Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2021 Update
Abstract
Background:
Despite the COVID-19 pandemic, there has been considerable activity in the clinical development of novel and improved drug-based therapies for the neurodegenerative condition of Parkinson’s disease (PD) during 2020. The agents that were investigated can be divided into “symptomatic” (alleviating the features of the condition) and “disease modifying” (attempting to address the underlying biology of PD) treatments, ST and DMT respectively, with further categorisation possible based on mechanism of action and class of therapy.
Objective:
Our goal in this report was to provide an overview of the pharmacological therapies –both ST and DMT - in clinical trials for PD during 2020–2021, with the aim of creating greater awareness and involvement in the clinical trial process. We also hope to stimulate collaboration amongst commercial and academic researchers as well as between the research and patient communities.
Methods:
We conducted a review of clinical trials of drug therapies for PD using trial data obtained from the ClinicalTrials.gov and World Health Organisation (WHO) registries, and performed a breakdown analysis of studies that were active as of February 18th 2021. We also assessed active drug development projects that had completed one clinical phase but were yet to start the next.
Results:
We identified 142 trials on ClinicalTrials.gov and 14 studies on the WHO registries that met our analysis criteria. Of these 156 trials, 91 were ST and 65 were DMT, Of the 145 trials registered on ClinicalTrials.gov in our 2020 analysis, 45 fell off the list and 42 were added. Despite this change, the balance of ST to DMT; the distribution across phases; the profile of therapeutic categories; and the proportion of repurposed therapies (33.5%); all remained very similar. There are only two DMTs in phase 3, and we identified 33 in-between-phase projects.
Conclusions:
Despite the effects of the coronavirus pandemic, investment and effort in clinical trials for PD appears to remain strong. There has been little change in the profile of the clinical trial landscape even though, over the past year, there has been considerable change to the content of the list.
ABBREVIATIONS
AAV-GAD | Adeno-associated virus encoding glutamic acid decarboxylase |
AMSC | Autologous Mesenchymal stem cells |
APM-CT1 | Australian Parkinson’s Mission –Clinical Trial 1 |
c-Abl | Abelson tyrosine kinase |
CCR3 | C–C chemokine receptor 3 |
CDNF | Cerebral dopamine neurotrophic factor |
COVID-19 | Coronavirus disease 2019 |
CNS | Central Nervous system |
CSF | Cerebrospinal fluid |
DIVE | Dopaminergic restoratIon by intraVEntriculaire Administration |
DMT | Disease Modifying Therapies |
DPP-4 | Dipeptidyl peptidase-4 |
FDA | Food and Drug Administration |
GBA | Glucocerebrosidase |
GDNF | Glial cell-derived neurotrophic factor |
GIT | Gastrointestinal Tract |
GLP-1 | Glucagon-like peptide 1 |
hfVM | Human fetal ventral mesencephalic |
ICD | Impulse control disorder |
ICTRP | International Clinical Trials Registry Platform |
LID | Levodopa-induced dyskinesia |
LRRK2 | Leucine-rich repeat kinase 2 |
MAMS | Multi-Arm Multi-Stage |
MAO-B | Monoamine oxidase type B |
MCFS | Metabolic Cofactor Supplementation |
MCI | Mild Cognitive Impairment |
MOA | Mechanisms of Action |
MRI | Magnetic Resonance Imaging |
NIH | National Institutes of Health |
NINDS | National Institute of Neurological Disorders and Stroke |
NLRP3 | NLR family pyrin domain containing 3 |
NMDA | N-methyl-D-aspartate |
NMS | Non-motor symptoms |
NPC | Neuronal precursor cells |
nOH | Neurogenic orthostatic hypotension |
PD | Parkinson’s Disease |
ST | Symptomatic Therapy |
STEADY | PD Safety, Tolerability, and Efficacy Assessment of Dynacirc® CR for PD |
TOPAZ | Trial of Parkinson’s And Zoledronic Acid |
TZ-PD | Terazosin for PD |
UDCA | Ursodeoxycholic acid |
WHO | World Health Organization |
INTRODUCTION
Parkinson’s disease (PD) is the second most common neurodegenerative disease affecting approximately 6 million people worldwide with only symptomatic treatments available that have limited effect as the disease progresses [1, 2]. This devastating toll on human health also has a financial impact with a recent report that the total cost of PD to individuals, families and the U.S. government is $51.9 billion annually [3]. This makes it of utmost importance that clinical trials for PD are sufficiently robust to increase the chances of effective therapies reaching patients.
In 2020, the COVID-19 pandemic resulted in the closure of academic research institutes and clinical trial facilities around the world, and the postponement or halting of many studies. Despite this, there was considerable ongoing clinical development of symptomatic agents that sought to improve the quality of life of PD patients. In 2020 alone, three therapeutic approaches were approved by the U.S. FDA - a levodopa inhaler, a thin film apomorphine slip and a catechol-O-methyltransferase inhibitor were all approved for the treatment of “off” time, a disruptive period when PD symptoms return between medication doses. Although these treatments may improve the quality of life of PD patients, the ultimate goal to stop and/or reverse the progression of the disease remains elusive.
Increasingly, there are clinical trials evaluating agents that target the biological pathways related to specific genetic variants that increase the risk of PD [4, 5]. With higher throughput genetic screening methods, lowered costs, and multiple initiatives from non-profit organizations (such as the Michael J Fox Foundation’s “FoxInsight” [6], the Parkinson’s Foundation funded PD-GENEration [7], and the Cure Parkinson’s supported PD-Frontline [8]), among others, we are gradually witnessing more targeted clinical trials focused on genetic subtypes of the condition. By taking this more precision medicine-based approach, we will hopefully increase our chances of slowing or halting the progression of PD.
Patient participation in clinical research is crucial and in a collaborative effort to provide the research and patient communities with a better understanding of the current landscape of drug-based trials, we set up a team to deliver an annual report analysing ongoing activity. Our 2020 report on the PD clinical trial pipeline focused on the data contained in the US National Institutes of Health website, ClinicalTrials.gov, the most widely used clinical trial registry [9]. It enabled the comparison of several parameters related to clinical trials using a common dataset. This 2021 review updates that analysis but also extends the search to other clinical trial registries that are collated on the World Health Organization (WHO)’s International Clinical Trials Registry Platform (ICTRP). We have also gathered information on drug development projects that are in between phases, i.e. a clinical trial has completed one phase and the next is being planned, but there is currently no active registered study.
