Comparison of Different Platform Immunoassays for the Measurement of Plasma Alpha-Synuclein in Parkinson’s Disease Patients
Abstract
Background:
The identification of reliable biomarkers in Parkinson’s disease (PD) would provide much needed diagnostic accuracy, a means of monitoring progression, objectively measuring treatment response, and potentially allowing patient stratification within clinical trials. Whilst the assessment of total alpha-synuclein in biofluids has been identified as a promising biomarker, conflicting trends in these levels across patient plasma samples relative to controls has limited its use. Different commercially available assay platforms that have been used to measure alpha-synuclein may contribute to different study outcomes.
Objective:
To compare different platform immunoassays for the measurement of total alpha-synuclein using the same plasma samples from 49 PD patients and 47 controls.
Methods:
Total plasma alpha-synuclein concentrations were assessed using the BioLegend, MesoScale Discovery, and Quanterix platform in plasma samples from PD patients and matched controls.
Results:
A significant increase in total plasma alpha-synuclein was observed in PD patients using the Biolegend (10%), Mesoscale Discovery (13%) and Quanterix (39%) assays. The Mesoscale Discovery and Quanterix assays showed the strongest correlations (r = 0.78, p < 0.0001) with each other, whilst the Quanterix platform demonstrated the lowest variation and highest effect size. Inclusion of age, sex and hemoglobin levels as covariates in the analysis of total alpha-synuclein improved the ability of all three immunoassays to detect a significant difference between patients and controls.
Conclusion:
All three immunoassays were sensitive enough to detect group level differences between PD patients and controls, with the largest effect size observed with the Quanterix assay. These results may help inform assay choices in ongoing clinical trials.
INTRODUCTION
Mutations in the alpha-synuclein protein are a cause of inherited Parkinson’s disease (PD) [1], and alpha-synuclein misfolding and aggregation in brain tissue is considered as the pathological hallmark of disease [2]. Consequently, there is substantial interest in the measurement of alpha-synuclein as a PD biomarker. However, measurement of alpha-synuclein is complicated as it can exist as a monomer, in higher order complexes (e.g., tetramer), in post translationally modified forms (e.g., phosphorylated, nitrosylated), and in aggregated pathogenic forms (oligomers of different sizes and fibrils) [3–6]. While there is merit in detecting different forms of alpha-synuclein, assays to detect the post translationally modified and aggregated forms of alpha-synuclein in human biofluids remain largely in development (e.g., [7–20]), and/or have not yet been cross-validated by multiple research groups.
In contrast, assays to detect total alpha-synuclein are established and cross-validated [21]. Many studies have measured the concentration of total alpha-synuclein in cerebrospinal fluid (CSF), with the general finding that alpha-synuclein levels are reduced in PD patient CSF [22–28], leading to the inference that this results from increased accumulation of alpha-synuclein in PD brain tissue. However, the invasive nature of CSF collection, as well as limited accessibility in clinical settings, particularly in the context of a longitudinal study, has led to a desire for blood-based biomarkers [29]. Indeed, alpha-synuclein is present in plasma [30] and has been reported as higher in PD patient plasma using the newer technology ultrasensitive single molecule array (SIMOA) platform [31]. Longitudinal assessment of total alpha-synuclein also suggests its levels increase over time and it may be useful as a biomarker of disease progression [32]. Furthermore, changes in serum alpha-synuclein have been used to demonstrate efficacy in early clinical trials of alpha-synuclein antibody therapies indicating clinical utility in monitoring disease progression [33]. However, conflicting trends in total alpha-synuclein levels in PD patients relative to controls have been reported [19, 29–34]. At least one possibility for the discrepancies observed across different studies may be the sensitivity and accuracy of the assay platforms that are used to measure alpha-synuclein.
In addition to assay sensitivity, pre-analytical factors, limited age and sex matching and consideration of hemoglobin levels, have been identified as cofounders to the measurement of total alpha-synuclein [29, 38], complicating method comparisons between different cohort studies. Red blood cells, in particular, are a major source of endogenous alpha-synuclein, accounting for more than 99% of alpha-synuclein in whole blood [44]. Any red blood cell lysis during blood collection and/or processing can therefore lead to artificially elevated plasma alpha-synuclein levels. Controlling for potential red blood cell lysis, as assessed by hemoglobin levels, has therefore been suggested, yet not mentioned in most studies [29]. Therefore, the present study aimed to compare the ability of three independent assays of increasing sensitivity, namely the BioLegend sandwich ELISA, the Mesoscale Discovery (MSD) electrochemiluminescence ELISA, and Quanterix single molecule counting (SIMOA) assay respectively, to determine plasma levels of total alpha-synuclein in a common sample set. We hypothesised that increasing assay sensitivity would increase group level differences in total alpha-synuclein between PD and control samples.
MATERIALS AND METHODS
Plasma samples
Plasma aliquots from a prior study conducted by our group to investigate glucocerebrosidase activity in PD patients were used [39]. Participants were recruited with ethical approval from the University of Sydney Human Research Ethics Committee (#2016/363) and with informed consent. Venous blood was collected into 8 ml CPT vacutainers (BD Biosciences) in a non-fasted state, and centrifuged at 1800×g for 20 min at room temperature as previously described [39]. Plasma was then collected, snap-frozen into aliquots and stored at –80°C.
Participant details
PD cases were classified according to clinically established criteria [40], whereas controls were age and sex matched participants with no neurological, psychiatric or immunological conditions, and with no first-degree relatives diagnosed with PD. Demographic and clinical data are shown in Table 1.
Table 1
Demographic and clinical details. Demographic and clinical details of the participants who donated the plasma samples used in this study. Data are mean±standard error, with the range shown in parentheses. Disease severity was recorded using the Hoehn and Yahr (H&Y) scale. LEDD, L-Dopa equivalent daily dose; Hgb, hemoglobin; NA, not applicable
| Control (n = 47) | PD (n = 49) | p | |
| Age (y) | 64±1.1 (52–82) | 67±1.2 (45–87) | 0.074 |
| Sex (% M) | 53% | 65% | 0.231 |
| Years since diagnosis | N/A | 4±0.3 (0–8) | |
| Age at diagnosis (y) | N/A | 64±1.3 (44–87) | |
| H&Y stage | N/A | 2±0.1 (1–3) | |
| LEDD | N/A | 600±43 (38–1445) | |
| Hgb (μg/ml) | 53.6±8.3 (11.7–266.1) | 46±5 (5.3–214.4) | 0.680 |
Assessment of hemoglobin
Plasma hemoglobin (Hgb) levels were determined using a Human Hemoglobin ELISA Kit (Abcam; cat# ab157707) as per manufacturer instructions using a 1:1000 dilution of plasma.
Assessment of total alpha-synuclein using the BioLegend assay
We previously measured total alpha-synuclein levels using the LEGEND MAXtrademark Human α-Synuclein ELISA Kit (BioLegend; Cat. No. 844101) [39]. The assay was performed according to the manufacturer instructions. In brief, diluted plasma samples (1:40) were added into antibody coated wells and incubated overnight at 4°C. Plates were then washed and incubated with a biotinylated primary antibody for 2 h at room temperature. After an additional wash, plates were incubated with streptavidin-HRP for 1 h at room temperature. Following a final wash, chemiluminescent substrate was added, and luminescence was measured using a CLARIOstar plate reader (BMG Labtech). A 7-point calibration curve was also added to each plate. The dynamic range was 6.1–1500 pg/ml, and a 4-parameter curve fit was used to determine total alpha-synuclein concentrations in the plasma samples. All calibration points and samples were performed in duplicate with the average of the duplicates used in the final analysis. Further details are provided in Table 2.
