinese AD patients [41], which was consistent with the clustering of the NPI sub-domains shown in AD-MCI here.
Second, the clinical diagnosis may directly interfere with the level of caregiver stress at the MCI stage. The existing PD-MCI criteria emphasize the absence of significant functional impairment resulting from cognitive impairment as the primary feature [31]. In such a context, most patients with PD-MCI still manage their medications and/or finances, which might lead to a lower caregiver burden. There were significant correlations between the MMSE scores and the caregiver burden scores in AD and PD in this study. With higher MMSE scores in PD-MCI, the lower burden in PD-MCI might have reflected the relative intactness of general cognitive performance on the equivalent functional scale. In our PD-MCI patients, the relationship between total NPI scores and caregiver burden was also lower than that in the AD-MCI patients.
In fact, there are challenges in terms of separating contributions of cognitive, behavioral, and motor deficits to caregiver load. Theoretically, the progression from PD-MCI to PDD should increase caregiver burden incrementally, yet another interesting finding here was that caregiver burden in PD-MCI and PDD were not significantly different. These results could be related to the fewer case numbers in the PDD group. Alternatively, deterioration of motor function along with a decline of cognitive ability may not necessarily lead to a greater caregiver burden in PDD from the perspective of care load. Our study pointed out that only delusion, but not other subdomains or total NPI scores (r = 0.148, p = 0.462), was a significant predictive factor of greater caregiver burden in PDD (Table 5). Other studies have suggested that the presence of delusion and hallucinations (psychosis cluster), agitation or total NPI scores, instead, are associated with higher caregiver distress scores in PDD [18, 42].
Although our regression model emphasized the role of total NPI scores in PD caregiver burden, the correlation analysis also supported the clinical significance of the MMSE and Modified Trails B test scores. Both PD-MCI and PDD patients have various levels of memory and executive deficits [43–45]. The longitudinal brain-behavior study also pointed out that PD with more posterior cortical basis symptoms represents a cognitive syndrome that can be distinguished from the based symptoms [46]. Further study should include the subtypes of PD-MCI to understand whether caregiver burdens are driven by such differences.
Relationships of caregiver burden in FTD and differences from AD in the early dementia phase
Evidence has shown that caregivers of FTD patients report higher levels of general burden, and feel less competent than AD caregivers [12]. In our study, most patients with FTD received diagnostic consultations when the CDR scores were 1. Although the CDR may not be a suitable tool for functional evaluations of FTD patients, the criticism related to the use of the CDR was because the included items might lead to an under-estimation of functional ability in FTD patients. In such cases, patients with FTD received consultations at a later phase than those with AD. Focusing on both groups at the dementia phase, the caregiver burden scores for the patients with FTD were related to the delusion and hallucination subdomains, similar to those for the AD caregivers. By using multivariate linear regression models, we found that higher beta values (Supplementary Table 1) were seen in FTD compared with AD for predicting caregiver stress using NPI total scores. The higher proportions of other NPI subdomains in the FTD patients also implied stronger links among overall severities and frequencies in all NPI domains.
Recently, one report from China focusing on a total of 214 patients with FTD, diffuse Lewy body dementia, and AD pointed out the highest caregiver burden in bvFTD [47]. The same trend for higher caregiver burden in FTD was found in our study. Unique to this study, we divided the FTD patients based on the diagnostic criteria and found that the caregiver burden was not significantly different among the three subtypes of FTD.
The severity of agitation [19], apathy [12], and mood disturbances have been associated with greater caregiver burdens in bvFTD, while a lack of relationship between caregiver burden with degree of cognitive impairment or functional severities has also been reported [48]. We compared the behavioral changes among three FTD subtypes, where hallucination and appetite were two key features that distinguished the subtypes. However, only hallucination was related to greater caregiver burden.
For the cognitive domains, the FTD caregiver burden in this study was found to be correlated to the cognitive scores for digit forward, calculation, and visuospatial ability although the clinical weightings were less than the NPI scores (Table 2). Since the bvFTD, SD, and PNFA showed significant differences in the cognitive profiles, it is possible that the significant correlation between the cognitive tests and caregiver burden was not driven by the diagnosis per se.
