Cardiac Alpha-Synuclein Is Present in Alpha-Synucleinopathies
Abstract
Background:
Alpha-synucleinopathies (AS) are characterized by pathologic aggregations of alpha-synuclein (α-syn) in the central nervous system, and comprise dementia with Lewy bodies, Parkinson’s disease, and multiple system atrophy. Previous studies on AS have reported findings of α-syn pathology in the peripheral nervous system of multiple organs, including the heart.
Objective:
The aim of this study was to further investigate and confirm the presence of cardiac α-syn in AS compared to other major neurocognitive disorders in a neuropathologically confirmed cohort.
Methods:
All deceased patients with performed autopsy and with neuropathologically confirmed AS at the Clinical Department of Pathology in Lund 2010–May 2021 were evaluated for inclusion. Cases with insufficiently sampled cardiac tissue or only limited neuropathological investigation were excluded. An age-matched group of individuals with other neurodegenerative diseases, having no α-syn in the CNS, served as controls. In total, 68 AS and 32 control cases were included in the study. Immunohistochemistry for detection of cardiac α-syn aggregates was performed.
Results:
The AS group had a significantly higher prevalence of cardiac α-syn pathology (p≤0.001) than the control group, 82% and 0%, respectively.
Conclusion:
This study confirms the association between AS and the presence of cardiac α-syn in a neuropathologically confirmed cohort. This motivates further research on potential pathophysiological effects on cardiac function in AS patients.
INTRODUCTION
Alpha-synucleinopathies (AS) comprise the clinical disease entities dementia with Lewy bodies (DLB), Parkinson’s disease (PD), and multiple system atrophy (MSA), plus cognitively unimpaired individuals with alpha-synuclein (α-syn) pathology in the brainstem. DLB and PD are referred to as Lewy body disease (LBD) [1, 2]. While normal α-syn is believed to maintain synaptic integrity and play an important role in the regulation of dopamine biosynthesis [3–5], AS are all characterized by the presence of abnormal aggregates of misfolded α-syn in neurons and/or surrounding glial cells [1]. It is thought that the misfolded aggregates cause damage to mitochondria, subsequently leading to neuronal apoptosis [6].
The pathological alterations in LBD are believed to originate in one single anatomical location, and then spread to adjacent neuroanatomical regions [7]. One theory proposes transcellular spread of α-syn pathology in a caudo-rostral direction, where lesions initially occur in the enteric nervous system, the glossopharyngeal nerve, the olfactory nerves or the vagus nerve and advance in upward propagation, ultimately reaching the cerebral cortex [8–11]. For MSA, the origin of pathological alterations is not yet established [12].
During the last decades, several studies have found evidence of extra-axial engagement and involvement of nerves in multiple organs, including the heart [13–18]. Studies have reported findings of cardiac, i.e., epicardial nerve α-syn aggregates in most patients with LBD [15]. Moreover, cardiac sympathetic denervation is frequently observed in LBD [19]. An association between cardiac α-syn deposits and this denervation has been suggested [13, 20]. Cardiac sympathetic degeneration and α-syn pathology was shown to occur in pre-symptomatic and early-stage PD patients, and to concurrently increase with the severity and duration of the disease [21–23]. Contrarily, in MSA, the cardiac innervation appears to be better preserved and the α-syn aggregates seem to be less frequent [13, 19, 24].
While not all previous studies provided neuropathologic confirmation, some did, and, i.e., the early work of Orimo et al. (2008) [13] did meticulously explore the cardiac nerve proteinopathy which we also have encountered in the clinical investigation of synucleinopathies over the years. Hence, we felt compelled to report on our findings in a slightly larger cohort—cases accessible from clinical diagnostic investigations over 12 years.
Aim of the study
The aim of this study was to investigate the presence of cardiac α-syn pathology in deceased subjects with neuropathologically confirmed AS and non-AS individuals.
MATERIAL AND METHODS
Study design
We analyzed the prevalence of α-syn in epicardial nerves of deceased patients neuropathologically diagnosed with AS. A control group consisted of age-matched subjects neuropathologically diagnosed with other cognitive diseases/dementia disorders without α-syn in the central nervous system (CNS).
An approval was obtained from the Regional Ethical Review Board, Lund University, currently the Swedish Ethical Review Authority, nr 631-2015, 944-2017, 00051-2019 and 06582-2019.
Subjects
Neuropathological diagnoses
All deceased patients with neuropathologically confirmed AS at the Clinical Department of Pathology in Lund 2010-May 2021 were evaluated for inclusion (n = 92). Cases between 2010 and 2019 were analyzed retrospectively and cases between 2020 and May 2021 were collected prospectively. Exclusion criteria were either a limited neuropathological examination due to minor investigation of a few brain regions, or lack of archival heart tissue.
The neuropathological diagnosis of AS was defined by findings of α-syn pathology in the CNS (brainstem or above) in the immunohistochemical analysis following autopsy, irrespective of other concurrent disease. LBD and MSA pathologies were identified, using current criteria for the brain diseases [12, 24]. The different stages of LBD were judged according to the regional extent of pathology [25]. MSA was evaluated for predominant pathology distribution as MSA-C (cerebellar), MSA-P (parkinsonian), or MSA-C + P (mixed pathology) [12].
