Cognitive Behavioral Therapy for Anxiety in Parkinson’s Disease Induces Functional Brain Changes
Abstract
Background:
Cognitive behavioral therapy (CBT) reduces anxiety symptoms in patients with Parkinson’s disease (PD).
Objective:
The objective of this study was to identify changes in functional connectivity in the brain after CBT for anxiety in patients with PD.
Methods:
Thirty-five patients with PD and clinically significant anxiety were randomized over two groups: CBT plus clinical monitoring (10 CBT sessions) or clinical monitoring only (CMO). Changes in severity of anxiety symptoms were assessed with the Parkinson Anxiety Scale (PAS). Resting-state functional brain MRI was performed at baseline and after the intervention. Functional networks were extracted by an Independent Component Analysis (ICA). Functional connectivity (FC) changes between structures involved in the PD-related anxiety circuits, such as the fear circuit (involving limbic, frontal, and cingulate structures) and the cortico-striato-thalamo-cortical limbic circuit, and both within and between functional networks were compared between groups and regressed with anxiety symptoms changes.
Results:
Compared to CMO, CBT reduced the FC between the right thalamus and the bilateral orbitofrontal cortices and increased the striato-frontal FC. CBT also increased the fronto-parietal FC within the central executive network (CEN) and between the CEN and the salience network. After CBT, improvement of PAS-score was associated with an increased striato-cingulate and parieto-temporal FC, and a decreased FC within the default-mode network and between the dorsal attentional network and the language network.
Conclusion:
CBT in PD-patients improves anxiety symptoms and is associated with functional changes reversing the imbalance between PD-related anxiety circuits and reinforcing cognitive control on emotional processing.
INTRODUCTION
Parkinson’s disease (PD) is characterized by both motor and non-motor symptoms. Anxiety is one of the most common neuropsychiatric symptoms in PD, with an average point prevalence around 31% [1]. Anxiety is associated with increased motor disability and a reduced quality of life [2, 3]. Recently, we showed that a cognitive behavioral therapy (CBT) module tailored to treat anxiety in PD patients was more effective than clinical monitoring only (CMO) in reducing symptoms of anxiety [4]. In a systematic review, we reported that PD-related anxiety was associated with structural and functional changes in the limbic cortico-striato-thalamo-cortical (CSTC) circuit and the fear circuit and hypothesized that anxiety in PD would be due to an imbalance between these two circuits [5]. Moreover, a recent study suggested that anxiety in PD patients would be associated with a reduced cognitive control of emotional processes [6]. Hence, the reduction of anxiety symptoms induced by CBT could result from restoring the balance between these two circuits and reinforcing cognitive control on emotion.
The aim of the present study was to identify functional changes in the brain occurring after CBT for anxiety in PD patients. We hypothesized that CBT would induce functional changes in the PD-related anxiety circuits, with also FC changes both within and between networks.
MATERIALS AND METHODS
Study design
This study is a prospective, open, randomized controlled trial. PD patients with anxiety were randomized to either ‘CBT plus clinical monitoring’ (the intervention group) or ‘CMO’ (control group). All participants underwent standardized clinical, cognitive, and behavioral assessment as well as MRI scanning (except in case of contra-indications such as deep brain stimulation leads or claustrophobia) at baseline and at the end of the intervention. The duration of the intervention was approximately 10–12 weeks. For more details on the design, we refer to Mulders et al. [7].
Study population
Patients were recruited among outpatients of the movement disorders clinics in Maastricht University Medical Centre, Maastricht (the Netherlands) and University Hospital of Lille (France). Patients included in this study had a diagnosis of idiopathic PD according to the Queens Square Brain Bank diagnostic criteria [8], were between 35 and 80 years old, had clinically relevant anxiety symptoms, defined as a score on the Parkinson Anxiety Scale (PAS) subscale for persistent anxiety (PAS-A) >9 and/or a score on the avoidance subscale (PAS-C)>3 [9], and were not receiving any other current psychological treatment for anxiety (psychopharmacotherapy was allowed if a stable dose was used at least two months prior to participation. During the trial the dosage should not be changed. Medication use and mental health care were tracked throughout the study). They were on a stable dose of antiparkinsonian medication for at least one month and provided informed consent. Patients with other neurological disorders, dementia or severe cognitive impairment operationalized as a Montreal Cognitive Assessment (MoCA) score <24 [10], contra-indications for MRI, major depressive disorder or abuse of alcohol, drugs or benzodiazepines were excluded.
At baseline, demographic and clinical variables were collected including sex, age, years of formal education, year of PD onset, side of onset, type and dose of antiparkinsonian medication, and other medication including psychopharmacotherapy. Motor symptoms and disease severity were respectively assessed during “ON” phases (in case of motor and non-motor fluctuations) with the Movement Disorder Society – unified Parkinson’s disease rating scale (MDS-UPDRS) part 3 and the Hoehn & Yahr staging system [11]. Depression was assessed using Hamilton depression rating scale (HDRS) [12] (See published design [7]).
The study is carried out in compliance with the Helsinki Declaration. The local ethics committee of Maastricht University Medical Centre (July 2016) and Lille University Hospital (September 2016) have approved the study protocol. Written informed consents was obtained from all participants. The study is registered at the ClinicalTrials.gov database under registration number NCT02648737, as well at FoxTrialFinder under ID number 004701 (See published design [7]).
Assessment of anxiety
The PAS, a scale specifically developed to detect and measure anxiety in PD patients, was used to assess anxiety symptoms at baseline (t = 0) and post-intervention (t = 1). It is insensitive for motor and depressive symptoms and has subsections forpersistent anxiety (PAS-A), episodic/situational anxiety (PAS-B), and avoidance behavior (PAS-C) [9].
CBT and clinical monitoring
The CBT consisted of 10 weekly individual sessions of 60–75 min, tailored to the preferences and needs of each patient. All patients received clinical monitoring which involved an information sheet about anxiety and a phone call by an independent psychologist one month after baseline assessment to inquire about current anxiety symptoms. More details are reported in the study design paper [7].
