The Role of Parkinson Nurses for Personalizing Care in Parkinson’s Disease: A Systematic Review and Meta-Analysis
Abstract
Background:
Quality of life (QoL) of persons with Parkinson’s disease (PD) is diminished by (non-)motor symptoms, that require personalized care. Parkinson Nurses (PN) may be pivotal promoting tailored care offerings. This systematic review and meta-analysis investigates PD care models and aims at furnishing current concepts of PN to offer personalized care.
Objective:
The purpose of this study is to identify the various roles and functions that PN may hold for personalized PD care.
Methods:
We performed a systematic literature review, utilizing: PubMed, Web of Science, The Cochrane Library, and PsycINFO. The review qualitatively evaluated articles, which described personalized care models involving PNs and was guided by the personalized care management model. A meta-analysis compared patient-reported QoL (quantified using the 39-item Parkinson’s Disease Questionnaire) between personalized care interventions involving PN versus standard care with.
Results:
Twenty-seven publications were identified, including six randomized, controlled trials ascertaining with health related QoL (n = 1830 PwPs). The qualitative evaluation revealed that PN contribute to all aspects of personalized care. The meta-analysis showed no improved QoL in personalized care models compared to standard care, thought a great heterogeneity among study design and interventions was outlined (Standardized Mean Difference = –0.8935; 95% Confidence Interval, –2.1177 to 0.3307; z = –1.43, p = 0.1526).
Conclusion:
PN fulfil important functions in personalized PD care. For the future, a clear role definition will be necessary to adjust training for PN across healthcare systems and care settings but especially to realize their full potential for PD care.
INTRODUCTION
Parkinson’s disease (PD), is an incurable neurodegenerative disorder presenting with a heterogenous phenotype including motor- and non-motor symptoms [1]. With increasing disease duration, the majority of patients experience an increased level of symptom burden [2], and a growing demand for medical and social care services. From a healthcare resources demand perspective, PD already ranks among the top ten most resource-intensive brain disorders in Europe with 1.2 million affected people to date [3]. An increasing incidence among older people and the population development forecasts in mind, it is foreseeable, that persons with Parkinson’s disease (PwPs) will have doubled by 2030 [4].
Traditional physician-centered care models for PwPs may reach their limits when degenerative processes disperse and multiple psychosocial needs require individualized treatment strategies. In addition, the increasing number of PwPs is in conflict with the scarcity of medical resources. Ideal and sustainable care services are nowadays considered to be those that are comprehensive but, above all, that are coined to the patients and their environment [5]. The term “personalized care” embodies such care services and has already found its place in science and practice alike [6]. In the broadest sense, can, “personalized care” be defined as “tailored to the needs and preferences of each individual” [7]. Regarding PD care, van Halteren et al. defined the five core elements of personalized care management for PwPs, which are summarized in Table 1.
Table 1
Five dimensions of the personalized care management model for people with Parkinson’s disease
| Dimension | Description |
| Care Coordination | Ensuring that all relevant health information is shared across all healthcare layers, creating a common understanding of care needs of each patient, aligning treatment plans to prevent contradictory disease. Managing and assuring that each discipline is certain about their responsibilities in the management process. |
| Patient Navigation | Proactively guiding and supporting PwPs to find their way through the complex health care system and referring them timely to the appropriate health care provider. |
| Information Provision | Providing PD-related information in oral, written or other form. |
| Proactive Monitoring | Timely detecting first changes in signs or symptoms, allowing for pre-emptive interventions to prevent further worsening of problems and to avoid complications that might lead to emergency department visits, hospital admission and use of unnecessary resources. |
| Process Monitoring | Routinely reviewing and evaluating the care management process regarding adherence to care plans. |
cf. (van Halteren et al. [7]), p. S14.
One might argue that an implementation of these elements in PwPs, requires expertise, networking skills and good oversight of the care process. While no doubt exists that PD care improves with multi-professional collaboration, the question arises which profession may drive the implementation of personalized care elements. There is no uniform definition of the sufficient or ideal composition of a multidisciplinary care team, and a strong heterogeneity exists in practice [8]. Nevertheless, when it comes to achieving care personalization, the role of specialized nurses has been recognized as a pivotal facilitator [9, 10].
In research and practice, such nurses are called Parkinson’s Nurses (PN). In a broad sense, PN are specially trained professionals who deal with individual issues related to the care and support of PwPs [11]. However, a precise description of the tasks PN are supposed to master is ill-defined, and wide range of assignments and roles has been described [12–14]. This heterogeneity is problematic because role clarity bolsters effective interprofessional collaboration, adequate training, and the future perpetuation of job profiles. The aim of this paper is therefore to provide an overview of the role of PN in care personalization for PwPs and explore its impact on quality of life (QoL) in PwPs, using a systematic review approach.
MATERIALS AND METHODS
Review question
This present systematic review and meta-analysis aim to examine the following research questions:
1) What are existing models of personalized care for PwPs in which PN are involved?
2) What is the role of involved PN within existing models of personalized care for PwPs?
3) Can complex, personalized care models improve the QoL of PwPs compared to standard care?
Search
The following databases were utilized: PubMed, The Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science (science and social science citation index), and PsycINFO. The study protocol was registered with PROSPERO on February 11, 2021, and confirmed for registration on March 15, 2021 (CRD42021236755). The search followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) reporting guidelines [15]. The initial search of the databases was performed in April 2021 and a follow-up search was conducted in December 2021 to update the review in a timely manner. Literature search was based on several MeSH terms adapted to the databases to be employed, including: “parkinson’s disease”, “parkinsonian disorders”, “nurse”, “management”, “rehabilitation”, “quality of life”, with individual search terms adapted according to each database (cf. Supplementary Table 1 for PubMed’s exemplary search strategy).
The flow chart of the study selection process is displayed in Fig. 1.
Studies published in English and German language or with English and German language translation available were included. There were no restrictions for inclusion based on date of publication and/or geographical location.
Inclusion/exclusion
For the systematic review, we adopted the following inclusion criteria: 1) Studies qualitatively describing or quantitively evaluating a care model which is tailored towards individual needs and preferences; 2) Studies that addressed the role of PN in the context of personalized care; 3) Setting either inpatient, outpatient or community-based; 4) Sample population comprised adults (≥18 years of age) diagnosed with PD (following United Kingdom Parkinson’s disease Society Brain Bank criteria [16] and the Movement Disorder Society’s clinical diagnostic criteria [17]); any gender included. Exclusion criteria were: 1) Failure meeting the language requirements; 2) Studies not providing primary data (i.e., comments, case series, case reports); 3) full text was unavailable; 4) not relevant to the subject of the review. The following inclusion criteria applied additionally to the meta-analysis: 1) Eligible studies had to be controlled randomized or quasi-randomized; 2) The intervention had to be tailored towards patients’ individual needs and preferences; 3) Use of the PD validated QoL scale (8-/ or 39-item Parkinson’s Disease Questionnaire –PDQ-8/-39).
Outcomes
For the systematic review, we collected the following information: identification of care settings, team composition, descriptions of the care model, intervention and the PN roles/tasks. The dimensions of personalized care delivered by the PN were described based on the five dimensions of the model of personalized care management for PwPs (cf. Table 1) [7, 18]. The identification of dimensions is qualitative in nature [18], which is why there was no quantitative data analysis. For our analysis, models and interventions that documented at least one dimension of personalized care were considered for the review. We added a sixth dimension for tasks that do not match one of the five dimensions but still aims to adapt care to the needs and preferences of PwPs, termed “other”.
The main outcome of the meta-analysis was patient-reported health-related (HR)-QoL. The PDQ-39 and the shortened version (PDQ-8) are widely utilized and validated tools ascertaining QoL in PwPs [19]. Eligible studies had to report PDQ-39/-8 results at 6–24 weeks after randomization. This period was chosen because the power of randomized controlled trials is related to the observation period [20] and the defined period is a common observation period in PD health care studies [8, 21–23]. Since none of the included studies reported the PDQ-8 as an outcome, we will only address the PDQ-39 as an outcome in the following.
Data extraction
Every step of the data extraction process was conducted by two authors independently (M.vM., J.S.). Discrepancies during each step were resolved through discussion with a third researcher (F.T.) and mutual agreement was reached. First, the two authors (M.vM., J.S.) screened independently titles and/or abstracts of studies retrieved using the search strategy. Based on the first screening, full texts of studies meeting inclusion criteria were retrieved and independently assessed for eligibility.
After removal of duplicates, the authors (M.vM.; J.S.) extracted data from every eligible article to a standardized prespecified template. Missing data was requested from study authors (this was once the case; the requested data could be provided by the authors). The template captured study information (authors’ names, year of publication, location), study population (sample size), the intervention (setting, involved professionals, duration (if applicable), and content) and the roles of PN according to the five dimensions of personalized care. For studies eligible for the meta-analysis, we collected additional information on the study population (baseline characteristics, in- and exclusion criteria) and the intervention (outcomes assessed, comparator). Since the review involved a systematic review and meta-analysis of previously published studies, approval by the institutional ethics committee and written informed consent from patients were not required.
Risk of bias assessment
The articles included in the meta-analysis were independently examined for internal validity utilizing the Cochrane risk-of-bias tool [24]. To ensure interrater reliability, the tool was applied independently by the two researchers (M.vM.; J.S.). Subsequently, the results were compared and discrepancies were discussed with a third researcher (F.T.) until a consensus was reached.
Data synthesis and publication bias assessment
For the systematic review, a narrative synthesis of the included studies was conducted. For the meta-analysis, we pooled the results from (quasi-)randomized control trials reporting the outcome measure (PD quality of life; PDQ-39) with a random-effects model of the standardized mean differences [25]. We calculated 95% CI and two-sided p-values for the outcome measure [25] and included studies were weighted according to the inverse-variance. Heterogeneity between studies were assessed using the χ2 test and the corresponding I2 statistic [25]. Substantial heterogeneity was defined as I2 statistic greater than 50% [25]. Additionally, we assessed for publication bias with the Egger’s regression intercept as per the small number of studies [26]. The meta-analysis was conducted in R 4.1.2 using the “meta” package version 5.1-1 [27].
RESULTS
Study characteristics
The combined searches retrieved 3,122 records (cf. Fig. 1 for details on the search strategy). After removal of duplicates and screening for titles/abstracts, 742 records remained for full-text review. In the next step, 511 of the 742 articles were excluded because the topic of the article did not address our primary research interest. The remaining 231 articles were then considered for a full-text review in which176 articles had to be excluded on the basis of poor-fit to the inclusion criteria and to the research question. Thus, 25 articles could be included into the presented systematic review. The excluded articles were related to care models disregarding PN (n = 18), neither reported primary data nor described an implemented care model (n = 83), reported findings for non-PD patients or involved a mixed study population in which the results for PD-patients were not disclosed separately (n 49), unavailable (n = 17) or published in another language as defined in the inclusion criteria (n = 17). Two additional articles were included after hand searching the reference lists of those 25 selected articles.
