Parkinsonism-Hyperpyrexia Syndrome and Dyskinesia-Hyperpyrexia Syndrome in Parkinson’s Disease: Two Cases and Literature Review
Abstract
Parkinsonism-hyperpyrexia syndrome (PHS) and dyskinesia-hyperpyrexia syndrome (DHS) are rare but exhibit life-threatening complications in Parkinson’s disease (PD). We herein presented two cases of PD patients and performed a comprehensive and comparative literature review for these two syndromes. The first case was diagnosed as PHS with cerebral salt wasting syndrome caused by abrupt withdrawal of antiparkinsonian medication. Her symptoms were gradually remitted with reinstitution of the medication. The second one was an early-stage PD patient diagnosed as DHS in association with abuse of antiparkinsonian drugs. Her symptoms were gradually remitted with reduced dosage of dopaminergic drugs. Results of literature reviews revealed a total of 56 and 13 cases of PHS and DHS, respectively, and they were more likely to occur in elderly and long-term PD patients. These two syndromes showed different female-to-male ratio, similar mortality, and different recovery time. There were stark differences between PHS and DHS, including triggers (abrupt drug stoppage versus drug abuse), symptoms (worsened tremor and rigidity versus continuous dyskinesia), and treatment (drug reinstitution versus drug reduction). In summary, our reports and the review provide new insights into PHS and DHS in association with PD and may facilitate rapid discrimination of the syndromes for timely and proper treatment to reduce mortality.
BACKGROUND
Parkinson’s disease (PD) is a common neurodegenerative disease characterized by classical motor features of parkinsonism and a progressive loss of dopaminergic neurons in the substantia nigra pars compacta. The clinical challenges of PD include difficulties to accurately diagnose at the earliest stage and to manage symptoms at later stages [1]. Acute critical syndromes may occur in PD patients [2]. For instance, parkinsonism-hyperpyrexia syndrome (PHS), also known as malignant syndrome or akinetic crisis [3], is often caused by abrupt withdrawal of dopaminergic drugs. Dyskinesia-hyperpyrexia syndrome (DHS), another acute complication of PD, was first defined as an emergency in 2010 [4] and often caused by antiparkinsonian drug abuse. Besides, there are a number of other factors that provoke PHS and DHS.
Both PHS and DHS are rare but life-threatening complications of PD. Compared with PHS, DHS is even rarer. Hyperthermia occurs in both PHS and DHS. Although hyperthermia is believed to be resulted from massive dyskinetic movements [4], it is also considered to be attributed to dysfunction of central thermoregulation [5]. Many pathological processes in PD may result in abnormal thermoregulation. Autonomic dysfunction, a common non-motor symptom, may lead to abnormal sweating and skin cooling in high temperature [6, 7]. Hypothalamic dopamine release, which is disturbed in PD patients, may increase when temperature rises [8]. Indeed, autonomic dysfunction and altered metal status are often observed in patients with PHS and DHS. However, pathological mechanisms by which PHS and DHS occur in PD remain unclear.
Herein, we reported two cases of PD patients who were diagnosed with PHS and DHS, as well as performed a comprehensive literature review and a comparative analysis of these two syndromes.
CASE DESCRIPTION
Case 1: PD with PHS
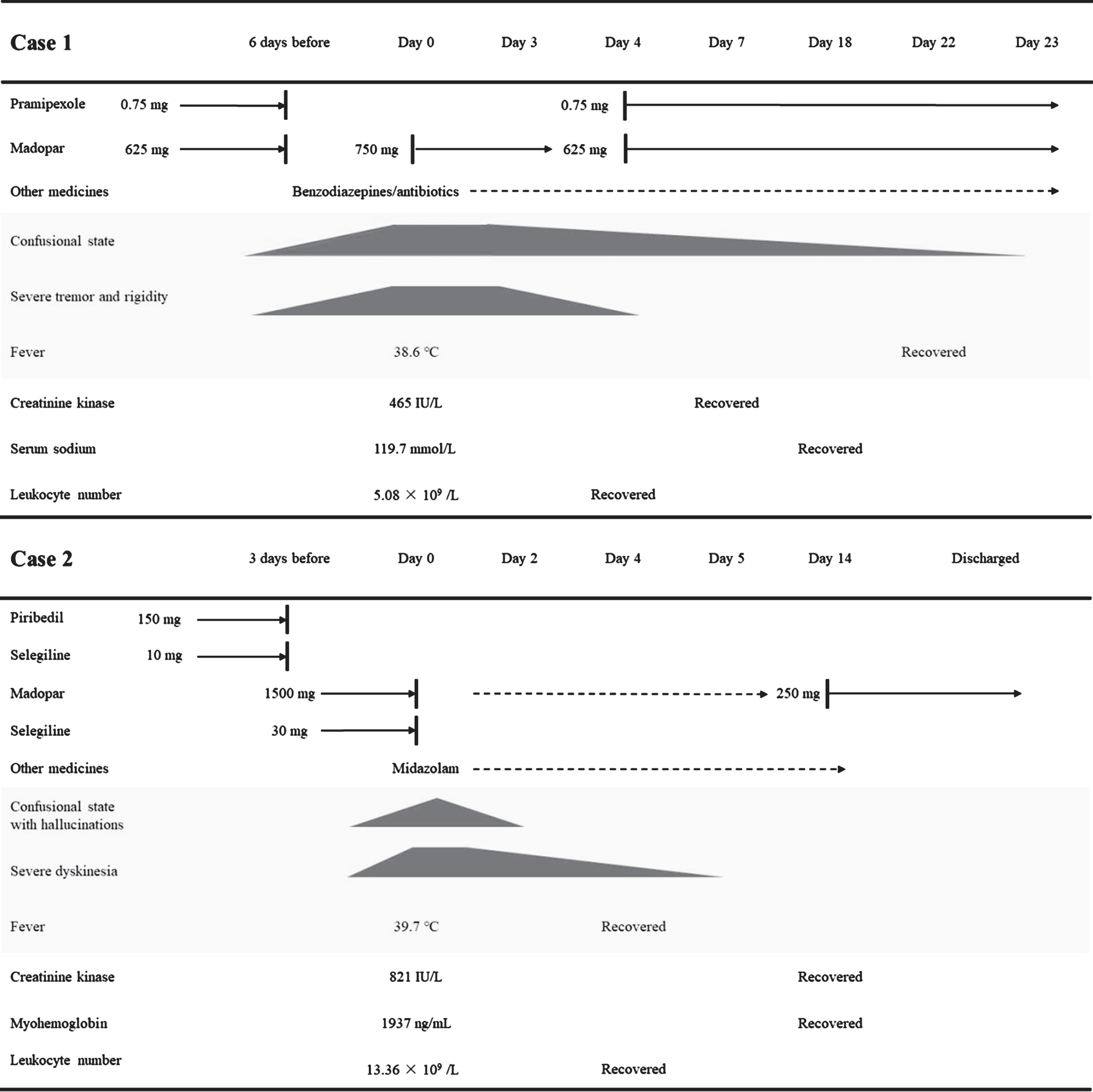
