Characteristics of Parkinson’s Disease in Patients with and without Cognitive Impairment
Abstract
Background:
Characterizing patients with Parkinson’s disease (PD) and cognitive impairment is important toward understanding their natural history.
Objective:
Understand clinical, treatment, and cost characteristics of patients with PD pre- and post-cognitive impairment (memory loss/mild cognitive impairment/dementia or dementia treatment) recognition.
Methods:
2,711 patients with PD newly diagnosed with cognitive impairment (index) were identified using administrative claims data. They were matched (1:1) on age and gender to patients with PD and no cognitive impairment (controls). These two cohorts were compared on patient characteristics, healthcare resource utilization, and total median costs for 3 years pre- and post-index using Chi-square tests, t-tests, and Wilcoxon rank-sum tests. Logistic regression was used to identify factors predicting cognitive impairment.
Results:
Comorbidity indices for patients with cognitive impairment increased during the 6-year study period, especially after the index. Enrollment in Medicare Advantage Prescription Drug plans vs. commercial (OR = 1.60), dual Medicare/Medicaid eligibility (OR = 1.36), cerebrovascular disease (OR = 1.24), and PD medication use (OR = 1.46) were associated with a new cognitive impairment diagnosis (all p < 0.05). A greater proportion of patients with cognitive impairment had hospitalizations and emergency department visits and higher median total healthcare costs than controls for each year pre- and post-index.
Conclusion:
In patients with PD newly diagnosed with cognitive impairment, comorbidity burden, hospitalizations, emergency department visits, and total costs peaked 1-year pre- and post-identification. These data coupled with recommendations for annual screening for cognitive impairment in PD support the early diagnosis and management of cognitive impairment in order to optimize care for patients and their caregivers.
INTRODUCTION
Parkinson’s disease (PD), a neurogenerative disease, is typically defined as a movement disorder characterized by tremor, rigidity, bradykinesia, and postural instability [1–3]. Non-motor symptoms (affective disorders, psychosis, sleep disturbance, autonomic dysfunction, and cognitive impairment) are also important features of PD [1, 4, 5]. It is estimated that nearly 80% of patients with PD will develop dementia (i.e., cognitive impairment with functional impairment) during the course of the disease [6–9].
Cognitive impairment is associated with increased disability, reduced quality of life, and distress for pat-ients with PD, as well as their caregivers [2, 10–12]. Detecting cognitive decline in patients with PD, namely, mild cognitive impairment (MCI) or dementia, is often challenging and largely based on recom-mended clinical and cognitive assessments [13]. MCI represents an intermediary cognitive deficit on the spectrum between normal cognition and dementia [2, 14, 15] with symptoms that are not severe eno-ugh to considerably impact the patient’s ability to function normally [6]. Unlike MCI associated with Alzheimer’s disease, which typically presents as an amnestic syndrome, MCI in patients with PD is often associated with a deficit in a single domain such as executive/attentional, visuospatial, and verbal fluency, albeit loss of memory may also occur [16]. PD-associated dementia, often identified within 20 years of the initial PD diagnosis [17], is insidious and progressive and defined as impairment in more than one cognitive domain, representing a decline from pre-morbid level, and deficits are severe enough to impair daily life (social, occupational, or personal care), independent of the impairment ascribable to motor or autonomic symptoms [1]. Clinical features associated with dementia in PD patients inc-lude impairment in attention, memory, executive and visuo-spatial functions, as well as behavioral symptoms such as affective changes, hallucinations, del-usions, apathy, and excessive daytime sleepiness [1].
Published prevalence estimates of cognitive impairment in patients with PD vary widely due to differences in diagnostic criteria, methodology, and study populations. Approximately 25.0% to 30.0% of patients with PD satisfy criteria for MCI following onset of disease [18, 19]. A recent meta-analysis reported that the pooled prevalence of MCI was 40.0% based on 7,053 patients with PD without dementia [20]. The prevalence of PD-associated dementia estimated from community-based studies is 30.0%–40.0%, although the range is wide (10.0%–80.0%) [9, 21]. Overall, the prevalence of dementia in patients with PD increases with age and duration of the disease symptoms [21]. Among industrialized nations, the estimated prevalence of PD rises with age, 1.0% in those older than 60 years and 3.0% in those older than 80 years [3]. For females and males, a meta-analysis reported that incidence rates rose with age from approximately 3.26 to 3.57 per 100,000 person-year at age 40–49 to 103.48 to 258.47 per 100,000 person-years at age 80+, respectively [22]. Due to aging of the United States (US) population, it is estimated that the number of patients with co-existent PD and dementia will triple in US, suggesting that future burden will escalate dramatically [23]. Proposed risk factors for development of PD-associated dementia include rigidity and gait instability, MCI, and the presence of visual hallucinations [24].
The primary goal of this study was to understand the clinical, treatment, and cost characteristics of patients with confirmed PD prior to and following identification of cognitive impairment. Importantly, this study sought to include all possible indicators of cognitive impairment in patients with PD (MCI through dementia). Characterizing patients with PD and cognitive impairment using real-world data sou-rces can help identify disease and treatment patterns, as well as predictors that may allow for early identification and management of this challenging aspect of PD. Overall, this analysis is hypothesis generating and may lead to interventions to reduce risk for cognitive impairment or to modify the impact of cognitive impairment on patients, families, and healthcare resource utilization.
METHODS
Study design and data source
This was a retrospective cohort study utilizing claims data for individuals with PD, enrolled in Humana, Inc.’s Medicare Advantage and Prescription Drug (MAPD) or commercial health plans. MAPD plans are insurance plans offered to US consumers through private companies that cover medical and hospital services included under Medicare Parts A and B and include additional coverage not available in Medicare, typically including a prescription drug plan [25]. Medicaid in the US is a jointly funded federal and state program that assists with medical costs for some individuals with limited income including eligible low-income adults, children, pregnant women, elderly adults, and individuals with disabilities [26]. The study did not include individuals enrolled only in Medicaid but those eligible for both Medicare and Medicaid. Commercial insurance, which is either employer-sponsored or privately purchased, is defined as any type of healthcare ben-efit not provided by the government (Medicare/Medicaid) [27].
PD diagnosis was defined as those individuals with > 2 medical claims with a diagnosis code for PD (332.x, G32.x) or > 1 medical claim with a diagnosis code for PD and > 1 prescription claim for a PD medication between January 1, 2007 to December 31, 2014. Specifically, the database was initially searched to identify patients with PD based on diagnostic co-des in medical claims or on the following PD medica-tions in pharmacy claims: amantadine, apomorphine, benzotropine, bromocriptine, carbidopa, levodopa, carbidopa-levodopa, entacapone, ethopropazine, per-golide, pramipexole, procyclidine, rasagiline, ropi-nirole, rotigotine, safinamide, selegiline, tolcapone, and trihexyphenidyl. Among patients identified with PD, those with or without cognitive impairment for the entire study period were subsequently identified. Cognitive impairment was defined as a diagnosis of MCI, memory loss, dementia, or treatment for dem-entia. Specifically, we identified those newly diagnosed with MCI/memory loss/dementia based on > 1 medical claim or > 1 pharmacy claim for a pre-scription drug used to treat dementia between January 1, 2010 and December 31, 2014. MCI (331.83, G31.84), memory loss (780.93, R41.2, R41.3), and dementia (290.0–290.4, 294.1x, 294.9x, 331.0, 331.1, F02.8, F03.9x, F05.x, F06.8, G30.x, G31.0x) diagnoses were identified using ICD-9 and ICD-10 CM codes. Dementia was also identified based on medication use (acetylcarnitine, donepezil, galanta-mine, memantine, rivastigmine, or tacrine). The date of first medical claim for MCI/memory loss/dementia or the date of first pharmacy claim for a prescription drug used to treat dementia was set as the index date. This index date was required to occur after or at the time of the first PD diagnosis or PD prescription claim date in order to be included in this analysis.