Ultimately, we could include 27 records in the qualitative review. Details of the included studies are displayed in Table 3. The study characterizes of 7 randomized, controlled trials (RCT) included in the quantitative meta-analysis and 14 other experimental studies included in the systematic review. The remaining 6 articles were nonexperimental and included description of practical care models or qualitative research.
Table 3
Detailed study characteristics
| Source | Study | Country | Setting | Parkinson Nurse or Team Composition | Intervention Description |
| Dorsey et al. (2010) [28] | Randomized-Controlled-Trial | United States | outpatient | Nurse Movement disorder specialist | A telemedicine intervention was implemented for 6 months at a community in the U.S. with the goal to provide care to patients that live far away from the clinic. Control group (usual care): saw their usual physician according to their routine schedule intervention group (telemedicine intervention): participants attended 3 telemedicine visits over 6 months, visits lasted 30–45 minutes patients underwent a routine clinical assessment (updates on medical history and medication usage; motor examination) which was performed by PD nurse |
| ∘ Based on the assessment, recommendations were reported by a Movement disorder specialist to the patients and by postal mail to the patients’ physicians. | |||||
| Eggers et al. (2018) [29, 30] | Randomized-Controlled-Trial | Germany | outpatient | Nurse, Movement disorder specialist, Community Neurologist | A nurse-led, patient-centered integrated healthcare model was implemented and evaluated after 6-months. |
| Control group (usual care): Baseline visit in the Parkinson’s consultation hour; continued regular German neurological treatment (visits at the community neurologists’ practice about every 3 months). Patients had access to regular physiotherapy, occupational or speech therapy. Access to different medications was the same for both treatment arms. | |||||
| intervention group (integrated care): Additionally, received an individual treatment plan, regular home visits of a PD nurse (every 3 months or whenever necessary on short notice) and access to a telephone hotline. Individual treatment plans were reviewed every 4 weeks and adapted according to individual patients’ needs. Furthermore, the PD nurse synchronized the therapeutic pharmacological intervention with the program of speech therapists or physiotherapists. | |||||
| Additionally: PD nurse obtained questionnaires and surveyed clinical parameters (e.g., part III of the UPDRS) | |||||
| Hurwitz et al. (2005); Jarman et al. (2002) [31, 32] | Randomized-Controlled-Trial | United Kingdom | outpatient | Nurse, General Practitioner | In 1997, a community-based PD nurse Service was implemented in 9 out of 57 eligible English health authorities that did not already have well-developed services; intervention lasted 2 years |
| Control group (usual care): standard care from their general practitioner in the community | |||||
| intervention group (community nurse): Community PD nurse had an advisory role to general practitioner and was guided by an experienced PD nurse | |||||
| Role of the Nurse: | |||||
| • counselling and educating patients and carers about PD –in their homes, at health center and GP clinics, in hospital outpatients and on the telephone | |||||
| • provision of drug information to patients under the auspices of physicians and consultants | |||||
| • monitoring clinical well-being and response to treatment (minimum of two assessments per year), reporting to physicians and consultants where appropriate | |||||
| • instigating respite and day hospital care where appropriate; seeing patients in hospital if admitted, and liaising with hospital staff when discharged | |||||
| • assessing social security benefit entitlement | |||||
| • liaison with members of local multidisciplinary primary care teams for ongoing assessment and therapy where appropriate | |||||
| • not empowered to change patient medication unilaterally but could (and did) make suggestions to physicians about altering dose regimens, change in medication preparation and addition of new drugs | |||||
| van der Marck et al. (2013) [33] | Randomized-Controlled-Trial | Canada | outpatient | Movement disorder specialist, Social worker, Nurse | Care provided by a multidisciplinary/specialist team within a Movement Disorder Specialist Center was compared to stand-alone care from a general neurologist from baseline to 8 months. |
| Control group (usual care): received standard care from a general neurologist outside the center | |||||
| intervention group (multidisciplinary care team): Patients received ongoing individually tailored care from the Movement disorders specialist, supported by PD nurse and social worker within the same physical location. Tasks within the care team: | |||||
| • Movement disorder specialist: performed visits at baseline, 4 months, and 8 months. | |||||
| • social worker: available for psychosocial and homecare issues, directed patients towards professionals in certain circumstances, telephone support | |||||
| • PD nurse: overseeing changes in symptoms, discussing medication issues and available for other PD-related questions, telephone support | |||||
| Wang & Zhang (2020) [34] | Quasi-experimental | China | inpatient | Nurse | After being administered to a hospital, patients either underwent a conventional or comprehensive nursing intervention; evaluation was performed after 3 months (length of the nursing cycle). |
| Control group (conventional nursing): Conventional disease education for patients, guiding patients to take their drugs and guiding the patients’ diets, helping the patients carry out the relevant physical or limb movements and exercises, keeping the ward clean and sanitary, educating the patients’ families about the disease, and guiding the families in supervising the patients’ medi–cation use and their daily lives. | |||||
| intervention group (comprehensive nursing): consisted of 3 central pillars: | |||||
| •psychological intervention: establishing a trustful relationship, gaining insight into patients’ emotion, evaluating the patient's psychological situation | |||||
| •nutritional intervention: formulating a personalized nutrition plan and supervising it implementation | |||||
| •physical intervention: delivering guidance on rehabilitation training and medication; for early onset: guidance how to participate in outdoor sports; for advanced: guidance on balance/ limb training; massaging patients after each rehabilitation training, supervising their drug regime and helping patients to adhere to it | |||||
| Aye et al. (2020) [35] | Descriptive | Singapore | outpatient | Nurse, multidisciplinary coordination team at the clinic | The Paper describes the purpose of the Integrated Community Care Programme for Parkinson’s Disease (ICCP) as one of the Community Care Partners Programme (CCPP) which was launched by the National Neuroscience Institute. |
| Aim of the CCPP: | |||||
| •professional education (including PD nurses) | |||||
| •joint care delivery | |||||
| •community resources Aim of the ICCP: | |||||
| •specifically targeted towards patients with more severe motor impairment or those without caregivers | |||||
| •patients are visited by a specialist PD nurse who refers them onwards to relevant community services if required | |||||
| •future goals: | |||||
| (1) establishing a 3-months program of interprofessional training to community care partners | |||||
| (2) providing patient-centered care by delivering care through community care partners, including joint consultations at the patient’s home with Movement disorder neurologists and the provision of care delivered by telemedicine | |||||
| (3) providing ongoing training in the form of monthly interprofessional learning, yearly access to training programs, and biennial access to a symposium | |||||
| Connor et al. (2019) [36] | Randomized-Controlled-Trial | United States | outpatient | Nurse, PD Specialist | This 2-group stratified randomized trial involved veterans with PD in southwestern United States. Guided care management, led by PD nurses, was compared to usual care. |
| Control group (usual care): not described | |||||
| intervention group (guided care management): Care management was performed by Nurse Care Managers (NCM) that collaborated with responsible PD specialists. After an initial assessment, they developed an action plan together with the patient that included problem-specific interventions. Other tasks: | |||||
| •telephone-administered structured and comprehensive assessment with embedded algorithms for identifying 28 problem areas | |||||
| •implementing an evidence-based practice containing 3 components: | |||||
| –ensuring that care protocols as derived from practice guidelines are followed (where they exist) | |||||
| –ensuring that expert consensus is followed where no guidelines are available | |||||
| –ensuring that veteran priorities and preferences are addressed | |||||
| •facilitating the use of communication tools (explaining patient the digital patient portal and personalized health care notebook) | |||||
| •filling out documentation templates to provide coordinated, patient-centered care | |||||
| Fleisher et al. (2020) [37] | Pilot-Study | United States | outpatient/ home-bound care | Movement disorder neurologist, Social worker, Nurse | The pilot-study aimed to determine whether facilitating expert in-home care could improve professionals understanding of disease progression, treatment options, and unmet needs in this vulnerable population, and whether such a model could mitigate decline in quality of life. Therefore, home-visits with 27 homebound patients were performed over 12 months. |
| The care team (nurse, social worker and neurologist) traveled to the patient’s home approximately every 4 months for a total of 4 visits over 1 year. | |||||
| Role of the Nurse: | |||||
| •gathered orthostatic vital signs | |||||
| •reviewed parts I and II of the UPDRS to identify impairments in activities of daily living | |||||
| •conducted a real-time medication reconciliation | |||||
| → social worker and neurologist initiated a conversation regarding the patient’s goals of care and advance directives | |||||
| → team formulated a comprehensive assessment and plan, including medication, dietary, and home safety recommendations and referrals | |||||
| → documented on a health literacy-friendly after-visit template, and confirmed with teach-back. | |||||
| Fleisher et al. (2020) [38] | Pilot-Study | United States | outpatient/ home-bound care | Movement disorder neurologist, Social worker, Nurse | The goal of this pilot-study was to improve efficiency and cost-effectiveness compared to the initial model (see Fleisher et al. 2020a). New structure: nurse, study coordinator, and social worker travel in-person to the initial visit; the Movement disorder specialist is present via telemedicine for all visits; the social worker is present via telemedicine for visits 2–4. |
| Role of the Nurse: | |||||
| •gathered orthostatic vital signs | |||||
| •reviewed parts I and II of the UPDRS to identify impairments in ADL | |||||
| •conducted a real-time medication reconciliation | |||||
| → after briefing, the team rejoins the patient-caregiver-dyad and connects to the neurologist via telemedicine and neurologist reviews the dyad’s chief concerns | |||||
| → team formulates a comprehensive assessment and plan, including medication, dietary, and home safety recommendations and referrals | |||||
| → Following the visit, each team member records their findings in the electronic medical record, which is compiled into a comprehensive note shared with all relevant health care providers | |||||
| → dyad receives contact information to reach the team between visits | |||||
| Giladi et al. (2014) [39] | Descriptive | Israel | inpatient | Neurologist, Nurse, Therapists (social worker, speech and language therapist, psychiatrist, genetic counselor, sexologist, geriatrician, dietitian, occupational therapist) | The paper describes the Tel Aviv Sourasky Medical Center model of interdisciplinary care. The model was designed to create a coordinated multidisciplinary team in the Movement Disorders Unit. Concept: three neurologists, all the therapists, and all the other team members conduct their work in the same facility and share the same secretarial services, including data access. The team meets formally once a month but there is constant informal sharing of information, special meetings are held when necessary. |
