Reduced Range of Gait Speed: A Parkinson’s Disease-Specific Symptom?
Abstract
Reduced range of gait speed (RGS) may lead to decreased environmental adaptability in persons with Parkinson’s disease (PwPD). Therefore, lab-measured gait speed, step time, and step length during slow, preferred, and fast walking were assessed in 24 PwPD, 19 stroke patients, and 19 older adults and compared with 31 young adults. Only PwPD, but not the other groups, showed significantly reduced RGS compared to young adults, driven by step time in the low and step length in the high gait speed range. These results suggest that reduced RGS may occur as a PD-specific symptom, and different gait components seem to contribute.
INTRODUCTION
Gait speed is directly associated with quality of life and social participation [1]. Moreover, reduced gait speed predicts adverse health outcomes [2]. This has primarily been investigated in participants walking at their preferred gait speed [1–3]. However, these may also hold true for fast gait speed [4, 5]. Less is known about the relationship between slow gait speed and the above-mentioned parameters, although there are indications that slow gait speed also changes over the course of life [6] and in various neurological diseases [7, 8]. Furthermore, it has been shown that people tend to walk slower in unsupervised settings, such as in the home environment [9], and walk at an entire range of gait speed (RGS) in daily life [10]. An adequate RGS may be highly relevant to adapt to environmental requirements. For example, slowing down gait speed allows to explore the environment, whereas the ability to increase speed allows reaching a certain target or escape from dangerous situations.
This raises the question whether RGS defined as the difference between slow and fast gait speed, also changes, for example, in the course of specific diseases. RGS could be seen as a measure of physiological adaptability in the locomotor system, potentially comparable to adaptive processes occurring in other domains, such as the body cell level (e.g., in immunological cells [11]), the organ level (e.g., in cardiovascular system [12]), or the psychological level [13]. Changes of such adaptabilities due to, e.g., aging have already been shown for many processes [14], including immune response [15], stem cell development [16], and the locomotor system performance in general [17, 18]. As persons with Parkinson’s disease (PwPD) show reduced adaptability at different levels (e.g., weaker adaptive responses to balance perturbations during quiet standing [19] or freezing of gait when gait needs to be adapted to external requirements [20]), we hypothesized that PD may specifically lead to reduced RGS.
Based on the above data, which was also primarily collected in the laboratory, we investigated in a lab experiment RGS in PwPD, persons with stroke (PwS), and older adults (OA) and compared results with those of young adults (YA). PwS serve as a control-like group in this study, to investigate whether there may be potentially disease-specific differences in PwPD.
MATERIALS AND METHODS
Study design and participants
Thirty-one YA, 19 OA, 19 PwS, and 24 PwPD according to the Movement Disorder Society (MDS) criteria [21] were included. The study was approved by the local ethical committee (University of Kiel; D438/18). All participants gave their written informed consent. Concerning study design, participants, recruitment strategy including inclusion and exclusion criteria and sampling methods, we refer to a previous publication [22]. In brief, sociodemographic data were collected, including age, height, weight, and current medication. The clinical assessment comprised the motor part of the MDS-sponsored Unified Parkinson’s Disease Rating Scale (MDS-UPDRS, Part III) [23], and the Montreal Cognitive Assessment (MoCA).
Afterwards, participants were instructed to walk 5 m on a 1-m-wide straight walkway, starting at least two strides before they crossed the starting line and stopping at least two strides after the end line to omit acceleration and deceleration phases within the 5 m measurement. The assessment included three different walking conditions (standardized: slow, preferred, fast). For slow walking, participants were instructed to walk at 50% of their preferred speed. For preferred walking, participants were instructed to walk at their comfortable speed. For fast walking, participants were instructed to walk with their individual maximum gait speed without feeling unsafe. Gait parameters were collected using an optical motion capture system (Qualisys AB, Gothenburg, Sweden; 12 cameras, sampling frequency 200 Hz). Passive markers were attached on both sides to the anterior superior iliac spine, posterior superior iliac spine, third metatarsal bone and the calcaneal tuber. Gait speed (from the pelvic markers), step length, and step time (both from the lower leg markers) were extracted.