Patients with PD with and without cognitive impairment were matched (1:1) on age (±3 years) and gender. The index date for the control group, PD without cognitive impairment, was based on the index date for the matched patient with cognitive impairment. Patients in all cohorts were 19–89 years of age on the index date and were enrolled in a MAPD or commercial plan for at least 3 years pre- and post-index date.
The study protocol was reviewed and approved by Advarra Institutional Review Board. The study was conducted according to Good Clinical Practice and the Declaration of Helsinki guidelines.
Measures
Demographic characteristics included age, gender, race/ethnicity, and plan type (MAPD or commercial). Among those enrolled in MAPD, we identified individuals eligible for Medicare and Medicaid (dual eligible) and those eligible for low-income subsidy (Medicare beneficiaries with income below 150% of poverty and limited resources are eligible for add-itional premium and cost-share assistance for prescription drugs under the Medicare Part D program).
Comorbidity indices Deyo Charlson Comorbidity index (DCCI) [28–30] and RxRisk V score [31] were used to understand the comorbidity burden for these patients. The prevalence of specific comorbidities, in-cluding myocardial infarction (MI), congestive heart failure (CHF), peripheral vascular disease (PVD), cerebrovascular disease (CVD), chronic obstructive pulmonary disease (COPD), diabetes mellitus with and without complications, renal disease, and cancer, was reported. The number of unique medications for all patients was calculated for each year. Additionally, use of specific classes of medications including PD medications, antipsychotics, antidepressants, and benzodiazepines/psychostimulants was evaluated se-parately. The proportion of patients in long-term care and skilled nursing facilities each year was identified. For healthcare resource use and costs, we calculated the proportion of patients with hospitalization and emergency department (ED) visits and median total median costs per person per year. Total cost is the sum of total medical and pharmacy costs (medications filled in retail or mail order pharmacy), adjusted to 2019 dollars.
Statistical analyses
Descriptive analyses were used to compare demographic characteristics of patients with PD with and without cognitive impairment. Clinical characteristics, including comorbidity indices, prevalence of specific comorbidities, medication use, long-term care, and skilled nursing facility use, were evaluated for the 3 years pre-and post-index (post-cognitive impairment identification). The Wilcoxon signed-rank test was used to assess differences in medians of continuous variables between baseline (pre-index) and post-index periods. The McNemar’s test and the kappa coefficient were used to assess the statistical significance of paired categorical variables and the paired t-test for paired continuous variables. Comparisons between cohorts (PD with and without cognitive impairment) on these clinical characteristics were conducted using Chi-square tests for categorical variables and t-tests and Wilcoxon rank-sum tests for continuous variables as appropriate.
Logistic regression was utilized to identify factors (pre-index demographic and clinical characteristics) associated with cognitive impairment. For potential prognostic factors, univariate logistic regression models were used to estimate the odds ratio (OR) for each of the demographic and clinical factors of interest. Variables with a p-value of < 0.05 in the univariate model were then fit into a multivariate logistic regression model. Variables responsible for multicollinearity were removed from consideration in the final model.
The proportion of patients hospitalized or with an ED visit in the cognitively impaired cohort was compared to the non-cognitively impaired cohort. Median total costs (adjusted to 2019) per year for each of the 3 years pre- and post-index were calculated and compared between the two cohorts.
RESULTS
We identified 2,711 patients with PD and newly identified cognitive impairment (Fig. 1). Among these 2,711 patients with PD, 751 (27.7%) were newly diagnosed with MCI/memory loss only, 1,498 (55.3%) with dementia, and 462 (17.0%) with both MCI/memory loss and dementia.
Demographic and clinical characteristics
Fig. 1
Study Population Identification. MAPD, Medicare Advantage and Prescription Drug; MCI, mild cognitive impairment.
Patients with PD with and without cognitive im-pairment, respectively, were 75.3 years (standard deviation [SD] 8.1) and 75.1 years (SD 8.1) of age, mostly White (84.0% and 85.0%), living in urban areas (69.3% and 67.0%), and largely enrolled in MAPD plans (98.3% and 97.2%) (Table 1). Among patients with PD and cognitive impairment, a greater proportion were eligible for low-income subsidy (22.5% vs. 20.4%) and/or dual eligible (18.0% vs. 15.0%) compared to the non-cognitively impaired cohort (p < 0.05). Of note, during the 3 years prior to the index date, the proportions of patients with CVD were significantly greater in the PD with cognitive impairment cohort (9.3% to 17.6%) vs. the PD without cognitive impairment cohort (7.1% to 9.1%; p < 0.02) but similar for diabetes (with and without complications) between PD patients with and without co-gnitive impairment. In addition, a higher proportion of patients with PVD was observed in the PD with cognitive impairment cohort vs. the PD without cognitive impairment cohort 1 year prior to the index date (16.5% vs. 13.6%, respectively; p = 0.002).