Clinical diagnoses
The clinical diagnoses were retrieved from the medical records or referrals to the neuropathological examination. Most individuals had a clinical diagnosis of PD, DLB, or MSA, as determined from a specialist. In a few individuals with AS in the brainstem only, there was cognitive disease of other type.
Control group
The age-matched control group included 32 subjects with any major neurocognitive disorder without α-syn in the brain and brainstem and having representative samples of heart tissue with epicardial nerves. All control group individuals had been diagnosed clinically with a cognitive disease, as in the AS group.
Disease duration
Disease duration was defined as the year of diagnosed cognitive impairment of any type or, when not available, date of first symptoms described in the medical records until time of death. Brainstem LBD and in many cases limbic LBD do not generally exhibit clinical symptoms of the AS, particularly not in the presence of other neurocognitive disorders [26]. Information regarding disease duration was retrieved for these cases as well, but their symptoms were likely due to other pathologies than α-syn pathology.
Cardiac alpha-synuclein pathology
The myocardial samples were taken from several different locations. The sections stained with hematoxylin-eosin were retrieved and analyzed for selection of the section most clearly harboring nerves. The correlating paraffin-embedded tissue block was re-sectioned at 3 micrometer and immunohistochemically stained with a monoclonal mouse anti-α-syn concentrate antibody (Invitrogen by Life technologies, Synuclein clone LB509, Lot no. 2068148) to visualize α-syn deposits. For the prospectively sampled cases, a tissue block containing the epicardium was sectioned and stained with hematoxylin-eosin and alpha-synuclein at one occasion. The same α-syn antibody was applied in brain sections during the neuropathologic diagnostic work prior to this investigation.
Cardiac α-syn pathology was defined as convincing findings of α-syn deposits (granular or filamentous positivities or LBs) within one or multiple cardiac nerves. We did not attempt to quantify deposits numerically or grade the staining intensity, since the sampling was non-homogenous. When positivities were minimal or questionable, they were discarded and judged not convincingly positive (see Figs. 1–4). Study subjects in which no cardiac nerves could be identified were excluded from the study. These were all from the large, retrospectively sampled cohort—no case from the prospectively sampled cohort (in which epicardial tissue was specifically targeted) was excluded.
Fig. 1
Cardiac (epicardial) nerve strongly positive for alpha-synuclein pathology, with Lewy bodies present.
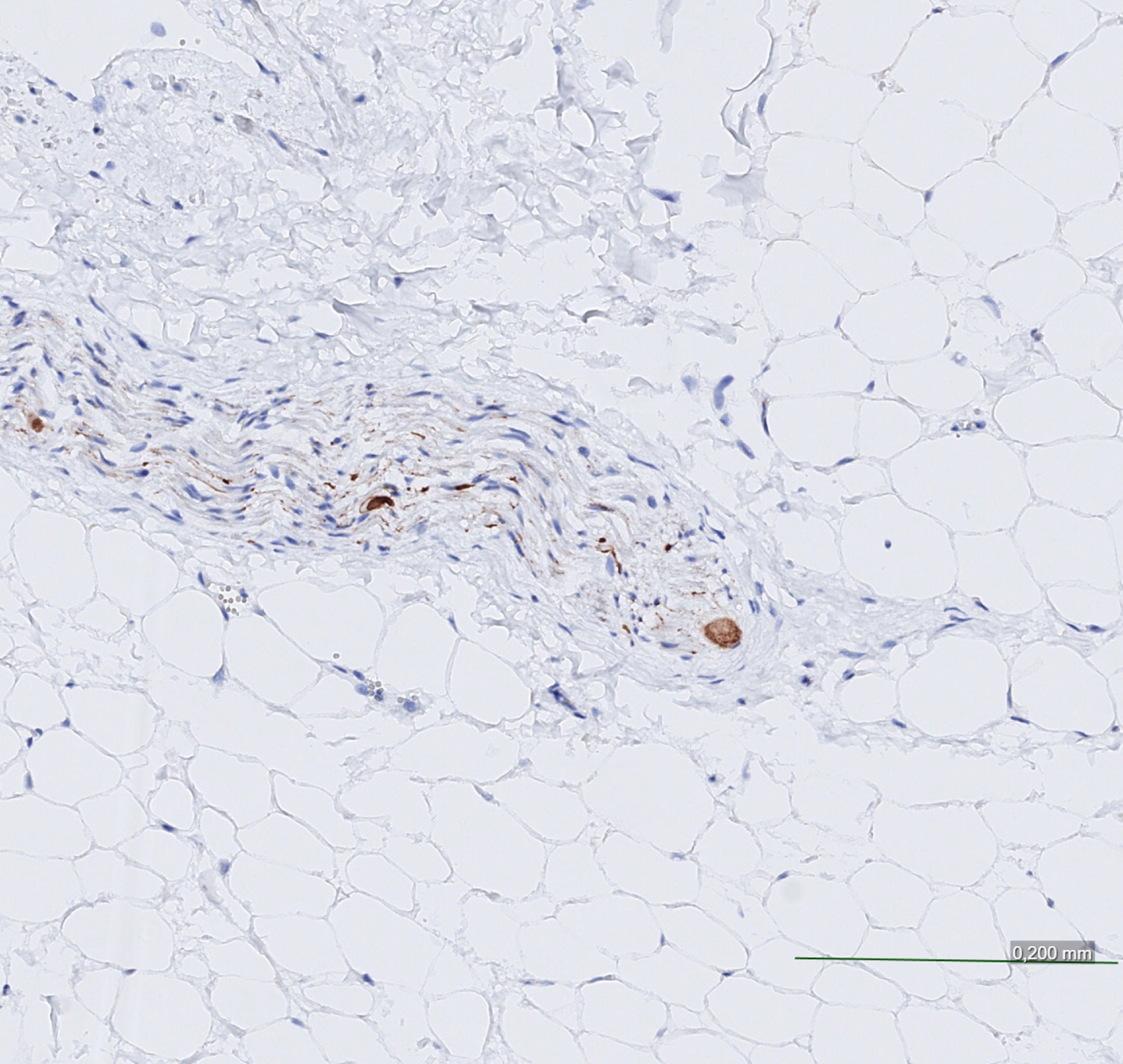
Fig. 2
Cardiac nerve weakly positive for alpha-synuclein pathology.