One-way analysis of variance for group differences (nine ANOVAs for the absolute and relative parameters, respectively) and Tukey test for post hoc analysis were calculated. The Welch correction was applied in case of significantly different variances. Within the PD group, clinical parameters were compared between “good” and “bad” RGS performers (median split) using Mann-Whitney U test. The data was analyzed using JASP, version 0.12.2 (JASP, Amsterdam, Netherlands).
RESULTS
OA, PwS, and PwPD did not differ significantly in age. All these groups showed a significantly higher BMI and lower score in MoCA compared to YA. As expected, PwPD differed significantly from all other groups in MDS-UPDRS III (Table 1).
Table 1
Demographics, clinical and gait parameters of participants
| YA | OA | PwS | PwPD | p | |
| N (female in %) | 31 (45) | 19 (37) | 19 (21) | 24 (29) | – |
| Age [y] | 30±9 | 72±7* | 66±17* | 66±10* | < 0.001 |
| Height [m] | 1.79±0.09 | 1.73±0.09 | 1.74±0.1 | 1.76±0.09 | 0.13 |
| Weight [kg] | 74±13 | 78±15 | 77±20 | 83±19 | 0.243 |
| BMI [kg/m2] | 23±3 | 26±5* | 26±5* | 26±5* | 0.004 |
| MoCA (0–30) | 28±2 | 24±4* | 22±4* | 23±3* | <0.001 |
| MDS-UPDRS III (0–132) | 2±2 | 5±5 | 6±6 | 29±21*#° | < 0.001 |
| Hoehn &Yahr Score (0–5) | – | – | – | 2.2±1.0 | – |
| Disease duration [y] | – | – | – | 9.0±6.3 | – |
| NIHSS (0–42) | – | – | 1.0±1.5 | – | – |
| Gait speed [m/s] | |||||
| S | 0.75 (±0.24) | 0.66 (±0.18) | 0.57* (±0.16) | 0.73 (±0.21) | 0.019 |
| P | 1.36 (±0.19) | 1.12* (±0.24) | 0.92*# (±0.29) | 1.06* (±0.22) | <0.001 |
| F | 2.07 (±0.26) | 1.59* (±0.4) | 1.36* (±0.41) | 1.40* (±0.32) | <0.001 |
| Step time [s] | |||||
| S | 0.79 (±0.18) | 0.73 (±0.09) | 0.79 (±0.17) | 0.70 (±0.1) | 0.058 |
| P | 0.54 (±0.04) | 0.56 (±0.05) | 0.62*# (±0.11) | 0.56° (±0.04) | 0.018 |
| F | 0.44 (±0.04) | 0.45 (±0.07) | 0.48* (±0.06) | 0.48 (±0.06) | 0.013 |
| Step length [m] | |||||
| S | 0.56 (±0.08) | 0.48* (±0.11) | 0.43* (±0.09) | 0.50 (±0.12) | <0.001 |
| P | 0.74 (±0.07) | 0.62* (±0.11) | 0.56* (±0.11) | 0.60* (±0.12) | <0.001 |
| F | 0.91 (±0.13) | 0.71* (±0.12) | 0.66* (±0.15) | 0.67* (±0.15) | <0.001 |
If not specified data is presented with mean±standard deviation. One-way analysis of variance for group differences, Welch correction in case of different variances (italics), and Tukey test for post hoc analysis were calculated. Significant p-values (<0.05) are in bold. *p< 0.05 compared to young adults (YA); #p< 0.05 compared to older adults (OA); °p< 0.05 compared to persons with stroke (PwS). BMI, body mass index; F, fast; MDS-UPDRS III, Movement Disorder Society sponsored Unified Parkinson’s Disease Rating Scale, motor part; MoCA, Montreal Cognitive Assessment; NIHSS, National Institutes of Health Stroke Scale; P, preferred; PwPD, persons with Parkinson’s disease; S, slow.