Limitations
There were some limitations in this study. First, because this was a two-center observational study, the inter-rater reliability of the NPI may not have been completely consistent, and thus some important associations might have been lost. However, we used the NPI because it is a highly structured instrument with high test-retest reliability, and our raters completed a training session before the study which minimized the potential bias. Second, we stated that the clinical profiles were collected at the drug-naïve phase to avoid possible confounding by medications, but strictly speaking, the definition may not have been applicable to the PD-MCI or PDD groups. Dopaminergic agents and several antiparkinsonian agents, commonly used for motor function restoration in PD, have behavioral effects and may thus have contributed to neuropsychiatric presentation in our PD-MCI and PDD groups. Drug-related influences on the NPI domains in PD-MCI or PDD should be considered clinically relevant, in particular, visual hallucinations [49]. Meanwhile, those using antipsychotic drugs were not considered to be eligible in this study. Taken together, NPI symptoms may have been more common in our PD cohorts. Third, the results for PD and FTD could have been driven by the PD-MCI and FTD with dementia. The selection of cases showing sample inhomogeneity in terms of functional severity may limit the general application to FTD at the non-dementia stage and in PDD. However, the sample distribution pattern may reflect the real-world situation for patients searching for behavior neurologist consultations. The statistical analyses were performed based on two functional severities, and the discussion only emphasized the PD-MCI or FTD, using MCI or dementia due to AD as a contrast, to delineate possible coping strategies. Fourth, the mean total NPI scores of the patients in our studies vary from 6.5 in AD to 19.3 in FTD, caregiver burden ranging from 0.9 in PD to 9.7 in FTD and CDR were limited to 0.5 and 1. It indicated that many of the enrolled patients were at the relatively earlier clinical stage and the generalization of our study results to the whole PD, AD, and FTD population should be cautious. Finally, there was clinical heterogeneity in the AD-MCI and PD-MCI, but our study did not classify them into different subtypes due to sample size considerations. The evaluation of functional performance in PD-MCI using CDR = 0.5 may not have been fully consistent with those for AD. Although we had selected PD-MCI or PDD at earlier cognitive stages, the disabilities in PD-MCI or PDD may cause greater caregiver burden from motor symptoms rather than cognition. The use of NPI-D may underestimate the overall burden in PD compared with AD or FTD which had predominantly cognition or behavior symptoms. Further development of applicable functional scores combining the impacts of motor, behavioral, and cognitive deficits for AD and PD may better delineate the weighting of each factor.
CONCLUSION
Disease-related neuropsychiatric comorbidities may be important determinants for relieving caregiver distress in terms of proposing treatment strategies. Clinical diagnosis, NPI total scores, NPI symptom clusters, and cognitive profiles predicted caregiver burden differently among the three common degenerative disorders under investigation in this study. At the MCI stage, PD caregivers had a lower caregiver burden although the total NPI scores were higher than those for MCI due to AD. After progression to the dementia phase, FTD caregivers encountered the highest burden, but the differences in the burden scores among the three FTD subtypes were not found to be significantly different.
ACKNOWLEDGMENTS
This work was supported by grants CMRPG8J0524, CMRPG8J0843, CMRPG8K1533, CORPG8N0041 from Kaohsiung Chang Gung Memorial Hospital, and 104-2314-B-182A-026-MY2 from the National Science Council to CC Chang.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-223550.