Table 1
Baseline Demographics and Clinical Characteristics Pre- and Post-Diagnosis of Cognitive Impairment Among Patients With Parkinson’s Disease
| Demographic Characteristics N | With Cognitive Impairment n = 2,711 | No Cognitive Impairment n = 2,711 | ||||
| Age in years | ||||||
| Mean (SD) | 75.3 (8.1) | 75.1 (8.1) | ||||
| Median [Q1-Q3] | 76 [71–81] | 76 [71–81] | ||||
| Min, Max | 35, 89 | 36, 89 | ||||
| Age categories, n (%) | ||||||
| <65 | 228 (8.4) | 239 (8.8) | ||||
| 65–74 | 942 (34.8) | 968 (35.7) | ||||
| 75–89 | 1,541 (56.8) | 1,504 (55.5) | ||||
| Sex, n (%) | ||||||
| Female | 1,271 (46.9) | 1,271 (46.9) | ||||
| Male | 1,440 (53.1) | 1,440 (53.1) | ||||
| Race/ethnicity, n (%) | ||||||
| White | 2,279 (84.0) | 2,304 (85.0) | ||||
| Black | 225 (8.3) | 207 (7.6) | ||||
| Other/unknown | 207 (7.6) | 200 (7.4) | ||||
| Population density, n (%) | ||||||
| Urban | 1,879 (69.3) | 1,815 (67.0) | ||||
| Suburban | 536 (19.8) | 591 (21.8) | ||||
| Rural | 225 (8.3) | 235 (8.7) | ||||
| Unknown | 71 (2.6) | 70 (2.6) | ||||
| Region, n (%) | ||||||
| Northeast | 56 (2.1) | 66 (2.4) | ||||
| Midwest | 630 (23.2) | 638 (23.5) | ||||
| South | 1,859 (68.6) | 1,804 (66.5) | ||||
| West | 166 (6.1) | 203 (7.5) | ||||
| Plan type: MAPD or commercial, n (%) | ||||||
| MAPD | 2,665 (98.3) | 2,634 (97.2) | ||||
| Commercial | 46 (1.7) | 77 (2.8) | ||||
| Low-income subsidy eligible, n (%¥) | 599 (22.5) | 538 (20.4) | ||||
| Dual eligibility, n (%¥) | 479 (18.0) | 394 (15.0) | ||||
| Clinical Characteristics of the Cognitively Impaired Cohort | ||||||
| Prior to Index | Post-Index | |||||
| N | Year 3 n = 2,711 | Year 2 n = 2,711 | Year 1 n = 2,711 | Year 1 n = 2,711 | Year 2 n = 2,711 | Year 3 n = 2,711 |
| DCCI | ||||||
| Mean [SD] | 1.3 [1.9] | 1.5 [2.1] | 1.9 [2.3] | 2.4 [2.4] | 2.3 [2.4] | 2.5 [2.6] |
| Median [Q1–Q3] | 1 [0–2] | 1 [0–2] | 1 [0–3] | 2 [1–4] | 2 [0–4] | 2 [0–4] |
| Cumulative mean [SD] | 1.3 [1.9] | 2.1 [2.4] | 2.8 [2.7] | 3.7 [3.0] | 4.3 [3.2] | 4.9 [3.8] |
| Specific comorbidities, n (%) | ||||||
| MI | 134 (4.9) | 216 (8.0) | 297 (11.0) | 370 (13.7) | 419 (15.5) | 482 (17.8) |
| CHF | 199 (7.3) | 332 (12.3) | 471 (17.4) | 619 (22.8) | 723 (26.7) | 845 (31.2) |
| PVD | 269 (9.9) | 480 (17.7) | 677 (25.0) | 917 (33.8) | 1,115 (41.1) | 1,272 (46.9) |
| CVD | 253 (9.3) | 469 (17.3) | 776 (28.6) | 1,076 (39.7) | 1,225 (45.2) | 1,375 (50.7) |
| COPD | 361 (13.3) | 547 (20.2) | 721 (26.6) | 884 (32.6) | 999 (36.9) | 1,106 (40.8) |
| Diabetes w/o complications | 679 (25.1) | 826 (30.5) | 931 (34.4) | 1,030 (38.0) | 1,087 (40.1) | 1,146 (42.3) |
| Diabetes with complications | 260 (9.6) | 361 (13.3) | 460 (17.0) | 556 (20.5) | 633 (23.4) | 697 (25.7) |
| Renal disease | 310 (11.4) | 480 (17.7) | 689 (25.4) | 866 (31.9) | 991 (36.6) | 1,107 (40.8) |
| Cancer | 174 (6.4) | 298 (11.0) | 379 (14.0) | 437 (16.1) | 497 (18.3) | 547 (20.2) |
| RxRisk V score | ||||||
| Mean [SD] | 5.7 [3.3] | 6.0 [3.4] | 6.4 [3.4] | 7.1 [3.5] | 7.0 [3.5] | 6.7 [3.6] |
| Median [Q1–Q3] | 6 [3–8] | 6 [4–8] | 6 [4–9] | 7 [5–9] | 7 [5–9] | 7 [5–9] |
| Number of unique medications (class level) | ||||||
| Mean [SD] | 8.4 [6.2] | 8.9 [6.4] | 10.0 [6.5] | 11.7 [6.7] | 12.0 [6.7] | 12.1 [6.8] |
| Median [Q1–Q3] | 8 [4–12>] | 8 [4–13>] | 9 [5–14] | 11 [7–16] | 11 [7–16] | 11 [7–16] |
| Use of PD medications, n (%) | 1,231 (45.4) | 1,437 (53.0) | 1,796 (66.3) | 1,936 (71.4) | 1,835 (67.7) | 2,365 (87.2) |
| Other drug classes of interest, n (%) | ||||||
| Anti-psychotics | 187 (6.9) | 203 (7.5) | 262 (9.7) | 379 (14.0) | 519 (9.6) | 865 (31.9) |
| Anti-depressants | 957 (35.3) | 1,023 (37.7) | 1,145 (42.2) | 1,263 (46.6) | 2,156 (39.8) | 1,948 (71.9) |
| Benzodiazepines/psychostimulants | 715 (26.4) | 787 (29.0) | 876 (32.3) | 938 (34.6) | 1,592 (29.4) | 1,741 (64.2) |
| Long-term care, n (%) | 60 (2.2) | 90 (3.3) | 193 (7.1) | 338 (12.5) | 364 (13.4) | 480 (17.7) |
| Skilled nursing facility, n (%) | 81 (3.0) | 120 (4.4) | 216 (8.0) | 337 (12.4) | 257 (9.5) | 315 (11.6) |
DCCI scores and prevalence of comorbidities represent the prevalence in each year based on presence of a claim with the diagnosis code. CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; DCCI, Deyo Charlson Comorbidity index; MAPD, Medicare Advantage and Prescription Drug Plan; Max, maximum; Min, minimum; MI, myocardial infarction; PD, Parkinson’s disease; PVD, peripheral vascular disease; Q, quartile; SD, standard deviation; w/o = without.¥ Calculated as a percentage of individuals enrolled in MAPD.
The DCCI and RxRisk V score comorbidity indices for patients with cognitive impairment inc-reased during the 6 years, especially after the index date (Table 1). The most common comorbidities included CVD, PVD, diabetes mellitus without complications, COPD, and renal disease. For these patients, the use of medications for PD increased from 45.4% during Year 3 pre-index to 87.2% in Year 3 post-index, whereas the proportion of patients treated with antidepressants increased from 35.3% to 71.9% and benzodiazepines/psychostimulants increased from 26.4% to 64.2% over the same time period. For patients with PD and cognitive impairment, the proportion of patients in a long-term care facility increased from 7.1% during the year prior to index date to 12.5% during the year immediately following index. Similarly, the proportion of patients with cognitive impairment in a skilled nursing facility increased from 8.0% during the year prior to index to 12.4% during the year immediately following index. By Year 3 post-index, 17.7% of patients with cognitive impairment were in a long-term care facility and 11.6% of patients were in a skilled nursing facility. Table 2 presents the clinical characteristics for the PD plus cognitive impairment cohort compared with control cohort (no cognitive impairment) at 1-, 2- and 3-years prior to index.
