Quantitative Assessment of Motor Response to a Low Subacute Levodopa Dose in the Differential Diagnosis of Parkinsonisms at Disease Onset: Data from the BoProPark Cohort
Abstract
Background:
Differential diagnosis between Parkinson’s disease (PD) and atypical parkinsonisms (APs) may be difficult at disease onset. The response to levodopa (LD) is a key supportive feature but its definition is largely empirical. Studies evaluating this issue by quantitative tests are scanty.
Objective:
We aimed to assess the utility of a subacute low LD dose kinetic-dynamic test in the differential diagnosis between PD and APs. It was applied at the baseline of a prospective follow-up in patients with parkinsonian signs within three years of disease motor onset (“BoProPark” cohort) and eventually diagnosed as PD or APs according to consensus criteria.
Methods:
Patients under at least 3-month LD therapy received a first morning fasting dose of LD/benserazide or carbidopa (100/25 mg) and underwent simultaneous serial assessments of plasma LD concentration and alternate finger tapping frequency up to 3 h. The main outcome was the extent of LD motor response, calculated by the area under the 3 h tapping effect–time curve (AUC_ETap). A receiver operating characteristic (ROC) curve analysis was performed to establish the optimal AUC_ETap cut-off to differentiate PD and APs.
Results:
The first 100 consecutive “BoProPark” patients were analyzed. Forty-seven patients were classified as possible, 37 as probable PD and 16 as APs. AUC_ETap medians were similar in the PD subgroups but reduced to a third in APs (p < 0.001). The optimal AUC_ETap cut-off value was >2186 [(tap/min) x min], with a sensitivity of 92% and a specificity of 75%. Accuracy of the test was 0.85 (95% CI 0.74–0.95), p < 0.0001.
Conclusion:
The estimation of 3 h AUC_ETap after a subacute low LD dose proved a reliable, objective tool to assess LD motor response in our cohort of patients. AUC_ETap value rounded to ≥2200 supports PD diagnosis, while lower values may alert to AP diagnoses.
INTRODUCTION
It is recognized that the differential diagnosis of Parkinson’s disease (PD) from other neurodegenerative diseases presenting with parkinsonism can be difficult at the early stages, when some of the more peculiar features may not yet have developed [1, 2]. The responsiveness of motor symptoms to levodopa (LD) is a key supportive characteristic included in different diagnostic criteria proposed over time [1, 3, 4]. According to these criteria, LD responsiveness generally rests on high dose, >600 mg/day, LD trials [4] coupled with Unified Parkinson’s Disease Rating Scale (UPDRS) –Part III motor assessments [5]. Its proposed definition is largely empirical: a “substantial and sustained response to LD” [1]; a “clear and beneficial response, which must be unequivocal and of large amplitude” [4]. Among the drawbacks, high dose LD trials can exacerbate cardiovascular symptoms which often complicate the clinical picture of some atypical parkinsonisms (APs), hampering a reliable evaluation of LD response. As far as UPDRS-III scoring is concerned, the 20–30% change from baseline used to define “LD responders” has been arbitrarily determined and has not been fully established across the different stages of the disease [6, 7]. Moreover, in AP patients UPDRS-III may be not adequate to score some motor tasks, such as gait, which may be influenced by factors unrelated to dopaminergic deficits (impairment of cerebellar function or orthostatic hypotension) [8].
Acute challenge tests with high LD/carbidopa (CD) or benserazide (BZ) dose (250/25 or 200/50 mg) combined with semiquantitative scale assessments have been repeatedly suggested in the past as a cli-nically practical tool in the differential diagnosis of parkinsonisms at their early courses [9, 10]. However, these tests proved to suffer from several flaws, including the frequent occurrence of gastrointestinal and cardiovascular adverse events, hindering their completion; heterogeneous and rater dependent assessments of motor effects; disagreement on res-ponse cut-off for the differential diagnosis. On the basis of this evidence and the relative high rates of false positive and false negative results acute dopaminergic drug challenge tests were not recommended for the diagnosis of de novo PD patients [11].
Our group has developed a subacute challenge test with a low (100/25 mg) oral dose of LD/carbidopa (CD) or benserazide (BZ) based on serial quantitative assessments of motor effect by means of computerized alternate index finger tapping test and simultaneous blood samples to measure drug plasma concentrations over 3 h [12]. From one of our pre-vious retrospective studies [8] the extent of motor response elicited by this LD challenge, estimated by the area under the 3 h tapping effect-time curve (AUC_ETap), proved helpful in distinguishing between PD and Multiple System Atrophy with predominant parkinsonism (MSA-P) early in the disease course.
The aim of this study was to further assess the utility of this objective approach in the differential diagnosis of PD. It was applied at the baseline of a prospective follow-up in a cohort of patients with a progressive neurodegenerative disease starting with parkinsonian signs within three years of disease motor onset (“Bologna Motor and Non-motor Pro-spective Study on Parkinsonism at Onset”, BoProPark study) [13] and eventually diagnosed as PD or APs according to international consensus criteria.
MATERIALS AND METHODS
Patients and protocol
All consecutive patients recruited from September 2007 up to November 2018 for the BoProPark study who completed a second evaluation by December 2018 were included in the present analysis. Only patients with pathological single-photon emission computerized tomography for imaging the dopamine transporter (DaTscan SPECT) were included in the study. Secondary causes of parkinsonism were also excluded before enrollment by means of appropriate investigations including brain magnetic resonance imaging. A further exclusion criterion to enter the study was a concurrent clinically severe medical or psychiatric disease that could interfere with study results [13]. The study was approved by the Independent Ethics Committee of the Bologna Health Authority (CE number: 09070). Patients gave their written informed consent to participate in the study and to publish the data.
