Effects of Oral Levodopa on Balance in People with Idiopathic Parkinson’s Disease
Abstract
Background:
Balance impairment is a frequent cause of morbidity and mortality in people with Parkinson’s disease (PD). As opposed to the effects of appendicular motor symptoms, the effects of Levodopa on balance impairment in idiopathic PD are less clear.
Objective:
To review the literature on the effects of oral Levodopa on clinical balance test performance, posturography, step initiation, and responses to perturbation in people with idiopathic PD (PwPD).
Methods:
A systematic search of three scientific databases (Pubmed, Embase, and Web of Science) was conducted in accordance with PRISMA guidelines. For the pilot meta-analysis, standardized mean differences with 95% confidence intervals were calculated using an inverse variance random effects model. Data not suitable for implementation in the meta-analysis (missing means or standard deviations, and non-independent outcomes) were analyzed narratively.
Results:
A total of 2772 unique studies were retrieved, of which 18 met the eligibility criteria and were analyzed, including data of 710 idiopathic PwPD. Levodopa had a significant positive effect on the Berg Balance Scale, the Push and Release test, and jerk and frequency parameters during posturography. In contrast, some significant negative effects on velocity-based sway parameters were found during posturography and step initiation. However, Levodopa had no significant effect on most step initiation- and all perturbation parameters.
Conclusion:
The effects of Levodopa on balance in PwPD vary depending on the outcome parameters and patient inclusion criteria. A systematic approach with well-defined outcome parameters, and prespecified, sensitive and reliable tests is needed in future studies to unravel the effects of oral Levodopa on balance.
INTRODUCTION
The diagnosis of Parkinson’s disease (PD) follows the observation of cardinal motor symptoms (tremor, rigidity, akinesia, and postural instability), leading to a reduction in active life participation and quality of life (QOL) [1–3]. Postural instability with increased postural sway and impaired reaction to external perturbations may result in impaired balance, especially in dual task conditions [4]. In addition, medication use, orthostatic hypotension, age, cognitive, vestibular, and visual impairments may aggravate reduced balance performance [5–9]. Studies on balance impairment and (other identifying factors of) recurrent falls indicated that about 60–80% of people with PD (PwPD) fall at least once and that 39% experience multiple fall episodes, with 23% sustaining a fracture as a result [10]. The impact of falls and fear of falling on QOL is highly recognized, which underlines the necessity to investigate the consequences of mediating factors on balance performance in PwPD [7,10–14].
Currently, oral Levodopa preparations, either in monotherapy or in combination therapy, are the most prescribed drugs to suppress motor symptoms in PD [15–17]. Levodopa has proven to significantly reduce the risk of death, independent of pre-Levodopa disease duration, with the strongest reduction in the first months after initiation of medication [18, 19]. In Levodopa naïve PwPD, Levodopa improves motor symptoms both in ON state (i.e., medication state of Levodopa with optimal effect) and in OFF state (i.e., overnight withdrawal), compared to the natural progression of PD [20]. It is important to note that the OFF state reported in most studies in fact refers to a partial OFF state, as motor symptoms can be (partially) suppressed up to two weeks after intake [21]. Furthermore, Levodopa treatment resulted in a 31% lower annual decline in UPDRS-III score compared to the natural progression [20]. Nevertheless, despite a substantial number of studies reporting generally positive effects of Levodopa to suppress motor symptoms, results from studies specifically investigating postural control are often conflicting. Some studies state that Levodopa improves balance performance in PwPD [22, 23], while others report a negative effect of Levodopa [24, 25]. For example, in a previous study Curtze et al. found that certain gait parameters like gait velocity and stride length can improve with Levodopa treatment, while indicators for balance like postural sway appeared to deteriorate. This could be attributable to differences in Levodopa responsiveness between the neutral circuits that control motor tasks such as gait and balance [26]. Ultimately, there is currently little to no consensus on the effects of Levodopa on balance during postural tasks and challenges specifically.
This systematic review with pilot meta-analysis aims to address these seemingly conflicting results regarding the effects of oral Levodopa intake on balance performance in idiopathic PwPD by providing a concise and comprehensive overview of the existing literature. To the best of our knowledge such a systematic review has not been published yet.
METHODS
Protocol and registration
This systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [27]. The review protocol was specified in advance and registered in PROSPERO (No. CRD42020212269).
Eligibility criteria
Eligibility criteria were defined by two authors (T.L., N.L.) and structured according to the Population Intervention Comparison Outcome (PICO) method (Supplementary Table 1) [27]. Peer reviewed original research studies written in English, French, or Dutch and published in internationally peer-reviewed journals that compared the effect of oral Levodopa intake (ON-medication state) with no medication intake (OFF-medication state) on balance performance in adults (older than 18 years) with idiopathic PD were considered for inclusion.
Case studies, review studies, meta-analyses, study protocols, conference abstracts, editorials, books, and letters were excluded. Furthermore, studies were excluded if they included individuals with secondary, juvenile, or atypical PD, persons who had surgical management of PD (e.g., deep brain stimulation or pallidotomy), persons with concurrent neurological or neurodegenerative disorders other than PD and individuals with diseases affecting balance performance not related to PD (e.g., vestibular disorders). Studies that reported the use of medication other than Levodopa were excluded as well. If the exclusive use of oral Levodopa was not clearly stated in the study, the corresponding author was contacted for confirmation (see below).
Information sources and search
Two review authors (T.L., N.L.) developed the search strategy using MeSH terms and keywords related to PD, Levodopa, and balance performance (Supplementary Table 2). Three electronic databases (Pubmed [Medline], Embase, and Web of Science) were searched to identify relevant studies published until June 28, 2022. In addition, reference lists of relevant systematic reviews (PROSPERO) and eligible or ongoing (https://www.clinicaltrials.gov https://www.clinicaltrials.gov/trials.gov/, https://apps.who.int/trialsearch) studies were searched for potential missed citations, using the keywords reported in Supplementary Table 2.
Study selection
After removal of duplicates using Endnote and Rayyan QCRI software, all remaining titles and abstracts were scanned for eligibility by three review authors (T.L., N.L., R.B.) in a blinded and standardized way using Rayyan QCRI software. Discrepancies were discussed and resolved by consensus.
Next, the same authors independently screened the full texts of the remaining references to check eligibility. Discrepancies were discussed and resolved by consensus.
Data collection process
A data collection form was designed according to the Cochrane Handbook for Systematic Reviews of Interventions version 6.1 [28]. Next, the data collection table was pilot-tested on three randomly selected studies and revised (T.L. and N.L.) before implementation. Finally, relevant data were extracted to complete the table with: 1) characteristics of the participants (e.g., UPDRS, Hoehn and Yahr scale [H&Y], disease duration), 2) intervention type (e.g., usual Levodopa dose, Levodopa intervention dose, time between OFF and ON), 3) outcome measures including posturography, step initiation parameters, responses to perturbation and clinical test batteries.
For eight original research papers authors were contacted by mail to request additional information to determine eligibility [29–36] (e.g., clarification of medication status). A reminder to provide the requested information was sent to the author and the head of the research group (if available) at one and two months after the initial request. Subsequently, four authors provided supplementary information [32, 33, 35, 36]. Data from the remaining four studies for which no response was received were excluded from the systematic review. Additionally, for some studies that were eligible according to our primary criteria, there was insufficient data to be included in the meta-analyses (e.g., only correlations reported). The results from these studies were subsequently moved to the residuals tables (see Results for details) and compared with the main results from the meta-analyses separately.
Quality assessment of included studies
Quality assessment of the selected studies was performed independently by two review authors (T.L. and R.B.) using the ‘NIH quality assessment tool for before-after (Pre-Post) study without control group’ [37]. This tool was adapted to the design of the selected studies by removing two parts of the questionnaire. Question nine was eliminated because all studies were performed on the same day without loss of participants. Question twelve was removed because the eligible studies could not include a group level intervention. This resulted in a possible scoring range from 0 to 10. The applied NIH quality assessment tool does not provide thresholds or guidelines to establish the overall quality of before-after studies. Therefore, only the objective scores are reported.
Summary measures and methods of analysis
Analysis of the outcome data from the selected studies was done in two different ways. First, all outcome data reported as mean (standard deviation [SD]) per study were imported in RevMan5 (5.4.1) to perform a pilot meta-analysis. These data were compared using standardized mean differences (SMD) in a random effects inverse variance weighted model. In this model, heterogeneity is calculated with an I2 test, which describes the percentage of the variance explained by the heterogeneity across studies rather than chance, with respect to the direction of the difference. In this model, negative scores favor Levodopa ON state and positive scores favor OFF state to reflect the inverse relationship between scores and improved balance for most of the outcomes. However, where necessary, some scores (e.g., clinical balance test scores) were multiplied by –1 to enable uniform reporting across all tables and figures. Additionally, and if needed, calculations were made to convert other statistical measures (e.g., median and range or standard error of the mean) into mean and standard deviation [38].
Second, studies that reported relevant data in graphs without giving exact mean and standard deviation values, were analyzed in a narrative way in the results section, listed in Tables 1 to 4, and compared with the results of the meta-analysis.
Table 1
Effect of Levodopa on clinical balance tests in people with idiopathic Parkinson’s disease residual outcome table
| Citation | Outcome parameter | Results | Effect on balance (mean difference) | Similar to meta-analysis |
| Nova 2004 [47] | Berg Balance Scale (BBS) | Significantly better Berg Balance Scale (BBS) in the ON state compared to the OFF state (p < 0.05). | +(10.9) | Yes |
| Franzén 2009 [44] | 180° turn (s) | Significantly better 180° turning in the ON state compared to the OFF state (p = 0.0118). | +(0.3) | ND |
| 360° turn (s) | No significant difference between ON and OFF state. | NS | ND | |
| Functional Reach Test (FRT) | No significant difference between ON and OFF state. | NS | ND |
ND, Not determined; NS, not significant.
