Personality Changes After Subthalamic Nucleus Stimulation in Parkinson’s Disease
Abstract
Background:
While deep brain stimulation of the subthalamic nucleus (STN-DBS) significantly improves motor deficits in patients with Parkinson’s disease (PD), it is still unclear whether it affects personality functioning.
Objective:
The objective of the present study was to examine personality changes in patients with PD after STN-DBS from the perspectives of both the patients and caregivers. Moreover, by assessing the premorbid personalities of the patients, we tried to determine individual vulnerability to STN-DBS-induced personality changes.
Methods:
In total, 27 patients and their caregivers participated in our retrospective observational study. They were asked to assess the patients’ personality changes with the Iowa Scale of Personality Changes (ISPC) and the patients’ premorbid personalities with the Big Five Inventory (BFI).
Results:
Caregivers reported significant personality changes in the ISPC domains of Executive Disturbance (p = 0.01) and Disturbed Social Behavior (p = 0.02). Most of the ISPC domains were positively correlated with Conscientiousness, while Executive Disturbance was negatively correlated with Neuroticism of the BFI scale.
Conclusion:
Our results show that executive and social functioning are the two most vulnerable domains in patients with PD after STN-DBS, especially in those patients who score higher for neuroticism and lower for conscientiousness on the BFI scale. The results of our study may provide movement disorder specialists with better counseling options and better selection of DBS candidates. Caregivers’ perspective might contribute significantly in understanding postoperative personality changes.
INTRODUCTION
After Alzheimer’s disease, Parkinson’s disease (PD) is the second most common neurodegenerative disease worldwide [1]. PD progressively destroys neurons in the substantia nigra, resulting in the classic tetrad of parkinsonian signs: tremor, rigidity, bradykinesia, and disturbances in posture and gait in later stages [2]. While PD is traditionally described as a movement disorder, a plethora of recent studies have shown that non-motor symptoms (e.g., mood disorders, sleep disturbances, and cognitive complaints) are a constituent part of PD and may play an important role in the symptomatic treatment of the disease [3, 4].
Patients with PD are traditionally treated with dopamine replacement therapy which, in some cases, fails to offer stable relief of motor symptoms or is associated with intolerable side effects [5]. Deep brain stimulation of the subthalamic nucleus (STN-DBS) has been established during the last 25 years as a highly effective therapy for advanced PD [6]. Numerous studies have consistently shown that STN-DBS significantly improves motor deficits in patients with PD [7]. An association between STN-DBS and non-motor symptoms in patients with PD has also been widely studied over the past two decades. There is strong evidence, that STN-DBS has beneficial effect on non-motor symptoms, which in turn is associated with an improvement in quality of life [8, 9]. For example, several studies on the cognitive consequences of DBS in patients with PD have shown that deterioration of one or more cognitive functions after STN-DBS is rare and unremarkable, with no negative impact on quality of life [10, 11]. When cognitive deterioration is observed after STN-DBS, it is usually mild to moderate and affects psychomotor speed, memory, attention, executive function, and verbal fluency [12]. It appears that globus pallidus stimulation leads to less neurocognitive impairment than STN-DBS [12], but studies are not entirely consistent [13].
Although cognitive abilities remain mostly unaffected after STN-DBS, it is still unclear whether this surgical procedure could affect other psychological domains such as mood or personality, in patients with PD. Combs et al. found that STN-DBS resulted in lower levels of depressive symptoms post-surgery [12]. STN-DBS was found to have a positive effect on subjective sleep/fatigue symptoms, while there were no significant changes in mood/apathy at 36 months after STN-DBS [8]. In a recent study, we also observed an overall improvement in non-motor symptoms, as well as improvement in anxiety, depression, sleep, and health-related quality of life up to 48 months after STN-DBS [9]. On the other hand, in a prospective study, Funkiewiez et al. found a slight increase in symptoms of apathy, a slight increase in depression, and rare transient behavioral changes [14].
Studies addressing a relationship between STN-DBS and personality changes are scarce. Pham et al. found increased levels of impulsivity in patients with PD after STN-DBS and that relatives were more sensitive to personality changes than patients [5]. In the study by Houeto et al., personality traits improved in eight patients, remained unchanged in seven, and worsened in eight. Among the worsened patients, the most impaired personality domains were lack of initiative, lack of persistence, and lack of planning [15]. It is not yet clear whether these STN-DBS-induced personality changes are transient or long-term. Some studies have found that STN-DBS does not produce long-term changes of patients’ personality [16]. By using personality disorder assessment tools, Castelli et al. found significant improvements in paranoid and obsessive-compulsive personality disorder symptoms in patients with PD after STN-DBS. Conversely, the level of thought disorder was significantly higher after STN-DBS [17].
The objective of the current study was to examine the personality changes in patients with PD after STN-DBS. Moreover, by assessing premorbid personality, we tried to determine individual susceptibility to personality changes after the operation. We hypothesized that (i) caregivers are more critical and aware of personality changes than patients and that (ii) patients with more maladaptive preoperative personality traits (e.g., high levels of neuroticism) are more vulnerable to personality changes after STN-DBS than patients with normal preoperative values.