Gait parameters and their changes at different walking conditions
Regarding absolute gait parameters, PwS walked slowest during the slow walking condition, which was significant against YA. Of note, PwPD showed almost the same gait speed as YA. During preferred and fast walking conditions, OA, PwS, and PwPD showed significantly slower gait speeds than YA. In addition, PwS walked significantly slower than OA during the preferred walking condition. Significant differences in step time and step length between the groups suggest that in all three walking conditions, step length contributes more than step time to the differences observed in OA, PwS, and PwPD, compared to YA. In addition, PwS showed larger step times particularly during the preferred and fast walking conditions compared to all other groups (Table 1).
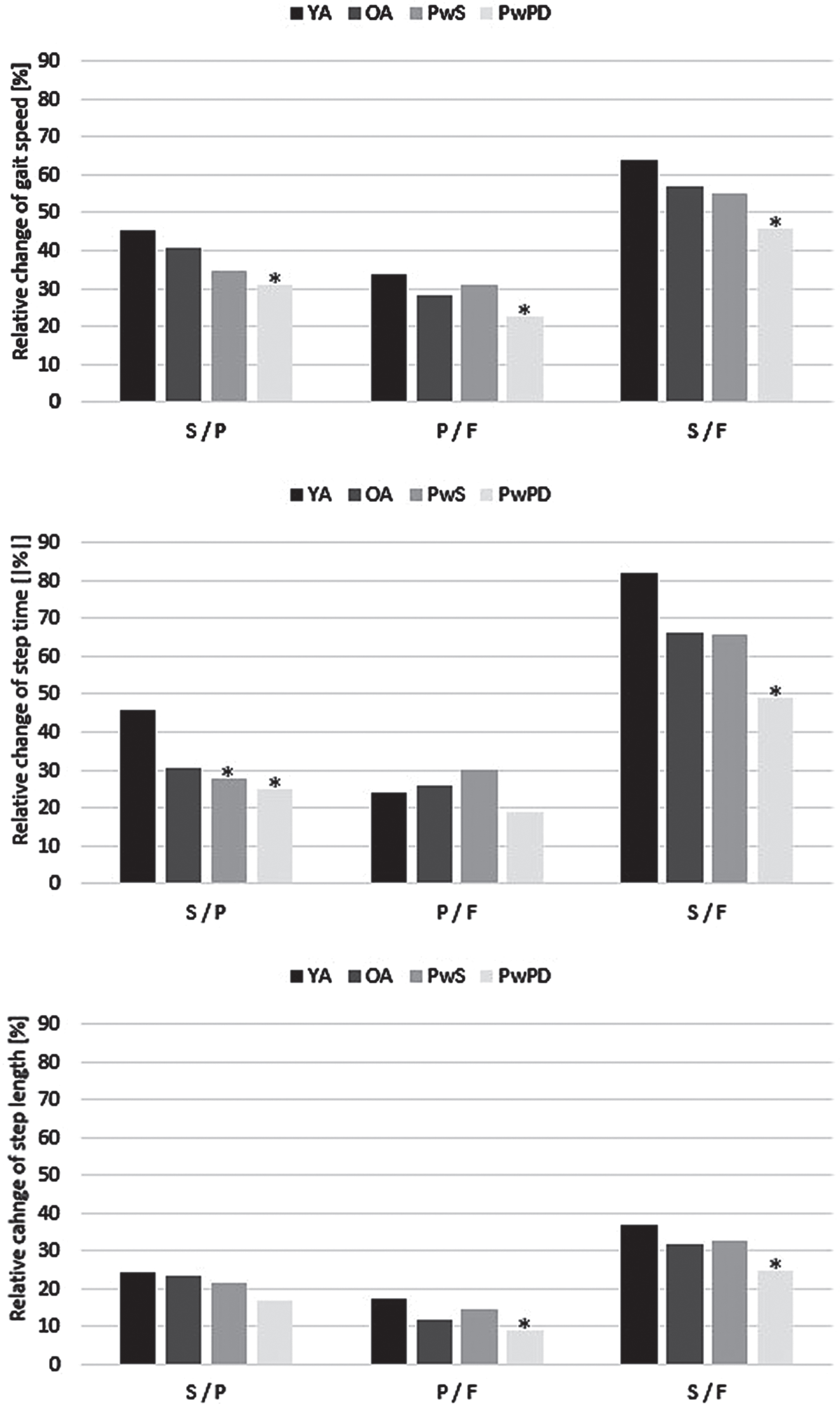
Regarding relative changes in gait parameters between the different walking conditions, PwPD showed the smallest changes in gait speed between the three conditions of all 4 groups, which reached significance compared to YA. The differences in gait speed changes were driven by a significantly smaller change of step time between the slow and preferred walking condition, and by a significantly smaller change of step length between the preferred and fast walking condition, compared to YA (Fig. 1). Within PwPD, although bad RGS performers were younger and had better MDS-UPDRS III values than the good RGS performers, their MOCA scores were worse (p = 0.12 for the slow versus preferred walking condition, p = 0.14 for the preferred versus fast walking condition).