Results of the data analyses were structured according to the method used to assess balance performance and divided into four groups: clinical tests, posturography, step initiation, and response to perturbation measurements. Sub-divisions were made according to their main defining characteristic (e.g., type of clinical test, based on medio-lateral or anterior-posterior sway). After final grouping into sub-categories, if there was more than one outcome parameter reported per population, the outcome with the most similar characteristics to the outcomes reported in other articles was maintained. The other outcomes were removed from the meta-analysis and reported in the residual outcome tables to avoid bias from non-independent effect sizes. In cases where the same outcome was reported within one article but for different populations (e.g., Hoehn & Yahr II vs. III-IV or fallers vs. non-fallers), data from both populations were included in the meta-analysis. Subsequently, the SMD was calculated with a 95% confidence interval (CI) and if the 95% CI did not cross zero, the result was classified as significant (p < 0.05). Heterogeneity of the results across grouped outcome parameters was determined by using χ2 and its degrees of freedom (df) to determine the index of heterogeneity (I2, <40% = low, <60% = moderate, <90% = substantial and≤100% = considerable heterogeneity) [28]. In accordance with Cochrane guidelines regarding the low number of studies for certain outcome measures and the relatively low number of participants in several studies, the level of significance for the χ2 test was set at p = 0.10 and used to determine the strength of evidence for heterogeneity [28].
RESULTS
Study selection
A total of 4,479 studies were retrieved. After removal of 1,707 duplicates, 2,772 unique studies were screened on title and abstract. Overall, 2,669 studies were excluded, leaving 103 studies for full text screening. Finally, all studies that did not meet the pre-determined eligibility criteria (e.g., intervention studies using medication other than Levodopa alone, studies including non-idiopathic PwPD) were excluded, resulting in a final selection of 18 studies for quantitative and qualitative analysis. The PRISMA flow chart is shown as Supplementary Fig. 1 [27].
In total, data from 710 PwPD (481 men, 223 women, and 6 unknown), with the number of participants per study ranging from 6 to 140 (pooled mean of 39.4 and median of 20.5 participants per study), and with a pooled mean age of 64.3 years (range 58.8–69.2), were included in this review. The disease duration ranged from 6.5 to 13.0 years with a pooled mean of 8.7 years. Finally, H&Y stage ranged from 1 (unilateral PD symptoms only) to 4 (balance impairment, moderate bilateral disease but physically independent), with a pooled mean of 2.4. Demographic data of individual studies are presented in Supplementary Table 3.
The results of the quality appraisal of all selected studies are displayed in Supplementary Table 4. Overall study quality scored 8 in four studies [22, 24–26] and below 8 in fourteen studies [23, 35, 47–50, 39–46]. No study scored above 8. The generally high risk of bias (e.g., selection bias, information bias, and confounding bias) was primarily the result of the observational design of the included studies. Furthermore, only one of the studies [41] was performed with researchers blinded to medication status of the participants and most of the studies had a relatively small sample size (median sample size = 20.5, Q1 = 12.5; Q3 = 73), which resulted in a low statistical power.
Synthesis of results
Clinical balance tests
Seven types of clinical balance tests were reported in seven different studies (323 PwPD) [22, 23, 41, 42, 44, 46, 50] of which five were included in this meta-analysis (Fig. 1). For descriptions and calculations of the individual outcomes per study, see Supplementary Table 5. It should be noted that, data from the Berg Balance Scale (BBS) of one study [47] was incomplete, while for the 180° and 360° turn and the FRT [44] only data from one population was available. Results from these studies were therefore left out of the meta-analysis.
Fig. 1
Effect of Levodopa on clinical balance tests in people with idiopathic Parkinson’s disease.
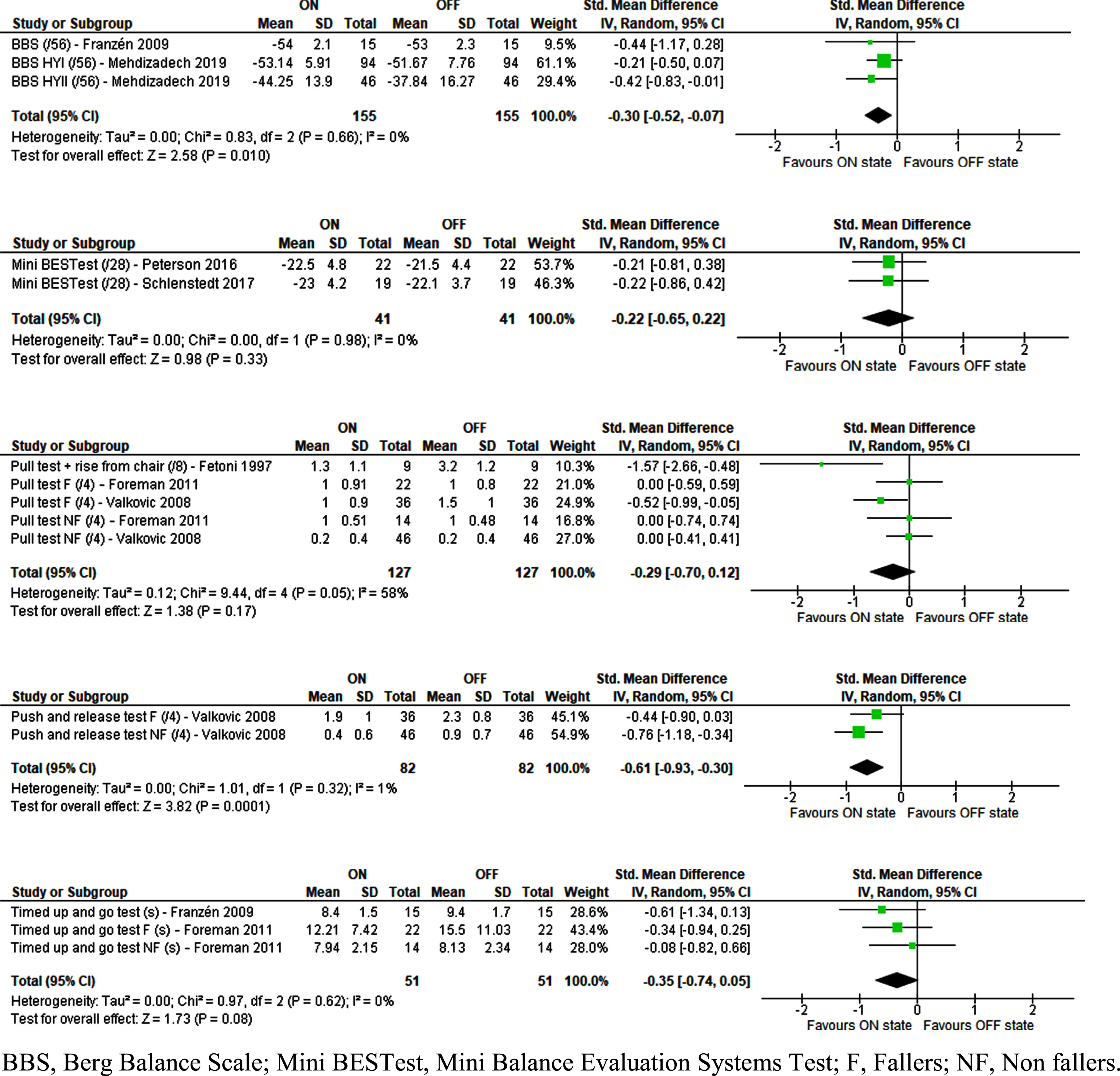
Levodopa caused a significant improvement in performance for the Push and Release Test (PRT) (SMD = –0.61 [–0.93;–0.30], Z = 3.82 [p < 0.001], I2 = 1% [p = 0.32]) and in the BBS (SMD = –0.30 [–0.52;–0.07], Z = 2.58 [p = 0.01], I2 = 0% [p = 0.66]). Nova et al. found similar results concerning the BBS [47], but these could not be included in the meta-analysis due to missing SDs and were reported separately as residual outcomes (Table 1).
All other clinical balance tests, including the Mini Balance Evaluation System Test, the Pull Test, and the Timed Up and Go test showed a trend towards improvement with Levodopa but these differences were not statistically significant. This was similar for the residual outcomes of the 360° turn and the FRT but the 180° turn showed significant improvement with levodopa in these individual studies.
Posturography
Posturography data were reported in four different studies (285 PwPD) [24, 26, 35, 39] and (partially) included in the meta-analysis (Fig. 2). Unfortunately, results from two studies, one with a standing posturography [24] and another with a rise to toes task [43], could not be included in the meta-analysis as only correlations between variables were reported and posturography data were lacking (reported as residual outcomes in Table 2). For descriptions and calculations of the individual outcomes per study, see Supplementary Table 5.
Fig. 2
Effect of Levodopa on posturography parameters in people with idiopathic Parkinson’s disease.