The aim of this report is to help people living with PD, caregivers, and researchers to navigate the growing number of clinical trials by providing an overview and analysis of the current PD drug development pipeline. Our hope is that this report will provide some insights into the different approaches to target PD and explore emerging novel treatment options. We also aim to raise awareness regarding the shape of the pipeline and the individual trials contained therein with the ultimate aim of contributing to greater participation and better trial outcomes.This paper also incorporates the views of people with PD as two of the authors have PD.
METHODS
Data collection
Data on active clinical trials of PD drug therapies were obtained from the ClinicalTrials.gov (https://clinicaltrials.gov/) and the WHO ICTRP (https://www.who.int/clinical-trials-registry-platform) websites for analysis in this review. ClinicalTrials.gov provides information on publicly and privately supported clinical studies conducted worldwide and is the world’s most comprehensive clinical trial registry. To date, over 2800 PD trials have been registered on the database since it was launched in 2000. Data on the registry are maintained directly by trial sponsors and include information about each trial’s structure and design, outcome measures, enrolment targets, recruiting status, locations, expected start and end dates, and more.
To generate our dataset for analysis of active PD drug trials, data were downloaded from the ClinicalTrials.gov website, on February 18th, 2021, based on the following search criteria:
• Condition: Parkinson disease
• Study type: Interventional
• Phase: Early Phase 1, Phase 1, Phase 2, Phase 3
• Status parameter: “Recruiting”, “Not yet recruiting”, “Active, not recruiting”, or “Enrolling by invitation”.
Since our pipeline review is focused on PD drug trials, we also included gene therapy and cell-based approaches. We filtered out trials that were evaluating devices, biomarkers, or behavioral interventions (for example, exercise), as well as drug trials i) for which no supporting information could be found online, ii) that were already completed, though still listed as active, or iii) applied to atypical Parkinson’s only. The remaining trials were included in our dataset for analysis of active phase 1, 2, and 3 PD drug trials. Trials whose phase was classified “as early phase 1” on ClinicalTrials.gov were grouped with phase 1 trials in this report. If a trial was classified as phase 1/2 or phase 2/3 on ClinicalTrials.gov, we included the study in the lower of the two phases in our analyses (for example, a Phase 1/2 was considered as Phase 1).
The World Health Organisation’s International Clinical Trials Registry Platform is a trial registry network that is composed of ‘Primary’ and ‘Partner’ registries, based on specific criteria. Only the ‘Primary’ registries were used in this analysis. For the WHO registries, different search criteria were required based on the varying search functionality (the list of search terms is provided in Supplementary File 1). The data used in this report were downloaded between February 17-18th, 2021. A significant number of trials were registered on multiple registries, and where this occurs we have used the larger registry with the most up to date data available. The full ‘master list’ of clinical trials included in our analysis is provided in Supplementary File 2.
Trial categorization
Each trial in our dataset was first determined to be ST or DMT, and then assigned to one of 15 categories in accordance with our previously used methods [9]. The categories used in the current study were expanded to include ‘anti-inflammatory’, which was applied to agents seeking to reduce inflammatory processes. We also removed the category of ‘botanical’ as this was considered to be too undefined. There is potential overlap between the immunotherapy and targeting α-synuclein categories, but it was decided to retain the distinction due to the scale of investment in immunotherapies.
We further assessed the type of therapeutic approach taken with each trial. Novel projects use active agents new to the world; repurposed therapies use molecules that are already in use in another disease indication; reformulation employs therapeutics that are already used in the treatment of PD in a different dosage form; and new claims are trials to add to the existing disease indications.
In-between-phase projects
Many drug development projects in the clinical phase are not registered on trial registries for the simple reason that they have completed one phase of development but have not yet started the next. Whilst the lack of trial information means that we are unable to include them in our analysis, we believe it is important to provide some details on these projects. We used information available on company websites and other online resources, including the Parkinson’s Hope List [10]. In some cases, we made direct contact with principal investigators, and finally used our collective judgment on which projects should be included.
RESULTS
There is considerable variability in the information provided across the different registries used in this analysis. To ensure a consistent dataset, we decided to only include trials that would enable the distinction between ST and DMT; allocation to therapeutic category; and clinical phase. The analysis was done at three levels, firstly those trials registered on clinicaltrials.gov; secondly, trials in registries collated on WHO ICTRP; and the sum of the two.
