Efficacy and Safety of Opicapone for Motor Fluctuations as an Adjuvant to Levodopa Therapy in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis
Abstract
Background:
Long-term levodopa administration for treating Parkinson’s disease (PD) may shorten the duration of effect and cause dyskinesias, inducing the need for catechol-O-methyltransferase (COMT) inhibitors as adjuvant therapy.
Objective:
We provide pooled scientific evidence highlighting the efficacy and safety of opicapone, a newly approved COMT inhibitor, as an adjuvant to levodopa.
Methods:
We searched Ovid Medline, Embase, and Cochrane databases for relevant reports. Efficacy and safety were evaluated as off-time reduction and risk ratio (RR) of dyskinesia, respectively. Data were independently extracted using predefined criteria. Selected placebo-controlled trials were divided into double-blind and open-label periods. Using a random-effects model, the mean difference (MD) of the off-time reduction (efficacy), RR for the occurrence of dyskinesia, and on-time without/with troublesome dyskinesia (TD; safety assessment) were compared between opicapone and placebo groups.
Results:
Five studies from three randomized controlled trials were included, and a meta-analysis was performed with 407 patients receiving opicapone 50 mg and 402 patients receiving placebo. Compared with the placebo, opicapone (50 mg) reduced off-time by 49.91 min during the double-blind period (95% confidence intervals [CIs] = –71.39, –28.43; I2 = 0%). The RR of dyskinesia was 3.43 times greater in the opicapone 50 mg group than in the placebo group (95% CI = 2.14, 5.51; I = 0%). Compared with the placebo, opicapone increased the on-time without TD by 44.62 min (95% CI = 22.60, 66.64; I2 = 0%); the on-time increase with TD did not differ between treatments.
Conclusion:
Opicapone can play a positive role as an adjuvant to levodopa in patients with PD by reducing off-time and prolonging on-time without PD.
INTRODUCTION
Parkinson’s disease (PD) is a neurodegenerative disease characterized by the loss of dopamine-producing (“dopaminergic”) neurons in the substantia nigra of the brain, resulting in motor dysfunction [1]. PD is the second most common neuropathological disorder worldwide, following Alzheimer’s disease, and has been associated with severe disability and poor quality of life. In 2020, the total number of patients with PD was estimated at 9.4 million globally, increasing by 55% from 2016 to 2020 [2]. According to a meta-regression analysis, the prevalence of PD increases with age, and males exhibit a 1.5 times higher risk of developing PD than females [3, 4].
Levodopa is the most commonly employed first-line drug therapy for PD, increasing dopamine levels by acting on nerve cells in the brain and consequently improving major cardinal symptoms, including resting tremor, rigidity, and bradykinesia [5]. These symptoms are well controlled by dopamine replacement therapy using levodopa. However, the efficacy of levodopa therapy decreases gradually, and most patients develop fluctuating responses and dyskinesia. It has been reported that approximately 30–50% of patients with PD experience troublesome motor fluctuation after long-term (five years) levodopa therapy, which could be attributed to the shortened half-life of levodopa [6]. Motor fluctuations are alterations between periods marked by a positive response to levodopa (“on”) and periods marked by reappearance of Parkinsonian symptoms (“off”). These motor fluctuations significantly impact the performance of day-to-day activities in patients with PD. Accordingly, catechol-O-methyltransferase (COMT) inhibitors are co-prescribed as an adjunct therapy to levodopa to reduce “off” time in patients with PD. COMT extends the duration of action of levodopa by inhibiting its peripheral metabolism, thus enhancing bioavailability [7].
In 2020, the US Food and Drug Administration (FDA) approved opicapone, a third-generation COMT inhibitor, and compared to second-generation COMT inhibitors such as entacapone, it non-inferiorly prolongs the duration of levodopa action [8] with a favorable safety profile [9]. Side effects known to occur with other COMT inhibitors (entacapone and tolcapone), such as severe diarrhea, urine discoloration, and hepatotoxicity, were not observed following opicapone administration [10]. Additionally, opicapone is relatively convenient than second-generation COMT inhibitors for maintaining efficacy as it requires administration only once-a-daily [11]. However, prolonging the duration of levodopa with opicapone could induce dopaminergic side effects such as “levodopa-induced dyskinesia (LID)” [12].
Several systematic reviews have been conducted to confirm the efficacy and safety of opicapone based on existing evidence [11, 13–17]. However, these earlier reviews are limited in scope, as they failed to comprehensively include existing studies or perform a quantitative synthesis of existing evidence. Accordingly, we conducted a systematic review and meta-analysis to summarize accumulated evidence on the efficacy and safety of opicapone treatment, measured by the decrease in off-time and increase in the risk of LID.
MATERIALS AND METHODS
Search strategy and data source
This study was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) [18]. We developed a study protocol that specified the objective, outcome, eligibility criteria, search strategy, methods for study selection, data extraction, and data synthesis for this meta-analysis.
We defined a first key question to examine the efficacy of opicapone as levodopa adjuvant therapy following the Population, Intervention, Comparison, Outcome, Study design (PICO-SD) format: “In advanced patients with Parkinson’s disease (Population), does taking third-generation COMT inhibitor, opicapone (Intervention), compared with placebo (Comparison) improve the off-time reduction effect (Outcome) in randomized controlled trial (RCT) settings (Study design)?” We also set up a second research question to determine the safety of opicapone. Safety outcomes were defined as three endpoints: incidence of dyskinesia, on-time without troublesome dyskinesia (TD), and on-time with TD. Except for defining the outcome, the PICO-SD format for the second question was the same as in the first research question.