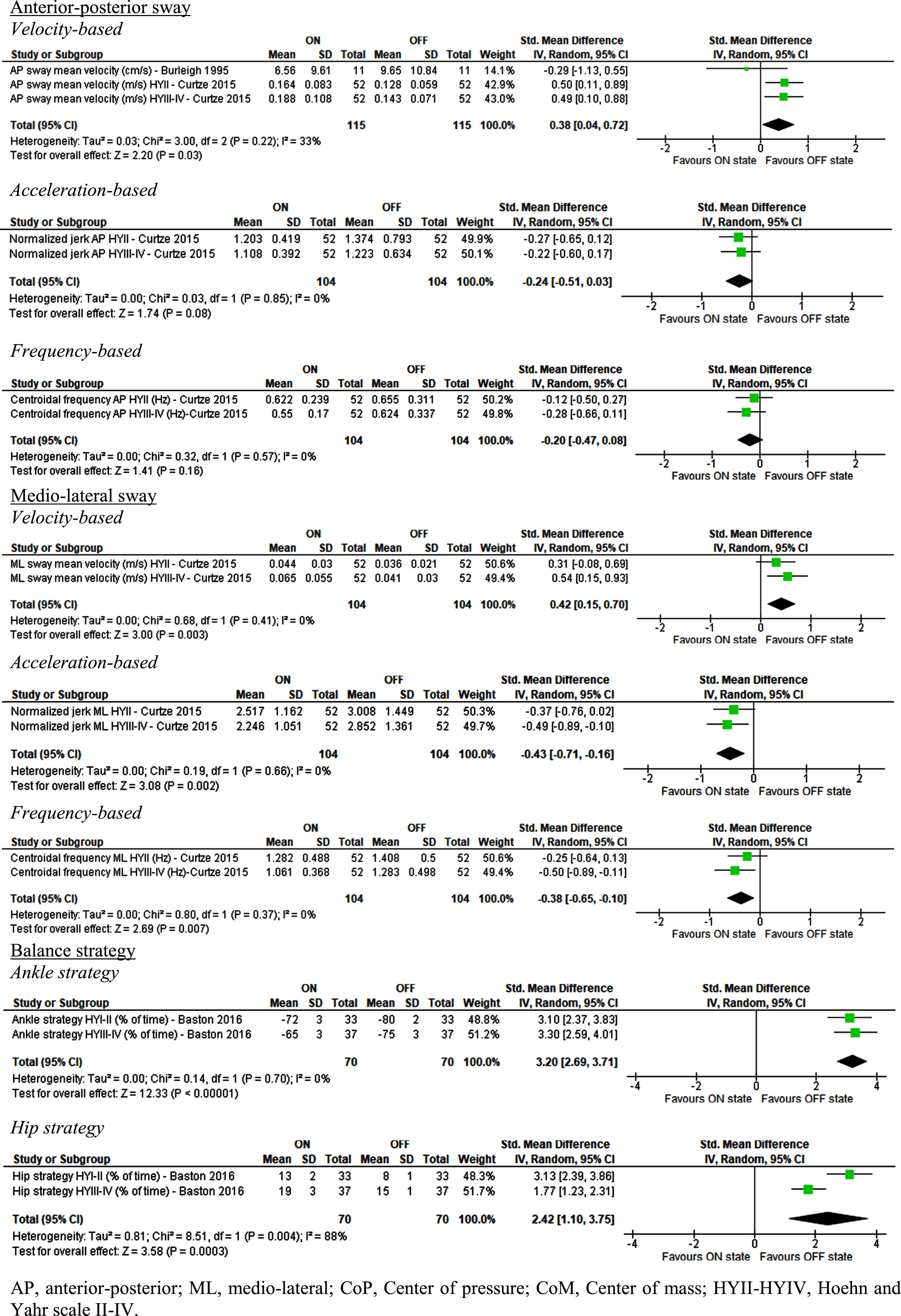
Table 2
Effect of Levodopa on posturography parameters in people with idiopathic Parkinson’s disease residual outcome table
| Citation | Outcome parameter | Result | Effect on balance (mean difference) | Similar to meta-analysis |
| AP sway | ||||
| Baston 2016 [24] | RMS AP sway CoP (m/s2) | Significantly larger RMS AP sway in the ON state compared to the OFF state (p = <0.05). | –(NR) | Yes |
| Burleigh 1995 [39] | AP sway mean amplitude (cm) | No significant difference between ON and OFF state. | NS | No |
| AP sway pathlength (cm) | No significant difference between ON and OFF state. | NS | No | |
| SD of AP COP sway amplitude | No significant difference between ON and OFF state. | NS | No | |
| Curtze 2015 [26]a | AP sway RMS velocity (m/s2) | Significantly faster AP sway RMS velocity in the ON state compared to the OFF state (p < 0.001). | –(NR) | Yes |
| Frequency dispersion AP | No significant difference between ON and OFF state. | NS | Yes | |
| Frank 2000 [43]b | Peak CoP AP displacement (cm) | No significant difference between ON and OFF state. | NS | Yes |
| Peak CoM AP displacement (cm) | No significant difference between ON and OFF state. | NS | Yes | |
| ML sway | ||||
| Curtze 2015 [26]a | ML sway RMS velocity (m/s2) | Significantly higher ML sway RMS velocity in the ON state compared to the OFF state (p = 0.008). | –(NR) | Yes |
| Frequency dispersion ML | No significant difference between ON and OFF state. | NS | No | |
| Combined sway | ||||
| Horak 2016 [35] | Acceleration range (m/s2) | Significantly higher acceleration rate in the ON state compared to the OFF state (p = 0.006). | –(0.061) | ND |
| CoM acceleration range (m) | Significantly larger CoM acceleration range in the ON state compared to the OFF state (p = 0.006). | –(0.006) | ND | |
| Jerk (m2/s5) | No significant difference between ON and OFF state. | NS | ND | |
| Mean frequency of sway (Hz) | Significantly lower mean frequency of sway in the ON state compared to the OFF state (p < 0.001). | +(0.17) | ND | |
| RMS of sway mean distance (m/s2) | Significantly larger RMS of sway mean distance in the ON state compared to the OFF state (p = 0.001). | –(0.013) | ND | |
| Sway area (mm2/s) | Significantly larger sway area in the ON state compared to the OFF state (p < 0.012). | –(0.028) | ND | |
| Sway mean amplitude (m/s2) | Significantly larger sway mean amplitude in the ON state compared to the OFF state (p = 0.001). | –(0.012) | ND | |
| Sway pathlength (m/s2) | No significant difference between ON and OFF state. | NS | ND | |
| Sway RMS of CoM (m) | Significantly larger sway RMS of CoM in the ON state compared to the OFF state (p = 0.001). | –(0.0011) | ND | |
| Others | ||||
| Frank 2000 [43]b | Onset of gastrocnemius contraction (ms) | Significantly earlier Gastrocnemius contraction in the ON state compared to the OFF state (p < 0.05). | +(28) | ND |
| Onset of tibialis anterior contraction (ms) | No significant difference between ON and OFF state. | NS | ND | |
| Time to reach peak CoP (ms) | No significant difference between ON and OFF state. | NS | ND | |
| Dorsiflexor torque | Significantly greater dorsiflexor torque in the ON state compared with OFF state (p < 0.05). | +(40) | ND |
AP, anterior-posterior; CoM, Center of mass; CoP, Center of pressure; NR, not reported; ND, not determined; NS, not significant; RMS, root mean square. aCurtze et al. only reported p values for a combination of groups (HYII and III/IV). The combined result and p value is displayed. b Frank et al. performed a rise to toes posturography.
The meta-analysis shows that Levodopa had statistically significant negative effects on mean sway velocity in both anterior-posterior (AP, SMD = 0.38 [0.04; 0.72], Z = 2.2 [p = 0.03], I2 = 33% [p = 0.22]) and medio-lateral (ML, SMD = 0.42 [0.15; 0.70], Z = 3 [p = 0.003], I2 = 0% [p = 0.41]) directions. These findings are in line with a number of findings from the residual outcomes which reported statistically significant larger AP sway and generally increased sway parameters for combined sway when PwPD were ON medication [24, 35].
Conversely, jerk (rate of change in acceleration) and centroidal frequency in ML direction were positively affected by Levodopa (SMD = –0.43 [–0.71;–0.16], Z = 3.08 [p = 0.002], I2 = 0% [p = 0.66] and SMD = –0.38 [–0.65;–0.10], Z = 2.69 [p = 0.007], I2 = 0% [p = 0.37] respectively). The residuals showed that for combined sway the mean sway frequency was also lower but no significant changes were found for jerk [35].
The meta-analysis also revealed that Levodopa caused a significant negative change in balance strategy, with a reduction in ankle strategy (SMD = 3.20 [2.69; 3.71], Z = 12.33 [p < 0.001], I2 = 0% [p = 0.70] and an increase in hip strategy (SMD = 2.42 [1.10; 3.75], Z = 3.58 [p < 0.001], I2 = 88% [p = 0.004), which could have a negative effect on balance.
None of the remaining outcomes extracted from posturography were significantly different between Levodopa ON and OFF state.
Step initiation
Six studies [26, 35, 40, 48–50] (266 PwPD) reported step initiation data (Fig. 3). However, not all data were entered into the meta-analysis as some studies were explorative in nature and reported several outcomes per population. In addition, one study by Burleigh et al. primarily reported results that could not be included in the meta-analysis due to a lack of quantifiable data as these were only represented visually (for all these, see residual data in Table 3) [40]. For descriptions and calculations of the individual outcomes per study, see Supplementary Table 5.
Fig. 3
Effect of Levodopa on step initiation parameters in people with idiopathic Parkinson’s disease.