MATERIALS AND METHODS
Subjects
Initially, 42 patients with PD who had been treated with bilateral STN-DBS were identified as potential candidates for our retrospective observational study. Exclusion criteria were (a) serious postoperative or medical complications, (b) presence of severe cognitive impairment (i.e.,≤2SD in more than one cognitive domain), (c) severe untreated mental disorder (e.g., major depressive disorder, psychosis, bipolar disorder), and (d) language problems. Of the 42 patients, seven patients refused to participate, and eight patients were excluded due to postoperative or other medical complications (i.e., two cases of postoperative intracerebral hemorrhage at the electrode puncture site, one case of postoperative depression, two cases of severe agitation, two cases of neurostimulator site infection and one case of scalp infection at the site of connection between the cable and the lead). 27 patients (11 women) and their caregivers finally participated in the study (see detailed information on their demographic and clinical characteristics in Table 1). Sixteen patients from our cohort had their STN-DBS implanted at Ludwig-Maximilian University Münich, Department of neurosurgery (between years 2008 and 2014) while 11 other patients had their implantation done at Ljubljana University Clinical Centre Ljubljana, Department of neurosurgery (between years 2015 and 2016). All participants provided written informed consent. The study was approved by the Medical Ethics Committee of the Republic of Slovenia.
Table 1
Clinical and demographic characteristics of patients with PD
| Age in years, mean (SD) | 61.7 (6.5) |
| Duration of disease in years, mean (SD) | 15.1 (4.6) |
| Modified Hoen-Yahr stage: | |
| Stage 2, number of patients | 16 |
| Stage 2.5, number of patients | 11 |
| LEDD before DBS in milligrams, mean (SD) | 1520.7 (692.6) |
| LEDD after DBS in milligrams, mean (SD) | 636.9 (404.6) |
| LEDD reduction in milligrams, mean (SD) | 785.6 (755.5) |
We assessed the severity of PD symptoms before the surgical procedure using the Modified Hoehn-Yahr Scale. 16 patients (57%) were assigned stage 2 and 11 patients (43%) stage 2.5. All patients had at least moderate troublesome motor fluctuations, ≥1 h of troublesome dyskinesia/day and/or≥2 h “off” symptoms/day, and most of them had ≥5 oral levodopa doses/day [18]. We calculated the levodopa equivalent daily dose (LEDD) [19, 20] (in mg/day) before and at least 6 months after surgery, when pharmacotherapy and DBS stimulation parameters had stabilized. The reduction of LEDD after the operation was 53.5% (SD = 28.9%), which was comparable to other studies [21–23].
2.2Personality assessments
Personality changes were evaluated by the officially translated Slovene version of the Iowa Scales of Personality Change (ISPC) [24, 25]. The ISPC provides a standardized assessment of 26 personality characteristics that might change as a result of a neurological condition. The assessed characteristics refer to emotional functioning, social and interpersonal behavior, decision-making and goal-directed behavior, behavior control, and insight. In the standard version of the ISPC, the information is provided by an informant-the caregiver who has regular and substantial contact with the patient. The caregiver assesses patient on seven-point scales, with 1 indicating very good functioning, 3 indicating the hypothetical average, 5 indicating that the characteristic is present to a problematic degree, and 7 indicating a severe problem. Ratings of personality functioning are provided for past (before STN-DBS) and current functioning (after STN-DBS). ISPC scales reflect personality disturbances along the following five dimensions: (1) Executive Deficits (Lack of Planning, Perseverance, Lack of Initiative, Lack of Persistence, Impulsivity, Poor Judgment, and Indecisiveness); (2) Disturbed Social Behavior (Social Inappropriateness, Insensitivity, Inappropriate Affect, Lack of Insight, Inflexibility, and Aggression); (3) Diminished Motivation/Hypo-emotionality (Apathy, Blunted Affect, and Social Withdrawal); (4) Emotional Reactivity/Irascibility (Irritability, Emotional Lability, and Impatience); and (5) Distress (Depression, Anxiety, Vulnerability to Pressure, and Dependency).
Personality traits were assessed by the officially translated Slovene version of the Big Five Inventory (BFI) [26, 27]. The BFI contains 44 items that measure personality traits defined by the Five Factor Theory of Personality (extraversion, neuroticism, agreeableness, openness, and conscientiousness). Caregivers were asked to assess patients’ personality characteristics on five-point scales (1: strongly disagree, 2: slightly disagree, 3: neither agree nor disagree, 4: slightly agree, 5: strongly agree).
ISPC and BFI were administered between January and July 2017, at least 6 months after surgical procedure when programming was completed, and patients were on stable medications. The average time between undergoing STN-DBS and personality diagnostics was 11 months. The aim of our study was not only to measure the degree of personality changes but also to assess potential discrepancies between the personality assessments of patients and caregivers. Therefore, ISPD and BFI were administered to patients with PD as well as their caregivers. For ISPC, both groups were asked to assess personality functioning before and at least 6 months after STN-DBS. For BFI, both groups were asked to assess retrospectively the patients’ premorbid personality traits (i.e., personality as it was before DBS treatment).
2.3Statistical analysis
All statistical analyses were conducted with PASW Statistics 18, and alpha was set to p < 0.05. Since the assumptions for using parametric statistical tests were not fulfilled, non-parametric statistical tests were used. The Wilcoxon signed-rank test was used to compare the ISPC test results pre- and post-STN-DBS. The Spearman’s correlation analysis (ρ) was used to analyze the relationship between personality changes (ISPC) and personality characteristics (BFI). Bonferroni correction was used to control for multiple comparisons.
3RESULTS
3.1ISPC personality changes
First, we assessed postoperative changes according to the caregivers’ ISPC ratings. Caregivers observed the most significant changes in Executive Functioning (p = 0.010), followed by Disturbed Social Behavior (p = 0.021), whereas they did not observe significant postoperative changes in personality functioning for the Irascibility, Diminished Motivation and Distress (Table 2). The changes in Executive Functioning are statistically significant at 5 % -alpha-level even after Bonferroni correction for multiple tests. Virtually no changes were found in the assessment of personality changes from the patients’ point of view after STN-DBS (Table 3). There was also a clear difference in effect sizes, which are between 0.2 and 0.3 for the caregivers’ perspective and around 0.0 for the patients’ perspective.