REFERENCES
[1] | Sun Y , Lee HJ , Yang SC , Chen TF , Lin KN , Lin CC , Wang PN , Tang LY , Chiu MJ ((2014) ) A nationwide survey of mild cognitive impairment and dementia, including very mild dementia, in Taiwan., PLoS One 9: , e100303. |
[2] | Wen H , Zhang Z , Huang J , Duan L , Wang Q ((2011) ) Mortality of dementia and its major subtypes in urban and rural communities of Beijing, Biomed Environ Sci 24: , 483–490. |
[3] | Zuliani G , Galvani M , Sioulis F , Bonetti F , Prandini S , Boari B , Guerzoni F , Gallerani M ((2012) ) Discharge diagnosis and comorbidity profile in hospitalized older patients with dementia, Int J Geriatr Psychiatry 27: , 313–320. |
[4] | Chen NC , Chang CC , Lin KN , Huang CW , Chang WN , Chang YT , Chen C , Yeh YC , Wang PN ((2013) ) Patterns of executive dysfunction in amnestic mild cognitive impairment, Int Psychogeriatr 25: , 1181–1189. |
[5] | Dubois B , Feldman HH , Jacova C , Hampel H , Molinuevo JL , Blennow K , DeKosky ST , Gauthier S , Selkoe D , Bateman R , Cappa S , Crutch S , Engelborghs S , Frisoni GB , Fox NC , Galasko D , Habert MO , Jicha GA , Nordberg A , Pasquier F , Rabinovici G , Robert P , Rowe C , Salloway S , Sarazin M , Epelbaum S , de Souza LC , Vellas B , Visser PJ , Schneider L , Stern Y , Scheltens P , Cummings JL ((2014) ) Advancing researchdiagnostic criteria for Alzheimer’s disease: The IWG-2 criteria, Lancet Neurol 13: , 614–629. |
[6] | Litvan I , Aarsland D , Adler CH , Goldman JG , Kulisevsky J , Mollenhauer B , Rodriguez-Oroz MC , Troster AI , Weintraub D ((2011) ) MDS Task Force on mild cognitive impairment in Parkinson’s disease: Critical review of PD-MCI, Mov Disord 26: , 1814–1824. |
[7] | Serrano-Aguilar PG , Lopez-Bastida J , Yanes-Lopez V ((2006) ) Impact on health-related quality of life and perceived burden of informal caregivers of individuals with Alzheimer’s disease, Neuroepidemiology 27: , 136–142. |
[8] | Papastavrou E , Kalokerinou A , Papacostas SS , Tsangari H , Sourtzi P ((2007) ) Caring for a relative with dementia: Family caregiver burden, J Adv Nurs 58: , 446–457. |
[9] | Varela G , Varona L , Anderson K , Sansoni J ((2011) ) Alzheimer’s care at home: A focus on caregivers strain, Prof Inferm 64: , 113–117. |
[10] | Huang CY , Musil CM , Zauszniewski JA , Wykle ML ((2006) ) Effects of social support and coping of family caregivers of older adults with dementia in Taiwan, Int J Aging Hum Dev 63: , 1–25. |
[11] | Riedijk SR , De Vugt ME , Duivenvoorden HJ , Niermeijer MF , Van Swieten JC , Verhey FR , Tibben A ((2006) ) Caregiver burden, health-related quality of life and coping in dementia caregivers: A comparison of frontotemporal dementia and Alzheimer’s disease, Dement Geriatr Cogn Disord 22: , 405–412. |
[12] | de Vugt ME , Riedijk SR , Aalten P , Tibben A , van Swieten JC , Verhey FR ((2006) ) Impact of behavioural problems on spousal caregivers: A comparison between Alzheimer’s disease and frontotemporal dementia, Dement Geriatr Cogn Disord 22: , 35–41. |
[13] | Zwijsen SA , Kabboord A , Eefsting JA , Hertogh CM , Pot AM , Gerritsen DL , Smalbrugge M ((2014) ) Nurses in distress? An explorative study into the relation between distress and individual neuropsychiatric symptoms of people with dementia in nursing homes, Int J Geriatr Psychiatry 29: , 384–391. |
[14] | Mioshi E , Hodges JR ((2009) ) Rate of change of functional abilities in frontotemporal dementia, Dement Geriatr Cogn Disord 28: , 419–426. |
[15] | Mioshi E , Foxe D , Leslie F , Savage S , Hsieh S , Miller L , Hodges JR , Piguet O ((2013) ) The impact of dementia severity on caregiver burden in frontotemporal dementia and Alzheimer disease, Alzheimer Dis Assoc Disord 27: , 68–73. |