Table 2
Characteristics of the assays used to measure plasma alpha-synuclein levels. Available details are provided to allow comparison of the different assays used to measure alpha-synuclein
| BioLegend | MesoScale | Quanterix | |
| Cat. No. | 844101 | K151WKP-2 | 102233 |
| Analyte | Total alpha-synuclein | Total alpha-synuclein | Total alpha-synuclein |
| Assay format | Sandwich immunoassay | Sandwich immunoassay | 2-step digital immunoassay |
| Detection type | Luminescence | Electro-chemiluminescence | Fluorescence |
| Instrument | CLARIOstar | MesoQuick Plex | Quanterix HD-1 |
| Automated | – | – | + |
| Capture Antibody (epitope) | Monoclonal (118–122) | Rabbit Monoclonal (110 to 125) | monoclonal mouse IgG Clone ADx301* (110–120) |
| Detection Antibody (epitope) | Monoclonal (103–108) | Mouse Monoclonal (epitope has not been mapped; it binds between 15 and 125) | Monoclonal mouse IgG, Clone: ADx302 coupled with biotin*(90–100) |
| Calibrator | Recombinant | Recombinant | Recombinant |
| Calibrator dynamic range | 6.1–1500 pg/ml | 0–10 500 pg/mL | 0–10 000 pg/mL |
| LLOD Median (range) | – | 0.900 pg/mL (0.464–1.68) | 0.955 pg/ml (0.202–1.76) |
| LLOQ | – | 8.00 pg/mL | 4.12 pg/ml, |
| ULOQ | – | 6 800 pg/mL | 10 000 pg/mL |
| Specificity/ Cross reactivity | Specific recognition of total alpha-synuclein. No cross-reactivity with beta or gamma synuclein | Specific recognition of total alpha-synuclein. R < 0.05% cross reactivity with beta or gamma synuclein | Specific recognition of total alpha-synuclein. No information about cross reactivity with beta/gamma synuclein |
Assessment of total alpha-synuclein using the MSD assay
Total plasma alpha-synuclein was additionally assessed using the U-PLEX Plus Human alpha-synuclein kit (Mesoscale Discovery; Cat. No. K151WKP-2), according to the manufacturer instructions. In brief, MSD GOLDTM Small Spot Streptavidin plates were coated with a biotinylated capture antibody and incubated for 1 h at room temperature (with shaking at 700 rpm). Plates were then washed three times with provided wash buffer, and a detection antibody conjugated with electrochemiluminescence (ECL) label (MSD GOLD SULFO-TAGtrademark) was added. Immediately after, diluted samples (1:8) were added, and the mixture was incubated for 2 h at room temperature with shaking at 700 rpm. Plates were then washed three times, diluted Read Buffer was added, and the plate was read using a MesoQuick Plex SQO 120 (Mesoscale Discovery). An 8-point calibration curve and 3 quality controls, provided in the kit were added to each plate. The calibrator ranged from 0–10,500 pg/mL; concentrations of alpha-synuclein in samples and controls were interpolated from the calibration curve using a 4-parameter logistic curve fit (1/Y2 weighted). All calibration points, samples and quality controls were performed in duplicate. Further details are provided in Table 2.
Assessment of total alpha-synuclein using the Quanterix assay
Total plasma alpha-synuclein was further assessed using the ultrasensitive single molecule array (SIMOA) Human Alpha-Synuclein DISCOVERY KIT (Quanterix; Cat. No. 102233) and Quanterix HD-1 Analyzer (Quanterix). Plasma aliquots were sent to GeneWorks (Adelaide, SA) on dry ice with the Quanterix assay performed on a fee for service basis following the manufacturer instructions. Briefly, anti-alpha-synuclein monoclonal antibody coated paramagnetic beads were incubated with thawed plasma samples (1:10 dilution) and biotin labelled detection antibody to allow the simultaneous binding of plasma alpha-synuclein molecules with antibody coated beads and detection antibody. Following a wash with Discovery Bead Reagent added to Bead Stock, streptavidin beta-galactosidase (SBG) was added and mixed, to allow binding of the detection antibody to the immunocomplex on beads. After a second wash, beads were resuspended in a resorufin beta-D-galactopyranoside (RGP) substrate solution, loaded onto the array, and sealed with oil. In the presence of beta-galactosidase, RGP substrate is hydrolysed into a fluorescent product that provides the signal for measurement. A 7-point calibration curve and two quality control samples were included. The calibration curve ranged from 0–10,000 pg/mL; concentrations of alpha-synuclein in samples and controls were determined from the calibration curve using a cubic curve fit (1/Y2 weighted). All calibrator points, samples and controls were performed in duplicate. Further details are provided in Table 2.
Statistical analysis
Where applicable, concentration values for Hgb and alpha-synuclein were log transformed to achieve a normal distribution as assessed by Shapiro-Wilk test (p > 0.05). Analysis of variance (ANOVA) was used to determine group level differences of age, sex and Hgb levels. Univariate analyses covarying for age, sex and Hgb concentration were used to determine differences between controls and PD patients. Correlational analyses were performed using Pearson or Spearman correlations as indicated. In all cases, significance was determined as p < 0.05. All statistical analysis was performed using the IBM SPSS Statistics 26 software. All plots were generated using GraphPad Prism v 8.4.3.