According to the BoProPark protocol each patient underwent the same battery of clinical and instrumental tests [13] at baseline (T0), after 16 months (T1) and 5 years (T2). Patients were also followed up according to clinical practice and were regularly assessed for routine visits every 6–12 months as needed. Diagnoses for each patient were carried out as previously detailed [13] with the aid of an ad hoc database (SparkBio Srl, Bologna, Italy) according to the international diagnostic criteria for PD [1], PD with dementia (PDD) [14, 15], MSA [16], dementia with Lewy bodies (DLB) [17], progressive supranuclear palsy (PSP) [18], and corticobasal syndrome (CBS) [19]. Patients not fulfilling any diagnostic criteria were diagnosed as unspecified atypical parkinsonism (uAP). All diagnoses were independently confirmed by three neurologists expert in movement disorders who were blinded to the diagnosis provided by the database. Diagnoses made at T0 were revisited at T1 based on the results obtained from these follow-up evaluations [13] (Fig. 1).
Fig. 1
A) Outline of the BoProPark study phase covered by the present analysis; B) protocol of the subacute challenge test with levodopa.
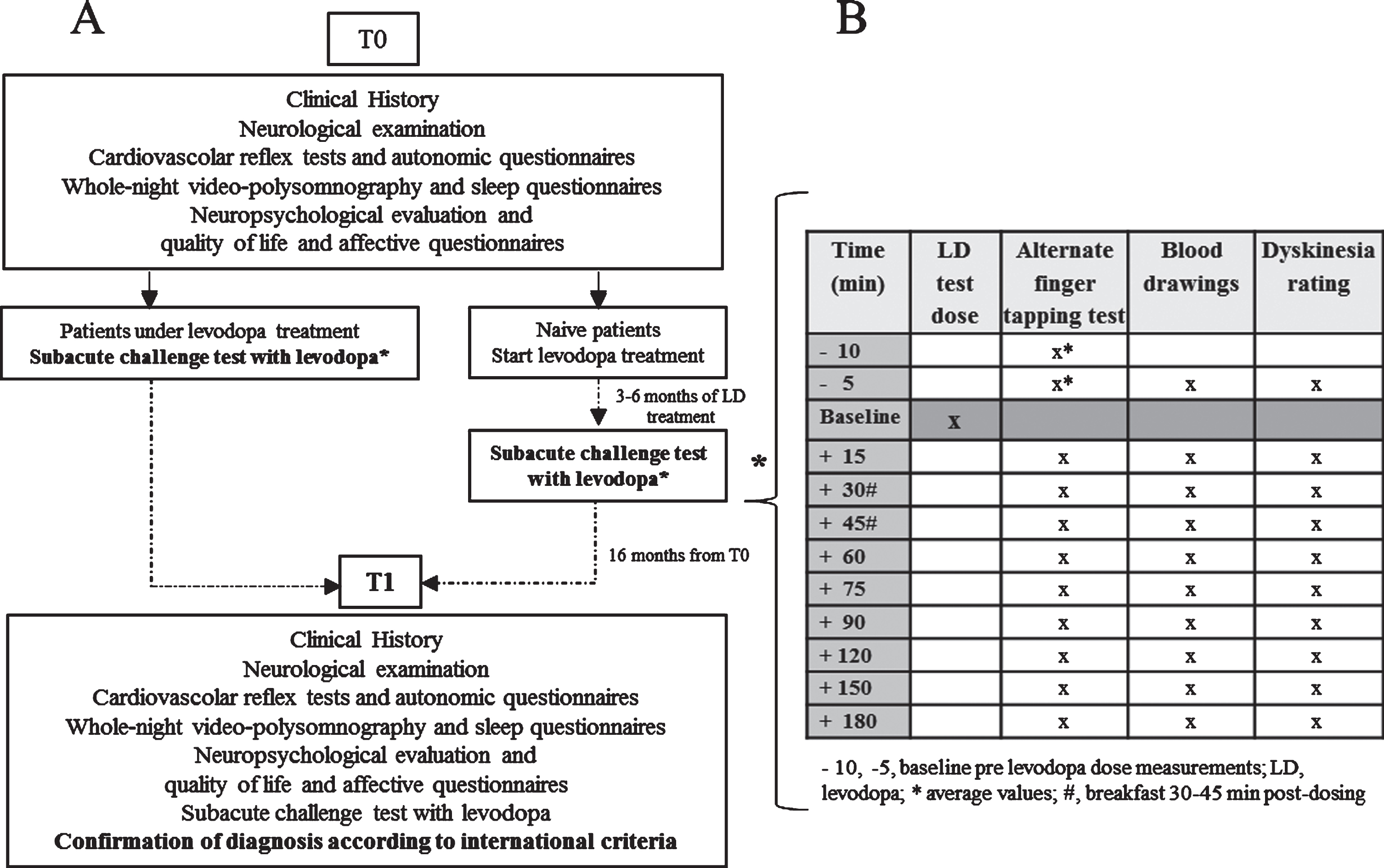
As far as LD kinetic-dynamic test is concerned, drug naïve patients at T0 started LD treatment titrated in one month up to 200 mg/day and underwent this examination within 3–6 months from drug introduction (Fig. 1). In the present report subacute LD test response obtained at T0 was matched with patients’ diagnosis carried out at T1, in order to increase confidence in the diagnosis (Fig. 1).
Levodopa kinetic-dynamic test
On the morning of the study the patients received an oral fasting dose of LD/BZ or CD (100/25 mg) after a 12 h washout of LD and any concomitant anti-parkinsonian drugs [12]. Blood venous samples for LD plasma concentration analysis were drawn by an indwelling catheter immediately before the LD dose, at 15 min intervals for the first 90 min, then half-hourly up to 3 h after dosing. Patients’ motor responses to the LD test dose were simultaneously assessed by the alternate index finger tapping test (the number of times the patient could alternately tap two buttons 20 cm apart in 60 s with the most affected hand), using a computerized touch screen system (mHealth Technologies srl, Bologna, Italy) (Supplementary Figure 1). The protocol of the subacute challenge test with levodopa is summarized in Fig. 1. Baseline pre-LD dosing finger tapping test was performed twice, 5 min apart, and tapping frequency values were averaged for further analyses. All pat-ients underwent a separate session of practice trial to acquaint themselves with the required movement.