Imaging acquisition
Patients were scanned at baseline and post-intervention with both sites using identical 3T Philips Achieva MRI scanners (Philips Healthcare, Best, The Netherlands) with identical software versions and MR sequences. High-resolution anatomical T1-weighted (T1w) images were acquired using a 3D inversion recovery MP-RAGE sequence (231 sagittal slices, no gap, TR/TE/flip angle = 12 ms/3.3 ms/9°, matrix 384 × 384, field of view 240 × 240 mm, voxel size 0.63 × 0.63 × 0.65 mm). Resting-state functional MRI (rs-fMRI) was performed using echo-planar imaging (40 axial slices, no gap, TR/TE/flip angle = 2400 ms/30 ms/90°, matrix 64 × 64, field of view 192 × 192 mm, voxel size 3 × 3 × 3 mm). The total MRI scan took about 45 min. Patients were scanned on “ON-dopamine medication state”.
Functional analyses
Functional analyses were performed using CONNv18 toolbox in MATLAB (SPM12) [13].
Preprocessing
rs-fMRI data were preprocessed using the CONNv18 toolbox (https://web.conn-toolbox.org/fmri-methods/preprocessing-pipeline). More details are provided in the Supplementary Material, section Ia.
ROI-based comparisons
A Brodmann atlas, created from the Talairach one [14], was used to define the cortical regions-of-interest (ROI). The FSL Harvard-Oxford Atlas was used to define the subcortical ROIs [15]. Based on the aforementioned atlases, the following twenty structures involved in the PD-related anxiety circuits were defined: the amygdala, striatum (caudate nucleus, putamen, nucleus accumbens), thalamus, the prefrontal cortex (lateral, medial, orbito-frontal), cingulate gyrus (anterior and posterior), parietal cortex (superior parietal lobule), temporal cortex (pole temporal, temporal gyri) and insular cortex [5]. Functional connectivity was computed by Pearson correlating time series data between every pair of ROI, resulting in 20 × 20 FC matrices.
Identification of functional networks: ICA
Group Independent Component Analyses (ICA) were performed to identify common functional networks in patients using group-level ICA approach with CONN toolbox. Twenty independent components have been identified. Among these components, the common functional resting-state networks were identified using a cross-correlation based on existing standardized templates (Human brain project) [13]: the default-mode network (DMN), the dorsal attentional network (DAN), the salience network, the central executive network (CEN), the sensorimotor network (SMN), the language network (LN), the visual network (VN). Each ICA component was divided into regions of interest (ROI) according to a standard anatomical atlas ((Human brain project) [13]. The process is defined in Supplementary Figure 1 and SuppData(ROI).xlsx. Functional connectivity was computed by Pearson correlating time series data between every pair of ROI, resulting in 113 × 113 FC matrices.
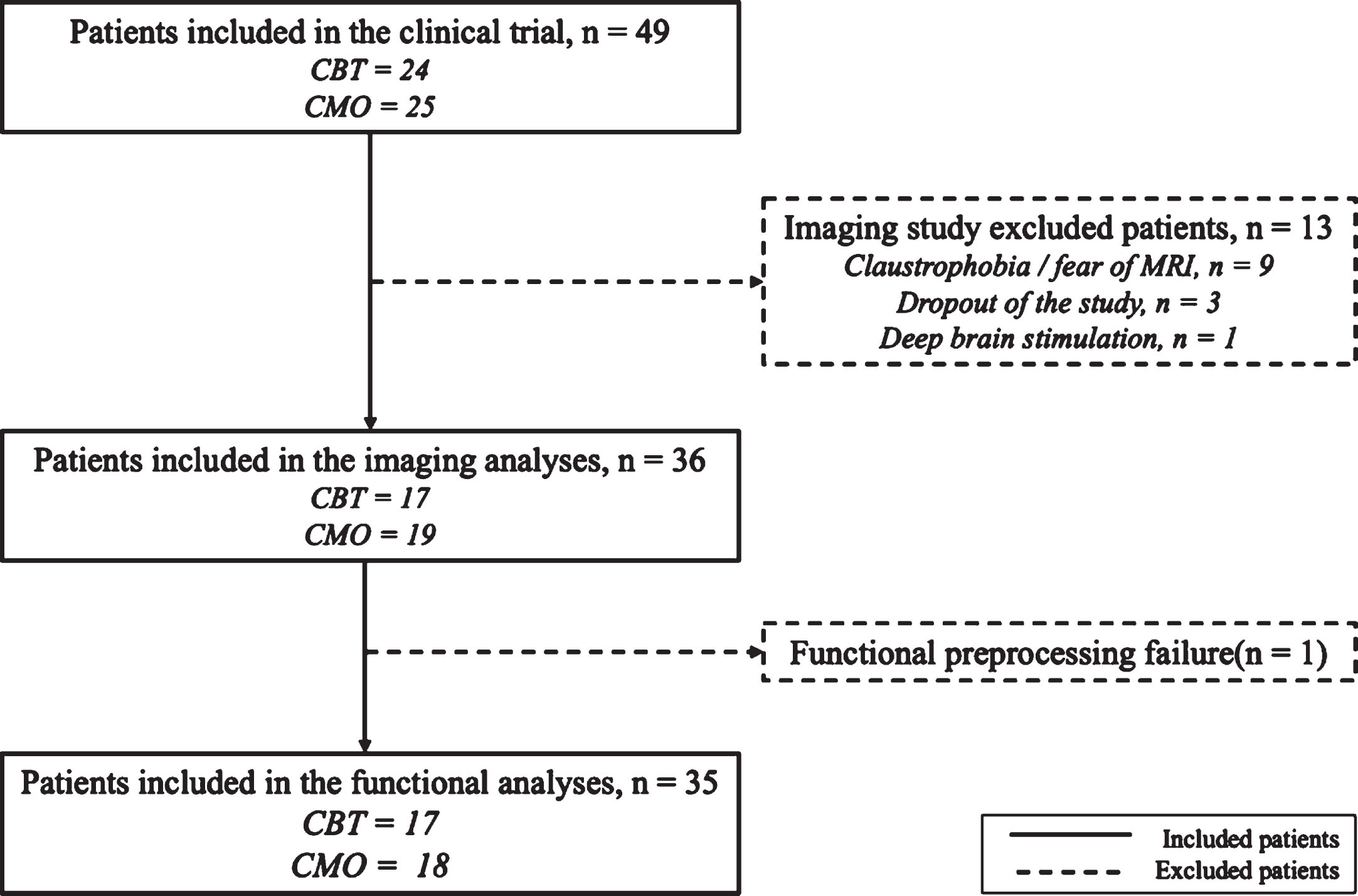
Fig. 1
Flow chart of the study. CBT, cognitive behavioral therapy; CMO, clinical monitoring only group.