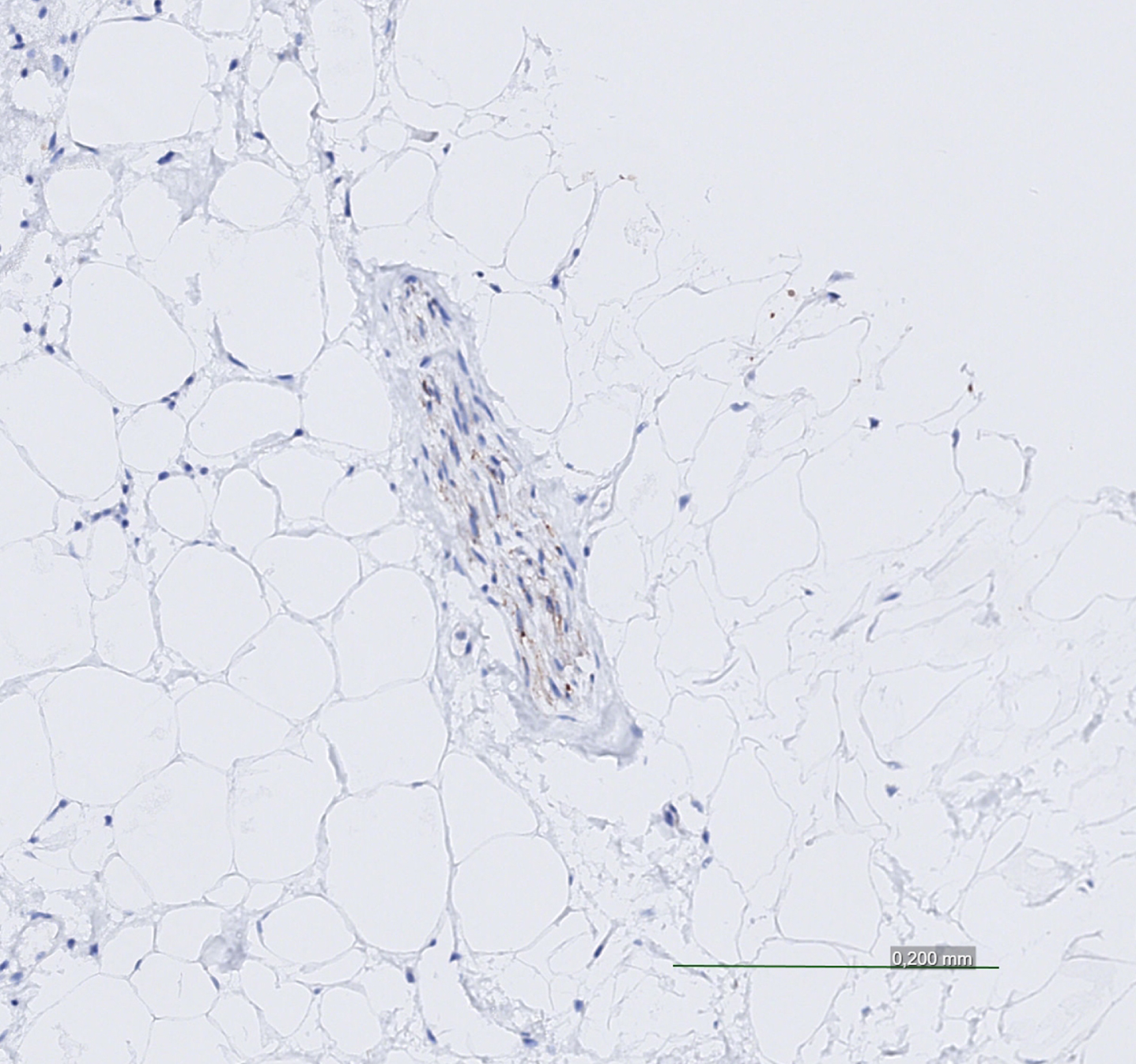
Fig. 3
Cardiac Lewy bodies, strong and focal positivity.