Possible LD induced dyskinesias (LIDs) were rated at the same times as motor responses by the Clinical Dyskinesia Rating Scale (CDRS) [20].
All patients were clinically assessed before LD subacute test (“off state”) by means of UPDRS- Part III motor scale [5] and Hoehn & Yahr (H&Y) scale [21]. One hour post-LD dosing UPDRS-III assessment (“on state”) was performed in a subset of patients.
LD kinetic-dynamic analysis
LD peak plasma concentration (Cmax) and time to LD peak (tmax) were the observed values.
The magnitude of the effect was estimated by the difference (ETapmax) between maximum tapping frequency (Tapmax) and baseline (Tap0) over baseline values, expressed as percentage (ΔTapmax%).
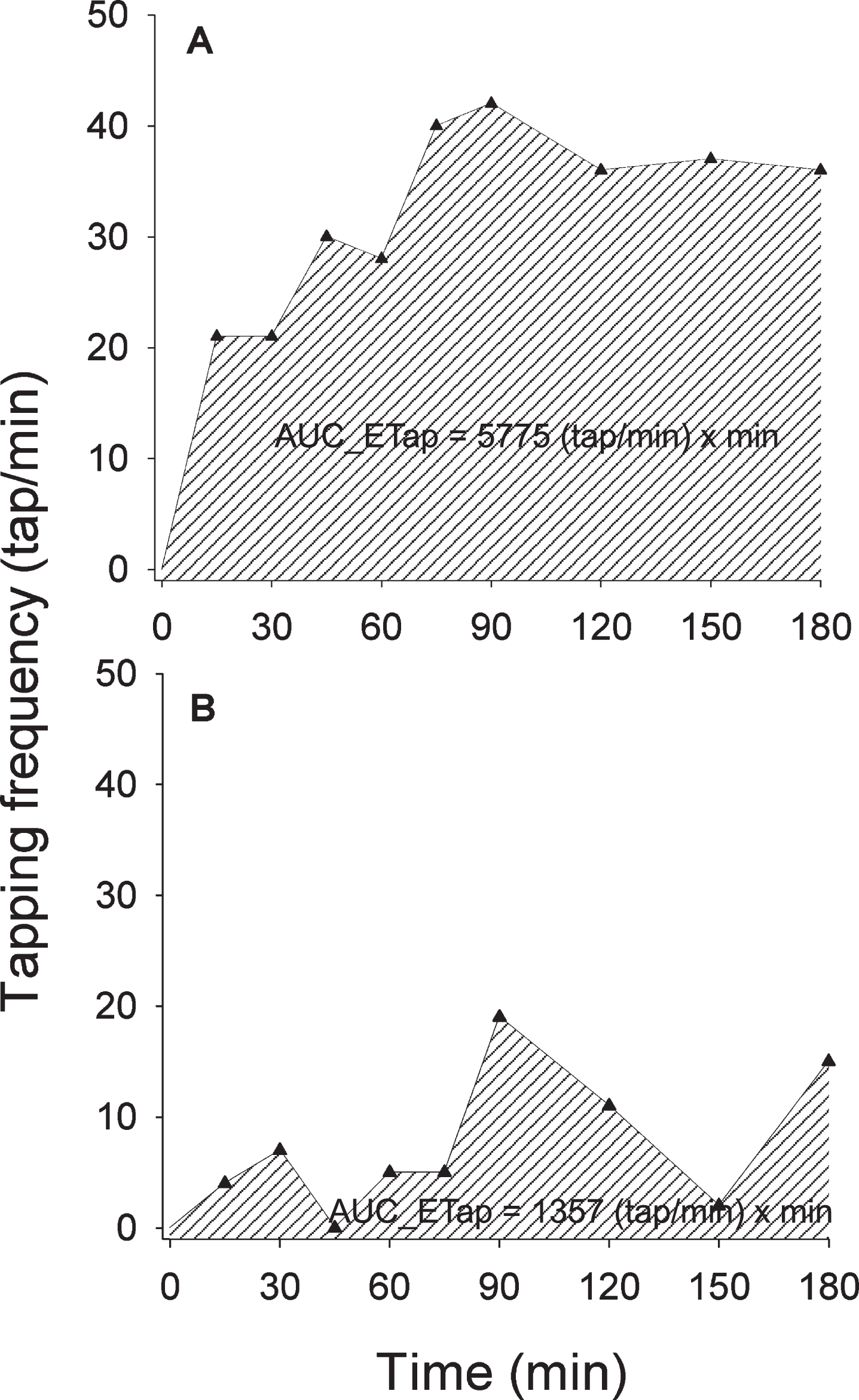
The main study outcome was the 3 h AUC_ETap calculated according to the linear trapezoidal rule by means of SigmaPlot 12.5 software (Systat Software, San Jose, CA, USA). Practically, for each patient the differences (ETap) between serial post-dosing tapping frequency absolute values and the average of two baseline, pre-dosing measurements were calculated and then plotted against matched assessment times, over the 3 h (Fig. 2).
Fig. 2
Graphs and computed values of the area under the 3 h tapping effect-time curve (AUC_ETap) elicited by the 100/25 levodopa/benserazide or carbidopa test dose in two representative patients: A) Parkinson’s disease; B) Atypical parkinsonism. Depicted tapping frequency values were obtained after correction for baseline values, i.e., expressed as the difference (ETap) between serial post-dosing tapping frequency absolute values and the average of two baseline, pre-dosing measurements.