RESULTS
Measurement of total alpha-synuclein levels in plasma samples from PD patients and controls using three independent assays
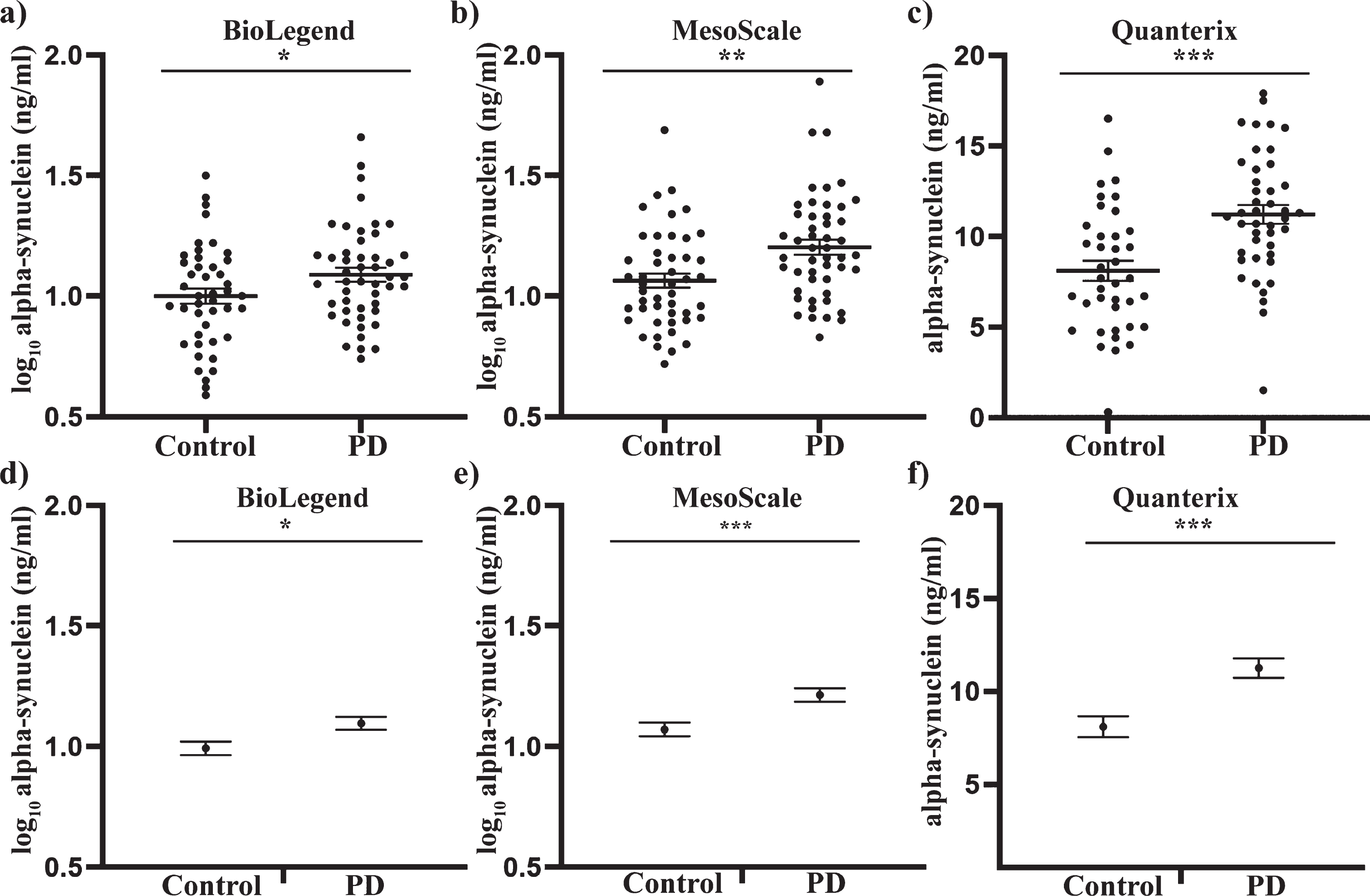
Analysis of the demographic data showed that the PD and control participants were matched for age and sex. There was also no significant difference in the Hgb levels between the groups (Table 1). An independent samples t-test was first performed to determine if alpha-synuclein differed between PD patients and controls. Alpha-synuclein was significantly increased in PD samples compared to controls with the BioLegend assay (8% increase, p < 0.05, Fig. 1a), MSD assay (13% increase, p < 0.01, Fig. 1b) and the Quanterix assay (39% increase, p < 0.001, Fig. 1c). To determine if a significant difference could still be observed when factoring in potential confounders, univariate analysis covarying for age, sex and Hgb levels was performed, as these covariates in particular can affect alpha-synuclein concentrations on an individual level [41]. Following univariate analysis, alpha-synuclein was still significantly increased in PD patient samples compared to controls with the Biolegend assay (10% increase, p < 0.05, Fig. 1d), MSD assay (13% increase, p < 0.001, Fig. 1e) and the Quanterix assay (39% increase, p < 0.001, Fig. 1d). Neither age nor sex had a significant effect on alpha-synuclein levels in any analysis (all p > 0.05), whereas hemoglobin levels had a significant effect in both the Biolegend and MSD assay (both p < 0.001), but not the Quanterix assay (p > 0.05). The inclusion of the covariates however, improved the p-value for discriminating between the two groups for all three assay platforms.
Fig. 1
Total alpha-synuclein concentrations in plasma samples of PD patients and healthy controls, using three independent assays. Where applicable, data were transformed to a log scale to achieve normality. An independent sample t-test revealed a significant increase in total alpha-synuclein in PD plasma samples relative to the control group, as determined using the Biolegend (a), MesoScale (b) and Quanterix (c) assays. Individual value graphs display mean±SEM. Univariate analysis was then used to compare control and PD alpha-synuclein concentrations after covarying for age, sex and Hgb levels. Post-analysis graphs display the estimated marginal mean±SEM. Significant differences between the control and PD groups were measured for the BioLegend (d), MesoScale (e) and Quanterix (f) assays. *p < 0.05, **p < 0.01, ***p < 0.001.
Comparison of total alpha-synuclein levels in plasma samples as determined using BioLegend, MSD and Quanterix assay
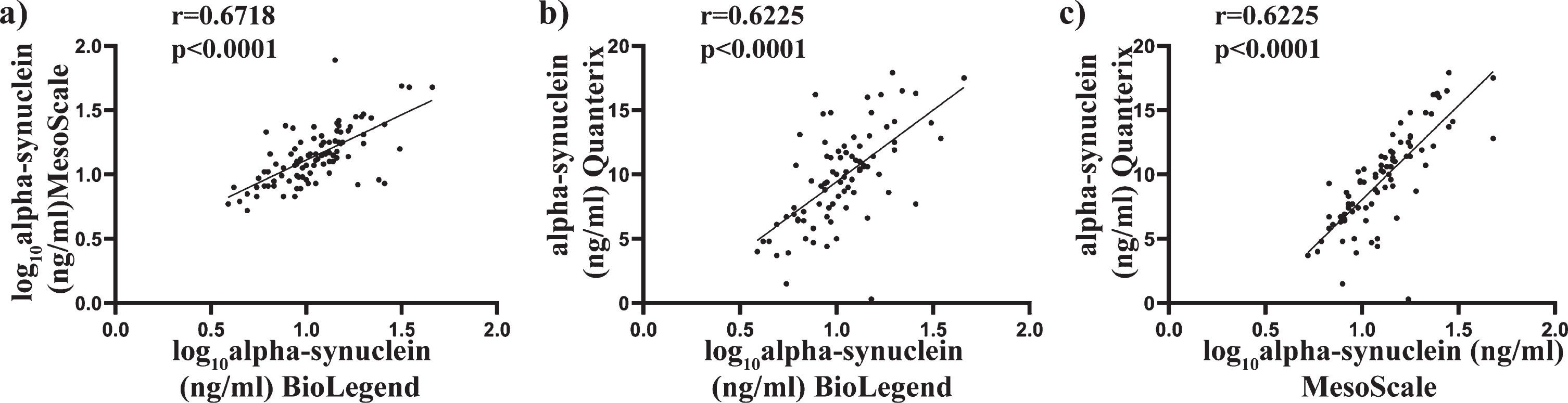
Scatter plots of alpha-synuclein concentration using the average of the duplicates were used to compare the assays against each other (Fig. 2a-c). Transformed data were used where applicable, with Pearson’s correlation used to determine associations between alpha-synuclein concentrations across all three assays. A significant positive correlation was found between the BioLegend assay and the MSD assay (r = 0.6718, p < 0.000, Fig. 2a), the BioLegend and the Quanterix (r = 0.6255, p < 0.0001, Fig. 2b), and the MSD and Quanterix (r = 0.7822, p < 0.0001, Fig. 2c). These results suggest a high level of correlation between the different assay platforms, giving confidence in the measurements.
Fig. 2
Comparison of total alpha-synuclein levels in plasma samples as determine using BioLegend, MSD and Quanterix assay. Scatter plots showing correlations between total alpha-synuclein (pg/mL) measured in the same patient plasma samples. Where required, data were transformed to achieve normality. Pearson analysis was used to assess significance at the 0.05 level. A significant correlation was observed between the BioLegend and MesoScale assay (a), BioLegend and Quanterix assay (b) and MesoScale and Quanterix assay (c).