We searched three core databases (Ovid Medline, Embase, and Cochrane) on April 21, 2021. The following search terms were used: “Parkinson’s disease” for population; “opicapone” and “Catechol O-methyltransferase inhibitors” for intervention; “Randomized controlled trial. Search items belonging to each group (population, intervention, and study design) were combined using “OR,” while population, intervention, and outcome were combined via “AND.” The detailed search strategy is provided in Supplementary Tables 1–3.
Study selection
Study selection was performed in two steps, according to predefined inclusion and exclusion criteria. After duplicate removal, titles and abstracts were independently screened by two reviewers (N.K. and J.P.). Subsequently, the full texts of identified studies that potentially met inclusion criteria were evaluated to decide on final inclusion for meta-analysis. Any disagreements were resolved by reaching a consensus through mutual discussion.
Studies were included if they met the following eligibility criteria: 1) studies on PD patients with motor fluctuations; 2) studies that employed opicapone as a therapeutic agent; 3) studies using placebo as a comparator; 4) studies including off-time as outcome indicators; 5) clinical research. Additionally, studies on animal experiments, reviews, gray studies, and duplicate publications were excluded, along with studies not written in English.
Data extraction and quality assessment
Two independent reviewers extracted data on the following items from selected articles: the first author, year of publication, country where the study was conducted, type of study design (i.e., double-blind or open-label study), comparator, study duration, and outcome (off-time and dyskinesia). Quality assessment was performed using the risk of bias (RoB) tool of the Cochrane group [19], which assesses the risk of bias in RCTs and consists of seven domains with different potential risks of bias as follows: randomization sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other risks that can threaten the validity [20]. Two reviewers independently assessed the risk of bias as ‘high risk,’ ‘low risk,’ and ‘unclear risk’ according to the contents of the text of studies for each domain. Any disagreements were resolved by reaching a consensus through mutual discussion.
Statistical analysis
From each of the included studies, we extracted the mean change of ‘duration of off-time (in min)’ before and after treatment for opicapone and placebo arms, respectively. We then calculated the mean difference
(1)
where, i = 1, . . . , nth patient.
To examine the safety of opicapone, the risk ratio (RR) for dyskinesia incidence was calculated as follows:
(2)
For other safety outcomes, ‘on-time without TD’ and ‘on-time with TD,’ MDs were computed as in Equation 1.
Meta-analysis was performed by applying a generic inverse-variance estimation method and a random-effects model. The inverse-variance method uses the inverse of the variance of the effect estimate to the weight of each study and is the most commonly used effect estimation method in meta-analysis. The random-effects model assumes that there is no single true value of the intervention effect in individual studies and that it follows a normal distribution centered on the average value of the intervention effect [21]. In addition, heterogeneity between studies was assessed using the I2 statistic, and a Chi-square test was performed. All analyses were performed using Review Manager software (version 5.3; Copenhagen, Denmark, Cochrane Collaboration), and results were presented in a forest plot.
RESULTS
Selection of studies
As shown in Fig. 1, 981 studies were initially screened using the predetermined electronic databases. After the two-step study selection procedure, only eight studies that met eligibility criteria were selected. For the final screening step to select studies for quantitative synthesis, three studies were excluded for the following reasons: the study was a pooled analysis; the study compared efficacy with other than placebo; the study did not include comparable opicapone doses. Finally, five studies were included in the meta-analysis.
Fig. 1
Flowchart for identifying relavant studies.
Study characteristics and quality assessment result
The final five included studies were based on the results of three clinical trials (BIPARK-I [8, 24], BIPARK-II [22], and ONO-2370 [23, 25]). All three trials provided efficacy and safety data measured during both double-blind and open-label periods (Table 1). The follow-up time for the double-blind period was consistently 14–15 weeks in all three trials. On pooling the three trials, 1,220 patients were included in the analysis for the double-blind period: 415 patients treated with opicapone 50 mg, 393 patients treated with opicapone 25 mg, and 412 patients receiving placebo. After completing the double-blind period, 1,056 subjects previously assigned to each dose (i.e., 348 with opicapone 50 mg, 342 with opicapone 25 mg, and 366 receiving placebo) were switched to opicapone 50 mg and followed-up for 52 weeks.