Statistical analysis
When data were consistent with a normal distribution and equal variances, means and standard deviations were calculated and comparisons of study variables among patients’ subgroups were carried out by One Way Analysis of Variance (ANOVA). Pairwise comparisons were performed by Holm-Sidak method when ANOVAs indicated a significant difference among subgroups. When deviation from a normal distribution was found, or when different var-iances were identified, medians and 25th-75th percentiles were calculated and comparisons were performed by Kruskal-Wallis One Way Analysis of Variance on Ranks (ANOVA on Ranks). Pairwise comparisons were performed by Dunn’s method when ANOVAs on Ranks indicated a significant difference among subgroups. Correlations between variables were assessed by Pearson’s product mom-ent coefficient. Sex and LIDs distribution was compared among groups by Chi square test. The optimal AUC_ETap cut-off value to differentiate PD and APs was determined by receiver operating characteristic (ROC) curve analysis. Analyses were carried out by SigmaPlot 12.5 software. Significance was set at p < 0.05.
RESULTS
Patients’ characteristics
Clinical characteristics of patients at the moment of their T0 LD test grouped by diagnoses carried out at T1 are reported in Table 1. Forty-seven patients were classified as possible and 37 as probable PD, while 16 patients were classified as APs (PSP, n = 3; MSA, n = 5; DLB, n = 2; CBS, n = 2; uAP, n = 4). The three groups were comparable for sex, age, weight and LD therapy duration. Possible PD patients had a shorter duration of parkinsonian symptoms (p < 0.001) compared with probable PD and AP subgroups. AP patients showed higher UPDRS-III [5] and Hoehn & Yahr (H&Y) score [21] than possible and probable PD (p < 0.001). LD daily dose significantly increased from possible and probable PD up to AP (p < 0.001, all pairwise comparisons). Three possible PD patients were co-treated with pramipexole. Six probable PD patients were also receiving pramipexole, three were on ropinirole and three on rasagiline. Two AP patients were co-treated with pramipexole and one with rasagiline. Possible PD patients had a lower LD equivalent daily dose (LEDD) [22] (p < 0.001) than probable PD and AP subgroups.
Table 1
Patients’ characteristics at their first prospective levodopa test matched with clinical classification made after 16 months of follow-up
| Patient group | Sex m/w | Age (y) | Weight (kg) | P symptoms’ duration (months)* | LD therapy duration (months) | LD dose (mg/d)# | LEDD (mg/d)* | UPDRS-III score§ | H&Y score§ |
| Possible PD | 24/23 | 61±10 | 73±13 | 12 (10–12) | 5.1 (4.0–8.0) | 200±38 | 205±43 | 12 (9–15) | 1 (1-2) |
| (n = 47) | |||||||||
| Probable PD | 26/11 | 60±9 | 75±15 | 24 (24–36) | 5.0 (4.0–7.1) | 227±61 | 272±103 | 15 (9–21) | 2 (1-2) |
| (n = 37) | |||||||||
| AP | 10/6 | 66±10 | 71±13 | 24 (24–36) | 4.1 (3.0–6.8) | 305±105 | 319±121 | 29 (22–33) | 2 (2-2) |
| (n = 16) |
Data are expressed as mean±standard deviation or median (25–75th percentiles).PD, Parkinson’s disease; AP, atypical parkinsonism; m, men; w, women; y, years; kg, kilogram; P, Parkinsonian; LD, levodopa; mg, milligram; d, day; LEDD, levodopa equivalent daily dose; UPDRS-III, Unified Parkinson’s Disease Rating Scale- Part III; H&Y, Hoehn and Yahr. UPDRS-III and H&Y scores were obtained before LD dosing (“off” state) during the LD challenge test.*p < 0.001, possible PD versus probable PD and AP; #p < 0.001, all pairwise multiple comparisons; §p < 0.001, AP versus possible and probable PD.
Clinical diagnoses carried out at the last available follow-up, at an overall median (25-75 percentiles) duration of parkinsonian symptoms of 64 (46–100) months are summarized in Supplementary Table 1. Patients’ main classification in PD or APs was substantially confirmed in all subjects but one (one uAP reclassified as PDD).
LD kinetic-dynamic test results
All patients completed the LD kinetic-dynamic test without any inconvenience or adverse effects. Alternate index finger tapping sessions were regularly carried out and completed by all patients without execution errors.
No difference in LD test dose expressed as mg/kg was observed among the three subgroups (Table 2). Mean LD Cmax and median tmax were similar among groups (Table 2).
Table 2
Levodopa kinetic-dynamic variables by patients’ subgroups
| Patients’ subgroups | |||
| Levodopa kinetic-dynamic variables | Possible PD | Probable PD | AP |
| Test dose (mg/kg) | 1.4±0.3 | 1.4±0.3 | 1.5±0.3 |
| tmax (min) | 30 (30–60) | 30 (30–45) | 30 (30–45) |
| Cmax (mg/L) | 2.0±0.8 | 2.1±0.8 | 1.9±0.7 |
| Tap0 (tap/min)* | 134±34 | 137±37 | 88±34 |
| Tapmax(tap/min)* | 179±45 | 187±49 | 112±50 |
| ETapmax (tap/min)* | 42 (29–59) | 43 (32–63) | 21 (13–33) |
| DeltaTapmax% | 30 (24–39) | 30 (22–41) | 23 (19–33) |
| AUC_ETap* [(tap/min)×min] | 5385 (3300–7410) | 5925 (3982–8261) | 1799 (442–4100) |
Data are expressed as mean±standard deviation or median (25-75th percentiles).PD, Parkinson’s disease; AP, Atypical parkinsonism; mg, milligram; kg, kilogram; L, liter; tmax, time to levodopa (LD) peak; Cmax, LD peak plasma concentration; Tap0, tapping frequency at baseline; Tapmax, maximum tapping frequency after LD dose; ETapmax, difference between Tapmax and Tap0; DeltaTapmax%, ETapmax over baseline values expressed as percentage; AUC_ETap, area under the 3 h tapping effect-time curve.*p < 0.001, AP versus Possible and Probable PD.