Relationship between total alpha-synuclein concentrations and age, Hgb, disease severity, disease duration and L-Dopa equivalent daily dose across three independent assays
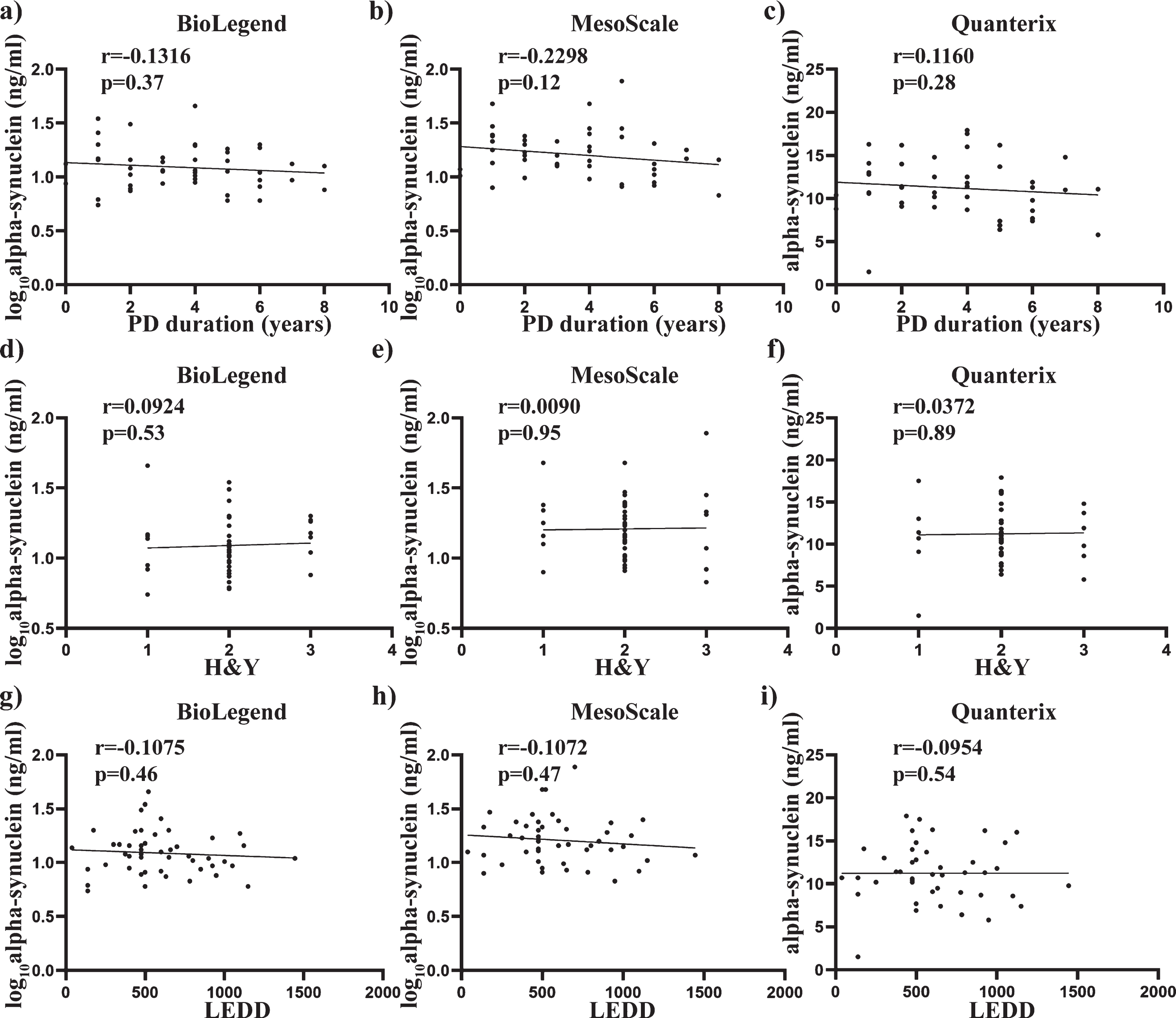
Pearson’s correlation was also used to determine any significant associations between alpha-synuclein concentrations and the age and Hgb covariates used across all three assays. No significant correlation with age was observed across all three assays (all p > 0.05) (Fig. 3 a-c), whereas alpha-synuclein levels showed a significant positive correlation with Hgb with the BioLegend assay (r = 0.4426, p < 0.0001, Fig. 3d), MSD assay (r = 0.4669, p < 0.0001, Fig. 3e) and the Quanterix assay (r = 0.2291, p < 0.05, Fig. 3f). Spearman’s correlation was used to assess the relationship between alpha-synuclein levels and the clinical measures across all three assays. No significant correlation with age at diagnosis, years since diagnosis, H&Y score or L-Dopa equivalent daily dose was observed across all three assays (all p > 0.05. Fig. 4a-i).
Fig. 3
Relationship between total alpha-synuclein concentrations and covariates age and Hgb across all three independent assays. Scatter plots showing correlations between total alpha-synuclein (pg/mL) and covariates age and Hgb (ug/mL). Pearson analysis was used to assess significance at the 0.05 level. No significant correlations were observed between age and total alpha-synuclein measured using the BioLegend (a), MesoScale (b) or Quanterix (c) assay. A significant correlation was observed between plasma levels of hemoglobin and total alpha-synuclein measured using the Biolegend (e), MesoScale (e) and Quanterix (f) assays
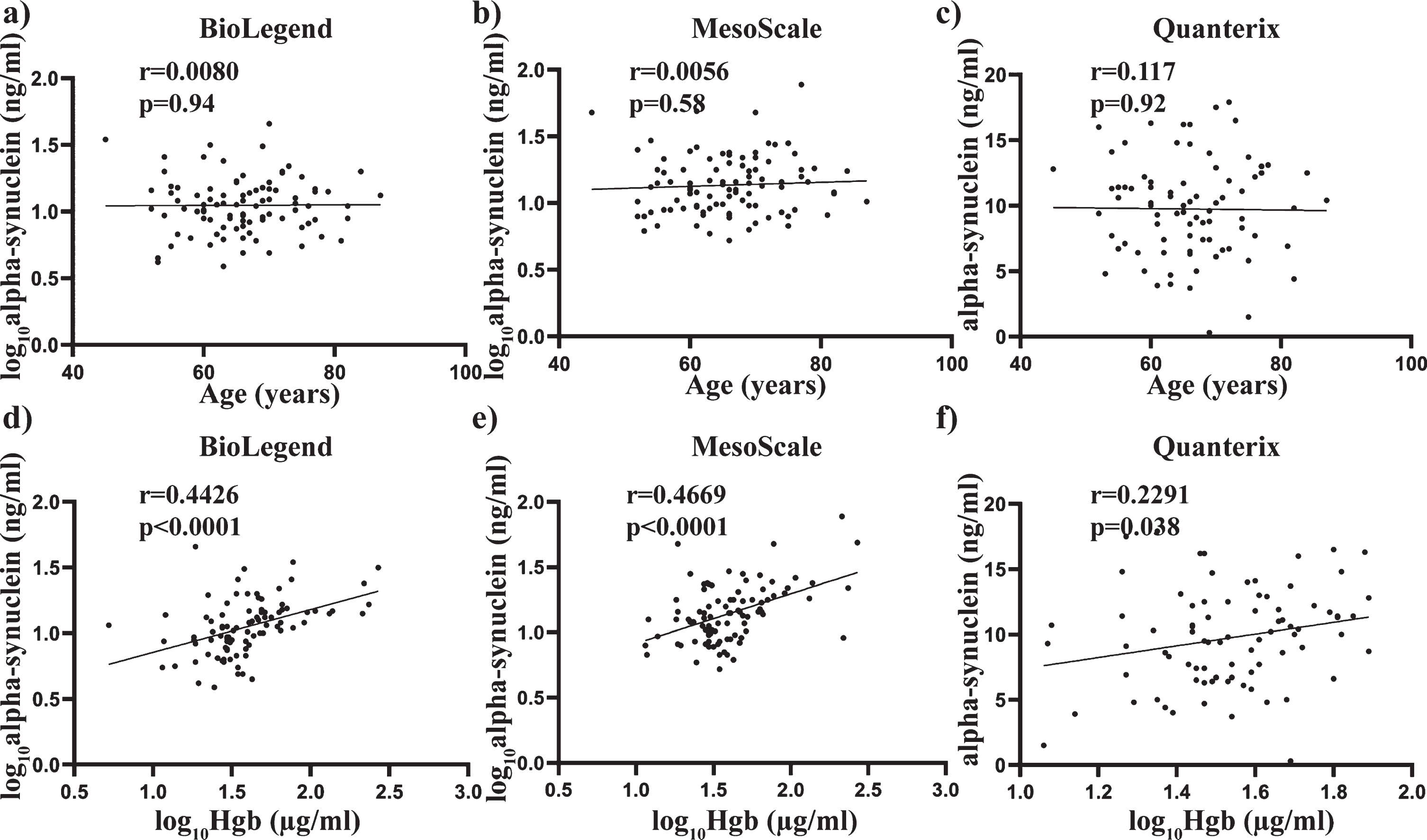
Fig. 4
Relationship between total alpha-synuclein concentrations and clinical measures, disease duration and H&Y scores. Scatter plots show correlations between total alpha-synuclein (pg/mL), disease duration (years) and H&Y scores. Spearman analysis was used to assess significance at the 0.05 level. No significant correlations were found between disease duration and total alpha-synuclein measured using BioLegend (a), MesoScale (b) and Quanterix (c) assays. No significant correlations were also found between H&Y scale and total alpha-synuclein measured using BioLegend (d), MesoScale (e) and Quanterix (f) assays. No significant correlations were further found between LEDD and total alpha-synuclein measured using BioLegend (g), MesoScale (h) and Quanterix (i) assays.
