Tracking Emergence of New Motor and Non-Motor Symptoms Using the MDS-UPDRS: A Novel Outcome Measure for Early Parkinson’s Disease?
Abstract
Background:
Summary scores of current clinical rating scales do not appear sensitive enough to quantify changes in disease progression in early Parkinson’s disease (PD) clinical trials. An alternate approach might be to track the appearance of new or emergent symptoms (ES) over time as a measure of disease progression.
Objective:
Explore the potential utility of patient reported ES as an outcome measure during the early phase of PD.
Methods:
We analyzed data from the MDS-UPDRS Parts IB (non-motor) and II (motor) Experiences of Daily Living scales over two years in the STEADY-PD3 study. We assessed the number of ES reported in each part of the scale in both participants who started symptomatic treatment and those who did not (STx-yes/no) in two periods: between 0 and 12-months (Year 1), and 13 and 24-months (Year 2).
Results:
Of 331 participants, 87% developed ES, and 55% started STx in Year 1. The median number of Part IB ES did not significantly differ between STx groups, but ES in Part II were significantly more frequent in the STx-yes group. Of 148 participants who remained STx-no into Year 2, 77% developed ES, and 42% started STx. Again, Part II, but not Part IB ES were more frequent the STx-yes group. Using these results, a sample size of ∼90 per group would be required to detect a 30% reduction in combined Part IB and II ES over 12 months.
Conclusion:
Assessing ES of patient-reported experiences of daily living may provide a useful marker for tracking PD progression.
INTRODUCTION
The Braak hypothesis holds that as Parkinson’s disease (PD) progresses, different areas of the brain become progressively impacted by the neurodegenerative process which manifests with a spectrum of clinical signs and symptoms [1]. Although the clinical impact of PD is obvious to most patients in the early stages of the disease, we do not have sensitive tools to assess disease progression specifically in early PD [2–4]. Currently, to assess progression we rely mainly on observations of symptoms and functionality, measured with clinician completed scales and patient self-report measures [5, 6]. The progression of functional impairment over the course of the disease, especially in its earliest stages, seems almost imperceptible as measured by the current scales. However, in daily practice, clinicians and researchers are commonly struck by patient statements such as: “Last time I saw you I could do “X”, but now I can’t (or I need help, or it takes me longer)”. Disease progression, as viewed through this patient-centric lens of ever-accumulating milestones of difficulty to the point of failure, is not a linear process, but a stepwise, saltatory one, with emerging symptoms (ES) or impairments piling on the old, one after another [7, 8].
Measuring the impact of therapies designed to slow disease progression is thus currently extremely challenging. Current clinical trials that aim to assess the clinical meaningfulness and statistical significance of interventions that might reduce by 30–50% an average disease progression rely on a background rate of change of 5% per year or less [9]. In this sense, determining how the occurrence of ES can impact the course of the disease, especially in patients with early-stage PD, may contribute to the development of new assessment methods based on patient centered outcomes [10].
A similar exploration in patients with early Alzheimer’s disease tracked the appearance of new neuropsychiatric symptoms, suggested that the number, rather than the severity of ES had the greatest impact on patients over time [11]. With this precedent, in the present study, we aimed to explore the utility of assessing ES impacting the daily experiences of patients with early PD, as measured by the Non-Motor (Part IB) and Motor (Part II) Experiences of Daily Living subscales of the Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [12] as a potentially novel patient relevant outcome applicable during the early phase of the disease. We also assessed the relationship between initiation of antiparkinson therapy (STx), to the rate of ES to determine whether this measure was affected by, or independent of, use of STx.
METHODS
We analyzed data from the Safety, Tolerability, and Efficacy Assessment of Isradipine for PD (STEADY-PD) study, a multicenter, randomized, parallel-group, double-blind, placebo-controlled trial (ClinicalTrials.gov: NCT02168842). The aims and methods of the STEADY-PD study have been published elsewhere [12], as well as results [13].
Data and sample
From the enrolled cohort of 336 participants of the STEADY-PD dataset, we limited the sample to PD participants with complete data from MDS-UPDRS Part IB (Non-motor Aspects of Experiences of Daily Living) and Part II (Motor Aspects of Experiences of Daily Living), and with at least one annual follow-up for two years, totaling 331 participants. Of note, the MDS-UPDRS was only administered 3 times in STEADY-PD3: at Baseline, and at the 12 and 24-month visits.