Correlation between FC matrices and change in anxiety scores
For each patient, the change in PAS-total score was calculated, corresponding to the difference in score between baseline (BL) and post-intervention (PI) (ΔPAS = PASBL – PASPI). The FC matrices were extracted for each patient and each session. A variable indicating the change in FC was calculated for each patient (ΔFC = FCPI – FCBL) and associated with the ΔPAS - total using multiple regression analyses.
Statistical analyses
The significance threshold was set at p < 0.05 and corrected for multiple comparisons (FDR - False Discovery Rate) when necessary.
Analyses of clinical data
The numerical variables were described as means and standard deviations, the ordinal variables as median and range, and the categorical variables as frequencies and percentages. The normality of distribution was assessed using a Kolmogorov-Smirnov test. Categorical data were compared with Chi2 and quantitative data with t-tests in case of normally distributed data and Mann-Whitney tests otherwise. A repeated mixed ANOVA test was performed to compare the PAS total score at baseline and after the intervention between the two groups (CMO and CBT). All these statistical tests were performed using SPSS, version 26 (SPSS, Chicago).
Functional MRI analyses
All the functional analyses were adjusted for time (in days) between BL and PI session and center. The ROI-based analysis consisted of comparing the FC matrices between the two groups and across the time [13]. Generalized linear models with permutation inference were calculated first to identify significant FC values for each group and each session and secondly to compare these connections between the groups and sessions [16]. Repeated mixed ANOVA tests with permutation inferences were then performed to compare the connectivity values longitudinally between groups [13].
Hierarchical multiple regression analyses were performed to examine the relationship between ΔPAS and ΔFC, using SPSS version 26. Center and time (in days) between BL and PI sessions were set as nuisance regressors in the first block (model 1) of all regression models, whereas ΔPAS score (independent variable) was separately added to the second block of the model (model 2). ΔFC was set as dependent variable. We ensured that all models met the assumptions for multiple regression analyses, including normality of the residuals, multicollinearity, and homoscedasticity.
Data availability
Data supporting the findings of this study are available from the principal investigator (AFGL), upon reasonable request.
RESULTS
Demographic and clinical characteristics
Among the 49 patients included in the clinical study, fourteen were excluded (13 due to the absence of an MRI scan mainly due to claustrophobia and one after preprocessing failure). Thirty-five patients were thus included in the analyses: 17 in the CBT group and 18 in the CMO group (Fig. 1). All participants were right-handed except for 2 participants in the CMO group and 1 ambidexter participant in the CBT group. There were no between-group differences regarding demographic variables and baseline clinical scores. Time between BL and PI was significantly longer in the CBT than in CMO group (p = 0.001; Table 1).
Table 1
Demographic variables, baseline clinical scores, and time between baseline and post-intervention comparisons between patients with CBT and patient with CMO
| BASELINE | CBT group (n = 17) | CMO group (n = 19) | p |
| Demographic variables | |||
| Age (y) | 62.8 (±7.8) | 64.5 (±8.9) | 0.15 |
| Female (men/women ratio) | 0.89 | 0.73 | 0.77 |
| Clinical center: | 0.43 | ||
| Maastricht (n = 13) | 5 (29%) | 8 (42%) | |
| Lille (n = 23) | 12 (71%) | 11 (58%) | |
| Right hand dominance (n = 33) | 16 (94%) | 17 (90%) | 0.49 |
| Formal education (y) | 13.5 (±3.4) | 14.2 (±4.0) | 0.59 |
| Illness duration (y) | 7.4 (±5.8) | 4.8 (±4.2) | 0.18 |
| First motor side (right, n = 19) | 10 (59%) | 9 (56%) | 0.88 |
| LEDD total (mg/day) | 592.6 (±374.0) | 770.3 (±583.6) | 0.40 |
| Antidepressant use (n = 8) | 4 (24%) | 4 (22%) | 0.99 |
| Benzodiazepine use (n = 7) | 3 (18%) | 4 (21%) | 0.99 |
| Baseline (BL) clinical variables | |||
| PAS | |||
| Part A. Persistent anxiety (/20) | 13.2 (±2.4) | 13.7 (±2.67) | 0.62 |
| Part B. Episodic anxiety (/16) | 6.4 (±3.8) | 4.4 (±2.8) | 0.06 |
| Part C. Avoidance (/12) | 5.1 (±3.0) | 4.11 (±2.7) | 0.33 |
| Total score (/48) | 25.0 (±6.9) | 22.7 (±6.3) | 0.27 |
| Hamilton DRS (/54) | 11.4 (±4.6) | 10.8 (±4.6) | 0.66 |
| MoCA (/30) | 26.9 (±2.1) | 25.9 (±2.9) | 0.40 |
| MDS-UPDRS part III total (/108) | 22.9 (±10.3) | 27.6 (±10.8) | 0.19 |
| Hoehn &Yahr stage (0–5) | 2 (1–3) | 2 (2-3) | 0.36 |
| Time baseline – post intervention (days) | 121.9 (±30.3) | 94.6 (±36.5) | 0.001* |
*p < 0.05; BL, baseline; CBT, cognitive behavioral therapy; CMO, clinical monitoring only; DRS, depression rating scale; ΔPAS, PASBL – PASPI; LEDD, levodopa equivalent daily dosages; MDS-UPDRS, Movement Disorder Society unified Parkinson’s disease rating scale; MoCA, Montreal cognitive assessment; PAS, Parkinson anxiety scale.
In the repeated mixed ANOVA test, the PAS total score was reduced in both groups after the intervention but significantly more in the CBT group (F-score = 4.58, p = 0.04; Supplementary Figure 2). These results were in line with the clinical study [4].