Table 2
Comparisons of ISPC personality traits before and after STN-DBS from the caregivers’ ratings
| Before treatment | After treatment | ||||||
| M | SD | M | SD | Z a | p | d | |
| Executive Disturbance | 3.05 | 0.87 | 3.61 | 1.43 | –2.321 | 0.010** | –0.316 |
| Disturbed Social Behavior | 2.52 | 0.97 | 2.99 | 1.22 | –2.095 | 0.021* | –0.271 |
| Irascibility | 3.29 | 1.10 | 3.51 | 1.53 | –0.855 | 0.393 | –0.116 |
| Diminished Motivation | 3.10 | 1.17 | 3.46 | 1.28 | –1.495 | 0.135 | –0.203 |
| Distress | 3.25 | 1.12 | 3.50 | 1.47 | –0.903 | 0.367 | –0.123 |
aWilcoxon signed rank test; *p < 0.05, **p < 0.01, without correction for multiple testing; d = effect size.
Table 3
Comparisons of ISPC personality traits before and after STN-DBS from the patients’ ratings
| Before treatment | After treatment | ||||||
| M | SD | M | SD | Z a | p | d | |
| Executive Disturbance | 3.06 | 1.14 | 2.99 | 0.91 | –0.228 | 0.819 | –0.031 |
| Disturbed Social Behavior | 2.50 | 0.94 | 2.53 | 0.73 | –0.071 | 0.943 | –0.010 |
| Irascibility | 2.84 | 1.12 | 3.00 | 1.22 | –0.374 | 0.708 | –0.051 |
| Diminished Motivation | 2.96 | 1.13 | 2.87 | 1.04 | –0.393 | 0.694 | –0.053 |
| Distress | 3.13 | 1.27 | 3.01 | 1.35 | –0.454 | 0.650 | –0.062 |
aWilcoxon signed rank test, d = effect size.
We also performed a direct comparison of ISPC score changes between caregivers and patients. Table 4 shows that caregivers reported statistically larger changes in Executive Disturbance and Disturbed Social Behavior compared to patients, but there were no significant differences in the remaining three ISPC domains. Although the differences failed to reach statistical significance after Bonferroni correction, Cohen’s effect size values suggested small to moderate differences between caregivers and patients.
Table 4
Differences between caregivers and patients in ISPC scores changes
| Caregivers | Patients | ||||||
| M | SD | M | SD | Z a | p | d | |
| Executive Disturbance | –0.57 | 1.29 | 0.07 | 1.40 | –2.072 | 0.038* | –0.282 |
| Disturbed Social Behavior | –0.47 | 1.10 | –0.03 | 0.99 | –2.160 | 0.031* | –0.294 |
| Irascibility | –0.22 | 1.41 | –0.16 | 1.54 | –0.202 | 0.840 | –0.027 |
| Diminished Motivation | –0.36 | 1.43 | 0.09 | 1.42 | –1.248 | 0.212 | –0.170 |
| Distress | –0.25 | 1.63 | 0.13 | 1.50 | –1.202 | 0.229 | –0.164 |
aWilcoxon signed rank test; *p < 0.05, **, without correction for multiple testing; d = effect size.
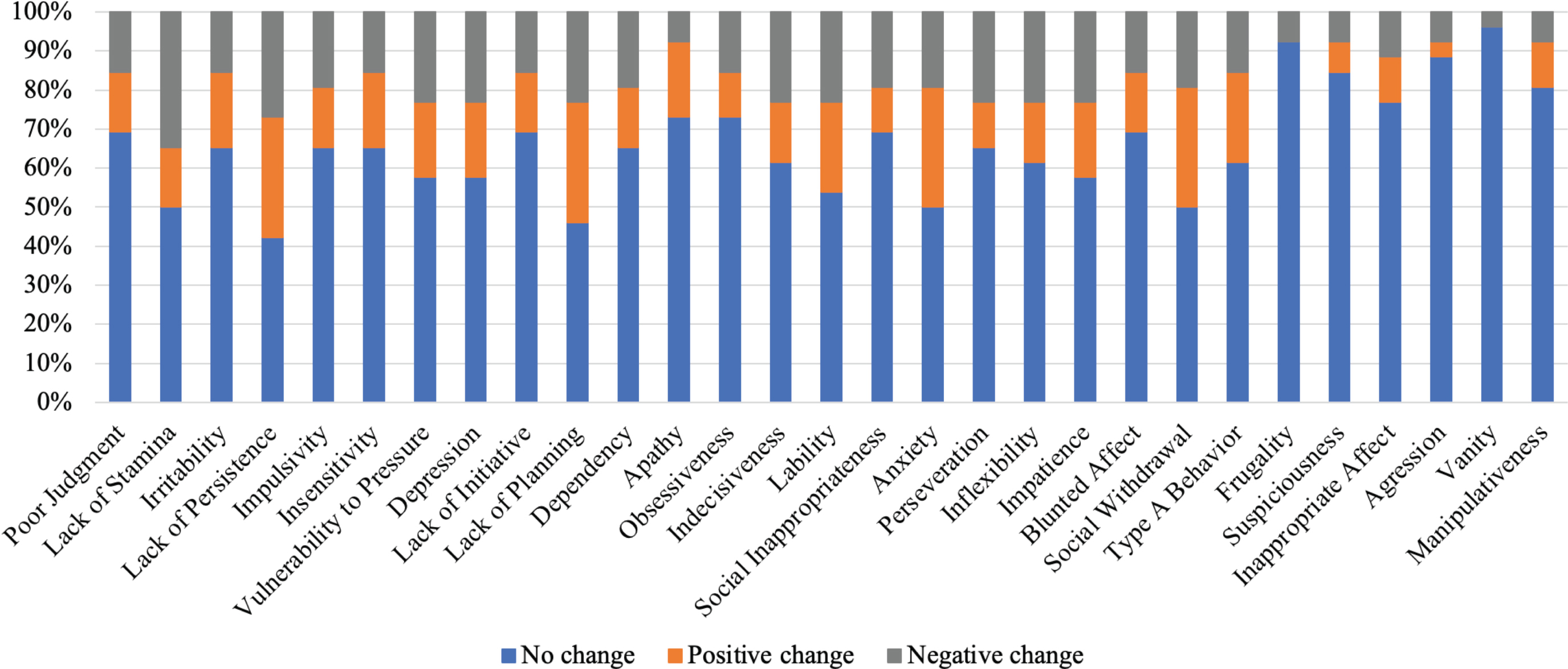
Figure 1 shows the caregivers’ ratings for the ISPC personality domains. The personality changes most frequently observed by caregivers were in the domains of Poor Judgment, Lack of Stamina, and Irritability, in which personality functioning deteriorated in more than 30% of patients. Conversely, minimal personality changes were observed in the domains of Aggression, Vanity, and Manipulativeness, in which personality functioning deteriorated in less than 10% of patients. On the other hand, according to the patients’ assessments, only Lack of Stamina was negatively affected in 30% or more of patients. For majority of the other personality domains, patients reported no or even positive changes (Fig. 2).