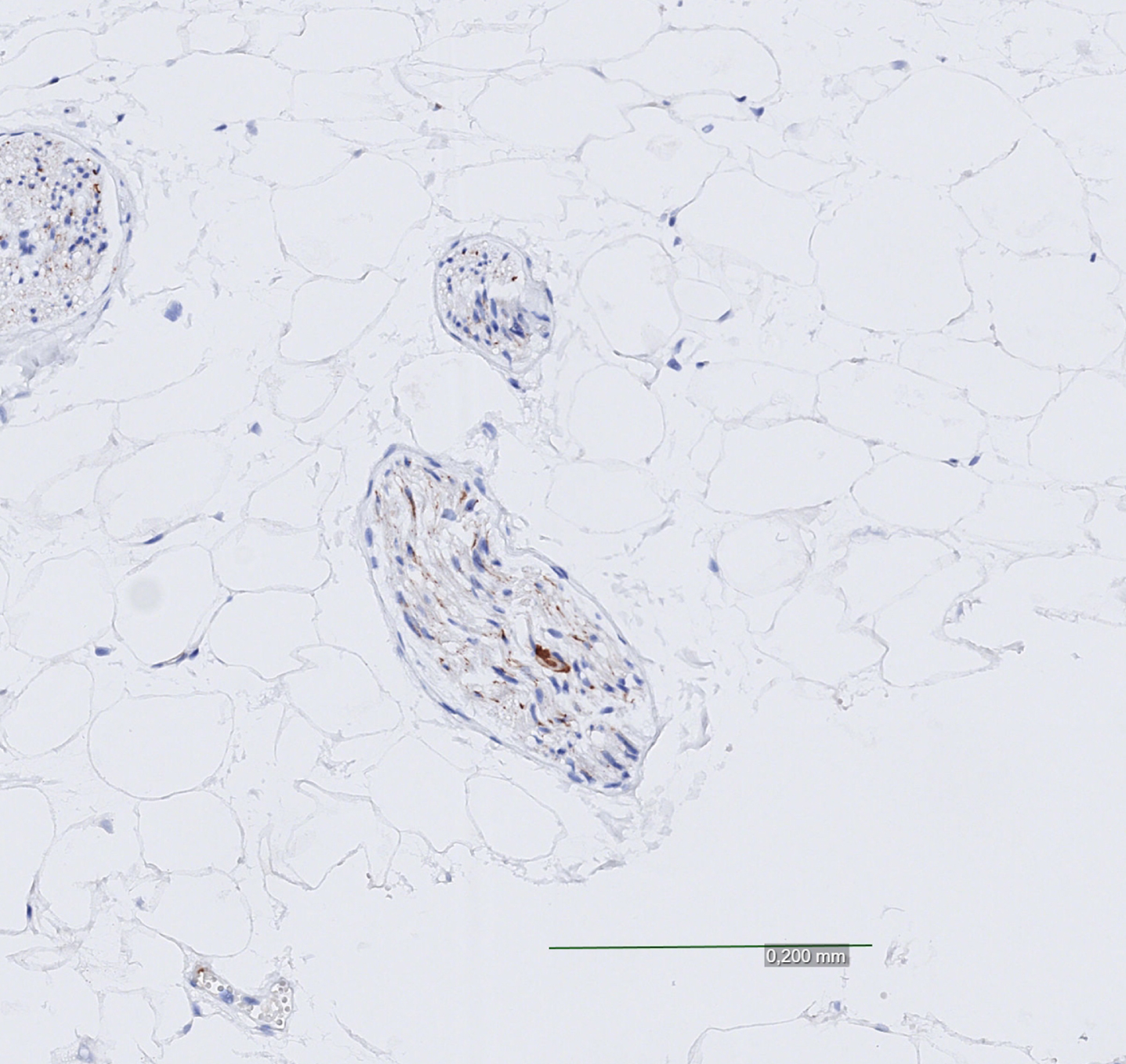
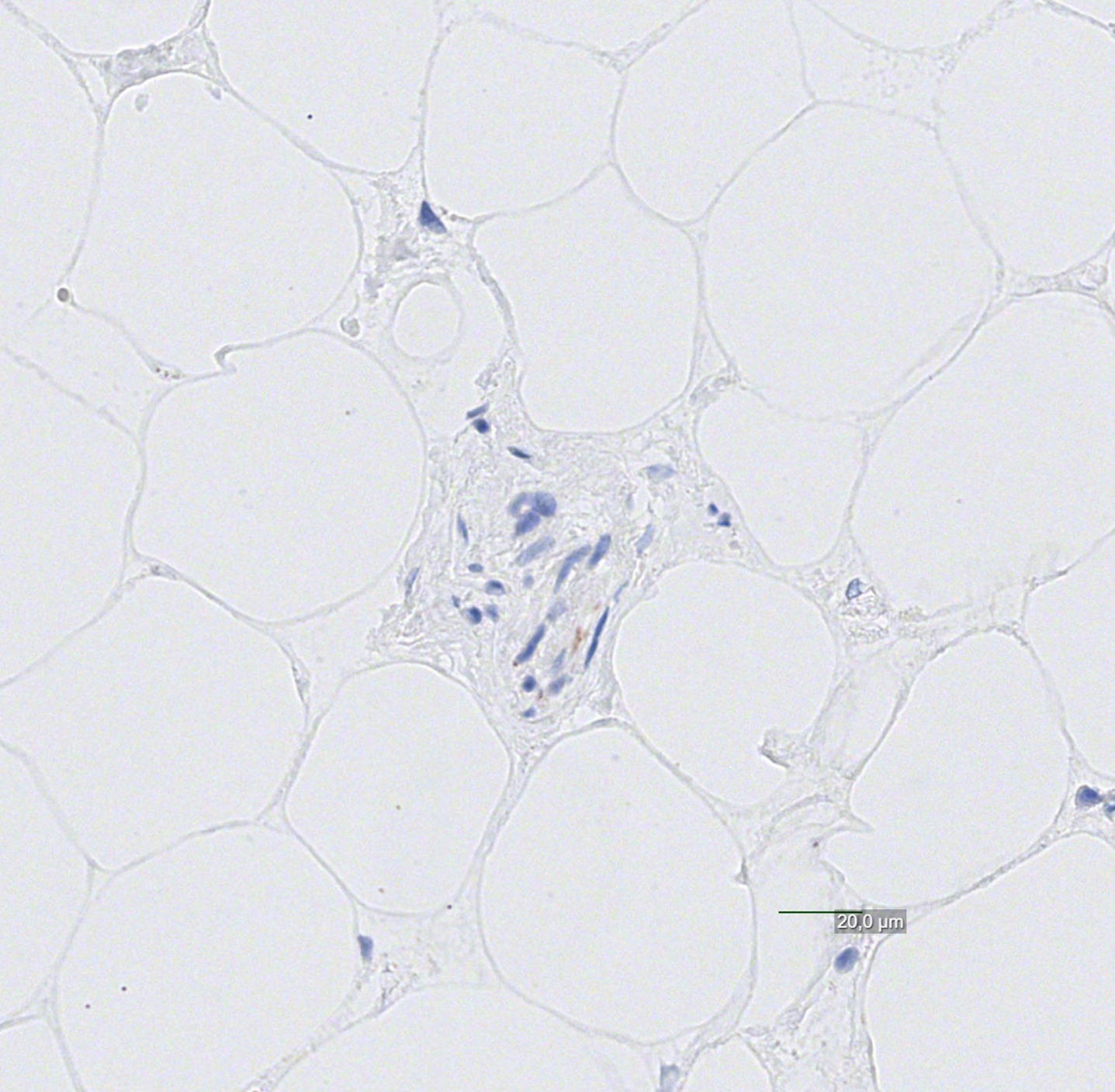
Fig. 4
Cardiac nerve negative for alpha-synuclein pathology with questionable/minimal findings.
Data analysis
Statistical analyses were carried out using Microsoft Excel and IBM SPSS Statistics 26. AS and the control group were compared regarding the presence of myocardial α-syn using crosstabs and Pearson Chi-Square test to test for significance and to investigate the variance in prevalence between the groups. A p-value of≤0.05 was considered statistically significant.
RESULTS
AS subjects
Among the total of 92 subjects reviewed for inclusion, 24 cases were excluded, mainly due to the lack of stainable nerve within the myocardial tissue (often myocardium without epicardial surface) and in a few the lack of sampled heart tissue. Hence, 68 study subjects were included for analysis. For demographic data on the study group, see Table 1.
Table 1
Demographic data and neuropathological diagnoses for all subjects
| Neuropathological diagnosis | n (%) | Sex (n%) Men | Age at Deatha |
| Total | 100 (100) | 66 (66) | 77 (10.0) |
| AS group | 68 (100) | 49 (72) | 76.0 (11.0) |
| LBD | 64 (94) | 47 (73) | 76.5 (10.2) |
| LBD brainstem | 13 | ||
| LBD limbic | 9 | ||
| LBD cortical | 42 | ||
| MSA | 4 (6) | 2 (50) | 64.5 (7.5) |
| Control group | 32 (100) | 17 (53) | 78.5 (10.0) |
| AD | 10 (31) | ||
| VaD | 6 (19) | ||
| FTLD* | 7 (22) | ||
| Mixed pathologies** | 9 (28) |