Because the outcome of the STEADY-PD study showed no effect of the investigational agent, we combined participants receiving both placebo and active treatment in our analysis.
Outcomes
Our primary outcome was ES for participants during the study. To perform this analysis, we divided the sample into categories according to the period of the follow-up visit, initiation of antiparkinson treatment or not (STx-yes or STx-no) during the observation period, and the presence of ES. We used STx as a proxy of patient- and clinician-perceived disease progression. We analyzed these outcomes for MDS- UPDRS Part IB and Part II. Part IB of the MDS-UPDRS has 7 items that assess non-motor functional impairment and Part II has 13 items that assess motor functional impairment.
We separated the follow-up visits in two distinct periods: the first observation period included the period between baseline and 12 months (Year 1), and the second observation period included the period between 13 months and 24 months of this 36-month clinical trial, beyond which time there was insufficient data for analysis.
We defined ES as the occurrence of a new symptom between the beginning of each period and the follow-up visit. That is, if participants were scored as “zero” on any given item in Part IB or Part II the MDS-UPDRS at the baseline and had any score other than zero at 12 months, they were classified as having an ES. Those who were scored zero at the baseline, zero at 13 months and any score different from zero at 24 months were classified as having ES in the period between 13 months and 24 months. Items scored as > 1 at baseline were not included in the analysis. Thus, increase in symptom severity was not assessed.
To assign STx categories, we used the date when antiparkinson therapy was initiated (the visit day) and analyzed it according to the time interval between follow-up visits. For example, if the participants started STx on day 105 of the study, they were allocated in year 1 to the “STx-yes” group for the entire interval. If a participant started STx on day 400 of the study, they were allocated STx-yes for Year 2 only.
Statistical analyses
We used tables and histograms with distribution of frequencies, medians, and percentages to summarize the descriptive statistics. To compare the proportion of participants with or without ES and the proportion of STx-yes versus STx-no as well as the interaction of ES and STx status we used binomial tests assuming equal proportions. Mann-Whitney U test was used to compare differences between the two groups of participants with or without ES, regardless of STx status. Statistical significance was set at alpha < 0.05 and analyses were corrected for multiple comparisons, where appropriate, using a Bonferroni correction. Finally, we estimated required sample size to detect at least a 30% change in ES over a 12-month period. All statistical analyses were performed using SPSS® Statistics version 28 (IBM reference)
RESULTS
Demographics
At baseline, the 331 STEADY-PD participants included in this study had a mean age of 62.4 years (± 9.0), with a preponderance of males (72%). The mean disease duration from diagnosis was 10 months (± 8.8), and the Hoehn and Yahr stage median score was 2 (ranging from 0 to 3). The mean total for the Motor Examination (Part III) of the MDS-UPDRS was 25.4 (SD 10.4). For the MDS-UPDRS Parts that were analyzed for this study (Parts IB and II) the means were 4.1 (SD 3.02) and 5.24 (SD 3.95).
Emergent symptoms in year 1
Of 331 participants evaluable at Month 12, significantly more participants developed ES (n = 288) than did not (n = 43) (p≤0.0005) (Table 1). This difference held for both Part IB, where 190 out of 331 experienced ES (p = 0.008) and Part 2, where 250 out of 331 experiencing ES (p≤0.0005). There was a non-significant difference in those who started symptomatic therapy (STx-yes = 182) from those who did not (STx-no = 149) (p = 0.08). The differences in experiencing an ES between STx were not significant for Part 1B (p = 0.44), Part 2 (p = 0.25) or Part IB and II combined (p = 0.25). The number of Part IB ES was not significantly different between the STx-yes group (median 1, range 0–6) and STx-no group (median 1, range 0–4) (Z = –0.861, p = 0.389). However, the STx-yes group had a significantly greater number of Part II ES (median 2, range 0–7) compared to the STx-no group (median 1, range 0–9) (Z = –2.38, p = 0.017). When Part IB and Part II ES were combined, the difference in total number of ES between STx-yes and STx-no remained significant (Z = –2.19, p = 0.028).