| Role of the Nurse: | |||||
| •clinical coordinator | |||||
| •initiates the discussion about sexual issues refers the patient to the sexologist | |||||
| •ensures that the patient’s main problems are identified and discussed with the patient, the patient’s caregiver, and the patient’s physician and nurse, and that a plan of action is agreed •schedules the appointments with the various specialists and follow-ups on those consults | |||||
| •serves as a link between the patient/family and the specialists | |||||
| Sessions with therapists are scheduled in advance (also available for caregivers) | |||||
| Guo et al. (2020) [40] | Randomized-Controlled-Trial | China | inpatient | Nurse | This study was designed to explore the improvements of sleep quality and quality of life by implementing the Roy Adaptation Model for nursing of patients with PD. The Roy model is an integrated adaption system emphasizing the interactions between humans and their environment. By using stimulation, medical workers can promote patients’ adaptation reaction and apply more energy to help patients recover. PD patients admitted to the hospital from March 2017 to March 2019 were included as the study subjects and equally divided into 2 groups (intervention/ control). |
| Control group (routine nursing): received health education; medication nursing; guidance on exercise; healthy diet provided by the nurses | |||||
| intervention group (Roy Adaption Model): The intervention was based on 4 pillars: | |||||
| 1. Physiological intervention –goal was to improve patients’ physiological functions; actions: therapeutic operations and timely attention in the night (followed the 4 principles of “gentle operations, speaking, walking and door shutting”); improvement in ward environment by regulating indoor temperature and humidity; advise patients to avoid any food containing caffeine 4–6 h before sleep, take a hot water foot bath if conditions permit; reduce night treatments or concentrate them in a certain time period to avoid disturbing patients’ rest during the night | |||||
| 2. Role intervention: goal was to help the patients to adapt to their new arrangements; actions: patients’ habits were maintained as far as possible; patients’ requirements were responded to if appropriate; enhancing communication between medical workers and patients to help them build confidence | |||||
| 3. Dependence intervention: goal was to reduce patient’s dependence on external help actions: enhanced communication and psychological support, especially in the night when family members stay with patients to eliminate their loneliness | |||||
| 4. Self-concept intervention: goal was to improve patient’s self-confidence actions: establishing amicable relationship with the patient; delivering proper education and setting up an individualized health education plan; cooperating with patients’ family members for ideological work | |||||
| Hellqvist (2021) [41] | Qualitative | Sweden | outpatient | Nurse | The paper describes qualitative findings from an evaluation of the Swedish National Parkinson School (NPS). The NPS is a dyadic self-management intervention for persons with PD and their care partners, which is delivered by PD nurses. The program was developed in 2013, and has been provided in clinical practice since 2014 in neurologic and geriatric outpatient clinics across Sweden. Focus of NPS: enabling patients and care partners to handle symptoms of PD in everyday life, by introducing techniques and strategies for self-monitoring, planning ahead, taking action, positive thinking, communication and resource utilization |
| → participants meet in a small group once a week for total of seven sessions (2h) Content of NPS: | |||||
| ∘ providing information about a topic relevant to everyday life | |||||
| ∘ group discussion stimulating interaction and peer-support between the participants | |||||
| ∘ home-assignments to give participants the opportunity to reflect on, and try out, what has been discussed during the sessions | |||||
| Kluger et al. (2020) [42] | Randomized-Controlled-Trial | United States | outpatient | Nurse, Palliative neurologist, Social worker, Chaplain with PD experience, Certified palliative medicine physician | In this study an integrated palliative care model provided by a neurologist, social worker, chaplain, and nurse using PC checklists, with guidance and selective involvement from a palliative medicine specialist was compared to standard care, which was provided by a neurologist and a primary care practitioner. |
| Control group (standard care): provided by the patient’s primary care physician and a neurologist as usual | |||||
| intervention group (integrated palliative care): patients received aside their standard care | |||||
| –outpatient visits every 3 months for 1 year (in person or by telemedicine every 3 months); visits were supplemented with phone calls &participants could contact the team as needed Visits: | |||||
| •performed by the interdisciplinary team •standardized using checklists for each team member | |||||
| •duration: 2–2.5 hours | |||||
| •topics: nonmotor symptoms, goals of care, anticipatory guidance, difficult emotions, and caregiver support | |||||
| After-visit: | |||||
| -> summaries were sent to the patient | |||||
| -> standard clinic notes provided to primary care physician + neurologist | |||||
| -> Suggestions for care outside of palliative care to the patient’s standard care team | |||||
| Role of the Nurse: Tasks for the nurse according to the checklist: •Medication reconciliation at beginning of visit | |||||
| •Primary concerns for patient (What should we focus on today?) | |||||
| •Primary concerns for caregiver (if present) •Health care proxy designation and documentation | |||||
| •Advanced care planning and documentation | |||||
| •Home safety and home health care needs •Assess for home palliative care or hospice needs | |||||
| •Nutritional status and diet •MoCA (for baseline, 6- and 12-month visits) | |||||
| •For high-risk patients [e.g., bedbound or incontinent]: assessing skin integrity and need for home care for wound or skin care | |||||
| Liu et al. (2019) [43] | Randomized-Controlled-Trial | China | inpatient | Nurse | The goal of the study was to implement a comprehensive nursing care for Parkinson’s patients, and to explore the psychological state of patients, changes in the quality of life and patients’ satisfaction with the nursing service. |
| Control group (conventional nursing): nurse cooperates with doctors to complete the treatment of patients and instructs patients to take medication on time and reasonably. | |||||
| intervention group (comprehensive nursing): | |||||
| 1. Nurse performed a comprehensive assessment: based on the patient’s family, economic situation and lifestyle ->reasonable care plan was created based on the results | |||||
| 2. Nurse provided patients with medication guidelines: carefully explained the reasons for the medication, importance of rational drug use, role of the drug and possible adverse reactions | |||||
| 3. Nurse provided psychological support: paying close attention to the patients’ psychological state all the time; fostering active communication; providing timely psychological counseling and psychological comfort; alleviating patients’ negative emotions, help to build up confidence maintain an optimistic attitude | |||||
| 4. Nurse provided sports guidance: helping patients to do simple exercises, and massage patients’ muscles and joints every day. | |||||
| 5. Caring for the patient’s safety: paying attention to the needs of patients and help patients finish part of their life needs; instructing patients’ family | |||||
| 6. Discharge management: giving nursing guidance by a virtual chat and telephone after discharge from hospital | |||||
| Pretzer-Aboff et al. (2015) [44] | Descriptive | United States | outpatient | Movement disorder specialist, Psychologists, Nurse, Researcher, Physical and speech therapists Exercise physiologists, Nutritionists, Graduate students | The paper describes the PD Telehealth Clinic Model which is implemented at the University of Delaware Nurse Managed Health Center (NMHC). |
| Concept of the Clinic: the clinic is based on a collaborative framework that uses synchronous videoconferencing telehealth technology to bring together out-of-state clinicians and scientists with expertise in PD to help deliver specialized care to PD patients and their caregivers. | |||||
| Role of the Nurse: | |||||
| •identify PD motor and nonmotor problems from both physical exam and the patients’ perspective | |||||
| •helping to facilitate care and provide a consistent patient-provider relationship | |||||
| •reviewing the team’s recommendations and providing a written summary of the recommendations to the patient | |||||
| Wu et al. (2020) [45] | Randomized-Controlled-Trial | China | inpatient | Nurse | The goal of the study was to explore the effect of positive psychological nursing intervention on anxiety and depression symptoms in older adults with Parkinson’s disease compared to routine psychological intervention. |
| control group (routine intervention): nurses provided psychological support, empowered patients to speak about their emotions and tried to change adverse thinking. | |||||
| intervention group (positive psychology): | |||||
| •Nurses tried to foster gratitude for three things: asking patients to write down three things that were worthy of gratitude at the end of each day and explain the reasons | |||||
| •Nurses empowered patients to utilize advantages: discovering hobbies of patients through communication and organize some similar activities based on these activities on a regular basis, so that patients could find their own positive points in the activities and enhance these points to reflect their self-value | |||||
| •Nurses actively guided patients to recall every little thing in life, learn to taste, and know how to be satisfied. Asking patients to record these pleasures every day and share it with everyone. | |||||
| •Nurses encouraged patients to write an autobiography: encouraging patients to recall their glorious moments and record how they faced frustration and achieved glory in the form of 1–2 pages of an autobiography | |||||
| •Nurses encouraged patients to recall things that made them proud or happy to replace the existing negative emotions | |||||
| Carne et al. (2005) [46] | Quasi-Experimental | United States | outpatient | Movement disorder specialist, Neurology nurse, Physiatrists, Psychologist, Neurosurgeon trained in deep brain stimulation surgery | The study examined the impact of a multidisciplinary clini–cal management of the Parkinson’s Disease Research, Educa–tion, and Clinical Center program on Parkinson’s disease progression. |
| control group (not present): / | |||||
| intervention group (care program): The Parkinson’s Disease Research, Education, and Clinical Center (PADRECC) program consists of the following pillars: | |||||
| 1. PD medication management (recommended dosing of levodopa, dopamine agonists, catechol-O-methyltransferase inhibitors, and other symptom-specific agents [e.g., amantadine sulphate]). | |||||
| 2. Physiatrist/ Nursing and Neurologist visits. | |||||
| 3. Neuropsychological evaluation. | |||||
| 4. Functional diagnostic testing (i.e., gait laboratory, computerized posturography). | |||||
| 5. Rehabilitation therapy (i.e., physical, occupational- or kinesiotherapy, speech-language therapy). | |||||
| 6. Home exercise program. | |||||
| 7. Support group. | |||||
| 8. Health and wellness education Role of the Nurse: performed patient visits; involved in clinical assessments (UPDRS, MMST, Vital signs) and team conferences where suggestions to community providers are made | |||||