AS, alpha-synucleinopathy; LBD, Lewy body disease; MSA, multiple system atrophy, including one MSA-C, MSA-P, MSA-C + P and one undetermined; AD, Alzheimer’s disease; VaD, vascular dementia; FTLD, frontotemporal lobar degeneration. *Including five cases with progressive supranuclear palsy and one with corticobasal degeneration. **Including AD, VaD, and FTLD, whereof 6 had a combination of AD + VaD. aPresented in median years, interquartile range within brackets.
In the AS group, 64 (94%) had LBD and 4 (6%) had MSA. Within the LBD group, 42 had cortical, 9 had limbic and 13 had brainstem LBD (Table 1). In addition to fulfilling the criteria for AS, a total of 25 cases had concomitant AD, VaD, FTLD pathology, or admixtures of these, most frequently concurrent AD pathology (Table 2A). The four MSA cases were of cerebellar, parkinsonian and mixed type, respectively, whereas one could not be specified further due to the lack of representatively sampled brain regions (Table 1). The clinical diagnoses did not indicate an AS in all individuals of the studied group and the clinicopathological concordance was 66% (45 cases with a concordant clinical-neuropathologic diagnosis). The clinical diagnoses were distributed as follows: 17 (25.0%) PD, 12 (17.6%) DLB, 5 (7.4%) MSA, 11 (16.1%) AD, 5 (7.4%) vascular dementia (VaD), 5 (7.4%) frontotemporal lobar degeneration (FTLD), 2 (2.9%) with a mix of AD and VaD, 1 (1.5%) with a mix of AD and LBD, and 1 (1.5%) with a mix of AD and PD. Six individuals (8.8%) were labeled with dementia not otherwise specified (DNOS) and three (4.4%) had no diagnosis at all (Table 2B). All three cases with no clinical diagnosis had a neuropathologically confirmed cortical LBD. All cases with brainstem LBD had been clinically diagnosed with other neurocognitive disorders, which were verified as specific neuropathologic conditions in each case.