Fig. 1
The percentages of patients with changes in personality domains observed with ISPC. The ratings were provided by the caregivers.
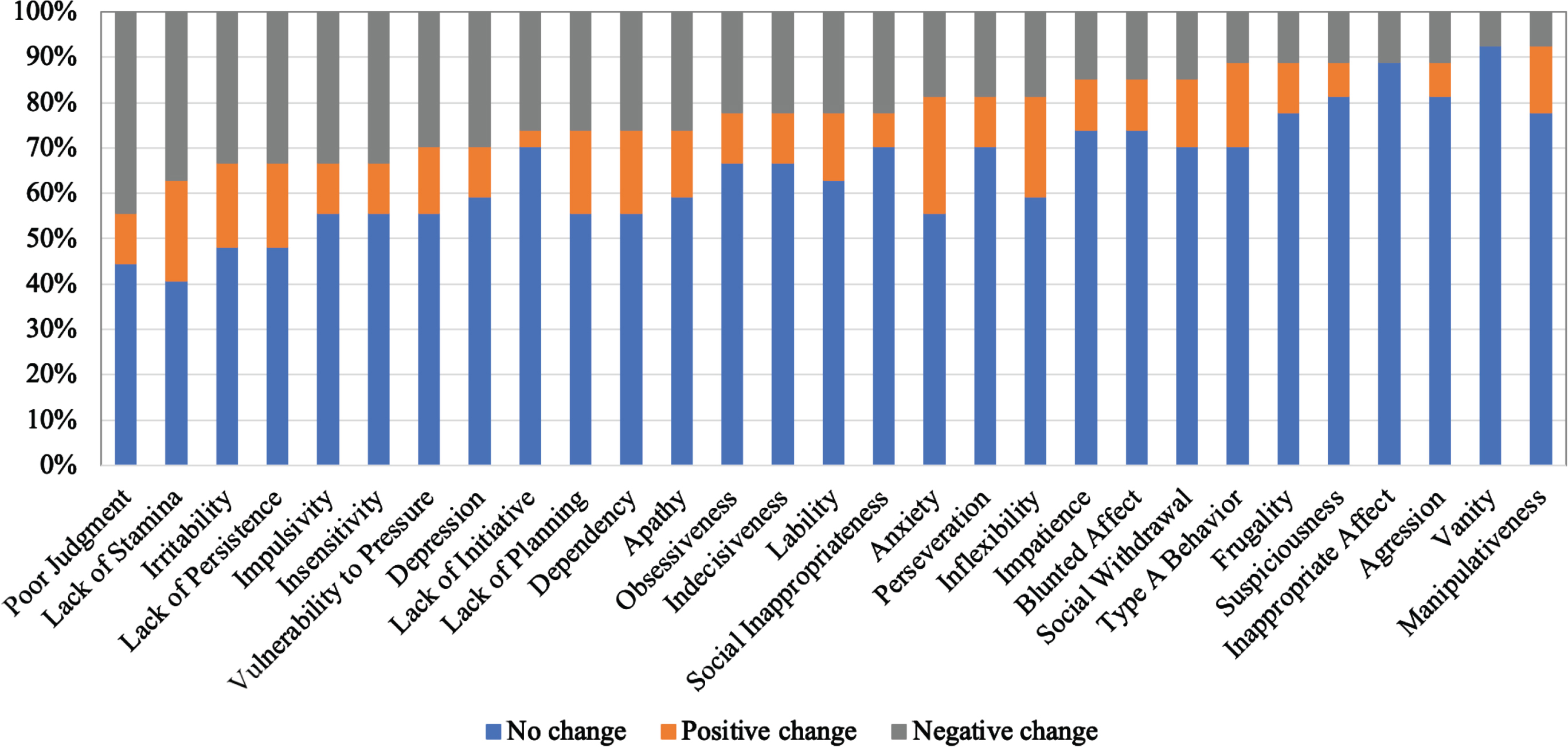
Fig. 2
The percentages of patients with changes in personality domains observed with ISPC. The ratings were provided by the patients.