Table 1
Characteristics of the studies included
| Type | Author (y) | Clinical study (duration) | Number of Patients | Intervention (# of patients)† | Comparator (# of patients) | Sex (male %) / mean Age (y) | Outcome | |
| Efficacy | Safety | |||||||
| (change from baseline in absolute OFF-time (min), SE) | Event (Dyskinesia) | |||||||
| Double-blind period | Ferreira et al. [8] | BIPARK-I (14-15 weeks) | 600 PD patients | capsule OPC (5, 25, 50 mg): (122, 119, 116) | Placebo (121) | Placebo: 59% / 64.3 | Placebo: –56.0 (13.4) | Placebo: 5 |
| ENT (122) | ENT: 62% / 63.7 | ENT: –96.3 (13.4) | ENT: 10 | |||||
| 5 mg: 58% / 63.6 | 5 mg: –91.3 (13.5) | 5 mg: 17 | ||||||
| 25 mg: 56% / 64.4 | 25 mg: –85.9 (13.7) | 25 mg: 9 | ||||||
| 50 mg: 60% / 63.5 | 50 mg: –116.8 (14.0) | 50 mg: 18 | ||||||
| Lees et al. [22] | BIPARK-II (14-15 weeks) | 427 PD patients | capsule OPC (25, 50 mg): (129, 154) | Placebo (144) | Placebo: 52.6% / 61.5 | Placebo: –64.5 (14.4) | Placebo: 11 | |
| 25 mg: 65.6% / 62.5 | 25 mg: –101.7 (14.9) | 25 mg: 30 | ||||||
| 50 mg: 60.5% / 65.5 | 50 mg: –118.8 (13.8) | 50 mg: 36 | ||||||
| Takeda et al. [23] | ONO-2370 (14-15 weeks) | 437 PD patients | tablet OPC (25, 50 mg): (145, 145) | Placebo (147) | Placebo: 38.1% / 68.5 | Placebo: –25.2 (12.6) | Placebo: 4 | |
| 25 mg: 40% / 67.9 | 25 mg: –69.6 (13.2) | 25 mg: 13 | ||||||
| 50 mg: 41.4% / 67.4 | 50 mg: –62.4 (12.6) | 50 mg: 18 | ||||||
| Open-label period | Ferreira et al. [24] | BIPARK-I (52 weeks) | 495 PD patients | capsule OPC 50 mg | Placebo (99) | - | previous Placebo: –64.9(14.8) | - |
| ENT (100) | previous ENT: -39.9 (14.4) | |||||||
| 5 mg (100) | previous 5 mg: –27.5 (14.3) | |||||||
| 25 mg (98) | previous 25 mg: –23.0 (14.4) | |||||||
| 50 mg (98) | previous 50 mg: –1.8 (14.6) | |||||||
| Lees et al. [22] | BIPARK-II (52 weeks) | 376 PD patients | capsule OPC 50 mg | Placebo (130) | - | –126.3 min at 52 weeks ‡ | - | |
| 25 mg (118) | ||||||||
| 50 mg (128) | ||||||||
| Takeda et al. [25] | ONO-2370 (52 weeks) | 385 PD patients | tablet OPC 50 mg | Placebo (137) | - | previous Placebo: –75.6(11.4) | - | |
| 25 mg (126) | previous 25 mg: –22.2 (12.0) | |||||||
| 50 mg (122) | previous 50 mg: –4.2 (12.6) | |||||||
†ITT, Intention to treatment; ‡No report of open-label off-time by treatment group. Off-time reduction was sustained throughout the open-label. DB, double-blind; OPC, opicapone; ENT, entecapone; SE, standard error; CI, confidence interval; PD, Parkinson’s disease.
Table 3
Change from baseline to endpoint of the different on-time state in Double-blind period
| Parameter | Study | Treatment group | ||
| Placebo | OPC 25 mg | OPC 50 mg | ||
| On-time without dyskinesia or with non-troublesome dyskinesia (min) | Ferreira et al. (2016) [8] | 46.5 (14.2) | 84.7 (14.6) | 109.1 (14.9) |
| LS mean change from baseline (SE) | Lees et al. (2017) [22] | 48.1 (15.3) | 84.1 (14.8) | 85.6 (14.1) |
| Takeda et al. (2021) [23] | 23.4 (12.6) | 66.6 (12.6) | 60 (12.6) | |
| On-time with troublesome dyskinesia (min) | Ferreira et al. (2016) [8] | 0.6 (6.0) | 1.4 (6.2) | 9.9 (6.3) |
| LS mean change from baseline (SE) | Lees et al. (2017) [22] | 11.2 (8.2) | 19.4 (8.6) | 25.6 (7.9) |
| Takeda et al. (2021) [23] | –0.6 (3.0) | 1.8 (3.0) | 4.8 (3.0) | |
LS mean, least square mean; SE, standard error; OPC, opicapone.
Table 2 presents the results of the risk of bias assessment. In studies with double-blind periods, there was a low risk of bias associated with blinding of participants and personnel, whereas in open-label studies, the risk of bias was rated as high.