Fig. 1
Relative changes of gait parameters between slow, preferred, and fast walking condition, separated by groups. *p < 0.05 compared to young adults (YA); F, fast; OA, older adults; P, preferred; PwPD, persons with Parkinson’s disease; PwS, persons with stroke; S, slow.
DISCUSSION
This study assessed whether RGS is reduced in specific diseases or due to the aging process and evaluated how step time and step length contribute to these effects. Differences in absolute gait parameters between the groups studied were comparable to those reported in previous studies [24–26], which proves the representativeness of the present results. This study now provides, to our knowledge for the first time, marked evidence that RGS is reduced in PwPD, as hypothesized, and that step time and step length contribute to this in diverse ways.
Non-preferred gait speeds are accompanied by reduced dynamic balance [27, 28]. It could be, therefore, that PwPD reduce their RGS to maintain dynamic stability and keep the risk of falling as low as possible. It seems unlikely that RGS is associated with, or “part” of bradykinesia, hypokinesia or rigidity, respectively. For example, the relatively high gait speed (“YA-like") under the slow walking condition speaks against this. Moreover, RGS may be associated with specific central pathophysiology (for example, with involvement of basal ganglia-related networks), as there was no significantly reduced RGS in PwS. Furthermore, RGS does not appear to be relevantly age-related, as OA did not show a significantly reduced RGS, compared to YA. This observation also supports, at least indirectly, the existence of a specific pathophysiology associated with RGS. Indeed, results obtained from the comparison between “good” and “bad” RGS performers within the PD group suggest that the RGS effect, as observed here, is due to restriction of cognitive (e.g., cholinergic) rather than motor (e.g., dopaminergic) networks.
In this context, it seems interesting that step time and step length contributed differently to the reduced RGS in PwPD. Therefore, it does not seem unlikely that various pathophysiological processes contribute to RGS in PwPD. It is already known from several studies investigating gait in PwPD, that regulation of step length is impaired in PD [29, 30]. Moreover, the reduction of step length is strongly associated with disease progression [31] and is particularly associated with pathology of the dopaminergic system [32, 33].
Less is known about the association of step time with specific neurotransmitter systems and cerebral structures, although the growing literature on the cholinergic system makes it particularly promising to investigate this system in more detail in this context [34–36]. Interestingly, a recent review highlighted the association of postural instability and gait difficulties with cholinergic system changes in thalamic, caudate, limbic, neocortical, and cerebellar nodes [36]. Further, a correlation between degeneration of cholinergic nuclei and increased step time variability was recently demonstrated [35]. It could also be that the smaller change of step time under slow walking condition in PwPD is due to an increase in disinhibition associated with changes in frontal lobe function [37, 38], as PwPD often show changes in this brain area [39].
As a limitation, it must be assumed that RGS is primarily relevant in the real world, but the study was conducted under laboratory conditions. Future studies should therefore evaluate the observations presented here not only in the laboratory but also under real life conditions.
Based on the observation presented here of reduced RGS in PwPD, which is explained at different levels by reduced adaptation of step length and step time, the potential relevance of this symptom to everyday life, and the given likelihood that reduced RGS is treatable by pharmacological and allied health-associated and behavioral therapies, we propose to define RGS as a separate symptom in PD. This will allow research on RGS within an overall concept, considering pathophysiology, clinical and everyday presentation, and its treatability.