Table 2
Clinical Characteristics for the PD plus Cognitive Impairment Cohort Compared With Control Cohort (No Cognitive Impairment)
| Year 3 prior to Index | Year 2 prior to Index | 1 Year Prior to Index | |||||||
| MCI/Memory Loss/Dementia | Matched Controls | p | MCI/Memory Loss/Dementia | Matched Controls | p | MCI/Memory Loss/Dementia | Matched Controls | p | |
| N | n = 2,711 | n = 2,711 | n = 2,711 | n = 2,711 | n = 2,711 | n = 2,711 | |||
| DCI | |||||||||
| Mean [SD] | 1.3 [1.9] | 1.3 [1.8] | 0.533 | 1.5 [2.1] | 1.5 [1.9] | 0.481 | 1.9 [2.3] | 1.6 [2.0] | <0.001 |
| Median [Q1–Q3] | 1 [0–2] | 1 [0–2] | 1 [0–2] | 1 [0–2] | 1 [0–3] | 1 [0–3] | |||
| Specific Comorbidities, n (%) | |||||||||
| MI | 134 (4.9) | 121 (4.5) | 0.404 | 138 (5.1) | 127 (4.7) | 0.488 | 159 (5.9) | 135 (5.0) | 0.15 |
| CHF | 199 (7.3) | 186 (6.9) | 0.492 | 250 (9.2) | 224 (8.3) | 0.211 | 318 (11.7) | 260 (9.6) | 0.011 |
| PVD | 269 (9.9) | 293 (10.8) | 0.285 | 353 (13.0) | 335 (12.4) | 0.463 | 448 (16.5) | 368 (13.6) | 0.002 |
| CVD | 253 (9.3) | 193 (7.1) | 0.003 | 287 (10.6) | 236 (8.7) | 0.019 | 478 (17.6) | 248 (9.1) | <0.001 |
| COPD | 361 (13.3) | 348 (12.8) | 0.600 | 423 (15.6) | 418 (15.4) | 0.851 | 499 (18.4) | 427 (15.6) | 0.009 |
| Diabetes w/o complications | 679 (25.1) | 699 (25.8) | 0.533 | 699 (25.8) | 707 (26.1) | 0.804 | 763 (28.1) | 727 (26.8) | 0.273 |
| Diabetes with complications | 260 (9.6) | 262 (9.7) | 0.927 | 288 (10.6) | 277 (10.2) | 0.625 | 345 (12.7) | 318 (11.7) | 0.263 |
| Renal disease | 310 (11.4) | 303 (11.2) | 0.764 | 377 (13.9) | 395 (14.5) | 0.484 | 515 (19.0) | 469 (17.3) | 0.105 |
| Cancer | 174 (6.4) | 179 (6.6) | 0.783 | 214 (7.9) | 211 (7.8) | 0.879 | 230 (8.5) | 216 (8.0) | 0.489 |
| RxRisk V score | |||||||||
| Mean [SD] | 5.7 [3.3] | 5.3 [3.2] | 0.006 | 6.0 [3.4] | 5.5 [3.2] | 0.002 | 6.4 [3.4] | 5.8 [3.2] | 0.001 |
| Median [Q1–Q3] | 6 [3–8] | 5 [3–7] | 6 [4–8] | 5 [3–8] | 6 [4–9] | 6 [4–8] | |||
| Number of unique medications (class level) | |||||||||
| Mean [SD] | 8.4 [6.2] | 7.6 [5.6] | <0.001 | 8.9 [6.4] | 8.1 [5.7] | <0.001 | 10.0 [6.5] | 8.8 [5.9] | <0.001 |
| Median [Q1–Q3] | 8 [4–12] | 7 [4–11] | 8 [4–13] | 8 [4–11] | 9 [5–14>] | 8 [5–12] | |||
| Use of PD medications, n (%) | 1,231 (45.4) | 958 (35.3) | <0.001 | 1,437 (53.0) | 1,128 (41.6) | <0.001 | 1,796 (66.3) | 1,319 (48.7) | <0.001 |
| Number of unique PD medication classes | |||||||||
| Mean [SD] | 0.60 [0.87] | 0.46 [0.77] | <0.001 | 0.71 [0.90] | 0.55 [>0.83] | <0.001 | 0.90 [0.93] | 0.65 [0.85] | <0.001 |
| Median [Q1–Q3] | 0 [0–1] | 0 [0–1] | 0 [0–1] | 0 [0–1] | 1 [0–1] | 0 [0–1] | |||
| Long-term care, n (%) | 60 (2.2) | 38 (1.4) | 0.025 | 90 (3.3) | 47 (1.7) | <0.001 | 193 (7.1) | 71 (2.6) | <0.001 |
| Skilled nursing facility, n (%) | 81 (3.0) | 62 (2.3) | 0.107 | 120 (4.4) | 69 (2.6) | <0.001 | 216 (8.0) | 95 (3.5) | <0.001 |
Note: Cell sizes less than 10 were suppressed. Where no comparisons could be made due to 1 or more cells being suppressed, p-values were removed (i.e., n/a). **matched on age (+/- 3 y) and gender CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; DCI, Deyo-Charlson Comorbidity index,; MI, myocardial infarction; PD, Parkinson’s disease; PVD, peripheral vascular disease; Q, quartile; SD, standard deviation; w/o, without.
Predictors of a new cognitive impairment diagnosis
Table 3 depicts the results of the logistic regression evaluating factors associated with the identification of cognitive impairment. For the overall population, diagnosis of CVD (OR = 1.24; 95% confidence interval [CI] 1.01–1.51; p = 0.036) and use of PD medication (OR = 1.46; 95% CI 1.30–1.63; p < 0.001) increased the odds of cognitive impairment by 24.0% and 46.0%, respectively. Enrollment in an MAPD plan increased the odds of having a cognitive impairment by 60.0% compared to those enrolled in a commercial plan (OR = 1.60; 95% CI 1.10–2.33; p = 0.013). Furthermore, individuals eligible for both Medicare and Medicaid had a 36.0% higher chance to have cognitive impairment than individuals who were not dual eligible (OR = 1.36; 95% CI 1.07–1.72; p = 0.012).
Table 3
Logistic Regression of Factors Associated With a New Diagnosis of Cognitive Impairment in Patients With Parkinson’s Disease
| Parameter | Odds Ratio (95% Confidence Interval) | Pr>ChiSq |
| CVD | 1.24 (1.01–1.51) | 0.036 |
| RxRisk V score | 1.01 (0.98–1.03) | 0.640 |
| Number of unique medications (class level) | 1.01 (1.00–1.03) | 0.127 |
| Use of Parkinson’s disease medications | 1.46 (1.30–1.63) | <0.0001 |
| Plan type (MAPD vs. commercial) | 1.60 (1.10–2.33) | 0.013 |
| Low-income subsidy eligible | 0.84 (0.68–1.05) | 0.126 |
| Dual eligibility (Medicare and Medicaid) | 1.36 (1.07–1.72) | 0.012 |
| Long-term care | 1.34 (0.88–2.03) | 0.173 |
ChiSq, Chi square; MAPD, Medicare Advantage and Prescription Drug plan; Pr, probability.