The patient was a 57-year-old woman with a 6-year history of PD. She had regular follow-up visits to the Second Affiliated Hospital of Wenzhou Medical University. The patient has been treated with pramipexole (0.75 mg/day) and madopar (625 mg/day) since the age of 56. Her medical history was unremarkable. The patient was transported to the emergency department because she developed a confusional state, fever, diaphoresis, and severe tremor. Six days before, she discontinued the antiparkinsonian prescription drugs herself because of slight dyskinesia.
In the emergency department with a confusional state, her body temperature was 38.6°C and heart rate was 102 beats per minute. Neurological examinations showed that she developed severe tremor and rigidity on her four limbs, dysphagia, and diaphoresis. Meningeal and other neurological signs were unremarkable. Blood tests showed high creatinine kinase level (465 IU/L), low serum sodium level (119.7 mmol/L), and low serum uric acid level (61μmol/L). Leukocyte number (5.08×109 /L) was within the normal range. Urinalysis showed normal urine specific gravity (1.024), high urine sodium level (415.2 mmol per 24 h), and high urine chloride level (415.7 mmol per 24 h). Bacteriological culture of blood was negative.
We diagnosed the patient as PHS. The diagnosis of cerebral salt wasting syndrome (CSWS) was based on low serum levels of sodium and uric acid, normal urine specific gravity, and high urine levels of sodium and chloride. For PHS, she was treated with madopar (250 mg; thrice daily) via a nasogastric feeding tube and sedated with benzodiazepines. For CSWS, she was given intravenous fluids and sodium supplementation. During the hospitalization, the patient developed pulmonary infection and urinary tract infection such that antibiotics were also administered. Her clinical conditions were worsened in the first few days but were then gradually improved. Her body temperature returned to 37.0°C on day 22 after the hospitalization. The leukocyte number rose in the first three days and returned to normal on day 4. The rise and recovery might be caused by the infection and the use of antibiotics, respectively. Creatinine kinase and serum sodium levels became normal on day 7 and day 18, respectively. As a note, serum sodium level was declined from day 2 to day 4 even with sodium supplementation. Because her tremor was remitted on day 4, we prescribed the patient with madopar (625 mg/day) and pramipexole (0.75 mg/day). Since then, her rigidity and mental state were improved steadily. On day 23, she was able to follow instructions and perform rehabilitation training. She has been taking antiparkinsonian medication as prescribed and returning for follow-up visits regularly since being discharged from the hospital.
Case 2: PD with DHS
The patient aged 74 when she visited the Second Affiliated Hospital of Wenzhou Medical University and was diagnosed as PD. We treated the patient with piribedil (150 mg/day) and selegiline (10 mg/day) and she did not show symptoms of motor fluctuation. She had displayed bradykinesia and resting tremor in her left limbs in the past 4 years. Her medical history included hypertension, diabetes, and osteoporosis. In a September afternoon, the patient was transported to the emergency department due to severe choreiform dyskinesia, hyperthermia, and hallucination in the past 9 hours. Prior to the emergency visit, the patient disregarded doctor’s prescription and took madopar (1500 mg/day; prescribed from another hospital) and selegiline (30 mg/day) by her own decision for 3 consecutive days.