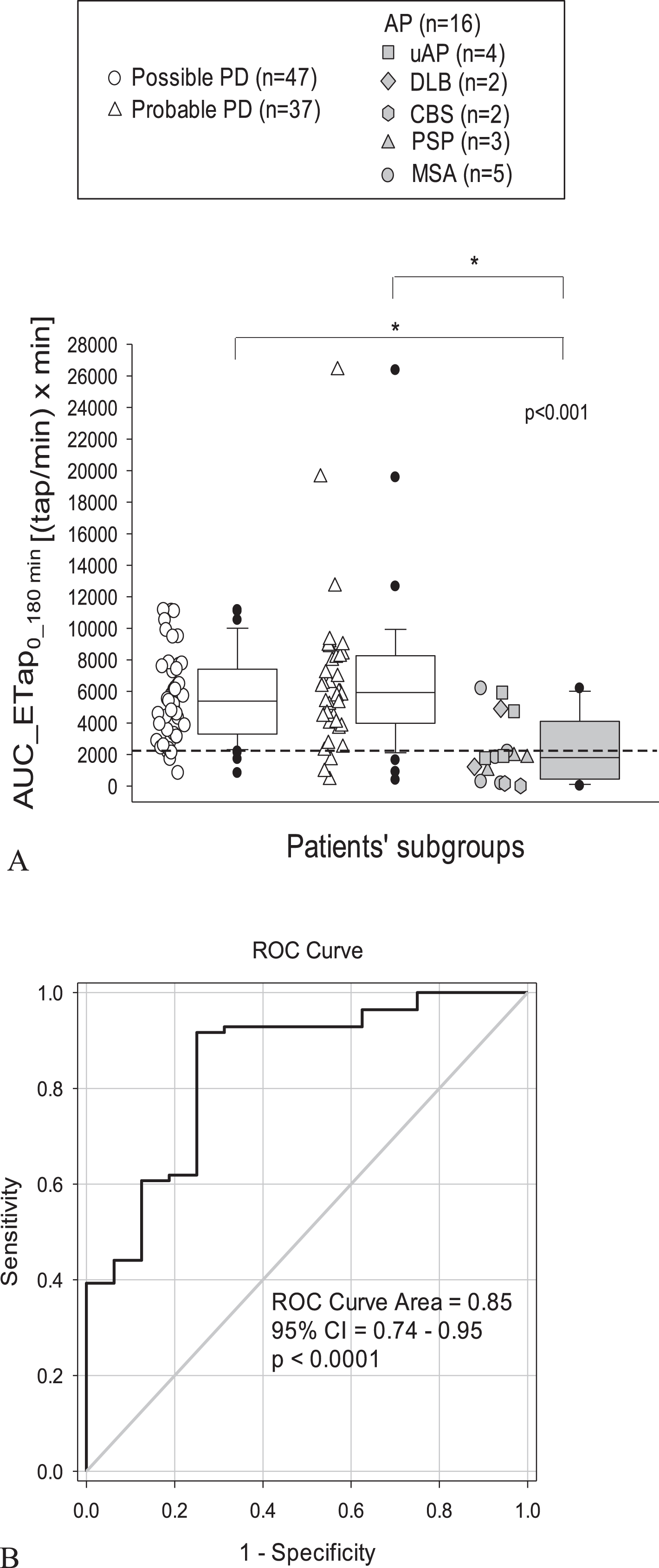
Median values of baseline, maximum tapping frequency and magnitude of tapping effect were significantly lower in AP patients than in possible and probable PD (p < 0.001). ΔTapmax% tended to be reduced in APs but failed to reach a statistical significance (Table 2). Median AUC_ETap values were similar in possible and probable PD but reduced to a third in AP (p < 0.001) (Table 2). Scatter and box plots of AUC_ETap values by patients’ groups are depicted in Fig. 3. Representative AUC_ETap graphs obtained in one PD and one AP patient are illustrated in Fig. 2.
Fig. 3
A) Scatter and box plots of area under the 3 h tapping effect–time curve (AUC_ETap) by patients’ groups. Box plots depict the range between the 25th and 75th percentiles of the data. The horizontal line marks the median value; capped bars indicate 10th-90th percentiles. Black circles represent outlying values. The dashed bold line indicates the optimal cut-off value for AUC_ETap according to the receiver operating characteristic (ROC) curve (B). p < 0.001, overall significance of comparisons by One Way Analysis of Variance; * Significance of pairwise comparisons by Dunn’s method (p < 0.05). PD, Parkinson’s disease; AP, atypical parkinsonisms; PSP, progressive supranuclear palsy; MSA, multiple system atrophy; uAP, undefined atypical parkinsonisms; DLB, dementia with Lewy bodies; CBS, corticobasal syndrome.
From LD plasma analyses, no impairment in LD bioavailability was observed in the AP subgroup. Overall, no significant correlation was observed between LD Cmax and matched AUC_ETap.
From ROC curve analysis (Fig. 3) an AUC_ETap value of 2186 [(taps/min) × min] proved to be the optimal cut-off (Table 3) in the differential diagnosis between overall PD patients and AP, with a sensitivity of 92% and a specificity of 75%. Accuracy of the test, expressed by the area under the ROC curve was 0.85 (95% CI 0.74–0.95), p < 0.0001.
Table 3
Report extract of different cutoff points for AUC_ETap values and matched sensitivity and specificity according to ROC curve analysis
| AUC_ETap cut-off> | Sensitivity | 95% CI | Specificity | 95% CI |
| 2058 | 0.93 | 0.85–0.97 | 0.69 | 0.41–0.89 |
| 2148 | 0.92 | 0.83–0.97 | 0.69 | 0.41–0.89 |
| 2186 | 0.92 | 0.83–0.96 | 0.75 | 0.48–0.93 |
| 2213 | 0.90 | 0.82–0.96 | 0.75 | 0.48–0.93 |
| 2288 | 0.89 | 0.81–0.95 | 0.75 | 0.48–0.93 |
| 2381 | 0.88 | 0.79–0.95 | 0.75 | 0.48–0.93 |
AUC_ETap, area under the 3 h tapping effect-time curve; ROC, receiver operating characteristic curve. In bold: optimal cut-off value.
Moderate LIDs were registered in 5 possible PD (11%), 7 probable PD (19%) and two AP (12%).