Healthcare resource utilization and cost
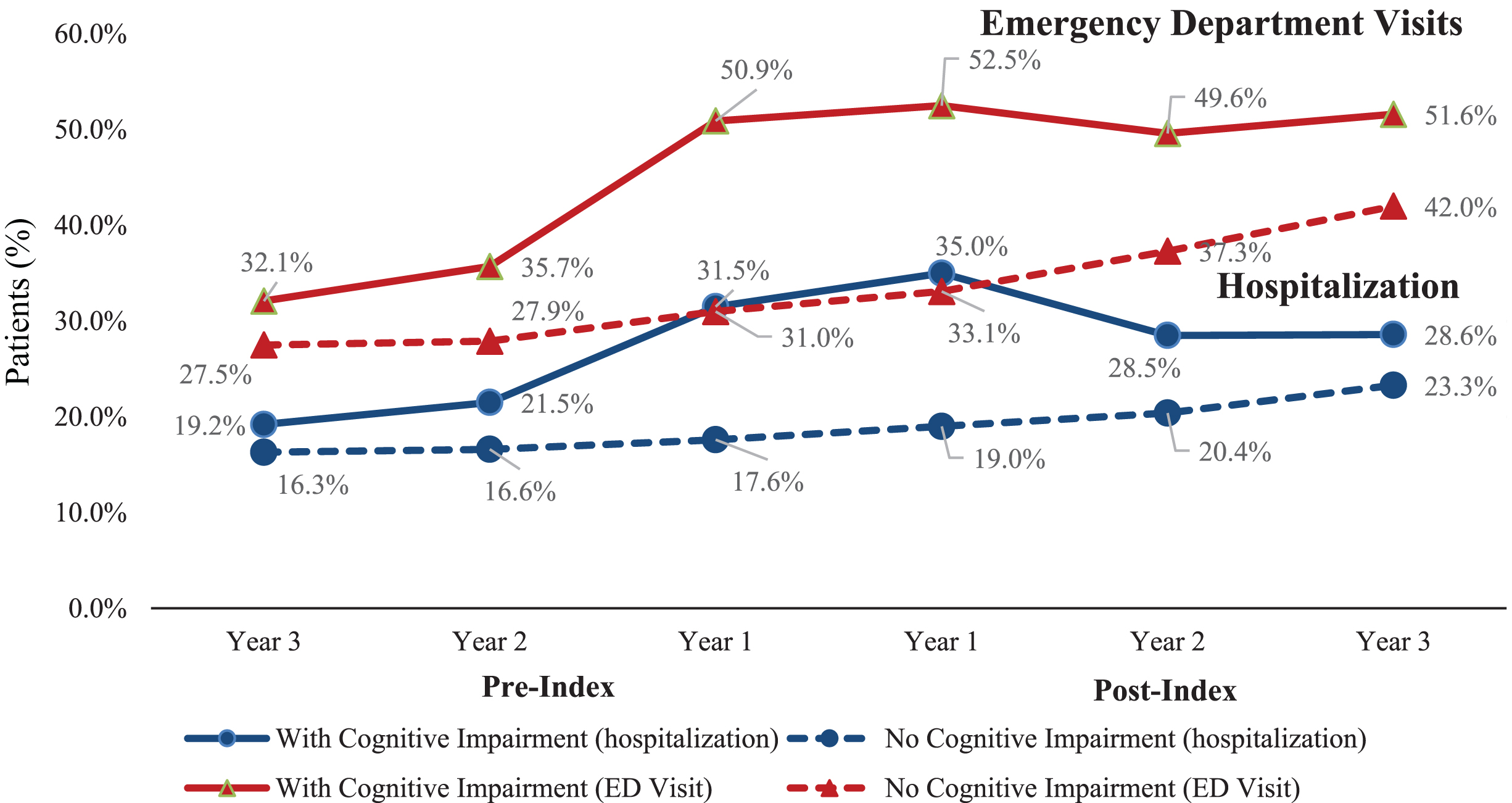
The cohort with newly identified cognitive impairment had a higher proportion of patients with at least one hospitalization than the cohort without cognitive impairment (Fig. 2). The proportion of patients with cognitive impairment and ED visits increased especially during the year prior to (50.9%) and following index date (52.5%) compared to those without cognitive impairment (31.0% and 33.1%, respectively). However, the proportion of patients with cognitive impairment and ED visits declined slightly by Years 2 and 3 post-index (49.6% and 51.6%, respectively). Similar results were observed for proportion of patients with hospitalization. The proportion of patients with hospitalization in the cohort with cognitive impairment was nearly 2-fold greater than that of patients without cognitive impairment during the year prior to (31.5% vs. 17.6%) and following index (35.0% vs. 19.0%) (Fig. 2). The cohort without cognitive impairment saw a steady increase in proportion of patients with a hospitalization or ED visit from Year 3 pre-index to Year 3 post-index.
Fig. 2
Proportion of Patients with Hospitalization or Emergency Department Visits Prior to and Following Cognitive Impairment Diagnosis. ED visit, Emergency department visit.
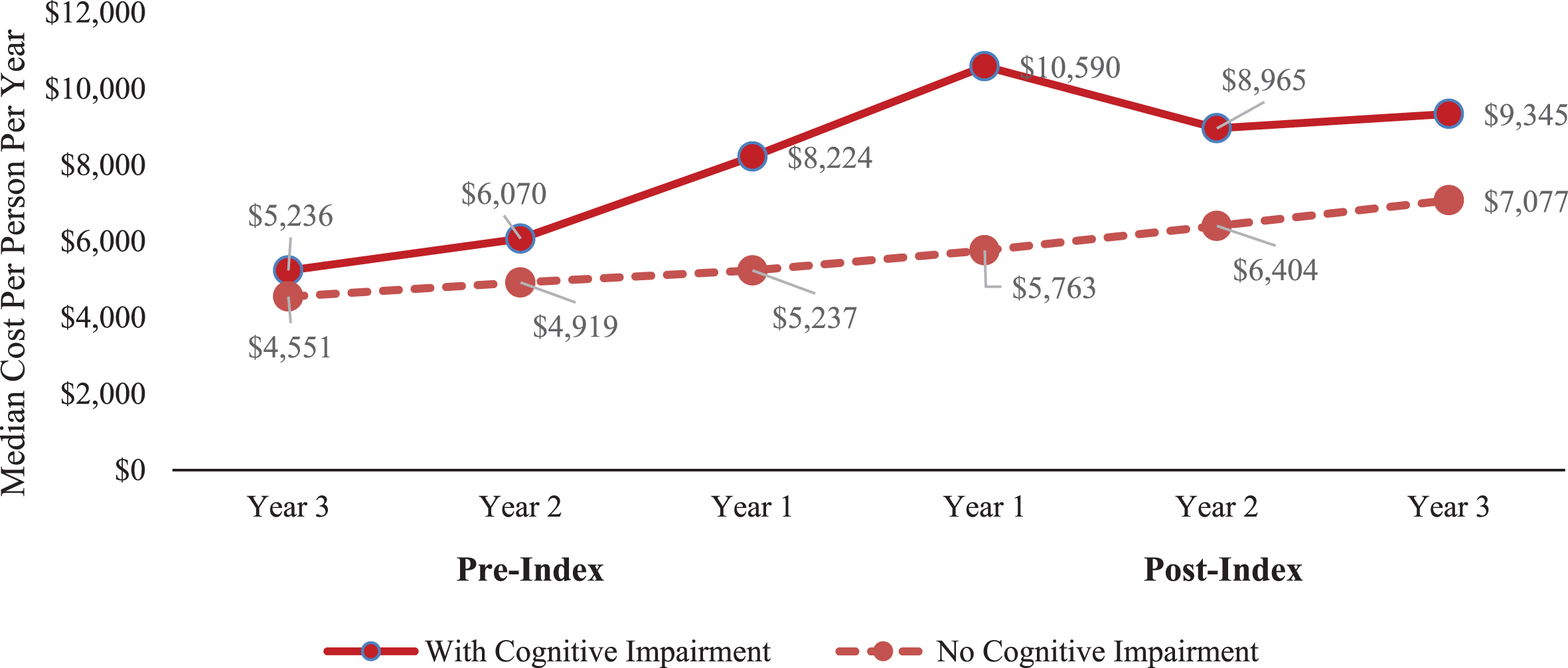
The cognitively impaired cohort had higher median total healthcare costs per patient compared with the non-cognitively impaired cohort for each year pre- and post-index (Fig. 3). For patients with a cognitive impairment, costs peaked during Year 1 post-index ($10,590) and declined during Years 2 ($8,965) and 3 post-index ($9,345), whereas the non-cognitively impaired cohort showed a much slower increase in costs over the observation period ($5,237 Year 1 pre-index, $5,763 Year 1 post-index, and $7,077 Year 3 post-index).