In the emergency department, she was in a confusional state with hallucination. Her body temperature was 39.7°C and heart rate was 123 beats per minute. Neurological examinations revealed continuous dyskinesia over her head, trunk, and four limbs, with her skin being sweaty. Meningeal and other neurological signs were unremarkable. Blood tests showed high levels of creatinine kinase (821 IU/L), myohemoglobin (1937 ng/mL), and leukocytes (13.36×109/L). Aspertate aminotransferase and creatinine levels were slightly elevated (49 U/L and 101μmol/L, respectively). Chest computerized tomography and cranial magnetic resonance imaging were negative. Bacteriological culture of blood was negative.
We diagnosed the patient as DHS. She was sedated with intravenous midazolam infusion and hydrated with normal saline. Oral antiparkinsonian drugs were suspended on day 0. In an attempt to prevent from being rebounded to PHS, we monitored the patient’s condition and gave her a small dose of madopar (62.5 mg) when mild parkinsonism symptoms appeared. Physical antipyretic measures were administered to lower her body temperature. Since then, her clinical condition had gradually improved. The hallucination disappeared on day 2. On day 4, her body temperature (37.2°C) and leukocyte number (7.79×109 /L) were back to normal. Her dyskinesia was remitted completely on day 5. Creatinine kinase and myohemoglobin levels became normal on day 14 (207 IU/L and 165 ng/mL, respectively). Before she was discharged from the hospital, we prescribed her with a low dose of madopar (250 mg/day). Since then, the patient has been taking antiparkinsonian medication as prescribed and showing stable conditions in the telephone follow-ups.
The study was approved by the Ethics Committee of the Second Affiliated Hospital and Yuying Children’s Hospital, Wenzhou Medical University. Written informed consents for publication were obtained from the patients.
LITERATURE REVIEW AND DISCUSSION
PD patients may experience life-threatening complications such as PHS and DHS. These two syndromes share significant similarities but also show major differences in several aspects including causes, clinical manifestations, and treatments. As summarized in Fig. 1, we herein present two cases of patients who developed PHS or DHS. We then review all known up-to-date cases of PHS and DHS in PD and provide a comparative analysis of these two syndromes.
Fig. 1
Summary of the symptoms and treatments for these two cases. Day 0, the day of hospitalization; dashed arrow line, taken when needed; solid arrow line, taken daily; dark patterns, reduced severity with lower height.
Literature related to PHS and DHS in PD were searched in Medline via PubMed up to May 1, 2022. The searching term for PHS was “(((((neuroleptic malignant-like syndrome) OR (Neuroleptic-like Malignant Syndrome)) OR (Parkinson hyperpyrexia syndrome)) OR (Parkinsonism hyperpyrexia syndrome)) OR ((((Parkinson’s Disease[Title/Abstract]) OR (Parkinson Disease[Title/Abstract])) OR (Parkinsonism[Title/Abstract])) AND (((((Fever) OR (Hyperpyrexia)) OR (Pyrexia)) OR (Pyrexias)) OR (malignant syndrome))))”. The searching term for DHS was “(((((fever[Title/Abstract]) OR (hyperpyrexia[Title/Abstract])) OR (pyrexia[Title/Abstract])) OR (pyrexias[Title/Abstract])) AND (((dyskinesia[Title/Abstract]) OR (dyskinesias[Title/Abstract])) OR (hyperkinetic[Title/Abstract])))”. To increase search hits, the “Parkinson”-associated terms were not included for the DHS search. As a result, 688 and 141 literatures on PHS and DHS were obtained, respectively. After additional title and abstract screening, we eventually retrieved 48 articles for PHS and 9 for DHS (Supplementary Tables 1 and 2).
PHS was first described as a neuroleptic malignant syndrome-like syndrome in a PD patient after discontinuation of his antiparkinsonian medication [9]. As summarized in Table 1 and Supplementary Table 1 including our case, 56 such cases have been reported since 1981 and twenty-four of them are women [10–56]. The onset of PHS ranges from 43 to 79 years of age with PD duration between 1 to 25 years. Seven of the reports with 10 patients recorded the onset of PHS in summertime [10, 11, 16, 17, 23, 26, 50]. The most common cause of PHS is determined to be the reduction or withdrawal of antiparkinsonian medication (26 cases), followed by battery depletion of deep brain stimulation (DBS) impulse generator (7 cases). Other triggers include experiencing the “Off” state, premenstrual period, diabetic coma, heatwave, cessation of fava bean intake, hyponatremia, infection, constipation, diarrhea, and drinking too little fluids. In recent years, the DBS surgery number for PD patients has been increasing dramatically. Thus, it should be brought into attention as to the likelihood of PHS due to perioperative antiparkinsonian drug discontinuation and DBS stimulator battery depletion. Given the relatively long operation time of DBS, anesthesia, surgery, and stress may become potential triggers for PHS. It may be important to maintain a certain dose of antiparkinsonian medication during the perioperative period. Compared with traditional drug therapy, the efficacy of DBS is more effective and stable to control parkinsonism. As a result, follow-up visits of such patients may become irregular. Therefore, clinicians should remind patients to have follow-up visits regularly as well as to warn them of potentially severe outcomes if the battery power is depleted.