Table 1
Emergent symptoms in participants with or without antiparkinsonian therapy measured by MDS-UPDRS Parts IB and II according to the follow-up visit
| 0 to 12 Months (n of patients = 331) | |||||||||
| MDS-UPDRS | Patients with ES (%) | Patients without ES (%) | p | STx-Yes (N = 182) | STx-No (N = 149) | Patients with ES p | ES Median p | ||
| Patients with ES (%) | ES Median (range) | Patients with ES (%) | ES Median (range) | ||||||
| ES-Part IB | 190 (57.4) | 141 (42.6) | 0.008 | 108 (56.8) | 1 (1–4) | 82 (43.2) | 1 (1–6) | 0.437 | 0.389 |
| ES-Part II | 250 (75.5) | 81 (24.5) | < 0.0005 | 142 (56.8) | 2 (1–7) | 108 (43.2) | 2 (1–9) | 0.251 | 0.017 |
| ES-Parts IB and II | 288 (87.0) | 43 (13.0) | < 0.0005 | 162 (56.3) | 3 (1–11) | 126 (43.8) | 2 (1–12) | 0.253 | 0.028 |
| 13 to 24 Months (n of patients = 148) | |||||||||
| MDS-UPDRS | Patients with ES (%) | Patients without ES (%) | p | STx-Yes (N = 62) | STx-No (N = 86) | Patients with ES p | ES Median p | ||
| Patients with ES (%) | ES Median (range) | Patients with ES (%) | ES Median (range) | ||||||
| ES-Part IB | 62 (41.9) | 86 (58.1) | 0.058 | 25 (40.3) | 1 (1–5) | 37 (59.7) | 1 (1–3) | 0.866 | 0.738 |
| ES-Part II | 99 (66.9) | 49 (33.1) | < 0.0005 | 46 (46.5) | 2 (1–7) | 53 (53.5) | 2 (1-6) | 0.116 | 0.024 |
| ES-Parts IB and II | 114 (77.0) | 34 (23.0) | < 0.0005 | 49 (43.0) | 3 (1–12) | 65 (57.0) | 2 (1-8) | 0.694 | 0.126 |
STx, symptomatic treatment starting during the interval; ES, emergent symptoms.
Emergent symptoms in year 2
Of 149 participants who had not started to receive treatment at Year 1 and who remained at the 24-month time point, 148 were evaluable (one participant contributed no data). Significantly more of these 148 participants developed any ES (n = 114) than not (n = 34) (p≤0.0005) and this difference was mostly due to differences in Part II where 99 developed ES but 49 did not (p≤0.0005), but not in Part IB where 62 developed ES but 86 did not (p = 0.058). 62 participants (42%) were STx-yes and 86 were STx-no (p = 0.058) (Table 1). The differences in experiencing an ES between STx-yes and STx-no were not significant for Part IB (p = 0.866), Part II (p = 0.116) or Part IB and II combined (p = 0.694). The number of Part IB ES was not significantly different between the STx-yes group (median 0, range 0–5) and STx-no group (median 0, range 0–3) (Z = –0.334, p = 0.738). However, the STx-yes group had a significantly greater number of Part II ES (median 1, range 0–7) compared to the STx-no group (median 1, range 0–6) (Z = –2.25, p = 0.024). When Part IB and Part II ES were combined, the difference in total number of ES between STx-yes and STx-no was no longer significant (Z = –1.529, p = 0.126).