Table 2
Risk of bias assessment results
| Study | Type of Bias | ||||||
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | |
| Ferreira et al. (2016) [8] | Low risk (randomized trial) | Low risk (randomization was performed by computer-generating scheme) | Low risk (double-blind study) | Low risk (double-blind study) | Low risk (outcome data were collected from all participants) | Low risk (all outcome measures reported) | Unclear risk |
| Ferreira et al. (2018) [24] | Low risk (randomized trial) | Low risk (randomization was performed by computer-generating scheme) | High risk (Open-label study) | High risk (Open-label study) | Low risk (outcome data were collected from all participants) | Low risk (all outcome measures reported) | Unclear risk |
| Lees et al. (2017) [22] | Low risk (randomized trial) | Low risk (randomization was performed by computer-generating scheme) | Low risk (double-blind study) | Unclear (includes both Double-blind and Open-label period) | Low risk (outcome data were collected from all participants) | Low risk (all outcome measures reported) | Unclear risk |
| Takeda et al. (2021) [23] | Low risk (randomized trial) | Low risk (permuted block method) | Low risk (double-blind study) | Low risk (double-blind study) | Low risk (outcome data were collected from all participants) | Low risk (all outcome measures reported) | Unclear risk |
| Takeda et al. (2021) [25] | Low risk (randomized trial) | Low risk (permuted block method) | High risk (Open-label study) | High risk (Open-label study) | Low risk (outcome data were collected from all participants) | Low risk (all outcome measures reported) | Unclear risk |
Main analysis results
Efficacy: Off-time reduction in the double-blind period
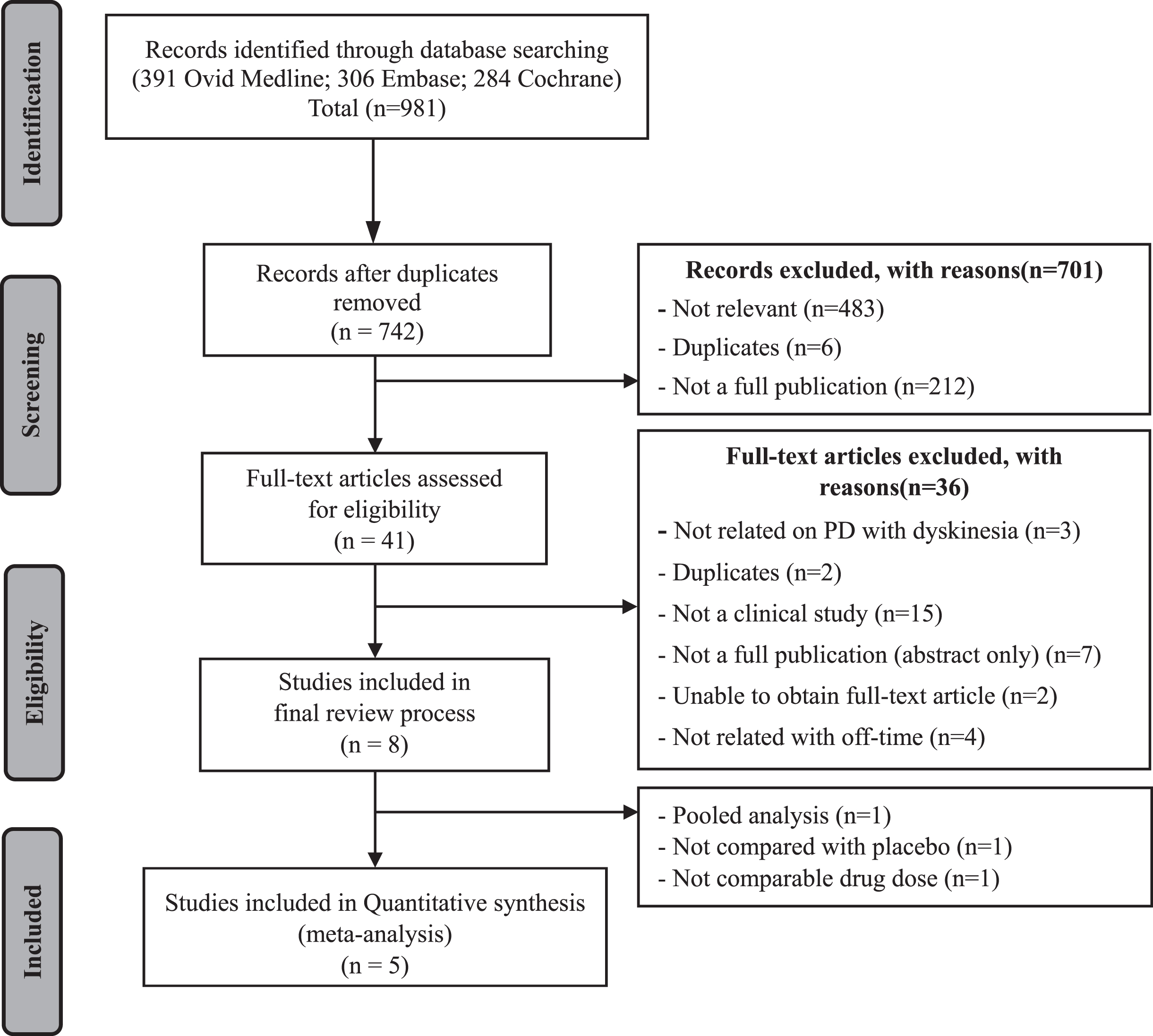
Three studies reported the off-time reduction with opicapone 50 mg treatment during the double-blind period, with 407 patients in the opicapone 50 mg group and 402 patients in the placebo group. As shown in Fig. 2, the meta-analysis revealed that the off-time reduction was higher in the opicapone 50 mg group than in the placebo group, and the MD was statistically significant (–49.91 min [95% CI: –71.39, –28.43], p < 0.001), without statistical heterogeneity (I2 = 0%).
Fig. 2
The mean difference of off-time change in opicapone 50 mg versus placebo group in double-blind period. (CI, confidence interval; OPC, opicapone; PLA, placebo).
For the 25 mg group, 384 patients in the opicapone group and 402 patients in the placebo group were included in three studies. Compared with placebo, opicapone 25 mg showed significant efficacy in terms of off-time reduction (–37.42 min [95% CI: –59.25, –15.58], p = 0.0008, I2 = 0%). (Supplementary Figure. 1A).
Efficacy: Off-time reduction in the open-label period
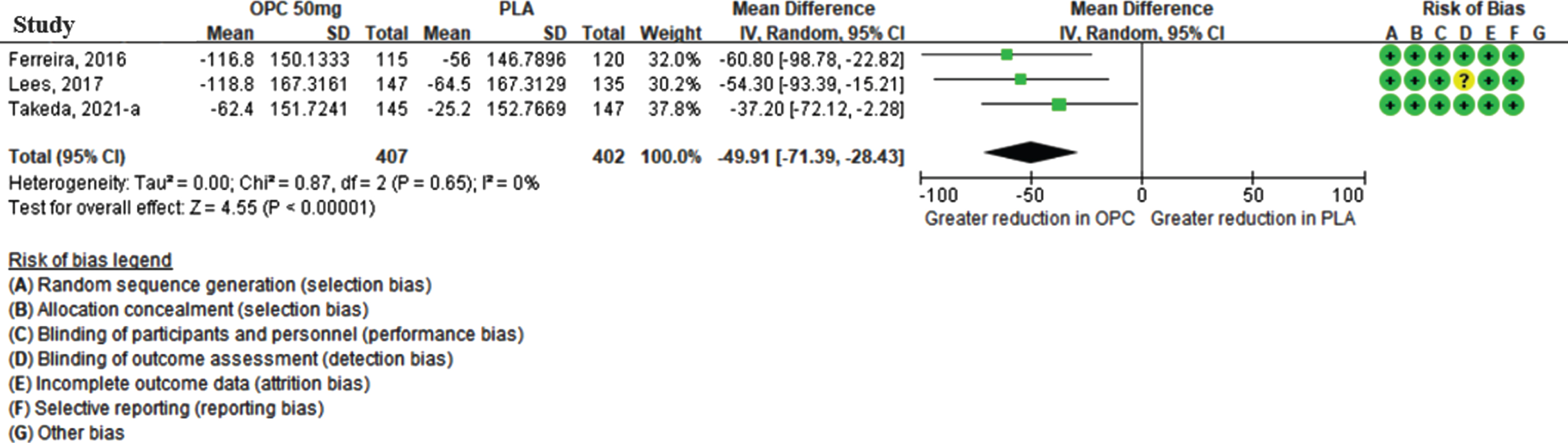
Although three studies reported off-time reduction during the open-label period, only two studies were quantitatively synthesized, as these provided dose-specific results after switching treatment to opicapone 50 mg (Ferreira [24], Takeda [25]). In total, 220 and 236 patients were previously assigned to the opicapone 50 mg group and placebo group, respectively. As shown in Fig. 3, the meta-analysis revealed that the reduction in the off-time during the open-label period was higher in the placebo group than in the group already taking opicapone 50 mg, and the MD was statistically significant (68.08 min [95% CI: 42.29, 93.86], p-value < 0.001) without statistical heterogeneity between studies (I2 = 0%) for the meta-analysis.