Fig. 3
Median Total Costs for Each Cohort Prior to and Following Cognitive Impairment Diagnosis. Note: In 2019 dollars.
DISCUSSION
This retrospective cohort study utilizing claims data from a national health plan provides an in-depth examination of demographic and clinical characteristics, healthcare resource utilization, and costs during the 3-year span prior to and following identification of cognitive impairment among 2,711 individuals with PD compared to individuals with PD who did not develop cognitive impairment. Overall, our study findings suggest that even before a cognitive impairment diagnosis, patients with PD had increased comorbidities (i.e., CVD), need for long-term care or skilled nursing facility care, increased healthcare resource utilization (hospitalization and ED visits), and increased costs.
Among patients with PD who developed cognitive impairment, comorbidity burden increased during the 3 years prior to identification of cognitive impairment, especially the year prior, and continued to increase after identification. The increased DCCI and RxRisk V scores parallel the increasing proportions of patients with comorbidities closer to the diagnosis of one or more of these cognitive conditions. Among the specified comorbidities, notably, a greater proportion of patients had cardiovascular-related diagnoses (MI, CHF, CVD, and PVD). This aligns with reports of increased heart disease-related risks among patients with PD and cognitive impairment or dementia [32, 33].
Among the comorbidities, CVD was significantly associated with a diagnosis of cognitive impairment (i.e., 24.0% greater odds of cognitive impairment). In the literature, CVD has been consistently associated with dementia [34, 35], and our findings support the potential role of CVD in the development of cognitive impairment in PD. The increase in CVD and other select comorbidities in the year prior to the diagnosis of cognitive impairment-related to PD could also represent surveillance bias. That is, patients beginning to experience cognitive impairment symptoms may have more healthcare provider visits, which in turn may result in additional diagnostic tests/scans that uncover new diagnoses or vice versa. In addition, this analysis cannot imply causality, and it is plausible that the increasing severity of cognitive dysfunction over time may play a role in a patient’s ability to manage chronic conditions and/or engage in health maintenance, resulting in the development of new physical ailments or the exacerbation of existing conditions. The increasing number of comorbidities in this cognitively impaired population with PD may indeed be greater with MCI or dementia and mandates clinical practice to focus more on risk factor management of these patients, as well as preventive care with appropriate medical support. In addition, earlier recognition of cognitive impairment in patients with PD may alter the management of care by the physician and caregiver to provide closer oversight of treatments and medications, thereby potentially reducing healthcare resource use such as ED visits.
In this study, admission to either long-term care or a skilled nursing facility increased each year prior to identification of cognitive impairment and was almost doubled by the third year after identification. These findings likely reflect the increasing care needs of these patients as the disease progresses, especially in those with dementia, which was high in our sample population. However, the increased admissions to long-term care or skilled nursing facilities may also be partly due to the increase of other comorbidities (e.g., CVD). Safarpour et al. reported that a quarter of Medicare-enrolled patients with PD and dementia resided in a long-term care facility and dementia was among the strongest predictors of use of long-term care facilities [36].
Cognitive impairment was associated with a gre-ater proportion of patients prescribed medications for PD, such as levodopa, compared to non-cognitively impaired cohort. While we did not evaluate the specific regimens and their respective doses, it has been suggested that higher daily doses of levodopa may be an independent risk factor for the development of dementia [37]. In contrast, several studies indicate that the use of levodopa is not associated with, or may even prevent, cognitive decline in pat-ients with PD [38, 39]. Greater healthcare utilization, such as increased hospitalizations/ED visits and long-term/skilled nursing facility care, due to comorbidities may account for more intensive interactions with prescribers and greater likelihood of being treated. The association of increased PD medications may not be related to a new diagnosis of cognitive impairment but rather other progressive disease signs and symptoms. It is also possible that patients with PD and cognitive impairment may have more severe disease and require more treatment. Published literature on the influence of PD medications on dementia risk is mixed, and further research is needed to elucidate the apparent association between PD treatment and cognitive impairment.
Enrollment in MAPD and dual eligibility (Medicare and Medicaid) were associated with increased detection of cognitive impairment. The risk associa-ted with MAPD enrollment may be due to the fact that > 90% patients with these diagnoses were enro-lled in MAPD in this study. Additionally, it may also be reflective of age and its influence on cognitive impairment risk. It is notable that patients with PD eligible for Medicare and Medicaid had a 36.0% greater chance of being diagnosed with a cognitive impairment. With the increased need for long-term care services and disabilities among patients with dementia, Medicaid is often needed to cover gaps in coverage with Medicare. This dual eligibility also could reflect the impact of socioeconomic factors on dementia risk and the impact of other illnesses that lead to disability.
Finally, these study findings suggest that during each of the 3 years prior to and following a cognitive impairment diagnosis, patients had increased hospitalizations and ED visits and higher costs compared to patients in the non-cognitively impaired cohort. The proportion of patients with hospitalization or ED visit peaked during the year pre- and post-identification of cognitive impairment. Healthcare costs for patients with PD and newly identified with cognitive impairment peaked during the year immediately following the identification and declined slightly during Years 2 and 3 post-identification. Overall, costs for patients with cognitive impairment were higher than those of the non-cognitively impaired cohort. Approaches to identify those at highest risk for cognitive impairment may allow interventions to minimize healthcare utilization and costs prior to dementia onset. Several studies have reported similar trends in healthcare resource utilization and costs in newly diagnosed Alzheimer’s patients [40–43]. Albrecht and coworkers reported that HCRU was highest in the outpatient setting, which in part was related to increased neuropsychiatric comorbidities during the year prior to dementia diagnosis [42]. Our study corroborates the findings of Desai et al. who reported increased costs secondary to all-cause hospitalizations and all-cause ED visits compared to the control group each year preceding a diagnosis of Alzheimer’s disease [43]. Marešová et al. reported costs in patients with PD and dementia to be 3 times higher than those in patients with PD and no dementia [44]. In the current study, costs in the cognitive impairment cohorts were 15% to 45% higher than the non-cognitively impaired cohort. This observation may be due to differences in the population, duration of disease (newly diagnosed), methodology, and the healthcare system.