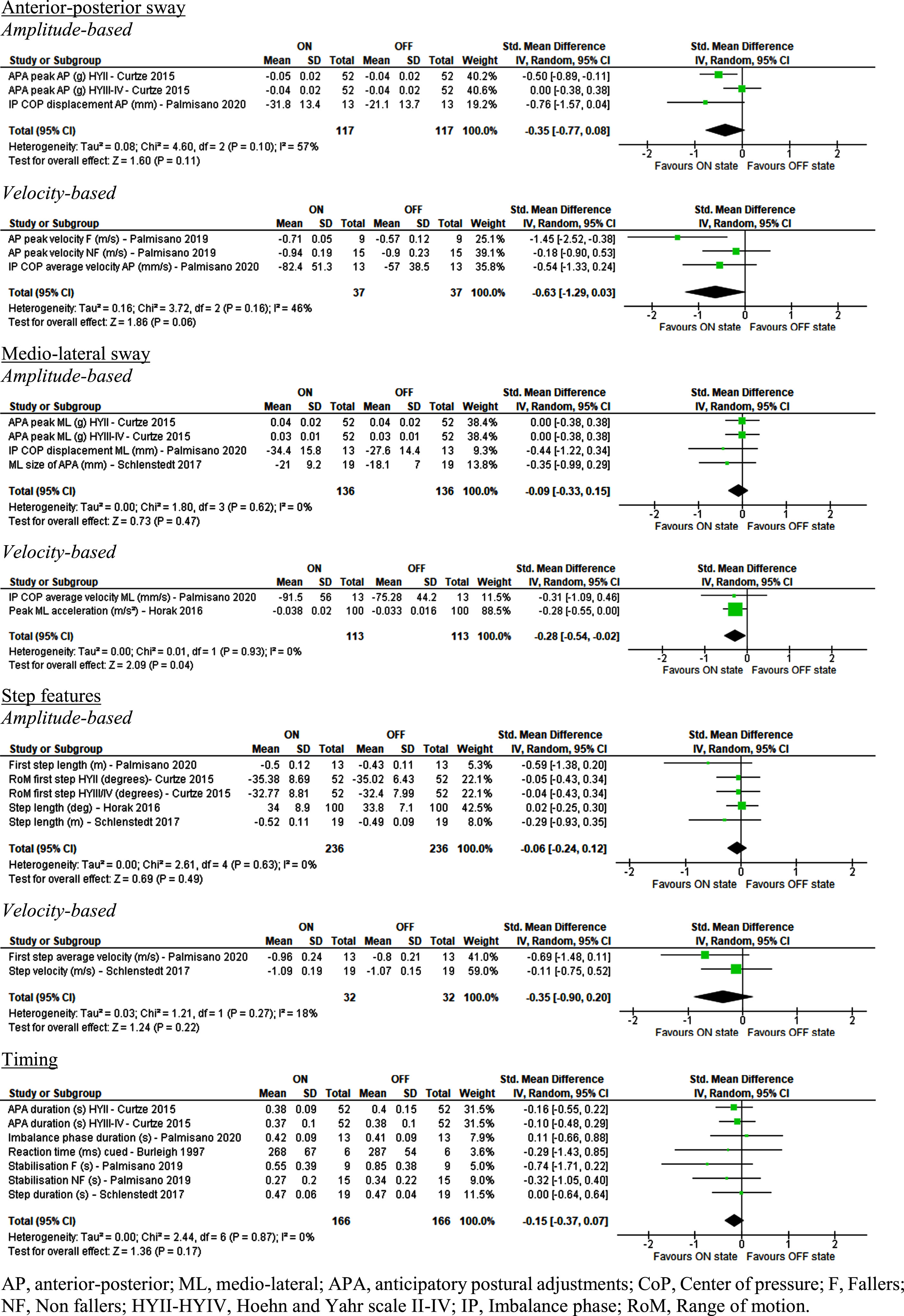
Table 3
Effect of Levodopa on step initiation parameters in people with idiopathic Parkinson’s disease residual outcome table
| Citation | Outcome parameter | Results | Effect on balance (mean difference) | Similar to meta-analysis |
| AP sway | ||||
| Burleigh 1997 [40] | AP sway CoM velocity (cm/s) – self initiated | No significant difference between ON and OFF state. | NS | Yes |
| AP sway CoM velocity (cm/s) – response to cue | No significant difference between ON and OFF state. | NS | Yes | |
| Palmisano 2019 [48] | Seat off velocity AP (m/s) F | No significant difference between ON and OFF state. (P = NR) | NS | Yes |
| Seat off velocity AP (m/s) NF | No significant difference between ON and OFF state. (P = NR) | NS | Yes | |
| Palmisano 2020 [49] | IP CoP maximal velocity AP (mm/s) | Significantly higher IP CoP maximal velocity in the ON state compared to the OFF state (p < 0.05). | –(10.7) | No |
| UP CoP average velocity (mm/s) | No significant difference between ON and OFF state. | NS | Yes | |
| UP CoP displacement AP (mm) | No significant difference between ON and OFF state. | NS | Yes | |
| UP CoP maximal velocity AP (mm/s) | No significant difference between ON and OFF state. | NS | Yes | |
| ML sway | ||||
| Palmisano 2020 [49] | IP CoP maximal velocity ML (mm/s) | No significant difference between ON and OFF state. | NS | No |
| Step features | ||||
| Burleigh 1997 [40] | Step length (cm) – response to cue | No significant difference between ON and OFF state. | NS | Yes |
| Timing | ||||
| Burleigh 1997 [40] | Anticipation phase duration (ms) – self initiated | A significant reduction in duration of the anticipation phase was observed (p = 0.004) in the ON state compared to the OFF state. | +(NR) | No |
| Push off phase duration (ms) – self initiated | A significant reduction in duration of the push off phase was observed (p = 0.03) in the ON state compared to the OFF state. | +(NR) | No | |
| Reaction time phase (ms) – response to cue | No significant difference between ON and OFF state. | NS | Yes | |
| Anticipation phase duration (ms) – response to cue | No significant difference between ON and OFF state. | NS | Yes | |
| Push off phase duration (ms) – response to cue | No significant difference between ON and OFF state. | NS | Yes | |
| Curtze 2015 [26]a | APA first step duration (s) | No significant difference between ON and OFF state. | NS | Yes |
| APA latency (s) | No significant difference between ON and OFF state. | NS | Yes | |
| Palmisano 2020 [49] | Unloading phase duration (s) | No significant difference between ON and OFF state. | NS | Yes |
AP, anterior-posterior; CoM, Center of mass; CoP, Center of pressure; NS, not significant; NR, not reported; IP, imbalance phase; UP, unloading phase. a Curtze et al. only reported p values for a combination of groups (HYII and III/IV). The combined result and p value is displayed.
Of all the outcomes reported for step initiation only velocity-based outcomes in ML direction showed a small but marginally statistically significant effect favoring Levodopa ON state (SMD = –0.28 [–0.54; –0.02], Z = 2.09 [p = 0.04], I2 = 0% [p = 0.93]).
None of the other sway-based outcomes, step features, or timing parameters revealed any significant difference between ON and OFF Levodopa state. The results for most of the timing parameters were supported by the residual findings [40]. Of the residual data, only the study of Burleigh et al. had contrasting findings with a significantly shorter anticipation phase duration in the ON state [40].
Response to perturbation
Five studies [22, 25, 40, 45, 50] (73 PwPD) reported various perturbation methods of which the outcome parameters are included in this meta-analysis (Fig. 4). Only one study in the meta-analysis reported on surface reactive torque responses. Three studies [25, 40, 45] reported parameters concerning response to perturbations that could not be included into the meta-analysis due to the lack of data and several results were moved to the residuals because they were obtained from the same population (residual data in Table 4). For descriptions and calculations of the individual outcomes per study, see Supplementary Table 5.
Fig. 4
Effect of Levodopa on perturbation parameters in people with idiopathic Parkinson’s disease.
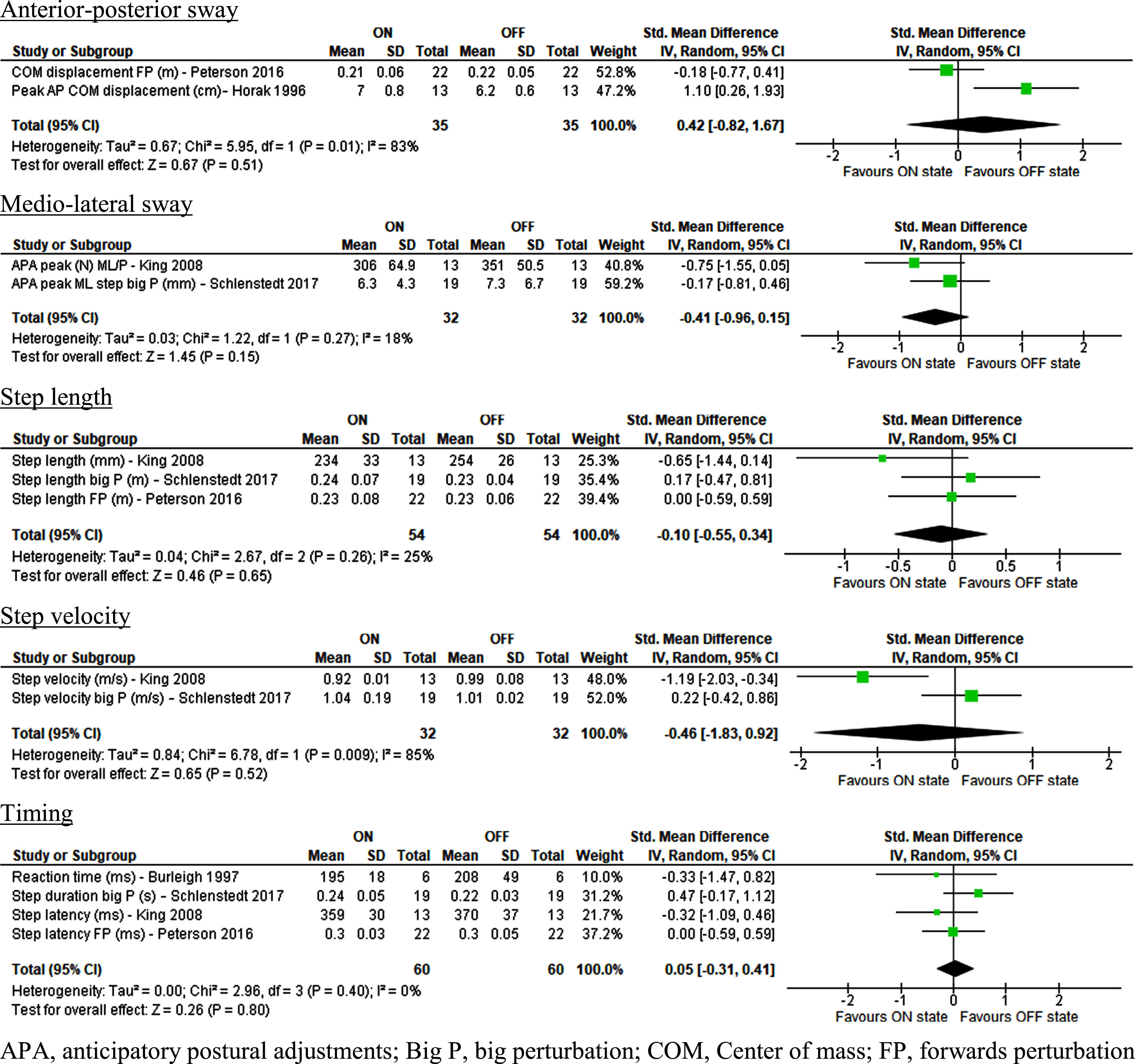
Table 4
Effect of Levodopa on perturbation parameters in people with idiopathic Parkinson’s disease residual outcome table
| Citation | Outcome parameter | Results | Effect on balance (mean difference) | Similar to meta-analysis |
| AP sway | ||||
| Burleigh 1997 [40] | AP sway CoM velocity (cm/s) | No significant difference between ON and OFF state. | NS | Yes |
| Horak 1996 [25] | CoM AP velocity (mm/s) | No significant difference between ON and OFF state. | NS | Yes |
| Peak AP COP displacement (cm) | No significant difference between ON and OFF state. | NS | Yes | |
| Peterson 2016 [22] | CoM AP displacement BP (m) | No significant difference between ON and OFF state. | NS | Yes |
| ML sway | ||||
| Schlenstedt 2017 [50] | APA peak ML step small P (mm) | No significant difference between ON and OFF state. | NS | Yes |
| Step length | ||||
| Burleigh 1997 [40] | Step length (cm) | No significant difference between ON and OFF state. | NS | Yes |
| Peterson 2016 [22] | Step length BP (m) | No significant difference between ON and OFF state. | NS | Yes |
| Schlenstedt 2017 [50] | Step length small P (m) | No significant difference between ON and OFF state. | NS | Yes |
| Step velocity | ||||
| Schlenstedt 2017 [50] | Step verlocity small P (m/s) | No significant difference between ON and OFF state. | NS | ND |
| Step strategy | ||||
| King 2008 [45] | Step strategy | No significant difference between ON and OFF state. | NS | ND |
| APA before side step (%) | No significant difference between ON and OFF state. | NS | ND | |
| Falls cross over strategy | No significant difference between ON and OFF state. | NS | ND | |
| Falls side step strategy | No significant difference between ON and OFF state. | NS | ND | |
| Number of steps | ||||
| Peterson 2016 [22] | Numer of steps BP | No significant difference between ON and OFF state. | NS | ND |
| Numer of steps FP | No significant difference between ON and OFF state. | NS | ND | |
| Margin of stability | ||||
| Peterson 2016 [22] | Margin of stability BP (m) | No significant difference between ON and OFF state. | NS | ND |
| Margin of stability FP (m) | No significant difference between ON and OFF state. | NS | ND | |
| Surface torque response | ||||
| Horak 1996 [25] | Slope of surface reactive torque | No significant difference between ON and OFF state. | NS | ND |
| Timing | ||||
| Burleigh 1997 [40] | Reaction time phase (ms) | No significant difference between ON and OFF state. | NS | Yes |
| Anticipation phase duration (ms) | No significant difference between ON and OFF state. | NS | Yes | |
| Push off phase duration (ms) | No significant difference between ON and OFF state. | NS | Yes | |
| Horak 1996 [25] | Time to reach peak CoP (ms) | No significant difference between ON and OFF state. | NS | Yes |
| King 2008 | APA latency (ms) | No significant difference between ON and OFF state. | NS | Yes |
| Peterson 2016 [22] | Step latency BP (ms) | No significant difference between ON and OFF state. | NS | Yes |
| Schlenstedt 2017 [50] | Step duration small P (s) | No significant difference between ON and OFF state. | NS | Yes |
AP, anterior-posterior; APA, anticipatory postural adjustments; BP, backwards perturbation; FP, forwards perturbation; CoM, Center of mass; CoP, Center of pressure; ND, not determined; NS, not significant.