| Su et al. (2017) [47] | Descriptive | United States | inpatient | Neurology clinicians, Physical therapist, Speech pathologist, Social worker, Nursing coordinator | The paper describes the Oregon Health & Science University Parkinson Center and Movement Disorders Clinic. |
| Concept of the Clinic: patient and caregiver are seen within the same visit by the different disciplines. Close communication is maintained between disciplines during the visit, and a comprehensive care plan is ultimately developed following dedicated discussions. | |||||
| Role of the Nurse: | |||||
| •primary case manager or care coordinator for the clinic ensures that the recommended treatment plan is implemented to the benefit of the patient and family | |||||
| •works to coordinate referrals, staff assignments, information triage, and health care plan implementation | |||||
| •communicating with referring providers on appropriateness of referrals scheduling | |||||
| •communicating to the patient and caregiver(s) the goals of the interdisciplinary clinic | |||||
| •helps to maintain the flow of the clinic and keeps the team members on track with time | |||||
| •finalizing and carrying out the recommendations utilizing a specialized and limited protocol to facilitate orders for referrals | |||||
| •researches healthcare resources nearby the patient and caregiver’s home such as home health services | |||||
| •serves as a liaison with patient and caregiver | |||||
| Gage et al. (2014) [48] | Randomized-Controlled-Trial | United Kingdom | outpatient | Parkinson’s nurse specialists, Physiotherapists, Occupational therapists, Speech and language therapists | The purpose of this study was to evaluate the effectiveness of a Specialist Parkinson’s Integrated Rehabilitation Team. |
| Concept of the Model: The multidisciplinary rehabilitation program coordinated by a Parkinson’s nurse specialist (PNS) and delivered to patients/ caregivers their own homes. It compromises multidisciplinary team assessment, care planning and treatment. | |||||
| control group (usual care): no coordinated multidisciplinary rehabilitation or ongoing support | |||||
| intervention group (care program): | |||||
| Two groups of patients were visited by a multidisciplinary team at home, which delivered a specialist rehabilitation package, tailored to individual needs. Educational materials were provided on aspects of Parkinson’s disease. A client record form was left in the participant’s home for the duration of the intervention, and was completed by each professional at each visit. There were two team meetings per cohort to discuss patient care plans and progress. Referrals to other professionals were made when indicated. In addition to the program support for 4 months from a care assistant. The care assistant received training in Parkinson’s disease, were embedded in the MDT and worked under the supervision of the Nurse. | |||||
| Role of the Nurse: | |||||
| •Supervision of the care assistant (group B) | |||||
| •To provide expert Parkinson’s management to maintain maximum independence for patients | |||||
| •To act as a reliable source of information about clinical and social issues that were of concern to people with Parkinson’s and their carers | |||||
| •To ensure appropriate timely referral to essential services such as therapy or social care | |||||
| •To empower and educate people with Parkinson’s and their carers | |||||
| •To identify the tolerance and efficacy of medication | |||||
| •To complete adverse event forms as per the project protocol | |||||
| •To reinforce all multidisciplinary team treatment programs | |||||
| Reynolds et al. (2000) [49] | Randomized-Controlled-Trial | United Kingdom | inpatient | Parkinson Nurse | The study investigated differences between care provided by the hospital-based Parkinson’s disease nurse specialist (PDNS) compared with the Consultant Neurologist. |
| control group (Neurologist): performed consultation hours as usual. | |||||
| intervention group (PDNS): The PDNS led care only in one study center. At the two other study centers, the PDNS provided care as an “add-on” service where patients saw the nurse mainly and the doctor briefly for follow up care. The following actions were performed by the PDNS: | |||||
| •taking patients’ history, performing an assessment of each patient case, explaining Parkinson’s disease illness and symptoms as well as answering patients’ questions about other illnesses | |||||
| •referred to patients’ family circumstances and general coping strategies | |||||
| •taking opportunities to give practical advice about coping with Parkinson’s disease | |||||
| •provided factual information on a range of other services available according to patients’ needs and choice and answered questions on alternative therapies | |||||
| Jones et al. (2016) [14] | Descriptive | Australia | outpatient | Nurse, Care coordinators (mostly social workers), Multidisciplinary care teams in hospitals | The paper describes the Canberra Hospital and Health Service Parkinson’s and Movement Disorder (PMD) Service. |
| Concept of the Service: The service compromises nurse-led education, clinical support, and care coordination for people with PD or related conditions and was established with 3 objectives: | |||||
| 1. improving patient care and self-management of their condition, patient quality of life, well-being, and caregiver burden | |||||
| 2. improving the knowledge and skills of healthcare professionals through secondary education | |||||
| 3. avoiding unnecessary hospital and nursing home admissions by providing community and home-based support | |||||
| Role of the Nurse: Nurse is responsible for designing, implementing and managing the service: | |||||
| •Works closely with a multidisciplinary team to ensure that people with PD admitted to the hospital were cared for appropriately | |||||
| •Provides education about PD to patients, caregivers and healthcare professionals | |||||
| •Works in close collaboration with care coordinators who could provide additional support | |||||
| Trend et al. (2002) [50] | Quasi-Experimental | United Kingdom | outpatient | multidisciplinary team of therapists, Nurse, Consultant neurologist | The purpose of the study was to evaluate the short-term effectiveness of an intensive multidisciplinary rehabilitation program for people with Parkinson’s disease and their caregivers. |
| control group (not applicable): / | |||||
| intervention group (rehabilitation program): pairs of participants (people with PD and their caregivers) attended a day care unit one day per week for six consecutive weeks; each weekly session from 10 am to 3:30 pm, | |||||
| •1st week: individual assessment by therapists & nurse •weeks 2–5: patients received treatment according to individual needs | |||||
| •6th week: patient’s condition was reassessed, and advice for the future was provided | |||||
| •At each visit, participants received 2 hours of individual attention. Group activities including relaxation were arranged for each afternoon. Talks from experts were designed to broaden participants’ knowledge about Parkinson’s disease. | |||||
| Role of the Nurse: | |||||
| •provide information, advice, support and counselling on issues related to Parkinson’s disease | |||||
| •optimizing medication; recognizing problems associated with side-effects of medication, e.g., dyskinesia and hallucinations | |||||
| •initiation of appropriate action | |||||
| •maintenance of bladder and bowel function monitoring blood pressure; pain control; sexual dysfunction; anxiety and depression | |||||
| Lindskov et al. (2007) [51] | Non-Randomized-Controlled-Trial | Sweden | outpatient | Nurse, Physician, Occupational therapist, Physiotherapist, Dietician, Psychologist, Speech therapist, Dental hygienist, Social worker | This study evaluated a patient-reported health outcomes of a multidisciplinary group educational PD program, delivered as part of routine clinical practice. |
| control group (no education): received educational program after the study | |||||
| intervention group (education program): The program consisted of a six-week outpatient educational program with weekly two-hour sessions; delivery and content of the sessions were planned by all participating session leaders together; first hour of each session was delivered by one or two healthcare professionals in the format of interactive dialogues; during the second hour, therapists demonstrated relaxation, speech and movement promoting exercises to patients, while family members took part in a nurse-led peer support group. | |||||
| Role of the Nurse: | |||||
| •teaching about general information on PD (e.g., symptoms, disease progression, day-to-day management, recent research) | |||||
| Wade et al. (2003) [52] | Randomized-Controlled-Trial | United Kingdom | outpatient | PD nurse, Physiotherapist, Speech and language therapist, Occupational therapist | The aim of the study was to determine whether a program of multidisciplinary rehabilitation and group support achieves sustained benefit for people with Parkinson’s disease or their carers. |
| control group (no program): received rehabilitation program after the study | |||||
| intervention group (program): patients participated in a multidisciplinary rehabilitation program; attended for whole days, 1x per week for six consecutive weeks | |||||
| •first week: patient assessment and individualized treatment program planning | |||||
| •week 2–5: rehabilitation provided by specialist team •Each week the patient received two hours of individual treatment in the morning, followed by group activities (for example, talks from experts and relaxation) in the afternoon | |||||
| Role of the Nurse: | |||||
| •provide information, advice, support and counselling on issues related to Parkinson’s disease | |||||
| •optimizing medication; recognizing problems associated with side-effects of medication, e.g., dyskinesia and hallucinations | |||||
| •initiation of appropriate action | |||||
| •maintenance of bladder and bowel function | |||||
| •monitoring blood pressure; pain control; sexual dysfunction; anxiety and depression | |||||
| Sitzia et al. (1998) [53] | Quasi-Experimental | United Kingdom | inpatient | Nurse, Dietitian, Occupational therapist, Physiotherapist, Speech and language therapist, Social worker, Discharge coordinator, Doctor | The aim of this study was to ascertain whether or not an inpatient multidisciplinary treatment program in a measurable change in patients’ health-related quality of life |
| control group (not applicable): / | |||||
| intervention group (program): admissions were arranged by the consultant or associate specialist in neurology, but nursing staff planned the admissions and coordinated the multidisciplinary team. Patients were followed up routinely in the hospital neurological outpatient clinic and by a community team. The treatment program lasted for one to two weeks, rarely three weeks. Patients returned home at weekends, so that patients and carers could evaluate the benefits of any changes. The hospital discharge scheme allowed extra help in the home for patients in the month following discharge. | |||||
| Role of the Nurse: | |||||
| •planning admissions |
The included articles were published between 1998 and 2021 (cf. Table 2), with most articles (n = 18) published after 2010. Of the 27 articles, 21 articles stemmed from quantitative research. Studies were conducted more frequently in Europe (n = 11).
Table 2
Characteristics of the articles (total n = 27)
| Characteristics | Number (%) |
| Publication Type | |
| Quantitative Study | 21(77.7%) |
| Qualitative Study | 1 (3.7%) |
| Descriptive Paper | 5 (18.5%) |
| Origin of Study | |
| Europe | 11 (40.7%) |
| America/Canada | 9 (33.3%) |
| Asia | 5 (18.5%) |
| Other Countries | 2 (7.4%) |
| Year of Publication | |
| 2010 and earlier | 9 (33.3%) |
| 2011–2021 | 18 (66.6%) |
Systematic review of personalized care models
We identified 25 individual reported models from 27 articles (cf. Table 3) that met our definition of personalized care. In total, 28 tasks performed by PN and covering all five domains of personalized care were identified. All actions and domains are summarized in Fig. 2.
Fig. 2
Tasks performed by Parkinson Nurses which relate to the model of personalized care management for people with Parkinson’s disease.