Limitations
The findings of these analyses need to be interpreted within the context of the study limitations described below. Limitations common to studies using administrative claims data, such as potential errors in coding, omissions in claims data, and unmeasured clinical, economic, or behavioral factors, may impact the results. This study utilized data with a Medicare-rich member population, thus the results may not be generalizable, particularly for younger patients in commercial plans. However, given the lower risk of PD and cognitive impairment for these younger individuals, this is unlikely to have a significant impact in the overall findings. No causal inference can be ascertained from this study since it was a non-interventional study using existing data. Accordingly, the root cause of cognitive impairment could not be addressed in this study. Yet, it is acknowledged that 1) cognitive impairment is a common sequelae in PD, and 2) etiology of cognitive impairment, as with many forms of dementias, may be multifactorial, including vascular and other risk factors as well as primary PD pathology.
The definitions of PD and cognitive impairment used in this study, while imperfect, were an attempt to identify the populations of interest. It is possible that patients with only 1 medical claim with a diagnosis code for PD and 1 prescription claim for a PD medication were misclassified as having PD. It is also recognized that PD medications were not completely captured and only included representative medications for each class. Likewise, it is also possible that the definition used to identify cognitive impairment in PD patients either missed some patients who had cognitive impairment or classified some patients with cognitive impairment who did not have true cognitive impairment issues. This analysis employed a lower threshold for identifying cognitive impairment for several reasons: 1) In a patient with PD, new onset cognitive impairment is likely to be relevant as they are at higher risk for cognitive impairment, and therefore, the finding is less likely to be a false negative despite being less strict; 2) Providers may be less likely to diagnose cognitive impairment or prescribe cognitive impairment medications in a PD patient for a variety of reasons; therefore, stricter criteria may have missed individuals with cognitive impairment; and 3) Since PD identification had to predate cognitive impairment in these analyses, we were interested in changes up to the initial cognitive impairment diagnosis and used the first cognitive impairment diagnosis to understand clinical and HCRU prior to and at the time of cognitive impairment diagnosis.
Lack of medical records makes it difficult to fully understand diagnosis and treatment choices and reasons behind physicians’ decisions. Although the study data were drawn from a large national health plan with persons enrolled throughout the US, the results may not be generalizable to the overall US population or to specific subpopulations in certain geographic regions. Moreover, the results may not be generalizable to all beneficiaries due to differences in benefit structures of MAPD vs. non-MAPD health plans. Finally, a potential bias is that this analysis was limited to PD patients with 6 years of follow-up in the database; thus, patients who disenrolled prior to 6 years may be different from those included in the analysis.
Despite these limitations, to the best of our knowledge, this is the first study to longitudinally examine the characteristics of patients with PD before and after being newly diagnosed with cognitive impairment. The findings from this study should be replicated using additional data sources that include other populations of patients with PD.
CONCLUSION
In patients with PD who were newly diagnosed with cognitive impairment, comorbidity burden and use of long-term care or skilled nursing facilities increased over the years, especially the year prior to the cognitive impairment diagnosis. Furthermore, HCRU and total costs peaked 1-year pre- and post-cognitive impairment identification. These data coupled with recommendations for annual screening for cognitive impairment in PD [13] support the early diagnosis and management of cognitive impairment in order to optimize care for patients and their caregivers.
ACKNOWLEDGMENTS
Eli Lilly and Company and Humana, Inc. collaboratively designed the study and interpreted results. Data analyses were performed by Humana. Writing support was provided by Teresa Tartaglione, PharmD (Synchrogenix, a Certara Company, Wilmington, Delaware).
CONFLICT OF INTEREST
JC, KB, and LM are employees and minor stockholders for Eli Lilly and Company; RN, EAF, and NP are employees of Humana Healthcare Research, Inc.; and TC is an employee of Humana, Inc.
REFERENCES
[1] | Emre M , Aarsland D , Brown R , Burn DJ , Duyckaerts C , Mizuno Y , Broe GA , Cummings J , Dickson DW , Gauthier S , Goldman J , Goetz C , Korczyn A , Lees A , Levy R , Litvan I , McKeith I , Olanow W , Poewe W , Quinn N , Sampaio C , Tolosa E , Dubois B ((2007) ) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22: , 1689–1707. |
[2] | Wada K , Nakashima K ((2012) ) Mild cognitive impairment in Parkinson’s disease. Brain Nerve 64: , 1365–1375. |
[3] | Balestrino R , Schapira AHV ((2020) ) Parkinson disease. Eur J Neurol 27: , 27–42. |
[4] | Keener AM , Paul KC , Folle A , Bronstein JM , Ritz B ((2018) ) Cognitive impairment and mortality in a population-based Parkinson’s disease cohort. J Parkinsons Dis 8: , 353–362. |
[5] | Shiraishi T , Murakami H , Iguchi Y ((2019) ) Diagnosis and treatment of cognitive impairment in Parkinson’s disease. Brain Nerve 71: , 869–874. |
[6] | Martinez-Horta S , Kulisevsky J ((2019) ) Mild cognitive impairment in Parkinson’s disease. J Neural Transm (Vienna) 126: , 897–904. |
[7] | Kjeldsen PL , Damholdt MF ((2019) ) Subjective cognitive complaints in patients with Parkinson’s disease. Acta Neurol Scand 140: , 375–389. |
[8] | Postuma RB , Berg D , Stern M , Poewe W , Olanow CW , Oertel W , Obeso J , Marek K , Litvan I , Lang AE , Halliday G , Goetz CG , Gasser T , Dubois B , Chan P , Bloem BR , Adler CH , Deuschl G ((2015) ) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30: , 1591–1601. |
[9] | Aarsland D , Perry R , Brown A , Larsen JP , Ballard C ((2005) ) Neuropathology of dementia in Parkinson’s disease: A prospective, community-based study. Ann Neurol 58: , 773–776. |
[10] | Vatter S , Stanmore E , Clare L , McDonald KR , McCormick SA , Leroi I ((2020) ) Care burden and mental ill health in spouses of people with Parkinson disease dementia and lewy body dementia. J Geriatr Psychiatry Neurol 33: , 3–14. |
[11] | Hermanowicz N , Jones SA , Hauser RA ((2019) ) Impact of non-motor symptoms in Parkinson’s disease: A PMD Alliance survey. Neuropsychiatr Dis Treat 15: , 2205–2212. |