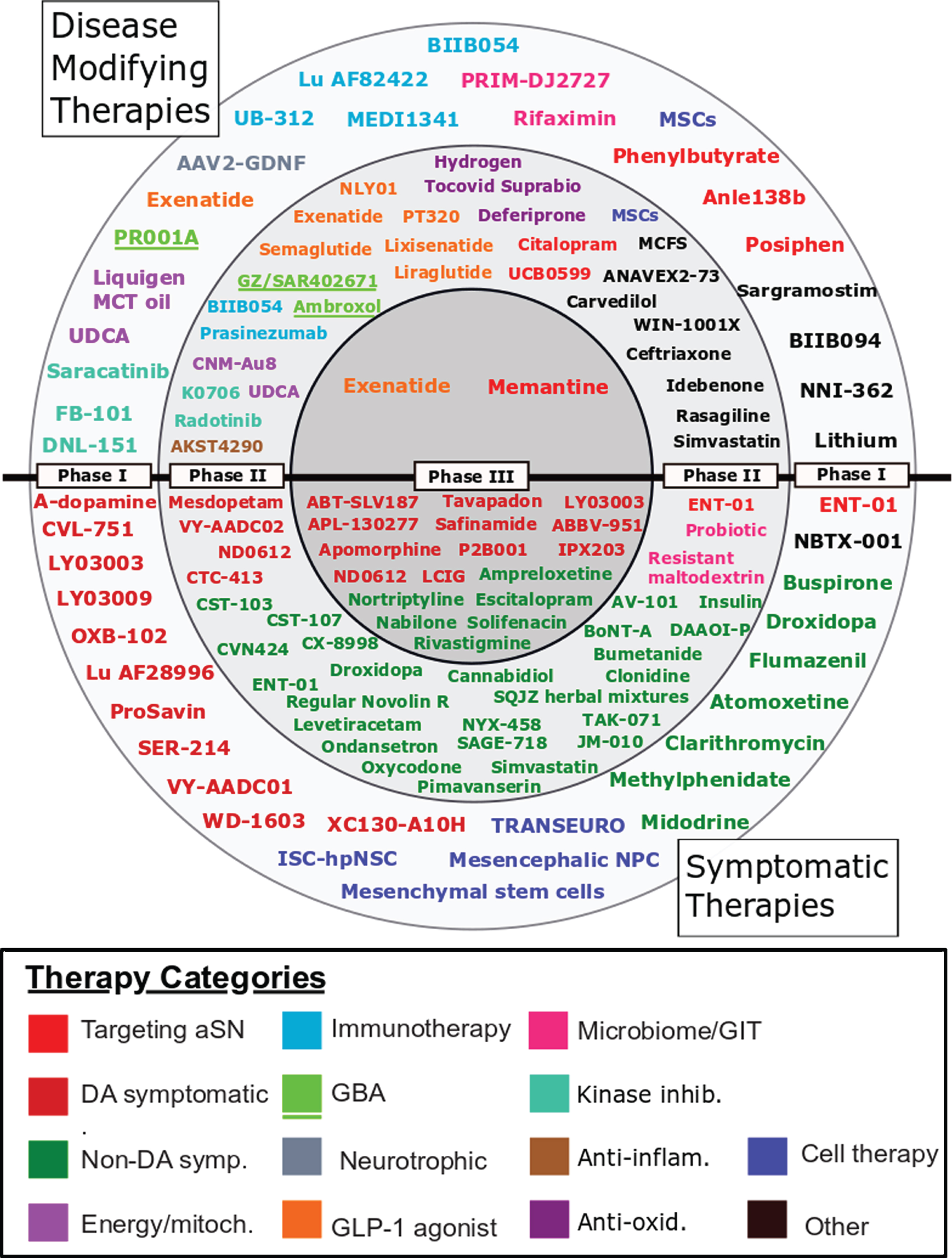
Using the criteria outlined in the Methods section above, 208 interventional trials were found to be registered on clinicaltrials.gov. Following the exclusion of trials that did not meet the criteria described in the Methods section, we identified 142 trials on clinicaltrials.gov, and despite finding 63 trials with phase information for Parkinson’s on the ICTRP’s search portal, only 14 provided sufficient information to enable comparable analysis to the clinicaltrials.gov entries. A further 42 trials registered on ICTRP did not indicate trial phase and were not included (Supplementary File 4). This gave us 156 trials for analysis. The full set of trials is in Supplementary File 2, the “Master List”. The 127 agents involved in these 156 trials are displayed in their respective phases in Fig. 1.
Fig. 1
A schematic of all of the agents in active clinical trials for PD, registered on clinicaltrials.gov as of February 18th 2021.
Trials registered on clinicaltrials.gov
To allow for a comparison with our 2020 report, we initially focused our analysis on just the trials registered on clinicaltrials.gov. Table 1 displays the classification of the clinicaltrials.gov trials by ST and DMT compared to our analysis from 2020 [9]. There has not been a major change in the numbers of trials listed on clinicaltrials.gov, nor in the distribution across phases. The 2021 review contains 83 ST trials, compared to 88 in 2020 with a slightly higher number of DMT trials (59), a total of 142 vs 145 in 2020. There has, however, been considerable change in the mix of trials, with 45 studies (31%) dropping off the list, of which 25 were completed, 4 terminated, 2 withdrawn and 14 moved to unknown status. While these studies were removed, 42 new trials have been added to the list.
Table 1
Comparison of ST and DMT studies listed on Clinicaltrials.gov by phase and year
| ST and DMT by Phase | Clinicaltrials.gov | |||||
| ST | DMT | #TRIALS | ||||
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | |
| 1 | 27 | 24 | 24 | 25 | 51 | 49 |
| 2 | 36 | 33 | 30 | 32 | 66 | 65 |
| 3 | 25 | 26 | 3 | 2 | 28 | 28 |
| TOTAL | 88 | 83 | 57 | 59 | 145 | 142 |
Many of the studies that have come off the list remain as active projects, waiting for the next trial in the sequence to start. They have not been included in our analysis as there are no current registry documents, but a list of the in-between-phase projects is provided in Supplementary File 3.
In Table 2, we present the therapeutic categories associated with the trials, and a comparison to that from 2020 is provided. The botanicals category used in 2020 was less useful than other categories so studies that were originally included here in 2020 have been allocated elsewhere based on the claims and/or properties of the medication. “Anti-inflammatories” has been added as a category because, although there is currently only one trial included on clinicaltrials.gov, there are a further two in the WHO dataset and the pre-clinical pipeline is quite active with more expected to move to the clinical stage in the next year or two.
Table 2
Comparison of therapeutic categories by phase and year from clinicaltrials.gov data
| Number of trials by therapeutic category | ||||||||
| Therapeutic category | Phase 1 | Phase 2 | Phase 3 | Total | ||||
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | |
| Anti-inflammatories* | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Antioxidants | 0 | 0 | 2 | 4 | 0 | 0 | 2 | 4 |
| Botanicals** | 0 | 0 | 3 | 0 | 1 | 0 | 4 | 0 |
| Cell therapy | 8 | 6 | 1 | 2 | 0 | 0 | 9 | 8 |
| Dopaminergic symptom relief | 12 | 13 | 6 | 4 | 16 | 19 | 34 | 37 |
| Energy and mitochondria | 1 | 2 | 3 | 2 | 0 | 0 | 4 | 4 |
| GBA | 1 | 1 | 3 | 2 | 0 | 0 | 4 | 3 |
| GLP-1 agonists | 1 | 1 | 4 | 6 | 1 | 1 | 6 | 8 |
| Immunotherapy | 5 | 5 | 2 | 2 | 0 | 0 | 7 | 7 |
| Kinase inhibitors | 3 | 3 | 1 | 2 | 0 | 0 | 4 | 5 |
| Microbiome/GIT | 3 | 3 | 3 | 2 | 0 | 0 | 6 | 5 |
| Neurotrophic factors | 3 | 2 | 0 | 0 | 0 | 0 | 3 | 2 |
| Non-dopaminergic symptom relief | 6 | 4 | 26 | 26 | 9 | 7 | 41 | 37 |
| Targeting alpha synuclein | 2 | 4 | 2 | 3 | 1 | 1 | 5 | 8 |
| Other | 6 | 5 | 10 | 9 | 0 | 0 | 16 | 13 |
| TOTAL | 51 | 49 | 66 | 65 | 28 | 28 | 145 | 142 |
*new for 2021. **2020 only.