Fig. 3
The mean difference of off-time change in previous opicapone 50 mg versus previous placebo group in open-label period. (CI, confidence interval; OPC, opicapone; PLA, placebo).
The efficacy of opicapone for off-time reduction was significant in two open-label studies conducted at a reduced dose (25 mg). The 236 patients who switched from placebo to 50 mg opicapone displayed an additional reduction in the off-time reduction of 48.18 min (95% CI: 20.90, 75.45; p = 0.0005; I2 = 0%) when compared with the 224 patients who switched from 25 mg to 50 mg opicapone. (Supplementary Figure 1B).
Safety of dyskinesia during the double-blind period
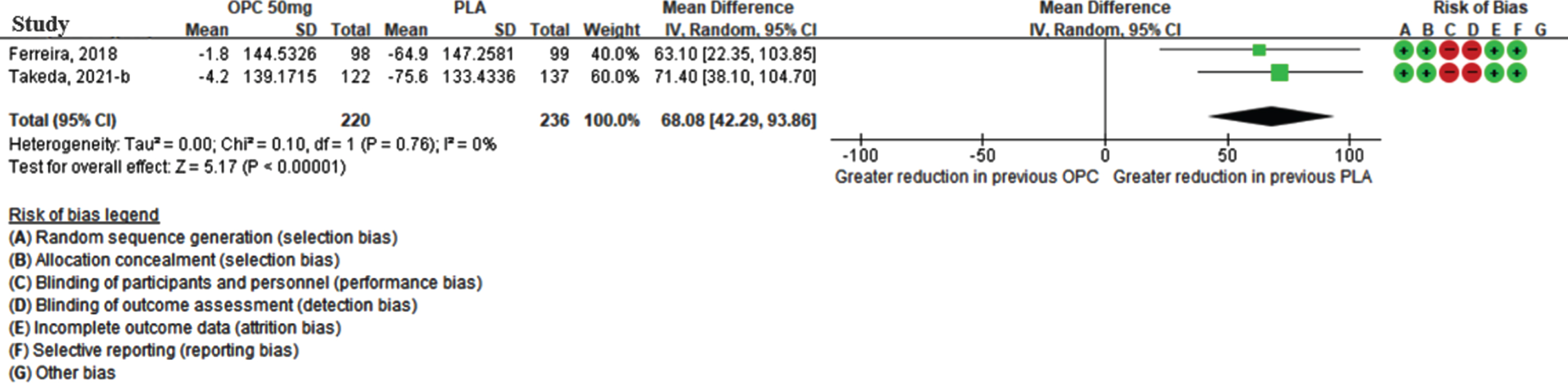
Three studies reported the development of dyskinesia during the double-blind period. The meta-analysis revealed that opicapone had a higher risk of dyskinesia than placebo at 50 mg (RR = 3.43 [95% CI: 2.14, 5.51]; p < 0.0001, I2 = 0%) (Fig. 4). Even in the opicapone 25 mg group, the risk of dyskinesia was statistically significantly higher than that of placebo group. (Supplementary Figure 1C).
Fig. 4
The relative risk of dyskinesia in opicapone 50 mg versus placebo group in double-blind period. (CI, confidence interval; OPC, opicapone; PLA, placebo).
According to a meta-analysis of the on-time according to accompanying TD, the on-time without TD was longer in the opicapone 50 mg group than in the placebo group, and the MD between the two groups was statistically significant (44.62 min [95% CI: 22.60, 66.64], p < 0.0001, I2 = 0%) (Fig. 5A). The results of opicapone 25 mg also revealed that on-time without TD was significantly increased in the opicapone group when compared with the placebo group (39.60 min [95% CI: 17.38, 61.82]; p < 0.001, I2 = 0%). (Supplementary Figure 1D).
Fig. 5
The mean difference of on-time change in opicapone 50 mg versus placebo group in double-blind period. Plot A (A), on-time without troublesome dyskinesia, minutes per day; plot B (B), on-time with troublesome dyskinesia, minutes per day. (CI, confidence interval; OPC, opicapone; PLA, placebo; TD, Troublesome Dyskinesia).