ACKNOWLEDGMENTS
There are no funders to report. No authors have anything to disclose related to the content of the article.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | van de Port IG , Kwakkel G , Lindeman E ((2008) ) Community ambulation in patients with chronic stroke: How is it related to gait speed? J Rehabil Med 40: , 23–27. |
[2] | Sanders JB , Bremmer MA , Comijs HC , van de Ven PM , Deeg DJH , Beekman ATF ((2017) ) Gait speed and processing speed as clinical markers for geriatric health outcomes, Am J Geriatr Psychiatry 25: , 374–385. |
[3] | Studenski S , Perera S , Patel K , Rosano C , Faulkner K , Inzitari M , Brach J , Chandler J , Cawthon P , Connor EB , Nevitt M , Visser M , Kritchevsky S , Badinelli S , Harris T , Newman AB , Cauley J , Ferrucci L , Guralnik J ((2011) ) Gait speed and survival in older adults, JAMA 305: , 50–58. |
[4] | Knapstad MK , Steihaug OM , Aaslund MK , Nakling A , Naterstad IF , Fladby T , Aarsland D , Giil LM ((2019) ) Reduced walking speed in subjective and mild cognitive impairment: A cross-sectional study, J Geriatr Phys Ther 42: , E122–E128. |
[5] | Costa P , De Jesus T , Torriani-Pasin C , Polese J ((2022) ) Functional capacity and walking speed reserve in individuals with chronic stroke: A cross-sectional study, Physiother Theory Pract 38: , 2563–2567. |
[6] | Fukuchi CA , Fukuchi RK , Duarte M ((2019) ) Effects of walking speed on gait biomechanics in healthy participants: A systematic review and meta-analysis, Syst Rev 8: , 153. |
[7] | Sidoroff V , Raccagni C , Kaindlstorfer C , Eschlboeck S , Fanciulli A , Granata R , Eskofier B , Seppi K , Poewe W , Willeit J , Kiechl S , Mahlknecht P , Stockner H , Marini K , Schorr O , Rungger G , Klucken J , Wenning G , Gaßner H ((2021) ) Characterization of gait variability in multiple system atrophy and Parkinson’s disease, J Neurol 268: , 1770–1779. |
[8] | Liang JN , Ho KY , Lee YJ , Ackley C , Aki K , Arias J , Trinh J ((2021) ) Slow walking in individuals with chronic post-stroke hemiparesis: Speed mediated effects of gait kinetics and ankle kinematics, Brain Sci 11: , 365. |
[9] | Warmerdam E , Hausdorff JM , Atrsaei A , Zhou Y , Mirelman A , Aminian K , Espay AJ , Hansen C , Evers LJW , Keller A , Lamoth C , Pilotto A , Rochester L , Schmidt G , Bloem BR , Maetzler W ((2020) ) Long-term unsupervised mobility assessment in movement disorders, Lancet Neurol 19: , 462–470. |
[10] | Atrsaei A , Corrà MF , Dadashi F , Vila-Chã N , Maia L , Mariani B , Maetzler W , Aminian K ((2021) ) Gait speed in clinical and daily living assessments in Parkinson’s disease patients: Performance versus capacity, NPJ Parkinsons Dis 7: , 24. |
[11] | Hausdorff JM ((2009) ) Gait dynamics in Parkinson’s disease: Common and distinct behavior among stride length, gait variability, and factarl-like scaling, Chaos 19: , 026113. |
[12] | Heusch G , Libby P , Gersh B , Yellon D , Böhm M , Lopaschuk G , Opie L ((2014) ) Cardiovascular remodelling in coronary artery disease and heart failure, Lancet 383: , 1933–1943. |
[13] | de Ridder D , Geenen R , Kuijer R , van Middendorp H ((2008) ) Psychological adjustment to chronic disease, Lancet 372: , 246–255. |
[14] | Lipsitz LA , Goldberger AL ((1992) ) Loss of ‘complexity’ and aging: Potential applications of factarls and chaos theory to senescence, JAMA 267: , 1806–1809. |