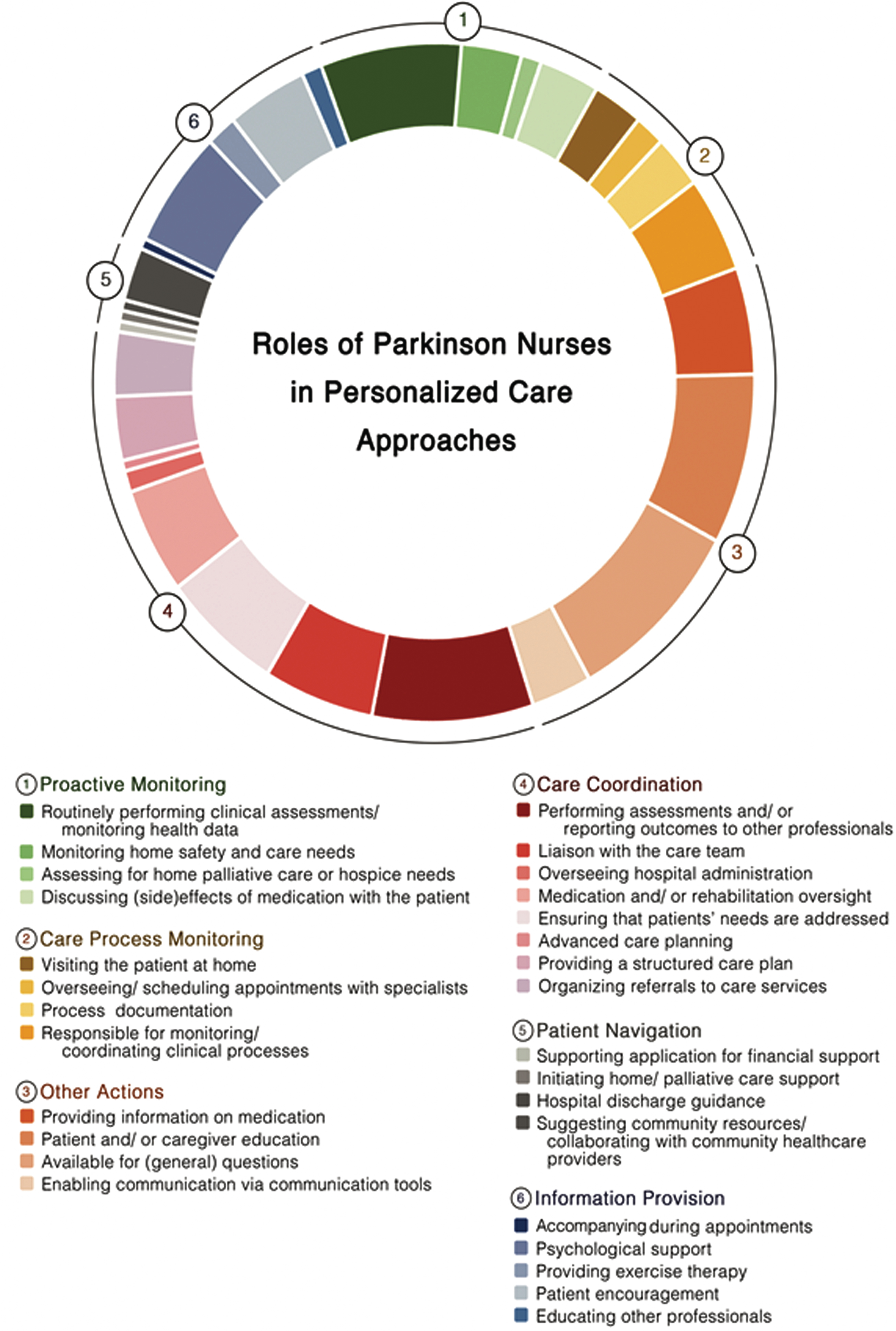
In most care models, PN performed tasks covering at least two domains of personalized care. The domain most frequently covered by PN was the provision of personalized information, with PN being especially available for general questions [14, 29–36, 41–53] and educated patients and/ or care partners [14, 31–36, 40–44, 48–53]. In 10 studies, PN provided personalized information to patient and care partners by providing information on medication [14, 31–34, 43, 48–50, 53]. Besides, PN enabled communication via tools such as virtual chats [29, 30, 33, 36–38, 44]. In several studies, PN coordinated care processes by reporting outcomes of clinical assessments to other professionals [28–30, 33–40, 42–45, 47–49], liaising with the care team [14, 31, 32, 39, 44, 46–48, 50, 52, 53], implementing structured care plan [31, 32, 36, 38, 39, 48], overseeing patients’ hospital admissions [48, 53] or their medication and rehabilitation schemes [31, 32, 34, 35, 37, 38, 42–44]. PN additionally ensured that all care providers were aware of the patients’ needs [34, 37–40, 42–48]. Moreover PN, facilitated advanced care planning [42] or organized referrals to specific care services [14, 31, 32, 39, 42, 47, 48]. In terms of monitoring the care process, PN performed home visits [29–32, 36–38], oversaw and scheduled appointments with specialists for patients [31, 32, 39, 40], documented the care process [38–40, 47, 48] and coordinated these processed within clinical evaluations [31, 32, 36, 40, 44, 45, 47, 48, 50, 53]. However, PN also performed proactive monitoring actions by routinely performing clinical assessments [14, 28–34, 37–39, 44, 46–49], monitoring patients home safety and care needs [39, 40, 42, 43, 46, 49], evaluation the demand for home palliative care or admissions to hospice care [42, 43] and by discussing (side-)effects of medication with patients [31, 32, 37, 38, 40, 46]. Actions attributed to patient navigation were only limitedly observable across the studies. In one trial PN’s supported patients in applying for financial support [31, 32], whereas another study mentioned the PN being responsible for initiating home/ palliative care support [42]. In another study the PN navigated the patient through the hospital discharge process [43]. Five studies reported that PN were responsible for suggesting community resources to patients and for collaborating with community healthcare providers [14, 35, 36, 47, 49]. Finally, six themes were identified across the studies which did not fit into one of the proposed dimensions of personalized care but also facilitate the personalization of care processes. Several studies reported that PN provided individual psychological support [34, 35, 40–43, 45, 48, 51–53] and encouraged patients throughout the care process [14, 39–41, 43, 45, 48, 49]. In three studies, PN implemented personalized exercise programs for patients [34, 35, 43], while in two studies PNs educated other healthcare professionals about patient needs [14, 50]. Finally, in one care model, PN accompanied patients during medical appointments [31, 32].
Impact of personalized care in PD-related quality of life (meta-analysis)
Description of studies
Study details are displayed in Tables 3 and 4.
Table 4
Study characteristics of randomized controlled trials
| Study | Sample Size (IG:CG)1 | Study Population | Setting | Primary Outcome(s) | Secondary Outcome(s) | Times of data collection | ||
| Age (mean±SD) | Disease duration (mean±SD)2 | Hoehn &Yahr (mean±SD) | ||||||
| Dorsey et al. (2010) [28] | 6:4 | 70.94 y (±7.54) | no data | 2.44 (±0.44) | outpatient | feasibility (proportion of telemedicine visits completed as scheduled; willingness of study participants to receive telemedicine care) | quality of life (PDQ-39; EuroQol); patient satisfaction (Modified Group Health Association of America’s Consumer Satisfaction Survey); motor performance (UPDRS, motor-subscale); mood (Geriatric Depression Scale); cognition (MoCA) | baseline 6 months |
| Eggers et al. (2018) [29] | 132:125 | IG: 69.8 y (±8.4) CG: 69.9 y (±7.8) | IG: 6.2 y (±6.2) CG: 5.5 y (±5.2) | IG: 2.5 (±0.8) CG: 2.6 (±0.8) | outpatient | quality of life (PDQ-39) | Changes in mood, motor and non-motor functioning and cognition (BDI-2, UPDRS III, NMS-Score, PANDA) | baseline 6 months |
| Hurwitz et al. (2005) / Jarman et al. (2002) [31, 32] | 1041:818 / 1028:808 | < 70 y IG: 34% CG: 32% 70–77 y IG: 35% CG: 36% > 77 y IG: 31% CG: 32% | 0–4 y IG: 53% CG: 52% 5–9 y IG: 22% CG: 24% > 9 y IG: 25% CG: 24% | No data | outpatient | Clinical: Stand-up test group, Dot-in-square score, Mortality, Proportion sustaining fracture Patient well-being: PDQ-39, EuroQol, Global subjective well-being question Health care costs: Institutional, respite, hospital, day care, medication, community and general practitioner care, social security benefits, home aids and adaptations PDNS | Medication: Median dose L-dopa, Proportion patients on L-dopa controlled release preparation, Proportion patients on more than monotherapy Referral: Proportion patients referred to ancillary therapy, Proportion of patients referred to PD specialist | baseline 6 months / baseline 1 year –2 y |
| van der Marck et al. (2013) [33] | 51:49 | IG: 65.9 y (±8.5) CG: 68.1 y (±8.8) | IG: 4.6 y (±3.9) CG: 3.7 y (±3.5) | No data | outpatient | change in quality of life (PDQ-39) | UPDRS depression (MADRS) psychosocial functioning (SCOPA-PS) caregiver strain (CSI) | baseline 8 months |
| Wade et al. (2003) [52] | 53:41 | IG: 71.3 y (±8.6) CG: 70.4 y (±7.6) | No data | No data | outpatient | Parkinson’s disease disability questionnaire Parkinson’s disease questionnaire (PDQ-39) Short Form 36 item health survey (SF-36) EuroQol -5D stand-walk-sit test Nine-hole-peg-Test (NHPT) Hospital Anxiety and Depression Scale (HADS) selected items concerning speech from the unified Parkinson’s disease rating scale (UPDRS) | not applicable | baseline 24 weeks |
| Wang & Zhang (2020) [34] | 58:57 | 61.35 | y (±4.28) | No data | No data | inpatient | SGA SAS SDS Barthel index PDSS MoCA modified Ashworth score PDQ-39 | baseline 3 months |
IG, intervention group/; CG, control group; SD, standard deviation; PDQ 39, 39-item Parkinson’s Disease Questionnaire; EuroQol, EuroQol Questionnaire; UPDRS, Unified Parkinson’s Disease Rating Scale; MoCA, Montreal Cognitive Assessment; BDI, Beck’s-Depressions-Inventory; NMS-Score, Non-motor Symptoms; PANDA, Parkinson Neuropsychometric Dementia Assessment; PDNS, Parkinson’s Disease Nurse Specialists; L- dopa, Levodopa; PD, Parkinson’s disease; MADRS, Montgomery–Åsberg Depression Rating Scale; SCOPA-PS, Scales for Outcomes in Parkinson’s disease- Psychosocial Functioning; CSI, Caregiver Strain Index; SF, Short Form 36 item health survey; NHPT, Nine-hole-peg-Test; HADS, Hospital Anxiety and Depression Scale; SGA, Subjective Global Assessment; SAS, Supervisory Attentional System; SDS, Self-Rating Depression Scale; PDSS, Parkinson Disease Sleep Scale.
In this subgroup of RCTs, included studies were conducted between 2002 and 2020 [28, 29, 31–34, 52]. There were 1830 participants overall, who were all community-dwelling adults with PD. Sample sizes of the individual studies ranged from 10 to 1254 PwP.
The settings for the interventions were: fully inpatient in one study [34] and delivered in an outpatient setting in the remaining ones [28, 29, 31–33, 52]. While most studies [28, 29, 31–33, 52] involved a multidisciplinary team for intervention delivery, one study solely involved PNs as care professionals [34]. The duration of the intervention program varied from three to twenty-four months, with two studies implementing weekly patient contact [34, 52], three studies implementing monthly patient contact or when desired [28, 29, 33] and one study disclosing no further information in this regard [31, 32].
Risk of bias assessment
The risk of bias assessment for included studies is presented in Supplementary Figure 1. The trials covered a wide range of methodological quality, but no study achieved low risk in all the seven criteria assessed. The Egger’s regression intercept test was not significant (p = 0.204), suggesting asymmetry and therefore publication bias in these results.
Effects of interventions on patient’s quality of life
QoL scores were available for 1830 participants, as measured by the PDQ-39. Pooling data from all the available RCTs (n = 6) resulted in substantial heterogeneity (I2 = 96.6%, χ2 = 146.31, Fig. 3) and suggested no significant effects of personalized care delivery on QoL in PwP if compared with standard care (Standardized Mean Difference [SMD] = –0.8935; 95% Confidence Interval [CI], –2.1177 to 0.3307; z = –1.43, p = 0.1526).
Fig. 3
Forest plot of meta-analysis.