DISCUSSION
Alpha-synuclein is a key protein implicated in the pathogenesis of PD and has been described as a promising biomarker candidate. Since its identification in peripheral body fluids, there has been much
interest in the measurement of total alpha-synuclein in plasma samples as a favourable alternative to the collection of CSF in clinical settings. In the present study, we aimed to compare the performance of three independent assays of increasing reported sensitivity for the measurement of total alpha-synuclein in plasma samples: the BioLegend, MSD and Quanterix assays respectively. A common sample set was used to ensure that potential pre-analytical cofounders, including sample collection and processing and storage conditions [38], did not interfere with the study objective. All assays were commercially available and designed with specificity for alpha-synuclein, although the exact extent to which different forms of alpha-synuclein can be detected by the different assay platforms is unknown. Post-translational modification of alpha-synuclein may also affect detection in the different assay platforms. However, none of antibody epitope regions overlapped at least with the serine 129 phosphorylation site, the most common disease-associated post-translational modification site in alpha-synuclein. Included quality controls further indicated that all immunoassays performed as expected with a high degree of positive correlation seen across all the assays for the measured levels of alpha-synuclein. These results suggest that despite using different assay chemistries and antibodies, all of the utilised assays were reliably detecting total alpha-synuclein. Interestingly, the p-value for discriminating PD patients from controls was markedly stronger for the Quanterix assay compared to the others. However, this improved result with the Quanterix assay was likely attributable to the reduced variance found with this assay rather than an increased sensitivity per se. A greater effect size for increased alpha-synuclein in the PD patients was also observed with the Quanterix assay. Why this occurred remains unknown but may be related to the above in that the increased sensitivity of this platform may facilitate the detection of different/lower abundant forms of alpha-synuclein.
Interestingly, we also noted improved p-values for discriminating PD patients from control across all three immunoassays when age, sex and Hgb levels were included as covariates in the analysis. The use of covariates as a statistical control for potential confounders is not always employed in the analysis of plasma alpha-synuclein levels [31, 32, 37, 42], and this in part, may contribute to inconsistencies reported in the literature [29, 41, 43]. In particular, Hgb levels were found to be significantly correlated with total alpha-synuclein levels across all three immunoassays in the current study. It is known that Hgb in plasma comes predominantly from the lysis of red blood cells during plasma collection, and given that red blood cells are a major source of alpha-synuclein in blood [44], any red blood cell lysis during blood collection and/or processing could consequently lead to artificially elevated plasma alpha-synuclein levels. Therefore, it is important to include the measurement of Hgb when interpreting plasma alpha-synuclein data. Total-alpha synuclein has also been assessed directly in red blood cells and reported as significantly higher in PD patients relative to healthy controls, which may circumvent the impact of hemolysis on plasma measures [45, 46]. Although increases in total alpha-synuclein in red blood cells may be associated with the membrane fraction, and it has been suggested that fractionation should be considered when interpreting results [47]. Additionally, side-by-side comparison of plasma and erythrocyte alpha-synuclein levels suggest that plasma alpha-synuclein may better correlate with clinical measures of PD [46].
Our finding of elevated alpha-synuclein in PD plasma samples is in agreement with the study of Ng et al. [31], who found a 16% increase in PD plasma using the SIMOA platform and controlling for age and sex as covariates. Additional studies using earlier platforms, including colormetric ELISA assays, have also demonstrated increases in total alpha-synuclein in plasma samples of PD patients relative to controls [37, 48], although conflicting studies using similar platforms also exist [42]. In light of our findings, this suggests that consideration of pre-analytical factors, including Hgb levels and cohort demographics may become even more important when platforms of lower sensitivity are being used. It is, however, noteworthy that even with the inclusion of covariates, the effect size of increased alpha-synuclein in PD plasma is relatively small, suggesting that total plasma alpha-synuclein levels may not make a definitive diagnostic biomarker on their own. Indeed, several lines of evidence have suggested that pathological forms of alpha-synuclein, including oligomeric and phosphorylated species may be better diagnostic aids, and demonstrate heightened sensitivity when normalised to total alpha-synuclein [49–52], although these have not yet been cross-validated by multiple groups. The diagnostic potential of total alpha-synuclein in plasma may therefore lie in its ability to normalise the measurement of toxic alpha-synuclein species, or as part of a biomarker panel, rather than as a stand-alone measure. Additionally, the assessment of alpha-synuclein to promote fibrillar aggregation using real-time quaking induced conversion (RT-Quic) and/or protein misfolding cyclic amplification (PMCA) is currently showing diagnostic promise for PD [12, 14–16, 53], albeit currently limited to CSF. Finally, the molecular basis of why plasma alpha-synuclein is increased in PD patients remains unclear. In this regard, plasma alpha-synuclein has been suggested to result from efflux from CSF [54]. However, there is also support that plasma alpha-synuclein may originate from peripheral tissues such as the enteric plexus which is also pathologically affected in PD [55, 56].