Therapy category analysis
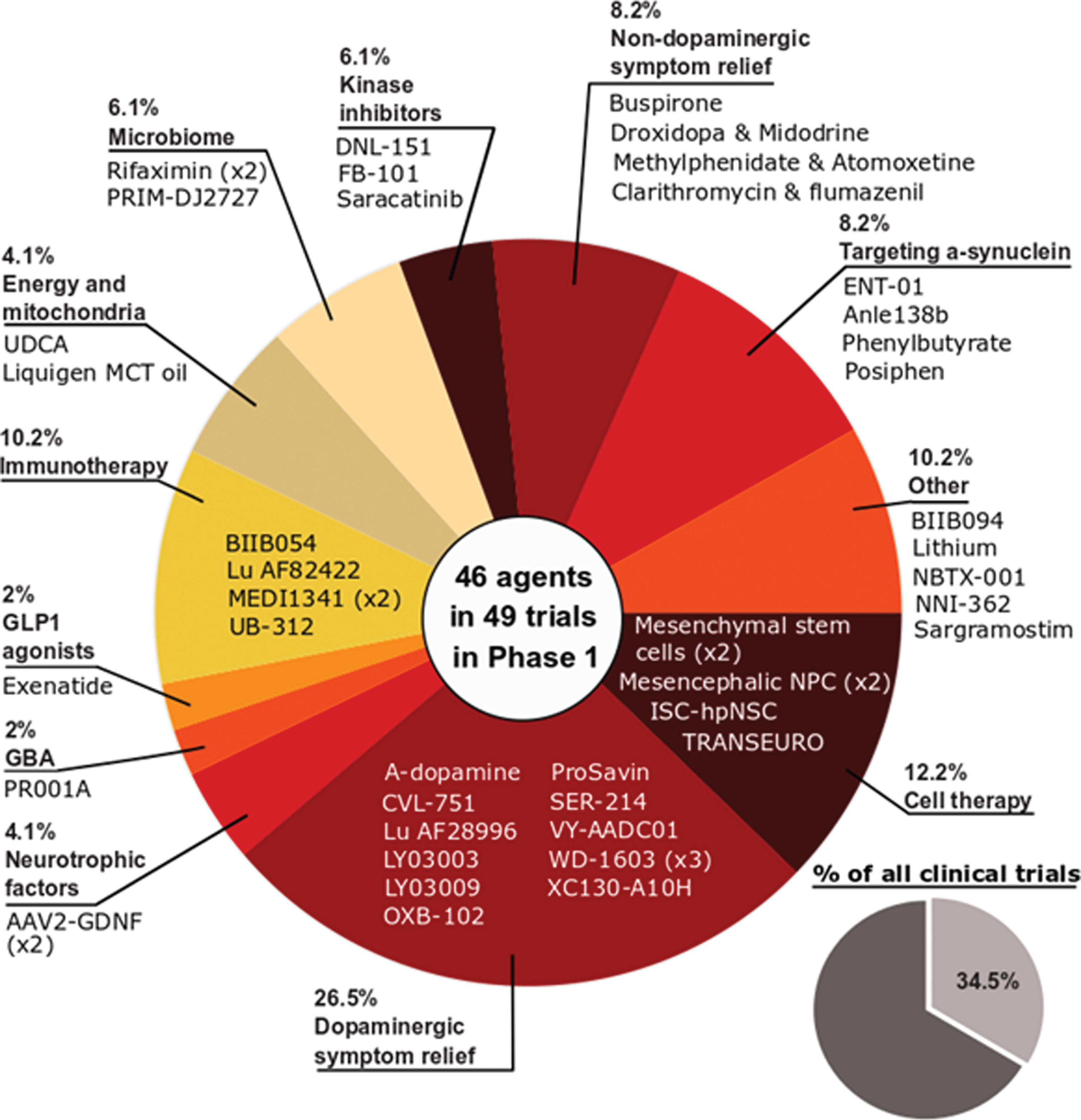
Phase 1 - Of the active trials registered on the clinicaltrials.gov website, 34.5% (49 trials of 46 agents) were listed as phase 1 or phase 1/2 (Fig. 2). Similar to the previous 2020 report, the phase 1 studies represented a broad spectrum of different therapeutic approaches. One quarter of the studies (26.5%) focused on agents targeting dopaminergic symptom relief, but cell and immunotherapies were the next largest classes of trials (12.2% and 10.2% of the total, respectively). While there were slightly fewer phase 1 trials in 2021 compared to 2020 (49 vs 51, respectively), one encouraging observation is the percentage of DMTs which was higher in 2021 (51%) compared to 2020 (47%).
Fig. 2
A pie chart of the agents in active phase1 trials for PD, registered on clinicaltrials.govas of February 18th 2021.