Table 2A
Neuropathological comorbidity in the AS group
| Neuropathological Diagnosis | n (%) |
| AS group | 68 (100) |
| Cases with only AS | 43 (63) |
| Cases with concomitant pathologies | 25 (37) |
| AD | 9 |
| VaD | 6 |
| FTLD | 4 |
| Mixed pathologies* | 6 |
AS, alpha-synucleinopathy; AD, Alzheimer’s disease; VaD, vascular dementia; FTLD, frontotemporal lobar degeneration. *≥2 of the pathologies mentioned above.
Table 2B
Clinical diagnoses in the AS group
| Clinical diagnosis | n (%) |
| AS group | 68 (100.0) |
| PD | 17 (25.0) |
| DLB | 12 (17.6) |
| MSA | 5 (7.4) |
| AD | 11 (16.1) |
| VaD | 5 (7.4) |
| AD + VaD | 2 (2.9) |
| AD + LBD | 2 (2.9) |
| FTLD | 5 (7.4) |
| DNOS | 6 (8.8) |
| No diagnosis | 3 (4.4) |
AS, alpha-synucleinopathy; PD, Parkinson’s disease; DLB, Dementia with Lewy bodies; MSA, multiple system atrophy; AD, Alzheimer’s disease; VaD, vascular dementia; LBD, Lewy body disease; FTLD, frontotemporal lobar degeneration; DNOS, dementia not otherwise specified.
Control subjects
A total of 32 subjects were included. For demographic data on the control group, see Table 1. The neuropathological diagnoses in the control group were AD in 10 (31%), VaD in 6 (19%), FTLD in 7 (22%), and mixed pathologies in 9 (28%) (Table 1). In the latter group, the majority had a combination of AD and VaD.
Cardiac alpha-synuclein pathology
Cardiac α-syn pathology was found in 56 (82%) cases of the study subjects, including 53/64 of LBD cases and 3/4 of MSA cases. Many cases displayed an irregular distribution of α-syn deposits, with both positive and negative nerves within the same tissue sample. In the 12 (18%) negative cases there was a notable scarcity of cardiac nerves in the sampled myocardial tissue seen in light microscopy. Among them, four exhibited minimal/questionable findings of α-syn positivity (such as solitary positive granules in a few locations), but not enough to be considered as convincingly positive. Examples from this assessment are demonstrated in Figs. 1–4.
The prevalence of cardiac α-syn pathology was significantly higher in the AS group, as all 32 subjects in the control group were negative to cardiac α-syn (p≤0.001) (Table 3). Cardiac α-syn pathology was found in all stages of LBD: brainstem, limbic and cortical. Cases judged negative to cardiac pathology (n = 12) included 9 cases of cortical LBD, 2 cases of limbic LBD, and 1 case of MSA-C + P.
Table 3
Cardiac alpha-synuclein pathology in AS and control cases
| Variables | AS group n (%) | Control group n (%) | pa |
| Total | 68 (100.0) | 32 (100.0) | |
| Cardiac α-syn pathology | 56 (82) | 0 (0) | 0.000 |
AS, alpha-synucleinopathy; α-syn, alpha-synuclein. aChi-square value between study group and control group. A p-value≤0.05 is considered statistically significant.
DISCUSSION
In this study we investigated the presence of cardiac α-syn in AS and a control group consisting of other major non-AS neurocognitive disorders. Our main finding was a significantly higher prevalence of cardiac α-syn pathology in the AS group compared to the control group. In the control group with no α-syn in the brain or brainstem, there was no α-syn in the cardiac nerves.
Our findings regarding AS are concordant with previous studies reporting involvement of nerves in multiple organs including the heart, suggesting that AS are systemic disorders with primarily neurological manifestations [13–18]. Further, our results lend support to the caudo-rostral spread of α-syn pathology in LBD [8–11], as we found cardiac α-syn among cases with only a scarcity of α-syn in the brainstem, but also in all subtypes of LBD: brainstem, limbic and cortical. Cardiac α-syn could thus be present before the AS has given rise to any cognitive symptoms. This is in line with previous studies reporting cardiac sympathetic denervation and α-syn pathology in pre-symptomatic and early-stage PD patients [21–23]. We found cardiac α-syn pathology in 3/4 MSA cases. This was a higher prevalence than previously observed [13], while the prior work consisted of a larger cohort of MSA cases.
The findings of cardiac α-syn in cases with isolated LBD in the brainstem (thus not necessarily with overt cognitive impairment) motivates further research on the pathophysiological effects of α-syn in cardiac nerves. It remains unclear whether the cardiac α-syn deposition plays a causative role for autonomic dysfunction such as orthostatic hypotension. Additionally, MIBG myocardial scintigraphy, which serves as a method for evaluating cardiac sympathetic denervation linked to PD and DLB [27–29], could possibly be used more frequently in diagnosing AS, and may be able to identify patients with or at risk of clinically severe autonomic dysfunction [30]. This investigation was applied in only exceptional/singular cases in our patient groups, not specifically reported.
Regarding disease duration, no striking nominal difference was observed between the different AS disorders. However, no results were presented, and no conclusions were drawn as information concerning onset of brainstem LBD and limbic LBD was not judged representative due to coexisting other neurocognitive disease contributing to clinical symptoms.