No significant difference was found between ON and OFF Levodopa state regarding balance parameters during perturbations. This applies for anterior-posterior sway, falls, margin of stability, medio-lateral sway, and surface reactive torque response. Step parameters such as step length, timing, step velocity and the number of steps were not significantly different between ON and OFF states. Finally, no significant differences between ON and OFF states were reported in the residual data.
DISCUSSION
Primary results of the systematic review
The results of this systematic review and pilot meta-analyses indicate statistically significant positive effects of oral Levodopa on performance of common clinical balance tests such as the BBS and the PRT, as well as a positive but non-significant trend for all other clinical balance tests. In contrast, findings from posturography studies were not unequivocal, with a significant negative effect of Levodopa on balance strategy and velocity-based sway parameters but positive effects on jerk and some frequency parameters. Finally, Levodopa was not associated with a change of balance indicated by all other outcome parameters in this meta-analysis. These mixed results are likely attributable to the heterogeneity in methodology to test balance performance combined with a lack of sufficiently powered studies. In addition, these results underline the importance of differentiating the underlying balance components that are being addressed by each testing methodology and the need for a consensus on the optimal balance test battery for PwPD. It is important to note that, due to the limited number of study outcomes and sample sizes of some studies, the meta-analyses in this review are performed as a pilot to guide future studies and more comprehensive reviews.
Interpretation of results
Clinical balance tests
Clinical balance tests such as the BBS are commonly used and offer good validity [51], test-retest reliability [52] and fair predictability of (recurrent) fall episodes over a period of six or twelve months [53, 54]. Our meta-analysis revealed a statistically significant positive effect of Levodopa on BBS performance. However, improvements in mean BBS scores from OFF to ON state ranged from 1 to 6.4 [44, 46] points, thus appearing to be too small to be clinically relevant. Indeed, this increase is more or less the same as the minimal detectable change (MDC) of 5 points [52] and the minimal clinically important difference (MCID), which is 5 points in people with early subacute stroke [55]. However, a ceiling effect for the BBS is reported, especially in PwPD with little disease progression [56]. Because all included studies involving the BBS focused on people with a H&Y score between 2 and 3, the positive effects of Levodopa on BBS-performance could be limited. Consequently, larger changes in BBS could be expected when assessing the effects of Levodopa on static balance in PwPD with longer disease duration and progressive motordisability [57].
Another clinical test, the PRT, showed significant positive effects of Levodopa as well, although this test type was reported in only one study with two subgroups [23]. The clinical importance of this improvement is still unknown and further research for this novel test is needed. Nevertheless, findings from this study are promising as the PRT was shown to be more sensitive in both ON and OFF states [23, 58] and consistently performed and correlated better with self-reported falls than the more commonly performed pull test [58].
Posturography
Levodopa is reported to induce a significant shift from ankle to hip strategy so that balance is preserved by reduced movement around the ankle joints and increased movement around the hip joints [59]. This shift in strategy was interpreted as a negative effect of Levodopa, because predominant use of hip strategies is a more conservative strategy, and associated with the decreased balance control of elderly people [60–62] and increased sway during quiet stance [63]. The increase of sway and sway velocity associated with altered balance strategy in ON state is in line with higher combined sway parameters, which were only reported by Horak et al. [35] and could therefore not be included in the meta-analysis. However, these findings are not supported by other studies that only analyzed a single sway direction or split analyses over AP and ML directions despite similar Hoehn & Yahr scores of their populations [26, 39]. This suggests that measures obtained from combined sway directions may be more sensitive to effects of Levodopa on balance during quiet stance. Ultimately, the relatively high heterogeneity of all grouped outcome parameters for posturography indicate that these results should be interpreted with care. Nevertheless, Okada et al. suggested that a shift from an ankle strategy to a hip strategy compensates the deterioration of processing sensory information [64] and fear of falling by reducing the forward translation of the center of mass [65]. The practical use of testing balance strategy, including the meaning of the Levodopa effect, remains unclear as a shift between ankle and hip strategies could be both the cause or the consequence of impaired postural balance.
Step initiation
The meta-analysis only showed a minor significantly positive effect on in the form of velocity-based sway in ML direction, a general trend towards increased sway can be seen. Although increased excursions of the body’s center of mass as a positive indicator for improved balance may seem counter-intuitive, this increase of sway can be considered positive as the magnitude of lateral anticipatory postural adjustments (APAs) during self-initiated stepping is higher in healthy controls compared to PwPD [62]. This is contrary to an increase of anterior-posterior sway in quiet standing posturography and perturbation conditions, which is correlated with disease severity and falls [66, 67]. The increased magnitude of spatial characteristics such as APAs during step initiation is in line with previous findings on balance [62] and could be relevant as it is a sign of reduced bradykinesia as well as reduced step initiation problems, which are frequent and related to freezing of gate (FoG) in PwPD [68, 69]. Similar positive effects of Levodopa have been reported on improved spatial characteristics (e.g., increased step length, stride velocity) during normal gait, while temporal characteristics (e.g., cadence) showed little to no improvements [70]. Further research is needed to examine the exact effect of the increase in both anterior-posterior and medio-lateral sway parameters during step initiation on balance and FoG.
Response to perturbation
None of the outcome parameters that assessed responses to perturbations showed significant effects of Levodopa. Although the methodology to assess responses to perturbations varies widely, the most common approaches involve the spatio-temporal aspects of the step response. Despite the known positive effects of Levodopa on bradykinesia [71] and rigidity [72], no effects were reported on any of the parameters related to spatial and temporal characteristics of the motor responses. This can be explained by the role of typically reduced automaticity of motor responses and increase reliance on slower cognitive strategies (e.g., vision and proprioception) to maintain balance in PwPD [73]. Moreover, these results are in line with findings from a study by Workman and Thrasher, who found that Levodopa did not improve automaticity [74].
Complexity of measuring balance in PD
The major obstacle for this review and balance research in general is the fact that balance is a complex, multi-dimensional function. As such, the definition of balance in the context of human locomotion varies and researchers tend to focus on single dimensions and associated methodologies, depending on their goals. As a result, a wide variety of measurement methods are used in concurrent studies to assess balance performance, potentially leading to high heterogeneity of the results as observed for the outcome parameters obtained from posturography. Even though it is crucial to avoid falls during everyday locomotor tasks, this heterogeneity makes it hard to quantify and compare (deficits in) balance. Nevertheless, there are some scales and parameters that are used frequently among balance researchers (e.g., BBS, TUG, sway parameters).
The four groups of outcome parameters defined within this review each have intrinsic strengths and limitations. In analyzing the results of this review, the struggle researchers experience in measuring balance was underlined. Clinical balance test are often subjective, sensitive to observer bias, and difficult to perform in an unblinded way (e.g., pull test) [58, 75]. However, these tests do provide an initial impression of one’s postural balance performance without the need of expensive equipment required to perform posturography or camera-based three-dimensional balance analysis. On the other hand, objective outcome measures are the most sensitive way to measure the effect of interventions on balance [76].
The selection of appropriate outcome measures is especially difficult in PD. Several disease-related factors may confound the measurements. As stated before, PD is associated with reduced automaticity [73], thus increasing the need for cognitive balance strategies which rely on input from the vestibular, visual and somatosensory systems. However, these systems can simultaneously be affected in PD. For example, vestibular function is reported to be affected more severely by PD compared to other forms of sensory input [77]. This may compound the effects of reduced automaticity during a perturbation task, likely skewing results for studies with a relatively large number of late-stage PwPD. Similarly, patients with impaired visual input may show relatively poorer performance during dual-tasking conditions. All these factors indicate the need for sufficiently sized and well-controlled studies involving PwPD.
Limitations of this systematic review and meta-analysis
Apart from the aforementioned general limitations and pitfalls in assessing balance in PwPD, the authors acknowledge some additional inherent limitations to this systematic review and meta-analysis.