Overall, a negative correlation was found between UPDRS-III score obtained before subacute LD dosing and matched baseline tapping frequency (r = –0.447, p < 0.001). UPDRS-III value 1 h after LD test was available in 51 patients: 24 (51%) of possible PD, 20 (54%) of probable PD and 7 (44%) of AP. Percentage difference between post-dosing and baseline UPDRS-III positively correlated with matched ΔTapmax% (r = 0.430, p < 0.001) in this subset of patients.
DISCUSSION
In our cohort of patients, the 3 h extent of alternate finger tapping motor response elicited by the subacute LD test was markedly lower in patients eventually diagnosed as APs compared to possible or probable PD. DeltaTapmax% did not significantly differ between PD and AP patients, partly due to the high intersubject variability and the limited number of the AP subgroup, including heterogeneous syndromes. The observed differences in LD pharmacodynamics were not explained by pharmacokinetics that were similar in the subgroups. These findings are in line with the results of one of our retrospective studies in a series of patients followed up for a parkinsonian syndrome and eventually diagnosed as MSA-P or PD [8]. As already pinpointed [8], it might be argued that quantitative differences in LD tapping effect result from the higher motor impairment (i.e., lower basal tapping values) in the AP group. However, in previous studies in PD patients the magnitude of LD motor response was unaltered [23] or even increased with the advancing of the disease [24], possibly as a result of compensatory postsynaptic-receptor upregulation in presence of extensive presynaptic lesions [24]. No significant relationship was observed between LD Cmax and matched AUC_ETap, in line with the notion that the response to LD is the result of a complex interplay between cerebral LD kinetic mechanisms and the degree of degeneration of the nigrostriatal dopaminergic system which accompanies the progression of symptoms [23, 24].
AUC_ETap was selected as the primary outcome to quantify the effect elicited by the LD test dose. Integrating over time rather than looking at individual effect measurements can be a more accurate approach to estimate the pharmacological response to a given dose of a drug [25]. AUC_ETap value rounded to ≥2200 [(taps/min)×min] proved an accurate cut-off in the differential diagnosis between PD and APs. The low severity of bradykinesia observed in five out of 84 overall possible and probable PD patients may partly account for the overlapping of AUC_ETap measures around the optimal cut-off value in this subgroup. The sensitivity of our test (92%) was better than that reported by most studies performed with acute high LD/CD or BZ (200/25 or 200/50 mg) challenge tests, ranging 70–81% [2, 26, 27]. Test specificity (75%) was of similar order of that obtained by high LD/CD or BZ acute challenges (71–82%) [2, 26, 27]. This finding is also in line with the notion that LD responsiveness is poorly specific to PD. As previously recalled by Gelb and coauthors [1], an at least initial and temporary response to LD treatment was documented in 22% to 35% of patients with PSP, 69% to 75% of MSA and up to 87% of DLB patients presenting parkinsonian features.
Taken overall, the following considerations can be drawn on the diagnostic value and clinical applicability of our subacute low LD dose objective test in parkinsonisms at disease onset:
a) Our patients were tested after median 4–5 months of LD therapy, at mean low daily doses ranging from 200 to 400 mg. As previously outlined [8], a chronic treatment of at least 3 months was considered necessary to accomplish with a safe slow titration of LD dose in de novo patients, patients’ acceptance and familiarization with a new chronic treatment and latency to experience long-duration response [28]. This low daily dose LD trial coupled with a low subacute test dose was well tolerated in all our patients and may be a more reliable and safer approach to test drug re-sponsiveness than acute high LD dose challenges, often complicated by gastrointestinal and cardiovascular adverse events hampering adequate assessments [2, 26], especially in AP patients. This approach is in line with the nowadays recognized notion that high LD doses are associated with an increased risk of developing both dyskinesias and wearing-off phenomena [29]. It is recommended that LD should be used from the beginning in the lowest dose that provides a satisfactory clinical response and dose increases should be made with caution [30].
b) Quantitative assessment of LD motor response by a simple test, such as the alternate index finger tapping, is not prone to the interobserver variations of clinical scale evaluations. This characteristic may be particularly useful in long-term intrasubject follow-ups, where patients are likely to be examined by different clinicians over time. Moreover, objective monitoring may promote a more standardized comparison of patients’ clinical and therapeutic assessment from different medical centers. At present UPDRS-III is still the common reference scale in the development and validation of both quantitative and semiquantitative tools for assessing PD features. Of note, in our patients both baseline UPDRS-III and percentage difference between post-dosing and baseline value proved to significantly correlate with matched baseline alternate tapping frequency and magnitude of the effect (ΔTapmax%). These findings are in keeping with reported evidence of a strong correlation between UPDRS-III, namely bradykinesia subscore, and tapping frequency [31].
c) Serial measurements of LD plasma concentrations enclosed in our kinetic-dynamic test allow checking for possible delayed, reduced or irregular patterns of drug absorption. We confirmed that AUC_ETaps below the optimal cut-off value were not ascribable to any impairment of LD test dose bioavailability. In the light of this observation and of our previous experience of intrapatient longitudinal LD kinetic-dynamic monitoring [23] our standardized LD test could be reliably performed without blood sampling in the first place, reserving the assessment of LD kinetics to cases of negative or doubtful motor response.
Our study has some limitations. First of all, the objective assessment of LD motor response was based only on the alternate finger tapping test. We are aware that this test can be not fully adequate to monitor LD effects in patients showing light bradykinesia or tremor as prevalent symptom [32]. We have experienced other simple motor tasks, such as the Timed Up and Go (TUG) test [33], but it proved less sensitive than finger tapping in detecting subtle subacute motor effects elicited by LD dosing at the early stages of PD [12, 32, 34]. Further, patient’s performance on the alternate finger tapping test could be affected by factors like cognitive impairment (mainly attention, executive and praxis dysfunction), affective and behavioral modifications (decreased motivation or impulsivity). On the other hand UPDRS-III, the common reference scale used to estimate motor LD response may be affected, in addition to the above factors, by symptomatic orthostatic hypotension and cerebellar dysfunctions.