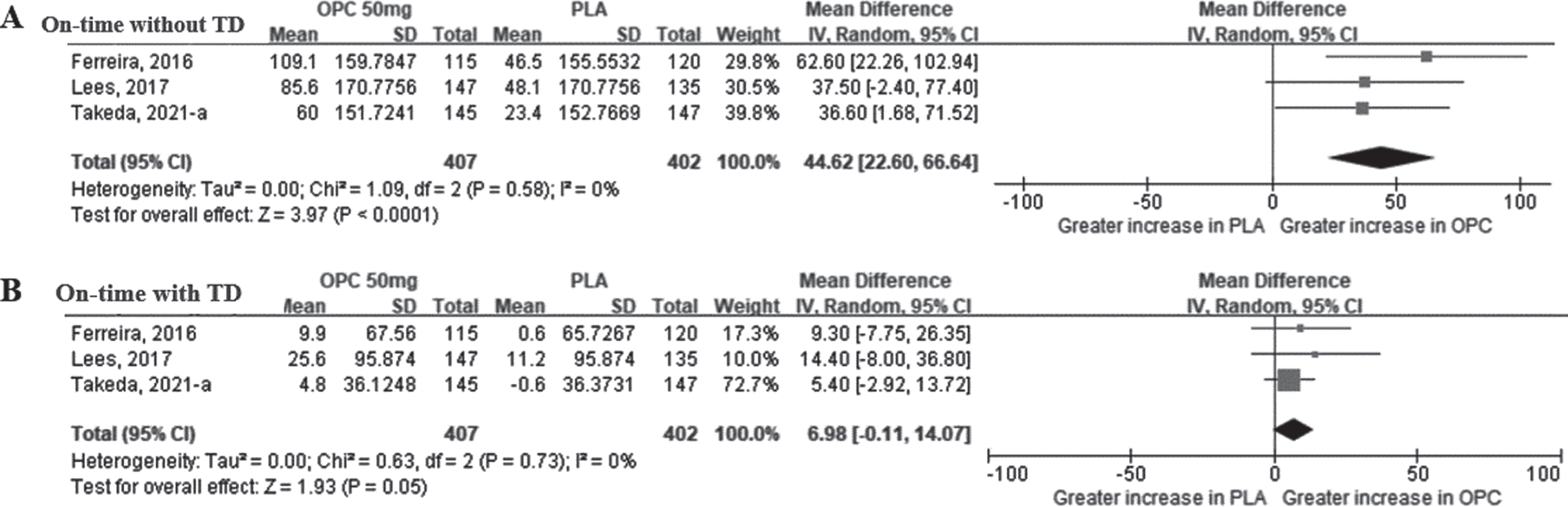
Values for the on-time with TD were extracted from the same three studies that reported on-time without TD. As shown in Fig. 5B, the meta-analysis revealed that the MD of the on-time with TD between the opicapone 50 mg and placebo groups was not statistically significant (6.98 min [95% CI: –0.11, 14.07], p = 0.05). As a result of additional analysis for the opicapone 25 mg dose, the on-time with TD was slightly increased in the opicapone group, with no significant difference observed between the two groups (2.64 min [95% CI: –4.20, 9.48], p = 0.45) (Supplementary Figure 1E).
DISCUSSION
Based on results of the present systematic review and meta-analysis, opicapone 50 mg, the standard dose, reduced the off-time of levodopa in patients with PD by 49.91 min (on average) when compared with the placebo (95% CI: –71.39, –28.43, heterogeneity: p = 0.87, I2 = 0%) during the double-blind period. In addition, patients who switched from placebo to opicapone 50 mg exhibited an off-time reduction of 68.08 min (95% CI: 42.29, 93.86, heterogeneity: p = 0.76, I2 = 0%) when compared with those who continued taking opicapone 50 mg. Although the RR of dyskinesia as a side effect of opicapone was 3.43 times (95% CI: 2.14, 5.51) higher in the opicapone 50 mg group than in the placebo group, the on-time without TD significantly increased in the opicapone group, whereas the on-time with TD did not differ among treatment groups.
Additional meta-analysis was performed for opicapone 25 mg dose in the double-blind period, and the use of opicapone significantly reduced the off-time. By altering the drug dose from that previously assigned (placebo, 25 mg, 50 mg) to opicapone 50 mg, the long-term effects of opicapone use were confirmed during the open-label period. Accordingly, the additional decrease in off-time in the placebo group and the maintenance of treatment effect in the 50 mg group ascertained the efficacy of opicapone.
However, the risk of dyskinesia, a side effect of opicapone, was higher in the opicapone group than in the placebo group. The secondary endpoint associated with this adverse event, the on-time without TD, was increased in the opicapone group, whereas the on-time with TD did not differ from that observed in the placebo group. COMT inhibitors can increase the half-life of levodopa and consequently increase blood levels of levodopa more rapidly [26]. Consequently, this could result in the most common dopaminergic adverse events, such as dyskinesia, which tend to occur transiently and immediately after taking COMT inhibitors. The reduced frequency of dyskinesias corresponds with a decrease in the levodopa dose [27]. Thus, the risk of dyskinesia can be considered a reflection of the levodopa-induced dopaminergic activity. Some previous studies recommended reducing the dose of levodopa when administering COMT inhibitors, such as tolcapone and entacapone, in patients who already had dyskinesia. [7, 28]. However, those studies only allowed dose reduction of levodopa within a certain-period (2–3 weeks after baseline) due to methodological necessity [8, 22, 23]. Therefore, further long-term studies are required to determine the optimal dose of levodopa and OPC in PD patients with pre-existing dyskinesia.