When assessed against clinical measures, no significant findings were identified with either disease duration or H&Y score across all three immunoassays. These findings could be attributed to the very small range of patients within these measures in our study as H&Y score has previously been correlated with plasma alpha-synuclein levels using the SIMOA platform [12]. In particular, an increase in alpha-synuclein levels were observed in milder stages of disease (H&Y 1-2), with a decrease in detectable total alpha-synuclein observed across later disease stages (H&Y 3-4). The authors proposed that this observation might be due to increased c-terminal truncation and aggregation in the later stages, which would escape detection in the current assays targeting the c-terminal of alpha-synuclein [31]. These findings support the longitudinal study by Foulds et al., who reported total alpha-synuclein as a potential marker of disease progression in milder stages of PD (H&Y 1-2) [32]. Increased levels of alpha-synuclein have also been measured in exosomes derived from the plasma of PD patients, and found to correlate with motor progression [57]. In contrast to the limited longitudinal studies using plasma samples, several groups have reported longitudinal assessment of total alpha-synuclein in CSF samples using different PD patient cohorts. From this work, most studies report no significant change in total alpha-synuclein in CSF overtime, suggesting this is not a marker of disease progression [58–61], although conflicting studies again exist suggesting an increase in CSF alpha-synuclein with PD [18, 62], or even a significant decrease [63]. Importantly however, total alpha-synuclein levels in CSF do not appear to correlate with total alpha-synuclein levels in corresponding plasma samples [64], suggesting that conclusions drawn from longitudinal studies using CSF may not directly apply to the ability of plasma alpha-synuclein to serve as a disease progression biomarker for PD. Thus, further longitudinal assessment of plasma alpha-synuclein is of merit, and an obvious limitation in our current cross-sectional work.
To the best of our knowledge, this is the first study to directly compare total plasma alpha-synuclein levels using the BioLegend, MSD and Quanterix assays. While the Quanterix assay was superior in detecting the highest group level difference of total alpha-synuclein PD and control plasma samples, the MSD assay performed similarly and may represent a suitable alternative in discriminating total alpha-synuclein plasma levels in PD patients from controls in settings where the SIMOA platform may not be feasible and/or accessible. Limitations to the current study include that we did not assess inter or intra assay variation to compare assay performance between batches or over time. The study also did not address the specificity of increased alpha-synuclein for PD, and this could be done by comparing to other neurodegenerative diseases such as Alzheimer’s disease. Whilst our study suggests that all three assays were sensitive enough to discriminate PD patients from controls, a larger PD cohort with a wider clinical range and data with a greater range of scores (such as MDS UPDRS motor scores and cognitive assessments) would have expanded the study by enabling the assessment of each immunoassay according to its progressive biomarker potential.
ACKNOWLEDGMENTS
This project received funding from the Australian Government as part of Australian Parkinson’s Mission, a collaboration between the Garvan Institute of Medical Research, The University of Sydney, The Cure Parkinson’s trust, The Michael J Fox Foundation and the Shake It Up Australia Foundation. SJGL is supported by an NHMRC Leadership Fellowship (#1195830). GMH holds a NHMRC senior leadership fellowship (#176607). The Dementia and Movement Disorders Laboratory is supported by ForeFront, a collaborative research group dedicated to the study of non-Alzheimer disease degenerative disorders, funded by NHMRC grants (#1037746, #1095127 and #1132524).
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare
REFERENCES
[1] | Polymeropoulos MH , Lavedan C , Leroy E , Ide SE , Dehejia A , Dutra A , Pike B , Root H , Rubenstein J , Boyer R , Stenroos ES , Chandrasekharappa S , Athanassiadou A , Papapetropoulos T , Johnson WG , Lazzarini AM , Duvoisin RC , Di Iorio G , Golbe LI , Nussbaum RL ((1997) ) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276: , 2045–2047. |
[2] | Meade RM , Fairlie D , Mason J ((2019) ) Alpha-synuclein structure and Parkinson’s disease lessons and emerging principles. Mol Neurodegener 14: , 29. |
[3] | Lucas H , Fernández R ((2020) ) Navigating the dynamic landscape of alpha-synuclein morphology: A review of the physiologically relevant tetrameric conformation. Neural Regen Res 15: , 407–415. |
[4] | Stefanis L ((2012) ) α-Synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med 2: , a009399. |
[5] | Ingelsson M ((2016) ) Alpha-synuclein oligomers—neurotoxic molecules in Parkinson’s disease and other Lewy body disorders. Front Neurosci 10: , 408. |
[6] | Zhang J , Li X , Li J-D ((2019) ) The roles of post-translational modifications on α-synuclein in the pathogenesis of Parkinson’s diseases. Front Neurosci 13: , 381. |
[7] | El-Agnaf OM , Salem SA , Paleologou KE , Curran MD , Gibson MJ , Court JA , Schlossmacher MG , Allsop D ((2006) ) Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J 20: , 419–425. |
[8] | Tokuda T , Qureshi MM , Ardah MT , Varghese S , Shehab SA , Kasai T , Ishigami N , Tamaoka A , Nakagawa M , El-Agnaf OM ((2010) ) Detection of elevated levels of α-synuclein oligomers in CSF from patients with Parkinson disease. J Neurol 75: , 1766–1772. |
[9] | Candelise N , Baiardi S , Franceschini A , Rossi M , Parchi P ((2020) ) Towards an improved early diagnosis of neurodegenerative diseases: The emerging role of in vitro conversion assays for protein amyloids. Acta Neuropathol Commu 8: , 117. |
[10] | Landeck N , Hall H , Ardah MT , Majbour NK , El-Agnaf OMA , Halliday G , Kirik D ((2016) ) A novel multiplex assay for simultaneous quantification of total and S129 phosphorylated human alpha-synuclein. Mol Neurodegener 11: , 61. |
[11] | Cariulo C , Martufi P , Verani M , Azzollini L , Bruni G , Weiss A , Deguire SM , Lashuel HA , Scaricamazza E , Sancesario GM , Schirinzi T , Mercuri NB , Sancesario G , Caricasole A , Petricca L ((2019) ) Phospho-S129 alpha-synuclein is present in human plasma but not in cerebrospinal fluid as determined by an ultrasensitive immunoassay. Front Neurosci 13: , 889. |
[12] | Bhumkar A , Magnan C , Lau D , Soh Wei Jun E , Dzamko N , Sierecki E , Gambin Y ((2021) ) Single molecule counting coupled to rapid amplification enables detection of αSynuclein aggregates in cerebrospinal fluid of PD patients. Angew Chem Int Ed Engl 60: , 11874–11883. |