Table 1
Comparative analysis of PHS and DHS in PD patients
| PHS | DHS | |
| Subject, n | 56 | 13 |
| Gender, F/M | 24/32 | 10/3 |
| Age, y (mean±SD) | 63.2±9.2 | 71.3±6.0 |
| PD duration, y (mean±SD) | 12.0±5.8 | 17.1±8.2 |
| Mortality, n (%) | 12 (21.4) | 2 (15.4) |
| Recovery ratio, n (%) | 44 (78.6) | 11 (84.6) |
| Recovery time, days (IR)a | 13 (5–22) | 4 (3.5–6) |
| Triggers (%)b | Reduction/withdrawal of antiparkinsonian medication (46.4) | Antiparkinsonian drug change/abuse (38.5) |
| Battery depletion of DBS impulse generator (12.5) | Heatwave (38.5) | |
| Heatwave (5.4) | Infection (23.1) | |
| Constipation/diarrhea (5.4) | Trauma (15.4) | |
| Infection (3.6) | Gastrointestinal dysmotility (7.7) | |
| Premenstrual period (1.8) | ||
| Diabetic coma (1.8) | ||
| Experience of the “Off” state (1.8) | ||
| Hyponatremia (1.8) | ||
| Without common trigger (16.1) | ||
| Manifestations (%)b | Hyperthermia (98.2) | Hyperthermia (100) |
| Worsened tremor and rigidity (94.6) | Continuous dyskinesia (100) | |
| Altered mental status (73.2) | Altered mental status (76.9) | |
| Autonomic dysfunction (76.8) | Autonomic dysfunction (46.2) | |
| Diaphoresis (48.2) | Diaphoresis (23.1) | |
| Myoclonus (7.1) | Dehydration (15.4) | |
| Rhabdomyolysis (5.4) | Rhabdomyolysis (15.4) | |
| Dystonia (3.6) | ||
| Dehydration (3.6) | ||
| Treatments | Reinstitution of antiparkinsonian medication, vital function support, intravenous fluids, antipyretic drugs, and physical antipyretic measures | Reduction of dopaminergic drugs, vital function support, intravenous fluids, antipyretic drugs, and physical antipyretic measures |
aThe recovery time with an exact number is included for calculation. bThe percentage numbers are calculated in relation to the total subjects. DBS, deep brain stimulation; DHS, dyskinesia-hyperpyrexia syndrome; F, female; IR, interquartile range; M, male; PHS, parkinsonism-hyperpyrexia syndrome; SD, standard deviation.
The main clinical manifestations of PHS are hyperthermia, worsened tremor and rigidity, altered mental status, autonomic dysfunction, and diaphoresis (Table 1 and Supplementary Table 1). Other less common symptoms include dysphagia, myoclonus, rhabdomyolysis, dystonia, and dehydration. Our patient is additionally diagnosed with CSWS. Hyponatremia has been reported in another case and considered as a cause of PHS [28]. We believe that hyponatremia is an outcome of CSWS resulting from PHS because serum sodium level continues to decline within the first four days even with sodium supplementation to the patient. The treatment of PHS includes vital function support, reinstitution of antiparkinsonian medication, intravenous fluids, empiric antibiotics, benzodiazepines, antipyretic drugs, and physical antipyretic measures. Among the 56 cases, 12 patients died shortly or in a few days and 44 patients were recovered from the symptoms within 2–32 days.
A total of 13 PD patients with DHS has been reported including our case [4, 5, 57–63] (Table 1 and Supplementary Table 2). The onset of DHS ranges from 62 to 80 years of age, and 10 of them are women. According to 4 reports, 6 patients developed the syndrome in summer [5, 59–61]. Two cases including ours occurred in early autumn when the ambient temperature might still be relatively high [57]. Indeed, heatwave is one of the common triggers of DHS. It has been conceived that DHS usually occurs in advanced PD patients [64], with disease duration ranging from 10 to 34 years. Nonetheless, our case suggests that DHS may also occur in early-stage PD patients.
Box 1.
Suggested diagnostic criteria and management schemes for PHS
Diagnosis
1. Clear triggers before the onset. These include reduction/withdrawal of antiparkinsonian medication, battery depletion of DBS impulse generator, and heatwave. Of note, a small percentage of patients may lack any of such triggers.
2. Core clinical manifestations are required. These include hyperthermia, worsened parkinsonism, and elevated creatinine kinase.
3. At least two of the following clinical manifestations are required. These include altered mental status, autonomic dysfunction, diaphoresis, myoclonus, rhabdomyolysis, dystonia, and dehydration.
4. The following conditions should be excluded: neuroleptic malignant syndrome, serotonin syndrome, dyskinesia-hyperpyrexia syndrome, heat stroke, intracranial infection, autoimmune encephalitis, septicemic shock, drug intoxication, and thyroid crisis.
5. An alternative syndrome should be considered if the expert physician, based on full clinical manifestations and auxiliary assessments, feels that an alternative condition is more likely than PHS.
Management
1. Treat the underlying triggers immediately.
2. Provide adequate supportive treatments including vital function support, intravenous fluids, antipyretic drugs, and antipyretic measures
3. Antibiotics treatment is not necessary, but spectrum antibiotics should be applied immediately if the patient is infected.
4. Oral or nasogastric dopaminergic drugs should be used immediately when the diagnosis of PHS is confirmed.
5. Delirium in patients should be treated with intravenous benzodiazepines infusion (Taken when needed).
6. If the patient develops multiple organ failure, intensive care unit treatment and multidisciplinary care should be initiated immediately.