A meta-analysis of some second-generation COMT inhibitors showed that tolcapone has greater efficacy than entacapone in increasing on-time and decreasing off-time [29, 30]. However, tolcapone has safety concerns owing to fulminant hepatotoxicity [31, 32]; thus, entacapone is currently more widely used than tolcapone in patients with advanced PD [33]. Another recent network meta-analysis showed that opicapone and entacapone were slightly inferior to tolcapone in on-time increase; however, the main outcomes of the present study, including off-time reduction and on-time increase without troublesome dyskinesia, were not analyzed [34]. According to the BIPARK-I trial, the reduction in off-time was higher in the opicapone 50 mg group (–116.8 min) than in the entacapone 200 mg group (–96.3 min) [8]. The MD between the two groups was statistically significant (–26.2, 95% CI: –63.8, 11.4, p = 0.005). The incidence of dyskinesia was higher in the opicapone 50 mg group (16%) than in the entacapone group (8%), but most dyskinesias were deemed non-troublesome by patients. However, since only one head-to-head comparison study of opicapone and entacapone (BIPARK-1) has been conducted [8], further studies on the efficacy and safety of several COMT inhibitors are required. According to a cost-effectiveness simulation study comparing opicapone with entacapone, opicapone was more effective in reducing off-time than entacapone and had a 60–65% probability of being cost-effective [35].
The present study had the following limitations. First, the statistical power may be weak owing to the small number of selected studies. However, the number of patients included in each study was large, which would have higher power than individual studies. Second, there were differences in opicapone formulations employed among studies. For example, Takeda et al. [23] used a tablet, and Ferreira et al. [8] dispensed a capsule formulation. Despite these differences in formulations, the direction of opicapone efficacy and the magnitude of safety were similar. Third, all studies included in this meta-analysis were supported by pharmaceutical companies; hence, potential bias in establishing study protocols may exist, necessitating careful attention while interpreting obtained data. Fourth, there were differences in the demographic characteristics of patients and study settings among studies included in this meta-analysis. For example, in the study by Takeda et al. [23], the average age of subjects, who were Japanese, was approximately 67–68 years, and the proportion of males was approximately 40%; however, in BIPARK-I and II, the study subjects were 2–5 years younger on average, and the proportion of males was 10–20% higher. Additionally, the Takeda study used a lower levodopa dosage (408–445 mg) than BIPARK-I and II (642–806 mg). The combined use rate of selegiline (a monoamine oxidase B inhibitor used in Japan) in the Japanese patient group was approximately 50% during the study period [23]; this rate was 11.1–14.7% for rasagiline in BIPARK-I [8] and II [22]. Although there was clinical heterogeneity, the statistical heterogeneity of most meta-analyses was reported as 0 (no heterogeneity), and accordingly, the effect of differences in study settings on results was deemed negligible. Despite the limitations of the present study, this is the first meta-analysis to quantitatively synthesize the effect of opicapone, a third-generation COMT inhibitor, on the off-time and safety aspects in patients with PD presenting dyskinesia. Therefore, the results of this study are expected to provide scientific evidence for the selection of levodopa adjuvant therapy in patients with advanced PD.
CONCLUSIONS
In the present study, a meta-analysis was performed to quantitatively synthesize the efficacy of the new third-generation COMT inhibitor, opicapone, and found that opicapone 50 mg shortened the off-time by 49.91 min when compared with placebo; however, the pooled RR of dyskinesia was 3.43 times higher in the opicapone 50 mg group than in the placebo group. In addition, opicapone increased the on-time without TD by 44.62 min when compared with the placebo group, whereas the on-time with TD did not differ from the placebo group. These findings indicated that opicapone is more effective than placebo for symptom control in patients with advanced PD without TD. Therefore, this study provides evidence to support the view that opicapone is a clinically useful treatment for patients with advanced PD [36] by quantitatively demonstrating the efficacy of opicapone. Furthermore, additional head-to-head trials with an existing COMT inhibitor (i.e., entacapone) are needed to compare efficacy with conventional COMT inhibitors.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-213057.
REFERENCES
[1] | Simon DK , Tanner CM , Brundin P ((2020) ) Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clin Geriatr Med 36: , 1–12. |
[2] | Maserejian N , Vinikoor-Imler L , Dilley A ((2020) ) Estimation of the 2020 global population of Parkinson’s disease (PD) [abstract]. Mov Disord 35: , 198. |
[3] | Pringsheim T , Jette N , Frolkis A , Steeves TD ((2014) ) The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 29: , 1583–1590. |
[4] | Wooten GF , Currie LJ , Bovbjerg VE , Lee JK , Patrie J ((2004) ) Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry 75: , 637–639. |
[5] | LeWitt PA ((2015) ) Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics. Mov Disord 30: , 64–72. |
[6] | Lee JJ ((2019) ) Pharmacological treatment in Parkinson’s disease. J Korean Neurol Assoc 37: , 335–344. |
[7] | Rivest J , Barclay CL , Suchowersky O ((1999) ) COMT inhibitors in Parkinson’s disease. Can J Neurol Sci 26 Suppl 2: , S34–38. |