[16] | Chang CC , Lin PH , Chang YT , Chen NC , Huang CW , Lui CC , Huang SH , Chang YH , Lee CC , Lai WA ((2015) ) The impact of admission diagnosis on recurrent or frequent hospitalizations in 3 dementia subtypes: A hospital-based cohort in Taiwan with 4 years longitudinal follow-ups., Medicine (Baltimore) 94: , e2091. |
[17] | Kaufer DI , Cummings JL , Christine D , Bray T , Castellon S , Masterman D , MacMillan A , Ketchel P , DeKosky ST ((1998) ) Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: The Neuropsychiatric Inventory Caregiver Distress Scale, J Am Geriatr Soc 46: , 210–215. |
[18] | Aarsland D , Bronnick K , Ehrt U , De Deyn PP , Tekin S , Emre M , Cummings JL ((2007) ) Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: Frequency, profile and associated care giver stress, J Neurol Neurosurg Psychiatry 78: , 36–42. |
[19] | Mourik JC , Rosso SM , Niermeijer MF , Duivenvoorden HJ , Van Swieten JC , Tibben A ((2004) ) Frontotemporal dementia: Behavioral symptoms and caregiver distress, Dement Geriatr Cogn Disord 18: , 299–306. |
[20] | Ballard CG , Margallo-Lana ML ((2004) ) The relationship between antipsychotic treatment and quality of life for patients with dementia living in residential and nursing home care facilities, J Clin Psychiatry 65 Suppl 11: , 23–28. |
[21] | Chang CC , Chang YY , Chang WN , Lee YC , Wang YL , Lui CC , Huang CW , Liu WL ((2009) ) Cognitive deficits in multiple system atrophy correlate with frontal atrophy and disease duration, Eur J Neurol 16: , 1144–1150. |
[22] | Huang CW , Chang WN , Lui CC , Chen CF , Lu CH , Wang YL , Chen C , Juang YY , Lin YT , Tu MC , Chang CC ((2010) ) Impacts of hyper-homocysteinemia and white matter hyper-intensity in Alzheimer’s disease patients with normal creatinine: An MRI-based study with longitudinal follow-up, Curr Alzheimer Res 7: , 527–533. |
[23] | Chang YT , Huang CW , Chang YH , Chen NC , Lin KJ , Yan TC , Chang WN , Chen SF , Lui CC , Lin PH , Chang CC ((2015) ) Amyloid burden in the hippocampus and default mode network: Relationships with gray matter volume and cognitive performance in mild stage Alzheimer disease., Medicine (Baltimore) 94: , e763. |
[24] | Huang CW , Tsai MH , Chen NC , Chen WH , Lu YT , Lui CC , Chang YT , Chang WN , Chang AY , Chang CC ((2015) ) Clinical significance of circulating vascular cell adhesion molecule-1 to white matter disintegrity in Alzheimer’s dementia, Thromb Haemost 114: , 1230–1240. |
[25] | Lu YT , Chang WN , Chang CC , Lu CH , Chen NC , Huang CW , Lin WC , Chang YT ((2016) ) Insula volume and salience network are associated with memory decline in Parkinson disease: Complementary analyses of voxel-based morphometry versus volume of interest, Parkinsons Dis 2016: , 2939528. |
[26] | Ross R (1994) Diagnostic and statistical manual of mental disorders: DSM-IV 4th ed. American Psychiatric Association, Washington, DC. |
[27] | McKhann G , Drachman D , Folstein M , Katzman R , Price D , Stadlan EM ((1984) ) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease, Neurology 34: , 939–944. |
[28] | Chang YT , Huang CW , Chen NC , Lin KJ , Huang SH , Chang WN , Hsu SW , Hsu CW , Chen HH , Chang CC ((2016) ) Hippocampal amyloid burden with downstream fusiform gyrus atrophy correlate with face matching task scores in early stage Alzheimer’s disease, Front Aging Neurosci 8: , 145. |
[29] | Neary D , Snowden JS , Gustafson L , Passant U , Stuss D , Black S , Freedman M , Kertesz A , Robert PH , Albert M , Boone K , Miller BL , Cummings J , Benson DF ((1998) ) Frontotemporal lobar degeneration: A consensus on clinical diagnostic criteria, Neurology 51: , 1546–1554. |
[30] | Gorno-Tempini ML , Hillis AE , Weintraub S , Kertesz A , Mendez M , Cappa SF , Ogar JM , Rohrer JD , Black S , Boeve BF , Manes F , Dronkers NF , Vandenberghe R , Rascovsky K , Patterson K , Miller BL , Knopman DS , Hodges JR , Mesulam MM , Grossman M ((2011) ) Classification of primaryprogressive aphasia and its variants, Neurology 76: , 1006–1014. |