A strength of this study is the relatively large sample size. Moreover, the confirmation of α-syn pathology in cardiac nerves was performed by one single neuropathologist, thus increasing the consistency of the evaluations and thereby the quality of the results. Also, since all cases with questionable cardiac α-syn pathology were counted as negative, the risk of overestimating this prevalence is probably low; instead we may rather have underestimated the prevalence of cardiac synuclein pathology. Another strength of this study is the inclusion of subjects with LBD solely in the brainstem. These subjects did not fulfill the criteria for probable LBD and might not have been included in other studies if other pathologies were present [26], since the neuropathological diagnosis for LBD is ordinarily based on pathological findings in the limbic system and above.
Optimally, the control group would be comprised of cases representing the age-matched “general population” instead of cases with major neurocognitive disorders. The brain is however not specifically investigated in cognitively healthy individuals, except for by selective tissue sampling for specific reasons. A control group representative of the general population could thus hardly be obtained. Hence a mere referral for autopsy means a selection of individuals.
A limitation of this study concerns the myocardial tissue sampling, which was targeted to include epicardial tissue only in the smaller, prospectively collected group, while tissue was evaluated retrospectively in most cases. Many heart tissue samples were originally taken with the intention to verify a suspected myocardial infarction, steered by macroscopic findings of discolored myocardium, rather than to analyze nerve tissue, which is best studied in the epicardial tissue [26]. Consequently, some AS cases were excluded from the study, and some cases were included and analyzed despite suboptimal tissue samples with minimal nerve components, likely leading to an under-reporting of cardiac α-syn pathology in the study group. For future studies, samples should be taken from cardiac regions with a high density of nerves, preferably the epicardial region, to increase the reliability of the collected data. There was a scarcity of cardiac nerves in all AS cases judged “negative”. Pathological α-syn involvement of these hearts would conceivably have been found if the original sampling of tissue and the subsequent sectioning had been more extensive and specifically directed. In all cases sampled “prospectively” from year 2020 and on, their cardiac samples were positive for cardiac α-syn pathology. Also, in view of the known cardiac sympathetic denervation in AS, and its proposed association with cardiac α-syn pathology, the cases presented as “negative” might in fact be the most severely affected, due to advanced cardiac nerve degeneration that precluded mere identification of stainable tissue [19]. Further, the findings of irregular distribution of α-syn in cardiac nerves within affected subjects may suggest that all nerves are not affected, at least not synchronously.
The finding however of 100% cardiac a-syn positivity in the prospectively sampled cases, indicates that we perhaps could have found cardiac a-syn positivity in all AS cases, had they been sampled in a prospective way.
CONCLUSION
We found an association between AS and cardiac α-syn pathology, confirming the findings of previously published work. Cardiac α-syn can be present even when the presentation of CNS α-syn is limited to the brainstem only, with probably no clinical cognitive symptoms. Further research is needed to establish clinical and pathologic correlates pertinent to cardiac α-syn and its implementation in the diagnostics, management, and treatment of these patients.
ACKNOWLEDGMENTS
The Trolle-Wachtmeister Foundation for Medical Research, as well as The Lund University Medical Faculty, are acknowledged for essential funding of this study.
CONFLICT OF INTEREST
MH is a former employee of Biogen and Eli Lilly but has no active financial interest in either company. The other authors have no conflict of interest to report.
REFERENCES
[1] | Sanford AM ((2018) ) Lewy body dementia. Clin Geriatr Med 34: , 603–615. |
[2] | Spillantini M , Crowther R , Jakes R ((1998) ) Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci U S A 95: , 6469–6473. |
[3] | Abeliovich A , Schmitz Y , Farinas I , Choi-Lundberg D ((2000) ) Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: , 239–252. |
[4] | Perez R , Waymire J , Lin E , Liu J , Guo F , Zigmond M ((2002) ) A role for alpha-synuclein in the regulation of dopamine biosynthesis. J Neurosci 22: , 3090–3099. |
[5] | Chandra S , Fornai F , Kwon H ((2004) ) Double-knockout mice for alpha- and beta-synucleins - effect on synaptic functions. Proc Natl Acad Sci U S A 101: , 14966–14971. |
[6] | Stefanis L ((2012) ) alpha-Synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med 2: , a009399. |
[7] | Kosaka K , Yoshimura M , Ikeda K , Budka H ((1984) ) Diffuse type of Lewy body disease: Progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree - a new disease? Clin Neuropathol 3: , 185–192. |