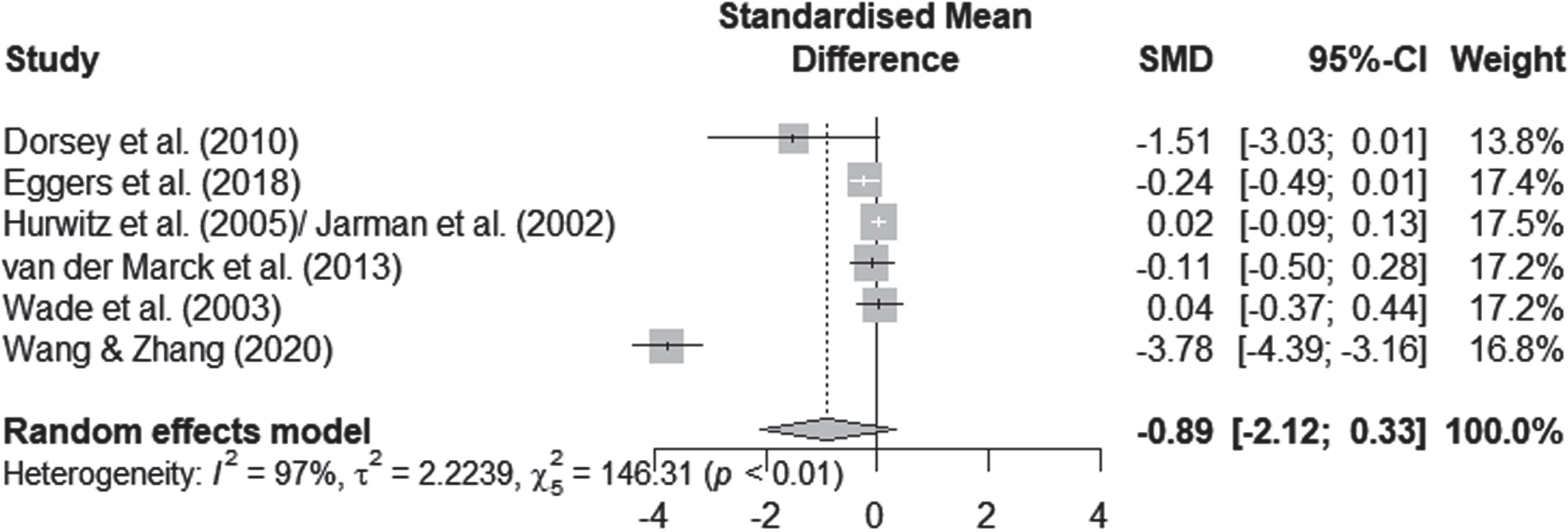
Various factors such as the nature of the intervention, the duration of follow-up and the overall quality of the studies may explain the heterogeneity of our results. One study reported the outcome of interest after a considerably shorter period of time (assessment after three months of intervention) [34]. The intensity and frequency of the intervention in the inpatient study differed considerably from the outpatient studies. In addition, it was the only study that showed a significant effect (cf. Fig. 3). We therefore performed an exploratory post-hoc subgroup analysis. In the subgroup analysis, we pooled data from the remaining five care models that reported the outcome of interest over a period of more than six months [28, 29, 31–33, 52]. According to the I2 statistic heterogeneity was 47% for this subgroup, whereas concerning the outcome of interest no significant effects for QoL improvements after personalized care versus standard care delivery were traceable (SMD, –0.076; 95% CI = [–0.235 to 0.084]; p = 0.353).
DISCUSSION
In this systematic review, we identified models of personalized PD care in which PN play primordial role. These models covered the entire spectrum of healthcare settings (inpatient, outpatient, and community-based) and originated from a broad gamut of healthcare systems. A trend can be seen in the meta-analysis which indicates that personalized care models may impact PwPs’ physical, mental, and social well-being (cf. Fig. 3). Nevertheless, the meta-analysis of pooled data from randomized, controlled trials identified no significant favorable effects of personalized care on QoL compared with usual care. Possibly, this reflects the significant differences in populations included, in the care settings scrutinized, and the timings of outcome assessments. In what follows, we will try to dissect why results of the RCTs do not necessarily imply that the models are ineffective or lack important practical implications but why more high-quality data is imperative.
Heterogeneity instead of a clear line: What do the studies tell us?
Personalized PD care describes a comparatively new approach and has therefore not yet been finally and uniformly established throughout literature. In the included studies, it remained at times unclear whether concepts of personalized care were actively pursued, not only as care models differed significantly, but also due to the applied terminology. These discrepancies are paralleled beyond the scope of this review, insofar as that some aspects of personalized PD care can be met in patient-centered or integrated care models with somehow differing nomenclature [54, 55]. In agreement with this blurred definition, we could identify tasks corresponding to but not matching exactly one of van Halteren’s five elements of personalized PD care which might nonetheless be attributable to their idea of care according to the individuals’ needs and preferences [7]. On the one hand, divergency of services may reflect strengths of care models incorporating specific, e.g., regional elements. It is hard to imagine anyone assuming personalized care approaches being a universal concept in the sense of a one-fits-all approach. Otherwise, great heterogeneity in implementation or nomenclature hinders scientific assessment and the development of binding and specific descriptions of professions like PN. In the future theoretical work, a balance between precision and reflection of theories and concepts in the international comparisons is desirable.
Against the background of distinct concepts of personalized care, peculiarities of healthcare systems are only comprehensible. The differences in the adopted approaches revolved around the implementation of personalized care and the role of PN being responsible for at least single elements, but at times also for two or more. This divergence is also found in everyday life: for example, Prell et al. observed that in practice there is a wide range of training, tasks and areas of work exists [56]. These results are likely to be related to the organization of healthcare systems with regard to professional collaboration. For example, while the German system is not geared towards cooperation [57], other healthcare systems are deemed collaborative [58–60]. Besides, the traditional values and training of staff might also play a role, as in countries where PN do not yet exist and no funding mechanisms are implemented [61], versus those in which these specialists are already an integral part of care approaches [8, 62, 63]. Cross-country analyses may help differentiate the roles of PN in personalized care delivery.
Regardless of the structure of healthcare systems, multispecialty care models with multiple healthcare professional working in a team are intuitive for PwPs’ personalized care. The relevance and effectiveness of a multi-professional collaboration is gaining momentum, as reflected in clinical recommendations, practical observations and scientific studies [11–13, 56, 64–68]. So, what should the role of PN be in the care process of PwPs?
In practice, PN represent an important member of multispecialty care teams, as they fulfill several functions. They may enable the integrated flow of care between patients and care teams, and between professionals in the care team [56]. Moreover, they may facilitate the implementation of PD-specific care in previously unspecialized healthcare facilities. Possible other roles of PN have been highlighted in recent clinical recommendations [13, 64, 66]. Thus, Radder et al. consider them a core team member and explicitly mention them as putative team coordinator, a role to which they attach central importance for its success [66]. MacMahon broaden the range of roles of PN to include assessments of patients’ concerns and challenges, the provision of educational and emotional support, and the facilitation of health or social services [64]. Finally, Lennaerts et al. bring up the facilitation of self-management in PwPs, the establishment of multidisciplinary collaboration among care professionals and the execution of specific nursing-technical interventions as tasks for PN [13]. All these recommendations nourish in particular the view that the profession of PN is not yet clearly defined.
Strength and weaknesses of the method
Thus far, our results have rendered evidence on existing models of personalized care for PwPs involving PN. We could elaborate their distinct roles within existing models of care and highlight, in which roles within healthcare teams the PN was favored. At the same time, some limitations must be acknowledged. Firstly, even though, full texts were requested from the authors, it was not possible to access all publications that were found during the systematic search process. The number of unavailable studies, as well as the language restrictions might have biased the results of this review.
Contrary to the expectations, no evidence was traceable that personalized care models improve the QoL of PwPs compared to standardized care. It would be tempting to restrict this to the relatively few studies eligible for our meta-analysis. Yet, some other aspects may also explain this apparent contradiction. Another source of variance is the considerable difference in sample sizes, modes and content of delivery for multidisciplinary programs for PwPs but also the intervention length. The occurrence of reporting bias may have led to a deceptively positive perception of the interventions’ efficacy and should therefore be addressed. Information was lacking in some studies, particularly about to the in-depth characterization of the concept of PN, so that future research should address this gap with detailed descriptions of each professional’s role within care teams.
Nevertheless, some important strengths of this work are to be highlighted. It is well-known that PD impacts severely the lives of PwPs and their care partners [69, 70] and that the PDQ-39 is a validated and widely-used tool assessing QoL [71]. Along with a rather large sample size, the use of the PDQ-39, a validated and widely-used tool assessing QoL [71], as primary outcome enabled greater statistical power and generalizability. The pre-specified analysis in this systematic review and the few exclusion criteria but especially the meticulous methods, underline our results as the current state of knowledge. Yet, a question to be addressed is whether a holistic view of QoL is possible [5, 72]. Further research should also investigate whether this primary outcome is adequate when examining the impact of personalized care approaches for the manifold limitations PD entails over its course.
Considerations for future research in the field of Parkinson’s care
Notwithstanding the heterogeneity and diversity of the study results to date, some recommendations for the future are possible. First, scientists should consider the complexity of (PD-)care research when designing studies and critically appraise which elements should be addressed. Focusing on single theoretical concepts may be helpful in planning studies, but limit the evaluation of results. Care models may be multidisciplinary, patient-centered, integrated, and personalized.
The planning of problem-orientated research is of central importance. From a methodological perspective, we follow previously made recommendations [8] advocating for a head-to-head design, comparing the standard of care with a complex, personalized care intervention or and for implementing cluster-randomized designs, as blinding participants may be hardly achievable. While randomized controlled study designs are considered the gold standard, more pragmatic research approaches also appear as relevant research approaches [73, 74]. Finally, explicit descriptions of the settings, the objectives, the mechanisms of cooperation, the responsibilities and the preliminary theoretical considerations might promote a universal definition of the roles of PN.
Conclusion
In summary, we provide evidence on existing models of personalized care for PwPs involving PN. The results of the present studies demonstrate a diverse set of tasks a PN can perform and confirm the picture of a manifold understanding of roles, but also show that scientific concepts such as personalized care can help to sort out and make tangible these tasks. For example, van Halteren’s concept of personalized PD care served as a structural basis for the qualitative analysis in this review. The review revealed that, these highly specialized professionals already assume various roles within the care team, some of which go beyond defined elements of personalized PD care, such as providing psychological support. Additionally, we could highlight the roles within healthcare teams that might become more prevalent in the future because they provide extraordinary benefits, such as including patients’ preferences in medical decisions. Overall, it seems important to further harmonize concepts of the PN across healthcare systems and to emphasize their role as team members of multidisciplinary care approaches and, perhaps, in leading the implementation of personalized care. Thus, further scientific evidence of the PN role in care models is desirable to generate a larger data pool supported by clearly stated theoretical concepts and well-designed evaluative studies.
ACKNOWLEDGMENTS
We would like to thank Beate Weber-Schicker and Elena Keuchel for their assistance in the collection of data. Special appreciation to Annika Dörrhöfer for her support in the preparation of charts and tables.
This review and meta-analysis were conducted as part of the research project “iCARE-PD”. This is an EU Joint Programme –Neurodegenerative Disease Research (JPND) project. The project is supported through the following funding organisations under the aegis of JPND –http://www.jpnd.eu (Canada –Canadian Institutes of Health Research; Czech Republik –Ministry of Education, Youth and Sport of the Czech Republic; France –Agence National de la Recherche; Germany –Bundesministerium für Bildung und Forschung; Spain –National Institute of Health Carlos III; United Kingdom –Medical Research Council).
M.vM. and J.S. are funded by the aforementioned research project “iCARE-PD”.