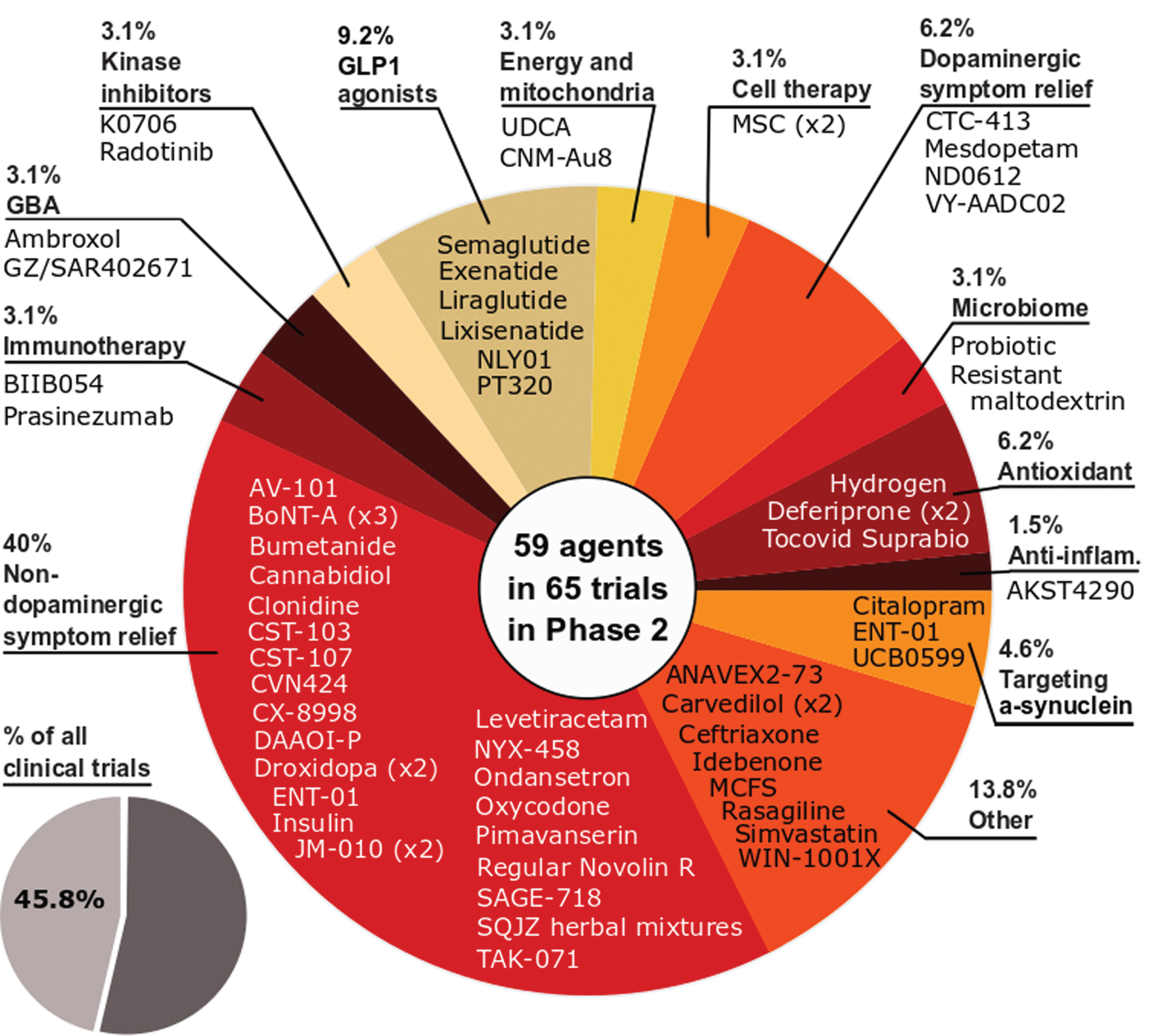
Phase 2 – As with 2020, phase 2 made up the largest number of clinical trials in our dataset (45.8% of the total number of trials, 65 studies involving 59 agents; Fig. 3). And similar to 2020, the largest category of trials in phase 2 was non-dopaminergic symptom relief (40% of the trials in phase 2).The breadth of the approaches being applied to better treating PD is displayed in phase 2 as the second largest category of trials was ‘Other’ (13.8% of the total), representing a group of agents which could not be clearly pooled with similar therapies. As with phase 1, the number of DMT studies increased in 2021 (32 vs 30 in 2020), while STs decreased (33 vs 36 in 2020), and this shift was apparent in our analysis as the third largest category of phase 2 studies in 2021 was GLP-1 agonists (9.2% of the total).
Fig. 3
A schematic of the agents in active phase 2 trials for PD, registered on clinicaltrials.gov as of February 18th 2021.
Phase 3 – This stage of clinical development represents the agents that were closest to potential regulatory approval, and while it was encouraging to see 3 new therapies approved by the U.S. FDA in 2020, there was no increase in the number of phase 3 studies in 2021 (28 studies in 2021 vs 28 in 2020; Fig. 4). Dopaminergic symptomatic relief once again represented the largest category of trials in phase 3 testing (67.9% of the total), with non-dopaminergic symptom relief making up another 25% of the studies. Only 2 DMT trials were active at phase 3 in 2021 (7.1%).This is contrast to the clinical trial pipeline for Alzheimer’s disease, where 17 of the 28 (60.1%) agents in phase 3 trials are DMTs [11].
Fig. 4
A schematic of the agents in active phase 3 trials for PD, registered on clinicaltrials.gov as of February 18th 2021.

Trials registered on WHO ICTRP
A total of 14 trials registered on WHO ICTRP reached the final criteria for inclusion, of which five are in phase 1, eight in phase 2 and one in phase 3. The breakdown of ST vs DMT and therapeutic category for these trials are provided in Supplementary File 2. The phase 1 group includes CST-2032 from Curasen, another adrenoreceptor modulator aimed at the relief of cognitive symptoms. PMX205 is an anti-inflammatory inhibitor of the complement cascade, currently in a phase 1 study in healthy volunteers, and another anti-inflammatory, azathioprine, is in phase 2. NeuroEPO, a nasal form of recombinant human erythropoietin with low sialic acid, is also in phase 2 in Cuba. Four of the trials are evaluating supplements; cannabidiol, melatonin and probiotics are in phase 2, and curcumin is in phase 3.
Summary of trials in the combined dataset
Combining the clinicaltrials.gov and WHO datasets provides a list of 156 trials, of which 91 trials were ST with 65 classed as DMT. The trials in phase 1 numbered 54 (representing 34.6% of the total) and more than half of them were DMTs (53.7%). Phase 2 was the largest portion of trials (73 trials, making up 46.8% of the total), and there were slightly more ST studies in this phase (53.4%). The combination of the datasets at the phase 3 stage had little impact on the data (with 29 trials, equivalent to 18.6% of the total; Table 3). The number of trials in the combined dataset by therapeutic category is shown in Table 4. The combination of the datasets did not significantly impact any particular category.