Firstly, care should be taken when interpreting the results of this systematic review for specific patient subgroups. Due to the inherent heterogeneity of disease progression with regards to deterioration of cognitive and motor function, and various alternative treatments available, several exclusion criteria were applied. Studies including PwPD undergoing or waiting for deep brain stimulation (DBS) were excluded from this review as in those cases it would have been impossible to distinguish Levodopa-mediated effects from the effects of DBS [78, 79]. In addition, task conditions (e.g., dual-tasking) and interactions with other PD related symptoms (e.g., dyskinesias, FoG) may have implications for the generalizability of our findings. On the one hand, many studies listed symptoms such as dyskinesias [42, 49], orthostatic hypotension [48, 49], or cognitive impairment [23, 41, 46, 48, 49] as exclusion criteria. On the other hand, not all studies that include PwPD report the presence of these symptoms. Therefore, it is hard to determine to what extent the results of this review are affected by these symptoms. All these factors can have negative effects on balance performance and potentially occlude positive effects achieved by Levodopa administration. For example, previous research has reported links between FoG and balance [4]. However, the implications of the presence of FoG on balance are not well known as FoG is difficult to evoke and assess in clinical or experimental conditions [80]. Moreover, the inclusion of data from freezers does not necessarily affect study outcomes [50], therefore no distinction was made between data from freezers and non-freezers in this pilot meta-analysis. Another example is the presence of dyskinesias, which can have a major impact on balance in PwPD since they occur in more than 50% of PwPD after 5 years of treatment [10] and in almost all PwPD after 15 years [81]. This is reflected by evidence from postural tasks showing higher postural sway [26, 81] and corresponding postural instability in patients that present dyskinesias [26, 82, 83]. Furthermore, a significant association between dyskinesias and falling is reported in large cohort studies [83, 84]. By excluding PwPD experiencing debilitating dyskinesias, the external validity of the results reported in the selected studies is reduced and care should be taken when translating these findings to late-stage PwPD. Finally, although cognitive impairment and orthostatic hypotension are common in PwPD [85, 86] and a crucial factor in balance and falling [11, 87], they were common exclusion criteria in some eligible studies [23, 41, 46, 48, 49]. Nevertheless, the effects of these factors should not be overlooked in future research as they are considered crucial for the treatment and management of falls in PwPD by the NPF (National Parkinson Foundation) task force [88]. This review only included pilot meta-analyses due to the limited number of studies included per meta-analysis of the separate categories and limited sample sizes for several studies. Consequently, it was not possible to properly assess possible funnel plot asymmetry. Since this method should only be used when there are at least 10 studies included in the meta-analysis [28], no funnel plots were included in this manuscript.
Additional limitations are apparent from the quality assessment (Supplementary Table 4) and include the unblinded nature and the relatively small sample sizes (median = 21.5) of many studies included in this review, resulting in limited statistical power [89]. Furthermore, eight studies [23, 39–42, 44, 46, 47] did not perform multiple trials per outcome despite the low reliability of results from single test protocols [90]. Two other studies with similar patient samples [25, 43] reported one patient “frozen”, and thus not able to stand or sit in OFF state while another study [47] reported problems in reaching a true OFF state in two patients. This could have resulted in skewed outcomes in favor of OFF state and a decrease of the observed positive effects of Levodopa on balance. Also, no RCT or prospective studies about the effect of oral Levodopa monotherapy on balance in PwPD, comparing ON vs. OFF state, were found. However, despite the possible fundamental insights long-term controlled studies can provide, the clinical relevance of such studies is questionable as Levodopa therapy is standard clinical practice. Therefore, it is unlikely and possibly unethical for Levodopa to be withheld in the long-term.
Finally, although most studies included a (variable) wash-out period of all dopaminergic medication, an influence of other (dopaminergic) medication than Levodopa cannot be ruled out. Apart from that, NICE guidelines from 2017 suggest Levodopa as monotherapy for motor symptoms only for early-stage PwPD. As PD progresses, other medication types like mono amino oxidase B (MAO-B) inhibitors and dopamine agonist, depending on the specific symptoms of the patients, are suggested [91]. However, the aim of this review and pilot meta-analysis was to focus on the effects of Levodopa alone as a starting point for future studies as there is still little to no consensus on the effects of Levodopa monotherapy on balance. The difficulties in reaching a first conclusion on these effects would only have been compounded by the various mediating effects of the various combinations of antiparkinsonian medication regimens. Nevertheless, it is important to acknowledge that this limits the ecological validity of these initial results for PwPD and clinicians and to highlight the need to expand on these findings in future studies.
Further research
To the best of our knowledge, this is the first systematic review and meta-analysis assessing the effects of oral Levodopa on balance in PwPD.
A major threshold to definitively establish not only the significance, but also the clinically relevant effects of Levodopa on balance in PwPD, is the lack of sufficiently powered studies with a low risk of bias. As such, future studies should aim to include larger sample sizes and ensure proper blinding of assessors to medication status. However, blinding to medication status may only be relevant in early-stage PwPD where motor symptoms are not as discernible. In late-stage PwPD unblinding due to the presence or absence of obvious motor symptoms is likely inevitable. Regarding the aforementioned research complexity, a standardized multimodal way to measure balance should be established to be able to mutually compare clinical studies [51, 92–94]. For example, within the included studies, several standard balance outcomes were slightly modified (e.g., rise to toes during posturography [43] or pull test with rising from a chair [41]). Although these choices were likely made to improve the differentiation between PwPD and healthy controls, they do add to the heterogeneity of the generally applied methodology, which makes it difficult to compare them with other studies. This recommendation to use standardized methods does not only apply to research of balance in PwPD but to all balance research.
Various authors have tried to find the ideal combination of tests to measure balance and predict future falls [51, 95–97]. For example, Latt et al. found FoG and abnormal axial posture to be strong PD-specific predictors of fall risk [97]. However, As mentioned before, FoG can be hard to assess in clinical and research settings [80], therefore it might be easier to assess the axial posture as a predictor of fall risk in PwPD. Abnormal axial posture can easily be assessed and can affect balance by reducing the limits of stability and may cause abnormal postural reflexes [97], but was not assessed in any of the studies included in this review. Based on the findings from this review, we suggest using either the BBS, as a more extensive clinical test with a substantial body of evidence, and/or the novel PRT, which is relatively short and easy to administer, and sensitive for both fallers and non-fallers [23]. Adding to this posturography data, whether measured with wearable sensors or not, during standing and as response to standardized perturbations, would give researchers and clinicians a good view on their patients’ balance performance. The disadvantage to this multimodal research strategy is the increase in time and resources needed. To avoid a decrease in applicability, a stepwise approach could be useful, by starting with the PRT and performing more tests when needed and affordable. Moreover, using less expensive and time-consuming wearable sensors to measure balance (and gait) and the effects of interventions in daily life conditions appears to be the most feasible choice [89, 98]. The measured variables from these wearable sensors consist of a combination of standing posturography variables (e.g., accelerations and sway), walking stability (e.g., step features) and even clinical balance tests (e.g., TUG, Rombergtest) [98].
Conclusion
Although Levodopa is widely used by idiopathic PwPD and some significant positive effects and trends are reported, the available literature offers no consensus regarding the effects of Levodopa on balance. Nevertheless, the results of this review and pilot meta-analysis indicate that oral Levodopa tends to improve performance as measured by clinical balance scales and shows a positive trend towards increased anterior-posterior and mediolateral sway during step initiation. Results from posturography are less unequivocal, with both significant positive and negative effects of Levodopa reported for various outcome parameters. Response to perturbation did not show any significant effects of Levodopa. Despite some positive indicators, the clinical importance of these findings requires further research. The greatest challenges for future research are the multidimensional aspect of balance, the heterogeneity of outcome parameters, and the search for reliable and useful tests and the heterogeneity of PwPD. Further research is needed to examine the relation between Levodopa and balance in PwPD and the mediating role of factors like dyskinesia, freezing of gait and dual-tasking conditions.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-223536.