F.R. is supported by the Helmholtz Association under the joint research school “Munich School for Data Science –MUDS"
CONFLICT OF INTEREST
M.v.M. declares no COI; J.S. declares no COI; F.T. declares no COI; F.R. declares no COI; T.C. declared no COI; K.C. declared no COI; D.P. received honoraria as a speaker on symposia sponsored by Boston Scientific and Desitin Pharma. The institution of D.P., not D.P. personally received funding by Boston Scientific, the German Research Foundation, the German Ministry of Education and Research and the Deutsche Parkinson Vereinigung.
T.M. has received personal compensation for serving as a Consultant for CHDI, Sunovion, Valeo Pharma, Roche, Biogen and nQ, received personal compensation serving on a Speakers Bureau for Abbvie, Valeo Pharma, and has received research support from the Canadian Institutes of Health Research, EU Joint Programme— Neurodegenerative Disease Research, the Ontario Research Fund, Michael J Fox Foundation, Parkinson Canada, uOBMRI/Parkinson Research Consortium, Parkinson Canada, Brain Canada, Ontario Brain Institute, and PSI Foundation.
O.R. has received personal compensation for serving as a Consultant for AbbVie, Adamas, Acorda, Addex, Aguettant, Alkahest, AlzProtect, Apopharma, Astrazeneca, Axovant, Bial, Biogen, Britannia, Buckwang, Cerevel, Clevexel, Contera, Handltherapeutic, Irlab, Lilly, Lundbeck, Lupin, Merck, MundiPharma, Neuratris, Neuroderm, Novartis, ONO Pharma, Orion Pharma, Osmotica, Oxford Biomedica, Parexel, Pfizer, Prexton Roche,Therapeutics, Quintiles, Sanofi, Servier, Sunovion, Théranexus, Takeda, Teva, UCB, Watermark Research, XenoPort, XO and Zambon and has received research support from Agence Nationale de la Recherche (ANR), CHU de Toulouse, France-Parkinson, INSERM-DHOS Recherche Clinique Translationnelle, MJFox Foundation, Programme Hospitalier de Recherche Clinique, European Commission (FP7, H2020).
SUPPLEMENTARY DATA
The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-223215.
REFERENCES
[1] | Armstrong MJ , Okun MS ((2020) ) Diagnosis and treatment of Parkinson disease: A review. JAMA 323: , 548–560. |
[2] | Macchi ZA , Koljack CE , Miyasaki JM , Katz M , Galifianakis N , Prizer NP , Sillau SH , Kluger BM ((2020) ) Patient and caregiver characteristics associated with caregiver burden in Parkinson’s disease: A palliative care approach. Ann Palliat Med 9: , 24–33. |
[3] | Gustavsson A , Svensson M , Jacobi F , Allgulander C , Alonso J , Beghi E , Dodel R , Ekman M , Faravelli C , Fratiglioni L , Gannon B , Hilton Jones D , Jennum P , Jordanova A , Jönsson L , Karampampa K , Kapp M , Kobelt G , Kurth T , Lieb R , Linde M , Ljungcrantz C , Maercker A , Melin B , Moscarelli M , Musayev A , Norwood F , Preisig M , Pugliatti M , Rehm J , Salvador-Carulla L , Schlehofer B , Simon R , Steinhausen H-C , Stovner LJ , Vallat J-M , van den Bergh P , van Os J , Vos P , Xu W , Wittchen H-U , Jönsson B , Oleen J , CDBE2010Study Group ((2011) ) Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21: , 718–779. |
[4] | Dorsey ER , Constantinescu R , Thompson JP , Biglan KM , Holloway RG , Kieburtz K , Marshall FJ , Ravina BM , Schifitto G , Siderowf A , Tanner CM ((2007) ) Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68: , 384–386. |
[5] | Thieken F , Timmermann L , Sohrabi K , Woopen C , Schmitz-Luhn B , Janhsen A , Eggers C ((2021) ) Development of a multidimensional assessment tool for the evaluation of holistic quality of life in Parkinson’s disease. J Parkinsons Dis 12: , 361–370. |
[6] | Nardini C , Osmani V , Cormio PG , Frosini A , Turrini M , Lionis C , Neumuth T , Ballensiefen W , Borgonovi E , D’Errico G ((2021) ) The evolution of personalized healthcare and the pivotal role of European regions in its implementation. Per Med 18: , 283–294. |
[7] | van Halteren AD , Munneke M , Smit E , Thomas S , Bloem BR , Darweesh SKL ((2020) ) personalized care management for persons with Parkinson’s disease. J Parkinsons Dis 10: (s1), S11–S20. |
[8] | Rajan R , Brennan L , Bloem BR , Dahodwala N , Gardner J , Goldman JG , Grimes DA , Iansek R , Kovács N , McGinley J , Parashos SA , Piemonte MEP , Eggers C ((2020) ) Integrated care in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 35: , 1509–1531. |
[9] | Bloem BR , Henderson EJ , Dorsey ER , Okun MS , Okubadejo N , Chan P , Andrejack J , Darweesh SKL , Munneke M ((2020) ) Integrated and patient-centred management of Parkinson’s disease: A network model for reshaping chronic neurological care. Lancet Neurol 19: , 623–634. |
[10] | Calabresi P , Nigro P , Schwarz HB ((2019) ) A nurse-led model increases quality of care in Parkinson disease. Neurology 92: , 739–740. |
[11] | MacMahon DG , Thomas S ((1998) ) Practical approach to quality of life in Parkinson’s disease: The nurse’s role. J Neurol 245: , 19–22. |
[12] | Bhidayasiri R , Boonpang K , Jitkritsadakul O , Calne SM , Henriksen T , Trump S , Chaiwong S , Susang P , Boonrod N , Sringean J , van Laar T , Drent M , Chaudhuri KR ((2016) ) Understanding the role of the Parkinson’s disease nurse specialist in the delivery of apomorphine therapy. Parkinsonism Relat Disord 33: , 49–55. |
[13] | Lennaerts H , Groot M , Rood B , Gilissen K , Tulp H , van Wensen E , Munneke M , van Laar T , Bloem BR ((2017) ) A guideline for parkinson’s disease nurse specialists, with recommendations for clinical practice. J Parkinsons Dis 7: , 749–754. |
[14] | Jones B , Hopkins G , Wherry SA , Lueck CJ , Das CP , Dugdale P ((2016) ) Evaluation of a regional Australian nurse-led Parkinson’s service using the context, input, process, and product evaluation model. Clin Nurse Spec 30: , 264–270. |
[15] | Page MJ , McKenzie JE , Bossuyt PM , Boutron I , Hoffmann TC , Mulrow CD , Shamseer L , Tetzlaff JM , Akl EA , Brennan SE , Chou R , Glanville J , Grimshaw JM , Hróbjartsson A , Lalu MM , Li T , Loder EW , Mayo-Wilson E , McDonald S , McGuinness LA , Stewart LA , Thomas J , Tricco AC , Welch VA , Whiting P , Moher D ((2021) ) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372: , n71. |
[16] | Hughes AJ , Daniel SE , Kilford L , Lees AJ ((1992) ) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: , 181–184. |
[17] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W , Obeso J , Marek K , Litvan I , Lang AE , Halliday G , Goetz CG , Gasser T , Dubois B , Chan P , Bloem BR , Adler CH , Deuschl G ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30: , 1591–1601. |
[18] | Elo S , Kyngäs H ((2008) ) The qualitative content analysis process. J Adv Nurs 62: , 107–115. |
[19] | Martinez-Martin P , Jeukens-Visser M , Lyons KE , Rodriguez-Blazquez C , Selai C , Siderowf A , Welsh M , Poewe W , Rascol O , Sampaio C , Stebbins GT , Goetz CG , Schrag A ((2011) ) Health-related quality-of-life scales in Parkinson’s disease: Critique and recommendations. Mov Disord 26: , 2371–2380. |
[20] | Ouyang Y , Qian H , Yatham LN , Wong H ((2020) ) Increasing the power of randomized trials comparing different treatment durations. Contemp Clin Trials Commun 19: , 100588. |
[21] | Pazzaglia C , Imbimbo I , Tranchita E , Minganti C , Ricciardi D , Lo Monaco R , Parisi A , Padua L ((2020) ) Comparison of virtual reality rehabilitation and conventional rehabilitation in Parkinson’s disease: A randomised controlled trial. Physiotherapy 106: , 36–42. |
[22] | Strouwen C , Molenaar EALM , Münks L , Keus SHJ , Zijlmans JCM , Vandenberghe W , Bloem BR , Nieuwboer A ((2017) ) Training dual tasks together or apart in Parkinson‘s disease: Results from the DUALITY trial. Mov Disord 32: , 1201–1210. |
[23] | van der Kolk NM , Vries de NM , Kessels RPC , Joosten H , Zwinderman AH , Prost B , Bloem BR ((2019) ) Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lanc Neur 18: , 998–1008. |
[24] | Sterne JAC , Savović J , Page MJ , Elbers RG , Blencowe NS , Boutron I , Cates CJ , Cheng HY , Corbett MS , Eldridge SM , Emberson JR , Hernán MA , Hopewell S , Hróbjartsson A , Junqueira DR , Jüni P , Kirkham JJ , Lasserson T , Li T , McAleenan A , Reeves BC , Shepperd S , Shrier I , Stewart LA , Tilling K , White IR , Whiting PF , Higgins JPT ((2019) ) RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 28: , l4898. |
[25] | Deeks JJ , Higgins JPT , Altman DG ((2019) ) Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions, Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, et al., eds.: JohnWiley & Sons Ltd., pp. 241–284. |
[26] | Egger M , Davey Smith G , Schneider M , Minder C ((1997) ) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: , 629–634. |
[27] | Balduzzi S , Rücker G , Schwarzer G ((2019) ) How to perform a meta-analysis with R: A practical tutorial. Evid Based Ment Health 22: , 153–160. |
[28] | Dorsey ER , Deuel LM , Voss TS , Finnigan K , George BP , Eason S , Miller D , Reminick JI , Appler A , Polanowicz J , Viti L , Smith S , Joseph A , Biglan KM ((2020) ) Increasing access to specialty care: A pilot, randomized controlled trial of telemedicine for Parkinson’s disease. Mov Disord 25: , 1652–1659. |
[29] | Eggers C , Dano R , Schill J , Fink GR , Hellmich M , Timmermann L ; CPN study group ((2018) ) Patient-centered integrated healthcare improves quality of life in Parkinson’s disease patients: A randomized controlled trial. J Neurol 265: , 764–773. |
[30] | Eggers C , Dano R , Schill J , Fink GR , Timmermann L , Voltz R , Golla H , Lorenzl S ((2018) ) Access to end-of life Parkinson’s disease patients through patient-centered integrated healthcare. Front Neurol 9: , 627. |
[31] | Hurwitz B , Jarman B , Cook A , Bajekal M ((2005) ) Scientific evaluation of community-based Parkinson’s disease nurse specialists on patient outcomes and health care costs. J Eval Clin Pract 11: , 97–110. |