3.2The relationship between premorbid personality traits and postoperative personality changes
Table 5 shows the relationships between personality traits measured with BFI and postoperative personality domains measured with ISPC. Due to multiple comparisons, we set alpha level on 0.01. According to the perspective of the caregivers, Conscientiousness is significantly related to Executive Disturbance, Disturbed Social Behavior, Irascibility, and Diminished Motivation. Conscientiousness was negatively correlated to these ISPC personality dimensions, indicating that patients who scored higher on the Conscientiousness dimension were less prone to personality changes. We also found significant positive correlation between Neuroticism and Executive Disturbance. Patients who were perceived to be more emotionally labile were more susceptible to difficulties in executive functioning according to caregivers’ perspective. In contrast, when BFI and ISPC patients’ ratings were compared, there were no statistically significant correlations at the 1% -alpha-level (Table 6).
Table 5
Premorbid personality traits and postoperative changes (caregivers’ perspective)
| Executive Disturbance | Disturbed Social Behavior | Irascibility | Diminished Motivation | Distress | |
| Extraversion | –0.15 | 0.05 | 0.02 | –0.24 | 0.01 |
| Agreeableness | –0.10 | –0.19 | –0.14 | –0.03 | –0.06 |
| Conscientiousness | –0.54* | –0.58* | –0.56* | –0.58* | –0.34 |
| Neuroticism | 0.45* | 0.33 | 0.26 | 0.36 | 0.40 |
| Openness | –0.03 | 0.11 | 0.06 | –0.10 | 0.14 |
Spearman correlation coefficient; *p < 0.01, without correction for multiple tests.
Table 6
Premorbid personality traits and postoperative changes (patients’ perspective)
| Executive Disturbance | Disturbed Social Behavior | Irascibility | Diminished Motivation | Distress | |
| Extraversion | 0.16 | 0.33 | 0.25 | 0.18 | 0.35 |
| Agreeableness | –0.05 | 0.08 | –0.05 | 0.17 | 0.09 |
| Conscientiousness | 0.40 | 0.47 | 0.47 | 0.20 | 0.38 |
| Neuroticism | –0.26 | –0.25 | –0.32 | –0.20 | –0.29 |
| Openness | 0.12 | 0.16 | 0.02 | 0.09 | 0.11 |
Spearman correlation coefficient.
In our final additional analysis, we compared the BFI ratings of caregivers and patients. After Bonferroni correction for multiple comparisons, there were no significant differences in BFI scores, indicating that patients and caregivers rated premorbid personality with similar scores. However, the small to moderate effect size in Neuroticism and Openness may suggest that these two traits may be perceived differently to some extent by caregivers and patients (Table 7).
Table 7
Comparison of caregivers’ and patients’ ratings of BFI personality traits
| Caregivers | Patients | ||||||
| M | SD | M | SD | Z a | p | d | |
| Extraversion | 26.49 | 5.81 | 26.35 | 5.08 | –0.131 | 0.896 | –0.018 |
| Agreeableness | 34.32 | 6.65 | 36.58 | 4.22 | –1.417 | 0.157 | –0.193 |
| Conscientiousness | 32.34 | 5.35 | 33.96 | 4.59 | –1.331 | 0.183 | –0.181 |
| Neuroticism | 22.99 | 5.33 | 20.31 | 5.38 | –2.320 | 0.020* | –0.316 |
| Openness | 31.10 | 5.75 | 33.46 | 6.60 | –2.301 | 0.021* | –0.313 |
aWilcoxon signed rank test; *p < 0.05, without correction for multiple testing; d = effect size.
4DISCUSSION
The aim of our research was to examine possible personality changes in patients with PD after STN-DBS. We examined the personality changes observed from both the perspectives of the patients and caregivers and assessed possible differences in their ratings. Moreover, we assessed possible relationships between personality changes and premorbid personality traits in patients with PD.
Regarding our first hypothesis, caregivers were more sensitive in observing potential personality changes. They reported the most significant changes in executive and social functioning. In contrast, patients did not observe any significant changes in their own personality. Our results are consistent with the findings of Pham’s study [5], who also reported that relatives are more sensitive to personality changes than patients. Consistent with our second hypothesis, we found significant correlations between conscientiousness and neuroticism and postoperative personality changes as assessed by caregivers.
According to the caregivers’ observations, personality dimension of Executive Disturbance (i.e., problems with planning, initiative, persistence, judgment, decision-making, and impulsivity) were the most affected by STN-DBS. Our results are in line with previous studies in which higher levels of impulsivity and greater problems with persistence, planning, and executive functions were observed after STN-DBS [5, 28]. One possible explanation for postoperative executive problems may be reduction of dopaminergic medication after STN-DBS [29]. STN stimulation can also lead to poor impulse inhibition and consequently a higher level of impulsivity [30–32]. Furthermore, STN plays an important role as a gating mechanism involved in response initiation [30]. All these mechanisms can be involved in personality changes after STN-DBS. The underlying cause of potential personality changes in patients with PD might be therefore directly related to the functional neuroanatomy of the STN. The STN, a crucial part of the basal ganglia circuitry, is not only an important structure of the motor system of the brain, but is also connected to the limbic and prefrontal regions of the brain [33]. STN stimulation may therefore affect patient behavior, observed as personality changes. Another possible explanation might be related to the number of microelectrode passes, which was significantly correlated to behavioral changes in a study of Burdick et al.. [34]. According to their hypothesis, personality changes are primarily the result of microlesions and not of stimulation per se.