REFERENCES
[1] | Tarolli CG , Zimmerman GA , Auinger P , McIntosh S , Horowitz RK , Kluger BM , Dorsey ER , Holloway RG ((2020) ) Symptom burden among individuals with Parkinson disease. Neurol Clin Pract 10: ,65–72. |
[2] | Armstrong MJ , Okun MS ((2020) ) Diagnosis and treatment of Parkinson disease: A review. JAMA 323: ,548–560. |
[3] | Goubault E , Bogard S , Blanchet PJ , Bézard E , Vincent C , Martino D , Sarna J , Monchi O , Duval C ((2020) ) Parkinsonian symptoms, not dyskinesia, negatively affect active life participation of dyskinetic patients with Parkinson’s disease. Tremor Other Hyperkinet Mov (N Y) 10: ,20. |
[4] | Bekkers EMJ , Dijkstra BW , Heremans E , Verschueren SMP , Bloem BR , Nieuwboer A ((2018) ) Balancing between the two: Are freezing of gait and postural instability in Parkinson’s disease connected? Neurosci Biobehav Rev 94: ,113–125. |
[5] | Boonstra TA , van der Kooij H , Munneke M , Bloem BR ((2008) ) Gait disorders and balance disturbances in Parkinson’s disease: Clinical update and pathophysiology. Curr Opin Neurol 21: ,461–471. |
[6] | de Souza Fortaleza AC , Mancini M , Carlson-Kuhta P , King LA , Nutt JG , Chagas EF , Freitas IFJ , Horak FB ((2017) ) Dual task interference on postural sway, postural transitions and gait in people with Parkinson’s disease and freezing of gait. Gait Posture 56: ,76–81. |
[7] | Allen NE , Schwarzel AK , Canning CG ((2013) ) Recurrent falls in parkinson’s disease: A systematic review. Parkinsons Dis 2013: ,906274. |
[8] | Beuter A , Hernández R , Rigal R , Modolo J , Blanchet PJ ((2020) ) Postural sway and effect of levodopa in early Parkinson’s disease. Can J Neurol Sci 35: ,65–68. |
[9] | J V , J M , BR B , WI V ((2016) ) Neurovestibular analysis and falls in Parkinson’s disease and atypical parkinsonism. Eur J Neurosci 43: ,1636–1646. |
[10] | Hely MA , Morris JGL , Reid WGJ , Trafficante R ((2005) ) Sydney multicenter study of Parkinson’s disease: Non-L-dopa-responsive problems dominate at 15 years. Mov Disord 20: ,190–199. |
[11] | Hiorth YH , Larsen JP , Lode K , Pedersen KF ((2014) ) Natural history of falls in a population-based cohort of patients withParkinson’s disease: An 8-year prospective study. Parkinsonsim Relat Disord 20: ,1059–1064. |
[12] | Brozova H , Stochl J , Roth J , Ruzicka E ((2009) ) Fear of falling has greater influence than other aspects of gait disorders on quality of life in patients with Parkinson’s disease. Neuroendocrinol Lett 30: ,453–456. |
[13] | Grimbergen YAM , Schrag A , Mazibrada G , Borm GF , Bloem BR ((2013) ) Impact of falls and fear of falling on health-related quality of life in patients with Parkinson’s disease. J Parkinsons Dis 3: ,409–413. |
[14] | Balash Y , Peretz C , Leibovich G , Herman T , Hausdorff JM , Giladi N ((2005) ) Falls in outpatients with Parkinson’s disease: Frequency, impact and identifying factors. J Neurol 252: ,1310–1315. |
[15] | Tan EK , Yeo AP , Tan V , Pavanni R , Wong MC , Eo APY , Tan V , Pavanni R , Wong MC ((2005) ) Prescribing pattern in Parkinson’s disease: Are cost and efficacy overriding factors? Int J Clin Pract 59: ,511–514. |
[16] | Rosa MM , Ferreira JJ , Coelho M , Freire R , Sampaio C ((2010) ) Prescribing patterns of antiparkinsonian agents in Europe. Mov Disord 25: ,1053–1060. |
[17] | Orayj K , Lane E ((2019) ) Patterns and determinants of prescribing for Parkinson’s disease: A systematic literature review. Parkinsons Dis 2019: ,9237181. |
[18] | Uitti RJ , Ahlskog JE , Maraganore DM , Muenter MD , Atkinson EJ , Cha RH , O’Brien PC ((1993) ) Levodopa therapy and survival in idiopathic Parkinson’s disease: Olmsted County project. Neurology 43: ,1918–1926. |
[19] | Maier Hoehn MM ((1983) ) Parkinsonism treated with levodopa: Progression and mortality. J Neural Transm Suppl 19: ,253–264. |
[20] | Cilia R , Cereda E , Akpalu A , Sarfo FS , Cham M , Laryea R , Obese V , Oppon K , Del Sorbo F , Bonvegna S , Zecchinelli AL , Pezzoli G ((2020) ) Natural history of motor symptoms in Parkinson’s disease and the long-duration response to levodopa. Brain 143: ,2490–2501. |
[21] | Dijkstra BW , Gilat M , Cofré Lizama LE , Mancini M , Bergmans B , Verschueren SMP , Nieuwboer A ((2021) ) Impaired weight-shift amplitude in people with Parkinson’s disease with freezing of gait. J Parkinsons Dis 11: ,1367–1380. |
[22] | Peterson DS , Horak FB ((2016) ) The effect of Levodopa on improvements in protective stepping in people with Parkinson’s disease. Neurorehabil Neural Repair 30: ,931–940. |
[23] | Valkovič P , Brožová H , Bötzel K , Růžička E , Benetin J ((2008) ) Push and release test predicts better Parkinson fallers and nonfallers than the pull test: Comparison in OFF and ON medication states. Mov Disord 23: ,1453–1457. |
[24] | Baston C , Mancini M , Rocchi L , Horak F ((2016) ) Effects of Levodopa on postural strategies in Parkinson’s disease. Gait Posture 46: ,26–29. |
[25] | Horak FB , Frank J , Nutt J ((1996) ) Effects of dopamine on postural control in parkinsonian subjects: Scaling, set, and tone. J Neurophysiol 75: ,2380–2396. |
[26] | Curtze C , Nutt JG , Carlson-Kuhta P , Mancini M , Horak FB ((2015) ) Levodopa is a double-edged sword for balance and gait in people with Parkinson’s disease. Mov Disord 30: ,1361–1370. |
[27] | Liberati A , Altman DG , Tetzlaff J , Mulrow C , Gøtzsche PC , Ioannidis JPA , Clarke M , Devereaux PJ , Kleijnen J , Moher D ((2009) ) The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med 6: ,e1000100. |
[28] | Higgins JPT , Thomas J , Chandler J , Cumpston M , Li T , Page MJ , Welch VA ((2022) ) Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane. Available from www.training.cochrane.org/handbook. |
[29] | Bartels AL , Balash Y , Gurevich T , Schaafsma JD , Hausdorff JM , Giladi N ((2003) ) Relationship between freezing of gait (FOG) and other features of Parkinson’s: FOG is not correlated with bradykinesia. J Clin Neurosci 10: ,584–588. |
[30] | Yelshyna D , Gago MF , Bicho E , Fernandes V , Gago NF , Costa L , Silva H , Rodrigues ML , Rocha L , Sousa N ((2016) ) Compensatory postural adjustments in Parkinson’s disease assessed via a virtual reality environment. Behav Brain Res 296: ,384–392. |
[31] | Gago MF , Fernandes V , Ferreira J , Silva H , Rodrigues ML , Rocha L , Bicho E , Sousa N ((2015) ) The effect of levodopa on postural stability evaluated by wearable inertial measurement units for idiopathic and vascular Parkinson’s disease. Gait Posture 41: ,459–464. |
[32] | Morris S , Morris ME , Iansek R , S M , ME M , R I ((2001) ) Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys Ther 81: ,810–818. |
[33] | Horak FB , Nutt JG , Nashner LM ((1992) ) Postural inflexibility in parkinsonian subjects. J Neurol Sci 111: ,46–58. |
[34] | Hall LM , Brauer SG , Horak F , Hodges PW ((2013) ) The effect of Parkinson’s disease and levodopa on adaptation of anticipatory postural adjustments. Neuroscience 250: ,483–492. |
[35] | Horak FB , Mancini M , Carlson-Kuhta P , Nutt JG , Salarian A ((2016) ) Balance and gait represent independent domains of mobility in Parkinson disease. Phys Ther 96: ,1364–1371. |
[36] | King LA , St George RJ , Carlson-Kuhta P , Nutt JG , Horak FB ((2010) ) Preparation for compensatory forward stepping in Parkinson’s disease. Arch Phys Med Rehabil 91: ,1332–1338. |
[37] | NIH ((2014) ) Quality Assessment Tool for Before-After (Pre-Post) Studies without Control Group. |
[38] | Hozo SP , Djulbegovic B , Hozo I ((2005) ) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5: ,13. |
[39] | Burleigh A , Horak F , Nutt J , Frank J. ((1995) ) Levodopa reduces muscle tone and lower extremity tremor in Parkinson’s disease. Can J Neurol Sci 22: ,280–285. |
[40] | Burleigh-Jacobs A , Horak FB , Nutt JG , Obeso JA ((1997) ) Step initiation in Parkinson’s disease: Influence of levodopa and external sensory triggers. Mov Disord 12: ,206–215. |
[41] | Fetoni V , Genitrini S , Monza D , Soliveri P , Testa D , Caraceni T , Girotti F ((1997) ) Variations in axial, proximal, and distal motor response to L-dopa in multisystem atrophy and Parkinson’s disease. Clin Neuropharmacol 20: ,239–244. |
[42] | Foreman KB , Addison O , Kim HS , Dibble LE ((2011) ) Testing balance and fall risk in persons with Parkinson disease, an argument for ecologically valid testing. Parkinsonism Relat Disord 17: ,166–171. |
[43] | Frank JS , Horak FB , Nutt J ((2000) ) Centrally initiated postural adjustments in parkinsonian patients on and off levodopa. J Neurophysiol 84: ,2440–2448. |
[44] | Franzén E , Paquette C , Gurfinkel VS , Cordo PJ , Nutt JG , Horak FB ((2009) ) Reduced performance in balance, walking and turning tasks is associated with increased neck tone in Parkinson’s disease. Exp Neurol 219: ,430–438. |
[45] | King LA , Horak FB ((2008) ) Lateral stepping for postural correction in Parkinson’s disease. Arch Phys Med Rehabil 89: ,492–499. |
[46] | Mehdizadeh M , Martinez-Martin P , Amirhasan Habibi S , Nikbakht N , Alvandi F , Bazipoor P , Panahi A , Taghizadeh G ((2019) ) The association of balance, fear of falling, and daily activities with drug phases and severity of disease in patients with Parkinson. Basic Clin Neurosci 10: ,355–361. |
[47] | Nova IC , Perracini MR , Ferraz HB ((2004) ) Levodopa effect upon functional balance of Parkinson’s disease patients. Parkinsonism Relat Disord 10: ,411–415. |
[48] | Palmisano C , Brandt G , Pozzi NG , Leporini A , Maltese V , Canessa A , Volkmann J , Pezzoli G , Frigo CA , Isaias IU ((2019) ) Sit-to-walk performance in Parkinson’s disease: A comparison between faller and non-faller patients. Clin Biomech 63: ,140–146. |
[49] | Palmisano C , Br , T G , Vissani M , Pozzi NG , Canessa A , Brumberg J , Marotta G , Volkmann J , Mazzoni A , Pezzoli G , Frigo CA , Isaias IU ((2020) ) Gait initiation in Parkinson’s disease: Impact of dopamine depletion and initial stance condition. Front Bioeng Biotechnol 8: ,137. |