Table 3
Combined breakdown of symptomatic and disease-modifying therapies for the combined dataset
| Clinicaltrials.gov + WHO | ||||
| ST and DMT | ||||
| Phase | ST | DMT | # Trials | % Trials |
| 1 | 25 | 29 | 54 | 34.6% |
| 2 | 39 | 34 | 73 | 46.8% |
| 3 | 27 | 2 | 29 | 18.6% |
| TOTAL | 91 | 65 | 156 | 100.0% |
Table 4
Combined breakdown of trials by therapeutic category for the combined dataset
| Clinicaltrials.gov + WHO | ||||
| Number of trials by therapeutic category | ||||
| Category | Phase 1 | Phase 2 | Phase 3 | Total |
| Anti-inflammatories | 1 | 2 | 0 | 3 |
| Antioxidants | 0 | 4 | 0 | 4 |
| Cell therapy | 6 | 2 | 0 | 8 |
| Dopaminergic symptom relief | 14 | 6 | 19 | 40 |
| Energy and mitochondria | 2 | 2 | 0 | 4 |
| GBA | 1 | 2 | 0 | 3 |
| GLP-1 agonists | 1 | 6 | 1 | 8 |
| Immunotherapy | 5 | 2 | 0 | 7 |
| Kinase inhibitors | 3 | 2 | 0 | 5 |
| Microbiome/GIT | 3 | 2 | 0 | 5 |
| Neurotrophic factors | 3 | 0 | 0 | 3 |
| Non-dopaminergic symptom relief | 4 | 27 | 8 | 39 |
| Targeting alpha synuclein | 4 | 3 | 1 | 8 |
| Other | 7 | 12 | 0 | 17 |
| TOTAL | 54 | 73 | 29 | 156 |
As a final step in our analysis, we sought to provide an indication of the origin of the agents being evaluated in our datasets. Table 5 shows the breakdown of the type of therapeutic agent by novel, repurposed, reformulation or new claim. Even in this combined dataset, the proportions of novel and repurposed trials are very similar to our 2020 analysis, while the percentage of reformulation projects has increased by almost 5%, with a small decline in new claims.
Table 5
The type of therapeutic approach for the combined dataset
| Clinicaltrials.gov + Who | ||||||
| Phase | 1 | 2 | 3 | Total | % in 2021 | % in 2020* |
| Novel | 35 | 26 | 7 | 68 | 43.6% | 45.5% |
| Repurposed | 13 | 35 | 5 | 53 | 34.0% | 34.5% |
| Reformulation | 7 | 7 | 15 | 29 | 18.6% | 13.8% |
| New claim | 0 | 4 | 2 | 6 | 3.8% | 6.2% |
| TOTAL | 55 | 72 | 29 | 156 | ||
*clinicaltrials.gov numbers only.
DISCUSSION
Despite the catastrophic events of 2020, our 2021 review of the drug development pipeline for PD reveals a similar number of trials in progress to the year before on clinicaltrials.gov (142 trials were active in 2021 compared to 145 in 2020). A change in the content would be expected as trials finish and others start, and indeed we found 45 studies had completed, been terminated or reached unknown status, with 42 new entries to the registry. The registration of this large number of new clinical trials in 2021 was rather remarkable considering the circumstances with the COVID-19 pandemic. Another encouraging feature in the clinicaltrials.gov data was the shift in the DMT/ST ratio at the phase 1 and 2 stages of clinical development, with more DMT studies at both of these stages.The distribution of novel, repurposed, reformulations and new claim therapies remained similar to 2020, but it is interesting to note that repurposed therapies still represent approximately a third of all the trials in our analysis, including the only two phase 3 DMTs. The fact that there are only two DMT phase 3 trials is, however, disappointing, as the study of Lingzhi (Ganoderma) from Xuanwu Hospital in Beijing reached unknown status, leaving only the exenatide study at UCL (NCT04232969) and a trial of memantine at Wayne State University (NCT03858270).
Additional registries
The dataset employed in our 2020 report consisted of active clinical trials registered only on the clinicaltrials.gov website. Upon review, we wondered whether limiting our analysis to this sole registry provided an accurate assessment of the landscape of drug development for PD. To address this question, and provide the reader with a more thorough review, we explored the ICTRP database of clinical trial registries provided by the WHO. Disappointingly, there were only 14 clinical trials in the ICTRP that we were able to add to our analysis. In addition, there were a further 42 studies that met the criteria for inclusion apart from the lack of phase data (Supplementary file 4). These 14 trials, albeit a relatively low number, showed a similar distribution of phases and split between ST and DMT to the clinicaltrials.gov dataset. The review of the WHO’s ICTRP trial registries revealed a large number of registered trials, but a wide variation in the information/details provided. We encountered limited phase information, retrospective registration of trials (where results had already been published), and a lack of description of trial details. It appears that the information requested by the individual country registries drives the detail entered by the sponsor, and we recommend trial sponsors to encourage the registry administrators in their countries to adopt some universal standards similar to clinicaltrials.gov. We also urge clinical trial sponsors to keep the information on trial registries complete and up to date. A number of studies were unable to be included due to their status reaching “unknown”. If participant involvement in trials is based on ‘informed’ consent, up to date information is required.
Newly registered trials
Among the newly registered trials on the dataset are numerous agents of interest, including an oral, brain-penetrant inhibitor of α-synuclein aggregation called anle138b which is being developed by the German biotech firm MODAG. Encouraging preclinical research in models of PD helped propel this molecule into clinical trial [12]. Phase 1 testing of anle138b is due to complete in 2021 (NCT04685265), and the company is currently planning a phase 2 study. AstraZeneca registered an immunotherapy agent called MEDI1341 (NCT04449484), which is an antibody aimed at stopping the pathological spread of α-synuclein aggregation. Immunotherapy agents represent a sizable portion of the phase 1 trials (10.2%).
In the new ST trials, Lundbeck’s Lu AF28996 is a D1/D2 agonist focused on symptom relief (NCT04291859). There is also the DIVE study (NCT04332276), which will evaluate the administration of aerobically-produced dopamine directly into cerebral ventricles and targeted towards more advanced PD. Work in non-human primates has shown symptom relief without troublesome dyskinesia [13].