[8] | Hardy J ((2005) ) Expression of normal sequence pathogenic proteins for neurodegenerative disease contributes to disease risk: “permissive templating” as a general mechanism underlying neurodegeneration. Biochem Soc Trans 33: , 578–581. |
[9] | Braak H , Del Tredici K , Rüb U ((2003) ) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24: , 197–211. |
[10] | Orimo S , Ghebremedhin E , Gelpi E ((2018) ) Peripheral and central autonomic nervous system: Does the sympathetic or parasympathetic nervous system bear the brunt of the pathology during the course of sporadic PD? Cell Tissue Res 373: , 267–286. |
[11] | Coon E , Cutsforth-Gregory J , Benarroch E ((2018) ) Neuropathology of autonomic dysfunction in synucleinopathies. Mov Disord 33: , 349–358. |
[12] | Seth Love AP , Ironside James , Budka Herbert ((2015) ) Greenfield’s Neuropathology, CRC Press. |
[13] | Orimo S , Uchihara T , Nakamura A , Mori F , Kakita A , Wakabayashi K , Takahashi H ((2008) ) Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 131: , 642–650. |
[14] | Beach T , Adler C , Sue L ((2010) ) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119: , 689–702. |
[15] | Gelpi E , Navarro-Otano J , Tolosa E , Gaig C , Compta Y , Rey MJ , Marti MJ , Hernandez I , Valldeoriola F , Rene R , Ribalta T ((2014) ) Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord 29: , 1010–1018. |
[16] | Kim JY , Illigens BM , McCormick MP , Wang N , Gibbons CH ((2019) ) Alpha-synuclein in skin nerve fibers as a biomarker for alpha-synucleinopathies. J Clin Neurol 15: , 135–142. |
[17] | Wakabayashi K , Takahashi H ((1997) ) Neuropathology of autonomic nervous system in Parkinson’s disease.(Suppl 2). Eur Neurol 38: , 2–7. |
[18] | Wakabayashi K , Takahashi H , Takeda S , Ohama E , Ikuta F ((1988) ) Parkinson’s disease: The presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 76: , 217–221. |
[19] | Orimo S , Amino T , Itoh Y , Takahashi A , Kojo T , Uchihara T , Tsuchiya K , Mori F , Wakabayashi K , Takahashi H ((2005) ) Cardiac sympathetic denervation precedes neuronal loss in the sympathetic ganglia in Lewy body disease. Acta Neuropathol 109: , 583–588. |
[20] | Isonaka R , Sullivan P , Jinsmaa Y , Corrales A , Goldstein DS ((2018) ) Spectrum of abnormalities of sympathetic tyrosine hydroxylase and alpha-synuclein in chronic autonomic failure. Clin Auton Res 28: , 223–230. |
[21] | Fujishiro H , Frigerio R , Burnett M , Klos KJ , Josephs KA , Delledonne A , Parisi JE , Ahlskog JE , Dickson DW ((2008) ) Cardiac sympathetic denervation correlates with clinical and pathologic stages of Parkinson’s disease. Mov Disord 23: , 1085–1092. |
[22] | Orimo S , Takahashi A , Uchihara T , Mori F , Kakita A , Wakabayashi K , Takahashi H ((2007) ) Degeneration of cardiac sympathetic nerve begins in the early disease process of Parkinson’s disease. Brain Pathol 17: , 24–30. |
[23] | Navarro-Otano J , Gelpi E , Mestres CA , Quintana E , Rauek S , Ribalta T , Santiago V , Tolosa E ((2013) ) Alpha-synuclein aggregates in epicardial fat tissue in living subjects without parkinsonism. Parkinsonism Relat Disord 19: , 27–31; discussion 27. |
[24] | Goldstein DS , Orimo S ((2009) ) Cardiac sympathetic neuroimaging: Summary of the First International Symposium. Clin Auton Res 19: , 137–148. |
[25] | Galasko D ((2017) ) Lewy body disorders. Neurol Clin 35: , 325–338. |
[26] | McKeith IG , Boeve BF , Dickson DW , Halliday G , Taylor JP , Weintraub D , Aarsland D , Galvin J , Attems J , Ballard CG , Bayston A , Beach TG , Blanc F , Bohnen N , Bonanni L , Bras J , Brundin P , Burn D , Chen-Plotkin A , Duda JE , El-Agnaf O , Feldman H , Ferman TJ , Ffytche D , Fujishiro H , Galasko D , Goldman JG , Gomperts SN , Graff-Radford NR , Honig LS , Iranzo A , Kantarci K , Kaufer D , Kukull W , Lee VMY , Leverenz JB , Lewis S , Lippa C , Lunde A , Masellis M , Masliah E , McLean P , Mollenhauer B , Montine TJ , Moreno E , Mori E , Murray M , O’Brien JT , Orimo S , Postuma RB , Ramaswamy S , Ross OA , Salmon DP , Singleton A , Taylor A , Thomas A , Tiraboschi P , Toledo JB , Trojanowski JQ , Tsuang D , Walker Z , Yamada M , Kosaka K ((2017) ) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89: , 88–100. |
[27] | Orimo S , Yogo M , Nakamura T , Suzuki M , Watanabe H ((2016) ) (123)I-meta-iodobenzylguanidine (MIBG) cardiac scintigraphy in alpha-synucleinopathies. Ageing Res Rev 30: , 122–133. |
[28] | Chung E , Kim S ((2015) ) (123)I-metaiodobenzylguanidine myocardial scintigraphy in Lewy body-related disorders: A literature review. J Mov Disord 8: , 55–66. |
[29] | Gabilondo I , Llorens V , Rodriguez T , Fernandez M , Concha TP , Acera M , Tijero B , Murueta-Goyena A , Del Pino R , Cortes J , Gomez-Esteban JC ((2019) ) Myocardial MIBG scintigraphy in genetic Parkinson’s disease as a model for Lewy body disorders. Eur J Nucl Med Mol Imaging 46: , 376–384. |
[30] | Jeong YJ , Jeong J-E , Cheon S-M , Yoon B-A , Kim JW , Kang D-Y ((2020) ) Relationship between the washout rate of I-123 MIBG scans and autonomic function in Parkinson’s disease. PloS One 15: , e0229860. |