[50] | Schlenstedt C , Mancini M , Horak F , Peterson D ((2017) ) Anticipatory postural adjustment during self-initiated, cued, and compensatory stepping in healthy older adults and patients with Parkinson disease. Arch Phys Med Rehabil 98: ,1316–1324.e1. |
[51] | Winser SJ , Kannan P , Bello UM , Whitney SL ((2019) ) Measures of balance and falls risk prediction in people with Parkinson’s disease: A systematic review of psychometric properties. Clin Rehabil 33: ,1949–1962. |
[52] | Steffen T , Seney M ((2008) ) Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys Ther 88: ,733–746. |
[53] | Duncan RP , Leddy AL , Cavanaugh JT , Dibble LE , Ellis TD , Ford MP , Foreman KB , Earhart GM ((2012) ) Accuracy of fall prediction in Parkinson disease: Six-month and 12-month prospective analyses. Parkinsons Dis 2012: ,237673. |
[54] | Almeida LRS , Valenca GT , Negreiros NN , Pinto EB , Oliveira-Filho J ((2016) ) Comparison of self-report and performance-based balance measures for predicting recurrent falls in people with Parkinson disease: Cohort study. Phys Ther 96: ,1074–84. |
[55] | Tamura S , Miyata K , Kobayashi S , Takeda R , Iwamoto H ((2022) ) The minimal clinically important difference in Berg Balance Scale scores among patients with early subacute stroke: A multicenter, retrospective, observational study. Top Stroke Rehabil 29: ,423–429. |
[56] | Downs S , Marquez J , Chiarelli P ((2013) ) The Berg Balance Scale has high intra- and inter-rater reliability but absolute reliability varies across the scale: A systematic review. J Physiother 59: ,93–99. |
[57] | Bloem BR , Marinus J , Almeida Q , Dibble L , Nieuwboer A , Post B , Ruzicka E , Goetz C , Stebbins G , Martinez-Martin P , Schrag A; Movement Disorders Society Rating Scales Committee ((2016) ) Measurement instruments to assess posture, gait, and balance in Parkinson’s disease: Critique and recommendations. Mov Disord 31: ,1342–1355. |
[58] | Jacobs JV , Horak FB , Van Tran K , Nutt JG ((2006) ) An alternative clinical postural stability test for patients with Parkinson’s disease. J Neurol 253: ,1404–1413. |
[59] | Horak FB , Nashner LM ((1986) ) Central programming of postural movements: Adaptation to altered support-surface configurations. J Neurophysiol 55: ,1369–1381. |
[60] | Manchester D , Woollacott M , Zederbauer-Hylton N , Marin O ((1989) ) Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol 44: ,M118–M127. |
[61] | Liaw M-Y , Chen C-L , Pei Y-C , Leong C-P , Lau Y-C ((2009) ) Comparison of the static and dynamic balance performance in young, middle-aged, and elderly healthy people. Chang Gung Med J 32: ,297–304. |
[62] | Schoneburg B , Mancini M , Horak F , Nutt JG ((2013) ) Framework for understanding balance dysfunction in Parkinson’s disease. Mov Disord 28: ,1474–1482. |
[63] | Kuo AD , Speers RA , Peterka RJ , Horak FB ((1998) ) Effect of altered sensory conditions on multivariate descriptors of human postural sway. Exp Brain Res 122: ,185–195. |
[64] | Horak FB , Shupert CL , Mirka A ((1989) ) Components of postural dyscontrol in the elderly: A review. Neurobiol Aging 10: ,727–738. |
[65] | Okada S , Hirakawa K , Takada Y , Kinoshita H ((2001) ) Age-related differences in postural control in humans in response to a sudden deceleration generated by postural disturbance. Eur J Appl Physiol 85: ,10–18. |
[66] | Matinolli M , Korpelainen JT , Korpelainen R , Sotaniemi KA , Virranniemi M , Myllylä VV ((2007) ) Postural sway and falls in Parkinson’s disease: A regression approach. Mov Disord 22: ,1927–1935. |
[67] | Frenklach A , Louie S , Koop MM , Bronte-Stewart H ((2009) ) Excessive postural sway and the risk of falls at different stages of Parkinson’s disease. Mov Disord 24: ,377–385. |
[68] | Amundsen Huffmaster SL , Lu C , Tuite PJ , MacKinnon CD ((2020) ) The transition from standing to walking is affected in people with Parkinson’s disease and freezing of gait. J Parkinsons Dis 10: ,233–243. |
[69] | Lamberti P , Armenise S , Castaldo V , Mari M de , Iliceto G , Tronci P , Serlenga L ((1997) ) Freezing gait in Parkinson’s disease. Eur Neurol 38: ,297–301. |
[70] | Smulders K , Dale ML , Carlson-Kuhta P , Nutt JG , Horak FB ((2016) ) Pharmacological treatment in Parkinson’s disease: Effects on gait. Parkinsonism Relat Disord 31: ,3–13. |
[71] | Bologna M , Paparella G , Fasano A , Hallett M , Berardelli A ((2020) ) Evolving concepts on bradykinesia. Brain 143: ,727. |
[72] | Klawans HL ((1986) ) Individual manifestations of Parkinson’s disease after ten or more years of levodopa. Mov Disord 1: ,187–192. |
[73] | Park J-H , Kang Y-J , Horak FB ((2015) ) What is wrong with balance in Parkinson’s disease? J Mov Disord 8: ,109. |
[74] | Workman CD , Thrasher TA ((2019) ) The influence of dopaminergic medication on balance automaticity in Parkinson’s disease. Gait Posture 70: ,98–103. |
[75] | Munhoz RP , Li J-Y , Kurtinecz M , Piboolnurak P , Constantino A , Fahn S , Lang AE ((2004) ) Evaluation of the pull test technique in assessing postural instability in Parkinson’s disease. Neurology 62: ,125–127. |
[76] | Hasegawa N , Shah VV , Harker G , Carlson-Kuhta P , Nutt JG , Lapidus JA , Jung SH , Barlow N , King LA , Horak FB , Mancini M ((2020) ) Responsiveness of objective vs. clinical balance domain outcomes for exercise intervention in Parkinson’s disease. Front Neurol 11: ,940. |
[77] | Beylergil SB , Petersen M , Gupta P , Elkasaby M , Kilbane C , Shaikh AG ((2021) ) Severity-dependent effects of Parkinson’s disease on perception of visual and vestibular heading. Mov Disord 36: ,360–369. |
[78] | Pollak P ((2013) ) Deep brain stimulation for Parkinson’s disease – patient selection. Handb Clin Neurol 116: ,97–105. |
[79] | Benabid AL , Chabardes S , Mitrofanis J , Pollak P ((2009) ) Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson’s disease. Lancet Neurol 8: ,67–81. |
[80] | Barthel C , Mallia E , Debû B , Bloem BR , Ferraye MU ((2016) ) The practicalities of assessing freezing of gait. J Parkinsons Dis 6: ,667–674. |
[81] | Chung KA , Lobb BM , Nutt JG , McNames J , Horak F ((2010) ) Objective measurement of dyskinesia in Parkinson’s disease using a force plate. Mov Disord 25: ,602–608. |
[82] | Armand S , Landis T , Sztajzel R , Burkhard PR ((2009) ) Dyskinesia-induced postural instability in Parkinson’s disease. Parkinsonism Relat Disord 15: ,359–364. |
[83] | Paul SS , Sherrington C , Canning CG , Fung VSC , Close JCT, Lord SR ((2014) ) The relative contribution of physical and cognitive fall risk factors in people with Parkinson’s disease: A large prospective cohort study. Neurorehabil Neural Repair 28: ,282–90. |
[84] | Ashburn A , Stack E , Pickering RM , Ward CD ((2001) ) A community-dwelling sample of people with Parkinson’s disease: Characteristics of fallers and non-fallers. Age Ageing 30: ,47–52. |
[85] | Aarsland D , Andersen K , Larsen JP , Lolk A , Kragh-Sørensen P ((2003) ) Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Arch Neurol 60: ,387–392. |
[86] | Magalhães M , Wenning GK , Daniel SE , Quinn NP ((1995) ) Autonomic dysfunction in pathologically confirmed multiple system atrophy and idiopathic Parkinson’s disease–a retrospective comparison. Acta Neurol Scand 91: ,98–102. |
[87] | Rascol O , Perez-Lloret S , Damier P , Delval A , Derkinderen P , Destée A , Meissner WG , Tison F , Negre-Pages L ((2015) ) Falls in ambulatory non-demented patients with Parkinson’s disease. J Neural Transm 122: ,1447–1455. |
[88] | van der Marck MA , Klok MPC , Okun MS , Giladi N , Munneke M , Bloem BR , Arney K , Browner NM , Caunter M , Cianci HJ , Dunlop B , Eggert K , Fisher B , Hass CJ , Hunter C , Jabre M , Kraakevik J , Lyons KE , Phibbs F , Scott BL , Shih L , Tan EK , Tan L , Varanese S , Voss T , Ashburn A , Ballinger C , Bhatti MT , Hausdorff J , Lindvall S , Morris ME , Nieuwboer A , Schwalb JM , Studenski S , Wood BH ((2014) ) Consensus-based clinical practice recommendations for the examination and management of falls in patients with Parkinson’s disease. Parkinsonism Relat Disord 20: ,360–369. |
[89] | Horak FB , Mancini M ((2013) ) Objective biomarkers of balance and gait for Parkinson’s disease using body-worn sensors. Mov Disord 28: ,1544–1551. |
[90] | Seuthe J , D’Cruz N , Ginis P , Blöbaum R , Weisser B , Deuschl G , Nieuwboer A , Schlenstedt C ((2021) ) How many gait initiation trials are necessary to reliably detect anticipatory postural adjustments and first step characteristics in healthy elderly and people with Parkinson’s disease? Gait Posture 88: ,126–131. |
[91] | National Institute for Health and Care Excellence (NICE) ((2017) ) Parkinson’s disease in adults: Diagnosis and management. NICE Guideline, No. 71. National Institute for Health and Care Excellence (NICE), London. |
[92] | Dibble LE , Lange M ((2006) ) Predicting falls in individuals with Parkinson disease: A reconsideration of clinical balance measures. J Neurol Phys Ther 30: ,60–67. |
[93] | Jacobs JV , Horak FB , Tran VK , Nutt JG ((2006) ) Multiple balance tests improve the assessment of postural stability in subjects with Parkinson’s disease. J Neurol Neurosurg Psychiatry 77: ,322. |
[94] | Bloem BR , Valkenburg VV , Slabbekoorn M , van Dijk JG ((2001) ) The multiple tasks test. Strategies in Parkinson’s disease. Exp Brain Res 137: ,478–486. |
[95] | Bloem BR , Grimbergen YA , Cramer M , Willemsen M , Zwinderman AH ((2001) ) Prospective assessment of falls in Parkinson’s disease. J Neurol 248: ,950–958. |
[96] | Michalska J , Kamieniarz A , Brachman A , Marszałek W , Cholewa J , Juras G , Słomka KJ ((2020) ) Fall-related measures in elderly individuals and Parkinson’s disease subjects. PLoS One 15: ,e0236886. |
[97] | Latt MD , Lord SR , Morris JGL , Fung VSC ((2009) ) Clinical and physiological assessments for elucidating falls risk in Parkinson’s disease. Mov Disord 24: ,1280–1289. |
[98] | Hubble RP , Naughton GA , Silburn PA , Cole MH ((2015) ) Wearable sensor use for assessing standing balance and walking stability in people with Parkinson’s disease: A systematic review. PLoS One 10: ,e0123705. |