Another limitation is the small and heterogeneous group of APs (n = 16) detected in our patient population, which is in any case of the same order of magnitude (n = 24) of the group of “other neuro-degenerative disorders” (ONDs) reported in the DeNoPa cohort [2]. It is noteworthy that the percentage of APs (16%) found in our study was identical to the percentage of ONDs (17%) found in the DeNoPa study.
Finally, all patients were diagnosed on the basis of clinical features. An autopsy study should be performed for a definite diagnosis.
In conclusion, the estimation of 3 h AUC_ETap after a subacute 100/25 mg LD/CD or BZ dose proved a reliable, objective tool to assess LD motor response in our cohort of patients. AUC_ETap value rounded to ≥2200 supports PD diagnosis, while lower values may alert to AP diagnoses. Further validation studies are required to confirm the accuracy of this new tool in a larger cohort of patients.
CONFLICT OF iNTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
The skillful nursing assistance of Monica Balboni is gratefully acknowledged. Cecilia Baroncini edited the English text.
This study was partly financed with contributions of “5×1000”- Support for Neuroscience Health Research –2015 of IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy.
SUPPLEMENTARY MATERIAL
[1] The supplementary material is available in the electronic version of this article: https://dx.doi.org/10.3233/JPD-202262
REFERENCES
[1] | Gelb DJ , Gilman S ((1999) ) Diagnostic criteria for Parkinson disease, Arch Neurol 56: , 33–39. |
[2] | Schade S , Sixel-Doring F , Ebentheuer J , Schulz X , Trenkwalder C , Mollenhauer B ((2017) ) Acute levodopa challenge test in patients with de novo Parkinson’s disease: Data from the DeNoPa cohort, Mov Disord Clin Pract 4: , 755–762. |
[3] | Hughes AJ , Colosimo C , Kleedorfer B , Daniel SE , Lees AJ ((1992) ) The dopaminergic response in multiple system atrophy, J Neurol Neurosurg Psychiatr 55: , 1009–1013. |
[4] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W , Obeso J , Marek K , Litvan I , Lang AE , Halliday G , Goetz CG , Gasser T , Dubois B , Chan P , Bloem BR , Adler CH , Deuschl G ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease, Mov Disord 30: , 1591–1599. |
[5] | Fahn S , Elton RL , Members of the UPDRS Development Committee Unified Parkinson’s disease rating scale (1987) Unified Parkinson’s disease Rating Scale. In Recent developments in Parkinson’s Disease, Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Macmillan Health Care Information, Florham Park, NJ, pp. 153-164. |
[6] | Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease ((2003) ) The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and recommendations, Mov Disord 18: , 738–750. |
[7] | Shulman LM , Gruber-Baldini AL , Anderson KE , Fishman PS , Reich SG , Weiner WJ ((2010) ) The clinically important difference on the Unified Parkinson’s disease Rating Scale, Arch Neurol 67: , 64–70. |
[8] | Calandra-Buonaura G , Doria A , Lopane G , Guaraldi P , Capellari S , Martinelli P , Cortelli P , Contin M ((2016) ) Pharmacodynamics of a low subacute levodopa dose helps distinguish between multiple system atrophy with predominant Parkinsonism and Parkinson’s disease, J Neurol 263: , 250–256. |
[9] | Clarke CE , Davies P ((2000) ) Systematic review of acute levodopa and apomorphine challenge tests in the diagnosis of idiopathic Parkinson’s disease, J Neurol Neurosurg Psychiatr 69: , 590–594. |
[10] | Albanese A , Bonuccelli U , Brefel C , Chaudhuri KR , Colosimo C , Eichhorn T , Melamed E , Pollak P , Van Laar T , Zappia M ((2001) ) Consensus statement on the role of acute dopaminergic challenge in Parkinson’s disease, Mov Disord 16: , 197–201. |
[11] | Berardelli A , Wenning GK , Antonini A , Berg D , Bloeme BR , Bonifati V , Brooks D , Burn DJ , Colosimo C , Fanciulli A , Ferreira J , Gasserd T , Grandas F , Kanovsky P , Kostic V , Kulisevsky J , Oertel W , Poewe W , Reese J-P , Relja M , Ruzicka E , Schrag A , Seppi K , Taba P , Vidailhet M ((2013) ) EFNS/MDS-ES recommendations for the diagnosis of Parkinson’s disease, Eur J Neurol 20: , 16–34. |
[12] | Contin M , Riva R , Martinelli P , Albani F , Avoni P , Baruzzi A ((2001) ) Levodopa therapy monitoring in patients with Parkinson disease: A kinetic-dynamic approach, Ther Drug Monit 23: , 621–629. |
[13] | Calandra-Buonaura G , Sambati L , Baschieri F , Vitiello M , Contin M , Tonon C , Capellari S , Provini F , Cortelli P , on behalf of the BoProPark Study ((2020) ) The Bologna motor and non-Motor Prospective study on Parkinsonism at onset (BoProPark): Study design and population, Neurol Sci 41: , 2531–2537. |
[14] | Emre M , Aarsland D , Brown R , Burn DJ , Duyckaerts C , Mizuno Y , Broe GA , Cummings J , Dickson DW , Gauthier S , Goldman J , Goetz C , Korczyn A , Lees A , Levy R , Litvan I , McKeith I , Olanow W , Poewe W , Quinn N , Sampaio C , Tolosa E , Dubois B ((2007) ) Clinical diagnostic criteria for dementia associated with Parkinson’s disease, Mov Disord 22: , 1689–1707. |