[13] | Shahnawaz M , Tokuda T , Waragai M , Mendez N , Ishii R , Trenkwalder C , Mollenhauer B , Soto C ((2017) ) Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol 74: , 163–172. |
[14] | van Rumund A , Green AJE , Fairfoul G , Esselink RAJ , Bloem BR , Verbeek MM ((2019) ) α-Synuclein real-time quaking-induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Ann Neurol 85: , 777–781. |
[15] | Groveman BR , Orrú CD , Hughson AG , Raymond LD , Zanusso G , Ghetti B , Campbell KJ , Safar J , Galasko D , Caughey B ((2018) ) Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol Commun 6: , 7. |
[16] | Fairfoul G , McGuire LI , Pal S , Ironside JW , Neumann J , Christie S , Joachim C , Esiri M , Evetts SG , Rolinski M , Baig F , Ruffmann C , Wade-Martins R , Hu MT , Parkkinen L , Green AJ ((2016) ) Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 3: , 812–818. |
[17] | Jang SJ , Lee CS , Kim TH ((2020) ) α-Synuclein oligomer detection with aptamer switch on reduced graphene oxide electrode. Nanomaterials (Basel) 10: , 832. |
[18] | Majbour NK , Vaikath NN , Eusebi P , Chiasserini D , Ardah M , Varghese S , Haque ME , Tokuda T , Auinger P , Calabresi P , Parnetti L , El-Agnaf OM ((2016) ) Longitudinal changes in CSF alpha-synuclein species reflect Parkinson’s disease progression. Mov Disord 31: , 1535–1542. |
[19] | Wang Y , Shi M , Chung KA , Zabetian CP , Leverenz JB , Berg D , Srulijes K , Trojanowski JQ , Lee VM , Siderowf AD , Hurtig H , Litvan I , Schiess MC , Peskind ER , Masuda M , Hasegawa M , Lin X , Pan C , Galasko D , Goldstein DS , Jensen PH , Yang H , Cain KC , Zhang J ((2012) ) Phosphorylated α-synuclein in Parkinson’s disease. Sci Transl Med 4: , 121ra20. |
[20] | O’Hara DM , Kalia SK , Kalia LV ((2020) ) Methods for detecting toxic α-synuclein species as a biomarker for Parkinson’s disease. Crit Rev Clin Lab Sci 57: , 291–307. |
[21] | Mollenhauer B , Bowman FD , Drake D , Duong J , Blennow K , El-Agnaf O , Shaw LM , Masucci J , Taylor P , Umek RM , Dunty JM , Smith CL , Stoops E , Vanderstichele H , Schmid AW , Moniatte M , Zhang J , Kruse N , Lashuel HA , Teunissen C , Schubert T , Dave KD , Hutten SJ , Zetterberg H ((2019) ) Antibody-based methods for the measurement of α-synuclein concentration in human cerebrospinal fluid - method comparison and round robin study. J Neurochem 149: , 126–138. |
[22] | Mollenhauer B , Locascio JJ , Schulz-Schaeffer W , Sixel-Döring F , Trenkwalder C , Schlossmacher MG ((2011) ) α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: A cohort study. Lancet Neurol 10: , 230–240. |
[23] | Tokuda T , Salem SA , Allsop D , Mizuno T , Nakagawa M , Qureshi MM , Locascio JJ , Schlossmacher MG , El-Agnaf OM ((2006) ) Decreased alpha-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson’s disease. Biochem Biophys Res Commun 349: , 162–166. |
[24] | Kang J-H , Irwin DJ , Chen-Plotkin AS , Siderowf A , Caspell C , Coffey CS , Waligórska T , Taylor P , Pan S , Frasier M , Marek K , Kieburtz K , Jennings D , Simuni T , Tanner CM , Singleton A , Toga AW , Chowdhury S , Mollenhauer B , Trojanowski JQ , Shaw LM , Parkinson’s Progression Markers Initiative ((2013) ) Association of cerebrospinal fluid β-amyloid 1-42, T-tau, P-tau181, and α-synuclein levels with clinical features of drug-naive patients with early parkinson disease. JAMA Neurol 70: , 1277–1287. |
[25] | Magdalinou NK , Paterson RW , Schott JM , Fox NC , Mummery C , Blennow K , Bhatia K , Morris HR , Giunti P , Warner TT , de Silva R , Lees AJ , Zetterberg H ((2015) ) A panel of nine cerebrospinal fluid biomarkers may identify patients with atypical parkinsonian syndromes. J Neurol Neurosurg Psychiatry 86: , 1240–1247. |
[26] | Hall S , Öhrfelt A , Constantinescu R , Andreasson U , Surova Y , Bostrom F , Nilsson C , Håkan W , Decraemer H , Någga K , Minthon L , Londos E , Vanmechelen E , Holmberg B , Zetterberg H , Blennow K , Hansson O ((2012) ) Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol 69: , 1445–1452. |
[27] | Mollenhauer B , Trautmann E , Taylor P , Manninger P , Sixel-Döring F , Ebentheuer J , Trenkwalder C , Schlossmacher MG ((2013) ) Total CSF α-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neurosci lett 532: , 44–48. |
[28] | Eusebi P , Giannandrea D , Biscetti L , Abraha I , Chiasserini D , Orso M , Calabresi P , Parnetti L ((2017) ) Diagnostic utility of cerebrospinal fluid α-synuclein in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord 32: , 1389–1400. |
[29] | Atik A , Stewart T , Zhang J ((2016) ) Alpha-synuclein as a biomarker for Parkinson’s disease. Brain Pathol 26: , 410–418. |
[30] | El-Agnaf OM , Salem SA , Paleologou KE , Cooper LJ , Fullwood NJ , Gibson MJ , Curran MD , Court JA , Mann DM , Ikeda S , Cookson MR , Hardy J , Allsop D ((2003) ) Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J 17: , 1945–1947. |
[31] | Ng ASL , Tan YJ , Lu Z , Ng EYL , Ng SYE , Chia NSY , Setiawan F , Xu Z , Tay KY , Prakash KM , Au WL , Tan E-K , Tan LCS ((2019) ) Plasma alpha-synuclein detected by single molecule array is increased in PD. Ann Clin Transl Neurol 6: , 615–619. |
[32] | Foulds PG , Diggle P , Mitchell JD , Parker A , Hasegawa M , Masuda-Suzukake M , Mann DM , Allsop D ((2013) ) A longitudinal study on α-synuclein in blood plasma as a biomarker for Parkinson’s disease. Sci Rep 3: , 2540. |
[33] | Schenk DB , Koller M , Ness DK , Griffith SG , Grundman M , Zago W , Soto J , Atiee G , Ostrowitzki S , Kinney GG ((2017) ) First-in-human assessment of PRX002, an anti-α-synuclein monoclonal antibody, in healthy volunteers. Mov Disord 32: , 211–218. |
[34] | Li QX , Mok SS , Laughton KM , McLean CA , Cappai R , Masters CL , Culvenor JG , Horne MK ((2007) ) Plasma alpha-synuclein is decreased in subjects with Parkinson’s disease. Exp Neurol 204: , 583–588. |
[35] | Smith LM , Schiess MC , Coffey MP , Klaver AC , Loeffler DA ((2012) ) α-Synuclein and anti-α-synuclein antibodies in Parkinson’s disease, atypical Parkinson syndromes, REM sleep behavior disorder, and healthy controls. PLoS One 7: , e52285. |
[36] | Besong-Agbo D , Wolf E , Jessen F , Oechsner M , Hametner E , Poewe W , Reindl M , Oertel WH , Noelker C , Bacher M , Dodel R ((2013) ) Naturally occurring α-synuclein autoantibody levels are lower in patients with Parkinson disease. J Neurol 80: , 169–175. |
[37] | Duran R , Barrero FJ , Morales B , Luna JD , Ramirez M , Vives F ((2010) ) Plasma α-synuclein in patients with Parkinson’s disease with and without treatment. Mov Disord 25: , 489–493. |
[38] | Mollenhauer B , Batrla R , El-Agnaf O , Galasko DR , Lashuel HA , Merchant KM , Shaw LM , Selkoe DJ , Umek R , Vanderstichele H , Zetterberg H , Zhang J , Caspell-Garcia C , Coffey C , Hutten SJ , Frasier M , Taylor P , Investigating Synuclein Consortium of the Michael J. Fox Foundation for Parkinson’s Research ((2017) ) A user’s guide for α-synuclein biomarker studies in biological fluids: Perianalytical considerations. Mov Disord 32: , 1117–1130. |