Fig. 2
Representation of the induced functional connectivity changes after cognitive behavioral therapy in Parkinson’s disease related anxiety circuits and corresponding tables of statistical results. BA, Brodmann area; CBT, cognitive behavioral therapy; CMO, clinical monitoring only; FC, functional connectivity; PAS, Parkinson anxiety scale; Unc., uncorrected. (image credits: http://www.fmriconsulting.com/brodmann/)
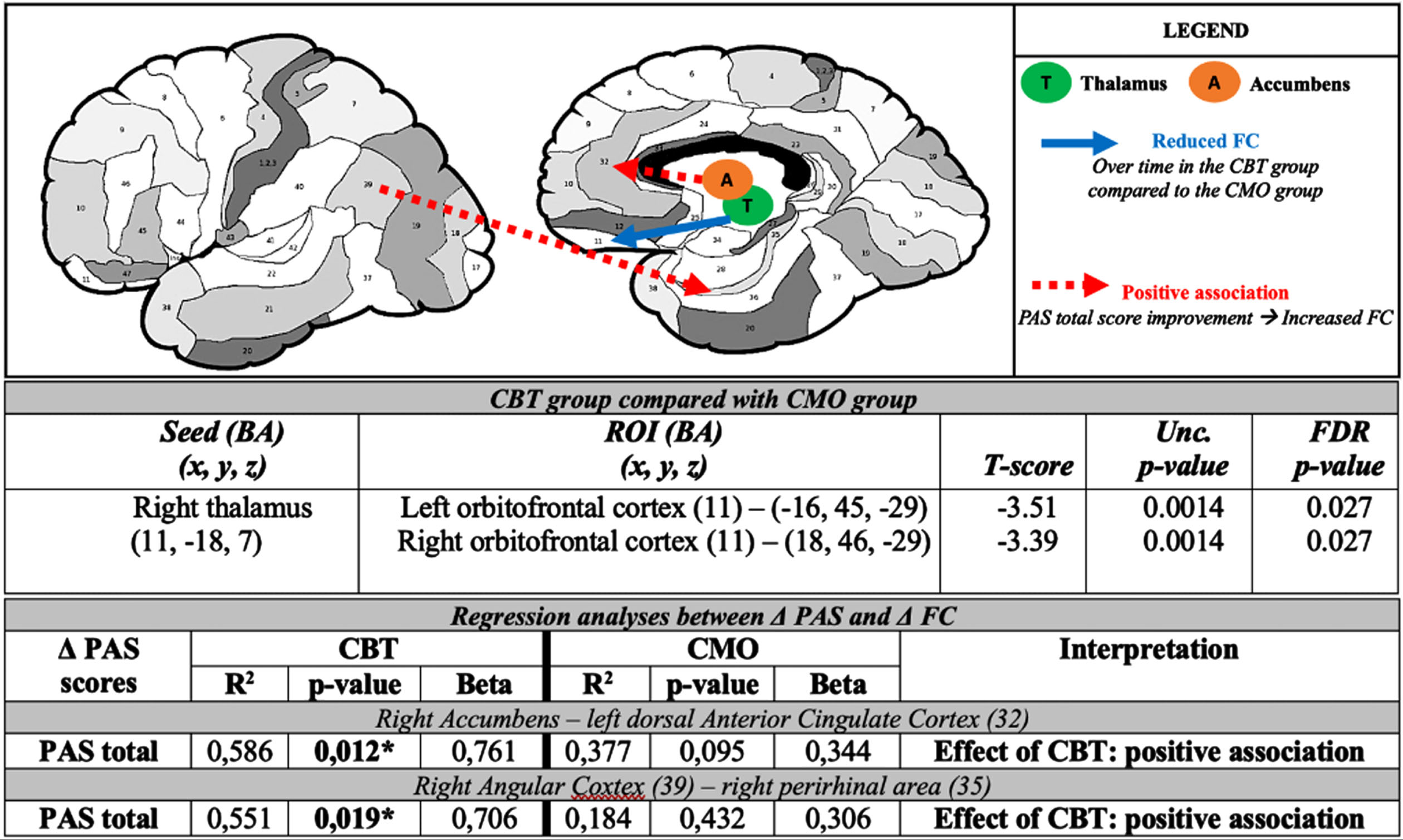
PD-related anxiety circuits
Functional connectivity analysis
After intervention, there was a significant reduction of FC between the right thalamus and the bilateral OFC in the CBT group compared to the CMO group (FDR p-value = 0.027) (Fig. 2).
Correlation of FC with anxiety scores
After CBT, improvement of PAS-total score was associated with increased FC between the right nucleus accumbens and the right dorsal anterior cingulate cortex (dACC) and between the right angular cortex and the right perirhinal area (of the medial temporal gyrus) (Fig. 2). Details are also provided on Supplementary Table 1.
Resting-state functional networks
Functional connectivity analysis
There was a significantly increased fronto-parietal FC within the CEN and striato-frontal FC within the language network in the CBT group compared with the CMO group.
There was also a significantly greater fronto-parietal FC between the CEN and the salience network in the CBT group compared with the CMO group (Table 2 and Fig. 3).
Fig. 3
Changes within and between resting-state functional networks in cognitive behavioral therapy group compared with clinical monitoring only group for anxiety in Parkinson’s disease. Axial, sagittal and frontal view; (z, x, y) = coordinates in the MNI space (Montreal Neurological Institute).