Newly registered phase 2 trials include the TEAL study of Alkahest’s AKST4290, an inhibitor of CCR3 (C-C chemokine receptor 3), which is an immunomodulatory protein that blocks the action of eotaxin (NCT04369430). Biotech company Curasen has registered a phase 2 study investigating the combination of CST-103 and CST-107 which aims to assess the impact of restoring noradrenergic function in key brain areas affected by neurodegenerative disease on cognitive fluctuations (NCT04739423). The effects of intranasal insulin administration on motor and non-motor symptoms of PD are being investigated by Shahid Beheshti University of Medical Sciences in Iran, and separately by Health Partners Institute in the US (NCT04687878 and NCT04251585, respectively). IRL’s mesdopetam is targeted to the reduction of dyskinesia in PD (NCT04435431). Peptron are investigating the effects of sustained-release exenatide with PT320, building on successful work with GLP-1 agonists, with immediate release exenatide being the most advanced (NCT04269642). Il-Yang Pharma is seeking to repurpose the cancer drug radotinib, a c-Abl tyrosine kinase inhibitor, for PD (NCT04691661). Sage Therapeutics’ SAGE-718, a NMDA receptor modulator, entered phase 2 for the treatment of cognitive impairment in PD (NCT04476017).
One trial of note in the WHO registry is APM-CT1, the first multi-arm, multi-stage (MAMS) study for PD (ACTRN12620000560998;[14]). This is a federally-funded collaboration between The Australian Parkinson’s Mission, Cure Parkinson’s and Shake It Up Australia, which studies drugs that have been closely evaluated and prioritised as potentially PD-neuroprotective by the International PD Linked Clinical Trials Committee. Three active agents in separate treatment arms are being compared with a single placebo arm in a MAMS-style double-blinded phase 2 study aiming to show DMT activity. The agents are alogliptin, a DPP-4 inhibitor used for treating type 2 diabetes; albuterol, a beta2 adrenergic agonist used in treating asthma (with evidence for an effect on alpha-synuclein); and nilvadipine, a calcium channel blocker used in treating hypertension (with evidence of additional anti-inflammatory properties).
The “in-betweeners”
This year’s analysis includes a list of clinical stage projects that did not have currently registered trials at the time of our data download. Other sources of information (such as company websites) enable the inclusion of these projects in this paper, but as a separate list. It may be argued that the lack of verification renders such information less reliable than trial registries. However, given that the information provided on such registries remains the responsibility of the sponsor, and the registry managers do not perform an editorial function, we believe it is entirely appropriate to include the list of “in-between-phase” projects. Many of the studies that have come off our master list of trials remain as active projects, waiting for the next trial in the sequence to start. They have not been included in our analysis as there are no current registry documents, but a list of these in-between-phase projects is provided in Supplementary File 3.
One project of interest in the inbetweeners list is the AFFiRiS sponsored alpha synuclein targeted vaccine (PD01A). In January 2020, the company announced that they were planning a phase 2 study which would be initiated in the US and Europe in the second half of 2020 [15]. At the time of writing, no study had been registered (presumably due to COVID-19), and as a result this project was placed on the inbetweeners list. Another ‘inbetweener’ project of interest is the NLRP3 inhibitor Inzomelid which was being developed by the biotech firm Inflazome, before the company was acquired by the pharmaceutical company Roche last year [16]. PD is one of the indications this brain penetrant agent was being aimed towards, but no trials are currently registered. A third agent of interest that is in between stages of clinical testing is the alpha-blocker terazosin. The terazosin for PD (TZ-PD; NCT03905811) study completed in November 2020, and the research team behind the study are preparing to publish the results. They are also further exploring this agent in a target engagement study in healthy volunteers (NCT04551040). We look forward to news of further progress in the development of these and other agents.
There is also intriguing evidence to suggest that the inbetweeners list may be broader than we have presented here as some previously abandoned agents are being re-assessed. One example of this is the gene therapy approach, AAV-GAD, which was originally developed by Neurologix Ltd, but failed to demonstrate robust results in Phase II studies ten years ago [17]. More recent follow up research has indicated a biological effect [18], which has led to the resuscitation of this therapy by the biotech firm MeiraGTx, which is seeking to re-start clinical trials [19]. Another more recent example is the calcium channel blocker isradipine. The phase 3 STEADY-PD clinical trial results indicated that this treatment had no impact on the progression of PD in newly diagnosed individuals [20], but recent post-hoc analysis of the phase 3 clinical trial data has provided some evidence to support a re-examination of this agent [21]. Accordingly, a similar reanalysis is now being conducted on the earlier Phase II clinical data.
Noteworthy DMTs
In addition to the announcement of the STEADY-PD phase 3 results, other trials reported results in the timeframe covered by our paper. The Roche prasinezumab phase 2 study (PASADENA, NCT03100149) was the first of several such α-synuclein antibody projects to report. The results were unclear, but with sufficient promise to support the initiation of an expanded phase 2 program (NCT04777331). The early months of 2021 produced two further phase 2 trial readouts, firstly of Biogen’s α-synuclein antibody, BIIB054 (NCT03318523), and Sanofi’s venglustat (NCT02906020). Unfortunately, both trials failed to meet their desired outcomes. In addition, the Voyager Therapeutics/Neurocrine Biosciences gene therapy phase 2 study of VY-AADC (NCT03562494), was placed on clinical hold by the FDA. Despite these setbacks, the phase 2 pipeline remains healthy and we look forward to the progression of strong candidates to phase 3.
The impact of COVID-19
It may be too early to determine the full extent of the damage to the PD clinical trial program caused by the COVID-19 pandemic, but it has certainly had a major impact. Heroic efforts were made by investigators and participants (and their families) in order for assessments and data points to be achieved. It is encouraging that only two PD trials on the ClinicalTrials.gov were withdrawn due to COVID-19. The situation has brought home the urgent need for better remote methods of assessment that provide real world measures of treatment efficacy with less requirement for clinical research centre visits. It represents an opportunity that the research community is actively exploring in new clinical trials that are designed to be conducted in a completely remote format (such as the ‘Trial of Parkinson’s And Zoledronic Acid’ (or TOPAZ) study - NCT03924414 [22]).