Pattern of reported ES
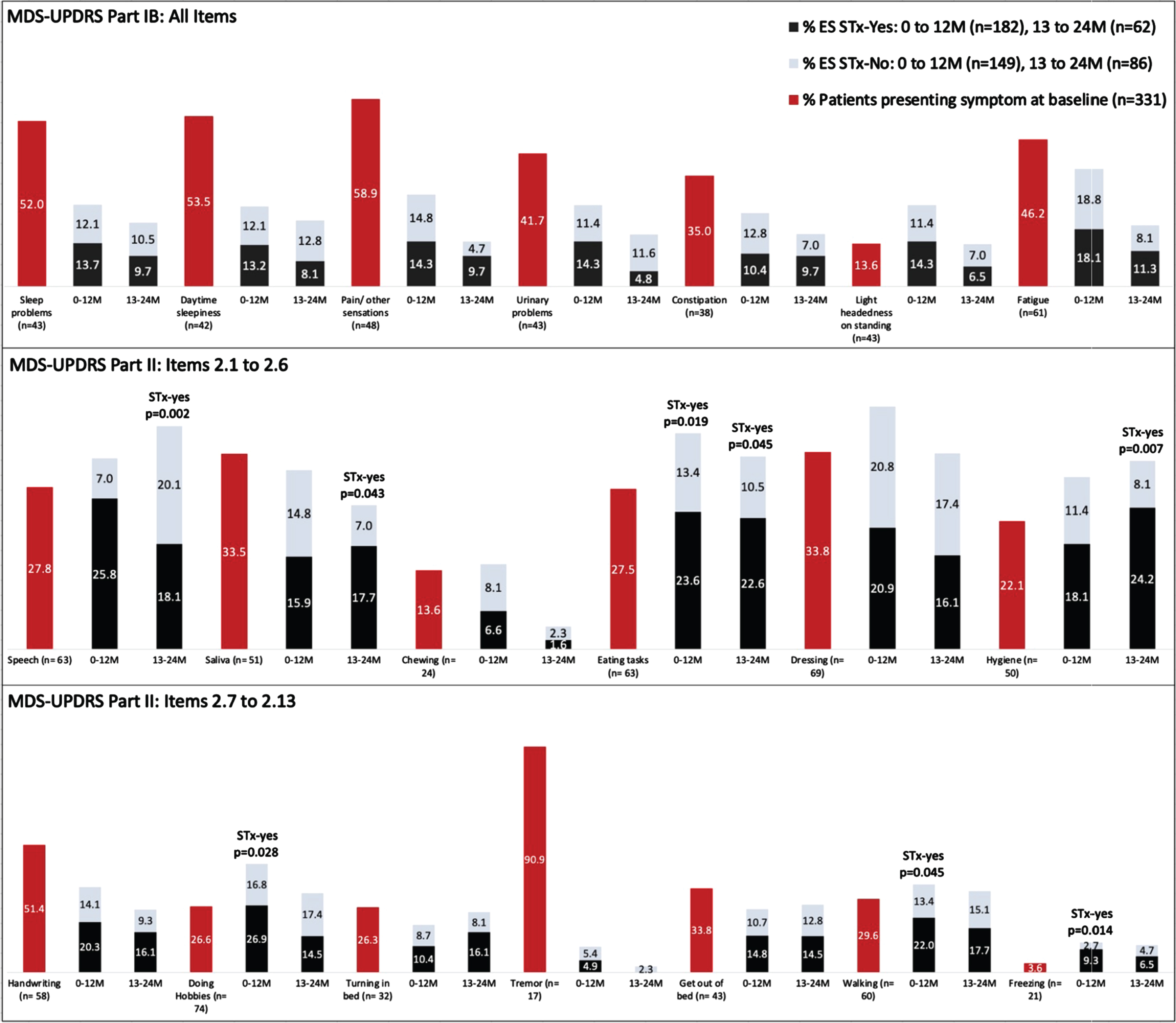
The pattern of individual symptoms experienced by the groups of participants is presented in Fig. 1. The percentage of participants with ES for each item of Part 1B (top row) and Part 2 (middle and bottom rows) are presented for baseline (red bars) and broken-out for the two treatment periods (gray and black bars). Participants who reported a given symptom at the previous timepoint are not included in the denominator in calculating the proportions; persons who did not report a given symptom during the observation period are not represented in the graph; thus totals for any given symptom do not add to 100%. The participants in the STx-yes group had significantly more ES related to Freezing (p = 0.014), Eating Tasks (p = 0.019), Walking and Balance (p = 0.045) and Doing Hobbies (p = 0.028) compared to the STx-no group in Year 1. In Year 2, participants in the STx-yes group had significantly more ES related to Speech (p = 0.002), Hygiene (p = 0.007), Saliva and Drooling (p = 0.043), and Eating Tasks (p = 0.045) compared to the STx-no group. Tremor, when present, was overwhelmingly present at baseline, and emerged only infrequently as a new symptom.
Fig. 1
Percentages of participants endorsing individual MDS-UPDRS Part IB and II scale items at baseline and at follow-up study visits. Emergent symptoms (ES) reported at the follow-up timepoints are divided according to use of antiparkinson therapy (STx-yes and STx-no). Once a participant endorsed a given symptom, they were no loger included in the denominator for that scale item. Thus, the numbers reflect the proportions of participants available at the respective time points who had not previously reported the symptom, so the totals for each item do not sum to 100%.
Potential utility of ES as a clinical trial outcome measure
Given these results, we were interested to see how the proportion of patients developing ES might perform as a clinical trial outcome measure. We estimate that a sample size of 98 would provide 0.80 power (1-β) to detect a 30% reduction in ES from baseline to 12-month follow-up, given an alpha of 0.05 and with equal assignment to treatment group and continuity correction when using Part IB alone. A sample size of 96 would be required for the same parameters when considering Part II alone. However, when both Parts IB and II are combined, a sample size of 82 would provide 0.80 power (1-β) to detect a 30% reduction in ES given the same parameters for alpha and participant assignment.