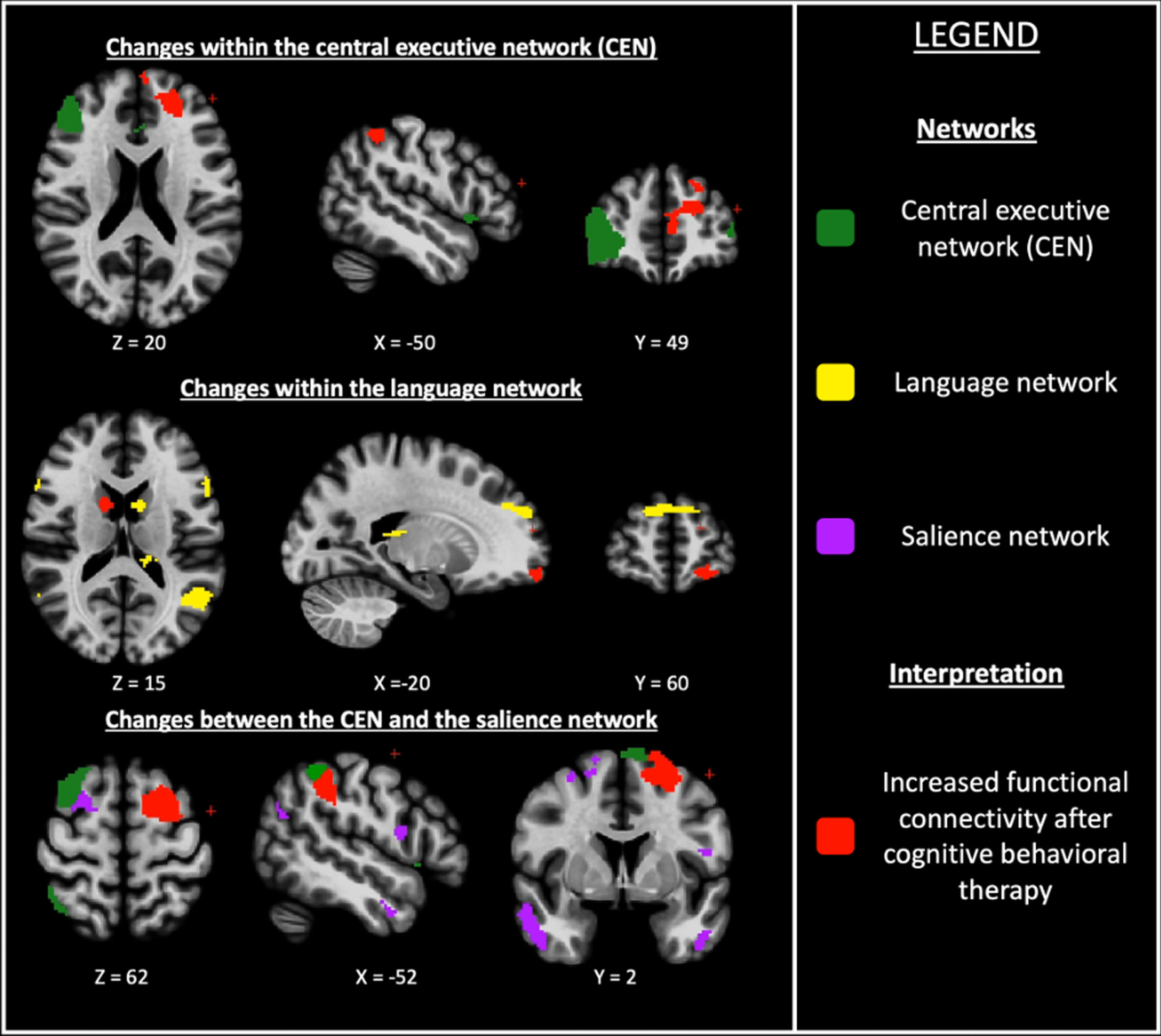
Table 2
Comparisons of ROIs extracted from ICA analyses within and between common functional networks after cognitive behavioral therapy for anxiety in Parkinson’s disease compared with clinical monitoring only
| Network | ROI / Network 1Localization,Brodmann area (BA),MNI coordinates (x, y, z) | ROI / Network 2Localization,Brodmann area (BA),MNI coordinates (x, y, z) | T-score | FDRp |
| Differences within networks | ||||
| CEN | L. inferior parietal lobule,BA 40, (–50, –47, 52) | L. anterior PFC,BA 10, (–4, 60, 30) | 3.29 | 0.044 |
| LN | R. caudate(13, 8, 25) | L. anterior PFC,BA 10, (–22, 61, –15) | 3.65 | 0.024 |
| L. orbito-frontal gyrus,BA 11, (–10, 64, –16) | 3.64 | 0.024 | ||
| Differences between networks | ||||
| CEN L. superior frontal gyrus, BA 6, (–12, 8, 61) | Salience network L. inferior parietal lobule, BA 40, (–53, –38, 41) | 4.11 | 0.031 | |
Coordinates in Montreal Neurological Institute (MNI) space. Only significant results are provided in this table. BA, Brodmann area; CEN, central executive network; FDR, false discovery rate; L., left; LN, language network; PFC, prefrontal cortex; R., right; ROI, region of interest; Unc., uncorrected.
Correlation of FC with anxiety scores
After CBT, improvement of PAS-total score was associated with a significantly decreased frontal FC within the DMN as well as an increased fronto-temporal FC and a decreased temporo-caudate FC between the DAN and the language network (Table 3).
Table 3
Regression analyses between ΔFC and ΔPAS-total in ROIs extracted from ICA analyses within and between common functional networks after cognitive behavioral therapy for anxiety in patients with Parkinson’s disease
| Network / ROI 1 Localization, Brodmann area (BA), coordinates (x, y, z) | Network / ROI 2 Localization, Brodmann area (BA), coordinates (x, y, z) | PAS-total | ||
| R2 | Std.β | p | ||
| Within network changes | ||||
| DMN | DMN | |||
| L. inferior frontal gyrus, pars orbit., BA 47, (–39, 41, –14) | R. anterior PFC BA 10, (3, 57, –11) | 0.59 | –0.43 | 0.007 |
| Between network changes | ||||
| DAN | LN | |||
| R. middle frontal gyrus, BA 6, (27, –7, 58) | R. inferior temporal gyrus BA 20, (50, –2, –44) | 0.89 | 0.26 | <0.0001* |
| DAN | LN | |||
| R. fusiform gyrus, BA 37, (48, –59, –10) | L. caudate BA 34, (27, 9, –17) | 0.74 | –0.81 | 0.0004 |
Coordinates in Montreal Neurological Institute (MNI) space. Only significant results are provided in this table. No significant change in CMO group. *significant change in both CBT and CMO groups; BA, Brodmann area; CBT, cognitive behavioral therapy; CEN, central executive network; CMO, clinical monitoring only; DAN, dorsal attentional network; L., left; LN, language network; R., right; ROI, region of interest; Std.β, standardized beta score.