Furthermore, caregivers reported significant personality deterioration in the ISPC dimension of Disturbed Social Behavior (i.e., aggression, insensitivity, lack of insight, and inflexibility). Two previous studies did not find any significant changes in social functioning after STN-DBS [35, 36]. On the contrary, a recent study by Baumann-Vogel et al. revealed that patients with PD became less prone to assume common duties and daily activities despite exhibiting better and more stable motor control. These changes following DBS might lead to conflicts and problems in interpersonal relationships and role allocations [37]. Since Executive Disturbance and Disturbed Social Behavior are closely intertwined dimensions, it is possible that the same underlying cause (i.e., changes in the fronto-striatal loop) may be responsible for personality changes in both dimensions [38].
Reduction of dopaminergic medication after STN-DBS can also affect mood and behavior. LEDD reduction in our patients is comparable to other studies [21–23]. In this regard the effect of LEDD reduction on mood and behavior in our study should not differ from other STN-DBS patient populations.
An important and still unanswered question remains why disruptive personality changes occur only in certain patients (see Fig. 1). This heterogeneity could be explained by differences in electrode positions. Since motor, associative, and limbic parts of the STN partially overlap anatomically, it is possible that in some patients the electrodes stimulate the associative part of the STN, which belongs to the dorsolateral and orbitofrontal loops of the basal ganglia [39, 40]. The dorsolateral prefrontal loop is closely related to executive functions, and the orbitofrontal loop is responsible for social functioning [41]. In addition, the heterogeneity of patients could be explained by the fact that some patients are pre-operatively endowed with less adaptive personality traits. High levels of neuroticism and low levels of conscientiousness are related to poor stress coping strategies [42]. Since emotional lability and conscientiousness are significantly correlated with most ISPC dimensions, it is possible that these premorbid personality traits could lead to unfavorable personality changes.
As already mentioned, negative personality changes are not uniformly observed in our study. Nevertheless, we still observed negative personality changes in six personality domains (i.e., poor judgment, lack of stamina, irritability, lack of persistence, impulsivity, insensitivity) in 30% of our PD patients. These executive functions are widely recognized as an important predictor of long-term cognitive and social developmental outcomes [43], and thus play a very important role with in an intact daily life. Since our results are consistent with some previous studies [5], we believe that this information can be part of preoperative counseling.
In addressing the limitations of our study, it is noteworthy to mention that the patients in our research sample differ significantly in terms of disease duration. Since our study was retrospective, the interval between STN-DBS and personality measurements differed significantly amongst our patients. These two factors could contribute to the interindividual variability in reported personality changes among study participants. Another limitation refers to our sample size which is still relatively small and should be increased in future studies. We used modified Hoehn and Yahr Scale to assess the motor symptoms of the disease. Unfortunately, we could not recuperate all the MDS-UPDRS data before and after the operation. However our inclusion criteria for surgery were not based on MDS-UPDRS scores, but on daily time spent with troublesome dyskinesias and “off” periods and advanced stage of the disease [18]. Finally, the retrospective nature of our study may have led to errors in the personality assessment. It is still questionable to what extent the post-operative BFI assessment truly reflects the premorbid personality in our operated patients. Therefore, future prospective studies are needed that include preoperative personality assessments, which would provide more reliable and valid information about personality changes and their influencing factors after STN-DBS. An important line of research that has received much attention recently is connectivity research [44]. By exploring different connections with the neuromodulation site one can explore changes in personality related to stimulation. This can help plan precise targeting and avoid the connections that lead to disturbed personality after STN-DBS.
Despite improvements in motor and several non-motor symptoms, changes in family roles, greater patient autonomy, false pre-operative expectations and, according to our study, behavioral and personality changes perceived by the caregivers are probably the four most important factors that can negatively influence social adaptation and interpersonal relationships in families after STN-DBS operation. All these factors must be adequately addressed in pre-operative counseling, since they can significantly affect the overall success of the operation.
4.1Conclusions
Although STN-DBS improves motor and even non-motor symptoms, it might also cause significant personality changes in some patients. Although subjective, the caregivers’ perspective indicates that executive and social functioning are the two most vulnerable personality domains, especially in patients with PD who score higher on the neuroticism scale and lower on the conscientiousness scale of the BFI. The results from our study and future prospective studies might help in recognizing patients who are more vulnerable to negative personality changes after DBS. Our results are in line with some previous studies, too [5]. Therefore, we wish to propose the integration of the assessment of behavior changes by the caregivers, to the pre- and post-operative patients’ management.