[39] | Atashrazm F , Hammond D , Perera G , Dobson-Stone C , Mueller N , Pickford R , Kim WS , Kwok JB , Lewis SJG , Halliday GM , Dzamko N ((2018) ) Reduced glucocerebrosidase activity in monocytes from patients with Parkinson’s disease. Sci Rep 8: , 15446. |
[40] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W , Obeso J , Marek K , Litvan I , Lang AE , Halliday G , Goetz CG , Gasser T , Dubois B , Chan P , Bloem BR , Adler CH , Deuschl G ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30: , 1591–1601. |
[41] | Shi M , Zabetian CP , Hancock AM , Ginghina C , Hong Z , Yearout D , Chung KA , Quinn JF , Peskind ER , Galasko D , Jankovic J , Leverenz JB , Zhang J ((2010) ) Significance and confounders of peripheral DJ-1 and alpha-synuclein in Parkinson’s disease. Neurosci Lett 480: , 78–82. |
[42] | Foulds PG , Mitchell JD , Parker A , Turner R , Green G , Diggle P , Hasegawa M , Taylor M , Mann D , Allsop D ((2011) ) Phosphorylated α-synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson’s disease. FASEB J 25: , 4127–4137. |
[43] | Fayyad M , Salim S , Majbour N , Erskine D , Stoops E , Mollenhauer B , El-Agnaf OMA ((2019) ) Parkinson’s disease biomarkers based on α-synuclein. J Neurochem 150: , 626–636. |
[44] | Barbour R , Kling K , Anderson JP , Banducci K , Cole T , Diep L , Fox M , Goldstein JM , Soriano F , Seubert P , Chilcote TJ ((2008) ) Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 5: , 55–59. |
[45] | Abd Elhadi S , Grigoletto J , Poli M , Arosio P , Arkadir D , Sharon R ((2019) ) α-Synuclein in blood cells differentiates Parkinson’s disease from healthy controls. Ann Clin Transl Neurol 6: , 2426–2436. |
[46] | Wang L , Wang G , Duan Y , Wang F , Lin S , Zhang F , Li H , Li A , Li H ((2019) ) A Comparative study of the diagnostic potential of plasma and erythrocytic α-synuclein in Parkinson’s disease. Neurodegener Dis 19: , 204–210. |
[47] | Tian C , Liu G , Gao L , Soltys D , Pan C , Stewart T , Shi M , Xie Z , Liu N , Feng T , Zhang J ((2019) ) Erythrocytic α-Synuclein as a potential biomarker for Parkinson’s disease. Transl Neurodegener 8: , 15. |
[48] | Lee PH , Lee G , Park HJ , Bang OY , Joo IS , Huh K ((2006) ) The plasma alpha-synuclein levels in patients with Parkinson’s disease and multiple system atrophy. J Neural Transm (Vienna) 113: , 1435–1439. |
[49] | Vaikath NN , Hmila I , Gupta V , Erskine D , Ingelsson M , El-Agnaf OMA ((2019) ) Antibodies against alpha-synuclein: Tools and therapies. J Neurochem 150: , 612–625. |
[50] | Daniele S , Frosini D , Pietrobono D , Petrozzi L , Lo Gerfo A , Baldacci F , Fusi J , Giacomelli C , Siciliano G , Trincavelli ML , Franzoni F , Ceravolo R , Martini C , Bonuccelli U ((2018) ) α-Synuclein heterocomplexes with β-amyloid are increased in red blood cells of Parkinson’s disease patients and correlate with disease severity. Front Mol Neurosci 11: , 53. |
[51] | Parnetti L , Chiasserini D , Persichetti E , Eusebi P , Varghese S , Qureshi MM , Dardis A , Deganuto M , De Carlo C , Castrioto A , Balducci C , Paciotti S , Tambasco N , Bembi B , Bonanni L , Onofrj M , Rossi A , Beccari T , El-Agnaf O , Calabresi P ((2014) ) Cerebrospinal fluid lysosomal enzymes and alpha-synuclein in Parkinson’s disease. Mov Disord 29: , 1019–1027. |
[52] | Hansson O , Hall S , Öhrfelt A , Zetterberg H , Blennow K , Minthon L , Nägga K , Londos E , Varghese S , Majbour NK , Al-Hayani A , El-Agnaf OMA ((2014) ) Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimers Res Therapy 6: , 25. |
[53] | Kang UJ , Boehme AK , Fairfoul G , Shahnawaz M , Ma TC , Hutten SJ , Green A , Soto C ((2019) ) Comparative study of cerebrospinal fluid α-synuclein seeding aggregation assays for diagnosis of Parkinson’s disease. Mov Disord 34: , 536–544. |
[54] | Bates CA , Fu S , Ysselstein D , Rochet JC , Zheng W ((2015) ) Expression and transport of α-synuclein at the blood-cerebrospinal fluid barrier and effects of manganese exposure. ADMET DMPK 3: , 15–33. |
[55] | Braak H , de Vos RA , Bohl J , Del Tredici K ((2006) ) Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396: , 67–72. |
[56] | Klingelhoefer L , Reichmann H ((2015) ) Pathogenesis of Parkinson disease–the gut-brain axis and environmental factors. Nat Rev Neurol 11: , 625–636. |
[57] | Niu M , Li Y , Li G , Zhou L , Luo N , Yao M , Kang W , Liu J ((2020) ) A longitudinal study on α-synuclein in plasma neuronal exosomes as a biomarker for Parkinson’s disease development and progression. Eur J Neurol 27: , 967–974. |
[58] | Mollenhauer B , Caspell-Garcia CJ , Coffey CS , Taylor P , Singleton A , Shaw LM , Trojanowski JQ , Frasier M , Simuni T , Iranzo A , Oertel W , Siderowf A , Weintraub D , Seibyl J , Toga AW , Tanner CM , Kieburtz K , Chahine LM , Marek K , Galasko D , study ftP ((2019) ) Longitudinal analyses of cerebrospinal fluid α-Synuclein in prodromal and early Parkinson’s disease. Mov Disord 34: , 1354–1364. |
[59] | Førland MG , Öhrfelt A , Dalen I , Tysnes O-B , Blennow K , Zetterberg H , Pedersen KF , Alves G , Lange J ((2018) ) Evolution of cerebrospinal fluid total α-synuclein in Parkinson’s disease. Parkinsonism Rel Disord 49: , 4–8. |
[60] | Mollenhauer B , Caspell-Garcia CJ , Coffey CS , Taylor P , Shaw LM , Trojanowski JQ , Singleton A , Frasier M , Marek K , Galasko D ((2017) ) Longitudinal CSF biomarkers in patients with early Parkinson disease and healthy controls. J Neurol 89: , 1959–1969. |
[61] | Mollenhauer B , Zimmermann J , Sixel-Döring F , Focke NK , Wicke T , Ebentheuer J , Schaumburg M , Lang E , Trautmann E , Zetterberg H , Taylor P , Friede T , Trenkwalder C , DeNoPa Study G ((2016) ) Monitoring of 30 marker candidates in early Parkinson disease as progression markers. J Neurol 87: , 168–177. |
[62] | Hall S , Surova Y , Öhrfelt A , Blennow K , Zetterberg H , Hansson O ((2016) ) Longitudinal Measurements of Cerebrospinal Fluid Biomarkers in Parkinson’s Disease. Mov Disord 31: , 898–905. |
[63] | Stewart T , Liu C , Ginghina C , Cain KC , Auinger P , Cholerton B , Shi M , Zhang J , Parkinson Study Group DI ((2014) ) Cerebrospinal fluid α-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am J Pathol 184: , 966–975. |
[64] | Goldman JG , Andrews H , Amara A , Naito A , Alcalay RN , Shaw LM , Taylor P , Xie T , Tuite P , Henchcliffe C , Hogarth P , Frank S , Saint-Hilaire M-H , Frasier M , Arnedo V , Reimer AN , Sutherland M , Swanson-Fischer C , Gwinn K , Fox Investigation of New Biomarker Discovery, Kang UJ ((2018) ) Cerebrospinal fluid, plasma, and saliva in the BioFIND study: Relationships among biomarkers and Parkinson’s disease Features. Mov Disord 33: , 282–288. |