DISCUSSION
CBT is an effective treatment to reduce symptoms of anxiety, especially for situational anxiety and avoidance behavior [4]. This analysis showed that a reduction of anxiety symptoms after CBT is associated with changes of FC in the PD-related anxiety circuits and both within and between functional networks. FC between the right thalamus and the bilateral OFC was reduced to a greater degree in the CBT group compared to the CMO group and the improvement of anxiety after CBT was associated with increased striato-cingulate and parieto-temporal FC. Moreover, the fronto-parietal FC within the CEN, the striato-frontal FC within the language network and the fronto-parietal FC between the CEN and the salience network were higher in the CBT group compared with the CMO group. The improvement of anxiety after CBT was associated with a decreased FC within the DMN as well as between the language network and the DAN. Therefore, CBT-induced reduction of anxiety in anxious PD patients is mediated by functional connectivity changes.
CBT reverses the imbalance between PD-related anxiety circuits
In PD, the striatal dopaminergic depletion leads to reduced activity in the cortico-striato-thalamocortical (CSTC) circuits, including the limbic one. Dysfunction of this limbic loop has been associated with psychiatric symptoms, such as anxiety. This circuit connects the anterior cingulate cortex (ACC), the medial PFC and brainstem nuclei with the basal ganglia such as the nucleus accumbens, the pallidum, the subthalamic nucleus (STN) and the thalamus in order to modulate mood and behavior [17, 18]. The fear circuit involves the amygdala and the ACC, the medial PFC, the insular cortex, the hippocampus, and the striatum. The fear circuit is involved in fear processing, while the limbic CSTC circuit is more involved in emotional and behavioral adaptations to fear [5]. In anxiety, the limbic CSTC circuit is mostly under-activated while the fear circuit is over-activated. We recently proposed that anxiety in PD could be due to this imbalance between these twocircuits [5].
In the present study, we showed that CBT induces an increased FC between the nucleus accumbens and the PFC and between the caudate and the dACC. These are parts of the limbic CSTC circuit which could be reactivated by CBT in anxious PD patients. This would be in line with our earlier hypothesis of imbalance between the limbic CSTC circuit and the fear circuit. Moreover, the FC between the thalamus and the OFC was lower in the CBT group than in the CMO group. Scarce studies of CBT for anxiety disorders in non-PD patients reported that the improvement of anxiety symptoms after CBT was associated with a lower activity of the OFC and the thalamus [19, 20]. A systematic review addressing the neurobiological basis of emotional processing in PD patients showed that the pathway between the thalamus and the OFC was involved in emotion recognition as well as the processing of intense emotional stimuli [21]. It was hypothesized that difficulties in emotion recognition in PD patients may arise from reduced dopaminergic input from structures that have close interconnections with OFC, such as the caudate nucleus [21]. Moreover, in PD the reduced dopaminergic projections to the frontal cortex, including the OFC, may prevent a disinhibition of the amygdala. This may lead to an inappropriate response to intense emotional stimuli [21]. In PD, anxiety symptoms could be associated with an imbalance between overactivity of the thalamus-OFC pathway and a reduced dopaminergic state between the PFC/OFC and striatal structures. By reducing the thalamo-orbitofrontal FC and increasing the striato-prefrontal and striato-cingulate FC, namely the limbic CSTC circuit, CBT could restore the balance between these circuits. This would reduce the abnormal representation of non-anxious stimuli that are wrongly interpreted as anxiogenic, and thus modulate the processing of intense emotional reaction. Reduction of thalamo-orbitofrontal activation would be a general effect of CBT on anxiety symptoms while reactivation of the limbic CSTC circuit could specifically act on PD-related anxiety. Finally, we did not find any CBT-induced changes in the fear circuit.
CBT reinforces the cognitive control on the emotional processing
Firstly, the fronto-parietal FC within the CEN was increased in the CBT group compared with the CMO group. The CEN, which includes the dorsal lateral PFC, the inferior parietal lobule and the anterior cingulate cortex, is involved in coordination of multiple domains of cognitive control such as attention, working memory, planning, and motor and behavioral inhibition [22, 23]. In a recent study, Micco et al. reported that anxiety in PD-patients was associated with a decreased fronto-parietal FC within the CEN [6]. In our study, by restoring the fronto-parietal FC within the CEN, CBT could improve cognitive control. Moreover, CBT induced an increased fronto-parietal FC between the CEN and the salience network. The salience network mainly includes the insular and cingulate cortices but also parts of the parietal and frontal cortices. It is therefore involved in “bottom-up” attentional processing and may cause hypervigilance in case of insufficient filtering of the captured stimuli [24]. Micco et al. reported disruptions in the salience network in PD patients with anxiety symptoms, with both a decreased and an increased FC within the ACC and insula. They also found a reduced FC between the salience network and the CEN at disease onset in patients with PD and anxiety symptoms. The authors hypothesized that an abnormal interplay within and between limbic and executive areas may impair the filtering role of the salience network over external and internal stimuli, leading to anxiety symptoms. Thus, abnormal interconnection between the salience network and the CEN may decrease the ability to modulate behavioral, as was also shown in anxious non-PD patients [6]. In our study, we found the opposite in the CBT compared with the CMO group. Hence, CBT could restore the FC between the CEN and the salience network in anxious PD-patients. By increasing cognitive control on the emotional process, it could reduce anxiety symptoms in PD patients. Besides, we found disrupted connections between the DAN and the language network, with an increased fronto-temporal FC and decreased temporo-caudate FC. The DAN, which includes the inferior parietal sulcus, the frontal eye fields, the visual cortex, and the temporal cortex, is involved in working memory, spatial attentional function, flexible coordination of cognitive control, and decision-making processes [25]. CBT could induce fronto-temporal FC changes in order to modulate the cognitive control of emotions. Moreover, the reduced temporo-caudate FC, which is also part of the fear circuit, could reflect its reduced activity after CBT. These results seem to be in line with our previous findings but as no previous work has studied the DAN activity in anxious PD patients, further studies are needed. Finally, we found that improvement of anxiety symptoms was associated with a reduced FC within the DMN. A similar decreased activity in the DMN has been described in anxious PDpatients [6].