[32] | Jarman B , Hurwitz B , Cook A , Bajekal M , Lee A ((2002) ) Effects of community based nurses specialising in Parkinson’s disease on health outcome and costs: Randomised controlled trial. BMJ 324: , 1072–1075. |
[33] | van der Marck MA , Bloem BR , Borm GF , Overeem S , Munneke M , Guttman M ((2013) ) Effectiveness of multidisciplinary care for Parkinson’s disease: A randomized, controlled trial. Mov Disord 28: , 605–611. |
[34] | Wang S , Zhang Z ((2020) ) The effects of comprehensive nursing intervention on the nutritional status and negative emotions of patients with Parkinson’s disease. Int J Clin Exp 13: , 5297–5304. |
[35] | Aye YM , Liew S , Neo SX , Li W , Ng HL , Chua ST , Zhou WT , Au WL , Tan EK , Tay KY , Tan LC , Xu Z ((2020) ) Patient-centric care for Parkinson’s disease: From hospital to the community. Front Neurol 11: , 502. |
[36] | Connor KI , Cheng EM , Barry F , Siebens HC , Lee ML , Ganz DA , Mittman BS , Connor MK , Edwards LK , McGowan MG , Vickrey BG ((2019) ) Randomized trial of care management to improve Parkinson disease care quality. Neurol 92: , e1831–e1842. |
[37] | Fleisher JE , Sweeney MM , Oyler S , Meisel T , Friede N , Di Rocco A , Chodosh J ((2020) ) Disease severity and quality of life in homebound people with advanced Parkinson disease: A pilot study. Neurol Clin Pract 10: , 277–286. |
[38] | Fleisher JE , Klostermann EC , Hess SP , Lee J , Myrick E , Chodosh J ((2020) ) Interdisciplinary palliative care for people with advanced Parkinson’s disease: A view from the home. Ann Palliat Med 9: , 80–89. |
[39] | Giladi N , Manor Y , Hilel A , Gurevich T ((2014) ) Interdisciplinary teamwork for the treatment of people with Parkinson’s disease and their families. Curr Neurol Neurosci Rep 14: , 493. |
[40] | Guo Y , Gao X , Ji S ((2020) ) Improvements of sleep quality and QOL by Roy Adaptation Model in nursing of patients with Parkinson’s disease. Int J Clin Exp Med 13: , 1033–1040. |
[41] | Hellqvist C ((2021) ) Promoting self-care in nursing encounters with persons affected by long-term conditions-a proposed model to guide clinical care. Int J Environ Res Public Health 18: , 2223. |
[42] | Kluger BM , Miyasaki J , Katz M , Galifianakis N , Hall K , Pantilat S , Khan R , Friedman C , Cernik W , Goto Y , Long J , Fairclough D , Sillau S , Kutner JS ((2020) ) Comparison of integrated outpatient palliative care with standard care in patients with Parkinson disease and related disorders: A randomized clinical trial. JAMA Neurol 77: , 551–560. |
[43] | Liu W , Zhang H , Du K ((2019) ) Effect of comprehensive nursing on quality of life and nursing satisfaction in Parkinson’s patients. Int J Clin Exp Med 12: , 12340–12347. |
[44] | Pretzer-Aboff I , Prettyman A ((2015) ) Implementation of an integrative holistic healthcare model for people living with Parkinson’s disease. Gerontologist 55: , 146–153. |
[45] | Wu T , Huang W , Wang Y ((2020) ) Effect of positive psychological nursing intervention on anxiety and depression symptoms in elderly patients with Parkinson’s disease. Int J Clin Exp Med 13: , 4337–4345. |
[46] | Carne W , Cifu DX , Marcinko P , Baron M , Pickett T , Qutubuddin A , Calabrese V , Roberge P , Holloway K , Mutchler B ((2005) ) Efficacy of multidisciplinary treatment program on long-term outcomes of individuals with Parkinson’s disease. J Rehabil Res Dev 42: , 779–786. |
[47] | Su K , Carter J , Tuck K , Borcich T , Bryans LA , Mann LL , Wilhelm JL , Fromme EK ((2016) ) Palliative care for patients with Parkinson’s disease: An interdisciplinary review and next step model. J Parkinsonism Restless Legs Syndrome 7: , 1–12. |
[48] | Gage H , Grainger L , Ting S , Williams P , Chorley C , Carey G , Borg N , Bryan K , Castleton B , Trend P , Kaye J , Jordan J , Wade D (2014) Specialist rehabilitation for people with Parkinson’s disease in the community: A randomised controlled trial. NIHR Journals Library, Southampton (UK). |
[49] | Reynolds H , Wilson-Barnett J , Richardson G ((2000) ) Evaluation of the role of the Parkinson’s disease nurse specialist. Int J Nurs Stud 37: , 337–349. |
[50] | Trend P , Kaye J , Gage H , Owen C , Wade D ((2002) ) Short-term effectiveness of intensive multidisciplinary rehabilitation for people with Parkinson’s disease and their carers. Clin Rehabil 16: , 717–725. |
[51] | Lindskov S , Westergren A , Hagell P ((2007) ) A controlled trial of an educational programme for people with Parkinson’s disease. J Clin Nurs 16: (11C), 368–376. |
[52] | Wade DT , Gage H , Owen C , Trend P , Grossmith C , Kaye J ((2003) ) Multidisciplinary rehabilitation for people with Parkinson’s disease: A randomised controlled study. J Neurol Neurosurg Psychiatry 74: , 158–162. |
[53] | Sitzia J , Haddrell V , Rice-Oxley M ((1998) ) Evaluation of a nurse-led multidisciplinary neurological rehabilitation programme using the Nottingham Health Profile. Clin Rehabil 12: , 389–394. |
[54] | McCormack B , Borg M , Cardiff S , Dewing J , Jacobs G , Janes N , Karlsson B , McCance T , Mekkin TE , Porock D , vam Lieshout F , Wilson V ((2015) ) Person-centredness –the ‘state’ of the art. Int Pract Dev J 5: , 1–15. |
[55] | Ferrer L (2015) Engaging patients, carers and communities for the provision of coordinated/integrated health services: Strategies and tools. World Health Organization: Copenhagen. |
[56] | Prell T , Siebecker F , Lorrain M , Tönges L , Warnecke T , Klucken J , Wellach I , Buhmann C , Wolz M , Lorenzl S , Herbst H , Eggers C , Mai T ((2020) ) Specialized staff for the care of people with Parkinson’s disease in Germany: An overview. J Clin Med 9: , 2581. |
[57] | Blümel M , Spranger A , Achstetter K , Maresso A , Busse R ((2020) ) Germany: Health system review 2020. Health systems in transition 22: , 1–296. |
[58] | Antunes V , Moreira JP ((2011) ) Approaches to developing integrated care in Europe: A systematic literature review. J Man Health Mark 4: , 129–135. |
[59] | De Baetselier E , Van Rompaey B , Batalha LM , Bergqvist M , Czarkowska-Paczek B , De Santis A , Dijkstra NE , Fernandes MI , Filov I , Grøndahl VA , Heczkova J , Helgesen AK , Isfort M , Jordan S , Karnjus I , Keeley S , Kolovos P , Langer G , Lillo-Crespo M , Logan V , Malara A , Meyer G , Olah A , Padysakova H , Prosen M , Pusztai D , Sino CG , Tziaferi S , Ziakova E , Dilles T ((2020) ) EUPRON: Nurses’ practice in interprofessional pharmaceutical care in Europe. A cross-sectional survey in 17 countries. BMJ Open 10: , e036269. |
[60] | Maier CB , Aiken LH ((2016) ) Task shifting from physicians to nurses in primary care in 39 countries: A cross-country comparative study. Eur J Public Health 26: , 927–934. |
[61] | Mai T ((2018) ) Stand und entwicklung der rolle als Parkinson nurse in Deutschland –eine online-befragung. Pflege 31: , 181–189. |
[62] | Rafferty AM , Busse R , Zander-Jentsch B , Sermeus W , Bruyneel L (2019) Strengthening health systems through nursing: Evidence from 14 European countries. Health Policy Series No. 51,World Health Organization: Copenhagen. |
[63] | Delamaire M , Lafortune G (2010) Nurses in Advanced Roles: A Description and Evaluation of Experiences in 12 Developed Countries. OECD Health Working Papers, No. 54, OECD Publishing: Paris. |
[64] | MacMahon DG ((1999) ) Parkinson’s disease nurse specialists: An important role in disease management. Neurology 52: , 21–25. |
[65] | Prell T , Siebecker F , Lorrain M , Eggers C , Lorenzl S , Klucken J , Warnecke T , Buhmann C , Tönges L , Ehret R , Wellach I , Wolz M ((2020) ) Recommendations for standards of network care for patients with Parkinson’s disease in Germany. J Clin Med 9: , 1455. |
[66] | Radder DLM , de Vries NM , Riksen NP , Diamond SJ , Gross D , Gold DR , Heesakkers J , Henderson E , Hommel ALAJ , Lennaerts HH , Busch J , Dorsey RE , Andrejack J , Bloem BR ((2019) ) Multidisciplinary care for people with Parkinson’s disease: The new kids on the block! . Expert Rev Neurother 19: , 145–157. |
[67] | Tod AM , Kennedy F , Stocks AJ , McDonnell A , Ramaswamy B , Wood B , Whitfield M ((2016) ) Good-quality social care for people with Parkinson’s disease: A qualitative study. BMJ Open 6: , e006813. |
[68] | van Munster M , Stümpel J , Thieken F , Pedrosa DJ , Antonini A , Côté D , Fabbri M , Ferreira JJ , Ružička E , Grimes D , Mestre TA ((2021) ) Moving towards integrated and personalized care in Parkinson’s disease: A framework proposal for training Parkinson nurses. J Pers Med 11: , 623. |
[69] | Edwards NE , Ruettiger KM ((2002) ) The influence of caregiver burden on patients’ management of Parkinson’s disease: Implications for rehabilitation nursing. Rehabil Nurs 27: , 182–186. |
[70] | Henry RS , Lageman SK , Perrin PB ((2020) ) The relationship between Parkinson’s disease symptoms and caregiver quality of life. Rehabil Psychol 65: , 137–144. |
[71] | Marinus J , Ramaker C , van Hilten JJ , Stiggelbout AM ((2002) ) Health related quality of life in Parkinson’s disease: A systematic review of disease specific instruments. J Neurol Neurosurg Psychiatry 72: , 241–248. |
[72] | van Uem JM , Marinus J , Canning C , van Lummel R , Dodel R , Liepelt-Scarfone I , Berg D , Morris ME , Maetzler W ((2016) ) Health-related quality of life in patients with Parkinson’s disease–A systematic review based on the ICF model. Neurosci Biobehav Rev 61: , 26–34. |
[73] | Chodankar D ((2021) ) Introduction to real-world evidence studies. Perspect Clin Res 12: , 171–174. |
[74] | Seers K , Critelton N ((2001) ) Quantitative research: Designs relevant to nursing and healthcare. NT Res 6: , 487–500. |

![Study flow diagram (ref. [15]).](https://content.iospress.com:443/media/jpd/2022/12-6/jpd-12-6-jpd223215/jpd-12-jpd223215-g001.jpg)