DISCUSSION
In this exploratory analysis we asked whether, like the Braak-staged progression of engagement of new brain areas based on prion-like spread of synuclein pathology, the clinical progression of PD can be characterized by progressive appearance of ES, independent of the worsening severity of symptoms already present. Using data from Parts IB and II of the MDS-UPDRS in the STEADY-PD clinical trial [12, 13], we found that the number of both motor and non-motor symptoms reported by participants increased over time in a clinical trial population, and that 87% of the study population reported at least one ES over the first 12 months of the study. Emergence of new motor symptoms was slightly more frequent than the emergence of non-motor symptoms. Interestingly, the incidence of both motor and non-motor ES was less in the group of participants who began STx after 13 months of the study. Thus, tracking self-reported ES may provide a novel means of assessing the progression of PD.
Of note was the fact that while a vast majority of study participants reported tremor as a symptom present at baseline, it rarely emerged as a new symptom. This observation is in keeping with the observation of that tremor and non-tremor items of the MDS-UPDRS Part III subscale bear different relationships to the underlying concept of PD severity [14] and that tremor as assessed in Part 3 of the MDS-UPDRS contributes to functional disability at baseline but newly emergent tremor is uncommon as the disease progresses, as assessed by Part II [15].
The results of our study demonstrate that Parts IB and II of the MDS-UPDRS, taken together as a single Patient-Reported Outcome (PRO) measure, can function as a record of milestone attainment in the form of appearance of new disease manifestations [16]. Arguably, especially early in disease, appearance of a new symptom, as occurred in 87% of our participants within the first year of observation, could be interpreted to represent a significant milestone for most persons suffering from PD. The sensitivity of tracking ES as an outcome measure is reflected by the sample size estimates that less than 100 participants/arm would be required to observe a statistically significant effect in a 1-year clinical trial. This contrasts with sample size requirements of 312 and 1240 per group to observe, with 80% power, a 50% or 25% reduction, in the rate of progression of the total Part II score. The required sample sizes using Part I would be 1090 and 4352 based on data from PPMI[17].
Kieburtz et al. recently suggested that tracking milestones of disease progression could provide a useful outcome measure for clinical trials of potential disease modifying therapies [18]. However, milestones previously proposed, such as need for symptomatic medication, significant falls, or recognizable cognitive impairment, either represent changes in patient status relevant to more advanced disease or represent subjective and/or socially determined states. In fact, only 20% of PPMI participants reached one of a set of designated milestones, by the end of 1 year of observation [19]. In early disease a milestone-based assessment of disease progression would of necessity need to be much more fine-grained. Tracking of ES may be a more useful method for monitoring evolution of early-stage PD in the short term.
Our work does have limitations. First, our observation is based on a study in which the MDS-UPDRS was administered only at yearly intervals. At this point in time, data are not available in the public domain from other clinical studies that have administered the MDS-UPDRS more frequently than once every 6 or 12 months. Thus, we were unable, for example to assess the stability of ES once recorded. For ES to constitute a truly useful outcome measure, one would need to verify stability of ES with observations at consecutive timepoints at least a month apart. Thus, it would be highly desirable to replicate our observations in a database with more-frequent MDS-UPDRS administration. With more frequently sampled data it would also be possible to evaluate the relative meaningfulness and consistency of various criteria for determining ES; for example, a 2 point, as opposed to the 1-point threshold employed in the current study. Secondly, even though Part 2 of the MDS-UPDRS is generally considered to be a validated instrument that corelates with other clinically meaningful measures [20], the clinical meaningfulness for participants of ES based on the MDS-UPDRS item inventory, while an attractive concept, has yet to be verified. Such verification could come either via the traditional scale validation and clinimetric methodology, e.g., correlating ES with other conventional outcome measures, use of Delphi panels, cognitive debriefing, and revalidation, or via correlation with patient self-reported experiences using approaches, such as the Patient Report of Problems (PROP) based on data in the Fox Insight database [21].