[8] | Ferreira JJ , Lees A , Rocha JF , Poewe W , Rascol O , Soares-da-Silva P ((2016) ) Opicapone as an adjunct to levodopa in patients with Parkinson’s disease and end-of-dose motor fluctuations: a randomised, double-blind, controlled trial. Lancet Neurol 15: , 154–165. |
[9] | Fabbri M , Ferreira JJ , Lees A , Stocchi F , Poewe W , Tolosa E , Rascol O ((2018) ) Opicapone for the treatment of Parkinson’s disease: A review of a new licensed medicine. Mov Disord 33: , 1528–1539. |
[10] | Haasio K ((2010) ) Toxicology and safety of COMT inhibitors. Int Rev Neurobiol 95: , 163–189. |
[11] | Greenwood J , Pham H , Rey J ((2021) ) Opicapone: A third generation COMT inhibitor. Clin Parkinsonism Relat Disord 4: , 100083. |
[12] | Thanvi B , Lo N , Robinson T ((2007) ) Levodopa-induced dyskinesia in Parkinson’s disease: clinical features, pathogenesis, prevention and treatment. Postgrad Med J 83: , 384–388. |
[13] | Feldman M , Margolesky J (2021) Opicapone for the treatment of Parkinson’s disease: a review. Int J Neurosci. doi: 10.1080/00207454.2021.1929217. |
[14] | Ferreira J , Lees A , Rocha JF , Poewe W , Rascol O , Soares-da-Silva P ((2019) ) Long-term efficacy of opicapone in fluctuating Parkinson’s disease patients: a pooled analysis of data from two phase 3 clinical trials and their open-label extensions. Eur J Neurol 26: , 953–960. |
[15] | Salamon A , Zádori D , Szpisjak L , Klivényi P , Vécsei L ((2019) ) Opicapone for the treatment of Parkinson’s disease: an update. Expert Opin Pharmacother 20: , 2201–2207. |
[16] | Scott LJ ((2016) ) Opicapone: a review in Parkinson’s disease. Drugs 76: , 1293–1300. |
[17] | Katsaiti I , Nixon J ((2018) ) Are there benefits in adding catechol-O methyltransferase inhibitors in the pharmacotherapy of Parkinson’s disease patients? A systematic review. J Parkinsons Dis 8: , 217–231. |
[18] | Moher D , Liberati A , Tetzlaff J , Altman DG ((2009) ) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: , e1000097. |
[19] | Higgins JPT , Savović J , Page MJ , Elbers RG , Sterne JAC (2019) Chapter 8: Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions, Second Edition, Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. The Cochrane Collaboration. |
[20] | Kim S , Park J , Seo H , Lee Y , Jang B , Son H , Suh H , Shin C (2011) NECA’s guidance for undertaking systematic reviews and meta-analyses for intervention. National Evidence-Based Healthcare Collaborating Agency, Seoul. |
[21] | Borenstein M , Hedges LV , Higgins JP , Rothstein HR ((2010) ) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1: , 97–111. |
[22] | Lees AJ , Ferreira J , Rascol O , Poewe W , Rocha J-F , McCrory M , Soares-da-Silva P , Investigators B-S ((2017) ) Opicapone as adjunct to levodopa therapy in patients with Parkinson disease and motor fluctuations: a randomized clinical trial. JAMA Neurol 74: , 197–206. |
[23] | Takeda A , Takahashi R , Tsuboi Y , Nomoto M , Maeda T , Nishimura A , Yoshida K , Hattori N ((2021) ) Randomized, controlled study of opicapone in Japanese Parkinson’s patients with motor fluctuations. Mov Disord 36: , 415–423. |
[24] | Ferreira JJ , Lees AJ , Poewe W , Rascol O , Rocha J-F , Keller B , Soares-da-Silva P ((2018) ) Effectiveness of opicapone and switching from entacapone in fluctuating Parkinson disease. Neurology 90: , e1849–e1857. |
[25] | Takeda A , Takahashi R , Tsuboi Y , Nomoto M , Maeda T , Nishimura A , Yoshida K , Hattori N ((2021) ) Long-term safety and efficacy of opicapone in Japanese Parkinson’s patients with motor fluctuations. J Neural Transm 128: , 337–344. |
[26] | Hsu A , Yao HM , Gupta S , Modi NB ((2015) ) Comparison of the pharmacokinetics of an oral extended-release capsule formulation of carbidopa-levodopa (IPX066) with immediate-release carbidopa-levodopa (Sinemet®), sustained-release carbidopa-levodopa (Sinemet® CR), and carbidopa-levodopa-entacapone (Stalevo®). J Clin Pharmacol 55: , 995–1003. |
[27] | Lees A , Ferreira JJ , Rocha J-F , Rascol O , Poewe W , Gama H , Soares-da-Silva P ((2019) ) Safety profile of opicapone in the management of Parkinson’s disease. J Parkinsons Dis 9: , 733–740. |
[28] | Martinez-Martin P , O’Brien C ((1998) ) Extending levodopa action: COMT inhibition. Neurology 50: , S27–S32. |
[29] | Deane KH , Spieker S , Clarke CE ((2004) ) Catechol-O-methyltransferase inhibitors for levodopa-induced complications in Parkinson’s disease. Cochrane Database Syst Rev CD004554. |
[30] | Lees AJ ((2008) ) Evidence-based efficacy comparison of tolcapone and entacapone as adjunctive therapy in Parkinson’s disease. CNS Neurosci Ther 14: , 83–93. |
[31] | EMEA (2004) European Medicines Agency Public Statement on the lifting of the suspension of the marketing authorisation for Tolcapone (Tasmar). |
[32] | Marsala SZ , Gioulis M , Ceravolo R , Tinazzi M ((2012) ) A systematic review of catechol-0-methyltransferase inhibitors: efficacy and safety in clinical practice. Clin Neuropharmacol 35: , 185–190. |
[33] | Gordin A , Kaakkola S , Teravainen H ((2004) ) Clinical advantages of COMT inhibition with entacapone - a review. J Neural Transm 111: , 1343–1363. |
[34] | Song Z , Zhang J , Xue T , Yang Y , Wu D , Chen Z , You W , Wang Z ((2021) ) Different catechol-O-methyl transferase inhibitors in Parkinson’s disease: a Bayesian network meta-analysis. Front Neurol 12: , 707723. |
[35] | Hansen RN , Suh K , Serbin M , Yonan C , Sullivan SD ((2021) ) Cost-effectiveness of opicapone and entacapone in reducing OFF-time in Parkinson’s disease patients treated with levodopa/carbidopa. J Med Econ 24: , 563–569. |
[36] | Fox SH , Katzenschlager R , Lim SY , Barton B , de Bie RMA , Seppi K , Coelho M , Sampaio C ; Movement Disorder Society Evidence-Based Medicine Committee ((2018) ) International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord 33: , 1248–1266. |