[15] | Deczkowska A , Matcovitch-Natan O , Tsitsou-Kampeli A , Ben-Hamo S , Dvir-Szternfeld R , Spinrad A , Singer O , David E , Winter DR , Smith LK , Kertser A , Baruch K , Rosenzweig N , Terem A , Prinz M , Villeda S , Citri A , Amit I , Schwartz M ((2017) ) Mef2C restrains microglial inflammatory response and is lost in brain ageing in an IFN-I-dependent manner, Nat Commun 8: , 717. |
[16] | Goodell MA , Rando TA ((2015) ) Stem cells and healthy aging, Science 350: , 1199–1204. |
[17] | Brown CJ , Flood KL ((2013) ) Mobility limitation in the older patient: A clinical review, JAMA 310: , 1168–1177. |
[18] | Bierbaum S , Peper A , Karamanidis K , Arampatzis A ((2011) ) Adaptive feedback potential in dynamic stability during disturbed walking in the elderly, J Biomech 44: , 1921–1926. |
[19] | Fransson PA , Nilsson MH , Rehncrona S , Tjernström F , Magnusson M , Johansson R , Patel M ((2021) ) Deep brain stimulation in the subthalamic nuclei alters postural alignment and adaptation in Parkinson’s disease, PLoS One 16: , e0259862. |
[20] | Schaafsma JD , Balash Y , Gurevich T , Bartels AL , Hausdorff JM , Giladi N ((2003) ) Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson’s disease, Eur J Neurol 10: , 391–398. |
[21] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W , Obeso J , Marek K , Litvan I , Lang AE , Halliday G , Goetz CG , Gasser T , Dubois B , Chan P , Bloem BR , Adler CH , Deuschl G ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease, Mov Disord 30: , 1591–1601. |
[22] | Warmerdam E , Romijnders R , Geritz J , Elshehabi M , Maetzler C , Otto JC , Reimer M , Stuerner K , Baron R , Paschen S , Beyer T , Dopcke D , Eiken T , Ortmann H , Peters F , von der Recke F , Riesen M , Rohwedder G , Schaade A , Schumacher M , Sondermann A , Maetzler W , Hansen C ((2021) ) Proposed mobility assessments with simultaneous full-body inertial measurement units and optical motion capture in healthy adults and neurological patients for future validation studies: Study protocol, Sensors 21: , 5833. |
[23] | Goetz CG , Tilley BC , Shaftman SR , Stebbins GT , Fahn S , Martinez-Martin P , Poewe W , Sampaio C , Stern MB , Dodel R , Dubois B , Holloway R , Jankovic J , Kulisevsky J , Lang AE , Lees A , Leurgans S , LeWitt PA , Nyenhuis D , Olanow CW , Rascol O , Schrag A , Teresi JA , van Hilten JJ , LaPelle N , Agarwal P , Athar S , Bordelan Y , Bronte-Stewart HM , Camicioli R , Chou K , Cole W , Dalvi A , Delgado H , Diamond A , Dick JP , Duda J , Elble RJ , Evans C , Evidente VG , Fernandez HH , Fox S , Friedman JH , Fross RD , Gallagher D , Goetz CG , Hall D , Hermanowicz N , Hinson V , Horn S , Hurtig H , Kang UJ , Kleiner-Fisman G , Klepitskaya O , Kompoliti K , Lai EC , Leehey ML , Leroi I , Lyons KE , McClain T , Metzer SW , Miyasaki J , Morgan JC , Nance M , Nemeth J , Pahwa R , Parashos SA , Schneider JSJS , Schrag A , Sethi K , Shulman LM , Siderowf A , Silverdale M , Simuni T , Stacy M , Stern MB , Stewart RM , Sullivan K , Swope DM , Wadia PM , Walker RW , Walker R , Weiner WJ , Wiener J , Wilkinson J , Wojcieszek JM , Wolfrath S , Wooten F , Wu A , Zesiewicz TA , Zweig RM ((2008) ) Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results, Mov Disord 23: , 2129–2170. |