Two most common provocation factors for DHS are antiparkinsonian drug change or abuse and heatwave (5 cases each; Table 1 and Supplementary Table 2). Excessive dopaminergic stimulation is destructive given that PD patients are defective in the maintenance of dopamine status. Other DHS triggers include infection, trauma, and gastrointestinal dysmotility. Clinical manifestations of DHS include hyperthermia, continuous dyskinesia, altered mental status, and to a less extent, autonomic dysfunction, diaphoresis, dehydration, and rhabdomyolysis. The treatment of DHS includes vital function support, reduction of dopaminergic drugs, intravenous infusions, antipyretic drugs, and physical antipyretic measures. Among the 13 cases, 2 patients died in a few days due to pneumonia and renal failure or acute pulmonary edema [60]. The remaining 11 patients were recovered within 2–10 days.
Both PHS and DHS are prone to occur in elderly PD patients with long disease duration (Table 1). Although DHS mainly occurs in females, PHS is predominantly found in males. The recovery rate for both syndromes is about 80% despite a faster recovery in DHS than in PHS patients. Clinical manifestations of PHS and DHS are mostly similar, including raised creatinine kinase. The raised kinase level may occur in rhabdomyolysis, myositis, myocardial infarction, muscular dystrophy, etc. Diagnosis of rhabdomyolysis requires not only high creatinine kinase but also elevated myohemoglobin in blood and urine. Thus, raised creatinine kinase alone does not mean the occurrence of rhabdomyolysis, which is only present in a small percentage of PHS and DHS cases. However, a stark difference between these two syndromes is that worsened tremor and rigidity is dominated in PHS, but continuous dyskinesia is exclusively found in DHS patients. PHS may be induced by abrupt stoppage in the antiparkinsonian treatment such as drug withdrawal or DBS stimulator power loss whereas DHS is plausibly induced by the abuse of antiparkinsonian drugs. Accordingly, the primary treatment for PHS is drug reinstitution and for DHS is drug reduction. Other auxiliary treatments are basically alike. Therefore, careful inquiry of medication history and appropriate neurological examinations are indispensable for rapid recognition and treatment of the syndromes. No diagnostic criteria are available for these two conditions to the best of our knowledge. We herein suggest diagnosis criteria for PHS (Box 1) and DHS (Box 2) based on the reported cases and our aforementioned rationales. Structured suggestions are also provided toward the management of PHS (Box 1) and DHS (Box 2) for the reference of clinicians.
Box 2.
Listing’s law
Suggested diagnostic criteria and management schemes for DHS
Diagnosis
1. Clear triggers before the onset. These include antiparkinsonian drug change/abuse, heatwave, infection, trauma, and gastrointestinal dysmotility. Of note, a small percentage of patients may lack any of such triggers.
2. Core clinical manifestations are required. These include hyperthermia, continuous dyskinesia, and elevated creatinine kinase.
3. At least one of the following clinical manifestations are required. These include altered mental status, autonomic dysfunction, diaphoresis, dehydration, and rhabdomyolysis.
4. The following conditions should be excluded: neuroleptic malignant syndrome, serotonin syndrome, parkinsonism-hyperpyrexia syndrome, heat stroke, intracranial infection, autoimmune encephalitis, septicemic shock, drug intoxication, and thyroid crisis.
5. An alternative syndrome should be considered if the expert physician, based on full clinical manifestations and auxiliary assessments, feels that an alternative condition is more likely than DHS.
Management
1. Treat the underlying triggers immediately.
2. Provide adequate supportive treatments including vital function support, intravenous fluids, antipyretic drugs, and antipyretic measures.
3. Antibiotics treatment is not necessary, but spectrum antibiotics should be applied immediately if the patient is infected.
4. Carefully reduce antiparkinsonian drugs while avoiding rebound to PHS.
5. Delirium in patients should be treated with intravenous benzodiazepines infusion (Taken when needed).
6. If the patient develops multiple organ failure, intensive care unit treatment and multidisciplinary care should be initiated immediately.
The mortality rates of the reported cases are 21.4% and 15.4% for PHS and DHS, respectively (Table 1). Among the 14 deceased patient cases (Supplementary Tables 1 and 2), there are a total of 12 aged 50 or older and 12 with more than 9 years of PD duration, respectively. The causes of death and number are hyperthermic coma (3), respiratory failure (10), renal failure (7), heart failure (3), disseminated intravascular coagulation (2), and septicemic shock (1). These data suggest that patients of older age and longer disease duration may be more susceptible to develop multisystem organ failure and malignant outcome. Because some cases may have died before being diagnosed as PHS or DHS and some may be reluctant to get the death endpoint to be published, the mortality is likely to be underreported. Patients may be treated on general wards in general. But with the mortality rate as high as it is, we recommend treating the underlying triggers immediately with adequate supportive treatments. Those with multiple organ failure should be immediately initiated for intensive care unit treatment and multidisciplinary care in monitored settings to reduce potential mortality (Boxes 1 and 2).
In summary, we herein present two cases of PD patients with hyperpyrexia. One was PHS coupled with CSWS caused by abrupt withdrawal of the antiparkinsonian medication, and the other was drug abuse-induced DHS occurred in early-stage PD. Our reports and the comparative review provide new and updated insights into PHS and DHS in PD and may facilitate rapid discrimination of the syndromes for timely and proper treatment to reduce mortality.