ACKNOWLEDGMENTS
This research work was supported by the Slovenian Research Agency through the research programme P1-0389, research project J7-2600. We thank Mr. David Sakić for translating the Iowa Scales of Personality Change into Slovenian. We also thank Dr. Eva Lasič for language editing of this manuscript. Last but not least, we thank the members of the multidisciplinary team at the Department of Neurology in Ljubljana and especially the patients with PD and their caregivers for their cooperation in our study.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
REFERENCES
[1] | Castonguay A-M , Gravel C , Lévesque M ((2021) ) Treating Parkinson’s disease with antibodies: Previous studies and future directions. J Parkinsons Dis 11: , 71–92. |
[2] | Conley SC , Kirchner JT ((1999) ) Parkinson’s disease—the shaking palsy. Postgrad Med 106: , 39–52. |
[3] | Poewe W ((2008) ) Non-motor symptoms in Parkinson’s disease. Eur J Neurol 15: (Suppl 1), 14–20. |
[4] | Pfeiffer RF ((2016) ) Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 22: (Suppl 1), S119–122. |
[5] | Pham U , Solbakk A-K , Skogseid I-M , Toft M , Pripp AH , Konglund AE , Andersson S , Haraldsen IR , Aarsland D , Dietrichs E , Malt UF ((2015) ) Personality changes after deep brain stimulation in Parkinson’s disease. Parkinsons Dis 2015: , 490507. |
[6] | Groiss SJ , Wojtecki L , Südmeyer M , Schnitzler A ((2009) ) Deep brain stimulation in Parkinson’s disease. Ther Adv Neurol Disord 2: , 20–28. |
[7] | Machado FA , Reppold CT ((2015) ) The effect of deep brain stimulation on motor and cognitive symptoms of Parkinson’s disease: A literature review. Dement Neuropsychol 9: , 24–31. |
[8] | Jost ST , Sauerbier A , Visser-Vandewalle V , Ashkan K , Silverdale M , Evans J , Loehrer PA , Rizos A , Petry-Schmelzer JN , Reker P , Fink GR , Franklin J , Samuel M , Schnitzler A , Barbe MT , Antonini A , Martinez-Martin P , Timmermann L , Ray-Chaudhuri K , Dafsari HS , EUROPAR and the International Parkinson and Movement Disorders Society Non-Motor Parkinson’s Disease Study Group ((2020) ) A prospective, controlled study of non-motor effects of subthalamic stimulation in Parkinson’s disease: Results at the 36-month follow-up. J Neurol Neurosurg Psychiatry 91: , 687–694. |
[9] | Georgiev D , Mencinger M , Rajnar R , Mušič P , Benedičič M , Flisar D , Boš R , Mehrkens J , Pirtošek Z , Boetzel K , Trošt M ((2021) ) Long-term effect of bilateral STN-DBS on non-motor symptoms in Parkinson’s disease: A four-year observational, prospective study. Parkinsonism Relat Disord 89: , 13–16. |
[10] | Foki T , Hitzl D , Pirker W , Novak K , Pusswald G , Lehrner J ((2018) ) Individual cognitive change after DBS-surgery in Parkinson’s disease patients using Reliable Change Index Methodology. Neuropsychiatr 32: , 149–158. |
[11] | Freund H-J , Kuhn J , Lenartz D , Mai JK , Schnell T , Klosterkoetter J , Sturm V ((2009) ) Cognitive functions in a patient with Parkinson-dementia syndrome undergoing deep brain stimulation. Arch Neurol 66: , 781–785. |
[12] | Combs HL , Folley BS , Berry DTR , Segerstrom SC , Han DY , Anderson-Mooney AJ , Walls BD , van Horne C ((2015) ) Cognition and depression following deep brain stimulation of the subthalamic nucleus and globus pallidus pars internus in Parkinson’s disease: A meta-analysis. Neuropsychol Rev 25: , 439–454. |
[13] | Mehanna R , Bajwa JA , Fernandez H , Wagle Shukla AA ((2017) ) Cognitive impact of deep brain stimulation on Parkinson’s disease patients. Parkinsons Dis 2017: , e3085140. |
[14] | Funkiewiez A , Ardouin C , Caputo E , Krack P , Fraix V , Klinger H , Chabardes S , Foote K , Benabid A , Pollak P ((2004) ) Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson’s disease. J Neurol Neurosurg Psychiatry 75: , 834–839. |
[15] | Houeto JL , Mesnage V , Mallet L , Pillon B , Gargiulo M , du Moncel ST , Bonnet AM , Pidoux B , Dormont D , Cornu P , Agid Y ((2002) ) Behavioural disorders, Parkinson’s disease and subthalamic stimulation. J Neurol Neurosurg Psychiatry 72: , 701–707. |
[16] | Houeto J-L , Mallet L , Mesnage V , Tezenas du Montcel S , Béhar C , Gargiulo M , Torny F , Pelissolo A , Welter M-L , Agid Y ((2006) ) Subthalamic stimulation in Parkinson disease: Behavior and social adaptation. Arch Neurol 63: , 1090–1095. |
[17] | Castelli L , Perozzo P , Zibetti M , Crivelli B , Morabito U , Lanotte M , Cossa F , Bergamasco B , Lopiano L ((2006) ) Chronic deep brain stimulation of the subthalamic nucleus for Parkinson’s disease: Effects on cognition, mood, anxiety and personality traits. Eur Neurol 55: , 136–144. |
[18] | Antonini A , Stoessl AJ , Kleinman LS , Skalicky AM , Marshall TS , Sail KR , Onuk K , Odin PLA ((2018) ) Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: A multi-country Delphi-panel approach. Curr Med Res Opin 34: , 2063–2073. |
[19] | Verber D , Novak D , Borovič M , Dugonik J , Flisar D ((2020) ) EQUIDOPA: A responsive web application for the levodopa equivalent dose calculator. Comput Methods Programs Biomed 196: , 105633. |
[20] | Tomlinson CL , Stowe R , Patel S , Rick C , Gray R , Clarke CE ((2010) ) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25: , 2649–2653. |