CONCLUSIONS
The clinical trial pipeline for PD has undergone a significant change in content in the last year but retained a similar profile of ST/DMT, therapeutic category and therapeutic approach. Clinicaltrials.gov is the predominant trial registry although some relevant trials are registered on the WHO IRCTP. We have also provided a list of in-between-phase projects.It is clear that a very large investment continues not just in financial terms and the dedication of health professionals, but also in the contribution of trial participants with PD, and healthy volunteers. We thank them all.
AUTHOR CONTRIBUTION
KMcF, GR, MB, RKW & SRWS performed the trial categorization & helped write/edit the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Sue Buff for helping with data retrieval and technical support. Also, Prof Tanya Simuni of Northwestern University and Helen Matthews of Cure Parkinson’s for reading the manuscript and providing constructive feedback. The authors would also like to thank all of the trial participants and their families, and researchers involved in the ongoing clinical research for PD.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-219006.
REFERENCES
[1] | Bloem BR , Okun MS , Klein C (2021) Parkinson’s disease. The Lancet. Apr 9; Online ahead of print. |
[2] | Dorsey ER , Sherer T , Okun MS , Bloemd BR ((2018) ) The emerging evidence of the Parkinson pandemic. Journal of Parkinson’s Disease 8: , S3–S8. |
[3] | Yang W , Hamilton JL , Kopil C , Beck JC , Tanner CM , Albin RL , Ray Dorsey E , Dahodwala N , Cintina I , Hogan P , Thompson T ((2020) ) Current and projected future economic burden of Parkinson’s disease in the U.S. npj Parkinson’s Disease 6: , 15. |
[4] | Chang D , Nalls MA , Hallgrímsdóttir IB , et al. Graham RR ((2017) ) A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nature Genetics 49: , 1511–1516. |
[5] | Nalls MA , Blauwendraat C , Vallerga CL , et al. Zhang F ((2019) ) Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. The Lancet Neurology 18: , 1091–1102. |
[6] | Fox Insights, https://foxinsight.michaeljfox.org/. |
[7] | PDGENEration, https://www.parkinson.org/PDGENEration. |
[8] | PD Frontline, https://pdfrontline.com/en. |
[9] | McFarthing K , Buff S , Rafaloff G , Dominey T , Wyse RK , Stott SRW ((2020) ) Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2020. Journal of Parkinson’s Disease 10: , 757–774. |
[10] | The Hope list, http://bit.ly/ParkinsonsHopeList. |
[11] | Cummings J , Lee G , Zhong K , Fonseca J , Taghva K ((2021) ) Alzheimer’s disease drug development pipeline: 2021, Alzheimer’s & Dementia: Translational Research & Clinical Interventions 7: (1), e12179. |
[12] | Wegrzynowicz M , Bar-On D , Calo’ L , Anichtchik O , Iovino M , Xia J , Ryazanov S , Leonov A , Giese A , Dalley JW , Griesinger C , Ashery U , Spillantini MG ((2019) ) Depopulation of dense α-synuclein aggregates is associated with rescue of dopamine neuron dysfunction and death in a new Parkinson’s disease model. ActaNeuropathologica 138: , 575–595. |
[13] | Moreau C , Rolland AS , Pioli E , Li Q , Odou P , Barthelemy C , Lannoy D , Demailly A , Carta N , Deramecourt V , Auger F , Kuchcinski G , Laloux C , Defebvre L , Bordet R , Duce J , Devedjian JC , Bezard E , Fisichella M , David D ((2020) ) Intraventricular dopamine infusion alleviates motor symptoms in a primate model of Parkinson’s disease. Neurobiology of Disease 139: , 104846. |
[14] | Australian Parkinson’s Mission - first trial: APM-CT1, http://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=375958. |
[15] | AFFiRiS Announces FDA Response to its pre-IND Submission for Phase 2 trial with AFFITOPE PD01 in Early Parkinson’s Disease Patients, https://uk.finance.yahoo.com/news/affiris-announces-fda-response-pre-090003312.html. |
[16] | Dives into NLRP3 Inflammasome Inhibition with Acquisition of Inflazome, https://www.biospace.com/article/roche-dives-into-nlrp3-inflammasome-inhibition-with-acquisition-of-inflazome/. |
[17] | Niethammer M , Tang CC , LeWitt PA , Rezai AR , Leehey MA , Ojemann SG , Flaherty AW , Eskandar EN , Kostyk SK , Sarkar A , Siddiqui MS , Tatter SB , Schwalb JM , Poston KL , Henderson JM , Kurlan RM , Richard IH , Sapan C v. , Eidelberg D , During MJ , Kaplitt MG , Feigin A ((2017) ) Long-term follow-up of a randomized AAV2-GAD gene therapy trial for Parkinson’s disease. JCI insight 2: , e90133. |
[18] | Niethammer M , Tang CC , Vo A , Nguyen N , Spetsieris P , Dhawan V , Ma Y , Small M , Feigin A , During MJ , Kaplitt MG , Eidelberg D ((2018) ) Gene therapy reduces Parkinson’s disease symptoms by reorganizing functional brain connectivity. Science Translational Medicine 10: (469), eaau0713. |
[19] | MeiraGTx Reports First Quarter 2020 Financial Results, https://www.globenewswire.com/news-release/2020/05/07/2029993/0/en/MeiraGTx-Reports-First-Quarter-2020-Financial-Results.html. |
[20] | Parkinson Study Group STEADY-PD III Investigators ((2020) ) Isradipine Versus Placebo in Early Parkinson Disease: A Randomized Trial. Annals of Internal Medicine 172: (9), 591–598. |
[21] | Venuto CS , Yang L , Javidnia M , Oakes D , James Surmeier D , Simuni T ((2021) ) Isradipine plasma pharmacokinetics and exposure-response in early Parkinson’s disease. Annals of Clinical and Translational Neurology 8: , 603–612. |
[22] | Tanner CM , Cummings SR , Schwarzschild MA , et al. Lyles KW ((2021) ) The TOPAZ study: A home-based trial of zoledronic acid to prevent fractures in neurodegenerative parkinsonism. npj Parkinson’s Disease 7: (1), 16. |