ACKNOWLEDGMENTS
The authors are grateful to hospital personnel who took care of the patients. The study was supported in part by fundings from Wenzhou Municipal Science and Technology Bureau (Y2020065, and Y20180136), Fundamental Research Funds for Wenzhou Medical University (KYYW202030), and Novel Technology Program of the Second Affiliated Hospital and Yuying Children’s Hospital (2022014).
CONFLICT OF INTEREST
The authors declare that there is no potential conflict of interest.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-223362.
REFERENCES
[1] | Kalia LV , Lang AE ((2015) ) Parkinson’s disease. Lancet 386: , 896–912. |
[2] | Simonet C , Tolosa E , Camara A , Valldeoriola F ((2020) ) Emergencies and critical issues in Parkinson’s disease. Pract Neurol 20: , 15–25. |
[3] | Thomas A , Onofrj M ((2005) ) Akinetic crisis, acute akinesia, neuroleptic malignant-like syndrome, Parkinsonism-hyperpyrexia syndrome, and malignant syndrome are the same entity and are often independent of treatment withdrawal.1671; author reply. Mov Disord 20: , 1671–1672. |
[4] | Gil-Navarro S , Grandas F ((2010) ) Dyskinesia-hyperpyrexia syndrome: Another Parkinson’s disease emergency. Mov Disord 25: , 2691–2692. |
[5] | Herreros-Rodriguez J , Sanchez-Ferro A ((2016) ) Summertime dyskinesia-hyperpyrexia syndrome: The “dual heat” hypothesis. Clin Neuropharmacol 39: , 210–211. |
[6] | Leclair-Visonneau L , Magy L , Volteau C , Clairembault T , Le Dily S , Preterre C , Peyre A , Damier P , Neunlist M , Pereon Y , Derkinderen P ((2018) ) Heterogeneous pattern of autonomic dysfunction in Parkinson’s disease. J Neurol 265: , 933–941. |
[7] | Wang JY , Wang MY , Liu RP , Li Y , Zhang WY , Ovlyakulov B , Zhang X , Zhu JH ((2020) ) Association analyses of autonomic dysfunction and sympathetic skin response in motor subtypes of Parkinson’s disease. Front Neurol 11: , 577128. |
[8] | Kao TY , Chio CC , Lin MT ((1994) ) Hypothalamic dopamine release and local cerebral blood flow during onset of heatstroke in rats.2483-2486; discussion. Stroke 25: , 2486–2487. |
[9] | Toru M , Matsuda O , Makiguchi K , Sugano K ((1981) ) Neuroleptic malignant syndrome-like state following a withdrawal of antiparkinsonian drugs. J Nerv Ment Dis 169: , 324–327. |
[10] | Sechi GP , Tanda F , Mutani R ((1984) ) Fatal hyperpyrexia after withdrawal of levodopa. Neurology 34: , 249–251. |
[11] | Friedman JH , Feinberg SS , Feldman RG ((1985) ) A neuroleptic malignantlike syndrome due to levodopa therapy withdrawal. JAMA 254: , 2792–2795. |
[12] | Pfeiffer RF , Sucha EL ((1989) ) “On-off”-induced lethal hyperthermia. Mov Disord 4: , 338–341. |
[13] | Reutens DC , Harrison WB , Goldswain PR ((1991) ) Neuroleptic malignant syndrome complicating levodopa withdrawal. Med J Aust 155: , 53–54. |
[14] | Keyser DL , Rodnitzky RL ((1991) ) Neuroleptic malignant syndrome in Parkinson’s disease after withdrawal or alteration of dopaminergic therapy. Arch Intern Med 151: , 794–796. |
[15] | Yamawaki Y , Ogawa N ((1992) ) Successful treatment of levodopa-induced neuroleptic malignant syndrome (NMS) and disseminated intravascular coagulation (DIC) in a patient with Parkinson’s disease. Intern Med 31: , 1298–1302. |
[16] | Mizuta E , Yamasaki S , Nakatake M , Kuno S ((1993) ) Neuroleptic malignant syndrome in a parkinsonian woman during the premenstrual period. Neurology 43: , 1048–1049. |
[17] | Saeki H , Muneta S , Kobayashi T ((1998) ) [Malignant syndrome associated with disseminated intravascular coagulation and a high level of amylase in serum, followed by diabetic coma in an elderly patient with Parkinson’s disease during L-dopa therapy]. Nihon Ronen Igakkai Zasshi 35: , 139–144. |
[18] | Cao L , Katz RH ((1999) ) Acute hypernatremia and neuroleptic malignant syndrome in Parkinson disease. Am J Med Sci 318: , 67–68. |
[19] | Gordon PH , Frucht SJ ((2001) ) Neuroleptic malignant syndrome in advanced Parkinson’s disease. Mov Disord 16: , 960–962. |
[20] | Linazasoro G ((2003) ) Malignant syndrome in Parkinson’s disease. Parkinsonism Relat Disord 10: , 115–116. |
[21] | Linazasoro G , Van Blercom N , Castro A , Dapena MD ((2004) ) Subthalamic deep brain stimulation masking possible malignant syndrome in Parkinson disease. Neurology 63: , 589–590. |