[15] | Dubois B , Burn D , Goetz C , Aarsland D , Brown RC , Broe GA , Dickson D , Duyckaerts C , Cummings J , Gauthier S , Korczyn A , Lees A , Levy R , Litvan I , Mizuno Y , McKeith IG , Olanow CW , Poewe W , Sampaio C , Tolosa E , Emre M ((2007) ) Diagnostic procedures for Parkinson’s disease dementia: Recommendations from the movement disorder society task force, Mov Disord 22: , 2314–2324. |
[16] | Gilman S , Wenning GK , Low PA , Mathias CJ , Trojanowski JQ , Wood NW , Colosimo C , Dürr A , Fowler CJ , Kaufmann H , Klockgether T , Lees A , Poewe W , Quinn N , Revesz T , Robertson D , Sandroni P , Seppi K , Vidailhet M ((2008) ) Second consensus statement on the diagnosis of multiple system atrophy, Neurology 71: , 670–676. |
[17] | McKeith IG , Dickson DW , Lowe J , Emre M , O’Brien JT , Feldman H , Cummings J , Duda JE , Lippa C , Perry EK , Aarsland D , Arai H , Ballard CG , Boeve B , Burn DJ , Costa D , Del Ser T , Dubois B , Galasko D , Gauthier S , Goetz CG , Gomez-Tortosa E , Halliday G , Hansen LA , Hardy J , Iwatsubo T , Kalaria RN , Kaufer D , Kenny RA , Korczyn A , Kosaka K , Lee VMY , Lees A , Litvan I , Londos E , Lopez OL , Minoshima S , Mizuno Y , Molina JA , Mukaetova-Ladinska EB , Pasquier F , Perry RH , Schulz JB , Trojanowski JQ , Yamada M , Consortium on DLB ((2005) ) Diagnosis and management of dementia with Lewy bodies: Third report of the DLB Consortium, Neurology 65: , 1863–1872. |
[18] | Litvan I , Agid Y , Calne D , Campbell G , Dubois B , Duvoisin RC , Goetz CG , Golbe LI , Grafman J , Growdon JH , Hallett M , Jankovic J , Quinn NP , Tolosa E , Zee DS ((1996) ) Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP international workshop, Neurology 47: , 1–9. |
[19] | Riley DE , Lang AE ((2000) ) Clinical diagnostic criteria. In Corticobasal degeneration and related disorders, Litvan I, Goetz CG, Lang AE, eds. Lippincott Williams & Wilkins, Philadelphia, pp. 29-39. |
[20] | Hagell P , Widner H ((1999) ) Clinical rating of dyskinesias in Parkinson’s disease: Use and reliability of a new rating scale, Mov Disord 14: , 448–455. |
[21] | Hoehn MM , Yahr MD ((1967) ) Parkinsonism: Onset, progression, and mortality, Neurology 17: , 427–442. |
[22] | Tomlinson CL , Stowe R , Patel S , Rick C , Gray R , Clarke CE ((2010) ) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease, Mov Disord 15: , 2649–2653. |
[23] | Contin M , Riva R , Martinelli P , Cortelli P , Albani F , Baruzzi A ((1994) ) Longitudinal monitoring of the levodopa concentration-effect relationship in Parkinson’s disease, Neurology 44: , 1287–1292. |
[24] | Nutt JG , Woodward WR , Carter JH , Gancher ST ((1992) ) Effect of long-term therapy on the pharmacodynamics of levodopa. Relation to on-off phenomenon, Arch Neurol 49: , 1123–1130. |
[25] | Scheff JD , Almon RR , Dubois DC , Jusko WJ , Androulakis IP ((2011) ) Assessment of pharmacologic area under the curve when baselines are variable, Pharm Res 28: , 1081–1089. |
[26] | Merello M , Nouzeilles MI , Arce GP , Leiguarda R ((2002) ) Accuracy of acute levodopa challenge for clinical prediction of sustained long-term levodopa response as a major criterion for idiopathic Parkinson’s Disease diagnosis, Mov Disord 17: , 795–798. |
[27] | Asayama S , Wate R , Kaneko S , Asayama T , Oki M , Tsuge A , Nagashima M , Morita J , Nakamura S , Nakamura M , Nishii M , Fujita K , Saito A , Nakano S , Ito H , Kusaka H ((2013) ) Levodopa challenge test and 123I-metaiodobenzyl guanidine scintigraphy for diagnosing Parkinson’s disease, Acta Neurol Scand 128: , 160–165. |
[28] | Fahn S , Oakes D , Shoulson I , Kieburtz K , Rudolph A , Lang A , Olanow CW , Tanner C , Marek K ((2004) ) Levodopa and the progression of Parkinson’s disease, N Engl J Med 351: , 2498–2508. |
[29] | Olanow CW , Kieburtz K , Rascol O , Poewe W , Schapira AH , Emre M , Nissinen H , Leinonen M , Stocchi F ((2013) ) Factors predictive of the development of Levodopa-induced dyskinesia and wearing-off in Parkinson’s disease, Mov Disord 28: , 1064–1071. |
[30] | Olanow CW , Stocchi F ((2018) ) Levodopa: A new look at an old friend, Mov Disord 33: , 859–866. |
[31] | Taylor Tavares AL , Jefferis GS , Koop M , Hill BC , Hastie T , Heit G , Bronte-Stewart HM ((2005) ) Quantitative measurements of alternating finger tapping in Parkinson’s disease correlate with UPDRS motor disability and reveal the improvement in fine motor control from medication and deep brain stimulation, Mov Disord 20: , 1286–1298. |
[32] | Lopane G , Mellone S , Corzani M , Chiari L , Cortelli P , Calandra-Buonaura G , Contin M ((2018) ) Supervised versus unsupervised technology-based levodopa monitoring in Parkinson’s disease: An intrasubject comparison, J Neurol 265: , 1343–1352. |
[33] | Podsiadlo D , Richardson S ((1991) ) The timed “Up & Go”: A test of basic functional mobility for frail elderly persons, J Am Geriatr Soc 39: , 142–148. |
[34] | Contin M , Riva R , Martinelli P , Procaccianti G , Cortelli P , Avoni P , Baruzzi A ((1990) ) Response to a standard oral levodopa test in parkinsonian patients with and without motor fluctuations, Clin Neuropharmacol 13: , 19–28. |