[31] | Litvan I , Goldman JG , Troster AI , Schmand BA , Weintraub D , Petersen RC , Mollenhauer B , Adler CH , Marder K , Williams-Gray CH , Aarsland D , Kulisevsky J , Rodriguez-Oroz MC , Burn DJ , Barker RA , Emre M ((2012) ) Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines, Mov Disord 27: , 349–356. |
[32] | Cummings JL , Mega M , Gray K , Rosenberg-Thompson S , Carusi DA , Gornbein J ((1994) ) The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia, Neurology 44: , 2308–2314. |
[33] | Chang CC , Kramer JH , Lin KN , Chang WN , Wang YL , Huang CW , Lin YT , Chen C , Wang PN ((2010) ) Validating the Chinese version of the Verbal Learning Test for screening Alzheimer’s disease, J Int Neuropsychol Soc 16: , 244–251. |
[34] | Reitan RM ((1955) ) The relation of the trail making test to organic brain damage, J Consult Psychol 19: , 393–394. |
[35] | Szeto JY , Mowszowski L , Gilat M , Walton CC , Naismith SL , Lewis SJ ((2016) ) Mild cognitive impairment in Parkinson’s disease: Impact on caregiver outcomes, J Parkinsons Dis 6: , 589–596. |
[36] | Wragg RE , Jeste DV ((1989) ) Overview of depression and psychosis in Alzheimer’s disease, Am J Psychiatry 146: , 577–587. |
[37] | Fuh JL , Liu CK , Mega MS , Wang SJ , Cummings JL ((2001) ) Behavioral disorders and caregivers’ reaction in Taiwanese patients with Alzheimer’s disease, Int Psychogeriatr 13: , 121–128. |
[38] | Chiu PY , Chung CL ((2006) ) Delusions in patients with very mild, mild and moderate Alzheimer’s disease, Acta Neurol Taiwan 15: , 21–25. |
[39] | Chow TW , Liu CK , Fuh JL , Leung VP , Tai CT , Chen LW , Wang SJ , Chiu HF , Lam LC , Chen QL , Cummings JL ((2002) ) Neuropsychiatric symptoms of Alzheimer’s disease differ in Chinese and American patients, Int J Geriatr Psychiatry 17: , 22–28. |
[40] | Lam LC , Tang WK , Leung V , Chiu HF ((2001) ) Behavioral profile of Alzheimer’s disease in Chinese elderly–a validation study of the Chinese version of the Alzheimer’s disease behavioral pathology rating scale, Int J Geriatr Psychiatry 16: , 368–373. |
[41] | Lee DR , McKeith I , Mosimann U , Ghosh-Nodyal A , Thomas AJ ((2013) ) Examining carer stress in dementia: The role of subtype diagnosis and neuropsychiatric symptoms, Int J Geriatr Psychiatry 28: , 135–141. |
[42] | Mahieux F , Fenelon G , Flahault A , Manifacier MJ , Michelet D , Boller F ((1998) ) Neuropsychological prediction of dementia in Parkinson’s disease, J Neurol Neurosurg Psychiatry 64: , 178–183. |
[43] | Levy G , Jacobs DM , Tang MX , Cote LJ , Louis ED , Alfaro B , Mejia H , Stern Y , Marder K ((2002) ) Memory and executive function impairment predict dementia in Parkinson’s disease, Mov Disord 17: , 1221–1226. |
[44] | Janvin CC , Aarsland D , Larsen JP ((2005) ) Cognitive predictors of dementia in Parkinson’s disease: A community-based, 4-year longitudinal study, J Geriatr Psychiatry Neurol 18: , 149–154. |
[45] | Williams-Gray CH , Evans JR , Goris A , Foltynie T , Ban M , Robbins TW , Brayne C , Kolachana BS , Weinberger DR , Sawcer SJ , Barker RA ((2009) ) The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort, Brain 132: , 2958–2969. |
[46] | Liu S , Jin Y , Shi Z , Huo YR , Guan Y , Liu M , Liu S , Ji Y ((2017) ) The effects of behavioral and psychological symptoms on caregiver burden in frontotemporal dementia, Lewy body dementia, and Alzheimer’s disease: Clinical experience in China, Aging Ment Health 21: , 651–657. |
[47] | Boutoleau-Bretonniere C , Vercelletto M , Volteau C , Renou P , Lamy E ((2008) ) Zarit burden inventory and activities of daily living in the behavioral variant of frontotemporal dementia, Dement Geriatr Cogn Disord 25: , 272–277. |
[48] | Wint DP , Okun MS , Fernandez HH ((2004) ) Psychosis in Parkinson’s disease, J Geriatr Psychiatry Neurol 17: , 127–136. |