[22] | Stotz M , Thummler D , Schurch M , Renggli JC , Urwyler A , Pargger H ((2004) ) Fulminant neuroleptic malignant syndrome after perioperative withdrawal of antiParkinsonian medication. Br J Anaesth 93: , 868–871. |
[23] | Gaig C , Marti MJ , Tolosa E , Gomez-Choco MJ , Amaro S ((2005) ) Parkinsonism-hyperpyrexia syndrome not related to antiparkinsonian treatment withdrawal during the 2003 summer heat wave. J Neurol 252: , 1116–1119. |
[24] | Ladha SS , Walker R , Shill HA ((2005) ) Case of neuroleptic malignant-like syndrome precipitated by abrupt fava bean discontinuance. Mov Disord 20: , 630–631. |
[25] | Ward C ((2005) ) Neuroleptic malignant syndrome in a patient with Parkinson’s disease: A case study. J Neurosci Nurs 37: , 160–162. |
[26] | Douglas A , Morris J ((2006) ) It was not just a heatwave! Neuroleptic malignant-like syndrome in a patient with Parkinson’s disease. Age Ageing 35: , 640–641. |
[27] | Meagher LJ , McKay D , Herkes GK , Needham M ((2006) ) Parkinsonism-hyperpyrexia syndrome: The role of electroconvulsive therapy. J Clin Neurosci 13: , 857–859. |
[28] | Factor SA ((2007) ) Fatal Parkinsonism-hyperpyrexia syndrome in a Parkinson’s disease patient while actively treated with deep brain stimulation. Mov Disord 22: , 148–149. |
[29] | Chandran CJ ((2008) ) Malignant syndrome in Parkinson’s disease without dopaminergic drug withdrawal. Ann Indian Acad Neurol 11: , 248–250. |
[30] | Rajabally YA , Ramlackhansingh A , Fraser M , Abbott RJ ((2009) ) Neuroleptic malignant syndrome and acute motor axonal neuropathy after Campylobacter jejuni infection. Neurophysiol Clin 39: , 135–138. |
[31] | Newman EJ , Grosset DG , Kennedy PG ((2009) ) The parkinsonism-hyperpyrexia syndrome. Neurocrit Care 10: , 136–140. |
[32] | Kim JH , Kwon TH , Koh SB , Park JY ((2010) ) Parkinsonism-hyperpyrexia syndrome after deep brain stimulation surgery: Case report. Neurosurgery 66: , E1029. |
[33] | Federico Landriel EG , Ajler P , Ciraolo C ((2011) ) Deep brain stimulation surgery complicated by Parkinson hyperpyrexia syndrome. Neurol India 59: , 911–912. |
[34] | Kadowaki T , Hashimoto K , Suzuki K , Watanabe Y , Hirata K ((2011) ) Case report: Recurrent parkinsonism-hyperpyrexia syndrome following discontinuation of subthalamic deep brain stimulation. Mov Disord 26: , 1561–1562. |
[35] | Themistocleous MS , Boviatsis EJ , Stavrinou LC , Stathis P , Sakas DE ((2011) ) Malignant neuroleptic syndrome following deep brain stimulation surgery: A case report. J Med Case Rep 5: , 255. |
[36] | Wu YF , Kan YS , Yang CH ((2011) ) Neuroleptic malignant syndrome associated with bromocriptine withdrawal in Parkinson’s disease–a case report.301 e. Gen Hosp Psychiatry 33: , 307–308. |
[37] | Ogawa E , Sakakibara R , Kishi M , Tateno F ((2012) ) Constipation triggered the malignant syndrome in Parkinson’s disease. Neurol Sci 33: , 347–350. |
[38] | Arora A , Fletcher P ((2013) ) Parkinsonism hyperpyrexia syndrome caused by abrupt withdrawal of ropinirole. Br J Hosp Med (Lond) 74: , 698–699. |
[39] | Hocker S , Kenney DL , Ramar K ((2013) ) Parkinsonism-hyperpyrexia syndrome: Broadening our differential diagnosis in the ICU. Neurol Clin Pract 3: , 535–538. |
[40] | Neuneier J , Barbe MT , Dohmen C , Maarouf M , Wirths J , Fink GR , Timmermann L ((2013) ) Malignant deep brain stimulation-withdrawal syndrome in a patient with Parkinson’s disease. Mov Disord 28: , 1640–1641. |
[41] | Urasaki E , Fukudome T , Hirose M , Nakane S , Matsuo H , Yamakawa Y ((2013) ) Neuroleptic malignant syndrome (parkinsonism-hyperpyrexia syndrome) after deep brain stimulation of the subthalamic nucleus. J Clin Neurosci 20: , 740–741. |
[42] | Wei L , Chen Y ((2014) ) Neuroleptic malignant-like syndrome with a slight elevation of creatine-kinase levels and respiratory failure in a patient with Parkinson’s disease. Patient Prefer Adherence 8: , 271–273. |
[43] | Artusi CA , Merola A , Espay AJ , Zibetti M , Romagnolo A , Lanotte M , Lopiano L ((2015) ) Parkinsonism-hyperpyrexia syndrome and deep brain stimulation. J Neurol 262: , 2780–2782. |