Both interventions induced changes in FC
The clinical study showed that even though CBT was more effective than CMO in improving situational anxiety and avoidance behavior, improvement was also observed in the CMO group [4]. In our study, in both groups, improvement of the PAS-total score was associated with increased parieto-cingulate and striato-cingulate FC and decreased temporo-insular FC. We suggest that both interventions are able to improve anxiety symptoms. Even a simple clinical monitoring was able to slightly improve anxiety symptoms, and this induced slight FC changes. Detecting, diagnosing and offering support for anxiety in PD patients is thus essential.
Strengths and limitations
This study has several strengths and limitations. A strength is that this is the first study to explore the neural bases of changes induced by CBT in PD patients with anxiety. Moreover, this study was done in the context of a randomized controlled trial with a control group. Finally, in order to validate the results, several statistical methods have been performed. Only the significant results for all the methods were considered for this paper. A limitation is the small sample size that could have reduced the statistical power and increase the chance of type II errors. However, our sample size is higher (n = 35) than that usually observed in the literature on neuroimaging of CBT [19, 20, 26–29]. Secondly, the control group is not really a placebo group, since clinical monitoring may also be seen as an intervention. Clinical monitoring has been recommended as a control situation when exploring the clinical effectiveness of a new or adjusted psychotherapeutic intervention [7, 30]. Thirdly, we choose to not introduce the improvement of depressive symptoms after the intervention as a nuisance factor. The CBT was tailored for anxiety symptoms and the PAS-score is insensitive for depressive symptoms [9]. In our study, the severity of depressive symptoms was assessed by the HDRS-score. At baseline this score was low in both groups. Moreover, this scale includes items assessing anxiety. Correcting our analyses by the change in the HDRS-score would cancel a great part of the effects due to anxiety improvement. There is also a frequent co-morbidity between anxiety and depression. These symptoms are often intermingled and separating them would distort reality. Fourth, we excluded 10 patients from the imaging analyses (and 3 patients dropout the clinical and imaging study) for lack of MRI scan at the 2 sessions (claustrophobia n = 9 and deep brain stimulation n = 1). We ensured that the excluded patients had no differences at baseline by comparing them with included patients using χ2 and Mann-Whitney tests. They were no differences (Supplementary Table 2). Then, as mentioned before, the CSCT circuit is classically divided in limbic, motor, and associative loop. We did not compare these three loops in the same analyze. A further work would be interesting to better understand the effect of CBT on the entire CSTC circuit. Finally, the OFC is classically susceptible to imaging artifacts. As mentioned in the Supplementary Material, the EPI double-echo sequence was not available for all the patients. We processed a distortion correction using the Susceptible Distortion Correction included in the CONN toolbox pipeline.
Conclusion
In this study, CBT induced cerebral changes in anxious PD patients that were associated with symptom reduction. CBT can restore the imbalance between PD-related anxiety circuits and thus reinforce cognitive control of emotional processing, leading to improvement of anxiety symptoms in PD patients. Our study also revealed that, in PD patients, the limbic CSTC circuit is more accessible to modulation by CBT than the fear circuit.
ACKNOWLEDGMENTS
The authors thank all participants of the study for their patience and cooperation; Marie Delliaux and Marine Brion for their help in neuropsychological assessment; Anne-Claire Leterme for the CBT sessions; the neurologists from the Parkinson expert centers of Lille University Medical Centre and Maastricht University Medical Centre for their help in clinical assessment and Romain Viard from “UMS 2014 – US 41 – PLBS – Plateformes Lilloises en Biologie & Santé, F-59000, Lille, France” (CI2C – http://www.ci2c.fr/ for his help and his advices in imaging preprocessing and processing.
This study was sponsored by the Michael J. Fox Foundation for Parkinson’s Research. The sponsor was not involved in the design of this analysis, nor in data interpretation, the writing of this paper or decision to submit this article for publication.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
Not related to this study:
G. Carey was employed by Lille University Medical Center and French Government (enseignement supérieur) and received a mobility grant from the EURON network.
Renaud Lopes was employed by Lille University Medical Center.
Anja J.H. Moonen was employed by Maastricht University Medical Center.
Anne E.P. Mulders was employed by Maastricht University.
Joost J.A. de Jong was employed by Maastricht University Medical Center.
Gregory Kuchcinski was employed by Lille University Medical Center and French Government
Luc Defebvre was employed by French Government (enseignement supérieur), gave consultancies to “Abbvie” and “Orkyn”’ and earned honoraria for lectures from “UCB” and “Abbvie”.
Mark L. Kuijf was employed by Maastricht University Medical Center
Kathy Dujardin was employed by French Government (enseignement supérieur).
Albert F.G. Leentjens was employed by Maastricht University Medical Center, received a grant from “Michael J Fox Foundation” and earned royalties from “Springer media” and “de Tijdstroom”.