Finally, we found it interesting to note that the appearance of ES was slightly more frequent in study participants, all of whom were untreated at the time of enrollment, who began to receive STx during the study. It should be noted, however that since MDS-UPDRS was performed only at the beginning and end of each interval, we cannot determine whether the emergence of new symptoms created a need for medication, or whether in fact new symptoms were masked by the initiation of treatment in this analysis. Based on the available data it cannot be determined whether either a) the newly emergent symptoms contributed to the initiation of treatment, or b) participants who started STx did so because they had a more rapidly progressive clinical course. However, as has been reported, initiation of STx is the result of a complex medical and often social and economic calculus for individual participants, and factors like social circumstances, continuation of employment etc., may be more powerful determinants of STx initiation than emergence of any one or combination of symptoms [22]. Importantly, it does appear that addition of STx did not completely mask the severity of ES in these individuals. Thus, tracking ES may be a robust method of gauging PD progression that is and relatively resistant to confounding effects of symptomatic treatment.
CONCLUSIONS
New symptoms continue to emerge in most PD participants in the first 2 years of PD. Motor ES (Part II) were more frequent than non-motor ES (Part IB) among participants initiating antiparkinsonian treatment in both Year 1 and Year 2 of the study. Assessing ES reflecting patient-reported experiences of daily living may provide a useful marker for tracking PD progression, especially in early PD. The accrual of ES may have a particular advantage as an efficient outcome in Proof-of-Concept clinical trials which, by their nature, require relatively small sample sizes. The concept of tracking ES as a milestone-based clinical trial outcome measure is worthy of exploration in future studies and alternative datasets.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
REFERENCES
[1] | Braak H , Del Tredici K , Rüb U , De Vos RAI , Jansen Steur ENH , Braak E ((2003) ) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24: , 197–211. |
[2] | Holden SK , Finseth T , Sillau SH , Berman BD ((2018) ) Progression of MDS-UPDRS scores over five years in de novo Parkinson disease from the Parkinson’s Progression Markers Initiative Cohort. Mov Disord Clin Pract 5: , 47–53. |
[3] | Regnault A , Boroojerdi B , Meunier J , Bani M , Morel T , Cano S ((2019) ) Does the MDS-UPDRS provide the precision to assess progression in early Parkinson’s disease? Learnings from the Parkinson’s progression marker initiative cohort. J Neurol 266: , 1927–1936. |
[4] | Vu TC , Nutt JG , Holford NHG ((2012) ) Progression of motor and nonmotor features of Parkinson’s disease and their response to treatment. Br J Clin Pharmacol 74: , 267–283. |
[5] | McCann H , Cartwright H , Halliday GM ((2016) ) Neuropathology of α-synuclein propagation and braak hypothesis. Mov Disord 31: , 152–160. |
[6] | Lawrence BJ , Gasson N , Kane R , Bucks RS , Loftus AM ((2014) ) Activities of daily living, depression, and quality of life in Parkinson’s disease. PLoS One 9: , e102294. |
[7] | Strupp J , Kunde A , Galushko M , Voltz R , Golla H ((2018) ) Severely affected by Parkinson disease: The patient’s view and implications for palliative care. Am J Hosp Palliat Med 35: , 579–585. |
[8] | Fox S , Cashell A , Kernohan WG , Lynch M , McGlade C , O’Brien T , O’Sullivan SS , Foley M , Timmons S ((2017) ) Palliative care for Parkinson’s disease: Patient and carer’s perspectives explored through qualitative interview. Palliat Med 31: , 634–641. |
[9] | Simuni T , Siderowf A , Lasch S , Coffey CS , Caspell-Garcia C , Jennings D , Tanner CM , Trojanowski JQ , Shaw LM , Seibyl J , Schuff N , Singleton A , Kieburtz K , Toga AW , Mollenhauer B , Galasko D , Chahine LM , Weintraub D , Foroud T , Tosun D , Poston K , Arnedo V , Frasier M , Sherer T , Chowdhury S , Marek K ; Parkinson’s Progression Marker Initiative ((2018) ) Longitudinal change of clinical and biological measures in early Parkinson’s disease: Parkinson’s Progression Markers Initiative Cohort. Mov Disord 33: , 771–782. |