[44] | Govindappa ST , Abbas MM , Hosurkar G , Varma RG , Muthane UB ((2015) ) Parkinsonism hyperpyrexia syndrome following deep brain stimulation. Parkinsonism Relat Disord 21: , 1284–1285. |
[45] | Campa D , Ariello M , Castaldo R , Zibella F , Ferrazza P , Del Gaudio S ((2016) ) A fatal case of neuroleptic malignant syndrome after paralytic bowel in a patient taking antiparkinson medication. Eur J Case Rep Intern Med 3: , 000368. |
[46] | Lee DH , Moon JM , Cho YS ((2017) ) Malignant syndrome in Parkinson disease similar to severe infection. Korean J Crit Care Med 32: , 359–362. |
[47] | Liu CJ , Crnkovic A , Dalfino J , Singh LY ((2017) ) Whether to proceed with deep brain stimulator battery change in a patient with signs of potential sepsis and Parkinson hyperpyrexia syndrome: A case report. A A Case Rep 8: , 187–191. |
[48] | Sauer T , Wolf ME , Blahak C , Capelle HH , Krauss JK ((2017) ) Neuroleptic-like malignant syndrome after battery depletion in a patient with deep brain stimulation for secondary parkinsonism. Mov Disord Clin Pract 4: , 629–631. |
[49] | Akcakaya MO , Akcakaya NH , Kasimcan MO , Kiris T ((2018) ) Life-threatening parkinsonism-hyperpyrexia syndrome following bilateral deep brain stimulation of the subthalamic nucleus. Neurol Neurochir Pol 52: , 289–292. |
[50] | Sahu H , Manjunath MB , Ray A , Vikram NK ((2018) ) Neuroleptic malignant-like syndrome causing thrombocytopaenia: A rare association. BMJ Case Rep 11: . |
[51] | Grover S , Sathpathy A , Reddy SC , Mehta S , Sharma N ((2018) ) Parkinsonism-hyperpyrexia syndrome: A case report and review of literature. Indian J Psychiatry 60: , 499–503. |
[52] | Azar J , Elinav H , Safadi R , Soliman M ((2019) ) Malignant deep brain stimulator withdrawal syndrome. BMJ Case Rep 12: . |
[53] | Matsuzono K , Baba M , Imai G , Imai H , Fujimoto S ((2019) ) Malignant syndrome triggered by influenza A virus infection in a patient with Parkinson’s disease with improvement after intravenous peramivir treatment. Neurol Sci 40: , 1291–1294. |
[54] | Ryu HS , Yang SY ((2020) ) A case of refractory hypernatremia in the setting of parkinsonism-hyperpyrexia syndrome. Acta Neurol Belg 120: , 989–991. |
[55] | Mori Y , Miura I , Nozaki M , Osakabe Y , Izumi R , Akama T , Kimura S , Yabe H ((2021) ) Electroconvulsive therapy for Parkinson’s disease with depression and neuroleptic malignant syndrome: A case report. Clin Psychopharmacol Neurosci 19: , 572–575. |
[56] | Dos Santos DT , Imthon AK , Strelow MZ , Pille A , Schumacher-Schuh AF ((2021) ) Parkinsonism-hyperpyrexia syndrome after amantadine withdrawal: Case report and review of the literature. Neurologist 26: , 149–152. |
[57] | Taguchi S , Niwa J , Ibi T , Doyu M ((2015) ) [Dyskinesia-hyperpyrexia syndrome in a patient with Parkinson’s disease: A case report]. Rinsho Shinkeigaku 55: , 182–184. |
[58] | Baek MS , Lee HW , Lyoo CH ((2017) ) A patient with recurrent dyskinesia and hyperpyrexia syndrome. J Mov Disord 10: , 154–157. |
[59] | Acebron Sanchez-Herrera F , Garcia-Barragan N , Estevez-Fraga C , Martinez-Castrillo JC , Lopez-Sendon Moreno JL ((2017) ) Dyskinesia-hyperpyrexia syndrome under continuous dopaminergic stimulation. Parkinsonism Relat Disord 36: , 103–104. |
[60] | Sarchioto M , Ricchi V , Melis M , Deriu M , Arca R , Melis M , Morgante F , Cossu G ((2018) ) Dyskinesia-hyperpyrexia syndrome in Parkinson’s disease: A heat shock-related emergency? Mov Disord Clin Pract 5: , 534–537. |
[61] | Novelli A , Di Vico IA , Terenzi F , Sorbi S , Ramat S ((2019) ) Dyskinesia-hyperpyrexia syndrome in Parkinson’s disease with deep brain stimulation and high-dose levodopa/carbidopa and entacapone. Parkinsonism Relat Disord 64: , 352–353. |
[62] | Zu J , Raza HK , Chansysouphanthong T , Xu C , Zhang W , Cui G ((2021) ) Dyskinesia and hyperpyrexia syndrome: A case report and review of the literature. Rev Neurol (Paris) 177: , 710–713. |
[63] | Pitakpatapee Y , Srikajon J , Sangpeamsook T , Srivanitchapoom P ((2021) ) Rhabdomyolysis associated with severe levodopa-induced dyskinesia in Parkinson’s disease: A report of two cases and literature review. Tremor Other Hyperkinet Mov (N Y) 11: , 39. |
[64] | Cossu G , Colosimo C ((2017) ) Hyperkinetic movement disorder emergencies. Curr Neurol Neurosci Rep 17: , 6. |