[21] | Alexoudi A , Shalash A , Knudsen K , Witt K , Mehdorn M , Volkmann J , Deuschl G ((2015) ) The medical treatment of patients with Parkinson’s disease receiving subthalamic neurostimulation. Parkinsonism Relat Disord 21: , 555–560; discussion 555. |
[22] | Fan S-Y , Wang K-L , Hu W , Eisinger RS , Han A , Han C-L , Wang Q , Michitomo S , Zhang J-G , Wang F , Ramirez-Zamora A , Meng F-G ((2020) ) Pallidal versus subthalamic nucleus deep brain stimulation for levodopa-induced dyskinesia. Ann Clin Transl Neurol 7: , 59–68. |
[23] | Fasano A , Appel-Cresswell S , Jog M , Zurowkski M , Duff-Canning S , Cohn M , Picillo M , Honey CR , Panisset M , Munhoz RP ((2016) ) Medical management of Parkinson’s disease after initiation of deep brain stimulation. Can J Neurol Sci 43: , 626–634. |
[24] | Barrash J , Tranel D , Anderson S ((2000) ) Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Dev Neuropsychol 18: , 355–81. |
[25] | Barrash J , Asp E , Markon K , Manzel K , Anderson SW , Tranel D ((2011) ) Dimensions of personality disturbance after focal brain damage: Investigation with the Iowa Scales of Personality Change. J Clin Exp Neuropsychol 33: , 833–852. |
[26] | John OP , Donahue EM , Kentle RL ((1991) ) The Big Five Inventory Versions 4a and 54, University of California, Berkeley, Institute of Personality and Social Research, Berkeley, CA. |
[27] | Avsec A , Sočan G (2007) Vprašalnik petih velikih faktorjev BFI. Psihodiagnostika Osebnosti, pp. 171-178. |
[28] | Denheyer M , Kiss ZH , Haffenden AM ((2009) ) Behavioral effects of subthalamic deep brain stimulation in Parkinson’s disease. Neuropsychologia 47: , 3203–3209. |
[29] | Ardouin C , Voon V , Worbe Y , Abouazar N , Czernecki V , Hosseini H , Pelissolo A , Moro E , Lhommée E , Lang AE , Agid Y , Benabid A-L , Pollak P , Mallet L , Krack P ((2006) ) Pathological gambling in Parkinson’s disease improves on chronic subthalamic nucleus stimulation. Mov Disord 21: , 1941–1946. |
[30] | Ballanger B , van Eimeren T , Moro E , Lozano AM , Hamani C , Boulinguez P , Pellecchia G , Houle S , Poon YY , Lang AE , Strafella AP ((2009) ) Stimulation of the subthalamic nucleus and impulsivity: Release your horses. Ann Neurol 66: , 817–824. |
[31] | Aulická ŠR , Jurák P , Chládek J , Daniel P , Halámek J , Baláž M , Bočková M , Chrastina J , Rektor I ((2014) ) Subthalamic nucleus involvement in executive functions with increased cognitive load: A subthalamic nucleus and anterior cingulate cortex depth recording study. J Neural Transm 121: , 1287–1296. |
[32] | Georgiev D , Dirnberger G , Wilkinson L , Limousin P , Jahanshahi M ((2016) ) In Parkinson’s disease on a probabilistic Go/NoGo task deep brain stimulation of the subthalamic nucleus only interferes with withholding of the most prepotent responses. Exp Brain Res 234: , 1133–1143. |
[33] | Alexander GE , Crutcher MD , DeLong MR ((1990) ) Basal ganglia-thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res 85: , 119–146. |
[34] | Burdick AP , Foote KD , Wu S , Bowers D , Zeilman P , Jacobson CE , Ward HE , Okun MS ((2011) ) Do patient’s get angrier following STN, GPi, and thalamic deep brain stimulation. Neuroimage 54: (Suppl 1), S227–232. |
[35] | Enrici I , Mitkova A , Castelli L , Lanotte M , Lopiano L , Adenzato M ((2017) ) Deep Brain Stimulation of the subthalamic nucleus does not negatively affect social cognitive abilities of patients with Parkinson’s disease. Sci Rep 7: , 9413. |
[36] | Boel JA , Odekerken VJJ , Geurtsen GJ , Schmand BA , Cath DC , Figee M , van den Munckhof P , de Haan RJ , Schuurman PR , de Bie RMA , NSTAPS study group ((2016) ) Psychiatric and social outcome after deep brain stimulation for advanced Parkinson’s disease. Mov Disord 31: , 409–413. |
[37] | Baumann-Vogel H , Bodenmann G , Schmid J , Waldvogel D , Ineichen C , Baumann CR ((2020) ) Partners’ view after subthalamic deep brain stimulation: Better relationships despite patients being less active. Clin Parkinsonism Relat Disord 3: , 100052. |
[38] | Albaugh DL , Shih Y-YI ((2014) ) Neural circuit modulation during deep brain stimulation at the subthalamic nucleus for Parkinson’s disease: What have we learned from neuroimaging studies? Brain Connect 4: , 1–14. |
[39] | Temel Y , Blokland A , Steinbusch HWM , Visser-Vandewalle V ((2005) ) The functional role of the subthalamic nucleus in cognitive and limbic circuits. Prog Neurobiol 76: , 393–413. |
[40] | Temel Y , Kessels A , Tan S , Topdag A , Boon P , Visser-Vandewalle V ((2006) ) Behavioural changes after bilateral subthalamic stimulation in advanced Parkinson disease: A systematic review. Parkinsonism Relat Disord 12: , 265–272. |
[41] | Duffy JD , Campbell JJ ((1994) ) The regional prefrontal syndromes: A theoretical and clinical overview. J Neuropsychiatry Clin Neurosci 6: , 379–387. |
[42] | Afshar H , Roohafza HR , Keshteli AH , Mazaheri M , Feizi A , Adibi P ((2015) ) The association of personality traits and coping styles according to stress level. J Res Med Sci 20: , 353–358. |
[43] | Carlson SM , Zelazo PD , Faja S ((2013) ) Executive function. In The Oxford Handbook of Developmental Psychology, Vol. 1, Zelazo PD, ed. Oxford University Press, pp. 705–743. |
[44] | Horn A , Fox MD ((2020) ) Opportunities of connectomic neuromodulation. Neuroimage 221: , 117180. |