REFERENCES
[1] | Broen MPG , Narayen NE , Kuijf ML , Dissanayaka NNW , Leentjens AFG ((2016) ) Prevalence of anxiety in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 31: , 1125–1133. |
[2] | Leentjens AFG , Dujardin K , Marsh L , Martinez-Martin P , Richard IH , Starkstein SE ((2011) ) Symptomatology and markers of anxiety disorders in Parkinson’s disease: A cross-sectional study. Mov Disord 26: , 484–492. |
[3] | Pontone GM , Williams JR , Anderson KE , Chase G , Goldstein SR , Grill S , Hirsch ES , Lehmann S , Little JT , Margolis RL , Rabins PV , Weiss HD , Marsh L ((2011) ) Anxiety and self-perceived health status in Parkinson’s disease. Parkinsonism Relat Disord 17: , 249–254. |
[4] | Moonen AJH , Mulders AEP , Defebvre L , Duits A , Flinois B , Köhler S , Kuijf ML , Leterme A , Servant D , Vugt M , Dujardin K , Leentjens AFG ((2021) ) Cognitive behavioral therapy for anxiety in Parkinson’s disease: A randomized controlled trial. Mov Disord 36: , 2539–2548. |
[5] | Carey G , Görmezoğlu M , Jong JJA de , Hofman PAM , Backes WH , Dujardin K , Leentjens Albert FG ((2020) ) Neuroimaging of anxiety in Parkinson’s disease: A systematic review. Mov Disord 36: , 327–339. |
[6] | Micco RD , Satolli S , Siciliano M , Nardo F , Caiazzo G , Russo A , Giordano A , Esposito F , Tedeschi G , Tessitore A ((2021) ) Connectivity correlates of anxiety symptoms in drug-naive Parkinson’s disease patients. Mov Disord 36: , 96–105. |
[7] | Mulders AEP , Moonen AJH , Dujardin K , Kuijf ML , Duits A , Flinois B , Handels RLH , Lopes R , Leentjens AFG ((2018) ) Cognitive behavioural therapy for anxiety disorders in Parkinson’s disease: Design of a randomised controlled trial to assess clinical effectiveness and changes in cerebral connectivity. J Psychosom Res 112: , 32–39. |
[8] | Rijk MC de , Rocca WA , Anderson DW , Melcon MO , Breteler MMB , Maraganore DM ((1997) ) A population perspective on diagnostic criteria for Parkinson’s disease. Neurology 48: , 1277–1281. |
[9] | Leentjens AFG , Dujardin K , Pontone GM , Starkstein SE , Weintraub D , Martinez-Martin P ((2014) ) The Parkinson Anxiety Scale (PAS): Development and validation of a new anxiety scale. Mov Disord 29: , 1035–1043. |
[10] | Nasreddine ZS , Phillips NA , Bédirian V , Charbonneau S , Whitehead V , Collin I , Cummings JL , Chertkow H ((2005) ) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: , 695–699. |
[11] | Goetz CG , Tilley BC , Shaftman SR , Stebbins GT , Fahn S , Martinez-Martin P , Poewe W , Sampaio C , Stern MB , Dodel R , Dubois B , Holloway R , Jankovic J , Kulisevsky J , Lang AE , Lees A , Leurgans S , LeWitt PA , Nyenhuis D , Olanow CW , Rascol O , Schrag A , Teresi JA , Hilten JJ van , LaPelle N , Movement Disorder Society UPDRS Revision Task Force ((2008) ) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 23: , 2129–2170. |
[12] | Hamilton M ((1960) ) A rating scale for depression. J Neurology Neurosurg Psychiatry 23: , 56–62. |
[13] | Whitfield-Gabrieli S , Nieto-Castanon A ((2012) ) Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2: , 125–141. |
[14] | Lancaster JL , Tordesillas-Gutiérrez D , Martinez M , Salinas F , Evans A , Zilles K , Mazziotta JC , Fox PT ((2007) ) Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp 28: , 1194–1205. |
[15] | Desikan RS , Ségonne F , Fischl B , Quinn BT , Dickerson BC , Blacker D , Buckner RL , Dale AM , Maguire RP , Hyman BT , Albert MS , Killiany RJ ((2006) ) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31: , 968–980. |
[16] | Winkler AM , Ridgway GR , Webster MA , Smith SM , Nichols TE ((2014) ) Permutation inference for the general linear model. Neuroimage 92: , 381–397. |
[17] | Obeso JA , Rodríguez-Oroz MC , Benitez-Temino B , Blesa FJ , Guridi J , Marin C , Rodriguez M ((2008) ) Functional organization of the basal ganglia: Therapeutic implications for Parkinson’s disease. Mov Disord 23: , S548–S559. |
[18] | Galvan A , Devergnas A , Wichmann T ((2015) ) Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front Neuroanat 9: , 5. |
[19] | Furmark T , Tillfors M , Marteinsdottir I , Fischer H , Pissiota A , Långström B , Fredrikson M ((2002) ) Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiat 59: , 425–433. |
[20] | Klumpp H , Fitzgerald DA , Phan KL ((2013) ) Neural predictors and mechanisms of cognitive behavioral therapy on threat processing in social anxiety disorder. Prog Neuropsychopharmacol Biol Psychiatry 45: , 83–91. |
[21] | Moonen AJH , Wijers A , Dujardin K , Leentjens AFG ((2017) ) Neurobiological correlates of emotional processing in Parkinson’s disease: A systematic review of experimental studies. J Psychosom Res 100: , 65–76. |
[22] | Niendam TA , Laird AR , Ray KL , Dean YM , Glahn DC , Carter CS ((2012) ) Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cognitive Affect Behav Neurosci 12: , 241–268. |
[23] | Marek S , Dosenbach NUF ((2018) ) The frontoparietal network: Function, electrophysiology, and importance of individual precision mapping. Dialogues Clin Neurosci 20: , 133–140. |
[24] | Menon V ((2015) ) Brain mapping: Salience network. Syst 2: , 597–611. |
[25] | Vossel S , Geng JJ , Fink GR ((2014) ) Dorsal and ventral attention systems. Neurosci 20: , 150–159. |
[26] | Månsson KNT , Salami A , Frick A , Carlbring P , Andersson G , Furmark T , Boraxbekk C-J ((2016) ) Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiat 6: , e727–e727. |
[27] | Yuan M , Zhu H , Qiu C , Meng Y , Zhang Y , Shang J , Nie X , Ren Z , Gong Q , Zhang W , Lui S ((2016) ) Group cognitive behavioral therapy modulates the resting-state functional connectivity of amygdala-related network in patients with generalized social anxiety disorder. BMC Psychiatry 16: , 198. |
[28] | Månsson KNT , Carlbring P , Frick A , Engman J , Olsson C-J , Bodlund O , Furmark T , Andersson G ((2013) ) Altered neural correlates of affective processing after internet-delivered cognitive behavior therapy for social anxiety disorder. Psychiatry Res Neuroimaging 214: , 229–237. |
[29] | Månsson KNT , Salami A , Carlbring P , Boraxbekk C-J , Andersson G , Furmark T ((2017) ) Structural but not functional neuroplasticity one year after effective cognitive behaviour therapy for social anxiety disorder. Behav Brain Res 318: , 45–51. |
[30] | Borkovec TD , Sibrava NJ ((2005) ) Problems with the use of placebo conditions in psychotherapy research, suggested alternatives, and some strategies for the pursuit of the placebo phenomenon. J Clin Psychol 61: , 805–818. |