[24] | Pistacchi M , Gioulis M , Sanson F , de Giovannini E , Filippi G , Rossetto F , Marsala SZ ((2017) ) Gait analysis and clinical correlations in early Parkinson’s disease, Funct Neurol 32: , 28–34. |
[25] | Hobert MA , Nussbaum S , Heger T , Berg D , Maetzler W , Heinzel S ((2019) ) Progressive gait deficits in Parkinson’s disease: A wearable-based biannual 5-year prospective study, Front Aging Neurosci 11: , 22. |
[26] | Peterson DS , Mancini M , Fino PC , Horak F , Smulders K ((2020) ) Speeding up gait in Parkinson’s disease, J Parkinsons Dis 10: , 245–253. |
[27] | Cole MH , Sweeney M , Conway ZJ , Blackmore T , Silburn PA ((2017) ) Imposed faster and slower walking speeds influence gait stability differently in Parkinson fallers, Arch Phys Med Rehabil 98: , 639–648. |
[28] | Lee M , Youm C , Noh B , Park H , Cheon SM ((2020) ) Gait characteristics under imposed challenge speed conditions in patients with parkinson’s disease during overground walking, Sensors (Basel) 20: , 2132. |
[29] | Zijlstra W , Rutgers AWF , Van Weerden TW ((1998) ) Voluntary and involuntary adaptation of gait in Parkinson’s disease, Gait Posture 7: , 53–63. |
[30] | Morris ME , Iansek R , Matyas TA , Summers JJ ((1994) ) Ability to modulate walking cadence remains intact in Parkinson’s disease, J Neurol Neurosurg Psychiatry 57: , 1532–1534. |
[31] | Welzel J , Wendtland D , Warmerdam E , Romijnders R , Elshehabi M , Geritz J , Berg D , Hansen C , Maetzler W ((2021) ) Step length is a promising progression marker in parkinson’s disease, Sensors 21: , 2292. |
[32] | Kalia LV , Lang AE ((2015) ) Parkinson’s disease, Lancet 386: , 896–912. |
[33] | Braak H , Braak E ((2000) ) Pathoanatomy of Parkinson’s disease, J Neurol Suppl 247: , 3–10. |
[34] | Henderson EJ , Lord SR , Brodie MA , Gaunt DM , Lawrence AD , Close JCT , Whone AL , Ben-Shlomo Y ((2016) ) Rivastigmine for gait stability in patients with Parkinson’s disease (ReSPonD): A randomised, double-blind, placebo-controlled, phase 2 trial, Lancet Neurol 15: , 249–258. |
[35] | Wilson J , Yarnall AJ , Craig CE , Galna B , Lord S , Morris R , Lawson RA , Alcock L , Duncan GW , Khoo TK , O’Brien JT , Burn DJ , Taylor JP , Ray NJ , Rochester L ((2021) ) Cholinergic basal forebrain volumes predict gait decline in Parkinson’s disease, Mov Disord 36: , 611–621. |
[36] | Bohnen NI , Yarnall AJ , Weil RS , Moro E , Moehle MS , Borghammer P , Bedard MA , Albin RL ((2022) ) Cholinergic system changes in Parkinson’s disease: Emerging therapeutic approaches, Lancet Neurol 21: , 381–392. |
[37] | Bang J , Spina S , Miller BL ((2015) ) Frontotemporal dementia, Lancet 386: , 1672–1682. |
[38] | Murley AG , Rouse MA , Simon Jones P , Ye R , Hezemans FH , O’Callaghan C , Frangou P , Kourtzi Z , Rua C , Adrian Carpenter T , Rodgers CT , Rowe JB ((2020) ) GABA and glutamate deficits from frontotemporal lobar degeneration are associated with disinhibition, Brain 143: , 3449–3462. |
[39] | Mosley PE , Robinson K , Coyne T , Silburn P , Barker MS , Breakspear M , Robinson GA , Perry A ((2020) ) Subthalamic deep brain stimulation identifies frontal networks supporting initiation, inhibition and strategy use in Parkinson’s disease, Neuroimage 223: , 117352. |