[10] | van Uem JMT , Marinus J , Canning C , van Lummel R , Dodel R , Liepelt-Scarfone I , Berg D , Morris ME , Maetzler W ((2016) ) Health-related quality of life in patients with Parkinson’s disease - A systematic review based on the ICF model. Neurosci Biobehav Rev 61: , 26–34. |
[11] | Tariot P , Lyketsos C , Crans G , Cedarbaum J , Hernandez C , Abushakra S ((2012) ) The effects of ELND005 (Scyllo-Inositol) on emergence of neuropsychiatric symptoms (NPS) in mild/moderate Alzheimer’s disease: Results from a 78-week phase 2 study (P04.215). Neurology 78: (1 Supplement), P04.215. |
[12] | Goetz CG , Tilley BC , Shaftman SR , Stebbins GT , Fahn S , Martinez-Martin P , Poewe W , Sampaio C , Stern MB , Dodel R , Dubois B , Haooloway R , Jankovic J , Kulisevsky J , Lang AE , Lees A , Leurgans S , LeWitt PA , Nyenhuis D , Olanow CW , Rascol O , Schrag A , Teresi JA , van Hilten JJ , Lapelle N , Movement Disorder Society UPDRS Revision Task Force ((2008) ) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov Disord 23: , 2129–2170. |
[13] | Biglan KM , Oakes D , Lang AE , Hauser RA , Hodgeman K , Greco B , Lowell J , Rockhill R , Shoulson I , Venuto C , Young D , Simuni T , Parkinson Study Group STEADY-PD III Investigators ((2017) ) A novel design of a Phase III trial of isradipine in early Parkinson disease (STEADY-PD III). Ann Clin Transl Neurol 4: , 360–368. |
[14] | Simuni T ((2020) ) Isradipine versus placebo in early Parkinson disease a randomized trial. Ann Intern Med 172: , 591–598. |
[15] | Tosin MHS , Goetz CG , Luo S , Choi D , Stebbins GT ((2020) ) Item response theory analysis of the MDS-UPDRS motor examination: Tremor vs. nontremor items. Mov Disord 35: , 1587–1595. |
[16] | Cedarbaum JM , Xiao J , Yang M ((2019) MDS-UPDRS Part III predictors of Part II item scores in PPMI. 34: (Supppl 2), S489–S490. |
[17] | Kane PB , Benjamin DM , Barker RA , Lang AE , Sherer T , Kimmelman J ((2001) ) Comparison of patient and expert perceptions of the attainment of research milestones in Parkinson’s disease. Mov Disord 36: , 171–177. |
[18] | Simuni T , Caspell-Garcia C , Seedorff N , Coffey C , Lasch S , Mollenhauer B , Tanner C , Kieburtz K . Marek K ((2017) ) Sample size estimation for clinical trials in de novo Parkinson’s disease (PD): Results from the Parkinson’s Progression Markers Initiative (PPMI) Study. Mov Disord 32: (Suppl 2), 449–450. |
[19] | Kieburtz K , Katz R , McGarry A , Olanow CW ((2021) ) A new approach to the development of disease-modifying therapies for PD; fighting another pandemic. Mov Disord 36: , 59–63. |
[20] | Brumm M , Siderowf A , Simuni T , Caspell-Garcia L , Chahine L , Foroud T , Arnedo V , Reimer A , Tanner C , Poston K , Weintraub D , Hutten S , Kieburtz K , Marek K , Coffey C ((2021) ) A Milestone-based approach to monitoring disease progression in Parkinson’s disease. Mov Disord 36: (Suppl 1), S163. |
[21] | Rodriguez-Blazquez C , Rojo Abuin JM , Alvarez-Sanchez M , Arakaki T , Bergareche-Yarza A , Chade A , Garetto N , Gershanik O , Kurtis MM , Martinez-Castrillo JC , Mendoza-Rodriguez A , Moore HP , Rodriguez-Violante M , Singer C , Tilley BC , Huang J , Stebbins GT , Goetz CG , Martinez-Martin P ((2013) ) The MDS-UPDRS Part II (motor experiences of daily living) resulted useful for assessment of disability in Parkinson’s disease. Parkinsonism Relat Disord 19: , 889–893. |
[22] | Vinikoor-Imler L , Arbatti L , Hosamath A , Sapir I , Shirvan J , Maserejian N , Shoulson I ((2021) ) Cross-sectional profile of most bothersome problems as reported directly by individuals with Parkinson’s disease. Neurology 96: (15 Supplement), 2697. |
[23] | LeWitt P , Oakes D , Cui L ((1997) ) The need for levodopa as an end point of Parkinson’s disease progression in a clinical trial of selegiline and α-tocopherol. Mov Disord 12: , 183–189. |