[12] | Aarsland D , Larsen JP , Karlsen K , Lim NG , Tandberg E ((1999) ) Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry 14: , 866–874. |
[13] | Cheng EM , Tonn S , Swain-Eng R , Factor SA , Weiner WJ , Bever CT , American Academy of Neurology Parkinson Disease Measure Development Panel ((2010) ) Quality improvement in neurology: AAN Parkinson disease quality measures: Report of the Quality Measurement and Reporting Subcommittee of the American Academy of Neurology. Neurology 75: , 2021–2027. |
[14] | Galtier I , Nieto A , Lorenzo JN , Barroso J ((2016) ) Mild cognitive impairment in Parkinson’s disease: Diagnosis and progression to dementia. J Clin Exp Neuropsychol 38: , 40–50. |
[15] | Geurtsen GJ , Hoogland J , Goldman JG , Schmand BA , Tröster AI , Burn DJ , Litvan I ; MDS Study Group on the Validation of PD-MCI Criteria ((2014) ) Parkinson’s disease mild cognitive impairment: Application and validation of the criteria. J Parkinsons Dis 4: , 131–137. |
[16] | Aarsland D , Bronnick K , Fladby T ((2011) ) Mild cognitive impairment in Parkinson’s disease. Curr Neurol Neurosci Rep 11: , 371–378. |
[17] | Hely MA , Reid WG , Adena MA , Halliday GM , Morris JG ((2008) ) The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov Disord 23: , 837–844. |
[18] | Litvan I , Aarsland D , Adler CH , Goldman JG , Kulisevsky J , Mollenhauer B , Rodriguez-Oroz MC , Tröster AI , Weintraub D ((2011) ) MDS Task Force on mild cognitive impairment in Parkinson’s disease: Critical review of PD-MCI. Mov Disord 15: , 1814–1824. |
[19] | Aarsland D , Bronnick K , Williams-Gray C , Weintraub D , Marder K , Kulisevsky J , Burn D , Barone P , Pagonabarraga J , Allcock L , Santangelo G , Foltynie T , Janvin C , Larsen JP , Barker RA , Emre M ((2010) ) Mild cognitive impairment in Parkinson disease: A multicenter pooled analysis. Neurology 75: , 1062–1069. |
[20] | Baiano C , Barone P , Trojano L , Santangelo G ((2020) ) Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: A meta-analysis. Mov Disord 35: , 45–54. |
[21] | Aarsland D , Kurz MW ((2010) ) The epidemiology of dementia associated with Parkinson disease. J Neurol Sci 289: , 18–22. |
[22] | Hirsch L , Jette Nathalie , Frolkis A , Steeves T , Pringsheim T ((2016) ) The incidence of Parkinson’s disease: A systematic review and meta-analysis. Neuroepidemiology 4: , 292–300. |
[23] | Savica R , Grossardt BR , Rocca WA , Bower JH ((2018) ) Parkinson disease with and without dementia: A prevalence study and future projections. Mov Disord 33: , 537–543. |
[24] | Goetz CG , Emre M , Dubois B ((2008) ) Parkinson’s disease dementia: Definitions, guidelines, and research perspectives in diagnosis. Ann Neurol 64: , S81–S92. |
[25] | What is Medicare Advantage. https://www.medicare.gov/Pubs/pdf/11474.pdf, Accessed on October 15, 2020. |
[26] | Medicaid. https://www.medicaid.gov/medicaid/index.html, Accessed on 15 October, 2020. |
[27] | What is commercial insurance and howdoes itwork? https://share.upmc.com/2018/06/what-is-commercial-health-insurance/, Accessed on October 15, 2020. |
[28] | Deyo RA , Cherkin DC , Ciol MA ((1992) ) Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45: , 613–619. |
[29] | Quan H , Sundararajan V , Halfon P , Fong A , Burnand B , Luthi JC , Saunders LD , Beck CA , Feasby TE , Ghali WA ((2005) ) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43: , 1130–1139. |
[30] | Klabunde CN , Potosky AL , Legler JM , Warren JL ((2000) ) Development of a comorbidity index using physician claims data. J Clin Epidemiol 53: , 1258–1267. |
[31] | Sloan KL , Sales AE , Liu CF , Fishman P , Nichol P , Suzuki NT , Sharp ND ((2003) ) Construction and characteristics of the RxRisk-V: A VA-adapted pharmacy-based case-mix instrument. Med Care 41: , 761–764. |
[32] | Jones JD , Tanner JJ , Okun M , Price CC , Bowers D ((2017) ) Are Parkinson’s patients more vulnerable to the effects of cardiovascular risk: A neuroimaging and neuropsychological study. J Int Neuropsychol Soc 23: , 322–331. |
[33] | Pilotto A , Turrone R , Liepelt-Scarfone I , Bianchi M , Poli L , Borroni B , Alberici A , Premi E , Formenti A , Bigni B , Cosseddu M , Cottini E , Berg D , Padovani A ((2016) ) Vascular risk factors and cognition in Parkinson’s disease. J Alzheimers Dis 51: , 563–570. |
[34] | Gorelick PB , Scuteri A , Black SE , Decarli C , Greenberg SM , Iadecola C , Launer LJ , Laurent S , Lopez OL , Nyenhuis D , Petersen RC , Schneider JA , Tzourio C , Arnett DK , Bennett DA , Chui HC , Higashida RT , Lindquist R , Nilsson PM , Roman GC , Sellke FW , Seshadri S ; American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia ((2011) ) Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42: , 2672–2713. |
[35] | Raz L , Knoefel J , Bhaskar K ((2016) ) The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab 36: , 172–186. |
[36] | Safarpour D , Thibault DP , DeSanto CL , Boyd CM , Dorsey ER , Racette BA , Willis AW ((2015) ) Nursing home and end-of-life care in Parkinson disease. Neurology 85: , 413–419. |
[37] | Zhu K , van Hilten JJ , Marinus J ((2014) ) Predictors of dementia in Parkinson’s disease; findings from a 5-year prospective study using the SCOPA-COG. Parkinsonism Relat Disord 20: , 980–985. |
[38] | Ikeda M , Kataoka H , Ueno S ((2017) ) Can levodopa prevent cognitive decline in patients with Parkinson’s disease? Am J Neurodegener Dis 6: , 9–14. |
[39] | Molloy SA , Rowan EN , O’Brien JT , McKeith IG , Wesnes K , Burn DJ ((2006) ) Effect of levodopa on cognitive function in Parkinson’s disease with and without dementia and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 77: , 1323–1328. |
[40] | Suehs BT , Davis CD , Alvir J , van Amerongen D , Patel NC , Joshi AV , Faison WE , Shah SN ((2013) ) The clinical and economic burden of newly diagnosed Alzheimer’s disease in a Medicare advantage population. Am J Alzheimers Dis Other Demen 28: , 384–392. |
[41] | Nair R , Haynes VS , Siadaty M , Patel NC , Fleisher AS , Van Amerongen D , Witte MM , Downing AM , Fernandez LAH , Saundankar V , Ball DE ((2018) ) Retrospective assessment of patient characteristics and healthcare costs prior to a diagnosis of Alzheimer’s disease in an administrative claims database. BMC Geriatr 18: , 243. |
[42] | Albrecht JS , Hanna M , Kim D , Perfetto EM ((2018) ) Increased health care utilization in dementia subtypes before diagnosis. Alzheimer Dis Assoc Disord 32: , 326–332. |
[43] | Desai U , Kirson NY , Ye W , Mehta NR , Wen J , Andrews JS ((2019) ) Trends in health service use and potentially avoidable hospitalizations before Alzheimer’s disease diagnosis: A matched, retrospective study of US Medicare beneficiaries. Alzheimers Dement (Amst) 11: , 125–135. |
[44] | Marešová P , Klimova B , Kuca K ((2017) ) Medical and non-medical costs of Parkinson disease - comparison of Europe, USA, Asia and Australia. Ceska Slov Farm 66: , 3–8. |




